Abstract
Background:
Air pollution has been linked to gestational diabetes mellitus (GDM) but no studies have evaluated impact of preconception and early pregnancy air pollution exposures on GDM risk.
Methods:
Electronic medical records provided data on 219,952 singleton deliveries to mothers with (n=11,334) and without GDM (n=208,618). Average maternal exposures to particulate matter (PM) <2.5 microns (PM2.5) and PM2.5 constituents, PM <10 microns (PM10), nitrogen oxides (NOx), carbon monoxide, sulfur dioxide (SO2) and ozone (O3) were estimated for the 3-month preconception window, first trimester, and gestational weeks 1-24 based on modified Community Multiscale Air Quality models for delivery hospital referral regions. Binary regression models with robust standard errors estimated relative risks (RR) for GDM per interquartile range (IQR) increase in pollutant concentrations adjusted for study site, maternal age and race/ethnicity.
Results:
Preconception maternal exposure to NOX (RR=1.09, 95% CI: 1.04, 1.13) and SO2 (RR=1.05, 1.01, 1.09) were associated with increased risk of subsequent GDM and risk estimates remained elevated for first trimester exposure. Preconception O3 was associated with lower risk of subsequent GDM (RR=0.93, 0.90, 0.96) but risks increased later in pregnancy.
Conclusion:
Maternal exposures to NOX and SO2 preconception and during the first few weeks of pregnancy were associated with increased GDM risk. O3 appeared to increase GDM risk in association with mid-pregnancy exposure but not in earlier time windows. These common exposures merit further investigation.
Keywords: preconception, pregnancy, air pollution, gestational diabetes
1. Introduction
Exposure to air pollution during pregnancy has been shown to adversely impact birth outcomes (Shah and Balkhair 2011; Sram et al. 2005) and may also affect maternal health during pregnancy and over the life course (Kampa and Castanas 2008; Basile and Bloch 2012). Epidemiological studies have linked air pollution to type 2 diabetes prevalence,(Brook et al. 2008) incidence (Kramer et al. 2010; Coogan et al. 2012) and mortality in non-pregnant women (Raaschou-Nielsen et al. 2013). Type 2 diabetes and GDM share some common risk factors and both are characterized by insulin resistance and impaired insulin secretion (Ben-Haroush et al. 2004). Approximately 50% of women experiencing GDM will develop type 2 diabetes within 5 years of the affected pregnancy, with pregnancy thought to unmask underlying beta cell dysfunction in susceptible women (American Diabetes Association 2003). Although the biologic mechanisms that link air pollution to the development of diabetes are still unclear, one pathway may be systemic inflammation that results in metabolic dysfunction (Rajagopalan and Brook 2012). Furthermore, obesity and overnutrition, risk factors for the development of diabetes, may render women more susceptible to the effects of air pollution (Sun et al. 2009) and also promote the development of GDM during pregnancy.
Recent studies have linked air pollutants to GDM (Malmqvist et al. 2013) and abnormal glucose tolerance during pregnancy (Fleisch et al. 2014) but exposure assessments were limited to one or two pollutants occurring during the first and/or second trimester of pregnancy. The objectives of the present study were to investigate the association between criteria air pollutants regulated by the US Environmental Protection Agency (EPA) and the risk of GDM in a contemporary obstetric cohort in the US. Exposure estimates were based on modified Community Multiscale Air Quality models that allowed for complete coverage of the study areas (Chen et al. 2014). Since GDM is typically diagnosed in the mid-late second trimester and may be a function of underlying maternal vulnerability, we chose to expand the time windows studied to include the 3 months prior to conception, the first and second trimesters and each gestational week of pregnancy from 1 to 24 in order to identify critical windows of exposure for GDM risk.
2. Methods
2.1. Study design, participants and outcome measurement
The Consortium on Safe Labor (CSL) was a retrospective cohort study of labor and delivery in the US conducted between 2002 and 2008 based in 12 clinical centers (with 19 hospitals) across 15 hospital referral regions. The study design and characteristics of subjects have been previously described in detail (Zhang et al. 2010). The names and locations of centers can be found in the acknowledgments. Briefly, participating hospitals had obstetric electronic medical records (EMRs) by design that allowed clinical data to be captured into pre-specified fields. This allowed uniform data collection strategies across study sites. Unlike administrative data, the use of EMRs in this study were a direct source of data rich in clinical and demographic details. Institutional review board approval was obtained from all participating institutions.
Data from EMRs of mothers and infants were obtained for 228,562 deliveries among 208,695 women and linked to hospital discharge records. The present analysis was restricted to singleton pregnancies without pregestational diabetes (n=220,264). Data were obtained on maternal characteristics including age, race/ethnicity, parity, marital status, type of insurance and pre-pregnancy body mass index (BMI). Since age was retained in the final model, we excluded women with missing data (n=302). Ten singleton pregnancies with preconception time windows prior to 2002 were also excluded because exposure models only covered the main study years. Analyses were conducted using data on the remaining singleton pregnancies (n=219,952) among 201,015 women.
While the information captured does not contain specific glucose screening methods, in the US, a 1 hour/50 gram oral glucose challenge test is routinely administered between 24-28 weeks gestation to screen pregnant women for GDM (American Diabetes Association 2003). If blood glucose is found to be between 135-199 mg/dl, women undergo a 3 hour/100 gram oral glucose challenge test for diagnosis of GDM (Metzger and Coustan 1998). GDM was recorded in the medical record or in discharge records (code 648.8) using the International Classification of Diseases, Ninth Revision.
2.2. Air pollution measurements
The Air Quality and Reproductive Health (AQRH) study linked pregnancies from the CSL to air pollutant exposures estimated using a modified Community Multi-scale Air Quality Model (CMAQ) version 4.7.1 (Community Multiscale Air Quality Overview 2013; K.M Foley et al. 2009). Since the CSL data are anonymous, maternal exposures are based on the average air pollutant levels for her delivery hospital referral region during each of the defined exposure windows (The Dartmouth Atlas of Health Care 2013). The size of hospital referral regions ranged from 415 to 312, 644 square kilometers. Observed data from air quality monitors in the EPA Air Quality System were used to correct estimates predicted with the CMAQ using an inverse distance weighting technique. Average hourly exposure estimates for each hospital referral region were weighted for population density to discount exposure in places where women were unlikely to live or work. This technique for generating average pollutant concentrations for the study population is described in detail elsewhere (Chen et al, 2014). Briefly, the CMAQ is a three-dimensional multi-pollutant air quality model developed by the EPA. The CMAQ predicts ambient pollutant levels using emissions and meteorological data (including temperature, relative humidity and wind characteristics) from the National Emission Inventories and from the Weather Research and Forecasting Model, respectively. Our final pollution prediction model was compared with four other exposure assessment methods, including observed data only, and found to best account for the spatial variation in pollutant concentrations and population density across hospital referral regions (Chen et al. 2014).
Hourly exposure estimates for each pollutant were averaged across the pregnancy exposure windows for each woman based on her last menstrual period (LMP). Since routine screening for GDM generally occurs in the second trimester (24-28 weeks) and preliminary analyses showed no association between mean levels of air pollutants across the whole second trimester average and GDM risk (data not shown), we focused on exposure windows prior to this time period. This analysis includes a preconception period window (91 days prior to last menstrual period (LMP), a first trimester average (LMP through 13 weeks of gestation) and weekly averages for gestational weeks 1 through 24. Criteria air pollutants, particulate matter (PM) with aerodynamic diameter ≤2.5 μm in μg/m3 (PM2.5) and ≤10 μm in μg/m3 (PM10), nitrogen oxides (NOxin parts per billion (ppb)), carbon monoxide (CO in ppm), sulfur dioxide (SO2 in ppb) and ozone (O3 in ppb) were estimated in each exposure window. Modeled ambient levels of PM2.5 constituents (μg/m3) were also estimated and included elemental carbon, organic compounds, ammonium ion, sulfate, nitrate and dust components.
2.3. Statistical analyses
Descriptive statistics summarized demographic characteristics and air pollution exposure for the analytic cohort of pregnancies with (n=l 1,334) and without GDM (n=208,618). Examination of the association between air pollution exposure quartiles on GDM risk in regression models suggested a linear relationship. Therefore, in regression models the pollutant concentrations were modeled in their original scale for ease of interpretation. Spearman rank correlations between each of the pollutants were calculated (Supplemental Table 1). Binary regression models with the log link function were fitted to estimate relative risks (RR) for GDM per interquartile range (IQR) increase for each air pollutant. The 95% confidence intervals (CI) were calculated using robust standard errors. A first order autoregressive covariance structure was used to account for within-cluster correlation for women with more than one singleton pregnancy during the study period. Separate models were created for air pollutants during each exposure window, including each gestational week from 1-24.
Models for constituents of fine particulate matter were adjusted for total (PM2.5). We assessed potential confounding of the association between air pollution and GDM by maternal characteristics (parity, marital status, insurance status, hospital type, prenatal history of smoking (yes/no) and alcohol use (yes/no)) and observed no substantive change (<10%) in estimates. Final models were adjusted for maternal age, race/ethnicity and study site as these were of interest a priori. Maternal age was treated as a continuous variable and race/ethnicity categories were created for White, Black, Hispanic, Asian/Pacific Islander, Other and Unknown groups. To examine the potential effect modification by maternal BMI, we conducted sensitivity analyses stratifying models by pre-pregnancy normal weight (18.5-24.9 kg/m2) and overweight/obese (BMI≥25 kg/m2) status. We also explored multi-pollutant models to examine the association between air pollutants and GDM accounting for concentrations of other pollutants. In multi-pollutant models for gases (NOX, SO2, CO and O3) we adjusted for all other gases and levels of PM2.5 and PM10. Models for constituents of particulate matter were adjusted for their respective total. While we observed attenuation of risk estimates in multi-pollutant models, the conclusions regarding the association between GDM and air pollutants did not change and we therefore present only estimates for single pollutant models. Sensitivity analyses including season of conception as a covariate were conducted. All analyses were performed using SAS (version 9.3; SAS Institute Inc., Cary, NC).
3. Results
There were 11,334 cases of GDM (5.2% of the study sample). GDM was more prevalent in pregnancies of women who were Hispanic or Asian/Pacific Islander, married, greater than 30 years of age and those who were more likely to be overweight or obese compared to women without GDM (Table 1). Socioeconomic factors such as insurance status and type of hospital where delivery occurred were similar by GDM status. Levels of all criteria air pollutants averaged over the 3-month preconception window in the CSL catchment areas among women with and without GDM are presented in Table 2. Generally criteria air pollutants were positively correlated with each other (0.21 ≤ r ≤ 0.67), with the exception of ozone and particulate matter which were often negatively correlated with other pollutants (−0.06 to −0.42) (Supplementary Table 1). Constituents of PM2.5 were also positively correlated with each other (0.46 ≤ r ≤ 0.86), as well as with total PM2.5 (0.65 ≤ r ≤ 0.97).
Table 1:
Demographic Characteristics by Gestational Diabetes Mellitus Status for Singleton Pregnancies From the Consortium of Safe Labor (n=219,952), 2002-2008
| Gestational Diabetes Mellitus |
||
|---|---|---|
| No (n = 208,618) |
Yes (n = 11,334) |
|
| n (%) | n (%) | |
| Race/Ethnicity | ||
| Non-Hispanic White | 104500 (50) | 4978 (44) |
| Non-Hispanic Black | 46993 (23) | 2154 (19) |
| Hispanic | 35574(17) | 2454 (22) |
| Asian/Pacific Islander | 8139 (4) | 901 (8) |
| Other | 4730 (2) | 389 (3) |
| Unknown | 8682 (4) | 458 (4) |
| Maternal age (years) | ||
| <20 | 20243 (10) | 324 (3) |
| 20 - 24 | 54640 (26) | 1498 (13) |
| 25 - 29 | 58571 (28) | 2833 (25) |
| 30 - 34 | 45853 (22) | 3405 (30) |
| ≥35 | 29311 (14) | 3274 (29) |
| Marital Status | ||
| Married | 122109 (58) | 7342 (65) |
| Divorced/Widowed | 3207 (2) | 258 (2) |
| Single | 76835 (37) | 3354 (30) |
| Unknown | 6467 (3) | 380 (3) |
| Body Mass Index (kg/m2) | ||
| <18.5 | 7793 (4) | 156 (1) |
| 18.5 - 24.9 | 76185 (37) | 2231 (20) |
| 25.0 - 29.9 | 31027 (15) | 1961 (17) |
| ≥30 | 23947 (11) | 2830 (25) |
| Unknown | 69666 (33) | 4156 (37) |
| Parity | ||
| Race/Ethnicity | ||
| 0 | 84096 (40) | 3938 (35) |
| 1 | 63661 (31) | 3556 (31) |
| ≥2 | 60861 (29) | 3840 (34) |
| Hospital Type | ||
| University-Affiliated Teaching Hospital | 88434 (42) | 4972 (44) |
| Teaching Community Hospital | 104332 (50) | 5814 (51) |
| Non-Teaching Community Hospital | 15852 (8) | 548 (5) |
| Insurance | ||
| Group Practice | 116644 (56) | 6609 (58) |
| Government | 67166 (32) | 3529 (31) |
| Other | 2767 (1) | 161 (1) |
| Unknown | 22041 (11) | 1035 (9) |
Table 2:
Levels of Criteria Air Pollutants and PM2.5 Subspecies during the 3 Months Prior to Conception and First Trimester for Singleton Pregnancies From the Consortium of Safe Labor (n=219,952)a, 2002-2008
| Preconception | First Trimester | |||||||
|---|---|---|---|---|---|---|---|---|
| 25th | 50th | 75th | IQR | 25th | 50th | 75th | IQR | |
| PM2.5 (μg/m3) | 8.98 | 11.71 | 14.52 | 5.54 | 9.25 | 11.85 | 14.53 | 5.28 |
| PM2.5 Sub-Species (μg/m3) | ||||||||
| Nitrate | 0.65 | 1.30 | 2.78 | 2.14 | 0.70 | 1.41 | 2.91 | 2.21 |
| Dust Components | 0.81 | 1.41 | 2.02 | 1.21 | 0.85 | 1.44 | 2.01 | 1.17 |
| Elemental Carbon | 0.39 | 0.57 | 0.85 | 0.47 | 0.39 | 0.57 | 0.84 | 0.45 |
| Ammonium Ion | 0.81 | 1.41 | 2.02 | 1.21 | 0.85 | 1.44 | 2.01 | 1.17 |
| Organic Compounds | 2.13 | 2.68 | 3.40 | 1.28 | 2.17 | 2.72 | 3.43 | 1.26 |
| Sulfate | 1.71 | 2.98 | 4.03 | 2.31 | 1.59 | 2.90 | 3.92 | 2.33 |
| PM10 (μg/m3) | 18.65 | 21.64 | 24.95 | 6.30 | 18.59 | 21.62 | 24.91 | 6.32 |
| Carbon Monoxide (ppm) | 0.42 | 0.54 | 0.67 | 0.26 | 0.42 | 0.55 | 0.68 | 0.26 |
| Nitrogen Oxides (ppb) | 14.50 | 24.27 | 43.05 | 28.55 | 14.97 | 25.18 | 45.18 | 30.21 |
| Ozone (ppb) | 23.49 | 29.71 | 35.82 | 12.33 | 22.81 | 29.21 | 35.17 | 12.36 |
| Sulfur Dioxide (ppb) | 2.06 | 3.36 | 5.37 | 3.30 | 2.04 | 3.34 | 5.35 | 3.31 |
Maternal residential addresses were not available and maternal exposure was estimated by averaging hourly air pollutant estimates for hospital referral regions were deliveries took place.
3.1. Air pollution and risk of GDM
Maternal exposure in the 3 months prior to conception to NOX (RR=1.09, 95% CI: 1.04, 1.13) and SO2 (RR=1.05, 95% CI: 1.01, 1.09) were associated with an increased risk of subsequent GDM, while preconception exposure to ozone was associated with decreased GDM risk (RR=0.93, 95% CI: 0.90, 0.96). Except for sulfate, that was associated with a decrease in GDM risk (RR=0.95, 95%CI: 0.92, 0.98), preconception maternal exposures to PM2.5 constituents were not associated with subsequent GDM.
Maternal exposure to NOX and SO2 during the first trimester remained significantly associated with subsequent GDM risk (Table 3). In models for constituents that accounted for PM2.5 levels, increasing levels of nitrate (RR=1.05, 95% CI: 1.05, 1.02, 1.09) were associated with increased GDM risk while sulfate was associated with a decrease in GDM risk (RR=0.96, 95% CI: 0.92, 0.99). Sensitivity analyses that included season of conception as a covariate yielded similar risk estimates but with less precision (Supplemental Table 4). Further exploration of the association between maternal exposure to criteria air pollutants and GDM risk by gestational week revealed that maternal exposure to SO2 (Figure 1) and NOX (Supplementary Figure 1) during preconception and during the first few weeks of the first trimester were associated with increased GDM risk after which risk estimates were attenuated. Although overall maternal exposure to ozone during the first trimester were associated with a decrease in GDM risk, examination of risk estimates by gestational week indicated that exposure to ozone was associated with a decreased GDM risk during early pregnancy but increased GDM risk later in the first trimester and through the second trimester (gestational weeks 13-24; see Supplementary Figure 1).
Table 3:
Adjusted Relative Risksa and 95% Confidence Intervals for Single-Pollutant Models Examining the Association Between Gestational Diabetes Mellitus and Each IQR Increase in Preconception and First Trimester Air Pollutant Levels Among Consortium of Safe Labor Singleton Pregnancies (n=219,952), 2002-2008
| Preconception | First Trimester | |
|---|---|---|
| Criteria Air Pollutanta,b | ||
| PM2.5 (μg/m3) | 0.97 (0.93, 1.02) | 0.98 (0.94, 1.03) |
| PM10 (μg/m3)b | 0.99 (0.96, 1.02) | 0.98 (0.95, 1.01) |
| NOX (ppb) | 1.09 (1.04, 1.13) | 1.06 (1.01, 1.10) |
| SO2 (ppb) | 1.05 (1.01, 1.09) | 1.04 (1.00, 1.08) |
| CO (ppm) | 1.00 (0.97, 1.03) | 0.99 (0.96, 1.03) |
| O3 (ppb) | 0.93 (0.90, 0.96) | 1.00 (0.97, 1.03) |
| PM2.5 Constituentsa,b (μg/m3) | ||
| Elemental Carbon | 1.02 (0.99, 1.06) | 0.97 (0.94, 1.01) |
| Organic Compounds | 1.00 (0.95, 1.02) | 0.98 (0.95, 1.02) |
| Ammonium Ion | 0.98 (0.94, 1.03) | 1.03 (0.99, 1.07) |
| Dust Components | 0.98 (0.94, 1.03) | 1.03 (0.99, 1.07) |
| Sulfate | 0.95 (0.92, 0.98) | 0.96 (0.92, 0.99) |
| Nitrate | 1.03 (0.99, 1.06) | 1.05 (1.02, 1.09) |
11,334 women were diagnosed with GDM and all estimates were adjusted for maternal age, race and study site. Pollutant abbreviations are designated for particulate matter (PM), nitrogen dioxides (NOX), sulfur dioxide (SO2), carbon monoxide (CO) and ozone (O3)
Single-pollutant models for sub-species are adjusted for PM2.5 concentrations
Figure 1:
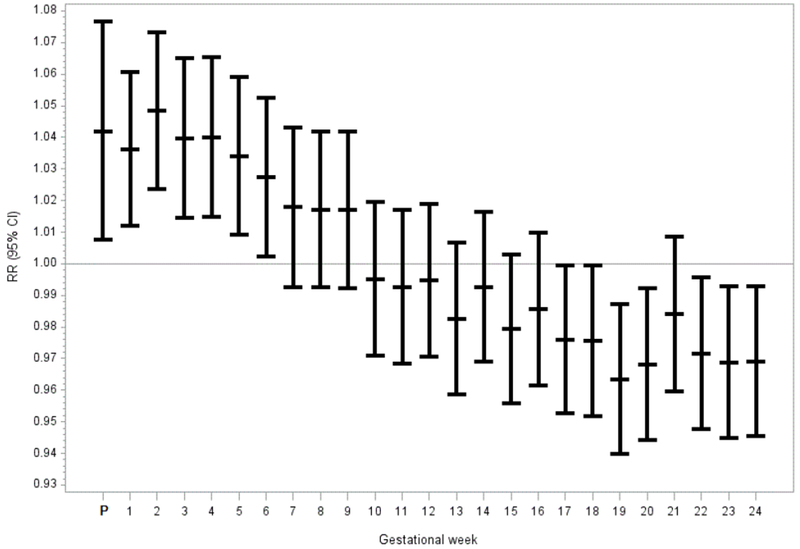
Adjusted relative risks and Their 95% Confidence Intervals for the Association Between Gestational Diabetes Mellitus and each IQR Increase in SO2 From 3 Months Prior to Conceptiona Through Gestational Week 24, Consortium of Safe Labor, 2002-2008
a P represents the relative risk estimate for the average of 3 months prior to conception. Each model was adjusted for maternal age, race and study site.
3.2. Air pollution and GDM risk by categories of BMI
Sensitivity analyses assessing whether air pollutant and GDM risk estimates were modified by BMI revealed similar relationships among both normal weight and overweight/obese women. Risk estimates were somewhat attenuated due to smaller sample size but generally of the same magnitude and direction for preconception (Supplementary Table 2) and first trimester (Supplementary Table 2) exposure windows.
4. Discussion
In this large, retrospective US cohort of singleton pregnancies, maternal exposure to NOX and SO2 during three months preconception and the first seven weeks of pregnancy were associated with subsequent GDM. Our findings for NOX and PM2.5 were consistent with prior studies of GDM and air quality (Malmqvist et al. 2013; Fleisch et al. 2014) but we add new information on the preconception exposure window and have evaluated the association between GDM with all criteria air pollutants, including constituents of PM2.5. We identified novel associations between preconception SO2 exposure and second trimester ozone exposure and increased GDM risk. While associations between GDM risk and some constituents of PM2.5 were found to be statistically significant, our findings for PM10 and PM2.5 were null. We also observed that ozone was associated with lower risk of GDM during the preconception period and during the early weeks of pregnancy but increased risk during mid-pregnancy. Our early time-window findings suggest that maternal exposure to air pollutants before pregnancy as well as during critical periods of placental implantation and early embryonic development may increase the development of gestational diabetes later in pregnancy. Although the absolute risks were small, extrapolating our results to the US population, where approximately 4 million births occur each year, we would expect 36,900 fewer cases of GDM each year if exposures were one IQR lower for NOX and SO2.
A Swedish Medical Birth Registry study of 81,110 singleton pregnancies, including 1,599 registered cases of GDM (Malmqvist et al. 2013) that occurred in Scania county, reported an association between increasing quartiles of NOX in the first and second trimesters with GDM prevalence. Furthermore, a cohort study of pregnant women living in the Boston area (n=2,093) reported an association between increasing levels of fine particulate matter and impaired glucose tolerance (n=65) but not GDM (n=118) (Fleisch et al. 2014). Our study identified the 3 months prior to conception and the first trimester, particularly the first seven weeks, as windows of susceptibility for the effects of air pollution on GDM. Our data on second trimester exposure was generally null with the exception of ozone which was unexplored in the Swedish and Boston studies. The differences in results by timing of exposure may be attributed to differences in exposure assessment between studies, such as the air pollution models utilized. The availability of air pollution data by gestational week in our study allowed us to explore potential critical windows for the association between air pollution and GDM risk.
Although not all studies of the association between air pollution and diabetes mellitus are confirmatory (Puett et al. 2011; Dijkema et al. 2011), our results are in agreement with the majority that have found associations between type 2 diabetes mellitus incidence and prevalence with exposure to particulate matter and traffic-related air pollution (NOX, nitrogen dioxide, and distance from roads) (Kramer et al. 2010; Coogan et al. 2012; Andersen et al. 2012; Pearson et al. 2010). Studies with both particulate matter and traffic-related air pollution data also confirm that the latter may largely account for the association between air pollution and diabetes (Puett et al. 2011; Kramer et al. 2010; Coogan et al. 2012; Andersen et al. 2012). These studies also demonstrated that women were more susceptible to the effects of air pollution than men. The susceptibility of women to air pollution exposures is attributed to gender differences in the anatomy and physiology of the respiratory system and differences in particle deposition in the lung (Kim and Hu 1998; Bennett et al. 1996).
Chronic exposure to air pollution alters endothelial function that disrupts insulin action or leads to insulin resistance, provoking metabolic dysfunction (Rajagopalan and Brook 2012). Animal studies provide support for this mechanism by demonstrating air pollution exposure can lead to inflammation in the lung (Tamagawa et al. 2008) that disrupts insulin action by inducing inflammation in adipose (Sun et al. 2009; Xu et al. 2011) and vascular tissue (Sun et al. 2005; Tamagawa et al. 2008). Recently, studies in human populations have also linked air pollution with elevated levels of C-reactive protein (CRP), a marker of systemic inflammation, in healthy young adults (Rich et al. 2012), a diabetic population (Khafaie et al. 2013) and in pregnant women (van den Hooven et al. 2012; Lee et al. 2011). CRP levels, in turn, are associated with increased risk of type 2 diabetes (Wang et al. 2013) and gestational glucose intolerance (Lowe et al. 2010). Although animal studies suggested that diet-induced obesity further promotes the effects of air pollution on diabetes risk (Sun et al. 2009; Yan et al. 2011), our results did not meaningfully differ after stratification by obesity status. Taken together, this evidence from previous type 2 diabetes and GDM literature demonstrates the biological plausibility as well as provides support for the observed associations in our study between air pollution and GDM.
Our study is limited by the use of electronic medical records as our source of cases. Although date of GDM diagnosis was unavailable, assuming it occurred in the second trimester (24-28 weeks gestation) for most pregnancies follows US standards and recommendations for GDM screening and diagnosis. Our analyses were restricted to women without a previous diagnosis of diabetes, but it is possible that some pre-gestational diabetes may have been reported as GDM in the medical record.
The use of hospital referral regions as a marker of maternal residence during pregnancy should also be considered when interpreting our study findings. Exposure misclassification may have occurred if mothers lived outside of the hospital referral region for some or all of pregnancy, but any misclassification of exposure is unlikely to be strongly related to GDM status and would likely bias our results towards the null. Previous research has demonstrated that 9-34% of mothers move during pregnancy and moves occur over short distances in the same region and more often in the second and third trimesters (Chen et al. 2010; Bell and Belanger 2012). We acknowledge that while all CSL hospitals were located in highly urban areas, the size of hospital referral regions varied greatly in our study and that they may not accurately reflect individual exposure. However, while distances from maternal residences to hospitals will vary with geography, in the US generally patients are admitted to hospitals close to where they live. In general, 91% of Americans live in a hospital referral region and more than 80% of hospitalizations occurred locally (The Dartmouth Atlas of Health Care 2013). Due to maternal mobility and time-varying activities during pregnancy, exposure estimates in our study may be somewhat less susceptible to exposure misclassification if we assume that women are more likely to live and work in the hospital referral region in which they delivered. In addition, because air pollution is regulated at the population level, studies identifying regional-level air pollution levels that are associated with increased risk in GDM will be informative for regulatory and public health agencies. We acknowledge that this exposure assessment strategy does not estimate risks associated with short-lived pollutants or those that are concentrated in small areas.
The large geographic area with which we defined maternal air pollution exposure and the collinearity between pollutants may also explain our null findings for pollutants or statistically significant protective associations between air pollutants and GDM. Given that air pollution is a mixture of pollutants that vary by geographic location, studies that examine the associations between specific air pollutants and pregnancy complications are needed. However, high collinearity among pollutants exists because of their common sources and photochemical interactions in the atmosphere. For example, when atmospheric levels of nitrogen species are high, ozone levels are low. Correlation between maternal exposures during the preconception and first trimester windows, particularly for NOx and SO2, hinders our ability to differentiate the risk of GDM attributable to each exposure window due to collinearity. However, we also note that risk appeared to vary over the smaller weekly exposure windows with early pregnancy a risk window for some pollutants, followed by a period of null or lower risk, while other pollutants had an opposite pattern with greater risk later in gestation. This variation explains the attenuated or null results for broader time windows but there is no biologic rationale for the protective effects that are occasionally observed. Potentially, the temporal correlation of the pollutants could be handled by functional regression methods to determine how the whole profile of pollutants during pregnancy is associated with GDM, which we will leave for future exploration. These findings should be interpreted with caution since some of the effects we observe could be due to chance or to the high correlation of pollutants that could be exerting an influence on GDM risk in different pregnancy time windows (Supplemental Tables 1 and 3).
The major strength of our study was the detailed assessment of multiple criteria air pollutants and PM2.5 constituents. The modified CMAQ models utilized in this study used both emission and meteorological data to estimate hourly levels of pollutants examined for grids that cover the entire US over the full study period. These models also considered complex atmospheric chemistry and mixing to develop a surface level exposure matrix. Within the hospital referral regions, we used monitor data to adjust the model output, essentially tying the surface level exposure model to the monitor data where available. Our study also benefitted from the availability of a large amount of clinical data from patient medical records. Therefore, this study is the most comprehensive examination of the association between air pollution exposure and risk of GDM to date. These data allowed us to examine the individual associations between each air pollutant and GDM risk. We confirmed previous findings that exposure to NOX is associated with GDM risk while exposure to PM2.5 is not. Additionally, this study is the first to demonstrate that maternal exposure to SO2 during the preconception time window and to ozone during the second trimester is associated with increased GDM risk.
Preconception is an often understudied critical exposure window for the effects of air pollution during pregnancy. This has been supported in literature with other environmental exposures and neonatal outcomes (Murphy et al. 2010). Our study demonstrates that maternal exposure to NOX and SO2 prior to conception and during early pregnancy is associated with GDM risk. This suggests that air pollution exposures, in addition to impacting fetal growth and development (Shah and Balkhair 2011), may also be detrimental to maternal health. Additionally, pregnant women represent a vulnerable group due to the fact that pregnancy is a naturally insulin-resistant state. The relationship between air pollution and related health outcomes should be explored in obstetric populations in order to elucidate the impact air pollution exposure may have on maternal health as well as adverse infant outcomes.
Supplementary Material
Highlights.
Air pollution may be related to gestational diabetes (GDM).
No prior studies have examined preconception exposure.
Maternal exposure to NOx and SO2 before conception increased subsequent GDM risk.
NOx and SO2 exposure in the first seven weeks of pregnancy also increased GDM risk.
Early exposure to O3 reduced GDM risk but risk increased after 15 weeks gestation.
Acknowledgements
Institutions involved in the Consortium on Safe Labor include, in alphabetical order: Baystate Medical Center, Springfield, MA; Cedars-Sinai Medical Center Burnes Allen Research Center, Los Angeles, CA; Christiana Care Health System, Newark, DE; Georgetown University Hospital, MedStar Health, Washington, DC; Indiana University Clarian Health, Indianapolis, IN; Intermountain Healthcare and the University of Utah, Salt Lake City, Utah; Maimonides Medical Center, Brooklyn, NY; MetroHealth Medical Center, Cleveland, OH.; Summa Health System, Akron City Hospital, Akron, OH; The EMMES Corporation.
Funding
This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health & Human Development, National Institutes of Health through contract number HHSN257200603425C for the Consortium on Safe Labor, and through contract number HHSN275200800002I, task number HHSN27500008 for the Air Quality and Reproductive Health Study.
List of abbreviations:
- AQRH
Air Quality and Reproductive Health Study
- BMI
body mass index
- CI
confidence interval
- CMAQ
Community Multi-scale Air Quality Model
- CO
carbon monoxide
- CSL
Consortium on Safe Labor Study
- EPA
US Environmental Protection Agency
- GDM
gestational diabetes mellitus
- LMP
last menstrual period
- PM10
particulate matter ≤ 10 microns
- PM2.5
fine particulate matter ≤ 2.5 microns
- RR
relative risk
- O3
ozone
- NOX
nitrogen oxides
- SO2
sulfur dioxides
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Community Multiscale Air Quality Overview. Center for Environmental Modeling for Policy Development at The University of North Carolina at Chapel Hill, 2013. [Google Scholar]
- The Dartmouth Atlas of Health Care. The Dartmouth Institute for Health Policy and Clinical Practice, 2013. [Google Scholar]
- American Diabetes Association. 2003. Gestational Diabetes Mellitus. Diabetes Care 26:S103–S105. [DOI] [PubMed] [Google Scholar]
- Andersen ZJ, Raaschou-Nielsen O, Ketzel M, Jensen SS, Hvidberg M, Loft S, Tjonneland A, Overvad K, Sorensen M. 2012. Diabetes incidence and long-term exposure to air pollution: a cohort study. Diabetes Care 35:92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile JN, Bloch MJ. 2012. Exposure to air pollution increases the incidence of hypertension and diabetes in black women living in Los Angeles. J Clin Hypertens (Greenwich ) 14:819–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML, Belanger K. 2012. Review of research on residential mobility during pregnancy: consequences for assessment of prenatal environmental exposures. J Expo Sci Environ Epidemiol 22:429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Haroush A, Yogev Y, Hod M. 2004. Epidemiology of gestational diabetes mellitus and its association with Type 2 diabetes. Diabet Med 21:103–113. [DOI] [PubMed] [Google Scholar]
- Bennett WD, Zeman KL, Kim C. 1996. Variability of fine particle deposition in healthy adults: effect of age and gender. Am J Respir Crit Care Med 153:1641–1647. [DOI] [PubMed] [Google Scholar]
- Brook RD, Jerrett M, Brook JR, Bard RL, Finkelstein MM. 2008. The relationship between diabetes mellitus and traffic-related air pollution. J Occup Environ Med 50:32–38. [DOI] [PubMed] [Google Scholar]
- Chen G, Li J, Ying Q, Sherman S, Perkins N, Rajeshwari S, Mendola P. 2014. Evaluation of observation-fused regional air quality model results for population air pollution exposure estimation. Sci Total Environ 485-486:563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Bell EM, Caton AR, Druschel CM, Lin S. 2010. Residential mobility during pregnancy and the potential for ambient air pollution exposure misclassification. Environ Res 110:162–168. [DOI] [PubMed] [Google Scholar]
- Coogan PF, White LF, Jerrett M, Brook RD, Su JG, Seto E, Burnett R, Palmer JR, Rosenberg L. 2012. Air pollution and incidence of hypertension and diabetes mellitus in black women living in Los Angeles. Circulation 125:767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkema MB, Mallant SF, Gehring U, van den HK, Alssema M, van Strien RT, Fischer PH, Nijpels G, Stehouwer CD, Hoek G, Dekker JM, Brunekreef B. 2011. Long-term exposure to traffic-related air pollution and type 2 diabetes prevalence in a cross-sectional screening-study in the Netherlands. Environ Health 10:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisch AF, Gold DR, Rifas-Shiman SL, Koutrakis P, Schwartz JD, Kloog I, Melly S, Coull BA, Zanobetti A, Gillman MW, Oken E. 2014. Air Pollution Exposure and Abnormal Glucose Tolerance during Pregnancy: The Project Viva Cohort. Environ Health Perspect 122:378–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- M Foley K, Roselle SJ, Appel KW, Bhave PV, Pleim JE, Otte TL, Mathur R, Sarwar G, Young JO, Gilliam RC, Nolte CG, Kelly JT, Gilliand AB, Bash JO. 2009. Incremental testing of the community multiscale air quality (CMAQ) modeling system version 4.7. Geoscientific Model Development Discussions 2:1245–1297. [Google Scholar]
- Kampa M, Castanas E. 2008. Human health effects of air pollution. Environ Pollut 151:362–367. [DOI] [PubMed] [Google Scholar]
- Khafaie MA, Salvi SS, Ojha A, Khafaie B, Gore SS, Yajnik CS. 2013. Systemic inflammation (C-reactive protein) in type 2 diabetic patients is associated with ambient air pollution in pune city, India. Diabetes Care 36:625–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CS, Hu SC. 1998. Regional deposition of inhaled particles in human lungs: comparison between men and women. J Appl Physiol 84:1834–1844. [DOI] [PubMed] [Google Scholar]
- Kramer U, Herder C, Sugiri D, Strassburger K, Schikowski T, Ranft U, Rathmann W. 2010. Traffic-related air pollution and incident type 2 diabetes: results from the SALIA cohort study. Environ Health Perspect 118:1273–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PC, Talbott EO, Roberts JM, Catov JM, Sharma RK, Ritz B. 2011. Particulate air pollution exposure and C-reactive protein during early pregnancy. Epidemiology 22:524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe LP, Metzger BE, Lowe WL Jr, Dyer AR, McDade TW, McIntyre HD. 2010. Inflammatory mediators and glucose in pregnancy: results from a subset of the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. J Clin Endocrinol Metab 95:5427–5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmqvist E, Jakobsson K, Tinnerberg H, Rignell-Hydbom A, Rylander L. 2013. Gestational Diabetes and Preeclampsia in Association with Air Pollution at Levels below Current Air Quality Guidelines. Environ Health Perspect 121:488–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger BE, Coustan DRE. 1998. Proceedings of the fourth international workshop conference on gestational diabetes mellitus. Diabetes Care B 1–B167. [PubMed] [Google Scholar]
- Murphy LE, Gollenberg AL, Buck Louis GM, Kostyniak PJ, Sundaram R. 2010. Maternal serum preconception polychlorinated biphenyl concentrations and infant birth weight. Environ Health Perspect 118:297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson JF, Bachireddy C, Shyamprasad S, Goldfine AB, Brownstein JS. 2010. Association between fine particulate matter and diabetes prevalence in the U.S. Diabetes Care 33:2196–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puett RC, Hart JE, Schwartz J, Hu FB, Liese AD, Laden F. 2011. Are particulate matter exposures associated with risk of type 2 diabetes? Environ Health Perspect 119:384–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raaschou-Nielsen O, Sorensen M, Ketzel M, Hertel O, Loft S, Tjonneland A, Overvad K, Andersen ZJ. 2013. Long-term exposure to traffic-related air pollution and diabetes-associated mortality: a cohort study. Diabetologia 56:36–46. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Brook RD. 2012. Air pollution and type 2 diabetes: mechanistic insights. Diabetes 61:3037–3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich DQ, Kipen HM, Huang W, Wang G, Wang Y, Zhu P, Ohman-Strickland P, Hu M, Philipp C, Diehl SR, Lu SE, Tong J, Gong J, Thomas D, Zhu T, Zhang JJ. 2012. Association between changes in air pollution levels during the Beijing Olympics and biomarkers of inflammation and thrombosis in healthy young adults. JAMA 307:2068–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah PS, Balkhair T. 2011. Air pollution and birth outcomes: a systematic review. Environ Int 37:498–516. [DOI] [PubMed] [Google Scholar]
- Sram RJ, Binkova B, Dejmek J, Bobak M. 2005. Ambient air pollution and pregnancy outcomes: a review of the literature. Environ Health Perspect 113:375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Wang A, Jin X, Natanzon A, Duquaine D, Brook RD, Aguinaldo JG, Fayad ZA, Fuster V, Lippmann M, Chen LC, Rajagopalan S. 2005. Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA 294:3003–3010. [DOI] [PubMed] [Google Scholar]
- Sun Q, Yue P, Deiuliis JA, Lumeng CN, Kampfrath T, Mikolaj MB, Cai Y, Ostrowski MC, Lu B, Parthasarathy S, Brook RD, Moffatt-Bruce SD, Chen LC, Rajagopalan S. 2009. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation 119:538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamagawa E, Bai N, Morimoto K, Gray C, Mui T, Yatera K, Zhang X, Xing L, Li Y, Laher I, Sin DD, Man SF, van Eeden SF. 2008. Particulate matter exposure induces persistent lung inflammation and endothelial dysfunction. Am J Physiol Lung Cell Mol Physiol 295:L79–L85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hooven EH, de KY, Pierik FH, Hofman A, van Ratingen SW, Zandveld PY, Lindemans J, Russcher H, Steegers EA, Miedema HM, Jaddoe VW. 2012. Chronic air pollution exposure during pregnancy and maternal and fetal C-reactive protein levels: the Generation R Study. Environ Health Perspect 120:746–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Bao W, Liu J, Ouyang YY, Wang D, Rong S, Xiao X, Shan ZL, Zhang Y, Yao P, Liu LG. 2013. Inflammatory markers and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 36:166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Liu C, Xu Z, Tzan K, Zhong M, Wang A, Lippmann M, Chen LC, Rajagopalan S, Sun Q. 2011. Long-term exposure to ambient fine particulate pollution induces insulin resistance and mitochondrial alteration in adipose tissue. Toxicol Sci 124:88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan YH, Chou CC, Lee CT, Liu JY, Cheng TJ. 2011. Enhanced insulin resistance in diet-induced obese rats exposed to fine particles by instillation. Inhal Toxicol 23:507–519. [DOI] [PubMed] [Google Scholar]
- Zhang J, Troendle J, Reddy UM, Laughon SK, Branch DW, Burkman R, Landy HJ, Hibbard JU, Haberman S, Ramirez MM, Bailit JL, Hoffman MK, Gregory KD, Gonzalez-Quintero VH, Kominiarek M, Learman LA, Hatjis CG, van VP. 2010. Contemporary cesarean delivery practice in the United States. Am J Obstet Gynecol 203:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


