Abstract
Introduction:
Central nervous system (CNS) metastases in lung cancer are a frequent cause of morbidity and mortality. There are conflicting data on the incidence of CNS metastases in stage IV ROS1+ non-small cell lung cancer (NSCLC) and rate of CNS progression on crizotinib.
Methods:
A retrospective review of 579 patients with stage IV NSCLC between June 2008 to December 2017 was performed. Brain metastases and oncogene status (ROS1, ALK, EGFR, KRAS, BRAF, and other) were recorded. We measured progression free survival (PFS) and time to CNS progression (P-CNS) in ROS1+ and ALK+ patients on crizotinib.
Results:
We identified 33 ROS1+ and 115 ALK+ patients with stage IV NSCLC. The incidence of brain metastases for treatment-naïve, stage IV ROS1+ and ALK+ NSCLC was 36% (12/33) and 34% (39/115) respectively. There were no statistically significant differences in incidence of brain metastases across ROS1, ALK, EGFR, KRAS, BRAF or other mutations. Complete survival data was available for 19 ROS1+ and 83 ALK+ patients. Median PFS for ROS1+ and ALK+ patients was 11 and 8 months (p = 0.304). The CNS was the first and sole site of progression in 47% (9/19) of ROS1+ and 33% ALK+ (28/83) patients with no differences between these groups (p = 0.610).
Conclusions:
Brain metastases are common in treatment-naïve stage IV ROS1+ NSCLC, though the incidence does not differ from other oncogene cohorts. The CNS is a common first site of progression in ROS1+ patients on crizotinib. This study reinforces the importance of developing CNS-penetrant TKIs for patients with ROS1+ NSCLC.
Keywords: brain metastasis, non-small cell lung cancer, crizotinib, ROS1
1. Introduction
Rearrangement of the ROS1 receptor tyrosine kinase gene has been identified as an oncogenic driver, occurring in approximately 1–2% of non-small cell lung cancer (NSCLC) cases.1, 2 Rearrangement commonly leads to the creation of chimeric fusion genes that promote an oncogenic phenotype through constitutive activation of the ROS1 kinase activity.3 Pre-clinical studies and clinical trials have demonstrated that crizotinib, a small molecule TKI, demonstrates activity against both ALK and ROS1 rearrangements.1, 3–6 Crizotinib is approved by regulatory agenices worldwide as a first line agent for treatment of ROS1+ NSCLC. Several next generation inhibitors with activity against ROS1 are also under investigation.7–9
Brain metastases remain a major cause of morbidity and mortality in patients with NSCLC. It is estimated that between 25% and 40% of NSCLC patients develop brain metastases during the course of their disease.10 Despite many clinical similarities between ALK+ and ROS1+ NSCLC patients, there remain conflicting data regarding the incidence of brain metastases in treatment-naïve ROS1+ NSCLC. In contrast to ALK+ NSCLC, where the incidence of brain metastases in the treatment-naïve population has been reported to range between 25–30%,11–15 there remains wide variability in the reported incidence of brain metastases for ROS1+ NSCLC.16–18
While excellent systemic responses are seen in patients with ALK+ NSCLC to crizotinib therapy, central nervous system (CNS) progression is common due to the reduced penetrance of crizotinib through the blood-brain barrier.4, 12 This can be mitigated by newer generation TKIs such as ceritinib,8 alectinib,19 and brigatinib,20 all of which are US FDA approved for ALK+ NSCLC. The median progression free survival (PFS) of crizotinib in advanced ALK+ NSCLC is consistently reported between 8 and 11 months across different studies.12–15, 21 The initial report of crizotinib in ROS1+ NSCLC demonstrated a median PFS of 19 months,6 though a recent large phase II study of patients with ROS1+ NSCLC on crizotinib reported a lower median PFS of 15 months.18 One potential explanation for the difference in PFS between the ROS1+ and ALK+ populations is that if ROS1+ NSCLC had a lower tropism for the CNS than ALK+ NSCLC, then the CNS liability of crizotinib would be less apparent.6, 16 An alternative hypothesis is that crizotinib has increased potency (lower IC50) against ROS1 compared to ALK, which would allow a lower dose of crizotinib in the CNS to inhibit ROS1+ cancer cells more effectively than ALK+ cancer cells.22, 23
In this single center retrospective series, we recorded the incidence of brain metastases in stage IV ROS1+ NSCLC and compared this incidence to other oncogene cohorts including ALK, EGFR, KRAS, BRAF and other mutations. Our hypothesis was that the incidence of brain metastases in ROS1+ NSCLC was not significantly different from other oncogene subtypes based on a prior study showing no difference in the incidence of brain metastases between EGFR, ALK, and KRAS patients with metastatic NSCLC.11 Additionally, we explored the rate of CNS progression in the ROS1+ and ALK+ cohorts while on crizotinib. We chose ALK+ NSCLC as a comparator to ROS1+ NSCLC because crizotinib is an approved first line therapy for both groups.
2. Material and methods
2.1. Incidence of brain metastases in treatment-naïve stage IV NSCLC
All patients with NSCLC (classified by TNM 7th edition) evaluated at University of Colorado from June 2008 to December 2017 were eligible for assessment. A protocol approved by the institutional review board (IRB) permits clinical correlates to be made on all patients in whom molecular analyses have been conducted within the Colorado Molecular Correlates Laboratory (CMOCO) or for whom we have written documentation of testing performed at outside facilities. Patients were identified through a database that included all patients treated through the University of Colorado health system. The patient population contained a mix of patients that were diagnosed and treated within the University of Colorado health system and patients that were treated in the community and subsequently referred to our institution.
Outcomes data and imaging results were collected by retrospective chart review. We captured age, sex, smoking status, histology, and clinical stage at diagnosis. We captured brain metastases at time of stage IV diagnosis through clinical review and review of baseline neuroimaging. All patients considered for analysis had magenetic resonance imaging (MRI) of the brain at time of stage IV diagnosis. For each patient included in this analysis, we captured oncogene status and divided patients into the following cohorts: ROS1, ALK, EGFR, KRAS, or BRAF. Patients with no identifiable oncogene or a driver mutation that did not fall into one of the categories listed above were grouped in a separate cohort. For the ROS1 cohort, we identified the method used to detect the ROS1 rearrangement. If available, we noted the ROS1 gene fusion partner for each patient. Patients with squamous histology, incomplete neuroimaging assessment at stage IV diagnosis, incomplete clinical data, or no documented mutation testing were excluded (Supplemental Figure 1).
2.2. CNS progression of ROS1+ and ALK+ NSCLC on crizotinib
We defined treatment-naïve as patients that had not received any systemic therapy and TKI-naïve as those patients that had not received TKI therapy. For ROS1+ and ALK+ cohorts on crizotinib, we noted whether patients first progressed in the CNS, systemically, or both. We captured progression free survival (PFS) and time to CNS progression (P-CNS). PFS was defined as duration of time from start of crizotinib therapy to first radiographic progression or death in the absence of documented progression. Patients alive without documented disease progression were censored on the last follow-up date. P-CNS was defined as duration of time from start of crizotinib to first radiographic documentation of CNS progression. We captured whether patients received brain radiotherapy and prior chemotherapy as these were potential confounders. When comparing CNS progression between ALK+ and ROS1+ cohorts, patients were excluded if they never received crizotinib, if there was no follow-up data after starting crizotinib, or if they received newer generation TKIs as first line therapy.
2.3. Molecular methods for mutation and rearrangement testing
Molecular testing was conducted via CLIA certified laboratories and performed using laboratory assays that were independently validated. Over the course of time of this study, the testing approach for standard of care laboratory testing evolved, and therefore testing was performed using a variety of assay platforms. These approaches included: Sanger sequencing of relevant targeted regions, single nucleotide base extension assay (SNaPshot™), real-time polymerase chain reaction (PCR), targeted Next Generation Sequencing (NGS) using a 26 gene panel (TruSight, Illumina), or Archer FusionPlex Solid Tumor library preparation kit (ArcherDx, Boulder, CO) with raw sequence data analyzed by using the Archer Analysis software package (version 4.1.1.7; ArcherDx). Evaluation of gene fusions such as ALK and ROS1 were performed with a variety of techniques, including: 1) fluorescence in situ hybridization (FISH) using break apart probes previously described;24–26 2) FISH testing using the Abbott Vysis ALK Break-Apart Probe or immunohistochemistry (IHC) using the Ventana ALK (D5F3) CDX assay; or 3) fusion NGS testing utilizing Archer FusionPlex run with analysis software 4.1.1.7. For internally tested cases, FISH was performed on 4μM (± 1μM) thick formalin-fixed, paraffin-embedded (FFPE) tumor sections. ROS1 FISH probes used for these cases included Vysis (Abbott Molecular, Abbott Park, Illinois) or custom designed probes. FISH assays were performed as previously described, or using the Vysis Paraffin Pretreatment IV and Post-Hybridization Wash Buffer Kit (Abbott) per the manufacturer’s instructions. Signals were evaluated in at least 50 tumor nuclei per specimen. Specimens were considered positive for ROS1 rearrangement if ≥15% of cells displayed a split 5’/3’ and/or single 3’ signal pattern. Separation between 5’ and 3’ ROS1 signals of ≥1 signal diameter was required to score as split. In some cases that were tested by outside laboratories, information regarding the specific FISH probes was not available.
2.4. Statistical testing
To compare treatment-naïve incidence of brain metastases between oncogene groups, a Fisher exact test was used to compare the proportion of patients with brain metastasis at stage IV diagnosis to the proportion of patients without brain metastasis at stage IV diagnosis. Similarly, a Fischer exact test was used to assess differences in incidence of brain metastases between CD74-ROS1 gene fusions and non-CD74-ROS1 gene fusions. Kaplan-Meier curves and estimates were used to assess PFS and P-CNS on crizotinib for ROS1+ and ALK+ NSCLC. Differences in PFS and P-CNS progression were assessed using a log-rank test. All statistics were generated using GraphPad Prism (Version 6.00 for Windows®, GraphPad Software, La Jolla, CA) and R (Version 3.4.3 for Windows®, R Project®, Vienna, Austria).
3. Results
3.1. Clinical characteristics
A total of 689 patients were considered for this study. Of these, 110 were excluded based on exclusion criteria described earlier, with 579 patients included for further analysis (Supplemental Figure 1). When sub-divided by oncogene, we identified 33 ROS1, 115 ALK, 192 EGFR, 102 KRAS, and 16 BRAF mutations. Patients with no identifiable driver oncogene or a mutation that did not fit into the categories listed above (n = 121) were grouped separately. Baseline clinical characteristics for each of the oncogene groups are listed in Table 1. Methods for detecting the ROS1 gene rearrangement were as follows: FISH 39% (13/33), reverse transcriptase PCR 3% (1/33), NGS 56% (19/33). Fusion partner data was available for 17 ROS1+ patients with the following distribution: CD74-ROS1 12/17, SLC34A2-ROS1 4/17, and ZCCHC8-ROS1 1/17. Of note, 45% (15/33) ROS1+ NSCLC had their ROS1 rearrangement diagnosed internally and 55% (18/33) were diagnosed at outside institutions. The median age of the ROS1+ cohort was 55 years. The median cigarette smoking pack-year was zero (range 0 – 45 years).
Table 1:
Baseline demographics and clinical characteristics of patients with NSCLC by oncogene.
| All Patients 579 (%) |
ROS1 (n= 33) |
ALK (n = 115) |
EGFR (n = 192) |
KRAS (n = 102) |
BRAF (n = 16) |
Other (n = 121) |
|
|---|---|---|---|---|---|---|---|
| Sex | |||||||
| Male | 244 (42) | 13 (39) | 56 (49) | 63 (33) | 41 (40) | 8 (50) | 63 (52) |
| Female | 335 (58) | 20 (61) | 59 (51) | 129 (67) | 61 (60) | 8(50) | 58 (48) |
| Age* | |||||||
| Median age (years) | 61 | 55 | 53 | 59 | 64 | 63 | 64 |
| Range | 22 - 96 | 22 - 76 | 21-81 | 34-96 | 37-87 | 43-74 | 31-83 |
| Smoking history | |||||||
| Never | 303 (52) | 24 (73) | 91 (79) | 145 (76) | 6 (6) | 3 (19) | 34 (28) |
| ≤ 10 pack years | 60 (10) | 2 (6) | 13 (11) | 24 (12) | 9 (9) | 2 (12) | 10 (8) |
| >10 pack years | 216 (37) | 7 (21) | 11 (10) | 23 (12) | 87 (85) | 11 (68) | 77 (58) |
| Histology | |||||||
| Adenocarcinoma | 565 (98) | 31 (94) | 114 (99) | 192 (100) | 101 (99) | 16 (100) | 111 (92) |
| Adenosquamous | 7 (1) | 1 (3) | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 5 (4) |
| Large cell | 7 (1) | 1 (3) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 5 (4) |
| Clinical stage at diagnosis | |||||||
| Stage I | 38 (6) | 0 (0) | 5 (4) | 15 (8) | 11 (11) | 2 (13) | 5 (4) |
| Stage II | 19 (4) | 0 (0) | 2 (2) | 6 (3) | 5 (5) | 1 (6) | 5 (4) |
| Stage III | 60 (10) | 5 (15) | 20 (17) | 7 (4) | 10 (10) | 3 (19) | 15 (12) |
| Stage IV | 462 (80) | 28 (85) | 88 (76) | 164 (85) | 76 (74) | 10 (62) | 96 (80) |
| Brain metastases† | |||||||
| Yes | 162 (28) | 12 (36) | 39 (34) | 53 (28) | 29 (28) | 3 (19) | 26 (21) |
| No | 417 (72) | 21 (64) | 76 (66) | 139 (72) | 73 (72) | 13 (81) | 95 (79) |
In reference to age at stage IV disease.
Brain metastases is in reference to time of stage IV disease, not at initial diagnosis.
Note: not all numbers may add up to one hundred percent due to rounding
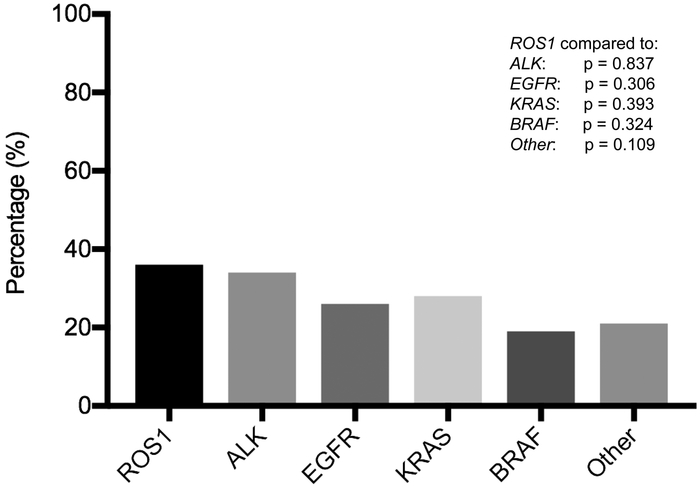
The percentage of brain metastases at stage IV diagnosis per oncogene group was as follows: ROS1 36% (12/33), ALK 34% (39/115), EGFR 28% (53/192), KRAS 28% (29/102), BRAF 19% (3/16), and other 22% (26/121). There were no statistically significant differences in the incidence of brain metastases in patients with metastatic ROS1+ NSCLC when compared to each of the other oncogene groups (Figure 1). Forty-two percent (5/12) CD74-ROS1 gene fusions and 60% (3/5) non-CD74-ROS1 fusions had brain metastases at stage IV diagnosis. There were no statistically significant differences in the percentage of brain metastases at stage IV diagnosis between the CD74-ROS1 and non-CD74-ROS1 fusions (p = 0.620).
Figure 1: Incidence of brain metastases across oncogene groups.
The incidence of brain metastases at stage IV diagnosis as separated by oncogene. A Fisher-Exact test was used to assess for statistically significant differences between ROS1 and other oncogene cohorts. The incidence of brain metastases in treatment-naïve ROS1+ NSCLC was 36%. There were no statistically significant differences in the incidence of brain metastases at stage IV diagnosis across oncogene cohorts.
3.2. CNS progression in ROS1+ and ALK+ NSCLC on crizotinib
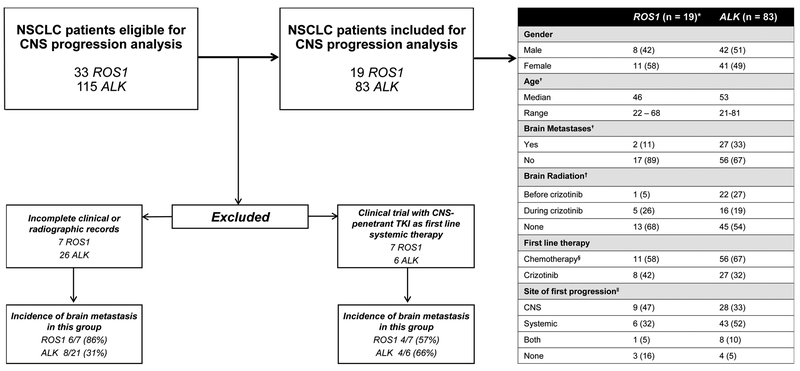
A total of 33 ROS1+ patients and 115 ALK+ patients were considered for this analysis. A consort diagram demonstrating exclusion criteria is shown in Figure 2. A total of 19 ROS1+ patients and 83 ALK+ patients were eligible for analysis of CNS progression on crizotinib. Baseline clinical characteristics for ROS1+ and ALK+ patients are shown in Figure 2. Median duration of follow up was 30 months and 47 months for the ROS1+ and ALK+ cohorts respectively. The percentage of patients who received chemotherapy and radiotherapy prior to crizotinib is shown in Figure 2. Pre-TKI incidence of brain metastases for the ROS1+ and ALK+ patients included in this analysis was 11% (2/19) and 33% (27/83) respectively. The incidence of pre-TKI brain metastases among patients excluded from this analysis is shown in Figure 2. Among the ROS1+ patients who were excluded due to enrollment in a clinical trial, the pre-TKI incidence of brain metastases was 57% (4/7).
Figure 2: Consort diagram for ROS1+ and ALK+ NSCLC included in analysis of CNS progression on crizotinib.
A total 33 ROS1+ and 115 ALK+ were eligible for this analysis. Characteristics of patients excluded are shown. Note: not all percentages may add to 100 due to rounding.
*These were ROS1+ and ALK+ patients for whom crizotinib was their first TKI, who had complete clinical follow up information, and for whom radiographic information to assess CNS progression was available.
† This is in reference to age and presence of brain metastases at time of stage IV diagnosis.
‡Brain radiation included either stereotactic radiosurgery (SRS) or whole-brain radiotherapy (WBRT).
§All patients that received chemotherapy received a combination of a platinum agent (cisplatin/carboplatin) in conjunction with either paclitaxel, etoposide, or pemetrexed prior to treatment with crizotinib.
‖All of these data are captured while patients were on crizotinib therapy.
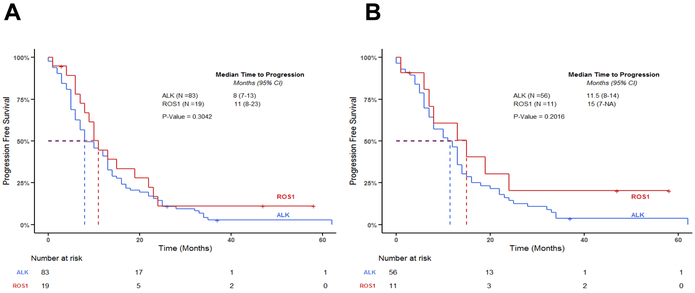
Ninety-five patients (16 ROS1+ and 79 ALK+) progressed on crizotinib. The median treatment duration for all ROS1+ and ALK+ patients in this analysis was 11 months and 13 months, respectively. PFS for the ROS1+ and ALK+ cohorts is shown in Figure 3. The median PFS for the ROS1+ cohort and ALK+ cohorts were 11 months (95% CI 8–23) and 8 months (95% CI 7–13) respectively, with no statistically significant differences between these groups (p = 0.304, Figure 3A). When focusing on patients who received crizotinib in the second line setting, the median PFS for ROS1+ NSCLC and ALK+ NSCLC was 15 months (95% CI 7 – NA) and 11.5 months (95% CI 8–14) respectively, with no significant differences between both groups (p = 0.202, Figure 3B). The upper limit of the 95% confidence interval was not reached for the second line ROS1+ NSCLC.
Figure 3: Median progression free survival in patients with ROS1+ and ALK+ NSCLC on crizotinib.
Progression free survival for ROS1 vs. ALK groups who received crizotinib. A log-rank test was used to compare survival between the two groups. (A): PFS of ROS1+ and ALK+ NSCLC on crizotinib at any line. There were no significant differences between groups (p = 0.304) (B): PFS of ROS1+ and ALK+ NSCLC who received crizotinib in the second line setting. There were no significant differences between groups (p = 0.202).
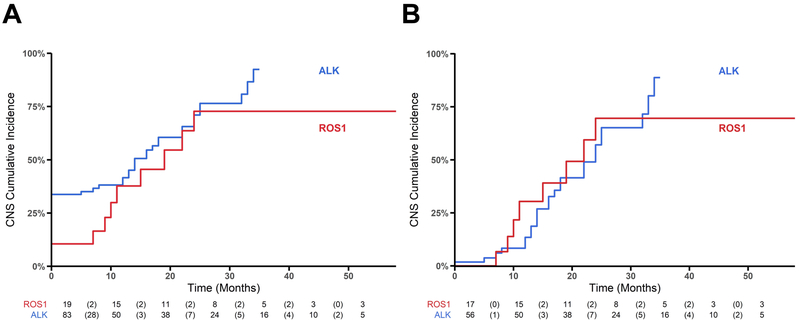
The CNS was the first and sole site of progression in 47% (9/19) of ROS1+ patients and 33% ALK+ (28/83) patients with no statistically significant differences between these groups (p = 0.610, Figure 2). P-CNS while on crizotinib is demonstrated in Figure 4. Among all patients, P-CNS was not significantly different between the ROS1+ and ALK+ groups (p =0.222, Figure 4A). When limiting analysis to patients who did not have brain metastases prior to receiving crizotinib, P-CNS was not significantly different between the ROS1+ and ALK+ groups (p = 0.908, Figure 4B). Within 24 and 21 months, 50% of ROS1+ and ALK+ patients without brain metastases prior to crizotinib had progressed within the CNS.
Figure 4: CNS progression in patients with ROS1+ and ALK+ NSCLC on crizotinib.
Cumulative incidence of CNS disease between ROS1 and ALK groups. The risk table below the graph reports (i) number at risk of CNS and death at the beginning of each time interval, (ii) in brackets the number of CNS events within a time interval. A log-rank test was used to compare differences between groups. (A): Cumulative incidence of brain metastases over time among all ROS1 and ALK NSCLC (p = 0.222). (B): Cumulative incidence of brain metastases over time in ROS1+ and ALK+ NSCLC with no brain metastases at time of stage IV diagnosis. There were no significant differences between the two groups (p = 0.908).
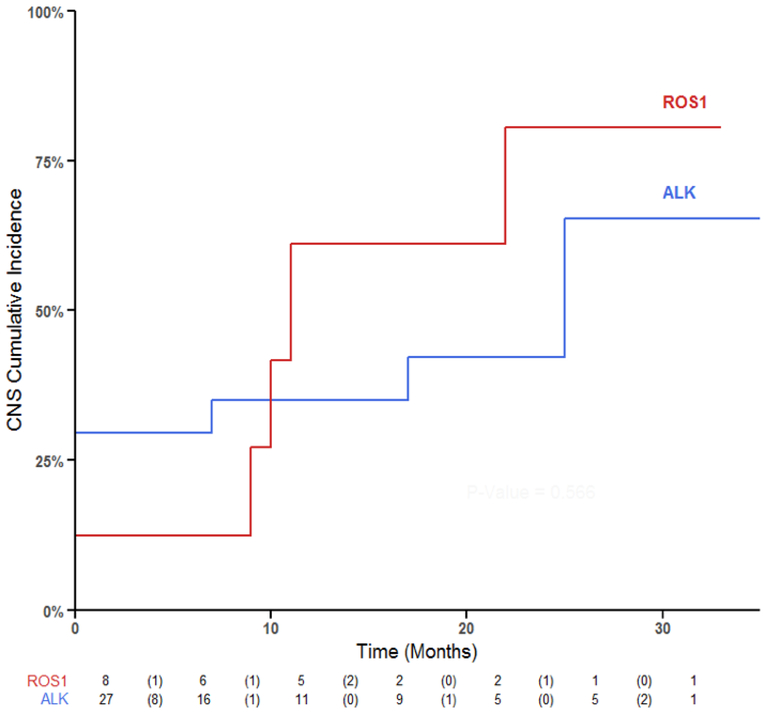
Given that prior chemotherapy could be a confounding variable, we ran a separate analysis to explore CNS progression in ROS1+ (n = 8) and ALK+ (n = 27) patients who received crizotinib as first line systemic therapy. In this subset, 12% (1/8) ROS1+ and 40% (8/20) ALK+ NSCLC patients had brain metastases at time of stage IV diagnosis. The percentage of ROS1+ and ALK+ patients on first line crizotinib who progressed within the CNS alone was 63% (5/8) and 22% (6/27) with no significant differences between the groups (p = 0.07, Figure 5). Brain radiosurgery was also a potential confounding variable in our data. We identified whether patients received brain radiosurgery at any point from time of diagnosis to cessation of crizotinib therapy. In this subset, none of the ROS1+ patients and 40% (8/20) of ALK+ patients received brain radiosurgery. There was no difference in cumulative incidence of CNS progression between these two groups (p = 0.556, Figure 5). The median duration of follow up for ROS1+ and ALK+ NSCLC who received first line crizotinib was 16 months and 8 months respectively.
Figure 5: CNS progression among ROS1+ and ALK+ NSCLC who received crizotinib as first line systemic therapy.
Cumulative incidence of CNS disease between ROS1 and ALK groups for the subset of individuals that received first line crizotinib. There were no differences between the two groups (p = 0.506).
4. Discussion
Our data demonstrate that brain metastases are common in stage IV, treatment-naïve, ROS1+ NSCLC, a finding in contrast with other studies that report a reduced incidence of brain metastasis.17, 27 In the EUROS1 Cohort, 31 ROS1+ NSCLC were retrospectively identified with 3.2% (1/31) patients having brain metastases at time of stage IV diagnosis.17 In a phase II study of ceritinib in patients with ROS1 rearrangements, 25% (8/32) of patients were found to have brain metastases prior to treatment with ceritinib, but the percentage of patients with brain metastases at time of stage IV disease was not reported.28 A multicenter phase II trial study of crizotinib in East Asian patients with ROS1+ NSCLC found that 18% (23/117) of patients had brain metastases at baseline.18 It is worth noting that in this study, patients with brain metastases were eligible only if they were asymptomatic or were neurologically stable for two weeks if treated. Another recent single institution series of 39 ROS1+ NSCLC cases found a lower prevalence of brain metastases at time of metastatic diagnosis when compared to an ALK+ cohort (19.4% versus 39.1% respectively).27
In our study, the baseline incidence of brain metastases in ROS1+ and ALK+ NSCLC was 36% and 34%, respectively. There were no statistically significant differences in the incidence of brain metastasis in ROS1+ NSCLC when compared to other oncogene subsets. While the percentage of ROS1+ NSCLC cases included in this study is higher than the reported population incidence (6% versus 1–2%), the number of ROS1+ patients in our study is comparable to other large scale studies at tertiary referral centers.17, 18, 27 Although differences in patterns of metastatic spread have been observed between oncogene subgroups in NSCLC,11 the data presented here suggest that the propensity towards brain metastases is independent of the oncogene, consistent with other studies demonstrating no statistically significant difference in the incidence of brain metastases between ALK, EGFR, KRAS or other oncogenes.11, 29 The data presented here extends this finding to ROS1 and BRAF oncogene subsets.
When we analyzed the incidence of brain metastases based on ROS1 fusion partners, we found that 42% percent (5/12) CD74-ROS1 gene fusions and 60% (3/5) non-CD74-ROS1 gene fusions had brain metastases at stage IV disease, a finding in contrast to a recent study that noted a higher incidence of brain metastases in patients with the CD74-ROS1 gene fusion.30 Since CD74-ROS1 is the most common gene rearrangement, it remains possible that the higher incidence of brain metastases noted may simply reflect the underlying prevelance of the CD74-ROS1 gene fusion. Given our limited sample size, our study was not powered to detect differences between gene fusions and time to CNS progression on crizotinib, though a recent study found no differences in CNS progression between CD74-ROS1 and non-CD74-ROS1 gene fusions.30 It is relevant to note that a univariate analysis in this study did identify brain metastases as a significant risk factor for PFS on crizotinib. Future studies examining differences in ROS1 gene fusions and propensity for CNS progression on TKI therapy are warranted.
The incidence of brain metastases for the ROS1+ patients included for the CNS progression analysis was markedly lower than the ALK+ group (11% vs 33%). We attribute this percentage difference to the significant number of otherwise eligible patients with ROS1+ NSCLC who were enrolled in clinical trials with CNS-penetrant TKIs, and were thereby excluded from this analysis. When we analyzed the excluded ROS1+ patients from this study, 71% (10/14) had brain metastases at stage IV disease and the exclusion of these patients from the crizotinib-treatment analyses accounts for the baseline differences in brain metastases between the ROS1+ and ALK+ cohorts.
Among patients without CNS metastases prior to crizotinib therapy, 50% of ROS1+ and ALK+ patients developed CNS metastases as only site of progression after 24 and 21 months of therapy suggesting that brain metastases can occur quite late in the course of treatment with crizotinib. This frequent CNS progression was observed in the ROS1 cohort despite the lower percentage of patients with brain metastases at initiation of crizotinib. This suggests that while crizotinib can be quite effective in maintaining systemic control, poor penetration across the blood brain barrier with resultant CNS metastatic spread remains a major source of morbidity for both ROS1+ and ALK+ NSCLC.
Prior systemic and radiotherapy were potential confounders in our analysis of CNS progression on crizotinib. We compared incidence of CNS metastases in ROS1+ and ALK+ patients who received crizotinib as first line systemic therapy. 63% of ROS1+ and 22% of ALK+ patients who received first line crizotinib had CNS progression as first and only site of progression, with no significant differences between these groups (p = 0.07). Our small sample size for these subset analyses limit the strength of these conclusions. Recent studies have shown improved outcomes for radiosurgery in patients with ALK or EGFR oncogene-addicted NSCLC.31 None of our ROS1+ patients who received first line crizotinib (n = 8) received brain radiotherapy. In this subset, 63% (5/8) of ROS1+ patients receiving crizotinib progressed in the CNS alone. In contrast, 40% (8/20) of ALK+ patients had received prior brain radiotherapy and 22% (6/27) of these patients progressed in the CNS alone. It is therefore possible that differences in CNS progression between our ROS1+ and ALK+ cohorts might be due to the percentage of ALK+ patients who received brain radiotherapy prior to crizotinib. This is consistent with prior studies where local ablative strategies in oncogene-addicted NSCLC have demonstrated disease control.32
There are several limitations to our study. Despite accruing a cohort of ROS1+ patients comparable to multiple other large studies, our sample size was not large enough to perform a multivariable analysis to adjust for potential confounders such as number of lines of prior therapy, duration of therapy, and treatment of brain metastases with radiation. We attempted to evaluate some of these potential confounders through stratified analyses but larger scale studies utilizing shared institutional databases would be helpful in clarifying the rate of CNS progression in ROS1+ NSCLC receiving TKI therapy. Given the retrospective nature of this series, small number of ROS1+ patients, and lack of standardized surveillance brain imaging while on crizotinib, it was not possible to accurately estimate intracranial overall response rate, intracranial disease control rate, or intracranial time to progression. These will be a valuable metrics in assessing efficacy of newer CNS-penetrant TKIs moving forward. Finally, given our small sample size, we were unable to generate conclusions regarding the association between ROS1 gene fusions and CNS progression while on crizotinib.
In the ALEX trial, alectinib has been shown to be superior to crizotinib as first line therapy for ALK+ NSCLC.19 Competing risk analysis of CNS progression within this trial demonstrated that much of the benefit in PFS was derived from a delay in CNS progression. Osimertinib has recently shown superiority to erlotinib or gefitinib in treatment-naïve EGFR+ NSCLC.33, 34 Like alectinib, osimertinib has shown CNS activity and thus one can speculate that some of the PFS benefit in this population was also due to a delay in CNS progression.33, 35 In the phase II study of ceritinib for ROS1+ NSCLC, intracranial response was 25% and disease control was achieved in 63% of patients in this study.8 Thus, it is likely that ROS1+ patients may also benefit from the use of a CNS-penetrant ROS1 inhibitor in the first line setting. In addition to ceritinib, several other TKIs with extended CNS penetration such as entrectinib,7 lorlatinib,9 and ropotrectinib,36 are being evaluated in clinical trials. Together, these observations reinforce the importance of CNS-penetrant TKIs for patients with ALK+ and ROS1+ NSCLC.
Supplementary Material
A total of 689 NSCLC patient charts with molecular testing were reviewed. 110 patients were excluded based on previously defined exclusion criteria. The number of patients per oncogene that were considered for analysis are shown.
Acknowledgements
Tejas Patil, Derek E. Smith, and Robert C. Doebele were involved with conception, data acquisition, design, and writing of the manuscript. Derek E. Smith assited in the statistical analysis of our data. D. Ross Camidge, Paul A. Bunn, Dara L. Aisner, and Daniel W. Bowles assisted in the revision of subsequent manuscripts and figures. All authors played a critical role in the appraisal of the final manuscript.
Sources of support: D. Ross Camidge MD PhD, Paul A. Bunn MD, and Robert C. Doebele MD PhD were supported in part by the University of Colorado Lung Cancer Specialized Program of Research Excellence (P50CA058187).
Footnotes
Competing Interests
Tejas Patil, Derek E. Smith, Mark Hancock, Daniel W. Bowles, and William T. Purcell have no conflict of interest. Paul A. Bunn received personal fees from Astrazenca and Guardant Health. Dara L. Aisner received personal frees from AbbVie, Bristol Myers Squibb, Inivata. Anh T. Le has a patent with Abbott Molecular with royalties paid. D. Ross Camidge has received honoraria or consulting fees from Ariad, Takeda, Ignyta, and Roche/Genentech, and a sponsored clinical trial research agreement with Takeda. Robert C. Doebele has received honoraria or consulting fees from Pfizer, Ariad, Takeda, Ignyta, AstraZeneca, Guardant Health, a sponsored research agreement with Ignyta, licensing fees for patent from Abbott Molecular and stock ownership in Rain Therapeutics.
References
- 1.Bergethon K, Shaw AT, Ou SH, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol 2012;30:863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies KD, Le AT, Theodoro MF, et al. Identifying and targeting ROS1 gene fusions in non-small cell lung cancer. Clin Cancer Res 2012;18:4570–4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies KD, Doebele RC. Molecular pathways: ROS1 fusion proteins in cancer. Clin Cancer Res 2013;19:4040–4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camidge DR, Pao W, Sequist LV. Acquired resistance to TKIs in solid tumours: learning from lung cancer. Nat Rev Clin Oncol 2014;11:473–481. [DOI] [PubMed] [Google Scholar]
- 5.Gainor JF, Shaw AT. Novel targets in non-small cell lung cancer: ROS1 and RET fusions. Oncologist 2013;18:865–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 2014;371:1963–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drilon A, Siena S, Ou SI, et al. Safety and Antitumor Activity of the Multitargeted Pan-TRK, ROS1, and ALK Inhibitor Entrectinib: Combined Results from Two Phase I Trials (ALKA-372–001 and STARTRK-1). Cancer Discov 2017;7:400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw AT, Engelman JA. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med 2014;370:2537–2539. [DOI] [PubMed] [Google Scholar]
- 9.Shaw AT, Felip E, Bauer TM, et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol 2017;18:1590–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorensen JB, Hansen HH, Hansen M, et al. Brain metastases in adenocarcinoma of the lung: frequency, risk groups, and prognosis. J Clin Oncol 1988;6:1474–1480. [DOI] [PubMed] [Google Scholar]
- 11.Doebele RC, Lu X, Sumey C, et al. Oncogene status predicts patterns of metastatic spread in treatment-naive nonsmall cell lung cancer. Cancer 2012;118:4502–4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johung KL, Yeh N, Desai NB, et al. Extended Survival and Prognostic Factors for Patients With ALK-Rearranged Non-Small-Cell Lung Cancer and Brain Metastasis. J Clin Oncol 2016;34:123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385–2394. [DOI] [PubMed] [Google Scholar]
- 14.Solomon BJ, Mok T. First-line crizotinib in ALK-positive lung cancer. N Engl J Med 2015;372:782. [DOI] [PubMed] [Google Scholar]
- 15.Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167–2177. [DOI] [PubMed] [Google Scholar]
- 16.Gainor JF, Tseng D, Yoda S, et al. Patterns of Metastatic Spread and Mechanisms of Resistance to Crizotinib in ROS1-Positive Non-Small-Cell Lung Cancer. JCO Precis Oncol 2017;2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazieres J, Zalcman G, Crino L, et al. Crizotinib therapy for advanced lung adenocarcinoma and a ROS1 rearrangement: results from the EUROS1 cohort. J Clin Oncol 2015;33:992–999. [DOI] [PubMed] [Google Scholar]
- 18.Wu YL, Yang JC, Kim DW, et al. Phase II Study of Crizotinib in East Asian Patients With ROS1-Positive Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:1405–1411. [DOI] [PubMed] [Google Scholar]
- 19.Ou SH, Ahn JS, De Petris L, et al. Alectinib in Crizotinib-Refractory ALK-Rearranged Non-Small-Cell Lung Cancer: A Phase II Global Study. J Clin Oncol 2016;34:661–668. [DOI] [PubMed] [Google Scholar]
- 20.Kim DW, Tiseo M, Ahn MJ, et al. Brigatinib in Patients With Crizotinib-Refractory Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer: A Randomized, Multicenter Phase II Trial. J Clin Oncol 2017;35:2490–2498. [DOI] [PubMed] [Google Scholar]
- 21.Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol 2012;13:1011–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davare MA, Vellore NA, Wagner JP, et al. Structural insight into selectivity and resistance profiles of ROS1 tyrosine kinase inhibitors. Proc Natl Acad Sci U S A 2015;112:E5381–5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ou SH, Tan J, Yen Y, et al. ROS1 as a ‘druggable’ receptor tyrosine kinase: lessons learned from inhibiting the ALK pathway. Expert Rev Anticancer Ther 2012;12:447–456. [DOI] [PubMed] [Google Scholar]
- 24.Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014;311:1998–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sholl LM, Aisner DL, Varella-Garcia M, et al. Multi-institutional Oncogenic Driver Mutation Analysis in Lung Adenocarcinoma: The Lung Cancer Mutation Consortium Experience. J Thorac Oncol 2015;10:768–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su Z, Dias-Santagata D, Duke M, et al. A platform for rapid detection of multiple oncogenic mutations with relevance to targeted therapy in non-small-cell lung cancer. J Mol Diagn 2011;13:74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gainor JF, Tseng D, Yoda S, Dagogo-Jack I, Friboulet L, Hubbeling HG, Farago AF, Schultz KR, Ferris LA, Piotrowska Z, Hardwick J, Huang D, Mino-Kenudson M, Iafrate AJ, Hata AN, Yeap BY, Shaw AT Patterns of Metastatic Spread and Mechanisms of Resistance to Crizotinib in ROS1-Positive Non–Small-Cell Lung Cancer. JCO Precis Oncol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim SM, Kim HR, Lee JS, et al. Open-Label, Multicenter, Phase II Study of Ceritinib in Patients With Non-Small-Cell Lung Cancer Harboring ROS1 Rearrangement. J Clin Oncol 2017;35:2613–2618. [DOI] [PubMed] [Google Scholar]
- 29.Rangachari D, Yamaguchi N, VanderLaan PA, et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer 2015;88:108–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Z, Shen L, Ding D, et al. Efficacy of Crizotinib among Different Types of ROS1 Fusion Partners in Patients with ROS1-Rearranged Non-Small Cell Lung Cancer. J Thorac Oncol 2018;13:987–995. [DOI] [PubMed] [Google Scholar]
- 31.Robin TP, Camidge DR, Stuhr K, et al. Excellent Outcomes with Radiosurgery for Multiple Brain Metastases in ALK and EGFR Driven Non-Small Cell Lung Cancer. J Thorac Oncol 2018;13:715–720. [DOI] [PubMed] [Google Scholar]
- 32.Weickhardt AJ, Scheier B, Burke JM, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol 2012;7:1807–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramalingam SS, Yang JC, Lee CK, et al. Osimertinib As First-Line Treatment of EGFR Mutation-Positive Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:841–849. [DOI] [PubMed] [Google Scholar]
- 34.Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113–125. [DOI] [PubMed] [Google Scholar]
- 35.Vansteenkiste J, Reungwetwattana T, Nakagawa K, et al. CNS response to osimertinib vs standard of care (SoC) EGFR-TKI as first-line therapy in patients (pts) with EGFR-TKI sensitising mutation (EGFRm)-positive advanced non-small cell lung cancer (NSCLC): Data from the FLAURA study. Ann Oncol 2017;28. [Google Scholar]
- 36.Drilon A, Ou SH, Cho BC, et al. A phase 1 study of the next-generation ALK/ROS1/TRK inhibitor ropotrectinib (TPX-0005) in patients with advanced ALK/ROS1/NTRK+ cancers (TRIDENT-1). J Clin Oncol 2018;36. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A total of 689 NSCLC patient charts with molecular testing were reviewed. 110 patients were excluded based on previously defined exclusion criteria. The number of patients per oncogene that were considered for analysis are shown.