Abstract
Patients with borderline personality disorder (BPD) often engage in dangerous self-injurious behaviors (SIBs) as a maladaptive technique to decrease heightened feelings of distress (e.g. negative feelings caused by social exclusion). The reward system has recently been proposed as a plausible neural substrate, which may influence the interaction between social distress and physical pain processing in patients that engage in SIBs. Using functional magnetic resonance imaging (fMRI) in 20 adult BPD patients with a history of SIBs and 23 healthy controls (HCs), we found a hyper-activation of the nucleus accumbens (NAcc) and amygdala when painful stimuli were presented to BPD patients (but not HCs) in a state of heightened distress, induced via social exclusion. This differential NAcc activity was mediated by anxious attachment style, which is a key developmental feature of the disorder. Altogether, these results suggest a neural mechanism underlying the pathophysiology of SIBs in these patients, which is likely reinforced via the reward system.
Keywords: Borderline Personality Disorder, self-injury, pain, social distress, nucleus accumbens, attachment style
Introduction
Borderline personality disorder (BPD) is a severe and debilitating disorder characterized by emotional instability, chaotic relationships and impulsive self-destructive behaviors (American Psychiatric Association, 2013). Affective and interpersonal difficulties are related to non-secure attachment patterns (reported in more than 80% of patients; Agrawal et al., 2004) and are associated with dangerous self-injurious behaviors (SIBs; Martin et al., 2017). Up to 80% of BPD patients engage in SIBs (e.g. cutting, burning, etc.), which could lead to increased risk of suicide (Perroud et al., 2012). These harmful behaviors are predicted by low levels of distress tolerance, i.e. the ability to tolerate daily distressing experiences (Ammerman et al., 2017). Negative affect and distress are often caused by (real or perceived) social rejection, exclusion or separation (Bohus et al., 2000; Ducasse et al., 2014). Fluctuations in negative affective states and perceived rejection/interpersonal distress are a large contributing factor to the urge to engage in SIBs, which, when acted upon, results in relief from this distress/inner tension (Kleindienst et al., 2008; Snir et al., 2015). Compared to healthy controls (HCs), BPD patients report a greater decrease of arousal immediately after nociceptive stimuli (skin incision or blade application) which also induces tension relief in the context of stress (Willis et al., 2017). Oddly, 60% of BPD patients report no pain (i.e. analgesia) while engaging in SIBs (Bohus et al., 2000), while all generally experience relief from emotional distress (Nock, 2010; Perroud et al., 2012; Bresin and Gordon, 2013).
SIBs represent an effective (yet maladaptive) emotion regulation strategy used by BPD patients. Several functional magnetic resonance imaging (fMRI) studies have aimed to better understand the neural mechanisms underlying the interaction between physical pain and heightened distress in these patients; however there are conflicting results within the literature. BPD patients showed a negative coupling between amygdala and the middle frontal gyrus as well as between the right insula and dorsolateral prefrontal cortex, only when negative emotional pictures were combined with painful temperature (Niedtfeld et al., 2012). More recently, Reitz et al. (2015) reported that inducing stress (via time-constrained arithmetic tasks) led to decreased pain-related amygdala activity in BPD patients (with respect to HCs) together with increased fronto-amygdalar connectivity, possibly reflecting an inhibition of limbic activity. Together, these results suggest that SIBs might directly contribute to the downregulation of heightened negative emotional states. Finally, in a longitudinal study Niedtfeld et al., (2017), BPD patients first showed a decrease in amygdalar response (as well as altered limbic connectivity) when negative emotional stimuli were paired with painful thermal stimulation, which then resolved following dialectical behavior therapy (a therapy which taught the patients emotional regulation techniques). Together, these neuroimaging results suggest an inhibition of limbic activity (possibly via prefrontal areas) by physical pain following heightened emotional distress; however in each of the abovementioned studies, stress was induced via negative emotional pictures or via an arithmetic task, but not directly via social rejection. Bungert et al. (2015) tested patients’ sensitivity to thermal pain after induction of social distress through a virtual ball-tossing game, i.e. the Cyberball game, a well-established paradigm of social exclusion (Eisenberger et al., 2003; Williams and Jarvis, 2006; Bungert et al., 2015). Using a region-of-interest (ROI) approach, they found that physical pain activated the posterior insula after exclusion but reduced amygdala activation mainly after inclusion in the BPD patients (compared to HCs). In view of these seemingly conflicting results, further research is awaited to clarify the neural mechanisms underlying the interaction between physical and emotional pain in BPD patients, to better understand the development and maintenance of SIBs and ultimately improve their therapeutic management.
SIBs do not only alleviate heightened distress, but can also result in an increase of positive emotions (Nock, 2010; Bresin and Gordon, 2013), which we suggest here may in turn reinforce these pathological behaviors in particular via an activation of the reward system. Intriguingly, BPD patients (independent of SIB status) show decreased nucleus accumbens (NAcc) responses to cues predictive of an upcoming reward (Enzi et al., 2013; Herbort et al., 2016). Similarly, reductions in NAcc and prefrontal cortex activity during reward anticipation in self-injuring adolescent girls (independent of BPD status) have also been reported (Sauder et al., 2016). Moreover, Vega et al. (2017) found that adult BPD patients that engage in SIBs (compared to both non-SIB patients and HCs) exhibited enhanced orbitofrontal cortex activation following an unexpected reward, suggesting an impairment in the ability to update reward associations. These studies converge to show dysfunctional neural responses within reward-related circuits in BPD.
SIBs are frequent, serious and often used as a diagnostic criterion of BPD. Yet, because SIBs alter physical pain perception in BPD patients (Ludäscher et al., 2009), and thus potentially neural responses to pain processing itself, it appears critical to recruit a homogeneous population of patients regarding SIBs (i.e. using tissue-damaging methods such as cutting, scratching, burning). To our knowledge, this has never been done before, and may explain some of the inconsistencies in the literature (see above). Therefore, in the present study, we recruited BPD patients with a recent and well-documented history of engaging in SIBs (e.g. self-cutting/burning). Our main hypothesis was to test if the reward system (in particular the NAcc) of these patients modulates the processing of physical pain (via thermal stimulation) following induction of social distress (elicited via the Cyberball game). Additionally, the use of a full-factorial brain model (with a specific stimulation temperature covariate) allowed us to control for the differential pain sensitivity, which is present between BPD patients and HCs. Further, during an explorative analysis, we utilized a mediation model to determine whether and how other key dimensions of this disorder (i.e. individual attachment styles and depression level) might contribute to the observed differences in neural activations. Finally, as a secondary aim, we sought to replicate/clarify the role of the limbic system in the interaction between social distress and physical pain processing in BPD (by replicating the results of Bungert et al.2015).
Materials and methods
Participants
Twenty-one female BPD patients with a history of engaging in SIBs (see below), who met the DSM-5-criteria for BPD and 24 healthy female participants, without any lifetime psychiatric diagnoses were included in our study. Due to technical issues, one patient and one HC were excluded from the fMRI analyses (BPD: n = 20, age 21–38: M = 27.0, s.d. = 4.86; HC: n = 23, age 19–36: M = 25.0, s.d. = 5.44). We recruited BPD outpatients from the specialized ambulatory service in Geneva (Switzerland), and the HCs were contacted by advertisements throughout the Geneva area.
Participants were first screened for inclusion criteria and then assessed by both a trained psychologist and a psychiatrist (see Supplemental Material for more detail). All patients had a history of tissue damaging SIBs (specifically including self-cutting, −burning and -hitting) within the past 1.5 years (median = 30 days). Diagnosis was established through medical records and standardized assessments [Diagnostic Interview for Genetic Studies (DIGS)] for axis-I psychopathology and Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II) BPD part. Three patients were on benzodiazepines. To control for potential effects of medication in the results, we computed a medication load for each patient (see Supplemental Material). Level of depression was assessed by the Montgomery and Asberg Depression Rating Scale (MADRS) (Montgomery and Asberg, 1979) and Beck Depression Inventory-II (BDI; Beck et al., 1988). We used the Relationship Scales Questionnaire (RSQ; Griffin and Bartholomew, 1994) to assess anxious and avoidant attachment styles (Kurdek, 2002). We additionally evaluated BPD symptom severity using the Borderline-Symptom-List (BSL-23) and the frequency of recent self-destructive behaviors using the BSL-Supplement (Nicastro et al., 2016), both of which were administered within 1 week before the scanning session (Table 1 and Supplemental Material).
Table 1.
Demographic and clinical characteristics
| Group | |||||||
| BPD (N = 20) | HC (N = 23) | ||||||
| Demographic characteristics and questionnaire ratings | |||||||
| Mean | s.d. | Mean | s.d. | t-Value | P-Value | ||
| Age | 26.95 | 4.86 | 25.00 | 5.44 | 1.23 | 0.225 | |
| SCID-II (BPD part) | 7.30 | 1.22 | -- | -- | -- | -- | |
| Last instance of SIB (in days) | 72.39 | 96.02 | -- | -- | -- | -- | |
| BSL-23 | 41.75 | 14.11 | 1.52 | 2.40 | 12.59 | 0.000 | |
| BSL-Supplement | 4.05 | 4.63 | 0.34 | 0.83 | 3.53 | 0.002 | |
| MADRS | 11.60 | 5.35 | 0.22 | 0.52 | 9.47 | 0.000 | |
| BDI | 24.60 | 7.92 | 1.61 | 1.95 | 12.65 | 0.000 | |
| RSQ: Attachment Avoidance | 23.75 | 5.18 | 20.09 | 4.87 | 2.39 | 0.022 | |
| RSQ: Attachment Anxiety | 19.55 | 4.43 | 9.39 | 3.14 | 8.76 | 0.000 | |
| Clinical Characteristics | |||||||
| N | % | ||||||
| Currently depressed | 7 | 35 | |||||
| Bipolar disorder | 3 | 15 | |||||
| Anxiety disorder | 12 | 60 | |||||
| ADHD | 7 | 35 | |||||
| Eating disorder | 2 | 10 | |||||
| Suicide attempt | 11 | 55 | |||||
| Medication | |||||||
| N | % | ||||||
| Antidepressants | 12 | 60 | |||||
| Antipsychotics | 5 | 25 | |||||
| Benzodiazepines | 3 | 15 | |||||
| Methylphenidate | 3 | 15 | |||||
ADHD, attention deficit hyperactivity disorder; BDI, Beck depression inventory; BPD, borderline personality disorder patients; BSL, borderline symptom list; HC, healthy controls; MADRS, Montgomery and Asberg Depression Scale; RSQ, relationship scales questionnaire; s.d., standard deviation; SCID-II, structured clinical interview for DSM Axis II personality disorders (BPD part); SIB, self-injurious behavior
The Ethics committee of the University Hospitals of Geneva approved the study. Each participant provided written informed consent.
Experimental overview
Participants played a total of 16 blocks of a modified version of the virtual ball-tossing game called Cyberball, which is a well-established paradigm used to evoke feelings of social inclusion or exclusion (Eisenberger et al., 2003; Williams and Jarvis, 2006; Bungert et al., 2015). Participants were told that they would randomly play the game in short blocks with two different pairs of anonymous players (i.e. players A&B, or players C&D; Figure 1). Unbeknownst to the participant, players A&B represented the inclusion pair that often threw the ball to the participant while C&D represented the exclusion pair, which only threw the ball to the participant once, then threw it exclusively to each other (see Supplemental Material). Thus, from the participant’s point of view, she was not randomly included and excluded by the same ‘people’, but instead the behavior of each player was consistent throughout the experiment thus, increasing the believability of the exclusion condition.
Fig. 1.

Schematic representation of the experimental paradigm. Participants played the Cyberball game with blocks of either inclusion or exclusion. These were followed by either a warm, non-painful stimulation or a hot, painful thermal stimulation. Finally, participants rated on a 5-point Likert scale their subjective pain intensity and pain unpleasantness.
Each Cyberball block was followed by the administration of either a hot, subjectively painful or a warm, non-painful thermal stimulation (41°C). The temperature of the hot stimuli was delivered with a 70% subjective pain rating which was determined before scanning using a multiple random staircase algorithm (see the Supplemental Material). Thermal stimuli were delivered through a computer-controlled thermal stimulator with an MRI-compatible 25 × 50 mm fluid-cooled Peltier probe (MSA, Thermal Stimulator-Somedic AB, Sweden), attached to the participants’ inner side of the left upper arm. Each stimulation (hot and warm) consisted of 2 seconds of raising temperature, 2 seconds of plateau and 2 seconds of decrease. Following each thermal stimulation, participants were asked to report pain intensity and unpleasantness on 5-point Likert scales (Figure 1). At the end of the scanning session, participants answered questions about their levels of social distress experienced during the inclusion and exclusion blocks (i.e. the Need–Threat Scale; Williams and Jarvis, 2006). Thus, separate distress scores were obtained for the inclusion and exclusion conditions, i.e. when playing with the A&B pair and C&D pair, respectively.
MRI data acquisition and analysis
Functional images of the whole brain were acquired using a multiplexed echo planar imaging (EPI) sequence (Feinberg et al., 2010) with repetition time (TR) = 650 ms, echo time (TE) = 30 ms, flip angle = 50°, 36 slices, 64 × 64 pixels, voxel size = 3 × 3 × 3 mm. The multiband acceleration factor was 4, and parallel acquisition technique (PAT) was not used. Structural images were acquired with a T1 weighted 3D sequence (MPRAGE, TR/inversion time/TE = 1900/900/2.27 ms, flip angle = 9°, PAT factor = 2, matrix size = 256 × 256 × 192, voxel size = 1 × 1 × 1 mm).
All MRI data preprocessing and analyses were performed using SPM8 software (http://www.fil.ion.ucl.ac.uk/spm) implemented in Matlab R2012b (Mathworks, Natick, MA). During preprocessing, the functional volumes were first realigned to the mean image. Next, the resliced mean image and realigned functional images were normalized using the EPI template provided with the SPM toolbox, which is in MNI space. Finally, images were smoothed with an 8 mm3 Gaussian kernel. To account for residual movement artefacts after realignment, Artefact Detection Toolbox (ART; http://web.mit.edu/swg/software.htm) was used (see Supplemental Material).
A general linear model (GLM) was then used to compute parameter estimates of activity at each voxel, for each experimental condition, in each participant. Regressors included in this GLM were as follows (Figure 1): two regressors corresponding to the Cyberball blocks (i.e. inclusion and exclusion; 43 sec blocks); four regressors corresponding to the thermal stimulations (i.e. hot stimulation following inclusion, warm following inclusion, hot following exclusion and warm following exclusion; 6 sec mini-blocks); two regressors corresponding to the pain ratings (i.e. intensity and pleasantness; 5 sec mini-blocks); six motion parameters and outlier scans detected by the ART toolbox were modeled as nuisance regressors (see Supplemental Material). A high-pass filter of 128 sec was also applied.
To determine the effects of social exclusion on physical pain processing, linear contrasts for the four different thermal stimulation conditions (i.e. hot stimulations following inclusion and exclusion and warm stimulations following inclusion and exclusion) were entered into a second-level 2 × 2 × 2 full factorial model containing two within factors: ‘Previous Cyberball Condition’ (inclusion, exclusion) × ‘Stimulation Temperature’ (hot, warm) and the between factor ‘Group’ (HC, BPD). Because the BPD patients required a significantly higher stimulation temperature in order to reach a similar subjective threshold of physical pain as the HC group (see the Behavioral results), we added the individual stimulation temperatures as nuisance regressors to the GLM. Thus, for warm stimulations the corresponding regressor was always equal to 41°, while for hot stimulations the corresponding regressor contained the stimulation temperature used for that condition for each participant, as determined by the individual subjective pain thresholds (see the Supplemental Material for more information).
In order to investigate our main hypothesis about the role of reward circuits, in particular those involved in reward anticipation (see Introduction) on the processing of physical pain under heightened distress (i.e. following social exclusion), we created a single mask image from an automated term-based meta-analysis using Neurosynth (neurosynth.org; Yarkoni et al., 2011). To create this mask, we searched for the term ‘reward anticipation’ and downloaded the activation map based on reverse inference from 64 studies (and 2355 reported activations). This automated activation map was created using a chi-squared test to generate P-value maps which were FDR-corrected (see Yarkoni et al., 2011 for more details). This single mask contained several well-defined structures including the bilateral NAcc, VTA and caudate.
As we were mainly interested in interaction between physical pain (i.e. hot vs warm stimulations) following heightened social distress (i.e. exclusion vs inclusion) in BPD patients (vs HCs), we focused on the contrast representing this three-way interaction. Because the reward anticipation mask included rather small, well-defined structures (e.g. the bilateral NAcc, VTA and caudate) we utilized a small volume correction (SVC) approach by reporting activations that survive family-wise error (FWE) correction for multiple comparisons at the voxel level. Both SVC analyses were conducted applying a statistical threshold corresponding to P < 0.05 FWE corrected for the whole volume of interest.
Finally, given that the experimental design used here shared similarities with the one used in the Bungert et al. (2015) study, our secondary aim was to replicate their results in the posterior insula and amygdala. To this end, we created a second mask containing 10 mm spheres over coordinates in bilateral insula [±30, −28, 19] and amygdala [±21, −7, −14] reported in this previous study (Bungert et al., 2015). The mask was crafted using the WFU PickAtlas toolbox (Maldjian et al., 2003, 2004), and was also applied to the same above mentioned three-way contrast using SVC.
We extracted and plotted the beta estimates from the significantly activated brain regions in order to visualize the results (as well as to run the mediation analysis described below). This was done using the MarsBaR toolbox (http://marsbar.sourceforge.net) from a 4 mm sphere around the peaks identified during the SVCs.
Mediation analysis
To better characterize the relationship between the significantly activated regions during the three-way interaction (i.e. Previous Cyberball Condition by Stimulation Temperature by Group) and the clinical properties of our patient population, we ran a mediation analysis. Specifically, we conducted a group-level multiple mediation analysis using ordinary least squares path analysis (according to Hayes, 2013) using the PROCESS macro (processmacro.org) implemented in IBM SPSS 22 (SPSS Inc., Chicago, IL). The categorical variable ‘group’ (HC = 0, BPD = 1) was entered as the independent variable, the beta estimates extracted from significantly activated regions in the reward-anticipation mask during the three-way interaction (i.e. the right NAcc) were entered as the dependent variable. The mediators entered were the attachment anxiety and avoidance subscales from the RSQ as well as the total score from the BDI. The significance of the indirect effect (i.e. part of the relationship between the dependent and independent variables explained by the mediators) was assessed using a bias-corrected bootstrap technique with 10 000 re-samplings.
Psychometric and statistical analysis
To compare group differences for variables with repeated measures (i.e. distress ratings and pain ratings) and to check for any effects of medication load, we performed analyses of variance and covariance (ANOVA/ANCOVAs) using SPSS. For other variables (e.g. subjective stimulation temperatures, questionnaire ratings), we used independent t-tests. Finally, Spearman correlations were used where appropriate (see below). For these analyses, significance threshold was set to a = 0.05, and all tests were two-tailed.
Results
Behavioral results
Subjective pain thresholds
BPD patients had higher subjective pain thresholds (M = 49.08 °C, s.d. = 1.17) compared with HCs (M = 47.48 °C, s.d. = 1.23; t(43) = −4.20, P < 0.001). In the BPD group, pain thresholds correlated negatively with the number of days since last SIB (ρ = −0.48, P = 0.044; i.e. the more recent the SIB, the higher the pain threshold) and positively with frequency of self-destructive behaviors (i.e. the BSL-Supplement; ρ = 0.46, P = 0.034).
Pain ratings
The ANOVA for the pain intensity ratings collected after each stimulation during scanning showed a main effect of Stimulation Temperature [F(1,41) = 929.38, P < 0.001, ηp2 = 0.96] because all participants rated hot stimulations as more intense than the warm ones. There was also a main effect of Group [F(1,41) = 4.17, P = 0.048, ηp2 = .092] because BPD patients as compared to HCs rated stimulations as being generally less intense (Supplementary Table S2). The main effect of Previous Cyberball Condition and all interactions were not significant.
The ANOVA for the pain unpleasantness ratings also collected during scanning showed a main effect of Stimulation Temperature (F(1,41) = 181.17, P < 0.001, ηp2 = 0.82) because hot stimulations were rated as more unpleasant than warm stimulations (Supplementary Table S2). The main effect of Group, Previous Cyberball Condition and all interactions were not significant. Interestingly, BSL-23 score negatively correlated with differential pain unpleasantness for hot minus warm in the BPD group following exclusion (ρ = −0.50, P = 0.027), but not inclusion (ρ = −0.25, P = 0.30), suggesting that patients with a more severe symptomatology experienced post-exclusion pain as less unpleasant.
Distress ratings
At the very end of the scanning session, both groups rated the exclusion condition as more distressing than the inclusion condition (Supplementary Table S2), resulting in a main effect of Previous Cyberball Condition (F(1,40) = 169.68, P < 0.001, ηp2 = 0.81). BPD patients also were more distressed overall, as evidenced by a main effect of Group (F(1,40) = 15.01, P < 0.001, ηp2 = 0.27). The interaction between Previous Cyberball Condition and Group was not significant (P = 0.10).
fMRI results
SVC using the reward anticipation mask in the three-way interaction
SVC analysis revealed a significant three-way interaction in the right NAcc [4 consecutive voxels, peak (15, 8, −14), t = 3.28, PSVC-FWE = 0.017; Figure 2A], and there was a statistical trend in the left NAcc [peak (−15, 11, −11), t = 2.88, PSVC-FWE = 0.051].
Fig. 2.

NAcc mean activation during the interaction Cyberball Condition by Stimulation Temperature by Group. (A) NAcc activation (yellow) during the three-way interaction (Cyberball Condition × Stimulation Temperature × Group) overlaid on the reward anticipation mask (red). Activations are shown with P = 0.005 uncorrected (for illustrative purposes) and overlaid on the averaged normalized T1-weighted anatomical images created from all participants. (B) Mean beta estimates extracted from the right NAcc [15 8–14]. Error bars, standard error of the mean (SEM); a.u., arbitrary units; BPD, borderline personality disorder patients; HC, healthy controls; NAcc, nucleus accumbens.
To better understand the interaction effect in the right NAcc, beta estimates were extracted and entered into a 2 × 2 within-subject ANOVA in SPSS for each group separately. In the HCs, there was solely a significant main effect of Stimulation Temperature [F(1,22) = 8.94, P = 0.007, ηp2 = 0.29] whereby the NAcc was more activated during the hot stimulations than the warm (Figure 2B left graph). Similar to the HCs, there was also a main effect of Stimulation Temperature [F(1,19) = 5.54, P = 0.029, ηp2 = 0.23], whereby the NAcc was more activated during the hot stimulations. In addition, there was also a main effect of Previous Cyberball Condition [F(1,19) = 4.84, P = 0.040, ηp2 = 0.2], as well as an interaction between both factors (F(1,19) = 5.70, P = 0.025, ηp2 = 0.24). Figure 2B (right graph) shows that the interaction was driven by a large increase in NAcc activity for hot stimulations following exclusion compared to the other conditions.
Mediation analysis
A multiple mediation analysis revealed that individual RSQ anxiety scores contributed to the effect of Group (BPD patients, HCs) on NAcc activity for the critical interaction between Previous Cyberball Condition and Stimulation Temperature. As can be seen from Figure 3 (Supplementary Table S3), BPD patients (compared to HCs) expressed significantly higher attachment anxiety (a1 = 10.2, P < 0.001), attachment avoidance (a2 = 3.7, P = 0.02) and BDI (a3 = 23.0, P < 0.001) scores. The bias-corrected bootstrap confidence interval (CI) for the total indirect effect was entirely above 0 [CI (0.13, 2.53)], but the indirect path of attachment anxiety was the only significant mediator [attachment anxiety CI (0.011, 2.47); attachment avoidance CI (−0.06, 0.38); BDI CI (−0.91, 1.37)]. There was no longer an effect of BPD group (vs HC) on the differential NAcc activity after accounting for the mediation (c’ = −0.46, P = 0.56).
Fig. 3.
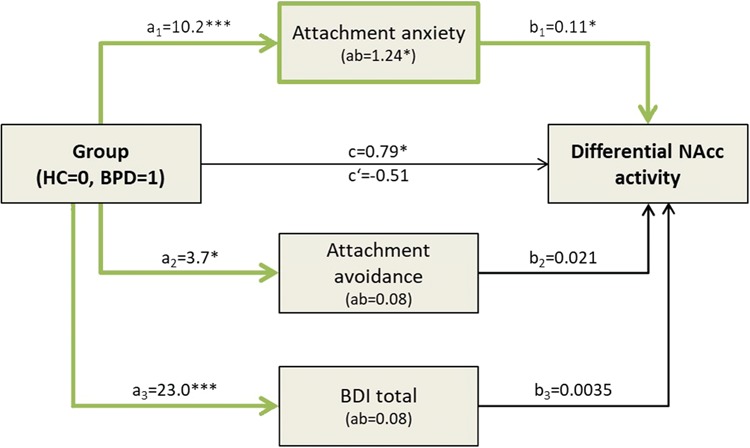
Right NAcc Mediation Analysis Schematic Representation. Multiple mediation model testing the relationship between group and beta estimates extracted from the right NAcc (three-way interaction, Figure 2). Beta estimates for each participant correspond to the difference of activity between Cyberball conditions (Inclusion > Exclusion) and stimulation temperatures (Hot > Warm). The bias-corrected bootstrap CI for the total indirect effect was entirely above 0 [CI (0.13, 2.53)], but the indirect path of attachment anxiety was the only significant mediator [attachment anxiety CI (0.011, 2.47); attachment avoidance CI (−0.06, 0.38); BDI CI (−0.91, 1.37)]. Significant pathways/mediators are shown by the thickened green lines. *P < 0.05, **P < 0.01, ***P < 0.001. BDI, Beck Depression Inventory; BPD, borderline personality disorder; HC, healthy controls; NAcc, nucleus accumbens; RSQ, Relationships Scale Questionnaire.
SVC using the bilateral insula and amygdala mask
SVC analysis revealed a significant three-way interaction in the left amygdala [3 consecutive voxels, peak (−15, −4, −17), t = 3.49, PSVC-FWE = 0.029; Figure 4A]. There were no significant voxels in the insula.
Fig. 4.

Amygdala mean activation during the interaction Cyberball Condition by Stimulation Temperature by Group. (A) Amygdala activation (yellow) during the three-way interaction (Cyberball Condition × Stimulation Temperature × Group) overlaid on the ROI mask created from Bungert et al.2015 (red). Activations are shown with P = 0.005 uncorrected (for illustrative purposes) and overlaid on the averaged normalized T1-weighted anatomical images created from all participants. (B) Mean beta estimates extracted from the left amygdala [−15, −4, −17]. Error bars, standard error of the mean (SEM); a.u., arbitrary units; BPD, borderline personality disorder patients; HC, healthy controls.
To better understand the interaction effect in the left amygdala, beta estimates were extracted and entered into a 2 × 2 within-subject ANOVA in SPSS for each group separately. The ANOVA in the HCs did not reveal any significant main effects or interactions (all P > 0.09; Figure 4B left graph). In the BPD patients, there was a main effect of Stimulation Temperature [F(1,19) = 47.96, P < 0.001, ηp2 = 0.72] where the amygdala was more activated for the hot stimulations than the warm, and an interaction between Previous Cyberball Condition and Stimulation Temperature [F(1,19) = 6.69, P = 0.018, ηp2 = 0.26] where the difference between hot and warm stimulations in the exclusion condition was much larger than the difference in the warm (Figure 4B, right graph).
Discussion
We believe that the present study represents a strong, comprehensive and substantial addition to our understanding of the neural dysfunctions underlying BPD, specifically the interaction between physical pain and emotional distress. Experimentally induced social exclusion affects physical pain processing in the NAcc (and amygdala) in BPD patients compared to HCs. Additionally, this group-dependent NAcc activation was mediated by anxious attachment style.
At the behavioral level, patients required higher temperature stimuli to feel comparable levels of pain like HCs. The individual stimulation temperatures negatively correlated with the number of days since the last instance of SIB and positively with the BSL-Supplemental questionnaire, suggesting that recent and frequent self-destructive behaviors relate to increased pain thresholds in BPD patients. BPD patients have previously been shown to express reduced pain sensitivity (Schmahl et al., 2006; Schmahl and Baumgärtner, 2015), in the absence of peripheral somatosensory deficits (normal detection thresholds; Ludäscher et al., 2007; Pavony and Lenzenweger, 2014). Also in line with previous literature (Renneberg et al., 2011), BPD patients felt more overall distress throughout the Cyberball game as compared to HCs. This effect is consistent with the observation that BPD patients have greater tendency to expect and perceive rejection in social situations and are more concerned about these experiences compared with healthy individuals or patients with mood or anxiety disorders (Staebler et al., 2011; Domsalla et al., 2014). We also found that BPD patients did not differ from HCs in how they rated pain unpleasantness (following exclusion vs inclusion). However, symptom severity negatively correlated with unpleasantness for painful hot stimulations (vs warm) but only in the exclusion condition, suggesting that social exclusion decreased perceived pain unpleasantness selectively for patients with higher BPD symptom severity. Because of such dampened affective impact of physical pain, these patients may be deprived from important internal signals that would prevent them from seriously injuring themselves. These results show that BPD patients who engage in SIBs experience a state-independent decrease in pain sensitivity, whose affective component is further suppressed by heightened distress (e.g. social exclusion; Bohus et al.2000).
Confirming our hypothesis, the NAcc, which is an important component of the brain reward pathway (Diekhof et al., 2012), was activated for hot stimulations following exclusion in BPD patients, but not the HC group. Activity extracted from this region showed solely a main effect of Stimulation Temperature in the HCs, whereas in the BPD group, there was also a main effect of Previous Cyberball Condition as well as an interaction between both factors. A similar interaction was also found in the patients’ left amygdala. This pattern of results provides a neural substrate for the distinctive interaction between physical and emotional pain in BPD patients, whereby physical pain applied during social distress boosts NAcc and amygdala responses. In addition, we found that group differences in NAcc activity for the interaction between the Previous Cyberball Conditions and Stimulation Temperatures were mediated by anxious attachment style. Anxious attachment, which relates to a high fear of abandonment/rejection coupled with a negative self-representation, is highly associated with the development of BPD (Scott et al., 2009). Based on these findings, we may ask whether changes in NAcc functional responses to pain follow a similar developmental trajectory—a hypothesis that could be tested in future work.
In addition to conveying reward-related dopaminergic signals, the NAcc is also dense in opioidergic receptors (Castro and Berridge, 2014). The opioidergic system is known to be involved in physical pain processing, and also in social distress, social bonding (Herman and Panksepp, 1978) and attachment style (Eisenberger, 2012). Based on the observation that BPD patients express abnormal patterns of opioidergic activity (Prossin et al., 2010), several authors proposed an opioid-deficiency theory of BPD (New and Barbara, 2010; Stanley and Siever, 2010; Herpertz and Bertsch, 2015). According to this theory, patients engage in SIBs as a means to stimulate this dysfunctional opioidergic system (New and Barbara, 2010; Brüne, 2015). Even very speculative, our findings of increased NAcc reactivity to painful stimuli during social distress may be understood at light of the hypothesis that the emotion regulation function of SIBs may also be partly attributable to an ensuing increase in endogenous opioid release (see Bresin and Gordon 2013). Nevertheless, conclusions about the neurochemistry of SIBs cannot be confirmed based on our fMRI results. This field of investigation deserves further studies involving pharmacological imaging.
A secondary aim of our paper was to replicate the previous results reported by Bungert et al. (2015). Specifically, the authors found that BPD patients expressed a hypo-activation of the amygdala for hot (vs warm) stimuli following inclusion (and a trending hypo-activation following exclusion). In contrast, our results show that our BPD patients expressed an interaction between Previous Cyberball Condition and Stimulation Temperature wherein there was a greater increase in amygdala activity between hot (vs warm) stimulations following exclusion compared to inclusion. The amygdala is part of subcortical structures in the limbic system which has been shown to be particularly responsive to relevant stimuli (Sander et al., 2003), and has been extensively implicated in the pathophysiology of BPD (Ruocco and Carcone, 2016). One possible explanation for these discrepant results might be due to differences in the studied patient populations. Unlike Bungert et al. who recruited patients independently of their SIB status (i.e. 50–50% a mix between patients that do and do not engage in SIBs), we only included patients engaging in SIBs. As previously stated, presenting painful stimuli in a state of social distress may be particularly relevant for BPD patients presenting with SIBs, because these behaviors represent a means to alleviate current feelings of social rejection (for a more in-depth discussion, see the Supplementary Material). Thus, increased amygdala activity in our study would signal higher affective relevance of the painful stimuli. Further supporting this interpretation, the recent BPD literature is starting to suggest differences in neuronal processing between patients who do and do not engage in SIBs (e.g. Vega et al.2017), which may also be consistent with the clinical heterogeneity of this pathology. However, it is also possible that the duration and intensity of the stimulations could have had an effect on these results, as the stimulations we used were shorter and subjectively more painful than those used in previous studies. Moreover, we did not find an activation of posterior insula during physical pain following exclusion, presumably because we included stimulation temperature in our model as previously explained (Methods, and Supplemental Material). Taken together, our results confirm that BPD patients differ from HCs in how they process physical pain following social exclusion, both at the behavioral and brain levels.
One main strength of our study is the inclusion of patients with a recent history of SIBs. This is also the first time that a direct link has been established between specific dysfunctional neural activity and insecure attachment, the latter being one of the main proposed contributing factors to the etiology of BPD (Crowell et al., 2009). Nevertheless, our study also has limitations. First, painful stimulations were not self-inflicted, unlike what happens in the patients’ real life. Yet, self-inflicted physical pain involves cognitive processes, influencing pain processing. This aspect provides an interesting future direction for BPD and SIB research. A second possible concern relates to the fact that we included medicated patients. Even though we checked for interactions between medication load and NAcc (and amygdala) activations, we cannot fully rule it out as a confounding factor. However, it should be noted that our differential neural activity in the NAcc was mediated by anxious attachment style, which is a stable trait-like measure, unlike individual prescriptions for medication, which vary over time. Thus, the results from the mediation analysis, at least partially, further imply that medication load did not interact with the NAcc activity.
To conclude, our results suggest a neural mechanism whereby physical pain interacts with social distress in the NAcc of BPD patients presenting with SIBs and anxious attachment. Together with a plausible implication of the opioidergic system in physical pain reducing emotional distress, these findings may explain why these patients often engage in SIBs.
Supplementary Material
Acknowledgements
We would like to thank Rosetta Nicastro and Paco Prada for their role in recruiting the BPD patients as well as Emilie Douine for her help in assessing all participants. Lastly, thank you to Amanda Buckley (amanda@notinsidethebox.com) for her consultation with the figures in this manuscript.
Funding
This work was supported by the National Center of Competence in Research (NCCR) Affective Sciences financed by the Swiss National Science Foundation (grant number 51NF40-104897) and hosted by the University of Geneva, and by the grant SFETD-IUD 2012 from Institut UPSA de la Douleur (to E.O.).
Financial disclosures
None.
References
- Agrawal H.R., Gunderson J., Holmes B.M., et al. (2004). Attachment Studies with Borderline Patients: A Review. Harvard Review of Psychiatry, 12, 94–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders (5th ed.). Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Ammerman B.A., Olino T.M., Coccaro E.F., et al. (2017). Predicting nonsuicidal self-injury in borderline personality disorder using ecological momentary assessment. Journal of Personality Disorders, 31, 1–12. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Epstein N., Brown G., et al. (1988). An inventory for measuring clinical anxiety: psychometric properties. Journal of Consulting and Clinical Psychology, 56, 893–7. [DOI] [PubMed] [Google Scholar]
- Bohus M., Limberger M., Ebner U., et al. (2000). Pain perception during self-reported distress and calmness in patients with borderline personality disorder and self-mutilating behavior. Psychiatry Research, 95, 251–60. [DOI] [PubMed] [Google Scholar]
- Bresin K., Gordon K.H. (2013). Endogenous opioids and nonsuicidal self-injury: a mechanism of affect regulation. Neuroscience and Biobehavioral Reviews, 37, 374–83. [DOI] [PubMed] [Google Scholar]
- Brüne M. (2015). On the role of oxytocin in borderline personality disorder. British Journal of Clinical Psychology, 1–18. [DOI] [PubMed] [Google Scholar]
- Bungert M., Koppe G., Niedtfeld I., et al. (2015). Pain processing after social exclusion and its relation to rejection sensitivity in borderline personality disorder. PloS One, 10, e0133693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro D.C., Berridge K.C. (2014). Opioid hedonic hotspot in nucleus accumbens shell: mu, delta, and kappa maps for enhancement of sweetness ‘liking’ and ‘wanting’. Journal of Neuroscience, 34, 4239–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell S.E., Beauchaine T.P., Linehan M.M. (2009). A biosocial developmental model of borderline personality: elaborating and extending Linehan’s theory. Psychological Bulletin, 135, 495–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof E.K., Kaps L., Falkai P., et al. (2012). The role of the human ventral striatum and the medial orbitofrontal cortex in the representation of reward magnitude—an activation likelihood estimation meta-analysis of neuroimaging studies of passive reward expectancy and outcome processing. Neuropsychologia, 50, 1252–66. [DOI] [PubMed] [Google Scholar]
- Domsalla M., Koppe G., Niedtfeld I., et al. (2014). Cerebral processing of social rejection in patients with borderline personality disorder Social Cognitive and Affective Neuroscience, 9, 1789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducasse D., Courtet P., Olié E. (2014). Physical and social pains in borderline disorder and neuroanatomical correlates: a systematic review. Current Psychiatry Reports, 16, 443. [DOI] [PubMed] [Google Scholar]
- Eisenberger N.I. (2012). The pain of social disconnection: examining the shared neural underpinnings of physical and social pain. Nature Reviews. Neuroscience, 13, 421–34. [DOI] [PubMed] [Google Scholar]
- Eisenberger N.I., Lieberman M.D., Williams K.D. (2003). Does rejection hurt? An FMRI study of social exclusion. Science (New York, N.Y.), 302, 290–2. [DOI] [PubMed] [Google Scholar]
- Enzi B., Doering S., Faber C., et al. (2013). Reduced deactivation in reward circuitry and midline structures during emotion processing in borderline personality disorder. The World Journal of Biological Psychiatry: The Official Journal of the World Federation of Societies of Biological Psychiatry, 14, 45–56. [DOI] [PubMed] [Google Scholar]
- Feinberg D.A., Moeller S., Smith S.M., et al. (2010). Multiplexed echo planar imaging for sub-second whole brain FMRI and fast diffusion imaging. PLoS One, 5, e15710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin D., Bartholomew K. (1994). The metaphysics of measurement: the case of adult attachment. Journal of Personality and Social Psychology, 67, 17–52. [Google Scholar]
- Hayes A.F. (2013). Methodology in the social sciences. Introduction to Mediation, Moderation, and Conditional Process Analysis: A regression-based approach. New York, NY, US: Guilford Press. [Google Scholar]
- Herbort M.C., Soch J., Wüstenberg T., et al. (2016). A negative relationship between ventral striatal loss anticipation response and impulsivity in borderline personality disorder. NeuroImage: Clinical, 12, 724–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman B.H., Panksepp J. (1978). Effects of morphine and naloxone on separation distress and approach attachment: evidence for opiate mediation of social affect. Pharmacology, Biochemistry and Behavior, 9, 213–20. [DOI] [PubMed] [Google Scholar]
- Herpertz S.C., Bertsch K. (2015). A new perspective on the pathophysiology of borderline personality disorder: a model of the role of oxytocin. American Journal of Psychiatry, 172, 840–51. [DOI] [PubMed] [Google Scholar]
- Kleindienst N., Bohus M., Ludäscher P., et al. (2008). Motives for nonsuicidal self-injury among women with borderline personality disorder. Journal of Nervous and Mental Disease, 196, 230–6. [DOI] [PubMed] [Google Scholar]
- Kurdek L.A. (2002). On being insecure about the assessment of attachment styles. Journal of Social and Personal Relationships, 19, 811–34. [Google Scholar]
- Ludäscher P., Bohus M., Lieb K., et al. (2007). Elevated pain thresholds correlate with dissociation and aversive arousal in patients with borderline personality disorder. Psychiatry Research, 149, 291–6. [DOI] [PubMed] [Google Scholar]
- Ludäscher P., Greffrath W., Schmahl C., et al. (2009). A cross-sectional investigation of discontinuation of self-injury and normalizing pain perception in patients with borderline personality disorder. Acta Psychiatrica Scandinavica, 120, 62–70. [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Burdette J.H. (2004). Precentral gyrus discrepancy in electronic versions of the Talairach atlas. NeuroImage, 21, 450–5. [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., et al. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage, 19, 1233–9. [DOI] [PubMed] [Google Scholar]
- Martin J., Bureau J.F., Lafontaine M.F., et al. (2017). Preoccupied but not dismissing attachment states of mind are associated with nonsuicidal self-injury. Development and Psychopathology, 29, 379–88. [DOI] [PubMed] [Google Scholar]
- Montgomery S.A., Asberg M. (1979). A new depression scale designed to be sensitive to change. The British Journal of Psychiatry: The Journal of Mental Science, 134, 382–9. [DOI] [PubMed] [Google Scholar]
- New A.S., Barbara S. (2010). An opioid deficit in borderline personality disorder: self-cutting, substance abuse, and social dysfunction. American Journal of Psychiatry, 167, 882–5. [DOI] [PubMed] [Google Scholar]
- Nicastro R., Prada P., Kung A.-L., et al. (2016). Psychometric properties of the French borderline symptom list, short form (BSL-23). Borderline Personality Disorder and Emotion Dysregulation, 3, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedtfeld I., Kirsch P., Schulze L., et al. (2012). Functional connectivity of pain-mediated affect regulation in borderline personality disorder. PloS One, 7, e33293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedtfeld I., Schmitt R., Winter D., et al. (2017). Pain-mediated affect regulation is reduced after dialectical behavior therapy in borderline personality disorder: a longitudinal fMRI study. Social Cognitive and Affective Neuroscience, 12, 739–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock M.K. (2010). Self-injury. Annual Review of Clinical Psychology, 6, 339–63. [DOI] [PubMed] [Google Scholar]
- Pavony M.T., Lenzenweger M.F. (2014). Somatosensory processing and borderline personality disorder: pain perception and a signal detection analysis of proprioception and exteroceptive sensitivity. Personality Disorders: Theory, Research, and Treatment, 5, 164–71. [DOI] [PubMed] [Google Scholar]
- Perroud N., Dieben K., Nicastro R., et al. (2012). Functions and timescale of self-cutting in participants suffering from borderline personality disorder. Journal of Personality Disorders, 26, 267–79. [DOI] [PubMed] [Google Scholar]
- Prossin A.R., Love T.M., Koeppe R.A., et al. (2010). Dysregulation of regional endogenous opioid function in borderline personality disorder. American Journal of Psychiatry, 167, 925–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz S., Kluetsch R., Niedtfeld I., et al. (2015). Incision and stress regulation in borderline personality disorder: neurobiological mechanisms of self-injurious behaviour. The British Journal of Psychiatry, 1–8. [DOI] [PubMed] [Google Scholar]
- Renneberg B., Herm K., Hahn A., et al. (2011). Perception of social participation in borderline personality disorder Clinical Psychology & Psychotherapy, 19, 473–80. [DOI] [PubMed] [Google Scholar]
- Ruocco A.C., Carcone D. (2016). A neurobiological model of borderline personality disorder. Harvard Review of Psychiatry, 24, 311–29. [DOI] [PubMed] [Google Scholar]
- Sander D., Grafman J., Zalla T. (2003). The human amygdala: an evolved system for relevance detection. Reviews in the Neurosciences, 14, 303–16. [DOI] [PubMed] [Google Scholar]
- Sauder C.L., Derbidge C.M., Beauchaine T.P. (2016). Neural responses to monetary incentives among self-injuring adolescent girls. Development and Psychopathology, 28, 277–91. [DOI] [PubMed] [Google Scholar]
- Schmahl C., Baumgärtner U. (2015). Pain in borderline personality disorder. Modern Trends in Pharmacopsychiatry, 30, 166–75. [DOI] [PubMed] [Google Scholar]
- Schmahl C., Bohus M., Esposito F., et al. (2006). Neural correlates of antinociception in borderline personality disorder. Archives of General Psychiatry, 63, 659–67. [DOI] [PubMed] [Google Scholar]
- Scott L.N., Levy K.N., Pincus A.L. (2009). Adult attachment, personality traits, and borderline personality disorder features in young adults. Journal of Personality Disorders, 23, 258–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snir A., Rafaeli E., Gadassi R., et al. (2015). Explicit and inferred motives for nonsuicidal self-injurious acts and urges in borderline and avoidant personality disorders. Personality Disorders, 6, 267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staebler K., Helbing E., Rosenbach C., et al. (2011). Rejection sensitivity and borderline personality disorder. Clinical Psychology and Psychotherapy, 18, 275–83. [DOI] [PubMed] [Google Scholar]
- Stanley B., Siever L.J. (2010). The interpersonal dimension of borderline personality disorder: toward a neuropeptide model. American Journal of Psychiatry, 167, 24–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega D., Ripolles P., Soto A., et al. (2017). Orbitofrontal overactivation in reward processing in borderline personality disorder: the role of non-suicidal self-injury. Brain Imaging and Behavior, 1–12. [DOI] [PubMed] [Google Scholar]
- Williams K.D., Jarvis B. (2006). Cyberball: a program for use in research on interpersonal ostracism and acceptance. Behavior Research Methods, 38, 174–80. [DOI] [PubMed] [Google Scholar]
- Willis F., Kuniss S., Kleindienst N., et al. (2017). The role of nociceptive input and tissue injury on stress regulation in borderline personality disorder. Pain, 158, 479–87. [DOI] [PubMed] [Google Scholar]
- Yarkoni T., Poldrack R.A., Nichols T.E., et al. (2011). Large-scale automated synthesis of human functional neuroimaging data. Nature Methods, 8, 665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


