Abstract
Adherence to tenofovir disoproxil fumarate/emtricitabine (TDF/FTC, Truvada®) is the primary determinant of HIV pre-exposure prophylaxis (PrEP) efficacy. Despite its importance, limitations exist in current methods of adherence quantification, restricting their implementation in the clinic. Proteus Discover (Proteus Digital Health®) can measure the time of each dose using an ingestible sensor that is coencapsulated with medication. In this study, the bioequivalence of coencapsulated TDF/FTC with the Proteus sensor was compared relative to unencapsulated drug. This was a 1:1 randomized cross-over study in which healthy participants received a single dose of unencapsulated and coencapsulated TDF/FTC. A 14-day washout separated each period. Blood was collected at predose and at 0.25, 0.5, 1, 2, 4, 6, 10, 24, 48, and 72 h postdose. Plasma concentrations were determined by LC-MS/MS methods, with a 10 ng/mL lower limit of quantitation (LLOQ) for both tenofovir (TFV) and FTC. Noncompartmental analysis was carried out with Phoenix® WinNonlin® for maximum concentrations (Cmax), area under the concentration–time curve from time 0 to the last measured time point (AUClast) and AUC extrapolated to infinity (AUCinf). Geometric mean ratios were calculated for each parameter and bioequivalence was defined as the 90% confidence interval (CI) of each ratio being within 80%–125%. Twenty-four participants (11 males; 19 white, 3 African American, and 2 Hispanic) completed both visits. Mean ± SD age was 28 ± 4 years and weight was 74 ± 14 kg. The 90% CIs for TFV Cmax, AUClast, and AUCinf were 89%–119%, 94%–111%, and 96%–111%, respectively. The 90% CIs for FTC Cmax, AUClast, and AUCinf were 96%–120%, 96%–108%, and 96%–108%, respectively. Bioequivalence was observed for the coencapsulation of TDF/FTC with the Proteus ingestible sensor, as assessed by a rigorously conducted pharmacokinetic study. Future studies will evaluate the utility and effectiveness of the sensor system as a tool to monitor PrEP adherence in clinical settings.
Keywords: : PrEP, adherence, pharmacokinetics, bioequivalence, digital medicine
In 2012, the U.S. Food and Drug Administration (FDA) approved daily oral tenofovir disoproxil fumarate plus emtricitabine (TDF/FTC, Truvada®) for HIV pre-exposure prophylaxis (PrEP), primarily based on the results of two large clinical trials.1,2 The iPrEx and Partners PrEP studies reported 44% (95% confidence interval [CI], 15%–63%) and 75% (95% CI, 55%–87%) HIV risk reduction with daily TDF/FTC relative to placebo. Both studies showed that quantifiable drug concentrations in plasma were strongly associated with prophylactic effect. For example, in the iPrex trial, the HIV risk reduction increased from 44% to 92% in those with quantifiable plasma drug concentrations. Low adherence rates (21%–37%) were later discovered in the VOICE and FEM-PrEP trials, which failed to demonstrate efficacy.3 Adherence, therefore, plays a key role in the efficacy of TDF/FTC for PrEP.
Several subjective and objective measures exist for assessing adherence, such as self-report, pill counts, clinician assessment, pharmacy refill records, and drug concentration measurements.4,5 Although these methods have been long-standing, several drawbacks limit their use, including lack of accuracy and precision, expense, “white coat” effects, and difficult implementation into clinical practice.6
To address some of these challenges, a unique approach to objectively and rapidly measure drug ingestion has been developed using Proteus Discover (Proteus Digital Health, Inc.). This digital medicine program consists of an FDA-approved ingestible sensor pill that transmits a small electrical signal after interaction with gastric fluid, an adhesive sensor patch—worn on the torso and replaced weekly—that detects the signal from the ingestible sensor, and an application on a smartphone or tablet device that captures all transmitted data. In this way, time-stamped and longitudinal records of drug adherence can be measured in a precise manner. The ingestible sensor pills can be coencapsulated with medications to create digital medicines. The sensor is the size of a grain of sand and contains minute quantities of silicon, copper, and magnesium that pass through the body naturally. Several studies have found that coencapsulated versions of medicine had similar dissolution or pharmacokinetics profiles as the unencapsulated versions.7 Abilify® MyCite, a fully integrated digital medicine with the ingestible sensor inside of the drug tablet, was approved for use by the FDA, encouraging further investigation in other fields, including PrEP.8 Proteus Discover® has also been extensively evaluated in other therapeutic areas, including hypertension, hypercholesterolemia, diabetes, heart failure, tuberculosis, HIV, and other diseases.9 The objective of this study was to confirm that the rate and extent of absorption of TDF/FTC coencapsulated with Proteus Discover are unchanged relative to unencapsulated drug.
The study took place on the University of Colorado–Anschutz Medical Campus. The study protocol was approved by the Institutional Review Board and all participants signed an informed consent. The study design was a single-dose randomized (1:1) cross-over study conducted in healthy volunteers. Key exclusion criteria included a positive HIV EIA or suspected acute HIV infection, creatinine clearance <60 mL/(min ·1.73 m2) (modification of diet in renal disease criteria for impaired kidney function), and contraindicated concomitant medications that could interfere with TDF/FTC disposition.
Participants were randomized to begin with a single dose of the unencapsulated or coencapsulated TDF/FTC. Tablets were encapsulated with Capsugel DBcaps® (AAel) and microcrystalline cellulose was used to fill empty space. TDF/FTC (300/200 mg) was administered with 250 mL of water after an overnight fast of at least 10 h. A nonstandardized meal was provided 4 h after the dose. Blood was collected at predose and at 0.25, 0.5, 1, 2, 4, 6, 10, 24, 48, and 72 h postdose. The rationale behind these sampling times was to capture the maximum concentration (Cmax) of tenofovir (TFV) in plasma, and to include a total collection time exceeding 3.3 half-lives of TFV (∼17 h).10 A 14-day washout (range 13–21 days; >7 half-lives) separated each period.
A noncompartmental analysis was carried out using a built-in function in Phoenix® WinNonlin® (Certara). Values less than the lower limit of quantitation (BLQ) were evaluated by imputing half of the lower limit of quantitation (LLOQ). Sensitivity analyses treated BLQ values as missing. Noncompartmental parameters that were estimated included Cmax, area under the concentration–time curve (AUC) from time 0 to the last measured time point (AUClast), AUC extrapolated to infinity (AUCinf), and half-life. Linear up/log down trapezoidal methods were utilized to calculate AUC. Cmax was observed from the concentration–time curves and half-life was calculated from the elimination constant, which was derived from points in the terminal declining slope. Parameters were log-transformed to normalize distributions for statistical analysis, then were back-transformed to the original scale. Geometric mean ratios (GMRs) were calculated for each parameter and bioequivalence was defined as the 90% CI of each ratio being within 80%–125%. GMR comparisons for AUClast, AUCinf, and Cmax were modeled using a mixed effects model with treatment, treatment sequence, and period as fixed effects and patient nested in treatment sequence as the random effect, using the built-in bioequivalence function in Phoenix WinNonlin.
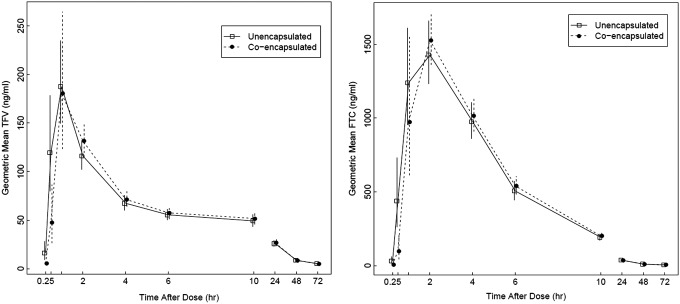
Twenty-four participants (11 males; 19 Caucasian, 3 African American, and 2 Hispanic) completed both visits. Mean ± SD age was 28 ± 4 years and weight was 74 ± 14 kg. From a total of 477 samples, 105 (22%) were BLQ, primarily at the 48- and 72-h time points. Geometric mean concentration vs. time plots for plasma TFV and FTC are shown in Figure 1. TFV geometric mean AUCinf (%CV) for the unencapsulated and coencapsulated formulations was 1,978 (27) and 2,042 (26) ng × h/mL, respectively. Geometric mean FTC AUCinf (%CV) estimates were 9,342 (23) and 9,512 (20) ng × h/mL for the unencapsulated and coencapsulated formulations, respectively. TFV geometric mean Cmax (%CV) for the unencapsulated and coencapsulated drugs was 222 (37) and 229 (32) ng/mL, respectively. For FTC, geometric mean Cmax for the unencapsulated and coencapsulated formulations were 1,567 (33) and 1,684 (29) ng/mL, respectively.
FIG. 1.
Geometric mean concentration vs time plots of coencapsulated (●) and unencapsulated (□) TFV (left) and FTC (right). FTC, emtricitabine; TFV, tenofovir.
When imputing half the LLOQ, the 90% CIs for TFV Cmax, AUClast, and AUCinf ratios were 89%–119%, 94%–111%, and 96%–111%, respectively. The half-life comparison also satisfied the CI requirement (95%–108%). The 90% CIs for FTC Cmax, AUClast, and AUCinf were 96%–120%, 96%–108%, and 96%–108%, respectively. The 90% CI for FTC half-life was 83%–112%. CI estimates also satisfied bioequivalence requirements when BLQ results were considered missing (<5% change in CI estimates for AUC and Cmax calculations and a 12% increase in the upper limit of FTC half-life CI). Sequence and period effects did not have a significant effect on bioequivalence results, as assessed by the mixed effects model.
In general, TDF/FTC and the sensor tablet were safe and well tolerated. Fourteen adverse events were recorded and reviewed by the study team, approximately equal numbers after the encapsulated versus nonencapsulated formulations (11/14 Grade 1—cold symptoms, nausea, vomiting; 2/14 Grade 2—facial swelling, respiratory infection; 1/14 Grade 3—headache). The headache and facial swelling were subjective reports from two different participants. The relationship with study drugs was unclear and both resolved during the study. Other AEs were as expected. One subject became pregnant and was removed from the study before her second visit and was not included in the pharmacokinetic analysis.
Taken together, the results of this study demonstrate bioequivalence for TDF/FTC coencapsulation with the Proteus ingestible sensor pill. These results support future clinical research that seeks to over-encapsulate TDF/FTC with the Proteus ingestible sensor. As with all adherence measures, Proteus Discover has some limitations, including the added component of adherence to the patch that must be replaced weekly, and potential cost and patient privacy concerns. These considerations, along with patient and provider satisfaction, should also be assessed in future studies.
Acknowledgments
We wish to thank the study participants, the principal investigators, and staff at CTRC and members of the Colorado Antiviral Pharmacology Laboratory. Grants: CTRC UL1TR001082, NIH R01 A1122308, and K23AI104315 (J.R.C.-M.).
Author Disclosure Statement
Study drug was donated by Gilead and the sensor was provided by Proteus. P.L.A. receives grant and contract funding from Gilead Sciences, paid to his institution. No competing financial interests exist.
References
- 1.Baeten JM, Donnell D, Ndase P, et al. : Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012;367:399–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant RM, Lama JR, Anderson PL, et al. : Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010;363:2587–2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haberer JE: Current concepts for PrEP Adherence in the PrEP revolution: From clinical trials to routine practice. Curr Opin HIV AIDS 2016;11:10–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lam WY, Fresco P: Medication adherence measures: An overview. Biomed Res Int 2015;2015:217047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gutierrez EB, Sartori AM, Schmidt AL, et al. : Measuring adherence to antiretroviral treatment: The role of pharmacy records of drug withdrawals. AIDS Behav 2012;16:1482–1490 [DOI] [PubMed] [Google Scholar]
- 6.Berg KM, Arnsten JH: Practical and conceptual challenges in measuring antiretroviral adherence. J Acquir Immune Defic Syndr 2006;43 Suppl 1:S79–S87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Browne SH, Peloquin C, Santillo F, et al. : Digitizing medicines for remote capture of oral medication adherence using co-encapsulation. Clin Pharmacol Ther 2018;103:502–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peters-Strickland T, Pestreich L, Hatch A, et al. : Usability of a novel digital medicine system in adults with schizophrenia treated with sensor-embedded tablets of aripiprazole. Neuropsychiatr Dis Treat 2016;12:2587–2594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Virdi NS: Digital medicines to measure drug ingestion adherence. In: Drug Adherence in Hypertension and Cardiovascular Protection. Updates in Hypertension and Cardiovascular Protection (Burnier M, ed.) Springer, Cham, Switzerland, 2018 [Google Scholar]
- 10.Kearney BP, Flaherty JF, Shah J: Tenofovir disoproxil fumarate: Clinical pharmacology and pharmacokinetics. Clin Pharmacokinet 2004;43:595–612 [DOI] [PubMed] [Google Scholar]