Abstract
We sought to estimate the prevalence of hypertension and characteristics of hypertensive adults in the United States according to blood pressure thresholds used for diagnosis and estimate their associated CVD risk. Analyses included adults 20 years of age or older in the 2013-2014 National Health and Nutrition Examination Survey (NHANES) (N=5,389), and enrolled participants in the Systolic Blood Pressure Intervention Trial (SPRINT) (N=9,361) and the Action to Control Cardiovascular Risk in Diabetes-BP Trial (ACCORD-BP) (N=4,733) trials. In NHANES, prevalence estimates incorporated the probability of observing elevated blood pressure on two separate occasions. Using the new BP thresholds of 130/80 mm Hg or greater, approximately 24 million new American adults would be diagnosed as having hypertension and 4.3 million would be recommended to start antihypertensive medications. These individuals would have a lower mean atherosclerotic CVD risk (17%) than participants in SPRINT and ACCORD-BP (22% and 27%) and would be less likely to have prevalent cardiovascular disease (9% versus 17% and 34%). In SPRINT and ACCORD-BP, only a minority (9% and 13%) of participants were not on antihypertensive medications at baseline, and, rates of incident CVD in these participants were substantially lower compared to those on baseline BP medications. We conclude that adopting the ACC/AHA guidelines would lead to a substantial increase in the prevalence of hypertension and in the number of American adults recommended to start antihypertensive medications. These individuals would have a substantially lower cardiovascular risk than most participants previously studied in two large BP trials.
Keywords: Hypertension, cardiovascular disease, epidemiology, guidelines, diagnosis
Introduction
Hypertension is an independent, modifiable risk factor for the development of cardiovascular disease and the leading cause of disability worldwide1,2. Guidelines have traditionally defined hypertension as the current use of an antihypertensive medication and/or a systolic BP (SBP) ≥140 mm Hg or a diastolic BP (DBP) ≥90 mmHg, based on at least two BP readings obtained on two or more occasions3,4. Recently, the American College of Cardiology and American Heart Association (ACC/AHA) BP guidelines recommended changing the BP threshold used to diagnose hypertension to ≥ 130/80 mm Hg5.
It is estimated that under these new lower BP thresholds, 31 million US adults with SBP 130-139 mm Hg or DBP 80-89 mm Hg not on BP medications will be labeled as having hypertension6. Of these, the ACC/AHA guidelines recommend starting BP therapy on individuals with a 10-year atherosclerotic cardiovascular risk (ASCVD) greater than 10%. It is estimated that 4.2 million US adults would meet this recommendation6. These estimates, however, have been based on BP readings taken in one occasion; an important consideration since most BP guidelines recommend establishing a diagnosis of hypertension only after BP is measured on at least two occasions. It is critical to properly characterize the individuals that would be newly recommended to start a BP medication since recent research has shown a net benefit from intensive BP therapy only among individuals with a 10-year ASCVD risk greater than 18%7.
The Systolic Blood Pressure Intervention Trial (SPRINT) and the Action to Control Cardiovascular Risk in Diabetes-BP Trial (ACCORD-BP) are the largest clinical trials evaluating intensive BP targets among individuals with SBP >130 mm Hg at high CVD risk8,9. Even though the newly recommended thresholds for hypertension were largely informed by results from SPRINT, most patients in this trial (and in ACCORD-BP) were on BP medications at baseline. Given that the CVD risk profile of patients on long-standing BP medications may be different than that of patients not on medications despite similar BP values, it is important to determine whether individuals that would be newly recommended to start BP therapy were well represented in these trials, and therefore, establish if there is evidence to support the recommendation for BP treatment initiation in these patients.
We sought to estimate how adopting these newly recommended BP thresholds would impact the prevalence of hypertension among US adults accounting for persistent hypertension (elevated BP on more than two occasions). In addition, we sought to characterize the population that would be newly recommended for initiation of antihypertensive medications and compare them to the demographic and clinical characteristics of participants enrolled in the SPRINT and ACCORD-BP trials.
Methods
Data source
Individual level data from SPRINT and ACCORD-BP were obtained from the National Heart, Lung, and Blood Institute (NHLBI) biologic specimen and data repositories information coordinating center (BioLINCC). To request access to the BioLINCC data repository, a completed application form and research proposal should be submitted to NHLBI. Data can be accessed through the BioLINCC web site at https://biolincc.nhlbi.nih.gov/home/. NHANES data can be accessed through the Centers for Disease Control and Prevention (CDC) National Center for Health Statistics website at https://www.cdc.gov/nchs/nhanes/index.htm. The present study was deemed exempt from review by the Institutional Review Board at the University of Washington.
Study Populations
The National Health and Nutrition Examination Survey (NHANES)10 is a program of studies conducted by the National Center for Health Statistics to evaluate the health of non-institutionalized adults and children in the United States. The NHANES sample is selected through a complex multistage design with oversampling for persons of black race, Hispanic ethnicity, or both.
The current study includes 5,380 participants of the 2013-2014 NHANES who were aged 20 years or older, who underwent a health examination at an NHANES mobile examination center (MEC) with available data for prescription medication use and at least two BP readings.
SPRINT enrolled 9,361 adults 50 years of age or older with a SBP of at least 130 mm Hg and an increased CVD risk8. High CVD risk was defined as history of clinical or subclinical CVD, chronic kidney disease with an estimated glomerular filtration rate of 20 to less than 60 ml/min/1.73 m2, a 10-year CVD risk on the basis of the Framingham risk score of 15% or greater, or an age of 75 years or older. Patients with diabetes mellitus or prior stroke were excluded. ACCORD-BP enrolled 4,733 participants 40 years of age or older with type 2 diabetes mellitus (T2DM) and a glycated hemoglobin level of 7.5% or greater, a SBP of 130 to 180 mm Hg, and history of CVD9. Participants with a body-mass index of 45 kg/m2 or greater, a serum creatinine of more than 1.5 mg/dl, and other serious illnesses were excluded.
Blood pressure measurement
In NHANES, blood pressure was measured by a physician certified in blood pressure measurement using the standardized protocol of the AHA during a single MEC visit11,12. The first BP measurement was recorded after a 5-minute period of rest with two additional readings obtained 30 seconds apart using the same arm. A fourth reading was recorded in case one or more of the readings were unobtainable.
SPRINT and ACCORD-BP used automated devices to measure BP and used very similar classes and numbers of antihypertensive agents. Although SPRINT measured BP in the absence of an observer for the majority of participants (as opposed to ACCORD-BP), similar results for the effect of the intensive BP intervention have been found when analyses have been performed separately in observed or unobserved participants13.
Hypertension definition in NHANES
Prevalent hypertension was defined as current use of an antihypertensive medication or mean systolic blood pressure (SBP) or diastolic blood pressure (DBP) higher than the diagnostic threshold accounting for the probability of having elevated blood pressure on two occasions (persistent hypertension). We used two different BP thresholds to diagnose hypertension: the traditional threshold of ≥140/90 mm Hg and the newly recommended threshold of ≥130/80 mm Hg.
Prevalent hypertension estimates incorporate the probability of having persistent hypertension because most guidelines recommend diagnosing hypertension only if elevated BP is present on at least two separate days3–5. We considered any participant treated with an antihypertensive medication to have persistent hypertension. For participants not treated with an antihypertensive medication but with mean BP above diagnostic threshold, we incorporated into our statistical analyses the estimated probability that mean SBP or DBP would be elevated when next measured, following an approach we used previously to estimate the prevalence of persistent chronic kidney disease14. We based our estimates that BP would be persistently elevated on data collected during NHANES III (1988-1994), during which 1,853 participants underwent repeat BP measurements two weeks apart (Supplemental material).
Cardiovascular disease endpoints in SPRINT and ACCORD-BP
SPRINT and ACCORD-BP evaluated effect of intensive compared to standard BP treatment targets (<120 versus <140 mm Hg SBP) on a primary composite CVD end-point of non-fatal myocardial infarction or stroke, or death from cardiovascular causes. SPRINT also included unstable angina and acute heart failure as part of its composite endpoint8,9. We conducted separate analyses with the original primary pre-specified composite CVD endpoint for each trial.
Other Clinical Characteristics
In NHANES, we defined diabetes mellitus as use of a glucose lowering medication or hemoglobin A1C% ≥6.5%14 and prevalent cardiovascular disease (CVD) as a self-reported history of congestive heart failure, coronary artery disease, or stroke. Estimated glomerular filtration rate (eGFR) was calculated from serum creatinine using the CKD-EPI formula15, and estimated atherosclerotic CVD (ASCVD) risk was calculated using the 2013 ACC/AHA ASCVD pooled cohort equation16.
In SPRINT and ACCORD-BP, we used the CKD-EPI formula to estimate the glomerular filtration rate and estimated the ASCVD risk with the 2013 ACC/AHA pooled cohort equation. In ACCORD-BP, all included patients had a history of T2DM and a baseline glycated hemoglobin of 7.5% or greater.
Statistical methods
In NHANES, analyses incorporated sampling weights to account for non-response bias and the sampling design. We used a bootstrap approach with 500 replicates to account for the variability in the estimate of the probability of persistent hypertension9. We estimated the prevalence of hypertension as the prevalence of hypertension in the bootstrap sample multiplied by the bootstrap probability of persistence; the final estimate and 95% CI were the mean and 2.5/97.5th percentiles across bootstrap replicates, respectively (Supplement).
Incident CVD rates were calculated separately in SPRINT and ACCORD-BP as the number of events in the primary composite endpoint divided by the total time at risk stratifying by BP value and prior use of BP medication. Confidence intervals were derived with a bootstrap approach using 500 replicates.
Analysis were performed using STATA version 11.0 (StataCorp) and R version 3.4.0 (R foundation for Statistical Computing).
Results
Prevalence of Hypertension
The estimated prevalence of hypertension among US adults was 44.0% (95% CI, 42.2%, 45.7%) using newly recommended BP thresholds as compared to 34.2% (95% CI, 32.5%, 35.8%) using traditional thresholds. This represents an estimated absolute increase in hypertension prevalence of 9.8% (95% CI, 8.7%, 11.1%) and a relative increase of 28.6% (95% CI, 25%, 33.1%) (Table 1).
Table 1.
Prevalence of hypertension among US adults with traditional and new criteria for the diagnosis of hypertension in NHANES 2013-2014
| Characteristic | Prevalence with traditional criteria* (95% CI) |
Prevalence with new criteria† (95% CI) |
Absolute difference in prevalence (95% CI) |
Relative percent difference in prevalence (95% CI) |
|---|---|---|---|---|
| Overall | 34.2 (32.5, 35.8) | 44.0 (42.2, 45.7) | 9.8 (8.7, 11.1) | 28.6 (25.0, 33.1) |
| Age | ||||
| 20-39 | 8.7 (7.3, 10.0) | 17.1 (15.4, 18.9) | 8.4 (7.3, 9.8) | 97.1 (76.5, 122.7) |
| 40-59 | 34.7 (31.9, 37.5) | 47.6 (44.7, 50.7) | 12.9 (11.0, 15.0) | 37.3 (31.4, 45.5) |
| ≥60 | 72.4 (69.8, 75.5) | 79.7 (77.2, 82.1) | 7.2 (5.5, 9.0) | 10.0 (7.5, 12.8) |
| Gender | ||||
| Male | 35.0 (32.6, 37.5) | 46.7 (44.1, 49.4) | 11.7 (10.1, 13.4) | 33.2 (28.4, 39.6) |
| Female | 33.5 (31.1, 35.4) | 41.5 (39.3, 43.5) | 8.1 (6.9, 9.2) | 24.2 (20.3, 28.7) |
| Race/Ethnicity | ||||
| White, non-Hispanic | 36.8 (34.5, 39.2) | 46.2 (44.0, 48.7) | 9.4 (8.0, 10.9) | 25.5 (21.0, 30.5) |
| Black, non-Hispanic | 41.1 (38.0, 44.0) | 52.3 (49.4, 55.4) | 11.1 (9.4, 13.2) | 27.1 (21.9, 32.8) |
| Asian, non-Hispanic | 25.9 (22.0, 29.5) | 34.3 (30.3, 38.2) | 8.4 (6.5, 10.7) | 32.7 (24.3, 43.3) |
| Hispanic | 21.5 (19.6, 23.8) | 31.9 (29.4, 34.6) | 10.4 (8.7, 12.2) | 48.2 (38.7, 58.5) |
| Diabetes | ||||
| No | 28.7 (27.0, 30.5) | 38.8 (37.0, 40.9) | 10.1 (8.9, 11.5) | 35.2 (30.3, 41.2) |
| Yes | 75.6 (72.0, 79.1) | 82.2 (79.1, 85.0) | 6.5 (4.7, 8.8) | 8.7 (6.0, 12.0) |
| Prevalent CVD | ||||
| No | 29.9 (28.1, 31.6) | 40.2 (38.4, 42.1) | 10.4 (9.2, 11.7) | 34.6 (30.3, 40.3) |
| Yes | 82.0 (77.3, 86.5) | 85.4 (81.1, 89.2) | 3.4 (1.9, 5.4) | 4.2 (2.3, 6.8) |
| ASCVD Risk | ||||
| < 10% | 20.4 (18.6, 22.1) | 30.5 (28.6, 32.7) | 10.2 (8.9, 11.6) | 49.9 (42.0, 59.2) |
| ≥ 10% | 76.7 (74.1, 79.4) | 84.4 (82.2, 86.4) | 7.6 (5.6, 9.6) | 9.9 (7.1, 12.8) |
| BMI (kg/m2) | ||||
| < 25 | 21.6 (19.2, 24.1) | 29.0 (26.4, 31.6) | 7.3 (6.0, 8.8) | 34.0 (26.8, 43.1) |
| 25 - < 30 | 33.0 (30.4, 36.0) | 42.8 (40.0, 45.8) | 9.7 (8.0, 11.4) | 29.3 (23.3, 35.7) |
| ≥ 30 | 44.4 (41.7, 47.2) | 56.3 (53.7, 59.1) | 11.9 (10.4, 13.8) | 26.7 (22.7, 32.4) |
| eGFR < 60 mL/min/1.73m2 | ||||
| ≥ 60 | 30.0 (28.2, 31.7) | 40.2 (38.5, 42.2) | 10.3 (9.1, 11.6) | 34.4 (29.7, 39.8) |
| < 60 | 84.9 (80.8, 88.6) | 87.3 (83.5, 90.3) | 2.3 (0.8, 4.2) | 2.7 (1.0, 5.1) |
| Urine ACR (mg/g) | ||||
| < 30 | 30.9 (29.1, 32.6) | 40.7 (39.0, 42.6) | 9.8 (8.7, 11.1) | 31.6 (27.4, 37.2) |
| ≥ 30 | 59.2 (54.4, 63.4) | 68.8 (63.9, 72.8) | 9.5 (7.3, 12.0) | 16.2 (11.5, 21.0) |
Cell contents are proportions (%) (95% CI).
Abbreviations: CVD, cardiovascular disease; ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; eGFR, estimated glomerular filtration rate; ACR, albumin to creatinine ratio.
All prevalence estimates account for the persistence of hypertension (supplement).
Defined as SBP ≥140 mmHg, DBP≥90 mm Hg, and/or current use of antihypertensive medication.
Defined as SBP≥130 mmHg, DBP≥80 mm Hg, and/or current use of antihypertensive medication.
The absolute increase in hypertension prevalence was largest for adults aged 40-59 years, men, those who were obese (Quetelet index ≥ 30 kg/m2) or free of significant comorbidities such as diabetes or CVD, and in those with eGFR ≥60 mL/min/1.73m2 (Table 1 and Figure 1)
Figure 1. Prevalence of hypertension in US adults with traditional and new criteria for diagnosis by age and clinical characteristics.
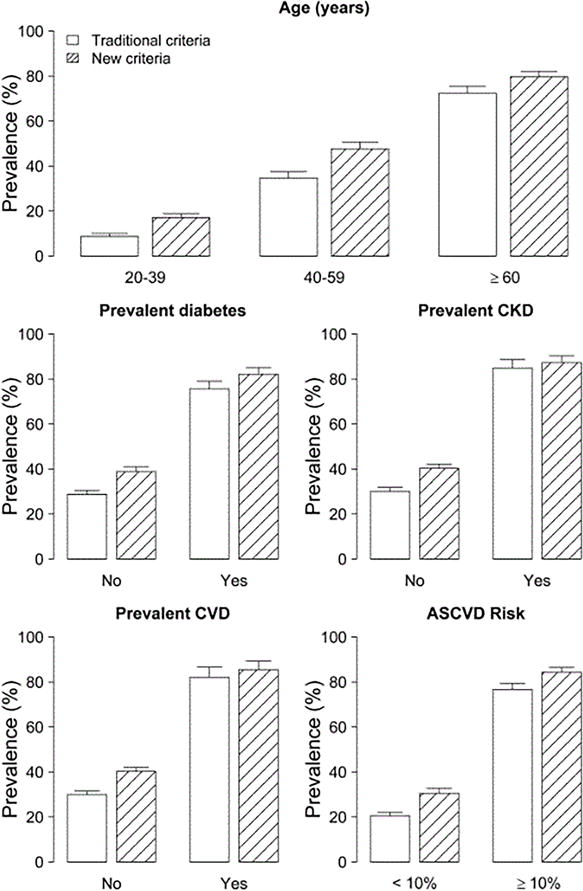
Traditional criteria for hypertension diagnosis: SBP ≥140 mmHg, DBP≥90 mm Hg, and/or current use of antihypertensive medication. New criteria for hypertension diagnosis: SBP≥130 mmHg, DBP≥80 mm Hg, and/or current use of antihypertensive medication
Error bars indicate 95% confidence intervals
Characteristics of individuals classified as hypertensive under newly recommended BP thresholds
Compared to US adults classified as hypertensive using traditional BP thresholds (≥ 140/90 mm Hg), participants re-classified as hypertensive (BP ≥ 130/80 and <140/90 mm Hg) were younger; less likely to have diabetes mellitus, CVD, or an estimated glomerular filtration rate < 60 ml/min/1.73 m2; and had a lower estimated 10-year risk of ASCVD (Table 2).
Table 2.
Demographic and clinical characteristics of NHANES 2013-2014 population
| Characteristic | No hypertension* (N = 2,617) |
Newly classified with hypertension† (N=618) |
Hypertension based on traditional criteria‡ (N = 2,145) |
|||
|---|---|---|---|---|---|---|
| N | Weighted mean or percent (95% CI) | N | Weighted mean or percent (95% CI) | N | Weighted mean or percent (95% CI) | |
| Demographic variables | ||||||
| Age (years) | 38.9 (38.0, 39.8) | 46.4 (44.5, 48.3) | 60.0 (59.2, 60.9) | |||
| Women | 1,455 | 55 (52, 57) | 255 | 43 (37, 49) | 1,085 | 50 (47, 53) |
| Race/ethnicity | ||||||
| White, non-Hispanic | 1,097 | 63 (56, 71) | 249 | 63 (55, 71) | 966 | 71 (64, 77) |
| Black, non-Hispanic | 413 | 10 (6, 13) | 135 | 13 (9, 17) | 561 | 14 (10, 18) |
| Asian, non-Hispanic | 358 | 6 (4, 8) | 63 | 5 (3, 6) | 190 | 4 (3, 5) |
| Hispanic | 655 | 18 (12, 24) | 148 | 16 (11, 21) | 388 | 10 (5, 14) |
| Medical history | ||||||
| Diabetes | 112 | 3.4 (2.6, 4.1) | 59 | 8 (5, 11) | 611 | 25 (23, 27) |
| CVD | 60 | 2 (1, 3) | 19 | 3 (1, 4) | 438 | 19 (17, 21) |
| Atherosclerotic CVD risk (%), mean | 2.6 (2.3, 2.9) | 5.5 (4.7, 6.2) | 16.5 (15.6, 17.4) | |||
| ASCVD> 10% | 167 | 6 (4, 7) | 118 | 18 (14, 23) | 1,191 | 55 (51, 59) |
| Medications | ||||||
| Antihypertensive medications | 0 | 0 | 0 | 0 | 1,723 | 80 (77, 84) |
| Lipid-lowering medications | 143 | 6 (5, 8) | 53 | 10 (6, 14) | 931 | 45 (41, 48) |
| Statins | 127 | 6 (4, 7) | 48 | 9 (5, 12) | 867 | 41 (37, 45) |
| Fibrates | 15 | 0.5 (0.1, 0.9) | 4 | 1.5 (0, 3.2 | 74 | 4.0 (2.5, 5.4) |
| Aspirin | 1 | 0.02 (0, 0.07) | 2 | 0.4 (0, 1.0) | 48 | 1.5 (0.9, 2.0) |
| Physical examination | ||||||
| BMI (kg/m2), mean | 27.5 (27.1, 27.9) | 30.3 (29.5, 31.2) | 31.0 (30.6, 31.4) | |||
| Systolic BP | 112.2 (111.7, 112.6) | 128.8 (127.8, 129.7)§ | 133.4 (131.8, 135.0)║ | |||
| Diastolic BP | 66.7 (65.9, 67.5) | 77.7 (76.7, 78.8)§ | 71.6 (70.5, 72.7)║ | |||
| Laboratory data | ||||||
| eGFR (mL/min/1.73 m2), mean | 101 (99, 103) | 96 (94, 98) | 81 (80, 83) | |||
| eGFR < 60 | 46 | 1.7 (1.0, 2.3) | 10 | 1.7 (0.5, 3.0) | 392 | 18 (16, 20) |
Cell contents are N, mean (95% CI), or proportion (%) (95% CI).
Abbreviations: CVD, cardiovascular disease; ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; eGFR, estimated glomerular filtration rate.
No hypertension was defined as SBP <130 mmHg, DBP<80 mmHg, and no treatment with antihypertensive medication.
Newly classified with hypertension was defined as SBP≥130 mmHg or DBP≥80 mmHg and, SBP<140 mmHg and DBP<90 mmHg, and no treatment with antihypertensive medication.
Hypertension based on traditional criteria was defined as SBP≥140 mmHg, DBP≥90 mm Hg, and/or current use of an antihypertensive medication.
In this group, 39% were classified as hypertensive based on SBP only, 43% based on DBP only, and 18% based on SBP and DBP.
In this group, 13% were classified as hypertensive based on SBP only, 2% on DBP only, 4% based on SBP and DBP, and 80% based on use of antihypertensive medications.
Compared to SPRINT and ACCORD-BP, individuals that would be reclassified as having hypertension and be recommended for initiation of BP therapy based on 10-year ASCVD risk greater than 10%, were younger and more likely to be male; less likely to have a history of CVD, or an estimated glomerular filtration rate <60 ml/min/m2; and had a lower estimated 10-year ASCVD risk (Table 3).
Table 3.
Demographic and clinical characteristics of US adults newly recommended to start antihypertensive medications and baseline characteristics of SPRINT and ACCORD-BP participants.
| Characteristic | NHANES Newly classified with hypertension and 10-year ASCVD risk >10% (N = 118) |
SPRINT (N = 9361) |
ACCORD (N = 4733) |
|---|---|---|---|
| Weighted mean (SD) or N (weighted percent) | Mean (SD) or N (percent) | Mean (SD) or N (percent) | |
| Demographic variables | |||
| Age (years) | 63.2 (12.3) | 67.9 (9.4) | 62.7 (6.7) |
| Women | 32 (31) | 3332 (36) | 2258 (48) |
| Race/ethnicity | |||
| White, non-Hispanic | 49 (72) | 5399 (58) | 2781 (59) |
| Black, non-Hispanic | 24 (11) | 2802 (30) | 1127 (24) |
| Hispanic | 34 (12) | 984 (11) | 330 (7) |
| Other | 11 (6) | 176 (2) | 495 (10) |
| Medical history | |||
| Diabetes | 24 (17) | 0 (0) | 4733 (100) |
| CVD | 10 (9) | 1562 (17) | 1593 (34) |
| Atherosclerotic CVD risk (%), mean | 16.9 (7.7) | 22.0 (14.5) | 27.2 (14.5) |
| ASCVD > 10% | 118 (100) | 7602 (81) | 4226 (89) |
| Medications | |||
| Antihypertensive medications | 0 (0) | 8479 (91) | 4132 (87) |
| Lipid-lowering medications | 19 (18) | NA | 3235 (68) |
| Statins | 18 (18) | 4054 (43) | 3063 (65) |
| Fibrates | 0 (0) | NA | 311 (7) |
| Aspirin | 4756 (51) | 2472 (52) | |
| Physical examination | |||
| BMI (kg/m2), mean | 28.5 (7.2) | 29.9 (5.8) | 32.1 (5.5) |
| Systolic BP | 133.1 (4.8) | 139.7 (15.6) | 144.2 (10.5) |
| Diastolic BP | 72.6 (12.3) | 78.1 (11.9) | 78.3 (9.6) |
| Laboratory data | |||
| eGFR (mL/min/1.73 m2), mean | 82.2 (17.8) | 71.7 (20.6) | 91.6 (28.8) |
| eGFR < 60 | 7 (6) | 2650 (28) | 403 (9) |
Cell contents are N, mean (95% CI), or proportion (%) (95% CI). Abbreviations: CVD, cardiovascular disease; ASCVD, atherosclerotic cardiovascular disease; BP, blood pressure; BMI, body mass index; eGFR, estimated glomerular filtration rate.
Endpoints in SPRINT and ACCORD-BP
The cumulative incidence of the primary composite cardiovascular endpoint in SPRINT and ACCORD-BP were 6% and 9% during a median follow-up time of 3.2 years and 4.7 years, respectively. Of participants not on BP medications at baseline and with a SBP ≥ 130 or DBP ≥ 80 mm Hg, and a SBP <140 and DBP<90 mm Hg, there were 10 events (2% of total) and 14 events (3% of total), in SPRINT and ACCORD-BP, respectively.
In each trial, incident rates of the primary endpoint in participants not on antihypertensive medications at baseline were substantially lower than rates observed for participants on baseline BP medications (Table 4).
Table 4.
Primary composite CVD endpoints in SPRINT and ACCORD-BP
| Study | N at risk* | N events | Proportion of total events* | IR per 1000 person-years (95% bootstrapped CI) |
|---|---|---|---|---|
| SPRINT | ||||
| SBP≥130 or DBP≥80 and SBP<140 and DBP<90, not on BP medications at baseline | 247 | 10 | 2% | 12.9 (5.3,22.0) |
| SBP ≥ 140 or DBP ≥ 90, not on BP medications at baseline | 561 | 21 | 4% | 12.2 (7.5, 17.9) |
| SBP≥130 or DBP≥80 and SBP<140 and DBP<90, on BP medications at baseline | 2599 | 133 | 24% | 16.2 (13.5, 19.0) |
| SBP ≥ 140 or DBP ≥ 90, on BP medications at baseline | 4092 | 285 | 51% | 22.4 (19.9, 25.0) |
| ACCORD-BP | ||||
| SBP≥130 or DBP≥80 and SBP<140 and DBP<90, not on BP meds at baseline | 227 | 14 | 3% | 12.8 (6.8, 20.2) |
| SBP≥140 or DBP≥90, not on BP meds at baseline | 373 | 25 | 6% | 13.9 (8.8, 19.5) |
| SBP≥130 or DBP≥80 and SBP<140 and DBP<90, on BP meds at baseline | 1584 | 147 | 33% | 20.0 (16.9, 23.5) |
| SBP≥140 or DBP≥90, on BP meds at baseline | 2549 | 259 | 58% | 21.8 (19.3, 24.5) |
Composite primary outcome for ACCORD is nonfatal MI, nonfatal stroke, or CVD death; composite primary outcome for SPRINT is MI, HF, stroke, angina, or CVD death.
Abbreviations: BP, blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; IR, incident rate.
An additional 113 events occurred in 1,749 participants with a SBP <130 and a DBP <80 mm Hg at randomization. Only 74 participants in this category were not on medications at baseline.
Discussion
Implementation of the new ACC/AHA guidelines for hypertension diagnosis would lead to an estimated prevalence of hypertension of 44% among US adults, representing an absolute increase of nearly 10%. Newly reclassified hypertensive individuals would be younger and have a lower prevalence of major comorbid conditions compared to adults traditionally diagnosed with hypertension. Furthermore, individuals that would be recommended to start BP therapy have a substantial lower CVD risk profile than most participants enrolled in the SPRINT and ACCORD-BP trials. In each of these trials, participants that were not on antihypertensive medications at baseline had a substantially lower CVD risk than participant on baseline antihypertensive medications.
We estimate that applying the newly recommended BP thresholds to hypertension diagnosis would reclassify an estimated 24 million adults with SBP 130-139 mmHg or DBP 80-89 who are not currently treated for hypertension, and based on a 10-year ASCVD risk of ≥10%, would recommend starting BP therapy on approximately 4.5 million American adults. These estimates are lower than prior reports that have estimated the population impact of adopting the ACC/AHA guidelines in US adults. Muntner et al, estimated that 31 million American adults would be reclassified as having hypertension6. The reason for the discrepancy is that in our analyses we account for the probability of the persistence of hypertension on our prevalence estimates, an approach recommended by most BP guidelines.
The new guidelines recommend treating hypertension to a target BP of <130/80 mmHg based largely on results of the SPRINT trial, which demonstrated that targeting SBP <120 mmHg reduced CVD events among participants at high CVD risk5. However, treatment targets are distinct from, and need not be identical to, thresholds used for diagnosis which we evaluate in this study. For example, hemoglobin A1c ≥6.5% is used to diagnose diabetes, while hemoglobin A1c <7% is a common treatment target14. This consideration is critical to interpret the results of SPRINT and ACCORD-BP who were designed to evaluate the effectiveness of intensive treatment targets (but not of different diagnostic thresholds) in participants who were for the most part on baseline antihypertensive medications.
American adults that would be newly recommended to start antihypertensive medications have a lower CVD risk than participants in SPRINT and ACCORD-BP. This is supported by our findings of lower CVD risk-factor profile in NHANES participants compared to those in SPRINT and ACCORD-BP. Furthermore, CVD event rates in participants in these trials that were not on baseline antihypertensive medications, and, thus, that would most closely resemble the population recommended to start antihypertensive medications, were markedly lower than those of participants on baseline antihypertensive medications. These observations are important since prior research has shown a net benefit to risk balance of intensive BP reduction that is dependent on CVD risk, with individuals at highest CVD risk deriving most benefit7,17.
There is insufficient evidence to support the recommendation of starting antihypertensive medications in the newly reclassified population. As we show, only a minority of CVD events in in SPRINT and ACCORD-BP occurred among participants in the lower BP threshold category who were not on baseline antihypertensive medications. In the HOPE-3 trial of adults with intermediate CVD risk and no history of CVD, participants with baseline BP <140/90 mmHg had no significant benefit from treatment with candesartan plus hydrochlorothiazide, compared with placebo, on a composite primary CVD endpoint18. As opposed to SPRINT and ACCORD-BP, only 22% of HOPE-3 participants were taking BP medications. Although data from HOPE-3 may apply more closely to the population that would be recommended to start BP therapy compared to SPRINT and ACCORD-BP, no prior clinical trial has primarily evaluated the effect of BP therapy in this population.
Our study is limited in that NHANES 2013-2014 did not have repeated BP measurements to accurately classify persistent hypertension at the individual level. It is possible that our estimates of persistence may be imprecise due to secular trends in BP treatment and control. In addition, NHANES relied on self-report of CVD which may underestimate the prevalence of CVD. Moreover, the cross-sectional study design in NHANES precludes assessing associations of hypertension diagnoses with CVD events. Finally, owing to the small number of events in SPRINT and ACCORD-BP, we were unable to provide reliable estimates for the effect of intensive BP therapy in the newly reclassified population recommended for BP therapy.
In conclusion, the newly recommended thresholds for blood pressure diagnosis would lead to a substantial increase in the prevalence of hypertension with the largest increase among adults at a low cardiovascular risk. Individuals that would be recommended to start BP therapy were not well represented and had a markedly lower CVD risk profile than participants in SPRINT and ACCORD-BP.
Supplementary Material
Perspectives.
There is insufficient evidence to recommend initiation of BP therapy among US adults newly labeled as hypertensives using the ACC/AHA recommended thresholds. Future research should establish if lowering diagnostic thresholds for hypertension is associated with a reduction in cardiovascular disease burden.
Novelty and Significance.
What is new?
-
-
We estimate the prevalence of hypertension in US adults under the new ACC/AHA diagnostic blood pressure thresholds accounting for the probability of observing an elevated blood pressure on two occasions.
-
-
We characterize the adult US population that would be recommended to start antihypertensive medications.
-
-
We estimate the cardiovascular disease risk of adults that would be recommended to start antihypertensive medications in two large contemporary blood pressure trials.
What is relevant?
-
-
Proper characterization and estimation of cardiovascular risk of adults that would be recommended to start antihypertensive medications under the new guidelines has significant clinical and public health implications.
Summary
-
-
The newly recommended thresholds for hypertension diagnosis would lead to a substantial increase in the prevalence of hypertension. There is insufficient evidence to recommend initiation of BP therapy among US adults newly classified as hypertensives.
Acknowledgments
This research was supported by an unrestricted gift from the Northwest Kidney Centers to the Kidney Research Institute.
Footnotes
Conflicts of Interest Disclosure:
Dr. de Boer reported receiving grant funding from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Heart, Lung, and Blood Institute (NHLBI), research support from Medtronic and Abbott, and consulting for Boehringer-Ingelheim and Ironwood. Dr. Hall reported serving on the board of Trustees of the American Kidney Fund. No other disclosures were reported.
References
- 1.Rosendorff C, Lackland DT, Allison M, et al. Treatment of hypertension in patients with coronary artery disease: a scientific statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension. Circulation. 2015;131(19):e435–e470. doi: 10.1161/CIR.0000000000000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2016 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1345–1422. doi: 10.1016/S0140-6736(17)32366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 4.de Boer IH, Bangalore S, Benetos A, et al. Diabetes and Hypertension: A Position Statement by the American Diabetes Association. Diabetes Care. 2017;40(9):1273–1284. doi: 10.2337/dci17-0026. [DOI] [PubMed] [Google Scholar]
- 5.Whelton PK, Karey RM, Aronow WS, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. J Am Coll Cardiol. 2017;2018:71e127–248. doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Muntner P, Carey RM, Gidding SM, et al. Potential U.S. Population Impact of the 2017 American College of Cardiology/American Heart Association Blood Pressure Guideline. Circulation. 2018;137(2):109–118. doi: 10.1161/CIRCULATIONAHA.117.032582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillips RA, Xu Jiaqiong Xu, Peterson LF, et al. Impact of Cardiovascular Risk on the Relative Benefit and Harm of Intensive Treatment of Hypertension. J Am Coll Cardiol. 2018;17:1601–10. doi: 10.1016/j.jacc.2018.01.074. [DOI] [PubMed] [Google Scholar]
- 8.SPRINT Research Group. Wright JT, Williamson JD, Whelton PK, Snyder JK, Sink KM, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med Massachusetts Medical Society. 2015 Nov 26;373(22):2103–16. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ACCORD Study Group. Cushman WC, Evans GW, Byington RP, Goff DC, Grimm RH, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010 Apr 29;362(17):1575–85. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.US Centers for Disease Control and Prevention; National Center for Health Statistics. National Health and Nutrition Examination Survey 1988-1994, and 2013-2014 documentation files. http://www.cdc.gov/nchs/nhanes.htm. Accessed November 2017.
- 11.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45(1):142–161. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 12.US Centers for Disease Control and Prevention; National Center for Health Statistics. National Health and Nutrition Examination Survey 2013-2014 examination and laboratory procedures files. https://wwwn.cdc.gov/nchs/nhanes/ContinuousNhanes/Manuals.aspx?BeginYear=2013. Accessed November 2017.
- 13.Johnson KC, Whelton PK, Cushman WC, Cutler JA, Evans GW, Snyder JK, et al. Blood Pressure Measurement in SPRINT (Systolic Blood Pressure Intervention Trial) Hypertension. 2018 doi: 10.1161/HYPERTENSIONAHA.117.10479. HYPERTENSIONAHA.117.10479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Afkarian M, Zelnick LR, Hall YN, et al. Clinical Manifestations of Kidney Disease Among US Adults with Diabetes, 1988-2014. JAMA. 2016;316(6):602–610. doi: 10.1001/jama.2016.10924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–9. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 17.The Blood Pressure Lowering Treatment Trialists’ Collaboration. Blood-pressure lowering Treatment based on cardiovascular risk: a meta-analysis of individual patient data. Lancet. 2014;384(9943):591–598. doi: 10.1016/S0140-6736(14)61212-5. [DOI] [PubMed] [Google Scholar]
- 18.Lonn EM, Bosch J, López-Jaramillo P, et al. Blood-Pressure Lowering in Intermediate-Risk Persons without Cardiovascular Disease. N Engl J Med. 2016;374(21):2009–2020. doi: 10.1056/NEJMoa1600175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


