Abstract
Cardiovascular disease is a leading cause of death worldwide and accounts for greater than 17.3 million deaths per year, with an estimated increase in incidence to 23.6 million by 2030 1. Cardiovascular death represents 31% of all global deaths 2 - with stroke, heart attack, and ruptured aneurysms predominantly contributing to these high mortality rates. A key risk factor for cardiovascular disease is hypertension. Although treatment or reduction in hypertension can prevent the onset of cardiovascular events, existing therapies are only partially effective. A key pathological hallmark of hypertension is increased peripheral vascular resistance due to structural and functional changes in large (conductive) and small (resistance) arteries. In this review, we discuss the clinical implications of vascular remodeling, compare the differences between vascular smooth muscle cell (VSMC) remodeling in conductive and resistance arteries, discuss the genetic factors associated with VSMC function in hypertensive patients, and provide a prospective assessment of current and future research and pharmacological targets for the treatment of hypertension.
Keywords: vascular smooth muscle cells, hypertension, resistance arteries, conductive arteries, functional genomics
I. Introduction
The arterial vascular wall contains multiple cellular components including endothelial cells, layers of vascular smooth muscle cells (VSMCs) and an extracellular matrix, and can be subdivided into three distinct layers or tunics: the tunica intima, tunica media and tunica externa. The innermost tunica intima is comprised of a single layer of endothelial cells, while the tunica externa is the outermost layer of the vessel wall and forms a sheath of connective tissue primarily comprised of collagen and elastin fibers. Between the intima and externa is the tunica media - the largest of the three layers and the site of key differences as we move down the arterial tree from large conducting vessels, to medium sized distributing vessels, and finally to resistance arteries. Conducting or elastic arteries (e.g. the aorta or carotid) have a tunica media with greater elastic than smooth muscle content, which facilitates vascular compliance in response to high pressure blood flow from the heart. In comparison, muscular distributing arteries such as the radial artery have decreased elastic material but higher smooth muscle content with contribute to increased contractility in these vessels 3,4. Lastly, in resistance arteries, the tunica media lacks elastin and is made up of one to two layers of VSMCs.
In the healthy artery, changes in the extravascular environment, local signaling molecules, or hemodynamic demands, initiate structural and functional adaptations within the different cell types and layers of the vessel wall designed for blood pressure homeostasis. However, in disease states, these adaptive changes do not return to baseline levels but instead initiate pathological vascular alterations observed with cardiovascular disease. This maladaptive change is defined as “vascular remodeling”.
In hypertension, vascular remodeling involves changes to VSMCs in the vessel wall of both large and small arteries, as well as other cellular components of the vascular wall including endothelial cells 5,6, and elastin and collagen content 7,8. Vascular remodeling is a heterogeneous process and differs depending on the vessel type and specific disease state or progression (Figure 1). For example, vascular remodeling may increase or decrease the arterial lumen diameter in processes defined as outward and inward remodeling respectively. Vessels that undergo eutrophic remodeling have no net change in vessel wall material or media cross-sectional area. Conversely, hypo- and hypertrophic remodeling result in a net decrease or increase in vessel cellular material, respectively. In larger conductive vessels, VSMCs primarily undergo hypertrophy, which results in an increased intima-media thickness and contributes to increased arterial stiffness and blood pulse wave pressure. By comparison, small resistance vessel remodeling may present as either eutrophic or hypertrophic remodeling, depending on the form of hypertension.
Figure 1:
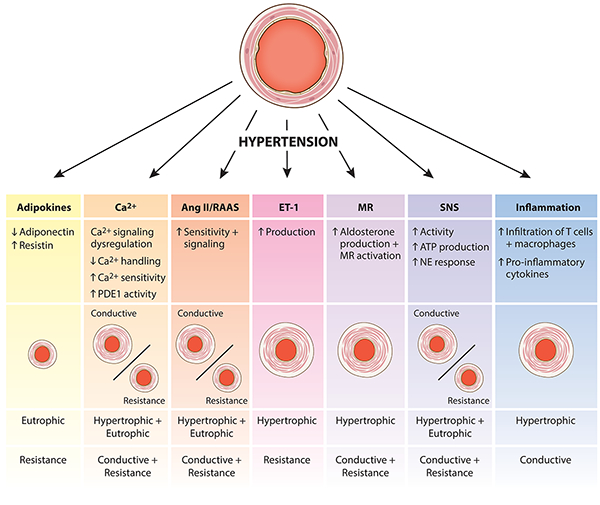
Differential physiological effects on smooth muscle based arterial remodeling (conductive and resistance).
Mechanical forces on the blood vessel wall also greatly contribute to hypertensive vascular remodeling. Blood vessels respond to altered fluid shear stress and circumferential strain through changes in vascular extracellular matrix composition, cellular secretion of endogenous growth factors and cytokines, and vascular sensitivity to circulating humoral factors. These dynamic changes are further regulated by temporal variables such as force duration, load, and force traits (e.g. cyclic stretch versus static strain) which cause differential responses on the vasculature. Disruption of the vascular-mechanical homeostasis may in fact initiate early pathways involved in pathological remodeling. The mechanobiological mechanisms that govern organ, tissue, and cell level changes in organisms, and how these structures adapt to mechanical insults, is an area of intense research interest and is too large for the scope of this vascular specific review. Extensive data exists and is expertly reviewed by others 9,10.
In this review article, we discuss the clinical relevance of vascular remodeling, emphasizing the relationship between current hypertensive drugs and the reversal of remodeling. Next, we outline the functional and structural alterations to VSMCs in both large and small arteries, highlighting similarities and differences in the pattern and mechanisms of remodeling. We examine genome-wide association data to address the genomic underpinnings of VSMC remodeling in hypertension, and lastly, assess future research areas and drug targets for treating hypertension.
II. Clinical Relevance
Clinical studies in patients with cardiovascular diseases find correlations between vascular remodeling and cardiovascular disease progression 11–13. These findings extend from cardiovascular disease in general, to hypertension specifically, as vascular remodeling, particularly in the small arteries, is a hallmark of disease progression in hypertension and is highly correlated with disease severity 14,15. Therefore, improved understanding of the pathological mechanisms of vascular remodeling holds high relevance for the clinical consequences and treatment of hypertension. In Table 1, we provide a comprehensive summary of the features of a number of clinical studies that investigate the clinical relationship between hypertension and vascular remodeling.
Table 1:
Basic properties of clinical studies referenced in manuscript
| Age | Sex | Race | Cohort Size | Type of Hypertension | Reference |
|---|---|---|---|---|---|
| ≥65 | Female & Male | 88.4% White, 11.6% Black | 4476 | 65 years of age or older without known clinical cardiovascular disease; 40% with essential hypertension | 11 |
| 54 (SD 5.8) | 56.6% Female, 43.4% Male | 74.5% White, 25.5% Black | 12576 | Normotensives from Atherosclerosis Risk in Communities (ARIC) Cohort | 12 |
| 40–66 | 35% Female, 65% Male | Patients: 81% White, 19% Black; Controls: 74% White, 26% Black | 86 (43 hypertensive, 43 normotensive) | Asymptomatic Hypertension | 16 |
| 30–59 & 57–80 | Undisclosed | Undisclosed | Undisclosed | Isolated Systolic Hypertension | 17 |
| 40–75 | 55.9% Female, 44.1 Male | 78% White, 22% Nonwhite | 2845 | Essential Hypertension | 18 |
| 18–60+ | 51.5% Female, 48.5% Male | 72.2% Non-Hispanic White, 10.9% Non-Hispanic Black, 7.2% Mexican American, 9.7% Other | 14653 | Essential Hypertension | 21 |
| ≥70 | 74.7% Female, 25.3% Male | Japanese | 164 | Essential Hypertension | 23 |
| 52±12.7 | 47.2% Female, 52.8% Male | Chinese | 644 | Mild Hypertension | 24 |
| 74±9 | 56% Female, 44% Male | Japanese | 211 (161 Hypertensive, 50 normotensive) | Essential Hypertension | 25 |
| 42–74 | 38.2% Female, 61.8% Male | Undisclosed | 424 (314 Hypertensive, 110 Normotensive) | Essential Hypertension | 26 |
| 40–99 | 67% Female, 33% Male | Undisclosed | 212 | Isolated Systolic Hypertension | 28 |
| 20–81 | 42.4% Female, 57.6% Male | Undisclosed | 151 (128 Hypertensive, 23 Normotensive) | Essential Hypertension, Renovascular Hypertension, Primary Aldosteronism, Pheochromocytoma, NIDDM | 29 |
| Mean 51±4 | 27% Female, 73% Male | Undisclosed | 30 (15 Hypertensive, 15 Normotensive) | Essential Hypertension | 30 |
| 25–60 | 33% Female, 67% Male | Undisclosed | 21 (14 Hypertensive, 7 Normotensive) | Essential Hypertension | 31 |
| 18–65 | 100% Male | Undisclosed | 50 (21 Hypertensive, 29 Normotensive) | Essential Hypertension | 32 |
| 46–72 | 35.6% Female, 64.4% Male | Undisclosed | 500 | Essential Hypertension | 33 |
| 25–70 | 40% Female, 60% Male | Undisclosed | 66 | Mild Essential Hypertension | 34 |
| 35–65 | 32% Female, 68% Male | Undisclosed | 50 (25 Hypertensive, 25 Normotensive) | Essential Hypertension | 38 |
| 60–80 | 68.8% Female, 31.2% Male | Undisclosed | 77 | Essential Hypertension | 40 |
| 27–72 | Undisclosed | White | 12 | Essential Hypertension | 41 |
| 26–67 | 27% Female, 73% Male | Undisclosed | 55 | Essential Hypertension | 42 |
| 18–75 | 21% Female, 79% Male | White | 114 | Essential Hypertension | 43 |
| 25–50 | 100% Male | Undisclosed | 29 (17 Hypertensive, 12 Normotensive) | Mild Essential Hypertension | 44,45 |
| 30–65 | 43% Female, 57% Male | Undisclosed | 28 (19 Hypertensive, 9 Normotensive) | Mild Essential Hypertension | 46 |
| 38–65 | 25% Female, 75% Male | Undisclosed | 11 | Essential Hypertension | 47 |
| Undisclosed | Undisclosed | Undisclosed | 21 (10 Hypertensive, 11 Normotensive) | Essential Hypertension | 52 |
a. Conductive Artery Remodeling in Clinical Hypertension
In larger conductive arteries, vessel remodeling in hypertension is characterized by increased intima-media thickness, which contributes to overall increased vessel wall thickness 16. Conductive wall thickening and hypertrophy during hypertension ultimately lead to increased vessel stiffness and decreased arterial compliance, which could negatively impact myocardial work capacity and coronary perfusion 17. The changes observed in large arteries during hypertension are similar to those seen as a consequence of physiological aging in elderly patients 18,19 leading to one theory that increased blood pressure accelerates age-related alterations to large arteries 20–22.
In this regard, a cross-sectional study of elderly patients with at least one risk factor for cardiovascular disease showed an association between carotid arterial stiffness, high average systolic blood pressures and high visit-to-visit systolic blood pressure 23. In another clinical study, increased aortic stiffness (measured by pulse wave velocity) correlated significantly with negative end organ effects in the heart and kidney in patients with mild hypertension 24. Specifically, pulse wave velocity correlated with changes in left ventricular mass and wall thickness, left atrial diameter, and negatively with creatinine clearance, indicative of renal function decline. These findings have been reported in several other studies investigating structural and functional changes in the heart 25–27 and kidney 28 of hypertensive patients with increased arterial stiffness.
b. Resistance Artery Remodeling in Clinical Hypertension
The smaller diameter arteries of the resistance vasculature play a vital role in the control of systemic blood pressure. As such, resistance arteries are particularly vulnerable to vascular remodeling during hypertension and small artery changes have been well-correlated with clinical disease progression and severity 29. Numerous groups report an increased media-to-lumen (M/L) ratio in small subcutaneous resistance arteries collected during a gluteal biopsy 30,31. More recently developed non-invasive techniques, including microscopic and clinical analysis of the retinal vascular bed, confirm findings of increased M/L ratio and highlight novel non-invasive tools for diagnosing pathophysiological vascular changes in hypertension 32,33.
Hypertensive patients have significantly increased wall-to-lumen ratio in retinal arterioles compared to normotensive controls as measured using scanning laser Doppler flowmetry 32. Similarly, hypertensive patients with advanced microvascular retinal damage (retinopathy) have a higher incidence of left atrial enlargement, reduced left ventricular ejection fraction, and consequently congestive heart failure 33. Comparison of resistance arteries from the forearm and coronary microvasculatures suggests that these two parameters are remodeled in parallel in untreated patients with mild hypertension 34. These findings provide examples of non-invasive determination of changes to resistance arteries in hypertension and support the clinical significance of small artery remodeling in hypertension. These techniques may also be useful to clinically assess whether vascular remodeling can be reversed by effective treatment of hypertension.
c. Clinical Interventions and Vascular Remodeling
Treatment for individuals with hypertension currently includes a multipronged approach including pharmacological antihypertensive agents and/or lifestyle changes in both diet and exercise. The five major classes of antihypertensive drugs (β-blockers, diuretics, angiotensin-converting enzyme (ACE) inhibitors, angiotensin II (Ang II) receptor blockers (ARB) and calcium-channel blockers (CCBs)) 35 all promote blood pressure lowering, but have different effects on vascular remodeling depending on their mechanisms of action. Specifically, a comprehensive review of the literature shows that drugs that target vessel dilation are typically more successful in correcting resistance artery remodeling than those that primarily manipulate cardiac output 36–39.
Clinical studies have shown that drugs targeting the renin-angiotensin-aldosterone system (RAAS), specifically the synthesis of Ang II (ACE inhibitors) or the binding of the ligand to its receptor (ARBs), are beneficial in reversing conductive vascular remodeling due to hypertension. For example, the ACE inhibitor (ACE-I) perindopril significantly decreases vessel thickness in the conductive radial artery in hypertensive patients after 9 months of treatment 40. In another randomized cross-over study, patients were treated with the ARB valsartan, the ACE inhibitor captopril or a combination of both 41. Both single and combination treatments decreased brachial artery pulse wave velocity, an indirect measure of arterial wall stiffness 41. An 8 week treatment with the ARB irbesartan decreased radial, but not carotid, artery wall thickness with no correlation to degree of blood pressure lowering or the levels of circulating RAAS circulating hormones 42. In support of a role for VSMC remodeling, the authors hypothesize that these heterogeneous findings are due to differences in vessel wall structure as the radial artery has a higher proportion of VSMCs to extracellular matrix than the carotid 42
Pharmacological agents blocking the RAAS have similar beneficial effects on remodeling in the resistance vasculature. Treatment with the direct renin inhibitor aliskiren, in concert with the ARB valsartan, significantly improved small artery remodeling in hypertensive patients, as assessed by retinal arteriole structure 43. The works of Thybo et al. and Schiffrin and colleagues similarly report that in clinical studies ACE-Is 38,44,45 and ARBs 46,47, but not β-blockers, improved resistance vasculature structure. These data support the notion that the RAAS is a relevant and important clinical target for reversing vascular remodeling in the conductive and resistance vasculature. Many of these studies show a 2–3 mmHg lowering of systolic blood pressure in patients treated with ACE-Is or ARBs compared to those treated with β-blockers or other classes, raising the question of whether a direct effect of RAAS or the level of blood pressure (with implied direct effect of mechanical stretch) is responsible for vascular remodeling.
The beneficial effects of RAAS blockade in reversing large and small artery vascular remodeling are recapitulated in animal models of hypertension. Treatment with the ACE-I perindopril decreased aortic wall thickness in two-kidney, one-clip hypertensive rats, by reversing VSMC hypertrophy without altering vessel collagen content 48. In a comparison between low dose or high dose ACE-I quinapril versus hydralazine, both doses of quinapril was more effective in lowering aortic collagen content in the spontaneously hypertensive rats (SHRs), despite less effective blood pressure lowing with low dose quinapril compared to hydralazine 49. Lastly, the ARB losartan is superior to the CCB amlodipine at improving vascular remodeling in prehypertensive stroke-prone spontaneously hypertensive (SHRSP) rats 50. Taken together, these studies suggest a dominant role of the RAAS in vascular remodeling in hypertension.
Cardiovascular training is also an effective treatment against hypertension and vascular remodeling, likely attributable in part to attenuating the RAAS. Spontaneously hypertensive rats (SHRs) that engaged in moderate exercise training over 12 weeks had lower blood pressure and ACE expression, and attenuated aortic remodeling 51. Hypertensive patients with decreased capillary area and capillary lumen area had an improvement in both parameters following aerobic exercise training 52. The beneficial effects of aerobic exercise training and pharmaceutical interventions on remodeling demonstrate a clinical relevance of vascular remodeling in the pathology and cardiovascular consequences of hypertension.
III. Conductive Artery Remodeling
Vascular smooth muscle cells (VSMC) in conductive arteries maintain vessel tone and structure by balancing forces between vasoconstrictive and vasodilatory signals, and regulate the production of extracellular matrix. Yet, the adaptations of conductive arterial VSMCs to rising intravascular pressure have not been particularly well studied in the literature. Included in this section are several seminal works that have expanded our current understanding of the structural changes and signaling pathways involved in vascular remodeling in large conductive arteries during hypertension.
a. Structural and Functional Changes of Large Arteries and VSMCs in Hypertension
The conductive (or elastic) arteries of the vascular system are critically important in regulating pulse wave pressures generated by the left ventricle during a heart cycle. Conductive arteries are located closest to the heart and their large lumen, high content of elastic fibers, and low compliance allows for regulation of the pulse wave pressures generated by the contractile motions of the heart, a feature known as the Windkessel effect. During hypertension, large elastic artery remodeling is characterized by a decrease in arterial compliance and an increase in wall thickness, due to an increase in intima-media thickness in the range of 15–40% 3.
Studies in patients with untreated essential hypertension show that local pulse pressure is correlated with overall wall thickening and increased intima-media thickness in the common carotid artery, a representative conductive artery 53. Similarly, aortic stiffening is associated with increased blood pressure in a longitudinal community-based cohort study of the relationship between arterial stiffening and incidence of hypertension 54. Medium sized distal muscular arteries are also remodeled during hypertension, and present with increased intima-media thickness but no overall changes in vessel or lumen diameter 53,55. In contrast to the correlation in conductive arteries, there is no significant relationship between local pulse pressure and intima-media thickness in the distal radial artery 3,53. This highlights a key difference in remodeling in proximal elastic arteries (such as the carotid) compared to distal medium sized arteries like the radial artery, likely due to differences in their structural composition as briefly discussed earlier in this review.
The increased lumen in proximal elastic arteries during hypertension is typically attributed to the breakdown of elastin fibers in response to increased pulsatile strength 56. Simultaneously, hypertension is also thought to increase collagen deposition in large vessels and it is hypothesized that the alteration of the elastin/collagen ratio is a significant contributor to increased arterial stiffness. However, data from patients with essential hypertension, as well as rodent models of hypertension, show conflicting findings where arterial wall thickening does not always result in enhanced arterial stiffness or a change in elastin/collagen ratio 55,57–60. This suggests that remodeling is not uniform in all large vessels and that other variables are at play. For example, other extracellular matrix proteins such as fibronectin and integrins may be involved in the structural changes in conductive arteries in hypertension 58,59. This demonstrates that our current understanding of potential (mal)adaptive cellular and molecular mechanisms that contribute to essential hypertension are limited by the relatively small cohorts of human studies and the inherent limitations of animal models.
In light of this, new tools are being applied to the field to better understand the cellular mechanisms contributing to hypertension. For example, Sehgel et al. measured stiffness of aortic VSMCs using atomic force microscopy and an in vitro reconstituted aortic tissue model in SHRs that have increased blood pressure and aortic stiffness and in normotensive Wistar Kyoto rats (WKYs), to define the direct contribution of VSMCs to increased conductive artery stiffness 61. The authors also show that SHR aortic VSMCs exhibit increased cellular stiffness due to the increased expression of contractile proteins including phosphorylated myosin light chain and myosin light chain kinase. These alterations in VSMC stiffness are also present due to physiological aging, and vessel and cell stiffness are amplified when hypertension and aging are both present 62. Researchers have also identified a role for apoptosis or programmed cell death in hypertension-induced remodeling in conductive vessels. SHRs 63 and deoxycorticosterone acetate–salt induced hypertensive rats 64 have increased apoptosis in the aorta or isolated aortic VSMCs compared to control animals/cells. Sharifi et al. hypothesize that increased apoptosis may be a countermeasure against cellular hypertrophy during hypertension 64.The molecular mechanisms responsible for these cellular changes are not yet fully defined but the findings presented above begin to provide evidence for direct changes in VSMCs in conductive arteries during hypertension.
Further evidence for direct changes to VSMCs is presented in the in vitro culture system studies by Leung et al. (1976) where cells are plated on matrix and exposed to cyclic stretch 65. Rabbit aortic VSMCs, cultured on elastin membranes, increased their production of collagen after exposure to a cyclic stretch paradigm65. Cyclic stretch also induces significant changes in smooth muscle myosin protein and mRNA expression 66. This effect appeared to be dependent on the extracellular matrix as stretch-induced myosin expression was only present when VSMCs were plated on collagen type 1 or laminin, but not when cells were plated on fibronectin. Thus, cyclic stretch combined with signals from the extracellular matrix seems critical for the regulation of smooth muscle myosin activity 66. Although these studies do not address the molecular pathways between cyclical stretch and protein synthesis, they begin to address the effects of mechanical strain on conductive VSMCs and highlight the need for further investigation in the area of VSMC-extracellular matrix interaction.
Recently, a relationship between senescence (aging) of VSMCs and hypertension has emerged. Klotho is a recently discovered anti-aging gene and its expression typically decreases with age. However, a study of elderly hypertensive, elderly non-hypertensive and non-elderly hypertensive patients by Su et al. showed that non-elderly hypertensive patients had lower circulating levels of Klotho protein than elderly non-hypertensive patients 67. Further, Klotho protein in elderly hypertensive patients was the lowest among all groups 67. The decreased circulating levels of Klotho in non-elderly hypertensive patients suggest a role of cell senescence in vascular remodeling in hypertension. In support of this, middle-aged heterozygous Klotho+/− mice have increased aortic stiffness (measured by pulse wave velocity) and aldosterone levels compared to age-match wild-type littermate controls 68. Hu et al. have proposed that Klotho decreases phosphate uptake in VSMCs, thus decreasing vascular calcification 69. Additional studies modulating Klotho protein levels show positive effects with increased Klotho (or negative effects with decreased Klotho) but do not specifically investigate changes to VSMCs 70–73.
b. Signaling and Neurohumoral Pathways
VSMC and vessel function in conductive arteries are typically self-regulated via internal signaling pathways. In addition, conductive arteries are subject to peripheral nervous system oversight via well-defined neurohumoral pathways. In this section, we will discuss alterations in intrinsic signaling pathways related to VSMC function, as well as changes in the peripheral nervous system regulation of conductive artery function, during hypertension.
As previously mentioned, the RAAS is a major therapeutic target for reducing blood pressure and reversing vascular remodeling in hypertension. This is partially because VSMCs in conductive arteries have altered reactivity to RAAS signaling components during hypertension. For example, cultured aortic VSMCs from SHRs have enhanced activation of MAP kinase in response to Ang II, compared to normotensive WKYs 74. Treatment of cultured VSMCs from the pulmonary artery of hypertensive patients with Ang II also induces cell proliferation via the Ang II Type 1 receptor (AT1R) 75. Several studies have shown that the interaction of aldosterone with the VSMC-mineralocorticoid receptor (MR) promotes pathways that contribute to development of large artery stiffness 76–79. In patients with primary hyperaldosteronism, arterial wall stiffness is increased as measured by pulse wave velocity 78 and ultrasonography 79. Supporting this, Galmiche et al. show that VSMC-MR knockout mice are protected against changes in arterial stiffness following aldosterone/salt challenge, by preventing the induction of changes in extracellular matrix proteins such as fibronectin and α5-integrin 76.
The handling of intra and extracellular calcium (Ca2+) is an important mechanism by which VSMCs control arterial lumen diameter and blood flow. A clear dysfunction in Ca2+ handling during hypertension was established as early as the 1960s and 70s 80,81. Femoral artery strips collected from SHRs, renal hypertensive, and deoxycorticosterone acetate (DCA) hypertensive rats required higher concentrations of Ca2+ to produce a maximal vasoconstriction in response to KCl than normotensive rats 81. Since then, subsequent studies have firmly established that VSMCs in hypertensive patients have enhanced reactivity to Ca2+ compared to cells from normotensive human and animals 82–87. Interestingly, alterations in multiple intracellular signaling components are implicated in Ca2+ sensitivity in hypertensive VSMCs. These include increased expression and sensitivity of IP3 receptors 88,89, altered Rho-kinase signaling 90, enhanced phospholipase C activity 85, abnormal expression and function of plasma membrane Ca2+ 91,92 and K+ channels 93, and hypoxia 94.
Sympathetic nerve activity is long known to play a vital role in the regulation of cardiovascular function. Unsurprisingly, increased sympathetic nerve activity is associated with hypertension in animal models with increased blood pressure 95 and cellular changes in VSMCs response to neurohumoral factors have been reported. Isolated thoracic aorta from SHRs have an increased endothelium-independent contractile response to noradrenaline and phenylephrine, compared to normotensive controls 96. Further, cultured aortic VSMCs and intact rat aorta are sensitive to noradrenergic-induced VSMC proliferation 97. Noradrenergic-induced cell proliferation, in conjunction with increased sympathetic nerve activity in hypertension, suggests that altered SNS activity may directly impact VSMCs structure and reactivity in hypertension.
Beyond the signaling pathways discussed above, transgenic mouse models with VSMC specific targeting have been used to identify additional signaling molecules and pathways that are critical to the proper regulation of blood pressure. In Table 2, we have summarized seminal studies describing gene targets that can increase or decrease blood pressure when specifically deleted from smooth muscle cells. Included among them are G12-G13 and their primary effector leukemia associated Rho-GEF (LARG) 98, the antioxidant/antiaging protein SIRT1 99, PPAR-γ 100,101 and the mineralocorticoid receptor (MR) 102.
Table 2:
Smooth muscle specific targeting of genes that regulate blood pressure in mice
| Smooth muscle target protein | Cre or Promoter | Knockout or Overexpression | Hypertension or Hypotension | Resistance or Conductive arteries analyzed | Reference |
|---|---|---|---|---|---|
| Ang II Type 1A receptors | i) SM22-Cre ii) KISM22-Cre |
i) KO ii) KO |
i) No change ii) Hypotension |
Conductive and resistance – aorta and mesenteric arteries | 217,218 |
| Arhgef1 | SMMHC-CreERT2 | KO | Attenuates Ang II hypertension | Conductive – aorta | 219 |
| Atp2b1 | SM22-Cre | KO | Hypertension | Conductive – femoral artery | 220 |
| Connexin 43 | SMMHC-CreERT2 | KO | No Change | In vivo blood pressure only; carotid wire injury | 221 |
| COX2 | SM22-Cre | KO | No change | Conductive – aorta | 222 |
| Dicer | SMMHC-CreERT2 | KO | Hypotension | Resistance – saphenous and mesenteric arteries | 223 |
| EGFR | SMMHC-CreERT2 | KO | Hypotension | Conductive – aorta | 224 |
| EPHB4 | SMMHC-Cre | KO | Hypotension | Resistance – mesenteric arteries | 225 |
| Filamin A | SMMHC-CreERT2 | KO | Hypotension | Resistance arteries – caudal artery | 226,137 |
| G12-G13-LARG | SMMHC-CreERT2 | KO | Attenuates salt-induced hypertension | Conductive – aorta | 98 |
| Guanylyl cyclase-A | SM22-Cre | KO | No Change | Conductive and resistance – aorta, femoral, pulmonary, and renal arteries | 227 |
| NO-sensitive guanylyl cyclase | SMMHC-CreERT2 | KO | Hypertension | Conductive - aorta | 228 |
| IP3R1, IP3R2, IP3R3 | SMMHC-CreERT2 | KO (all 3 together) | No Change in Basal BP Attenuates Ang II hypertension |
Conductive and resistance - aorta and mesenteric arteries | 89 |
| L-type Ca2+ channel Cav1.2 | SM22-CreERT2 | KO | Hypotension | Conductive and resistance – aorta and tibialis | 229 |
| Mineralocorticoid receptor | SMactin-CreERT2 | KO | Hypotension | Resistance – mesenteric arteries | 102 |
| Myosin Light Chain Kinase (MLCK) | SM22-Cre | KO | Hypotension | Resistance – mesenteric arteries | 230,231 |
| Myosin phosphatase target subunit 1 (MYPT1) | SMα-actin promoter | KO | Hypertension | Resistance – mesenteric arteries | 232 |
| Na+/Ca2+ exchanger | SMMHC-Cre | KO | Hypotension | Resistance – mesenteric arteries | 233 |
| Na+-K+-ATPase | SMα-actin promoter | OE | Hypotension | Conductive – aorta | 234 |
| Ndst1 | SM22α-Cre | KO | No change | Conductive and resistance – aorta and thoracodorsal arteries | 235 |
| Nox1 | SMMHC-Cre | OE | Potentiates Ang II hypertension | Conductive – aorta | 236 |
| Pannexin 1 | SMMHC-CreERT2 | KO | Hypotension | Resistance – thoracodorsal artery | 175 |
| Piezo1 | SM22α-Cre | KO | No change | Resistance arteries – caudal and rostral cerebellar artery | 137 |
| PPAR-γ | i) SM22-Cre ii) SMMHC promoter |
i) KO ii) OE of inactive mutation |
i) Hypotension ii) Hypertension |
Conductive and resistance - aorta and cerebral | 100,101 |
| Rac1 | SMMHC‐CreERT2 | KO | Hypertension | Conductive and resistance – aorta and mesenteric arteries | 237,238 |
| SIRT1 | SM22α promoter | OE | Attenuates hypertension | Conductive – aorta | 99 |
c. Inflammatory Pathways
Vascular inflammation is a key feature in the development of cardiovascular diseases, including hypertension, and the infiltration of inflammatory cells such as T cells and macrophages into large conductive arteries is a hallmark feature of hypertension in animal models 103,104. A series of studies using genetic knockout animals and the adoptive transfer of immune cells provide compelling evidence for a role for vascular inflammation in the development of hypertension. For example, the injection of immunosuppressive regulatory T cells (Tregs) 105 or T and B cell deficiency (Rag−/−) 106 are both protective against Ang II induced hypertension, while the adoptive transfer of T cells into Rag−/− mice is sufficient to produce a full hypertensive response to Ang II 106. However, a more recent study reported that this Ang II resistance in Rag−/− mice has been lost, and is independent of T cells, suggesting genetic drift can influence the effect of vascular inflammation in hypertension 107. The signaling of individual pro-inflammatory cytokines is also implicated in hypertension pathology where increased concentrations of the pro-inflammatory cytokine IL-17 is observed in hypertensive mice and in smooth muscle cells from hypertensive patients, and IL-17−/− mice are resistant to sustained hypertension following Ang II infusion 108. Treatment with small hairpin RNA against interleukin-6 (IL-6) is protective against vascular inflammation, vascular remodeling and increased blood pressure in a model of cold-induced hypertension 109.
Atherosclerosis is another cardiovascular disease that is closely associated with hypertension incidence and severity 110, and is characterized by loss of vessel wall elasticity and plaque formation in the intima. Inflammatory signaling and inflammation-induced vascular remodeling are thought to be key players in the pathology of atherosclerosis. As in hypertension, Treg cells play a key role in disease progression as the depletion of Treg cells promotes the development of atherosclerosis 111–113. Important roles have also been identified for the cytokines IL-6 and IL-17 in the progression of atherosclerotic disease. IL-17/IL-17A/IL-17A receptor depletion in animals model of atherosclerosis decreased the systemic inflammation during disease progression but did not significantly decrease aortic plaque burden 114,115. Differential findings exist for the role of IL-6 in atherosclerosis. Animals genetically lacking IL-6 in combination with ApoE deficient (IL-6−/−ApoE−/−116 and IL-6−/−AprE+/−117) had enhanced atherosclerotic lesion formation in a high fat and pathogen induced atheroscelorsis respectively 116,117. Conversely, weekly IL-6 injections increased lesion size in both wild-type and ApoE−/− animals on high fat diet 118. Taken together, these data from both human and animal models impressively demonstrate the multiple functions inflammatory signaling molecules can play in large artery remodeling in both hypertension and atherosclerosis, perhaps playing a role in parallel in disease development.
IV. Resistance Artery Remodeling
Small diameter (<250 μm) arteries in the peripheral vasculature are traditionally responsible for the regulation of blood pressure by controlling peripheral vascular resistance and blood flow. Structural and functional changes in these resistance arteries are a classic measure of cardiovascular pathology and disease progression. In this section, we discuss an extensive body of literature focused on resistance arterial VSMC changes during hypertension.
a. Structural and Functional of Resistance Arteries and VSMCs in Hypertension
Structural remodeling in the small arteries of the resistance vasculature is a hallmark of hypertension pathophysiology and is associated with increased risks of cardiovascular events 15. In essential (idiopathic) hypertension, resistance arteries undergo inward eutrophic remodeling, which significantly increases the media/lumen ratio without a change in the media cross-sectional area 3,14,15,30,119–121. In studies where resistance arteries were isolated from the subcutaneous fat of essential hypertensive patients, the rearrangement of VSMCs and extracellular matrix observed were characteristic of eutrophic remodeling, rather than VSMC hypertrophy and hyperplasia 15. Alternatively, resistance arteries can undergo differential patterns of structural remodeling, depending on causative or modifying factors for hypertension. In hypertensive patients with diabetes mellitus or patients diagnosed with pulmonary hypertension, resistance arteries (from the systemic and pulmonary circulations respectively) undergo inward hypertrophic remodeling, which results in an increased media cross-sectional area and media/lumen ratio, typically due to VSMC hypertrophy or hyperplasia 119,121,122.
Hypertension-dependent structural changes in VSMCs are thought to have short-term protective, but long-term pathological, effects. For example, initial structural changes in forearm small arteries increased vascular resistance at maximal vasodilation in response to increased blood flow, so as to normalize wall stress 3. On the other hand, long-term increases in vascular resistance lead to reductions in maximal dilation over time (such as reduced coronary flow reserve), reduced vascular perfusion, and impaired capillary rarefaction 3,15,123–125. Furthermore, increased collagen levels did not correlate with increased stiffness during early stages of hypertension; yet, in later stages the vascular wall continues to compensate for elevated blood pressures by depositing more collagen and increasing wall stiffness 125. These studies demonstrate that the temporal effects of vessel adaptations can differentially influence physiological outcomes.
Although intrinsic changes to the arterial wall influence blood pressure adaptation, circumferential wall stress (i.e. pressure forces) reciprocally impact vessel structure 126. In vein graft experiments, exposure to high arterial pressure induced hypertrophic remodeling to a similar level as those observed in secondary forms of hypertension 127–129. This suggests a pressure dependent, but vessel independent contribution. These conclusions were reached when pressure reduction using antihypertensive drugs, such as β-blockers, failed to normalize artery structure or reduce peripheral resistance in humans 119,130. These data suggest two things: 1. targeted reversal of resistance artery remodeling by simply lowering blood pressure in essential hypertension may fail due to pressure independent mechanisms, and 2. different etiologies of hypertension, either primary or secondary, should be treated differently as the main drivers for structural vessel changes may come from different mechanisms 131. Importantly, the latter is a strategy that is already employed clinically.
Hypertensive patients, and animal models of essential hypertension, exhibit increased myogenic reactivity (the inherent constriction/dilation responses of VSMCs to changes in luminal pressure) which may contribute to inward remodeling in hypertension 3,132–134. In line with this, current evidence exploring the role of ion channels in myogenic tone points to an important new role for their regulation of myogenic constriction in the development of hypertension 135–138. Finally, the role of apoptosis in small artery remodeling is an emerging field of research where one hypothesis suggests that inward eutrophic remodeling is due to simultaneous inward growth of VSMC layers and cell death in the media periphery 139–141. However, Dickhout et al. report decreased levels of apoptotic markers in their study of young prehypertensive SHRs 142. These divergent findings demonstrate a need for further work to conclusively determine a role for apoptosis of VSMC in resistance artery remodeling.
Together, the structural and functional changes described above highlight the complex properties underlying vascular remodeling in the resistance vasculature in hypertension.
b. Signaling and Neurohumoral Pathways.
The cellular changes underlying differential VSMC remodeling patterns in hypertension (eutrophic or hypertrophic) have also been attributed to the differential activation of signaling pathways important for vasoconstriction, cellular migration, VSMC hypertrophy, apoptosis, and inflammation 3,15,143. Imbalances between vasoconstrictive and vasodilatory intracellular signaling, or altered sensitivity to these signaling molecules, may contribute to the development and/or progression of hypertension. Here, we examine a number of key signaling pathways that are altered in resistance arteries during hypertension.
Altered responses to neurohumoral vasopressors have been well documented in studies of hypertensive humans who show some degree of vascular remodeling. It is unclear whether VSMCs become more sensitive to vasoconstrictor molecules such as norepinephrine, or if vasoconstriction pathways remain activated due to increased sympathetic nerve activity, as both events have been observed in hypertensive humans 144–150. In humans, counter-regulatory vasodilatory events have also been tested using endothelium-independent agents, such as sodium nitroprusside (SNP), and were not different between control and hypertensive subjects 149,151. Conversely, endothelial-dependent vasodilation by acetylcholine was impaired in hypertensive patients and animal models, but the amount of experimental variability in these observations may be a red-herring in interpreting the outcome of studies of VSMC function in hypertension 149,152,153. Nonetheless, it remains critically important to test the complex signaling pathways used to balance vasoconstriction and counteracting vasodilatory forces, which, under pathological conditions cause hyperconstriction, increased vascular resistance, and high blood pressure.
The RAAS plays a key angioadaptive role during hypertension 154, in part through the binding of Ang II to the AT1R which results in vasoconstriction and the activation of cellular growth pathways mediated by tyrosine kinases 155. In particular, c-Src acts as both a regulator of vasoconstriction and growth through transactivation of receptor tyrosine kinases 155–158, acting through mitogen activated protein kinases (MAPKs) which influence cell growth, apoptosis, and cell survival 154. Treatment with antihypertensive drugs targeting the RAAS reverse pathologic VSMC phenotypes in resistance arteries, indicating that alterations in this key pathway can influence structural remodeling of VSMCs in resistance arteries 42,44,46,145,159,160.
Other signaling mediators influencing VSMCs during hypertension include aldosterone, endothelin 1 (ET-1), and NADPH which regulate hypertrophy, fibrosis, and inflammation of small arteries. Aldosterone infusion increases expression of ET-1, which directly induces hypertrophic remodeling of VSMCs 161–163. These effects can be ameliorated by mineralocorticoid receptor antagonism 151,164. Moreover, both aldosterone and Ang II induce NADPH oxidase activity in VSMCs, which causes ROS generation and concomitant activation of MAPKs, redox sensitive transcription factors (i.e. AP-1), inflammatory mediators, and matrix remodeling enzymes 125,165,166. Because aldosterone is synthesized and secreted following Ang II stimulation of adrenal cortical cells via AT1 receptor, imbalances in RAAS activation may have compounded negative effects as both aldosterone and Ang II increase blood pressure systemically, while also having direct effects on the vascular wall.
Sympathetic nerve innervation and purinergic signaling also have profound effects on VSMCs during hypertension. Neural-derived purinergic stimuli (ATP) regulate the beneficial short-term control of vascular tone, but may also drive long-term negative changes during small artery remodeling 167–169. Clinical cases of hypertension typically present with enhanced sympathetic nerve activation 170, which has been shown to result in hypertrophic VSMC remodeling 169,171,172. Moreover, local extracellular release of ATP and other purine nucleotides, which are derived from sympathetic nerve terminals, local immune cells, and adjacent VSMCs, can exert direct pathogenic changes on VSMCs 173–175 despite their normal functionality of coordinating minute-to-minute vasoconstriction events. In one study, sympathetic-driven vasoconstriction was increased in SHR, which was dependent upon ATP activation of purinergic receptors 176. Further, mesenteric beds of SHR show potentiated responses to ATP compared to controls 177. These observations demonstrate that hyper-stimulation of resistance arteries, due to increased sympathetic drive and purinergic signaling, directly promotes the progression of VSMCs remodeling changes observed in hypertension.
In contrast to the direct constrictive effects of neural-purinergic stimuli on vasoconstriction, ATP can also act as a mitogenic signal, stimulating both VSMC growth and proliferation in hypertensive models 178,179. The effects of ATP and its metabolic breakdown products are mediated by the direct binding of ligand to a diverse class of ionotropic and metabotropic cell surface receptors (e.g. G-protein coupled P2Y or cation channel P2X) 160. Alterations in purinergic receptor subtypes, expression pattern, or abundance might also influence VSMC phenotypes during hypertension. In one study, proliferative VSMCs were correlated with increased expression of P2Y receptors, while differentiated contractile VSMCs primarily express P2X1 receptors 180. Together, this evidence establishes a novel neural-purinergic signaling nexus, which acts synergistically with other angioadaptive cellular pathways.
Phosphodiesterase 1 (PDE1) is a calcium/calmodulin dependent enzyme that hydrolyzes cyclic nucleotides such as cGMP and cAMP when intracellular Ca2+ is high 181. These cyclic nucleotides play important secondary messenger roles in the vasodilatory effect of nitric oxide (NO) and impairment of their actions enables VSMC constriction. PDE1A is upregulated in VSMCs following stimulation with Ang II and transforming growth factor beta-1 (TGF-β1) 181,182, and is increased in animal models of pulmonary hypertension 183. Treatment with PDE1 specific inhibitors improved endpoint measurements in animal models of cardiovascular disease 181–185. Similarly, inhibition of PDE1A halted the progression of pulmonary arterial hypertension and reversed pulmonary artery remodeling and right heart hypertrophy 183.
Increased blood pressure in essential hypertension is often associated with obesity and increased whole body adiposity. Adipose tissue has gained recognition as an important endocrine organ, and adipokines (the primary signaling molecules released from adipocytes) have varying and important effects on VSMC function. Adiponectin is protective in the vasculature in part due to its anti-proliferative and anti-migratory effects on VSMCs 186. In fact, hypoadiponectinemia (reduced circulating levels of adiponectin) is considered a risk factor for the development of hypertension and its consequent vascular remodeling 187. Conversely, adipokines such as resistin 188,189, or treatment of VSMCs with adipocyte-conditioned media 190,191, promote the migration and/or proliferation of VSMCs in both human and animal models. Through the release of adipokines, and their direct signaling on VSMCs, the perivascular adipose tissue that is in direct contact with the vasculature can have significant effects on vascular remodeling during hypertension.
Here, we provided a comprehensive, but not exhaustive, examination of key pathways whose signaling is dysregulated in resistance arteries during hypertension (Figure 1).
V. Genetic Factors Underlying the Hypertensive Phenotype in VSMC
Adaptive changes in VSMCs resulting from high blood pressure are often underwritten by genetic factors 3,192,193. The heritability of blood pressure phenotypes and the regulation of blood pressure by polygenic and monogenic traits reveal a strong influence of genetics on hypertension pathophysiology 194–197. For example, offspring from hypertensive patients already have increased renal vascular resistance at an early age 14. These changes influence the long-term maintenance of blood flow and cardiovascular homeostasis by VSMCs.
Genome wide association studies (GWAS) have become increasingly important in discerning candidate genes involved in modulating VSMC function during hypertension. Within large hypertensive cohorts, GWAS have identified ATP2B1, a vascular calcium/calmodulin-dependent membrane ATPase, as a gene of interest in hypertension 198–202. Atp2b1 mRNA expression levels were elevated in aortic VSMCs of SHRs compared to the normotensive WKYs, suggesting that changes in Atp2b influence cellular calcium homeostasis 203. Similarly, the CSK gene reached genome wide significance in hypertensive cohorts 199–202. CSK encodes a cytoplasmic tyrosine kinase, which is directly involved in Ang II-dependent VSMC proliferation 204. In another study, gene variants near the GNAS-EDN3 alleles also reached genome wide significance in hypertensive cohorts 201,202. GNAS-EDN3 encodes the G-alpha subunit of G-protein receptor complexes and the vasoactive peptide endothelin 3, two proteins involved in vasoconstriction signaling pathways. Risk association scores for essential hypertension was found to be associated with SNPs in TRIC-A, a gene that encodes an intracellular monovalent cation channel involved in myogenic tone regulation 136.
Another gene family found to be involved in hypertension and playing a role in the VSMC are the phosphodiesterases (PDEs), discussed earlier in this review. In an Ang II rat model of hypertension, higher expression levels of PDE1A were correlated with decreased cyclic guanosine monophosphate homeostasis, which is associated with vasodilatory signaling pathways 181. Further, PDE1A single nucleotide polymorphisms in humans were significantly associated with increased diastolic blood pressure and carotid intima-media thickening in genome-wide associated studies 184. Recently, single gene variants in the PDE3A were identified as the primary cause of a rare autosomal dominant form of hypertension 205. These gene variants encoded missense mutations in PDE3A resulting in gain of function mutations in protein kinase A/cyclic AMP signaling, increased cAMP-hydrolytic activity, and enhanced VSMC proliferation 206.
Non-coding genetic alterations (e.g. microRNAs) have also been shown to modulate VSMC function in hypertension. Mice lacking miR-143 and miR-145 exhibit reduced blood pressure, reduced VSMC migration, disorganized VSMC actin stress fibers, and reduced vascular tone 207. In SHRs, up regulation of miR-130a was detected in remodeled superior mesenteric arteries and inhibition of miR-130a reduced VSMC proliferation in vitro 208. Additionally, miR-133 expression was found to repress VSMC differentiation and proliferation by silencing the transcription factor SP-1 209. Lastly, the down regulation of Krüppel-like factor 4 (KLF4) by miR-146a was found to promote VSMC proliferation and neointimal hyperplasia in VSMCs 210. These experiments focus on effects in large arteries and require validation in VSMCs of resistance arteries. Taken together, these studies highlight the importance of genetic factors in regulating VSMC remodeling and vascular homeostasis in hypertension 211.
VI. Look to the Future
Hypertension is a strong and independent predictor of risk and future incidence of cardiovascular events (e.g. stroke, myocardial infarction, end-organ damage) 15. In particular, resistant hypertension pathologies are a persistent and growing problem, with an increasing number of patients unable to achieve adequate control their blood pressure despite taking three or more anti-hypertensive drugs, 212,213. Thus, finding ways to prevent hypertension progression by reversing vascular remodeling associated with hypertension is vital for global health. As discussed in this review, distinct hypertension etiologies cause differential vascular remodeling which depend on the type of pathology/origin, disease progression, and vessel type/size. An important goal for future research in vascular remodeling would be to find ways to more accurately characterize different classes of vascular remodeling phenotypes as defined above. Current research primarily focuses on remodeling within the resistance vasculature, since changes to resistance arteries are highly predictive of disease progression. However, the field would benefit from more detailed research on hypertension-dependent remodeling in the larger conductive arteries since maladaptive changes in conductive arteries (changes in vessel stiffness and increased pulse wave pressure/velocity) influence resistance vessels. Briefly alluded to earlier in this review, changes in medium-sized muscular arteries (skeletal and cardiac) in hypertension are also vastly understudied and may present a novel area for research in vascular remodeling in hypertension. Lastly, sex differences in regards to hypertension are becoming more apparent (as reviewed in 214); it is likely that with further work, our view of SMC biology may change as research progresses in this area.
Although beyond the scope of this review, VSMC remodeling may be therapeutically targeted through improved endothelial cell function. Endothelial cells and VSMCs closely interact through direct cell-to-cell contact, and via soluble signaling molecules. Endothelial-dependent vasodilation is significantly impaired in hypertension and may contribute to VSMC remodeling. Thus, improvement of hypertensive-dependent endothelial dysfunction may in turn have beneficial effects on the VSMC. Vascular remodeling in hypertension involves changes in a wide host of signaling molecules and pathways (see Table 2). Thus, researchers have opportunities to target different pathways to reverse pathological vascular remodeling. The most effective therapeutic strategy likely requires a multivariable approach.
Lastly, reducing aberrant inflammatory and neurohumoral pathways to homeostatic levels in hypertension may also prove effective in reversing pathological VSMC remodeling, while simultaneously improving overall disease pathology. For example, therapeutic alterations in purinergic signaling would improve both inflammatory and neurohumoral pathways 148. One interesting and potential novel target of the neural-purinergic axis in the peripheral vasculature is the ATP release channel Pannexin1 (Panx1). Panx1 has been shown to regulate small arterial VSMC constriction responses to NE and α1-adrenergic receptor stimulation with the NE mimetic phenylephrine. Recent evidence shows that inhibition of Panx1, either pharmacologically or genetically, significantly blunts adrenergic induced vasoconstriction and significantly reduces blood pressure in mice 175,215. Another potential target is the gene collectrin (Tmem27), an amino acid transport regulator. Deletion of collectrin results in hypertension, augmented salt-sensitivity, and vascular remodeling 216. Further evaluation of these and other targets may prove beneficial for treating essential hypertension and progression into resistant hypertension made worse by pathological vascular remodeling.
Supplementary Material
Highlights.
Structural and functional changes in large (conductive) and small (resistance) arteries accompany hypertension.
There are important clinical implications of smooth muscle remodeling that are different in conductive and resistance arteries.
This review discusses current and future research in smooth muscle remodeling, and how it could be a target for treatment of hypertension.
Acknowledgments:
None.
Sources of Funding:
This work was supported by NIH grants HL088554 (BEI), HL120840 (BEI) and DK113632 (THL).
Abbreviations
- Ang II
Angiotensin II
- ACE
Angiotensin converting enzyme
- ACE-I
ACE inhibitor
- ARB
Angiotensin receptor blocker/antagonist
- CCB
Calcium channel blocker
- ECM
Extracellular matrix
- HT
Hypertension
- RAAS
renin-angiotensin-aldosterone system
- SHR
Spontaneously hypertensive rat
- Panx
Pannexin
- VSMCs
Vascular smooth muscle cells
- WKY
Wistar Kyoto rat
Footnotes
Disclosures:
None
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 3.Laurent S, Boutouyrie P. The structural factor of hypertension: large and small artery alterations. Circ Res. 2015;116:1007–1021. doi: 10.1161/CIRCRESAHA.116.303596. [DOI] [PubMed] [Google Scholar]
- 4.Leloup AJA, Van Hove CE, Heykers A, Schrijvers DM, De Meyer GRY, Fransen P. Elastic and Muscular Arteries Differ in Structure, Basal NO Production and Voltage-Gated Ca2+-Channels. Front Physiol. 2015;6:674–679. doi: 10.3389/fphys.2015.00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Savoia C, Sada L, Zezza L, Pucci L, Lauri FM, Befani A, Alonzo A, Volpe M. Vascular inflammation and endothelial dysfunction in experimental hypertension. Int J Hypertens. 2011;2011:281240. doi: 10.4061/2011/281240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong WT, Wong SL, Tian XY, Huang Y. Endothelial dysfunction: the common consequence in diabetes and hypertension. J Cardiovasc Pharmacol. 2010;55:300–307. [DOI] [PubMed] [Google Scholar]
- 7.Duca L, Blaise S, Romier B, Laffargue M, Gayral S, Btaouri El H, Kawecki C, Guillot A, Martiny L, Debelle L, Maurice P. Matrix ageing and vascular impacts: focus on elastin fragmentation. Cardiovasc Res. 2016;110:298–308. doi: 10.1093/cvr/cvw061. [DOI] [PubMed] [Google Scholar]
- 8.Wagenseil JE, Mecham RP. Elastin in large artery stiffness and hypertension. J Cardiovasc Transl Res. 2012;5:264–273. doi: 10.1007/s12265-012-9349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Humphrey JD. Mechanisms of arterial remodeling in hypertension: coupled roles of wall shear and intramural stress. Hypertension. 2008;52:195–200. doi: 10.1161/HYPERTENSIONAHA.107.103440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang JHC, Thampatty BP. An introductory review of cell mechanobiology. Biomech Model Mechanobiol. 2006;5:1–16. doi: 10.1007/s10237-005-0012-z. [DOI] [PubMed] [Google Scholar]
- 11.O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 12.Nambi V, Chambless L, He M, Folsom AR, Mosley T, Boerwinkle E, Ballantyne CM. Common carotid artery intima-media thickness is as good as carotid intima-media thickness of all carotid artery segments in improving prediction of coronary heart disease risk in the Atherosclerosis Risk in Communities (ARIC) study. Eur Heart J. 2012;33:183–190. doi: 10.1093/eurheartj/ehr192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magnussen CG. Carotid artery intima-media thickness and hypertensive heart disease: a short review. Clin Hypertens. 2017;23:7. doi: 10.1186/s40885-017-0063-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mulvany MJ. Small Artery Remodeling and Significance in the Development of Hypertension. Physiology. 2002;17:105–109. doi: 10.1152/nips.01366.2001. [DOI] [PubMed] [Google Scholar]
- 15.Mulvany MJ. Small artery remodelling in hypertension. Basic Clin Pharmacol Toxicol. 2012;110:49–55. doi: 10.1111/j.1742-7843.2011.00758.x. [DOI] [PubMed] [Google Scholar]
- 16.Roman MJ, Saba PS, Pini R, Spitzer M, Pickering TG, Rosen S, Alderman MH, Devereux RB. Parallel cardiac and vascular adaptation in hypertension. Circulation. 1992;86:1909–1918. [DOI] [PubMed] [Google Scholar]
- 17.Kingwell BA. Large artery stiffness: implications for exercise capacity and cardiovascular risk. Clin Exp Pharmacol Physiol. 2002;29:214–217. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell GF, Guo C-Y, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Cross-sectional correlates of increased aortic stiffness in the community: the Framingham Heart Study. Circulation. 2007;115:2628–2636. doi: 10.1161/CIRCULATIONAHA.106.667733. [DOI] [PubMed] [Google Scholar]
- 19.Harvey A, Montezano AC, Touyz RM. Vascular biology of ageing-Implications in hypertension. J Mol Cell Cardiol. 2015;83:112–121. doi: 10.1016/j.yjmcc.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aging Sun Z., arterial stiffness, and hypertension. Hypertension. 2015;65:252–256. doi: 10.1161/HYPERTENSIONAHA.114.03617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ong KL, Cheung BMY, Man YB, Lau CP, Lam KSL. Prevalence, Awareness, Treatment, and Control of Hypertension Among United States Adults 1999–2004. Hypertension. 2006;49:69–75. doi: 10.1161/01.HYP.0000252676.46043.18. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Agnoletti D, Xu Y, Wang J-G, Blacher J, Safar ME. Carotid-femoral pulse wave velocity in the elderly. J Hypertens. 2014;32:1572–6–discussion1576. doi: 10.1097/HJH.0000000000000187. [DOI] [PubMed] [Google Scholar]
- 23.Nagai M, Dote K, Kato M, Sasaki S, Oda N, Kagawa E, Nakano Y, Yamane A, Kubo Y, Higashihara T, Miyauchi S, Harada W, Masuda H. Visit-to-visit blood pressure variability, average BP level and carotid arterial stiffness in the elderly: a prospective study. J Hum Hypertens. 2017;31:292–298. doi: 10.1038/jhh.2016.77. [DOI] [PubMed] [Google Scholar]
- 24.Tan J, Pei Y, Hua Q, Xing X, Wen J. Aortic pulse wave velocity is associated with measures of subclinical target organ damage in patients with mild hypertension. Cell Biochem Biophys. 2014;70:167–171. doi: 10.1007/s12013-014-9876-9. [DOI] [PubMed] [Google Scholar]
- 25.Iida M, Yamamoto M, Ishiguro Y, Yamazaki M, Ueda N, Honjo H, Kamiya K. Association of left atrial phasic volumes with systemic arterial stiffness and ankle-brachial index in hypertensive patients. J Hum Hypertens. 2017;31:270–277. doi: 10.1038/jhh.2016.74. [DOI] [PubMed] [Google Scholar]
- 26.Bruno RM, Cartoni G, Stea F, Armenia S, Bianchini E, Buralli S, Giannarelli C, Taddei S, Ghiadoni L. Carotid and aortic stiffness in essential hypertension and their relation with target organ damage: the CATOD study. J Hypertens. 2017;35:310–318. doi: 10.1097/HJH.0000000000001167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frohlich ED, Apstein C, Chobanian AV, Devereux RB, Dustan HP, Dzau V, Fauad-Tarazi F, Horan MJ, Marcus M, Massie B. The heart in hypertension. N Engl J Med. 1992;327:998–1008. doi: 10.1056/NEJM199210013271406. [DOI] [PubMed] [Google Scholar]
- 28.Verhave JC, Fesler P, Cailar du G, Ribstein J, Safar ME, Mimran A. Elevated pulse pressure is associated with low renal function in elderly patients with isolated systolic hypertension. Hypertension. 2005;45:586–591. doi: 10.1161/01.HYP.0000158843.60830.cf. [DOI] [PubMed] [Google Scholar]
- 29.Rizzoni D, Porteri E, Boari GEM, De Ciuceis C, Sleiman I, Muiesan ML, Castellano M, Miclini M, Agabiti-Rosei E. Prognostic significance of small-artery structure in hypertension. Circulation. 2003;108:2230–2235. doi: 10.1161/01.CIR.0000095031.51492.C5. [DOI] [PubMed] [Google Scholar]
- 30.Aalkjaer C, Heagerty AM, Petersen KK, Swales JD, Mulvany MJ. Evidence for increased media thickness, increased neuronal amine uptake, and depressed excitation--contraction coupling in isolated resistance vessels from essential hypertensives. Circ Res. 1987;61:181–186. [DOI] [PubMed] [Google Scholar]
- 31.Intengan HD, Deng LY, Li JS, Schiffrin EL. Mechanics and composition of human subcutaneous resistance arteries in essential hypertension. Hypertension. 1999;33:569–574. [DOI] [PubMed] [Google Scholar]
- 32.Ritt M, Harazny JM, Ott C, Schlaich MP, Schneider MP, Michelson G, Schmieder RE. Analysis of retinal arteriolar structure in never-treated patients with essential hypertension. J Hypertens. 2008;26:1427–1434. doi: 10.1097/HJH.0b013e3282ffdc66. [DOI] [PubMed] [Google Scholar]
- 33.Varghese M, Adhyapak SM, Thomas T, Sunder M, Varghese K. The association of severity of retinal vascular changes and cardiac remodelling in systemic hypertension. Ther Adv Cardiovasc Dis. 2016;10:224–230. doi: 10.1177/1753944716630869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eftekhari A, Mathiassen ON, Buus NH, Gotzsche O, Mulvany MJ, Christensen KL. Changes in blood pressure and systemic vascular resistance do not predict microvascular structure during treatment of mild essential hypertension. J Hypertens. 2012;30:794–801. doi: 10.1097/HJH.0b013e328350e4ff. [DOI] [PubMed] [Google Scholar]
- 35.Laurent S Antihypertensive drugs. Pharmacol Res. 2017;124:116–125. doi: 10.1016/j.phrs.2017.07.026. [DOI] [PubMed] [Google Scholar]
- 36.Christensen KL, Mulvany MJ. Vasodilatation, not hypotension, improves resistance vessel design during treatment of essential hypertension: a literature survey. J Hypertens. 2001;19:1001–1006. [DOI] [PubMed] [Google Scholar]
- 37.Agabiti-Rosei E, Heagerty AM, Rizzoni D. Effects of antihypertensive treatment on small artery remodelling. J Hypertens. 2009;27:1107–1114. doi: 10.1097/HJH.0b013e328329272e. [DOI] [PubMed] [Google Scholar]
- 38.Thybo NK, Stephens N, Cooper A, Aalkjaer C, Heagerty AM, Mulvany MJ. Effect of antihypertensive treatment on small arteries of patients with previously untreated essential hypertension. Hypertension. 1995;25:474–481. [DOI] [PubMed] [Google Scholar]
- 39.Schiffrin EL. Remodeling of resistance arteries in essential hypertension and effects of antihypertensive treatment. Am J Hypertens. 2004;17:1192–1200. doi: 10.1016/j.amjhyper.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 40.Girerd X, Giannattasio C, Moulin C, Safar M, Mancia G, Laurent S. Regression of radial artery wall hypertrophy and improvement of carotid artery compliance after long-term antihypertensive treatment in elderly patients. J Am Coll Cardiol. 1998;31:1064–1073. doi: 10.1016/S0735-1097(98)00043-6. [DOI] [PubMed] [Google Scholar]
- 41.Mahmud A, Feely J. Reduction in arterial stiffness with angiotensin II antagonist is comparable with and additive to ACE inhibition. Am J Hypertens. 2002;15:321–325. [DOI] [PubMed] [Google Scholar]
- 42.Benetos A, Gautier S, Laflèche A, Topouchian J, Frangin G, Girerd X, Sissmann J, Safar ME. Blockade of angiotensin II type 1 receptors: effect on carotid and radial artery structure and function in hypertensive humans. J Vasc Res. 2000;37:8–15. [DOI] [PubMed] [Google Scholar]
- 43.Jumar A, Ott C, Kistner I, Friedrich S, Schmidt S, Harazny JM, Schmieder RE. Effect of aliskiren on vascular remodelling in small retinal circulation. J Hypertens. 2015;33:2491–2499. doi: 10.1097/HJH.0000000000000735. [DOI] [PubMed] [Google Scholar]
- 44.Schiffrin EL, Deng LY, Larochelle P. Effects of a beta-blocker or a converting enzyme inhibitor on resistance arteries in essential hypertension. Hypertension. 1994;23:83–91. [DOI] [PubMed] [Google Scholar]
- 45.Schiffrin EL, Deng LY. Comparison of effects of angiotensin I-converting enzyme inhibition and beta-blockade for 2 years on function of small arteries from hypertensive patients. Hypertension. 1995;25:699–703. [DOI] [PubMed] [Google Scholar]
- 46.Schiffrin EL, Park JB, Intengan HD, Touyz RM. Correction of arterial structure and endothelial dysfunction in human essential hypertension by the angiotensin receptor antagonist losartan. Circulation. 2000;101:1653–1659. [DOI] [PubMed] [Google Scholar]
- 47.Schiffrin EL, Park JB, Pu Q. Effect of crossing over hypertensive patients from a beta-blocker to an angiotensin receptor antagonist on resistance artery structure and on endothelial function. J Hypertens. 2002;20:71–78. [DOI] [PubMed] [Google Scholar]
- 48.Levy BI, Michel JB, Salzmann JL, Azizi M, Poitevin P, Safar M, Camilleri JP. Effects of chronic inhibition of converting enzyme on mechanical and structural properties of arteries in rat renovascular hypertension. Circ Res. 1988;63:227–239. [DOI] [PubMed] [Google Scholar]
- 49.Albaladejo P, Bouaziz H, Duriez M, Gohlke P, Levy BI, Safar ME, Benetos A. Angiotensin converting enzyme inhibition prevents the increase in aortic collagen in rats. Hypertension. 1994;23:74–82. [DOI] [PubMed] [Google Scholar]
- 50.He D-H, Lin J-X, Zhang L-M, Xu C-S, Xie Q. Early treatment with losartan effectively ameliorates hypertension and improves vascular remodeling and function in a prehypertensive rat model. Life Sci. 2017;173:20–27. doi: 10.1016/j.lfs.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 51.Gu Q, Wang B, Zhang X-F, Ma Y-P, Liu J-D, Wang X-Z. Contribution of renin–angiotensin system to exercise-induced attenuation of aortic remodeling and improvement of endothelial function in spontaneously hypertensive rats. Cardiovascular Pathology. 2014;23:298–305. doi: 10.1016/j.carpath.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 52.Gliemann L, Buess R, Nyberg M, Hoppeler H, Odriozola A, Thaning P, Hellsten Y, Baum O, Mortensen SP. Capillary growth, ultrastructure remodelling and exercise training in skeletal muscle of essential hypertensive patients. Acta Physiol (Oxf). 2015;214:210–220. doi: 10.1111/apha.12501. [DOI] [PubMed] [Google Scholar]
- 53.Boutouyrie P, Bussy C, Lacolley P, Girerd X, Laloux B, Laurent S. Association between local pulse pressure, mean blood pressure, and large-artery remodeling. Circulation. 1999;100:1387–1393. [DOI] [PubMed] [Google Scholar]
- 54.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308:875–881. doi: 10.1001/2012.jama.10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laurent S, Hayoz D, Trazzi S, Boutouyrie P, Waeber B, Omboni S, Brunner HR, Mancia G, Safar M. Isobaric compliance of the radial artery is increased in patients with essential hypertension. J Hypertens. 1993;11:89–98. [DOI] [PubMed] [Google Scholar]
- 56.Wagenseil JE, Mecham RP. Vascular extracellular matrix and arterial mechanics. Physiol Rev. 2009;89(3):957–989. doi: 10.1152/physrev.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hayoz D, Rutschmann B, Perret F, Niederberger M, Tardy Y, Mooser V, Nussberger J, Waeber B, Brunner HR. Conduit artery compliance and distensibility are not necessarily reduced in hypertension. Hypertension. 1992;20:1–6. [DOI] [PubMed] [Google Scholar]
- 58.Bézie Y, Lamazière JM, Laurent S, Challande P, Cunha RS, Bonnet J, Lacolley P. Fibronectin expression and aortic wall elastic modulus in spontaneously hypertensive rats. Arterioscler Thromb Vasc Biol. 1998;18:1027–1034. [DOI] [PubMed] [Google Scholar]
- 59.Boumaza S, Arribas SM, Osborne-Pellegrin M, McGrath JC, Laurent S, Lacolley P, Challande P. Fenestrations of the Carotid Internal Elastic Lamina and Structural Adaptation in Stroke-Prone Spontaneously Hypertensive Rats. Hypertension. 2001;37:1101–1107. doi: 10.1161/01.HYP.37.4.1101. [DOI] [PubMed] [Google Scholar]
- 60.Keeley FW, Johnson DJ. The effect of developing hypertension on the synthesis and accumulation of elastin in the aorta of the rat. Biochem Cell Biol. 1986;64:38–43. [DOI] [PubMed] [Google Scholar]
- 61.Sehgel NL, Zhu Y, Sun Z, Trzeciakowski JP, Hong Z, Hunter WC, Vatner DE, Meininger GA, Vatner SF. Increased vascular smooth muscle cell stiffness: a novel mechanism for aortic stiffness in hypertension. Am J Physiol Heart Circ Physiol. 2013;305:H1281–H1287. doi: 10.1152/ajpheart.00232.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sehgel NL, Sun Z, Hong Z, Hunter WC, Hill MA, Vatner DE, Vatner SF, Meininger GA. Augmented vascular smooth muscle cell stiffness and adhesion when hypertension is superimposed on aging. Hypertension. 2015;65:370–377. doi: 10.1161/HYPERTENSIONAHA.114.04456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sharifi AM, Schiffrin EL. Apoptosis in aorta of deoxycorticosterone acetate-salt hypertensive rats: effect of endothelin receptor antagonism. J Hypertens. 1997;15:1441–1448. [DOI] [PubMed] [Google Scholar]
- 64.Devlin AM, Clark JS, Reid JL, Dominiczak AF. DNA synthesis and apoptosis in smooth muscle cells from a model of genetic hypertension. Hypertension. 2000;36:110–115. [DOI] [PubMed] [Google Scholar]
- 65.Leung DY, Glagov S, Mathews MB. Cyclic stretching stimulates synthesis of matrix components by arterial smooth muscle cells in vitro. Science. 1976;191:475–477. [DOI] [PubMed] [Google Scholar]
- 66.Reusch P, Wagdy H, Reusch R, Wilson E, Ives HE. Mechanical Strain Increases Smooth Muscle and Decreases Nonmuscle Myosin Expression in Rat Vascular Smooth Muscle Cells. Circ Res. 1996;79:1046–1053. doi: 10.1161/01.RES.79.5.1046. [DOI] [PubMed] [Google Scholar]
- 67.Su X-M, Yang W. Klotho protein lowered in elderly hypertension. Int J Clin Exp Med. 2014;7:2347–2350. [PMC free article] [PubMed] [Google Scholar]
- 68.Chen K, Zhou X, Sun Z. Haplodeficiency of Klotho Gene Causes Arterial Stiffening via Upregulation of Scleraxis Expression and Induction of Autophagy. Hypertension. 2015;66:1006–1013. doi: 10.1161/HYPERTENSIONAHA.115.06033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hu MC, Shi M, Zhang J, Quiñones H, Griffith C, Kuro-o M, Moe OW. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2011;22:124–136. doi: 10.1681/ASN.2009121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Y, Sun Z. Klotho gene delivery prevents the progression of spontaneous hypertension and renal damage. Hypertension. 2009;54:810–817. doi: 10.1161/HYPERTENSIONAHA.109.134320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lin Y, Chen J, Sun Z. Antiaging Gene Klotho Deficiency Promoted High-Fat Diet-Induced Arterial Stiffening via Inactivation of AMP-Activated Protein Kinase. Hypertension. 2016;67:564–573. doi: 10.1161/HYPERTENSIONAHA.115.06825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou X, Chen K, Lei H, Sun Z. Klotho gene deficiency causes salt-sensitive hypertension via monocyte chemotactic protein-1/CC chemokine receptor 2-mediated inflammation. J Am Soc Nephrol. 2015;26:121–132. doi: 10.1681/ASN.2013101033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maltese G, Karalliedde J. The putative role of the antiageing protein klotho in cardiovascular and renal disease. Int J Hypertens. 2012;2012:757469. doi: 10.1155/2012/757469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lucchesi PA, Bell JM, Willis LS, Byron KL, Corson MA, Berk BC. Ca2+-Dependent Mitogen-Activated Protein Kinase Activation in Spontaneously Hypertensive Rat Vascular Smooth Muscle Defines a Hypertensive Signal Transduction Phenotype. Circ Res. 1996;78:962–970. doi: 10.1161/01.RES.78.6.962. [DOI] [PubMed] [Google Scholar]
- 75.Morrell NW, Upton PD, Kotecha S, Huntley A, Yacoub MH, Polak JM, Wharton J. Angiotensin II activates MAPK and stimulates growth of human pulmonary artery smooth muscle via AT1 receptors. Am J Physiol. 1999;277:L440–L448. [DOI] [PubMed] [Google Scholar]
- 76.Galmiche G, Pizard A, Gueret A, Moghrabi El S, Ouvrard-Pascaud A, Berger S, Challande P, Jaffe IZ, Labat C, Lacolley P, Jaisser F. Smooth muscle cell mineralocorticoid receptors are mandatory for aldosterone-salt to induce vascular stiffness. Hypertension. 2014;63:520–526. doi: 10.1161/HYPERTENSIONAHA.113.01967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Riet Te L, van Esch JHM, Roks AJM, van den Meiracker AH, Danser AHJ. Hypertension: renin-angiotensin-aldosterone system alterations. Circ Res. 2015;116:960–975. doi: 10.1161/CIRCRESAHA.116.303587. [DOI] [PubMed] [Google Scholar]
- 78.Strauch B, Petrák O, Wichterle D, Zelinka T, Holaj R, Widimský J. Increased arterial wall stiffness in primary aldosteronism in comparison with essential hypertension. Am J Hypertens. 2006;19:909–914. doi: 10.1016/j.amjhyper.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 79.Bernini G, Galetta F, Franzoni F, Bardini M, Taurino C, Bernardini M, Ghiadoni L, Bernini M, Santoro G, Salvetti A. Arterial stiffness, intima-media thickness and carotid artery fibrosis in patients with primary aldosteronism. J Hypertens. 2008;26:2399–2405. doi: 10.1097/HJH.0b013e32831286fd. [DOI] [PubMed] [Google Scholar]
- 80.Tobian L, Chesley G. Calcium Content of Arteriolar Walls in Normotensive and Hypertensive Rats. Experimental Biology and Medicine. 1966;121:340–343. doi: 10.3181/00379727-121-30773. [DOI] [PubMed] [Google Scholar]
- 81.Holloway ET, Bohr DF. Reactivity of vascular smooth muscle in hypertensive rats. Circ Res. 1973;33:678–685. [DOI] [PubMed] [Google Scholar]
- 82.Bohr DF. Reactivity of vascular smooth muscle from normal and hypertensive rats: effect of several cations. Fed Proc. 1974;33:127–132. [PubMed] [Google Scholar]
- 83.Goulopoulou S, Webb RC. Symphony of vascular contraction: how smooth muscle cells lose harmony to signal increased vascular resistance in hypertension. Hypertension. 2014;63:e33–e39. doi: 10.1161/HYPERTENSIONAHA.113.02444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tostes RC, Wilde DW, Bendhack LM, Webb RC. Calcium handling by vascular myocytes in hypertension. Braz J Med Biol Res. 1997;30:315–323. [DOI] [PubMed] [Google Scholar]
- 85.Uehara Y, Ishii M, Ishimitsu T, Sugimoto T. Enhanced phospholipase C activity in the vascular wall of spontaneously hypertensive rats. Hypertension. 1988;11:28–33. [DOI] [PubMed] [Google Scholar]
- 86.Aqel MB, Sharma RV, Bhalla RC. Increased Ca2+ sensitivity of alpha 1-adrenoceptor-stimulated contraction in SHR caudal artery. Am J Physiol. 1986;250:C275–C282. [DOI] [PubMed] [Google Scholar]
- 87.Sharma RV, Bhalla RC. Calcium and abnormal reactivity of vascular smooth muscle in hypertension. Cell Calcium. 1988;9:267–274. [DOI] [PubMed] [Google Scholar]
- 88.Abou-Saleh H, Pathan AR, Daalis A, Hubrack S, Abou-Jassoum H, Al-Naeimi H, Rusch NJ, Machaca K. Inositol 1,4,5-trisphosphate (IP3) receptor up-regulation in hypertension is associated with sensitization of Ca2+ release and vascular smooth muscle contractility. J Biol Chem. 2013;288:32941–32951. doi: 10.1074/jbc.M113.496802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lin Q, Zhao G, Fang X, Peng X, Tang H, Wang H, Jing R, Liu J, Lederer WJ, Chen J, Ouyang K. IP3 receptors regulate vascular smooth muscle contractility and hypertension. JCI Insight. 2016;1:e89402. doi: 10.1172/jci.insight.89402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 91.Sonkusare S, Palade PT, Marsh JD, Telemaque S, Pesic A, Rusch NJ. Vascular calcium channels and high blood pressure: pathophysiology and therapeutic implications. Vascul Pharmacol. 2006;44:131–142. doi: 10.1016/j.vph.2005.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kubo T, Taguchi K, Ueda M. L-type calcium channels in vascular smooth muscle cells from spontaneously hypertensive rats: effects of calcium agonist and antagonist. Hypertens Res. 1998;21:33–37. [DOI] [PubMed] [Google Scholar]
- 93.Huang K-C, Li T-M, Liu X, et al. KCNQ1 variants associate with hypertension in type 2 diabetes and affect smooth muscle contractility in vitro. J Cell Physiol. 2017;232:3309–3316. doi: 10.1002/jcp.25775. [DOI] [PubMed] [Google Scholar]
- 94.Barnes EA, Chen C-H, Sedan O, Cornfield DN. Loss of smooth muscle cell hypoxia inducible factor-1α underlies increased vascular contractility in pulmonary hypertension. FASEB J. 2017;31:650–662. doi: 10.1096/fj.201600557R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grassi G, Cattaneo BM, Seravalle G, Lanfranchi A, Mancia G. Baroreflex control of sympathetic nerve activity in essential and secondary hypertension. Hypertension. 1998;31:68–72. [DOI] [PubMed] [Google Scholar]
- 96.Lograno MD, Daniele E, Galli C. Changes of vascular smooth muscle reactivity in hypertensive rats. Pharmacol Res. 1989;21:719–728. [DOI] [PubMed] [Google Scholar]
- 97.Chen L, Xin X, Eckhart AD, Yang N, Faber JE. Regulation of vascular smooth muscle growth by alpha 1-adrenoreceptor subtypes in vitro and in situ. J Biol Chem. 1995;270:30980–30988. [DOI] [PubMed] [Google Scholar]
- 98.Wirth A, Benyó Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, Örsy P, Horváth B, Maser-Gluth C, Greiner E, Lemmer B, Schütz G, Gutkind JS, Offermanns S. G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med. 2008;14:64–68. doi: 10.1038/nm1666. [DOI] [PubMed] [Google Scholar]
- 99.Gao P, Xu T-T, Lu J, Li L, Xu J, Hao D-L, Chen H-Z, Liu D-P. Overexpression of SIRT1 in vascular smooth muscle cells attenuates angiotensin II-induced vascular remodeling and hypertension in mice. J Mol Med. 2014;92:347–357. doi: 10.1007/s00109-013-1111-4. [DOI] [PubMed] [Google Scholar]
- 100.Chang L, Villacorta L, Zhang J, Garcia-Barrio MT, Yang K, Hamblin M, Whitesall SE, D’Alecy LG, Chen YE. Vascular smooth muscle cell-selective peroxisome proliferator-activated receptor-gamma deletion leads to hypotension. Circulation. 2009;119:2161–2169. doi: 10.1161/CIRCULATIONAHA.108.815803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Halabi CM, Beyer AM, de Lange WJ, Keen HL, Baumbach GL, Faraci FM, Sigmund CD. Interference with PPAR gamma function in smooth muscle causes vascular dysfunction and hypertension. Cell Metab. 2008;7:215–226. doi: 10.1016/j.cmet.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.McCurley A, Pires PW, Bender SB, Aronovitz M, Zhao MJ, Metzger D, Chambon P, Hill MA, Dorrance AM, Mendelsohn ME, Jaffe IZ. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat Med. 2012;18:1429–1433. doi: 10.1038/nm.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kasal DA, Barhoumi T, Li MW, Yamamoto N, Zdanovich E, Rehman A, Neves MF, Laurant P, Paradis P, Schiffrin EL. T regulatory lymphocytes prevent aldosterone-induced vascular injury. Hypertension. 2012;59:324–330. doi: 10.1161/HYPERTENSIONAHA.111.181123. [DOI] [PubMed] [Google Scholar]
- 104.Rodríguez-Iturbe B, Pons H, Quiroz Y, Johnson RJ. The immunological basis of hypertension. Am J Hypertens. 2014;27:1327–1337. doi: 10.1093/ajh/hpu142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Barhoumi T, Kasal DA, Li MW, Shbat L, Laurant P, Neves MF, Paradis P, Schiffrin EL. T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension. 2011;57:469–476. doi: 10.1161/HYPERTENSIONAHA.110.162941. [DOI] [PubMed] [Google Scholar]
- 106.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ji H, Pai AV, West CA, Wu X, Speth RC, Sandberg K. Loss of Resistance to Angiotensin II-Induced Hypertension in the Jackson Laboratory Recombination-Activating Gene Null Mouse on the C57BL/6J Background. Hypertension. 2017;69:1121–1127. doi: 10.1161/HYPERTENSIONAHA.117.09063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension. 2010;55:500–507. doi: 10.1161/HYPERTENSIONAHA.109.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Crosswhite P, Sun Z. Ribonucleic acid interference knockdown of interleukin 6 attenuates cold-induced hypertension. Hypertension. 2010;55:1484–1491. doi: 10.1161/HYPERTENSIONAHA.109.146902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.van Rooy M-J, Pretorius E. Obesity, hypertension and hypercholesterolemia as risk factors for atherosclerosis leading to ischemic events. Curr Med Chem. 2014;21:2121–2129. [DOI] [PubMed] [Google Scholar]
- 111.Ait-Oufella H, Salomon BL, Potteaux S, Robertson A-KL, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S, Flavell RA, Hansson GK, Klatzmann D, Tedgui A, Mallat Z. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med. 2006;12:178–180. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 112.van Es T, van Puijvelde GHM, Foks AC, Habets KLL, Bot I, Gilboa E, Van Berkel TJC, Kuiper J. Vaccination against Foxp3(+) regulatory T cells aggravates atherosclerosis. Atherosclerosis. 2010;209:74–80. doi: 10.1016/j.atherosclerosis.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 113.Klingenberg R, Gerdes N, Badeau RM, et al. Depletion of FOXP3+ regulatory T cells promotes hypercholesterolemia and atherosclerosis. J Clin Invest. 2013;123:1323–1334. doi: 10.1172/JCI63891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Butcher MJ, Gjurich BN, Phillips T, Galkina EV. The IL-17A/IL-17RA axis plays a proatherogenic role via the regulation of aortic myeloid cell recruitment. Circ Res. 2012;110:675–687. doi: 10.1161/CIRCRESAHA.111.261784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Madhur MS, Funt SA, Li L, Vinh A, Chen W, Lob HE, Iwakura Y, Blinder Y, Rahman A, Quyyumi AA, Harrison DG. Role of interleukin 17 in inflammation, atherosclerosis, and vascular function in apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol. 2011;31:1565–1572. doi: 10.1161/ATVBAHA.111.227629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schieffer B, Selle T, Hilfiker A, Hilfiker-Kleiner D, Grote K, Tietge UJF, Trautwein C, Luchtefeld M, Schmittkamp C, Heeneman S, Daemen MJAP, Drexler H. Impact of interleukin-6 on plaque development and morphology in experimental atherosclerosis. Circulation. 2004;110:3493–3500. doi: 10.1161/01.CIR.0000148135.08582.97. [DOI] [PubMed] [Google Scholar]
- 117.Madan M, Bishayi B, Hoge M, Amar S. Atheroprotective role of interleukin-6 in diet- and/or pathogen-associated atherosclerosis using an ApoE heterozygote murine model. Atherosclerosis. 2008;197:504–514. doi: 10.1016/j.atherosclerosis.2007.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Huber SA, Sakkinen P, Conze D, Hardin N, Tracy R. Interleukin-6 exacerbates early atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 1999;19:2364–2367. [DOI] [PubMed] [Google Scholar]
- 119.Rizzoni D, Agabiti-Rosei E. Small artery remodeling in hypertension and diabetes. Curr Hypertens Rep. 2006;8:90–95. [DOI] [PubMed] [Google Scholar]
- 120.Mulvany MJ. Peripheral vasculature in essential hypertension. Clin Exp Pharmacol Physiol. 1996;23:S6–S10. [DOI] [PubMed] [Google Scholar]
- 121.Rizzoni D, Agabiti-Rosei E. Structural abnormalities of small resistance arteries in essential hypertension. Intern Emerg Med. 2012;7:205–212. doi: 10.1007/s11739-011-0548-0. [DOI] [PubMed] [Google Scholar]
- 122.Bloodworth NC, West JD, Merryman WD. Microvessel mechanobiology in pulmonary arterial hypertension: cause and effect. Hypertension. 2015;65:483–489. doi: 10.1161/HYPERTENSIONAHA.114.04652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Antonios TF, Singer DR, Markandu ND, Mortimer PS, MacGregor GA. Rarefaction of skin capillaries in borderline essential hypertension suggests an early structural abnormality. Hypertension. 1999;34:655–658. [DOI] [PubMed] [Google Scholar]
- 124.Noon JP, Walker BR, Webb DJ, Shore AC, Holton DW, Edwards HV, Watt GC. Impaired microvascular dilatation and capillary rarefaction in young adults with a predisposition to high blood pressure. J Clin Invest. 1997;99:1873–1879. doi: 10.1172/JCI119354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Intengan HD, Schiffrin EL. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension. 2001;38:581–587. [DOI] [PubMed] [Google Scholar]
- 126.Pries AR. Vascular Adaptation in Hypertension In: Vascular Adaptation in Hypertension. Vol 19 Berlin, Heidelberg: Springer; Berlin Heidelberg; 2015:1619–1624. doi: 10.1007/978-3-642-37078-6_48. [DOI] [Google Scholar]
- 127.Lawrie GM, Lie JT, Morris GC, Beazley HL. Vein graft patency and intimal proliferation after aortocoronary bypass: early and long-term angiopathologic correlations. Am J Cardiol. 1976;38:856–862. [DOI] [PubMed] [Google Scholar]
- 128.Seidel CL, Lewis RM, Bowers R, Bukoski RD, Kim HS, Allen JC, Hartley C. Adaptation of canine saphenous veins to grafting. Correlation of contractility and contractile protein content. Circ Res. 1984;55:102–109. [DOI] [PubMed] [Google Scholar]
- 129.Spray TL, Roberts WC. Changes in saphenous veins used as aortocoronary bypass grafts. Am Heart J. 1977;94:500–516. [DOI] [PubMed] [Google Scholar]
- 130.Park JB, Charbonneau F, Schiffrin EL. Correlation of endothelial function in large and small arteries in human essential hypertension. J Hypertens. 2001;19:415–420. doi: 10.1097/00004872-200103000-00009. [DOI] [PubMed] [Google Scholar]
- 131.Mulvany MJ. Vascular structure and smooth muscle contractility in experimental hypertension. J Cardiovasc Pharmacol. 1987;10 Suppl 6:S79–S85. [PubMed] [Google Scholar]
- 132.Koller A Signaling pathways of mechanotransduction in arteriolar endothelium and smooth muscle cells in hypertension. Microcirculation. 2002;9:277–294. doi: 10.1038/sj.mn.7800142. [DOI] [PubMed] [Google Scholar]
- 133.Miller FJ, Dellsperger KC, Gutterman DD. Myogenic constriction of human coronary arterioles. Am J Physiol. 1997;273:H257–H264. [DOI] [PubMed] [Google Scholar]
- 134.Hughes JM, Bund SJ. Arterial myogenic properties of the spontaneously hypertensive rat. Exp Physiol. 2002;87:527–534. [DOI] [PubMed] [Google Scholar]
- 135.Tajada S, Cidad P, Moreno-Domínguez A, Pérez-García MT, López-López JR. High blood pressure associates with the remodelling of inward rectifier K+ channels in mice mesenteric vascular smooth muscle cells. J Physiol (Lond). 2012;590:6075–6091. doi: 10.1113/jphysiol.2012.236190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yamazaki D, Tabara Y, Kita S, et al. TRIC-A channels in vascular smooth muscle contribute to blood pressure maintenance. Cell Metab. 2011;14:231–241. doi: 10.1016/j.cmet.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 137.Retailleau K, Duprat F, Arhatte M, Ranade SS, Peyronnet R, Martins JR, Jodar M, Moro C, Offermanns S, Feng Y, Demolombe S, Patel A, Honoré E. Piezo1 in Smooth Muscle Cells Is Involved in Hypertension-Dependent Arterial Remodeling. Cell Rep. 2015;13:1161–1171. doi: 10.1016/j.celrep.2015.09.072. [DOI] [PubMed] [Google Scholar]
- 138.Orlov SN, Koltsova SV, Tremblay J, Baskakov MB, Hamet P. NKCC1 and hypertension: role in the regulation of vascular smooth muscle contractions and myogenic tone. Ann Med. 2012;44 Suppl 1:S111–S118. doi: 10.3109/07853890.2011.653395. [DOI] [PubMed] [Google Scholar]
- 139.Intengan HD, Schiffrin EL. Structure and mechanical properties of resistance arteries in hypertension: role of adhesion molecules and extracellular matrix determinants. Hypertension. 2000;36:312–318. [DOI] [PubMed] [Google Scholar]
- 140.Vega F, Panizo A, Pardo-Mindán J, Diez J. Susceptibility to apoptosis measured by MYC, BCL-2, and BAX expression in arterioles and capillaries of adult spontaneously hypertensive rats. Am J Hypertens. 1999;12:815–820. [DOI] [PubMed] [Google Scholar]
- 141.Rizzoni D, Rodella L, Porteri E, Rezzani R, Guelfi D, Piccoli A, Castellano M, Muiesan ML, Bianchi R, Rosei EA. Time course of apoptosis in small resistance arteries of spontaneously hypertensive rats. J Hypertens. 2000;18:885–891. doi: 10.1097/00004872-200018070-00010. [DOI] [PubMed] [Google Scholar]
- 142.Dickhout JG, Lee RMKW. Apoptosis in the muscular arteries from young spontaneously hypertensive rats. J Hypertens. 1999;17:1413–1419. doi: 10.1097/00004872-199917100-00008. [DOI] [PubMed] [Google Scholar]
- 143.Rizzoni D, Porteri E, Castellano M, Bettoni G, Muiesan ML, Muiesan P, Giulini SM, Agabiti-Rosei E. Vascular hypertrophy and remodeling in secondary hypertension. Hypertension. 1996;28:785–790. [DOI] [PubMed] [Google Scholar]
- 144.Meier A, Weidmann P, Grimm M, Keusch G, Glück Z, Minder I, Ziegler WH. Pressor factors and cardiovascular pressor responsiveness in borderline hypertension. Hypertension. 1981;3:367–372. [DOI] [PubMed] [Google Scholar]
- 145.Schiffrin EL. Vascular remodeling in hypertension: mechanisms and treatment. Hypertension. 2012;59:367–374. doi: 10.1161/HYPERTENSIONAHA.111.187021. [DOI] [PubMed] [Google Scholar]
- 146.Anderson EA, Sinkey CA, Lawton WJ, Mark AL. Elevated sympathetic nerve activity in borderline hypertensive humans. Evidence from direct intraneural recordings. Hypertension. 1989;14:177–183. [DOI] [PubMed] [Google Scholar]
- 147.Egan B, Panis R, Hinderliter A, Schork N, Julius S. Mechanism of increased alpha adrenergic vasoconstriction in human essential hypertension. J Clin Invest. 1987;80:812–817. doi: 10.1172/JCI113138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Parati G, Esler M. The human sympathetic nervous system: its relevance in hypertension and heart failure. Eur Heart J. 2012;33:1058–1066. doi: 10.1093/eurheartj/ehs041. [DOI] [PubMed] [Google Scholar]
- 149.Falloon BJ, Heagerty AM. In vitro perfusion studies of human resistance artery function in essential hypertension. Hypertension. 1994;24:16–23. [DOI] [PubMed] [Google Scholar]
- 150.Kiowski W, Bühler FR, van Brummelen P, Amann FW. Plasma noradrenaline concentration and alpha-adrenoceptor-mediated vasoconstriction in normotensive and hypertensive man. Clin Sci. 1981;60:483–489. [DOI] [PubMed] [Google Scholar]
- 151.Savoia C, Touyz RM, Amiri F, Schiffrin EL. Selective mineralocorticoid receptor blocker eplerenone reduces resistance artery stiffness in hypertensive patients. Hypertension. 2008;51:432–439. doi: 10.1161/HYPERTENSIONAHA.107.103267. [DOI] [PubMed] [Google Scholar]
- 152.Gündüz F, Baskurt OK, Meiselman HJ. Vascular dilation responses of rat small mesenteric arteries at high intravascular pressure in spontaneously hypertensive rats. Circ J. 2009;73:2091–2097. [DOI] [PubMed] [Google Scholar]
- 153.Tesfamariam B, Halpern W. Endothelium-dependent and endothelium-independent vasodilation in resistance arteries from hypertensive rats. Hypertension. 1988;11:440–444. [DOI] [PubMed] [Google Scholar]
- 154.Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev. 2000;52:639–672. [PubMed] [Google Scholar]
- 155.Touyz RM, Wu XH, He G, Park JB, Chen X, Vacher J, Rajapurohitam V, Schiffrin EL. Role of c-Src in the regulation of vascular contraction and Ca2+ signaling by angiotensin II in human vascular smooth muscle cells. J Hypertens. 2001;19:441–449. doi: 10.1097/00004872-200103000-00012. [DOI] [PubMed] [Google Scholar]
- 156.Qin B, Zhou J. Src Family Kinases (SFK) Mediate Angiotensin II-Induced Myosin Light Chain Phosphorylation and Hypertension. PLoS ONE. 2015;10:e0127891. doi: 10.1371/journal.pone.0127891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Saito Y, Berk BC. Transactivation: a novel signaling pathway from angiotensin II to tyrosine kinase receptors. J Mol Cell Cardiol. 2001;33:3–7. doi: 10.1006/jmcc.2000.1272. [DOI] [PubMed] [Google Scholar]
- 158.Eguchi S, Inagami T. Signal transduction of angiotensin II type 1 receptor through receptor tyrosine kinase. Regul Pept. 2000;91:13–20. [DOI] [PubMed] [Google Scholar]
- 159.Rizzoni D, Porteri E, De Ciuceis C, Sleiman I, Rodella L, Rezzani R, Paiardi S, Bianchi R, Ruggeri G, Boari GEM, Muiesan ML, Salvetti M, Zani F, Miclini M, Rosei EA. Effect of treatment with candesartan or enalapril on subcutaneous small artery structure in hypertensive patients with noninsulin-dependent diabetes mellitus. Hypertension. 2005;45:659–665. doi: 10.1161/01.HYP.0000153308.91043.97. [DOI] [PubMed] [Google Scholar]
- 160.Schiffrin EL, Deng LY, Larochelle P. Progressive improvement in the structure of resistance arteries of hypertensive patients after 2 years of treatment with an angiotensin I-converting enzyme inhibitor. Comparison with effects of a beta-blocker. Am J Hypertens. 1995;8:229–236. [DOI] [PubMed] [Google Scholar]
- 161.Pu Q, Neves MF, Virdis A, Touyz RM, Schiffrin EL. Endothelin antagonism on aldosterone-induced oxidative stress and vascular remodeling. Hypertension. 2003;42:49–55. doi: 10.1161/01.HYP.0000078357.92682.EC. [DOI] [PubMed] [Google Scholar]
- 162.Amiri F, Virdis A, Neves MF, Iglarz M, Seidah NG, Touyz RM, Reudelhuber TL, Schiffrin EL. Endothelium-restricted overexpression of human endothelin-1 causes vascular remodeling and endothelial dysfunction. Circulation. 2004;110:2233–2240. doi: 10.1161/01.CIR.0000144462.08345.B9. [DOI] [PubMed] [Google Scholar]
- 163.Schiffrin EL, Larivi egrave re R, Li J-S, Sventek P. Enhanced Expression of the Endothelin-1 Gene in Blood Vessels of DOCA-Salt Hypertensive Rats: Correlation with Vascular Structure. J Vasc Res. 1996;33:235–248. doi: 10.1159/000159151. [DOI] [PubMed] [Google Scholar]
- 164.Neves MF, Virdis A, Schiffrin EL. Resistance artery mechanics and composition in angiotensin II-infused rats: effects of aldosterone antagonism. J Hypertens. 2003;21:189–198. doi: 10.1097/01.hjh.0000045515.82010.5a. [DOI] [PubMed] [Google Scholar]
- 165.Martinez-Lemus LA, Galiñanes EL. Matrix metalloproteinases and small artery remodeling. Drug Discov Today Dis Models. 2011;8:21–28. doi: 10.1016/j.ddmod.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Taniyama Y, Griendling KK. Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension. 2003;42:1075–1081. doi: 10.1161/01.HYP.0000100443.09293.4F. [DOI] [PubMed] [Google Scholar]
- 167.Burnstock G Control of vascular tone by purines and pyrimidines. Br J Pharmacol. 2010;161:527–529. doi: 10.1111/j.1476-5381.2010.00937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Burnstock G Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 169.Burnstock G, Ralevic V. Purinergic signaling and blood vessels in health and disease. Pharmacol Rev. 2014;66:102–192. doi: 10.1124/pr.113.008029. [DOI] [PubMed] [Google Scholar]
- 170.Grassi G, Mark A, Esler M. The sympathetic nervous system alterations in human hypertension. Circ Res. 2015;116:976–990. doi: 10.1161/CIRCRESAHA.116.303604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hart MN, Heistad DD, Brody MJ. Effect of chronic hypertension and sympathetic denervation on wall/lumen ratio of cerebral vessels. Hypertension. 1980;2:419–423. [DOI] [PubMed] [Google Scholar]
- 172.Fisher JP, Paton JFR. The sympathetic nervous system and blood pressure in humans: implications for hypertension. J Hum Hypertens. 2012;26:463–475. doi: 10.1038/jhh.2011.66. [DOI] [PubMed] [Google Scholar]
- 173.Burnstock G Historical review: ATP as a neurotransmitter. Trends Pharmacol Sci. 2006;27:166–176. doi: 10.1016/j.tips.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 174.Adamson SE, Leitinger N. The role of pannexin1 in the induction and resolution of inflammation. FEBS Lett. 2014;588:1416–1422. doi: 10.1016/j.febslet.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Billaud M, Chiu Y-H, Lohman AW, Parpaite T, Butcher JT, Mutchler SM, DeLalio LJ, Artamonov MV, Sandilos JK, Best AK, Somlyo AV, Thompson RJ, Le TH, Ravichandran KS, Bayliss DA, Isakson BE. A molecular signature in the pannexin1 intracellular loop confers channel activation by the α1 adrenoreceptor in smooth muscle cells. Sci Signal. 2015;8(364):ra17–ra17. doi: 10.1126/scisignal.2005824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Goonetilleke L, Ralevic V, Dunn WR. Influence of pressure on adenosine triphosphate function as a sympathetic neurotransmitter in small mesenteric arteries from the spontaneously hypertensive rat. J Hypertens. 2013;31:312–320. doi: 10.1097/HJH.0b013e32835bd74d. [DOI] [PubMed] [Google Scholar]
- 177.Naito Y, Yoshida H, Konishi C, Ohara N. Differences in Responses to Norepinephrine and Adenosine Triphosphate in Isolated, Perfused Mesenteric Vascular Beds Between Normotensive and Spontaneously Hypertensive Rats. J Cardiovasc Pharmacol. 1998;32:807–818. doi: 10.1097/00005344-199811000-00018. [DOI] [PubMed] [Google Scholar]
- 178.Zhang S, Remillard CV, Fantozzi I, Yuan JX-J. ATP-induced mitogenesis is mediated by cyclic AMP response element-binding protein-enhanced TRPC4 expression and activity in human pulmonary artery smooth muscle cells. Am J Physiol, Cell Physiol. 2004;287:C1192–C1201. doi: 10.1152/ajpcell.00158.2004. [DOI] [PubMed] [Google Scholar]
- 179.Chang K, Hanaoka K, Kumada M, Takuwa Y. Molecular cloning and functional analysis of a novel P2 nucleotide receptor. J Biol Chem. 1995;270:26152–26158. [DOI] [PubMed] [Google Scholar]
- 180.Erlinge D, Hou M, Webb TE, Barnard EA, Möller S. Phenotype changes of the vascular smooth muscle cell regulate P2 receptor expression as measured by quantitative RT-PCR. Biochem Biophys Res Commun. 1998;248:864–870. doi: 10.1006/bbrc.1998.9083. [DOI] [PubMed] [Google Scholar]
- 181.Giachini FR, Lima VV, Carneiro FS, Tostes RC, Webb RC. Decreased cGMP level contributes to increased contraction in arteries from hypertensive rats: role of phosphodiesterase 1. Hypertension. 2011;57:655–663. doi: 10.1161/HYPERTENSIONAHA.110.164327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhou H-Y, Chen W-D, Zhu D-L, Wu L-Y, Zhang J, Han W-Q, Li J-D, Yan C, Gao P-J. The PDE1A-PKCalpha signaling pathway is involved in the upregulation of alpha-smooth muscle actin by TGF-beta1 in adventitial fibroblasts. J Vasc Res. 2010;47:9–15. doi: 10.1159/000231716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Schermuly RT, Pullamsetti SS, Kwapiszewska G, et al. Phosphodiesterase 1 upregulation in pulmonary arterial hypertension: target for reverse-remodeling therapy. Circulation. 2007;115:2331–2339. doi: 10.1161/CIRCULATIONAHA.106.676809. [DOI] [PubMed] [Google Scholar]
- 184.Bautista Niño PK, Durik M, Danser AHJ, et al. Phosphodiesterase 1 regulation is a key mechanism in vascular aging. Clin Sci. 2015;129:1061–1075. doi: 10.1042/CS20140753. [DOI] [PubMed] [Google Scholar]
- 185.Yan C Cyclic nucleotide phosphodiesterase 1 and vascular aging. Clin Sci. 2015;129:1077–1081. doi: 10.1042/CS20150605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hui X, Lam KSL, Vanhoutte PM, Xu A. Adiponectin and cardiovascular health: an update. Br J Pharmacol. 2012;165:574–590. doi: 10.1111/j.1476-5381.2011.01395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Chow W-S, Cheung BMY, Tso AWK, Xu A, Wat NMS, Fong CHY, Ong LHY, Tam S, Tan KCB, Janus ED, Lam T-H, Lam KSL. Hypoadiponectinemia as a predictor for the development of hypertension: a 5-year prospective study. Hypertension. 2007;49:1455–1461. doi: 10.1161/HYPERTENSIONAHA.107.086835. [DOI] [PubMed] [Google Scholar]
- 188.Jiang C, Zhang H, Zhang W, Kong W, Zhu Y, Zhang H, Xu Q, Li Y, Wang X. Homocysteine promotes vascular smooth muscle cell migration by induction of the adipokine resistin. Am J Physiol, Cell Physiol. 2009;297:C1466–C1476. doi: 10.1152/ajpcell.00304.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hirai H, Satoh H, Kudoh A, Watanabe T. Interaction between resistin and adiponectin in the proliferation of rat vascular smooth muscle cells. Mol Cell Endocrinol. 2013;366:108–116. doi: 10.1016/j.mce.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 190.Schlich R, Lamers D, Eckel J, Sell H. Adipokines enhance oleic acid-induced proliferation of vascular smooth muscle cells by inducing CD36 expression. Arch Physiol Biochem. 2015;121:81–87. doi: 10.3109/13813455.2015.1045520. [DOI] [PubMed] [Google Scholar]
- 191.Lamers D, Schlich R, Greulich S, Sasson S, Sell H, Eckel J. Oleic acid and adipokines synergize in inducing proliferation and inflammatory signalling in human vascular smooth muscle cells. Journal of Cellular and Molecular Medicine. 2010;15:1177–1188. doi: 10.1111/j.1582-4934.2010.01099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104:545–556. [DOI] [PubMed] [Google Scholar]
- 193.Padmanabhan S, Caulfield M, Dominiczak AF. Genetic and molecular aspects of hypertension. Circ Res. 2015;116:937–959. doi: 10.1161/CIRCRESAHA.116.303647. [DOI] [PubMed] [Google Scholar]
- 194.Kupper N, Willemsen G, Riese H, Posthuma D, Boomsma DI, de Geus EJC. Heritability of daytime ambulatory blood pressure in an extended twin design. Hypertension. 2005;45:80–85. doi: 10.1161/01.HYP.0000149952.84391.54. [DOI] [PubMed] [Google Scholar]
- 195.Levy D, DeStefano AL, Larson MG, O’Donnell CJ, Lifton RP, Gavras H, Cupples LA, Myers RH. Evidence for a gene influencing blood pressure on chromosome 17. Hypertension. 2000;36:477–483. [DOI] [PubMed] [Google Scholar]
- 196.Padmanabhan S, Newton-Cheh C, Dominiczak AF. Genetic basis of blood pressure and hypertension. Trends Genet. 2012;28:397–408. doi: 10.1016/j.tig.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 197.Pickering GW. The role of the genetic factor in hypertension. Acta Med Scand Suppl. 1956;312:18–27. doi: 10.1111/j.0954-6820.1956.tb16946.x. [DOI] [PubMed] [Google Scholar]
- 198.Ehret GB, Munroe PB, Rice KM, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478:103–109. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Johnson T, Gaunt TR, Newhouse SJ, et al. Blood pressure loci identified with a gene-centric array. Am J Hum Genet. 2011;89:688–700. doi: 10.1016/j.ajhg.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kato N, Takeuchi F, Tabara Y, et al. Meta-analysis of genome-wide association studies identifies common variants associated with blood pressure variation in east Asians. Nature Publishing Group. 2011;43:531–538. doi: 10.1038/ng.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Levy D, Ehret GB, Rice K, et al. Genome-wide association study of blood pressure and hypertension. Nature Publishing Group. 2009;41:677–687. doi: 10.1038/ng.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Padmanabhan S, Melander O, Hastie C, Menni C, Delles C, Connell JM, Dominiczak AF. Hypertension and genome-wide association studies: combining high fidelity phenotyping and hypercontrols. J Hypertens. 2008;26:1275–1281. doi: 10.1097/HJH.0b013e3282ff634f. [DOI] [PubMed] [Google Scholar]
- 203.Monteith GR, Kable EP, Kuo TH, Roufogalis BD. Elevated plasma membrane and sarcoplasmic reticulum Ca2+ pump mRNA levels in cultured aortic smooth muscle cells from spontaneously hypertensive rats. Biochem Biophys Res Commun. 1997;230:344–346. doi: 10.1006/bbrc.1996.5956. [DOI] [PubMed] [Google Scholar]
- 204.Sayeski PP, Ali MS. The critical role of c-Src and the Shc/Grb2/ERK2 signaling pathway in angiotensin II-dependent VSMC proliferation. Exp Cell Res. 2003;287:339–349. doi: 10.1016/S0014-4827(03)00154-X. [DOI] [PubMed] [Google Scholar]
- 205.Bähring S, Rauch A, Toka O, et al. Autosomal-dominant hypertension with type E brachydactyly is caused by rearrangement on the short arm of chromosome 12. Hypertension. 2004;43:471–476. doi: 10.1161/01.HYP.0000111808.08715.ec. [DOI] [PubMed] [Google Scholar]
- 206.Maass PG, Aydin A, Luft FC, et al. PDE3A mutations cause autosomal dominant hypertension with brachydactyly. Nature. 2015;47:647–653. doi: 10.1038/ng.3302. [DOI] [PubMed] [Google Scholar]
- 207.Xin M, Small EM, Sutherland LB, Qi X, McAnally J, Plato CF, Richardson JA, Bassel-Duby R, Olson EN. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev. 2009;23:2166–2178. doi: 10.1101/gad.1842409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wu W-H, Hu C-P, Chen X-P, Zhang W-F, Li X-W, Xiong X-M, Li Y-J. MicroRNA-130a mediates proliferation of vascular smooth muscle cells in hypertension. Am J Hypertens. 2011;24:1087–1093. doi: 10.1038/ajh.2011.116. [DOI] [PubMed] [Google Scholar]
- 209.Torella D, Iaconetti C, Catalucci D, Ellison GM, Leone A, Waring CD, Bochicchio A, Vicinanza C, Aquila I, Curcio A, Condorelli G, Indolfi C. MicroRNA-133 controls vascular smooth muscle cell phenotypic switch in vitro and vascular remodeling in vivo. Circ Res. 2011;109:880–893. doi: 10.1161/CIRCRESAHA.111.240150. [DOI] [PubMed] [Google Scholar]
- 210.Sun S-G, Zheng B, Han M, Fang X-M, Li H-X, Miao S-B, Su M, Han Y, Shi H-J, Wen J-K. miR-146a and Krüppel-like factor 4 form a feedback loop to participate in vascular smooth muscle cell proliferation. Nature. 2011;12:56–62. doi: 10.1038/embor.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ohanyan V, Yin L, Bardakjian R, et al. Requisite Role of Kv1.5 Channels in Coronary Metabolic Dilation. Circ Res. 2015;117:612–621. doi: 10.1161/CIRCRESAHA.115.306642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kumbhani DJ, Steg PG, Cannon CP, Eagle KA, Smith SC, Crowley K, Goto S, Ohman EM, Bakris GL, Perlstein TS, Kinlay S, Bhatt DL, REACH Registry Investigators. Resistant hypertension: a frequent and ominous finding among hypertensive patients with atherothrombosis. Eur Heart J. 2013;34:1204–1214. doi: 10.1093/eurheartj/ehs368. [DOI] [PubMed] [Google Scholar]
- 213.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT, Roccella EJ, National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 214.Reckelhoff JF. Gender differences in hypertension. Curr Opin Nephrol Hypertens. 2018;27:176–181. doi: 10.1097/MNH.0000000000000404. [DOI] [PubMed] [Google Scholar]
- 215.Billaud M, Lohman AW, Straub AC, Looft-Wilson R, Johnstone SR, Araj CA, Best AK, Chekeni FB, Ravichandran KS, Penuela S, Laird DW, Isakson BE. Pannexin1 regulates α1-adrenergic receptor-mediated vasoconstriction. Circ Res. 2011;109:80–85. doi: 10.1161/CIRCRESAHA.110.237594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Cechova S, Zeng Q, Billaud M, et al. Loss of collectrin, an angiotensin-converting enzyme 2 homolog, uncouples endothelial nitric oxide synthase and causes hypertension and vascular dysfunction. Circulation. 2013;128:1770–1780. doi: 10.1161/CIRCULATIONAHA.113.003301. [DOI] [PubMed] [Google Scholar]
- 217.Sparks MA, Parsons KK, Stegbauer J, Gurley SB, Vivekanandan-Giri A, Fortner CN, Snouwaert J, Raasch EW, Griffiths RC, Haystead TAJ, Le TH, Pennathur S, Koller B, Coffman TM. Angiotensin II type 1A receptors in vascular smooth muscle cells do not influence aortic remodeling in hypertension. Hypertension. 2011;57:577–585. doi: 10.1161/HYPERTENSIONAHA.110.165274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Sparks MA, Stegbauer J, Chen D, Gomez JA, Griffiths RC, Azad HA, Herrera M, Gurley SB, Coffman TM. Vascular Type 1A Angiotensin II Receptors Control BP by Regulating Renal Blood Flow and Urinary Sodium Excretion. J Am Soc Nephrol. 2015;26:2953–2962. doi: 10.1681/ASN.2014080816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Guilluy C, Brégeon J, Toumaniantz G, Rolli-Derkinderen M, Retailleau K, Loufrani L, Henrion D, Scalbert E, Bril A, Torres RM, Offermanns S, Pacaud P, Loirand G. The Rho exchange factor Arhgef1 mediates the effects of angiotensin II on vascular tone and blood pressure. Nat Med. 2010;16:183–190. doi: 10.1038/nm.2079. [DOI] [PubMed] [Google Scholar]
- 220.Kobayashi Y, Hirawa N, Tabara Y, et al. Mice lacking hypertension candidate gene ATP2B1 in vascular smooth muscle cells show significant blood pressure elevation. Hypertension. 2012;59:854–860. doi: 10.1161/HYPERTENSIONAHA.110.165068. [DOI] [PubMed] [Google Scholar]
- 221.Liao Y, Regan CP, Manabe I, Owens GK, Day KH, Damon DN, Duling BR. Smooth muscle-targeted knockout of connexin43 enhances neointimal formation in response to vascular injury. Arterioscler Thromb Vasc Biol. 2007;27:1037–1042. doi: 10.1161/ATVBAHA.106.137182. [DOI] [PubMed] [Google Scholar]
- 222.Yu Y, Ricciotti E, Scalia R, Tang SY, Grant G, Yu Z, Landesberg G, Crichton I, Wu W, Puré E, Funk CD, FitzGerald GA. Vascular COX-2 modulates blood pressure and thrombosis in mice. Sci Transl Med. 2012;4: 132ra54–132ra54. doi: 10.1126/scitranslmed.3003787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Albinsson S, Skoura A, Yu J, DiLorenzo A, Fernández-Hernando C, Offermanns S, Miano JM, Sessa WC. Smooth muscle miRNAs are critical for post-natal regulation of blood pressure and vascular function. PLoS ONE. 2011;6:e18869. doi: 10.1371/journal.pone.0018869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Schreier B, Hünerberg M, Rabe S, Mildenberger S, Bethmann D, Heise C, Sibilia M, Offermanns S, Gekle M. Consequences of postnatal vascular smooth muscle EGFR deletion on acute angiotensin II action. Clin Sci. 2016;130:19–33. doi: 10.1042/CS20150503. [DOI] [PubMed] [Google Scholar]
- 225.Wang Y, Thorin E, Luo H, Tremblay J, Lavoie JL, Wu Z, Peng J, Qi S, Wu J. EPHB4 Protein Expression in Vascular Smooth Muscle Cells Regulates Their Contractility, and EPHB4 Deletion Leads to Hypotension in Mice. J Biol Chem. 2015;290:14235–14244. doi: 10.1074/jbc.M114.621615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Retailleau K, Arhatte M, Demolombe S, Peyronnet R, Baudrie V, Jodar M, Bourreau J, Henrion D, Offermanns S, Nakamura F, Feng Y, Patel A, Duprat F, Honoré E. Arterial Myogenic Activation through Smooth Muscle Filamin A. Cell Rep. 2016;14:2050–2058. doi: 10.1016/j.celrep.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 227.Holtwick R, Gotthardt M, Skryabin B, Steinmetz M, Potthast R, Zetsche B, Hammer RE, Herz J, Kuhn M. Smooth muscle-selective deletion of guanylyl cyclase-A prevents the acute but not chronic effects of ANP on blood pressure. Proc Natl Acad Sci USA. 2002;99:7142–7147. doi: 10.1073/pnas.102650499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Groneberg D, König P, Wirth A, Offermanns S, Koesling D, Friebe A. Smooth muscle-specific deletion of nitric oxide-sensitive guanylyl cyclase is sufficient to induce hypertension in mice. Circulation. 2010;121:401–409. doi: 10.1161/CIRCULATIONAHA.109.890962. [DOI] [PubMed] [Google Scholar]
- 229.Moosmang S, Schulla V, Welling A, Feil R, Feil S, Wegener JW, Hofmann F, Klugbauer N. Dominant role of smooth muscle L-type calcium channel Cav1.2 for blood pressure regulation. EMBO J. 2003;22:6027–6034. doi: 10.1093/emboj/cdg583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.He W-Q, Peng Y-J, Zhang WC, et al. Myosin light chain kinase is central to smooth muscle contraction and required for gastrointestinal motility in mice. Gastroenterology. 2008;135:610–620. doi: 10.1053/j.gastro.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.He W-Q, Qiao Y-N, Zhang C-H, et al. Role of myosin light chain kinase in regulation of basal blood pressure and maintenance of salt-induced hypertension. Am J Physiol Heart Circ Physiol. 2011;301:H584–H591. doi: 10.1152/ajpheart.01212.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Qiao Y-N, He W-Q, Chen C-P, Zhang C-H, Zhao W, Wang P, Zhang L, Wu Y-Z, Yang X, Peng Y-J, Gao J-M, Kamm KE, Stull JT, Zhu M-S. Myosin phosphatase target subunit 1 (MYPT1) regulates the contraction and relaxation of vascular smooth muscle and maintains blood pressure. J Biol Chem. 2014;289:22512–22523. doi: 10.1074/jbc.M113.525444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Zhang J, Ren C, Chen L, Navedo MF, Antos LK, Kinsey SP, Iwamoto T, Philipson KD, Kotlikoff MI, Santana LF, Wier WG, Matteson DR, Blaustein MP. Knockout of Na+/Ca2+ exchanger in smooth muscle attenuates vasoconstriction and L-type Ca2+ channel current and lowers blood pressure. Am J Physiol Heart Circ Physiol. 2010;298:H1472–H1483. doi: 10.1152/ajpheart.00964.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Pritchard TJ, Parvatiyar M, Bullard DP, Lynch RM, Lorenz JN, Paul RJ. Transgenic mice expressing Na+-K+-ATPase in smooth muscle decreases blood pressure. Am J Physiol Heart Circ Physiol. 2007;293:H1172–H1182. doi: 10.1152/ajpheart.00279.2007. [DOI] [PubMed] [Google Scholar]
- 235.Montaniel KRC, Billaud M, Graham C, Kim SK, Carlson M, Zeng W, Zeng O, Pan W, Isakson BE, Hall JL, Adhikari N. Smooth muscle specific deletion of Ndst1 leads to decreased vessel luminal area and no change in blood pressure in conscious mice. J Cardiovasc Transl Res. 2012;5:274–279. doi: 10.1007/s12265-012-9369-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Dikalova A, Clempus R, Lassègue B, Cheng G, McCoy J, Dikalov S, San Martin A, Lyle A, Weber DS, Weiss D, Taylor WR, Schmidt HHHW, Owens GK, Lambeth JD, Griendling KK. Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation. 2005;112:2668–2676. doi: 10.1161/CIRCULATIONAHA.105.538934. [DOI] [PubMed] [Google Scholar]
- 237.André G, Sandoval JE, Retailleau K, Loufrani L, Toumaniantz G, Offermanns S, Rolli-Derkinderen M, Loirand G, Sauzeau V. Smooth muscle specific Rac1 deficiency induces hypertension by preventing p116RIP3-dependent RhoA inhibition. J Am Heart Assoc 2014;3:e000852. doi: 10.1161/JAHA.114.000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Loirand G, Pacaud P. The role of Rho protein signaling in hypertension. Nat Rev Cardiol 2010;7:637–647. doi: 10.1038/nrcardio.2010.136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


