Abstract
Background
Mental disorders are characterized by a high likelihood of recurrence. Thus, aftercare and follow-up interventions aim to maintain treatment gains and to prevent relapse. Internet- and mobile-based interventions (IMIs) may represent promising instruments in tertiary prevention. This systematic review summarizes and evaluates the research on the efficacy of IMIs as aftercare or follow-up interventions for adults with mental health issues.
Methods
A systematic database search (PsycInfo, MEDLINE, CENTRAL) was conducted and studies selected according to predefined eligibility criteria (RCTs, adult population, clinical symptoms/disorder, assessed with validated instruments, clinical-psychological intervention rationale, aftercare/follow-up intervention, web-/mobile-based, minimum follow-up measurement of three months, inclusion of a control group). Inspected outcomes were symptom severity, recurrence- and rehospitalization rates, functioning, quality of life and adherence to primary treatment.
Systematic review registration: PROSPERO CRD42017055289.
Results
Sixteen RCTs met the inclusion criteria, covering trials on depression (n = 5), eating disorders (n = 4) and transdiagnostic interventions (n = 7). The majority of the interventions were based on Cognitive Behavioral Therapy (CBT) principles and were web-based (n = 11). Methodological quality of included studies was suboptimal. Limitations included attrition bias and non-specification of routine care co-interventions. IMIs yielded small to medium post-treatment effects for symptom severity (d = −0.08 – d = −0.45) in comparison to control groups. Best evidence base was found for symptom severity of depression and anxiety. Study results regarding recurrence and rehospitalization were inconsistent.
Discussion
There is some evidence, that IMIs are feasible instruments for maintaining treatment gains for some mental disorders. However, further high quality, large-scale trials are needed to expand research fields, improve adherence to and uptake of IMIs and facilitate implementation of effective interventions into routine care.
Keywords: Systematic review, Aftercare, Relapse prevention, Mental health, Internet- and mobile-based interventions
Highlights
-
•
Systematic overview of Internet- and mobile-based interventions as aftercare or relapse prevention across mental disorders.
-
•
Included studies are predominantly transdiagnostic, web- and CBT-based and feature some form of human guidance.
-
•
Small effects were found on study level for symptom severity of depression and anxiety.
-
•
Methodological quality and attrition rates of included studies were suboptimal.
-
•
Further high quality, large-scale RCTs, and strategies to make IMIs impactful are needed.
1. Introduction
Most mental disorders are characterized by a high risk of recurrence or chronic courses (Olmsted et al., 1994; Paykel et al., 2005; Yonkers et al., 2003). Short- and long-term recurrence rates for common mental disorders such as 21% to 55% for eating disorders (McFarlane et al., 2008; Olmsted et al., 2005), 40% for generalized anxiety disorder (Yonkers et al., 2003) or up to 85% for depression (Keller and Boland, 1998) have been reported. Adverse implications of chronicity and recurrence include an increased risk for comorbid somatic diseases (Fleischhacker et al., 2008; Prince et al., 2007), early retirement (Mykletun et al., 2006), a reduced quality of life (Simon, 2003) and elevated mortality (Joukamaa et al., 2001).
Therefore, tertiary prevention aims to reduce symptom severity or disability, to promote functioning and quality of life and to identify, prevent and cope with recurrence or rehospitalization (Caplan, 1964; Witkiewitz and Marlatt, 2004). Tertiary prevention thus plays an essential role in the intermediate or continuous care of mental disorders. It can be delivered through various forms, such as pharmacological or psychosocial treatments, medical or occupational rehabilitation, as aftercare, follow-up or maintenance treatment. A key sector of tertiary prevention is the post-discharge transition period following acute care, in which convalescents face various challenges regarding the transfer, adoption, and stabilization of health behavior changes and are confronted with individual, social or occupational difficulties (Blank et al., 2008). Extensive research has documented a heightened risk of relapse or rehospitalization in the first months after acute treatment (Halmi et al., 2002; Olmsted et al., 2005; Vittengl et al., 2007). Risk factors include individual aspects (e.g. residual symptoms, compliance to treatment), implementation of and access to aftercare services, or contextual factors such as proximity to services or social support (Judd et al., 1998).
Meta-analytic evidence indicates the effectiveness of aftercare for mental disorders including psychotherapy (Carter et al., 2009; Vittengl et al., 2007), psychosocial interventions (Beynon et al., 2008; Scott et al., 2007), pharmacological maintenance treatment (Geddes et al., 2003) or psychosomatic rehabilitation (Steffanowski, 2007) in reducing symptom severity, recurrence and in promoting functionality or treatment compliance (Barkhof et al., 2012; MacDonald et al., 2016).
However, although chronicity and recurrence of mental disorders represent a significant societal and economic burden (Walker et al., 2015), the effectiveness and implementation of aftercare instruments in routine care are limited for various reasons: reduced adoption of and compliance with aftercare services (Kampman et al., 2003; Lingam and Scott, 2002; Mitchell et al., 2004; Ramana et al., 2003), organizational barriers such as long waiting times, limited local or temporal accessibility or pessimistic treatment expectancies (Schulz et al., 2008; Sibold et al., 2011), as well as insufficient prescription or initiation by clinicians (Ehrenreich et al., 2012; Klinkenberg and Calsyn, 1997; Sibold et al., 2011). Eventually, limited resources of healthcare systems and high medical cost of aftercare services further impede their widespread implementation (Adair et al., 2005; Klinkenberg and Calsyn, 1996).
In an effort to reduce the threshold to health care utilization and to improve care along health sectors, Internet- and mobile-based interventions (IMIs) have been developed extensively, in particular within the last decade. IMIs can be administered cost-effectively (Paganini et al., 2018) and may represent widely accessible instruments of tertiary prevention regarding increasing Internet access and use (Internet Society, 2016). IMIs may vary with regard to intervention strategy (e.g. monitoring, psychoeducation, behavior-change), technical implementation (e.g. mobile phone- app, website), localization in the healthcare process (e.g. prevention, stand-alone interventions, blended- or aftercare), or in their amount of human support (Ebert et al., 2018). Guidance may range from self-administered or automated interventions (unguided) over varying intensity of human support through personalized feedback or contact with online-coaches up to regular synchronous contact, mirroring face-to-face therapy (Newman et al., 2011).
Evidence from several meta-analytic studies suggests the effectiveness of psychological IMIs as stand-alone interventions for a broad spectrum of mental disorders in adults such as affective and anxiety disorders (Karyotaki et al., 2017; Olthuis et al., 2015), posttraumatic stress disorder (PTSD) (Kuester et al., 2016), eating disorders (Hedman et al., 2012) or chronic pain (Eccleston et al., 2014). With regard to the prevention of mental disorders, a recent meta-analysis of eight studies by Sander et al. (2016) found a small effect size for IMIs in the primary prevention of depression (standardized mean difference, SMD = 0.35). However, the evidence for the effectiveness of IMIs in the primary prevention of further mental disorders is still limited and inconsistent (Ebert et al., 2017).
Previous research on Internet- or mobile-based aftercare focused on guided, web- or mobile based self-help, mirroring existing treatment rationales in modular, interactive treatment elements, combined with a certain amount of asynchronous (written) therapist contact (Ebert et al., 2013; Zwerenz et al., 2013). Others investigated mobile-based (Schmädeke and Bischoff, 2015) or rather synchronous, chat- or video-based aftercare (Bauer et al., 2011b; Fichter et al., 2013), highlighting the broad spectrum of implementation of IMIs in tertiary prevention. A growing body of research indicates the applicability of IMIs in tertiary prevention of chronic or recurrent mental disorders (Barnes et al., 2015; Hunkeler et al., 2012) and as step-down interventions after inpatient treatment for eating disorders (Bauer et al., 2012; Gulec et al., 2014), depression (Holländare et al., 2011; Schmädeke and Bischoff, 2015) or transdiagnostic approaches (Bauer et al., 2011b; Ebert et al., 2013).
Hence, the purpose of this article is to systematically review the literature regarding the application of IMIs as aftercare or follow-up interventions for adults with mental health issues, to draw conclusions about their efficacy and to outline future directions for research and implementation into routine care.
2. Method
2.1. Registration and study protocol
This systematic review was reported according to the guidelines of the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) (Liberati et al., 2009), see Appendix A and was registered in the international prospective register of systematic reviews (PROSPERO: CRD42017055289). The methodical procedure is described in detail in a correspondant study protocol (Hennemann et al., 2017b).
2.2. Eligibility criteria
Studies were eligible for inclusion if they (a) focused on adults (≥18 years), who (b) have received treatment for a mental disorder or a somatic condition with comorbid mental symptoms within the previous six months on average. Mental disorder or clinical symptoms under study needed to be assessed with (c) standardized or validated instruments. Only (d) randomized controlled trials (RCTs) that were available in full text and published in English or German language were taken into consideration. Interventions under study should have been based on (e) distinguishable clinical-psychological elements and rationales, as described by Kampling et al. (2014), and implemented as (f) aftercare or follow-up interventions. Interventions needed to be provided (g) predominantly in an online setting (web- or mobile -based) and (h) report a minimum follow-up assessment of the main outcome of three months. Mandatory control groups (CG) (i) could be active (e.g. attention control website, treatment as usual (TAU), other treatment) or inactive (e.g. waiting list or no treatment).
2.3. Search strategy and study selection
A systematic search in three electronic databases (Medline, PsycInfo, Cochrane Central Register of Controlled trials, CENTRAL) was conducted in March 2018, based on a sensitive search term (see Appendix B). Furthermore, the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) was hand searched to identify ongoing trials. In addition, we examined reference lists of included publications. Study authors were contacted in case of unclear eligibility, unpublished or missing data and if no succeeding publication could be retrieved for published study protocols.
Two independent researchers [SH, SF] screened titles and abstracts of retrieved studies to identify eligible studies in a first step. In a second step, full texts of these studies were screened against eligibility criteria. Disagreement at both stages was resolved through discussion and consultation of a third researcher [LS].
2.4. Data extraction
The following data were extracted from each study: (a) study identification items, (b) study design characteristics, (c) intervention characteristics, (d) technical characteristics, (e) type of mental disorder or clinical symptom to be treated, (f) target population items, (g) setting, (h) treatment engagement, (i) assessment of additional outcome variables, and (j) clinical outcome.
Regarding the latter, inspected outcomes were: symptom severity, symptom recurrence or incidence rate of mental disorder under study, rehospitalization rate, indicators of functionality or quality of life and adherence to primary treatment (e.g. medication compliance) from post-treatment to latest available follow-up. Follow-up periods up to 6 months were categorized as ‘short’, 6 to 12 months as ‘medium’ and beyond as ‘long-term’, as adapted from previous research (Sander et al., 2016). A second reviewer [SH] reexamined the extraction process and quality assessment to control for an investigator bias.
2.5. Evaluation of methodological quality
The methodological quality of each study was analyzed according to the of Cochrane Risk of Bias tool (Higgins and Green, 2011) in the following domains: (1) selection bias, (2) performance and detection bias, (3) attrition and reporting bias, including incomplete outcome data and availability of intention-to-treat analysis. For guided interventions, thresholds for acceptable dropout rates were determined as ≤20% for short-term, ≤ 30% for medium and ≤ 35% for long-term follow-up periods and up to 40% for unguided interventions based on average dropout rates reported in previous reviews (Melville et al., 2010; Richards and Richardson, 2012; Sander et al., 2016; van Ballegooijen et al., 2014). Other threats to validity (4) included assessment of co-interventions, similar groups at baseline, intervention compliance, and identical timing for outcome assessments. Each domain was rated as having a ‘low’, ‘unclear’ or ‘high’ risk of bias according to the abovementioned criteria. Above that, the cumulative quality of evidence on the predefined outcomes was rated according to the ‘Grading of Recommendations Assessment, Development and Evaluation (GRADE)’ (Guyatt et al., 2008) (see Appendix C). Dimensions of the GRADE rating included (a) study limitations, (b) inconsistency of results, (c) indirectness of evidence, and (d) imprecision of effect estimates reporting bias. Evidence was graded following guidelines from the Cochrane Collaboration and supported by a checklist by Meader et al. (2014) into ‘very low’, ‘low’, ‘moderate’, or ‘high’.
2.6. Data analyses
Outcome variables were differentiated in terms of ‘short’, ‘medium’ or ‘long-term’ efficacy according to follow-up classification. For continuous outcomes (e.g. symptom severity), standardized effect sizes were calculated for the between-group comparison at the respective follow-up. Dichotomous outcomes (e.g. recurrence, rehospitalization) were transformed into odds or risk ratios (OR, RR). Because of the considerable heterogeneity of intervention, diagnostic and clinical characteristics of the included studies, meta-analytic pooling of effect sizes was not feasible. Furthermore, possible publication biases could not be estimated, due to the limited number of studies per outcome. However, mixed sample sizes, significant and non-significant effects reported may indicate a low risk for publication bias.
3. Results
3.1. Study selection
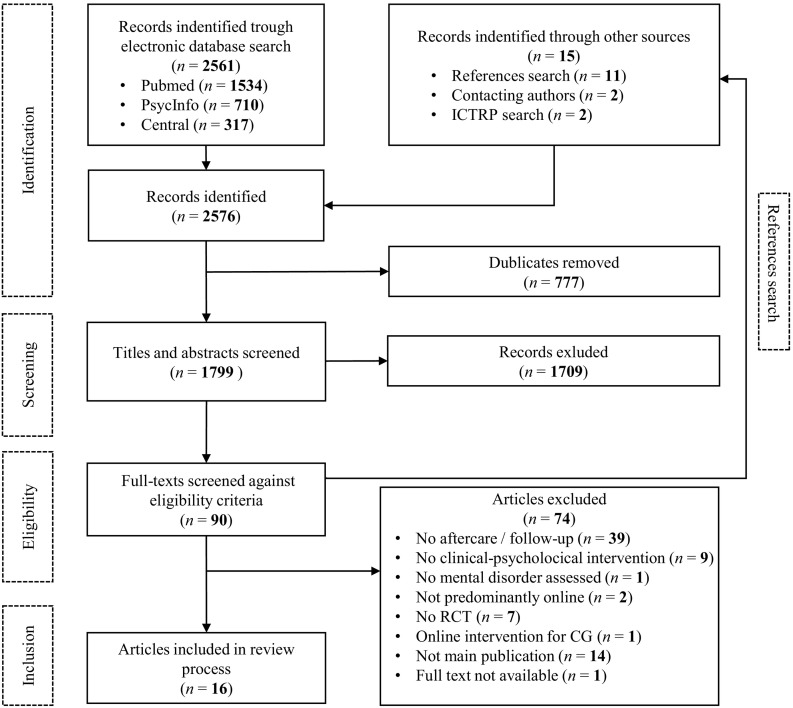
The database search provided a total of 2576 results. After removing duplicates, screening titles, abstracts and full-texts against inclusion criteria, a complementary hand search of trial registers, reference lists of eligible studies and contacting study authors, a total of 16 studies were included in this review. Fig. 1 illustrates the search and selection process and reasons for exclusion according to the PRISMA-guidelines.
Fig. 1.
PRISMA flow chart of study inclusion process.
3.2. Data extraction
3.2.1. Overview
Included studies mainly targeted the tertiary prevention of depression (n = 5) and eating disorders (n = 4) or were designed as transdiagnostic interventions (n = 7), including aftercare for comorbid mental symptoms of somatic disorders. For three studies on eating disorders in an adolescent target population (Fichter et al., 2012; Gulec et al., 2014; Jacobi et al., 2017), we were able to retrieve unpublished age-stratified data of adult participants provided by study authors.
3.2.2. Intervention characteristics
The majority of interventions (n = 11) were based on principles of Cognitive Behavioral Therapy (CBT). One study (Bischoff et al., 2013) was based on general psychotherapy (Grawe, 1997), two studies on psychodynamic (Zwerenz et al., 2017a; Zwerenz et al., 2017b) and one on mindfulness-based cognitive therapy (MBCT) (Kraft et al., 2017) or focused on disease management (Välimäki et al., 2017). Eleven IMIs were web- and 5 predominantly mobile-based. Most interventions (n = 11) included some form of human support, all based on a written communication or additional phone contact. Five interventions were unguided (Bischoff et al., 2013; Kraft et al., 2017; Välimäki et al., 2017; Willems et al., 2017a; Zwerenz et al., 2017c). Intensity and mode of contact with participants varied across studies, including (semi-) automated pre-formulated messages or reminders to participants (Bauer et al., 2012; Kordy et al., 2016; Kraft et al., 2017) or individual, monthly to weekly forms of human support through written feedback in the majority of interventions (see Table 1). Here, guidance was provided by various occupational groups (e.g. research assistants, psychotherapists, clinical psychologists, nurses). Six studies provided an online or phone crisis management system (Bauer et al., 2012; Ebert et al., 2013; Fichter et al., 2012; Gulec et al., 2014; Holländare et al., 2011; Kordy et al., 2016). Interventions differed regarding the sequence of intervention elements with consecutive (Fichter et al., 2012; Holländare et al., 2011; Zwerenz et al., 2017b) or flexible order (Norlund et al., 2018; Willems et al., 2017a).
Table 1.
Study characteristics.
| Study [country] |
Program name | Target Disorder/symptom | Target population |
Intervention type [duration] |
Condition | Instruments | Follow-up | Study dropouta |
|---|---|---|---|---|---|---|---|---|
|
Bauer et al. (2012)b [Germany] |
[SMS-BRIDGE] | Bulimia Nervosa (BN) or Eating disorder not otherwise specified (EDNOS) | Female adults, at least two episodes of binge eating a week for minimum of one month, after inpatient psychosomatic treatment | CBT Mobile-based; guided SMS-based weekly self-monitoring + semi-automated supportive feedback [16 weeks] |
IG: SMS-BRIDGE (n = 82) CG: TAU (n = 83) |
LIFE EDI-2 SEED |
8 months | IG: 13.4% CG: 16.9% |
|
Jacobi et al. (2017) [Germany] |
IN@ | BN | Female adolescents (≥ 17 yrs)c after inpatient psychosomatic treatment | CBT Web-based; guided Active website; monitoring log + body image + personal diary + weekly feedback (e-mail) and individual chat (on demand) [9 months] |
IG: IN@ (n = 122) CG: TAU (n = 122) |
SIAB-EX | 9/18 months | IG: 38.8% CG: 31.2% |
|
Gulec et al. (2014) [Hungary] |
EDINAd | BN or EDNOS |
Female adolescents (≥ 16 yrs)c after inpatient psychosomatic treatment | CBT Web-based; guided Psychoeducational online platform + weekly peer/individual chat (on demand) [4 months] |
IG: EDINA (n = 50) CG: TAU (n = 51) |
EDE-Q DASS-21 |
4 months | IG: 32.0% CG: 21.6% |
|
Fichter et al. (2012)e [Germany] |
VIAf | Anorexia Nervosa (AN) | Female adults (≥16 yrs)c after inpatient treatment | CBT Web-based; guided Active website for relapse prevention + moderated monthly peer chat + contact on demand [9 months] |
IG: VIA (n = 113) CG: TAU (n = 117) |
SIAB-EX MROAS EDI-2 BIS-11 BSI |
9/18 months | IG: 5.3% CG: 6.0%g |
|
Zwerenz et al., 2017b [Germany] |
KENh | Transdiagnostic | Adults after inpatient/day-clinic psychosomatic treatment | Psychodynamic Web-based; guided Active website with affect-focused/mindfulness exercises + individual encouraging feedback on homework [10 weeks] |
IG: KEN (n = 42) CG = Waiting list (n = 40) |
ERSQ PHQ-9 GAD-7 EUROHIS-QOL RSE SSS-8 CDS-2 SPE |
10/18/20 weeksi | IG: 38.1% CG: 37.5% |
|
Bischoff et al. (2013) [Germany] |
E-COACH | Transdiagnostic | Formerly employed adults after inpatient psychosomatic rehabilitation with excessive work-related self-demands | General psychotherapy Mobile-based; unguided Self-monitoring + daily automated feedback [6 months (2 weekly intervention phases)] |
IG: E-COACH (n = 158) CG: Intention therapy (n = 121) |
AVEM SSI-K3 MUM Health-49 |
12 months | IG: 63.9% CG: 64.5% |
|
Ebert et al. (2013) [Germany] |
TIMTj | Transdiagnostic | Adults after inpatient psychosomatic treatment | CBT Web-based; guided Personal development plan + self-monitoring + web diary + peer support + weekly individual feedback on web-diary [3 months] |
IG: TIMT + TAU (n = 200) CG: TAU (n = 200) |
Health-49 PANAS SEK-27 |
12 months | IG: 34.5% CG: 27.0% |
|
Zwerenz et al., 2017a [Germany] |
GSA-ONLINEk | Transdiagnostic | Formerly employed patients (18–59 yrs) after inpatient cardiologic, psychosomatic/ orthopedic rehabilitation | Psychodynamic Web-based; guided Weekly expressive writing + individual feedback [3 months] |
IG: GSA-ONLINE (n = 319) CG: Placebo website (n = 345) |
PHQ-9 PHQ-15 PHQ-10 SF-12 GAD-7 AVEM-44 |
6 months | IG: 32.0% CG: 30.4% |
|
Välimäki et al. (2017) [Finland] |
MOBILE.NET | Tansdiagnostic | Adults (18–65 yrs) after inpatient psychiatric treatment, continuing antipsychotic medication | Self-determination theory Mobile-based; unguided self-selected, automated SMS to support treatment adherence/functioning [12 months] |
IG: MOBILE.NET (n = 569) CG: TAU (n = 570) |
CSQ-8 Q-LES-Q GAS |
12 months | IG: 52.9% CG: 54.0%m |
|
Holländare et al. (2011)l [Sweden] |
iCBTn | Major Depression Disorder (MDD) | Adults after previous psychotherapy/pharmacological therapy, ≥ 1 MDD in the last 5 years, in remission at recruitment | CBT Web-based; guided Active-website + individual feedback to weekly homework [10 weeks] |
IG: iCBT (n = 42) CG: Generic e-mail support on demand (n = 42) |
MADRS-S SCID-I BDI-II BAI WHOQOL-BREF |
6/12/24 months | IG: 23.8% CG: 16.7% |
|
Kordy et al. (2016) [Germany] |
SUMMIT/ SUMMIT-PERSONo |
Recurrent MDD | Adults (18–65 yrs) after inpatient treatment for depression, ≥ 3 previous depressive episodes | CBT Web-based; guided Self-monitoring + crisis management plan + self-management skills, automated supportive feedback + moderated peer forum (SUMMIT-PERSON: + monthly peer/individual chat) [12 months] |
IG1: SUMMIT + TAU (n = 77) IG2: SUMMIT-PERSON + TAU (n = 79) CG: TAU (n = 80) |
LIFE | 6/12/18/24 months | IG1: 16.9% IG2: 17.7% CG: 18.8% |
|
Zwerenz et al., 2017c [Germany] |
DEPREXIS | Depression, adjustment disorder |
Adults (18–65 yrs) after inpatient psychodynamic treatment for depression | CBT Web-based; unguided Active website + simulated dialogues in consecutive self-help modules [3 months] |
IG: DEPREXIS (n = 115) CG: placebo website (n = 114) |
BDI-II PHQ-9 GAD-7 RSE EUROHIS-QOL WAI HAQ DAS |
3 months | IG: 26.1% CG: 25.4% |
|
Schmädeke and Bischoff (2015) [Germany] |
eATROS | Depression | Adults with (partially) remitted depression after inpatient psychosomatic rehabilitation | CBT Mobile- based; guided Daily activity structuring/evaluation + relaxation exercises + stimulation of euthymic activities + medication plan + emergency strategies + standardized feedback (via phone on demand) [3 months, 3 active phases] |
IG: eATROS (n = 81) CG: TAU (n = 64) |
SSI-K3 BDI |
3 months | IG: 42.0% CG: 34.4% |
|
Kraft et al. (2017) [Germany] |
MIND-Sp | Depression | Adults (18–75 yrs) after mindfulness group sessions in inpatient psychiatric treatment | Mindfulness-based cognitive therapy (MBCT) Mobile-based; unguided SMS monitoring of mindfulness exercise + weekly reinforcing feedback [4 months] |
IG: MIND-S + TAU (n = 70) CG: TAU (n = 70) |
PHQ-9 PTQ FFA-14 SCS-D |
4 months | IG: 14.3% CG: 15.0% |
|
Willems et al. (2017a)q [Netherlands] |
KNWr | Cancer diagnosis with comorbid anxiety/depression/fatigue | Adults after primary treatment for cancer without signs of recurrence | PST-/CBT Web-based; unguided Self-management training for fatigue, anxiety, and depression [6 months] |
IG: KNW (n = 253) CG: Waitinglist + TAU (n = 265) |
HADS CIS EORTC QLQ-C30 global health status scale, functional scales (self-con-structed) |
3/6/12 months | IG: 16.2% CG: 36.2% |
|
Norlund et al. (2018) [Sweden] |
U-CARE Heart | Myocardial infarction (MI) with comorbid depression and anxiety | Adult inpatients (< 75 years) with MI within last three months | CBT Web- and mobile-based; guided Tailored online-platform; psychoeducation + assignments + library (videos, supplementary material) + discussion board + weekly feedback (e-mail) [14 weeks] |
CG: TAU (n = 122) IG: U-CARE (n = 117) |
HADS MADRS-S BADS-SF CAQ |
14 weeks | IG: 17.9% CG: 5.7% |
Dropout-rate from baseline to the longest available follow-up.
Additional publications: Bauer et al. (2011a),Bauer et al. (2013).
Age-stratified data were provided study author.
EDINA: [Eve'si Rendellenesse'gek Internetre Adapta'lt Uto´ kezele'se].
Additional publication: Fichter et al. (2013).
VIA: Virtuelles Interventionsprogramm bei Anorexia Nervosa.
Dropout for age-stratified sample for the 9 months follow-up.
KEN: [Die Kraft der eigenen Emotionen Nutzen].
For 18/20-week follow-up only within group comparisons available.
TIMT: Transdiagnostic Internet-Based Maintenance Treatment.
GSA-ONLINE: Gesundheitstraining Stressbewältigung am Arbeitsplatz-Online.
Additional publication: Holländare et al. (2013).
Drop-out rates from patient survey.
iCBT: Internet-based Cognitive Behavioral Therapy.
SUMMIT: Supportive Monitoring and Depression Management over the Internet (SUMMIT-PERSON: with guidance by clinical expert).
MIND-S: SMS-Assisted Mindfulness-based Intervention for Relapse Prevention in Depression.
Additional publication: Willems et al., 2017b
KNW: Kanker Nazorg Wijzer.
3.2.3. Study characteristics
In the sixteen included studies, a total of 4680 adults participated. Study dropout varied between 5.6% (Fichter et al., 2012) and 64.2% (Bischoff et al., 2013) with an average of 26.3%. About two third of the studies included passive CGs, one-third active CGs (Bischoff et al., 2013; Holländare et al., 2011; Zwerenz et al., 2017a; Zwerenz et al., 2017c). The majority of studies (n = 10) reported at least a medium follow-up period (6–12 months). Six studies reported a short (<6 months) (Gulec et al., 2014; Kraft et al., 2017; Norlund et al., 2018; Schmädeke and Bischoff, 2015; Zwerenz et al., 2017b; Zwerenz et al., 2017c), and four studies a long-term follow-up period (>12 months) (Fichter et al., 2013; Holländare et al., 2013; Jacobi et al., 2017; Kordy et al., 2016). Table 1 provides an overview of relevant characteristics of included studies ordered by the diagnostic focus of the studies (for a list of abbreviations of instruments, see Appendix D).
3.3. Mental disorders and symptoms
3.3.1. Eating disorders
Four studies targeted eating disorders in a female, mostly adolescent population. Fichter et al. (2012) investigated an IMI for anorexia nervosa (AN), the other three studies examined the efficacy of an IMI for bulimia nervosa (BN) or eating disorders, not otherwise specified (EDNOS) (Bauer et al., 2012; Gulec et al., 2014; Jacobi et al., 2017). All interventions were CBT-based and provided some form of human guidance. The web-based program VIA (Fichter et al., 2012) was based on well-established treatment manuals for anorexia and related disorders (Garner et al., 1997; Jacobi et al., 2000, Jacobi et al., 2004). The intervention used common CBT techniques such as cognitive restructuring, behavioral analysis, stimulus control and action plans to prevent relapse after inpatient treatment. This included psychoeducational information, a web-diary on body-related and eating behaviors, exercises, symptom monitoring and optional therapist moderated monthly group chats. The SMS-BRIDGE by Bauer et al. (2012) investigated the efficacy of a 4-months text messaging intervention for the relapse prevention and symptom reduction of BN and EDNOS. Participants indicated bulimic symptoms weekly and received a tailored, semi-automated feedback including reinforcement of self-management strategies. The 4-months, web-based program EDINA (Gulec et al., 2014) was developed as a step-down intervention to maintain treatment gains and to prevent relapse in BN and EDNOS. Guidance was provided through weekly moderated chat-groups of 5–8 participants, on-demand individual therapist contact and a monitoring and feedback system. The 9-months intervention IN@ (Jacobi et al., 2017) used a similar therapeutic rationale for adolescents with BN following inpatient treatment with 11 lectures including symptom monitoring and a diary that was commented on by therapists and on-demand individual live-chats.
3.3.2. Depression
The search yield five studies on CBT-based aftercare or follow-up interventions for depression (Holländare et al., 2011; Kordy et al., 2016; Kraft et al., 2017; Schmädeke and Bischoff, 2015; Zwerenz et al., 2017c). Three interventions were therapist-guided and two unguided (Kraft et al., 2017; Zwerenz et al., 2017c). The 10-week Swedish intervention by Holländare et al. (2011) focused on preventing relapse by treating residual symptoms and developing preventive strategies. Participants worked through web-based self-help material (e.g. behavioral activation, cognitive restructuring and relapse prevention) and received personalized feedback regarding homework and prompts when inactive. The web-based, 12-month program SUMMIT (Kordy et al., 2016) focused on maintaining a remissive state of depression through symptom monitoring, an individual crisis management plan, psychoeducation and reinforcement of self-management strategies. SUMMIT included automated, pre-formulated feedback on symptom monitoring, whereas the variation SUMMIT-PERSON provided additional guidance through a monthly expert group or individual chat in case of symptom deterioration. The guided 3-months intervention eATROS by Schmädeke and Bischoff (2015) included three active phases with degrading intensity based on an intermittent reinforcement of self-management. Core elements comprised activity structuring, relaxation exercises, a crisis management plan and guidance by an online-coach. Participants were asked to plan, structure and evaluate daily activities based on CBT-techniques and received standardized supportive feedback. Additional telephone-contact to the online-coach was available on demand and in case of critical health issues. The unguided, web-based program DEPREXIS was implemented as blended- and aftercare (Zwerenz et al., 2017c) following inpatient psychosomatic treatment. Program use was initiated during inpatient psychosomatic treatment and was continued for 3 months after discharge. Using automated and simulated dialogues, the program lead participants through 12 consecutive self-help modules. The intervention makes use of common CBT-techniques (e.g. activation, cognitive restructuring) as well as elements of positive psychology, emotion-focused therapy, and dream work. The text message-based intervention MIND-S (Kraft et al., 2017) aimed to support daily mindfulness practice for depressive symptoms in former psychiatric inpatients (76.2% major depression diagnosis). Over 4 months, participants were asked to regularly indicate the type and duration of mindfulness exercises and received a pre-formulated, automated reinforcing feedback.
3.3.3. Transdiagnostic interventions
The search yield seven studies with a transdiagnostic approach (Bischoff et al., 2013; Ebert et al., 2013; Norlund et al., 2018; Välimäki et al., 2017; Willems et al., 2017a; Zwerenz et al., 2017a; Zwerenz et al., 2017b). Of these, three studies excluded individuals with acute suicidality, alcohol or drug addiction or psychiatric disorders (Norlund et al., 2018; Ebert et al., 2013; Willems et al., 2017a; Zwerenz et al., 2017b) and another study excluding eating and personality disorders (Bischoff et al., 2013).
The web-based self-help GSA-ONLINE (Zwerenz et al., 2017a) aimed to promote long-term workability following inpatient rehabilitation and was based on a psychodynamic (supportive-expressive) rationale. In a weekly expressive writing task, participants were asked to reflected recurrent, maladaptive relationship patterns at their workplace following Luborsky's Core Conflictual Relationship Theme (Luborsky, 1984) and received an individual feedback from an online-coach. The same study group investigated the web-based program KEN (Zwerenz et al., 2017b) that adapted the self-help book ‘Living Like You Mean It’ (Frederick, 2013). Based on the psychodynamic model of affect phobia (McCullough and Andrews, 2001) and mindfulness-based-therapy, the 10-week intervention included consecutive information and exercises on enhancing emotional awareness, regulating anxiety, and experiencing and expressing emotions to other people mindfully. Participants received a weekly, supportive feedback on their exercise completion.
The unguided Finish intervention MOBILE.NET by Välimäki et al. (2017) makes use of principles of self-determination theory (Deci and Ryan, 2008) to prevent rehospitalization and to increase the quality of life in schizophrenic patients (40% of study participants) and other psychiatric disorders. Over the course of 12 months, participants received pre-selected short messages supporting treatment adherence and self-management strategies. Contents and frequency of messages were selected a-priori by participants.
The CBT-based program TIMT by Ebert et al. (2013) supported the implementation of individual self-management strategies and health goals in patients after psychosomatic rehabilitation. Participants generated a web-based personal development plan (goal setting, implementation strategies and -challenges) and evaluated implementation over the course of 3 months through a weekly web-diary. The program further included weekly peer- and expert feedback referring to diary entries as well as a symptom monitoring and emergency phone contact.
The mobile-based aftercare E-COACH by Bischoff et al. (2013) followed inpatient rehabilitation in patients with dysfunctional occupational expenditure using handheld devices. Based on general psychotherapy (Grawe, 1997, Grawe, 2000), the intervention aimed to promote action competence in functional health behaviors. Participants were asked four times per day to reflect, modify and evaluate modification of current behavior and experiences according to their personal goals. The control group was advised via telephone contact to implement goal-setting and action-planning strategies.
The Dutch program KNW by Willems et al., 2017a, Willems et al., 2017b is an unguided psychosocial aftercare for cancer survivors that makes use of common CBT and problem-solving therapy (PST) tools such as problem identification, goal setting, action planning and monitoring behavioral changes. Informative contents of the eight modules were tailored and focused on self-management training regarding residual symptoms (fatigue, depression, anxiety), healthy lifestyle, social and occupational aspects. Modules could be used in a self-determined order following personal recommendations based on self-report data.
The 14-week guided intervention U-CARE (Norlund et al., 2018) was offered as a tailored web- or mobile-based program for comorbid symptoms of depression and anxiety in patients after myocardial infarction including psychoeducation, assignments (e.g. self-monitoring, skills training, exercises) followed by a written therapist feedback.
3.4. Quality assessment
Detailed ratings of the risk of bias domains for each study are listed in Table 2. Random sequence generation was met in n = 12 studies and rated as high risk of bias in one study (Schmädeke and Bischoff, 2015) and as unclear in three studies (Fichter et al., 2012; Gulec et al., 2014; Bischoff et al., 2013). Allocation concealment was mostly fulfilled (n = 12) with two studies that were categorized as unclear (Bischoff et al., 2013; Gulec et al., 2014; Norlund et al., 2018) and one study as having a high risk of bias (Schmädeke and Bischoff, 2015). None of the studies met criteria for blinding of participants or personnel, formerly resulting in a high risk of bias. One study by Willems et al. (2017a) was rated as unclear. However, since blinding of health care providers/systems or participants concerning treatment allocation is not regularly warranted in psychological interventions, performance and detection bias may be indicated commonly. Twelve studies did not report blinding of outcome assessment. Study dropout rates exceeded acceptable cut-offs in relation to latest available follow-up in 9 out of 16 included studies. Most studies (n = 11) provided ITT-data for primary outcome. However, two studies (Willems et al., 2017a; Zwerenz et al., 2017a) implemented baseline measurement after allocation, which can be considered a methodical constraint of ITT-analyses. Regarding selective outcome reporting, all studies were classified as having a low risk of bias. In terms of other threats to validity, ten studies assessed type and intensity of co-interventions during the course of the study (e.g. medication, outpatient therapy). For the other studies, comparability of groups at baseline could not be determined definitely or showed high risk of bias (Schmädeke and Bischoff, 2015). Since outcome assessments for intervention and control groups were parallel in the majority of included studies (n = 14), measurement timing was categorized as low risk of bias except in two studies (unclear): In the study by Zwerenz et al. (2017b) the wait-list CG was transformed into the IG and in Kordy et al. (2016), timing of follow-up interviews varied with intervention completion and participants were censored. Intervention compliance was determined acceptable in eight studies. Risk of bias was rated high in six studies with exceeding dropout rates and as unclear in two studies (Bauer et al., 2012; Välimäki et al., 2017)
Table 2.
Risk of bias assessment (+ low risk of bias; − high risk of bias;? unclear risk of bias).
| Study | Selection bias |
Performance and detection bias |
Attrition bias |
Reporting bias |
Other sources of bias |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Random sequence generationa | Allocation con-cealmentb | Blinding: Participantsc | Blinding: Personneld | Blinding: Outcome assessmente | Dropoutf | ITTg | Selective reportingh | Co-interventionsi | Similar Groupsj | Compliancek | Timingl | |
| Bauer et al. (2012)m | + | + | – | – | ? | + | + | + | + | + | ? | + |
| Zwerenz et al. (2017a) | + | + | − | − | − | − | + | + | + | + | − | ? |
| Bischoff et al. (2013) | ? | ? | – | – | − | − | − | + | + | + | + | + |
| Ebert et al. (2013) | + | + | – | − | − | − | + | + | + | + | + | + |
| Fichter et al. (2012)n | ? | + | – | − | + | + | − | + | + | + | + | + |
| Gulec et al. (2014) | ? | ? | – | − | − | − | − | + | + | ? | – | + |
| Holländare et al. (2011)o | + | + | – | − | ? | + | + | + | − | ? | + | + |
| Jacobi et al. (2017) | + | + | − | − | + | − | + | + | + | + | + | + |
| Kordy et al. (2016) | + | + | − | − | + | + | + | + | + | ? | + | ? |
| Kraft et al. (2017) | + | + | − | − | − | + | − | + | − | − | − | + |
| Norlund et al. (2018) | + | ? | − | − | − | + | + | + | − | ? | − | + |
| Schmädeke and Bischoff (2015) | − | − | − | − | − | − | − | + | − | − | + | + |
| Välimäki et al. (2017) | + | + | − | − | + | –p | + | + | + | ? | ? | + |
| Willems et al. (2017a)q | + | + | − | ? | − | + | +r, s | + | − | + | – | + |
| Zwerenz et al. (2017a) | + | + | − | − | − | − | +s | + | − | + | + | + |
| Zwerenz et al. (2017c) | + | + | − | − | − | − | + | + | + | ? | − | + |
Adequate generation of a randomized sequence.
Participants and investigators could not foresee assignment.
Intervention and control group are indistinguishable for the participants.
Intervention and control group are indistinguishable for the care providers.
Intervention and control group are indistinguishable for the outcome assessors for primary outcome (for patient-reported outcomes, it is adequate if patients are blinded).
Dropout must be described and reasons must be given. Dropout should not exceed 20% for short-term follow-up (3–6 months), 30% for medium-term follow-up (6–12 months), 35% for long term follow-up (>12 months) in guided and 40% in unguided interventions.
ITT: intention-to-treat analyses. All randomized patients are reported and analyzed in the group they were allocated to by randomization.
Results of all pre-specified outcomes have to be adequately and completely reported.
Cointerventions must be specified.
Groups should not differ significantly at baseline regarding outcomes and main demographics.
Acceptable compliance with the main component(s) of the intervention (e.g. intensity, duration, frequency).
Identical timing of outcome assessments for intervention and control groups.
Additional publications: Bauer et al. (2011a),Bauer et al. (2013).
Additional publication: Fichter et al. (2013).
Additional publication: Holländare et al. (2013).
Drop-out rates from patient survey.
Additional publication: Willems et al. (2017b).
Additional ITT Data provided by study author.
ITT-analyses provided, but baseline-measurement after allocation.
3.5. Effects of the interventions
3.5.1. Symptom severity
The effects of the interventions on the main outcomes of each included study at the study's primary endpoint are presented in Table 3. Evidence of clinical symptom severity was reported in 14 studies. Studies yielded small to medium sized post-treatment between-group effects of symptom severity (d = −0.08–d = −0.45) for the intervention under study. One study investigating an unguided mobile-based self-help yielded contrary results in favor of the CG (Bischoff et al., 2013). Small effect sizes could be observed in studies, in which depressive symptoms were measured for a medium follow-up period (6–12 months) in guided, web-based interventions ranging from d = −0.03 to d = −0.37(Ebert et al., 2013; Holländare et al., 2011; Zwerenz et al., 2017a). For the same studies effects for self-rated symptom severity of anxiety varied between d = 0.05 and d = −0.35 (medium follow-up period).
Table 3.
Main outcomes of studies on Internet-or mobile-based interventions for mental health problems.
| Study | Health condition | Comparison | Main outcome [Instrument] | Outcomea [follow-up]b | 95% CI |
|---|---|---|---|---|---|
| Bauer et al. (2012) | Bulimia Nervosa | Guided text messaging intervention vs. TAU | Remission rates [LIFE] |
RR = 1.42c [8 months] |
0.99; 2.02 |
| Fichter et al. (2012) | Anorexia Nervosa (AN) | Internet-based guided self- help vs. TAU | Body Mass Index [BMI] |
d = −0.19d [9 months] |
−0.08; 0.46 |
| Gulec et al. (2014) | Bulimia Nervosa/EDNOS | Internet-based guided self-help vs. TAU | Severity of eating disorder [EDE-Q] |
d = −0.28d [4 months] |
−0.73; 0.18 |
| Jacobi et al. (2017) | Bulimia Nervosa (BN) | Internet-based guided self- help vs. TAU | abstinence from core symptoms of BN [SIAB-EX] |
OR = 1.29 [9 months] OR = 1.49 [18 months] |
0.68; 2.44 0.77; − 2.86 |
| Holländare et al. (2011) | Depression | Internet-based guided self- help vs. TAU | Relapse rates [SCID] |
RR = 0.28 [6 months] RR = 0.24 [24 months]e |
0.10; 0.77 0.10; 0.55 |
| Kordy et al. (2016) | Recurrent depression | Internet-based guided intervention vs. unguided intervention vs. TAU | Transition from to ‘well’ to ‘unwell’ [LIFE] | unguided: RR = 0.91 [12 months] RR = 0.69 [24 months] guided: RR = 1.11 [12 months] RR = 0.92 [24 months] |
0.51; 1.64 0.44; 1.09 0.63; 1.95 0.59; 1.43 |
| Kraft et al. (2017) | Depression | Text message self-help vs. TAU | Severity of depressive symptoms [PHQ-9] |
d = −0.45 [4 months] |
−1.11; −0.23 |
| Norlund et al. (2018) | Myocardial Infarction (comorbid depressive/anxiety symptoms) |
Web-based guided self- help vs. TAU | Severity of depression and anxiety [HADS-T] |
d = −0.13 [14 weeks] |
−0.38; 0.13 |
| Schmädeke and Bischoff (2015) | Depression | Mobile-based self-help vs. TAU | Severity of depression symptoms [BDI] |
d = −0.08 [3 months] |
−0.49; 0.33 |
| Zwerenz et al. (2017a) | Depression | Web-based self-help vs. attention control website | depressive symptoms [BDI-II] |
d = −0.44 [6 months] |
−0.71; −0.17 |
| Bischoff et al. (2013) | Transdiagnostic | Mobile-based self-help vs. phone contact | Work-related Behaviour and Experience Patterns [AVEM] |
d = 0.18 [6 months] d = 0.08 [12 months] |
−0.21; 0.56 −0.32; 0.48 |
| Ebert et al. (2013) | Transdiagnostic | Web-based guided self- help vs. TAU | general psychopathological symptom severity [HEALTH-49] |
d = −0.38c [3 months] d = −0.44c [12 months] |
−0.18; −0.58 −0.64; −0.24 |
| Välimäki et al. (2017) | Transdiagnostic | Text message self-help vs. TAU | patient readmission to psychiatric hospital [Finnish national Care Register for Health Care (HILMO)] |
RR = 1.11 [12 months] |
0.92; 1.33 |
| Willems et al. (2017a) | Cancer (transdiagnostic) | Web-based self-help vs. WLCG+TAU | Various, e.g. depression [PHQ-9] |
d = −0.21 [6 months] d = −0.10 [12 months]f |
−0.40; −0.01 −0.30; 0.11 |
| Zwerenz et al. (2017b) | Transdiagnostic | Web-based guided self-help vs. WLCG | Various, e.g. depression [PHQ-9] |
d = −0.34c [10 weeks] |
−0.81; 0.14 |
| Zwerenz et al. (2017a) | Transdiagnostic | Web-based guided self-help vs. TAU | subjective prognosis of work ability [SPE] |
d = −0.13 [3 months] d = −0.20 [12 months] |
−0.28; 0.03 −0.36; −0.04 |
Note. Own calculations based on study data. EDNOS: Eating disorder not otherwise specified; OR: Odd's ratio; RR: Risk ratio; TAU: Treatment as usual; WLCG: Waitlist control group. Abbreviations of measurement instruments can be found in Appendix D.
Note. Own calculations based on study data. EDNOS: Eating disorder not otherwise specified; OR: Odd's ratio; RR: Risk ratio; TAU: Treatment as usual; WLCG: Waitlist control group. Abbreviations of measurement instruments can be found in Appendix D.
Between-group comparisons.
Post-treatment- and latest available follow-up.
ITT-analyses.
Unpublished age stratified data provided by PI.
Additional publication: Holländare et al. (2013).
Additional publication: Willems et al. (2017b).
3.5.2. Relapse and rehospitalization
Data on relapse rates were reported by three studies with various target disorders (Bauer et al., 2012; Holländare et al., 2011; Kordy et al., 2016). Relapse rates ranged from 10.5% to 43.0% in the interventions under study for a medium follow-up period. Risk ratios were heterogeneous and included positive effect of interventions in contrast to the control groups (Bauer et al., 2012; Holländare et al., 2011), whereas the web-based intervention for recurrent depression by Kordy et al. (2016) reported an elevated risk of relapse in the IG (RR = 1.02) at a twelve month follow-up. Rehospitalization rates were reported in five studies (Fichter et al., 2012; Gulec et al., 2014; Jacobi et al., 2017; Kordy et al., 2016; Välimäki et al., 2017). Results were inconsistent in studies targeting eating disorders: Gulec et al. (2014) found a reduced risk of rehospitalization (RR = 0.12) at a 4-months follow-up for adults with BN or EDNOS. In contrast, age-stratified data on AN by Fichter et al. (2012) showed higher rehospitalization rates for participants in the IG (RR = 1.55) at a 9-month follow-up. The transdiagnostic intervention by Välimäki et al. (2017) showed higher rates of rehospitalization in of participants receiving a mobile-based self-help (RR = 1.11), whereas Kordy et al. (2016) yielded a lower risk of rehospitalization in the intervention group (RR = 0.79). Rehospitalization rates in the study of Jacobi et al. (2017) were similar in both groups after 18 months. See Table 3 for studies investigating relapse and rehospitalization rates as main outcomes.
3.5.3. Quality of life and functioning
Quality of life was assessed as a secondary outcome in five studies (Holländare et al., 2011; Välimäki et al., 2017; Willems et al., 2017a; Zwerenz et al., 2017b; Zwerenz et al., 2017c) using different self-administered instruments (WHOQOL-BREF, Q-LES-Q, EUROHIS-QOL, QLQ-C30). Välimäki et al. (2017) reported a null-effect of an unguided mobile-based intervention on quality at a 12-months follow-up. Similarly, Holländare et al. (2011) yielded no effect of a web-based CBT for recurrent depression in the short follow-up period. However, the authors could show that effect sizes slightly improved (d = 0.0–d = −0.20) in the course of 24 months follow-up measurements (Holländare et al., 2013). Other effect sizes for a medium follow-up period were small (d = 0.08 to d = −0.35), indicating a minor improvement in quality of life through web-based interventions.
Various domains of functioning (e.g. psychological, social, emotional, health-related, occupational) were assessed with self-rated (SF-12, QLQ-C30) and one clinician-rated instrument (GAS) in three studies (Välimäki et al., 2017; Willems et al., 2017a; Zwerenz et al., 2017a). Effect sizes were small to medium (d = −0.08 to d = −0.43), indicating a beneficial effect on functioning of IMIs in the post-discharge phase over control conditions. Interestingly, Välimäki et al. (2017) could demonstrate, that participants receiving a mobile-based intervention were less disabled than control participants at hospital readmission (Odd's Ratio, OR = 0.68, 95% CI [0.47; 0.97]). However, due to the considerable heterogeneity of operationalization and measurement, meta-analytic pooling of effect sizes was not feasible for other study outcomes.
3.5.4. Additional findings
None of the included RCTs explicitly reported data on adherence to pretreatment (e.g. medication compliance). None of the four predominantly mobile-based interventions yielded significant results for the efficacy of the respective intervention, with small effect sizes for studies investigating primarily symptom severity (d = −0.08–d = −0.45) (Bischoff et al., 2013; Kraft et al., 2017; Schmädeke and Bischoff, 2015) and increased risk ratios in trials on relapse prevention (RR = 1.11–RR = 1.42) (Bauer et al., 2012; Välimäki et al., 2017). With regard to human guidance, only two out of five studies using unguided interventions yielded significant results with small to medium effect sizes for a medium follow-up period (d = −0.21–d = −0.44) (Willems et al., 2017a; Zwerenz et al., 2017c).
3.5.5. Cumulated quality of evidence (GRADE)
Confidence in the cumulative quality of evidence per outcome was rated using GRADE (Guyatt et al., 2008), see Appendix C. Ratings indicated a moderate cumulated quality of evidence for symptom severity of depression and anxiety. However, the quality of evidence for other inspected outcomes such as quality of life and level of functioning was rated as ‘low’ and as ‘very low’ for relapse and rehospitalization rates. Main reasons for downgrading included the indirectness of results (e.g. heterogeneous target disorders), imprecision (e.g. small number of studies, low median sample size) and methodological risk of bias (see Table 2). With regard to outcomes regarding relapse/rehospitalization rates and quality of life, study results were partially inconsistent. The estimation of mean effect sizes was not feasible for the majority of outcomes. Furthermore, possible publication biases could not be estimated, due to the limited number of studies.
3.6. Ongoing trials
A search for ongoing trials was conducted in the ICTRP database. The search yielded 718 records of 700 trials (years 2003–2018), of which n = 24 were identified as potentially relevant. Targeted conditions were mostly affective disorders or comorbid mental symptoms in somatic conditions (cancer, osteoarthritis, diabetes, cardiac disease). However, the majority (n = 12) described interventions adjunct to primary care (ISRCTN56908625, NCT03404583), as standalone interventions for mental disorders (ISRCTN64953693) or for comorbid mental disorders of serious somatic disorders (ACTRN12613001198718, ACTRN12613001174774, ISRCTN45945396, NTR793, ACTRN12613001026718, ACTRN12613000001796, ACTRN12613001170718, ACTRN12611000278932, ISRCTN32477700) that did not classify as aftercare or follow-up interventions. The main publications of four of these were included in this review included in this review (Ebert et al., 2013; Fichter et al., 2012; Välimäki et al., 2017; Zwerenz et al., 2017b; Zwerenz et al., 2017c). Seven studies that planned to investigate web- or mobile-based aftercare for various mental disorders (affective, eating disorders, cancer-related anxiety/depression) and were still ongoing or unpublished (ISRCTN32477700; DRKS00008847; DRKS00009272; NCT02258711; ISRCTN18274621; ISRCTN08870215; NTR2599) or a pilot study not fulfilling the criteria of RCT (ACTRN12617001447347) at the time of the systematic search.
4. Discussion
4.1. Summary of evidence
This review is the first to systematically summarize and examine the existing empirical evidence of Internet- and mobile-based aftercare and follow-up interventions for the tertiary prevention of a broad range of mental disorders and related symptoms. It therefore adds to previous reviews on primary prevention (Ebert et al., 2017; Sander et al., 2016) and provides a comprehensive overview of modern instruments of continuous care of mental disorders.
Sixteen RCTs were included in this review, all of them were published within the last six years. The selected studies corroborate previous research, highlighting that the implementation of IMIs is mostly web-based, that CBT-techniques are more common than other therapeutic interventions and that some form of asynchronous guidance is frequently used (Baumeister et al., 2014; Ebert et al., 2017; Sander et al., 2016). In this regard, studies comprised mainly minimal guidance, which has been shown to be a (cost-) effective strategy in most mental disorders (Baumeister et al., 2014; Newman et al., 2011). Mirroring the frequent comorbidity and commonalities of mental disorders (Kessler et al., 2011a; Kessler et al., 2011b), most studies were designed transdiagnostic, targeting occupational rehabilitation (Bischoff et al., 2013; Zwerenz et al., 2017a), individual self-management strategies (Ebert et al., 2013; Välimäki et al., 2017) or comorbid mental symptoms of severe somatic diseases (Norlund et al., 2018; Willems et al., 2017a). Disorder-specific interventions targeted affective and eating disorders, reflecting their frequent recurrences and risk of chronification (Keller and Boland, 1998; McFarlane et al., 2008).
The limited amount of studies eligible for inclusion did not allow to draw conclusions about the efficacy of IMIs as aftercare or follow-up interventions for mental disorders. Small effects were found for depressive and symptoms of anxiety in guided, web-based interventions. However, considering that tertiary prevention aims to sustain health and functioning and thus treatment gains of previous (main) interventions, smaller effect sizes can be expected and are consequently lower than moderate to high effect sizes observed in IMIs for in the context of the treatment of (full-blown) mental disorders (Königbauer et al., 2017). The small effects found for symptom severity can be compared to findings from Sander et al. (2016), showing a small effect of IMIs in the treatment of subclinical depressive symptoms (n = 8, SMD = −0.22). Also, since the included studies did not target anxiety disorders explicitly, our findings corroborate previous research, showing small effects of IMIs on subclinical anxiety (Heber et al., 2017; Rollman et al., 2005). Furthermore, some of the interventions included in our meta-analyses primarily aimed to prevent relapse or rehospitalization (Bauer et al., 2012; Fichter et al., 2012; Holländare et al., 2011), which may further explain small effects on symptom severity as a secondary outcome measure. In summary, the meta-analytic results must be interpreted cautiously considering various sources of heterogeneity (e.g. target mental disorders, intervention characteristics, methodological limitations). Nevertheless, the present findings illustrate the potential of IMIs in the field of tertiary prevention as aftercare and follow-up in maintaining treatment gains of depressive and anxiety symptoms.
Study results on relapse and rehospitalization rates were inconsistent, including reduced risk ratios for interventions targeting eating disorders or recurrent depression (Bauer et al., 2012; Gulec et al., 2014; Holländare et al., 2011; Kordy et al., 2016) in contrast to studies reporting similar or elevated risk of relapse and rehospitalization between experimental groups (Fichter et al., 2012; Kordy et al., 2016; Välimäki et al., 2017). However, as continuous care improves the detection of early warnings signs of recurrence, rehospitalization can also be seen as an indicator of an early and adequate treatment initiation. Further research is needed, to explore the potential of IMIs in accelerated care and establish the effectiveness if IMIs in relapse prevention in relevant mental disorders.
Included studies reported mixed evidence of the efficacy of IMIs on quality of life and functioning, including nearly zero (Holländare et al., 2011; Välimäki et al., 2017) to medium effect sizes (Zwerenz et al., 2017b; Zwerenz et al., 2017c). From a methodical point of view, multidimensional and complex measures of quality of life may be less accurate and responsive than symptom- or disorder-specific outcome measures, in particular when the latter is the primary outcome of a treatment (Higginson and Carr, 2001). Also, the course of quality of life or functioning may be more discontinuous in comparison to clinical change, due to various adaption processes in the immediate post-discharge phase. Results by Holländare et al. (2013) support the and delayed effect of IMIs on quality of life. Future research thus should include adequately powered sample sizes and long-term follow-up periods to determine the effectiveness of IMIs on quality of life and functioning.
Moreover, our results indicate a limited effectiveness of unguided predominantly mobile-based interventions as aftercare. This corroborates previous research, demonstrating a beneficial effect of guidance on adherence and effectiveness (Baumeister et al., 2014; Newman et al., 2011). Although automated mobile-based interventions can be easily implemented as low-intensity maintenance interventions, future research should aim to expand their potential in the post-discharge phase.
4.2. Methodological quality of studies
Methodological quality of included studies was suboptimal in total, as reflected in mostly low-quality GRADE ratings (Guyatt et al., 2008) with moderate quality of evidence only for symptom severity of depression. Several methodical shortcomings and consequent recommendations for future studies can be outlined: Firstly, as expected, no study met quality criteria of blinding of personnel and participants, which may be considered as inevitable in psychological trials. However, we identified multiple studies without proper blinding of outcome assessors, demonstrating abundant room for methodical improvement in future studies. Secondly, we observed critical treatment fidelity (dropout rates, intervention adherence) in about two-thirds of the studies. However, average dropout rates of guided (31.6%) and unguided (34.7%) interventions are comparable to evidence from previous meta-analyses (Melville et al., 2010; Richards and Richardson, 2012; van Ballegooijen et al., 2014). We could not observe a definite cause for reduced treatment fidelity. Previous research indicates that treatment fidelity is influenced by various factors, including study design (e.g. amount of guidance, intervention duration), intervention characteristics (e.g. interactivity, adaptability, usability), as well as user- or contextual characteristics (e.g. symptom severity, treatment credibility, co-interventions) (Andersson and Cuijpers, 2009; Baumeister et al., 2014; Donkin and Glozier, 2012; Kelders et al., 2012). Additionally, reduced treatment fidelity in various instruments of tertiary prevention has been documented previously (Kobelt et al., 2004; Mitchell et al., 2004). In this regard, reduced treatment adherence is not only an indicator for limited effectiveness or feasibility, but can also express recovery, since participants do not necessarily need to complete all intervention parts to benefit from treatments. Future studies are thus advised to differentiate reasons for and timing of reduced adherence or attrition, to better predict and prevent dropout in IMIs.
Thirdly, assessment of type and frequency of co-interventions was often missing or limited to the assessment of medication prescription. For three studies, differentiated reports on type and intensity of co-interventions were available (Bauer et al., 2013; Kordy et al., 2016; Välimäki et al., 2017). Bauer et al. (2013) for example could demonstrate that the relapse risk for BN in participants of the IG (mobile-based intervention) was slightly lower for those, who exclusively used the intervention (24.2%) than for the combination with outpatient therapy (28.9%). Similar results have been documented by for therapist-guided chat groups in relapse prevention of BN (Bauer et al., 2011b). These controversial findings underline the need for further studies to determine the differentiated indication for IMIs in terms of stepped or continuous care research on moderators of their effectiveness in routine care. Fourthly, CONSORT-standards regarding the inclusion of ITT-analyses were not evident in all the included studies, increasing the risk of biased results and thus limiting interpretability of study results. Therefore, future studies should include ITT-analyses and report type, amount and methods for handling of missing data (Schulz et al., 2008).
4.3. Limitations
Firstly, we did not limit our review to certain mental disorders, to illustrate the diversity of interventions in the field of tertiary prevention. Consequently, we included a broad spectrum of mental disorders, as reflected by the majority of transdiagnostic interventions. Furthermore, interventions differed regarding study objectives (e.g. relapse prevention, symptom reduction, occupational integration), therapeutic rationales, design, and mode of guidance. Also, type (e.g. self- or clinician-rated) and timing of measurement, as well as the choice of comparators, varied across studies. In this regard, some studies implemented waiting list CGs, others different types of active control groups (e.g. face-to-face treatment, placebo website). Therefore, subsequent heterogeneity must be considered carefully. Although the limited amount of studies eligible for inclusion did not allow for robust conclusions about the efficacy of IMIs as aftercare or follow-up interventions for mental health issues, our specific inclusion criteria (see 2.2) yielded a meaningful study selection. However, this review's focus on the transition phase after acute treatment does not allow to generalize the effectiveness of included IMIs to the entire field of tertiary prevention. Beyond step-down interventions, this may include long-term disease management or instruments focusing on the reduction of disability. Particularly for mental disorders with high risk for chronic courses IMIs may accompany existing medical strategies as low-threshold and flexible instruments.
Above that, the existence of a publication bias needs to be considered as a major concern in social sciences (Cuijpers et al., 2010; Rothstein, 2005). We could not examine possible publication bias with elaborated statistical methods (e.g. funnel plots), due to the limited number of studies and considerable heterogeneity (Sterne et al., 2011).
4.4. Practical implications and future research
Although a growing body of research illustrates the feasibility and effectiveness of IMIs for various mental disorders, this review reveals important directions for future research and necessary efforts in clinical implementation.
4.4.1. Tertiary prevention of psychiatric and chronic mental disorders
Our search yielded a broad spectrum of target disorders of IMIs in tertiary prevention, particularly addressing multiple disorders through transdiagnostic interventions. Trials investigating web- or mobile-based aftercare for further mental disorders that are characterized by chronic courses such as psychiatric, somatoform or personality disorders were underrepresented in our search results. However, a growing body of research recognizes the applicability of IMIs in the tertiary prevention of severe or chronic mental disorders. A recent review by Westermann et al. (2017) documents the potential of IMIs in preventing relapse in schizophrenic disorders, e.g. in promoting adherence to medical treatment using mobile-based symptom monitoring and automated notification of practitioners (Montes et al., 2012; Španiel et al., 2012). Yet, there is still abundant room for the implementation of psychosocial intervention elements in IMIs in the field of tertiary prevention of psychiatric disorders or severe clinical conditions such as suicidality (Christensen et al., 2014). For instance, the study by Välimäki et al. (2017) examined a low-intensity, mobile-based self-help to promote self-management strategies in a psychiatric target population but could not demonstrate its effectiveness with regard to recurrence or quality of life in contrast to usual care. For chronic depression, a study by Hunkeler et al. (2012) provides positive evidence for a web-based intervention adjunct to standard community care in symptom and relapse reduction. Furthermore, several studies have investigated web- and mobile-based relapse prevention in bipolar disorder (Barnes et al., 2007; Depp et al., 2015; Lobban et al., 2017; Smith et al., 2011). However, these studies could not demonstrate a long-term effectiveness of IMIs on functioning or relapse rates in contrast to active or passive control groups. Together, these results highlight the need for further studies addressing the challenges of continuous care in chronic mental disorders. Moreover, since most mental disorders show high incidence rates in childhood and adolescence (Bor et al., 2014) research on the effectiveness of web- or mobile-based aftercare or intermediate care in this target group represents another promising field of study.
4.4.2. Strategies for implementation in routine care
Besides rigorous and large-scaled RCTs, further research is also needed to establish the effectiveness of IMIs in routine care. Previous studies in naturalistic, e.g. community care settings show mixed results (Christensen et al., 2004; Gilbody et al., 2015; Hedman et al., 2013; Månsson et al., 2017). Implementation of IMIs would require foremost structural efforts such as sufficient resources in health care systems, clinical guidelines and quality criteria (Klein et al., 2016; Proudfoot et al., 2011), a participative approach in the development and integration of IMIs with existing treatments and strategies to facilitate access and reach of IMIs in relevant target groups.
Given the increased access and use of the Internet in health issues (Eichenberg et al., 2013), IMIs could reach people, that do not access established mental health care but deliberately seek for low-threshold support. Implementation strategies should therefore include multiple contact points and collaboration across healthcare-, education- or occupational- systems and institutions. Also, the possibility for self-reference, which has been associated with elevated adherence and effectiveness (Johansson et al., 2013; Schueller et al., 2013), could facilitate the implementation of IMIs. Another promising approach is the integration of IMIs in existing treatments (blended-care), where certain treatment elements (e.g. psychoeducation, exercises) can be augmented with or transferred to a web- or mobile-based application (Erbe et al., 2017). A meta-analysis of 25 studies by Lindhiem et al. (2015) found positive evidence that mobile-based interventions could boost the effect of primary psychotherapy. Blended-care may also help to concentrate therapeutic resources or to promote adherence and self-efficacy in patients. An innovative design is reported in the study by Zwerenz et al. (2017c) who combined blended- and aftercare and proved the effectiveness of a web-based intervention for the reduction of depression. Andersson et al. (2014) found that adding a 3-week, web-based booster program to a ten-week iCBT for patients with obsessive-compulsive disorder (OCD) could further improve the long-term outcome and prevent relapse. This may also point to a beneficial application of IMIs as aftercare or intermediate care following outpatient psychotherapy.
Regarding individual barriers to access and adoption of IMIs, previous studies have shown limited acceptance of IMIs in the general population (Eichenberg et al., 2013; Gun et al., 2011), in various patient groups (Hennemann et al., 2016; Waller and Gilbody, 2009) or in health professionals (Hennemann et al., 2017a). Person-centered barriers to acceptance include negative expectations towards outcome and usability, unfavorable attitudes of the social environment, as well as limited knowledgeability and eHealth literacy (Hennemann et al., 2016, Hennemann et al., 2017a, Hennemann et al., 2017b). Recent studies provide promising evidence that brief video-based presentations about areas of application, effectiveness, data security or utilization, combined with first hands-on experience can be an economical way to facilitate acceptance (Baumeister et al., 2015; Ebert et al., 2015). This would imply the systematic measurement of barriers to access and acceptance of IMIs. Promising instruments include the assessment of eHealth literacy (Norman and Skinner, 2006; van der Vaart and Drossaert, 2017) or attitudes towards psychological IMIs (Schröder et al., 2015). However, the identification and facilitation of acceptance need to be expanded to health professionals (Donovan et al., 2015) and other stakeholders in the healthcare system (Topooco et al., 2017).
4.4.3. Outcome assessment and research design
Considering the importance of participatory approaches in improving the adoption and impact of IMIs in health care (van Gemert-Pijnen et al., 2011; Yardley et al., 2015), patient-relevant outcomes are an important indicator of the practical and clinical relevance of IMIs. However, only half of the studies included in this review reported outcomes related to quality of life or functioning. Missing information on post-intervention employment status can be seen as critical as well. Future studies should thus cover relevant domains of functioning.
Furthermore, systematic assessment of cost-effectiveness is highly relevant for public health policy in order to compare the savings of IMIs in comparison with costs related to traditional health care utilization (e.g. rehospitalization), work loss or mortality. Of the included studies, only two (Hunkeler et al., 2012; Välimäki et al., 2017) included instruments for economic evaluation and demonstrated a relative cost-effectiveness in contrast to the control group. Cost-effectiveness also relates to intervention design, where previous research has documented the superiority of guided in comparison to unguided IMIs (Donker et al., 2015), while on a public health level, low-maintenance interventions may be associated with a higher range and effect in relation to the population level. More research is needed to differentiate moderators of cost-effectiveness in different healthcare settings.
4.4.4. Making use of technological innovations
Although this review found a broad spectrum of mostly web- and less frequently used mobile-based interventions, it seems, that the technological potential of IMIs is not yet sufficiently used. Since web- and mobile technologies advance rapidly, research on IMIs may benefit from technical progress in various domains. In future, advances in machine learning and intelligent algorithms may add to the development of more elaborated and tailored intervention strategies such as just-in-time interventions (Nahum-Shani et al., 2016), artificial intelligence in guidance and interaction or red flag systems that may identify treatment loss or failure and adapt intervention content accordingly. IMIs may also benefit from the increasing availability of sensor data (e.g. activity, health, context data) through smartphones, wearables or other devices that could be used to refine and adapt intervention strategies to various context and risk variables (Torous et al., 2016). Therefore, it is likely that tailored- or adaptive IMIs will add substantially to the progress and implementation of personalized medicine. However, elevated intervention flexibility and complexity would initially increase development costs and expense. Thus, the superiority of tailored IMIs in contrast to standardized or generic interventions remains to be investigated.
4.5. Conclusion
This review provides researchers, clinicians and public health policymakers with a valuable overview of the current state of research on the efficacy and clinical feasibility of IMIs as aftercare or follow-up interventions of mental disorders. IMIs can be effective instruments in maintaining treatment gains in the post-discharge phase. Despite small effects on clinical outcomes such as symptom severity, the flexibility, high range and facilitated monitoring in IMIs in relation to conventional aftercare are promising qualities for routine care. However, the current evidence regarding symptom severity, relapse prevention or quality of life is mixed, demonstrating the need of further, high-quality, large-scale RCTs to establish a solid evidence base for the effects of IMIs in tertiary prevention. Above that, future research should broaden the range of targeted mental disorders and also include severe and chronic conditions. Further efforts are needed regarding treatment adherence and identifying of critical courses in the vulnerable post-discharge phase. Advancements in machine-based learning and mobile-based technologies can improve adaptability and tailoring of interventions. Eventually, effective and feasible IMIs need to be implemented into routine care settings to make them impactful to people's lives.
The following are the supplementary data related to this article.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
Search term.
GRADE ratings for selected outcomes.
List of abbreviations of measurement instruments of included studies.
Role of funding sources
This study did not receive funding.
Contributors
SH, SF and LS were involved in the concept and design of the study. SH and SF performed the study selection, data extraction and analyses with the collaboration of LS. SH and SF wrote the draft of this manuscript. LS provided valuable revisions. All authors approved the final version of the manuscript.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
The authors would like to thank the researchers who provided unpublished or additional data.
Contributor Information
Severin Hennemann, Email: s.hennemann@uni-mainz.de.
Lasse Sander, Email: lasse.sander@psychologie.uni-freiburg.de.
References
- Adair C.E., McDougall G.M., Mitton C.R., Joyce A.S., Wild T.C., Gordon A., Costigan N., Kowalsky L., Pasmeny G., Beckie A. Continuity of care and health outcomes among persons with severe mental illness. PS. 2005;56(9):1061–1069. doi: 10.1176/appi.ps.56.9.1061. [DOI] [PubMed] [Google Scholar]
- Andersson G., Cuijpers P. Internet-based and other computerized psychological treatments for adult depression: a meta-analysis. Cogn. Behav. Ther. 2009;38(4):196–205. doi: 10.1080/16506070903318960. [DOI] [PubMed] [Google Scholar]
- Andersson E., Steneby S., Karlsson K., Ljotsson B., Hedman E., Enander J., Kaldo V., Andersson G., Lindefors N., Ruck C. Long-term efficacy of internet-based cognitive behavior therapy for obsessive-compulsive disorder with or without booster: a randomized controlled trial. Psychol. Med. 2014;44(13):2877–2887. doi: 10.1017/S0033291714000543. [DOI] [PubMed] [Google Scholar]
- Barkhof E., Meijer C.J., de Sonneville L.M.J., Linszen D.H., de Haan L. Interventions to improve adherence to antipsychotic medication in patients with schizophrenia—a review of the past decade. Eur. Psychiatry. 2012;27(1):9–18. doi: 10.1016/j.eurpsy.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Barnes C., Harvey R., Mitchell P., Smith M., Wilhelm K. Evaluation of an online relapse prevention program for bipolar disorder: an overview of the aims and methodology of a randomized controlled trial. Dis. Manag. Health Out. 2007;15(4):215–224. [Google Scholar]
- Barnes C.W., Hadzi-Pavlovic D., Wilhelm K., Mitchell P.B. A web-based preventive intervention program for bipolar disorder: outcome of a 12-months randomized controlled trial. J. Affect. Disord. 2015;174:485–492. doi: 10.1016/j.jad.2014.11.038. [DOI] [PubMed] [Google Scholar]
- Bauer S., Okon E., Meermann R. Nachsorge nach stationärer Psychotherapie für Essstörungen: Wirksamkeit eines SMS-basierten programms. [Aftercare following inpatient psychotherapy for eating disorders. Efficacy of a program based on text messaging] Psychotherapeut. 2011;56(6):509–515. [Google Scholar]
- Bauer S., Wolf M., Haug S., Kordy H. The effectiveness of internet chat groups in relapse prevention after inpatient psychotherapy. Psychother. Res. 2011;21(2):219–226. doi: 10.1080/10503307.2010.547530. [DOI] [PubMed] [Google Scholar]
- Bauer S., Okon E., Meermann R., Kordy H. Technology-enhanced maintenance of treatment gains in eating disorders: efficacy of an intervention delivered via text messaging. J. Consult. Clin. Psychol. 2012;80(4):700–706. doi: 10.1037/a0028030. [DOI] [PubMed] [Google Scholar]
- Bauer S., Okon E., Meermann R., Kordy H. SMS-nachsorge: Sektorenübergreifende versorgung für patientinnen mit bulimia nervosa. [Aftercare based on text messaging: services across health care sectors for patients with bulimia nervosa] Verhaltenstherapie. 2013;23(3):204–209. [Google Scholar]
- Baumeister H., Reichler L., Munzinger M., Lin J. The impact of guidance on internet-based mental health interventions — a systematic review. Internet Interv. 2014;1(4):205–215. [Google Scholar]
- Baumeister H., Seifferth H., Lin J., Nowoczin L., Lüking M., Ebert D. Impact of an acceptance facilitating intervention on patients' acceptance of internet-based pain interventions: a randomized controlled trial. Clin. J. Pain. 2015;31(6):528–535. doi: 10.1097/AJP.0000000000000118. [DOI] [PubMed] [Google Scholar]
- Beynon S., Soares-Weiser K., Woolacott N., Duffy S., Geddes J.R. Psychosocial interventions for the prevention of relapse in bipolar disorder: systematic review of controlled trials. Br. J. Psychiatry. 2008;192(1):5–11. doi: 10.1192/bjp.bp.107.037887. [DOI] [PubMed] [Google Scholar]
- Bischoff C., Schmädeke S., Adam M., Dreher C., Bencetic D., Limbacher K. Wirksamkeit von handheld-gestütztem Selbstmanagement (E-coaching) in der Rehabilitationsnachsorge. [Efficacy of handheld-computer-supported self-management (E-coaching) in rehabilitation aftercare] Verhaltenstherapie. 2013;23(4):243–251. [Google Scholar]
- Blank L., Peters J., Pickvance S., Wilford J., Macdonald E. A systematic review of the factors which predict return to work for people suffering episodes of poor mental health. J. Occup. Rehabil. 2008;18(1):27–34. doi: 10.1007/s10926-008-9121-8. [DOI] [PubMed] [Google Scholar]
- Bor W., Dean A.J., Najman J., Hayatbakhsh R. Are child and adolescent mental health problems increasing in the 21st century? A systematic review. Aust. N. Z. J. Psychiatry. 2014;48(7):606–616. doi: 10.1177/0004867414533834. [DOI] [PubMed] [Google Scholar]
- Caplan G. Basic Books; New York: 1964. Principles of Preventive Psychiatry. [Google Scholar]
- Carter J.C., McFarlane T.L., Bewell C., Olmsted M.P., Woodside D.B., Kaplan A.S., Crosby R.D. Maintenance treatment for anorexia nervosa: a comparison of cognitive behavior therapy and treatment as usual. Int. J. Eat. Disord. 2009;42(3):202–207. doi: 10.1002/eat.20591. [DOI] [PubMed] [Google Scholar]
- Christensen H., Griffiths K.M., Korten A.E., Brittliffe K., Groves C. A comparison of changes in anxiety and depression symptoms of spontaneous users and trial participants of a cognitive behavior therapy website. J. Med. Internet Res. 2004;6(4) doi: 10.2196/jmir.6.4.e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen H., Batterham P.J., O'Dea B. E-health interventions for suicide prevention. Int. J. Environ. Res. Public Health. 2014;11(8):8193–8212. doi: 10.3390/ijerph110808193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuijpers P., Smit F., Bohlmeijer E., Hollon S.D., Andersson G. Efficacy of cognitive-behavioural therapy and other psychological treatments for adult depression: meta-analytic study of publication bias. Br. J. Psychiatry. 2010;196(3):173–178. doi: 10.1192/bjp.bp.109.066001. [DOI] [PubMed] [Google Scholar]
- Deci E.L., Ryan R.M. Self-determination theory: a macrotheory of human motivation, development, and health. Can. Psychol. 2008;49(3):182–185. [Google Scholar]
- Depp C.A., Ceglowski J., Wang V.C., Yaghouti F., Mausbach B.T., Thompson W.K., Granholm E.L. Augmenting psychoeducation with a mobile intervention for bipolar disorder: a randomized controlled trial. J. Affect. Disord. 2015;174:23–30. doi: 10.1016/j.jad.2014.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donker T., Blankers M., Hedman E., Ljotsson B., Petrie K., Christensen H. Economic evaluations of internet interventions for mental health: a systematic review. Psychol. Med. 2015;45(16):3357–3376. doi: 10.1017/S0033291715001427. [DOI] [PubMed] [Google Scholar]
- Donkin L., Glozier N. Motivators and motivations to persist with online psychological interventions: a qualitative study of treatment completers. J. Med. Internet Res. 2012;14(3) doi: 10.2196/jmir.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan C.L., Poole C., Boyes N., Redgate J., March S. Australian mental health worker attitudes towards cCBT: what is the role of knowledge? Are there differences? Can we change them? Internet Interv. 2015;2(4):372–381. [Google Scholar]
- Ebert D., Tarnowski T., Gollwitzer M., Sieland B., Berking M. A transdiagnostic internet-based maintenance treatment enhances the stability of outcome after inpatient cognitive behavioral therapy: a randomized controlled trial. Psychother. Psychosom. 2013;82(4):246–256. doi: 10.1159/000345967. [DOI] [PubMed] [Google Scholar]
- Ebert D., Berking M., Cuijpers P., Lehr D., Pörtner M., Baumeister H. Increasing the acceptance of internet-based mental health interventions in primary care patients with depressive symptoms. A randomized controlled trial. J. Affect. Disord. 2015;176:9–17. doi: 10.1016/j.jad.2015.01.056. [DOI] [PubMed] [Google Scholar]
- Ebert D., Cuijpers P., Muñoz R.F., Baumeister H. Prevention of mental health disorders using internet- and mobile-based interventions: a narrative review and recommendations for future research. Front. Psych. 2017;8:116. doi: 10.3389/fpsyt.2017.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert D.D., van Daele T., Nordgreen T., Karekla M., Compare A., Zarbo C., Brugnera A., Øverland S., Trebbi G., Jensen K.L., Kaehlke F., Baumeister H. Internet- and mobile-based psychological interventions: applications, efficacy, and potential for improving mental health. Eur. Psychol. 2018;23(2):167–187. [Google Scholar]
- Eccleston C., Palermo T.M., Williams A.C.d.C., Lewandowski Holley A., Morley S., Fisher E., Law E. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst. Rev. 2014;5 doi: 10.1002/14651858.CD003968.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich M.J., Robinson C.T., Glovinsky D.B., Dixon L.B., Medoff D.R., Himelhoch S.S. Medical inpatients' adherence to outpatient psychiatric aftercare: a prospective study of patients evaluated by an inpatient consultation liaison psychiatry service. Int. J. Psychiatry Med. 2012;44(1):1–15. doi: 10.2190/PM.44.1.a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenberg C., Wolters C., Brähler E. The internet as a mental health advisor in Germany—results of a national survey. PLoS One. 2013;8(11) doi: 10.1371/journal.pone.0079206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbe D., Eichert H.-C., Riper H., Ebert D.D. Blending face-to-face and internet-based interventions for the treatment of mental disorders in adults: systematic review. J. Med. Internet Res. 2017;19(9) doi: 10.2196/jmir.6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichter M.M., Quadflieg N., Nisslmuller K., Lindner S., Osen B., Huber T., Wunsch-Leiteritz W. Does internet-based prevention reduce the risk of relapse for anorexia nervosa? Behav. Res. Ther. 2012;50(3):180–190. doi: 10.1016/j.brat.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Fichter M.M., Quadflieg N., Lindner S. Internet-based relapse prevention for anorexia nervosa: nine- month follow-up. J. Eat. Disord. 2013;1:23. doi: 10.1186/2050-2974-1-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischhacker W.W., Cetkovich-Bakmas M., de Hert M., Hennekens C.H., Lambert M., Leucht S., Maj M., McIntyre R.S., Naber D., Newcomer J.W., Olfson M., Osby U., Sartorius N., Lieberman J.A. Comorbid somatic illnesses in patients with severe mental disorders: clinical, policy, and research challenges. J. Clin. Psychiatry. 2008;69(4):514–519. doi: 10.4088/jcp.v69n0401. [DOI] [PubMed] [Google Scholar]
- Frederick R.J. Jossey-Bass; San Francisco (CA): 2013. Living Like You Mean It: Use the Wisdom and Power of Your Emotions to Get the Life You Really Want. [Google Scholar]
- Garner D.M., Vitousek K.M., Pike K.M. Cognitive-behavioral therapy for anorexia nervosa. In: Garner D.M., Garfinkel P.E., editors. Handbook of Treatment for Eating Disorders. The Guilford Press; London, New York: 1997. pp. 94–144. [Google Scholar]
- Geddes J.R., Carney S.M., Davies C., Furukawa T.A., Kupfer D.J., Frank E., Goodwin G.M. Relapse prevention with antidepressant drug treatment in depressive disorders: a systematic review. Lancet. 2003;361(9358):653–661. doi: 10.1016/S0140-6736(03)12599-8. [DOI] [PubMed] [Google Scholar]
- Gilbody S., Littlewood E., Hewitt C., Brierley G., Tharmanathan P., Araya R., Barkham M., Bower P., Cooper C., Gask L. Computerised cognitive behaviour therapy (cCBT) as treatment for depression in primary care (REEACT trial): large scale pragmatic randomised controlled trial. BMJ. 2015;351 doi: 10.1136/bmj.h5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grawe K. Research-informed psychotherapy. Psychother. Res. 1997;7(1):1–19. [Google Scholar]
- Grawe K. Hogrefe; Göttingen: 2000. Psychologische Therapie. [Google Scholar]
- Gulec H., Moessner M., Túry F., Fiedler P., Mezei A., Bauer S. A randomized controlled trial of an internet-based posttreatment care for patients with eating disorders. Telemed. J. E Health. 2014;20(10):916–922. doi: 10.1089/tmj.2013.0353. [DOI] [PubMed] [Google Scholar]
- Gun S.Y., Titov N., Andrews G. Acceptability of internet treatment of anxiety and depression. Australas. Psychiatry. 2011;19(3):259–264. doi: 10.3109/10398562.2011.562295. [DOI] [PubMed] [Google Scholar]
- Guyatt G.H., Oxman A.D., Vist G.E., Kunz R., Falck-Ytter Y., Alonso-Coello P., Schünemann H.J. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halmi K.A., Agras W.S., Mitchell J., Wilson G.T., Crow S., Bryson S.W., Kraemer H. Relapse predictors of patients with bulimia nervosa who achieved abstinence through cognitive behavioral therapy. Arch. Gen. Psychiatry. 2002;59(12):1105–1109. doi: 10.1001/archpsyc.59.12.1105. [DOI] [PubMed] [Google Scholar]
- Heber E., Ebert D.D., Lehr D., Cuijpers P., Berking M., Nobis S., Riper H. The benefit of web- and computer-based interventions for stress: a systematic review and meta-analysis. J. Med. Internet Res. 2017;19(2) doi: 10.2196/jmir.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedman E., Ljótsson B., Lindefors N. Cognitive behavior therapy via the internet: a systematic review of applications, clinical efficacy and cost–effectiveness. Expert Rev. Pharmacoecon. Outcomes Res. 2012;12(6):745–764. doi: 10.1586/erp.12.67. [DOI] [PubMed] [Google Scholar]
- Hedman E., Ljótsson B., Rück C., Bergström J., Andersson G., Kaldo V., Jansson L., Andersson E., Blom K., El Alaoui S., Falk L., Ivarsson J., Nasri B., Rydh S., Lindefors N. Effectiveness of internet-based cognitive behaviour therapy for panic disorder in routine psychiatric care. Acta Psychiatr. Scand. 2013;128(6):457–467. doi: 10.1111/acps.12079. [DOI] [PubMed] [Google Scholar]
- Hennemann S., Beutel M.E., Zwerenz R. Drivers and barriers to acceptance of web-based aftercare of patients in inpatient routine care: a cross-sectional survey. J. Med. Internet Res. 2016;18(12) doi: 10.2196/jmir.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennemann S., Beutel M.E., Zwerenz R. Ready for eHealth? Health professionals' acceptance and adoption of eHealth interventions in inpatient routine care. J. Health Commun. 2017;22(3):274–284. doi: 10.1080/10810730.2017.1284286. [DOI] [PubMed] [Google Scholar]
- Hennemann S., Farnsteiner S., Sander L. Internet- and mobile-based aftercare and follow-up for mental disorders: protocol of a systematic review and meta-analysis. BMJ Open. 2017;7(6) doi: 10.1136/bmjopen-2017-016696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J., Green S. 2011. The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions - Version 5.1.0. URL: http://handbook.cochrane.org/ [accessed 2018-10-24] [WebCite Cache ID 6jU6DK1il] [Google Scholar]
- Higginson I.J., Carr A.J. Using quality of life measures in the clinical setting. BMJ. 2001;322(7297):1297–1300. doi: 10.1136/bmj.322.7297.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holländare F., Johnsson S., Randestad M., Tillfors M., Carlbring P., Andersson G., Engstrom I. Randomized trial of internet-based relapse prevention for partially remitted depression. Acta Psychiatr. Scand. 2011;124(4):285–294. doi: 10.1111/j.1600-0447.2011.01698.x. [DOI] [PubMed] [Google Scholar]
- Holländare F., Anthony S.A., Randestad M., Tillfors M., Carlbring P., Andersson G., Engstrom I. Two-year outcome of internet-based relapse prevention for partially remitted depression. Behav. Res. Ther. 2013;51(11):719–722. doi: 10.1016/j.brat.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Hunkeler E.M., Hargreaves W.A., Fireman B., Terdiman J., Meresman J.F., Porterfield Y., Lee J., Dea R., Simon G.E., Bauer M.S., Unutzer J., Taylor C.B. A web-delivered care management and patient self-management program for recurrent depression: a randomized trial. Psychiatr. Serv. 2012;63(11):1063–1071. doi: 10.1176/appi.ps.005332011. [DOI] [PubMed] [Google Scholar]
- Internet Society Global Internet Report. 2016. http://bit.ly/2fQDYzm [Online]. Available at.
- Jacobi C., Paul T., Thiel A. Beltz; Weinheim: 2000. Kognitive Verhaltenstherapie bei Anorexia und Bulimia nervosa [Cognitive Behavioral Therapy for Anorexia Nervosa and Bulimia Nervosa] [Google Scholar]
- Jacobi C., Paul T., Thiel A. Hogrefe; Göttingen: 2004. Essstörungen [Eating disorders] [Google Scholar]
- Jacobi C., Beintner I., Fittig E., Trockel M., Braks K., Schade-Brittinger C., Dempfle A. Web-based aftercare for women with bulimia nervosa following inpatient treatment: randomized controlled efficacy trial. J. Med. Internet Res. 2017;19(9) doi: 10.2196/jmir.7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson R., Nyblom A., Carlbring P., Cuijpers P., Andersson G. Choosing between internet-based psychodynamic versus cognitive behavioral therapy for depression: a pilot preference study. BMC Psychiatry. 2013;13:268. doi: 10.1186/1471-244X-13-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joukamaa M., Heliövarra M., Knekt P., Aromaa A., Raitasalo R., Lehtinen V. Mental disorders and cause-specific mortality. Br. J. Psychiatry. 2001;179(6):498–502. doi: 10.1192/bjp.179.6.498. [DOI] [PubMed] [Google Scholar]
- Judd L.L., Akiskal H.S., Maser J.D., Zeller P.J., Endicott J., Coryell W., Paulus M.P., Kunovac J.L., Leon A.C., Mueller T.I., Rice J.A., Keller M.B. Major depressive disorder: a prospective study of residual subthreshold depressive symptoms as predictor of rapid relapse. J. Affect. Disord. 1998;50(2–3):97–108. doi: 10.1016/s0165-0327(98)00138-4. [DOI] [PubMed] [Google Scholar]
- Kampling H., Baumeister H., Jäckel W.H., Mittag O. Prevention of depression in chronically physically ill adults. Cochrane Database Syst. Rev. 2014;8 doi: 10.1002/14651858.CD011246.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampman O., Illi A., Poutanen P., Leinonen E. Four-year outcome in non-compliant schizophrenia patients treated with or without home-based ambulatory outpatient care. Eur. Psychiatry. 2003;18(1):1–5. doi: 10.1016/s0924-9338(02)00006-8. [DOI] [PubMed] [Google Scholar]
- Karyotaki E., Riper H., Twisk J., Hoogendoorn A., Kleiboer A., Mira A., Mackinnon A., Meyer B., Botella C., Littlewood E., Andersson G., Christensen H., Klein J.P., Schroder J., Breton-Lopez J., Scheider J., Griffiths K., Farrer L., Huibers M.J.H., Phillips R., Gilbody S., Moritz S., Berger T., Pop V., Spek V., Cuijpers P. Efficacy of self-guided internet-based cognitive behavioral therapy in the treatment of depressive symptoms: a meta-analysis of individual participant data. JAMA Psychiat. 2017;74(4):351–359. doi: 10.1001/jamapsychiatry.2017.0044. [DOI] [PubMed] [Google Scholar]
- Kelders S.M., Kok R.N., Ossebaard H.C., van Gemert-Pijnen J.E. Persuasive system design does matter: a systematic review of adherence to web-based interventions. J. Med. Internet Res. 2012;14(6) doi: 10.2196/jmir.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M.B., Boland R.J. Implications of failing to achieve successful long-term maintenance treatment of recurrent unipolar major depression. Biol. Psychiatry. 1998;44(5):348–360. doi: 10.1016/s0006-3223(98)00110-3. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Ormel J., Petukhova M., McLaughlin K.A., Green J.G., Russo L.J., Stein D.J., Zaslavsky A.M., Aguilar-Gaxiola S., Alonso J. Development of lifetime comorbidity in the World Health Organization world mental health surveys. Arch. Gen. Psychiatry. 2011;68(1):90–100. doi: 10.1001/archgenpsychiatry.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Petukhova M., Zaslavsky A.M. The role of latent internalizing and externalizing predispositions in accounting for the development of comorbidity among common mental disorders. Curr. Opin. Psychiatry. 2011;24(4):307. doi: 10.1097/YCO.0b013e3283477b22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J.P., Gerlinger G., Knaevelsrud C., Bohus M., Meisenzahl E., Kersting A., Röhr S., Riedel-Heller S.G., Sprick U., Dirmaier J., Härter M., Hegerl U., Hohagen F., Hauth I. Internetbasierte Interventionen in der Behandlung psychischer Störungen. Nervenarzt. 2016;87(11):1185–1193. doi: 10.1007/s00115-016-0217-7. [DOI] [PubMed] [Google Scholar]
- Klinkenberg W.D., Calsyn R.J. Predictors of receipt of aftercare and recidivism among persons with severe mental illness: a review. Psychiatr. Serv. 1996;47(5):487–496. doi: 10.1176/ps.47.5.487. [DOI] [PubMed] [Google Scholar]
- Klinkenberg W.D., Calsyn R.J. Race as a moderator of the prediction of receipt of aftercare and psychiatric hospitalization. Int. J. Soc. Psychiatry. 1997;43(4):276–284. doi: 10.1177/002076409704300405. [DOI] [PubMed] [Google Scholar]
- Kobelt A., Nickel L., Grosch E.V., Lamprecht F., Kunsebeck H.-W. Inanspruchnahme psychosomatischer Nachsorge nach stationarer rehabilitation [participation in psychosomatic outpatient care after inpatient rehabilitation] Psychother. Psychosom. Med. Psychol. 2004;54(2):58–64. doi: 10.1055/s-2003-812612. [DOI] [PubMed] [Google Scholar]
- Königbauer J., Letsch J., Doebler P., Ebert D., Baumeister H. Internet- and mobile-based depression interventions for people with diagnosed depression: a systematic review and meta-analysis. J. Affect. Disord. 2017;223:28–40. doi: 10.1016/j.jad.2017.07.021. [DOI] [PubMed] [Google Scholar]
- Kordy H., Wolf M., Aulich K., Bürgy M., Hegerl U., Hüsing J., Puschner B., Rummel-Kluge C., Vedder H., Backenstrass M. Internet-delivered disease management for recurrent depression: a multicenter randomized controlled trial. Psychother. Psychosom. 2016;85(2):91–98. doi: 10.1159/000441951. [DOI] [PubMed] [Google Scholar]
- Kraft S., Wolf M., Klein T., Becker T., Bauer S., Puschner B. Text message feedback to support mindfulness practice in people with depressive symptoms: a pilot randomized controlled trial. JMIR Mhealth Uhealth. 2017;5(5) doi: 10.2196/mhealth.7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuester A., Niemeyer H., Knaevelsrud C. Internet-based interventions for posttraumatic stress: a meta-analysis of randomized controlled trials. Clin. Psychol. Rev. 2016;43:1–16. doi: 10.1016/j.cpr.2015.11.004. [DOI] [PubMed] [Google Scholar]
- Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gotzsche P.C., Ioannidis J.P.A., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindhiem O., Bennett C.B., Rosen D., Silk J. Mobile technology boosts the effectiveness of psychotherapy and behavioral interventions: a meta-analysis. Behav. Modif. 2015;39(6):785–804. doi: 10.1177/0145445515595198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingam R., Scott J. Treatment non-adherence in affective disorders. Acta Psychiatr. Scand. 2002;105(3):164–172. doi: 10.1034/j.1600-0447.2002.1r084.x. [DOI] [PubMed] [Google Scholar]
- Lobban F., Dodd A.L., Sawczuk A.P., Asar O., Dagnan D., Diggle P.J., Griffiths M., Honary M., Knowles D., Long R., Morriss R., Parker R., Jones S. Assessing feasibility and acceptability of web-based enhanced relapse prevention for bipolar disorder (ERPonline): a randomized controlled trial. J. Med. Internet Res. 2017;19(3) doi: 10.2196/jmir.7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luborsky L. Basic Books; New York: 1984. Principles of Psychoanalytic Psychotherapy: A Manual for Supportive-Expressive (SE) Treatment. [Google Scholar]
- MacDonald L., Chapman S., Syrett M., Bowskill R., Horne R. Improving medication adherence in bipolar disorder: a systematic review and meta-analysis of 30 years of intervention trials. J. Affect. Disord. 2016;194:202–221. doi: 10.1016/j.jad.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Månsson K.N., Klintmalm H., Nordqvist R., Andersson G. Conventional cognitive behavioral therapy facilitated by an internet-based support system: feasibility study at a psychiatric outpatient clinic. JMIR Res. Protoc. 2017;6(8) doi: 10.2196/resprot.6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough L., Andrews S. Assimilative integration: short-term dynamic psychotherapy for treating affect phobias. Clin. Psychol. Sci. Pract. 2001;8(1):82–97. [Google Scholar]
- McFarlane T., Olmsted M.P., Trottier K. Timing and prediction of relapse in a transdiagnostic eating disorder sample. Int. J. Eat. Disord. 2008;41(7):587–593. doi: 10.1002/eat.20550. [DOI] [PubMed] [Google Scholar]
- Meader N., King K., Llewellyn A., Norman G., Brown J., Rodgers M., Moe-Byrne T., Higgins J.P.T., Sowden A., Stewart G. A checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation. Syst. Rev. 2014;3(1):82. doi: 10.1186/2046-4053-3-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melville K.M., Casey L.M., Kavanagh D.J. Dropout from internet-based treatment for psychological disorders. Br. J. Clin. Psychol. 2010;49(Pt 4):455–471. doi: 10.1348/014466509X472138. [DOI] [PubMed] [Google Scholar]
- Mitchell J.E., Agras W.S., Wilson G.T., Halmi K., Kraemer H., Crow S. A trial of a relapse prevention strategy in women with bulimia nervosa who respond to cognitive-behavior therapy. Int. J. Eat. Disord. 2004;35(4):549–555. doi: 10.1002/eat.10265. [DOI] [PubMed] [Google Scholar]
- Montes J.M., Medina E., Gomez-Beneyto M., Maurino J. A short message service (SMS)-based strategy for enhancing adherence to antipsychotic medication in schizophrenia. Psychiatry Res. 2012;200(2):89–95. doi: 10.1016/j.psychres.2012.07.034. [DOI] [PubMed] [Google Scholar]
- Mykletun A., Overland S., Dahl A.A., Krokstad S., Bjerkeset O., Glozier N., Aarø L.E., Prince M. A population-based cohort study of the effect of common mental disorders on disability pension awards. Am. J. Psychiatry. 2006;163(8):1412–1418. doi: 10.1176/ajp.2006.163.8.1412. [DOI] [PubMed] [Google Scholar]
- Nahum-Shani I., Smith S.N., Spring B.J., Collins L.M., Witkiewitz K., Tewari A., Murphy S.A. Just-in-Time Adaptive Interventions (JITAIs) in mobile health: key components and design principles for ongoing health behavior support. Ann. Behav. Med. 2016;52(6):1–17. doi: 10.1007/s12160-016-9830-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman M.G., Szkodny L.E., Llera S.J., Przeworski A. A review of technology-assisted self-help and minimal contact therapies for anxiety and depression: is human contact necessary for therapeutic efficacy? Clin. Psychol. Rev. 2011;31(1):89–103. doi: 10.1016/j.cpr.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Norlund F., Wallin E., Olsson G.E.M., Wallert J., Burell G., von Essen L., Held C. Internet-based cognitive behavioral therapy for symptoms of depression and anxiety among patients with a recent myocardial infarction: the U-CARE heart randomized controlled trial. J. Med. Internet Res. 2018;20(3) doi: 10.2196/jmir.9710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman C.D., Skinner H.A. eHEALS: the eHealth literacy scale. J. Med. Internet Res. 2006;8(4) doi: 10.2196/jmir.8.4.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted M.P., Kaplan A.S., Rockert W. Rate and prediction of relapse in bulimia nervosa. Am. J. Psychiatry. 1994;151(5):738–743. doi: 10.1176/ajp.151.5.738. [DOI] [PubMed] [Google Scholar]
- Olmsted M.P., Kaplan A.S., Rockert W. Defining remission and relapse in bulimia nervosa. Int. J. Eat. Disord. 2005;38(1):1–6. doi: 10.1002/eat.20144. [DOI] [PubMed] [Google Scholar]
- Olthuis J.V., Watt M.C., Bailey K., Hayden J.A., Stewart S.H. Therapist-supported internet cognitive behavioural therapy for anxiety disorders in adults. Cochrane Database Syst. Rev. 2015;3 doi: 10.1002/14651858.CD011565. [DOI] [PubMed] [Google Scholar]
- Paganini S., Teigelkotter W., Buntrock C., Baumeister H. Economic evaluations of internet- and mobile-based interventions for the treatment and prevention of depression: a systematic review. J. Affect. Disord. 2018;225:733–755. doi: 10.1016/j.jad.2017.07.018. [DOI] [PubMed] [Google Scholar]
- Paykel E.S., Brugha T., Fryers T. Size and burden of depressive disorders in Europe. Eur. Neuropsychopharmacol. 2005;15(4):411–423. doi: 10.1016/j.euroneuro.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Prince M., Patel V., Saxena S., Maj M., Maselko J., Phillips M.R., Rahman A. No health without mental health. Lancet. 2007;370(9590):859–877. doi: 10.1016/S0140-6736(07)61238-0. [DOI] [PubMed] [Google Scholar]
- Proudfoot J., Klein B., Barak A., Carlbring P., Cuijpers P., Lange A., Ritterband L., Andersson G. Establishing guidelines for executing and reporting internet intervention research. Cogn. Behav. Ther. 2011;40(2):82–97. doi: 10.1080/16506073.2011.573807. [DOI] [PubMed] [Google Scholar]
- Ramana R., Paykel E.S., Melzer D., Mehta M.A., Surtees P.G. Aftercare of depressed inpatients—service delivery and unmet needs. Soc. Psychiatry Psychiatr. Epidemiol. 2003;38(3):109–115. doi: 10.1007/s00127-003-0613-8. [DOI] [PubMed] [Google Scholar]
- Richards D., Richardson T. Computer-based psychological treatments for depression: a systematic review and meta-analysis. Clin. Psychol. Rev. 2012;32(4):329–342. doi: 10.1016/j.cpr.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Rollman B.L., Belnap B.H., Mazumdar S., Houck P.R., Zhu F., Gardner W., Reynolds C.F., Schulberg H.C., Shear M.K. A randomized trial to improve the quality of treatment for panic and generalized anxiety disorders in primary care. Arch. Gen. Psychiatry. 2005;62(12):1332–1341. doi: 10.1001/archpsyc.62.12.1332. [DOI] [PubMed] [Google Scholar]
- Rothstein H., editor. Publication Bias in Meta-Analysis: Prevention, Assessment and Adjustments. Wiley; Chichester: 2005. [Google Scholar]
- Sander L., Rausch L., Baumeister H. Effectiveness of internet-based interventions for the prevention of mental disorders: a systematic review and meta-analysis. JMIR Ment. Health. 2016;3(3) doi: 10.2196/mental.6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmädeke S., Bischoff C. Wirkungen smartphonegestützer psychosomatischer Rehabilitationsnachsorge (eATROS) bei depressiven Patienten [Effects of Smartphone-supported Rehabilitation Aftercare (eATROS) for depressive patients] Verhaltenstherapie. 2015;25(4):277–286. [Google Scholar]
- Schröder J., Sautier L., Kriston L., Berger T., Meyer B., Späth C., Köther U., Nestoriuc Y., Klein J.P., Moritz S. Development of a questionnaire measuring Attitudes towards Psychological Online Interventions-the APOI. J. Affect. Disord. 2015;187:136–141. doi: 10.1016/j.jad.2015.08.044. [DOI] [PubMed] [Google Scholar]
- Schueller S.M., Leykin Y., Pérez-Stable E.J., Muñoz R.F. Selection of intervention components in an internet stop smoking participant preference trial: beyond randomized controlled trials. Psychiatry Res. 2013;205(1):159–164. doi: 10.1016/j.psychres.2012.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz H., Barghaan D., Harfst T., Koch U. 2008. Psychotherapeutische Versorgung [Mental Healthcare]., Robert Koch-Institut, Gesundheitsberichterstattung des Bundes [Health report of Germany] [Google Scholar]
- Scott J., Colom F., Vieta E. A meta-analysis of relapse rates with adjunctive psychological therapies compared to usual psychiatric treatment for bipolar disorders. Int. J. Neuropsychopharmacol. 2007;10(1):123–129. doi: 10.1017/S1461145706006900. [DOI] [PubMed] [Google Scholar]
- Sibold M., Mittag O., Kulick B., Muller E., Opitz U., Jackel W.H. Pradiktoren der Teilnahme an einer Nachsorge nach ambulanter Rehabilitation bei erwerbstatigen Rehabilitanden mit chronischen Rückenschmerzen. Rehabilitation (Stuttg) 2011;50(6):363–371. doi: 10.1055/s-0031-1271815. [DOI] [PubMed] [Google Scholar]
- Simon G.E. Social and economic burden of mood disorders. Biol. Psychiatry. 2003;54(3):208–215. doi: 10.1016/s0006-3223(03)00420-7. [DOI] [PubMed] [Google Scholar]
- Smith D.J., Griffiths E., Poole R., Di Florio A., Barnes E., Kelly M.J., Craddock N., Hood K., Simpson S. Beating bipolar: exploratory trial of a novel internet-based psychoeducational treatment for bipolar disorder. Bipolar Disord. 2011;13(5–6):571–577. doi: 10.1111/j.1399-5618.2011.00949.x. [DOI] [PubMed] [Google Scholar]
- Španiel F., Hrdlička J., Novák T., Kožený J., Höschl C., Mohr P., Motlová L.B. Effectiveness of the information technology-aided program of relapse prevention in schizophrenia (ITAREPS): a randomized, controlled, double-blind study. J. Psychiatr. Pract. 2012;18(4):269–280. doi: 10.1097/01.pra.0000416017.45591.c1. [DOI] [PubMed] [Google Scholar]
- Steffanowski A. Huber; Bern: 2007. Meta-Analyse der Effekte stationärer psychosomatischer Rehabilitation: Mesta-Studie. [Google Scholar]
- Sterne J.A.C., Sutton A.J., Ioannidis J.P.A., Terrin N., Jones D.R., Lau J., Carpenter J., Rücker G., Harbord R.M., Schmid C.H., Tetzlaff J., Deeks J.J., Peters J., Macaskill P., Schwarzer G., Duval S., Altman D.G., Moher D., Higgins J.P.T. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- Topooco N., Riper H., Araya R., Berking M., Brunn M., Chevreul K., Cieslak R., Ebert D.D., Etchmendy E., Herrero R. Attitudes towards digital treatment for depression: a European stakeholder survey. Internet Interv. 2017;8:1–9. doi: 10.1016/j.invent.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torous J., Kiang M.V., Lorme J., Onnela J.-P., Eysenbach G. New tools for new research in psychiatry: a scalable and customizable platform to empower data driven smartphone research. JMIR Ment. Health. 2016;3(2) doi: 10.2196/mental.5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Välimäki M., Kannisto K.A., Vahlberg T., Hätönen H., Adams C.E. Short text messages to encourage adherence to medication and follow-up for people with psychosis (Mobile.Net): randomized controlled trial in Finland. J. Med. Internet Res. 2017;19(7) doi: 10.2196/jmir.7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ballegooijen W., Cuijpers P., van Straten A., Karyotaki E., Andersson G., Smit J.H., Riper H. Adherence to internet-based and face-to-face cognitive behavioural therapy for depression: a meta-analysis. PLoS One. 2014;9(7) doi: 10.1371/journal.pone.0100674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vaart R., Drossaert C. Development of the digital health literacy instrument: measuring a broad spectrum of health 1.0 and health 2.0 skills. J. Med. Internet Res. 2017;19(1) doi: 10.2196/jmir.6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gemert-Pijnen J.E., Nijland N., van Limburg M., Ossebaard H.C., Kelders S.M., Eysenbach G., Seydel E.R. A holistic framework to improve the uptake and impact of eHealth technologies. J. Med. Internet Res. 2011;13(4) doi: 10.2196/jmir.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittengl J.R., Clark L.A., Dunn T.W., Jarrett R.B. Reducing relapse and recurrence in unipolar depression: a comparative meta-analysis of cognitive-behavioral therapy's effects. J. Consult. Clin. Psychol. 2007;75(3):475–488. doi: 10.1037/0022-006X.75.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker E.R., McGee R.E., Druss B.G. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry. 2015;72(4):334–341. doi: 10.1001/jamapsychiatry.2014.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller R., Gilbody S. Barriers to the uptake of computerized cognitive behavioural therapy: a systematic review of the quantitative and qualitative evidence. Psychol. Med. 2009;39(5):705–712. doi: 10.1017/S0033291708004224. [DOI] [PubMed] [Google Scholar]
- Westermann S., Moritz S., Berger T. Internet- und mobilbasierte Interventionen bei Schizophrenie [Internet- and mobile-based intervention in schizophrenia] Verhaltenstherapie. 2017;27 [Google Scholar]
- Willems R.A., Bolman C.A.W., Mesters I., Kanera I.M., Beaulen A.A.J.M., Lechner L. Short-term effectiveness of a web-based tailored intervention for cancer survivors on quality of life, anxiety, depression, and fatigue: randomized controlled trial. Psycho-Oncology. 2017;26(2):222–230. doi: 10.1002/pon.4113. [DOI] [PubMed] [Google Scholar]
- Willems R.A., Mesters I., Lechner L., Kanera I.M., Bolman C.A.W. Long-term effectiveness and moderators of a web-based tailored intervention for cancer survivors on social and emotional functioning, depression, and fatigue: randomized controlled trial. J. Cancer Surviv. 2017;11(6):691–703. doi: 10.1007/s11764-017-0625-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K., Marlatt G.A. Relapse prevention for alcohol and drug problems: that was Zen, this is Tao. Am. Psychol. 2004;59(4):224–235. doi: 10.1037/0003-066X.59.4.224. [DOI] [PubMed] [Google Scholar]
- Yardley L., Morrison L., Bradbury K., Muller I. The person-based approach to intervention development: application to digital health-related behavior change interventions. J. Med. Internet Res. 2015;17(1) doi: 10.2196/jmir.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonkers K.A., Bruce S.E., Dyck I.R., Keller M.B. Chronicity, relapse, and illness—course of panic disorder, social phobia, and generalized anxiety disorder: findings in men and women from 8 years of follow-up. Depress. Anxiety. 2003;17(3):173–179. doi: 10.1002/da.10106. [DOI] [PubMed] [Google Scholar]
- Zwerenz R., Gerzymisch K., Edinger J., Holme M., Knickenberg R.J., Spörl-Dönch S., Kiwus U., Beutel M.E. Evaluation of an internet-based aftercare program to improve vocational reintegration after inpatient medical rehabilitation: study protocol for a cluster-randomized controlled trial. Trials. 2013;14(1):26. doi: 10.1186/1745-6215-14-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwerenz R., Becker J., Gerzymisch K., Siepmann M., Holme M., Kiwus U., Spörl-Dönch S., Beutel M.E. Evaluation of a transdiagnostic psychodynamic online intervention to support return to work: a randomized controlled trial. PLoS One. 2017;12(5) doi: 10.1371/journal.pone.0176513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwerenz R., Becker J., Johansson R., Frederick R.J., Andersson G., Beutel M.E. Transdiagnostic, psychodynamic web-based self-help intervention following inpatient psychotherapy: results of a feasibility study and randomized controlled trial. JMIR Ment. Health. 2017;4(4) doi: 10.2196/mental.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwerenz R., Becker J., Knickenberg R.J., Siepmann M., Hagen K., Beutel M.E. Online self-help as an add-on to inpatient psychotherapy. Efficacy of a new blended treatment approach. Psychother. Psychosom. 2017;86(6):341–350. doi: 10.1159/000481177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
Search term.
GRADE ratings for selected outcomes.
List of abbreviations of measurement instruments of included studies.