Abstract
The concept of nanomedicine is not new. For instance, some nanocrystals and colloidal drug molecules are marketed that improve pharmacokinetic characteristics of single-agent therapeutics. For the past two decades, the number of research publications on single-agent nanoformulations has grown exponentially. However, formulations advancing to pre-clinical and clinical evaluations that lead to therapeutic products has been limited. Chronic diseases such as cancer and HIV/AIDS require drug combinations, not single agents, for durable therapeutic responses. Therefore, development and clinical translation of drug combination nanoformulations could play a significant role in improving human health. Successful translation of promising concepts into pre-clinical and clinical studies requires early considerations of the physical compatibility, pharmacological synergy, as well as pharmaceutical characteristics (e.g. stability, scalability and pharmacokinetics). With this approach and robust manufacturing processes in place, some drug-combination nanoparticles have progressed to non-human primate and human studies. In this article, we discuss the rationale and role of drug-combination nanoparticles, the pre-clinical and clinical research progress made to date and the key challenges for successful clinical translation. Finally, we offer insight to accelerate clinical translation through leveraging robust nanoplatform technologies to enable implementation of personalised and precision medicine.
Keywords: Nanoparticle, combination therapy, HIV, AIDS, cancer, targeted drug delivery, long-acting, drug-combination nanoparticle, drug targeting
Introduction
The overarching goal of a drug dosage form is to provide patients with sufficient exposure of therapeutic agents to create a pharmacologic action in target organs, tissues or cells that is both effective and safe [1]. Advances are being made towards defining potential drug targets linked to specific disease states and the subsequent development of compounds highly selective for these targets. However, most of these drug targets are typically expressed intracellularly and may not be readily accessible following parenteral or oral administration. Moreover, drug exposure, a measure of time and concentration at a site of action, dictates the efficacy and safety of drug products. It is the exposure of drugs at a site of action that generates therapeutic effect, while exposure in off-target tissues is often linked to adverse effects. Thus, in order to deliver active pharmaceutical ingredients (APIs) in a formulation from the site of administration, such as the gastrointestinal (GI) tract for oral dosing or the subcutaneous space for parenteral dosing, to the target, the formulation should be engineered in a manner that provides a consistent dose with predictable exposure [2].
It is commonly thought that therapeutic compounds must be sufficiently hydrophilic (water soluble) to dissolve in aqueous physiological milieus and also carry hydrophobic (water insoluble) domains or motifs to absorb through lipid membranes of cells that line sites of absorption into the blood circulation [3]. Pharmaceutical innovation and excipients have been used to attenuate some of the challenging properties of certain compounds. For example, the use of surfactants improves the solubility profile of hydrophobic APIs, while the use of permeation enhancers improves the absorption of hydrophilic APIs [4]. Unfortunately, even after overcoming these initial hurdles, the toxicity and safety profiles of these compounds as well as the permeation enhancers can still be limiting. Such limitations may manifest as off-target toxicity in vital organs associated with drug excretion and elimination, such as the liver and kidneys. It is in this context that targeted drug delivery could provide the greatest value. Targeted drug delivery is defined as the distribution of therapeutic agents to specific sites of interest where higher concentrations are achieved relative to peripheral or off-target tissues [5,6]. By specifically targeting drug delivery, the fraction of drug exposure in the off-target tissue can be minimised while the on-target exposure is maximised to provide optimal efficacy with high safety margins.
Recent technological advances have allowed for the development of sophisticated drug targeting and delivery systems aimed at impacting a number of disease states. Various approaches – including molecular engineering, molecular optimisation, nanoformulation and sustained release products – have improved clinical outcomes and have become major businesses of interest [7]. Such innovative technologies not only play an important role in patient life and quality-of-life extension, but have also been clinically impactful. For instance, tyrosine kinase inhibitors are classic examples of molecular engineering that improved drug targeting. Chronic myeloid leukaemia (CML) is a disease characterised by a chromosomal rearrangement that results in the overexpression of a fusion kinase protein known as BCR-ABL [8]. Overexpressed BCR-ABL kinase is linked to the dysregulated and proliferative spread of CML cells. The development of kinase inhibitors for CML was the result of combining this growing understanding of the pathobiology of CML together with molecular engineering approaches to target drug towards BCR-ABL receptors. The success of this approach is evident in the fact that since the approval of the first kinase inhibitor (imatinib) in 2001, the overall 8-year survival rate for CML has increased by about 20% from 0.6 in the 1990s to 0.8 in the 2000s [9].
However, although the developments of targeted and sustained release products are innovative approaches to drug delivery, the majority of these formulations are single agents. Because drug response in target cells may rapidly evolve to develop drug resistance, monotherapy with these formulations is a significant limitation. Separate administration of multiple sustained release products may overcome drug resistance, but this may be challenging to implement due to factors such as competing clearances and different cellular and tissue distribution of each compound. Moreover, the physiochemical properties of pharmaceutical compounds can also vary widely, which can impede sufficient accumulation of all compounds at the same target site if they each distribute through the body independently. In this regard, an important distinction could be made between sustained release and long-acting products. While sustained release products have the potential to ameliorate plasma fluctuations of drugs, tissue concentrations at the sites of interest may not always reflect plasma levels. Thus, a drug product can have sustained release into the plasma but not a long-acting profile at the site of action within a cell or target tissue. Both sustained release and targeted therapy approaches have been used in the context of HIV and cancer, but tissue drug insufficiency and drug resistance have persisted as a barrier to disease eradication [10,11].
Despite the innovations to date in drug therapies, HIV and cancer have persisted as chronic and major diseases where targeted and long-acting drug delivery systems could make significant impacts. In order to further improve the clinical outcomes of these difficult-to-treat diseases with fairly well-defined molecular targets, a combination of multiple agents delivered together (as a single combined unit) to target tissues and cells is of interest. The delivery of these agents must be done with sufficient quantity and duration to maximally reduce the risk of drug resistance and to clear the occult virus or cells within the body. In this review, we discuss the traditional approaches to targeted drug delivery and the aforementioned need for combination long-acting therapy. We describe the state of current nanoparticle (NP)-based combination chemotherapy, the advent of long-acting drug combination nanoparticles (DcNPs), and propose future directions of nanomedicines and their application in precision medicine.
Traditional approaches to drug delivery and the need for long-acting drug combination therapy
Obstacles of oral dosage forms
Oral dosage forms have long been the predominant focus in the formulation of APIs. Solid tablets and capsules have inherent advantages over parenteral products, which include long shelf-life and patient preference. However, these dosage forms also carry a number of technical and practical limitations. Many pharmaceutical compounds do not have compatible physicochemical properties for dissolution in the GI tract. Following dissolution, pharmaceutical compounds in the GI tract must be able to penetrate cell membranes for absorption into the systemic circulation. Free drug is also subject to first-pass metabolism which may occur in the GI and liver, and a sufficient fraction of drug must make it into the systemic circulation (and ultimately the site of action) for therapeutic effect to occur. Several technologies have been developed to ameliorate these limitations. For example, amorphous solid dispersions have been used extensively to improve the aqueous solubility of hydrophobic compounds [12]. Permeation enhancers can be used to improve absorption across cell membranes [4]. Pharmacokinetic boosting, where a drug is taken concomitantly with a cytochrome P450 inhibitor, can be used to reduce first-pass metabolism and has been useful in the context of HIV therapy with lopinavir/ritonavir combination [13]. These methods have been successful in addressing barriers that prevent APIs from accessing the systemic circulation. However, in many chronic conditions, access to the systemic circulation is not the only factor to consider. Oncologic and infectious diseases were responsible for a combined total of 24% deaths in the US in 2015 [14]. These two disease states are inherently difficult to treat due to the high prevalence of drug resistance. In order to counteract resistance, a combination chemotherapeutic approach (e.g. fixed-dose oral HIV drug combinations or oral and IV combination chemotherapy) is often used. By targeting multiple points of infectious or oncogenic replication cycles, the likelihood of developing drug resistance mutations is decreased.
Another source of potential drug resistance can be due to variance in expression of transporter proteins in the GI such as Pglycoprotein (P-gp), which was initially discovered as a multi-drug resistance gene in leukaemia [15,16]. These proteins can cause overall reduction in drug bioavailability. Cancer cells may also overexpress efflux transporters under selection pressure that lead to drug resistance. P-gp inhibitors have been used to overcome drug resistance and improve intracellular drug levels, but these compounds have been associated with significant liver toxicity [17]. Other approaches include associating P-gp substrates to nanoparticles, which has been shown in vitro to reduce drug efflux [18].
Challenges in combination cancer therapy
To overcome drug resistance, a more common practice in clinic is the combination of multiple agents that have diverse molecular targets. These drugs are administered separately into the body and improve the overall therapeutic outcome compared to monotherapy [19]. However, certain challenge still exists for combination therapy. For cancer therapy, the administration of multiple cytotoxic agents can be difficult to bear for patients. The delicate balance between minimum effective dose and maximum tolerable dose becomes challenging to maintain when multiple agents can be responsible for the observed toxicity. Concomitant administration of multiple agents is further complicated by the divergent physicochemical properties of the cytotoxic agents. These divergent properties translate into variable disposition and clearance mechanisms and thus different concentration–time courses for each cytotoxic agent. As a result, the use of combination therapy may not produce the intended results because simultaneous exposure may occur in the systemic circulation after IV administration but synchronised exposure in the tumour and in cancerous cells may be transient due to distinct biodistribution, cellular uptake and clearance profiles of each drug. To date, several techniques have been employed to improve the delivery of single chemotherapeutic agents. For example, incorporation of anthracycline derivatives into liposomes has been used to increase the plasma half-life and reduce off-target tissue exposure [20–23]. The development of tyrosine kinase inhibitors in conjunction with tumour specific genotyping has been used to minimise toxicity by limiting binding to those receptors that are known to be upregulated in a tumour [24–26]. However, while these monotherapy approaches have been promising, drug resistance persists as an issue. As a result, additional innovation in drug delivery that harnesses the advantages of drug combination therapy may be particularly beneficial for cancers [27].
Challenges in combination anti-infective therapy
As with anti-cancer therapy, drug resistance is also a common phenomenon in anti-infective therapy. Based on the understanding of the drug resistance mechanisms of bacteria and viruses, therapeutic regimens are modified accordingly [28,29]. A good example of an antimicrobial formulation that has been modified to overcome drug resistance is the fixed dose combination or amoxicillin and clavulanic acid. This drug combination addresses the well-known mechanism of resistance for gram positive bacteria that involves the expression of beta-lactamase. This enzyme degrades beta lactam-based antibiotics prior to any therapeutic effect. To prevent this, clavulanic acid was combined with amoxicillin (a beta lactam antibiotic) to produce a fixed dose combination where clavulanic acid irreversibly inhibits beta lactamase and improves the efficacy of amoxicillin [30]. Similarly, this concept of inhibiting the metabolic clearance of an API has also been used in the treatment of HIV, as mentioned above. Although combination therapy has improved the clinical outcomes of patients with infections, the rise of multi-drug resistant organisms still poses a significant threat to public health. While new chemical entities are being developed to address these organisms, novel drug delivery approaches may also aid in attenuating this issue. As with cancer therapy, concomitant administration of anti-infective agents may not distribute to the same tissues and may also be eliminated through various clearance mechanisms at different rates. Moreover, anti-infective agents can vary in the pharmacokinetic parameters that constitute efficacy (antibiotics, for instance, can have time-dependent, concentration-dependent or AUC-dependent killing). In addition, treatment of chronic infections relies heavily on patient adherence, and as pill burden increases, this can also become a factor in treatment failure [31].
In the context of cancer and infectious diseases, drug efficacy is a function of both concentration and time, not only in the plasma, but also at the specific site of a tumour or infection. Although the practice of combination therapy has been applied widely, achieving synchronous peak and trough concentrations in plasma of combination regimens in either disease state is challenging; examples are given in Table 1. Furthermore, sustained therapeutic levels of drugs in plasma and at sites of action may also be difficult to achieve through oral and IV administration. Therefore, long-acting targeted combination therapies that leverage a single DcNP entity may improve the current paradigm by sustaining the levels of multiple APIs at specific sites of interest.
Table 1.
Asynchronous pharmacokinetic profile of drug combinations typically used to treat chronic disease states.
| Asynchronous pharmacokinetic parameters | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Disease indication | Drug | Log P | Dose | Regimen | Tmax | T1/2 |
| HIV | Emtricitabine | −0.6 | 200 mg | PO QD | 3 h | 10 h |
| Rilpivirine | 4.5 | 25 mg | PO QD | 4 h | 50 h | |
| Tenofovir alafenamide | 1.9 | 25 mg | PO QD | 1 h | 0.51 h | |
| Cancer | Doxil (liposomal doxorubicin) | 1.3 | 60 mg/m2 | IV | – | 55 h at 20 mg/m2 |
| Cyclophophamide | 0.6 | 600 mg/m2 | IV | – | 3–12 h | |
| Hepatitis C | Sofosbuvir | 1 | 400 mg | PO QD | 0.8–1 h (prodrug); 3.5–4 h (active) | 0.4 h (prodrug); 27 h (active) |
| Ledipasvir | 7.4 | 90 mg | PO QD | 4–4.5 h | 47 h | |
Current status of nanoparticle-based combination chemotherapy
The assembly of multiple drugs into a single particle may enable a synchronous combination therapy with an improved likelihood of API persistence in target cells. This approach may require assembly of drugs with different physicochemical characteristics (e.g. hydrophobic and hydrophilic molecules) together in one particle. This is often the case as many of the nucleoside and nucleotide analogues for anti-HIV therapy (e.g. tenofovir) or chemotherapeutics (e.g. 5-flurouricil) are water soluble (hydrophilic), and other drugs used in combination (e.g. raltegravir or paclitaxel) are highly water insoluble (hydrophobic). Therefore, a DcNP platform must be able to accommodate both water soluble and insoluble drugs to produce a stable and scalable pharmaceutical product that exhibits targeted and long-acting characteristics in vivo. NP drug delivery systems with such characteristics can then produce optimal long-acting pharmacokinetic profiles and consistent delivery of APIs to defined cancer or infected occult target cells. These DcNPs could help overcome drug resistance that may occur in monotherapy, regardless of whether single agents are administered orally, as an injectable, or as other long-acting dosage forms [32,33].
Analysis of nanoparticle drug delivery systems in literature and clinical trials
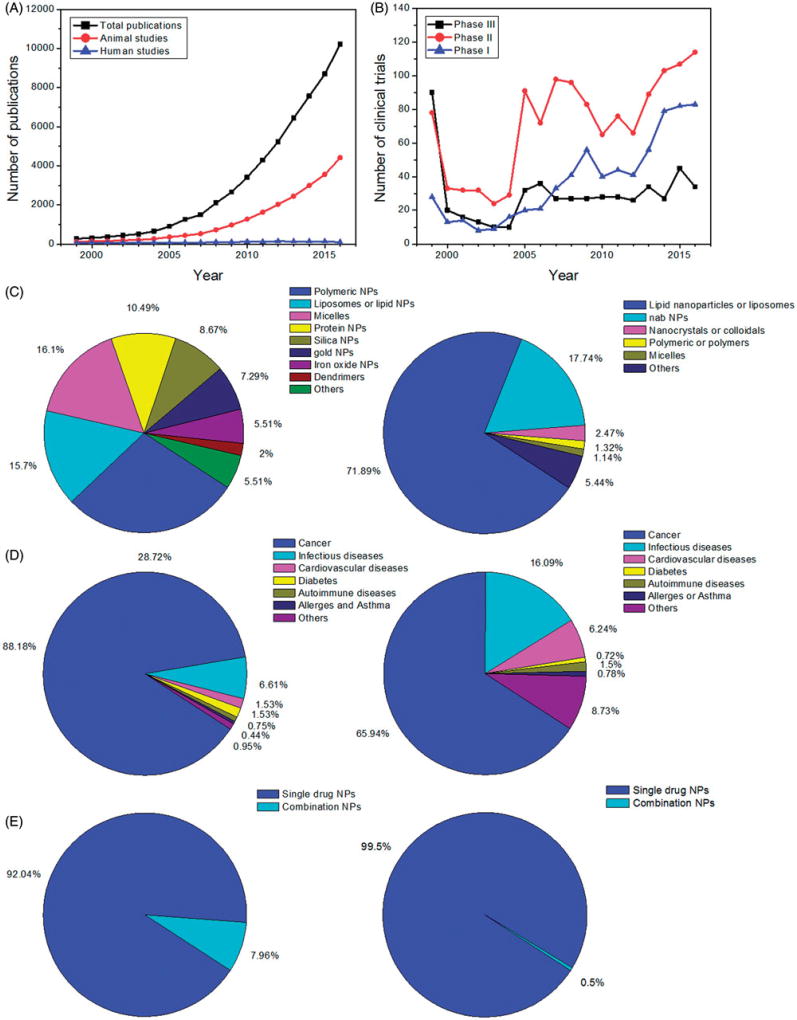
Through recognising the potential application of nano-drug particle technology to deliver and target drugs to specific sites of action, and also more recently the potential of long-acting targeted drug delivery systems, there has been substantial research on these topics as reflected by the continued growth in the number of peer reviewed manuscripts published over the past few years. The number of scientific publications in drug delivery using NPs has increased exponentially, from less than 300 in 1999 to over 10,000 per year in 2016 (Figure 1(A)). Publications that include in vivo studies, which indirectly reflect some degree of optimisation and scalability of nano-drug delivery systems, accounted for ~40% of NP drug delivery publications in 2016. Overall, the number of publications citing clinical trials, which suggests progression to clinical development, are low and have been around 150 per year for the past two decades. Thus, publications with clinical data have not increased over time, while in contrast non-clinical study reports continue to increase at an exponential rate.
Figure 1.
Literature and clinical trial analysis of NP-based drug delivery studies. (A) Numbers of scientific publications from 1999 to 2016 (data obtained from Web of Science and Pubmed). (B) Numbers of clinical trials from 1999 to 2016 (data obtained from clinicaltrials.gov). (C–E) Types of materials (C), diseases (D) and single/combination NPs (E) in NP drug delivery studies (left: publications; right: clinical trials). Keywords for search in clinical trials: nanoparticles OR liposomes OR lipid nanoparticles OR polymeric nanoparticles OR polymer nanoparticles OR micelles OR nanocrystals OR nano OR nab. For search in Web of Science, the term ‘drug delivery’ was added in combination with above keywords (with ‘AND’ command).
This exponential rate of increase in materials innovation and in vivo demonstration of NP utility, and yet stagnant output of related clinical data, could be due to a number of factors including the stability, scalability, reproducibility, robustness and cost of nanomaterials. Biocompatibility and rapid clearance could reduce the likelihood of advancing a particular NP formulation into clinical development, which often takes many years and the investment of multiple millions of dollars. It also takes several years before the results from clinical studies are published. For these reasons, only a few and the most promising nanomedicine candidates are developed and eventually appear in clinical publications.
Taking advantage of unique physicochemical properties of nanoscale materials with the promise to provide sustained or targeted drug delivery, many researchers have focussed on chemical and biophysical approaches to produce new nanomaterials. These materials are generally less than 1000 nm in diameter and most are 200 nm or less. They are often spherical in shape, although some have investigated the effect of various shapes and sizes on cell uptake and disposition. The nanomaterials are derived from assembly of proteins, lipids, carbohydrates, dendrimers and other polymers or inorganic materials such as mesoporous carbon, clay, iron, gold, etc. Other material innovations include added ligands, as chelates and receptors for selectivity, specificity and multifunctionality for concurrent multimodal diagnostic (e.g. via CT/MRI/PET imaging) and therapeutic entities (so called ‘theranostics’). The most common nanocomposite materials in publications include polymers, liposomes or lipid membrane vesicles, micelles and protein (and peptide) aggregates. The relative distribution of these materials is presented in Figure 1(C). Some of these materials could add value to APIs in traditional drug delivery systems by extending their blood circulation time, controlling or sustaining their release from NPs, targeting APIs to select tissue and providing biocompatibility for APIs [34–36].
In vivo models (e.g. solid tumours in mice) are often used as a proof-of-principle for evaluating the effectiveness of nano-drug delivery systems (Figure 1(D)). Some of the most promising nano-drug delivery systems have progressed to in vivo studies with appropriate animal models. Among these reported studies, only ~8% contain more than one drug, and could be considered as combination therapeutic approaches (Figure 1(E)). It is important to note that this article focuses only on small molecule chemical drugs. There are other therapeutics such as siRNA, anti-sense, miRNA, proteins and other therapeutic combinations assembled into a single or multiple formulations that can be considered as a nanomedicine. Such studies were reviewed recently by Huang and colleagues and readers are referred there for additional details [37]. If one excludes those combination therapies that contain drug treatment in conjunction with other modalities such as radio-, immuno-, gene- and photothermal-therapy, the percentage of DcNP studies that have actually progressed to in vivo characterisation would likely be lower than the 8% cited above.
Clinical translation of nanomedicine requires considerations of product scale-up, diverse disease phenotypes in human populations, safety, cost and meeting prescribed FDA regulatory standards for efficacy and safety [38]. Some believe that the diverging criteria and challenges in product scale-up (as well as other factors associated with developing nano-drug products) have widened the gap between materials engineering and clinical translation of promising nano-products from bench-top to clinical trials (Figure 1(B)). There have been no significant increases in clinical trials per year in the past decade, highlighting the importance of shortening the gap between academic pre-clinical research and approval for use in humans (Figure 1(B)). Due to a higher degree of complexity in engineering and scaling as well as evaluation in developing drug-combination pharmaceutical product candidates, very few DcNPs (~0.5%, Figure 1(E)) are undergoing clinical testing. While drug efficacy has improved over the years, primarily through molecular optimisation, chronic disease such as cancer and HIV still require novel treatment regimens composed of multiple agents.
As noted above, many NP systems contain a single drug agent that targets only one mechanism, and thus progressive diseases (cancer, chronic infections, etc.) can easily develop resistance through survival pathways [39,40]. Therefore, combination regimens are commonly used to treat cancer and HIV/AIDS. For example, due to high rate of viral mutation and escape of immune control, monotherapy for inhibition of HIV replication is not sufficient to provide durable viral suppression. Drug-resistant viruses emerge soon after chronic monotherapy is initiated [40]. Thus, HIV patients are treated with a drug combination often referred to as combination anti-retroviral therapy (cART). cART involves a therapeutic regimen that combines together two or more of the following APIs: nucleoside reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), protease inhibitors (PIs) or integrase strand transfer inhibitors (INSTIs) [41,42]. These drugs, in combination (either in separate tablets or in fixed-dose combination tablets), are taken one or more times daily due to rapid and differing rates of drug clearance. Currently, various drug combinations in cART provide durable viral suppression in HIV patients; implementation of cART has resulted in extending life-expectancy by ~10 years with HIV/AIDS now being a chronically managed disease [43,44]. However, residual viruses remain in tissues in patients who are taking cART and are adherent to the daily oral cART regimen. It is well-documented that viral rebound occurs upon cessation of drug therapy. Importantly, viral rebound following cART is not solely attributed to drug resistance, but it is also linked to residual HIV in tissues such as lymph nodes, where a majority of HIV host T cells reside, and insufficient drug exposure is present in these tissues [10,45,46]. Another limiting factor that negates the success of cART is that patients on daily, life-long oral therapy often experience pill fatigue and thus adherence to such drug regimens is challenging. Thus, it is of great interest to develop DcNPs as potent therapeutics to maximally improve patient care and clinical outcomes. To address pill fatigue, ideally DcNP therapy would not only be designed to improve drug persistence and penetration in tissues, but it would also exhibit long-acting pharmacokinetics so that patient adherence could improve.
Considerations in research and development of drug combination nanoparticles
The above analysis highlights the importance and the need for research and development of combination NP systems that are robust, stable and scalable with sufficient safety so that these systems could be translated into product candidates for preclinical and clinical studies. The incorporation two or more APIs into one nano-composite requires consideration of (1) physical, (2) pharmacological and (3) pharmaceutical aspects of the drug and excipient constituents.
For physical considerations, the challenges have been how to incorporate drugs with varying degrees of water solubility (which is related to pH and lipophilicity as well as degree of ionisation at physiological pH). While hydrophobic excipients such as fatty acids, lipids and poly(lactate-co-glycolate) (PLGA) polymers are used to encapsulate or incorporate water insoluble drugs such as paclitaxel, and lipid membranes and hydrogels are used to encapsulate water soluble drugs such as cytarabine, formulation of both soluble and insoluble drugs into these nanomaterials has been challenging due to their disparate physical characteristics.
-
Pharmacological considerations for drug combinations include overall drug potency, the development of drug resistance and synergistic effects on target cells. Evaluation of drug resistance is challenging and requires long-term evaluation in pre-clinical and clinical settings. However, the synergistic impact on target cells can be evaluated and optimised pre-clinically via selection of appropriate drug combinations (e.g. drugs that have distinct and complementary pharmacological mechanisms and pharmacokinetic profiles) and ratios (e.g. optimised for the best additive or synergistic effect). Regarding the later, the combination index (CI) parameter is used to evaluate drug interactions with respect to whether two drugs exhibit synergy at a specific molar ratio. CI can be calculated by the following equation:
In Equation (1), IC50,A(combi) and IC50,B(combi) are the concentrations of drug A and drug B used in combination to achieve 50% inhibition. IC50,A(single) and IC50,B(single) are the concentrations for single agents to achieve the same effect. A CI of less than, equal to and more than 1 indicates synergy, additivity and antagonism, respectively [47]. This equation can also be expanded to combinations of three or more drugs.(1) While the pharmacology of drug targets could provide a theoretical basis for synergy and qualitative estimation of drug resistance, there are other factors, including cell uptake, transport, elimination, as well as cellular drug exposure and stability, that may modify these actual pharmacological effects. Thus, evaluations using cell-based drug-combination assays are a critical component of early DcNP testing. In this context, the CI provides a quantitative metric in evaluating drug combination synergy assessment. Varying the ratios of selected drug combinations could similarly be evaluated and their resultant CI could inform the final drug combination selection. After successful assembly of the chosen drug combination at a specific molar ratio in NP carriers, they should be tested both in vitro and in vivo to verify and validate the synchronous uptake and synergistic effects in the NP form.
Pharmaceutical (stability, scalability and pharmacokinetic) characteristics of DcNPs are key to place a promising drug-combination nanoformulation on the path for clinical translation and product development. The method of preparation for initial physicochemical studies of DcNPs must be suitable for translation into a protocol-driven, scalable pharmaceutical manufacturing process so that clinical translation is feasible. Some of the more complex, multiple-emulsion and harsh solvents associated with DcNP formulation may not be suitable for product scale-up. In addition, the cost of complex, multiple-step processes that may include solvent disposal and drug wastage concerns may lead to premature project termination, which highlights the necessity of pharmaceutical characteristic consideration early in the course of clinical development.
Although the concept of combination therapy in nanomedicine research has existed for several years, only a few formulations are on the path for clinical translation, and some have recently been approved. CPX-1 and CPX-351 are examples of liposome-based DcNP formulations for cancer chemotherapy that are being translated into nanomedicine. CPX-1 contains drugs irinotecan and floxuridine and CPX-351 contains daunorubicin and cytarabine [48]. Both CPX-1 and CPX-351 employ a similar liposomal platform (enclosed lipid bilayer capable of encapsulating water soluble drugs and insoluble drug precipitates) to carry two drugs. These liposomes are composed of distearoylphosphatidycholine (DSPC) and distearolyphosphatidygylcerol (DSPG) as excipients to produce DcNPs exhibiting ~100 ± 20 nm diameters (Table 2). In human studies, the accumulation ratio of the chemotherapeutics irinotecan and floxuridine was found to be proportional to the initial formulation ratio (1:1 molar) [49].
Table 2.
Examples of NP-based combination chemotherapy in chronic diseases in mice, rats, primates and humans.
| Drug combinationa (Log P) | Drug ratio (w/w)b | NP composition | NP size (nm)c | Disease state | References |
|---|---|---|---|---|---|
| Human studies | |||||
| Cytarabine (−2.8), Daunorubicin HCl (1.83) | ~2.27/1 | DSPC, DSPG, cholesterol | 100 ± 20 | Acute myeloid leukaemia | [48,74] |
| Floxuridine (−1.16), Irinotecan HCl (3.2) | ~1/2.75 | DSPC, DSPG, cholesterol | 100 ± 20 | Colorectal cancer | [48,49] |
| Primate studies | |||||
| Lopinavir (5.94), Ritonavir (4.24), Tenofovir (−1.6) | 1/1/0.5 | DSPC, DSPE-mPEG2000 | ~52 | HIV/AIDS | [53,57] |
| Rat studies | |||||
| Cisplatin (−2.19), Docetaxel (2.4) | 2/1 | Poly(l-glutamic acid)-PEG | 28 ± 9 | Melanoma | [75] |
| Doxorubicin HCl (1.27), Gemcitabine (−1.4) | 1/1.12 | HPMA | 23.5 kDa | Prostate carcinoma | [76] |
| Mouse studies | |||||
| 10-hydroxycamptothecin (1.69), Doxorubicin HCl (1.27) | 1/1.24 | PBA-PEG-ss-PCL | 143 ± 3 | Breast cancer, lung metastasis | [77] |
| 5-Fluorouracil (−0.89), Oxaliplatin (−0.47) | 5/1 | PLA | ~300 | Colorectal cancer | [78] |
| 5-Fluorouracil (−0.89), Paciltaxel (3.0) | 3.75/1 | Vitamin E, TPGS | 82 | Cervical cancer | [79] |
| Axitinib (4.56), Celastrol (5.9) | N/A | Mesoporous silica, DSPE-mPEG2000 | 120 | Squamous carcinoma | [80] |
| BLZ-945 (3.4), Cisplatin (−2.19) | 2.5/1 | PEG-b-PAEMA-PAMAM | ~90 | Breast cancer and metastasis | [81] |
| Cisplatin (−2.19), Doxorubicin HCl (1.27) | 1/1 | PAA | ~100 | Breast cancer | [82] |
| Cisplatin (−2.19), Gemcitabine monophosphate (−0.12) | 1/5.8 | DOPA, PLGA-PEG | ~120 | Bladder cancer | [50] |
| Cisplatin (−2.19), Paclitaxel (3.0) | 3.78/1 | PEG, poly(l-Glu), poly(l-Phe) | 95 ± 3 | Ovarian cancer | [83] |
| Cisplatin (−2.19), Paclitaxel (3.0) | 2/1 | PEG5K(COOH)8-L-CA8 | 16.9 ± 4.8 | Lymphoma, ovarian cancer | [84] |
| Cisplatin (−2.19), Rapamycin (4.3) | 2/1 | PLGA-PEG-MBA, DOPA | 80 | Melanoma | [51] |
| Camptothecin (1.74), Doxorubicin HCl (1.27), Oxaliplatin (−0.47) | 3/2/5 | PC and PEG macromonomers | 21 ± 6 | Ovarian cancer | [85] |
| Combretastatin-A4 (3.7), Doxorubicin (1.27) | 100/1 | PLGA, PC, DSPE-PEG-2000, cholesterol | 180–200 | Melanoma, Lewis lung carcinoma | [86] |
| Combretastatin-A4 (3.7), Doxorubicin HCl (1.27) | 1/4 | DSPE-PEG5000, oleic acid, Fe3O4 | 53.7 ± 5.3 | Breast cancer | [87] |
| Combretastatin-A4 (3.7), Methotrexate (−1.85) | 1/1.67 | Pullulan | 200–250 | Hepatocellular carcinoma | [88] |
| Combretastatin-A4 (3.7), Paclitaxel (3.0) | 4/1 | PLGA | 244.1 ± 4.3 | Melanoma | [89] |
| Combretastatin-A4 (3.7), Paclitaxel (3.0) | 4/1 | mPEG2000-PLA2000 | 68.3 ± 1.4 | Lewis lung carcinoma, melanoma liver metastasis | [90] |
| Dasatinib (1.8), Doxorubicin HCl (1.27) | 3.37/1 | POEG | 200.8 | Breast cancer | [91] |
| Daunorubicin (1.83), Oxaliplatin (−0.47) | 1/2 | MPEG-P(LA-co-MCC) | 208 | Hepatocarcinoma | [92] |
| Diclofenac (4.51), Docetaxel (2.4) | 1.34/1 | PLGA, DPPC, DSPE-PEG2000 | 155.7 ± 1.2 | Glioblastoma | [93] |
| Docetaxel (2.4), Doxorubicin HCl (1.27) | 3.24/1 | PBS/PBDL, HPMA | 122 | T-cell lymphoma | [94] |
| Docetaxel (2.4), Mertansine (2.2) | 1/1.7 | PEG-TMC | 49 | Melanoma | [95] |
| Doxorubicin HCl (1.27), Paclitaxel (3.0) | 1/1 | DOPC, DOPG, MPB | 220 | Breast cancer | [96] |
| Gemcitabine (−1.4), Oxaliplatin (−0.47) | 1/2.5 | DOPA, DOPC, DSPE-PEG2000, cholesterol | 49.5 ± 0.6 | Pancreatic cancer | [97] |
| Irinotecan (3.2), Oxaliplatin (−0.47) | 1.5/1 | Egg PC, cholesterol | 194.6 ± 4.0 | Colorectal cancer | [98] |
| Irinotecan (3.2), Oxaliplatin (−0.47) | 2.5/1 | DPPC, triglyceride, Oleic acid, and Pluronic F68 | 126.9 ± 2.7 | Colorectal cancer | [99] |
| Lonidamine (4.3), Paclitaxel (3.0) | 4/1 | PCL, PLGA-PEG | 120–160 | Breast cancer | [100] |
| Paclitaxel (3.0), Rapamycin (4.3) | 1/3 | PEG-PCL | 9.2 ± 3.8 | Breast cancer | [52] |
| Paclitaxel (3.0), Tamoxifen (7.1) | 1/2 | PEO-PCL | 100–300 | Ovarian adenocarcinoma | [101] |
| Quercetin (1.5), Sorafenib (3.8) | 1.26/1 | DSPE-PEG2000, PLGA | 136.5 ± 3.2 | Hepatocellular carcinoma | [77] |
| Quercetin (1.5), Topotecan (0.8) | 1/1.5 | Mesoporous silica, PAA-chitosan | 72.9 | Breast cancer | [102] |
LogP values are included in brackets. The values are obtained from Drugbank.com or Pubchem.com. Computed values are used where experimental values are not available.
Drug ratios are obtained from authors’ claims if available or by calculating dosages of their in vivo administrations. Some studies investigated several ratios. The ratios in this table were used in in vivo studies.
NP size values are hydrodynamic size or EM size, or molecular weight where specified.
Some other DcNPs and their physiochemical, pharmacological and pharmaceutical characteristics and clinical progression status are listed in Table 2. It can be seen that lipids and polymers are the major excipients of nano-drug carriers on this list. The diameters of DcNPs, mostly in spherical shapes, are in the range of less than 10 nm to about 300 nm. Cancer is the predominant therapeutic indication and some formulations are currently in clinical trials. DcNP-based therapies for HIV/AIDS, on the other hand, are currently in pre-clinical non-human primate studies and human trials are not yet approved.
Drugs loaded into NPs, via a number of different approaches such as adsorption, encapsulation or chemical conjugation, have distinct pharmacological, pharmacokinetic and physicochemical properties. More importantly, the incorporation of APIs into NPs may alter their pharmacokinetic properties, impacting their delivery to target sites and allowing the maintenance of initial drug ratios for more synchronous action. For example, the Huang laboratory developed multiple DcNPs for cancer therapy. They encapsulated cisplatin and gemcitabine monophosphate (both hydrophilic) into PLGA NPs with precise ratiometric co-loading for co-delivery into bladder tumours in mice [50]. They also encapsulated cisplatin and rapamycin (hydrophilic and hydrophobic) into single PLGA NPs for synergistic treatment of melanoma [51]. The Ferrari group co-loaded rapamycin and paclitaxel (both hydrophobic) into poly(ethylene glycol)-block-poly(ε-caprolactone) copolymer NPs for co-delivery into breast cancer cells in mice. Precise drug ratios were maintained in tumours 48 h after NPs administration, with drug concentrations in tumours 2-fold higher than those in the liver and spleen, resulting in potent anti-tumour activity compared to free drug controls [52].
However, in the current pre-clinical paradigm, the transition from lab scale and rodent proof-of-principle (i.e. pre-clinical) studies to human or non-human primate proof-of-concept (i.e. for the clinic) evaluation is oftentimes considered a challenging and significant step. This challenge could be addressed, and the risk of project termination mitigated, by early consideration of pharmaceutical characteristics of the DcNPs. These characteristics include the complexity of formulation, API wastage during production, cost and disposal of reagents. In the literature (Table 2), few studies have been successful in pharmaceutical scale-up of NP formulations for non-human primate or human studies. Achieving long-acting and targeted cell and tissue exposure in vivo is another level of challenge for DcNPs in the development process. Applying nanomedicine to conquer not only drug resistance but also sustained suppression on targeted tissues is an important pharmaceutical characteristic essential for clinical translation. Filling the gaps between pre-clinical academic research and clinical studies by using innovations in pharmaceutical technology is imperative for the success of current combination nanomedicines. Therefore, the high number of reported innovations in nanomaterial research with proof-of-principle pre-clinical in vivo studies should be evaluated for feasibility, both in technical and economical aspects and for translation into nanomedicine for treating major diseases where drug resistance is a chronic and common impediment of eradication.
Progression of drug combination NPs to primate and human testing
An example in HIV/AIDS therapy (TLC-ART 101)
In particular, HIV/AIDS is indeed challenging to eradicate, even with the most potent oral cART, partly due to residual virus- infected cells in tissues. Lymph node cells taken from patients with no detectable plasma viremia were shown to harbour virus that was still sensitive to the prescribed oral medications [45]. We and others have described the lymphatic drug insufficiency phenomenon whereby virus is able to persist in the lymph system due to insufficient drug levels associated with conventional oral anti-retroviral therapy [10,45,53]. To overcome this, antiviral regimens must be efficiently taken up into the lymphatics and retained in lymph nodes in order to achieve therapeutic drug concentrations. Moreover, as has also been discussed earlier, to be efficacious, targeted therapies must be composed of a combination of agents to reduce the risk of developing disease resistance. Monotherapy carries a high likelihood of generating drug-resistant virus and is contraindicated for HIV treatment. The combination of APIs utilised in oral fixed dose combination therapy needs to inhibit or interfere with multiple targets to minimise the resistance risk while also exhibiting pharmacological activity. Finally, there is great interest amongst clinicians and patients alike for the development of not only targeted drug combinations but also long-acting therapies for a wide variety of therapeutic indications.
Advances in nanomedicine are rapidly evolving with several therapeutics in early clinical trials. For HIV treatment, two injectable single- drug formulations, long-acting rilpivirine and long-acting cabotegravir, are both solid drug NP formulations and have shown efficacy in early clinical trials [54–56]. The expected dosing interval for these formulations is monthly or perhaps even less frequently, which carries a clear advantage over currently available cART. However, both are single agent formulations and are at risk for development of viral resistance; thus, they will need to be co-administered with other agents to be efficacious for HIV treatment.
In 2003, the Ho laboratory developed an injectable lipid-nanoparticle formulation, containing the protease inhibitor indinavir, which exhibited much higher lymphatic drug levels than previously achieved [45]. However, as with the long-acting agents described above, this was a single agent formulation and presented resistance risks. We have since published studies on a similar nano-drug particle formulation containing three antiretroviral agents, with proven clinical efficacy and safety profiles, that are directed at two viral targets (protease and reverse transcriptase of HIV) in the presence of lipid excipients (DSPC and DSPE-mPEG2000) [53,57]. In both reports, drug-lipid NP formulations were delivered via subcutaneous injection and relatively high concentrations of all three drugs in the HIV host cells of lymph nodes and blood were observed. More specifically, drug levels were readily detectable in both peripheral blood mononuclear cells (PBMC) and plasma two weeks following administration, indicating the long-acting potential of this nanoparticle formulation (Figure 2). Furthermore, cell-specific targeting was observed; the concentrations of drugs observed in lymph nodes were much higher relative to those in plasma following administration of anti-retroviral agents in the DcNP formulation. These data are depicted in Table 3, which compares the lymph node mononuclear cells (LNMC) drug concentrations to both plasma and PBMC concentrations for three drugs determined 24 h after a single subcutaneous administration of DcNPs. Notably, there is a dramatic increase in the concentration of drugs in the LNMC cells when compared to plasma levels, indicating a targeting advantage of this particular formulation. An important advance with this DcNPs formulation is that the drugs utilised were of varying physiochemical characteristics and yet were co-formulated and efficiently delivered to the target sites. The agents in this case are tenofovir, a relatively water soluble compound (LogP = −1.6), in addition to lopinavir and ritonavir, both relatively hydrophobic (LPV-LogP = 5.94, RTV-LogP = 4.24) at physiological pH. The ability to co-formulate drugs with disparate physical chemical characteristics and subsequently deliver them in a targeted manner in a fixed-dose combination represents significant progress in drug design and nanomedicine. With validated processes in place, and verified long-acting plasma and cellular pharmacokinetics, this anti-HIV drug combination nanoformulation is now referred to as TLC-ART 101 and is a candidate for further clinical development [57].
Figure 2.
Long acting characteristics of three anti-retroviral agents in a drug-combination (DcNP) nanosuspension. These data represent the time course data of each anti-retroviral agents delivered simultaneously in a drug lipid-nanosuspension. Four macaques each received a subcutaneous injection of the nanoformulated drug and the time course of Lopinavir, Ritonavir and Tenofovir were evaluated in PBMCs and plasma up to 2 weeks by LC-MS/MS. PMBC, peripheral blood mononuclear cell.
Table 3.
Higher LNMC and PBMC relative to plasma for lopinavir, ritonavir and tenofovir 8 days following a single subcutaneous dose of 3-in-1 drug combination DcNPs in primates.
| LNMC/Plasma | LNMC/PBMC | |
|---|---|---|
| Lopinavir | 197.78 (93.7) | 79.20 (65.9) |
| Ritonavir | 3068.15 (111.2) | 531.37 (NA) |
| Tenofovir | 6.77 (165.3) | 4.41 (198.0) |
Four macaques received a single subcutaneous administration of the DcNP formulation containing lopinavir (25 mg/kg), ritonavir (6.96 mg/kg) and tenofovir (10.58 mg/kg). Data are the mean (% coefficient of variation) and are the concentration ratios at 192 h (8 days) following drug administration. NA indicates not available due to ritonavir concentrations < LLOQ in the validated LC-MS/MS assay.
LNMC: lymph node mononuclear cell; PBMC: peripheral blood mononuclear cell.
An example in cancer therapy (CPX-351)
Advances in combination nanomedicine are similarly occurring in oncology with particularly noteworthy clinical trial results for CPX-351 as a treatment for acute myeloid leukaemia (AML) (clinicaltrials.gov). As mentioned, CPX-351 is a liposomal, or enclosed lipid membrane vesicle-based, nanoformulation containing DSPC, DSPG and cholesterol in addition to the APIs cytarabine and daunorubicin (Tables 2 and 4). Pre-clinical testing of the drug ratios in this formulation led to interesting efficacy findings that indicated the most potent combination is not necessarily associated with the highest dose of either drug. It is thus critical to characterise and understand potential drug–drug interactions in nanoscale combination therapeutics [58]. The formulation with a 5:1 molar ratio of cytarabine:-daunorubicin has been evaluated in several clinical trials with very promising results in improvement of overall survival rates. Based on these data, CPX-351 was approved for first line treatment of therapy-related acute myeloid leukaemia (t-AML) or AML with myelodysplasia-related changes (AML-MRC) by the FDA in August 2017.
Table 4.
A comparison of drug-combination nanoparticle products CPX-351 and TLC-ART 101 with respect to physical and pharmacokinetic characteristics and manufacturing process.
| CPX-351a | TLC-ART 101b | |
|---|---|---|
| Disease indication | Acute myeloid leukaemia | HIV/AIDS |
| Physical properties | ||
| Characteristics | liposomes | Drug combination particles (excipient stabilised drug particles) |
| Hydrodynamic size | 100 ± 20 nm | ~52 nm |
| Excipients | DSPC, DSPG, cholesterol | DSPC, DSPE-mPEG2000 |
| Drugs and solubility | Daunorubicin (LogP = 1.83); Cytarabine (LogP = −2.8) | Lopinavir (LogP = 5.94); Ritonavir (LogP = 4.24); Tenofovir (LogP = −1.6) |
| Drug ratios (molar)c | 1:5 | 4:1:5 |
| Manufacturing process | ||
| Manufacturing stepsd |
|
|
| Pharmacokinetic characteristics | ||
| t1/2 | 31.5 h (Daunorubicin), 40.4 h (Cytarabine) | 476.9 h (Lopinavir), 44.1 h (Ritonavir), 65.3 h (Tenofovir) |
CPX-351 is a liposomal formulation consisting daunorubicin and cytarabine with fixed ratio for treatment of AML.
TLC-ART 101 is a lipid nanoparticle formulation consisting lopinavir, ritonavir and tenofovir with fixed ratio for treatment of HIV/AIDS.
Drug ratios in these formulations molar ratios of daunorubicin:cytarabine and lopinavir:ritonavir:tenoforvir, respectively.
The steps indicate simplified key procedures in manufacturing of CPX-351 and TLC-ART 101.
A comparison of TLC-ART 101 and CPX-351 from pharmaceutical aspects
From pharmaceutical drug development and regulatory perspectives, DcNPs are considered complex injectable formulations. Therefore, they are typically developed based on QbD (quality-by-design) principles. As more complex manufacturing processes often lead to a higher risk of product failure, designing simplified processes while achieving the necessary product complexity is desired. Table 4 shows a comparison of CPX-351 and TLC-ART 101, two relatively advanced DcNP drug delivery systems. While CPX-351 is a liposomal formulation, TLC-ART 101 is a DcNP stabilised by lipid excipients and has a smaller mean particle diameter which enables targeted lymphatic delivery. Moreover, the lipid constituents in TLC-ART 101 allow unique interactions with both hydrophobic and hydrophilic drugs. The complex formulation process for CPX-351 includes encapsulating water-soluble cytarabine within liposomes, followed by buffer exchange and the determination of encapsulated liposomal cytarabine concentration. Based on these data, a specified amount of more hydrophobic daunorubicin is added to form a drug precipitate inside liposomes to produce the target 5:1 molar ratio of cytarabine-to-daunorubicin [48]. Unlike the complex process to manufacture CPX 351, TLC-ART 101 uses a simple and less complex formulation strategy (solubilizing drugs and lipid excipient in a suitable organic solvent, the controlled removal of the solvent (drying), hydration of the stabilised drug–lipid combination and homogenisation to produce specified drug-particle size), keeping all components intact during the process without exchanging buffer systems [59]. Such simplified manufacturing processes reduce both sample loss and the risk of contamination while providing consistent and predictable drug loading efficiency.
In sum, the above studies highlight the potential for DcNPs to improve upon conventional therapeutic modalities. Importantly, the substitution of other APIs for a variety of therapeutic indications in these delivery systems presents many exciting opportunities for translating innovations in DcNPs research into in nanomedicines and for the individualisation of nanomedicine therapeutics and diagnostics.
Future outlook – personalised drug combination particles in precision medicine
Until recently, new drugs in development were evaluated in and intended for specific populations, rather than for individuals with a specific magnitude of disease or symptoms that make up the range in the population. These studies have not focussed on the precise differences among the individuals in a population and how these differences may correlate to the tested outcome [60]. For example, oncology patients have been traditionally classified as having one of about 200 different cancers according to anatomic and histologic criteria [61,62]. However, decades ago, it became clear that the detailed makeup of cancer cells in patients with the same cancer (e.g. lung) are highly variable, and cancer cells differ at different tumour sites in the same patient and within a tumour [63–66]. Moreover, the patient’s immune system and the specific tumour microenvironment, which themselves have high inter-individual variability, are now known to be key factors in whether a metastatic cancer cell develops into a metastatic tumour [67]. Because these inter- and intra-patient differences in tumour cell phenotypes, tissue microenvironments and immune responses can lead to variable prognosis and treatment outcomes, it is now recognised that the practice of medicine needs to adopt a more personalised approach. Such an approach would define and treat the precise characteristics of each patient’s cancer and their own unique biology, since cancer cells and host cells form a unique symbiotic ecosystem [68].
Thus, successful treatment of cancer may require cracking two halves of a puzzle. Half lies in the cancer’s intrinsic biology – its genes, its morphology, its nutritional preferences, its reproductive habits. The other half involves the match between that biology and the patient’s own ‘host factors’ being sufficient to harbour metastatic niches in their body. Integration of patient profiles and chemotherapeutic regimens based on specific cell biology is generally referred to as ‘precision medicine’. In this new era of precision medicine, the vision is that prevention and treatment strategies will take individual variability into account by incorporating genomic, proteomic and metabolomic information into disease diagnosis, prognosis and treatment [68]. In other words, matching the phenotype/genotype of the specific cancer and the physiology of patients with appropriate drug combination ratios as well as the optimal nano-delivery system that can provide persistent, long-acting pharmacology and selective multi-agent exposure to target cells, could greatly improve treatment efficacy. Thus, doctors will no longer be treating 200 different types of cancer, but they will potentially be treating thousands of unique diseases wherein the molecular lesions that drive each cancer are well understood, and the propensity of each patient’s specific biology and microenvironments to harbour cancer metastases is predicted.
This individualised approach to disease prevention and treatment first requires the efficient collection, management and access to an explosion of biomedical information (bioinformatics) [69]. With the advent and maturation of ‘-omics’ disciplines (e.g. genomics, proteomics, metabolomics, etc.), large-scale biologic databases and more detailed mechanistic knowledge, we now have tools (with more constantly being invented) to begin assigning detailed phenotypes to diseased cells, healthy cells and the immune cell arsenal in each individual patient. However, as this plethora of data materialises, the first step is to ensure that this information is consistent, reliable, independently validated and accessible; accurate evidence-based data must first be curated before using it to guide individualised clinical practice [70]. For the promise of precision medicine to be fully realised, the importance of this bioinformatics challenge and need for scientific rigour cannot be overstated.
Currently, there is limited knowledge about specific drug combinations and the drug ratios that would be most effective [60]; and even less is known about the personalisation and fine-tuning of DcNPs tailored to a specific patient. The traditional polypharmacy approach of combining several different single-agent formulations into a drug combination regimen for a patient may be impeded by systemic dose-limiting toxicity when only a fraction of the dose reaches target cells. This approach may not allow sustained ratios and concentrations of API at the target sites while minimising drug levels at off-target sites. As discussed above, drug combinations co-formulated into one NPs platform specifically targeted to diseased cells may maintain effective molar ratios of drug combinations at target sites by honing the drug distribution of multiple API to a common target location.
As more insights into pharmacogenomics are gained through studies of large research cohorts exposed to many kinds of therapies (e.g. the allofus.nih.gov longitudinal cohort of 1 million or more Americans) [68], the knowledge gained will guide selection of the right drug(s) at the right dose(s) to the right patient. In order for this knowledge and technology to be integrated and make progress towards clinical translation, DcNPs will need to be properly and efficiently formulated for the APIs to effectively and safely treat the patient’s precise disease phenotype and interact safely with the patient’s specific biology. This work will need to go beyond mere dose and drug selection, and will require incorporating DcNP formulation and cell targeting knowledge and understanding. This will require efficient and versatile DcNP delivery platforms that can stably combine multiple hydrophilic and hydrophobic APIs at different molar ratios, such as the platform we have developed [53,57]. Furthermore, a rapid, robust, reproducible and scalable manufacturing process, such as the microfluidic systems being marketed by Precision NanoSystems Inc., for commercial large batch production of drug-loaded nanoparticles, would be beneficial [71]. The ultimate goal is to efficiently link a tailored DcNP formulation to each patient; this approach promises to create safer medicines by overcoming dose-limiting toxicities (by targeting APIs specifically to diseased sites) and addressing drug resistance (through delivering consistent molar ratios of API combinations to targets). Such an approach could be used for not only targeted small molecules (e.g. kinase inhibitors) but also for highly potent small molecules (e.g. MMAE). Attaching ligands to the surface of DcNPs that selectively and tightly bind to unique molecules on diseased cells could further enhance drug combination delivery to diseases cells.
Thus, in the future, each personalised DcNP would account for both how the patient’s body would handle and eliminate the API and how the API would interact with the precise biology of the patient’s healthy and diseased cells. Organoids could be grown ex vivo using a patient’s own tumour cells and DcNP formulations could be tested on these [72]; tumour biopsies that are properly handled so they are most representative of the tumours in a patient could be used to assess the efficacy of multiple formulations and drug compositions simultaneously [73]. With appropriate pharmaceutical nanotechnology platforms in place, novel nano-drug manufacturing would be closely linked to the clinic and the individualised care of each patient [60]. In the coming decades, rigorous research studies will need to be designed and executed to investigate the details involved in personalisation of DcNPs for individual patients. Developing a framework for this work first in infectious disease and oncology would likely serve as a useful blueprint for personalising DcNPs in the future for other diseases such as metabolic and neurological conditions that require chronic therapeutic treatments.
Acknowledgments
We thank Samuel H. Griffin for his editorial assistance.
Funding
We acknowledge the support from NIH grants UM1 AI120176 and P51OD010425. Yu Gao is also supported by the Natural Science Foundation of China (81571802), Natural Science Foundation of Fujian Province (2016J06020), the Fujian Provincial Youth Topnotch Talent Support Program, and the China Scholarship Council (No. 201706655015).
Footnotes
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Banker GS. Drug products: their role in the treatment of disease, their quality, and their status and future as drug-delivery systems. In: Banker GS, Rhodes CT, editors. Modern pharmaceutics. 4. New York (NY): Marcel Dekker Inc.; 2002. pp. 1–21. revised and expanded. [Google Scholar]
- 2.Kawakami K. Modification of physicochemical characteristics of active pharmaceutical ingredients and application of supersaturatable dosage forms for improving bioavailability of poorly absorbed drugs. Adv Drug Deliv Rev. 2012;64:480–495. doi: 10.1016/j.addr.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Lipinski CA, Lombardo F, Dominy BW, et al. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 4.Aungst BJ. Intestinal permeation enhancers. J Pharm Sci. 2000;89:429–442. doi: 10.1002/(SICI)1520-6017(200004)89:4<429::AID-JPS1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 5.Singh R, Lillard JW., Jr Nanoparticle-based targeted drug delivery. Exp Mol Pathol. 2009;86:215–223. doi: 10.1016/j.yexmp.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koo OM, Rubinstein I, Onyuksel H. Role of nanotechnology in targeted drug delivery and imaging: a concise review. Nanomedicine. 2005;1:193–212. doi: 10.1016/j.nano.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Sastry SV, Nyshadham JR, Fix JA. Recent technological advances in oral drug delivery - a review. Pharm Sci Technol Today. 2000;3:138–145. doi: 10.1016/s1461-5347(00)00247-9. [DOI] [PubMed] [Google Scholar]
- 8.Druker B. Imatinib (Gleevec) as a paradigm of targeted cancer therapies. Keio J Med. 2010;59:1–3. doi: 10.2302/kjm.59.1. [DOI] [PubMed] [Google Scholar]
- 9.Hochhaus A, Larson RA, Guilhot F, et al. Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med. 2017;376:917–927. doi: 10.1056/NEJMoa1609324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fletcher CV, Staskus K, Wietgrefe SW, et al. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc Natl Acad Sci USA. 2014;111:2307–2312. doi: 10.1073/pnas.1318249111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holohan C, Van Schaeybroeck S, Longley DB, et al. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13:714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 12.Sareen S, Mathew G, Joseph L. Improvement in solubility of poor water-soluble drugs by solid dispersion. Int J Pharm Investig. 2012;2:12–17. doi: 10.4103/2230-973X.96921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larson KB, Wang K, Delille C, et al. Pharmacokinetic enhancers in HIV therapeutics. Clin Pharmacokinet. 2014;53:865–872. doi: 10.1007/s40262-014-0167-9. [DOI] [PubMed] [Google Scholar]
- 14.Health, United States, 2015. With special feature on racial and ethnic health disparities. Hyattsville (MD): Health, United States; 2016. [PubMed] [Google Scholar]
- 15.Endicott JA, Ling V. The biochemistry of p-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- 16.Deffie AM, Alam T, Seneviratne C, et al. Enhanced transcription of p-glycoprotein gene in adriamycin-resistant P388 leukemia-cells. Clin Investig Med. 1987;10:B112. [Google Scholar]
- 17.Kong LL, Zhuang XM, Yang HY, et al. Inhibition of P-glycoprotein gene expression and function enhances triptolide-induced hepatotoxicity in mice. Sci Rep. 2015;5:11747. doi: 10.1038/srep11747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaur V, Garg T, Rath G, et al. Therapeutic potential of nanocarrier for overcoming to P-glycoprotein. J Drug Target. 2014;22:859–870. doi: 10.3109/1061186X.2014.947295. [DOI] [PubMed] [Google Scholar]
- 19.LoRusso PM, Canetta R, Wagner JA, et al. Accelerating cancer therapy development: the importance of combination strategies and collaboration. Summary of an Institute of Medicine Workshop. Clin Cancer Res. 2012;18:6101–6109. doi: 10.1158/1078-0432.CCR-12-2455. [DOI] [PubMed] [Google Scholar]
- 20.Waterhouse DN, Tardi PG, Mayer LD, et al. A comparison of liposomal formulations of doxorubicin with drug administered in free form: changing toxicity profiles. Drug Saf. 2001;24:903–920. doi: 10.2165/00002018-200124120-00004. [DOI] [PubMed] [Google Scholar]
- 21.Gabizon A, Shmeeda H, Barenholz Y. Pharmacokinetics of pegylated liposomal Doxorubicin: review of animal and human studies. Clin Pharmacokinet. 2003;42:419–436. doi: 10.2165/00003088-200342050-00002. [DOI] [PubMed] [Google Scholar]
- 22.Gabizon AA. PEGylated liposomal doxorubicin: metamorphosis of an old drug into a new form of chemotherapy. Cancer Investig. 2001;19:424–436. doi: 10.1081/cnv-100103136. [DOI] [PubMed] [Google Scholar]
- 23.Rivera E. Liposomal anthracyclines in metastatic breast cancer: clinical update. Oncologist. 2003;8(Suppl 2):3–9. doi: 10.1634/theoncologist.8-suppl_2-3. [DOI] [PubMed] [Google Scholar]
- 24.McDermott U, Sharma SV, Dowell L, et al. Identification of genotype-correlated sensitivity to selective kinase inhibitors by using high-throughput tumor cell line profiling. Proc Natl Acad Sci USA. 2007;104:19936–19941. doi: 10.1073/pnas.0707498104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li T, Kung HJ, Mack PC, et al. Genotyping and genomic profiling of non-small-cell lung cancer: implications for current and future therapies. J Clin Oncol. 2013;31:1039–1049. doi: 10.1200/JCO.2012.45.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDermott U, Settleman J. Personalized cancer therapy with selective kinase inhibitors: an emerging paradigm in medical oncology. J Clin Oncol. 2009;27:5650–5659. doi: 10.1200/JCO.2009.22.9054. [DOI] [PubMed] [Google Scholar]
- 27.Barouch-Bentov R, Sauer K. Mechanisms of drug resistance in kinases. Expert Opin Investig Drugs. 2011;20:153–208. doi: 10.1517/13543784.2011.546344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. P T. 2015;40:277–283. [PMC free article] [PubMed] [Google Scholar]
- 29.Davies J, Davies D. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev. 2010;74:417–433. doi: 10.1128/MMBR.00016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Worthington RJ, Melander C. Combination approaches to combat multidrug-resistant bacteria. Trends Biotechnol. 2013;31:177–184. doi: 10.1016/j.tibtech.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin LR, Williams SL, Haskard KB, et al. The challenge of patient adherence. Ther Clin Risk Manag. 2005;1:189–199. [PMC free article] [PubMed] [Google Scholar]
- 32.Hu C-MJ, Zhang L. Nanoparticle-based combination therapy toward overcoming drug resistance in cancer. Biochem Pharmacol. 2012;83:1104–1111. doi: 10.1016/j.bcp.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 33.Owen A, Rannard S. Strengths, weaknesses, opportunities and challenges for long acting injectable therapies: insights for applications in HIV therapy. Adv Drug Deliv Rev. 2016;103:144–156. doi: 10.1016/j.addr.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeetah R, Bhaw-Luximon A, Jhurry D. Nanopharmaceutics: phytochemical-based controlled or sustained drug-delivery systems for cancer treatment. J Biomed Nanotechnol. 2014;10:1810–1840. doi: 10.1166/jbn.2014.1884. [DOI] [PubMed] [Google Scholar]
- 35.Mei L, Zhang ZP, Zhao LY, et al. Pharmaceutical nanotechnology for oral delivery of anticancer drugs. Adv Drug Deliv Rev. 2013;65:880–890. doi: 10.1016/j.addr.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Lu HJ, Wang JK, Wang T, et al. Recent progress on nanostructures for drug delivery applications. J Nanomaterials. 2016;2016:5762431. [Google Scholar]
- 37.Miao L, Guo S, Lin CM, et al. Nanoformulations for combination or cascade anticancer therapy. Adv Drug Deliv Rev. 2017;115:3–22. doi: 10.1016/j.addr.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi J, Kantoff PW, Wooster R, et al. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17:20–37. doi: 10.1038/nrc.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng HC. The molecular mechanisms of chemoresistance in cancers. Oncotarget. 2017;8:59950–59964. doi: 10.18632/oncotarget.19048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paxton WA, Koup RA. Mechanisms of resistance to HIV infection. Springer Semin Immunopathol. 1997;18:323–340. doi: 10.1007/BF00813501. [DOI] [PubMed] [Google Scholar]
- 41.Bock C, Lengauer T. Managing drug resistance in cancer: lessons from HIV therapy. Nat Rev Cancer. 2012;12:494–501. doi: 10.1038/nrc3297. [DOI] [PubMed] [Google Scholar]
- 42.Ogunwuyi O, Kumari N, Smith KA, et al. Antiretroviral drugs-loaded nanoparticles fabricated by dispersion polymerization with potential for HIV/AIDS treatment. Infect Dis (Auckl) 2016;9:21–32. doi: 10.4137/IDRT.S38108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trickey A, May MT, Vehreschild J-J, et al. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV. 2017;4:e349–e356. doi: 10.1016/S2352-3018(17)30066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382:1525–1533. doi: 10.1016/S0140-6736(13)61809-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kinman L, Brodie SJ, Tsai CC, et al. Lipid-drug association enhanced HIV-1 protease inhibitor indinavir localization in lymphoid tissues and viral load reduction: a proof of concept study in HIV-2287-infected macaques. J Acquir Immune Defic Syndr. 2003;34:387–397. doi: 10.1097/00126334-200312010-00005. [DOI] [PubMed] [Google Scholar]
- 46.Freeling JP, Ho RJY. Anti-HIV drug particles may overcome lymphatic drug insufficiency and associated HIV persistence. Proc Natl Acad Sci USA. 2014;111:E2512–E2513. doi: 10.1073/pnas.1406554111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao L, Wientjes MG, An JLS. Evaluation of combination chemotherapy: integration of nonlinear regression, curve shift, isobologram, and combination index analyses. Clin Cancer Res. 2004;10:7994–8004. doi: 10.1158/1078-0432.CCR-04-1087. [DOI] [PubMed] [Google Scholar]
- 48.Mayer LD, Harasym TO, Tardi PG, et al. Ratiometric dosing of anticancer drug combinations: controlling drug ratios after systemic administration regulates therapeutic activity in tumor-bearing mice. Mol Cancer Ther. 2006;5:1854–1863. doi: 10.1158/1535-7163.MCT-06-0118. [DOI] [PubMed] [Google Scholar]
- 49.Batist G, Gelmon KA, Chi KN, et al. Safety, pharmacokinetics, and efficacy of CPX-1 liposome injection in patients with advanced solid tumors. Clin Cancer Res. 2009;15:692–700. doi: 10.1158/1078-0432.CCR-08-0515. [DOI] [PubMed] [Google Scholar]
- 50.Miao L, Guo ST, Zhang J, et al. Nanoparticles with precise ratiometric co-loading and co-delivery of gemcitabine monophosphate and cisplatin for treatment of bladder cancer. Adv Funct Mater. 2014;24:6601–6611. doi: 10.1002/adfm.201401076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guo ST, Lin CM, Xu ZH, et al. Co-delivery of cisplatin and rapamycin for enhanced anticancer therapy through synergistic effects and microenvironment modulation. ACS Nano. 2014;8:4996–5009. doi: 10.1021/nn5010815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blanco E, Sangai T, Wu SH, et al. Colocalized delivery of rapamycin and paclitaxel to tumors enhances synergistic targeting of the PI3K/Akt/mTOR pathway. Mol Ther. 2014;22:1310–1319. doi: 10.1038/mt.2014.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Freeling JP, Koehn J, Shu C, et al. Long-acting three-drug combination anti-HIV nanoparticles enhance drug exposure in primate plasma and cells within lymph nodes and blood. AIDS. 2014;28:2625–2627. doi: 10.1097/QAD.0000000000000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet. 2017;390:1499–1510. doi: 10.1016/S0140-6736(17)31917-7. [DOI] [PubMed] [Google Scholar]
- 55.Markowitz M, Frank I, Grant RM, et al. Safety and tolerability of long-acting cabotegravir injections in HIV-uninfected men (ECLAIR): a multicentre, double-blind, randomised, placebo-controlled, phase 2a trial. Lancet HIV. 2017;4:e331–e340. doi: 10.1016/S2352-3018(17)30068-1. [DOI] [PubMed] [Google Scholar]
- 56.Han Y, Mesplede T, Wainberg MA. Investigational HIV integrase inhibitors in phase I and phase II clinical trials. Expert Opin Investig Drugs. 2017;26:1207–1213. doi: 10.1080/13543784.2017.1378643. [DOI] [PubMed] [Google Scholar]
- 57.Kraft JC, McConnachie LA, Koehn J, et al. Long-acting combination anti-HIV drug suspension enhances and sustains higher drug levels in lymph node cells than in blood cells and plasma. AIDS. 2017;31:765–770. doi: 10.1097/QAD.0000000000001405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dicko A, Mayer LD, Tardi PG. Use of nanoscale delivery systems to maintain synergistic drug ratios in vivo. Expert Opin Drug Deliv. 2010;7:1329–1341. doi: 10.1517/17425247.2010.538678. [DOI] [PubMed] [Google Scholar]
- 59.Freeling JP, Koehn J, Shu C, et al. Anti-HIV drug-combination nanoparticles enhance plasma drug exposure duration as well as triple-drug combination levels in cells within lymph nodes and blood in primates. AIDS Res Hum Retroviruses. 2015;31:107–114. doi: 10.1089/aid.2014.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schellekens H, Aldosari M, Talsma H, et al. Making individualized drugs a reality. Nat Biotechnol. 2017;35:507–513. doi: 10.1038/nbt.3888. [DOI] [PubMed] [Google Scholar]
- 61.Jameson JL, Longo DL. Precision medicine–personalized, problematic, and promising. N Engl J Med. 2015;372:2229–2234. doi: 10.1056/NEJMsb1503104. [DOI] [PubMed] [Google Scholar]
- 62.Fleck LM. Just caring: assessing the ethical and economic costs of personalized medicine. Urol Oncol. 2014;32:202–206. doi: 10.1016/j.urolonc.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 63.Ding L, Ellis MJ, Li S, et al. Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature. 2010;464:999–1005. doi: 10.1038/nature08989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fidler IJ. Commentary on “ "Tumor heterogeneity and the biology of cancer invasion and metastasis"”. Cancer Res. 2016;76:3441–3442. doi: 10.1158/0008-5472.CAN-16-1330. [DOI] [PubMed] [Google Scholar]
- 65.Kandoth C, McLellan MD, Vandin F, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502:333–339. doi: 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vignot S, Frampton GM, Soria JC, et al. Next-generation sequencing reveals high concordance of recurrent somatic alterations between primary tumor and metastases from patients with non-small-cell lung cancer. J Clin Oncol. 2013;31:2167–2172. doi: 10.1200/JCO.2012.47.7737. [DOI] [PubMed] [Google Scholar]
- 67.Langley RR, Fidler IJ. The seed and soil hypothesis revisited-the role of tumor-stroma interactions in metastasis to different organs. Int J Cancer. 2011;128:2527–2535. doi: 10.1002/ijc.26031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372:793–795. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shortliffe EH. Biomedical informatics in the education of physicians. JAMA. 2010;304:1227–1228. doi: 10.1001/jama.2010.1262. [DOI] [PubMed] [Google Scholar]
- 70.Hewitt JA, Brown LL, Murphy SJ, et al. Accelerating biomedical discoveries through rigor and transparency. ILAR J. 2017;58:115–128. doi: 10.1093/ilar/ilx011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Garg S, Heuck G, Ip S, et al. Microfluidics: a transformational tool for nanomedicine development and production. J Drug Target. 2016;24:821–835. doi: 10.1080/1061186X.2016.1198354. [DOI] [PubMed] [Google Scholar]
- 72.Gao D, Vela I, Sboner A, et al. Organoid cultures derived from patients with advanced prostate cancer. Cell. 2014;159:176–187. doi: 10.1016/j.cell.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Klinghoffer RA, Bahrami SB, Hatton BA, et al. A technology platform to assess multiple cancer agents simultaneously within a patient's tumor. Sci Transl Med. 2015;7:284ra258. doi: 10.1126/scitranslmed.aaa7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin TL, Newell LF, Stuart RK, et al. CPX-351 ((Cytarabine: Daunorubicin) liposome injection, (Vyxeos)) does not prolong QTCF intervals, requires no dose adjustment for impaired renal function and induces high rates of complete remission in acute myeloid leukemia. Blood. 2015;126:2510. [Google Scholar]
- 75.Song WT, Tang ZH, Zhang DW, et al. Anti-tumor efficacy of c(RGDfK)-decorated polypeptide-based micelles co-loaded with docetaxel and cisplatin. Biomaterials. 2014;35:3005–3014. doi: 10.1016/j.biomaterials.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 76.Lammers T, Subr V, Ulbrich K, et al. Simultaneous delivery of doxorubicin and gemcitabine to tumors in vivo using prototypic polymeric drug carriers. Biomaterials. 2009;30:3466–3475. doi: 10.1016/j.biomaterials.2009.02.040. [DOI] [PubMed] [Google Scholar]
- 77.Wei X, Wang Y, Xiong X, et al. Codelivery of a pi-pi stacked dual anticancer drug combination with nanocarriers for overcoming multidrug resistance and tumor metastasis. Adv Funct Mater. 2016;26:8266–8280. [Google Scholar]
- 78.Zhang JY, Wang X, Liu TJ, et al. Antitumor activity of electrospun polylactide nanofibers loaded with 5-fluorouracil and oxaliplatin against colorectal cancer. Drug Deliv. 2016;23:794–800. doi: 10.3109/10717544.2014.916768. [DOI] [PubMed] [Google Scholar]
- 79.Ma Y, Liu D, Wang D, et al. Combinational delivery of hydrophobic and hydrophilic anticancer drugs in single nanoemulsions to treat MDR in cancer. Mol Pharm. 2014;11:2623–2630. doi: 10.1021/mp400778r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Choi JY, Ramasamy T, Kim SY, et al. PEGylated lipid bilayer-supported mesoporous silica nanoparticle composite for synergistic co-delivery of axitinib and celastrol in multi-targeted cancer therapy. Acta Biomater. 2016;39:94–105. doi: 10.1016/j.actbio.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 81.Shen S, Li HJ, Chen KG, et al. Spatial targeting of tumor-associated macrophages and tumor cells with a pH-sensitive cluster nanocarrier for cancer chemoimmunotherapy. Nano Lett. 2017;17:3822–3829. doi: 10.1021/acs.nanolett.7b01193. [DOI] [PubMed] [Google Scholar]
- 82.Wu HQ, Jin HJ, Wang C, et al. Synergistic cisplatin/doxorubicin combination chemotherapy for multidrug-resistant cancer via polymeric nanogels targeting delivery. ACS Appl Mater Interfaces. 2017;9:9426–9436. doi: 10.1021/acsami.6b16844. [DOI] [PubMed] [Google Scholar]
- 83.Desale SS, Soni KS, Romanova S, et al. Targeted delivery of platinum-taxane combination therapy in ovarian cancer. J Control Release. 2015;220:651–659. doi: 10.1016/j.jconrel.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cai LQ, Xu GF, Shi CY, et al. Telodendrimer nanocarrier for co-delivery of paclitaxel and cisplatin: A synergistic combination nanotherapy for ovarian cancer treatment. Biomaterials. 2015;37:456–468. doi: 10.1016/j.biomaterials.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barnes JC, Bruno PM, Nguyen HVT, et al. Using an RNAi signature assay to guide the design of three-drug-conjugated nanoparticles with validated mechanisms, in vivo efficacy, and low toxicity. J Am Chem Soc. 2016;138:12494–12501. doi: 10.1021/jacs.6b06321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sengupta S, Eavarone D, Capila I, et al. Temporal targeting of tumour cells and neovasculature with a nanoscale delivery system. Nature. 2005;436:568–572. doi: 10.1038/nature03794. [DOI] [PubMed] [Google Scholar]
- 87.Liu HM, Xie YD, Zhang YF, et al. CA4-loaded doxorubicin prodrug coating Fe3O4 nanoparticles for tumor combination therapy. RSC Adv. 2016;6:113933–113939. [Google Scholar]
- 88.Wang YS, Chen HL, Liu YY, et al. pH-sensitive pullulan-based nanoparticle carrier of methotrexate and combretastatin A4 for the combination therapy against hepatocellular carcinoma. Biomaterials. 2013;34:7181–7190. doi: 10.1016/j.biomaterials.2013.05.081. [DOI] [PubMed] [Google Scholar]
- 89.Wang Z, Chui WK, Ho PC. Nanoparticulate delivery system targeted to tumor neovasculature for combined anticancer and antiangiogenesis therapy. Pharm Res. 2011;28:585–596. doi: 10.1007/s11095-010-0308-2. [DOI] [PubMed] [Google Scholar]
- 90.Wang Z, Ho PC. A nanocapsular combinatorial sequential drug delivery system for antiangiogenesis and anticancer activities. Biomaterials. 2010;31:7115–7123. doi: 10.1016/j.biomaterials.2010.05.075. [DOI] [PubMed] [Google Scholar]
- 91.Sun JJ, Liu YH, Chen YC, et al. Doxorubicin delivered by a redox-responsive dasatinib-containing polymeric prodrug carrier for combination therapy. J Control Release. 2017;258:43–55. doi: 10.1016/j.jconrel.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xiao HH, Li WL, Qi RG, et al. Co-delivery of daunomycin and oxaliplatin by biodegradable polymers for safer and more efficacious combination therapy. J Control Release. 2012;163:304–314. doi: 10.1016/j.jconrel.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 93.Lee A, Di Mascolo D, Francardi M, et al. Spherical polymeric nanoconstructs for combined chemotherapeutic and anti-inflammatory therapies. Nanomedicine Nanotechnol Biol Med. 2016;12:2139–2147. doi: 10.1016/j.nano.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 94.Jager E, Jager A, Chytil P, et al. Combination chemotherapy using core-shell nanoparticles through the self-assembly of HPMA-based copolymers and degradable polyester. J Control Release. 2013;165:153–161. doi: 10.1016/j.jconrel.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 95.Zhong P, Qiu M, Zhang J, et al. cRGD-installed docetaxel-loaded mertansine prodrug micelles: redox-triggered ratiometric dual drug release and targeted synergistic treatment of B16F10 melanoma. Nanotechnology. 2017;28:295103. doi: 10.1088/1361-6528/aa76cc. [DOI] [PubMed] [Google Scholar]
- 96.Liu YR, Fang JX, Joo KI, et al. Codelivery of chemotherapeutics via crosslinked multilamellar liposomal vesicles to overcome multidrug resistance in tumor. PLoS One. 2014;9:e110611. doi: 10.1371/journal.pone.0110611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Poon C, He CB, Liu DM, et al. Self-assembled nanoscale coordination polymers carrying oxaliplatin and gemcitabine for synergistic combination therapy of pancreatic cancer. J Control Release. 2015;201:90–99. doi: 10.1016/j.jconrel.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang B, Wang TQ, Yang SM, et al. Development and evaluation of oxaliplatin and irinotecan co-loaded liposomes for enhanced colorectal cancer therapy. J Control Release. 2016;238:10–21. doi: 10.1016/j.jconrel.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 99.Zhang B, Song YM, Wang TQ, et al. Efficient co-delivery of immiscible hydrophilic/hydrophobic chemotherapeutics by lipid emulsions for improved treatment of cancer. Int J Nanomedicine. 2017;12:2871–2886. doi: 10.2147/IJN.S129091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Milane L, Duan Z-F, Amiji M, et al. of Combination lonidamine and paclitaxel delivery in an orthotopic animal model of multi-drug resistant breast cancer using EGFR-targeted polymeric nanoparticles. Nanomedicine Nanotechnol Biol Med. 2011;7:435–444. doi: 10.1016/j.nano.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Devalapally H, Duan ZF, Seiden MV, et al. Modulation of drug resistance in ovarian adenocarcinoma by enhancing intracellular ceramide using tamoxifen-loaded biodegradable polymeric nanoparticles. Clin Cancer Res. 2008;14:3193–3203. doi: 10.1158/1078-0432.CCR-07-4973. [DOI] [PubMed] [Google Scholar]
- 102.Murugan C, Rayappan K, Thangam R, et al. Combinatorial nanocarrier based drug delivery approach for amalgamation of anti-tumor agents in breast cancer cells: an improved nanomedicine strategies. Sci Rep. 2016;6:34053. doi: 10.1038/srep34053. [DOI] [PMC free article] [PubMed] [Google Scholar]