Abstract
Regulatory T cells are critical for the generation and maintenance of peripheral tolerance. Conditional deletion of the transcriptional repressor NKAP in Tregs using Foxp3-YFP-cre NKAP conditional knockout mice causes aggressive autoimmunity characterized by thymic atrophy, lymphadenopathy, peripheral T cell activation, generation of autoantibodies, immune infiltration into several organs, and crusty skin at 3 weeks of age, similar to that of “scurfy” Foxp3-mutant mice. While Treg development in the thymus proceeds normally in the absence of NKAP, there is a severe loss of thymically-derived Tregs in the periphery. NKAP-deficient Tregs have a recent thymic emigrant phenotype, and are attacked by complement in a cell-intrinsic manner in the periphery. Previously, we demonstrated that NKAP is required for conventional T cell maturation as it prevents complement-mediated attack in the periphery. We now show that Tregs undergo a similar maturation process as conventional T cells, requiring NKAP to acquire complement resistance after thymic egress.
Keywords: NKAP, scurfy, Tregs, complement
1. Introduction
During T cell development, random gene rearrangement and the addition of non-template nucleotides to produce the TCR leads to a diverse repertoire of T cells that includes auto-reactive clones1. Not all auto-reactive thymocytes undergo thymic clonal deletion and, hence, mechanisms of peripheral tolerance are needed to prevent autoimmunity. In the thymus, some CD4 T cells bearing high affinity TCRs for their self peptide-MHC class II complexes are diverted into the regulatory T (Treg) cell lineage in the presence of appropriate external cues2–7. These regulatory cells predominantly suppress autoreactive T cells that escape clonal deletion, preventing autoimmunity and preserving tissue homeostasis8–10.
Foxp3 is the master regulator of Treg development and suppressive phenotype11,12. Loss-of-function Foxp3 mutations in either the forkhead or DNA binding domains result in a rapid and lethal autoimmune disorder, initially described as the “scurfy” phenotype in mice11,12. Scurfy mice lack CD4+ Foxp3+ Tregs, and develop severe dermatitis, splenomegaly, lymphadenopathy, and extensive immune infiltration into vital organs leading to runting and death by the age of three weeks13. Preventing Treg development by T-cell specific deletion of Foxp3 or Treg ablation with diphtheria toxin culminates in autoimmunity of a similar severity14,15. Transferring CD4+ CD25+ regulatory cells to 1–2 day old scurfy neonates prevents the onset of autoimmunity12. In humans, mutations in Foxp3 lead to Immunodysregulation Polyendocrinopathy Enteropathy X-linked syndrome (IPEX), a rare and severe multiorgan autoimmune disorder that results in death at infancy16,17. Together, these studies have demonstrated the role of Foxp3 in Treg development, and the importance of Treg persistence in neonates and adults in ensuring peripheral tolerance.
Long-term persistence of T cells in peripheral lymphoid organs is dependent on the process of T cell maturation. T cell maturation begins in the thymus, and consists of processes distinct from positive selection. Maturation endows T cells with the ability to egress into secondary lymphoid organs, to proliferate, and to produce cytokines upon encountering antigens in the periphery18–22. As part of T cell maturation, thymocytes gradually become resistant to complement proteins in serum in order to persist in circulation and to become a part of the long-lived T cell pool23, 24, 25, 26. Although the mechanisms orchestrating complement resistance are not well characterized, NKAP, Runx-1, and HDAC3 are known to be important for this process24–26. NKAP is a highly conserved X chromosome encoded transcriptional regulator essential for conventional T cell development and maturation23, 27. NKAP-deficient recent thymic emigrants (RTEs) fail to mature, exhibit defects in proliferation and in vitro cytokine (IL-2 and TNF-α) production upon TCR stimulation compared to WT RTEs, and are rapidly eliminated by complement proteins in circulation23,27. While it is well appreciated that conventional T cells undergo maturation, whether Tregs undergo maturation in the periphery as well remains unanswered. In this study, NKAP was deleted in the Treg lineage using Foxp3-YFP-cre NKAP conditional knockout (cKO). We found that NKAP is critical for Treg maintenance in peripheral lymphoid organs. Conditional deletion of NKAP in Tregs caused peripheral Treg loss, recapitulating the scurfy phenotype. NKAP-deficient splenic Tregs are NRP1lo CD62L+ CD44− Qa2lo, indicating that they are Treg RTEs that cannot persist in the periphery, and also show greater abundance of signal joint TCR excision circle (TREC) formed during TCR rearrangement. NKAP-deficient Tregs were targeted by complement proteins in a cell intrinsic manner, suggesting the involvement of complement mediated clearance of Tregs.
2. Materials and Methods
2.1. Mice
Foxp3-YFP-cre mice and NKAP fl mice have been previously described27,28. Mice were housed in a barrier facility and all experiments were performed adhering to guidelines and approval of the Mayo Clinic IACUC committee. Male animals were analyzed between 4–7 days of age or around 3 weeks. Female mice were analyzed between 6–10 weeks of age. Foxp3-YFP-cre WT chimeric females have a Foxp3-YFP-cre allele on one X chromosome and are WT on the other X chromosome. Foxp3-YFP-cre NKAP cKO chimeric females have Foxp3-YFP-cre and NKAP fl alleles on one X chromosome and NKAP fl only on the other X chromosome. Scurfy (sf) mice were generated by crossing Foxp3sf female heterozygotes (Jax stock #000664) with male C57BL/6J mice29.
2.2. Flow Cytometry
Single-cell suspensions were prepared from thymus, spleen or pooled lymph nodes. Cell surface markers, CD4, CD8, CD44, CD62L, GITR, NRP-1 and CD25 were detected using fluorescent conjugated antibodies from BioLegend, Tonbo and eBioscience diluted in (FACS) buffer. Intracellular staining for Foxp3 was done after fixing and permeabilizing cells (Tonbo Foxp3 staining kit), using antibodies from eBioscience and Tonbo. Complement deposition was detected using biotinylated antibodies for complement C1q, complement C3 and complement C4 from Cedarlane and PE or APC conjugated streptavidin from Biolegend and eBioscience. For complement experiments, freshly harvested splenocytes were incubated in GVB++ buffer (Complement Technology) for one hour at room temperature and cells were washed with FACs buffer and stained with appropriate antibodies. All experiments included fixable viability dye (Tonbo) for analysis of live cells. Stained cells were analyzed using an Attune NxT flow cytometer (Thermofisher). Data was analyzed using FlowJo (Tree Star) 9.8 or 10. In all experiments, doublets and dead cells were excluded using permeable to fixable viability dye.
2.3. Detection of autoantibodies
Antibodies to double stranded DNA were detected using a kit from BioRad (Autoimmune EIA Anti-dsDNA Test). Serum was diluted 100-fold in phosphate buffered saline with 1% bovine serum albumin (PBS/BSA). The assay was completed according to the manufacturer’s instructions except for the substitution of horseradish peroxidase coupled goat anti-mouse IgG for the detection antibody (Southern Biotechnology Associates, diluted 2000 fold in PBS/BSA). Absorbance at 450 nM was measured using a Molecular Devices Spectromax microplate reader. Background signal from samples lacking sera was subtracted from the absorbance measured for each sera sample.
Anti-nuclear antibodies were detected by applying the same diluted sera to slides from a kit from BioRad (Kallestad HEp-2 Cell Line Substrate). The assay was completed according to the manufacturer’s instructions except for the substitution of AF488 coupled goat anti-mouse IgG for the detection antibody (InVitrogen, diluted to 4 µg/ml in PBS/BSA). Each well was examined at room temperature without immersion medium using a Leica DMI3000B fluorescence microscope with a 20× lens (Plan Fluotar type, numerical aperture of 0.4) and a FITC/EGFP filter cube. Images were acquired using a Q-Imaging Q1-Click camera and Q Capture Pro 6 software and saved as TIFF files. Adobe Illustrator was used to prepare the representative field shown.
2.4. Histology
Lungs, liver and kidneys were isolated from WT or Foxp3-YFP-cre NKAP cKO mice and kept in 10% formalin for fixation. Fixed samples were processed by paraffin embedding, sectioning and H&E stained as per standard procedures. Sections were viewed on a Leica DMI3000B microscope, at 40X magnification and captured using the Leica EC3 camera.
2.5. TCR excision circle assay
CD4 T cells were enriched from total splenocytes by negative selection. Splenocytes were isolated from Foxp3-YFP-cre WT chimeric female and Foxp3-YFP-cre NKAP cKO female mice. Fc receptors were blocked using TruStain fcX anti-CD16/32 Fc blocking antibody from BioLegend. Splenocytes were then labeled with biotinylated antibodies from Tonbo or BioLegend against CD8α, CD19, B220, CD11b, CD11c, Gr1, NK1.1, and γδ TCR and subsequently incubated with EasySep Streptavidin Mouse RapidSpheres (STEMCELL technologies). EasySep magnet was used to negatively select CD4+ splenocytes to a purity of greater than 90 percent. Cells were then labeled with anti-CD4, anti- CD25 and fixable viability dye, and CD4+ CD25+ YFP+ Tregs were sorted using a BD FACSAria. Genomic DNA was purified from sorted cells using a QIAamp DNA Blood Mini Kit (Qiagen). Quantitative PCR was then performed using a Step One Plus instrument and SYBR Green master mix (Applied Biosystems). TRECs were detected using PCR conditions and primers flanking the recombination site as reported30. Primers that detect the mouse p16 promoter (5'ACACTCCTTGCCTACCTGAA3' and 5'CGAACTCGAGGAGAGCCATC3') were used to control for total DNA concentration. Difference in TREC content between samples was then determined using the ΔΔCt method. This analysis was performed on cells sorted, on four separate occasions, from a pair of mice (one Foxp3-YFP-cre WT chimeric female and one Foxp3-YFP-cre NKAP cKO chimeric female). Increased TREC content in the cKO was apparent within each pair. Thus, for the final analysis, DNA from one of the WT mice was chosen as a normalizer and a paired Student’s T test was used to determine significance.
2.6. Statistical Analysis
All statistical analysis involved enumeration of absolute cell counts or MFI, calculation of standard error of the mean, and statistical significance determined using a two-tailed unpaired or paired Student T test using Microsoft Excel or GraphPad Prism as stated in the figure legends.
3. Results and Discussion
3.1. Foxp3-YFP-cre NKAP cKO mice develop lethal autoimmunity similar to ‘scurfy’ phenotype
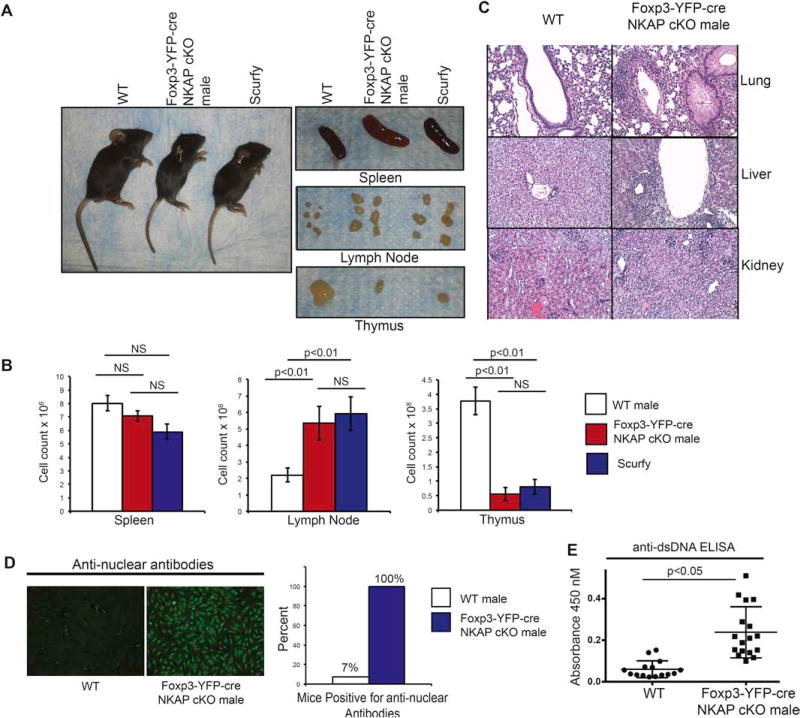
To examine the role of NKAP in Treg-mediated immune system homeostasis, we conditionally deleted NKAP in Tregs by crossing Foxp3-YFP-cre mice to NKAP floxed mice. As both Foxp3-YFP-cre and floxed NKAP are located on the X chromosome, the generation of Foxp3-YFP-cre NKAP conditional knock out (cKO) mice required a crossover event to place both Foxp3-YFP-cre and floxed NKAP on the same X chromosome due to random X inactivation in females and hemizygosity in males. The breeding scheme to produce Foxp3-YFP-cre NKAP cKO mice is shown in Fig S1. Foxp3-YFP-cre NKAP cKO male mice spontaneously developed early onset, systemic autoimmunity similar to scurfy mice (Fig 1). They failed to thrive, developed severe dermatitis as evidenced by thickening and crusting of skin on ears, tail and paws, and became moribund by three weeks of age, similar to scurfy mice (Fig 1A). Both Foxp3-YFP-cre NKAP cKO male mice and scurfy mice developed splenomegaly and lymphadenopathy, indicating lymphoproliferation (Fig 1B). We observed no significant change in splenocyte number despite an increase in the size of spleens in Foxp3-YFP-cre NKAP cKO male mice. However, there was a significant increase in lymph node cellularity, comparable to increased lymph node cellularity in scurfy mice. Thymic atrophy was observed in Foxp3-YFP-cre NKAP cKO male mice and scurfy mice by three weeks of age (Fig 1B). The thymus is known to be acutely susceptible to atrophy under non-infectious or infectious inflammatory conditions via two possible mechanisms of stress-induced thymic involution, i.e., increased apoptosis of double positive (DP) thymocytes and programmed loss of function of medullary thymic epithelial cells (mTECs)31. It has been shown that the loss of Foxp3+ Tregs in scurfy mice results in a loss of DP thymocytes cells which make up the majority of thymocytes, mediating rapid thymic involution by three weeks of age as a byproduct of unchecked and escalating inflammation32. While the exact mechanism of DP thymocyte disappearance is unclear, this is a cell-extrinsic outcome of systemic inflammation. Thymic atrophy observed in Foxp3-YFP-cre NKAP cKO male mice is likely secondary to systemic inflammation as well, phenocopying the scurfy mice. In addition, Foxp3-YFP-cre NKAP cKO male mice showed lymphocytic infiltration into multiple vital organs, particularly the liver and the lungs (Fig 1C). This is consistent with previous records of scurfy mice exhibiting striking lymphocytic infiltration into lungs and liver33. Previous studies have reported that the production of autoantibodies mediates autoimmunity and morbidity of scurfy mice, and the loss of B cells in scurfy mice prolongs their health and survival34–36. Both anti-double stranded DNA and anti-nuclear autoantibodies were detectable in sera of Foxp3-YFP-cre NKAP cKO male mice, indicating B cell activation and peripheral tolerance breakdown (Fig 1C–E). Thus, the loss of NKAP in Tregs leads to severe, systemic inflammation similar to the scurfy phenotype in Foxp3 mutant mice.
Figure 1. Treg-specific deletion of NKAP recapitulates the “scurfy” phenotype by the age of three weeks.
(A) Foxp3-YFP-cre NKAP cKO male mice juxtaposed with wildtype (WT) and Foxp3 mutant scurfy mice as well as spleens, lymph nodes (LN) and thymi. (B) Examination of absolute cell counts of splenocytes, lymph node cells and thymocytes. Bar graphs are averaged absolute cell counts per organ from at least 5 independent experiments with at least 5 mice per group, and error bars are standard error of mean (SEM). (C) Hematoxylin and Eosin staining from sections from Foxp3-YFP-cre NKAP cKO male mice and WT littermate lungs, liver and kidneys at three weeks of age. Data is representative of three organs per genotype. (D) Presence of antinuclear antibodies (ANA) in WT or cKO serum was determined by incubating serum with irradiated HepG2 cells, followed by detection using fluorescent anti-mouse secondary antibody. Data is representative of 15 WT and 17 Foxp3-YFP-cre NKAP cKO male mice. (E) Detection of anti double stranded DNA (dsDNA) antibodies. Data is averaged from of 15 WT and 17 Foxp3-YFP-cre NKAP cKO male mice.
3.2. Treg-specific loss of NKAP results in increased activation of peripheral T cells
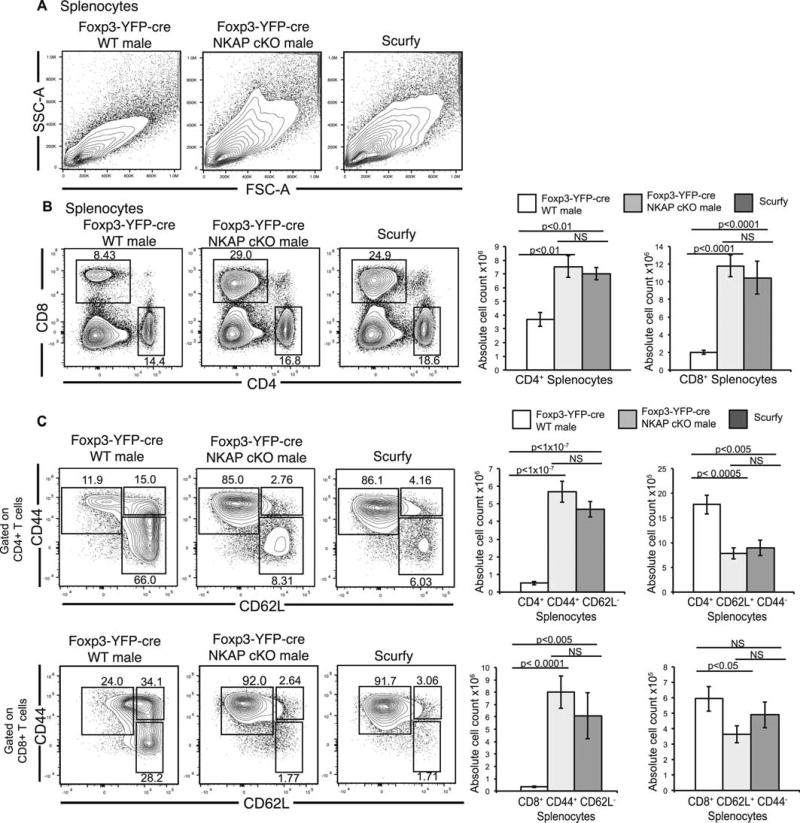
Activated CD4 T cells are thought to be the main driver of pathogenesis in scurfy mice, contributing to the expansion and activation of CD8 T cells, B cells, and myeloid cells37,38. Enlargement of lymph nodes and increased numbers of lymph node cells can be observed in scurfy mice as early as 5 days of age38. Scurfy mice exhibit increased size of lymphocytes in spleen as a result of increased blasting, increased conventional T cell number, and conventional T cells acquire a CD44hi CD62Llo activated/memory phenotype12,38. Splenocytes in Foxp3-YFP-cre NKAP cKO male mice also increased in size as determined by increased forward and side scatter (Fig 2A). By the age of three weeks, there was a significant increase in the frequencies and absolute cell counts of splenic CD4 and CD8 T cells, similar to scurfy mice (Fig 2B). Furthermore, CD4 and CD8 T cells in Foxp3-YFP-cre NKAP cKO male mice and scurfy mice acquired a CD44hi CD62Llo activated/memory phenotype compared to Foxp3-YFP-cre WT males (Fig 2C) and WT littermates (data not shown), suggesting dysregulated T cell activation. These observations suggest that expression of NKAP in Tregs is crucial for immune homeostasis and peripheral tolerance.
Figure 2. Treg-specific NKAP deletion results in lymphoproliferation and conventional T cell activation.
(A) Forward scatter and side scatter analysis of splenocytes from Foxp3-YFP-cre WT, Foxp3-YFP cre NKAP cKO and scurfy mice. (B) Analysis of frequency and absolute numbers of CD4 and CD8 T cells from spleens of Foxp3-YFP-cre WT, Foxp3-YFP-cre NKAP cKO and scurfy mice. (C) Analysis of frequency and absolute numbers of activated memory (CD44+ CD62L−) and naïve (CD44+ CD62L−) CD4+ and CD8+ T cells from spleens of Foxp3-YFP-cre WT, Foxp3-YFP-cre NKAP cKO and scurfy mice. All data shown are representative of at least 6 independent experiments with 6–7 mice in each group. Bar graphs illustrate mean absolute cell counts and error bars are SEM.
3.3. NKAP deficient thymically derived Tregs fail to persist in the periphery although thymic development of NKAP deficient Tregs is normal
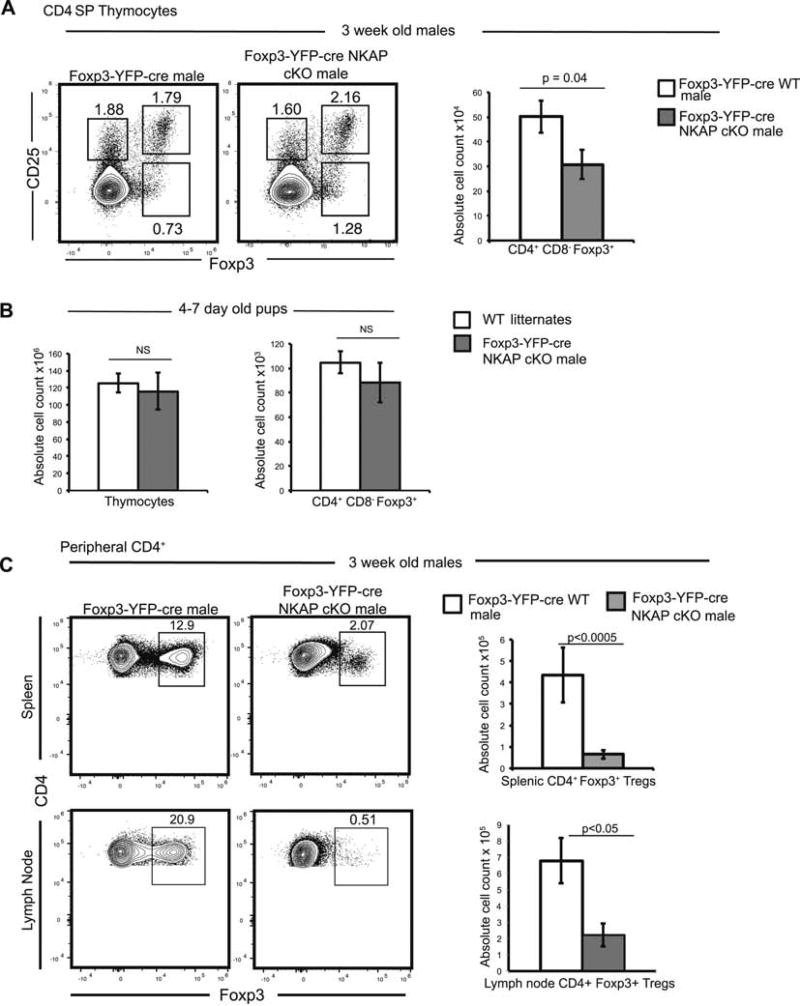
The thymus is the main source of Tregs (thymic Tregs or tTregs) but Tregs can also arise from conventional T cells in the periphery (induced Tregs or iTregs) when naïve conventional T cells are stimulated by self and foreign antigens in the presence of TGF-β, IL-10, retinoic acid, and IL-27,39. Foxp3 is absolutely required for the development of tTregs and iTregs, as the deletion of Foxp3 in T cells leads to a block in Treg development12. Thymic Treg development has been proposed to occur in a two-step manner, whereby strong TCR interactions with cognate pMHC drive increased expression of the high affinity IL-2 receptor (CD25), which then induces Foxp3 expression7. Recent studies indicate that Tregs arise from alternate Foxp3+ CD25− progenitors as well6. In the absence of NKAP, Treg development proceeded normally in the thymus (Fig 3A). However, there was a significant reduction in absolute cell counts of CD4+ CD8− Foxp3+ cells in Foxp3-YFP-cre NKAP cKO males in the thymus (Fig 3A). Reduced Treg cellularity could be a result of decrease in total thymocytes as a result of thymic atrophy, a byproduct of systemic inflammation. Thus, we examined 4–7 day old pups, prior to onset of visible characteristics of inflammation or autoimmunity, when thymi of WT littermates and male cKO mice were comparable in size and cellularity (Fig 3B). 4–7 day old cKO and WT littermates showed similar absolute cell count of CD4+ CD8− Foxp3+ Tregs in the thymus (Fig 3B), demonstrating that NKAP is not required for thymic Treg development. In contrast, Tregs in the spleen are severely decreased by 3 weeks of age, indicating that NKAP deficient Tregs are unable to persist in the periphery (Fig 3C). Similarly, very few CD4+ Foxp3+ Tregs were present in the lymph nodes (Fig 3B). Thus, our data indicates that NKAP is not required for thymic Treg development, but is a key determinant of Treg persistence in the periphery.
Figure 3. NKAP deficient Tregs develop in the thymus but fail to persist in the periphery.
(A) Examination of thymic development of Tregs at three weeks of age. Analysis of frequency of CD25+ Foxp3−, CD25− Foxp3+ and CD25+ Foxp3+ cells and absolute cell count of CD4+ CD8− Foxp3+ thymic Tregs. Data is from at least 6 mice per genotype from 3 independent experiments, and bar graphs show mean absolute cell count and SEM. (B) Examination of Treg development in pups aged 4–7 days. Data is from a total of 5 experiments with at least 5 mice in each genotype, and bar graphs show average absolute cell count and SEM. (C) Examination of Treg frequency and absolute cell counts in peripheral lymphoid organs (spleen, and pooled brachial, inguinal and cervical LN). Data is from at least 4 representative independent experiments with at least 4 mice per group, and bar graphs show mean absolute cell count and SEM.
3.4. Inability of NKAP-deficient Tregs to persist in the periphery is a cellintrinsic defect and residual NKAP deficient Tregs are recent thymic emigrants
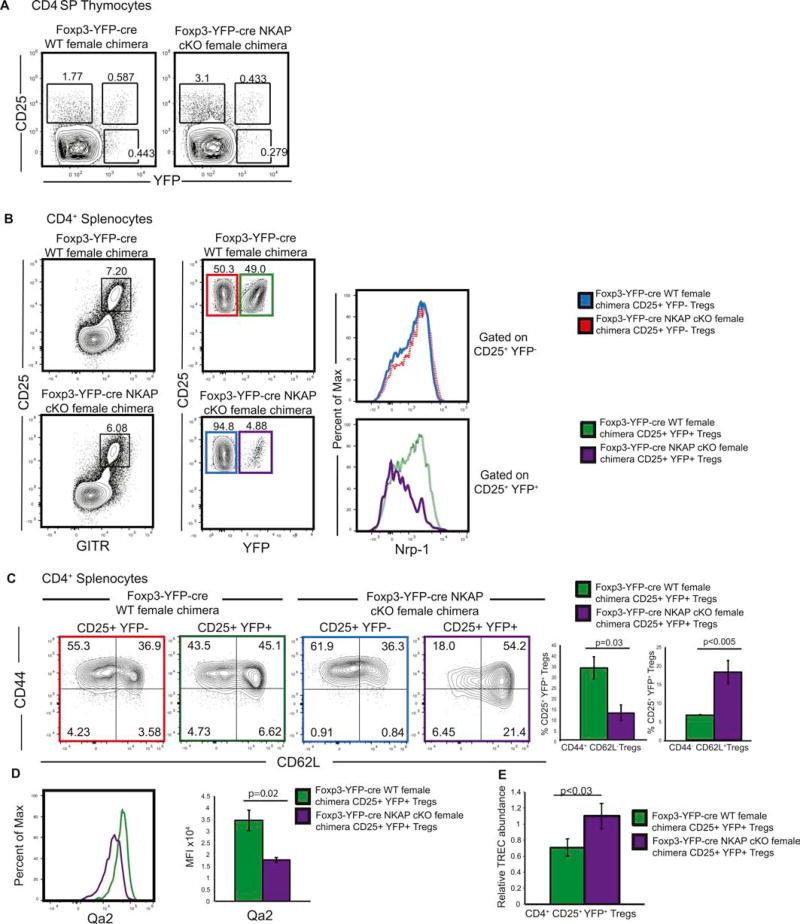
Next, we assessed whether the disappearance of NKAP deficient Tregs was a cell intrinsic effect or an outcome, of rampant inflammation. To bypass any influence due to inflammation, we compared Foxp3-YFP-cre NKAP cKO chimeric females to Foxp3-YFP-cre WT chimeric females. Foxp3-YFP-cre WT chimeric females have Foxp3-YFP-cre allele on one X chromosome and are WT on the other X chromosome. Foxp3-YFP-cre NKAP cKO chimeric females have Foxp3-YFP-cre and NKAP fl allele on one X chromosome and NKAP fl only on the other X chromosome. Therefore, in Foxp3-YFP-cre NKAP cKO chimeric females, due to random X-chromosome inactivation, half of the Tregs were YFP+ NKAP deficient and half were YFP− NKAP sufficient, allowing us to compare both WT and NKAP deficient Tregs from the same mice. Foxp3-YFP-cre NKAP cKO female mice did not show any overt signs of inflammation or stress, thrived, reproduced and survived into adulthood, thus permitting us to examine cell intrinsic effects of NKAP’s deletion on Tregs in the absence of an inflammatory environment. Expression of YFP was analyzed in CD4+ CD25hi GITRhi Tregs. Both Foxp3-YFP-cre WT and Foxp3-YFP-cre NKAP cKO chimeric female mice developed similar frequencies of YFP+ Tregs in the thymus, indicating that the generation of Tregs in the thymus does not require NKAP (Fig 4A). However, while Foxp3-YFP-cre WT chimeric female mice harbored approximately similar frequencies of YFP+ and YFP− Tregs in the periphery, Foxp3-YFP-cre NKAP cKO chimeric mice showed a dramatic skewing towards the YFP− NKAP sufficient population over a YFP+ NKAP deficient Treg population. This demonstrates that the disappearance of Tregs in the periphery is a cell-intrinsic defect (Fig 3B). NKAP-deficient Tregs in Foxp3-YFP-cre NKAP cKO chimeric females also expressed lower levels of Nrp-1. Nrp-1lo NKAP-deficient Tregs may represent either peripherally derived iTregs or thymically derived RTE Tregs40. To examine these possibilities, we examined CD44 and CD62L expression in splenic Tregs. Using Rag2GFP mice, it has been shown that CD44lo CD62Lhi Tregs are recent thymic emigrants (RTEs) and are precursors to CD44hi CD62Llo Tregs41. NKAP deficient splenic Tregs and lymph node Tregs have a CD44lo CD62Lhi phenotype, consistent with RTEs. It has also been shown that Qa2 is a phenotypic marker of maturation of Tregs42. We found that NKAP deficient Tregs expressed lower levels of Qa2 (Fig 4C), suggesting that NKAP deficient Tregs are eliminated at the RTE stage24. We also measured the relative abundance of TCR excision circles (TREC) as readout for recent thymic emigrant (RTE) status and find a statistically significant increase in relative abundance of TRECs in NKAP deficient Tregs in Foxp3-YFP-cre NKAP cKO chimeric females compared to Foxp3-YFP-cre WT females, consistent with NKAP-deficient splenic Tregs being RTES. Therefore, our analysis indicates that NKAP deficient Tregs in Foxp3- YFP-cre NKAP cKO chimeric females are RTEs.
Figure 4. Loss of Tregs as a result of NKAP deficiency is a cell-intrinsic defect.
(A) Analysis of Treg development in Foxp3-YFP-cre NKAP cKO chimeric females and Foxp3-YFP-cre WT chimeric females. Data is representative of 7 independent experiments with 7 mice in each group. (B) Analysis of splenic CD4+ CD25+ GITR+ YFP+ Treg and CD4+ CD25+ GITR+ YFP− Treg frequencies and examination of expression of Nrp-1 in CD4+ CD25+ YFP+ Tregs, and CD4+ CD25+ YFP− Tregs in Foxp3-YFP-cre NKAP cKO chimeric females and Foxp3-YFP-cre WT chimeric females. Data is representative of 6 experiments with 6 mice in each group. (C) Examination of CD44 and CD62L expression in CD4+ CD25+ YFP+ and CD4+ CD25+ YFP− Tregs in Foxp3-YFP-cre WT and Foxp3-YFP-cre NKAP cKO female mice. Use of color to identify samples refers to gated populations in (B). Data is representative from 3 independent experiments with at least 3 mice per group. (D) Examination of Qa2 in CD4+ CD25+ YFP+ Tregs in Foxp3-YFP-cre NKAP cKO chimeric females and Foxp3-YFP-cre WT chimeric females. Use of color to identify samples refers to gated populations in (B). Data is representative from 3 independent experiments with at least 3 mice per group. (E) Analysis of TCR excision circle (TREC) performed on CD4+ CD25+ YFP+ cells sorted, from four pairs of mice, a Foxp3- YFP-cre NKAP cKO female chimeric mouse and a Foxp3-YFP cre WT female chimeric mouse. For the final analysis, DNA from one of the WT mice was chosen as a normalizer and a paired Student’s T test was used to determine significance.
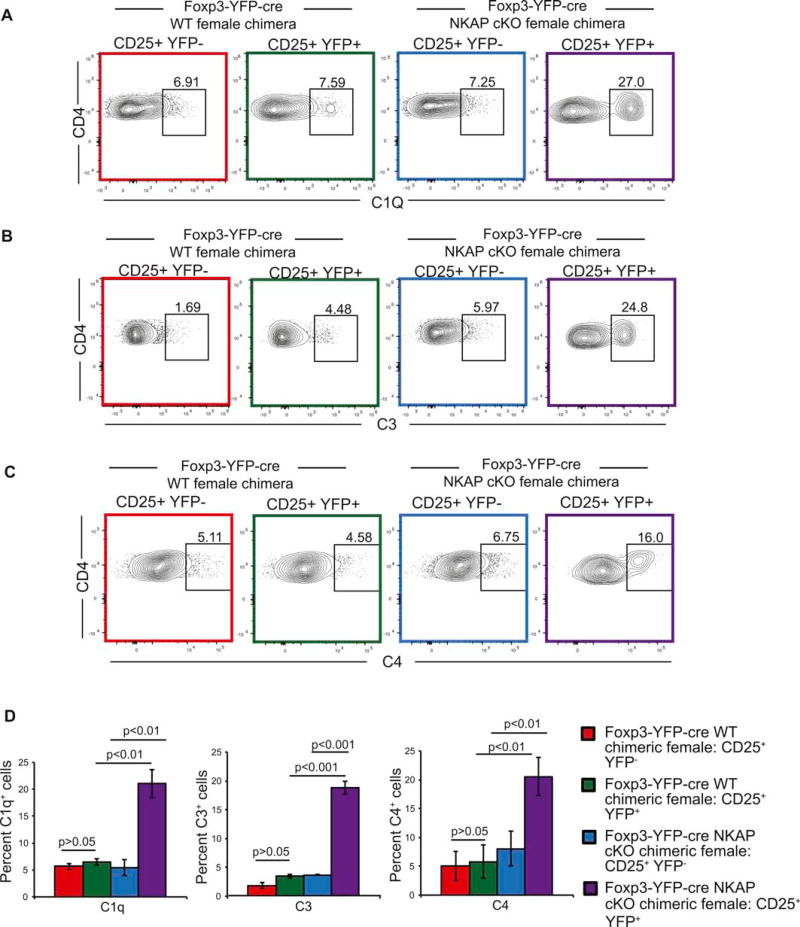
3.5. NKAP-deficient Tregs are targeted by complement proteins
Thymocytes undergo post-positive selection maturation when they gain protection from complement proteins in the circulation. NKAP deficient conventional T cells fail to undergo appropriate maturation and are rapidly eliminated by complement. Therefore, thymically derived NKAP deficient Tregs may not persist due to a failure of protection from complement24. As previous studies revealed heightened complement activation in the periphery of scurfy mice, we examined whether NKAP deficient Tregs were targeted by complement in healthy Foxp3-YFP-cre NKAP cKO female chimeric mice34. Increased deposition of C1q, C3 and C4 was observed on NKAP deficient YFP+ Tregs but not YFP− NKAP sufficient Tregs from the same mouse. This is consistent with our observation in CD4-cre NKAP cKO mice24. No appreciable C1q, C3 and C4 deposition was observed in the YFP+ or YFP− Treg populations in Foxp3-YFP-cre female chimeric control mice, demonstrating that the deposition of complement was due to the lack of NKAP. Our results indicate that NKAP deficient Tregs fail to gain resistance to complement, and are eliminated by complement in the periphery.
4. Discussion
Whether Tregs emerge from the thymus fully mature and able to mediate peripheral tolerance and immune homeostasis remains an unanswered question in the field. Current evidence suggests that conventional T cell maturation programs are distinct from positive selection, initiate in the thymus, and reach completion in the periphery21. These programs enable thymocyte egress, proliferation and cytokine production upon TCR stimulation, and survival in the long-lived T cell pool. NKAP is required for the acquisition of functional competency by conventional T cells, as well as resistance to serum complement proteins as demonstrated by the CD4-cre NKAP cKO mouse model where the deletion of NKAP occurs at the DP stage. Loss of NKAP at the DP stage occurs prior to positive selection, lineage commitment, and negative selection but selectively imposes a failure of late T cell maturation as defined by the inability of conventional T cells to produce cytokines and the inability of both conventional and regulatory T cells to persist in the long-lived T cell pool although their development is unaltered. CD4-cre NKAP cKO mice do not succumb to the scurfy phenotype despite peripheral loss of Tregs, revealing the fundamental importance of functional maturation of conventional T cells in driving the scurfy phenotype. These observations also raise the question of the requirement for NKAP at a specific time during T cell development in preparation for maturation, i.e., whether NKAP is required at the DP stage for maturation initiation or whether a later deletion of NKAP can lead to a similar outcome.
In this study, we used Foxp3-YFP-cre NKAP cKO mice in which the deletion of NKAP occurs following the expression of Foxp3 upon agonist selection; hence, this is a later deletion of NKAP, occurring well after positive selection and commitment to the CD4 lineage, and specifically in Tregs. The observation that NKAP-deficient Tregs undergo complement opsonization similar to immature NKAP-deficient conventional T cells suggests that Tregs also undergo a program of maturation and must acquire the ability to protect themselves from complement prior to thymic egress. This similar outcome although deletion of NKAP happens at a later stage demonstrates that continued expression of NKAP is required, perhaps up until egress of the cells into the periphery or continued expression after egress, to ensure protection of cells from complement proteins. Our findings raise two interesting questions. Firstly, is maturation is a state that must be actively maintained rather than a singular irreversible step in the lifetime of a T cell and hence, a critical determinant of T cell mediated homeostasis and autoimmunity? Secondly, is NKAP is specifically required at the time point prior to egress and once, T cell have achieved maturation, it is dispensable thereafter? Our data does not allow for discrimination between these two possibilities. Other systems such as the distal-Lck cre whereby the deletion of NKAP would happen in the periphery can be used in the future to gain insight into the temporal requirements of NKAP in maturation.
It is currently unclear what is the initiating event that drives the deposition of complement on peripheral NKAP-deficient Tregs. Importantly, it is well appreciated that thymocytes are susceptible to binding by complement proteins and the amount of complement binding is inversely proportional to development, i.e, as T cell mature, complement deposition decreases24–26. NKAP deficient Tregs are CD62Lhi CD44lo and Qa2lo, demonstrating that they are RTEs. An increased relative abundance of TCR excision circles (TRECs), an indicator of RTE status, further supports that NKAP deficient Tregs are eliminated at the RTE stage. CD4-cre NKAP cKO RTEs have altered sialylation as demonstrated by decreased expression of ligands for Siglec-E, which may stimulate binding of natural IgM to initiate the classical complement pathway, as well as decreased expression of the complement regulatory protein DAF (also known as CD55)24. However, there is minimal difference in Siglec-E binding to, or DAF/CD55 expression on splenic NKAP-deficient Tregs (Figure S2), indicating that other molecular mechanisms may be driving selective opsonization of NKAP deficient Tregs by complement. Because the loss of NKAP does not lead to perturbations of well-characterized cell survival pathways as knocking out the pro-apoptotic protein Bax or transgenic overexpression of the pro-survival protein Bcl2 in CD4-cre NKAP cKO T cells did not rescue the defect of cell survival, we probed the alternative mechanism of complement-mediated elimination. Interestingly, a recent publication notes that human recent thymic emigrants express higher levels of complement receptors 1 and 2 although the relevance of these proteins is not clear43.
Previous studies have suggested that the time taken for conventional RTEs to fully mature and gain functional competency in the periphery is 2–3 weeks18. The loss of Tregs results in rapid autoimmunity that is initiated between the second and third week after birth in male mice and Tregs emerge at around day 3 postpartum44. This suggests that the maturation time of Tregs in murine neonates must be temporally shorter than that of conventional T cells Intriguingly, the requirement of Tregs early on in life in order to keep conventional T cells in check, as evidenced by tolerance breakdown in scurfy mice and Foxp3-YFP-cre NKAP cKO male mice, also suggests that conventional T cells in neonates, which are predominantly RTEs, may undergo maturation at a rate different than in young adults. Interestingly, serum complement levels are decreased 10–80% in both neonatal mice and humans45. It has been suggested that maturation is highly context dependent, shaped by cell intrinsic factors and extrinsic cues, and thus, may occur differently in a lymphopenic environment and at different stages in life18. Our work raises the question of whether conventional RTE maturation kinetics in secondary lymphoid organs is altered in the absence of peripheral Tregs. Although beyond the scope of this study, the differences in neonatal and adult RTEs, both conventional and regulatory, merit future investigation.
5. Conclusions
Loss of NKAP specifically in Tregs resulted in systemic autoimmunity similar to the ‘scurfy’ phenotype seen in Foxp3 mutant mice, although development of thymic Tregs (tTregs) occurred unimpeded. In female chimeras, which generated NKAP sufficient and NKAP deficient Tregs in approximately equal proportions, NKAP deficient recent thymic emigrant (RTEs) Tregs were specifically targeted by complement proteins leading to their disappearance. Thus, NKAP is required for long-term persistence of Tregs in the periphery and prevention of autoimmunity.
Supplementary Material
Figure 5. Tregs are opsonized by complement proteins.
(A–C) Increased deposition of complement components C1q (A), C3 (B) and C4 (C) on NKAP deficient Tregs. Data is from at least 3 independent experiments with at least 3 mice per group). (D) Enumeration of mean frequency of cells bound by complement. Data is from 3 independent experiments with 3 mice per genotype. Bar graphs are mean percent complement positive cells, and error bars are SEM. Throughout the figure, use of color to identify samples refers to gated populations in Figure 5(B).
Highlights.
The transcriptional regulator NKAP is required for Treg survival in the periphery.
NKAP deletion in Tregs leads to lethal autoimmunity.
NKAP deficient mice resemble of the ‘scurfy’ phenotype of Foxp3 mutant mice.
NKAP-deficient Tregs are targeted for elimination by complement.
Acknowledgments
We thank Michael Lehrke and Paul Belmonte for critical reading of the manuscript. This was work supported by NIH R01 AI083279 to V.S.S.
Abbreviations
- cKO
conditional knockout
- IPEX
Immunodysregulation Polyendocrinopathy Enteropathy X-linked syndrome
- tTReg
Thymic Tregs
- iTreg
Induced Tregs
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
B.D. designed and performed experiments and wrote the manuscript, M.S. designed and performed experiments and edited the manuscript, J.Y.C. and S.R.A. maintained the mouse colony and genotyped the mice, V.S.S. designed experiments and edited the manuscript.
The authors declare no competing financial interests.
References
- 1.Hogquist KA, Jameson SC. The self-obsession of T cells: how TCR signaling thresholds affect fate ‘decisions’ and effector function. Nat. Immunol. 2014;15:815–23. doi: 10.1038/ni.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jordan MS, et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat. Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 3.Hsieh C-S, Zheng Y, Liang Y, Fontenot JD, Rudensky AY. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat. Immunol. 2006;7:401–410. doi: 10.1038/ni1318. [DOI] [PubMed] [Google Scholar]
- 4.Hsieh C-SS, et al. Recognition of the Peripheral Self by Naturally Arising CD25+ CD4+ T Cell Receptors. Immunity. 2004;21:267–277. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Moran AE, et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J. Exp. Med. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahmud SA, et al. Costimulation via the tumor-necrosis factor receptor superfamily couples TCR signal strength to the thymic differentiation of regulatory T cells. Nat. Immunol. 2014;15:473–81. doi: 10.1038/ni.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lio CWJ, Hsieh CS. A Two-Step Process for Thymic Regulatory T Cell Development. Immunity. 2008;28:100–111. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T Cells and Immune Tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Stritesky GL, Jameson SC, Hogquist KA. Selection of Self-Reactive T Cells in the Thymus. Annu. Rev. Immunol. 2012;30:95–114. doi: 10.1146/annurev-immunol-020711-075035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malchow S, et al. Aire Enforces Immune Tolerance by Directing Autoreactive T Cells into the Regulatory T Cell Lineage. Immunity. 2016;44:1102–1113. doi: 10.1016/j.immuni.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunkow ME, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat. Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 12.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 13.Lyon MF, Peters J, Glenister PH, Ball S, Wright E. The scurfy mouse mutant has previously unrecognized hematological abnormalities and resembles Wiskott-Aldrich syndrome. Proc. Natl. Acad. Sci. U. S. A. 1990;87:2433–2437. doi: 10.1073/pnas.87.7.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fontenot JD, et al. Regulatory T cell lineage specification by the forkhead transcription factor Foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 15.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 16.d’Hennezel E, et al. FOXP3 Forkhead Domain Mutation and Regulatory T Cells in the IPEX Syndrome. N. Engl. J. Med. 2009;361:1710–1713. doi: 10.1056/NEJMc0907093. [DOI] [PubMed] [Google Scholar]
- 17.Wildin RS, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat. Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 18.Fink PJ, Hendricks DW. Post-thymic maturation: young T cells assert their individuality. Nature. 2011;11:544–549. doi: 10.1038/nri3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boursalian TE, Golob J, Soper DM, Cooper CJ, Fink PJ. Continued maturation of thymic emigrants in the periphery. Nat Immunol. 2004;5:418–425. doi: 10.1038/ni1049. [DOI] [PubMed] [Google Scholar]
- 20.Priyadharshini B, et al. Maturation-Dependent Licensing of Naive T Cells for Rapid TNF Production. PLoS One. 2010;5 doi: 10.1371/journal.pone.0015038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hogquist KA, Xing Y, Hsu F-C, Shapiro VS. T Cell Adolescence: Maturation Events Beyond Positive Selection. J. Immunol. 2015;195:1351–1357. doi: 10.4049/jimmunol.1501050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Houston EG, Higdon LE, Fink PJ. Recent thymic emigrants are preferentially incorporated only into the depleted T-cell pool. Proc. Natl. Acad. Sci. 2011;108:5366–5371. doi: 10.1073/pnas.1015286108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu F, Pajerowski AG, Nelson-Holte M, Sundsbak R, Shapiro VS. NKAP is required for T cell maturation and acquisition of functional competency. J. Exp. Med. 2011;208:1291–304. doi: 10.1084/jem.20101874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu F, et al. Immature recent thymic emigrants are eliminated by complement. J. Immunol. 2014;193:6005–15. doi: 10.4049/jimmunol.1401871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsu F, et al. An Essential Role for the Transcription Factor Runx1 in T Cell Maturation. Sci. Rep. 2016;6:23533. doi: 10.1038/srep23533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsu F, et al. Histone Deacetylase 3 Is Required for T Cell Maturation. J. Immunol. 2015;195:1578–1590. doi: 10.4049/jimmunol.1500435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pajerowski AG, Nguyen C, Aghajanian H, Shapiro MJ, Shapiro VS. NKAP Is a Transcriptional Repressor of Notch Signaling and Is Required for T Cell Development. Immunity. 30:696–707. doi: 10.1016/j.immuni.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rubtsov YP, et al. Regulatory T Cell-Derived Interleukin-10 Limits Inflammation at Environmental Interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 29.Godfrey VL, Wilkinsonf JE, Rinchik EM, Russell LB. Fatal lymphoreticular disease in the scurfy (sf) mouse requires T cells that mature in a sf thymic environment: Potential model for thymic education. Med. Sci. 1991;88:5528–5532. doi: 10.1073/pnas.88.13.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phillips JA, et al. IL-7 Gene Therapy in Aging Restores Early Thymopoiesis without Reversing Involution. J. Immunol. 2004;173:4867–4874. doi: 10.4049/jimmunol.173.8.4867. [DOI] [PubMed] [Google Scholar]
- 31.Dooley J, Liston A. Molecular control over thymic involution: From cytokines and microRNA to aging and adipose tissue. Eur. J. Immunol. 2012;42:1073–1079. doi: 10.1002/eji.201142305. [DOI] [PubMed] [Google Scholar]
- 32.Liston A, et al. Lack of Foxp3 function and expression in the thymic epithelium. J. Exp. Med. 2007;204:475–480. doi: 10.1084/jem.20062465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huter EN, et al. TGF-beta-induced Foxp3+ regulatory T cells rescue scurfy mice. Eur. J. Immunol. 2008;38:1814–21. doi: 10.1002/eji.200838346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aschermann S, et al. B cells are critical for autoimmune pathology in Scurfy mice. Proc. Natl. Acad. Sci. U. S. A. 2013;110:19042–19047. doi: 10.1073/pnas.1313547110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hadaschik EN, et al. Regulatory T cell deficient scurfy mice develop systemic autoimmune features resembling lupus-like disease. Arthritis Res. Ther. 2015;17:35. doi: 10.1186/s13075-015-0538-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wildin RS, Freitas A. IPEX and FOXP3: Clinical and research perspectives. Journal of Autoimmunity. 2005;25:56–62. doi: 10.1016/j.jaut.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 37.Blair PJ, et al. CD4+CD8-T cells are the effector cells in disease pathogenesis in the scurfy (sf) Mouse. J. Immunol. 1994;153:3764–3774. [PubMed] [Google Scholar]
- 38.Clark L, et al. Cellular and Molecular Characterization of the scurfy Mouse Mutant. J. Immunol. 1999;162:2546–2554. [PubMed] [Google Scholar]
- 39.Shevach EM, Thornton AM. tTregs, pTregs, and iTregs: Similarities and differences. Immunol. Rev. 2014;259:88–102. doi: 10.1111/imr.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yadav M, et al. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J. Exp. Med. 2012;209:1713–1722. doi: 10.1084/jem.20120822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smigiel KS, et al. CCR7 provides localized access to IL-2 and defines homeostatically distinct regulatory T cell subsets. J. Exp. Med. 2014;211:121–36. doi: 10.1084/jem.20131142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cowan JE, McCarthy NI, Anderson G. CCR7 Controls Thymus Recirculation, but Not Production and Emigration, of Foxp3+ T Cells. Cell Rep. 2016;14:1041–1048. doi: 10.1016/j.celrep.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pekalski ML, et al. Neonatal and adult recent thymic emigrants produce IL-8 and express complement receptors CR1 and CR2. JCI insight. 2017;2 doi: 10.1172/jci.insight.93739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fontenot JD, Dooley JL, Farr AG, Rudensky AY. Developmental regulation of Foxp3 expression during ontogeny. J. Exp. Med. 2005;202:901–906. doi: 10.1084/jem.20050784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mcgreal EP, Hearne K, Spiller OB. Off to a slow start: Underdevelopment of the complement system in term newborns is more substantial following premature birth. Immunobiology. 2012;217:176–186. doi: 10.1016/j.imbio.2011.07.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.