Abstract
Hypertension, an important cause of chronic kidney disease, is characterized by peritubular capillary (PTC) loss. Circulating levels of endothelial microparticles (EMPs) reflect systemic endothelial injury. We hypothesized that systemic and urinary PTC-EMPs levels would reflect renal microvascular injury in hypertensive patients. We prospectively measured by flow-cytometry renal vein, inferior vena cava, and urinary levels of EMPs in essential (EH, n=14) and renovascular (RVH, n=24) hypertensive patients, and compared them with peripheral blood and urinary levels in healthy volunteers (HVs, n=14). PTC-EMPs were identified as urinary exosomes positive for the PTC marker plasmalemmal-vesicle-associated protein. In 7 RVH patients, PTC and fibrosis were also quantified in renal biopsy, and in 18 RVH patients, PTC-EMPs were measured again 3 months after continued medical therapy with or without stenting (n=9 each). Renal vein and systemic PTC-EMPs levels were not different among the groups, whereas their urinary levels were elevated in both RVH and EH vs. HVs (56.8±12.7 and 62.8±10.7 vs. 34.0±17.8%, both p≤0.001). Urinary PTC-EMPs levels correlated directly with blood pressure and inversely with estimated glomerular filtration rate. Furthermore, in RVH, urinary PTC-EMPs levels correlated directly with stenotic-kidney hypoxia, histological PTC count, and fibrosis, and inversely with cortical perfusion. Three months after treatment, the change in urinary PTC-EMPs levels correlated inversely with a change in renal function (r=−0.582, p=0.011). Therefore, urinary PTC-EMPs levels are increased in hypertensive patients, and may reflect renal microcirculation injury, whereas systemic PTC-EMPs levels are unchanged. Urinary PTC-EMPs may be useful as novel biomarkers of intrarenal capillary loss.
Keywords: urine, exosomes, hypertension, chronic kidney disease, microvascular rarefaction
INTRODUCTION
Peritubular capillary (PTC) damage and rarefaction characterizes chronic kidney disease (CKD), and accompanies declining renal function in diverse renal disorders.1–3 PTC loss has been associated with inappropriate and sustained activation of the peritubular endothelium.4 The ability to detect PTC injury non-invasively could allow monitoring of renal disease progression. However, to date there is no method to assess non-invasively PTC dropout in human subjects.
Extracellular vesicles (EVs) are membrane-bound vesicles produced and released by cells under duress, and are classified as exosomes, microparticles, or apoptotic bodies depending on their origin, size and content.5 Within the kidney, EVs can originate from diverse cell types such as podocytes, tubular epithelial cells, or endothelial cells, and changes in the numbers or characteristics of EVs may be associated with development of disease.6 Therefore, levels of urinary EVs have been used as biomarkers in various kidney disorders, including acute kidney injury,7 polycystic kidney disease,8 and glomerular diseases.9 We have previously shown that urine of hypertensive patients contains EVs reflecting podocyte injury and differential expression of microRNA.10,11 However, EVs reflecting endothelial damage of PTC have not been identified.
Endothelial microparticles (EMPs) are EVs that are shed by the endothelium as a result of activation, injury, or apoptosis of endothelial cells, and are considered important biomarkers of the status of endothelial cells and vascular function.12,13 Thus, levels of EMPs are increased in microvascular diseases with endothelial injury.14 CD31 and CD144 are endothelial markers,15–17 but can also be expressed on platelets, neutrophils, lymphocyte subset and hematopoietic stem cells.14,18–20 Plasmalemmal-vesicle-associated protein (PL-VAP) is expressed on the vascular endothelium of various organs, predominantly in the lung, kidney, spleen, endocrine glands and digestive tract.21 In the human kidney, PL-VAP is abundantly expressed in the endothelium of PTC and vasa recta, but is undetectable in glomerular and arterial endothelium.22,23 Hence, urinary EVs expressing PL-VAP likely originate from PTC or vasa recta endothelium.
Hypertension may lead to renal injury and capillary loss, but these are difficult to detect in the intact kidney. Thus, the purpose of this study was to test the hypothesis that PTC-EMPs would be increased in patients with essential (EH) and renovascular (RVH) hypertension compared with HVs, and correlate with indices of renal function.
MATERIALS AND METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Patient selection
We prospectively enrolled 38 hypertensive patients ≥18 years of age with EH (n=14) or RVH (n=24) and with relatively preserved renal function to participate in the studies. Atherosclerotic renal artery stenosis was defined as cross sectional luminal obstruction >60% as per computed tomography (CT) (Figure 1A) or magnetic resonance angiography, or by Doppler velocities ≥300 cm/s. Exclusion criteria included serum creatinine >2.5 mg/dL, diabetes mellitus, recent cardiovascular events (myocardial infarction, stroke or congestive heart failure) within the past 6 months, fibromuscular dysplasia, pregnancy and kidney transplant. Normotensive healthy volunteers (HVs) [systolic blood pressure (BP) <130 mmHg and diastolic BP <80mmHg] were prospectively recruited through the Mayo Clinic Biobank, and matched to patients with EH and RVH according to age and body mass index (BMI). This study was approved by the institutional review board of the Mayo Clinic and informed, written consent was obtained from each patient. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki.
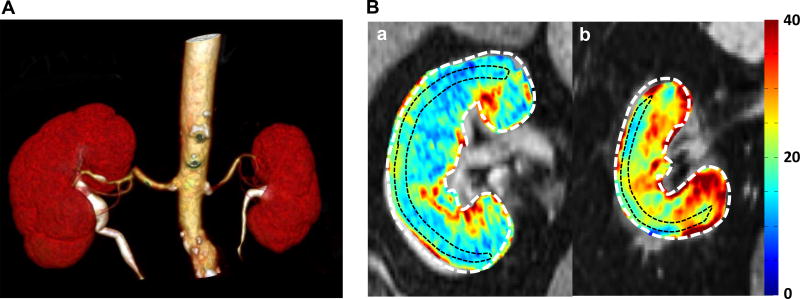
Figure 1.
(A) Computed tomography (CT) angiogram with a renal artery stenosis in left kidney. 3D-CT angiography shows left renal artery stenosis in the proximal portion. The left kidney has smaller volume than that of right kidney. (B) Representative T2* images and R2* parametric maps for subjects with essential hypertension (EH) (a) or renovascular hypertension (RVH) (b) obtained using the same color scale for R2*. Red areas represent hypoxic regions. Fractional hypoxia >30/s in RVH was greater than in EH (29.8% vs 12.1%).
Patients participated in this study during a 3-day inpatient protocol in the clinical research unit, as previously described.24 During this time, dietary sodium intake was maintained at 150 mEq/day; protein levels (in 24-h collection of freely voided urine) and serum creatinine were measured on the first day of the inpatient protocol. BP was measured using automated oscillometric recording and estimated glomerular filtration rate (eGFR) was calculated using the CKD Epidemiology Collaboration (CKD-EPI) equation.25 On the second day, blood oxygen level-dependent magnetic resonance imaging (BOLD MRI) scanning was performed on a Twin Speed Signa EXCITE 3.0T system (GE Medical Systems, Waukesha, WI) using a 12-channel torso phased array coil.26 On the third day of the protocol, renal blood flow (RBF) was measured using multidetector computed tomography (MDCT), and blood samples were collected from the inferior vena cava (IVC) and both renal veins (RVs).
All patients received antihypertensive drugs that block the renin-angiotensin system (angiotensin-converting enzyme inhibitors or angiotensin receptor blockers). RVH patients subsequently underwent renal stenting, or continued standard medical therapy, according to clinical indications and management decisions (including resistant hypertension, or progressive decline in renal function). Eighteen RVH patients (9 medically-treated and 9 stented following standard procedures) returned for repeat measurements 3 months later. Of the 24 patients with RVH, 7 patients underwent transjugular renal biopsy during RV blood collection, and 5 mm sections subsequently stained with H&E and trichrome.
Tissue oxygenation determined by BOLD MRI
Renal oxygenation was detected using R2* maps using BOLD MRI, and intrarenal hypoxia expressed as R2*, an index of deoxyhemoglobin concentration in the kidney.26 Scans were performed under suspended respiration, consisting of a 2D fast spoiled gradient echo sequence with multiple echo times, as previously described.27 Analysis of BOLD data from axial images was performed in regions of interest (ROI) traced through the midpole hilar region of each kidney on T2*-weighted images, which were then transferred to the corresponding R2* parametric image. Then, MRI BOLD data were processed using Matlab 7.10 (The Math Works, Natick, MA). To determine the fraction of the kidney area in which tissue hypoxia was present (fractional tissue hypoxia), we measured the percentage of voxels from the whole-kidney regions of interest with R2* values > 30/s averaging all available slices, as described previously.28
Blood sampling and measurement of renal blood flow
To measure single-kidney RBF, on the third day of the inpatient protocol the common femoral vein was cannulated. First, blood samples were selectively collected from the IVC and the right and left RVs with 5F pigtail Cobra catheter (Cook, Inc., Bloomington, IN, USA). The catheter was then moved to the right atrium for central venous injection of contrast media for flow studies using MDCT. MDCT imaging was performed using a dual source 64-slices helical scanner (SOMATOM Definition, Siemens Medical Solution, Forchheim, Germany) after a bolus injection of iopamidol 370 (0.5 ml/kg), as described previously.29 Kidney volume (5-mm-thick slices) was assessed in the helical mode after a second similar contrast injection to determine both cortical and medullary volumes. MDCT images were analyzed using the ANALYZE™ (Biomedical Imaging Resource Center, Mayo Clinic, Rochester, MN). ROI were selected from the aorta, renal cortex, and medulla of both kidneys. Average tissue attenuation was plotted versus time and the curves were fitted by algorithms to calculate hemodynamics in cortical and medullary regions of the kidney. Single-kidney RBF was calculated by multiplying kidney volume (cc tissue) by renal perfusion (ml/min per cc tissue), as previously described.30 In HVs, blood samples from a peripheral (antecubital) vein and a spot urine sample were collected during a single visit, and dietary sodium intake was not regulated.
EMPs isolation and analysis
EVs were isolated from whole urine using Total Exosome Isolation reagent (Invitrogen, Waltham, MA, USA) according to the manufacturer’s guidelines. Urine samples (1000 µL) were centrifuged at 2000 g for 30 min at 4°C to remove cells and debris. Supernatants (800 µL) were mixed with 1 volume of the Total Exosome Isolation reagent and incubated for 1 h at room temperature. After incubation, samples were centrifuged at 10000 g for 1h at 4°C. Pelleted exosomes were resuspended in extracellular vesicle buffer (2M sucrose and 500mM MES solution). Isolated EMPs were stained for 2 hours at 37°C with 0.5 mM Tag-it violet (TIV) (Biolegend, USA) cell labeling solution. Labeled EMPs were stained with cell-specific antibodies including PL-VAP (LSBio), CD31 (Biolegend) and CD144 (Biolegend). EMPs were quantified using a FlowSight (Amnis, Seattle, WA, USA) imaging flow cytometry, as previously described,31,32 by acquiring at least 50,000 TIV positive events. EMPs were measured using flow-gating strategy including a first positive gate for TIV events followed by positive gate for PL-VAP and positive or negative gate for other antibodies, and their combination evaluated for relationship with renal parameters. Acquisition was performed using Image StreamX Imaging Flow Cytometer (Amnis Corporation, Seattle, WA) equipped with INSPIRE software. The level of EMPs was expressed as a percentage.
Histological Analyses
Capillary density was quantitated on H&E stained slides by counting the total number of PTC enclosed within 10 random 0.25-mm2 fields (each of which was delineated by a 1-cm2 ocular grid viewed at 400 magnification) and expressed as mean per field. Capillaries were identified by the presence of lumen, red blood cells, and/or an endothelial cell lining.33 The degree of fibrosis in the renal cortex and medulla was semi-automatically quantified in trichrome stained slides in 10–15 fields using a masking algorithm based on color thresholding and edge detection in AxioVisionc (Carl Zeiss MicroImaging) and expressed as an average of percent fibrosis to total field area.
Statistical Analyses
Data were analyzed using the JMP software package version 10.0 (SAS institute, Cary, NC, USA). Normally distributed data were expressed as mean ± SD and non-normally distributed data as median (range). Comparisons among the HVs, EH and RVH groups were performed using ANOVA followed by an unpaired two-tailed t-test (or the Wilcoxon rank-sum test for skewed data) and correlation coefficients using least-square fit. Spearman rank correlation analysis was used to test for association between EMPs and other variables. The stenotic kidney in RVH was compared to the average of the left and right kidneys in EH patients. Comparisons between individual kidneys before and after treatment and changes over time in the 2 treatments groups were performed using paired t-test. P-values ≤0.5 was considered statistically significant.
RESULTS
Patient characteristics
The characteristics of the 52 study participants are presented in Table 1. There were no clinical differences among the groups, except for BP and renal function. Mean arterial pressure (MAP) of EH and RVH patients was higher compared to those in HVs (p=0.025 and p=0.003, respectively), whereas eGFR of EH and RVH patients was lower (p=0.008 and p<0.001, respectively). RBF and cortical perfusion in stenotic kidneys of patients with RVH were lower than in kidneys of EH patients (p<0.001 and p<0.001, respectively). Renal tissue hypoxia levels, defined by both R2* and fractional hypoxia (R2*>30/s), were higher in RVH than in EH kidneys (Table 1, Figure 1B).
Table 1.
linical characteristics of HVs and patients with EH or RVH†,
| Parameter | HV (n=14) |
EH (n=14) |
RVH (n=24) |
|---|---|---|---|
| Age, years | 72.2±5.2 | 70.0±4.7 | 70.5±5.8 |
| Men, (%) | 6 (43) | 7 (50) | 11 (46) |
| BMI, kg/m2 | 27.0±5.1 | 29.6±5.2 | 27.7±5.9 |
| MAP (mmHg) | 86.9±5.2 | 95.7±9.5* | 95.9±9.9* |
| Serum creatinine (mg/dL) | 0.95±0.16 | 1.03±0.27 | 1.29±0.39* |
| eGFR (mL/min/1.73m2) | 80.5±10.5 | 62.7±15.8* | 50.4±15.2* |
| Proteinuria (mg/mL or mg/mL/24 hour)†† | 68 (4–196) | 51 (29–213) | 76 (26–255) |
| Single- (or stenotic) RBF (mL/min) | 378.0 (191.5–710.5) | 143.2 (10.3–519.0)† | |
| Cortical perfusion (mL/min/mL) | 3.68±0.92 | 2.25±0.88† | |
| Medullary perfusion (mL/min/mL) | 1.21±0.31 | 0.97±0.26 | |
| Cortex R2* (sec−1) | 18.6±1.5 | 21.3±4.7† | |
| Fractional Hypoxia (% R2* > 30 sec−1) | 9.8±5.1 | 20.4±19.1† |
Values are presented as n (%), mean ± SD or median (range). HVs: healthy volunteers, EH: essential hypertension, RVH: renovascular hypertension, BMI: body mass index, MAP: mean arterial pressure, eGFR: estimated glomerular filtration rate, RBF: renal blood flow
p <0.05 vs. HV,
p <0.05 vs. EH
Protein levels were measured in HV in spot urine, and in EH and RAS in a 24h collection
PTCs-EMPs levels in hypertensive patients
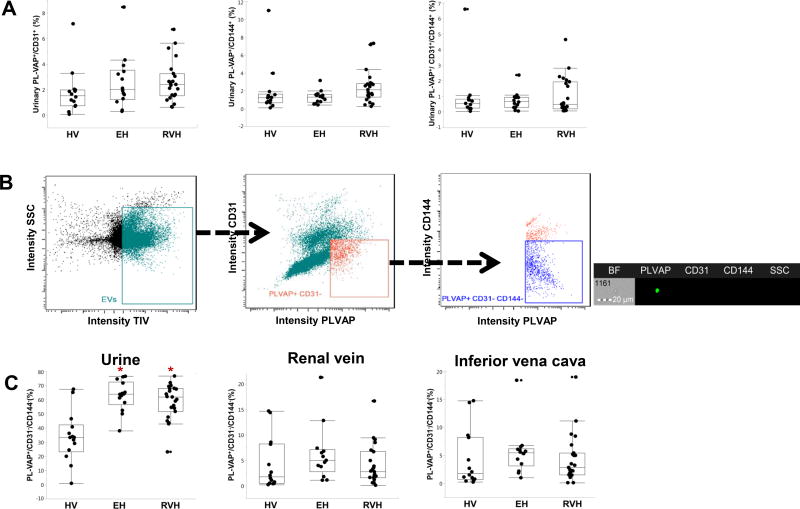
There were no difference among the patient groups in levels of plasma or urinary PL-VAP+/CD31+, PL-VAP+/CD144+, or PL-VAP+/CD31+/CD144+ EVs (Figure 2A). On the other hand, urinary levels of PL-VAP+/CD31−/CD144− EVs were elevated in EH and RVH compared with HV subjects (p<0.001 and p=0.001, respectively), as shown in both intensity graphs and representative fluorescent images (Figure 2B–C). These were considered to represent PTC-EMPs. Renal and systemic venous levels were not different among the groups.
Figure 2.
Levels of exosomes in urine of hypertensive patients. (A) There were no differences among the groups in percent of urinary PL-VAP+/CD31+, PL-VAP+/CD144+, and PL-VAP+/CD31+/CD144+ exosomes. (B) PTC-EMPs were identified using flow cytometry as PL-VAP+/CD31−/CD144− as shown in representative fluorescent images. Scale bar =20 µm. (C) Renal vein and systemic levels of PL-VAP+/CD31−/CD144− EMPs were not different among the groups, whereas their urinary levels were elevated in both EH and RVH compared to HVs (p<0.001 and p=0.001, respectively). EMPs, endothelial microparticles; HVs, healthy volunteers; EH, essential hypertension; RVH; renovascular hypertension *p <0.05 versus HV
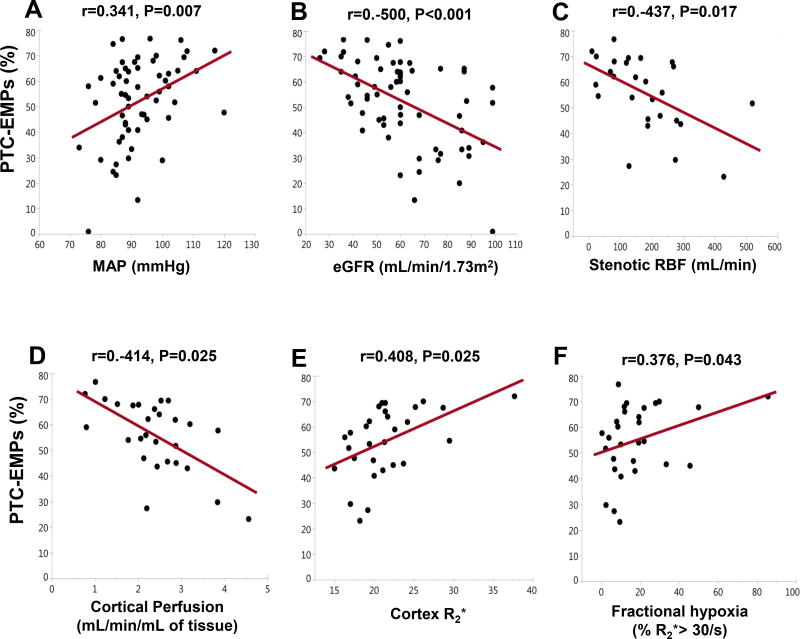
Among all participants, urinary PTC-EMPs levels correlated directly with MAP and inversely with eGFR, but not with proteinuria (Figure 3A–B). Additionally, in RVH patients, PTC-EMPs showed inverse correlation with stenotic RBF and cortical perfusion, and direct correlation with single stenotic kidney R2* and fractional hypoxia (Figure 3C–E).
Figure 3.
Correlation of PTC-EMPs with clinical parameters in hypertensive patients (n=38). Urinary PTC-EMPs levels correlated directly with MAP and inversely with eGFR. In patients with RVH (n=24), PTC-EMPs showed inverse correlation with stenotic RBF and cortical perfusion, and direct correlation with cortex R2* and fractional hypoxia. PTC-EMPs, peritubular capillary-derived endothelial microparticles; eGFR, estimated glomerular filtration rate; RVH, renovascular hypertension; RBF, renal blood flow
Correlation between PTC-EMPs levels and histologic findings
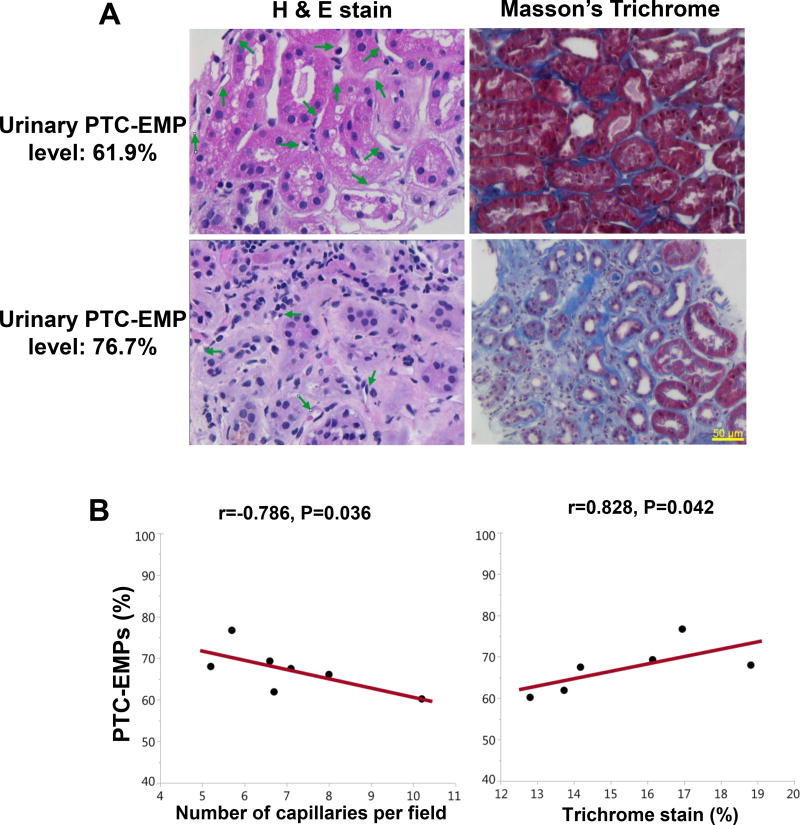
Similar to the entire RVH study population, a negative correlation was observed within the 7 biopsied RHV patients between urinary PTC-EMPs levels and RBF and cortical perfusion of the stenotic kidney (spearman correlation=−0.785, p=0.036, spearman correlation=−0.787, p=0.036, respectively). Furthermore, urinary PTC-EMPs levels were inversely correlated with the number of PTC and directly with interstitial fibrosis (Figure 4).
Figure 4.
Correlation of PTC-EMPs with renal histology. (A) Representative H&E and trichrome staining in stenotic kidney biopsies. The PTC number and the degree of interstitial fibrosis were higher and lower, respectively, in patients with low compared to patients with high urinary PTC-EMPs percent. (B) Urinary PTC-EMPs levels were inversely correlated with the number of PTC and directly with interstitial fibrosis in this subset of seven RVH patients. PTC-EMPs, peritubular capillary-derived endothelial microparticles; RVH, renovascular hypertension
No correlations were observed between histologic findings and any other EV levels, including PLVAP+/CD31+, PLVAP+/CD144+ or PLVAP+/CD31+/CD144+ (data not shown).
A change in PTC-EMPs levels with treatment
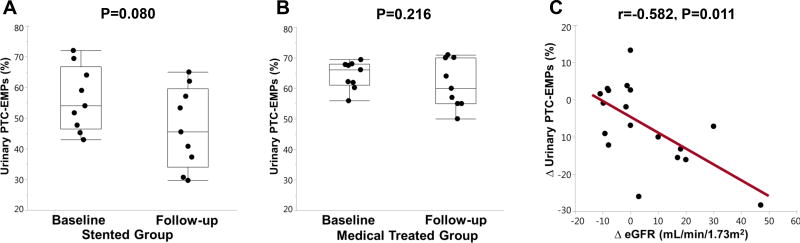
Stenting had no significant effect on MAP (from 99.4±13.3 to 95.5±11.1 mmHg, p=0.599), but slightly increased eGFR at follow-up (from 46.1±21.7 to 60.2±22.1 mL/min/1.73m2, p=0.031). There were no significant changes in MAP or renal function in medically-treated RVH patients (from 91.2±9.5 to 88.7±15.8 mmHg, p=0.612, from 54.1±8.6 to 54.1±15.2 mL/min/1.73m2, p=0.281). In repeated measurements of urinary PTC-EMPs in RVH patients 3 months after stenting (n=9) or continued medical therapy (n=9), the change in urinary PTC-EMPs levels was not significant in either group compared to baseline (Figure 5A, 5B), and was not significantly different between them (−10.44 versus −2.93, p=0.15). However, we observed a significant inverse correlation between the changes in urinary PTC-EMPs level and renal function (spearman correlation= −0.582, p=0.011) (Figure 5C).
Figure 5.
Change in the levels of PTC-EMPs 3 months after medical therapy (n=9) or stenting (n=9). Changes in individual PTC-EMPs from baseline to 3 months follow-up were not statistically significant in either stented (A) or medically treated (B) patients with RVH. (C) Change of urinary PTC-EMPs inversely correlated with change of eGFR. PTC-EMPs, peritubular capillary-derived endothelial microparticles; eGFR, estimated glomerular filtration rate; RVH, renovascular hypertension
DISCUSSION
This study demonstrates that urinary levels of PTC-EMPs, assessed using flow cytometry, are elevated in hypertensive patients compared with HVs, and in patients with RVH are correlated with histologic findings and clinical parameters such as MAP, eGFR, RBF, cortical perfusion, and tissue hypoxia of the stenotic kidney. Since sustained endothelial activation and cellular injury may lead to release of EMPs in the urine,4 we interpret these results as indicating that urinary PTC-EMPs may reflect renal microvascular capillary injury in hypertensive patients.
Hypertension is an important cause of CKD, and evolution of hypertensive kidney injury may involve peritubular capillary damage.34 Possibly, detection of PTC injury at the early phase of hypertension might direct management decisions. PTC rarefaction, along with interstitial fibrosis and tubular atrophy, is one of the major hallmarks of CKD, and predicts renal outcome in these patients.1–3 In response to kidney injury, endothelial cells of PTC may initially proliferate and subsequently dropout.35 Such capillary loss typically precedes development of prominent tubulo-interstitial fibrosis.36,37 In addition, a loss of postglomerular capillaries leads to a reduction in oxygen supply, which is one of the common pathways of CKD.38
EVs detected in urine might be released from different cell types along the renal nephron and urinary tract.6 Such EV release is due to cellular activation and/or cell stress, which induce shedding from plasma membrane of EV, called micropartilces.39,40 For example, Burger et al. showed that urinary podocyte-derived microparticles are generated by exposure to mechanical stretch and high glucose and may reflect early glomerular injury in diabetic nephropathy.41 Circulating EMPs are also increased in hypertension, diabetes and CKD.40 Therefore, analysis of urinary EVs bearing renal cellular markers may be useful to probe the status of kidney cells without need for invasive renal biopsy. However, EVs that reflect the status of PTC have not been identified.
In the present study, to measure PTC-EMPs, we compared several endothelial cell markers including PL-VAP, CD31 and CD144. Interestingly, we found that urinary PL-VAP+/CD31−/CD144− EMPs levels were higher in hypertensive patients compared to HVs, and correlated with both clinical and histologic parameters. There are several possible reasons for the closer association of CD31− and CD144− with PTC injury. First, besides endothelial cells, CD31 is also expressed on the surface of platelets, neutrophils, and lymphocytes.14,18–20 Second, compared to endothelial cells in the glomeruli, arteries, and venules, the expression of CD31 is lower in PTC, and might therefore constitute a less robust marker of PTC.42 Third, CD144, or vascular endothelial-cadherin, is expressed on endothelial cells and functions as a gatekeeper of endothelial junction, but is also expressed on hematopoietic stem cells.20 Furthermore, loss of CD144 expression may indicate endothelial cells that have lost their permeability barrier or have transitioned to mesenchymal cells.43,44 Notably, CD144 expression is relatively weak or absent in extraglomerular vessels.45 Consequently, urinary PL-VAP+/CD31−/CD144− EMPs are likely released from and may reflect PTC injury. Indeed, their urinary levels correlated well with renal function.
Vascular occlusion in patients with renal artery stenosis reduces blood flow and GFR.46,47 Subsequently, stenotic lesions not only induce hypoxia in the kidney, as observed in this study, but also activate the renin-angiotensin-aldosterone system and evoke oxidative stress, inflammation and microvascular rarefaction.48,49 In this study, urinary PTC-EMPs levels were inversely correlated with RBF, and cortical perfusion of stenotic kidney. Importantly, significant correlation between urinary PTC-EMPs and histologic findings of capillary rarefaction was also observed. The significant inverse correlation between a fall in urinary PTC-EMP concentration and an increase in eGFR following revascularization further underscores the potential functional significance of this measure. Contrarily, proteinuria was not correlated with urinary PTC-EMPs in this study, possibly because the proteinuria of our study groups was within a normal range. Thus, urinary PTC-EMPs levels in hypertension might provide an early marker of renal injury independent of proteinuria.
There are currently no biomarkers reflecting PTC injury in renovascular hypertension. In the present study, although there was no change urinary PTC-EMPs level after treatment, we observed a significant inverse correlation between the changes in urinary PTC-EMPs level and renal function. Notably some or our patient had unilateral disease or asymmetric bilateral disease, but only was the more severely stenotic kidney was revascularized. Therefore, the contralateral (non-stenotic or less stenotic) kidney could have masked differences in absolute numbers of EMP among the groups. These differences might have been better captured by paired changes in urinary PTC-EMPs level within patients. Furthermore, PTC-EMPs might be more useful as biomarkers in patients with CKD in who both kidneys are similarly injured or treated. Thus, we believe that urinary PTC-EMPs could serve as biomarkers reflecting PTC injury and renal function, although additional and larger studies are needed to address this point.
Interestingly, renal vein and systemic PTC-EMPs levels were not different among the groups in this study, probably because most EVs found in the urine originate from genitourinary tract rather than systemic blood.6,50 Because PL-VAP is expressed in the vascular endothelium of other organs,21 the renal vein and systemic PTC-EMPs might not reflect specifically renal PTC injury. Therefore, urinary EVs may serve as a more sensitive biomarker than systemic EVs to reflect renal injury.
EV isolation presents an important technical challenge. We used a commercial isolation kit, which is relatively quick and does not require laborious ultracentrifugation or large urine volumes, which might be impractical in the clinical setting.51,52 However, the cost of isolation kits is not insignificant, especially when applied to a large number of samples.52 Another potential confounder is proteinuria that can interfere with detection of urinary EV protein, owing to exosome-like particle of albumin-containing solution.53 However, urinary protein levels were unaltered in our patients. In addition, EVs loss from the sample might occur during sample processing. Therefore, we expressed PTC-EMPs as a fraction of total EV count. Indeed, longitudinal measurement of PTC-EMPs levels in medically-treated RVH patients demonstrated the reproducibility of our approach.
Our study has some limitations. First, this study has a relatively modest sample size and the numbers of patients who underwent renal biopsy or revascularization were small. Thus, larger studies are needed to confirm our results. Second, urinary PTC-EMPs also might originate from the bladder epithelium, which expresses low level of PL-VAP mRNA.21 Third, inhibitors of the renin-angiotensin system may modulate urinary levels of PTC-EMPs in hypertensive patients, by either increasing54 or decreasing PTC loss. Finally, urine samples from HVs were obtained in untimed collection. Although useful to assess proteinuria, their compatibility to timed urine collection is limited. In addition, for ethical reasons, blood was collected from a peripheral vein in HVs.
In conclusion, we observed elevated urinary levels of PTC-EMPs in hypertensive patients compared with HVs. PTC-EMPs might be useful markers reflecting capillary rarefaction in hypertension and may enable non-invasive monitoring of the renal microcirculation and the success of therapeutic strategies. Future studies are needed to evaluate their levels in other diseases and their utility for monitoring success of therapy.
PERSPECTIVES
Peritubular capillary (PTC) rarefaction is one of the major hallmarks of chronic kidney disease (CKD) and predicts renal outcome in CKD patients. Extracellular vesicles (EVs) are membrane-bound vesicles produced and released by cells. Urinary EVs have gained important recognition as potential diagnostic biomarkers in renal disease. However, EVs reflecting PTC injury have not been identified. This manuscript describes the characterization of a endothelial microparticles (EMPs) reflecting PTC loss in hypertensive patients. Compared to healthy controls, urinary PTC-EMPs levels were elevated in hypertensive patients, and correlated directly with blood pressure and inversely with renal function. Furthermore, urinary PTC-EMPs levels correlated directly with renal histological PTC count and fibrosis. Urinary PTC-EMPs may be useful as novel biomarkers of intrarenal capillary loss.
NOVELTY AND SIGNIFICANCE.
1. What is New?
Extracellular vesicles reflecting damage of peritubular capillary endothelial cells have not been identified.
Our study demonstrates that urinary peritubular capillary-derived endothelial microparticle levels are increased in hypertensive patients, and may reflect renal microcirculatory injury.
2. What is Relevant?
Hypertension, an important cause of chronic kidney disease, is characterized by peritubular capillary loss.
Urinary peritubular capillary-derived endothelial microparticle may reflect renal microvascular capillary injury in hypertensive patients.
3. Summary
Urinary peritubular capillary-derived endothelial microparticle might be useful markers reflecting capillary rarefaction in hypertension and may enable non-invasive monitoring of the renal microcirculation and the success of therapeutic strategies.
Acknowledgments
Sources of Funding
This study was partly supported by NIH grant numbers: DK100081, DK104273, HL123160, DK102325, and DK106427.
Footnotes
Disclosures
All the authors declare no competing interests.
References
- 1.Mayer G. Capillary rarefaction, hypoxia, VEGF and angiogenesis in chronic renal disease. Nephrol Dial Transplant. 2011;26:1132–1137. doi: 10.1093/ndt/gfq832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bohle A, Mackensen-Haen S, Wehrmann M. Significance of postglomerular capillaries in the pathogenesis of chronic renal failure. Kidney Blood Press Res. 1996;19:191–195. doi: 10.1159/000174072. [DOI] [PubMed] [Google Scholar]
- 3.Steegh FM, Gelens MA, Nieman FH, van Hooff JP, Cleutjens JP, van Suylen RJ, Daemen MJ, van Heurn EL, Christiaans MH, Peutz-Kootstra CJ. Early loss of peritubular capillaries after kidney transplantation. J Am Soc Nephrol. 2011;22:1024–1029. doi: 10.1681/ASN.2010050531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rabelink TJ, Wijewickrama DC, de Koning EJ. Peritubular endothelium: the Achilles heel of the kidney? Kidney Int. 2007;72:926–930. doi: 10.1038/sj.ki.5002414. [DOI] [PubMed] [Google Scholar]
- 5.van der Pol E, Boing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64:676–705. doi: 10.1124/pr.112.005983. [DOI] [PubMed] [Google Scholar]
- 6.Krause M, Samoylenko A, Vainio SJ. Exosomes as renal inductive signals in health and disease, and their applications as diagnostic markers and therapeutic agents. Front Cell Dev Biol. 2015;3:65. doi: 10.3389/fcell.2015.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.dU Cheyron D, Daubin C, Poggioli J, Ramakers M, Houillier P, Charbonneau P, Paillard M. Urinary measurement of Na+/H+ exchanger isoform 3 (NHE3) protein as new marker of tubule injury in critically ill patients with ARF. Am J Kidney Dis. 2006;42:497–506. doi: 10.1016/s0272-6386(03)00744-3. [DOI] [PubMed] [Google Scholar]
- 8.Hogan MC, Bakeberg JL, Gainullin VG, et al. Identification of biomarkers for PKD1 using urinary exosomes. J Am Soc Nephrol. 2015;26:1661–1670. doi: 10.1681/ASN.2014040354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou H, Kajiyama H, Tsuji T, Hu X, Leelahavanichkul A, Vento S, Frank R, Kopp JB, Trachtman H, Star RA, Yuen PS. Urinary exosomal Wilms’ tumor-1 as a potential biomarker for podocyte injury. Am J Physiol Renal Physiol. 2013;305:F553–F559. doi: 10.1152/ajprenal.00056.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwon SH, Woollard JR, Saad A, Garovic VD, Zand L, Jordan KL, Textor SC, Lerman LO. Elevated urinary podocyte-derived extracellular microvesicles in renovascular hypertensive patients. Nephrol Dial Transplant. 2017;32:800–807. doi: 10.1093/ndt/gfw077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwon SH, Tang H, Saad A, Woollard JR, Lerman A, Textor SC, Lerman LO. Differential Expression of microRNAs in urinary extracellular vesicles obtained from hypertensive patients. Am J Kidney Dis. 2016;68:331–332. doi: 10.1053/j.ajkd.2016.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyle LJ, Credeur DP, Jenkins NT, Padilla J, Leidy HJ, Thyfault JPK, Fadel PJ. Impact of reduced daily physical activity on conduit artery flow-mediated dilation and circulating endothelial microparticles. J Appl Physiol. 2013;115:1519–1525. doi: 10.1152/japplphysiol.00837.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berezin A, Zulli A, Kerrigan S, Petrovic D, Kruzliak P. Predictive role of circulating endothelial-derived microparticles in cardiovascular diseases. Clin Biochem. 2015;48:562–568. doi: 10.1016/j.clinbiochem.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Deng F, Wang S, Zhang L. Endothelial microparticles act as novel diagnostic and therapeutic biomarkers of circulatory hypoxia-related disease: a literature review. J Cell Mol M. 2017;21:1698–1710. doi: 10.1111/jcmm.13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berezin AE, Kremzer AA, Berezina TA, Martovitskaya YV. Pattern of circulating microparticles in chronic heart failure patients with metabolic syndrome: Relevance to nuerohumoral and inflammatory activation. BBA Clin. 2015;4:69–75. doi: 10.1016/j.bbacli.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boulanger CM, Amabile N, Tedgui A. Circulating microparticles: a potential prognostic marker for atherosclerotic vascular disease. Hypertension. 2006;48:180–186. doi: 10.1161/01.HYP.0000231507.00962.b5. [DOI] [PubMed] [Google Scholar]
- 17.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immuno. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 18.Jenkins NT, Padilla J, Boyle LJ, Credeur DP, Laughlin MH, Fadel PJ. Disturbed blood flow acutely induces activation and apoptosis of the human vascular endothelium. Hypertension. 2013;61:615–621. doi: 10.1161/HYPERTENSIONAHA.111.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Craenenbroeck EM, Frederix G, Pattyn N, Beckers P, Van Craenenbroeck AH, Gevaert A, Possemiers N, Cornelissen V, Goetschalckx K, Vrints CJ, Vanhees L, Hoymans VY. Effects of aerobic interval training and continuous training on cellular markers of endothelial integrity in coronary artery disease: a SAINTEX-CAD substudy. Am J Physiol Heart Circ Physiol. 2015;309:1876–1882. doi: 10.1152/ajpheart.00341.2015. [DOI] [PubMed] [Google Scholar]
- 20.Kim I, Yilmaz OH, Morrison SJ. CD144 (VE-cadherin) is transiently expressed by fetal liver hematopoietic stem cells. Blood. 2005;106:903–905. doi: 10.1182/blood-2004-12-4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stan RV, Arden KC, Palade GE. cDNA and protein sequence, genomic organization, and analysis of cis regulatory elements of mouse and human PLVAP genes. Genomics. 2001;72:304–313. doi: 10.1006/geno.2000.6489. [DOI] [PubMed] [Google Scholar]
- 22.Stan RV. Structure of caveolae. Biochim Biophys Acta. 2005;1746:334–348. doi: 10.1016/j.bbamcr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Niemela H, Elima K, Henttinen T, Irjala H, Salmi M, Jalkanen S. Molecular identification of PAL-E, a widely used endothelial-cell marker. Blood. 2005;106:3405–3409. doi: 10.1182/blood-2005-01-0254. [DOI] [PubMed] [Google Scholar]
- 24.Saad A, Herrmann SM, Crane J, Glockner JF, McKusick MA, Misra S, Eirin A, Ebrahimi B, Lerman LO, Textor SC. Stent revascularization restores cortical blood flow and reverses tissue hypoxia in atherosclerotic renal artery stenosis but fails to reverse inflammatory pathways or glomerular filtration rate. Circ Cardiovasc Interv. 2013;6:428–435. doi: 10.1161/CIRCINTERVENTIONS.113.000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gloviczki ML, Glockner J, Gomez SI, Romero JC, Lerman LO, McKusick M, Textor SC. Comparison of 1.5 and 3 T BOLD MR to study oxygenation of kidney cortex and medulla in human renovascular disease. Invest Radiol. 2009;44:566–571. doi: 10.1097/RLI.0b013e3181b4c1e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gloviczki ML, Lerman LO, Textor SC. Blood oxygen level-dependent (BOLD) MRI in renovascular hypertension. Curr Hypertens Rep. 2011;13:370–377. doi: 10.1007/s11906-011-0218-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saad A, Crane J, Glockner JF, Herrmann SM, Friedman H, Ebrahimi B, Lerman LO, Textor SC. Human renovascular disease: Estimating fractional tissue hypoxia to analyze blood oxygen level-dependent MR. Radiology. 2013;268:770–778. doi: 10.1148/radiol.13122234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daghini E, Juillard L, Haas JA, Krier JD, Romero JC, Lerman LO. Comparison of mathematic models for assessment of glomerular filtration rate with electron-beam CT in pigs. Radiology. 2007;242:417–424. doi: 10.1148/radiol.2422052144. [DOI] [PubMed] [Google Scholar]
- 30.Lerman LO, Taler SJ, Textor SC, Sheedy PF, 2nd, Stanson AW, Romero JC. Computed tomography-derived intrarenal blood flow in renovascular and essential hypertension. Kidney Int. 1996;49:846–854. doi: 10.1038/ki.1996.117. [DOI] [PubMed] [Google Scholar]
- 31.Jayachandran M, Lugo G, Heiling H, Miller VM, Rule AD, Lieske JC. Extracellular vesicles in urine of women with but not without kidney stones manifest patterns similar to men: a case control study. Biol Sex Differ. 2015;6:2. doi: 10.1186/s13293-015-0021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turco AE, Lam W, Rule AD, Denic A, Lieske JC, Miller VM, Larson JJ, Kremers WK, Jayachandran M. Specific renal parenchymal-derived urinary extracellular vesicles identify age-associated structural changes in living donor kidneys. J Extracell Vesicles. 2016;5:29642. doi: 10.3402/jev.v5.29642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ward WK, Quinn MJ, Wood MD, Tiekotter KL, Pidikiti S, Gallagher JA. Vascularizing the tissue surrounding a model biosensor: how localized is the effect of a subcutaneous infusion of vascular endothelial growth factor (VEGF)? Biosens Bioeletron. 2003;9:155–163. doi: 10.1016/s0956-5663(03)00180-5. [DOI] [PubMed] [Google Scholar]
- 34.Johnson RJ, Schreiner GF. Hypothesis: the role of acquired tubulointerstitial disease in the pathogenesis of salt-dependent hypertension. Kidney Int. 1997;52:1169–1179. doi: 10.1038/ki.1997.442. [DOI] [PubMed] [Google Scholar]
- 35.Kida Y, Tchao BN, Yamaguchi I. Peritubular capillary rarefaction: a new therapeutic target in chronic kidney disease. Pediatr Nephrol. 2014;29:333–342. doi: 10.1007/s00467-013-2430-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol. 2001;281:F887–F899. doi: 10.1152/ajprenal.2001.281.5.F887. [DOI] [PubMed] [Google Scholar]
- 37.Li B, Cohen A, Hudson TE, Motlagh D, Amrani DL, Duffield JS. Mobilized human hematopoietic stem/progenitor cells promote kidney repair after ischemic/reperfusion injury. Circulation. 2010;121:2211–2220. doi: 10.1161/CIRCULATIONAHA.109.928796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fine LG, Bandyopadhay D, Norman JT. Is there a common mechanism for the progression of different types of renal diseases other than proteinuria? Towards the unifying theme of chronic kidney hypoxia. Kidney Int. 2000;57:S22–S26. [PubMed] [Google Scholar]
- 39.Karpman D, Stahl AL, Arvidsson I. Extracellular vesicles in renal disease. Nat Rev Nephrol. 2017;13:545–562. doi: 10.1038/nrneph.2017.98. [DOI] [PubMed] [Google Scholar]
- 40.Burger D, Schock S, Thompson CS, Montezano AC, Hakim AM, Touyz RM. Microparticles: Biomarkers and beyond. Clin Sci (Lond) 2013;124:423–442. doi: 10.1042/CS20120309. [DOI] [PubMed] [Google Scholar]
- 41.Burger D, Thibodeau JF, Holterman CE, Burns KD, Touyz RM, Kennedy CR. Urinary podocyte microparticles identify prealbuminuric diabetic glomerular injury. J Am Soc Nephrol. 2014;25:1401–1407. doi: 10.1681/ASN.2013070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pusztaszeri MP, Seelentag W, Bosman FT. Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues. J Histochem Cytochem. 2006;54:385–395. doi: 10.1369/jhc.4A6514.2005. [DOI] [PubMed] [Google Scholar]
- 43.Corada M, Liao F, Lindgren M, Lampugnani MG, Breviario F, Frank R, Muller WA, Hicklin DJ, Bohlen P, Dejana E. Monoclonal antibodies directed to different regions of vascular endothelial cadherin extracellular domain affect adhesion and clustering of the protein and modulate endothelial permeability. Blood. 2001;97:1679–1684. doi: 10.1182/blood.v97.6.1679. [DOI] [PubMed] [Google Scholar]
- 44.Frid MG, Kale VA, Stenmark KR. Mature vascular endothelium can give rise to smooth muscle cells via endothelial-mesenchymal transdifferentiation: in vitro analysis. Circ Res. 2002;90:1189–1196. doi: 10.1161/01.res.0000021432.70309.28. [DOI] [PubMed] [Google Scholar]
- 45.Prandini MH, Dreher I, Bouillot S, Benkerri, Moll T, Huber P. The human VE-cadherin promoter is subjected to organ-specific regulation and is activate in tumour angiogenesis. Oncogene. 2005;24:2992–3001. doi: 10.1038/sj.onc.1208483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gloviczki ML, Keddis MT, Garovic VD, Friedman H, Herrmann S, McKusick MA, Misra S, Grande JP, Lerman LO, Textor SC. TGF expression and macrophage accumulation in atherosclerotic renal artery stenosis. Clin J Am Soc Nephrol. 2013;8:546–553. doi: 10.2215/CJN.06460612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keddis MT, Garovic VD, Bailey KR, Wood CM, Raissian Y, Grande JP. Ischaemic nephropathy secondary to atherosclerotic renal artery stenosis: clinical and histopathological correlates. Nephrol Dial Transplant. 2010;25:3615–3622. doi: 10.1093/ndt/gfq269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lerman LO, Nath KA, Rodriguez-Porcel M, Krier JD, Schwartz RS, Napoli C, Romero JC. Increased oxidative stress in experimental renovascular hypertension. Hypertension. 2001;37:541–546. doi: 10.1161/01.hyp.37.2.541. [DOI] [PubMed] [Google Scholar]
- 49.Eirin A, Gloviczki ML, Tang H, Gossl M, Jordan KL, Woollard JR, Lerman A, Grande JP, Textor SC, Lerman LO. Inflammatory and injury signals released from the post-stenotic human kidney. Eur Heart J. 2013;34:540–548a. doi: 10.1093/eurheartj/ehs197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yáñez-Mó M, Siljander PR, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schagman J, Zeringer E, Li M, Barta T, Lea K, Gu J, Magdaleno S, Setterquist R, Vlassov AV. The complete exosome workflow solution: from isolation to characterization of RNA cargo. Biomed Res Int. 2013;2013:253957. doi: 10.1155/2013/253957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Musante L, Tataruch DE, Holthofer H. Use and isolation of urinary exosomes as biomarkers for diabetic nephropathy. Front Endocrinol (Lausanne) 2014;5:149. doi: 10.3389/fendo.2014.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gleadle J, McNicholas K, Li J, Michael M, Rojas-Canales D. Nanoparticle tracking analysis of urine to detect exosomes can be confounded by albuminuria. J Am Soc Nephrol. 2018;29:1784. doi: 10.1681/ASN.2018020115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoo KH, Yim HE, Bae ES, Hong YS. Capillary rarefaction and altered renal development: the imbalance between pro- and anti-angiogenic factors in response to angiotensin II inhibition in the developing rat kidney. J Mol Histol. 2018;49:219. doi: 10.1007/s10735-018-9762-7. [DOI] [PubMed] [Google Scholar]