Abstract
Determining causal relationships between distinct chromatin features and gene expression, and ultimately cell behavior, remains a major challenge. Recent developments in targetable epigenome-editing tools enables us to assign direct transcriptional and functional consequences to locus-specific chromatin modifications. This review discusses the unprecedented opportunity that CRISPR/(d)Cas9 technology offers for investigating and manipulating the epigenome to facilitate further understanding of stem cell biology and engineering of stem cells for therapeutic applications. We also provide technical considerations for standardization and further improvement of the CRISPR/(d)Cas9 tools.
Introduction
Epigenetic factors contribute to the diverse phenotypes found in cells that share the same DNA. In mammals, there have been three reported types of epigenetic modifications: methylation of DNA, post-translational modifications of histones, and additional regulation mediated by non-coding RNAs (ncRNAs) (Jenuwein and Allis, 2001). A simplified view of the complex epigenetic regulation involves the so-called effector (writer and eraser) and reader proteins that deposit, remove, and detect specific chromatin modifications, respectively. Epigenetic features, composed of multiple chromatin marks, may be maintained after cellular division as a result of self-reinforcing feedback mechanisms (Allis and Jenuwein, 2016). Pioneering epigenetic studies have uncovered numerous correlations between chromatin marks, changes in gene expression, and cellular and organismal phenotypes through three main strategies: cataloging epigenetic marks and features during developmental or disease states, mutating candidate chromatin modifiers to globally alter specific chromatin marks, and modifying putative DNA regulatory sequences in transgenic assays (Allis et al., 2006). The prevailing methodologies involve either global epigenetic perturbations, or correlation of specific chromatin marks with transcription factor occupancy and mRNA levels (ENCODE Project Consortium, 2012; Ernst and Kellis, 2012) (www.roadmapepigenomics.org). It remains a major challenge to assign causal relationships between distinct chromatin marks at specific loci and gene expression, and ultimately cell behavior and function.
The engineering of programmable enzymes with DNA-binding domains such as zinc finger proteins (ZFPs) and transcription activator-like effectors (TALEs), have opened the door to site-specific chromatin modifications (Perez-Pinera et al., 2012). However, the laborious process of protein engineering limits large-scale application of ZFPs and TALEs. A major breakthrough came from the advent of the CRISPR/Cas9 system, in which the Cas9 endonuclease can be targeted to specific DNA sequences by guide RNAs (gRNAs) that are easily programmable (Jinek et al., 2012). The CRISPR/Cas9 system and its derivatives are becoming the most widely used genome-editing tool thanks to the high efficiency, specificity, versatility and ease of use. The utility of Cas9 is further expanded with the engineering of the nuclease-deficient version (dCas9), which can be tethered to a diverse array of epigenetic effector domains for site-specific epigenome modifications (Gilbert et al., 2013).
Here, we review the use of CRISPR/(d)Cas9 tools for epigenome engineering and investigation in the context of development and pluripotent stem cell self-renewal and differentiation. First, we discuss the use of the Cas9 system to examine regulatory sequences that can modulate gene expression and which may themselves be regulated by epigenetic mechanisms. We next discuss approaches that adapt CRISPR/dCas9 for chromatin modifications and visualization of chromatin dynamics. Based on previous studies, we propose technical considerations for the appropriate design and use of these tools. Finally we discuss the potential impact of the technology on future epigenetics studies, stem cell research, and regenerative medicine.
Cas9-mediated identification of cis-regulatory regions
In eukaryotes, four types of regulatory regions have been described: promoters, enhancers, silencers, and insulators. Enhancers and silencers interact with gene promoters to either stimulate or inhibit gene transcription. Insulator sequences block interactions between enhancers, silencers and promoters to protect genes from inappropriate regulation (Riethoven et al., 2010). These regions can display dynamic epigenetic states that correlate with changes in gene expression and cell behavior (Bulger and Groudine, 2010). This review will focus on promoters and enhancers, which have been the most vigorously investigated using Cas9 genome and epigenome engineering tools.
Systematically identifying functional regulatory regions, especially distal enhancers, is a challenging task. While promoters can have well characterized genetic sequences (such as the TATA box) and are adjacent to the transcription start site (TSS) of the gene they regulate, enhancers have no such identifiable sequence and can be located megabases away from their associated gene. Different criteria such as interspecies sequence conservation, chromatin accessibility, transcription factor recruitment and the presence of histone modifications have been used in order to identify and characterize DNA regulatory regions in mouse and human embryonic stem cells (mESCs and hESCs) and cell lines (Mikkelsen et al., 2007; Chen et al., 2008; Gifford et al., 2013; Xie et al., 2013). However, such genomic annotation does not fully predict functional regulatory elements, nor does it offer sufficient resolution. Furthermore, the current methods typically assign putative enhancers to nearest neighbor genes, which could have false discovery rates ranging from 40% to 73% (Li et al., 2012; Sanyal et al., 2012).
Although first used primarily to disrupt protein-coding regions (Koike-Yusa et al., 2014; Wang et al., 2014; Zhou et al., 2014; Shalem et al., 2015), the Cas9 protein can also be targeted to non-coding putative regulatory regions. Mutations in these regions may disrupt protein binding and DNA interactions and thus affect transcription of associated genes. In this way the CRISPR/Cas9 technology offers an easily scalable approach to define enhancers at high resolution within their native context (Canver et al., 2015; Diao et al., 2016; Korkmaz et al., 2016; Rajagopal et al., 2016; Sanjana et al., 2016). A general strategy involves designing gRNAs to introduce small indel mutations across the 5’ and 3’ regions of a preselected gene. However, the large number of gRNAs required to create gRNA libraries tilling across large genomic regions remains a significant bottleneck. For instance, the entire human coding sequence can be interrogated with libraries containing ~100,000 gRNAs (Shalem et al., 2014; Wang et al., 2014), whereas investigating just 715 Kb of noncoding sequence (approximately 0.02% of total noncoding sequence in the human genome) requires ~18,000 gRNAs (Sanjana et al., 2016). Thus it is often necessary to narrow down the non-coding sequences to be interrogated. A common approach has been to utilize genomic annotations including relevant transcription factor binding (Korkmaz et al., 2016), interaction with a gene promoter using Chromatin Interaction Analysis by Paired-End Tag Sequencing (ChIA-PET) (Rajagopal et al., 2016), a gene’s Topological Associated Domains (TADs) (Diao et al., 2016), and transcriptomic profiling of enhancer RNAs (eRNA) (Korkmaz et al., 2016). Alternatively, interrogating large genomic regions without relying on genomic annotations is possible through generating larger deletions using paired gRNAs, a method already used for screening long ncRNAs (lncRNAs) in liver cancer cells (Wu et al., 2016).
Despite being a relatively new method for identifying cis-regulatory sequences, CRISPR/Cas9 screening has already revealed previously unappreciated features of enhancer architecture and mechanism. For instance, most of the gRNAs directed to produce indel mutations in putative enhancer regions had no effect on the regulation of gene expression (Canver et al., 2017) suggesting that only a small number of critical domains are essential for enhancer function (Maurano et al., 2012; Oldridge et al., 2015). Unbiased screening efforts have identified regulatory regions for 6 ESC-associated genes, including novel enhancers and super-enhancers (Li et al., 2014; Diao et al., 2016; Rajagopal et al., 2016). Notably, some of these newly discovered DNA regulatory regions, such as the Mirc35hg promoter found to regulate Nanog in mESCs, have no identifiable correlative genomic annotation (Rajagopal et al., 2016), further emphasizing the need for screens based on unbiased genome editing to identify functional genomic elements.
Most of CRISPR/Cas9 screenings are based on single detectable features (proliferation, expression of reporter proteins, membrane markers) to isolate cell populations upon genetic disruption. However, recent efforts have been made to combine CRISPR/Cas9 with pooled gRNA libraries and single-cell RNA sequencing technology (Dixit et al., 2016; Jaitin et al., 2016; Datlinger et al., 2017). These new approaches will help us better understand how genome regulatory regions affect the transcriptome at the single-cell level.
The use of gRNA libraries have also been tested with dCas9 linked to chromatin modifiers to identify novel regulatory regions and genetic interactions (to be discussed later) (Kearns et al., 2015; Fulco et al., 2016; Du et al., 2017; Klann et al., 2017; Simeonov et al., 2017). An advantage of the dCas9-based tools is the high potency to induce chromatin changes, which could reduce the number of gRNAs necessary for scanning regulatory regions compared to the Cas9-mediated tiling mutagenesis screens. Indeed, the dCas9-p300 fusion was found to activate gene expression with a single gRNA at promoters and characterized enhancers (Hilton et al., 2015). Ultimately, the identification of regulatory regions could provide targets for epigenetic engineering either to manipulate gene expression or to study the mechanisms underlying transcriptional regulation.
Cas9-mediated chromatin modifications
Chromatin is a complex structure mainly composed of DNA, RNA and proteins, which is segmented into nucleosomes (Rodriguez-Campos and Azorin, 2007; Allis and Jenuwein, 2016). Each nucleosome consists of two copies of four histone proteins (H2A, H2B, H3 and H4) that wrap 147bp of DNA and actively interacts with non-coding RNAs (Allis et al., 2006). Engineered chromatin modifiers, consisting of an epigenetic effector tethered to a DNA binding protein domain such as ZFPs or TALEs, have been developed to produce targeted modifications of chromatin, and in some cases significant changes in the expression of the associated gene (Keung et al., 2015). Recent developments with the CRISPR/Cas9 system have greatly expanded the toolbox for targeted chromatin modification, from transcriptional regulation, to DNA and histone modification and ncRNA relocation.
Targeted transcriptional regulation
The first demonstration of the utility of Cas9 beyond genome editing comes from fusion of dCas9 to known functional domains such as VP64 and KRAB for transcriptional regulation (Fig. 1A). After the initial proof-of-concept experiment (Cheng et al., 2013; Farzadfard et al., 2013; Gilbert et al., 2013; Maeder et al., 2013; Mali et al., 2013a; Perez-Pinera et al., 2013; Qi et al., 2013), numerous studies have validated this approach and further refined the experimental systems utilized (Perez-Pinera et al., 2013; Chakraborty et al., 2014; Gilbert et al., 2014; Kearns et al., 2014; Balboa et al., 2015; Kearns et al., 2015; Konermann et al., 2015; Nihongaki et al., 2015; Polstein and Gersbach, 2015; Shechner et al., 2015; Thakore et al., 2015; Zalatan et al., 2015; Black et al., 2016; Braun et al., 2016; Chavez et al., 2016; Gao et al., 2016; Garcia-Bloj et al., 2016; Genga et al., 2016; Himeda et al., 2016; Mandegar et al., 2016; Pradeepa et al., 2016; Radzisheuskaya et al., 2016; Klann et al., 2017).
Figure 1. CRISPR/Cas9-based applications to study and manipulate the epigenome.
Main applications of the CRISPR/Cas9 system to study and manipulate the epigenome are based on the feasibility to allocate chromatin modifiers and fluorescent molecules in a very precise way. This includes the targeted reposition of transcriptional regulators, histone modifiers, enzymes responsible for changes in the DNA methylation, chromatin-interacting ncRNAs, and fluorescent molecules. (Grey dotted-boxes can be replaced with the colored-boxes corresponding to reported examples of chromatin-modifiers and fluorescent molecules). HDM: histone demethylase, HMT: histone methyltransferase, HAT: histone acetyltransferase, HDAC: histone deacyetylase, HUbq: histone ubiquitin ligase, DNMT: DNA methyltransferase, TET: ten-eleven translocation enzymes. Ac: acetylation, Me: methylation, Ub: ubiquitination.
There are two main effectors currently used to repress gene expression, the KRAB and the mSin3 interaction domain (SID) (see Supplementary Table 1). The KRAB domain recruits KAP1/TRIM28 and HP1 proteins, hindering the positioning of RNA polymerase II (Pol II). These factors also promote the recruitment of histone methyltransferases, which increase the levels of tri-methylation of histone H3 at lysine 9 (H3K9me3) and cause local compaction of chromatin (Pengue and Lania, 1996; Groner et al., 2010). The SID domain causes the recruitment of histone deacetylases via interaction with the transcriptional repressor domain PAH2. dCas9 fused to KRAB or SID has been shown to repress gene transcription by 50 to 99% in different cell lines, primary cultures and pluripotent stem cells (Gao et al., 2013; Kearns et al., 2015; Shechner et al., 2015; Thakore et al., 2015; Amabile et al., 2016; Pradeepa et al., 2016). Similarly, dCas9 has been linked to the activation domains of several transcription factors such as p65, HSF1 and MyoD, and of the viral transactivators Rta and VP16, to induce gene expression (see Supplementary Table 1). These transactivators promote the recruitment of chromatin modifiers, which cause chromatin decondensation, accumulation of histone marks such as acetylation of histone H3 at lysine 27 (H3K27ac) and tri-methylation of histone H3 at lysine 4 (H3K4me3), binding of Pol II and subsequent mRNA transcription. In this way, by using modular systems to recruit multiple activation domains, dCas9 can induce robust gene activation (Chavez et al., 2016).
These transcriptional regulation tools recruit several transcription factor-like elements and chromatin-modifying effectors to produce strong chromatin compaction or decompaction. Several studies have addressed how transactivator/repressor domains refashion the chromatin landscape (Gilbert et al., 2014; Himeda et al., 2016; Pradeepa et al., 2016; Klann et al., 2017). Although these changes are typically transient, persistent transcriptional change and cell fate conversion can occur if the newly introduced chromatin modifications are reinforced by the endogenous epigenetic machinery (Chakraborty et al., 2014; Balboa et al., 2015; Black et al., 2016). These strategies can be a useful alternative to transgene overexpression or antisense-mediated gene repression. In fact, several studies have already demonstrated its applicability in the validation of gene functionality (Gilbert et al., 2014; Chavez et al., 2016; Radzisheuskaya et al., 2016), interrogation of promoter and enhancer regions (Kearns et al., 2015; Zalatan et al., 2015; Himeda et al., 2016; Klann et al., 2017), induction of direct cell fate conversion (Chakraborty et al., 2014; Balboa et al., 2015; Black et al., 2016), reprogramming to pluripotency (Balboa et al., 2015; Xiong et al., 2016), genome-scale transcriptional modifications (Gilbert et al., 2014; Konermann et al., 2015) and proof of concept therapeutic approaches against various diseases (Bialek et al., 2016; Braun et al., 2016; Garcia-Bloj et al., 2016).
In addition to dramatically altering the transcriptional state, there is the need to fine-tune individual epigenetic marks for mechanistic investigation or correction of disease-associated epigenetic aberrations. These systems may not be as consistently efficient in modulating gene expression or producing stable epigenetic features, but they can offer the advantage of precision. Next we describe new dCas9-based tools for targeted histone modifications, DNA methylation, and interaction between ncRNAs and chromatin.
Targeted histone modification
An extensive set of histone post-translational modifications has been found to be associated with specific gene expression profiles in the context of pluripotency, cellular differentiation and disease modeling (see Supplementary Table 1) (Kouzarides, 2007; Huang et al., 2009; Stark et al., 2011; Roadmap Epigenomics Consortium, 2015). Effector proteins add or remove covalent groups, causing the phosphorylation, acetylation, methylation (mono, di- and tri-), and ubiquitination -among other modifications- of specific histone residues. In particular, the presence of specific histone modifications, such as H3K4me3 and H3K27me3, are often associated with gene activation or repression, respectively (Kouzarides, 2007). On the other hand, the exact transcriptional outcome associated with each histone modification is dependent on the cellular and chromatin context. For instance, seemingly counteracting H3K4me3 and H3K27me3 marks coexist in defined chromatin regions, known as bivalent domains, and have been reported in ESCs and progenitor cells (Bernstein et al., 2006; Chen and Dent, 2014). In pluripotent stem cells, bivalent domains are thought to support low basal expression levels of a large set of developmental genes, and upon differentiation, they facilitate the rapid recruitment of factors to either induce or repress gene expression.
dCas9 fusion proteins have been successfully used in ESC and cancer cell lines to target a number of histone modifiers to specific genomic loci, including a histone demethylase (LSD1/KDM1A) (Kearns et al., 2015), a transcriptional coactivator and histone acetyltransferase (p300) (Hilton et al., 2015; Klann et al., 2017) and histone methyltransferases (SMYD3, PRDM9 and DOT1L) (Kim et al., 2015; Cano-Rodriguez et al., 2016; Kwon et al., 2017) (Fig. 1B). These chromatin modifiers have been shown to play diverse roles from ESC self-renewal and differentiation to cell proliferation in cancer and development (Adamo et al., 2011) (Goodman and Smolik, 2000). Thus the dCas9 approach can help tackle a broad range of epigenetic questions in these contexts, including the exact role of each histone modifying enzyme, the interaction between different chromatin modifiers, and the effect of these modifications on local chromatin architecture and gene regulation. Moreover, this system has demonstrated effectiveness in discovering and validating complex DNA regulatory regions that may affect gene expression (Kearns et al., 2015; Klann et al., 2017).
Targeted DNA methylation and demethylation
In mammalian cells, DNA methylation refers to the addition of a methyl group to the 5th carbon of cytosine (5mC) or the less common 6th nitrogen of adenine (6mA) (Smith and Meissner, 2013; Wu et al., 2016). This review will focus on 5mC due to its well-documented role in gene expression regulation. Differentially methylated regions (DMR) between different cell types are often enriched for regulatory regions of the genome and CpG dinucleotides, which can constitute DNA stretches known as CpG islands (Rakyan et al., 2011). Promoter regions of mammalian genes are enriched for the CpG islands, typically showing a high frequency (higher than 50%) of CpG nucleotides within the promoter (Gardiner-Garden and Frommer, 1987). Methylation of these CpG islands loci located near the TSS of a gene has been linked to transcriptional repression (Rakyan et al., 2011).
Cytosine methylation is introduced and maintained by a set of DNA methytransferases (DNMTs), comprised of the de novo methyltransferases DNMT3A and DNMT3B, and the maintenance methyltransferase DNMT1, respectively. DNMT3L lacks methyltransferase activity but functions as a co-factor to enhance DNA methylation (Chedin et al., 2002). On the other hand, active DNA demethylation can occur in two steps. First the ten-eleven translocation (TET) family of enzymes (TET1, TET2 and TET3) oxidize the 5-methylcytosines (5mC) to 5-hydroxymethylcytosines (5hmC), 5-formylcytosine (5fC) and 5-carboxylcytosine (5CaC). Next, the highly oxidized products derived from this reaction are recognized and substituted with unmethylated cytosines by the DNA-repair mechanism mediated by thymine DNA glycosylase (TDG) (Pastor et al., 2013; Wu and Zhang, 2014; Rasmussen and Helin, 2016). Of note, DNA methylation and demethylation are often associated with the recruitment of histone modifiers and profound chromatin modifications (Rasmussen and Helin, 2016).
Targeted DNA methylation and demethylation has been attempted using TALEs and ZFPs with relatively modest effects (Keung et al., 2015). More recently several groups have engineered dCas9 fused to the catalytic domain of distinct DNMTs to methylate CpG islands, CTCF binding sites, and promoter and enhancer regions in cancer cell lines, ESCs and primary cells (Liu et al., 2016; McDonald et al., 2016; Vojta et al., 2016; Lei et al., 2017; Xiong et al., 2017) (Fig. 1C). Importantly the dCas9 approach induces higher methylation changes in larger genomic regions as well as greater repression of gene expression when compared to TALEs (Liu, 2016). In addition, targeted positioning of DNMTs has been also used to study how these effector proteins interact and contribute to modify the chromatin. For example, through targeted deposition of dCas9-DNMTs, multimerization of DNMT3a/DNMT3L was shown to promote processive methylation (DNA methylation spreading after binding to a specific DNA site) (Stepper et al., 2017).
Several groups have also engineered dCas9 fused with the catalytic domain of TET1 (Choudhury et al., 2016; Liu et al., 2016; Morita et al., 2016; Xu et al., 2016). This strategy has been used to investigate the impact of targeted hydroxylation of methylated CpG in contexts such as gene expression activation to induce cell fate conversion in ESCs, active demethylation in post-mitotic neurons in murine brains, and reactivation of tumor suppressor genes in cancer cells. In some cases, directing TET1 to certain DMRs may cause significant demethylation of the DNA and detectable changes in mRNA expression (Liu et al., 2016; Morita et al., 2016). However, targeted methylation or demethylation through transient dCas9 expression does not necessarily have to be sufficient to induce sustained changes in gene expression, which may require the modification of additional chromatin marks. The targeted DNA methylation or de-methylation systems can be further improved by including the concomitant recruitment of co-factors known to enhance the expected results, such as DNMT3L for de novo methylation (Amabile et al., 2016; Stepper et al., 2017) and TDG for demethylation.
Targeted relocation of non-coding RNAs
The discovery of DNA sequences that code for RNAs not involved in peptide synthesis has led to the emergence of the ncRNA field. ncRNAs can be classified according to their size (Holoch and Moazed, 2015). They have been shown to regulate gene expression at the transcriptional and post-transcriptional level, and some can directly influence chromatin remodeling. For example, some short ncRNAs (typically <30 nucleotides) can regulate the imprinting process of several genes (Koerner et al., 2009), as well as promote gene silencing by binding to specific DNA sequences and inducing heterochromatin formation (Holoch and Moazed, 2015). Similarly, some long ncRNAs (lncRNAs, >200 nucleotides) can directly recruit chromatin-modifying effectors, establish RNA-protein complexes, and induce profound changes in the surrounding chromatin structure. The prototypical example is X chromosome inactivation by the lncRNA Xist, which binds to its corresponding DNA sequence and recruits DNA compacting factors such as the polycomb repressive complex (Panning et al., 1997; Pennisi, 2013). In addition, specific ncRNAs transcribed from enhancer regions known as enhancer RNAs (eRNAs), can regulate transcriptional activity at their site of synthesis or in distant enhancer regions (Wang et al., 2011a; Melo et al., 2013).
The generation of tools to position non-coding RNAs in a targeted manner will be instrumental to better understand their role as epigenetic cis-regulatory elements in different biological contexts. However, the targeted relocation of RNAs cannot be achieved using DNA binding proteins such as ZFPs and TALEs. In contrast, the versatility of the CRISPR/(d)Cas9 system allows repurposing of not only the Cas9 protein, but also the gRNA. ncRNAs can be incorporated into the gRNA sequence without affecting its ability to bind to target DNA and recruit dCas9, as shown in the CRISPR-display system for targeting ncRNAs such as the NoRC-binding pRNA stem loop, the Xist-A repeat, e-RNAs, and the lncRNA HOTTIP to a genomic locus (Shechner et al., 2015) (Fig. 1D). Accurate positioning of the ncRNAs was confirmed by RNA immunoprecipitation experiments and changes in expression. Further development of these tools would significantly help us understand the role of ncRNAs in the establishment and maintenance of DNA and histone modifications.
Cas9-mediated chromatin visualization
Regulation of gene expression is multilayered and includes the organization of the entire genomic chromatin within the nucleus. This spatiotemporal organization enables distal regulatory elements, such as enhancers, to physically interact with their associated promoters and thus influence gene expression. Dynamic chromatin organization has been associated with cell fate transitions that occur during ESC differentiation and embryo development (Essers et al., 2005; Misteli, 2007; Kind et al., 2013; Misteli, 2013).
Currently the most widely used methods to study chromatin organization are chromosome conformation capture (3C) and its derived methods, such as Hi-C and DNA fluorescence in situ hybridization (FISH) (Croft et al., 1999; Cremer et al., 2001; de Wit and de Laat, 2012; Dekker et al., 2013). These techniques enable the identification of the genomic interactions for a locus of interest; however, they all require the fixation and denaturation of samples prior to analysis. Live cell imaging of chromatin organization has been explored using fluorescently tagged DNA-binding proteins, including the engineered ZFPs and TALEs, however, the imaging was restricted to repetitive sequences (Robinett et al., 1996; Hellwig et al., 2008; Wang et al., 2011b) (Lindhout et al., 2007; Ma et al., 2013; Miyanari et al., 2013; Thanisch et al., 2014; Yuan et al., 2014).
Recently, CRISPR/Cas9-based tools have been adapted to visualize native genomic loci in living cells. For this, dCas9 is fused with a fluorescent protein, either as a part of a fusion protein (Chen et al., 2013; Anton et al., 2014; Ma et al., 2015; Anton et al., 2016; Chen et al., 2016) or through an RNA scaffold (McDonald et al., 2016; Shao et al., 2016) (Fig. 1E). A unique advantage of the CRISPR/Cas9 system comes from the ease to multiplex. Non-repetitive sequences can now be targeted using an array of gRNAs that target adjacent sequences. In this manner, a dCas9-eGFP fusion protein, along with 26 to 36 gRNAs, was used to visualize a non-repetitive region of the MUC4 locus (Chen et al., 2013). Another study used an alternative enzymatic approach to generate gRNA libraries to label selected non-repetitive regions in living cells and ex-vivo primary samples (Lane et al., 2015). As non-disruptive tools, the dCas9 fusion proteins do not displace telomere-binding proteins (Chen et al., 2013; Anton et al., 2014; Shao et al., 2016) or centromere-binding proteins (Shao et al., 2016), nor do they affect the movement dynamics of telomeres (Chen et al., 2013). CRISPR/(d)Cas9-based imaging also provides unique benefits that will complement Hi-C studies. For instance, CRISPR/(d)Cas9 enables live-imaging to monitor the timing and persistence of chromatin configurations and how they correlate with dynamic changes in cell morphology, cell behavior and environmental perturbations (Essers et al., 2005; Misteli, 2007; Kind et al., 2013; Misteli, 2013). Furthermore CRISPR/Cas9 imaging would enable the investigation of the heterogeneity of chromatin configurations by visualizing these genomic interactions in individual cells.
Technical considerations
To facilitate future studies utilizing Cas9 for epigenome interrogation, this section describes key technical points to help researchers avoid the hurdles described in previous reports. Furthermore, we attempt to define the essential criteria necessary for the appropriate use the CRISPR/Cas9 system in an effort to standardize its use to induce targeted chromatin modifications and investigate the epigenome.
General considerations
Many reports have focused on changes in gene expression to validate new epigenome editing tools. Further development of the field will likely require additional efforts to confirm the targeted modifications and verify the specificity. We propose five levels of measurement. The first would be recruitment of dCas9 to the target genomic loci, which could be verified using ChIP-qPCR for dCas9. The second level would be directly assessing the appearance of the desired epigenetic mark at the appropriate locus. The assay used for this purpose would depend on the specific epigenetic modification introduced but could include: bisulfite sequencing (for targeted DNA methylation or demethylation), ChIP-qPCR (for targeted histone modifications), and RNA immunoprecipitation (for ncRNA targeting). The next level would be testing for expression changes of the associated gene after targeted epigenetic modification using RT-PCR. To fully characterize the specificity of these tools, it would then be advantageous to study the effect of the targeted chromatin modification at a genome-wide level by ChIP-seq, genome-wide-bisulfite sequencing and/or RNA-Seq. Finally, analysis of cellular phenotypic changes (cell type-specific assays) should be performed, if the goal of the targeted epigenetic modification is to induce changes in cell behavior or identity.
Moreover, to validate that any observed changes in gene expression or phenotype are due to the intended epigenetic modification, we propose to routinely include several controls such as experiments in which the dCas9 effectors are expressed in the target cell population along with scrambled gRNAs as well as without gRNAs in order to validate the specificity of the induced effect with the targeted gRNAs. It is also relevant to design experiments based on catalytic dead epigenetic effectors to assess the specific impact of the chromatin modifiers in comparison with the deposition of the dCas9 enzyme in a confined DNA region.
Two important parameters to keep in mind are the timing at which the acquisition of chromatin marks is assessed and the persistence of the chromatin modifications induced with dCas9. In transient transfection experiments, most of the expected chromatin modifications were assessed 48–72 hours post-transfection, while in stable transduction experiments, this assessment was performed 2–7 days after infection. Significantly in all studies performed so far, unless the targeted epigenetic modifications produced cellular phenotypic changes, the chromatin modifications were lost when the gRNAs or the dCas9-effector molecules ceased to be expressed (Gilbert et al., 2013; Chakraborty et al., 2014; Gilbert et al., 2014; Hu et al., 2014; Kearns et al., 2014; Black et al., 2016; Liu et al., 2016; Mandegar et al., 2016; McDonald et al., 2016; Thakore et al., 2016; Vojta et al., 2016). These results reveal the dynamic nature of these events and suggest performing analysis at earlier time points after induction.
dCas9-effector and gRNA design
To design dCas9-based epigenetic tools, one must consider how the effector protein will be constructed and how it will be linked to the dCas9 protein. When pursuing modification of specific chromatin marks, the use of the catalytic domain of the epigenetic effector protein, rather than the full-length protein itself, has been shown to be more efficient (Hilton et al., 2015; Kim et al., 2015; Choudhury et al., 2016; Liu et al., 2016; McDonald et al., 2016; Morita et al., 2016; Vojta et al., 2016; Xu et al., 2016; Stepper et al., 2017). Additionally, the majority of epigenetic engineering tools use amino acid linkers between the dCas9 protein and the effector domain to create a fusion protein. Several papers have found that the structure and length of the linkers is a key determinant for robust targeted chromatin modifications (Guilinger et al., 2014; Morita et al., 2016), though no general rule has been identified that applies to all the possible effectors. Optimization of the nuclear transport of dCas9 fusion proteins has been investigated by varying the number and position of nuclear localization signal (NLS) sequences with respect to the dCas9 protein and/or the fused eGFP. Constructs in which an NLS was placed at the N-terminus of dCas9 and a second NLS either at the C-terminus or the N-terminus of eGFP resulted in close to 100% localization of dCas9-eGFP to the nucleus (Chen et al., 2013) (Fig. 2A).
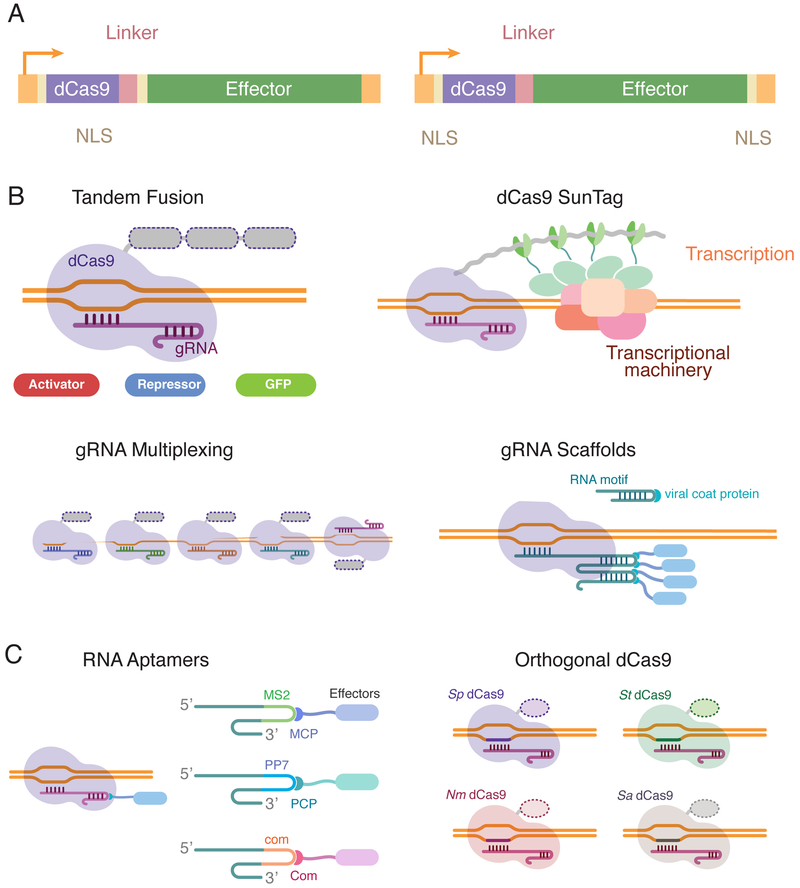
Figure 2. Technical considerations to improve CRISPR/Cas9 versatility.
Several factors can improve the efficiency of the epigenetic targeted modifications by CRISPR/Cas9. A) Using only the effector domain of the chromatin-modifiers, as well as binding peptidic linkers and nuclear localization signals (NLS) to the dCas9 enzyme, significantly increases the precision and efficiency of the intended chromatin modifications. B) The CRISPR/Cas9 system offers 4 different options to amplify the effect of single chromatin modifications, based on the possibility to multiplex the amount of effectors positioned in a specific locus. C) The CRISPR/Cas9 system offers the possibility to simultaneously induce several targeted chromatin modifications, as the gRNA molecules can function as scaffolds to recruit different RNA-binding proteins linked to various effector domains. Another option to target multiple loci is to exploit the orthogonality of this system, using Cas9 enzymes from different species and their corresponding gRNAs. Sp (Streptococcus pyogenes), St (Streptococcus thermophiles), Nm (Neisseria meningitides), Sa (Staphylococcus aureus).
An additional key point to be taken into account is the selection of the chromatin region for epigenetic modification. Taking the TSS as a reference point, the most common regions to be interrogated are the gene bodies, the promoter regions, and the enhancer regions. Based on comparative studies (Radzisheuskaya et al., 2016), we suggest the use of tested databases such as FANTOM5 to accurately determine the TSS and the corresponding promoter sequences prior to designing gRNAs. Candidate gRNAs can then be tested for off-targets using available resources (Hsu et al., 2014). The identification of the most efficient gRNA is still an empirical process. We thus recommend testing multiple gRNAs covering a specific genomic region to identify the most efficacious one(s). In addition to gRNA design optimization, increasing the gRNA expression level or its association with dCas9 may also increase targeting efficiency. Two different structural modifications of the targeting gRNA, an A-U base pair flip in the gRNA stem-loop and an increase in the dCas9-binding hairpin structure, may improve also the final effect (Chen et al., 2013). Of note, it is recently demonstrated that (d)Cas9 binding to the target DNA may be affected by nucleosome occupancy (Horlbeck et al., 2016b). This observation has led to improved gRNA libraries for gene activation or repression (Horlbeck et al., 2016a). However, it remains unclear to which degree this steric impediment may be circumvented by the use of multiple repressors or activators (e.g. dCas9-SAM).
Expression of dCas9-effector and gRNA
For epigenome engineering purposes, most experiments performed to date are validated on cell lines such as HEK 293, HeLa or NIH/3T3, after transient transfection of DNA vectors. However, for robust epigenome modifications, stable transduction of the dCas9-effector tool is often necessary, in particular when applied to primary cells or pluripotent stem cells (Gilbert et al., 2013; Chakraborty et al., 2014; Balboa et al., 2015; Konermann et al., 2015; Thakore et al., 2015; Black et al., 2016; Mandegar et al., 2016; McDonald et al., 2016; Morita et al., 2016; Vojta et al., 2016). Transduced cells can then be identified through the use of reporter or selectable markers, such as fluorescent proteins or drug resistance.
Several inducible approaches have been developed to add versatility to the dCas9-effector expression system. These include the classical approach utilizing the tetracycline-inducible expression system (Gonzalez et al., 2014; Mandegar et al., 2016). Inducible activation/repression can also be achieved by exploiting the versatility of the dCas9 protein and the use of radiation-, heat-, dimerizer-, and/or light-inducible systems (Nihongaki et al., 2015; Polstein and Gersbach, 2015; Gao et al., 2016). Among these, light-inducible systems hold great promise as they offer spatiotemporal precision for both basic science and therapeutic applications.
Regarding the expression of the gRNAs, an ideal system should have high transduction efficiency into the target cells and preferably, be amenable to multiplexing. Accordingly, lentiviral vectors have been used extensively for constitutive expression of single gRNAs, for both genome and epigenome engineering. This strategy can be combined with single-vector systems validated to express multiple gRNAs driven by polymerase-III promoters (Sakuma et al., 2014; Tsai et al., 2014), which may be useful to increase the effectiveness and flexibility of the gRNAs. Another noteworthy variation is to express gRNAs using polymerase-II promoters, a system that has proven highly efficient and adjustable for inducible expression (Nissim et al., 2014; Yoshioka et al., 2015).
An interesting alternative that has already been tested is the use of RNA oligonucleotides transcribed in vitro and transfected into the cells. The use of straightforward protocols to produce the gRNAs, and the high transfection efficiencies achieved for most of the tested primary and pluripotent stem cells, makes this strategy a very interesting option (Mandal and Rossi, 2013; Gonzalez et al., 2014).
Signal amplification
One of the most important advantages of the CRISPR/Cas9 system to interrogate the epigenome is the possibility to specifically amplify the intended epigenetic effect based on the versatility of the Cas9 enzyme and the gRNA molecules. So far, four strategies have been devised to achieve this goal (Fig. 2B). The first approach consists of fusing the dCas9 protein to multiple copies of an effector domain, or to several effectors with similar functions in tandem. This approach dramatically increases chromatin visualization and the efficiency of transcriptional expression and repression (Balboa et al., 2015; Ma et al., 2015; Pradeepa et al., 2016). Another strategy is to use multiple gRNAs that target a discrete DNA region in order to amplify the intended effect, as well as to promote epigenetic spreading of the targeted modification to adjacent chromatin (Maeder et al., 2013; Mali et al., 2013b; Hu et al., 2014; Hilton et al., 2015; Kim et al., 2015; Liu et al., 2016; McDonald et al., 2016; Morita et al., 2016; Pradeepa et al., 2016). A third strategy is based on the so-called SunTag system, an array of GCN4 peptide epitopes, separated by linker sequences attached to the dCas9 protein. These peptide epitopes are recognized by a single chain antibody (scFv), which is in turn linked to the effector protein of choice. Upon binding of the dCas9-GCN4 array to its target genomic locus, multiple copies of the desired effector are recruited as scFv-effector fusions (Tanenbaum et al., 2014; Himeda et al., 2016). As only a few effectors have thus far been tested using the SunTag system, further optimization may be needed for extending its utility to additional effectors. Finally, gRNAs can be engineered to harbor RNA motifs that are recognized by their corresponding RNA binding proteins, such as the MS2, PPC, boxB, PUF and COM motifs. These RNA binding peptides can be easily fused to multiple effector molecules and greatly increase the final expected epigenetic modifications (Konermann et al., 2015; Zalatan et al., 2015; Cheng et al., 2016). Of note, the use of the RNA binding protein Pumilio and its RNA recognition domain PUF offers remarkable adaptability for multiplexing and multimerization (Cheng et al., 2016). In general, these systems outperform those with dCas9 tethered to a single effector domain (Konermann et al., 2015; Chavez et al., 2016; Garcia-Bloj et al., 2016), and we strongly advise using multimerization systems to achieve robust targeted epigenetic changes.
Utilizing multiple epigenetic effectors within the same cell
In order to dissect relationships between epigenetic marks or to drastically manipulate gene expression it is necessary to be able to modify various epigenetic marks simultaneously in the same cell. This can be accomplished if each gRNA, in addition to the encoding information about the target genomic locus, also specifies which epigenetic modifier is to be recruited to that locus. This can be achieved through the use of multiple Cas9-gRNA orthologs or multiple gRNAs with different scaffolds (Fig. 2C).
Cas9 orthologs from different bacterial species use distinct PAM sequences, thus gRNAs can be designed to recruit only a particular Cas9 ortholog to the targeted genomic site (Esvelt et al., 2013). By associating each Cas9 ortholog to a unique epigenetic modifier, one can direct a particular epigenetic effector to the desired genomic site based on the targeting sequence of its cognate gRNA (Kearns et al., 2015; Gao et al., 2016). Expressing multiple Cas9 ortholog fusions in the same cell would then enable the manipulation of numerous epigenetic marks simultaneously. The current compilation of Cas9 orthologs is a promising resource to expand the capability and flexibility of epigenome engineering (Cong et al., 2013; Esvelt et al., 2013; Hou et al., 2013; Chylinski et al., 2014; Fonfara et al., 2014). Furthermore, the development of Streptococcus pyogenes Cas9 (Sp Cas9) variants that show altered PAM specificities could further expand the dCas9 toolset (Kleinstiver et al., 2015). Prior to the use of dCas9 orthologs or Sp dCas9 variants, a number of factors must be considered. First, the prevalence of the required PAM sequences in the genome must be assessed for each ortholog system. Second, the expression and targeting efficiency of the different dCas9 orthologs needs to be standardized, so that the epigenetic modifications are made at the appropriate levels relative to each other. Finally, the use of robust delivery systems (previously mentioned) is required to achieve the concomitant and efficient expression of multiple dCas9 proteins.
Similarly, efficient modification of multiple different epigenetic modifiers can also be accomplished by exploiting the possibility to engineer the gRNAs as a scaffold for different RNA viral motifs. In this case, instead of using multiple copies of a single effector linked to a single viral peptide (as for signal amplification), distinct epigenetic modifiers can be linked to specific viral proteins and directed to different genomic locations (Zalatan et al., 2015; Ma et al., 2016; Shao et al., 2016). In this way, the gRNA scaffold would encode information on both the target genomic site and the type of epigenetic effector being recruited to this location.
Specific considerations for relocation of gene transactivators/repressors
In order to standardize the use of dCas9-based systems to promote or repress gene expression, various technical details should be considered. First, it is important to define rules for designing the most efficient set of gRNAs. Most studies indicate that designing multiple gRNAs that target between −200bp and the TSS is optimal for gene activation, whereas gRNAs targeting between −200pb and +800bp are the most efficient for gene repression (Chakraborty et al., 2014; Gilbert et al., 2014; Balboa et al., 2015; Zalatan et al., 2015; Black et al., 2016; Braun et al., 2016; Chavez et al., 2016; Garcia-Bloj et al., 2016; Hu et al., 2016; Mandegar et al., 2016; Radzisheuskaya et al., 2016). In addition, it is important to assess whether off-target genes also show changes in their expression profile. Most previous studies include genome-wide RNA-Seq experiments to validate the specificity of the intended gene modulation (Gilbert et al., 2013; Gilbert et al., 2014; Hilton et al., 2015; Polstein et al., 2015; Thakore et al., 2015; Zalatan et al., 2015; Chavez et al., 2016). In general, off-target gene activation/repression is not observed, although some authors note that the mere presence of the dCas9-based tools, even in the absence of targeting gRNAs, can slightly affect the overall gene expression profile (Mali et al., 2013b; Thakore et al., 2015).
The number of studies using dCas9 to induce gene activation allows us to deduce and define optimized settings. Different arrays of activator domains have been tested to induce gene activation, including the use of multiple repeats of the VP16 domain (up to 12 repeats) and combinations of modular domains and transcription-like factors, such as p65 and HSF-1. Among these strategies, the synergistic activation mediator (SAM) system based on the modular coupling of dCas9-linked VP64 and the MS2 viral protein linked to the activation domains p65 and HSF1, induces the highest expression levels in numerous settings and cell lines (Konermann et al., 2015; Milite et al., 2015; Chavez et al., 2016; Garcia-Bloj et al., 2016). This system has been successfully tested for cell fate conversion (transdifferentiation, reprogramming, differentiation, etc.), lncRNA expression, and endogenous gene expression in highly complex chromatin regions.
Based on these observations we believe that the use of dCas9 linked to transactivators/repressors should be the first option to induce strong transcriptional changes with a detectable phenotype, while relocation of direct chromatin modifiers can be used to understand the role of specific chromatin marks and their meaning in different biological contexts.
Specific considerations for relocation of DNMT and TET enzymes
The extent of DNA methylation or demethylation induced by dCas9-based tools is not always proportional to the levels of gene repression or activation (Liu et al., 2016; McDonald et al., 2016). This highlights the importance of using more specific and accurate assays to assess DNMT-mediated DNA methylation as well as TET-mediated oxidation and subsequent DNA demethylation. Single-molecule real time (SMRT) sequencing is particularly attractive due to its ability to sequence every DNA molecule in a cell population and differentiate among several modified nucleotides including 5hmC, 5mC, and 6mA (Flusberg et al., 2010). In addition, it is possible that the DNMT and TET catalytic domains can induce epigenetic modifications without the dCas9-mediated targeted deposition. Thus, it is important to include control conditions using catalytically inactive TET1 and DNMT domains or non-targeting gRNAs.
Specific considerations for relocation of histone modifiers
In addition to the general technical considerations previously described, particular attention should be paid to the strategies to validate the ability of these tools to produce the desired histone modifications at the appropriate genomic location. The strategies used to verify correct targeting are comprised of ChIP-seq against the dCas9 protein and the specific histone modifier, ChIP to detect the expected histone modification, and ChIP against other histone marks, along with ATAC-seq or DNase-Seq, to identify secondary chromatin alterations (Hilton et al., 2015; Kearns et al., 2015; Kim et al., 2015; Cano-Rodriguez et al., 2016). A complementary approach to validate the accuracy of dCas9-associated histone modifiers is to perform functional studies in vitro using purified dCas9 protein on recombinant histone octamers (Kim et al., 2015; Horlbeck et al., 2016b).
Specific considerations for chromatin visualization
The ultimate goal for visualization studies of chromatin organization is to be able to visualize and track multiple loci simultaneously for time periods spanning hours or even days. The visualization of multiple genomic loci can be achieved using different dCas9 orthologs, or with gRNA scaffolds. At least three orthologs of the commonly used Streptococcus pyogenes dCas9 have been adapted for dCas9-based imaging (Ma et al., 2015; Chen et al., 2016). Similarly, researchers have been able to simultaneously visualize 6 independent genomic loci using gRNA scaffolds (Ma et al., 2016).
Most studies thus far have used CRISPR/dCas9 for imaging over relatively short intervals, ranging from seconds (Ma et al., 2016) to up to 2 hours (Chen et al., 2013). Tracking of single dCas9 molecules in living cells showed that dCas9 binds tightly to regions that have complete complementarity to the targeting gRNA (Knight et al., 2015). Photo-bleaching of the fluorescent proteins attached to these tightly bound dCas9 molecules would prevent long-term imaging of the labeled genomic locus. Significantly, a recent study found that dCas9 associated with a fluorescent protein through an RNA scaffold recovers fluorescence about 40 times faster than a dCas9-fluorescent protein fusion, and this strategy allows the continuous visualization of the chromatin for more approximately 26 hours. This result was attributed to the fast exchange between the MS2 RNA hairpin and the MCP peptide bound to the fluorescent protein (Shao et al., 2016).
The majority of studies using dCas9 to visualize chromatin thus far have focused on satellite regions, telomeres and repetitive regions within genes. These regions have tandem arrays of repetitive sequences that allow multiple dCas9-eGFP fusions to be recruited to the target locus using a single gRNA. Signal amplification methods such as SunTag and RNA scaffolds should facilitate the labeling of non-repetitive sequences. Depending on the kinetics of exchange between scFv and GCN4, it is conceivable that the SunTag system will also be less vulnerable to photo-bleaching than a dCas9 fluorescent protein fusion.
Applications in stem cell biology and regenerative medicine
Standardization of the CRISPR/(d)Cas9 technology to engineer and interrogate the epigenome will greatly contribute to the advance of the regenerative medicine and stem cell biology fields. In particular, this system has the potential to become a powerful tool to dissect in detail a broad range of epigenetic mechanisms, to manipulate cell-fate transitions, and to study and eventually cure diseases with an epigenetic basis (Fig. 3).
Figure 3. Future applications.
The use of the CRISPR/Cas9 system to interrogate and manipulate the epigenome will pave the road for the study and modification of chromatin at different levels of complexity. This system will significantly help us comprehend and control a broad range of epigenetic events based on its capacity to modify single chromatin marks, control endogenous gene expression, dissect the establishment of epigenetic features, and trace chromatin events inside the nucleus.
Studying epigenetic mechanisms
The generation of catalytic domain-mutated chromatin modifiers has been useful to corroborate the participation of effectors and readers when a chromatin feature is established in vivo. On this point, the development of dCas9-based systems to target specific chromatin modifiers in any specific cellular context, individually or in combination, will be invaluable for dissecting potential additive, synergistic, dominant, or antagonistic relationships between different chromatin marks. This tool will also be useful to define in detail the set of chromatin readers/effectors primarily recruited in the context of mRNA transcription, the secondary chromatin marks derived thereafter, and the effectors directly responsible for gene silencing or activation. These goals can be facilitated by characterizing the molecules associated with the target genomic sequence upon targeted chromatin modifications, utilizing dCas9-based ChIP (Fujita and Fujii, 2013, 2015) and chromatin affinity purification coupled with mass spectrometry (Byrum et al., 2012; Waldrip et al., 2014).
The use of dCas9 as a platform to induce targeted chromatin alterations will be instrumental to understand the development of complex epigenetic events, not necessarily related to mRNA transcription, which are currently viewed as the accumulation of multiple marks based on associative studies such as gene priming, conditional repression, or bivalency acquisition and resolution. Similarly, further development of dCas9-based techniques will elucidate how ncRNAs interact with specific DNA domains, transcription-like factors, histone modifiers and protein factors to change the chromatin conformation (Zalatan et al., 2015).
In addition to the above-mentioned strategies to understand the establishment of epigenetic marks, we foresee the use of genome-wide functional screenings using dCas9-based tools, to discover and validate how unknown DNA regulatory regions interact with specific chromatin effectors and alter gene activation (Gilbert et al., 2014; Klann et al., 2017). Moreover, we expect future development efforts to further generate dCas9-based tools to read chromatin states, functioning as reporters of the epigenetic events associated with specific cellular processes. This will be particularly useful for tracking epigenetic events occurring during cell fate conversion in lineage differentiation and in disease models.
Further development of CRISPR/(d)Cas9-based tools will enable not only understanding of how chromatin marks are established, but also how they are inherited after cell division. The mechanisms for epigenetic inheritance are commonly associated with the methylation of DNA, where DNMT3A and DNMT3B are able to methylate cytosine residues as a response to specific stimuli, and DNMT1 can “read” hemi-methylated DNA strands after cellular division and return the DNA back to its previous methylation status (Bestor, 2000). However, classic studies in S. pombe (Grewal and Klar, 1996; Audergon et al., 2015; Ragunathan et al., 2015) and Drosophila (Cavalli and Paro, 1998) suggest that histone modifications and regulatory proteins are sufficient to establish stable chromatin modifications across generations. Thus, we believe that the use of dCas9 to directly relocate chromatin readers and effectors will help identify self-reinforcing mechanisms. These efforts can be valuable not only to determine epigenetic inheritance at the cellular level, but also to understand epigenetic inheritance across generations in complex organisms (Huypens et al., 2016; Klosin et al., 2017)
Engineering cell identity
A large number of strategies to differentiate stem cells or transdifferentiate somatic cells are based on the overexpression of transcription factors (so-called master regulators) and microRNAs (Graf and Enver, 2009; Atala, 2011; Pulecio et al., 2014; Caiazzo et al., 2015; Capellera-Garcia et al., 2016; Pulecio et al., 2016). dCas9 can be used to target strong transactivators or repressors to induce robust changes in the expression levels of endogenous genes, which has been shown to yield similar or better results compared to the expression of exogenous factors (Chakraborty et al., 2014; Balboa et al., 2015; Konermann et al., 2015; Black et al., 2016; Chavez et al., 2016; Mandegar et al., 2016). The dCas9 system allows multiple and specific chromatin changes with high temporal precision, which is not easily achievable using the classical transduction system. This versatility opens up the possibility for inducing cell fate transitions based on complex regulatory networks, where specific dynamics of gene upregulation and downregulation are required to obtain the desired phenotype.
Particularly, in the context of reprogramming to pluripotency, it has been suggested that reprogrammed cells may retain epigenetic traits from the donor cells and bias the differentiation ability of the generated iPSCs (Kim et al., 2010; Nashun et al., 2015; Nishizawa et al., 2016; Ramos-Mejia et al., 2012; Vitaloni et al., 2014). These epigenetic features can be identified as factors that either cause partial reprogramming or facilitate the differentiation towards the lineage of the donor cell. With the use of dCas9-based tools, it will be possible to specifically manipulate those features in order to increase the efficiency of reprogramming and differentiation.
The CRISPR/dCas9 system can also be instrumental in producing phenotypic changes not strictly related with the expression of an identifiable set of genes, but related to specific epigenetic features. This is the case of naïve pluripotency, a ground phenotypic state in embryonic stem cells that confers the potential to differentiate in vivo and in vitro and to contribute to form chimeras in rodents, unlike primed pluripotent stem cells (Weinberger et al., 2016). Main differences between naïve and primed stem cells are correlated with several epigenetic features, such as global DNA methylation levels, chromosome X inactivation, and variations in the genome regions occupied by enhancers. Moreover, global modification of the epigenetic landscape of primed pluripotent stem cells, such as the ablation of histone methyl-transferase MLL1 or the ectopic expression of Tet2, have been shown to reprogram mouse or human primed stem cells to a naïve state (Fidalgo et al., 2016; Zhang et al., 2016). Thus, the use of dCas9-based targeted epigenome editing tools may allow the identification and subsequent modification of the specific chromatin features responsible for naïve pluripotency.
Modeling disease mechanisms and developing therapeutics
A large set of diseases is strongly correlated to abnormal regulation of epigenetic features. Occurrence of these aberrant states is mainly caused by DNA mutations in non-coding regions (Scacheri and Scacheri, 2015) and deregulation of specific chromatin modifications. Currently, non-coding mutations are identified by whole genome sequencing and then traced to regulatory regions by overlapping patient-specific mutations with epigenetic marks typical of such regions. However, this strategy does not reveal whether the identified non-coding mutation actually disrupts the function of the regulatory region, making it difficult to uncover its mechanism of action. As an alternative, wild-type Cas9 can be used to generate disease-mimicking cell lines that harbor patient-specific non-coding mutations. One may also perform mutagenesis screens to identify novel variants associated with gene expression changes.
Non-coding mutations can disrupt the 3D-structure of chromatin and thus deregulate gene expression. For instance, congenital malformations have been associated with the disruption of TADs, which organize chromatin and constrain the regulatory interactions between enhancers and promoters (Scacheri and Scacheri, 2015). It is conceivable that previously unappreciated mutations occurring in gene deserts but associated with disease could be functioning in a similar way to disrupt chromatin organization. This phenomenon could be tested by using Cas9 mediated mutagenesis to disrupt the sequences of TAD boundaries or points of contact between chromatin, as well as by using dCas9 fused to fluorescent proteins to monitor chromatin organization in real time. In addition to identifying sequences necessary for the formation of chromatin loops and TAD boundaries, one would also be able to observe the immediate consequences of this disruption on gene expression and cell phenotype.
Another promising application of the dCas9 technology is its potential as a method to study and eventually cure diseases correlated with alterations in the pattern of epigenetic modifications such as erroneous genomic imprinting (e.g Prader-Willi, Angelman), disorders with genetic anticipation (e.g. Huntington’s disease, X syndrome, Friedreich’s ataxia) and cancer (Cassidy and Schwartz, 1998; Kelly et al., 2010). In the case of cancer, most of the studies reporting the use of dCas9 to cause epigenome modifications include a set of experiments to ameliorate the conditions of this disorder in vitro (Braun et al., 2016; Choudhury et al., 2016; Garcia-Bloj et al., 2016; Morita et al., 2016) and in vivo (Braun et al., 2016). This has been attempted using dCas9 fused to strong transactivators or repressors to either reactivate the expression of endogenous tumor suppressor genes inhibited during cancer progression, or inhibit the expression of oncogenes (Braun et al., 2016; Garcia-Bloj et al., 2016). In addition, the use of dCas9 linked to strong transactivators, coupled to efficient transfection of pooled libraries of gRNAs, has been successfully tested as a tool to perform genome-scale screenings to discover genes that may promote or inhibit cancer cell growth or response to cancer treatment (Gilbert et al., 2014; Konermann et al., 2015).
Moreover, the use of strong transactivators and repressors coupled to dCas9 has recently been demonstrated as a powerful tool to evaluate the role of candidate genes to model diseases in vitro, based on iPSC differentiation and culture of primary samples (Ho et al., 2017; Lopez Rodriguez et al., 2017). The next step is to apply epigenome engineering in vivo, and explore its potential for therapeutic applications. Cas9 packaged into adeno-associated viruses (AAVs) has previously been used for in vivo genome editing of muscle, liver, and brain tissues to either produce desired mutations or to correct disease causing mutations (Swiech et al., 2015; Long et al., 2016; Nelson et al., 2016; Tabebordbar et al., 2016; Yang et al., 2016). However, a series of technical challenges need to be overcome for therapeutic applications. We refer the readers to an excellent review for more information in this area (Komor et al., 2017).
In summary, these works have paved the path for new strategies to induce the targeted modification of more complex epigenetic events, including changes in the methylation and hydroxymethylation status of specific DNA loci, chromatin looping and modification of multiple histone marks associated with disease onset and progress. In parallel, the possibility to efficiently manipulate complex chromatin marks with dCas9 provides a tool to model the onset and progression of disorders correlated with epigenetic events in vitro. These developments contribute a reliable model for in vitro drug testing, as well as a platform to understand and dissect the contribution and hierarchy of each single chromatin mark towards a specific diseased condition.
Conclusion and Future Directions
CRISPR/Cas9 genome and epigenome editing has the potential to revolutionize the field of epigenetics. Whereas previously the epigenetics field focused on profiling epigenetic states in different cell types to elucidate biological function, the robustness and ease of CRISPR/Cas9 editing tools enables direct manipulation of specific regulatory sequences and epigenetic marks to determine their significance for proper transcriptional activity and cellular function. Moreover, the multiplexing ability of Cas9 and the use of gRNA scaffolds or Cas9 orthologs enable the simultaneous investigation of multiple genomic loci and epigenetic marks, a prerequisite for dissecting the often complex layers of epigenetic regulation. Finally, the components for CRISPR/Cas9 genome and epigenome editing can be easily introduced into live cells and then used to precisely and dynamically manipulate the epigenetic state, the effects of which can then be followed in real time. Although currently confined to in vitro experiments, it seems reasonable that in the future CRISPR/Cas9 epigenome engineering could be employed in living organisms, a milestone that would facilitate the exploration of areas in development and disease that were previously beyond reach.
Supplementary Material
Acknowledgements
The work in the Raya laboratory is supported by the Spanish Ministry of Economy and Competitiveness-MINECO (SAF2015–69706-R), Instituto de Salud Carlos III-ISCIII/FEDER (FIS PI14/01682; Red de Terapia Celular - TerCel RD16/0011/0024), Generalitat de Catalunya-AGAUR (2014-SGR-1460), Fundació La Marató de TV3 (201534–30), and CERCA Programme / Generalitat de Catalunya. The work in the Huangfu laboratory is supported by NIH/NIDDK (R01DK096239), New York State Stem Cell Science (NYSTEM C029567 and C029156), Tri-Institutional Stem Cell Initiative (#2016–004, 2016–032), and the MSKCC Cancer Center Support Grant (P30 CA008748). JP was partially supported by the Juan de la Cierva program (MINECO). Wenjing Wu assisted with the graphics.
References:
- Adamo A, Sese B, Boue S, Castano J, Paramonov I, Barrero MJ, and Izpisua Belmonte JC (2011). LSD1 regulates the balance between self-renewal and differentiation in human embryonic stem cells. Nat Cell Biol 13, 652–659. [DOI] [PubMed] [Google Scholar]
- Allis CD, and Jenuwein T (2016). The molecular hallmarks of epigenetic control. Nat Rev Genet 17, 487–500. [DOI] [PubMed] [Google Scholar]
- Allis CD, Jenuwein T, and Reinberg D (2006). Epigenetics (Cold Spring Harbor, N.Y., Cold Spring Harbor Laboratory Press; ). [Google Scholar]
- Amabile A, Migliara A, Capasso P, Biffi M, Cittaro D, Naldini L, and Lombardo A (2016). Inheritable silencing of endogenous genes by hit-and-run targeted epigenetic editing. Cell 167, 219–232 e214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton T, Bultmann S, Leonhardt H, and Markaki Y (2014). Visualization of specific DNA sequences in living mouse embryonic stem cells with a programmable fluorescent CRISPR/Cas system. Nucleus 5, 163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton T, Leonhardt H, and Markaki Y (2016). Visualization of genomic loci in living cells with a fluorescent CRISPR/Cas9 system. Methods Mol Biol 1411, 407–417. [DOI] [PubMed] [Google Scholar]
- Atala A (2011). Principles of regenerative medicine (Amsterdam ; Boston, Elsevier/Academic Press,), pp. 1 online resource (online resource (xix, 1182 p.). [Google Scholar]
- Audergon PN, Catania S, Kagansky A, Tong P, Shukla M, Pidoux AL, and Allshire RC (2015). Epigenetics. Restricted epigenetic inheritance of H3K9 methylation. Science 348, 132–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balboa D, Weltner J, Eurola S, Trokovic R, Wartiovaara K, and Otonkoski T (2015). Conditionally stabilized dCas9 activator for controlling gene expression in human cell reprogramming and differentiation. Stem Cell Reports 5, 448–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, et al. (2006). A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125, 315–326. [DOI] [PubMed] [Google Scholar]
- Bestor TH (2000). The DNA methyltransferases of mammals. Hum Mol Genet 9, 2395–2402. [DOI] [PubMed] [Google Scholar]
- Bialek JK, Dunay GA, Voges M, Schafer C, Spohn M, Stucka R, Hauber J, and Lange UC (2016). Targeted HIV-1 Latency Reversal Using CRISPR/Cas9-Derived Transcriptional Activator Systems. PLoS One 11, e0158294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JB, Adler AF, Wang HG, D’Ippolito AM, Hutchinson HA, Reddy TE, Pitt GS, Leong KW, and Gersbach CA (2016). Targeted Epigenetic Remodeling of Endogenous Loci by CRISPR/Cas9-Based Transcriptional Activators Directly Converts Fibroblasts to Neuronal Cells. Cell Stem Cell 19, 406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun CJ, Bruno PM, Horlbeck MA, Gilbert LA, Weissman JS, and Hemann MT (2016). Versatile in vivo regulation of tumor phenotypes by dCas9-mediated transcriptional perturbation. Proc Natl Acad Sci U S A 113, E3892–3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulger M, and Groudine M (2010). Enhancers: the abundance and function of regulatory sequences beyond promoters. Dev Biol 339, 250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrum SD, Raman A, Taverna SD, and Tackett AJ (2012). ChAP-MS: a method for identification of proteins and histone posttranslational modifications at a single genomic locus. Cell Rep 2, 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caiazzo M, Giannelli S, Valente P, Lignani G, Carissimo A, Sessa A, Colasante G, Bartolomeo R, Massimino L, Ferroni S, et al. (2015). Direct conversion of fibroblasts into functional astrocytes by defined transcription factors. Stem Cell Reports 4, 25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano-Rodriguez D, Gjaltema RA, Jilderda LJ, Jellema P, Dokter-Fokkens J, Ruiters MH, and Rots MG (2016). Writing of H3K4Me3 overcomes epigenetic silencing in a sustained but context-dependent manner. Nat Commun 7, 12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canver MC, Smith EC, Sher F, Pinello L, Sanjana NE, Shalem O, Chen DD, Schupp PG, Vinjamur DS, Garcia SP, et al. (2015). BCL11A enhancer dissection by Cas9-mediated in situ saturating mutagenesis. Nature 527, 192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capellera-Garcia S, Pulecio J, Dhulipala K, Siva K, Rayon-Estrada V, Singbrant S, Sommarin MN, Walkley CR, Soneji S, Karlsson G, et al. (2016). Defining the Minimal Factors Required for Erythropoiesis through Direct Lineage Conversion. Cell Rep 15, 2550–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy SB, and Schwartz S (1998). Prader-Willi and Angelman syndromes. Disorders of genomic imprinting. Medicine (Baltimore) 77, 140–151. [DOI] [PubMed] [Google Scholar]
- Cavalli G, and Paro R (1998). The Drosophila Fab-7 chromosomal element conveys epigenetic inheritance during mitosis and meiosis. Cell 93, 505–518. [DOI] [PubMed] [Google Scholar]
- Chakraborty S, Ji H, Kabadi AM, Gersbach CA, Christoforou N, and Leong KW (2014). A CRISPR/Cas9-based system for reprogramming cell lineage specification. Stem Cell Reports 3, 940–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez A, Tuttle M, Pruitt BW, Ewen-Campen B, Chari R, Ter-Ovanesyan D, Haque SJ, Cecchi RJ, Kowal EJ, Buchthal J, et al. (2016). Comparison of Cas9 activators in multiple species. Nat Methods 13, 563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chedin F, Lieber MR, and Hsieh CL (2002). The DNA methyltransferase-like protein DNMT3L stimulates de novo methylation by Dnmt3a. Proc Natl Acad Sci U S A 99, 16916–16921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Gilbert LA, Cimini BA, Schnitzbauer J, Zhang W, Li GW, Park J, Blackburn EH, Weissman JS, Qi LS, et al. (2013). Dynamic imaging of genomic loci in living human cells by an optimized CRISPR/Cas system. Cell 155, 1479–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Hu J, Almeida R, Liu H, Balakrishnan S, Covill-Cooke C, Lim WA, and Huang B (2016). Expanding the CRISPR imaging toolset with Staphylococcus aureus Cas9 for simultaneous imaging of multiple genomic loci. Nucleic Acids Res 44, e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, and Dent SY (2014). Chromatin modifiers and remodellers: regulators of cellular differentiation. Nat Rev Genet 15, 93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Xu H, Yuan P, Fang F, Huss M, Vega VB, Wong E, Orlov YL, Zhang W, Jiang J, et al. (2008). Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell 133, 1106–1117. [DOI] [PubMed] [Google Scholar]
- Cheng AW, Jillette N, Lee P, Plaskon D, Fujiwara Y, Wang W, Taghbalout A, and Wang H (2016). Casilio: a versatile CRISPR-Cas9-Pumilio hybrid for gene regulation and genomic labeling. Cell Res 26, 254–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng AW, Wang H, Yang H, Shi L, Katz Y, Theunissen TW, Rangarajan S, Shivalila CS, Dadon DB, and Jaenisch R (2013). Multiplexed activation of endogenous genes by CRISPR-on, an RNA-guided transcriptional activator system. Cell Res 23, 1163–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury SR, Cui Y, Lubecka K, Stefanska B, and Irudayaraj J (2016). CRISPR-dCas9 mediated TET1 targeting for selective DNA demethylation at BRCA1 promoter. Oncotarget 7, 46545–46556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chylinski K, Makarova KS, Charpentier E, and Koonin EV (2014). Classification and evolution of type II CRISPR-Cas systems. Nucleic Acids Res 42, 6091–6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, et al. (2013). Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer M, von Hase J, Volm T, Brero A, Kreth G, Walter J, Fischer C, Solovei I, Cremer C, and Cremer T (2001). Non-random radial higher-order chromatin arrangements in nuclei of diploid human cells. Chromosome Res 9, 541–567. [DOI] [PubMed] [Google Scholar]
- Croft JA, Bridger JM, Boyle S, Perry P, Teague P, and Bickmore WA (1999). Differences in the localization and morphology of chromosomes in the human nucleus. J Cell Biol 145, 1119–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datlinger P, Rendeiro AF, Schmidl C, Krausgruber T, Traxler P, Klughammer J, Schuster LC, Kuchler A, Alpar D, and Bock C (2017). Pooled CRISPR screening with single-cell transcriptome readout. Nat Methods 14, 297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit E, and de Laat W (2012). A decade of 3C technologies: insights into nuclear organization. Genes Dev 26, 11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker J, Marti-Renom MA, and Mirny LA (2013). Exploring the three-dimensional organization of genomes: interpreting chromatin interaction data. Nat Rev Genet 14, 390–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao Y, Li B, Meng Z, Jung I, Lee AY, Dixon J, Maliskova L, Guan KL, Shen Y, and Ren B (2016). A new class of temporarily phenotypic enhancers identified by CRISPR/Cas9-mediated genetic screening. Genome Res 26, 397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit A, Parnas O, Li B, Chen J, Fulco CP, Jerby-Arnon L, Marjanovic ND, Dionne D, Burks T, Raychowdhury R, et al. (2016). Perturb-Seq: Dissecting Molecular Circuits with Scalable Single-Cell RNA Profiling of Pooled Genetic Screens. Cell 167, 1853–1866 e1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du D, Roguev A, Gordon DE, Chen M, Chen SH, Shales M, Shen JP, Ideker T, Mali P, Qi LS, et al. (2017). Genetic interaction mapping in mammalian cells using CRISPR interference. Nat Methods 14, 577–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENCODE Project Consortium. (2012). An integrated encyclopedia of DNA elements in the human genome. Nature 489, 57–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst J, and Kellis M (2012). ChromHMM: automating chromatin-state discovery and characterization. Nat Methods 9, 215–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essers J, van Cappellen WA, Theil AF, van Drunen E, Jaspers NG, Hoeijmakers JH, Wyman C, Vermeulen W, and Kanaar R (2005). Dynamics of relative chromosome position during the cell cycle. Mol Biol Cell 16, 769–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esvelt KM, Mali P, Braff JL, Moosburner M, Yaung SJ, and Church GM (2013). Orthogonal Cas9 proteins for RNA-guided gene regulation and editing. Nat Methods 10, 1116–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzadfard F, Perli SD, and Lu TK (2013). Tunable and multifunctional eukaryotic transcription factors based on CRISPR/Cas. ACS Synth Biol 2, 604–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidalgo M, Huang X, Guallar D, Sanchez-Priego C, Valdes VJ, Saunders A, Ding J, Wu WS, Clavel C, and Wang J (2016). Zfp281 Coordinates Opposing Functions of Tet1 and Tet2 in Pluripotent States. Cell Stem Cell 19, 355–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flusberg BA, Webster DR, Lee JH, Travers KJ, Olivares EC, Clark TA, Korlach J, and Turner SW (2010). Direct detection of DNA methylation during single-molecule, real-time sequencing. Nat Methods 7, 461–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonfara I, Le Rhun A, Chylinski K, Makarova KS, Lecrivain AL, Bzdrenga J, Koonin EV, and Charpentier E (2014). Phylogeny of Cas9 determines functional exchangeability of dual-RNA and Cas9 among orthologous type II CRISPR-Cas systems. Nucleic Acids Res 42, 2577–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T, and Fujii H (2013). Efficient isolation of specific genomic regions and identification of associated proteins by engineered DNA-binding molecule-mediated chromatin immunoprecipitation (enChIP) using CRISPR. Biochem Biophys Res Commun 439, 132–136. [DOI] [PubMed] [Google Scholar]
- Fujita T, and Fujii H (2015). Isolation of specific genomic regions and identification of associated molecules by engineered DNA-binding molecule-mediated chromatin immunoprecipitation (enChIP) using CRISPR. Methods Mol Biol 1288, 43–52. [DOI] [PubMed] [Google Scholar]
- Fulco CP, Munschauer M, Anyoha R, Munson G, Grossman SR, Perez EM, Kane M, Cleary B, Lander ES, and Engreitz JM (2016). Systematic mapping of functional enhancer-promoter connections with CRISPR interference. Science 354, 769–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R, Dong R, Du J, Ma P, Wang S, and Fan Z (2013). Depletion of histone demethylase KDM2A inhibited cell proliferation of stem cells from apical papilla by de-repression of p15INK4B and p27Kip1. Mol Cell Biochem 379, 115–122. [DOI] [PubMed] [Google Scholar]
- Gao Y, Xiong X, Wong S, Charles EJ, Lim WA, and Qi LS (2016). Complex transcriptional modulation with orthogonal and inducible dCas9 regulators. Nat Methods 13, 1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Bloj B, Moses C, Sgro A, Plani-Lam J, Arooj M, Duffy C, Thiruvengadam S, Sorolla A, Rashwan R, Mancera RL, et al. (2016). Waking up dormant tumor suppressor genes with zinc fingers, TALEs and the CRISPR/dCas9 system. Oncotarget 7, 60535–60554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner-Garden M, and Frommer M (1987). CpG islands in vertebrate genomes. J Mol Biol 196, 261–282. [DOI] [PubMed] [Google Scholar]
- Genga RM, Kearns NA, and Maehr R (2016). Controlling transcription in human pluripotent stem cells using CRISPR-effectors. Methods 101, 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford CA, Ziller MJ, Gu H, Trapnell C, Donaghey J, Tsankov A, Shalek AK, Kelley DR, Shishkin AA, Issner R, et al. (2013). Transcriptional and epigenetic dynamics during specification of human embryonic stem cells. Cell 153, 1149–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert LA, Horlbeck MA, Adamson B, Villalta JE, Chen Y, Whitehead EH, Guimaraes C, Panning B, Ploegh HL, Bassik MC, et al. (2014). Genome-Scale CRISPR-Mediated Control of Gene Repression and Activation. Cell 159, 647–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert LA, Larson MH, Morsut L, Liu Z, Brar GA, Torres SE, Stern-Ginossar N, Brandman O, Whitehead EH, Doudna JA, et al. (2013). CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell 154, 442–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez F, Zhu Z, Shi ZD, Lelli K, Verma N, Li QV, and Huangfu D (2014). An iCRISPR platform for rapid, multiplexable, and inducible genome editing in human pluripotent stem cells. Cell Stem Cell 15, 215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman RH, and Smolik S (2000). CBP/p300 in cell growth, transformation, and development. Genes Dev 14, 1553–1577. [PubMed] [Google Scholar]
- Graf T, and Enver T (2009). Forcing cells to change lineages. Nature 462, 587–594. [DOI] [PubMed] [Google Scholar]
- Grewal SI, and Klar AJ (1996). Chromosomal inheritance of epigenetic states in fission yeast during mitosis and meiosis. Cell 86, 95–101. [DOI] [PubMed] [Google Scholar]
- Groner AC, Meylan S, Ciuffi A, Zangger N, Ambrosini G, Denervaud N, Bucher P, and Trono D (2010). KRAB-zinc finger proteins and KAP1 can mediate long-range transcriptional repression through heterochromatin spreading. PLoS Genet 6, e1000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilinger JP, Thompson DB, and Liu DR (2014). Fusion of catalytically inactive Cas9 to FokI nuclease improves the specificity of genome modification. Nat Biotechnol 32, 577–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellwig D, Munch S, Orthaus S, Hoischen C, Hemmerich P, and Diekmann S (2008). Live-cell imaging reveals sustained centromere binding of CENP-T via CENP-A and CENP-B. J Biophotonics 1, 245–254. [DOI] [PubMed] [Google Scholar]
- Hilton IB, D’Ippolito AM, Vockley CM, Thakore PI, Crawford GE, Reddy TE, and Gersbach CA (2015). Epigenome editing by a CRISPR-Cas9-based acetyltransferase activates genes from promoters and enhancers. Nat Biotechnol 33, 510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himeda CL, Jones TI, and Jones PL (2016). CRISPR/dCas9-mediated Transcriptional Inhibition Ameliorates the Epigenetic Dysregulation at D4Z4 and Represses DUX4-fl in FSH Muscular Dystrophy. Mol Ther 24, 527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SM, Hartley BJ, Flaherty E, Rajarajan P, Abdelaal R, Obiorah I, Barretto N, Muhammad H, Phatnani HP, Akbarian S, et al. (2017). Evaluating synthetic activation and repression of neuropsychiatric-related genes in hiPSC-derived NPCs, neurons, and astrocytes. Stem Cell Reports 9, 615–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holoch D, and Moazed D (2015). RNA-mediated epigenetic regulation of gene expression. Nat Rev Genet 16, 71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horlbeck MA, Gilbert LA, Villalta JE, Adamson B, Pak RA, Chen Y, Fields AP, Park CY, Corn JE, Kampmann M, et al. (2016a). Compact and highly active next-generation libraries for CRISPR-mediated gene repression and activation. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horlbeck MA, Witkowsky LB, Guglielmi B, Replogle JM, Gilbert LA, Villalta JE, Torigoe SE, Tjian R, and Weissman JS (2016b). Nucleosomes impede Cas9 access to DNA in vivo and in vitro. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Z, Zhang Y, Propson NE, Howden SE, Chu LF, Sontheimer EJ, and Thomson JA (2013). Efficient genome engineering in human pluripotent stem cells using Cas9 from Neisseria meningitidis. Proc Natl Acad Sci U S A 110, 15644–15649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu PD, Lander ES, and Zhang F (2014). Development and applications of CRISPR-Cas9 for genome engineering. Cell 157, 1262–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Luo C, and Zheng YG (2016). Transient Kinetics Define a Complete Kinetic Model for Protein Arginine Methyltransferase 1. J Biol Chem 291, 26722–26738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Lei Y, Wong WK, Liu S, Lee KC, He X, You W, Zhou R, Guo JT, Chen X, et al. (2014). Direct activation of human and mouse Oct4 genes using engineered TALE and Cas9 transcription factors. Nucleic Acids Res 42, 4375–4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Maertens AM, Hyland EM, Dai J, Norris A, Boeke JD, and Bader JS (2009). HistoneHits: a database for histone mutations and their phenotypes. Genome Res 19, 674–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huypens P, Sass S, Wu M, Dyckhoff D, Tschop M, Theis F, Marschall S, Hrabe de Angelis M, and Beckers J (2016). Epigenetic germline inheritance of diet-induced obesity and insulin resistance. Nat Genet 48, 497–499. [DOI] [PubMed] [Google Scholar]
- Jaitin DA, Weiner A, Yofe I, Lara-Astiaso D, Keren-Shaul H, David E, Salame TM, Tanay A, van Oudenaarden A, and Amit I (2016). Dissecting Immune Circuits by Linking CRISPR-Pooled Screens with Single-Cell RNA-Seq. Cell 167, 1883–1896 e1815. [DOI] [PubMed] [Google Scholar]
- Jenuwein T, and Allis CD (2001). Translating the histone code. Science 293, 1074–1080. [DOI] [PubMed] [Google Scholar]
- Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, and Charpentier E (2012). A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns NA, Genga RM, Enuameh MS, Garber M, Wolfe SA, and Maehr R (2014). Cas9 effector-mediated regulation of transcription and differentiation in human pluripotent stem cells. Development 141, 219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns NA, Pham H, Tabak B, Genga RM, Silverstein NJ, Garber M, and Maehr R (2015). Functional annotation of native enhancers with a Cas9-histone demethylase fusion. Nat Methods 12, 401–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly TK, De Carvalho DD, and Jones PA (2010). Epigenetic modifications as therapeutic targets. Nat Biotechnol 28, 1069–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keung AJ, Joung JK, Khalil AS, and Collins JJ (2015). Chromatin regulation at the frontier of synthetic biology. Nat Rev Genet 16, 159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JM, Kim K, Schmidt T, Punj V, Tucker H, Rice JC, Ulmer TS, and An W (2015). Cooperation between SMYD3 and PC4 drives a distinct transcriptional program in cancer cells. Nucleic Acids Res 43, 8868–8883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kind J, Pagie L, Ortabozkoyun H, Boyle S, de Vries SS, Janssen H, Amendola M, Nolen LD, Bickmore WA, and van Steensel B (2013). Single-cell dynamics of genome-nuclear lamina interactions. Cell 153, 178–192. [DOI] [PubMed] [Google Scholar]
- Klann TS, Black JB, Chellappan M, Safi A, Song L, Hilton IB, Crawford GE, Reddy TE, and Gersbach CA (2017). CRISPR-Cas9 epigenome editing enables high-throughput screening for functional regulatory elements in the human genome. Nat Biotechnol 35, 561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinstiver BP, Prew MS, Tsai SQ, Topkar VV, Nguyen NT, Zheng Z, Gonzales AP, Li Z, Peterson RT, Yeh JR, et al. (2015). Engineered CRISPR-Cas9 nucleases with altered PAM specificities. Nature 523, 481–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klosin A, Casas E, Hidalgo-Carcedo C, Vavouri T, and Lehner B (2017). Transgenerational transmission of environmental information in C. elegans. Science 356, 320–323. [DOI] [PubMed] [Google Scholar]
- Knight SC, Xie L, Deng W, Guglielmi B, Witkowsky LB, Bosanac L, Zhang ET, El Beheiry M, Masson JB, Dahan M, et al. (2015). Dynamics of CRISPR-Cas9 genome interrogation in living cells. Science 350, 823–826. [DOI] [PubMed] [Google Scholar]
- Koerner MV, Pauler FM, Huang R, and Barlow DP (2009). The function of non-coding RNAs in genomic imprinting. Development 136, 1771–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike-Yusa H, Li Y, Tan EP, Velasco-Herrera Mdel C, and Yusa K (2014). Genome-wide recessive genetic screening in mammalian cells with a lentiviral CRISPR-guide RNA library. Nat Biotechnol 32, 267–273. [DOI] [PubMed] [Google Scholar]
- Komor AC, Badran AH, and Liu DR (2017). CRISPR-Based Technologies for the Manipulation of Eukaryotic Genomes. Cell 168, 20–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konermann S, Brigham MD, Trevino AE, Joung J, Abudayyeh OO, Barcena C, Hsu PD, Habib N, Gootenberg JS, Nishimasu H, et al. (2015). Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature 517, 583–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkmaz G, Lopes R, Ugalde AP, Nevedomskaya E, Han R, Myacheva K, Zwart W, Elkon R, and Agami R (2016). Functional genetic screens for enhancer elements in the human genome using CRISPR-Cas9. Nat Biotechnol 34, 192–198. [DOI] [PubMed] [Google Scholar]
- Kouzarides T (2007). Chromatin modifications and their function. Cell 128, 693–705. [DOI] [PubMed] [Google Scholar]
- Kwon DY, Zhao YT, Lamonica JM, and Zhou Z (2017). Locus-specific histone deacetylation using a synthetic CRISPR-Cas9-based HDAC. Nat Commun 8, 15315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane AB, Strzelecka M, Ettinger A, Grenfell AW, Wittmann T, and Heald R (2015). Enzymatically Generated CRISPR Libraries for Genome Labeling and Screening. Dev Cell 34, 373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y, Zhang X, Su J, Jeong M, Gundry MC, Huang YH, Zhou Y, Li W, and Goodell MA (2017). Targeted DNA methylation in vivo using an engineered dCas9-MQ1 fusion protein. Nat Commun 8, 16026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Ruan X, Auerbach RK, Sandhu KS, Zheng M, Wang P, Poh HM, Goh Y, Lim J, Zhang J, et al. (2012). Extensive promoter-centered chromatin interactions provide a topological basis for transcription regulation. Cell 148, 84–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Rivera CM, Ishii H, Jin F, Selvaraj S, Lee AY, Dixon JR, and Ren B (2014). CRISPR reveals a distal super-enhancer required for Sox2 expression in mouse embryonic stem cells. PLoS One 9, e114485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindhout BI, Fransz P, Tessadori F, Meckel T, Hooykaas PJ, and van der Zaal BJ (2007). Live cell imaging of repetitive DNA sequences via GFP-tagged polydactyl zinc finger proteins. Nucleic Acids Res 35, e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XS, Wu H, Ji X, Stelzer Y, Wu X, Czauderna S, Shu J, Dadon D, Young RA, and Jaenisch R (2016). Editing DNA Methylation in the Mammalian Genome. Cell 167, 233–247 e217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long C, Amoasii L, Mireault AA, McAnally JR, Li H, Sanchez-Ortiz E, Bhattacharyya S, Shelton JM, Bassel-Duby R, and Olson EN (2016). Postnatal genome editing partially restores dystrophin expression in a mouse model of muscular dystrophy. Science 351, 400–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez Rodriguez M, Kaminska D, Lappalainen K, Pihlajamaki J, Kaikkonen MU, and Laakso M (2017). Identification and characterization of a FOXA2-regulated transcriptional enhancer at a type 2 diabetes intronic locus that controls GCKR expression in liver cells. Genome Med 9, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Naseri A, Reyes-Gutierrez P, Wolfe SA, Zhang S, and Pederson T (2015). Multicolor CRISPR labeling of chromosomal loci in human cells. Proc Natl Acad Sci U S A 112, 3002–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Reyes-Gutierrez P, and Pederson T (2013). Visualization of repetitive DNA sequences in human chromosomes with transcription activator-like effectors. Proc Natl Acad Sci U S A 110, 21048–21053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Tu LC, Naseri A, Huisman M, Zhang S, Grunwald D, and Pederson T (2016). Multiplexed labeling of genomic loci with dCas9 and engineered sgRNAs using CRISPRainbow. Nat Biotechnol 34, 528–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeder ML, Linder SJ, Cascio VM, Fu Y, Ho QH, and Joung JK (2013). CRISPR RNA-guided activation of endogenous human genes. Nat Methods 10, 977–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali P, Aach J, Stranges PB, Esvelt KM, Moosburner M, Kosuri S, Yang L, and Church GM (2013a). CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nat Biotechnol 31, 833–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali P, Esvelt KM, and Church GM (2013b). Cas9 as a versatile tool for engineering biology. Nat Methods 10, 957–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal PK, and Rossi DJ (2013). Reprogramming human fibroblasts to pluripotency using modified mRNA. Nat Protoc 8, 568–582. [DOI] [PubMed] [Google Scholar]
- Mandegar MA, Huebsch N, Frolov EB, Shin E, Truong A, Olvera MP, Chan AH, Miyaoka Y, Holmes K, Spencer CI, et al. (2016). CRISPR Interference Efficiently Induces Specific and Reversible Gene Silencing in Human iPSCs. Cell Stem Cell 18, 541–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurano MT, Humbert R, Rynes E, Thurman RE, Haugen E, Wang H, Reynolds AP, Sandstrom R, Qu H, Brody J, et al. (2012). Systematic localization of common disease-associated variation in regulatory DNA. Science 337, 1190–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JI, Celik H, Rois LE, Fishberger G, Fowler T, Rees R, Kramer A, Martens A, Edwards JR, and Challen GA (2016). Reprogrammable CRISPR/Cas9-based system for inducing site-specific DNA methylation. Biol Open 5, 866–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo CA, Drost J, Wijchers PJ, van de Werken H, de Wit E, Oude Vrielink JA, Elkon R, Melo SA, Leveille N, Kalluri R, et al. (2013). eRNAs are required for p53-dependent enhancer activity and gene transcription. Mol Cell 49, 524–535. [DOI] [PubMed] [Google Scholar]
- Mikkelsen TS, Ku M, Jaffe DB, Issac B, Lieberman E, Giannoukos G, Alvarez P, Brockman W, Kim TK, Koche RP, et al. (2007). Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature 448, 553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milite C, Feoli A, Sasaki K, La Pietra V, Balzano AL, Marinelli L, Mai A, Novellino E, Castellano S, Tosco A, et al. (2015). A novel cell-permeable, selective, and noncompetitive inhibitor of KAT3 histone acetyltransferases from a combined molecular pruning/classical isosterism approach. J Med Chem 58, 2779–2798. [DOI] [PubMed] [Google Scholar]
- Misteli T (2007). Beyond the sequence: cellular organization of genome function. Cell 128, 787–800. [DOI] [PubMed] [Google Scholar]
- Misteli T (2013). The cell biology of genomes: bringing the double helix to life. Cell 152, 1209–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyanari Y, Ziegler-Birling C, and Torres-Padilla ME (2013). Live visualization of chromatin dynamics with fluorescent TALEs. Nat Struct Mol Biol 20, 1321–1324. [DOI] [PubMed] [Google Scholar]
- Morita S, Noguchi H, Horii T, Nakabayashi K, Kimura M, Okamura K, Sakai A, Nakashima H, Hata K, Nakashima K, et al. (2016). Targeted DNA demethylation in vivo using dCas9-peptide repeat and scFv-TET1 catalytic domain fusions. Nat Biotechnol 34, 1060–1065. [DOI] [PubMed] [Google Scholar]
- Nelson CE, Hakim CH, Ousterout DG, Thakore PI, Moreb EA, Castellanos Rivera RM, Madhavan S, Pan X, Ran FA, Yan WX, et al. (2016). In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science 351, 403–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nihongaki Y, Yamamoto S, Kawano F, Suzuki H, and Sato M (2015). CRISPR-Cas9-based photoactivatable transcription system. Chem Biol 22, 169–174. [DOI] [PubMed] [Google Scholar]
- Nissim L, Perli SD, Fridkin A, Perez-Pinera P, and Lu TK (2014). Multiplexed and programmable regulation of gene networks with an integrated RNA and CRISPR/Cas toolkit in human cells. Mol Cell 54, 698–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldridge DA, Wood AC, Weichert-Leahey N, Crimmins I, Sussman R, Winter C, McDaniel LD, Diamond M, Hart LS, Zhu S, et al. (2015). Genetic predisposition to neuroblastoma mediated by a LMO1 super-enhancer polymorphism. Nature 528, 418–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panning B, Dausman J, and Jaenisch R (1997). X chromosome inactivation is mediated by Xist RNA stabilization. Cell 90, 907–916. [DOI] [PubMed] [Google Scholar]
- Pastor WA, Aravind L, and Rao A (2013). TETonic shift: biological roles of TET proteins in DNA demethylation and transcription. Nat Rev Mol Cell Biol 14, 341–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pengue G, and Lania L (1996). Kruppel-associated box-mediated repression of RNA polymerase II promoters is influenced by the arrangement of basal promoter elements. Proc Natl Acad Sci U S A 93, 1015–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennisi E (2013). Long noncoding RNAs may alter chromosome’s 3D structure. Science 340, 910. [DOI] [PubMed] [Google Scholar]
- Perez-Pinera P, Kocak DD, Vockley CM, Adler AF, Kabadi AM, Polstein LR, Thakore PI, Glass KA, Ousterout DG, Leong KW, et al. (2013). RNA-guided gene activation by CRISPR-Cas9-based transcription factors. Nat Methods 10, 973–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Pinera P, Ousterout DG, and Gersbach CA (2012). Advances in targeted genome editing. Curr Opin Chem Biol 16, 268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polstein LR, and Gersbach CA (2015). A light-inducible CRISPR-Cas9 system for control of endogenous gene activation. Nat Chem Biol 11, 198–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polstein LR, Perez-Pinera P, Kocak DD, Vockley CM, Bledsoe P, Song L, Safi A, Crawford GE, Reddy TE, and Gersbach CA (2015). Genome-wide specificity of DNA binding, gene regulation, and chromatin remodeling by TALE- and CRISPR/Cas9-based transcriptional activators. Genome Res 25, 1158–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradeepa MM, Grimes GR, Kumar Y, Olley G, Taylor GC, Schneider R, and Bickmore WA (2016). Histone H3 globular domain acetylation identifies a new class of enhancers. Nat Genet 48, 681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulecio J, Alejo-Valle O, Capellera-Garcia S, Vitaloni M, Rio P, Mejia-Ramirez E, Caserta I, Bueren JA, Flygare J, and Raya A (2016). Direct conversion of fibroblasts to megakaryocyte progenitors. Cell Rep 17, 671–683. [DOI] [PubMed] [Google Scholar]
- Pulecio J, Nivet E, Sancho-Martinez I, Vitaloni M, Guenechea G, Xia Y, Kurian L, Dubova I, Bueren J, Laricchia-Robbio L, et al. (2014). Conversion of human fibroblasts into monocyte-like progenitor cells. Stem Cells 32, 2923–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi LS, Larson MH, Gilbert LA, Doudna JA, Weissman JS, Arkin AP, and Lim WA (2013). Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 152, 1173–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radzisheuskaya A, Shlyueva D, Muller I, and Helin K (2016). Optimizing sgRNA position markedly improves the efficiency of CRISPR/dCas9-mediated transcriptional repression. Nucleic Acids Res 44, e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragunathan K, Jih G, and Moazed D (2015). Epigenetics. Epigenetic inheritance uncoupled from sequence-specific recruitment. Science 348, 1258699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal N, Srinivasan S, Kooshesh K, Guo Y, Edwards MD, Banerjee B, Syed T, Emons BJ, Gifford DK, and Sherwood RI (2016). High-throughput mapping of regulatory DNA. Nat Biotechnol 34, 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakyan VK, Down TA, Balding DJ, and Beck S (2011). Epigenome-wide association studies for common human diseases. Nat Rev Genet 12, 529–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen KD, and Helin K (2016). Role of TET enzymes in DNA methylation, development, and cancer. Genes Dev 30, 733–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roadmap Epigenomics Consortium, Kundaje A, Meuleman W, Ernst J, Bilenky M, Yen A, Heravi-Moussavi A, Kheradpour P, Zhang Z, Wang J, et al. (2015). Integrative analysis of 111 reference human epigenomes. Nature 518, 317–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinett CC, Straight A, Li G, Willhelm C, Sudlow G, Murray A, and Belmont AS (1996). In vivo localization of DNA sequences and visualization of large-scale chromatin organization using lac operator/repressor recognition. J Cell Biol 135, 1685–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Campos A, and Azorin F (2007). RNA is an integral component of chromatin that contributes to its structural organization. PLoS One 2, e1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma T, Nishikawa A, Kume S, Chayama K, and Yamamoto T (2014). Multiplex genome engineering in human cells using all-in-one CRISPR/Cas9 vector system. Sci Rep 4, 5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjana NE, Wright J, Zheng K, Shalem O, Fontanillas P, Joung J, Cheng C, Regev A, and Zhang F (2016). High-resolution interrogation of functional elements in the noncoding genome. Science 353, 1545–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal A, Lajoie BR, Jain G, and Dekker J (2012). The long-range interaction landscape of gene promoters. Nature 489, 109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scacheri CA, and Scacheri PC (2015). Mutations in the noncoding genome. Curr Opin Pediatr 27, 659–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelson T, Heckl D, Ebert BL, Root DE, Doench JG, et al. (2014). Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 343, 84–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalem O, Sanjana NE, and Zhang F (2015). High-throughput functional genomics using CRISPR-Cas9. Nat Rev Genet 16, 299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao S, Zhang W, Hu H, Xue B, Qin J, Sun C, Sun Y, Wei W, and Sun Y (2016). Long-term dual-color tracking of genomic loci by modified sgRNAs of the CRISPR/Cas9 system. Nucleic Acids Res 44, e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechner DM, Hacisuleyman E, Younger ST, and Rinn JL (2015). Multiplexable, locus-specific targeting of long RNAs with CRISPR-Display. Nat Methods 12, 664–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeonov D, Gowen B, Boontanrart M, Roth T, Gagnon J, Mumbach M, Satpathy A, Lee Y, Bray N, Chan A, et al. (2017). Discovery of stimulation-responsive immune enhancers with CRISPR activation. Nature In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ZD, and Meissner A (2013). DNA methylation: roles in mammalian development. Nat Rev Genet 14, 204–220. [DOI] [PubMed] [Google Scholar]
- Stark C, Breitkreutz BJ, Chatr-Aryamontri A, Boucher L, Oughtred R, Livstone MS, Nixon J, Van Auken K, Wang X, Shi X, et al. (2011). The BioGRID Interaction Database: 2011 update. Nucleic Acids Res 39, D698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepper P, Kungulovski G, Jurkowska RZ, Chandra T, Krueger F, Reinhardt R, Reik W, Jeltsch A, and Jurkowski TP (2017). Efficient targeted DNA methylation with chimeric dCas9-Dnmt3a-Dnmt3L methyltransferase. Nucleic Acids Res 45, 1703–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiech L, Heidenreich M, Banerjee A, Habib N, Li Y, Trombetta J, Sur M, and Zhang F (2015). In vivo interrogation of gene function in the mammalian brain using CRISPR-Cas9. Nat Biotechnol 33, 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabebordbar M, Zhu K, Cheng JKW, Chew WL, Widrick JJ, Yan WX, Maesner C, Wu EY, Xiao R, Ran FA, et al. (2016). In vivo gene editing in dystrophic mouse muscle and muscle stem cells. Science 351, 407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanenbaum ME, Gilbert LA, Qi LS, Weissman JS, and Vale RD (2014). A protein-tagging system for signal amplification in gene expression and fluorescence imaging. Cell 159, 635–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakore PI, Black JB, Hilton IB, and Gersbach CA (2016). Editing the epigenome: technologies for programmable transcription and epigenetic modulation. Nat Methods 13, 127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakore PI, D’Ippolito AM, Song L, Safi A, Shivakumar NK, Kabadi AM, Reddy TE, Crawford GE, and Gersbach CA (2015). Highly specific epigenome editing by CRISPR-Cas9 repressors for silencing of distal regulatory elements. Nat Methods 12, 1143–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanisch K, Schneider K, Morbitzer R, Solovei I, Lahaye T, Bultmann S, and Leonhardt H (2014). Targeting and tracing of specific DNA sequences with dTALEs in living cells. Nucleic Acids Res 42, e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SQ, Wyvekens N, Khayter C, Foden JA, Thapar V, Reyon D, Goodwin MJ, Aryee MJ, and Joung JK (2014). Dimeric CRISPR RNA-guided FokI nucleases for highly specific genome editing. Nat Biotechnol 32, 569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojta A, Dobrinic P, Tadic V, Bockor L, Korac P, Julg B, Klasic M, and Zoldos V (2016). Repurposing the CRISPR-Cas9 system for targeted DNA methylation. Nucleic Acids Res 44, 5615–5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldrip ZJ, Byrum SD, Storey AJ, Gao J, Byrd AK, Mackintosh SG, Wahls WP, Taverna SD, Raney KD, and Tackett AJ (2014). A CRISPR-based approach for proteomic analysis of a single genomic locus. Epigenetics 9, 1207–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Garcia-Bassets I, Benner C, Li W, Su X, Zhou Y, Qiu J, Liu W, Kaikkonen MU, Ohgi KA, et al. (2011a). Reprogramming transcription by distinct classes of enhancers functionally defined by eRNA. Nature 474, 390–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Wei JJ, Sabatini DM, and Lander ES (2014). Genetic screens in human cells using the CRISPR-Cas9 system. Science 343, 80–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Li GW, Chen C, Xie XS, and Zhuang X (2011b). Chromosome organization by a nucleoid-associated protein in live bacteria. Science 333, 1445–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger L, Ayyash M, Novershtern N, and Hanna JH (2016). Dynamic stem cell states: naive to primed pluripotency in rodents and humans. Nat Rev Mol Cell Biol 17, 155–169. [DOI] [PubMed] [Google Scholar]
- Wu H, and Zhang Y (2014). Reversing DNA methylation: mechanisms, genomics, and biological functions. Cell 156, 45–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TP, Wang T, Seetin MG, Lai Y, Zhu S, Lin K, Liu Y, Byrum SD, Mackintosh SG, Zhong M, et al. (2016). DNA methylation on N(6)-adenine in mammalian embryonic stem cells. Nature 532, 329–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Schultz MD, Lister R, Hou Z, Rajagopal N, Ray P, Whitaker JW, Tian S, Hawkins RD, Leung D, et al. (2013). Epigenomic analysis of multilineage differentiation of human embryonic stem cells. Cell 153, 1134–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong K, Zhou Y, Hyttel P, Bolund L, Freude KK, and Luo Y (2016). Generation of induced pluripotent stem cells (iPSCs) stably expressing CRISPR-based synergistic activation mediator (SAM). Stem Cell Res 17, 665–669. [DOI] [PubMed] [Google Scholar]
- Xiong T, Meister GE, Workman RE, Kato NC, Spellberg MJ, Turker F, Timp W, Ostermeier M, and Novina CD (2017). Targeted DNA methylation in human cells using engineered dCas9-methyltransferases. Sci Rep 7, 6732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Tao Y, Gao X, Zhang L, Li X, Zou W, Ruan K, Wang F, Xu GL, and Hu R (2016). A CRISPR-based approach for targeted DNA demethylation. Cell Discov 2, 16009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Wang L, Bell P, McMenamin D, He Z, White J, Yu H, Xu C, Morizono H, Musunuru K, et al. (2016). A dual AAV system enables the Cas9-mediated correction of a metabolic liver disease in newborn mice. Nat Biotechnol 34, 334–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka S, Fujii W, Ogawa T, Sugiura K, and Naito K (2015). Development of a mono-promoter-driven CRISPR/Cas9 system in mammalian cells. Sci Rep 5, 18341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan K, Shermoen AW, and O’Farrell PH (2014). Illuminating DNA replication during Drosophila development using TALE-lights. Curr Biol 24, R144–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalatan JG, Lee ME, Almeida R, Gilbert LA, Whitehead EH, La Russa M, Tsai JC, Weissman JS, Dueber JE, Qi LS, et al. (2015). Engineering complex synthetic transcriptional programs with CRISPR RNA scaffolds. Cell 160, 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Gayen S, Xiong J, Zhou B, Shanmugam AK, Sun Y, Karatas H, Liu L, Rao RC, Wang S, et al. (2016). MLL1 Inhibition Reprograms Epiblast Stem Cells to Naive Pluripotency. Cell Stem Cell 18, 481–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Zhu S, Cai C, Yuan P, Li C, Huang Y, and Wei W (2014). High-throughput screening of a CRISPR/Cas9 library for functional genomics in human cells. Nature 509, 487–491. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.