Abstract
Objectives.
Basal interventricular septal (IVS) hypertrophy (BSH) with reduced basal IVS contraction and IVS to aorta angle is frequently associated with aortic stenosis (AS). The shape of BSH suggests compression from longitudinally elongated ascending aorta, causing basal IVS thickening and contractile dysfunction, which further suggests the possibility of aortic wall shortening to improve the BSH. Surgical aortic valve replacement (SAVR), as opposed to transcatheter AVR (TAVR), includes aortic wall shortening by incision and stitching on the wall and may potentially improve BSH. We hypothesized that BSH configurations and its contraction improves after SAVR, as opposed to TAVR, in patients with AS and associated BSH.
Methods.
In 32 patients with SAVR and 36 with TAVR for AS, regional wall thickness and systolic contraction (longitudinal strain) of 18 left ventricular (LV) segments as well as IVS to aorta angle were measured by echocardiography.
Results.
After SAVR, basal IVS to averaged LV wall thickness ratio, basal IVS strain, and IVS to aorta angle significantly improved (1.11±0.24 to 1.06±0.17, −6.2±5.7 to −9.1±5.2 %, 115±22 to 123±14 degree, p<0.001, respectively). Contractile improvement in basal IVS was correlated with pre-SAVR BSH (basal IVS to averaged LV wall thickness ratio or IVS to aorta angle: r=0.47 and 0.49, p<0.01, respectively). In contrast, these BSH indices did not improve after TAVR.
Conclusions.
In patients with AS, SAVR as opposed to TAVR improves associated BSH and its functional impairment.
Keywords: Aortic valve stenosis, Basal septal hypertrophy, Aortic valve replacement
Introduction
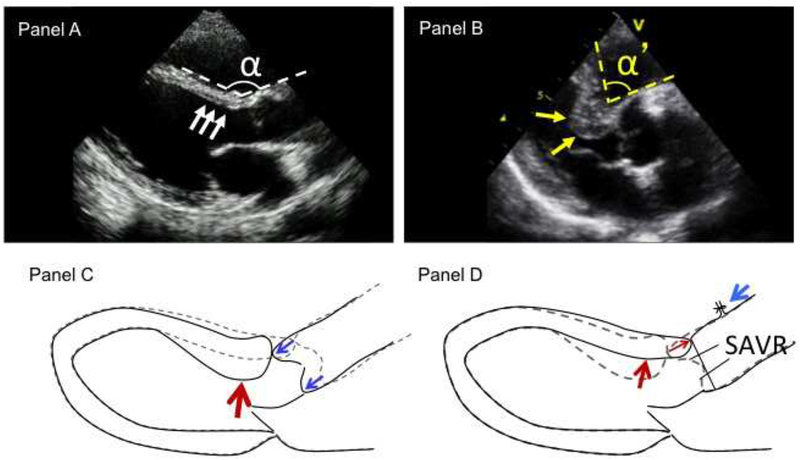
Basal interventricular septal (IVS) hypertrophy (BSH) is characterized with reduced angle between basal IVS and aorta and thickened basal IVS (Figure 1, panels A and B). This is common in elderly patients and is associated with reduced contraction in the region [1]. Aortic valvular stenosis (AS) develops mainly in aged population [2], therefore BSH is also common in patients with AS. BSH may potentially contribute to left ventricular (LV) outflow tract obstruction following surgical or transcatheter aortic valve replacement (SAVR and TAVR, respectively) with unfavorable outcome [3–5] and may increase the difficulty in accurate positioning and implantation of the prosthetic valve by confounding coaxial alignment of the guide wire and/or the valve or by resulting in superior displacement of the prosthetic valve during deployment [6]. Therefore, BSH is especially important in patients with AS. SAVR or TAVR are the standard intervention for symptomatic patients with AS. However, effects of AVR on the associated BSH have not yet been clarified.
Figure 1.
Study hypothesis suggested from configurations of basal septal hypertrophy (BSH). Panel A: normal interventricular septum (IVS) and aorta configurations. Panel B: Typical image of BSH. BSH is characterized with reduced angle α between basal IVS and ascending aorta and thickened basal IVS (yellow arrows). Panel C: potential mechanism of BSH. Configurations of BSH suggest compression on IVS from longitudinally elongated ascending aorta. Panel D: potential effects of surgical aortic valve replacement (SAVR) on BSH (hypothesis). SAVR includes incision and suture of the anterior wall of ascending aorta. By taking margin to seam, SAVR shortens the anterior wall of ascending aorta. In our study, aortic wall shortening was approximately 1 cm with 0.5 cm margin for the stitch. This can potentially reduce compression on IVS from ascending aorta, leading to attenuated post-SAVR BSH and improved contraction.
Although the mechanism of BSH is not yet established, its configuration suggests augmented compression on the IVS by the longitudinally elongated ascending aorta (Figure 1C). This concept has been suggested by previous imaging studies [7]. These further suggest a potential of aortic wall shortening to improve the BSH. SAVR includes incision and suture of the anterior wall of ascending aorta. By taking margin to seam, SAVR shortens the aortic wall. The anterior aortic wall shortening in this study was approximately 1 cm with 0.5 cm margin for the stitch (Figure 1D). This aortic wall shortening may exert a superiorly directed force on the basal IVS, causing the myocardium to be less compressed and therefore less thick, resulting in attenuated post-SAVR BSH configuration and improved contraction of the basal IVS (Figure 1D). These potential effects by SAVR on BSH may not be expected from TAVR without aortic wall shortening. We therefore hypothesized that SAVR as opposed to TAVR may potentially improve associated BSH configurations and its reduced contraction in patients with AS. The purpose of this study is to test the hypothesis by using comprehensive echocardiography with speckle tracking analysis in patients who undergo SAVR or TAVR for AS. This is important because post-procedural LV outflow tract obstruction following SAVR or TAVR and superior displacement of implanted prosthetic valve following TAVR are significant problems, affecting the patients [3–5] and derived data by this study may offer useful information to perform SAVR or TAVR and to figure out strategy for concomitant procedures at the time of SAVR for treating AS.
Materials and Methods
Study Population
Consecutive patients with SAVR or transfemoral TAVR were retrospectively included at the echocardiographic laboratories of 4 collaborating institutions: University of Occupational and Environmental Health (n=34), Asan Medical Center (n=4), Sakakibara Heart Institute of Okayama (n=12) and Kokura Memorial Hospital (n=18). All patients had significant AS, defined as aortic valve area <1.0 cm2 by the continuity equation. Patients with SAVR did not undergo concomitant myectomy or myotomy. Patients with transapical TAVR were not included due to the potential influences on LV contraction. Exclusion criteria were 1) concomitant other structural heart disease and 2) inadequate echocardiographic images. Consequently, 32 patients who underwent SAVR (16 men; mean age, 72±11 years) and 36 who underwent transfemoral TAVR (14 men; mean age, 85±5 years) were included. Post-SAVR and post-TAVR echocardiography was performed 113±147 and 113±142 days after the procedure, respectively. Images were analyzed by a single physician (H. Y.) with 20 years’ experience in echocardiography. This study was approved by the ethics committee of each institution, and informed consent was obtained from all subjects.
General Echocardiographic Measurements
Comprehensive echocardiographic study was performed using commercially available equipments (iE-33, Philips Medical Systems, Andover, MA USA; Vivid 7, GE Medical Systems, Milwaukee, WI, USA; Artida, Toshiba Medical Systems, Otawara, Japan). Pre- and post-procedural echocardiography was performed using a same scanner from the same vendor to minimize measurement differences by vendors [8]. In order to avoid error in measuring stroke volume in the presence of BSH, LV end-diastolic and end-systolic volumes (LVEDV and LVESV) were measured by the biplane Simpson’s method to derive ejection fraction and stroke volume [9]. The aortic valve area was then calculated by the continuity equation with continuous wave Doppler. Images with regular R-R intervals in the 2 preceding cardiac cycles were used for measurements in patients with atrial fibrillation [10].
BSH Specific Analysis by Echocardiography
Regional LV wall thickness was measured in 18 segments in the apical 4-chamber, 2-chamber and long axis views in end-diastole and averaged LV wall thickness of the 18 segments was calculated. Basal IVS to averaged LV wall thickness ratio was calculated as an index to express BSH (Figure 1B). BSH was defined as basal IVS to averaged LV wall thickness ratio > 1.05 (upper normal range in the control group). LV mass was measured at end-diastole as (LV epicardial volume – LV endocardial volume) ×1.05 by the area-length method [11]. The IVS to aorta angle was also measured in end-diastole between basal IVS line and anterior ascending aortic line (Figure 1B) [12]. LV longitudinal strain of 18 segments were measured by speckle tracking analysis using EchoPac PC BT12, GE Healthcare, QLAB 9, Phillips Medical Systems, or 2D Wall Motion Tracking, Toshiba Medical Systems. Global LV longitudinal strain was calculated as the average strain of the 18 segments. The ratio of basal IVS strain to global LV longitudinal strain was obtained as an index to express segmental dysfunction in the region of BSH [1].
Statistical Analysis
Categorical variables were presented as frequencies and continuous variables as mean ± standard deviation. Differences between proportions were assessed by Fisher exact test. Unpaired continuous variables were compared using the unpaired t test or Mann–Whitney U test according to the data distribution. Pre- and post-procedure results were compared by the paired t test. Quantitative results in 3 groups were analyzed by applying one way ANOVA and post hoc Turkey’s test. Spearman correlation coefficient was performed to investigate the relationship between echocardiographic measurements. P<0.05 was considered significant.
Interobserver variability for the measurements of longitudinal strain was obtained by analysis of measurements in 10 randomly selected patients by 2 independent blinded observers. Intraobserver variability was evaluated by analysis of measurements in the other 10 patients by the same observer at 2 different time points. Results were analyzed by both least squares fit linear regression analysis and Bland-Altman method.
Results
Clinical Characteristics
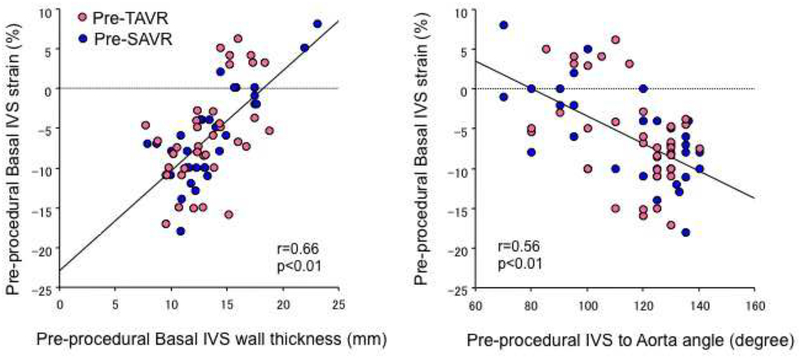
Patients’ clinical and echocardiographic characteristics are summarized in Tables 1 and 2. As expected, patients with SAVR were younger and physically larger compared to those with TAVR. Indexed LVEDV, LVESV were larger, LV ejection fraction was not augmented and LV stroke volume was comparable in patients with SAVR compared to those with TAVR. After AVR, indexed LVEDV and LVESV were reduced in SAVR, but not in TAVR [13]. Both AS patients who subsequently underwent either SAVR or TAVR had significantly increased basal IVS thickness, increased basal IVS to averaged LV wall thickness ratio, reduced IVS to aorta angle, reduced basal IVS strain, and reduced basal IVS to global LV strain ratio compared to controls (p<0.01, respectively) (Table 2) but without statistical differences between the 2 groups. Twelve patients with SAVR and 17 with TAVR met BSH criteria without statistical difference in its incidence (Table 2). Incidence of BSH tended to be reduced after SAVR but without statistical significance. Reduced basal IVS strain was correlated with increased thickness of the region and decreased IVS to aorta angle (r=0.66, p<0.01, r=0.56, p<0.01, respectively) (Figure 2).
Table 1.
Clinical Characteristics of studied patients
| AS | |||
|---|---|---|---|
| SAVR | TAVR | Control | |
| Variable | (n=32) | (n=36) | (n=20) |
| Clinical | |||
| Age, y | 72 ± 11*† | 85 ± 5* | 28 ± 9 |
| Male, n (%) | 16 (50) | 14 (39) | 11 (55) |
| BSA (m2) | 1.5±0.1*† | 1.4±0.2* | 1.6±0.2 |
| Systolic blood pressure, mmHg | 137 ± 21* | 131 ± 18* | 118 ± 10 |
| Diastolic blood pressure, mmHg | 74 ± 13 | 63 ± 10 | 67 ± 8 |
| Heart rate, bpm | 69 ± 13 | 64 ± 9 | 64 ± 7 |
| Hypertension, n (%) | 25 (78) | 28 (78) | 0 (0) |
| Hyperlipidemia, n (%) | 13 (41) | 11 (31) | 0 (0) |
| Diabetes mellitus, n (%) | 8 (25) | 12 (33) | 0 (0) |
AS = aortic stenosis; BSA = body surface area; SAVR = surgical aortic valve replacement; TAVR = transcatheter aortic valve replacement.
P<0.05 versus control,
P<0.05 versus TAVR.
Table 2.
Echocardiographic data before and after SAVR/TAVR
| SAVR (N=32) | TAVR (N=36) | Control (N=20) | ||||
|---|---|---|---|---|---|---|
| Before | After | Before | After | |||
| AS severity | ||||||
| AVA (cm2) | 0.8±0.2 | 1.4±0.5‡ | 0.7±0.1 | 1.8±0.5‡ | ||
| Mean PG (mmHg) | 52±16 | 20±10‡ | 50±14 | 10±4‡ | ||
| LVEDVI (mL/m2) | 65±28† | 55±22‡ | 50±17 | 49±14 | 55±22 | |
| LVESVI (mL/m2) | 29±24† | 22±17‡ | 16±12 | 14±8* | 20±4 | |
| LVEF (%) | 61±19† | 63±17 | 71±12* | 73±10* | 64±6 | |
| LVSVI (mL/m2) | 36±13 | 32±10 | 35±8 | 35±7 | 35±8 | |
| Indexed LV mass (g/m2) | 97±22*† | 91±20*‡ | 81 ±18* | 75±15*‡ | 49±9 | |
| Averaged LV wall thickness (mm) | 12.1±1.4* | 11.5±1.4*‡ | 12.0±2.1* | 11.4±2.0*‡ | 6.9±0.9 | |
| Basal IVS wall thickness (mm) | 13.5±3.5* | 12.2±2.6*‡ | 13.3±2.8* | 12.7±2.9*‡ | 6.8±1.1 | |
| Basal IVS to averaged LV wall thickness ratio | 1.11±0.24* | 1.06±0.17‡ | 1.11±0.17* | 1.12±0.17* | 0.99±0.03 | |
| Incidence of BSH | 12/32 | 10/32 | 17/36 | 18/36 | ||
| IVS to aorta angle (degree) | 115±22* | 123±14*‡ | 116±17* | 116±17* | 147±8 | |
| Global LV longitudinal strain (%) | −12.9±3.9* | −13.9±3.2* | −13.8±3.2* | −13.8±2.9* | −20.2±1.7 | |
| Basal IVS strain (%) | −6.2±5.7* | −9.1±5.2*‡ | −6.0±6.2* | −5.9±5.8* | −17.5±2.9 | |
| Basal IVS to global LV strain ratio (%) | 42±58* | 66±38*‡ | 41±47* | 41±42* | 87±12 | |
AS = aortic stenosis; AVA = aortic valve area; BSH = basal interventricular septal hypertrophy; IVS = interventricular septum; LV = left ventricle; LVEDVI = indexed left ventricular end me; LVESVI = indexed left ventricular end systolic volume; LVEF = left diastolic volume-ventricular ejection fraction; LVSVI = indexed left ventricular stroke volume; PG = pressure gradient; SAVR = surgical aortic valve replacement.
P<0.05 versus control,
P<0.05 versus TAVR,
P<0.05 versus replacement; TAVR = transcatheter aortic valve replacement procedural value.
Figure 2.
Correlation between pre-procedural morphological degree of basal septal hypertrophy (BSH) and basal interventricular septum (IVS) strain. Greater morphological abnormalities in BSH was associated with greater reduction in basal IVS strain.
Changes in Basal IVS Thickening and Reduced Contraction after SAVR and TAVR in the Entire Patients (Table 2)
All patients showed improvement in aortic valve function and symptom after SAVR or TAVR. While LVEDV significantly decreased after SAVR (p<0.01), it was not changed after TAVR. Indexed LV mass was significantly reduced after procedures in both groups (p<0.01). Following SAVR, averaged LV wall thickness decreased, basal IVS thickness showed greater reduction, resulting in reduced basal IVS to averaged LV wall thickness ratio (p<0.01). In contrast, averaged LV wall thickness and basal IVS thickness showed similar reduction after TAVR, resulting in no significant reduction in their ratio. IVS to aorta angle significantly increased after SAVR (p<0.01), while it remained unchanged following TAVR. Although global LV longitudinal strain did not change after SAVR, basal IVS strain and basal IVS to global LV strain ratio significantly improved following SAVR (p<0.01). In contrast, all global LV longitudinal strain, basal IVS strain, and their ratio did not change after TAVR. In general, basal IVS thickening and reduced contraction significantly improved after SAVR while these did not improve after TAVR.
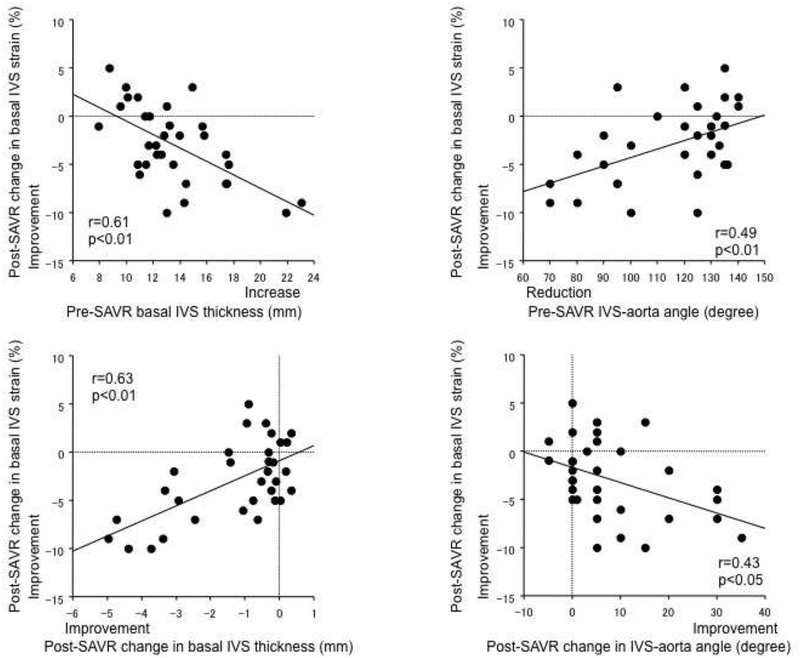
Improvement in basal IVS strain after SAVR was correlated with pre-SAVR basal IVS thickening (Figure 3, upper left), its post-SAVR reduction (lower left), pre-SAVR reduced IVS to aorta angle (upper right), and its post-SAVR increase (lower right). Therefore, greater post-SAVR improvements in basal IVS strain were associated with greater pre-SAVR BSH morphology and its greater post-SAVR improvement.
Figure 3.
Correlations between improvements in the basal interventricular septum (IVS) strain after surgical aortic valve replacement (SAVR) and pre-SAVR degree or post-SAVR hange in BSH morphology. Greater post-SAVR improvements in basal IVS strain was associated with greater pre-SAVR BSH and greater post-SAVR improvement of BSH morphology.
Changes in Basal IVS Thickening and Reduced Contraction in Patients with Associated BSH (Table 3)
Table 3.
Echocardiographic data before and after SAVR/TAVR in patients with preprocedural basal septal hypertrophy
| SAVR(N=12) | TAVR (N=17) | |||
|---|---|---|---|---|
| Before | After | Before | After | |
| AS severity | ||||
| AVA (cm2) | 0.8±0.2 | 1.4±0.4‡ | 0.7±0.2 | 1.7±0.5‡ |
| Mean PG (mmHg) | 52±9 | 20±9‡ | 44±11 | 10±3‡ |
| LVEDVI (mL/m2) | 58±28 | 51±17 | 52±20 | 50±16 |
| LVESVI (mL/m2) | 25±28 | 19±16 | 15±15 | 14±10 |
| LVEF (%) | 64±20 | 65±18 | 74±12 | 74±11 |
| LVSVI (mL/m2) | 33±9 | 31±9 | 37±8 | 35±8 |
| Indexed LV mass (g/m2) | 91±12 | 84±15‡ | 85±22 | 78±18‡ |
| Averaged LV wall thickness (mm) | 12.5±1.6 | 11.6±1.3‡ | 11.5±1.3 | 10.9±1.4‡ |
| Basal IVS wall thickness (mm) | 16.8±3.2 | 14.1±2.5‡ | 14.3±2.6 | 13.7±3.0‡ |
| Basal IVS to averaged LV wall thickness ratio | 1.35±0.21 | 1.22±0.18‡ | 1.24±0.15 | 1.24±0.16 |
| IVS to aorta angle (degree) | 90±14 | 108±11‡ | 104±16 | 103±16 |
| Global LV longitudinal strain (%) | −12.5±4.7 | −13.4±3.7 | −14.2±3.4 | −14.0±2.6 |
| Basal IVS strain (%) | −1.2±5.1 | −6.3±4.9‡ | −3.2±7.1 | −3.5±7.2 |
| Basal IVS to global LV strain ratio (%) | −4.4±70 | 46±31‡ | 16±51 | 20±51 |
AS = aortic stenosis; AVA = aortic valve area; IVS = interventricular septum; LVEDVI = indexed left ventricular end-diastolic volume; LVESVI = indexed left ventricular end-systolic volumeLVEF = left ventricular ejection-fraction; LVSVI= indexed left ventricular stroke volume PG = pressure gradient; SAVR = surgical aortic valve replacement; TAVR = transcatheter aortic valve replacement.
P<0.05 vs. pre-procedural value.
In general, BSH indices similarly improved only after SAVR in 12 patients with BSH. Improvements in BSH after SAVR tended to be greater in selected patients with pre-procedural BSH compared to whole patients (reduction in basal IVS thickening: −2.7±1.5 vs. −1.3±1.6 mm; improvement in basal IVS contraction: −5.1±3.8 vs. −3.0±4.0 %; improvement in IVS to aorta angle: 18±12 vs. 8.1±10.8 degree).
Representative 2 Patients
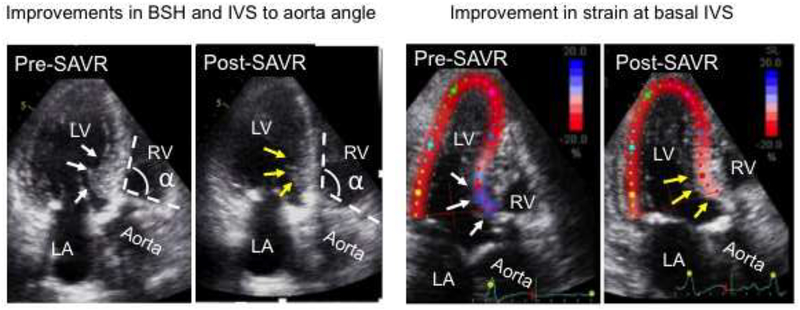
The patient in Figure 4 had severe AS and associated BSH as shown by basal IVS thickening (white arrows in the left pre-SAVR panel), reduced IVS to aorta angle (α in the left pre-SAVR panel) and reduced strain of the region (blue signals pointed by white arrows in the right pre-SAVR panel) and subsequently underwent SAVR. Post-SAVR echocardiography showed clear improvements in basal IVS thickness (yellow arrows in the left post-SAVR panel), IVS to aorta angle (α in the left post-SAVR panel) and strain of the region (pink signals pointed by yellow arrows in the right post-SAVR panel). Supplemental movie 1 showed morphological and functional improvements of the BSH, respectively.
Figure 4.
A representative patient who underwent surgical aortic valve replacement (SAVR). Pre-SAVR, this patient had severe aortic stenosis and associated basal septal hypertrophy (BSH) as shown in increased wall thickness in basal interventricular septum (IVS), reduced IVS to aorta angle and reduced strain of the region (white arrows and angle α in the left pre-SAVR panel and blue color strain signals in basal IVS in the right pre-SAVR panel, respectively). After SAVR, echocardiography showed clear improvements in all basal IVS thickness, IVS to aorta angle and strain of the region (yellow arrows and angle α in the left post-SAVR panel and pink color strain signals in the right post-SAVR panel, respectively). LA indicates left atrium; LV, left ventricle; and RV, right ventricle.
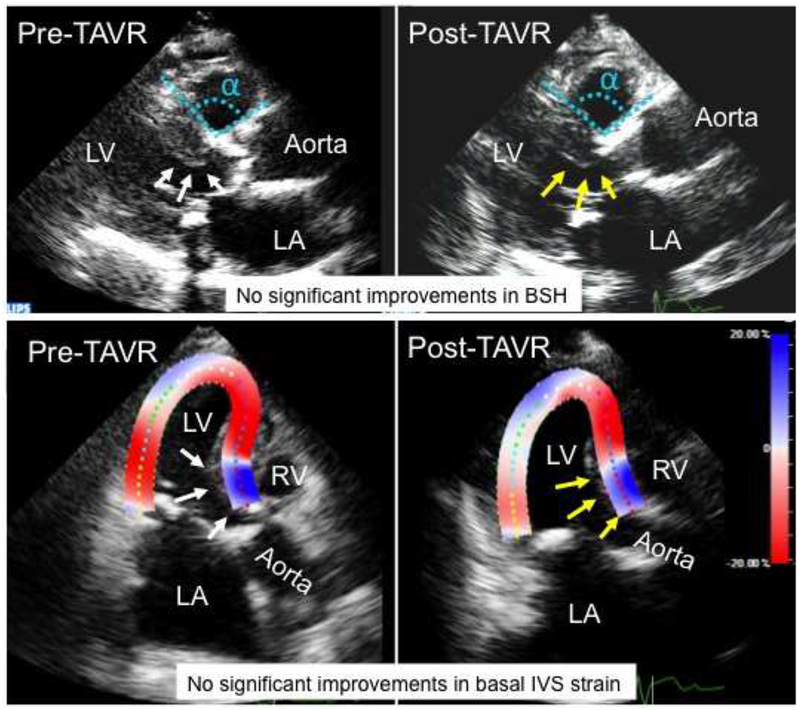
The patient in Figure 5 had severe AS and associated BSH as shown by basal IVS thickening, reduced IVS to aorta angle, and reduced basal IVS strain (white arrows and angle α in the upper pre-TAVR panel and blue strain signals in the lower pre-TAVR panel, respectively) and subsequently underwent TAVR. In contrast to the patient with SAVR, post-TAVR echocardiography in this patient did not show clear improvements in these BSH indices. Supplemental movie 2 showed no clear morphological or functional improvements of the BSH, respectively.
Figure 5.
A representative patient who underwent transcatheter aortic valve replacement (TAVR). Before the TAVR, this patient had severe aortic stenosis and associated basal septal hypertrophy (BSH) as shown in increased wall thickness in basal interventricular septum (IVS), reduced IVS to aorta angle and reduced basal IVS contraction (white arrows and angle α in the upper pre-TAVR panel and blue strain signals in the lower pre-TAVR panel, respectively). In contrast to the patient with SAVR, post-TAVR echocardiography in this patient did not show clear improvements in all basal IVS thickness, IVS to aorta angle and basal IVS strain (yellow arrows and angle α in the upper post-TAVR panel and blue strain signals in the lower post-TAVR panel, respectively). LA indicates left atrium; LV, left ventricle; and RV, right ventricle.
Measurement Variability
Good correlations were observed in inter- and intraobserver variability of the LV global longitudinal strain (r=0.97 and 0.93). From Bland-Altman method, inter- and intraobserver variability were 4.9% and 6.8%, respectively.
Discussion
This study demonstrated that abnormalities in morphology and contraction of basal IVS improves after SAVR but not after TAVR in patients with AS. Although both SAVR and TAVR are highly effective to improve aortic valve function [14,15], different effects of SAVR and TAVR on regions other than aortic valve have not been previously evaluated. The present study demonstrated beneficial effects of SAVR, as opposed to TAVR, on associated basal IVS abnormality and may provide a unique perspective to evaluate different effects of SAVR and TAVR, which may help to perform SAVR or TAVR and to consider strategy for concomitant procedures at the time of SAVR in patients with AS.
Correlation with Previous Investigations and Clinical Implications
High incidence of BSH and reduced contraction in the region of BSH in patients with AS has been reported [1], consistent with our findings. The present study further suggested beneficial influences of conventional SAVR on a structure other than the aortic valve itself, the associated BSH.
Main invasive procedures for symptomatic patients with AS are SAVR and TAVR. SAVR is a safe and effective procedure and has long history and established data, including those of improved long-term durability of prosthetic valve [16]; it requires thoracotomy and the use of extracorporeal cardiopulmonary pump. TAVR is also a safe and effective procedure, and is less invasive without the use of cardiopulmonary pump and thoracotomy is also not necessary for transfemoral TAVR [17], which is its major approach and the target of this study. TAVR is a relatively recent innovation with excellent outcomes and satisfactory intermediate-term durability of the implanted prosthetic valve [17]. Therefore, both SAVR and TAVR are established treatments for AS. The present study demonstrated beneficial influences of SAVR on regions other than the aortic valve itself, which is BSH in this case. BSH is a potential risk for post-procedural LV outflow tract obstruction following both SAVR and TAVR with unfavorable outcome [3–5] and potential risk at the time of TAVR [18]. Information derived from this investigation is a note to perform SAVR and TAVR.
One of additional merits of SAVR is its availability for concomitant procedures, such as coronary artery bypass grafting or mitral valve surgery. When BSH is associated with AS, myectomy in addition to SAVR is often performed, which reduces basal IVS thickening and offers favorable influences [19]. Our study also demonstrated that SAVR with aortic wall shortening mainly reduced basal IVS thickening. Reduction of basal IVS thickening by SAVR in the present study was −1.3±1.6 mm in the whole 32 patients and −3.0±1.4 −2.7±1.5 mm in 9 12 patients with significant BSH. This reduction of basal IVS thickening was comparable or even greater in comparison to those by concomitant myectomy with mean reduction of −0.7 to −2.0 mm [19,20]. Therefore, this study may offer an additional option as a concomitant procedure in case of AS with BSH.
BSH has been described as a phenotype of hypertrophic cardiomyopathy, however, it is still controversial whether BSH with its low prevalence of gene abnormalities represents primary cardiomyopathy or not [21]. We have conducted the present study based on the proposed mechanism of BSH as a secondary compression on the IVS from a longitudinally elongated ascending aorta. Although the mechanism of BSH is not yet established, morphological and functional improvement of BSH after SAVR and lack of the beneficial influence by TAVR in the present study suggests that the proposed mechanism may work in patients with AS and associated BSH. To confirm the proposed mechanism of BSH, further studies including computational finite element modeling and others are required [22]. However, confirmation of the proposed mechanism is not necessary for the validity of findings of our study. This study demonstrated beneficial influences of SAVR on associated BSH configurations and function and lack of such influences of TAVR, which might be a consideration to perform SAVR or TAVR.
Limitations
This study demonstrated beneficial influences on BSH by SAVR. However, morphological and functional abnormalities in the region of BSH still remained after the procedure. Even after SAVR, basal IVS thickness was greater and basal IVS strain was reduced compared to other segments (p<0.01, respectively), and IVS to aorta angle was also smaller than that of normal controls (p<0.01). The length of the prosthetic vessel to replace aneurysmal aorta is often considerably shorter than that of the resected aneurysm [23]. Aortic wall shortening of 1.0 cm only in the anterior wall in our study may not fully correct the whole aortic wall elongation. Individually tailored and whole, as opposed to only anterior, aortic wall shortening based on the evaluation of whole wall elongation seems preferable. Results of the present study may promote such surgical strategies. Effects of SAVR and TAVR on the associated BSH were evaluated several months and relatively early after the procedure. Longer term effects need to be further evaluated. Additional effects of SAVR on the BSH were evaluated but influences on patients’ outcome by these effects were not evaluated. Functional improvement in the basal IVS was evaluated with longitudinal strain in this study. Ideally speaking, all longitudinal, circumferential and radial strains are important, which requires analysis in the short axis view. Due to the deformity of basal IVS, analysis in the short axis view is difficult. In other studies of BSH, functional evaluation was also performed mainly by longitudinal strain with analysis on apical views [1]. LV outflow tract obstruction is one of important complications after SAVR and TAVR and BSH is a potential risk for this obstruction [3–5]. Attenuated BSH, especially with more aggressive aortic wall shortening, by SAVR may have favorable influences for the post-operative LV outflow tract obstruction. However, this was not evaluated.
Conclusions
In patients with AS, SAVR as opposed to TAVR improves associated BSH and its functional impairment. These can be considerations in the strategies for SAVR and TAVR.
Supplementary Material
Acknowledgments
The authors thank Drs. Yasufumi Nagata and Victor Chien-Chia Wu for their statistical assistance.
Funding
Y.O. was supported by Grants-in-aid for Scientific Research from the Japan Society of the Promotion of Science (17K09538).
Footnotes
Conflict of interest: We do not have any conflict of interest to disclose.
References
- [1].Baltabaeva A, Marciniak M, Bijnens B, Moggridge J, He FJ, Antonios TF, et al. Regional left ventricular deformation and geometry analysis provides insights in myocardial remodelling in mild to moderate hypertension. Eur J Echocardiogr 2008;9:501–8. [DOI] [PubMed] [Google Scholar]
- [2].Otto CM, Lind BK, Kitzman DW, Gersh BJ, Siscovick DS. Association of aortic valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med 1999;341:142–7. [DOI] [PubMed] [Google Scholar]
- [3].Aurigemma G, Battista S, Orsinelli D, Sweeney A, Pape L, Cuénoud H. Abnormal left ventricular intracavitary flow acceleration in patients undergoing aortic valve replacement for aortic stenosis. A marker for high postoperative morbidity and mortality. Circulation 1992; 86: 926–36. [DOI] [PubMed] [Google Scholar]
- [4].Leya F, Tuchek JM, Coats W. Abnormal distortion of aortic corevalve bioprosthesis with suicide left ventricle, aortic insufficiency, and severe mitral regurgitation during transcatheter aortic valve replacement. Catheter Cardiovasc Interv 2016;88:1181–1187. [DOI] [PubMed] [Google Scholar]
- [5].Abramowitz Y, Maeno Y, Chakravarty T, Kazuno Y, Takahashi N, Kawamori H, et al. Aortic angulation attenuates procedural success following self-expandable but not balloon-expandable TAVR. JACC Cardiovasc Imaging 2016;9:964–72. [DOI] [PubMed] [Google Scholar]
- [6].Hahn RT, Little SH, Monaghan MJ, Kodali SK, Williams M, Leon MB, et al. Recommendations for comprehensive intraprocedural echocardiographic imaging during TAVR. JACC Cardiovasc Imaging. 2015;8:261–87. [DOI] [PubMed] [Google Scholar]
- [7].Swinne CJ, Shapiro EP, Jamart J, Fleg JL. Age-associated changes in left ventricular outflow tract geometry in normal subjects. Am J Cardiol 1996;78:1070–3. [DOI] [PubMed] [Google Scholar]
- [8].Nagata Y, Takeuchi M, Mizukoshi K, Wu VC, Lin FC, Negishi K, et al. Intervendor variability of two-dimensional strain using vendor-specific and vendor-independent software. J Am Soc Echocardiogr 2015;28:630–41. [DOI] [PubMed] [Google Scholar]
- [9].Poh KK, Levine RA, Solis J, Shen L, Flaherty M, Kang YJ, et al. Assessing aortic valve area in aortic stenosis by continuity equation: a novel approach using real-time three-dimensional echocardiography. Eur Heart J 2008;29:2526–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tabata T, Grimm RA, Greenberg NL, Agler DA, Mowrey KA, Wallick DW, et al. Assessment of LV systolic function in atrial fibrillation using an index of preceding cardiac cycles. Am J Physiol Heart Circ Physiol 2001;281:H573–H580. [DOI] [PubMed] [Google Scholar]
- [11].Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- [12].Bernstein RF, Tei C, Child JS, Shah PM. Angled interventricular septum on echocardiography: anatomic anomaly or technical artifact? J Am Coll Cardiol. 1983;2:297–304. [DOI] [PubMed] [Google Scholar]
- [13].Ngo A, Hassager C, Thyregod HGH, Søndergaard L, Olsen PS, Steinbrüchel D, et al. Differences in left ventricular remodelling in patients with aortic stenosis treated with transcatheter aortic valve replacement with corevalve prostheses compared to surgery with porcine or bovine biological prostheses. Eur Heart J Cardiovasc Imaging. 2018;19:39–46. [DOI] [PubMed] [Google Scholar]
- [14].Kondur A, Briasoulis A, Palla M, Penumetcha A, Mallikethi-Reddy S, Badheka A, et al. Meta-analysis of transcatheter aortic valve replacement versus surgical aortic valve replacement in patients with severe aortic valve stenosis. Am J Cardiol 2016;117:252–7. [DOI] [PubMed] [Google Scholar]
- [15].Thyregod HG, Steinbrüchel DA, Ihlemann N, Nissen H, Kjeldsen BJ, Petursson P, et al. Transcatheter versus surgical aortic valve replacement in patients with severe aortic valve stenosis: 1-year results from the All-Comers NOTION randomized clinical trial. J Am Coll Cardiol 2015;65:2184–94. [DOI] [PubMed] [Google Scholar]
- [16].Thourani VH, Suri RM, Gunter RL, Sheng S, O’Brien SM, Ailawadi G, et al. Contemporary real-world outcomes of surgical aortic valve replacement in 141,905 low-risk, intermediate-risk, and high-risk patients. Ann Thorac Surg 2015;99:55–61. [DOI] [PubMed] [Google Scholar]
- [17].Zajarias A, Cribier AG. Outcomes and safety of percutaneous aortic valve replacement. J Am Coll Cardiol 2009;53:1829–36. [DOI] [PubMed] [Google Scholar]
- [18].Hahn RT, Kodali S, Tuzcu EM, Leon MB, Kapadia S, Gopal D, et al. Echocardiographic imaging of procedural complications during balloon-expandable transcatheter aortic valve replacement. JACC Cardiovasc Imaging. 2015;8:288–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Di Tommaso L, Stassano P, Mannacio V, Russolillo V, Monaco M, Pinna G, et al. Asymmetric septal hypertrophy in patients with severe aortic stenosis: The usefulness of associated septal myectomy. J Thorac Cardiovasc Surg 2013;145:171–175. [DOI] [PubMed] [Google Scholar]
- [20].Kayalar N, Schaff HV, Daly RC, Dearani JA, Park SJ. Concomitant septal myectomy at the time of aortic valve replacement for severe aortic stenosis. Ann Thorac Surg 2010;89:459–64. [DOI] [PubMed] [Google Scholar]
- [21].Spirito P, Maron BJ, Bonow RO, Epstein SE. Severe functional limitation in patients with hypertrophic cardiomyopathy and only mild localized left ventricular hypertrophy. J Am Coll Cardiol 1986;8:537–44. [DOI] [PubMed] [Google Scholar]
- [22].Morrel WG, Ge L, Zhang Z, Grossi EA, Guccione JM, Ratcliffe MB. Effect of mitral annuloplasty device shape and size on leaflet and myofiber stress following repair of posterior leaflet prolapse: a patient-specific finite element simulation. J Heart Valve Dis 2014;23:727–34. [PMC free article] [PubMed] [Google Scholar]
- [23].Hetzer R, Solowjowa N, Knosalla C, Kuckuka M, Delmo Walter EM. Surgical correction of ascending aortic aneurysm and aortic valve incompetence by relocation of the aortic valve plane using a short aortic replacement graft. Ann Thorac Surg 2012;94:1983–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.