Abstract
Delayed postpolypectomy bleeding (DPPB) is the most common complication of colonoscopic polypectomy. Prophylactic clipping after an uncomplicated polypectomy is increasingly used, but it is unclear if this results in the prevention of DPPB. This study aimed to review prophylactic clip use and its effect on the rates of DPPB. MEDLINE, Embase, and the Cochran Library were systematically searched for studies (1995–March 2017) that used prophylactic hemoclips and assessed DPPB as an outcome. Of 1402 articles identified, nine papers were eligible for inclusion, evaluating 4311 patients and 7783 polyps; 118 patients experienced a DPPB, and 49 of these patients received prophylactic clips. There was no significant difference in DPPB rates in patients who received prophylactic clipping compared to those who did not (odd ratio: 0.8; 95% confidence interval: 0.36–1.77; P = 0.56). There was also no significant difference in the DPPB of polyps <20 mm compared with polyps ≥20 mm. Clip application for prophylactic management of an uncomplicated polypectomy has not been demonstrated to reduce the risk of DPPB, casting doubt on the use of this costly practice.
Keywords: bleeding, clip, polypectomy, prophylactic, systematic review
Introduction
Colonoscopic polypectomy reduces the incidence and mortality of colorectal cancer and is standard practice during screening and surveillance colonoscopy. Colonoscopy is considered a safe and well‐tolerated procedure,1, 2 but rates of serious adverse events increase by up to sevenfold following polypectomy.3 Hemorrhage is by far the most common adverse event, with incidence ranging from 0.6% to 8.6%,4 with the risk of hemorrhage increasing by 9% with every 1 mm increase in polyp diameter.4 Multiple other factors, such as sessile form, right colonic location, unfavorable histology (villous or tubulovillous type), use of anticoagulants or dual‐antiplatelet therapy, presence of coagulopathy, or cardiovascular disease, as well as limited experience of the interventional endoscopist, increase the risk of bleeding.5, 6, 7, 8
Several techniques, including epinephrine–saline solution9, 10 and use of detachable nylon loop,11 are used prior to polypectomy to reduce the risk of hemorrhage. Significant immediate postpolypectomy bleeding can usually be controlled by application of hemostatic clips, with a high success rate.12, 13 However, delayed postpolypectomy bleeding (DPPB), defined as per rectal bleeding that occurs after completion of colonoscopy, poses serious clinical concerns, particularly when it occurs outside of hospitals as it can be associated with a relatively large volume blood loss and delay in appropriate treatment.3 Hospital readmission, transfusion, repeat endoscopy, and—rarely—radiological or surgical intervention may be required.
In attempts to minimize the risk of DPPB, the placement of hemostatic clips at nonbleeding polypectomy mucosal defects is becoming increasingly common; however, there is limited evidence to support the efficacy and safety of such “prophylactic” clipping. There has been only one very recent meta‐analysis assessing the efficacy of prophylactic clip placement, but this study was incomplete in its coverage of the published trials and included data from abstracts, which may be unreliable and vary in quality. The aim of this systematic review was to evaluate whether prophylactic clip placement reduces DPPB rates.
Methods
Search strategy and studies selection
A comprehensive systematic review of the medical literature was performed. MEDLINE (April 2016), EMBASE (April 2016), and the Cochrane Library (April 2016) were searched. MeSH index and free‐text terms were used. OVID Medline search terms were identified and combined using the set operator OR, by searching for the MeSH terms “colonic polyps,” “adenomatous polyps,” “polyps,” “colorectal neoplasms,” “colonoscopy,” “gastrointestinal hemorrhage,” and “hemorrhage,” and the keyword searches for “polyp*,” “endoscop*,” “colonoscop*,” “bleed*,” and “h?emorrhag*”. These studies were combined using the set operator AND with the keyword studies for “clip*” and “h?emoclip*”. A similar process was performed with OVID Embase MeSH search terms, which included “polyp,” “colon,” “adenomatous polyp,” “colorectal polyp,” “colonoscopy,” “bleeding,” “gastrointestinal hemorrhage,” with keyword searchers for “polyp*,” “endoscop*,” “colonoscop*,” “clip*,” “h?emoclip*,” “bleed*,” and “h?emorrhag*”. The Cochrane Library was also searched for polyp and clip with zero results. The search was limited to human studies. No language restriction was applied.
The primary outcome was to evaluate DPPB in patients who received prophylactic colonoscopic polypectomy clipping compared to those who did not. The inclusion criteria required the study to be a published randomized controlled trial (RCT) or observational study (cohort and case–controlled design) that compared prophylactic polypectomy clip use and no prophylactic clip use with DPPB as an outcome. Studies were excluded if they compared prophylactic clipping with another endoscopic hemostatic modality or combined prophylactic clipping with other measures, such as thermal therapy or adrenaline injection. All the citations and abstracts derived from the electronic searches were reviewed and screened for relevancy by two authors (SNK and DM) independently. The full text of identified relevant studies was then reviewed by the same two reviewers against the inclusion and exclusion criteria. A third reviewer (GB) confirmed these results and resolved disagreements or discrepancies.
A standard data extraction form was developed. Two review authors then independently undertook data extraction from the included trials, with any disagreements or discrepancies resolved by consensus or after discussion with the third author. Given the heterogeneous nature of the study types, a qualitative analysis was performed rather than a meta‐analysis. Quality assessment of included studies was performed using the Cochrane's Collaboration Risk of Bias Tool.14, 15 A score was assigned to each component based on the quality of available evidence. This score can be very low, low, moderate, or high quality based on various quality specifications.14, 15
Statistical analysis
The odds ratios of bleeding events with or without clipping were calculated from the number of patients and events in the individual studies and pooled with the metan command in Stata software version 13 (Stata Corp, College Station, TX, USA), using random effect modeling. The model weighs data intrinsically by using the individual study variance. A Forest plot was used to graphically display the odds ratios from the individual studies with their confidence intervals (CIs). The plot was constructed using SAS, version 9.4 (SAS Institute Inc., Cary, NC, USA).
Statistical heterogeneity was assessed using the I 2 values. I 2 values of 25%, 50%, and 75% were reported as low, moderate, and high level of heterogeneity, respectively. The value of I 2 was obtained from the metan command in Stata.
A funnel plot was obtained using the metan command in Stata software version 13 (Stata Corp) as a visual tool for the investigation of publication bias. The plot is a scatterplot of the log odds ratios obtained from the individual studies (horizontal axis) against the standard error in the vertical axis as a measure of the study size.
Results
The search strategy identified 1640 articles. The flow of citations is outlined in the PRISMA flow diagram (Fig. 1). After duplicates were removed, 1330 articles clearly did not meet inclusion criteria. Seventy‐two full‐text articles were further assessed. Of these, six were considered eligible for inclusion.16, 17, 18, 19, 20, 21 The earliest study included was published in 2002. Two studies were retrospective observational cohort studies, one was a case–control trial, and three were RCTs. Given the endoscopist performs the clipping intervention in all three RCTs (Table 1), it should be noted that it is not possible to blind the proceduralist, and this should be noted with regard to the study quality assessment. One study18 included partially clipped lesions. These data were removed from the analysis as they are not comparable to the interventions in other studies; the data on polypectomy sites that were fully clipped and those that did not receive prophylactic clipping were retained. One retrospective study22 published in 2012 was not included in this review as it was deemed that the indication for clipping was not prophylactic.
Figure 1.
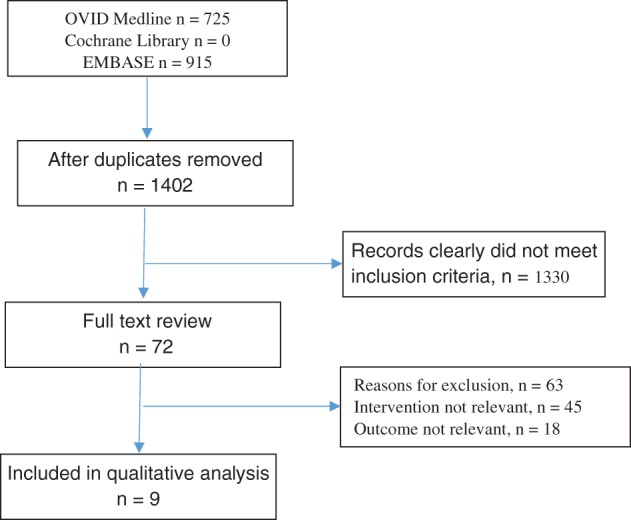
Trial search flow chart.
Table 1.
Characteristics and quality of RCTs included
| Study design | Year of publication | Period of data collection | Random sequence generation | Allocation concealment | Blinding | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other sources of bias | Quality assessment | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Matsumoto et al.22 | RCT | 2016 | January 2012–July 2013 | Adequate | Adequate | Single‐blinded | Adequate | None | None | Mild | High |
| Dokoshi et al.21 | RCT | 2015 | March 2012–December 2014 | Adequate | Adequate | Single‐blinded | Adequate | None | None | None | High |
| Zhang et al.20 | RCT | 2015 | October 2011–April 2014 | None | Adequate | Single‐blinded | None | None | None | None | High |
| Quintanilla et al.10 | RCT | 2012 | January 2007–December 2010 | Adequate | Adequate | Single‐blinded | Adequate | None | None | None | High |
| Shioji et al.17 | RCT | 2003 | November 1998–March 2001 | Inadequate | Yes | No | No | None | No | No | High |
| Feagins et al.19 | Restrospective observational | 2014 | July 2008–December 2009 | None | None | None | None | None | None | None | Moderate |
| Liaquat et al.18 | Restrospective observational | 2013 | January 2000–February 2012 | None | None | None | None | None | None | None | Moderate |
| Sobrino‐Faya et al.23 | Restrospective observational | 2002 | January 2001–October 2001 | None | None | None | None | None | None | None | Moderate |
| Fukata et al.16 | Restrospective observational | 2002 | January 1995–December 1999 | NR | NR | No | No | None | No | No | Moderate |
NR, not relevant; RCT, randomized controlled trial.
A total of 4311 subjects were included. The mean age was 64.5 (range: 21–90) years. Of the 4311 subjects, 2888 (67%) were male. A total of 7783 polypectomies were performed; of these, 3572 polypectomy sites were prophylactic clipped. Of the 4311 (2.7%) subjects, 118 (2.7%) experienced a DPPB, with 49 of 118 (41.5%) participants having received prophylactic clips and 69 of 118 (58.5%) with DPPB not having received prophylactic clips. Despite two papers demonstrating a significant benefit in reducing DPPB after prophylactic clipping,18, 20 there was no significant difference in DPPB when an overall random effect model was applied (odd ratio: 0.8; 95% CI: 0.36–1.77; P = 0.56). Six studies reported 3121 polyps <20 mm and 766 polyps ≥20 mm.10, 16, 18, 19, 20, 21 However, prophylactic clippings based on polyp size did not result in any overall significant benefit in DPPB reduction (for polyps <20 mm: odd ratio: 1.16; 95% CI: 0.7–1.92; P = 0.56 and for polyps >20 mm: odd ratio: 0.73; 95% CI: 0.08–7.04; P = 0.79) (Fig. 2).
Figure 2.
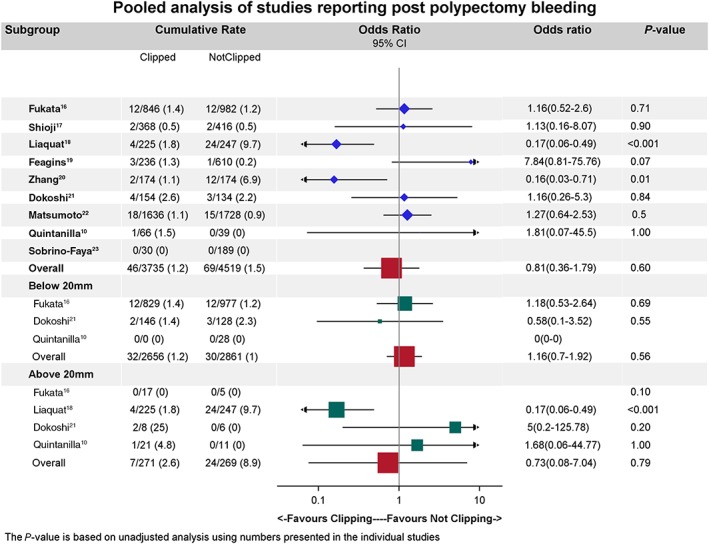
Forest plot showing the comparison of delayed postpolypectomy bleeding of prophylactically clipped and nonclipped polypectomy defects in all studies.
The majority of polyps (3152/7940, 39.7%) were removed using the endoscopic mucosal resection (EMR) technique. The remaining polyps were removed by hot polypectomy, circumferential EMR, endoscopic submucosal dissection, or the method of polyp removal was not specified.
Five studies contained polyp location, with 3758 of 7462 polyps being in the proximal colon. Two studies16, 21 included the location associated with DPPB: 18 of 1814 (1%) proximal polypectomy sites that were clipped, 8 of 1798 (0.4%) proximal polypectomy sites that were not clipped, 12 of 1698 (0.7%) distal polypectomy sites that were clipped, and 19 of 1904 (1%) polypectomy sites that were not clipped.
Histology was described in four papers17, 18, 19, 21 for 2427 polyps removed (Graph 1b). Of the 2427 polyps, 1834 (75.6%) were adenomas, 92 (3.8%) sessile serrated polyps, 110 (4.5%) high‐grade dysplasia or adenocarcinoma, 252 (10.4%) hyperplastic, and 139 (5.7%) other. The histology of the polyps removed associated with DPPB were high‐grade dysplasia or adenocarcinoma in 3 of 64 (4.7%), 3 of 61 (4.9%) had sessile serrated polyps, and 35 of 1085 (3.2%) were tubular adenomas. For these cases, data for prophylactic clipping were incomplete, and thus, statistical analysis was unable to be performed.
Assessment of heterogeneity
The odds ratio estimates for the six studies demonstrated a high level of heterogeneity using the random effect analysis (I 2: 71%; P = 0.004). The subanalyses of the population with polyp size larger than 20 mm also demonstrated significant heterogeneity (I 2: 74%; P = 0.05), while the heterogeneity was lower for the population of polyps smaller than 20 mm (I 2: 0%; P = 0.48).
Publication bias
The Funnel plot (Fig. 3) showed the distribution of log odds ratio obtained from the individual studies (horizontal axis) against the standard error in the vertical axis. Due to the small number of studies included in the analysis, accurate assessment of the degree of publication bias was difficult to obtain. However, the observed asymmetry in the Funnel plot (Fig. 3) indicates possible publication bias.
Figure 3.
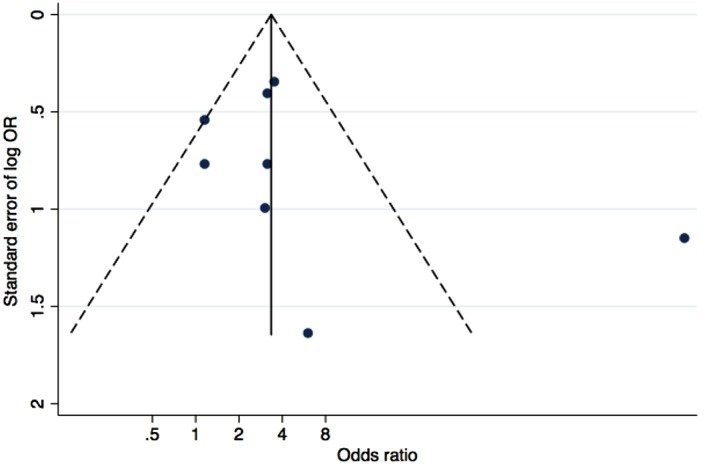
The Funnel plot horizontal axis represents the log odds ratio from the individual studies. The vertical axis represents the standard error of the log odds ratios. Studies with larger standard errors have a wider confidence interval caused by smaller sample size. The graphed vertical line represents the pooled odds ratio. The points represent the observed distribution of the published studies.
Other considerations
Subgroup analysis was planned for differences in total duration of hospitalization, number of packed red blood cells transfused, number of further interventions (endoscopic, radiological, or surgical), and anticoagulation/antiplatelet use, but there were insufficient data to perform any subgroup analysis.
One paper reported 1 gastric polypectomy out of 233 polypectomies, and this polyp has been excluded from the final analysis.
Discussion
In this study, we describe the current evidence for prophylactic clipping postpolypectomy and its effect on DPPB. Despite the growing pool of published research, there remains insufficient evidence to support the routine use of prophylactic hemoclips after an uncomplicated polypectomy in attempts to prevent DPPB. The number of DPPB complications was found to be within the expected range, at 2.9%, and despite an increased risk in DPPB if a prophylactic clip was not applied, this did not result in an overall statistically significant difference (odd ratio: 0.71; 95% CI: 0.25–2.03; P = 0.52).
Several studies with large numbers of small, subcentimeter polyps that, in current practice, would not conventionally warrant prophylactic clipping were included. Increasing polyp size greater than 1 cm is a risk factor for DPPB.24, 25 Two studies included in this review18, 20 suggested that the prophylactic clipping of these larger polyps reduced DPPB. However, an overall statistically significant difference could not be demonstrated when comparing polyps <20 mm with polyps ≥20 mm in size. A total of 734 polyps ≥20 mm were included, but the sizes of polyps that were complicated by DPPB was not included in some studies, making the number of DPPB events in this subgroup small. Thus, insufficient sample size is likely to have contributed to the nonsignificant benefit of prophylactic clipping of ≥20 mm polyps.
Other factors that increased the risk of DPPB include sessile form, right colonic location, unfavorable histology (villous or tubulovillous type), and use of anticoagulants or dual‐antiplatelet therapy. From the current review, there is insufficient evidence from which to draw a conclusion on the use of clips in these scenarios. However, these patients have an increased risk of DPPB, and the authors suggest that these may be situations in which prophylactic clipping could be considered. It is likely that strong evidence in these specific areas will remain difficult to obtain because of the low events rate of DPPB and the risk reduction associated with prophylactic clipping that would be required to power any prospective studies.
Endoscopic hemoclips are readily available and have become even easier to use with advances in improved rotatability. As their use increases, concurrent cost effectiveness assessment is also required. For the universal clip placement strategy to be cost effective and offset the cost of DPPB management, a relatively high DPPB rate and low cost of clips is a prerequisite. However, this is not currently the case. Bahin et al.26 assessed the cost effectiveness of the prophylactic clip placement strategy by applications of an economic model to the outcomes of an Australian colonic endoscopic mucosal resection study. This model demonstrated that it would cost a minimum of €1500 to prevent one DPPB event with presumed 100% clip efficacy. A cost of € 10 per clip at 100% efficacy and €7 per clip at 75% efficacy would result in cost equivalence. At current clip costs, the risk of DPPB would need to be at least 45% for prophylactic clip placement for there to be economic equilibrium. Similar findings were also seen in another study that used a decision tree model in a hypothetical reference case.27 Based on current costs of approximately $150 AUD per clip, routine prophylactic placement of any number of clips in the low‐risk case or placement of more than one prophylactic clip in patients receiving antiplatelets and anticoagulant therapy is not cost effective.27 However, it should be interpreted with caution as they carry several limitations. Cost analyses from the study by Bahin et al. were based on a single tertiary referral center in an Australian setting, which limits the universal applicability of the findings.26 In addition, the authors measured only direct costs resulting from the clinical management of DPPB; nonfinancial costs and indirect costs incurred by the individual or society were not considered.26, 27
In conclusion, this systematic review of RCTs and case‐control studies has found that prophylactic clip closure after uncomplicated polypectomy did not significantly reduce the risk of DPPB, although there is a trend toward benefit in polyps ≥20 mm. Prophylactic clip application in scenarios where risk of DPPB is increased may be beneficial, although there is little evidence to currently support its routine use. There is a need for further well‐designed, properly powered studies in which polyp size and clinical phenotyping are defined.
Dileep Mangira and Shara N Ket are equal first co‐authors.
References
- 1. Macrae FA, Tan KG, Williams CB. Towards safer colonoscopy: a report on the complications of 5000 diagnostic or therapeutic colonoscopies. Gut. 1983; 24: 376–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Singh R, Mangira D, Kawano H, Matsuda T. Screening colonoscopy in Australia. Dig. Endosc. 2015; 27 (Suppl. 1): 30–4. [DOI] [PubMed] [Google Scholar]
- 3. Waye JD, Lewis BS, Yessayan S. Colonoscopy: a prospective report of complications. J. Clin. Gastroenterol. 1992; 15: 347–51. [PubMed] [Google Scholar]
- 4. Rosen L, Bub DS, Reed JF 3rd, Nastasee SA. Hemorrhage following colonoscopic polypectomy. Dis. Colon Rectum. 1993; 36: 1126–31. [DOI] [PubMed] [Google Scholar]
- 5. Heldwein W, Dollhopf M, Rosch T et al The Munich Polypectomy Study (MUPS): prospective analysis of complications and risk factors in 4000 colonic snare polypectomies. Endoscopy. 2005; 37: 1116–22. [DOI] [PubMed] [Google Scholar]
- 6. Burgess NG, Metz AJ, Williams SJ et al Risk factors for intraprocedural and clinically significant delayed bleeding after wide‐field endoscopic mucosal resection of large colonic lesions. Clin. Gastroenterol. Hepatol. 2014; 12: 651–61.e1–3. [DOI] [PubMed] [Google Scholar]
- 7. Parra‐Blanco A, Kaminaga N, Kojima T, Endo Y, Tajiri A, Fujita R. Colonoscopic polypectomy with cutting current: is it safe? Gastrointest. Endosc. 2000; 51: 676–81. [DOI] [PubMed] [Google Scholar]
- 8. Singh M, Mehta N, Murthy UK, Kaul V, Arif A, Newman N. Postpolypectomy bleeding in patients undergoing colonoscopy on uninterrupted clopidogrel therapy. Gastrointest. Endosc. 2010; 71: 998–1005. [DOI] [PubMed] [Google Scholar]
- 9. Shirai M, Nakamura T, Matsuura A, Ito Y, Kobayashi S. Safer colonoscopic polypectomy with local submucosal injection of hypertonic saline‐epinephrine solution. Am. J. Gastroenterol. 1994; 89: 334–8. [PubMed] [Google Scholar]
- 10. Quintanilla E, Castro Jé L, Rábago L et al Is the use of prophylactic hemoclips in the endoscopic resection of large pedunculated polyps useful? A prospective and randomized study. J. Interv. Gastroenterol. 2012; 2: 183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hachisu T. A new detachable snare for hemostasis in the removal of large polyps or other elevated lesions. Surg. Endosc. 1991; 5: 70–4. [DOI] [PubMed] [Google Scholar]
- 12. Jacques J, Legros R, Chaussade S, Sautereau D. Endoscopic haemostasis: an overview of procedures and clinical scenarios. Dig. Liver Dis. 2014; 46: 766–76. [DOI] [PubMed] [Google Scholar]
- 13. Arezzo A, Verra M, Cravero F, Reddavid R, Morino M. How to place hemoclips to achieve hemostasis of a bleeding diverticulum. Dig. Dis. Sci. 2011; 56: 1589–90; author reply 1590–1. [DOI] [PubMed] [Google Scholar]
- 14. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. [updated March 2011]. The Cochrane Collaboration, 2011. Available from URL: http://handbook.cochrane.org [Google Scholar]
- 15. Guyatt GH, Oxman AD, Vist GE et al GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008; 336: 924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fukata M, Kijima H, Sanjo A, Sugisaka H, Inoue T, Takagi I. Prophylactic clipping may not eliminate delayed hemorrhage in colonoscopic polypectomies. Jikeikai Med. J. 2002; 49: 133–42. [Google Scholar]
- 17. Shioji K, Suzuki Y, Kobayashi M et al Prophylactic clip application does not decrease delayed bleeding after colonoscopic polypectomy. Gastrointest. Endosc. 2003; 57: 691–4. [DOI] [PubMed] [Google Scholar]
- 18. Liaquat H, Rohn E, Rex DK. Prophylactic clip closure reduced the risk of delayed postpolypectomy hemorrhage: experience in 277 clipped large sessile or flat colorectal lesions and 247 control lesions. Gastrointest. Endosc. 2013; 77: 401–7. [DOI] [PubMed] [Google Scholar]
- 19. Feagins LA, Nguyen AD, Iqbal R, Spechler SJ. The prophylactic placement of hemoclips to prevent delayed post‐polypectomy bleeding: an unnecessary practice? A case control study. Dig. Dis. Sci. 2014; 59: 823–8. [DOI] [PubMed] [Google Scholar]
- 20. Zhang QS, Han B, Xu JH, Gao P, Shen YC. Clip closure of defect after endoscopic resection in patients with larger colorectal tumors decreased the adverse events. Gastrointest. Endosc. 2015; 82: 904–9. [DOI] [PubMed] [Google Scholar]
- 21. Dokoshi T, Fujiya M, Tanaka K et al A randomized study on the effectiveness of prophylactic clipping during endoscopic resection of colon polyps for the prevention of delayed bleeding. Biomed. Res. Int. 2015; 2015: 490272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Matsumoto M, Kato M, Oba K et al Multicenter randomized controlled study to assess the effect of prophylactic clipping on post‐polypectomy delayed bleeding. Dig. Endosc. 2016; 28: 570–6. [DOI] [PubMed] [Google Scholar]
- 23. Sobrino‐Faya M, Martinez S, Gomez Balado M et al Clips for the prevention and treatment of postpolypectomy bleeding (hemoclips in polypectomy). Rev. Esp. Enferm. Dig. 2002; 94: 457–62. [PubMed] [Google Scholar]
- 24. Kim HS, Kim TI, Kim WH et al Risk factors for immediate postpolypectomy bleeding of the colon: a multicenter study. Am. J. Gastroenterol. 2006; 101: 1333–41. [DOI] [PubMed] [Google Scholar]
- 25. Watabe H, Yamaji Y, Okamoto M et al Risk assessment for delayed hemorrhagic complication of colonic polypectomy: polyp‐related factors and patient‐related factors. Gastrointest. Endosc. 2006; 64: 73–8. [DOI] [PubMed] [Google Scholar]
- 26. Bahin FF, Rasouli KN, Williams SJ, Lee EY, Bourke MJ. Prophylactic clipping for the prevention of bleeding following wide‐field endoscopic mucosal resection of laterally spreading colorectal lesions: an economic modeling study. Endoscopy. 2016; 48: 754–61. [DOI] [PubMed] [Google Scholar]
- 27. Parikh ND, Zanocco K, Keswani RN, Gawron AJ. A cost‐efficacy decision analysis of prophylactic clip placement after endoscopic removal of large polyps. Clin. Gastroenterol. Hepatol. 2013; 11: 1319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]


