To the Editor:
The use and cost of critical care medicine have risen steadily since 2000 (1, 2). Nearly one-third of patients who survive their critical illness will experience newly acquired or accelerated post–intensive care unit (post-ICU) cognitive impairment (3). One-third to one-half of all survivors of critical illness are newly unemployed at 12 months, likely attributable to newfound cognitive deficits (4–6). This public health problem is growing, has been poorly addressed, and requires innovation to reduce suffering and help patients return to normal functioning. By monitoring delirium (7, 8), we have previously shown that delirium duration (3, 9) is associated with white matter disruption after a critical illness (10). Although therapeutic approaches are emerging in the context of critical care delivery to reduce delirium and enhance patient safety by reducing the duration of delirium and coma (11), few therapeutic approaches have been tested in this population to regain acquired cognitive deficits. What remains unknown is whether patients can be helped to recover cognitive abilities once lost.
A need exists to develop cognitive rehabilitation approaches for patients with critical illnesses who, despite receiving best care, go on to acquire post-ICU cognitive impairment even without prior stroke or head injury. Although cognitive rehabilitation has historically been employed with predominantly brain-injured populations (traumatic brain injury, stroke), it has only recently been trialed in primary medical populations such as individuals with “chemo-fog” and human immunodeficiency virus–associated neurologic dysfunction, and only minimally among those with post–intensive care syndrome (12). Computerized cognitive rehabilitation (CCR) is a novel approach of traditional cognitive rehabilitation through the use of computerized “brain exercises.” Authors of a recent systematic review of 16 computerized cognitive training trials found moderate effect sizes in improvement of attention, executive function, and memory in individuals with cognitive impairment, which persisted at follow-up (13). Despite early promising results, many in the scientific community have questioned whether computerized cognitive exercises have any real impact (14, 15). To the contrary, others suggest that CCR holds the promise of accelerating recovery by harnessing neuroplasticity (16–18). We hypothesized that application of CCR would improve key cognitive abilities in ICU survivors even years after the original brain injury.
Methods
The RETURN-CCR (Returning to Everyday Tasks Using Rehabilitation Networks–Computerized Cognitive Rehabilitation) pilot investigation was designed by Posit Science to test the feasibility and proof of principle of specially designed computer exercises in addressing cognitive impairment in ICU survivors (19). Institutional Review Board approval was obtained, and patients from the BRAIN-ICU (Bringing to Light the Risk Factors and Incidence of Neuropsychological Dysfunction in ICU Survivors) observational cohort study (3) as well as our group’s ongoing studies were enrolled after we obtained their informed consent. These patients had survived ICU treatment with mechanical ventilation or vasopressors in the context of severe critical illness and had since shown persistent long-term cognitive impairment. A convenience sample (N = 33) of patients was recruited to participate in RETURN-CCR. In general, if patients had completed their participation in one of the aforementioned studies, were alive, and were believed to be able to participate in this trial (i.e., had previously followed up with former study assessments), they were approached for participation in RETURN-CCR.
Patients received the CCR program, which was comprised of adaptive exercises focused on optimizing speed and memory accuracy and auditory verbal processing (16, 17). Patients completed seven cognitive exercises daily (∼42 min), 5 days per week, over the 12-week trial period, with core therapeutic tasks built into a gamelike experience. Use of the program was tracked from Posit Science BrainHQ’s experimental portal, where the experimenter can easily track the progress of each participant, number of training days, and overall performance.
During a session, patients performed trials with auditory and visual feedback and rewards to indicate if the trial was performed correctly or incorrectly. After each session, the difficulty of the next session was titrated to ensure appropriate difficulty based on immediately preceding performance. That is, patients excelling experienced progressively more challenging tasks, whereas patients performing poorly engaged in tasks that were easier.
The brain-training program included 18 computerized training exercises. Core training targets include visual and auditory processing accuracy, speed, and sequencing; phasic, sustained, and divided attention; memory and memory association; and executive control abilities. For each session, participants were given seven computer exercises, and each game lasted about 6 minutes. During the 6-minute time window, participants played a certain level of game multiple times (usually between two and four times, varying on the basis of participants’ response speed and number of trials). Baseline was set by the performance of the very first attempt. Upon repetition, the initial difficulty was set by the previous trial’s best performance to promote performance improvement. As each session progresses, improvements in processing speed and/or performance accuracy and in their controlled, higher-order performance operations are challenged at progressively more cognitively demanding task levels to maintain approximately 75–85% accuracy. By this adaptive training strategy, virtually all trainees, regardless of learning rate, were continuously challenged at an appropriate difficulty level as their abilities improved.
We compared the baseline (pre-CCR) scores with post-CCR scores to assess improvement in performance. Each computerized cognitive exercise has multiple levels, presented to participants in fixed order (i.e., from easy to more complex levels). Participants set the baseline or “pre” cognitive training score from their individual performance on the initial trial and their “post” score representing their individual best performance within the trial period. Pre and post scores for each exercise were calculated by averaging the normed pre and post scores of all levels. Normed pre and post scores from each exercise were averaged to produce a composite score for each cognitive domain. Mixed-effects linear regression was performed for each cognitive domain separately with time (pre and post performance) as a factor. Mixed linear regression models were used to assess the effect of user group (super, high, normal) on improvement (pre and post) of untrained neuropsychological tests (including Digit Span, Spatial Span, and Trail Making Test A and B). To assess for group effect, we then used one-way analysis of variance (ANOVA) with group as a factor to assess for differences between groups. All statistical analyses were run in Python (version 3.5.3; Python Software Foundation).
Results
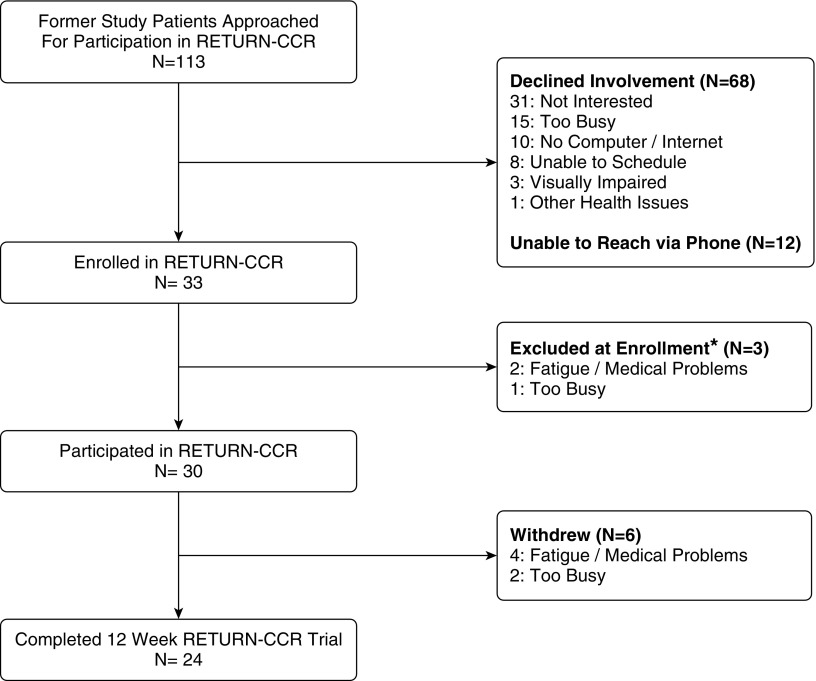
Between April 2015 and April 2016, 113 former study participants were approached for participation in RETURN-CCR (Figure 1). Thirty-three patients were initially enrolled; however, of these, three participants were excluded at enrollment because they declined to participate in the full program (Figure 1). Ultimately, 24 of the 30 (80%) who engaged in the program completed both a baseline and a post-training assessment at 12 weeks after initiation of the trial.
Figure 1.
Flow diagram of patients through the RETURN-CCR (Returning to Everyday Tasks Using Rehabilitation Networks–Computerized Cognitive Rehabilitation) investigation from recruitment through the 12-week pilot trial. *Patients excluded at enrollment were enrolled into RETURN-CCR, however afterwards declined participation and never engaged in the program.
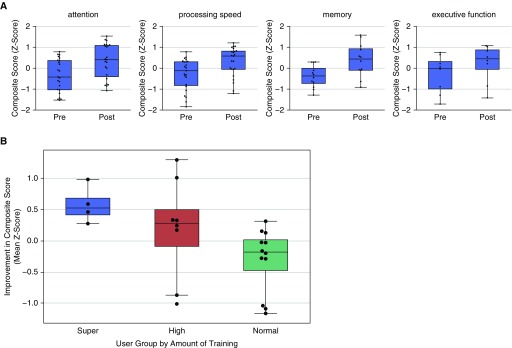
At the time of their original critical illness, patients who remained in the pilot at the 12-week mark had a median (interquartile range [IQR]) age of 60 (52–70) years. Ninety-six percent were white, and 52% were male. They had a median of 16 (12–16) years of education, were not cognitively impaired, and spent a median (IQR) of 15 (7–21) days in the hospital and 6 (3–15) days in the ICU (Table 1). Among those who completed the 12-week assessment, 75% had experienced at least 1 day of delirium, and 58% had experienced at least 1 day of coma. All but one patient required mechanical ventilation for at least 1 day during their original critical illness. Patients (n = 24) completed a median of 517.5 levels of rehabilitation (IQR, 240–862) (each daily rehabilitation session was comprised of a number of “levels”) and showed a significant (P < 0.01) improvement between baseline pre-CCR and post-CCR composite z-score performance in four key domains of cognitive functioning: attention, processing speed, memory, and executive function (Figure 2A).
Table 1.
Demographic and baseline characteristics
| Variable | Entire RETURN-CCR Cohort (N = 30) | Participation Cohort (N = 24) |
|---|---|---|
| Age at enrollment, yr | 60 (50–67) | 60 (52–70) |
| Sex (%) | ||
| Male | 53 | 50 |
| Female | 47 | 50 |
| Race (%) | ||
| White | 97 | 96 |
| Black | 3 | 4 |
| Years of education at enrollment | 15 (12–16) | 16 (12–16) |
| IQCODE at enrollment* | 3 (3–3.063) | 3 (3–3.063) |
| Days in the hospital | 17 (8–21) | 15 (7–21) |
| Days in the ICU | 6 (3–13) | 6 (3–15) |
| Admission diagnosis (%) | ||
| Sepsis or septic shock† | 40 | 42 |
| Hepatobiliary/pancreatic surgery | 13 | 13 |
| Airway protection/upper airway obstruction | 13 | 17 |
| Acute myocardial infarction | 10 | 13 |
| Gastric surgery | 7 | 4 |
| Acute lung injury/ARDS without infection | 3 | 4 |
| CHF/cardiogenic shock | 3 | 4 |
| Orthopedic surgery | 3 | 4 |
| Vascular surgery | 3 | 0 |
| GI bleed | 3 | 0 |
| Mean SOFA score at enrollment‡ | 11 (8–13) | 12 (8–13) |
| Mean Charlson comorbidity index score at enrollment§ | 2 (0–3) | 2 (0–3) |
| At least 1 d of delirium in the ICU, % | 73 | 75 |
| At least 1 d of coma in the ICU, % | 53 | 58 |
| Days spent in the ICU | 6 (3–13) | 6 (3–15) |
| Days spent in the hospital | 17 (8–21) | 15 (7–21) |
| Ventilator support, % | ||
| Mechanical ventilation (at least 1 d) | 97 | 96 |
| Noninvasive positive pressure ventilation | 3 | 4 |
Definition of abbreviations: ARDS = acute respiratory distress syndrome; CHF = congestive heart failure; GI = gastrointestinal; ICU = intensive care unit; IQCODE = Informant Questionnaire on Cognitive Decline in the Elderly; RETURN-CCR = Returning to Everyday Tasks Using Rehabilitation Networks–Computerized Cognitive Rehabilitation investigation; SOFA = Sequential Organ Failure Assessment.
Values are expressed as median (interquartile range) unless otherwise noted.
The scores on the short version of the IQCODE range from 1 to 5, with a score of 3 indicating no change in cognition over the past 10 years, a score less than 3 indicating improvement, and a score greater than 3 indicating decline in cognition, as compared with 10 years before. A score greater than or equal to 3.6 indicates preexisting cognitive impairment.
Sepsis or septic shock diagnosis includes individuals with ARDS with infection.
Scores on the SOFA range from 0 to 24 (from 0 to 4 for each of six organ systems), with higher scores indicating more severe organ dysfunction.
Scores on the Charlson comorbidity index range from 0 to 33, with higher scores indicating a greater burden of illness.
Figure 2.
(A) Cognitive performance over time (pre– vs. post–computerized cognitive rehabilitation [CCR]) by neuropsychological domain. Change from baseline (“pre-CCR”) performance (displayed as a composite z-score) to “post-CCR” score for each cognitive domain after the 3-month trial period is shown (N = 24). Error bars indicate standard error (SE). For all four cognitive domains, a statistically significant improvement (P < 0.01) in performance was demonstrated as compared with baseline performance. (B) Improvement based on cognitive assessment, by user group (super, >1,000 levels played; high, <1,000 and >500 levels played; normal, <500 levels played). Error bars and bands are SEs.
Next, the improvement of untrained cognitive exercises (i.e., a carryover effect of training) was measured by comparing baseline (before use of computerized cognitive exercises) and post-training cognitive assessment measures (such as the Digit Span, Spatial Span, and Trail Making Test A and B). At protocol completion, ICU survivors demonstrated improvement on some of the measures of cognitive ability (Digit Span, t = −1.91, P = 0.06; Spatial Span, t = −1.39, P > 0.05; Trail Making Test A, t = −2.26, P < 0.05; Trail Making Test B, t = −0.78, P > 0.05; Trails B-A [cost], t = 0.62, P > 0.05).
In a linear regression model, the amount of improvement was positively correlated with the number of training hours, suggesting that benefits from training will transfer to general untrained cognitive abilities [F(1,22) = 3.0; P = 0.097] (see Figure 2B). Super users (or those who played at least 1,000 levels) had a significantly higher mean z-score on untrained neuropsychological tasks than normal users (<500 levels) after participating in the 12-week trial (P = 0.046). When we analyzed the improvement in the untrained neuropsychological tests by user group (super, high, normal) in a one-way ANOVA model, with group as a factor, the group effect was significant at P = 0.03. This effect was driven mainly by the difference between the super and normal groups. In ANOVA with group (super and normal) as a factor, the group difference was significant (P = 0.005). There was no significant difference between super and high (high users, <1,000 and >500 levels played) and high and normal user groups.
Discussion
In this limited proof-of-concept pilot study, we showed that survivors of critical illness with cognitive impairment had significant improvement in important neuropsychological domains and that improvement in untrained cognitive abilities was positively correlated with the amount of levels played. This investigation is the first, to our knowledge, to evaluate feasibility of a computer gaming approach of cognitive rehabilitation in survivors of nonneurologic/nontrauma critical illness. Such an approach is appealing because it is more scalable than traditional cognitive rehabilitation interventions that require intensive face-to-face interaction between patients and clinical professionals.
Although the results of the individual untrained neuropsychological assessments before and after the 12-week trial did not reach statistical significance, the study was underpowered to do so. To answer questions of efficacy, a randomized clinical trial would be required. We did, however, find a statistically significant effect of user group (super, high, and normal) on untrained neuropsychological assessments, suggesting that the most “robust” improvement in higher-order cognitive tasks occurs in the super user group.
These pilot data were designed to help generate hypotheses to shape appropriately designed randomized trials. Future work should consider a time point more proximal to ICU stay for maximal impact and clinical outcome data inclusive of “real-world” evaluations, such as handling money, driving simulators, workplace capacity, and social interactions (14). In addition, future work should explore the impact of CCR on other important outcomes of critical illness, including depression, post-traumatic stress disorder, frailty, life space, and quality of life.
Supplementary Material
Footnotes
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Halpern NA, Pastores SM. Critical care medicine beds, use, occupancy, and costs in the United States: a methodological review. Crit Care Med. 2015;43:2452–2459. doi: 10.1097/CCM.0000000000001227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halpern NA, Goldman DA, Tan KS, Pastores SM. Trends in critical care beds and use among population groups and Medicare and Medicaid beneficiaries in the United States: 2000–2010. Crit Care Med. 2016;44:1490–1499. doi: 10.1097/CCM.0000000000001722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, et al. BRAIN-ICU Study Investigators. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369:1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norman BC, Jackson JC, Graves JA, Girard TD, Pandharipande PP, Brummel NE, et al. Employment outcomes after critical illness: an analysis of the bringing to light the risk factors and incidence of neuropsychological dysfunction in ICU survivors cohort. Crit Care Med. 2016;44:2003–2009. doi: 10.1097/CCM.0000000000001849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamdar BB, Huang M, Dinglas VD, Colantuoni E, von Wachter TM, Hopkins RO, et al. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Network. Joblessness and lost earnings after acute respiratory distress syndrome in a 1-year national multicenter study. Am J Respir Crit Care Med. 2017;196:1012–1020. doi: 10.1164/rccm.201611-2327OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamdar BB, Sepulveda KA, Chong A, Lord RK, Dinglas VD, Mendez-Tellez PA, et al. Return to work and lost earnings after acute respiratory distress syndrome: a 5-year prospective, longitudinal study of long-term survivors. Thorax. 2018;73:125–133. doi: 10.1136/thoraxjnl-2017-210217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 8.Ely EW, Truman B, Shintani A, Thomason JW, Wheeler AP, Gordon S, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289:2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 9.Girard TD, Jackson JC, Pandharipande PP, Pun BT, Thompson JL, Shintani AK, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38:1513–1520. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morandi A, Rogers BP, Gunther ML, Merkle K, Pandharipande P, Girard TD, et al. VISIONS Investigation, VISualizing Icu SurvivOrs Neuroradiological Sequelae. The relationship between delirium duration, white matter integrity, and cognitive impairment in intensive care unit survivors as determined by diffusion tensor imaging: the VISIONS prospective cohort magnetic resonance imaging study. Crit Care Med. 2012;40:2182–2189. doi: 10.1097/CCM.0b013e318250acdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pandharipande PP, Pun BT, Herr DL, Maze M, Girard TD, Miller RR, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298:2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 12.Needham DM, Davidson J, Cohen H, Hopkins RO, Weinert C, Wunsch H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med. 2012;40:502–509. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
- 13.Coyle H, Traynor V, Solowij N. Computerized and virtual reality cognitive training for individuals at high risk of cognitive decline: systematic review of the literature. Am J Geriatr Psychiatry. 2015;23:335–359. doi: 10.1016/j.jagp.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Simons DJ, Boot WR, Charness N, Gathercole SE, Chabris CF, Hambrick DZ, et al. Do “brain-training” programs work? Psychol Sci Public Interest. 2016;17:103–186. doi: 10.1177/1529100616661983. [DOI] [PubMed] [Google Scholar]
- 15.Stanford Center on Longevity A consensus on the brain training industry from the scientific community 2014. Oct 20 [accessed 2017 Sep 9]. Available from: http://longevity.stanford.edu/a-consensus-on-the-brain-training-industry-from-the-scientific-community-2/
- 16.Ball K, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M, et al. Advanced Cognitive Training for Independent and Vital Elderly Study Group. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA. 2002;288:2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosen AC, Sugiura L, Kramer JH, Whitfield-Gabrieli S, Gabrieli JD. Cognitive training changes hippocampal function in mild cognitive impairment: a pilot study. J Alzheimers Dis. 2011;26:349–357. doi: 10.3233/JAD-2011-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cognitive Training Data Cognitive Training Data response letter[accessed 2017 Sep 9]. Available from: https://www.cognitivetrainingdata.org/the-controversy-does-brain-training-work/response-letter/
- 19.Jackson JC, Ely EW, Morey MC, Anderson VM, Denne LB, Clune J, et al. Cognitive and physical rehabilitation of intensive care unit survivors: results of the RETURN randomized controlled pilot investigation. Crit Care Med. 2012;40:1088–1097. doi: 10.1097/CCM.0b013e3182373115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.