Abstract
Human CO2 respiration requires rapid conversion between CO2 and HCO3−. Carbonic anhydrase II facilitates this reversible reaction inside red blood cells, and band 3 [anion exchanger 1 (AE1)] provides a passage for HCO3− flux across the cell membrane. These 2 proteins are core components of the CO2 transport metabolon. Intracellular H2O is necessary for CO2/HCO3− conversion. However, abundantly expressed aquaporin 1 (AQP1) in erythrocytes is thought not to be part of band 3 complexes or the CO2 transport metabolon. To solve this conundrum, we used Förster resonance energy transfer (FRET) measured by fluorescence lifetime imaging (FLIM-FRET) and identified interaction between aquaporin-1 and band 3 at a distance of 8 nm, within the range of dipole–dipole interaction. Notably, their interaction was adaptable to membrane tonicity changes. This suggests that the function of AQP1 in tonicity response could be coupled or correlated to its function in band 3-mediated CO2/HCO3− exchange. By demonstrating AQP1 as a mobile component of the CO2 transport metabolon, our results uncover a potential role of water channel in blood CO2 transport and respiration.—Hsu, K., Lee, T.-Y., Periasamy, A., Kao, F.-J., Li, L.-T., Lin, C.-Y., Lin, H.-J., Lin, M. Adaptable interaction between aquaporin-1 and band 3 reveals a potential role of water channel in blood CO2 transport.
Keywords: anion exchanger-1, erythrocyte, FLIM-FRET, Miltenberger subtype III
More than two-thirds of CO2 in human circulation is in the form of soluble bicarbonate (HCO3−). Carbonic anhydrase II (CAII) is the main enzyme that facilitates constant exchange of CO2(g) ⇌ HCO3−(aq) (1, 2). Physiologically, the rate-limiting factor for this reversible process CO2(g) + H2O ⇌ HCO3−(aq) + H+(aq) inside human erythrocytes is not CAII, but band 3, a bidirectional Cl−/HCO3− transporter protein also known as anion exchanger 1 (AE1) (2–5). This is due to the fact that HCO3− conductance through band 3 on the red blood cell (RBC) membrane is 10 times slower than the rate of CAII enzymatic activity (2, 6). For HCO3−(aq)/CO2(g) exchange to take place inside RBCs, HCO3− must first enter or exit erythrocytes via band 3, whereas CO2 primarily passes through the RBC membrane by diffusion and perhaps also by Rh-associated glycoprotein (RhAG), aquaporin 1 (AQP1), or both (7–10). H2O, the other substrate necessary for CO2/HCO3− conversion inside erythrocytes, enters or exits RBCs through AQP1 (11, 12).
However, erythrocyte AQP-1 has not been implicated as part of the band 3 complex or the CO2 transport metabolon (13). Despite that AQP1 expresses abundantly in erythrocytes (160,000–200,000 molecules per human RBC), its function in RBCs remains unclear (14).
We stumbled over this conundrum when we uncovered significantly higher band 3 expression in human erythrocytes expressing a type of glycophorin B-A-B hybrid protein known as Miltenberger subtype III (Mi.III/GP.Mur) (15). This Mi.III erythrocyte phenotype is important in blood transfusion in Southeast Asia (prevalence between 2 and 10%), but is rare in other parts of the world (16, 17). GP.Mur (the protein entity of Mi.III) is the product of homologous gene recombination from glycophorin B (GYPB) and its homolog glycophorin A (GYPA) and presents as a glycophorin B-A-B fusion protein (18, 19). The inserted piece of glycophorin A (GPA) sequence in GP.Mur enables GP.Mur to function like GPA to enhance band 3 surface expression (15, 20). In a recent human study, we found that healthy persons with the Mi.III blood type bear a larger capacity for CO2 respiration and superior respiratory tolerance after they were given a moderate exercise (step) challenge (21). Intriguingly, we also observed substantially more protein–protein interaction between AQP1 and band 3 in erythrocyte samples from subjects with Mi.III phenotypes (15).
To probe into the nature of AQP1–band 3 interaction in RBCs, we used the biochemical and biophysical approaches coimmunoprecipitation (co-IP) and fluorescence-lifetime imaging microscopy–Förster resonance energy transfer (FLIM-FRET). Co-IP identified protein components that interacted both directly and indirectly. FRET measured by FLIM further validated protein–protein interaction at single-molecule levels (22). FRET results from delocalized excitation and transfer of excitation energy in a dipole–dipole interaction (23). It may occur when the distance between interacting components is within the range of dipole–dipole interaction, or 10 nm (24, 25). FLIM-FRET allows live cell measurements, and so we were able to assess physiologic relevance of their protein–protein interaction. In the current study, we identified adjustable physical interaction between AQP1 and band 3 in human erythrocytes. Our findings suggest an active role of AQP1 in blood CO2 transport.
MATERIALS AND METHODS
Ethics statement
The study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Institutional Review Board (IRB) of Taiwan Mackay Memorial Hospital (MMH) (MMH-IRB registration number: 13MMHIS273). Written informed consent was obtained from all participating subjects.
Co-IP and Western blot analysis
Mi.III/GP.Mur RBC phenotype was serologically determined by anti-Mur, anti-Hil, anti-Anek, and anti-Mia antisera, and confirmed by DNA sequencing, as described previously (26). The erythrocyte membrane fraction (ghosts) was obtained by hypotonic lysis of RBCs. RBC ghosts were then solubilized in PBS-based lysis buffer, containing 1% NP-40, 1% CHAPS, 0.025% SDS, and Complete Protease Inhibitor Cocktail (Roche, Basel, Switzerland), on ice. Ghost lysates were subjected to Western blot analysis or co-IP.
Co-IP experiments used antibody-crosslinked Dynabeads (Thermo Fisher Scientific, Waltham, MA, USA) to capture protein complexes. Anti-band 3 mAb BRIC170 [Bristol Institute for Transfusion Sciences (BITS), Bristol, United Kingdom] and anti-AQP1 mAb (clone 1/A5F6; Bio-Rad, Hercules, CA, USA) both recognize the cytoplasmic regions of band 3 and AQP1 (Fig. 1A), and they were individually chemically crosslinked to Dynabeads with dimethyl pimelimidate. Antibody-coated Dynabeads were then mixed with ghost lysates at 4°C for 12–16 h, followed by extensive high-salt washes and elution of bound proteins. The eluents were analyzed by immunoblot.
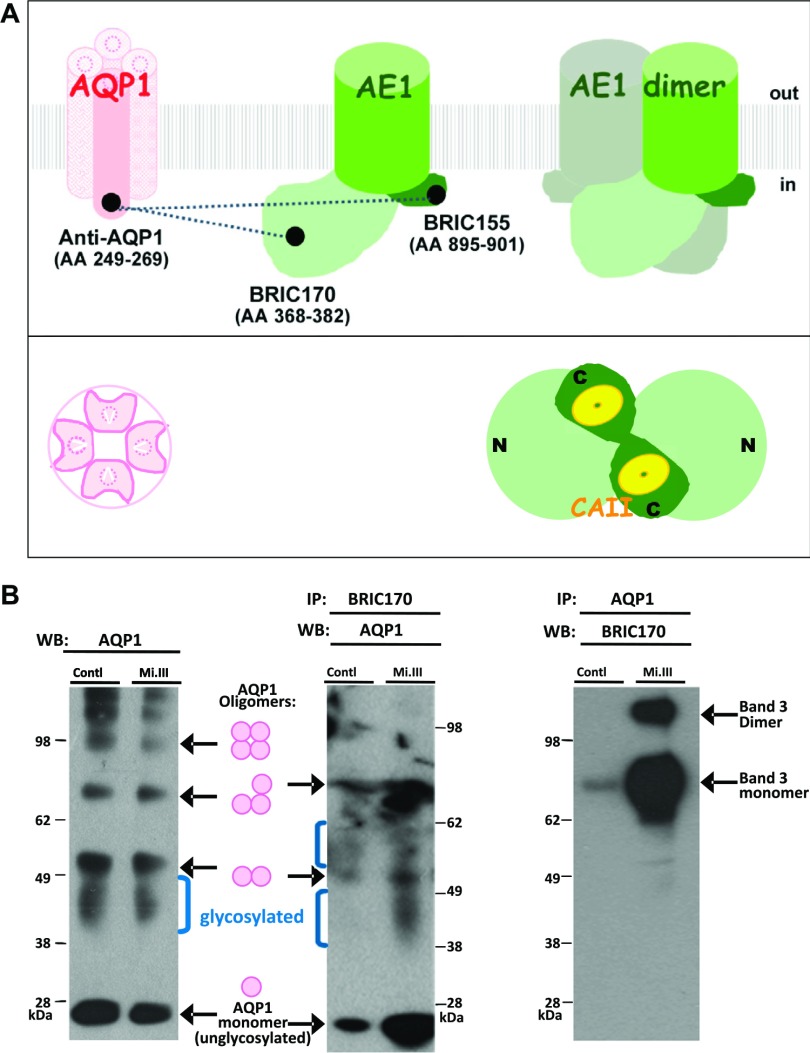
Figure 1.
Protein–protein interaction between AQP1 and band 3 in human RBCs. A) A simple illustration of AQP1 and band 3 viewed from the transmembrane cross-section (side view) and from inside an erythrocyte (bottom view). mAbs used in this study are labeled, with their corresponding amino acid epitopes indicated in parentheses. In the band 3 monomer, light green marks the N-terminal cytoplasmic domain of band 3 (aa 1–408), including a flexible linker structure (aa 357–408) connecting to the transmembrane domain; dark green: the short, C-terminal, cytoplasmic region of band 3 (27–29). The transmembrane domain of band 3 (green cylinder), responsible for anion conductance, connects the N- and C-terminal cytoplasmic regions. Dotted lines connecting the epitopes of AQP1 and band 3 represent the molecular interactions tested by FLIM. The bottom view shows an AQP1 tetramer and a band 3 dimer. Although AQP1 exists as a homotetramer, each AQP1 monomer contains a passage for water (30). On the other hand, the Cl−/HCO3− bidirectional transport requires dimeric composition of AE1. N, N-terminal; C, C-terminal cytoplasmic domains of band 3. Yellow: CAII, binding the C-terminal, cytoplasmic region of band 3. B) Co-IP experiments demonstrated stronger AQP1-band 3 interaction in Mi.III than in non-Mi.III (control) erythrocyte membrane. Each group (control/Mi.III) contained mixed ghost samples from 6 individual donors. Left: Western blot analysis with an anti-AQP1 mAb (clone 1/A5F6) showed similar expression levels of AQP in Mi.III and non-Mi.III erythrocytes. Non- and glycosylated AQP1 monomer, dimer, trimer, and tetramer (AQP1 oligomers) were labeled in an AQP1 Western blot. The molecular mass of the nonglycosylated AQP1 monomer was ∼26 kDa, and the molecular masses of glycosylated AQP1 monomers ranged from 34 to 40 kDa (31–33). Middle: co-IP, with anti-band 3 mAb (BRIC170), pulled down much more AQP1 in Mi.III ghosts than in non-Mi.III ghosts. Right: Co-IP by anti-AQP1 mAb pulled down much more band 3 protein in the Mi.III ghosts, compared to the control.
Preparation of RBC vesicles
To prepare erythrocyte right-side-out vesicles (ROVs) and inside-out vesicles (IOVs), we followed the methods described by Marchesi and Palade (34) and Bennett (35). In brief, fresh human RBCs were disrupted in ice-cold hypotonic buffer [5 mM Tris-HCl (pH 8), 1 mM EDTA, and protease inhibitor cocktail], followed by centrifugation to obtain the membrane fraction. RBC ghosts were then resuspended in high-salt buffer [50 mM Tris-HCl (pH 8), 500 mM NaCl, and 1 mM EDTA] to allow resealing in the same orientation as intact erythrocytes. These resealed vesicles were ROVs, which were validated by positive staining of the anti-band 3 mAb BRAC17 or -18 (BITS), which targets an extracellular region of band 3, and by negative staining of BRIC170, which targets the cytoplasmic region of band 3.
To make IOVs, previously prepared ROVs were washed with ice-cold spectrin extraction buffer (0.2 mM Na EDTA, pH 8), which disrupted the ROVs. To dissociate spectrins and endovasculature, the membrane fraction was incubated with spectrin extraction buffer containing 0.2 mM DTT and protease inhibitor cocktail at 37°C for 20 min and then washed. To further remove nonintegral membrane proteins, such as ankyrin, RBC vesicles were incubated in potassium iodide (KI) extraction buffer [1 M KI, 7.5 mM Na phosphate, 1 mM Na EDTA, 1 mM DTT (pH 7.5), and the protease inhibitor cocktail] at 37°C for 20 min, followed by washes. The resultant vesicles were IOVs, which were validated by positive staining of BRIC170 and negative staining of BRAC17 or BRAC18. For clear visualization, IOVs and ROVs were stained with 2 μM of lipid-bound fluorophore Di-8-ANEPPS (Thermo Fisher Scientific). Freshly prepared vesicles could be stored at 4°C for a day in PBS-based storage buffer containing 2 mM DTT and the protease inhibitor cocktail, before imaging experiments.
Immunofluorescence labeling of RBC vesicles
Anti-AQP1 antibody (1/A5F6) was chemically conjugated to fluorophore Alexa Fluor 568 with the Alexa Fluor 568 Antibody Labeling kit (Thermo Fisher Scientific). Similarly, band 3 mAbs (BRIC170 and BRIC155, both from BITS) were each conjugated to Alexa Fluor 488 by using the Alexa Fluor 488 Antibody Labeling kit (Thermo Fisher Scientific). RBC vesicles were labeled with BRIC170-Alexa Fluor 488 conjugate alone or BRIC155-Alexa Fluor 488 conjugate alone, or together with anti-AQP1-Alexa Fluor 568 conjugate. Freshly labeled vesicles were suspended in HBSS [137.9 mM NaCl, 5.33 mM KCl, 0.441 mM KH2PO4, 4.17 mM NaHCO3, 0.338 mM Na2HPO4, 5.56 mM glucose, and 20 mM HEPES (pH 7.5)] for immediate imaging studies. Confocal imaging was captured by a TCS SP equipped with an argon/krypton laser (Leica Microsystems, Wetzlar, Germany). Fluorescence colocalization in the doubly labeled samples was estimated using the measure colocalization application in the MetaMorph program (Molecular Devices, Sunnyvale, CA, USA).
FLIM recording and calculation
Immunofluorescence-labeled RBC vesicles were loaded into a microscope slide containing 6 parallel channels (μ-slide-VI-Flat; Ibidi, Martinsried, Germany). FLIM measurements were obtained primarily with the TCS-SP5-AOBS-MP microscope (Leica), which was equipped with single-molecule detection (SMD) and a multiphoton laser (680–1080 nm). Alexa Fluor 488, which labeled band 3, was designated the FRET donor; Alexa Fluor 568, which labeled AQP1 was the FRET acceptor. In the 2-photon setting, Alexa Fluor 488 was excited at 900 nm, and its emission collected at 500–560 nm. Calibration using Alexa Fluor 488 suspended in PBS, HBSS, or half-Cl− HBSS, all gave the same reference fluorescence lifetime, ∼3.3 ns. For each FLIM recording of fresh RBC vesicles, 500 photons per pixel were collected.
To perturb Cl− fluxes mediated by band 3, HBSS was modified to contain only half the Cl− content [half-Cl− HBSS: 69 mM NaCl, 5.33 mM KCl, 0.441 mM KH2PO4, 4.17 mM NaHCO3, 0.338 mM Na2HPO4, 69 mM Na gluconate, 5.56 mM glucose, and 20 mM HEPES (pH 7.5)]. In this formulation, gluconate (an impermeant anion for band 3) was supplemented to replace Cl− in equal moles in the composition of half-Cl− HBSS, in order to minimize osmotic differences resulting from Cl− depletion. Immunofluorescence-labeled vesicles were mixed in half-Cl− HBSS, before loading into a μ-slide channel. On the other hand, perturbation of H2O flux was performed on vesicles suspended in HBSS that had been loaded into a μ-slide channel and scanned for fluorescence lifetimes on the microscope stage. Additional double-distilled (dd)H2O was then gently injected into a μ-slide channel prefilled with erythrocyte vesicles in HBSS for further FLIM measurements.
We analyzed FLIM data with the SimFCS phasor software developed by Dr. Enrico Gratton (University of California, Irvine, Irvine, CA, USA) (36). Individual lifetime measurements were compiled and analyzed by 2-sample Student’s t tests. The efficiency of fluorescence energy transfer from the donor (D) to the acceptor (A) was estimated by using the phasor plot program or calculated as follows (Eq. 1) (22):
where E is the percentage efficiency of energy transfer; τDA is the donor lifetime in the presence of the acceptor; and τD is the lifetime of the donor alone.
The molecular distance (r) between the donor and the acceptor in a FRET pair could then be estimated with Eq. 2:
where R0 is Förster distance or Förster radius. For our FRET pair composed of Alexa Fluor 488 and Alexa Fluor 568, R0 is 6.2 nm (37).
RESULTS
Strong AQP1–band 3 interaction identified in GP.Mur erythrocytes
In this study, we tested whether AQP1 could interact with band 3 directly. In our previous proteomic study, in which we dissected band 3–glycophorin complexes in human RBCs (15), we uncovered a unique protein–protein interaction between band 3 and AQP1 in Mi.III RBCs (15). Forward immunoprecipitation using BRIC170 (an anti-band 3 mAb) pulled down much more AQP1 in Mi.III than in the control RBC membrane (Fig. 1B). Reverse co-IP using anti-AQP1 antibody also pulled down significantly more band 3 from Mi.III cells. AQP1 did not appear to be substantially involved in band 3 protein complexes in the non-Mi.III RBCs (Fig. 1). Although the expression levels of band 3 were 25% or more in Mi.III than non-Mi.III ghosts (15, 38), the expression levels of unglycosylated and glycosylated AQP1 monomers and oligomers were similar (Fig. 1B).
In an AQP1 tetramer, only 1 subunit is N-glycosylated (31–33). AQP1 glycosylation did not seem to be affected by Mi.III expression (Fig. 1B). However, anti-band 3 antisera were able to coimmunoprecipitate more glycosylated AQP1 in the Mi.III samples. This finding hints at an involvement of N-glycans in AQP1–band 3 interaction in Mi.III RBCs, which probably resulted from differential expressions and complex organization of band 3 in Mi.III erythrocytes (20).
AQP1–band 3 colocalization in erythrocyte vesicles
To test whether AQP1 could interact with band 3, we chose FLIM-FRET for its high sensitivity and capacity to measure live samples. Initially, for FLIM measurements, we attempted to label AQP1 and band 3 directly with antibody–fluorophore conjugates in intact RBCs. Those lifetime recordings, however, revealed strong interference from hemoglobin autofluorescence. To eliminate the source of autofluorescence, we then made ghosts and had them resealed into vesicles of comparable sizes to the RBCs: ROVs with diameters of 5.4–7.3 μm and IOVs with diameters of 5.4–7.9 μm (Fig. 2A). We also validated their membrane topology by using mAbs against cytoplasmic and extracellular regions of band 3 (i.e., clones BRIC170 and BRAC17, respectively). The membrane topology of ROVs was identical to that of RBCs, and the topology of IOVs was in the inverse orientation, as expected. However, after testing several commercially available anti-AQP1 mAbs, we could not find one capable of labeling AQP1 on the surface of ROVs. Only an anti-AQP1 mAb (clone 1/A5F6; Bio-Rad) that targets a cytoplasmic region of AQP1 bound well to the surface of IOVs (Fig. 1A). We thus used RBC IOVs for the following imaging experiments.
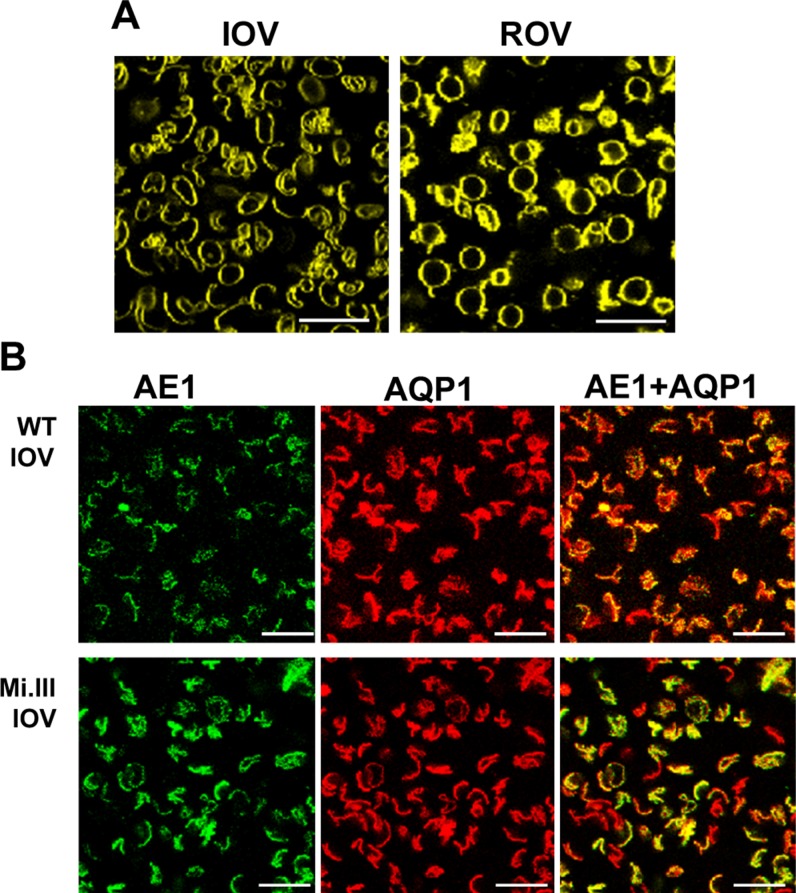
Figure 2.
Confocal imaging showed colocalization of AQP1 and band 3 on the surface of erythrocyte vesicles. A) Human RBC ghosts were resealed to form IOVs or ROVs. These vesicles were stained with the lipid-bound fluorophore Di-8-ANEPPS for visualization. B) Band 3 on the surface of IOVs was labeled with BRIC170-Alexa Fluor 488 (green fluorescence) and anti-AQP1-Alexa Fluor 568 (red fluorescence). Immunofluorescence-labeled IOVs from non-Mi.III (WT) and Mi.III RBC-IOVs showed substantial expressions of band 3 and AQP1. Various shades of orange and yellow fluorescence indicate different degrees of colocalization between band 3 and AQP1 on the surface of IOVs. Scale bars, 20 μm.
Double labeling of RBC-IOVs with BRIC170-Alexa Fluor 488 conjugate and anti-AQP1-Alexa Fluor 568 conjugate showed variable degrees of spatial overlaps (Fig. 2B). Noticeably, IOVs prepared from Mi.III RBCs exhibited higher band 3 expression, as well as higher degrees of colocalization of AQP1 and band 3, than did IOVs prepared from non-Mi.III (wild-type, WT) RBCs [63% colocalization (Mi.III) vs. 41% (non-Mi.III)]. These confocal imaging data supported previous co-IP results (Fig. 1B).
AQP1–band 3 physical interaction measured by FLIM-FRET
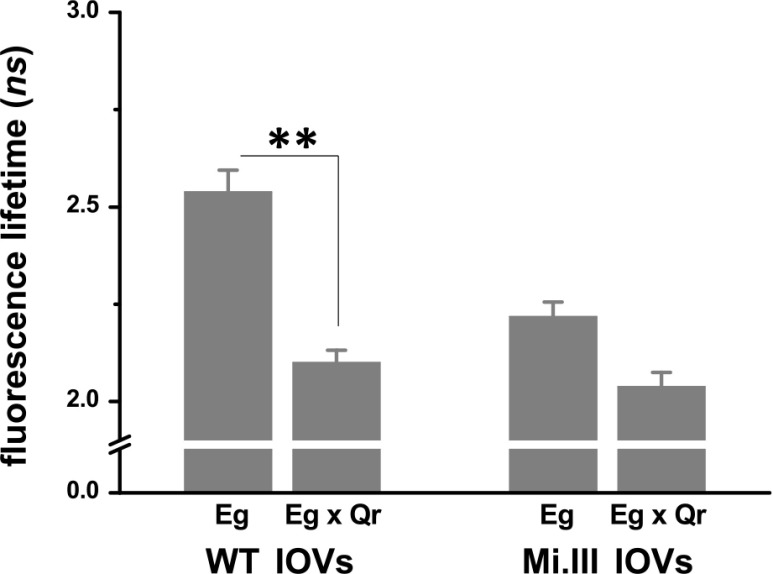
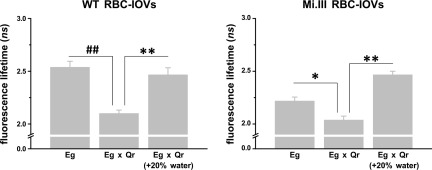
Next, we measured AQP1–band 3 interaction at single-molecule levels by FLIM-FRET. If the distance between the 2 probes (Alexa Fluor 488 and 568) was within 10 nm, FRET could occur and result in decreased lifetimes of the FRET donor (Alexa Fluor 488). Compared with IOVs labeled only with the anti-AE1-Alexa Fluor 488 conjugate (Eg), IOVs colabeled with anti-AQP1-Alexa Fluor 568 conjugate (Eg × Qr, AQP1 with red fluorescence) showed significantly lower fluorescence lifetimes of Alexa Fluor 488. For validation, we performed 2 sets of FLIM recordings with 2 different mAb conjugates as the FRET donors (Fig. 1A): Alexa Fluor 488 conjugates with BRIC170 (anti-N-terminal AE1 mAb) and with BRIC155 (anti-C-terminal AE1 mAb). In both sets of the lifetime recordings, colabeling with the FRET Qr acceptor significantly reduced the lifetimes of the FRET donor Eg: the BRIC170-Alexa Fluor 488 conjugate dropped from 2.54 ± 0.06 to 2.10 ± 0.03 ns in the presence of Qr (Fig. 3B), whereas BRIC155-Alexa Fluor 488 conjugate decreased from 3.11 ± 0.06 to 2.66 ± 0.17 ns in the presence of Qr (Fig. 3C). In the FRET pair using BRIC170-conjugated Alexa Fluor 488 as the donor, the energy transfer efficiency (%E) from FRET donor to FRET acceptor was estimated as 17.3% on average, which corresponded to a molecular distance (r) of 8 nm. In the other FRET pair using BRIC155-Alexa Fluor 488 conjugate as the donor, %E was 14.5%, and the distance between Eg and Qr was ∼8.3 nm. The occurrence of FRET in both wild-type and Miltenberger RBC membrane indicates that the molecular distance between band 3 and AQP1 was generally within 10 nm, the upper limit for the range of dipole–dipole interaction (Fig. 3B, C).
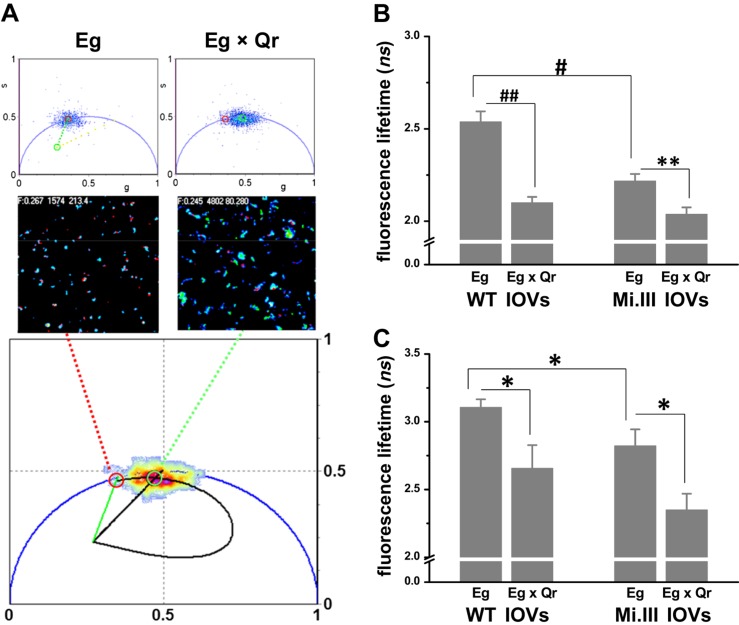
Figure 3.
FLIM-FRET revealed molecular interaction between AQP1 and band 3 on the RBC membrane. BRIC170-Alexa Fluor 488 conjugate labeled N-terminal band 3/AE1 with green fluorescence (Eg; the FRET donor). Anti-AQP1-Alexa Fluor 568 conjugate labeled AQP1 with red fluorescence (Qr; the FRET acceptor). A) Top: the 2 phasor plots showed fluorescence lifetimes (τ) of Alexa Fluor 488 emitted from IOVs, singly labeled with FRET donor Eg or doubly labeled with FRET donor and acceptor (Eg × Qr). Middle: their lifetime images. The bottom phasor plot estimated the degree of energy transfer from Eg to Qr using a trajectory line crossing Eg (red circle; 2.7 ns) to Eg × Qr (green circle; 2.0 ns) (36). B) FLIM-FRET experiments using BRIC170-Alexa Fluor 488 conjugate as the FRET donor demonstrated AQP1–band 3 interaction. Significant decreases in fluorescence lifetimes of Alexa Fluor 488 from Eg singly labeled to Eg × Qr doubly labeled IOVs indicated the occurrence of FRET. Mi.III IOVs showed FRET, but to a lesser degree than WT IOVs. Each group was compiled from 13 to 27 independent sample measurements (mean ± sem). C) FLIM-FRET experiments using BRIC155-Alexa Fluor 488 conjugated as the FRET donor also showed AQP1–band 3 interaction. Eg represents labeling of band 3 with BRIC155-Alexa Fluor 488 conjugate; Qr represents labeling with the same probe. Significant differences in the lifetimes of Alexa Fluor 488 between Eg and Eg × Qr were again observed. Their lifetime differences were similar in both Mi.III and non-Mi.III IOVs. Each group was compiled from 7 to 15 samples. *P < 0.05, **P < 0.005; #P < 0.0001, ##P < 10−6.
In the absence of the FRET acceptor Qr, we observed a significantly lower mean lifetime of Alexa Fluor 488 conjugated with BRIC170 in Mi.III membrane (2.22 ± 0.04 ns) vs. mean lifetime in the non-Mi.III membrane (2.54 ± 0.06 ns). Labeling by BRIC155-Alexa Fluor 488 conjugate also showed lower lifetimes in Mi.III IOVs than in non-Mi.III IOVs: 2.82 ± 0.12 ns vs. 3.11 ± 0.06 ns (Fig. 3C). The significant differences in the lifetime of the FRET donor alone (Eg) in Mi.III vs. non-Mi.III IOVs were the result of Eg homo-FRET, as Mi.III RBCs contain 25% or more band 3 than did non-Mi.III cells (15). Moreover, the significant differences in FLIM-FRET in Mi.III vs. non-Mi.III membrane reflected their structural differences in band 3 complex organization (Fig. 3) (20, 39).
AQP1–band 3 interaction not affected by band 3 permeation
To test whether AQP1–band 3 interaction in the RBC membrane could be physiologically relevant to their individual functions, we challenged their individual transport activities in the following FLIM-FRET experiments. Band 3-mediated Cl−/HCO3− exchange is bidirectional and is solely driven by the concentration gradients of its permeable anions across the cell membrane. We reduced Cl− conductance of band 3 by immersing IOVs in a modified HBSS containing only 50% Cl− of the original content (half-Cl− HBSS). Reduction of extracellular Cl− drove efflux of intracellular Cl− to a new equilibrium, which redistributed HCO3− across the membrane and changed the kinetics of anion exchange. RBC IOVs also shrank slightly in half-Cl− HBSS, because of Cl− efflux followed by water efflux. When experimenting in half-Cl− HBSS (Fig. 4), the lifetime differences between the donor alone (Eg) and the donor in the presence of the acceptor (Eg × Qr) remained significant (Eg: 2.40 ± 0.04 ns; Eg × Qr: 2.17 ± 0.03 ns), and corresponded to a molecular distance of 9 nm between band 3 and AQP1. This result indicates that reduced AE1-mediated Cl−/HCO3− transport did not drastically affect band 3 interaction with AQP1.
Figure 4.
Changes in Cl− flux did not affect AQP1-AE1 interaction in erythrocyte IOVs. In this set of experiments, IOVs singly labeled with BRIC170-Alexa Fluor 488 conjugate, or colabeled with anti-AQP1-Alexa Fluor 568, were suspended in half-Cl− HBSS for FLIM measurements. Despite the reduced Cl−, fluorescence lifetimes of FRET donor Eg decreased significantly in the presence of the acceptor Qr. Each group was compiled from 6 to 12 sample measurements (means ± sem). **P < 0.001.
AQP1–band 3 interaction disrupted by hypotonic conditioning
To test whether AQP1–band 3 physical coupling could be affected by AQP functionality, we forced water transport by adding extra 20% of ddH2O to a slide channel prefilled with IOVs immersed in HBSS. This corresponded to a change in solution osmolarity from 398 to 248 mOsM/kg. We observed significantly increased lifetimes of Alexa Fluor 488 in Eg × Qr-colabeled IOVs when ddH2O was added (Eg × Qr in HBSS: 2.10 ± 0.03 ns; Eg × Qr in ddH2O-supplemented HBSS: 2.47 ± 0.07 ns) (Fig. 5). IOVs made from Mi.III RBCs exhibited similar degrees of distance between band 3 and AQP1 in this hypotonically challenged condition. By increasing the concentration of water that stimulated water transport, the lifetime of FRET donor Eg in the presence of the acceptor Qr became as high as the lifetime of the FRET donor alone, indicating parting of AQP1 from band 3 protein complexes.
Figure 5.
Hypotonic conditioning dissociated AQP1 interaction from band 3 in erythrocyte IOVs. RBC-IOVs colabeled with BRIC170-Alexa Fluor 488 and anti-AQP1-Alexa Fluor 568 conjugates were first suspended in HBSS and loaded into a microscope slide channel. Additional ddH2O was then injected into the channel. When the milieu changed to HBSS containing 20% more ddH2O, fluorescence lifetimes of Eg in the presence of acceptor Qr (Eg × Qr) were significantly higher than in HBSS. Eg × Qr interaction in Mi.III IOVs was similarly affected by hypotonic conditioning. Each group was compiled from 3 to 27 sample measurements (means ± sem). *P < 0.05, **P < 0.001; ##P < 10−6.
DISCUSSION
In this study, we uncovered AQP1 interaction with band 3—the central component of the CO2 transport metabolon (Figs. 1–3). To probe into the physiologic role of AQP1–band 3 interaction, we disturbed their individual transport activities and measured their physical interaction by FLIM-FRET. We found that AQP1–band 3 interaction in human erythrocytes was adaptable to changes of concentration gradients for permeable anions and H2O (Figs. 4–5). The latter changes in our experiments (Fig. 5) directly lowered solution osmolarity and increased intracellular water content, resulting in segregation of AQP1 and band 3 complexes. This transient nature of AQP1–band 3 interaction could explain the residual protein–protein interaction observed in co-IP experiments (Fig. 1) and the previous seemingly negative results (13). In the fluorescence recovery after photobleaching (FRAP) experiments reported by Cho and coworkers (13), immobilization of band 3 by anti-band 3 mAb does not immobilize AQP1 to the same degree as for GPA, indicating that AQP1 and band 3 do not form “stable” protein complexes such as GPA and band 3. In their study, the lateral diffusion coefficients and the fractional mobility of AQP1 both decreased when either band 3 or GPA on the erythrocyte membrane was immobilized (13). Because FRAP is an intensity-based measurement, these phenomena likely resulted from transient or partial structural linkages between AQP1 and band 3 and do not contradict our data.
From the perspective of protein biosynthesis, AQP1 protein expression and N-glycosylation are both affected in human and mouse band 3-deficient RBCs (40). As N-glycosylation is initiated in the endoplasmic reticulum (41), the structural association between band 3 and AQP1 likely begins as early as in the endoplasmic reticulum. In the other end of the spectrum of band 3 expression, Mi.III RBCs express more band 3. Our recent human study of healthy people with Mi.III found faster CO2 respiration in the Mi.III-bearing subjects than in the controls, when they were physically challenged (21). We previously attributed this respiratory benefit only to the higher band 3 expression in people with Mi.III (15, 21). Our current finding showed that band 3–AQP1 interaction was much stronger in Mi.III RBCs than in the control cells (Fig. 1). Thus, current evidence from several Mi.III studies points to the finding the function of band 3 in CO2 respiration involves AQP1.
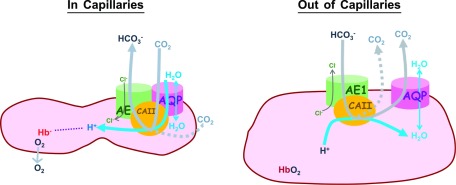
What is the potential physiologic role of the adaptable interaction between AQP1 and band 3? Similar to this work, binding between CAII and band 3 is also influenced by concentration gradients of the substrates for CO2/HCO3− conversion (6). Interaction between AQP1 and CAII also enhances water transport (42). All these findings identify AQP1 as a functionally active component of the CO2 respiratory metabolon (2). The concept of the CO2 respiratory metabolon was developed from important discoveries that demonstrated that CAII–band 3 structural interaction facilitates their individual functions in blood CO2 respiration (2, 3, 5, 6, 40, 43). Within the metabolon, individual reactions carried out by CAII and band 3 (i.e., CO2 ⇌ HCO3−; HCO3−in ⇌ HCO3−out) are “channeled” or spatially linked, thus minimizing the loss of substrates or intermediates and maximizing the rate of physiologic CO2 exchange (44). Similarly, AQP1–band 3 interaction may support channeling of water into CO2 conversion as RBCs circulate to the capillaries (Fig. 6).
Figure 6.
A proposed model describes the mobile localization of AQP1 relative to band 3 on human erythrocyte membrane. Arrows: the main direction of fluxes of ions, water, and CO2 across the human RBC membrane. Band 3, which transports HCO3− across the cell membrane and CAII, which facilitates CO2(g)/HCO3−(aq) conversion, are 2 core components of the CO2 transport metabolon. Left: when erythrocytes circulate and squeeze through systemic capillaries in as short a time as 50 ms (45), CO2 metabolite generated by tissues enters RBCs via diffusion (dashed arrow) and via passage through AQP1 (solid arrow), as indicated in the model (8–10, 46), Intracellular CO2 is converted into HCO3− by CAII. The other reaction product, H+, is stabilized by deoxyhemoglobin as O2 exits from the RBCs. In systemic capillaries, RBCs undergo rheological changes and increased intracellular CO2 conversion. Water channel AQP1 physically interacts with band 3/CAII complexes to facilitate hydration of CO2. Right: when erythrocytes leave systemic capillary beds, they resume cell shapes and volume with water influx. As erythrocytes circulate toward the lungs for CO2 expiration, blood HCO3− is converted into CO2 and H2O. When RBCs are well hydrated, AQP1 is segregated from band 3/CAII complexes.
In slightly dehydrated RBC vesicles, AQP1–band 3 interaction persisted (Fig. 4). In well-hydrated RBC vesicles, AQP1 became structurally separated from band 3 complexes (Fig. 5). Although the amount of water inside the RBCs for hydration of CO2 is not limited, because protein concentrations inside RBCs are higher than in plasma, osmotic pressure is slightly higher inside RBCs than outside: the concentration of water inside RBCs [H2O]in ∼17 M, and the concentration of water in plasma [H2O]out ∼52 M (detailed calculation in the Supplemental Data) (47). There are ∼2 × 1012 H2O molecules inside an RBC, and intraerythrocytic CO2 conversion/min consumes ∼2.4 × 108 H2O molecules per RBC (detailed calculation in the Supplemental Data) (48–50). When RBCs squeeze through systemic capillaries, [H2O]in is predicted to be lower than 17 M, which further increases the concentration gradient of water, or the osmotic pressure, across the RBC membrane during the capillary transit time (1–3.5 s) (51, 52).
To assess whether AQP1–band 3/CAII coupling could be meaningful for intraerythrocytic CO2 conversion, we estimated the changes of intraerythrocytic reaction rates, with or without AQP1–band 3 functional coupling (detailed calculation in the Supplemental Data):
Consider the reaction taking place inside RBCs as cells circulate from arteries to systemic capillaries (Eq. 3):
Forward reaction rate (Rf) = Kf [CO2]in [H2O]in, where Kf is the forward-reaction coefficient attributed mainly by the enzymatic activity of CAII. Next, we calculated whether AQP1–band 3 coupling could affect the reaction rate or Kf.
We found that as RBCs enter from systemic arterioles to capillaries, the change in the reaction rate for intraerythrocytic CO2 conversion is as follows (Eq. 4):
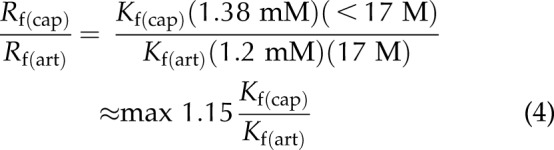 |
If Kf does not account for AQP1–band 3 functional coupling, then Kf(cap)/Kf(art) ∼ 1, and the intracellular reaction rate would increase a maximum of 15% when RBCs enter systemic capillaries. On the other hand, if AQP1 and band 3 are structurally and functionally coupled in systemic capillaries and uncoupled or less coupled in arteries, it results in larger Kf(cap) than Kf(art). Thus, Rf(cap)/Rf(art) > 1.15, indicating that the reaction rate will increase more than 15% when RBCs enter the systemic capillary bed.
Is AQP1–band 3 transient coupling functionally meaningful? When RBCs squeeze through systemic capillaries, the water concentration inside RBCs [(H2O)in] drops transiently. If AQP1–band 3 coupling is not physiologically meaningful [Kf(cap)/Kf(art)∼1], a 10% decrease in [H2O]in (e.g., 17 M→15 M) would yield (Eq. 5):
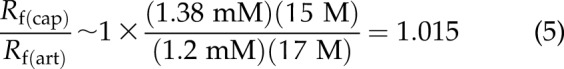 |
In the example above, a 10% drop in [H2O]in theoretically would result in 14% reduction of the reaction rate; a further decrease in [H2O]in would make Rf (cap) smaller than Rf (art). On the other hand, if osmotically driven AQP1–band 3 coupling promotes intraerythrocytic CO2 conversion, then Kf(cap)/Kf(art) > 1, which could compensate for the negative effect from erythrocyte size reduction [or (H2O)in reduction] on the reaction rate.
Circulating RBCs become more dehydrated toward the end of systemic capillaries, and RBCs return to a hydrated state as they leave the zone. Together with our current finding that AQP1–band 3 interaction diminished in well-hydrated RBCs (Fig. 5), the reaction coefficients for intraerythrocytic CO2 conversion in systemic capillaries [Kf (cap)] vs. that in larger blood vessels [Kf (art)] could be adjusted by varying degrees of RBC hydration during circulation. Moreover, although the enzymatic activity of CAII increases the reaction rate by 13,000–25,000-fold (46) and is the dominant contributor for Kf, we think that osmotically driven AQP1–band 3 coupling/decoupling most likely serves as a safeguard mechanism for the essential physiologic process.
We propose a model to describe the role of AQP1 in blood CO2 respiration (Fig. 6). We learned from this study that band 3 interacts with AQP1, even when band 3-mediated anion transport was slowed down. AQP1 and band 3 were segregated when RBCs were well hydrated. In this model, when the demand for CO2→HCO3− conversion is high in systemic capillary beds, physical interaction between AQP1 and band 3/CAII allows channeling of substrate H2O to the CO2 transport metabolon. As RBCs leave the capillary zones, AQP1 becomes distanced from band 3/CAII. Mobile AQP1 responds more effectively to osmotic stress and cell volume changes in the absence of spatial restriction imposed by the band 3–cytoskeletal network. Osmotically driven, alternate AQP1–band 3 coupling and decoupling may support timely and scalable removal of CO2 metabolites during circulation (45).
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Dr. John Yu (Chang Gung University, Taoyuan City, Taiwan and Academia Sinica-Taiwan, Taipei, Taiwan) and Dr. Hsin-Hung Lin (University of California, San Diego, San Diego, CA, USA and Academia Sinica-Taiwan) for support with the FLIM instrumentation, Dr. Yu-Jun Lai (MMH) for support with the MetaMorph software. This work was supported by Grant EX103-10122SI from National Health Research Institute (NHRI) of Taiwan; Grant 103-2320-B-195-001-MY3 from the Taiwan Ministry of Science & Technology (MOST); and Mackay Memorial Hospital Grants 104-49, 105-52, and 106-23 (to K.H.). K. H. and T.-Y.L. share equal first authorship. The authors declare no conflicts of interest.
Glossary
- AE1
anion exchanger 1 (band 3)
- AQP1
aquaporin 1
- band 3
anion exchanger 1 (AE1)
- BITS
Bristol Institute for Transfusion Sciences
- CAII
carbonic anhydrase II
- co-IP
coimmunoprecipitation
- ddH2O
double-distilled H2O
- FLIM
fluorescence-lifetime imaging microscopy
- FRET
Förster resonance energy transfer
- GPA
glycophorin A
- GP.Mur
Miltenberger subtype III (Mi.III)
- IOV
inside-out vesicle
- Mi.III
Miltenberger subtype III (GP.Mur)
- RBC
red blood cell
- ROV
right-side-out vesicle
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
K. Hsu and T.-Y. Lee conceived the projects; K. Hsu, T.-Y. Ling, and H.-J. Lin carried out laboratory experiments and analyzed the data; A. Periasamy, F.-J. Kao, L.-T. Li, C.-Y. Lin, and M. Lin supported FLIM instrumentation and experimental design; and K. Hsu wrote the paper.
REFERENCES
- 1.Forster R. E., and Crandall E. D. (1975) Time course of exchanges between red cells and extracellular fluid during CO2 uptake. J. Appl. Physiol. 38, 710–718 [DOI] [PubMed] [Google Scholar]
- 2.Reithmeier R. A. (2001) A membrane metabolon linking carbonic anhydrase with chloride/bicarbonate anion exchangers. Blood Cells Mol. Dis. 27, 85–89 [DOI] [PubMed] [Google Scholar]
- 3.Reithmeier R. A., Casey J. R., Kalli A. C., Sansom M. S., Alguel Y., and Iwata S. (2016) Band 3, the human red cell chloride/bicarbonate anion exchanger (AE1, SLC4A1), in a structural context. Biochim. Biophys. Acta 1858 (7 Pt A), 1507–1532 [DOI] [PubMed] [Google Scholar]
- 4.Steck T. L. (1978) The band 3 protein of the human red cell membrane: a review. J. Supramol. Struct. 8, 311–324 [DOI] [PubMed] [Google Scholar]
- 5.Sterling D., Reithmeier R. A., and Casey J. R. (2001) Carbonic anhydrase: in the driver’s seat for bicarbonate transport. JOP 2(4 Suppl)165–170 [PubMed] [Google Scholar]
- 6.Vince J. W., and Reithmeier R. A. (1998) Carbonic anhydrase II binds to the carboxyl terminus of human band 3, the erythrocyte C1-/HCO3- exchanger. J. Biol. Chem. 273, 28430–28437 [DOI] [PubMed] [Google Scholar]
- 7.Musa-Aziz R., Chen L. M., Pelletier M. F., and Boron W. F. (2009) Relative CO2/NH3 selectivities of AQP1, AQP4, AQP5, AmtB, and RhAG. Proc. Natl. Acad. Sci. USA 106, 5406–5411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Endeward V., Cartron J. P., Ripoche P., and Gros G. (2008) RhAG protein of the Rhesus complex is a CO2 channel in the human red cell membrane. FASEB J. 22, 64–73 [DOI] [PubMed] [Google Scholar]
- 9.Nakhoul N. L., Davis B. A., Romero M. F., and Boron W. F. (1998) Effect of expressing the water channel aquaporin-1 on the CO2 permeability of Xenopus oocytes. Am. J. Physiol. 274, C543–C548 [DOI] [PubMed] [Google Scholar]
- 10.Endeward V., Musa-Aziz R., Cooper G. J., Chen L. M., Pelletier M. F., Virkki L. V., Supuran C. T., King L. S., Boron W. F., and Gros G. (2006) Evidence that aquaporin 1 is a major pathway for CO2 transport across the human erythrocyte membrane. FASEB J. 20, 1974–1981 [DOI] [PubMed] [Google Scholar]
- 11.Agre P., Preston G. M., Smith B. L., Jung J. S., Raina S., Moon C., Guggino W. B., and Nielsen S. (1993) Aquaporin CHIP: the archetypal molecular water channel. Am. J. Physiol. 265, F463–F476 [DOI] [PubMed] [Google Scholar]
- 12.Preston G. M., Carroll T. P., Guggino W. B., and Agre P. (1992) Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 256, 385–387 [DOI] [PubMed] [Google Scholar]
- 13.Cho M. R., Knowles D. W., Smith B. L., Moulds J. J., Agre P., Mohandas N., and Golan D. E. (1999) Membrane dynamics of the water transport protein aquaporin-1 in intact human red cells. Biophys. J. 76, 1136–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verkman A. S. (2013) Aquaporins. Curr. Biol. 23, R52–R55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu K., Chi N., Gucek M., Van Eyk J. E., Cole R. N., Lin M., and Foster D. B. (2009) Miltenberger blood group antigen type III (Mi.III) enhances the expression of band 3. Blood 114, 1919–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu K., Lin Y. C., Chao H. P., Lee T. Y., Lin M., and Chan Y. S. (2013) Assessing the frequencies of GP.Mur (Mi.III) in several Southeast Asian populations by PCR typing. Transfus. Apheresis Sci. 49, 370–371 [DOI] [PubMed] [Google Scholar]
- 17.Issitt P. D. (1985) Applied Blood Group Serology, Montgomery Scientific Publications, Miami, FL, USA [Google Scholar]
- 18.Hsu K., Yao C. C., Lin Y. C., Chang C. L., and Lee T. Y. (2015) Dissecting alternative splicing in the formation of Miltenberger glycophorin subtype III (GYP.Mur). Vox Sang. 108, 403–409 [DOI] [PubMed] [Google Scholar]
- 19.Huang C. H., and Blumenfeld O. O. (1991) Molecular genetics of human erythrocyte MiIII and MiVI glycophorins: use of a pseudoexon in construction of two delta-alpha-delta hybrid genes resulting in antigenic diversification. J. Biol. Chem. 266, 7248–7255 [PubMed] [Google Scholar]
- 20.Hsu K. (2011) Physiological Implications of Miltenberger blood group antigen subtype III (Mi.III). ISBT Sci. Ser. 6, 302–305 [Google Scholar]
- 21.Hsu K., Kuo M. S., Yao C. C., Lee T. Y., Chen Y. C., Cheng H. C., Lin C. H., Yu T. H., and Lin H. J. (2015) Expedited CO2 respiration in people with Miltenberger erythrocyte phenotype GP.Mur. Sci. Rep. 5, 10327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Periasamy A., and Clegg R. M. (2010) FLIM Microscopy in Biology and Medicine, Taylor & Francis, Boca Raton, FL, USA [Google Scholar]
- 23.Sinanoglu O., North Atlantic Treaty Organization, Pure Science Bureau., and Orta Dogu Teknik Universitesi (Ankara Turkey) . (1965) Modern Quantum Chemistry, Istanbul Lectures, Academic Press, New York [Google Scholar]
- 24.Sun Y., Day R. N., and Periasamy A. (2011) Investigating protein-protein interactions in living cells using fluorescence lifetime imaging microscopy. Nat. Protoc. 6, 1324–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lakowicz J. R. (1999) Principles of Fluorescence Spectroscopy, Kluwer Academic, Plenum, New York [Google Scholar]
- 26.Hsu K., Lin Y. C., Chang Y. C., Chan Y. S., Chao H. P., Lee T. Y., and Lin M. (2013) A direct blood polymerase chain reaction approach for the determination of GP.Mur (Mi.III) and other Hil+ Miltenberger glycophorin variants. Transfusion 53, 962–971 [DOI] [PubMed] [Google Scholar]
- 27.Zhang D., Kiyatkin A., Bolin J. T., and Low P. S. (2000) Crystallographic structure and functional interpretation of the cytoplasmic domain of erythrocyte membrane band 3. Blood 96, 2925–2933 [PubMed] [Google Scholar]
- 28.Arakawa T., Kobayashi-Yurugi T., Alguel Y., Iwanari H., Hatae H., Iwata M., Abe Y., Hino T., Ikeda-Suno C., Kuma H., Kang D., Murata T., Hamakubo T., Cameron A. D., Kobayashi T., Hamasaki N., and Iwata S. (2015) Crystal structure of the anion exchanger domain of human erythrocyte band 3. Science 350, 680–684 [DOI] [PubMed] [Google Scholar]
- 29.Jiang J., Magilnick N., Tsirulnikov K., Abuladze N., Atanasov I., Ge P., Narla M., Pushkin A., Zhou Z. H., and Kurtz I. (2013) Single particle electron microscopy analysis of the bovine anion exchanger 1 reveals a flexible linker connecting the cytoplasmic and membrane domains. PLoS One 8, e55408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tani K., and Fujiyoshi Y. (2014) Water channel structures analysed by electron crystallography. Biochim. Biophys. Acta 1840, 1605–1613 [DOI] [PubMed] [Google Scholar]
- 31.Ma T., Frigeri A., Tsai S. T., Verbavatz J. M., and Verkman A. S. (1993) Localization and functional analysis of CHIP28k water channels in stably transfected Chinese hamster ovary cells. J. Biol. Chem. 268, 22756–22764 [PubMed] [Google Scholar]
- 32.Cabral P. D., and Herrera M. (2012) Membrane-associated aquaporin-1 facilitates osmotically driven water flux across the basolateral membrane of the thick ascending limb. Am. J. Physiol. Renal Physiol. 303, F621–F629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith B. L., and Agre P. (1991) Erythrocyte Mr 28,000 transmembrane protein exists as a multisubunit oligomer similar to channel proteins. J. Biol. Chem. 266, 6407–6415 [PubMed] [Google Scholar]
- 34.Marchesi V. T., and Palade G. E. (1967) The localization of Mg-Na-K-activated adenosine triphosphatase on red cell ghost membranes. J. Cell Biol. 35, 385–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bennett V. (1983) Proteins involved in membrane--cytoskeleton association in human erythrocytes: spectrin, ankyrin, and band 3. Methods Enzymol. 96, 313–324 [DOI] [PubMed] [Google Scholar]
- 36.Digman M. A., Caiolfa V. R., Zamai M., and Gratton E. (2008) The phasor approach to fluorescence lifetime imaging analysis. Biophys. J. 94, L14–L16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spence M. T. Z., and Johnson I. D. (2010) The Molecular Probes Handbook: A Guide to Fluorescent Probes and Labeling Technologies, Life Technologies Corporation, Carlsbad, CA, USA [Google Scholar]
- 38.Hsu K., Lin Y. C., Lee T. Y., and Lin M. (2011) Miltenberger blood group antigen subtype III (Mi.III) supports Wr(b) expression. Vox Sang. 100, 389–394 [DOI] [PubMed] [Google Scholar]
- 39.Hsu K., Lee T. Y., Chao H. P., Chan Y. S., Lin Y. C., and Lin M. (2012) Expression of the Rh/RhAG complex is reduced in Mi.III erythrocytes. Vox Sang. 102, 221–227 [DOI] [PubMed] [Google Scholar]
- 40.Bruce L. J., Beckmann R., Ribeiro M. L., Peters L. L., Chasis J. A., Delaunay J., Mohandas N., Anstee D. J., and Tanner M. J. (2003) A band 3-based macrocomplex of integral and peripheral proteins in the RBC membrane. Blood 101, 4180–4188 [DOI] [PubMed] [Google Scholar]
- 41.Aebi M. (2013) N-linked protein glycosylation in the ER. Biochim. Biophys. Acta 1833, 2430–2437 [DOI] [PubMed] [Google Scholar]
- 42.Vilas G., Krishnan D., Loganathan S. K., Malhotra D., Liu L., Beggs M. R., Gena P., Calamita G., Jung M., Zimmermann R., Tamma G., Casey J. R., and Alexander R. T. (2015) Increased water flux induced by an aquaporin-1/carbonic anhydrase II interaction. Mol. Biol. Cell 26, 1106–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sterling D., Reithmeier R. A., and Casey J. R. (2001) A transport metabolon: functional interaction of carbonic anhydrase II and chloride/bicarbonate exchangers. J. Biol. Chem. 276, 47886–47894 [DOI] [PubMed] [Google Scholar]
- 44.Miles E. W., Rhee S., and Davies D. R. (1999) The molecular basis of substrate channeling. J. Biol. Chem. 274, 12193–12196 [DOI] [PubMed] [Google Scholar]
- 45.Lodish H. F. (2000) Molecular Cell Biology, W.H. Freeman, New York [Google Scholar]
- 46.Heckwolf M., Pater D., Hanson D. T., and Kaldenhoff R. (2011) The Arabidopsis thaliana aquaporin AtPIP1;2 is a physiologically relevant CO2 transport facilitator. Plant J. 67, 795–804 [DOI] [PubMed] [Google Scholar]
- 47.Hall J. E., and Guyton A. C. (2011) Guyton and Hall Textbook of Medical Physiology, Saunders/Elsevier, Philadelphia [Google Scholar]
- 48.Beachey W. (2013) Respiratory Care Anatomy and Physiology: Foundations for Clinical Practice, Elsevier, St. Louis [Google Scholar]
- 49.Rhoades R., and Bell D. R. (2009) Medical Physiology: Principles for Clinical Medicine, Lippincott Williams & Wilkins, Philadelphia [Google Scholar]
- 50.Kuchel P. W., and Benga G. (2005) Why does the mammalian red blood cell have aquaporins? Biosystems 82, 189–196 [DOI] [PubMed] [Google Scholar]
- 51.Niizeki K., Mochizuki M., and Uchida K. (1983) Rate of CO2 diffusion in the human red blood cell measured with pH-sensitive fluorescence. Jpn. J. Physiol. 33, 635–650 [DOI] [PubMed] [Google Scholar]
- 52.Geers C., and Gros G. (2000) Carbon dioxide transport and carbonic anhydrase in blood and muscle. Physiol. Rev. 80, 681–715 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.