Abstract
Advancing age promotes cardiovascular disease (CVD), the leading cause of death in the United States and many developed nations. Two major age-related arterial phenotypes, large elastic artery stiffening and endothelial dysfunction, are independent predictors of future CVD diagnosis and likely are responsible for the development of CVD in older adults. Not limited to traditional CVD, these age-related changes in the vasculature also contribute to other age-related diseases that influence mammalian healthspan and potential lifespan. This review explores mechanisms that influence age-related large elastic artery stiffening and endothelial dysfunction at the tissue level via inflammation and oxidative stress, and at the cellular level via Klotho and energy sensing pathways (AMPK, Sirtuins, mTOR). We also discuss how long-term calorie restriction, a healthspan and lifespan extending intervention, can prevent many of these age-related vascular phenotypes through the prevention of deleterious alterations in these mechanisms. Lastly, we discuss emerging novel mechanisms of vascular aging, including senescence and genomic instability within cells of the vasculature. As the population of older adults steadily expands, elucidating the cellular and molecular mechanisms of vascular dysfunction with age is critical to better direct appropriate and measured strategies that utilize pharmacological and lifestyle interventions to prevent against CVD risk within this population.
Keywords: Aging, Endothelium, Large Artery Stiffness, Oxidative Stress, Inflammation, Senescence, Telomere, Genomic Instability, Cardiovascular Disease, Mechanisms, Vascular Disease
Cardiovascular Disease, Arterial Stiffness, Endothelial Function and Aging.
Cardiovascular diseases (CVDs), broadly defined as stroke, coronary artery disease (CAD), heart failure, and cardiac arrest, are the predominant killers of Americans, accounting for ~774,000 deaths per year according to current statistics from the Centers for Disease Control1. CVDs cause ~32% of all deaths for Americans 65 years of age and older, making them the leading causes of death in this age group1. Furthermore, with advancing age, the prevalence of CVDs among Americans increases progressively, from ~5.5% in 25–44 year-olds to ~41% in people 65 years of age and older2. Thus, CVDs can be considered true diseases of aging.
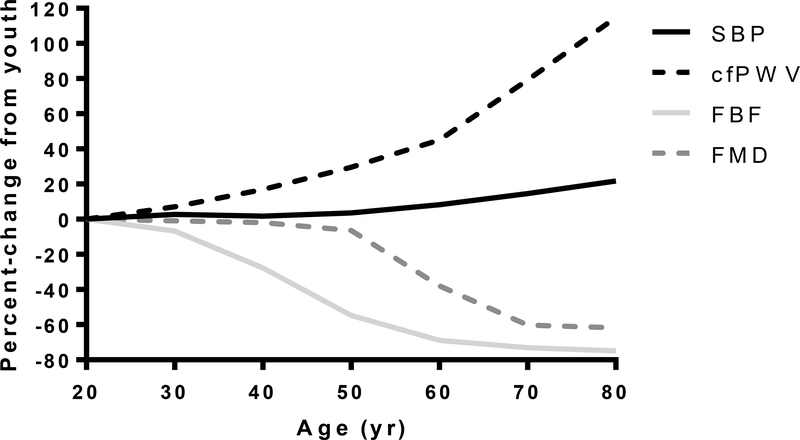
Arterial dysfunction plays an important causal role in the vast majority of CVDs3, 4. Age-related alterations in arteries ranging in size from the large elastic arteries to the conduit and small resistance arteries, and down to the microcirculation are thought to lead to a dysfunctional vascular phenotype that precedes CVDs3, 5. Interestingly, age-related arterial dysfunction is present in the absence of clinical CVD and conventional CVD risk factors6, supporting the concept that age-related arterial dysfunction is a primary effect of advancing age that may be a precursor to the development of clinical CVD. Although many functional and morphological characteristics of arteries are altered by advancing age (e.g. increase in the size of large and feed arteries, blunted angiogenesis, loss of vascular density7 and a diminished endothelial glycocalyx8), large longitudinal studies have demonstrated that two specific age-associated arterial phenotypes are potent risk factors for future CVD diagnosis and CVD-related morbidity and mortality: increased stiffness of the large elastic arteries and endothelial dysfunction5, 9. Figure 1 is the accumulated and normalized vascular function data from large cross-sectional studies of healthy (no frank CVD) humans. These data show that by the 5th decade of life, small artery endothelial dilation and pulse wave velocity (PWV; an indicator of arterial stiffness) are already altered, but that conduit artery endothelium dependent dilation (EDD) and systolic blood pressure (SBP) are maintained close to youthful levels, but decline substantially thereafter.
Figure 1. Age-Associated Cardiovascular Changes.
Predicted percent-change from youth in markers of vascular function: brachial systolic blood pressure (SBP)13; carotid-to-femoral pulse wave velocity (cfPWV)305; maximal forearm blood flow to acetylcholine (FBF)306; brachial flow-mediated dilation (FMD)307–309. All lines are the percent of the predicted fold-change from reference values at 20 yr. Reference values: SBP 110 mmHg; cfPWV 5.9 m/sec; FBF 897 Δ%; FMD 9%.
Large artery stiffening.
An important, early change seen with advancing age is stiffening of the large elastic arteries. For the purpose of this review, large artery stiffness is defined as increased conducted wave velocity and/or changes in the passive mechanical properties of the artery that reduce compliance. Longitudinal studies have shown a progressive increase in large elastic artery stiffness with aging (Figure 1) that contributes to CVD risk and is associated with pathophysiological conditions such as hypertension, stroke, left ventricular hypertrophy, sub-endocardial ischemia and cardiac fibrosis10. Large artery stiffening is postulated to impact CVDs in several ways. First, an important function of large elastic arteries (such as the aorta) is to act as a capacitance vessel to dampen the increase in pressure caused by the ejection of blood from the left ventricle during systole. The elasticity of the aorta allows it to distend as blood is pumped into it during systole, then to recoil, facilitating continuous blood flow and maintaining pressure during diastole. In this way, the elasticity of the aorta helps it to act as a secondary pump of blood during elastic recoil and to prevent large pulse pressures being conducted to the smaller blood vessels, the so called “Windkessel Effect”. As we age, the aorta loses elasticity through multiple mechanisms, becoming stiffer, and the capacitance function becomes impaired, increasing the systolic blood pressure per unit blood ejected from the left ventricle. Second, increased large artery stiffness leads to a greater velocity of the incident, systolic pressure waveform down the arterial tree, resulting in a quicker return of its reflected waveform11. In stiff arteries, the reflected waveform superimposes on the incident waveform, thereby augmenting systolic pressure and aortic impedance12, which increase the work imposed on the left ventricle during systole. Last, large artery stiffening leads to higher systolic pressure, a reduction in diastolic pressure and a concomitant widening of pulse pressure13. The widening of pulse pressure has also been hypothesized to augment transmission of pulsatility along the arterial tree. This has been measured in mid-sized and smaller conduit arteries, and pulsatility and higher pressure have been measured directly from the capillaries in the skin of older adults14. Increased pulsatility in muscular and resistance arteries with advancing age will deleteriously affect their ability to distribute blood flow and appropriately alter vascular resistance, a function necessary to reduce pressure in the microcirculation. These alterations could contribute to peripheral organ damage and dysfunction with aging15, particularly in vulnerable regions like the cerebral16–20 and renal circulations10.
The most commonly used method to measure large elastic artery stiffness in long term epidemiological studies is carotid-to-femoral PWV (cfPWV), due to its ease of use and high reproducibility21. Other techniques, like augmentation index (AI) and direct measurements of arterial distensibility, have also been utilized, depending on the questions of interest22. Higher cfPWV is indicative of greater large elastic artery stiffness and is a strong predictor of several age-related CVD outcomes23, 24 and cognitive impairment25. Many of the factors that alter aortic stiffness with age are plastic, such as modulators of arterial tone (i.e., vasodilators vs. vasoconstrictors), and can rapidly induce changes in arterial stiffness in different physiological states, such as in response to acute stress26. Other factors that affect aortic stiffness are less plastic and require more time (months to years). These include accumulation of advanced glycation end products (AGEs), alterations to collagen and elastin content and/or arrangement, remodeling and structural changes in the extracellular matrix. Importantly, changes in large artery stiffness with age not only affect end organ systems, but also modulate the cells within the vasculature, such as the endothelium, which in turn can further exacerbate large artery stiffness and subsequent global vascular dysfunction in older adults.
Endothelial dysfunction.
The arterial endothelium is extremely dynamic, performing many vital functions that vary from one segment of the arterial tree to another, as well as from one organ system to another27, 28. The vascular endothelium releases molecules that act in an autocrine and paracrine manner to regulate the function and health of the vascular network. These functions include the maintenance of blood in a fluid state, exchange of fluid and molecules between the blood and surrounding tissues, creation of new vascular networks, participation and facilitation of the immune response, and the control of vascular resistance in response to changes in blood flow through the regulation of arterial tone in resistance vessels27, 28. A healthy vascular endothelium must constantly maintain a balance between oxidants and antioxidants, vasodilators and vasoconstrictors, pro- and anti-inflammatory molecules, and pro- and anti-thrombotic signals. A dysfunctional endothelium has lost this tightly regulated balance, and displays oxidant, vasoconstrictor, pro-inflammatory and pro-thrombotic properties. Age-related dysfunction of the vascular endothelium is defined in this review as an age-associated impairment in flow- or agonist-induced vasodilation and/or a reduction in the critical endothelium-derived vasodilator, nitric oxide (NO). One hallmark of vascular endothelial dysfunction is impaired EDD, which is predictive of future CVD events3, 4. Increased age is the strongest independent correlate of EDD29, and the age-related decline in endothelial function in both the microcirculation and larger arteries (Figure 1) contributes to a host of hemodynamic changes. These include augmented large and resistance arterial tone, induction of greater oscillatory shear stress, and further elevations in large artery stiffness3, 30. By altering hemodynamics, EDD contributes to increases in the prevalence of hypertension13 and atherosclerosis4 with advancing age.
Interaction of arterial stiffness and endothelial dysfunction.
Although endothelial dysfunction and large artery stiffening are independent predictors of CVD, each of these can exacerbate the other, producing a cycle of damage and dysfunction in advanced age. For example, in vitro evidence suggests that increased matrix stiffness may impair flow-induced dilation by reducing the ability of the endothelium to effect a response to laminar shear stress31. Impaired EDD can also contribute to arterial stiffening by increasing the active tone in arteries32 and they may share common underlying mechanisms like oxidative stress and inflammation. As such, the mechanisms that underlie age-related arterial dysfunction provide an opportunity to develop and test interventions that may restore arterial function, prevent CVD and improve healthspan in middle-aged and older adults.
Goals of the Review.
Having considered the importance of age-related arterial stiffening and endothelial dysfunction on CVD the goals of this review are: first, we review two macro-mechanistic processes, oxidative stress and inflammation, that contribute to endothelial dysfunction in healthy older adults and animal models. Next, we discuss some of the cellular and molecular events that induce and contribute to the sustained cycle of inflammation and oxidative stress in the aged vasculature. We also review mechanistic insight gained from studies of calorie restriction (CR) and their relevance and how traditional aging and longevity pathways also appear to modulate arterial function with advancing age. Furthermore, we highlight important signaling and genomic changes with aging that impact cellular and tissue processes, ultimately leading to the functional vascular aging phenotype (Figure 2). Lastly we describe the role of the vasculature in the aging process (lifespan, healthspan), together with examples of non-canonical age-associated CVDs, specifically metabolic syndrome and Alzheimer’s disease (AD)/dementia.
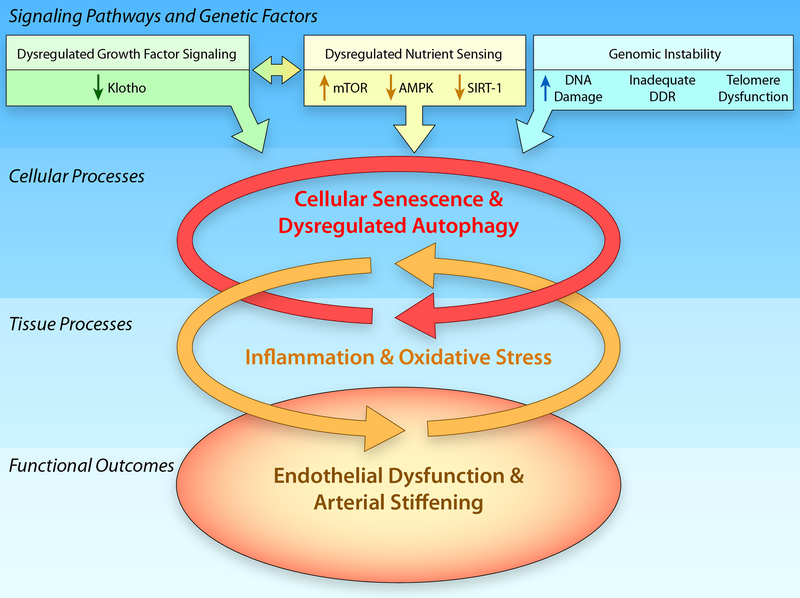
Figure 2. Hallmarks of Vascular Aging.
In addition to increases in genomic instability that are associated with increases in DNA damage, an inadequate DNA damage response (DDR) and telomere dysfunction, dysregulation of signaling pathways, including the growth factor signaling pathway, klotho, and the nutrient sensing pathways, mammalian Target of Rapamycin (mTOR), adenosine monophosphate protein kinase (AMPK), and sirtuin-1 (SIRT-1) will induce increases in cellular senescence and the dysregulation of autophagy that contribute to increased tissue oxidative stress and inflammation that ultimately result in the age-related arterial phenotype characterized by endothelial dysfunction and arterial stiffening. (Illustration Credit: Ben Smith).
Mechanisms of Vascular Dysfunction with Aging and Their Prevention by Calorie Restriction.
Reductions in EDD and increases in arterial stiffness can be reversed by lifestyle interventions such as CR, habitual exercise33, 34, sodium restriction35, 36, and weight loss37. Among these interventions, CR is the one most consistently associated with increases in lifespan. As such, insight from studies aimed at elucidating the molecular and cellular mediators of CRs beneficial effects on lifespan may provide novel targets for pharmacological intervention in the setting of CVD. In animal studies, CR is typically a lifelong intervention, initiated after sexual maturity, in which caloric intake is restricted by 40% of ad libitum (AL) intake without inducing malnutrition. CR improves maximal and/or median lifespan, as well as physiological function in rodents and non-human primates38. It has been associated with a dramatic reduction in CVD in non-human primates39 and protection against cardiac ischemia40. In addition, there is recent evidence for a vasoprotective effect of CR such that EDD and NO bioavailability are preserved41, 42 and increases in large artery stiffness are prevented43 in aged rodents. Although the translatability of CR to humans has often come in to question, the results of the CALERIE trial, in which non-obese young and middle-aged (21–51 yrs) adults were randomized to 25% CR for two years, indicated that CR was able to slow biological aging44 and reduce CVD risk factors45. Although the mechanisms underlying age-related arterial dysfunction and their prevention by CR are still being elucidated, the most widely explored mechanisms are oxidative stress and inflammation with modulation of autophagy emerging as a potential candidate mechanism.
Oxidative Stress.
Elevations in plasma markers of oxidative stress in older adults46 suggest that aging is a state of chronic, systemic oxidative stress47, a condition that ensues when the bioavailability of reactive oxygen species (ROS) is increased relative to antioxidant defenses48, 49. Oxidative stress in the vasculature is a primary mechanism underlying age-associated reductions in EDD and NO bioavailability46, 50, 51 as well as increases in large artery stiffness46, 50, and is characterized by increases in arterial ROS, including superoxide anion (O2-)52 and hydrogen peroxide (H2O2)53.
O2- reduces NO bioavailability49, 54 by interacting with NO to produce peroxynitrite (ONOO-). ONOO- is itself a ROS that nitrates cellular proteins to form nitrotyrosine (NT), a robust but reversible, marker of cellular oxidative stress and ONOO- production55–57. Increased abundance of arterial NT has been demonstrated in both animal models34, 58, 59 and ECs of humans60. In addition, markers of lipid peroxidation such as 4-hydroxynonenal (4-HNE) or malondialdehyde (MDA) and other oxidative stress markers such as glutathionylation at cysteine residues are also observed in aged arteries61, 62. Functionally, greater superoxide-mediated suppression of NO bioavailability leads to reductions in EDD34, 46, 50, 59 and contributes to arterial stiffening in rodent models63, 64 and older women65 (Figure 3). CR attenuates age-related elevations in arterial O2- production41–43, 52, which contributes to reduced vascular oxidative stress, as evidenced by lower arterial NT abundance after CR compared to age-matched AL controls42, 43.
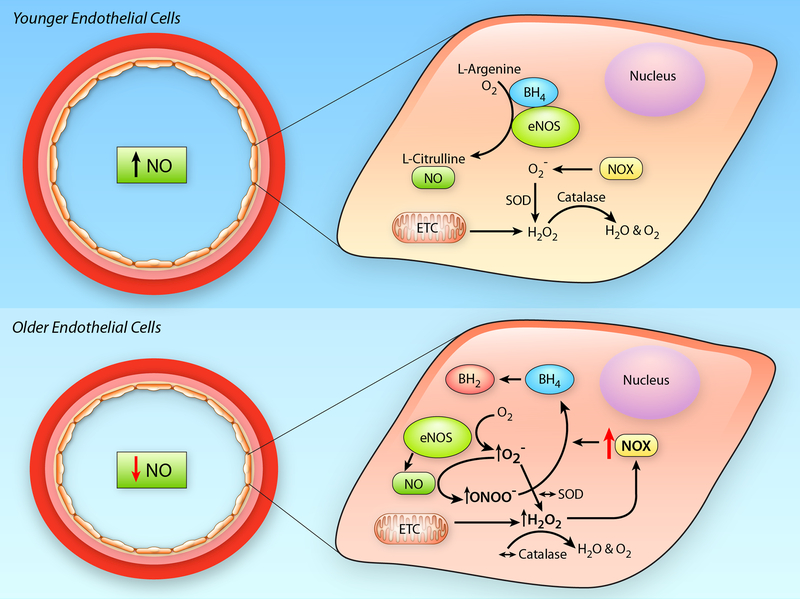
Figure 3. Impact of Aging on Endothelial Cells.
In younger endothelial cells (Upper Panel), endothelial nitric oxide synthase (eNOS) has adequate cofactor availability, e.g., tetrahydrobiopterin (BH4), and produces nitric oxide (NO) through the conversion of L-arginine to L-citrulline. Reactive oxygen species (ROS), e.g., superoxide (O2−) and hydrogen peroxide (H2O2), produced by the mitochondrial electron transport chain (ETC) or cytosolic oxidant enzymes, such as NADPH oxidase (NOX), are quenched by endogenous antioxidant enzymes (superoxide dismutase [SOD] and catalase). In older endothelial cells (Lower Panel), ROS produced in the mitochondria increase NOX mediated O2−, this quenches NO bioavailability, through its conversion to peroxynitrite (ONOO−), as well as uncouple eNOS by reducing BH4 availability. In the face of unchanged antioxidant defenses, these effects lead to a reduction in NO bioavailability and a pro-oxidant phenotype in the aged endothelium. (Illustration Credit: Ben Smith).
O2- is produced by oxidant enzyme systems such as NADPH oxidase-p67 phox, xanthine oxidase, cytochrome P450 2C9, and uncoupled eNOS48, 49, 66. This O2- acts to reduce NO bioavailability and impair EDD. With aging, there is an increased production of O2- that is associated with both elevated expression and activity of NADPH oxidase34 as well as with increased eNOS uncoupling67. NADPH oxidase derived O2- directly contributes to age-associated endothelial dysfunction, as evidenced by the restoration of EDD and NO after in vitro inhibition of this enzyme by apocynin in arteries from aged mice34. Likewise, eNOS uncoupling appears to play a role in age-related arterial dysfunction, as tetrahydrobiopterin (BH4) treatment, which reduces eNOS uncoupling, leads to improvements in EDD in older adults that are associated with a reduction in the oxidative stress-mediated suppression of EDD68 (Figure 3). Further supporting a role for eNOS uncoupling in age-related elevations in arterial O2-, it has been demonstrated that ex vivo administration of BH4 reduces O2- in arterioles from old rats69. In contrast to NADPH oxidase, other oxidant enzyme systems may be less critical to age-related arterial oxidative stress. Indeed, a preponderance of the data examining vascular function in NIA supported models of aged mice, rats (Fisher 344) and humans have found no age-related increase in expression and/or activity of xanthine oxidase or cytochrome P45034, 60, 70, 71, nor has pharmacological inhibition of xanthine oxidase or cytochrome P450 2C9 been found to be effective in improving EDD in older adults70, 72. Still, there is a single study that has suggested that xanthine oxidase is a source of augmented O2- in aged Sprague Dawley rats73 and there is also evidence for a critical role of xanthine oxidase in pathological settings such as heart failure74, 75. In summary, it is likely that NADPH oxidase and uncoupled eNOS are the major enzymatic sources of O2- in aged arteries and endothelial cells (ECs).
Reductions in O2- appear to be an important mechanism underlying the functional benefits of CR, as treatment of arteries with the superoxide scavenger TEMPOL improves EDD and NO bioavailability in old AL fed mice34, but not in old mice after CR43, 52, indicating that CR attenuates the superoxide-mediated suppression of EDD and NO42, 43, 52. This CR-mediated reduction in O2- may result from reduced production, as CR is also associated with reductions in NADPH oxidase expression and activity43, 52, 76. Although there is no direct evidence for a role of reduced eNOS uncoupling in the vascular benefits afforded by CR, association has been made between eNOS uncoupling and reductions in sirtuin (SIRT)-1, a deacetylase closely associated with CR-mediated lifespan extension77. Nevertheless, it remains to be determined if reductions in eNOS uncoupling or other oxidant enzymes play a role in the beneficial effects of CR.
In addition to the enzymatic production of O2-, mitochondrial O2- is another important source of arterial ROS in aging. Although O2- can be produced in abundance in the mitochondria at complex 1 and 378, it may be confined to the mitochondria. Mitochondrial O2- produced at complex 1 and 3 are released into the mitochondrial matrix or intermembrane space, respectively, and due to its highly reactive nature, O2- is unlikely to migrate to the cytosol or extracellular space without being transformed to a less reactive form of ROS, such as H2O2. Nevertheless, mitochondrial ROS, in the form of H2O279, contributes to the ROS production/spillover in arteries from old rodents and likely contributes to age-associated endothelial dysfunction. Indeed, genetic or pharmacologic targeting of mitochondrial O2- reduces arterial O2- and leads to improvements in NO and EDD80, 81. Furthermore, reducing mitochondrial ROS may also underlie the vasoprotective effects of CR, as elevations in mitochondrial O2- production are not present in aged primary cerebral microvascular ECs from aged mice after lifelong CR76.
To maintain an appropriate redox balance, elevations in superoxide production with aging should signal an increase in antioxidant defenses. However, total antioxidant capacity is reduced82 and the expression and/or activity of critical antioxidant enzymes do not increase in arteries of old rodents34. Thus, combined with the enhanced oxidant production, inadequate antioxidant defenses also contribute to age-related arterial oxidative stress and reductions in critical antioxidant enzymes, superoxide dismutases (SODs), catalase and glutathione (GSH), have been implicated in age-associated oxidative stress.
SODs are critical anti-oxidant enzymes that convert superoxide to H2O2, and thus play a primary role in ROS removal83. In the face of elevated superoxide production with aging, neither copper zinc SOD (CuZnSOD), the intracellular isoform, nor manganese SOD (MnSOD), the mitochondrial isoform34, 43, 52, increase in arteries of old mice34, 43. Although the expression of extracellular SOD (ecSOD) has been shown to be either unchanged43, 84 or increased34, there is no increase in activity of any of these SOD isoforms in arteries with advancing age34, 43, 52. Interestingly, there is experimental evidence that a lack of appropriate SOD response to O2- overproduction may have clinical significance, as these antioxidants play a critical role in the setting of CVD. For example, MnSOD may play a cardioprotective role. Evidence for this derives from genetic models in which deletion of MnSOD results in cardiomyopathy and heart failure85; whereas overexpression of ecSOD improves endothelial function in the setting of hypertension86. Likewise, old MnSOD haploinsufficient mice have impaired EDD compared with old wildtype mice87, further supporting the importance of this antioxidant and mitochondrial O2- in the function of aged arteries.
Interestingly, the inadequate response in SOD expression and activity with aging may contribute not only to the excess O2- that quenches NO bioavailability, but may also contribute to impaired vasodilation via reduced H2O2 production88. While at high concentrations H2O2 is itself a ROS that can impair vascular function89. H2O2 produced through the actions of SOD also promotes vasodilation via direct actions as an endothelium derived hyperpolarizing factor88, as well as by indirectly inducing eNOS expression, the enzyme responsible for the production of NO90. Interestingly, the importance of H2O2 as a vasodilator differs across the lifespan and with the emergence of CAD, such that the primary mediator of flow–induced dilation in coronary arteries shifts from prostacyclin in youth to NO in healthy adulthood and is again altered in the presence of CAD to favor H2O291.
Like SODs, aortic expression of catalase, the enzyme responsible for the reduction of H2O2 to oxygen and water, also fails to increase with advancing age in mice43. In advanced age, the lack of an increase in catalase combined with increased superoxide production may lead to excess accumulation of H2O2 and contribute to the impairments in vasodilation89. Still another antioxidant implicated in age-associated oxidative stress is GSH, an intracellular thiol that reduces disulfide bonds and acts as an electron donor, the expression of which is reduced in aortic lysates of old rats41. Although exogenous treatment with a GSH-depleting drug was without effect on EDD in aged rats92, evidence for deleterious effects of inadequate GSH expression can be found in disease states. Indeed, impaired GSH-related antioxidant effects are associated with CVD, such that atherosclerotic plaques demonstrate depressed antioxidant GSH effects93 and low serum GSH is associated with family history of CVD94. Furthermore, there is evidence that GSH supplementation can improve EDD in patients with atherosclerosis95.
In addition to lowering ROS production, reductions in oxidative stress after CR are also associated with improvements in antioxidant defenses. In contrast to AL aging, total antioxidant capacity in the serum of old CR rats does not differ from that of young rats82, and arterial expression of the antioxidants, MnSOD, CuZnSOD and ecSOD43, 52, as well as the activity of total SOD52 and catalase43 are all increased in aged mice after CR. Likewise, CR has also been demonstrated to increase GSH activity in human subjects96. Although it is not known if CR alters the reliance on H2O2 as a vasodilator, it does appear that a reduction in oxidative stress, which is mediated by both decreased oxidant production and improved antioxidant defenses, is a primary mechanism underlying the beneficial effects of CR on the vasculature.
Inflammation.
Chronic, low grade inflammation has been implicated in age-associated impairments in endothelial function and increases in large artery stiffness. Age-associated sterile inflammation is characterized by increases in circulating C-reactive protein (CRP), as well as pro-inflammatory cytokines and adhesion molecules including tumor necrosis factor alpha (TNF-α), interleukin (IL)-6, and vascular cell adhesion molecule (VCAM)-160, 71. These circulating markers of inflammation, in particular CRP and IL-6, have been positively related to aortic stiffness97 and inversely related to EDD in older adults98. While some inflammatory mediators are derived from vascular cells per se, including endothelial-derived TNF-α99, immune cells infiltrating the arterial adventitia may also be an important source of both inflammatory cytokines and ROS production100. This is supported by data demonstrating increased accumulation of both macrophages and lymphocytes in the adventitia of aged arteries101 and a role for adventitial-derived ROS in arterial inflammation in the setting of CVDs102.
Activation of the ROS-sensitive, pro-inflammatory transcription factor, Nuclear factor kappa B (NFκB), is believed to play a critical role in age-related vascular inflammation, with increased expression of NFκB observed in ECs from older human subjects60, 71. Through its interaction with the inhibitory protein, IκB-α, NFκB resides in the cytoplasm103. In response to ROS104 or other inflammatory stimuli103, IκB-α is phosphorylated by the inhibitor of NFκB kinase subunit beta (IKKβ) that will then release its inhibition on NFκB, leading to the nuclear translocation of NFκB and the activation of gene transcription of pro-inflammatory target genes such as TNF-α and IL-6103. Age-associated increases in NFκB activity have been directly implicated in arterial dysfunction in older rodents and humans, as NFκB inhibition by salicylate (salsalate in humans) improves EDD in aged mice105 and in overweight/obese middle-aged and older adults106. Furthermore, both salsalate treatment107 and inhibition of the NFκB-target TNF-α108 improve aortic stiffness in older adults.
Both vascular inflammation, via the cytokines IL-1β and TNF-α, and oxidative stress influence the primary mechanisms of extracellular matrix changes in the vessel wall with aging (Figure 4). Implicated in age-related arterial remodeling are an imbalance between matrix metalloproteinases (MMP) and their inhibitors, MMP-mediated arterial remodeling, elastin breakdown and fragmentation, differential changes in isoform expression and glycation of collagens, transforming growth factor-beta (TGF-β)-associated collagen accumulation109, and VSMC migration, proliferation and senescence (a more complete discussion of the specific role of the VSMC in arterial aging can be found in a recent review110). While the VSMCs of large arteries also directly impact arterial structure and stiffness by synthesizing and secreting ECM proteins, the interaction of the VSMCs and ECM components play a critical role in determining arterial stiffness. In addition to the VSMC per se, plasma MMP-9 is also associated with increased large elastic artery stiffness in both healthy individuals and individuals with isolated systolic hypertension111.
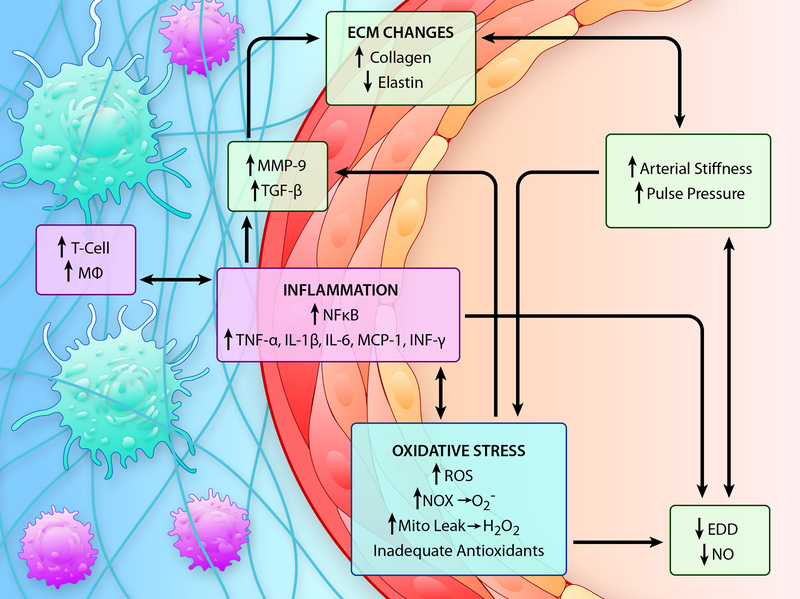
Figure 4. Mechanisms of Age-Associated Arterial Dysfunction.
The vascular aging phenotype is characterized by increased large artery stiffness and pulse pressure and reduced endothelium dependent dilation (EDD) and nitric oxide (NO) bioavailability. Oxidative stress, caused by increased production of reactive oxygen species (ROS) in the absence of an adequate antioxidant defense, and inflammation are two interconnected mechanisms that underlie arterial dysfunction in advanced age. Increases in oxidant production with aging are associated with increased NADPH oxidase (NOX)- and mitochondrial-produced ROS, such as superoxide (O2-) and hydrogen peroxide (H2O2). These ROS act to (1) quench bioavailable NO, thus limiting EDD, (2) induce inflammatory signaling through nuclear factor kappa B (NFκB) activation and (3) induce matrix metalloproteinase (MMP)-9 and transforming growth factor beta (TGF-β) that contribute to alterations in the extracellular matrix (ECM) including increases in collagen content and decreases or fragmentation of elastin that contribute to increases in arterial stiffening. In a vicious cycle, increased NFκB activation also exacerbates both oxidative stress and inflammation by transcribing oxidant enzymes such as NOX, pro-inflammatory cytokines and adhesion molecules, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6 and monocyte chemoattractant protein (MCP)-1, and this contributes to downstream increases in MMP-9 and TGF-β expression. Increased adhesion molecule expression contributes to the infiltration of immune cells such as T cells and macrophages (Mφ) to the perivascular tissues and these immune cells further exacerbate inflammation and oxidative stress through the production of cytokines, such as interferon(INF)-γ and TNF-α, as well as O2-. (Illustration Credit: Ben Smith).
MMP-9 can be directly transcribed via NFκB112 or indirectly via ROS-mediated activation of NFκB113. Interestingly, our group has demonstrated a substantial increases in MMP-9 in mouse aortic tissue with aging and life-long CR normalizes MMP-9 to near young values43. Experimentally, the simple viral overexpression of TGF-β is sufficient to induce fibrosis114. With aging, arterial expression of TGF-β is elevated in aortas of mice and this is associated with greater collagen I and III content in the adventitia and reduced medial elastin content in the aorta, as well as increased arterial superoxide115. In addition to modulation by oxidative stress and inflammation, both MMPs and TGF-β also appear to be influenced by NO bioavailability. For example, inhibition of NO via in vivo treatment with the NOS inhibitor, L-NAME, increased both markers of inflammation in the heart and coronary arteries of rats116. Likewise, it was demonstrated that shear stress-induced NO down-regulates MMP-2 expression in cultured ECs and further that treatment of ECs with an NO donor under static culture conditions will mimic this shear stress-induced reduction in MMP-2117. In addition, inducible NOS (iNOS)-associated NO inhibits MMP-9, as activity of this MMP is induced after iNOS inhibition and inhibited by SMC-produced NO118. Conversely, NO derived from macrophages can suppress tissue inhibitor of matrix metalloproteinases (TIMP)-1, leading to increased MMP-9 and VSMC migration when assessed in an in vitro wound healing assay119, supporting a role of immune cell derived ROS in vascular remodeling. Thus, increases in oxidative stress and inflammation and decreases in NO bioavailability with aging may act in concert to alter both TGF-β and MMPs, important determinants of the structural components of the artery, thereby influencing arterial stiffness. Thus, all of the three major layers of the artery; the intima, media and adventitia, contribute to oxidative stress and inflammation that impair vascular function (in-depth reviews of each of these vascular components are available110, 120, 121).
Interestingly, oxidative stress and inflammation act in a vicious cycle to negatively impact arterial function with aging. While ROS will act on NFκB to stimulate pro-inflammatory signaling, pro-inflammatory signaling itself can stimulate the local production of O2- by inducing transcription of redox-sensitive genes, such as those encoding subunits of NADPH oxidase122, and through the local recruitment of immune cells, which exacerbate the presence of inflammatory cytokines and also produce ROS to further promote a pro-oxidant environment. Indeed, advancing age is associated with increases in T and B cells as well as macrophages in the perivascular adipose tissue of both the aorta and mesenteric vascular arcade. Furthermore, aged splenic immune cells from these same mice demonstrate increased pro-inflammatory cytokine expression compared to cells isolated from young mice. Importantly, both the elevations in immune cell infiltration and the pro-inflammatory immune cell phenotype are prevented by CR123. Interestingly, both gene expression and activity of NFκB are also reduced in nuclear extracts of arteries and ECs from CR compared to AL fed old mice41, 76. Likewise, while the activity of the NFκB inhibitor IκB-α is decreased with aging105, 124, this disinhibition of NFκB is attenuated after CR124. Thus, a reduction in NFκB-associated inflammatory signaling may also contribute to improved vascular dysfunction in aged mice after CR. Taken together, it appears that inflammatory and oxidative pathways interact with advancing age to induce and perpetuate arterial dysfunction in a vicious cycle that can be broken by CR.
Autophagy.
Autophagy is a cellular housekeeping mechanism that helps to maintain homeostasis. Basal levels of autophagy are necessary to protect tissues from oxidative stress and inflammation by promoting the destruction of damaged proteins and organelles125. However, inadequate autophagy will lead to the accumulation of damaged proteins and promote further oxidative stress and inflammation. Flux through autophagic processes is stimulated by intra- and extracellular cues and is aimed at promoting survival and minimizing destruction of required proteins and organelles. Intriguingly, autophagy is reduced in animal models of aging126 and has recently been implicated as a modulator of longevity, with overexpression of an autophagy-related gene 5 (Atg5) leading to the induction of autophagy and an increase lifespan in mice127. Furthermore, nutrient deprivation is a well-established stressor that stimulates autophagy128, leading to the speculation that the effects of CR may be mediated by an induction of autophagy. This idea is supported by the finding that there is a lack of lifespan extension in response to dietary restriction in autophagy deficient, Beclin-1 knockdown nematodes129. Thus, there appears to be a critical role for autophagy in CR-mediated lifespan extension, although the role of autophagy in age-related vascular dysfunction or in the vasoprotection afforded by CR has only recently begun to be explored.
Within the vasculature, autophagy has been implicated in diverse physiological and patho-physiological processes including angiogenesis, paracrine responses in the endothelium, vascular wall calcification, and atherosclerosis (reviewed in Nussenzeig et al.130). In autophagy deficient, Atg3 knockdown ECs, there is a blunting of shear-induced eNOS activation and NO production that is associated with increases in both oxidative stress and inflammation131, providing evidence for a direct link between autophagy and endothelial function. Pharmacological evidence also supports this link, as inhibition of autophagy reduces, and activation enhances, the expression of eNOS in ECs132. Autophagy has also been associated with the modulation of eNOS uncoupling133 and NO appears to regulate EC mitophagy (mitochondrial specific autophagy)128.
Markers of autophagic flux are lower in both ECs of older adults and arteries of aged mice134. Induction of autophagy after treatment with trehalose restores autophagic flux, and is associated with improvements in EDD and NO bioavailability as well as reductions in markers of oxidative stress and inflammation in the arteries of aged mice134. Likewise, induction of autophagy with spermidine reduces arterial stiffness and improves EDD and NO bioavailability in aged mice. These vascular benefits are associated with a reduction in arterial O2-135. Although far from definitive, indirect evidence of a role for increased autophagy after CR comes from a study in cultured ECs. It was found that ECs treated with the CR mimetic, resveratrol, were protected against TNF-α induced inflammation through the induction of autophagy136. Although it remains unknown if the vasoprotection afforded by CR is dependent on increases in autophagy, there is growing evidence that impaired autophagic flux plays a critical role in age-related vascular dysfunction, oxidative stress and inflammation.
Countermeasures to Vascular Aging
mTOR. Rapamycin originally was discovered in a soil sample from Easter Island and used as an antifungal metabolite137. Through its inhibition of the mammalian target of rapamycin (mTOR), rapamycin also has anti-proliferative and immunosuppressant properties138. mTOR is a highly conserved serine/threonine kinase that responds to changes in energy balance and regulates many cellular functions, including translation, transcription, protein turnover, metabolism, and stress responses139. There are currently two known mTOR signaling complexes: the rapamycin “sensitive” mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2); the latter is less sensitive to rapamycin and less studied within the vasculature. As such, in this review we refer to either mTORC1 signaling or general mTOR activity140.
mTOR inhibition reduces mRNA translation and protein synthesis by activating ribosomal S6 protein kinase (S6K1) and inhibiting eukaryotic translation factor binding protein (4E-BP1)137, 141. In early studies, yeast, flies, and nematodes demonstrated lifespan extension through genetic manipulation or rapamycin treatment that inhibited TOR signaling142. Harrison et al. reported similar findings in mammals, with rapamycin-induced mTOR inhibition leading to lifespan extension and a delay in age-associated phenotypes143. These findings were further supported by a genetic model in which S6K1 knockout led to increased maximal lifespan and resistance to age-associated pathologies in mice144. Taken together, these studies implicate mTOR signaling in aging and its associated diseases.
While traditionally used as an immunosuppressant in organ transplant patients, rapamycin was recently found to act as a tumor suppressant in certain cancers145 and appears to have cardioprotective benefits146–149. To the best of our knowledge, the first evidence of rapamycin’s cardioprotective benefits in humans were found in kidney transplant patients, in which a lower incidence of hypertension has been reported in patients treated with rapamycin for immunosuppression146, 147. In a subsequent study, endothelial function, assessed by radial artery flow-mediated dilation, was also found to be greater in transplant patients treated with rapamycin148. In the setting of advancing age, our group has found increased mTOR activation in arteries of old mice that is accompanied by impaired EDD and reduced NO bioavailability43, 84, and that this can be reversed by 6 weeks of dietary rapamycin treatment84. We also observed reductions in superoxide production and NADPH oxidase expression after rapamycin treatment in arteries of old mice84. Thus, mTOR inhibition may exert some endothelial-protective effects by altering redox balance and/or inflammation in advanced age. Indeed, rapamycin treatment has also been shown to reduce liver mitochondrial ROS production in middle-aged mice150, as well as increased oxidative stress response gene expression of the antioxidants CuZnSOD, SOD, and GSH reductase151. Moreover, in a rat model of Parkinson’s disease, rapamycin treatment was shown to be associated with lower oxidative stress and increased glutathione peroxidase activity152. Lastly, rapamycin reduced the ratio of reduced/oxidized glutathione (GSH/GSSG) in type 2 diabetic rat hearts, indicative of an improvement in oxidative stress after mTOR inhibition153. In addition to its favorable effects on redox balance, rapamycin treatment also results in downregulation of the inflammatory cytokine, TNF-α151. These findings were further supported by pathway analysis that revealed an upregulation of free radical scavenging genes and a downregulation of NFκB signaling genes after rapamycin treatment151. Thus, it appears that inhibition of mTOR by rapamycin can impact both oxidative stress and inflammation, which may be one mechanism underlying greater NO bioavailability and EDD in rapamycin treated old mice (Figure 5).
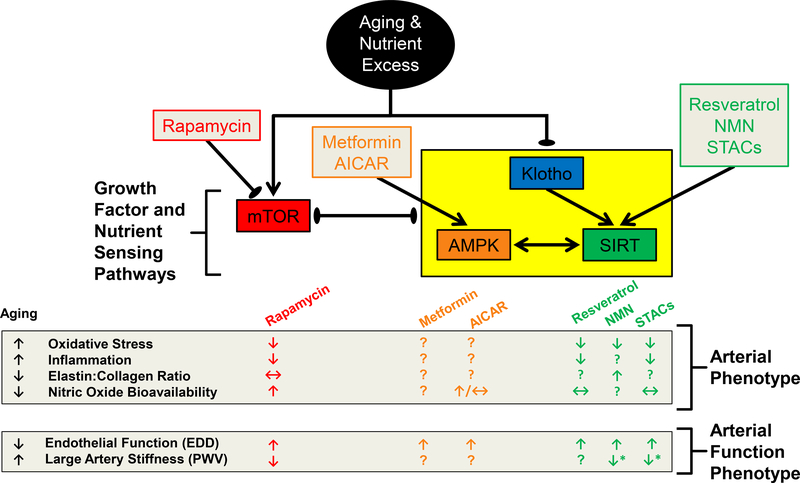
Figure 5. Summary of the Interdependence of Klotho and Nutrient Sensing Pathways in the Face of Advancing Age and Nutrient Excess, and Strategies to Reverse Age-Related Arterial Phenotypes.
↓ represents a reduction, ↑ represents an increase, ↔ represents weak or conflicting evidence and? Represents a lack of available data for the indicated outcome. Black arrows indicate pathway and drug interactions, with arrow points indicating induction and ovals indicating inhibition. The yellow box indicates that these pathways respond in a similar direction to the interacting pathway indicated by the black lines. AICAR, aminoimidazole carboxamide ribonucleotide; AMPK, AMP-activated protein kinase; mTOR, mammalian target of rapamycin; NMN, nicotamide mononucleotide; SIRT, sirtuin; STACs, sirtuin activating compounds.
mTOR inhibition via rapamycin treatment also exerts a beneficial effect on central arterial stiffness. Rapamycin treatment in kidney transplant patients results in lower central arterial stiffness, assessed by PWV and carotid AI, compared with patients that receive cyclosporine treatment for immunosuppression149. Lower central arterial stiffness in these patients was also accompanied by a reduction in blood pressure in the peripheral (brachial) and central (carotid) arteries149. In old mice, we have shown that age-related aortic stiffening is attenuated following 6 weeks of dietary rapamycin treatment84. In contrast to human data, rapamycin-mediated reductions in aortic stiffness in old mice were independent of blood pressure changes84. The reductions in aortic stiffness after mTOR inhibition in mice are likely related to structural changes in the aorta that are independent of blood pressure, as reductions in aortic stiffness in old mice following 6-weeks of rapamycin treatment were accompanied by lower aortic collagen and AGEs content, although no difference in aortic elastin content was present between groups84. Additionally, there may be other cardiac benefits associated with mTOR inhibition that may influence arterial stiffness, as rapamycin is a potential therapy for cardiac hypertrophy154 and vascular restenosis155. Although the results of studies utilizing mTOR inhibition to improve vascular aging phenotypes are promising, studies that directly target mTOR in the vasculature are scarce. Further study is warranted to determine if chronic inhibition of the mTOR pathway via rapamycin or other rapamycin analog treatments as well as whether more direct targeting of mTOR signaling in vascular cell types are efficacious to improve cardiovascular health in older adults.
AMPK.
AMPK is a highly conserved heterotrimeric serine-threonine kinase that is an important energy sensing signaling protein that integrates energy balance, metabolism, and stress resistance156. AMPK is made up of a catalytic α subunit, a structural β subunit and the AMPK binding site containing γ subunit157. AMPK activation requires phosphorylation of its α subunit, and occurs in response to increased AMP:ATP ratio, as well as physiological stimuli such as shear stress, heat shock, exercise, and hypoxia157. Interestingly, blunting age-related physiological dysfunction after mTOR inactivation also results in increased AMPK activity144; whereas AMPK activation results in direct mTOR inhibition via phosphorylation of Raptor, a critical mTOR adaptor protein158, 159. Additionally, AMPK signaling has been shown to be upregulated in S6K1 knockout mice that have reduced mTOR signaling144. Pharmacological AMPK activation can be achieved by aminoimidazole carboxamide ribonucleotide (AICAR) or metformin157. AICAR is a direct activator of AMPK via phosphorylation at the α2 subunit157, whereas metformin inhibits Complex I of the respiratory chain160, indirectly elevating the AMP:ATP ratio161. Importantly, indirect activation of AMPK by metformin also stimulates glycolysis, which may result in numerous AMPK-independent effects that impact the transcription factors and kinases involved in other cell cycle and metabolic pathways (p53, p38, MAPK, PKC and Akt)162–164. Therefore, metformin-mediated effects cannot solely be attributed to AMPK. Importantly, increased AMPK and decreased mTOR signaling are implicated in the longevity and physiological benefits associated with long term CR in mammals143, 144 and thus may be efficacious therapeutic targets to improve arterial function in advanced age.
AMPK activity has been shown to be reduced in the aorta165 and cerebral arteries166 of old rodents (Figure 5). In cultured ECs, AMPK is an activator of eNOS167, 168 that contributes to NO production via direct phosphorylation of eNOS169, 170, as well as through Rac1 and Akt signaling 168, 171. Using metformin treatment, AMPK activation has been shown to augment endothelial function in type 1 and 2 diabetic rodents172, 173. Similarly, AICAR treatment in old mice increases EDD, although this improvement is not mediated by increased NO bioavailability165. An NO independent effect of AICAR was also found after acute in vitro administration to isolated aortic rings174. AMPK activators may impact endothelial function through transcriptional regulation of proteins involved in inflammation, mitochondrial biogenesis, fatty acid and cholesterol synthesis, glucose metabolism, cell growth and oxidative stress signaling157. Indeed, after AMPK activation there is a reduction in inflammatory cytokines that is associated with blunted NFκB signaling in ECs175, 176. Thus, AMPK activators appear to improve endothelial function, but likely do so in an NO independent mechanism. There are few studies that have investigated the beneficial effects of AMPK activation on central arterial stiffness. In response to enhanced AMPK activity after metformin treatment, reductions in aortic PWV were observed in premenopausal women with polycystic ovary syndrome177. Although the effects of directly augmenting AMPK activity in old mice are unknown, accelerated high fat diet (HFD)-induced arterial stiffening and elevated collagen I expression are attenuated by AICAR in a mouse model deficient in the putative anti-aging gene, Klotho178. Taken together, these findings suggest that augmenting AMPK signaling increases bioavailability of NO and vasodilatory responses168, 171, 179, and although there is less direct evidence to suggest that increased AMPK activity lowers central arterial stiffness in advanced age, further study is warranted (Figure 5).
SIRTs.
SIRTs are a family of transcriptional regulators that play a major role in longevity129 and inflammation180. SIRTs were originally discovered in a screen for gene silencing factors in yeast and, therefore, given the name Sir2 (silent information regulator 2). Little research was conducted on the Sir2 family until these proteins were identified as critical regulators of longevity181. Thereafter, the mammalian Sir2 homologues, SIRTs 1–7, were quickly identified as nicotinamide adenine dinucleotide (NAD+)-dependent protein deacetylases182 and ADP-ribosyltransferases. SIRTs 1–4 have been implicated in the control of cellular metabolism with SIRT-2, 3 and 4 expressed in the mitochondria and SIRT-1 expressed predominately in the nucleus with some cytoplasmic expression183. The majority of the physiological benefits of CR on longevity can be attributed to the SIRT family and, specifically, SIRT-1183, 184. SIRT-1 acts by deacetylating histone and non-histone proteins185 to modify nuclear transcription factors, co-regulators and proteins to adapt gene expression in response to the cellular energy state and provide “stress resistance” by modulation of pro-inflammatory and oxidative stress pathways via deacetylating histone and non-histone proteins180, 185. Evidence for a critical role of SIRT-1 on longevity comes from studies in genetic models in which SIRT-1 deletion abolishes the lifespan extension afforded by CR186, while SIRT-1 overexpression recapitulates the CR phenotype187. These longevity-associated properties of SIRTs can also be ascribed to SIRT-6, which is also a NAD-dependent nuclear deacetylase that regulates lifespan188 and has been shown to be elevated in rats after CR189. Although emerging evidence suggests SIRT-6 appears to play a role in endothelial function190, the majority of vascular aging studies have focused on SIRT-1, which will be discussed further.
SIRT-1 activity has been associated with reductions in oxidative stress, inflammation and pro-apoptotic signaling, as well as improvements in insulin sensitivity, DNA damage repair, and telomere stability191. Age-related decreases in SIRT-1 expression are present in a multitude of tissues192, and our group has demonstrated age-related reductions in SIRT-1 expression and activity in the vasculature of humans and mice43, 52, 192, 193, which are associated with endothelial dysfunction in old mice43, 192. However, reduced SIRT-1 expression and endothelial dysfunction can be prevented by short and long-term calorie restriction41, 43, 52. Additionally, lifelong transgenic whole-body overexpression of SIRT-1 prevents the age-related decline in endothelium-dependent vasodilation194. SIRT-1 has been reported to modulate NO and endothelial function in small and large arteries directly via deacetylation and subsequent activation of eNOS192, 195. To investigate mechanisms for which activation of SIRT-1 prevents/ameliorates age-related vascular dysfunction, several SIRT-1 activators have been investigated, with the majority of studies utilizing the naturally occurring polyphenol, resveratrol196. Extremely high doses of resveratrol (2400 mg/kg of food) have been reported to improve EDD in isolated arteries from middle-aged animals after 1 year of treatment197. Although there appears to be an improvement in the vascular aging phenotype after resveratrol treatment198, there was no effect on lifespan197, 199, 200. Additionally, resveratrol activates up to 15 other unique pathways, and is a potent antioxidant and phytoestrogen196, 201, 202. Therefore, as with metformin, the pleiotropic resveratrol and other non-specific agents are not optimal to investigate mechanisms underlying the beneficial effects of SIRT-1, but these agents do afford insights as potential therapeutic agents.
Supplementation with nicotinamide mononucleotide (NMN), a key NAD+ intermediate, has also been shown to increase SIRT-1 activation203, resulting in reversal of mitochondrial dysfunction in advanced age204. In old mice, chronic NMN supplementation increases SIRT-1 activity and reverses age-related endothelial dysfunction and oxidative stress205, and has also recently been shown to increase skeletal muscle angiogenesis and blood flow leading to augmented endurance exercise capacity206. However, in middle-aged and older adult humans, augmented NAD+ activity via 6 weeks of supplementation with the NAD+ precursor, nicotinamide riboside, was not effective in improving EDD, as determined by brachial artery flow-mediated dilation207. In addition, direct activation of SIRT-1 can be achieved by treatment with the small molecule, SRT-1720, which has been shown to increase lifespan and preserve glucose tolerance in aged rodents208. In addition, our group has demonstrated that four weeks of SRT-1720 treatment restores SIRT-1 activity and reduces age-related NFκB acetylation, arterial inflammation and oxidative stress in old mice193. Yet surprisingly, SRT-1720 improvements in EDD are mediated not by increases in NO, but by increased reliance on COX-2 vasodilators193. Therefore, despite age-related reductions in vascular SIRT-1 that accompany reduced NO bioavailability and endothelial dysfunction, increased activation of SIRT-1 with SRT-1720 improves endothelial function and reduces inflammation and oxidative stress, but these effects are not associated with a restoration of NO.
SIRT-1 also plays a role in central arterial stiffness, as age-related reductions in aortic SIRT-1 expression are accompanied by elevated aortic stiffness in old mice (Figure 5)43, 192. Moreover, transgenic whole-body overexpression of SIRT-1 prevents aortic stiffening in old mice194, and, recently, our laboratory has observed lower aortic stiffness in all age groups (i.e., ages 3–24 mo), as well as a slower rate of aortic stiffening with advancing age in transgenic SIRT-1 overexpressing mice209. We also observed that a youthful aortic elastin:collagen ratio is maintained in 24 mo old transgenic SIRT-1 overexpressing mice, indicating that SIRT-1 overexpression prevents adverse structural changes in arteries, possibly through its negative regulation of MMP-9 and/or a positive regulation of TIMP-1210, 211. Age-related reductions in aortic SIRT-1 expression and the associated aortic stiffness43, 192 can be prevented by CR41, 43, 52. Pharmacologic SIRT-1 activation has also been shown to be beneficial for aortic stiffening. In non-human primates, high-fat and high-sucrose diet-induced increases in aortic stiffness can be prevented by resveratrol212. NMN supplementation-mediated increases in arterial SIRT-1 activity can reverse age-related aortic stiffening and normalize aortic collagen and elastin content in old mice205. Importantly, reductions in aortic stiffness, as well as blood pressure, have been reported in middle-age and older adults after 6 weeks of nicotinamide riboside supplementation207. Taken together these data suggest that SIRT-1 activation may hold significant promise for ameliorating endothelial dysfunction and aortic stiffening with aging or CVD. Future studies utilizing small molecule activators of SIRTs are needed to determine if they can ameliorate age-related CVD pathologies.
Klotho.
The Klotho gene was discovered in 1997 and named after Clotho, one of the three Fate sisters that was responsible for spinning the thread of human life in ancient Greek mythology. In the initial study, Kuro-o et al demonstrated that inactivation of Klotho resulted in a prematurely aged phenotype in mice characterized by infertility, arteriosclerosis, skin atrophy, osteoporosis, emphysema, as well as a short lifespan, with homozygous Klotho knockout (Klotho−/−) mice dying at 8–9 weeks of age213. Importantly, Klotho−/− mice were indistinguishable from wild-type mice until 3–4 weeks old, indicating that Klotho deletion-related effects were not due to incomplete development213. In follow up studies, transgenic overexpression of Klotho was shown to extend lifespan in mice by 20–30%214, promote cardioprotection215, and reduce oxidative stress216. The Klotho protein appears to exist in two forms, as a transmembrane protein that is bound to fibroblast growth factor receptor and as a circulating protein following cleavage from its transmembrane form217. As a circulating protein, Klotho appears to exert anti-aging effects by suppressing growth factor signaling and oxidative stress214, 216. In general, Klotho declines with advancing age218, resulting in a two-fold reduction in circulating Klotho concentrations from age 40 to 70 years in humans219. Additionally, genetic variants of Klotho can affect its secretion, as heterozygous expression of the KL-VS Klotho variant results in greater Klotho secretion220 and is associated with longevity and cardiovascular health221, 222. However, for unknown reasons, homozygous KL-VS Klotho variant results in lower Klotho secretion223, and is inversely associated with longevity and risk of CVD221, 222, 224. Still other Klotho genetic variants are associated with a greater risk of CVD225. Although Klotho variants are common and appear to have some relevance in aging and CVD by altering Klotho secretion, further discussion on the intricacies of functional Klotho variants is beyond the scope of this review.
Whether Klotho is expressed within the arterial vasculature remains a highly controversial topic226, but there are reports that Klotho is expressed in both human and mouse arteries227–229. Despite the controversy regarding arterial Klotho expression, whole-body Klotho haplodeficiency (Klotho+/−), which results in lower circulating Klotho protein230, reduces aortic eNOS expression 230 and impairs aortic and resistance arterial endothelial function in mice231 (Figure 5). Accordingly, Klotho deficiency-associated endothelial dysfunction can be reversed by parabiosis between wild-type and Klotho+/− mice231. Additionally, in Otsuka Long-Evans Tokshuma fatty (OLETF) rats, a model of type-2 diabetes and obesity, adenovirus-mediated Klotho gene delivery reverses aortic endothelium-mediated vasorelaxation and augments NO production232. In addition to its endothelial protective role, Klotho appears to also exert hypotensive effects, as Klotho gene delivery in OLETF rats led to a lower systolic blood pressure and prevented medial hypertrophy in the aorta232. Although this blood pressure lowering effect was not consistent between rodent models, as no reduction in blood pressure was observed after Klotho gene delivery in spontaneously hypertensive rats233, the underlying physiological mechanisms for elevated blood pressure in the spontaneously hypertensive rat may limit the generalizability of findings from that model. Nevertheless, there is an apparent inverse relationship between Klotho and aortic stiffness, as serum Klotho concentrations are ~45% lower in patients with elevated arterial stiffness and hypertension230. In mice, Klotho+/− results in greater aortic stiffness and blood pressure that are accompanied by a reduced elastin: collagen ratio, as well as elevated MMP-2 and MMP-9 expression230, 234. Despite equivocal findings on the ability of Klotho gene delivery to lower blood pressure, augmenting Klotho does appear to increase antioxidant defenses in rats and mice, and this is a likely mechanism underlying the improvements in endothelial function233.
In previous reviews we have commented on the interdependence of mTOR, AMPK, and SIRT-1 as they relate to the aging vasculature235, illustrating that decreased AMPK activity reduces SIRT-1 responses to low energy states236, whereas SIRT-1 directly interacts with the mTORC1 complex and SIRT-1 inhibition via nicotinamide increased mTOR activation237. We now expand on those interactions, update with recent findings, and also include a discussion of Klotho, as it appears to have some interaction with and/or governance over these pathways (Figure 5). Interestingly, Klotho+/− mice have reduced aortic SIRT-1 and AMPK expression230. Consequently, HFD-induced arterial stiffening and elevated collagen I expression are attenuated by AICAR in Klotho+/− mice178, while Klotho deficiency-induced aortic stiffness and hypertension are ameliorated after SRT-1720 treatment in Klotho+/− mice230. Moreover, vascular calcification, associated with increased mTOR activation, results in lower Klotho expression that can be reversed by rapamycin treatment237. Alternatively, rapamycin treatment is ineffective at reducing vascular calcification when Klotho is deleted in Klotho−/− mice229. Collectively, these studies suggest that Klotho deficiency downregulates AMPK and SIRT-1 expression, which may contribute to age-related arterial stiffening230. Further, they suggest that while Klotho is required for some of the vascular benefits afforded by mTOR inhibition, mTOR activity also impacts Klotho expression.
Cellular Senescence in Vascular Aging.
Cellular senescence is defined as irreversible cell cycle arrest, and occurs in response to critically short telomeres, excessive mitogenic signals238, increases in extracellular or intracellular stressors like oxidative stress239, chromatin disruptions240, and DNA damage 241. First described by Leonard Hayflick as an in vitro serial passage phenomenon in human fibroblasts, it is hypothesized that senescence develops in order to provide a critical tumor suppressive mechanism that prevents passing damaged DNA to daughter cells242, 243. Senescence is induced by the two major tumor suppressor pathways, known as the tumor suppressor protein, p53 (p53)/cyclin-dependent kinase inhibitor 1A (p21) and cyclin-dependent kinase inhibitor 2A p16/retinoblastoma-like protein 1 (pRB) pathways244. The preference toward one pathway versus another appears to be cell type-specific244, 245 with variation across species246. Currently, a preponderance of data suggests that senescence via the p53/p21 pathway is activated primarily by DNA damage and telomere dysfunction, while the p16/pRB pathway is linked primarily to mitogenic stress, chromatin disruptions, or general cellular stress244, 245, 247, although exceptions can be found. Senescent cells are characterized by a pro-inflammatory, oxidant senescence-associated secretory phenotype (SASP)242 that occurs within a few days of senescence induction and appears to be irreversible due to stable chromatin modifications around clusters of SASP genes248, 249. The release of classical SASP inflammatory mediators such as, IL-6, IL-1, IL-8, TNF-α, and monocyte chemoattractant protein-1 (MCP-1), as well as ROS production occurs when vascular cells are passaged to senescence250, 251 and can be found in ECs sampled from older adults43. The SASP likely reinforces cell cycle arrest in an autocrine fashion and activates immune cell surveillance of senescent cells242, 252. Furthermore, several studies support a major role for elevated NFκB activation in the induction and maintenance of the SASP in human and rodent cells, which, as previously mentioned, is a major inflammatory hub in age-related inflammation253, 254. Within the vascular compartment, senescent cells have been associated with a host of CVD states, such as atherosclerotic plaque255, abdominal aortic aneurysm256 and hypertension257. Furthermore, we and others have recently shown that senescent cells accumulate in normal arterial tissue over the lifespan of humans258, 259. We demonstrated that markers of senescence are increased in ECs taken from arteries and veins of older healthy adults. Importantly, there was a strong negative correlation between a marker of endothelial function (brachial artery flow-mediated dilation) and cyclin dependent kinase inhibitor, p16, such that higher expression of endothelial senescence markers was negatively associated with endothelial function. This demonstrated, for the first time, that in the absence of atherosclerosis, humans accumulate senescent ECs in vivo, which are in turn linked with attenuated endothelial function. This relation has been scientifically demonstrated in aged mice, where the clearance of senescent cells by genetic manipulation and removal of p16 positive cells or senolytic treatment to clear senescent cells leads to improved vascular endothelial function260.
Taken together, it is clear that vascular cell senescence is sufficient to blunt vascular function with advancing age. Conceptually, vascular cell senescence likely contributes to the spread of vascular inflammation and oxidative stress from senescent cells to healthy non-senescent neighboring cells via the SASP (Figure 6). Thus, promoting chronic low-grade inflammation and subsequent oxidative stress in non-senescent vascular cells. In summation, these alterations blunt NO and endothelial function and could lead to elevations in large artery stiffness through chronic matrix remodeling. Lastly, this chronic inflammation and oxidative stress could persist and accumulate indefinitely in the vasculature until removal of the senescent cells. Therefore, until clear mechanisms of age-related vascular senescence are elucidated, therapeutic options targeting senescence mediated dysfunction will be limited to senolytics or to the chronic treatment of the associated inflammation or oxidative stress pathways. While the precise mechanisms of cellular senescence induction are still unclear, several proof of concept hypotheses have been tested in relation to genomic instability which will be discussed further in the next section.
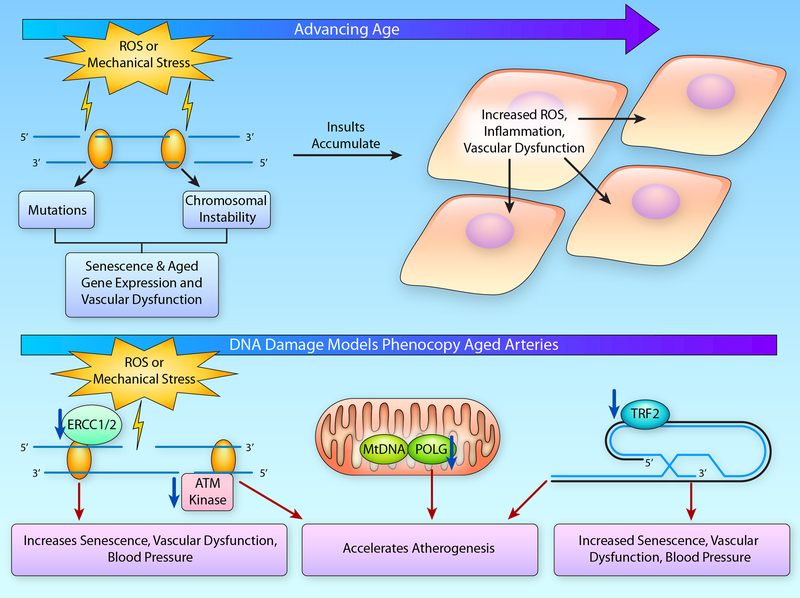
Figure 6. DNA Damage with Aging.
(A) With advancing age, DNA damage resulting from reactive oxygen species (ROS) or mechanical stress accumulates leading to mutations or chromosomal instability that ultimately contributes to cellular senescence or altered gene expression that drives the age-related pro-inflammatory and pro-oxidant vascular phenotype. This cellular dysfunction acts in an autocrine and paracrine manner affecting the local milieu and exacerbating endothelial dysfunction. (B) Indeed, DNA damage induced by ionizing radiation leads to cellular senescence and vascular dysfunction. Likewise, experimental manipulation genes involved in telomere capping, such as telomeric repeat binding factor (TRF)-2, and those involved in both nuclear DNA repair, including excision repair cross-complementation group (ERCC)-1/2 and ataxia-telangiectasia mutated (ATM) kinase, as well as in mitochondrial (Mt) DNA repair, such as polymerase gamma (POLG), will lead to increases in cellular senescence, vascular dysfunction and atherogenesis. (Illustration Credit: Ben Smith).
Age related Genomic Instability: Inducer of vascular senescence and dysfunction
One emerging molecular mechanism that explains the occurrence of senescent vascular smooth muscle cells and ECs in older adults is genomic instability. Genomic instability, or DNA damage acquired over time, is an important mechanism that may underlie the age-related accumulation of senescent cells reported in arterial tissues258, 259. Age-related genomic instability in vascular cells can occur from a variety of genotoxic insults, including oxidative stress261, 262 and mechanical stress263, resulting in potentially harmful mutations or chromosomal instability. It has been well described that augmented DNA damage via ionizing radiation leads to a succession of events including vascular apoptosis, cellular senescence, vascular dysfunction and subsequent end organ damage (reviewed in detail by Wang et al.264). In recent years, numerous studies have highlighted the role of dysregulated DNA damage repair pathways in the development of vascular cell senescence, dysfunction and/or pathology. Specifically, reduced expression of a major double stranded DNA (dsDNA) break repair protein, ataxia-telangiectasia mutated (ATM) protein kinase, via haploinsufficiency, accelerates the progression of atherosclerosis in an ApoE model265. This atheroprone phenotype is associated with elevated mitochondrial ROS production, highlighting the interplay between genomic damage and mitochondrial function265. Furthermore, mutations in two other DNA damage repair factors result in more subtle phenotypes typical of those seen in aged mice. Deficiency of the DNA excision repair proteins 1 and 2 (ERCC 1 and 2) and advanced age in mice both result in profound increases in senescent cells within vasculature, increases in large artery stiffening, systolic blood pressure and endothelial dysfunction266. Interestingly, the emergence of these phenotypes only occurs with chronological aging of the mice, evidence that it likely takes time for the DNA mutations and damage to accumulate, and it is through their accumulation that senescence and subsequent vascular dysfunction is induced. Similarly, mutations in mitochondrial DNA can influence vascular function. Accumulation of mitochondrial DNA damage in mice that lack a critical mitochondrial polymerase gamma (POLG), a catalytic unit of mitochondrial DNA polymerase, have accelerated atherogenesis in comparison to wild type POLG/APOE deficient mice267. This finding has been supported by human studies in which atheromas are more severe in arteries that display greater amounts of mitochondrial DNA damage267. Taken together, these data suggest that DNA damage is associated with vascular aging and associated CVDs, and that inducing DNA damage in rodent models is sufficient to produce an arterial aging phenotype.
Lastly, telomere dysfunction is another form of genomic instability that may lead to cellular senescence with advancing age. Telomere dysfunction, defined here as dsDNA damage response occurring at the telomere, can occur in several ways and will end in repair (if possible) or cell cycle arrest. First, telomere dysfunction can occur in response to critically short telomeres that are generated by numerous divisions, extensive genotoxic damage to the telomere, or by the cell being formed with very short telomeres. Some investigators estimate the critically short telomere length to be around 300–400 bp268, 269. A critically short telomere will elicit a dsDNA damage signal due the substantially shortened telomere being unable to form the T-loop structure at the end of the chromosome. Breakdown of the T-loop structure gives the appearance of a dsDNA break, which in turn elicits a response from the DNA damage machinery270. This is the mechanism hypothesized to induce senescence in primary cells that are serially passaged271. A second mechanism of telomere dysfunction is the occurrence of true dsDNA breaks at the telomeric DNA. Telomeric DNA is particularly guanine rich, making it particularly susceptible to oxidation272. Therefore, it is feasible that this may directly promote dsDNA breaks and the subsequent response. Last, alterations in the shelterin proteins that form and maintain the T-loop may be damaged, dysfunctional, or present in insufficient amounts. This would also result in uncapping of the T-loop and a dsDNA response at the telomeric DNA. Importantly, we do not define shortening of telomeres per se as dysfunctional. Telomere length may provide important information about the proliferation status of immune cells with aging or possibly vascular cells in atherogenesis, but it provides little information on the status of the canonical signaling pathway necessary to induce cellular senescence. Furthermore, data suggest that mean telomere length in the vasculature is not associated with classic cellular senescence signaling via p53 and p21259. Therefore, when examining mechanistic determinants of cellular senescence via telomere dysfunction, telomere length alone is not sufficient, and a secondary measure of DNA damage at the telomere (IF-FISH, ChIP assay etc.) is likely necessary to capture the complete picture of telomere dysfunction within a cell or tissue.
Currently, while several studies have demonstrated telomere attrition in the vasculature with aging and age-related diseases, only one has shown that healthy aging results in age-related telomere dysfunction with dsDNA breaks localized to telomeric DNA259. Furthermore, in arteries from hypertensive patients, telomere length was similar to normotensive patients, but the arteries of hypertensive patients demonstrated greater telomeric dsDNA damage. Recent proof of concept studies suggest that telomere dysfunction is sufficient to induce arterial dysfunction, as critically short telomeres may induce endothelial dysfunction273. However, these effects may be confounded by the established beneficial effects of telomerase on vascular function that is mediated through reductions in mitochondrial ROS, rather than through direct effects on telomere length274. Furthermore, a recent study that utilized inducible deletion of the shelterin protein, telomeric repeat binding factor 2 (TRF2), suggests that telomere dysfunction and subsequent vascular senescence is sufficient to induce large and small artery endothelial dysfunction, loss of NO, and increases in systolic blood pressure275. Similarly, an atherogenic mouse model of TRF2 loss of function in vascular smooth muscle cells demonstrated increased atherosclerotic plaque formation and markers of senescence276. Therefore, induction of telomere dysfunction is sufficient to promote atherogenesis as well as age-related arterial phenotypes.
Taken together, mouse models of augmented DNA damage, blunted DNA damage repair (genomic or mitochondrial) and dysfunctional telomeres demonstrate age like vascular phenotypes which include senescence, inflammation, oxidative stress, endothelial dysfunction, large artery stiffness and elevated blood pressure. While this is promising, these animal models still do not provide mechanistic proof that these genomic changes are responsible for arterial changes seen in older animals or humans. Future studies need to provide data to demonstrate that manipulation of these pathways can attenuate or reverse age-related changes.
Role of the Vasculature in the Rate of Biological Aging.
While age-related arterial dysfunction is heavily tied to CVD, emerging research is also interested in elucidating the role of ageing in different vascular cells types (e.g., ECs and vascular smooth muscle cells) in diseases beyond traditional CVDs. The potential for vascular cells to influence age-related non-CVDs and lifespan can be appreciated by realizing that the human vasculature is expansive, residing in nearly every tissue of the body. It is also estimated that ECs represent 2.1% of the total cell number (6×1011 total number of ECs) in the human body, making it one of the highest of the non-circulating cell types. For comparison, adipocytes are estimated at 0.2%, hepatocytes at 0.8% and red blood cells at ~84% of the total cells in the body277. Therefore, it is reasonable that the phenotype of ECs may independently influence the surrounding tissue, via alterations in blood flow, nutrient delivery, immune cell infiltration, permeability etc. and through their function as a secretory tissue, releasing bioactive molecules that could then alter the cellular homeostasis of the surrounding tissues. The latter is an interesting concept that we will explore in the next paragraphs.
Supporting a vascular-centric theory of biological aging, Hasegawa et al.278 reported reduced markers of cellular senescence (i.e. β galalactosidase staining) after reducing the pro-inflammatory NFκB pathway in ECs using the endothelial-specific (Tie2 Cre) dominant negative I kappa B-alpha (IkBα) in normal chow fed old mice. The mice also demonstrated blunted adhesion molecule expression and greater systemic NO bioavailability, and prevention of age-related increases in blood pressure. Interestingly, lifespan also increased, and while the group size was small (N=21–25) for a lifespan cohort study, these are promising data that should stimulate future studies to better understand the role of endothelial inflammation, senescence and NO in lifespan. An increased lifespan in this animal model is particularly compelling as the cause of death in aged rodents is not typically cardiovascular in nature, therefore implying that this manipulation may alter cancer and kidney diseases which are the major causes of death in laboratory rodents279. Hasegawa et al. also examined the role of attenuated endothelial NFκB signaling in high fat diet-induced insulin resistance, an expression of metabolic dysfunction that is similar to that which occurs as a consequence of aging in mouse models278.
Role of Vascular Aging in Metabolic Dysfunction.
Genetic blockade of pro-inflammatory signaling in the endothelium prevented obesity- and age-associated systemic insulin resistance, adipose inflammation, and was associated with increases in skeletal muscle blood flow278. These studies provide direct evidence of a role of endothelial function in the control of metabolic homeostasis and add to the evidence that dysfunction of the arteries contributes to age-associated metabolic syndrome/insulin resistance. The prevalence of metabolic syndrome, increases with advancing age, is associated with peripheral insulin resistance280 and increases patient risk for diabetes and CVDs, as well as CVD and all-cause mortality281.
In the setting of metabolic syndrome282 and aging283, the adipose is a metabolically important endocrine organ, contributing significantly to systemic inflammation. With aging and obesity, immune cells infiltrate adipose tissue and become important sources of circulating cytokines and adipokines that alter systemic insulin sensitivity. Because the endothelium is the site of immune cell adhesion, and is itself a source of inflammatory cytokine and adhesion molecules, the vascular endothelium plays a critical role in the maintenance of metabolic homeostasis via modulation of tissue inflammation284. In the setting of aging, reduced EDD and impaired angiogenic sprouting in adipose tissue have been associated with metabolic dysfunction and adipose tissue inflammation and oxidative stress285, suggesting that endothelial function in the adipose tissue may be an important determinant of metabolic homeostasis with advancing age.
Adipose tissue blood flow also influences metabolic and endocrine dysfunction in insulin resistant states. Both fasted and postprandial blood flow to adipose tissue is impaired in obesity, and may contribute to altered lipid metabolism and increased CVD risk286. There is also tight coupling between the vascular and metabolic functions in the adipose tissue, with adipose tissue blood flow and lipolysis increasing in response to β-adrenergic stimulation, and vasoconstriction and suppression of lipolysis in response to α-adrenergic stimulation287. Thus, adrenergic modulation of vascular tone and lipolysis has an important role in the normal control of metabolism. Taken together, these studies provide evidence that systemic metabolic homeostasis is importantly modulated by vascular control of tissue blood flow and by inflammation.
Influence of Vascular Aging on Alzheimer’s Disease and Cognitive Function.
AD pathology is another condition in which the role of vascular dysfunction is becoming increasingly appreciated. The pathology of AD has been well characterized with the accumulation of both amyloid beta (Aβ) plaques, neurofibrillary tangles (aggregates of Tau) and inflammation in neurons and in the neurovascular unit288. Although the vascular contribution to non-familial AD progression was overlooked for A long time, it is now clear that the vasculature and CVD risk factors influence the pathological progression of AD289. A recent meta-analysis demonstrated that AD risk is increased in patients who have had a prior stroke290, but stroke alone may not completely explain vascular links to AD. For example, autopsies performed on deceased patients in the Baltimore Longitudinal Study of Aging revealed that intracranial atherosclerosis increased dementia risk that was independent of the presence of AD-related pathologies or cerebral infarcts291. Furthermore, age-related elevations in large elastic artery stiffness are associated with greater cerebral artery pulse pressure, cognitive impairments, and AD diagnosis292–294. Taken together, these studies suggest that two of the major age-related arterial changes, large artery stiffness and cerebrovascular endothelial dysfunction, may play a critical role in the development of cognitive impairment and AD, both through increased stroke risk as well as through other, as of yet unknown, factors.
Emerging data that support the role of age-related large elastic artery stiffness and enhanced pulsatility in the development of cerebrovascular dysfunction and subsequent cognitive impairment and AD pathology has recently been reviewed in detail295. Briefly, increased large artery stiffness may propagate pulsatility to the cerebral circulation, disturbing auto-regulation via impaired myogenic tone296, with subsequent alterations in regional cerebral perfusion and impaired neurovascular coupling297. Other consequences include endothelial dysfunction, impairments in blood brain barrier function, altered angiogenesis, and perfusion-related outcomes298, 299. Age-related changes in moment-to-moment cerebral blood flow regulation have been associated with altered neuronal activity297, and abnormal neurovascular coupling is associated with cognitive decline with aging300. Furthermore, a direct link between impaired neurovascular coupling, functional hyperemia and measures of spatial and recognition memory has been shown in a mouse model301.
While all of these changes may underlie increasing neurovascular inflammation and neuronal damage, small artery cerebral endothelial dysfunction in particular may be involved in many etiologies involved in cognitive decline and AD. Indeed, endothelial dysfunction in the small cerebral arteries likely influences inflammatory/oxidative stress in the neurovascular unit, blood flow regulation via altered resistance artery tone, blood brain barrier function, and adhesion and/or infiltration of immune cells. Additionally, cerebral artery endothelial function may directly influence the β-amyloid precursor protein processing and the activity of critical Aβ production enzymes via endothelial NO production (for review, see302). Thus, age-related impairments in cerebral vascular function, due to enhanced large artery stiffness and endothelial dysfunction, are a likely initiation site for increased Tau and Aβ accumulation, neuroinflammation, and neurodegeneration, thereby contributing to future progression of cognitive impairment and AD.
Taking the evidence from metabolic syndrome and AD together, vascular dysfunction appears to be an important component of some chronic, age-related disease states in addition to those more traditionally thought of as CVDs. Gaining a better appreciation of the underlying mechanisms and regional differences in age-related vascular dysfunction may lead to improvements in treatments for these disorders with concomitant improvements in healthspan.
Conclusions
In summary, we have described the role of arterial aging in the progression of clinical CVD, which has a major societal impact via morbidity and mortality in the US, as well as the vast majority of developed nations. Also presented were several poignant examples of how the vasculature impacts non-CVD states, as well as evidence that the vasculature not only plays a role in mammalian healthspan but in lifespan as well. However, the role of the vasculature in non-CVD age-related diseases and lifespan is insufficiently understood, reinforcing the importance of research on the biological aging processes in this dynamic tissue. Age-related oxidative stress and inflammation in arteries interact to suppress vascular function, but this state can be prevented by lifestyle intervention to ultimately extend healthspan and lifespan. Emerging evidence suggests several promising pathways such as Klotho or energy-sensing pathways, e.g., SIRTs, mTOR and AMPK, that are attractive pharmacological targets for interventions, the manipulation of which have been demonstrated, in some cases, to restore normal youthful pathway activity in older mammals (Figure 2,5). While anti-aging therapies per se were not a sole focus of the present review, the reader is directed to a recent review expanding on this area of research303. Unfortunately, we as a research community still do not understand the critical initiating event(s) that alter the cellular machinery to induce the well described “vicious cycle” of oxidative stress and inflammation. While some focus on the effects of acute alterations in circulating lipids, glucose, or circulating factors associated with sedentary aging, others seek to define the role of molecular events as potential key initiators, as we described in detail with genomic instability and cellular senescence. Importantly, it is likely that these areas are related, suggesting the necessity for strong cross-field collaborations to best answer this critical question. Finally, treating older adults with prescription drugs to ameliorate age-related vascular dysfunction to prevent future CVD is not currently accepted in the absence of clinical disease. While clinical guidelines continue to be altered to allow earlier treatment (most recently, the hypertensive cutoff for systolic blood pressure being reduced from 140 to 130 mmHg304), the ethical and moral obligations to society to treat arterial dysfunction are complicated, as a single drug treatment or lifestyle intervention may not always be effective in reversing this state. Overall, despite impressive advancements in understanding the biology of the aging vasculature, as aging researchers, we must strive to better elucidate pathways to identify and develop appropriate countermeasures against age-related arterial dysfunction. Ultimately, to quote the phrase popularized by Sir William Osler in the late 19th century, “you are only as old as your arteries”.
Acknowledgements
We would like to thank Yu Liu for help with designing the preliminary figures, Sam Bloom and Torikul Islam for help with specific literature reviews and Dr. Phil Gates with helping scientifically and editing this review.
Sources of Funding
This work was supported in part by awards from the National Institute of Aging, R01 AG048366, R01 AG050238, R44 AG053131 and K02 AG045339, National Center for Complementary and Integrative Health, K99 AT010017, by Merit Review Award 1I01BX002151 from the United States (U.S.) Department of Veterans Affairs Biomedical Laboratory Research and Development Service, and by an Advanced Fellowship in Geriatrics from the U.S. Department of Veterans Affairs Geriatric Research, Education, and Clinical Center. The contents do not represent the views of the U.S. Department of Veterans Affairs, the National Institute on Aging or the United States Government.
Abbreviations
- 4E-BP1
Eukaryotic translation factor binding protein
- 4-HNE
4-hydroxynonenal
- AD
Alzheimer’s disease
- AGE
Advanced glycation end product
- AI
Augmentation index
- AICAR
Aminoimidazole carboxamide ribonucleotide
- AL
Ad libitum
- Atg5
Autophagy-related gene 5
- ATM
Ataxia-telangiectasia mutated
- Aβ
Amyloid β
- BH4
Tetrahydrobiopterin
- CAD
Coronary artery disease
- cfPWV
Carotid-to-femoral pulse wave velocity
- CR
Caloric restriction
- CRP
C-reactive protein
- CuZnSOD
Copper zinc superoxide dismutase
- CVD
Cardiovascular disease
- dsDNA
Double stranded DNA
- EC
Endothelial cell
- ecSOD
Extracellular superoxide dismutase
- EDD
Endothelium dependent dilation
- eNOS
Endothelial nitric oxide synthase
- ERCC
DNA excision repair protein
- GSH
Glutathione
- GSH/GSSG
Reduced/oxidized glutathione
- H2O2
Hydrogen peroxide
- HFD
High fat diet
- IKKβ
Inhibitor of nuclear factor kappa B kinase subunit beta
- IkBα
I kappa B-alpha
- IL
Interleukin
- iNOS
Inducible nitric oxide synthase
- MCP1
Monocyte chemoattractant protein-1
- MDA
Malondialdehyde
- miR
microRNA
- MMP
Matrix metalloproteinases
- MnSOD
Manganese superoxide dismutase
- mTOR
Mammalian target of rapamycin
- mTORC1
Mammalian target of rapamycin complex 1
- mTORC2
Mammalian target of rapamycin complex 2
- NAD+
Nicotinamide adenine dinucleotide
- NFkB
Nuclear factor kappa B
- NMN
Nicotinamide mononucleotide
- NO
Nitric oxide
- NT
Nitrotyrosine
- O2-
Superoxide anion
- OLETF
Otsuka Long-Evans Tokshuma Fatty
- ONOO-
Peroxynitrite
- p21
Cyclin-dependent kinase inhibitor 1A
- p53
Tumor suppressor protein p53
- POLG
Mitochondria polymerase gamma
- pRB
Cyclin-dependent kinase inhibitor 2A p16/retinoblastoma-like protein 1
- PWV
Pulse wave velocity
- RAAS
Renin-angiotensin-aldosterone system
- ROS
Reactive oxygen species
- S6K1
S6 protein kinase
- SASP
Senescence associated secretory phenotype
- SBP
Systolic blood pressure
- SIRT
Sirtuin
- SOD
Superoxide dismutase
- TGF-β
Transforming growth factor-beta
- TIMP
Tissue inhibitor of matrix metalloproteinases
- TNF-α
Tumor necrosis factor alpha
- TRF2
Telomere repeat binding factor 2
- VCAM
Vascular cell adhesion molecule
Footnotes
Disclosures:
None.
REFERENCES
- 1.Heron MP. Deaths: Leading causes for 2011. 2015
- 2.Health, united states, 2010: With special feature on death and dying. Hyattsville (MD); 2011. [PubMed] [Google Scholar]
- 3.Lakatta EG, Levy D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part i: Aging arteries: A “set up” for vascular disease. Circulation. 2003;107:139–146 [DOI] [PubMed] [Google Scholar]
- 4.Widlansky ME, Gokce N, Keaney JF, Jr., Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160 [DOI] [PubMed] [Google Scholar]
- 5.Franklin SS, Khan SA, Wong ND, Larson MG, Levy D. Is pulse pressure useful in predicting risk for coronary heart disease? The framingham heart study. Circulation. 1999;100:354–360 [DOI] [PubMed] [Google Scholar]
- 6.Lakatta EG, Wang M, Najjar SS. Arterial aging and subclinical arterial disease are fundamentally intertwined at macroscopic and molecular levels. Med Clin North Am. 2009;93:583–604, Table of Contents [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vlachopoulos C, O’Rourke M, Nichols WW. Mcdonald’s blood flow in arteries: Theoretical, experimental and clinical principles. CRC press; 2011. [Google Scholar]
- 8.Machin DR, Bloom SI, Campbell RA, Phuong TTT, Gates PE, Lesniewski LA, Rondina MT, Donato AJ. Advanced age results in a diminished endothelial glycocalyx. Am J Physiol Heart Circ Physiol. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Bussel BC, Schouten F, Henry RM, Schalkwijk CG, de Boer MR, Ferreira I, Smulders YM, Twisk JW, Stehouwer CD. Endothelial dysfunction and low-grade inflammation are associated with greater arterial stiffness over a 6-year period. Hypertension. 2011;58:588–595 [DOI] [PubMed] [Google Scholar]
- 10.Quinn U, Tomlinson LA, Cockcroft JR. Arterial stiffness. JRSM Cardiovasc Dis. 2012;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.London GM, Guerin AP. Influence of arterial pulse and reflected waves on blood pressure and cardiac function. Am Heart J. 1999;138:220–224 [DOI] [PubMed] [Google Scholar]
- 12.Mazzaro L, Almasi SJ, Shandas R, Seals DR, Gates PE. Aortic input impedance increases with age in healthy men and women. Hypertension. 2005;45:1101–1106 [DOI] [PubMed] [Google Scholar]
- 13.Franklin SS, Gustin Wt, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D. Hemodynamic patterns of age-related changes in blood pressure. The framingham heart study. Circulation. 1997;96:308–315 [DOI] [PubMed] [Google Scholar]
- 14.James MA, Tullett J, Hemsley AG, Shore AC. Effects of aging and hypertension on the microcirculation. Hypertension. 2006;47:968–974 [DOI] [PubMed] [Google Scholar]
- 15.Kass DA. Age-related changes in venticular-arterial coupling: Pathophysiologic implications. Heart Fail Rev. 2002;7:51–62 [DOI] [PubMed] [Google Scholar]
- 16.Tarumi T, Ayaz Khan M, Liu J, Tseng BY, Parker R, Riley J, Tinajero C, Zhang R. Cerebral hemodynamics in normal aging: Central artery stiffness, wave reflection, and pressure pulsatility. J Cereb Blood Flow Metab. 2014;34:971–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pase MP, Grima NA, Stough C, Scholey A, Pipingas A. Association of pulsatile and mean cerebral blood flow velocity with age and neuropsychological performance. Physiol Behav. 2014;130:23–27 [DOI] [PubMed] [Google Scholar]
- 18.Xu TY, Staessen JA, Wei FF, Xu J, Li FH, Fan WX, Gao PJ, Wang JG, Li Y. Blood flow pattern in the middle cerebral artery in relation to indices of arterial stiffness in the systemic circulation. Am J Hypertens. 2012;25:319–324 [DOI] [PubMed] [Google Scholar]
- 19.Webb AJ, Simoni M, Mazzucco S, Kuker W, Schulz U, Rothwell PM. Increased cerebral arterial pulsatility in patients with leukoaraiosis: Arterial stiffness enhances transmission of aortic pulsatility. Stroke. 2012;43:2631–2636 [DOI] [PubMed] [Google Scholar]
- 20.Zarrinkoob L, Ambarki K, Wahlin A, Birgander R, Carlberg B, Eklund A, Malm J. Aging alters the dampening of pulsatile blood flow in cerebral arteries. J Cereb Blood Flow Metab. 2016;36:1519–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H, European Network for Non-invasive Investigation of Large A. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605 [DOI] [PubMed] [Google Scholar]
- 22.O’Rourke MF, Nichols WW. Potential for use of pulse wave analysis in determining the interaction between sildenafil and glyceryl trinitrate. Clinical cardiology. 2002;25:295–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241 [DOI] [PubMed] [Google Scholar]
- 24.Mitchell GF, Hwang S-J, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: The framingham heart study. Circulation. 2010;121:505–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waldstein SR, Rice SC, Thayer JF, Najjar SS, Scuteri A, Zonderman AB. Pulse pressure and pulse wave velocity are related to cognitive decline in the baltimore longitudinal study of aging. Hypertension. 2008;51:99–104 [DOI] [PubMed] [Google Scholar]
- 26.Lim J, Pearman ME, Park W, Alkatan M, Machin DR, Tanaka H. Impact of blood pressure perturbations on arterial stiffness. Am J Physiol Regul Integr Comp Physiol. 2015;309:R1540–1545 [DOI] [PubMed] [Google Scholar]
- 27.R B IF. Vascular endothelium and blood flow. Handb. Exp. Pharmacol. 2006;176:43. [DOI] [PubMed] [Google Scholar]
- 28.Pober JS, Min W, Bradley JR. Mechanisms of endothelial dysfunction, injury, and death. Annual Review of Pathology: Mechanisms of Disease. 2009;4:71–95 [DOI] [PubMed] [Google Scholar]
- 29.Mitchell GF, Parise H, Vita JA, Larson MG, Warner E, Keaney JF Jr, Keyes MJ, Levy D, Vasan RS, Benjamin EJ. Local shear stress and brachial artery flow-mediated diation: The framigham heart study. Hypertension. 2004;44:134–139 [DOI] [PubMed] [Google Scholar]
- 30.Vaitkevicius PV, Fleg JL, Engel JH, O’Connor FC, Wright JG, Lakatta LE, Yin FC, Lakatta EG. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation. 1993;88:1456–1462 [DOI] [PubMed] [Google Scholar]
- 31.Kohn JC, Zhou DW, Bordeleau F, Zhou AL, Mason BN, Mitchell MJ, King MR, Reinhart-King CA. Cooperative effects of matrix stiffness and fluid shear stress on endothelial cell behavior. Biophys J. 2015;108:471–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bellien J, Favre J, Iacob M, Gao J, Thuillez C, Richard V, Joannides R. Arterial stiffness is regulated by nitric oxide and endothelium-derived hyperpolarizing factor during changes in blood flow in humans. Hypertension. 2010;55:674–680 [DOI] [PubMed] [Google Scholar]
- 33.Seals DR, DeSouza CA, Donato AJ, Tanaka H. Habitual exercise and arterial aging. J Appl Physiol (1985). 2008;105:1323–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Durrant JR, Seals DR, Connell ML, Russell MJ, Lawson BR, Folian BJ, Donato AJ, Lesniewski LA. Voluntary wheel running restores endothelial function in conduit arteries of old mice: Direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of nadph oxidase. J Physiol. 2009;587:3271–3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jablonski KL, Racine ML, Geolfos CJ, Gates PE, Chonchol M, McQueen MB, Seals DR. Dietary sodium restriction reverses vascular endothelial dysfunction in middle-aged/older adults with moderately elevated systolic blood pressure. J Am Coll Cardiol. 2013;61:335–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gates PE, Tanaka H, Hiatt WR, Seals DR. Dietary sodium restriction rapidly improves large elastic artery compliance in older adults with systolic hypertension. Hypertension. 2004;44:35–41 [DOI] [PubMed] [Google Scholar]
- 37.Pierce GL, Beske SD, Lawson BR, Southall KL, Benay FJ, Donato AJ, Seals DR. Weight loss alone improves conduit and resistance artery endothelial function in young and older overweight/obese adults. Hypertension. 2008;52:72–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weindruch R, Lane MA, Ingram DK, Ershler WB, Roth GS. Dietary restriction in rhesus monkeys: Lymphopenia and reduced mitogen-induced proliferation in peripheral blood mononuclear cells. Aging (Milano). 1997;9:304–308 [DOI] [PubMed] [Google Scholar]
- 39.Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edwards AG, Donato AJ, Lesniewski LA, Gioscia RA, Seals DR, Moore RL. Life-long caloric restriction elicits pronounced protection of the aged myocardium: A role for ampk. Mech Ageing Dev. 2010;131:739–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Csiszar A, Labinskyy N, Jimenez R, Pinto JT, Ballabh P, Losonczy G, Pearson KJ, de Cabo R, Ungvari Z. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: Role of circulating factors and sirt1. Mech Ageing Dev. 2009;130:518–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walker AE, Henson GD, Reihl KD, Nielson EI, Morgan RG, Lesniewski LA, Donato AJ. Beneficial effects of lifelong caloric restriction on endothelial function are greater in conduit arteries compared to cerebral resistance arteries. Age (Dordr). 2014;36:559–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Donato AJ, Walker AE, Magerko KA, Bramwell RC, Black AD, Henson GD, Lawson BR, Lesniewski LA, Seals DR. Life-long caloric restriction reduces oxidative stress and preserves nitric oxide bioavailability and function in arteries of old mice. Aging Cell. 2013;12:772–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Belsky DW, Huffman KM, Pieper CF, Shalev I, Kraus WE. Change in the rate of biological aging in response to caloric restriction: Calerie biobank analysis. J Gerontol A Biol Sci Med Sci. 2017;73:4–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Most J, Gilmore LA, Smith SR, Han H, Ravussin E, Redman LM. Significant improvement in cardiometabolic health in healthy non-obese individuals during caloric restriction-induced weight loss and weight loss maintenance. Am J Physiol Endocrinol Metab. 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J. Physiol. 2004;556:315–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.JI LL, LEEUWENBURGH C, LEICHTWEIS S, GORE M, FIEBIG R, HOLLANDER J, BEJMA J. Oxidative stress and aging: Role of exercise and its influences on antioxidant systems. Ann. N.Y. Acad. Sci. 1998;854:102–117 [DOI] [PubMed] [Google Scholar]
- 48.Griendling KK, Sorescu D, Ushio-Fukai M. Nad(p)h oxidase : Role in cardiovascular biology and disease. Circ. Res. 2000;86:494–501 [DOI] [PubMed] [Google Scholar]
- 49.Harrison DG. Endothelial function and oxidant stress. Clin Cardiol. 1997;20:II-11–17 [PubMed] [Google Scholar]
- 50.Moncada S, Palmer RM, Higgs EA. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142 [PubMed] [Google Scholar]
- 51.Persson MG, Gustafsson LE, Wiklund NP, Hedqvist P, Moncada S. Endogenous nitric oxide as a modulator of rabbit skeletal muscle microcirculation in vivo. Br J Pharmacol. 1990;100:463–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rippe C, Lesniewski L, Connell M, LaRocca T, Donato A, Seals D. Short-term calorie restriction reverses vascular endothelial dysfunction in old mice by increasing nitric oxide and reducing oxidative stress. Aging Cell. 2010;9:304–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou X, Bohlen HG, Unthank JL, Miller SJ. Abnormal nitric oxide production in aged rat mesenteric arteries is mediated by nad(p)h oxidase-derived peroxide. Am J Physiol Heart Circ Physiol. 2009;297:H2227–2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hamilton CA, Brosnan MJ, McIntyre M, Graham D, Dominiczak AF. Superoxide excess in hypertension and aging : A common cause of endothelial dysfunction. Hypertension. 2001;37:529–534 [DOI] [PubMed] [Google Scholar]
- 55.Quijano C, Castro L, Peluffo G, Valez V, Radi R. Enhanced mitochondrial superoxide in hyperglycemic endothelial cells: Direct measurements and formation of hydrogen peroxide and peroxynitrite. Am J Physiol Heart Circ Physiol. 2007;293:H3404–3414 [DOI] [PubMed] [Google Scholar]
- 56.Baker CS, Hall RJ, Evans TJ, Pomerance A, Maclouf J, Creminon C, Yacoub MH, Polak JM. Cyclooxygenase-2 is widely expressed in atherosclerotic lesions affecting native and transplanted human coronary arteries and colocalizes with inducible nitric oxide synthase and nitrotyrosine particularly in macrophages. Arterioscler Thromb Vasc Biol. 1999;19:646–655 [DOI] [PubMed] [Google Scholar]
- 57.Myatt L, Rosenfield RB, Eis AL, Brockman DE, Greer I, Lyall F. Nitrotyrosine residues in placenta. Evidence of peroxynitrite formation and action. Hypertension. 1996;28:488–493 [DOI] [PubMed] [Google Scholar]
- 58.Miller SJ, Watson WC, Kerr KA, Labarrere CA, Chen NX, Deeg MA, Unthank JL. Development of progressive aortic vasculopathy in a rat model of aging. Am J Physiol Heart Circ Physiol. 2007;293:H2634–2643 [DOI] [PubMed] [Google Scholar]
- 59.Lesniewski LA, Connell ML, Durrant JR, Folian BJ, Anderson MC, Donato AJ, Seals DR. B6d2f1 mice are a suitable model of oxidative stress-mediated impaired endothelium-dependent dilation with aging. J Gerontol A Biol Sci Med Sci. 2009;64:9–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: Relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappab. Circ Res. 2007;100:1659–1666 [DOI] [PubMed] [Google Scholar]
- 61.Oelze M, Kroller-Schon S, Steven S, Lubos E, Doppler C, Hausding M, Tobias S, Brochhausen C, Li H, Torzewski M, Wenzel P, Bachschmid M, Lackner KJ, Schulz E, Munzel T, Daiber A. Glutathione peroxidase-1 deficiency potentiates dysregulatory modifications of endothelial nitric oxide synthase and vascular dysfunction in aging. Hypertension. 2014;63:390–396 [DOI] [PubMed] [Google Scholar]
- 62.Ungvari Z, Bailey-Downs L, Gautam T, Sosnowska D, Wang M, Monticone RE, Telljohann R, Pinto JT, de Cabo R, Sonntag WE, Lakatta EG, Csiszar A. Age-associated vascular oxidative stress, nrf2 dysfunction, and nf-{kappa}b activation in the nonhuman primate macaca mulatta. J Gerontol A Biol Sci Med Sci. 2011;66:866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Soucy KG, Ryoo S, Benjo A, Lim HK, Gupta G, Sohi JS, Elser J, Aon MA, Nyhan D, Shoukas AA, Berkowitz DE. Impaired shear stress-induced nitric oxide production through decreased nos phosphorylation contributes to age-related vascular stiffness. J Appl Physiol. 2006;101:1751–1759 [DOI] [PubMed] [Google Scholar]
- 64.Fleenor BS, Eng JS, Sindler AL, Pham BT, Kloor JD, Seals DR. Superoxide signaling in perivascular adipose tissue promotes age-related artery stiffness. Aging Cell. 2014;13:576–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moreau KL, Gavin KM, Plum AE, Seals DR. Ascorbic acid selectively improves large elastic artery compliance in postmenopausal women. Hypertension %R 10.1161/01.HYP.0000165678.63373.8c. 2005;45:1107–1112 [DOI] [PubMed] [Google Scholar]
- 66.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ Res. 2000;87:840–844 [DOI] [PubMed] [Google Scholar]
- 67.Santhanam AV, d’Uscio LV, Smith LA, Katusic ZS. Uncoupling of enos causes superoxide anion production and impairs no signaling in the cerebral microvessels of hph-1 mice. J Neurochem. 2012;122:1211–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol. 2005;568:1057–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sindler AL, Delp MD, Reyes R, Wu G, Muller-Delp JM. Effects of ageing and exercise training on enos uncoupling in skeletal muscle resistance arterioles. J Physiol. 2009;587:3885–3897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eskurza I, Kahn Z, Seals D. Xanthine oxidase does not contribute to impaired peripheral artery conduit artery endothelium-dependent dilatation with aging. J Physiol. 2006;571:661–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Donato AJ, Black AD, Jablonski KL, Gano LB, Seals DR. Aging is associated with greater nuclear nfkappab, reduced ikappabalpha, and increased expression of proinflammatory cytokines in vascular endothelial cells of healthy humans. Aging Cell. 2008;7:805–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Donato AJ, Eskurza I, Jablonski KL, Gano LB, Pierce GL, Seals DR. Cytochrome p-450 2c9 signaling does not contribute to age-associated vascular endothelial dysfunction in humans. J Appl Physiol. 2008;105:1359–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Newaz MA, Yousefipour Z, Oyekan A. Oxidative stress-associated vascular aging is xanthine oxidase-dependent but not nad (p) h oxidase-dependent. J Cardiovasc Pharmacol. 2006;48:88–94 [DOI] [PubMed] [Google Scholar]
- 74.Farquharson CA, Butler R, Hill A, Belch JJ, Struthers AD. Allopurinol improves endothelial dysfunction in chronic heart failure. Circulation. 2002;106:221–226 [DOI] [PubMed] [Google Scholar]
- 75.Doehner W, Schoene N, Rauchhaus M, Leyva-Leon F, Pavitt DV, Reaveley DA, Schuler G, Coats AJ, Anker SD, Hambrecht R. Effects of xanthine oxidase inhibition with allopurinol on endothelial function and peripheral blood flow in hyperuricemic patients with chronic heart failure: Results from 2 placebo-controlled studies. Circulation. 2002;105:2619–2624 [DOI] [PubMed] [Google Scholar]
- 76.Csiszar A, Gautam T, Sosnowska D, Tarantini S, Banki E, Tucsek Z, Toth P, Losonczy G, Koller A, Reglodi D, Giles CB, Wren JD, Sonntag WE, Ungvari Z. Caloric restriction confers persistent anti-oxidative, pro-angiogenic, and anti-inflammatory effects and promotes anti-aging mirna expression profile in cerebromicrovascular endothelial cells of aged rats. Am J Physiol Heart Circ Physiol. 2014;307:H292–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lemarie CA, Shbat L, Marchesi C, Angulo OJ, Deschenes ME, Blostein MD, Paradis P, Schiffrin EL. Mthfr deficiency induces endothelial progenitor cell senescence via uncoupling of enos and downregulation of sirt1. Am J Physiol Heart Circ Physiol. 2011;300:H745–753 [DOI] [PubMed] [Google Scholar]
- 78.Dikalov SI, Ungvari Z. Role of mitochondrial oxidative stress in hypertension. Am J Physiol Heart Circ Physiol. 2013;305:H1417–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ungvari ZI, Labinskyy N, Gupte SA, Chander PN, Edwards JG, Csiszar A. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am J Physiol Heart Circ Physiol. 2008 [DOI] [PubMed] [Google Scholar]
- 80.Gioscia-Ryan RA, LaRocca TJ, Sindler AL, Zigler MC, Murphy MP, Seals DR. Mitochondria-targeted antioxidant (mitoq) ameliorates age-related arterial endothelial dysfunction in mice. J Physiol. 2014;592:2549–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Francia P, delli Gatti C, Bachschmid M, Martin-Padura I, Savoia C, Migliaccio E, Pelicci PG, Schiavoni M, Luscher TF, Volpe M, Cosentino F. Deletion of p66shc gene protects against age-related endothelial dysfunction. Circulation. 2004;110:2889–2895 [DOI] [PubMed] [Google Scholar]
- 82.Kalani R, Judge S, Carter C, Pahor M, Leeuwenburgh C. Effects of caloric restriction and exercise on age-related, chronic inflammation assessed by c-reactive protein and interleukin-6. J Gerontol A Biol Sci Med Sci. 2006;61:211–217 [DOI] [PubMed] [Google Scholar]
- 83.Fukai T, Ushio-Fukai M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid Redox Signal. 2011;15:1583–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lesniewski LA, Seals DR, Walker AE, Henson GD, Blimline MW, Trott DW, Bosshardt GC, LaRocca TJ, Lawson BR, Zigler MC, Donato AJ. Dietary rapamycin supplementation reverses age-related vascular dysfunction and oxidative stress, while modulating nutrient-sensing, cell cycle, and senescence pathways. Aging Cell. 2017;16:17–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, Noble LJ, Yoshimura MP, Berger C, Chan PH, Wallace DC, Epstein CJ. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995;11:376–381 [DOI] [PubMed] [Google Scholar]
- 86.Fennell JP, Brosnan MJ, Frater AJ, Hamilton CA, Alexander MY, Nicklin SA, Heistad DD, Baker AH, Dominiczak AF. Adenovirus-mediated overexpression of extracellular superoxide dismutase improves endothelial dysfunction in a rat model of hypertension. Gene Ther. 2002;9:110–117 [DOI] [PubMed] [Google Scholar]
- 87.Brown KA, Didion SP, Andresen JJ, Faraci FM. Effect of aging, mnsod deficiency, and genetic background on endothelial function: Evidence for mnsod haploinsufficiency. Arterioscler Thromb Vasc Biol. 2007;27:1941–1946 [DOI] [PubMed] [Google Scholar]
- 88.Kang LS, Chen B, Reyes RA, Leblanc AJ, Teng B, Mustafa SJ, Muller-Delp JM. Aging and estrogen alter endothelial reactivity to reactive oxygen species in coronary arterioles. Am J Physiol Heart Circ Physiol. 2011;300:H2105–2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Satoh K, Godo S, Saito H, Enkhjargal B, Shimokawa H. Dual roles of vascular-derived reactive oxygen species--with a special reference to hydrogen peroxide and cyclophilin a. J Mol Cell Cardiol. 2014;73:50–56 [DOI] [PubMed] [Google Scholar]
- 90.Drummond GR, Cai H, Davis ME, Ramasamy S, Harrison DG. Transcriptional and posttranscriptional regulation of endothelial nitric oxide synthase expression by hydrogen peroxide. Circ Res. 2000;86:347–354 [DOI] [PubMed] [Google Scholar]
- 91.Beyer AM, Zinkevich N, Miller B, Liu Y, Wittenburg AL, Mitchell M, Galdieri R, Sorokin A, Gutterman DD. Transition in the mechanism of flow-mediated dilation with aging and development of coronary artery disease. Basic Res Cardiol. 2017;112:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Denniss SG, Levy AS, Rush JW. Effects of glutathione-depleting drug buthionine sulfoximine and aging on activity of endothelium-derived relaxing and contracting factors in carotid artery of sprague-dawley rats. J Cardiovasc Pharmacol. 2011;58:272–283 [DOI] [PubMed] [Google Scholar]
- 93.Lapenna D, de Gioia S, Ciofani G, Mezzetti A, Ucchino S, Calafiore AM, Napolitano AM, Di Ilio C, Cuccurullo F. Glutathione-related antioxidant defenses in human atherosclerotic plaques. Circulation. 1998;97:1930–1934 [DOI] [PubMed] [Google Scholar]
- 94.Morrison JA, Jacobsen DW, Sprecher DL, Robinson K, Khoury P, Daniels SR. Serum glutathione in adolescent males predicts parental coronary heart disease. Circulation. 1999;100:2244–2247 [DOI] [PubMed] [Google Scholar]
- 95.Prasad A, Andrews NP, Padder FA, Husain M, Quyyumi AA. Glutathione reverses endothelial dysfunction and improves nitric oxide bioavailability. J Am Coll Cardiol. 1999;34:507–514 [DOI] [PubMed] [Google Scholar]
- 96.Meydani M, Das S, Band M, Epstein S, Roberts S. The effect of caloric restriction and glycemic load on measures of oxidative stress and antioxidants in humans: Results from the calerie trial of human caloric restriction. J Nutr Health Aging. 2011;15:456–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schnabel R, Larson MG, Dupuis J, Lunetta KL, Lipinska I, Meigs JB, Yin X, Rong J, Vita JA, Newton-Cheh C, Levy D, Keaney JF Jr., Vasan RS, Mitchell GF, Benjamin EJ. Relations of inflammatory biomarkers and common genetic variants with arterial stiffness and wave reflection. Hypertension. 2008;51:1651–1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vita JA, Keaney JF, Jr., Larson MG, Keyes MJ, Massaro JM, Lipinska I, Lehman BT, Fan S, Osypiuk E, Wilson PW, Vasan RS, Mitchell GF, Benjamin EJ. Brachial artery vasodilator function and systemic inflammation in the framingham offspring study. Circulation. 2004;110:3604–3609 [DOI] [PubMed] [Google Scholar]
- 99.Csiszar A, Wang M, Lakatta EG, Ungvari Z. Inflammation and endothelial dysfunction during aging: Role of nf-κb. J Appl Physiol (1985). 2008;105:1333–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Meijles DN, Pagano PJ. Nox and inflammation in the vascular adventitia. Hypertension. 2016;67:14–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lesniewski LA, Durrant JR, Connell ML, Henson GD, Black AD, Donato AJ, Seals DR. Aerobic exercise reverses arterial inflammation with aging in mice. American Journal of Physiology-Heart and Circulatory Physiology. 2011;301:H1025–H1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Konior A, Schramm A, Czesnikiewicz-Guzik M, Guzik TJ. Nadph oxidases in vascular pathology. Antioxidants & redox signaling. 2014;20:2794–2814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Karin M, Delhase M. The i kappa b kinase (ikk) and nf-kappa b: Key elements of proinflammatory signalling. Semin Immunol. 2000;12:85–98 [DOI] [PubMed] [Google Scholar]
- 104.Schreck R, Albermann K, Baeuerle PA. Nuclear factor kappa b: An oxidative stress-responsive transcription factor of eukaryotic cells (a review). Free Radic Res Commun. 1992;17:221–237 [DOI] [PubMed] [Google Scholar]
- 105.Lesniewski LA, Durrant JR, Connell ML, Folian BJ, Donato AJ, Seals DR. Salicylate treatment improves age-associated vascular endothelial dysfunction: Potential role of nuclear factor kappab and forkhead box o phosphorylation. J Gerontol A Biol Sci Med Sci. 2011;66:409–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pierce GL, Lesniewski LA, Lawson BR, Beske SD, Seals DR. Nuclear factor-{kappa}b activation contributes to vascular endothelial dysfunction via oxidative stress in overweight/obese middle-aged and older humans. Circulation. 2009;119:1284–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jablonski KL, Donato AJ, Fleenor BS, Nowlan MJ, Walker AE, Kaplon RE, Ballak DB, Seals DR. Reduced large elastic artery stiffness with regular aerobic exercise in middle-aged and older adults: Potential role of suppressed nuclear factor kappa b signalling. J Hypertens. 2015;33:2477–2482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Angel K, Provan SA, Gulseth HL, Mowinckel P, Kvien TK, Atar D. Tumor necrosis factor-alpha antagonists improve aortic stiffness in patients with inflammatory arthropathies: A controlled study. Hypertension. 2010;55:333–338 [DOI] [PubMed] [Google Scholar]
- 109.Yabluchanskiy A, Ma Y, Iyer RP, Hall ME, Lindsey ML. Matrix metalloproteinase-9: Many shades of function in cardiovascular disease. Physiology (Bethesda). 2013;28:391–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lacolley P, Regnault V, Avolio AP. Smooth muscle cell and arterial aging: Basic and clinical aspects. Cardiovasc Res. 2018;114:513–528 [DOI] [PubMed] [Google Scholar]
- 111.Wallace S, McEniery CM, Dakham Z, Pusalkar P, Maki-Petaja K, Ashby MJ, Cockcroft JR, Wilkinson IB. Matrix metalloproteinase-9 (mmp-9), mmp-2, and serum elastase activity are associated with systolic hypertension and arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:372–378 [DOI] [PubMed] [Google Scholar]
- 112.Bond M, Chase AJ, Baker AH, Newby AC. Inhibition of transcription factor nf-κb reduces matrix metalloproteinase-1,−3 and-9 production by vascular smooth muscle cells. Cardiovasc Res. 2001;50:556–565 [DOI] [PubMed] [Google Scholar]
- 113.Okamoto OK, Robertson DL, Fagan TF, Hastings JW, Colepicolo P. Different regulatory mechanisms modulate the expression of a dinoflagellate iron-superoxide dismutase. Journal of Biological Chemistry. 2001;276:19989–19993 [DOI] [PubMed] [Google Scholar]
- 114.Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest. 1997;100:768–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fleenor BS, Marshall KD, Durrant JR, Lesniewski LA, Seals DR. Arterial stiffening with ageing is associated with transforming growth factor-beta1-related changes in adventitial collagen: Reversal by aerobic exercise. J Physiol. 2010;588:3971–3982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Koyanagi M, Egashira K, Kubo-Inoue M, Usui M, Kitamoto S, Tomita H, Shimokawa H, Takeshita A. Role of transforming growth factor-beta1 in cardiovascular inflammatory changes induced by chronic inhibition of nitric oxide synthesis. Hypertension. 2000;35:86–90 [DOI] [PubMed] [Google Scholar]
- 117.Milkiewicz M, Kelland C, Colgan S, Haas TL. Nitric oxide and p38 map kinase mediate shear stress-dependent inhibition of mmp-2 production in microvascular endothelial cells. J Cell Physiol. 2006;208:229–237 [DOI] [PubMed] [Google Scholar]
- 118.Knipp BS, Ailawadi G, Ford JW, Peterson DA, Eagleton MJ, Roelofs KJ, Hannawa KK, Deogracias MP, Ji B, Logsdon C, Graziano KD, Simeone DM, Thompson RW, Henke PK, Stanley JC, Upchurch GR, Jr., Increased mmp-9 expression and activity by aortic smooth muscle cells after nitric oxide synthase inhibition is associated with increased nuclear factor-kappab and activator protein-1 activity. J Surg Res. 2004;116:70–80 [DOI] [PubMed] [Google Scholar]
- 119.Ridnour LA, Windhausen AN, Isenberg JS, Yeung N, Thomas DD, Vitek MP, Roberts DD, Wink DA. Nitric oxide regulates matrix metalloproteinase-9 activity by guanylyl-cyclase-dependent and -independent pathways. Proc Natl Acad Sci U S A. 2007;104:16898–16903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Monk BA, George SJ. The effect of ageing on vascular smooth muscle cell behaviour-a mini-review. Gerontology. 2015;61:416–426 [DOI] [PubMed] [Google Scholar]
- 121.Xu X, Wang B, Ren C, Hu J, Greenberg DA, Chen T, Xie L, Jin K. Age-related impairment of vascular structure and functions. Aging Dis. 2017;8:590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Manea A, Manea SA, Gafencu AV, Raicu M. Regulation of nadph oxidase subunit p22(phox) by nf-kb in human aortic smooth muscle cells. Arch Physiol Biochem. 2007;113:163–172 [DOI] [PubMed] [Google Scholar]
- 123.Trott DW, Henson GD, Ho MH, Allison SA, Lesniewski LA, Donato AJ. Age-related arterial immune cell infiltration in mice is attenuated by caloric restriction or voluntary exercise. Exp Gerontol. 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Weiss EP, Fontana L. Caloric restriction: Powerful protection for the aging heart and vasculature. Am J Physiol Heart Circ Physiol. 2011;301:H1205–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rubinsztein DC, Marino G, Kroemer G. Autophagy and aging. Cell. 2011;146:682–695 [DOI] [PubMed] [Google Scholar]
- 127.Pyo KH, Kim MK, Shin KS, Chun HS, Shin EH. Involvement of trypsin-digested silk peptides in the induction of raw264.7 macrophage activation. Nat Prod Commun. 2013;8:1755–1758 [PubMed] [Google Scholar]
- 128.Abada A, Elazar Z. Getting ready for building: Signaling and autophagosome biogenesis. EMBO Rep. 2014;15:839–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Morselli E, Maiuri MC, Markaki M, Megalou E, Pasparaki A, Palikaras K, Criollo A, Galluzzi L, Malik SA, Vitale I, Michaud M, Madeo F, Tavernarakis N, Kroemer G. Caloric restriction and resveratrol promote longevity through the sirtuin-1-dependent induction of autophagy. Cell Death Dis. 2010;1:e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nussenzweig SC, Verma S, Finkel T. The role of autophagy in vascular biology. Circ Res. 2015;116:480–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bharath LP, Mueller R, Li Y, Ruan T, Kunz D, Goodrich R, Mills T, Deeter L, Sargsyan A, Anandh Babu PV, Graham TE, Symons JD. Impairment of autophagy in endothelial cells prevents shear-stress-induced increases in nitric oxide bioavailability. Can J Physiol Pharmacol. 2014;92:605–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Guo F, Li X, Peng J, Tang Y, Yang Q, Liu L, Wang Z, Jiang Z, Xiao M, Ni C, Chen R, Wei D, Wang GX. Autophagy regulates vascular endothelial cell enos and et-1 expression induced by laminar shear stress in an ex vivo perfused system. Ann Biomed Eng. 2014;42:1978–1988 [DOI] [PubMed] [Google Scholar]
- 133.Shiroto T, Romero N, Sugiyama T, Sartoretto JL, Kalwa H, Yan Z, Shimokawa H, Michel T. Caveolin-1 is a critical determinant of autophagy, metabolic switching, and oxidative stress in vascular endothelium. PLoS One. 2014;9:e87871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.LaRocca TJ, Henson GD, Thorburn A, Sindler AL, Pierce GL, Seals DR. Translational evidence that impaired autophagy contributes to arterial ageing. J Physiol. 2012;590:3305–3316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.LaRocca TJ, Gioscia-Ryan RA, Hearon CM Jr., Seals DR. The autophagy enhancer spermidine reverses arterial aging. Mech Ageing Dev. 2013;134:314–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chen ML, Yi L, Jin X, Liang XY, Zhou Y, Zhang T, Xie Q, Zhou X, Chang H, Fu YJ, Zhu JD, Zhang QY, Mi MT. Resveratrol attenuates vascular endothelial inflammation by inducing autophagy through the camp signaling pathway. Autophagy. 2013;9:2033–2045 [DOI] [PubMed] [Google Scholar]
- 137.Sharp ZD, Strong R. The role of mtor signaling in controlling mammalian life span: What a fungicide teaches us about longevity. J Gerontol A Biol Sci Med Sci. 2010;65:580–589 [DOI] [PubMed] [Google Scholar]
- 138.Lieberthal W, Levine JS. The role of the mammalian target of rapamycin (mtor) in renal disease. J Am Soc Nephrol. 2009;20:2493–2502 [DOI] [PubMed] [Google Scholar]
- 139.Watanabe R, Wei L, Huang J. Mtor signaling, function, novel inhibitors, and therapeutic targets. J Nucl Med. 2011;52:497–500 [DOI] [PubMed] [Google Scholar]
- 140.Guertin DA, Sabatini DM. Defining the role of mtor in cancer. Cancer Cell. 2007;12:9–22 [DOI] [PubMed] [Google Scholar]
- 141.Laplante M, Sabatini DM. Mtor signaling at a glance. J Cell Sci. 2009;122:3589–3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fontana L, Partridge L, Longo VD. Extending healthy life span—from yeast to humans. Science. 2010;328:321–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, Woods A, Robinson IC, Schuster E, Batterham RL, Kozma SC, Thomas G, Carling D, Okkenhaug K, Thornton JM, Partridge L, Gems D, Withers DJ. Ribosomal protein s6 kinase 1 signaling regulates mammalian life span. Science. 2009;326:140–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Raymond E, Alexandre J, Faivre S, Vera K, Materman E, Boni J, Leister C, Korth-Bradley J, Hanauske A, Armand JP. Safety and pharmacokinetics of escalated doses of weekly intravenous infusion of cci-779, a novel mtor inhibitor, in patients with cancer. J Clin Oncol. 2004;22:2336–2347 [DOI] [PubMed] [Google Scholar]
- 146.Johnson RW, Kreis H, Oberbauer R, Brattstrom C, Claesson K, Eris J. Sirolimus allows early cyclosporine withdrawal in renal transplantation resulting in improved renal function and lower blood pressure. Transplantation. 2001;72:777–786 [DOI] [PubMed] [Google Scholar]
- 147.Lebranchu Y, Thierry A, Toupance O, Westeel PF, Etienne I, Thervet E, Moulin B, Frouget T, Le Meur Y, Glotz D, Heng AE, Onno C, Buchler M, Girardot-Seguin S, Hurault de Ligny B. Efficacy on renal function of early conversion from cyclosporine to sirolimus 3 months after renal transplantation: Concept study. Am J Transplant. 2009;9:1115–1123 [DOI] [PubMed] [Google Scholar]
- 148.Joannides R, Etienne I, Iacob M, Hurault de Ligny B, Barbier S, Bellien J, Lebranchu Y, Thuillez C, Godin M. Comparative effects of sirolimus and cyclosporin on conduit arteries endothelial function in kidney recipients. Transpl Int. 2010;23:1135–1143 [DOI] [PubMed] [Google Scholar]
- 149.Joannides R, Monteil C, de Ligny BH, Westeel PF, Iacob M, Thervet E, Barbier S, Bellien J, Lebranchu Y, Seguin SG, Thuillez C, Godin M, Etienne I. Immunosuppressant regimen based on sirolimus decreases aortic stiffness in renal transplant recipients in comparison to cyclosporine. Am J Transplant. 2011;11:2414–2422 [DOI] [PubMed] [Google Scholar]
- 150.Martinez-Cisuelo V, Gomez J, Garcia-Junceda I, Naudi A, Cabre R, Mota-Martorell N, Lopez-Torres M, Gonzalez-Sanchez M, Pamplona R, Barja G. Rapamycin reverses age-related increases in mitochondrial ros production at complex i, oxidative stress, accumulation of mtdna fragments inside nuclear DNA, and lipofuscin level, and increases autophagy, in the liver of middle-aged mice. Exp Gerontol. 2016;83:130–138 [DOI] [PubMed] [Google Scholar]
- 151.Kofman AE, McGraw MR, Payne CJ. Rapamycin increases oxidative stress response gene expression in adult stem cells. Aging (Albany NY). 2012;4:279–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jiang J, Jiang J, Zuo Y, Gu Z. Rapamycin protects the mitochondria against oxidative stress and apoptosis in a rat model of parkinson’s disease. Int J Mol Med. 2013;31:825–832 [DOI] [PubMed] [Google Scholar]
- 153.Das A, Durrant D, Koka S, Salloum FN, Xi L, Kukreja RC. Mammalian target of rapamycin (mtor) inhibition with rapamycin improves cardiac function in type 2 diabetic mice: Potential role of attenuated oxidative stress and altered contractile protein expression. J Biol Chem. 2014;289:4145–4160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Soesanto W, Lin HY, Hu E, Lefler S, Litwin SE, Sena S, Abel ED, Symons JD, Jalili T. Mammalian target of rapamycin is a critical regulator of cardiac hypertrophy in spontaneously hypertensive rats. Hypertension. 2009;54:1321–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wullschleger S, Loewith R, Hall MN. Tor signaling in growth and metabolism. Cell. 2006;124:471–484 [DOI] [PubMed] [Google Scholar]
- 156.Hardie DG, Hawley SA, Scott JW. Amp-activated protein kinase--development of the energy sensor concept. J Physiol. 2006;574:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fisslthaler B, Fleming I. Activation and signaling by the amp-activated protein kinase in endothelial cells. Circ Res. 2009;105:114–127 [DOI] [PubMed] [Google Scholar]
- 158.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. Ampk phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mori H, Inoki K, Munzberg H, Opland D, Faouzi M, Villanueva EC, Ikenoue T, Kwiatkowski D, MacDougald OA, Myers MG Jr., Guan KL. Critical role for hypothalamic mtor activity in energy balance. Cell Metab. 2009;9:362–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348 Pt 3:607–614 [PMC free article] [PubMed] [Google Scholar]
- 161.Wong AK, Howie J, Petrie JR, Lang CC. Amp-activated protein kinase pathway: A potential therapeutic target in cardiometabolic disease. Clin Sci (Lond). 2009;116:607–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Buzzai M, Jones RG, Amaravadi RK, Lum JJ, DeBerardinis RJ, Zhao F, Viollet B, Thompson CB. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67:6745–6752 [DOI] [PubMed] [Google Scholar]
- 163.Janjetovic K, Vucicevic L, Misirkic M, Vilimanovich U, Tovilovic G, Zogovic N, Nikolic Z, Jovanovic S, Bumbasirevic V, Trajkovic V, Harhaji-Trajkovic L. Metformin reduces cisplatin-mediated apoptotic death of cancer cells through ampk-independent activation of akt. Eur J Pharmacol. 2011;651:41–50 [DOI] [PubMed] [Google Scholar]
- 164.Saeedi R, Parsons HL, Wambolt RB, Paulson K, Sharma V, Dyck JR, Brownsey RW, Allard MF. Metabolic actions of metformin in the heart can occur by ampk-independent mechanisms. Am J Physiol Heart Circ Physiol. 2008;294:H2497–2506 [DOI] [PubMed] [Google Scholar]
- 165.Lesniewski LA, Zigler MC, Durrant JR, Donato AJ, Seals DR. Sustained activation of ampk ameliorates age-associated vascular endothelial dysfunction via a nitric oxide-independent mechanism. Mech Ageing Dev. 2012;133:368–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pu Y, Zhang H, Wang P, Zhao Y, Li Q, Wei X, Cui Y, Sun J, Shang Q, Liu D, Zhu Z. Dietary curcumin ameliorates aging-related cerebrovascular dysfunction through the ampk/uncoupling protein 2 pathway. Cell Physiol Biochem. 2013;32:1167–1177 [DOI] [PubMed] [Google Scholar]
- 167.Morrow VA, Foufelle F, Connell JM, Petrie JR, Gould GW, Salt IP. Direct activation of amp-activated protein kinase stimulates nitric-oxide synthesis in human aortic endothelial cells. J Biol Chem. 2003;278:31629–31639 [DOI] [PubMed] [Google Scholar]
- 168.Levine YC, Li GK, Michel T. Agonist-modulated regulation of amp-activated protein kinase (ampk) in endothelial cells. Evidence for an ampk -> rac1 -> akt -> endothelial nitric-oxide synthase pathway. J Biol Chem. 2007;282:20351–20364 [DOI] [PubMed] [Google Scholar]
- 169.Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witters LA, Power DA, Ortiz de Montellano PR, Kemp BE. Amp-activated protein kinase phosphorylation of endothelial no synthase. FEBS Lett. 1999;443:285–289 [DOI] [PubMed] [Google Scholar]
- 170.Chen Z, Peng IC, Sun W, Su MI, Hsu PH, Fu Y, Zhu Y, DeFea K, Pan S, Tsai MD, Shyy JY. Amp-activated protein kinase functionally phosphorylates endothelial nitric oxide synthase ser633. Circ Res. 2009;104:496–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nagata D, Mogi M, Walsh K. Amp-activated protein kinase (ampk) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress. J Biol Chem. 2003;278:31000–31006 [DOI] [PubMed] [Google Scholar]
- 172.Sartoretto JL, Melo GA, Carvalho MH, Nigro D, Passaglia RT, Scavone C, Cuman RK, Fortes ZB. Metformin treatment restores the altered microvascular reactivity in neonatal streptozotocin-induced diabetic rats increasing nos activity, but not nos expression. Life Sci. 2005;77:2676–2689 [DOI] [PubMed] [Google Scholar]
- 173.Katakam PV, Ujhelyi MR, Hoenig M, Miller AW. Metformin improves vascular function in insulin-resistant rats. Hypertension. 2000;35:108–112 [DOI] [PubMed] [Google Scholar]
- 174.Davis B, Rahman A, Arner A. Amp-activated kinase relaxes agonist induced contractions in the mouse aorta via effects on pkc signaling and inhibits no-induced relaxation. Eur J Pharmacol. 2012;695:88–95 [DOI] [PubMed] [Google Scholar]
- 175.Cacicedo JM, Yagihashi N, Keaney JF, Jr., Ruderman NB, Ido Y. Ampk inhibits fatty acid-induced increases in nf-kappab transactivation in cultured human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2004;324:1204–1209 [DOI] [PubMed] [Google Scholar]
- 176.Hattori Y, Nakano Y, Hattori S, Tomizawa A, Inukai K, Kasai K. High molecular weight adiponectin activates ampk and suppresses cytokine-induced nf-kappab activation in vascular endothelial cells. FEBS Lett. 2008;582:1719–1724 [DOI] [PubMed] [Google Scholar]
- 177.Agarwal N, Rice SP, Bolusani H, Luzio SD, Dunseath G, Ludgate M, Rees DA. Metformin reduces arterial stiffness and improves endothelial function in young women with polycystic ovary syndrome: A randomized, placebo-controlled, crossover trial. J Clin Endocrinol Metab. 2010;95:722–730 [DOI] [PubMed] [Google Scholar]
- 178.Lin Y, Chen J, Sun Z. Antiaging gene klotho deficiency promoted high-fat diet-induced arterial stiffening via inactivation of amp-activated protein kinase. Hypertension. 2016;67:564–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Li X, Han Y, Pang W, Li C, Xie X, Shyy JY, Zhu Y. Amp-activated protein kinase promotes the differentiation of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28:1789–1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of nf-kappab-dependent transcription and cell survival by the sirt1 deacetylase. Embo J. 2004;23:2369–2380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein sir2 is an nad-dependent histone deacetylase. Nature. 2000;403:795–800 [DOI] [PubMed] [Google Scholar]
- 182.Chang HC, Guarente L. Sirt1 and other sirtuins in metabolism. Trends Endocrinol Metab. 2014;25:138–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Guarente L Sirtuins as potential targets for metabolic syndrome. Nature. 2006;444:868–874 [DOI] [PubMed] [Google Scholar]
- 184.Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the sirt1 deacetylase. Science. 2004;305:390–392 [DOI] [PubMed] [Google Scholar]
- 185.Pillarisetti S A review of sirt1 and sirt1 modulators in cardiovascular and metabolic diseases. Recent Pat Cardiovasc Drug Discov. 2008;3:156–164 [DOI] [PubMed] [Google Scholar]
- 186.Boily G, Seifert EL, Bevilacqua L, He XH, Sabourin G, Estey C, Moffat C, Crawford S, Saliba S, Jardine K, Xuan J, Evans M, Harper ME, McBurney MW. Sirt1 regulates energy metabolism and response to caloric restriction in mice. PLoS One. 2008;3:e1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bordone L, Cohen D, Robinson A, Motta MC, van Veen E, Czopik A, Steele AD, Crowe H, Marmor S, Luo J, Gu W, Guarente L. Sirt1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007;6:759–767 [DOI] [PubMed] [Google Scholar]
- 188.Kanfi Y, Naiman S, Amir G, Peshti V, Zinman G, Nahum L, Bar-Joseph Z, Cohen HY. The sirtuin sirt6 regulates lifespan in male mice. Nature. 2012;483:218–221 [DOI] [PubMed] [Google Scholar]
- 189.Kanfi Y, Shalman R, Peshti V, Pilosof SN, Gozlan YM, Pearson KJ, Lerrer B, Moazed D, Marine JC, de Cabo R, Cohen HY. Regulation of sirt6 protein levels by nutrient availability. FEBS Lett. 2008;582:543–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Xu S, Yin M, Koroleva M, Mastrangelo MA, Zhang W, Bai P, Little PJ, Jin ZG. Sirt6 protects against endothelial dysfunction and atherosclerosis in mice. Aging (Albany NY). 2016;8:1064–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Rahman S, Islam R. Mammalian sirt1: Insights on its biological functions. Cell Commun Signal. 2011;9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Donato AJ, Magerko KA, Lawson BR, Durrant JR, Lesniewski LA, Seals DR. Sirt-1 and vascular endothelial dysfunction with ageing in mice and humans. J Physiol. 2011;589:4545–4554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Gano LB, Donato AJ, Pasha HM, Hearon CM, Jr., Sindler AL, Seals DR. The sirt1 activator srt1720 reverses vascular endothelial dysfunction, excessive superoxide production, and inflammation with aging in mice. Am J Physiol Heart Circ Physiol. 2014;307:H1754–1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wan YZ, Gao P, Zhou S, Zhang ZQ, Hao DL, Lian LS, Li YJ, Chen HZ, Liu DP. Sirt1-mediated epigenetic downregulation of plasminogen activator inhibitor-1 prevents vascular endothelial replicative senescence. Aging Cell. 2014;13:890–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno K, Irani K. Sirt1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2007;104:14855–14860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Das DK, Maulik N. Resveratrol in cardioprotection: A therapeutic promise of alternative medicine. Mol Interv. 2006;6:36–47 [DOI] [PubMed] [Google Scholar]
- 197.Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.da Luz PL, Tanaka L, Brum PC, Dourado PM, Favarato D, Krieger JE, Laurindo FR. Red wine and equivalent oral pharmacological doses of resveratrol delay vascular aging but do not extend life span in rats. Atherosclerosis. 2012;224:136–142 [DOI] [PubMed] [Google Scholar]
- 199.Bhullar KS, Hubbard BP. Lifespan and healthspan extension by resveratrol. Biochim Biophys Acta. 2015;1852:1209–1218 [DOI] [PubMed] [Google Scholar]
- 200.Strong R, Miller RA, Astle CM, Baur JA, de Cabo R, Fernandez E, Guo W, Javors M, Kirkland JL, Nelson JF, Sinclair DA, Teter B, Williams D, Zaveri N, Nadon NL, Harrison DE. Evaluation of resveratrol, green tea extract, curcumin, oxaloacetic acid, and medium-chain triglyceride oil on life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2013;68:6–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Stivala LA, Savio M, Carafoli F, Perucca P, Bianchi L, Maga G, Forti L, Pagnoni UM, Albini A, Prosperi E, Vannini V. Specific structural determinants are responsible for the antioxidant activity and the cell cycle effects of resveratrol. J Biol Chem. 2001;276:22586–22594 [DOI] [PubMed] [Google Scholar]
- 202.Labinskyy N, Csiszar A, Veress G, Stef G, Pacher P, Oroszi G, Wu J, Ungvari Z. Vascular dysfunction in aging: Potential effects of resveratrol, an anti-inflammatory phytoestrogen. Curr Med Chem. 2006;13:989–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Yoshino J, Mills KF, Yoon MJ, Imai S. Nicotinamide mononucleotide, a key nad(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011;14:528–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Gomes AP, Price NL, Ling AJ, Moslehi JJ, Montgomery MK, Rajman L, White JP, Teodoro JS, Wrann CD, Hubbard BP, Mercken EM, Palmeira CM, de Cabo R, Rolo AP, Turner N, Bell EL, Sinclair DA. Declining nad(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell. 2013;155:1624–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.de Picciotto NE, Gano LB, Johnson LC, Martens CR, Sindler AL, Mills KF, Imai S, Seals DR. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell. 2016;15:522–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Das A, Huang GX, Bonkowski MS, Longchamp A, Li C, Schultz MB, Kim LJ, Osborne B, Joshi S, Lu Y, Trevino-Villarreal JH, Kang MJ, Hung TT, Lee B, Williams EO, Igarashi M, Mitchell JR, Wu LE, Turner N, Arany Z, Guarente L, Sinclair DA. Impairment of an endothelial nad(+)-h2s signaling network is a reversible cause of vascular aging. Cell. 2018;173:74–89 e20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Martens CR, Denman BA, Mazzo MR, Armstrong ML, Reisdorph N, McQueen MB, Chonchol M, Seals DR. Chronic nicotinamide riboside supplementation is well-tolerated and elevates nad(+) in healthy middle-aged and older adults. Nat Commun. 2018;9:1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Mitchell SJ, Martin-Montalvo A, Mercken EM, Palacios HH, Ward TM, Abulwerdi G, Minor RK, Vlasuk GP, Ellis JL, Sinclair DA, Dawson J, Allison DB, Zhang Y, Becker KG, Bernier M, de Cabo R. The sirt1 activator srt1720 extends lifespan and improves health of mice fed a standard diet. Cell Rep. 2014;6:836–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Machin DR, Auduong Y, Henson GD, Lesniewski LA, Donato AJ. Sirt-1 overexpression mitigates large artery stiffening with advancing age. Physiologist. 2017;60:1310 [abstract] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Nakamaru Y, Vuppusetty C, Wada H, Milne JC, Ito M, Rossios C, Elliot M, Hogg J, Kharitonov S, Goto H, Bemis JE, Elliott P, Barnes PJ, Ito K. A protein deacetylase sirt1 is a negative regulator of metalloproteinase-9. FASEB J. 2009;23:2810–2819 [DOI] [PubMed] [Google Scholar]
- 211.Yao H, Hwang JW, Sundar IK, Friedman AE, McBurney MW, Guarente L, Gu W, Kinnula VL, Rahman I. Sirt1 redresses the imbalance of tissue inhibitor of matrix metalloproteinase-1 and matrix metalloproteinase-9 in the development of mouse emphysema and human copd. Am J Physiol Lung Cell Mol Physiol. 2013;305:L615–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Mattison JA, Wang M, Bernier M, Zhang J, Park SS, Maudsley S, An SS, Santhanam L, Martin B, Faulkner S, Morrell C, Baur JA, Peshkin L, Sosnowska D, Csiszar A, Herbert RL, Tilmont EM, Ungvari Z, Pearson KJ, Lakatta EG, de Cabo R. Resveratrol prevents high fat/sucrose diet-induced central arterial wall inflammation and stiffening in nonhuman primates. Cell Metab. 2014;20:183–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51 [DOI] [PubMed] [Google Scholar]
- 214.Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M. Suppression of aging in mice by the hormone klotho. Science. 2005;309:1829–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Xie J, Cha SK, An SW, Kuro OM, Birnbaumer L, Huang CL. Cardioprotection by klotho through downregulation of trpc6 channels in the mouse heart. Nat Commun. 2012;3:1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Hsieh CC, Kuro-o M, Rosenblatt KP, Brobey R, Papaconstantinou J. The ask1-signalosome regulates p38 mapk activity in response to levels of endogenous oxidative stress in the klotho mouse models of aging. Aging (Albany NY). 2010;2:597–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR. Insulin stimulates the cleavage and release of the extracellular domain of klotho by adam10 and adam17. Proc Natl Acad Sci U S A. 2007;104:19796–19801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Semba RD, Cappola AR, Sun K, Bandinelli S, Dalal M, Crasto C, Guralnik JM, Ferrucci L. Plasma klotho and cardiovascular disease in adults. J Am Geriatr Soc. 2011;59:1596–1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Xiao NM, Zhang YM, Zheng Q, Gu J. Klotho is a serum factor related to human aging. Chin Med J (Engl). 2004;117:742–747 [PubMed] [Google Scholar]
- 220.Dubal DB, Yokoyama JS, Zhu L, Broestl L, Worden K, Wang D, Sturm VE, Kim D, Klein E, Yu GQ, Ho K, Eilertson KE, Yu L, Kuro-o M, De Jager PL, Coppola G, Small GW, Bennett DA, Kramer JH, Abraham CR, Miller BL, Mucke L. Life extension factor klotho enhances cognition. Cell Rep. 2014;7:1065–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Arking DE, Atzmon G, Arking A, Barzilai N, Dietz HC. Association between a functional variant of the klotho gene and high-density lipoprotein cholesterol, blood pressure, stroke, and longevity. Circ Res. 2005;96:412–418 [DOI] [PubMed] [Google Scholar]
- 222.Arking DE, Krebsova A, Macek M Sr., Macek M Jr., Arking A, Mian IS, Fried L, Hamosh A, Dey S, McIntosh I, Dietz HC. Association of human aging with a functional variant of klotho. Proc Natl Acad Sci U S A. 2002;99:856–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Yokoyama JS, Marx G, Brown JA, Bonham LW, Wang D, Coppola G, Seeley WW, Rosen HJ, Miller BL, Kramer JH, Dubal DB. Systemic klotho is associated with klotho variation and predicts intrinsic cortical connectivity in healthy human aging. Brain Imaging Behav. 2017;11:391–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Majumdar V, Nagaraja D, Christopher R. Association of the functional kl-vs variant of klotho gene with early-onset ischemic stroke. Biochem Biophys Res Commun. 2010;403:412–416 [DOI] [PubMed] [Google Scholar]
- 225.Zhang H, Shi Y, Ma F, Wang I, Hou Y, Zhu Z. Association of klotho single nucleotide polymorphisms with cardiovascular diseases: A systematic review and meta-analysis. Int J Clin Exp Med. 2017;10:5721–5741 [Google Scholar]
- 226.Mencke R, Hillebrands JL, consortium N. The role of the anti-ageing protein klotho in vascular physiology and pathophysiology. Ageing Res Rev. 2017;35:124–146 [DOI] [PubMed] [Google Scholar]
- 227.Lim K, Groen A, Molostvov G, Lu T, Lilley KS, Snead D, James S, Wilkinson IB, Ting S, Hsiao LL, Hiemstra TF, Zehnder D. Alpha-klotho expression in human tissues. J Clin Endocrinol Metab. 2015;100:E1308–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lim K, Lu TS, Molostvov G, Lee C, Lam FT, Zehnder D, Hsiao LL. Vascular klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation. 2012;125:2243–2255 [DOI] [PubMed] [Google Scholar]
- 229.Zhao Y, Zhao MM, Cai Y, Zheng MF, Sun WL, Zhang SY, Kong W, Gu J, Wang X, Xu MJ. Mammalian target of rapamycin signaling inhibition ameliorates vascular calcification via klotho upregulation. Kidney Int. 2015;88:711–721 [DOI] [PubMed] [Google Scholar]
- 230.Gao D, Zuo Z, Tian J, Ali Q, Lin Y, Lei H, Sun Z. Activation of sirt1 attenuates klotho deficiency-induced arterial stiffness and hypertension by enhancing amp-activated protein kinase activity. Hypertension. 2016;68:1191–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Saito Y, Yamagishi T, Nakamura T, Ohyama Y, Aizawa H, Suga T, Matsumura Y, Masuda H, Kurabayashi M, Kuro-o M, Nabeshima Y, Nagai R. Klotho protein protects against endothelial dysfunction. Biochem Biophys Res Commun. 1998;248:324–329 [DOI] [PubMed] [Google Scholar]
- 232.Saito Y, Nakamura T, Ohyama Y, Suzuki T, Iida A, Shiraki-Iida T, Kuro-o M, Nabeshima Y, Kurabayashi M, Nagai R. In vivo klotho gene delivery protects against endothelial dysfunction in multiple risk factor syndrome. Biochem Biophys Res Commun. 2000;276:767–772 [DOI] [PubMed] [Google Scholar]
- 233.Ohta J, Rakugi H, Ishikawa K, Yang J, Ikushima M, Chihara Y, Maekawa Y, Oguro R, Hanasaki H, Kida I. Klotho gene delivery suppresses oxidative stress in vivo. Geriatrics & Gerontology International. 2007;7:293–299 [Google Scholar]
- 234.Chen K, Zhou X, Sun Z. Haplodeficiency of klotho gene causes arterial stiffening via upregulation of scleraxis expression and induction of autophagy. Hypertension. 2015;66:1006–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Donato AJ, Morgan RG, Walker AE, Lesniewski LA. Cellular and molecular biology of aging endothelial cells. J Mol Cell Cardiol. 2015;89:122–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Canto C, Jiang LQ, Deshmukh AS, Mataki C, Coste A, Lagouge M, Zierath JR, Auwerx J. Interdependence of ampk and sirt1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 2010;11:213–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ghosh HS, McBurney M, Robbins PD. Sirt1 negatively regulates the mammalian target of rapamycin. PLoS One. 2010;5:e9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16ink4a. Cell. 1997;88:593–602 [DOI] [PubMed] [Google Scholar]
- 239.Parrinello S, Samper E, Krtolica A, Goldstein J, Melov S, Campisi J. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nature cell biology. 2003;5:741–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Samper E, Nicholls DG, Melov S. Mitochondrial oxidative stress causes chromosomal instability of mouse embryonic fibroblasts. Aging Cell. 2003;2:277–285 [DOI] [PubMed] [Google Scholar]
- 241.Di Leonardo A, Linke SP, Clarkin K, Wahl GM. DNA damage triggers a prolonged p53-dependent g1 arrest and long-term induction of cip1 in normal human fibroblasts. Genes Dev. 1994;8:2540–2551 [DOI] [PubMed] [Google Scholar]
- 242.Coppé J-P, Desprez P-Y, Krtolica A, Campisi J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annual Review of Pathology: Mechanisms of Disease. 2010;5:99–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Campisi J, d’Adda di Fagagna F. Cellular senescence: When bad things happen to good cells. Nature reviews. Molecular cell biology. 2007;8:729–740 [DOI] [PubMed] [Google Scholar]
- 244.Campisi J Senescent cells, tumor suppression, and organismal aging: Good citizens, bad neighbors. Cell. 2005;120:513–522 [DOI] [PubMed] [Google Scholar]
- 245.Beausejour CM, Krtolica A, Galimi F, Narita M, Lowe SW, Yaswen P, Campisi J. Reversal of human cellular senescence: Roles of the p53 and p16 pathways. The EMBO journal. 2003;22:4212–4222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Jacobs JJ, de Lange T. Significant role for p16ink4a in p53-independent telomere-directed senescence. Curr Biol. 2004;14:2302–2308 [DOI] [PubMed] [Google Scholar]
- 247.Brookes S, Rowe J, Ruas M, Llanos S, Clark PA, Lomax M, James MC, Vatcheva R, Bates S, Vousden KH, Parry D, Gruis N, Smit N, Bergman W, Peters G. Ink4a-deficient human diploid fibroblasts are resistant to ras-induced senescence. The EMBO journal. 2002;21:2936–2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Narita M, Nunez S, Heard E, Narita M, Lin AW, Hearn SA, Spector DL, Hannon GJ, Lowe SW. Rb-mediated heterochromatin formation and silencing of e2f target genes during cellular senescence. Cell. 2003;113:703–716 [DOI] [PubMed] [Google Scholar]
- 249.Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic ras and the p53 tumor suppressor. PLoS biology. 2008;6:2853–2868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Gardner SE, Humphry M, Bennett MR, Clarke MC. Senescent vascular smooth muscle cells drive inflammation through an interleukin-1α–dependent senescence-associated secretory phenotype. Arterioscler Thromb Vasc Biol. 2015:ATVBAHA. 115.305896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Rippe C, Blimline M, Magerko KA, Lawson BR, LaRocca TJ, Donato AJ, Seals DR. Microrna changes in human arterial endothelial cells with senescence: Relation to apoptosis, enos and inflammation. Exp Gerontol. 2012;47:45–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Macip S, Igarashi M, Fang L, Chen A, Pan ZQ, Lee SW, Aaronson SA. Inhibition of p21-mediated ros accumulation can rescue p21-induced senescence. The EMBO journal. 2002;21:2180–2188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Chien Y, Scuoppo C, Wang X, Fang X, Balgley B, Bolden JE, Premsrirut P, Luo W, Chicas A, Lee CS, Kogan SC, Lowe SW. Control of the senescence-associated secretory phenotype by nf-kappab promotes senescence and enhances chemosensitivity. Genes Dev. 2011;25:2125–2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Salminen A, Kauppinen A, Kaarniranta K. Emerging role of nf-kappab signaling in the induction of senescence-associated secretory phenotype (sasp). Cellular signalling. 2012;24:835–845 [DOI] [PubMed] [Google Scholar]
- 255.Matthews C, Gorenne I, Scott S, Figg N, Kirkpatrick P, Ritchie A, Goddard M, Bennett M. Vascular smooth muscle cells undergo telomere-based senescence in human atherosclerosis: Effects of telomerase and oxidative stress. Circ Res. 2006;99:156–164 [DOI] [PubMed] [Google Scholar]
- 256.Cafueri G, Parodi F, Pistorio A, Bertolotto M, Ventura F, Gambini C, Bianco P, Dallegri F, Pistoia V, Pezzolo A, Palombo D. Endothelial and smooth muscle cells from abdominal aortic aneurysm have increased oxidative stress and telomere attrition. PLoS One. 2012;7:e35312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Morgan RG, Ives SJ, Walker AE, Cawthon RM, Andtbacka RH, Noyes D, Lesniewski LA, Richardson RS, Donato AJ. Role of arterial telomere dysfunction in hypertension: Relative contributions of telomere shortening and telomere uncapping. J Hypertens. 2014;32:1293–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Marchand A, Atassi F, Gaaya A, Leprince P, Le Feuvre C, Soubrier F, Lompre AM, Nadaud S. The wnt/beta-catenin pathway is activated during advanced arterial aging in humans. Aging Cell. 2011;10:220–232 [DOI] [PubMed] [Google Scholar]
- 259.Morgan RG, Ives SJ, Lesniewski LA, Cawthon RM, Andtbacka RH, Noyes RD, Richardson RS, Donato AJ. Age-related telomere uncapping is associated with cellular senescence and inflammation independent of telomere shortening in human arteries. Am J Physiol Heart Circ Physiol. 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Roos CM, Zhang B, Palmer AK, Ogrodnik MB, Pirtskhalava T, Thalji NM, Hagler M, Jurk D, Smith LA, Casaclang‐Verzosa G. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell. 2016;15:973–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Mihm MJ, Jing L, Bauer JA. Nitrotyrosine causes selective vascular endothelial dysfunction and DNA damage. J Cardiovasc Pharmacol. 2000;36:182–187 [DOI] [PubMed] [Google Scholar]
- 262.Ballinger SW, Patterson C, Yan CN, Doan R, Burow DL, Young CG, Yakes FM, Van Houten B, Ballinger CA, Freeman BA, Runge MS. Hydrogen peroxide- and peroxynitrite-induced mitochondrial DNA damage and dysfunction in vascular endothelial and smooth muscle cells. Circ Res. 2000;86:960–966 [DOI] [PubMed] [Google Scholar]
- 263.Mayr M, Hu Y, Hainaut H, Xu Q. Mechanical stress-induced DNA damage and rac-p38mapk signal pathways mediate p53-dependent apoptosis in vascular smooth muscle cells. FASEB J. 2002;16:1423–1425 [DOI] [PubMed] [Google Scholar]
- 264.Wang Y, Boerma M, Zhou D. Ionizing radiation-induced endothelial cell senescence and cardiovascular diseases. Radiat Res. 2016;186:153–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Mercer JR, Cheng K-K, Figg N, Gorenne I, Mahmoudi M, Griffin J, Vidal-Puig A, Logan A, Murphy MP, Bennett M. DNA damage links mitochondrial dysfunction to atherosclerosis and the metabolic syndromenovelty and significance. Circ Res. 2010;107:1021–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Durik M, Kavousi M, van der Pluijm I, Isaacs A, Cheng C, Verdonk K, Loot AE, Oeseburg H, Bhaggoe UM, Leijten F, van Veghel R, de Vries R, Rudez G, Brandt R, Ridwan YR, van Deel ED, de Boer M, Tempel D, Fleming I, Mitchell GF, Verwoert GC, Tarasov KV, Uitterlinden AG, Hofman A, Duckers HJ, van Duijn CM, Oostra BA, Witteman JC, Duncker DJ, Danser AH, Hoeijmakers JH, Roks AJ. Nucleotide excision DNA repair is associated with age-related vascular dysfunction. Circulation. 2012;126:468–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Yu E, Calvert PA, Mercer JR, Harrison J, Baker L, Figg NL, Kumar S, Wang JC, Hurst LA, Obaid DR, Logan A, West NE, Clarke MC, Vidal-Puig A, Murphy MP, Bennett MR. Mitochondrial DNA damage can promote atherosclerosis independently of reactive oxygen species through effects on smooth muscle cells and monocytes and correlates with higher-risk plaques in humans. Circulation. 2013;128:702–712 [DOI] [PubMed] [Google Scholar]
- 268.Capper R, Britt-Compton B, Tankimanova M, Rowson J, Letsolo B, Man S, Haughton M, Baird DM. The nature of telomere fusion and a definition of the critical telomere length in human cells. Genes Dev. 2007;21:2495–2508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Baird DM, Rowson J, Wynford-Thomas D, Kipling D. Extensive allelic variation and ultrashort telomeres in senescent human cells. Nat Genet. 2003;33:203–207 [DOI] [PubMed] [Google Scholar]
- 270.Doksani Y, de Lange T. The role of double-strand break repair pathways at functional and dysfunctional telomeres. Cold Spring Harb Perspect Biol. 2014;6:a016576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460 [DOI] [PubMed] [Google Scholar]
- 272.Oikawa S, Kawanishi S. Site-specific DNA damage at ggg sequence by oxidative stress may accelerate telomere shortening. FEBS Lett. 1999;453:365–368 [DOI] [PubMed] [Google Scholar]
- 273.Bhayadia R, Schmidt BM, Melk A, Homme M. Senescence-induced oxidative stress causes endothelial dysfunction. J Gerontol A Biol Sci Med Sci. 2016;71:161–169 [DOI] [PubMed] [Google Scholar]
- 274.Beyer AM, Freed JK, Durand MJ, Riedel M, Ait-Aissa K, Green P, Hockenberry JC, Morgan RG, Donato AJ, Peleg R. Critical role for telomerase in the mechanism of flow-mediated dilation in the human microcirculation. Circ Res. 2016;118:856–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Morgan RG, Ives SJ, Lesniewski LA, Cawthon RM, Andtbacka RHI, Noyes RD, Denchi EL, Richardson RS, Donato AJ. Telomere uncapping causes cellular senescence and inflammation in arteries: Implications for arterial aging. FASEB J. 2013;27:11311131 [abstract] [Google Scholar]
- 276.Wang J, Uryga A, Reinhold J, Figg N, Baker L, Finigan A, Gray K, Kumar S, Clarke M, Bennett M. Vascular smooth muscle cell senescence promotes atherosclerosis and features of plaque vulnerability. Circulation. 2015;132:1909. [DOI] [PubMed] [Google Scholar]
- 277.Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS biology. 2016;14:e1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Hasegawa Y, Saito T, Ogihara T, Ishigaki Y, Yamada T, Imai J, Uno K, Gao J, Kaneko K, Shimosawa T, Asano T, Fujita T, Oka Y, Katagiri H. Blockade of the nuclear factor-kappab pathway in the endothelium prevents insulin resistance and prolongs life spans. Circulation. 2012;125:1122–1133 [DOI] [PubMed] [Google Scholar]
- 279.Snyder J, Ward J, Treuting P. Cause-of-death analysis in rodent aging studies. Veterinary pathology. 2016;53:233–243 [DOI] [PubMed] [Google Scholar]
- 280.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among us adults: Findings from the third national health and nutrition examination survey. JAMA. 2002;287:356–359 [DOI] [PubMed] [Google Scholar]
- 281.Trevisan M, Liu J, Bahsas FB, Menotti A. Syndrome x and mortality: A population-based study. Risk factor and life expectancy research group. Am J Epidemiol. 1998;148:958–966 [DOI] [PubMed] [Google Scholar]
- 282.Maury E, Brichard S. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol. 2010;314:1–16 [DOI] [PubMed] [Google Scholar]
- 283.Wu D, Ren Z, Pae M, Guo W, Cui X, Merrill AH, Meydani SN. Aging up-regulates expression of inflammatory mediators in mouse adipose tissue. J Immunol. 2007;179:4829–4839 [DOI] [PubMed] [Google Scholar]
- 284.Karpe F, Fielding BA, Ilic V, Macdonald IA, Summers LK, Frayn KN. Impaired postprandial adipose tissue blood flow response is related to aspects of insulin sensitivity. Diabetes. 2002;51:2467–2473 [DOI] [PubMed] [Google Scholar]
- 285.Donato AJ, Henson GD, Hart CR, Layec G, Trinity JD, Bramwell RC, Enz RA, Morgan RG, Reihl KD, Hazra S, Walker AE, Richardson RS, Lesniewski LA. The impact of ageing on adipose structure, function and vasculature in the b6d2f1 mouse: Evidence of significant multisystem dysfunction. J Physiol. 2014;592:4083–4096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Sotornik R, Brassard P, Martin E, Yale P, Carpentier AC, Ardilouze JL. Update on adipose tissue blood flow regulation. Am J Physiol Endocrinol Metab. 2012;302:E1157–1170 [DOI] [PubMed] [Google Scholar]
- 287.Galitzky J, Lafontan M, Nordenström J, Arner P. Role of vascular alpha-2 adrenoceptors in regulating lipid mobilization from human adipose tissue. J Clin Invest. 1993;91:1997–2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T, Vitorica J, Ransohoff RM, Herrup K, Frautschy SA, Finsen B, Brown GC, Verkhratsky A, Yamanaka K, Koistinaho J, Latz E, Halle A, Petzold GC, Town T, Morgan D, Shinohara ML, Perry VH, Holmes C, Bazan NG, Brooks DJ, Hunot S, Joseph B, Deigendesch N, Garaschuk O, Boddeke E, Dinarello CA, Breitner JC, Cole GM, Golenbock DT, Kummer MP. Neuroinflammation in alzheimer’s disease. Lancet Neurol. 2015;14:388–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Faraco G, Iadecola C. Hypertension: A harbinger of stroke and dementia. Hypertension. 2013;62:810–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Zhou J, Yu JT, Wang HF, Meng XF, Tan CC, Wang J, Wang C, Tan L. Association between stroke and alzheimer’s disease: Systematic review and meta-analysis. J Alzheimers Dis. 2015;43:479–489 [DOI] [PubMed] [Google Scholar]
- 291.Dolan H, Crain B, Troncoso J, Resnick SM, Zonderman AB, Obrien RJ. Atherosclerosis, dementia, and alzheimer disease in the baltimore longitudinal study of aging cohort. Ann Neurol. 2010;68:231–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Lim JS, Lee JY, Kwon HM, Lee YS. The correlation between cerebral arterial pulsatility and cognitive dysfunction in alzheimer’s disease patients. J Neurol Sci. 2017;373:285–288 [DOI] [PubMed] [Google Scholar]
- 293.Pase MP, Beiser A, Himali JJ, Tsao C, Satizabal CL, Vasan RS, Seshadri S, Mitchell GF. Aortic stiffness and the risk of incident mild cognitive impairment and dementia. Stroke. 2016;47:2256–2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Hanon O, Haulon S, Lenoir H, Seux ML, Rigaud AS, Safar M, Girerd X, Forette F. Relationship between arterial stiffness and cognitive function in elderly subjects with complaints of memory loss. Stroke. 2005;36:2193–2197 [DOI] [PubMed] [Google Scholar]
- 295.Thorin-Trescases N, de Montgolfier O, Pincon A, Raignault A, Caland L, Labbe P, Thorin E. The impact of pulse pressure on cerebrovascular events leading to age-related cognitive decline. Am J Physiol Heart Circ Physiol. 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Springo Z, Toth P, Tarantini S, Ashpole NM, Tucsek Z, Sonntag WE, Csiszar A, Koller A, Ungvari ZI. Aging impairs myogenic adaptation to pulsatile pressure in mouse cerebral arteries. J Cereb Blood Flow Metab. 2015;35:527–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Park L, Anrather J, Girouard H, Zhou P, Iadecola C. Nox2-derived reactive oxygen species mediate neurovascular dysregulation in the aging mouse brain. Journal of Cerebral Blood Flow & Metabolism. 2007;27:1908–1918 [DOI] [PubMed] [Google Scholar]
- 298.Walker AE, Henson GD, Reihl KD, Morgan RG, Dobson PS, Nielson EI, Ling J, Mecham RP, Li DY, Lesniewski LA. Greater impairments in cerebral artery compared with skeletal muscle feed artery endothelial function in a mouse model of increased large artery stiffness. J Physiol. 2015;593:1931–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Faraci FM, Heistad DD. Regulation of large cerebral arteries and cerebral microvascular pressure. Circ Res. 1990;66:8–17 [DOI] [PubMed] [Google Scholar]
- 300.Fabiani M, Gordon BA, Maclin EL, Pearson MA, Brumback-Peltz CR, Low KA, McAuley E, Sutton BP, Kramer AF, Gratton G. Neurovascular coupling in normal aging: A combined optical, erp and fmri study. Neuroimage. 2014;85:592–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Toth P, Tarantini S, Ashpole NM, Tucsek Z, Milne GL, Valcarcel‐Ares NM, Menyhart A, Farkas E, Sonntag WE, Csiszar A. Igf‐1 deficiency impairs neurovascular coupling in mice: Implications for cerebromicrovascular aging. Aging Cell. 2015;14:1034–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Katusic ZS, Austin SA. Endothelial nitric oxide: Protector of a healthy mind. Eur Heart J. 2013;35:888–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Nowak KL, Rossman MJ, Chonchol M, Seals DR. Strategies for achieving healthy vascular aging. Hypertension. 2018;71:389–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KAJ, ones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr., Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 acc/aha/aapa/abc/acpm/ags/apha/ash/aspc/nma/pcna guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the american college of cardiology/american heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2017 [DOI] [PubMed] [Google Scholar]
- 305.Reference Values for Arterial Stiffness C. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘Establishing normal and reference values’. Eur Heart J. 2010;31:2338–2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation. 1995;91:1981–1987 [DOI] [PubMed] [Google Scholar]
- 307.Benjamin EJ, Larson MG, Keyes MJ, Mitchell GF, Vasan RS, Keaney JF Jr., Lehman BT, Fan S, Osypiuk E, Vita JA. Clinical correlates and heritability of flow-mediated dilation in the community: The framingham heart study. Circulation. 2004;109:613–619 [DOI] [PubMed] [Google Scholar]
- 308.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–476 [DOI] [PubMed] [Google Scholar]
- 309.Herrington DM, Fan L, Drum M, Riley WA, Pusser BE, Crouse JR, Burke GL, McBurnie MA, Morgan TM, Espeland MA. Brachial flow-mediated vasodilator responses in population-based research: Methods, reproducibility and effects of age, gender and baseline diameter. J Cardiovasc Risk. 2001;8:319–328 [DOI] [PubMed] [Google Scholar]