Abstract
Objective
To investigate the association between reported sleep duration and incident stroke in a US cohort of black and white adults, and evaluate race, age, and sex as potential effect modifiers.
Methods
From 2008 to 2010, 16,733 black and white adults, aged ≥45 years, without a history of stroke or sleep-disordered breathing from the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study, reported their habitual sleep duration (<6, 6.0–6.9, 7.0–8.9 [reference], ≥9 hours). Incident strokes were identified through biannual participant contact followed by physician adjudication of medical records. Cox proportional hazards analysis was conducted to calculate hazard ratios of interactions between sleep duration with race, age, sex, and 2-way combinations of these factors on incident stroke adjusting for stroke risk factors and sleep-disordered breathing risk.
Results
The sample comprised 10.4% (n = 1,747) short sleepers (<6 hours) and 6.8% (n = 1,134) long sleepers (≥9 hours). Over an average 6.1 years follow-up, 460 strokes occurred. There were significant interactions between sleep duration and race (p = 0.018) and sleep duration and race–sex groups (p = 0.0023) in association with incident stroke. Short sleep duration was significantly associated with decreased risk for stroke among black participants (hazard ratio [HR] 0.49 [95% confidence interval (CI) 0.28–0.85]), particularly black men (HR 0.21 [95% CI 0.07–0.69]), whereas long sleep duration was significantly associated with increased risk for stroke among white men (HR 1.71 [95% CI 1.06–2.76]).
Conclusions
The association of sleep duration with incident stroke differs by race and sex, with short sleep duration among black men associated with decreased risk, whereas long sleep duration among white men associated with increased risk for stroke.
Nontraditional risk factors such as sleep patterns, including duration of sleep, may be contributory to stroke risk.1 A meta-analysis indicated both short and long sleep duration to be associated with incident stroke and may vary by demographic factors such as age, sex, and race.2 Current evidence suggests that the association between long sleep duration and incident stroke may be strongest among older adults,2 though few age-stratified analyses have been conducted.3–7 Further, sex differences in the association between sleep duration and stroke are mixed depending on the length of sleep duration.2–4,7 Less studied is the modifying effect of race on the sleep duration–stroke relationship. Evidence suggests that black adults are significantly more likely to experience extremes in sleep duration and greater incidence of stroke.8–12 However, few studies have investigated the association between sleep duration and incident stroke by race. Our previous work suggested that short sleep duration partially mediated the relationship between race (black vs white adults) and incident stroke symptoms,13 and other studies also suggest that short or long sleep duration may be associated with excess risk for poor cardiometabolic risk status among black adults.14–19 However, studies addressing black–white differences on the influence of sleep duration on incident stroke are rare.
The present study investigated the relationship between reported sleep duration and incident stroke within the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study, a US population-based cohort of black and white adults, and determined whether age, sex, and race and combinations of these factors modified the association.
Methods
Study design and sample
The REGARDS study is a population-based national cohort investigating stroke incidence and mortality among 30,239 black and white participants aged 45 years or older enrolled from 2003 to 2007. REGARDS participants were randomly selected from a commercially available list (Genesys) and contacted by telephone and mail to complete a telephone interview and in-home assessment. The REGARDS cohort oversampled black adults and individuals living in the Southeastern Stroke Belt region of the United States. Telephone interviews are performed every 6 months thereafter to inquire about stroke events, with medical records on suspected stroke events retrieved for adjudication by dedicated study physicians. Further details about the study design can be found elsewhere.10,20
In the present analysis, eligible participants must have completed an ancillary sleep module (2008–2010) including questions about habitual sleep duration (n = 17,752) and be free of physician-diagnosed stroke and obstructive sleep apnea at the time of sleep module completion. The final analytic sample was 16,733 participants.
Standard protocol approvals, registrations, and participant consent
The REGARDS study was approved by all institutional review boards from participating universities, and all REGARDS participants provided written consent.
Measures
Sleep
From 2008 to 2010, participants responded to 2 questions about their habitual sleep duration on weekdays (work days) and weekends (nonwork days) with the following question format: How many hours of sleep do you usually get at night, or during your main sleep period, on your work days/nonwork days? Participants were not constrained to report values to the nearest integer. The weighted average for these values (5 weekdays to 2 nonweekdays) was computed and categorized as follows: <6, 6.0–6.9, 7.0–8.9 (reference group), 9+ hours. The sleep duration categories were chosen based on American Academy of Sleep Medicine21 and National Sleep Foundation22 guidelines for recommended and appropriate sleep durations among adults.
Stroke
Suspected stroke events, no matter the type, were identified every 6 months via telephone interview, and medical records associated with these events were retrieved and physician adjudicated. Physicians used the WHO definition of stroke as focal neurologic symptoms lasting more than 24 hours or those with neuroimaging data consistent with stroke.10,23
Covariates
The following covariates were adjusted for in subsequent models. Model 1 or the demographic model included age, sex, and race. Model 2 or the stroke risk factor model included the following: education (< high school, high school graduate, some college, ≥ college graduate); income (<$20,000, $20,000–$34,000, $35,000–$75,000, $75,000+ or refused); depressive symptoms (Center for Epidemiologic Studies–Depression 4-item scale)24; smoking status (current vs past or never); atrial fibrillation (defined by ECG evidence of atrial fibrillation or self-report of physician-diagnosed atrial fibrillation); heart disease (defined by ECG evidence of myocardial infarction or self-reported myocardial infarction, coronary artery bypass, angioplasty, or stent); left ventricular hypertrophy (defined using ECG evidence using the Sokolow criteria)25; diabetes (fasting glucose ≥126 mg/dL or nonfasting glucose ≥200 mg/dL, or on pills or insulin); and sleep-disordered breathing (high vs low risk as scored by the Berlin Questionnaire).26 The Berlin Questionnaire comprises 3 symptom categories: snoring (5 items), daytime sleepiness (3 items), and a history of high blood pressure or a body mass index (BMI) of ≥30 kg/m2 (2 items). High blood pressure was defined as systolic ≥140 mm Hg, diastolic ≥90 mm Hg, or self-report of taking antihypertensive medication at the baseline REGARDS assessment. BMI was calculated from weight and height measured during the baseline REGARDS assessment. Patients are identified as high risk for obstructive sleep apnea if they receive positive scores on at least 2 of the 3 symptom categories as indicated by greater than 2 positive responses in the snoring category, greater than 2 positive responses in the daytime sleepiness category, and either current hypertension or obesity in the last category.
Statistical analysis
Demographic and stroke risk factors for the overall analytic sample and by sleep duration category were examined. Continuous variables were summarized with means and SDs whereas categorical variables were summarized with count and proportions. Cox proportional hazards analysis was used to estimate hazard ratios (HRs) for incident stroke associated with sleep duration adjusting subsequently for demographic factors (model 1) and model 1 plus stroke risk factors (model 2). The follow-up period was defined from the date of sleep module completion to date of first stroke or last telephone contact. Interaction terms for sleep duration by race, sleep duration by age, sleep duration by sex, sleep duration by race–age groups, sleep duration by race–sex groups, and sleep duration by age–sex groups were entered separately into a demographic model (model 1). If statistically significant, then they were also entered into the fully adjusted model (model 2). Age groups were defined as <65 vs ≥ 65 years. Significance of interaction terms was determined a priori as α < 0.05. A sensitivity analysis was conducted to covary hypertension status in significant final models to address any residual confounding by hypertension status not captured by the Berlin Questionnaire. A further sensitivity analysis that eliminated participants at high risk for sleep-disordered breathing according to the Berlin Questionnaire was conducted in significant final models. All analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC).
Data availability
Our study uses data from the REGARDS cohort. In order to abide by its obligations with NIH/National Institute of Neurologic Disorders and Stroke and the institutional review board of the University of Alabama at Birmingham, REGARDS facilitates data sharing through formal data use agreements. Any investigator is welcome to access the REGARDS data through this process. Requests for data access may be sent to regardsadmin@uab.edu.
Results
Sample characteristics
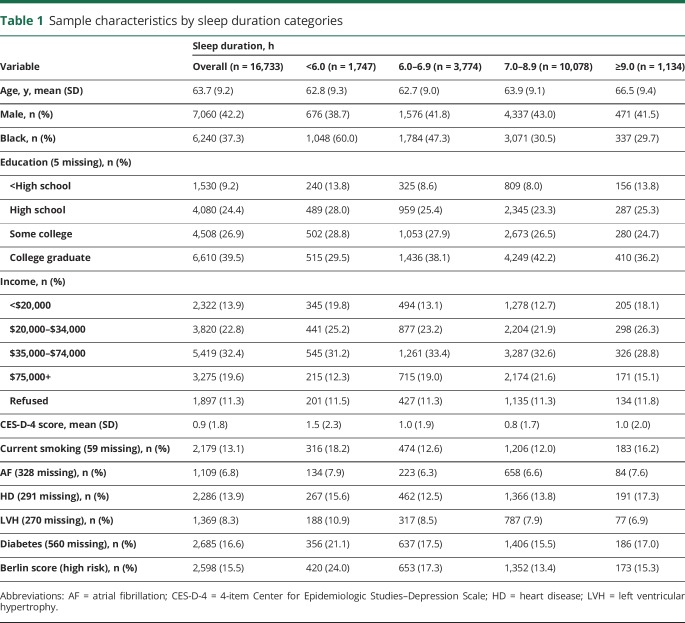
Of the 30,329 participants from the REGARDS baseline assessment, 16,733 completed the sleep module and had no history of stroke or obstructive sleep apnea. In table 1, sample characteristics of these participants by sleep duration categories are presented. Approximately 37.3% (n = 6,241) of the sample were black adults. At baseline, 10.4% (n = 1,747) of the sample were very short sleepers (<6 hours), of which black adults represented 60% of short sleepers, and 6.8% (n = 1,134) were long sleepers (9 + hours), of which black adults represented 29.7% of long sleepers. Over an average follow-up of 6.1 ± 2.4 years (range 0–8.6 years), 460 stroke events occurred (black participants = 172; white participants = 288).
Table 1.
Sample characteristics by sleep duration categories
Sleep duration and stroke events by age, sex, and race
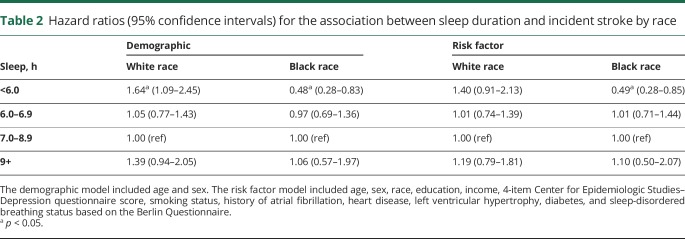
There was no interaction between sleep duration and age (p = 0.92) or sleep duration and sex in the demographic model (p = 0.31). There was an interaction between sleep duration and race in the demographic model (p = 0.006) and the fully adjusted model (p = 0.018). In the stratified, demographic model (table 2), shorter sleep duration was associated with decreased risk for stroke in black participants (HR 0.48, 95% confidence interval [CI] 0.28–0.83) and associated with increased risk for stroke in white participants (HR 1.64, 95% CI 1.09–2.45). After adjusting for stroke risk factors, the increased risk for stroke in white participants was attenuated (HR 1.39, 95% CI 0.91–2.13), but the decreased risk for stroke among black participants remained (HR 0.49, 95% CI 0.28–0.85).
Table 2.
Hazard ratios (95% confidence intervals) for the association between sleep duration and incident stroke by race
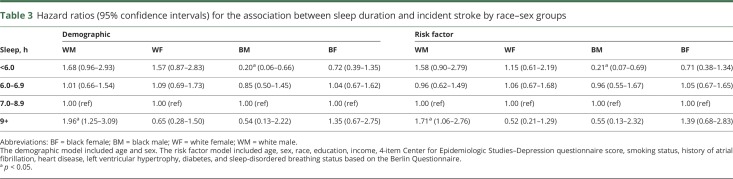
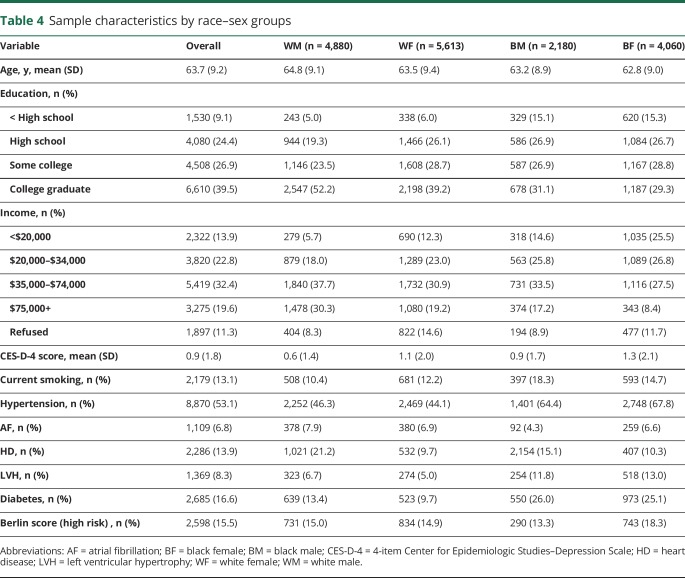
There were no interaction effects between sleep duration and age by sex groups or race by age groups. There was an interaction between sleep duration and race–sex groups in the demographic model (p = 0.015) and the fully adjusted model (p = 0.028). The stratified results are presented in table 3. In the stratified demographic model, short sleep duration was associated with decreased risk for stroke in black men (HR 0.20, 95% CI 0.06–0.66) and longer sleep duration was associated with increased risk for stroke in white men (HR 1.96, 95% CI 1.25–3.09). After adjusting for stroke risk factors, the increased risk for stroke in white men (HR 1.71, 95% CI 1.06–2.76) and the decreased risk for stroke in black men remained (HR 0.21, 95% CI 0.07–0.69). The sensitivity analysis adding in hypertension as a covariate revealed that the interaction between sleep duration and race–sex groups remained in the fully adjusted model (p = 0.032). In the stratified, fully adjusted model, the increased risk for stroke in white men (HR 1.69, 95% CI 1.05–2.72) and the decreased risk for stroke in black men remained (HR 0.21, 95% CI 0.06–0.69). The sensitivity analysis omitting participants at high risk for sleep-disordered breathing according to the Berlin Questionnaire revealed attenuation of the interaction term to nonsignificance (p = 0.074). Thus, the presence of sleep-disordered breathing may have partially explained the association between sleep duration and race–sex groups on incident stroke, or the attenuated interaction term may have been due to the reduction in power. To potentially clarify the association, demographic characteristics and stroke risk factors by race–sex groups were examined (table 4). Black men were less likely to have atrial fibrillation, but were comparably likely or more likely to have multiple other stroke risk factors compared to the other race–sex groups.
Table 3.
Hazard ratios (95% confidence intervals) for the association between sleep duration and incident stroke by race–sex groups
Table 4.
Sample characteristics by race–sex groups
Discussion
In the REGARDS cohort, counter to our hypothesis, short sleep duration (<6 hours) was associated with decreased risk for incident stroke among black, middle-aged to older adults, particularly black men, whereas agreeing with our hypothesis, long sleep duration (≥9 hours) among white men was associated with an increased risk of incident stroke. The findings suggest that resilience to insufficient sleep or vulnerability to extended sleep as risk factors for stroke may differ by race and sex groups. There were no indications of modification by age or sex alone in the association between sleep duration and incident stroke.
We report a prospective study to identify race by sex differences in susceptibility to stroke modified by extremes in sleep duration. Previous meta-analyses of prospective cohort studies examining the association between sleep duration and stroke did not test for interactions between sleep duration and race or stratify findings by race.2–7 Further, only a few studies included in these meta-analyses adjusted for race or ethnicity and none examined race-stratified or race by sex stratified results.27–29 Only 2 recent cross-sectional analyses using data from the 2004–2013 National Health Interview Survey examined sleep duration by race interactions on prevalent stroke.30,31 Though some of the results were similar to our findings, given the cross-sectional nature of the designs, reverse causality cannot be ruled out. In one preliminary analysis of patients with hypertension, when adding a race by age interaction term to their models, the authors found that short sleep duration (<6 hours) was not associated with prevalent stroke among white participants, yet was associated with prevalent stroke among black participants, but only among young black adults (ages 18–34 years).30 In contrast, long sleep duration (≥9 hours) was associated with higher prevalence of stroke similarly across all race and age groups. In the other analysis conducted among a subsample of patients with diabetes, the association between short sleep duration (≤6 hours) and prevalent stroke was only significant among white adults, whereas the association between long sleep duration (≥9 hours) and prevalent stroke was significant only among white adults, particularly if they were men.31 However, stronger evidence of the relationship between sleep duration and stroke by race is found in the present analysis, suggesting that short sleep duration among middle-aged to older black adults, particularly men, may be associated with decreased risk, whereas long sleep duration may be a risk factor among white men.
The underlying mechanisms that may explain the race and race by sex differences we found in the association between sleep duration and incident stroke are not well-understood. The alleged reduction in associated risk of incident stroke among black adults with short sleep duration, particularly black men, is perplexing, and conflicts with other studies examining race by sleep interactions on cardiometabolic risk factors. Most previous cross-sectional, nationally based cohort studies have found that short sleep duration was associated with prevalent hypertension (self-reported),16,18 hypercholesteremia,17 obesity,16 and diabetes14 among black adults but not white adults, with only one exception, when the opposite was found.19 However, these cross-sectional studies cannot inform about possible explanatory pathways. In a longitudinal study, actigraphy-estimated short sleep duration was associated with significant 5-year increases in diastolic blood pressure among black but not white adults.15 In contrast, another longitudinal study found that short sleep duration (<7 hours) was not associated with incident diabetes among black adults but was associated among white adults.32 In fact, the results suggested that short sleep duration is associated with decreased incident diabetes among black adults, though the association was not statistically significant. Why reported short sleep duration may be associated with reduced risk for diabetes and stroke among black adults is unclear. The reported construct of short sleep duration may actually be representing other sleep dimensions known to be associated with positive health outcomes such as consolidated sleep, less time spent sedentary in bed, and less variable sleep patterns. Indeed, there is some evidence that black and white adults may interpret questions about sleep duration differently.33,34 Another possibility is short sleep duration may be a marker for another confounding variable such as employment status. Full-time employed individuals tend to report short sleep durations.35 It might be the case that the black adults in our sample (particularly men) were more likely to be employed and thus perhaps more active. This counterintuitive result garnered from our prospective design highlights the importance of conducting further longitudinal research using both objective and subjective assessment of a variety of sleep metrics to substantiate these hypotheses.
Our finding of an association between long sleep duration and increased risk for incident stroke among middle-aged to older white men adds to a limited literature. Certainly, long sleep duration in association with incident stroke has been identified in several meta-analyses,2–7 thus supporting the external validity of the present study. However, few studies have been able to conduct age-, sex-, and race-specific subgroup analyses. It has been suggested that long sleep duration may be an early sign of underlying or developing cardiometabolic dysfunction among otherwise healthy older adults.2 Evidence provided in support of this hypothesis was from a large middle-aged to older cohort; participants with consistently long sleep duration or who had a substantive increase from short to long sleep duration over an approximate 4-year period were more likely to experience incident stroke. This cohort primarily comprised participants with European ancestry, and thus race-stratified analyses were not possible. Further, they were not able to exclude for a history of sleep-disordered breathing diagnosis or screen for risk of sleep-disordered breathing. In our sensitivity analysis that removed all participants with high risk for sleep-disordered breathing, the association between long sleep duration and increased risk for stroke among white men was attenuated, indicating perhaps that residual, undiagnosed sleep-disordered breathing may account for the association, or it may mean that the removal of these participants simply reduced power to be able to detect the association. In either case, the present study enhances the literature and lends credence that long sleep duration may be an early indicator of pathology whether due to sleep-disordered breathing or not, but only for certain subgroups. Mechanistic research examining different pathways between sleep and future stroke by demographic subpopulations should be implemented. Potential mechanisms that have previously been proposed, but rarely systematically investigated, include the following: long sleep duration is a proxy for other suboptimal sleep dimensions such as poor sleep quality, insomnia, sleep fragmentation, or reduction in slow-wave sleep known to be associated with cardiometabolic alterations; long sleep duration may be contributing to an overall sedentary lifestyle through greater time spent in bed and less energy expenditure; long sleep duration is associated with evening chronotype, with higher likelihood for circadian rhythm misalignment36; and long sleep duration may activate proinflammatory pathways.37 How these mechanisms may interact by different demographic subgroups is uncertain.
Our analysis has a number of strengths; most notably, it benefits from a prospective design. Other strengths are as follows. First, incident stroke events were confirmed through physician adjudication of medical records. Second, we adjusted for a comprehensive set of objectively measured stroke risk factors, as well as risk for sleep-disordered breathing, as determined by a validated screening questionnaire, a confounder often not considered in prior studies. Third, we utilized a large, US population-based sample of middle-aged to older adults, which increases generalizability, although our results do not extend to ethnic/racial groups beyond black and white adults. Finally, we assessed habitual sleep duration by computing the weighted average of sleep duration on work days (weekdays) and nonwork days (weekends). Previous literature suggests this method of assessment delivers estimates of sleep duration with greater validity than a single-item question.34 Further, self-report, though prone to biases in perception, is a convenient method of assessment in health care settings. We acknowledge the study also had limitations. Despite our use of a 2-item sleep duration assessment, the assessment remains self-reported and thus subject to recall biases. In addition, our sleep assessment was measured at one time point. Future investigations should use repeated overnight polysomnography as well as wrist actigraphy assessments of sleep. These assessments would also allow for ruling out other sleep diagnoses as confounding variables (e.g., insomnia), and evaluation of other health outcome–associated sleep dimensions. In addition to these limitations, we cannot rule out the possibility that other, unmeasured variables may confound the results, such as residual, undiagnosed sleep apnea. Finally, the reasons why a participant reported a particular habitual sleep duration were not assessed, such as whether the participant perceived that this sleep duration was sufficient or constrained by other factors such as time or environment.
In a population-based cohort of middle-aged to older black and white adults, in black men, reported short sleep duration was found to be associated with decreased risk of incident stroke, whereas long sleep duration among white men was associated with increased risk for stroke. The present study suggests that short and long sleep duration may have differing consequences depending on race and sex. These results underscore the need for mechanistic, longitudinal research of the sleep–stroke association by demographic subgroups. Further, it may be clinically advisable to assess and monitor middle-aged to older adult patients with long sleep duration, particularly white men, for cardiovascular risk.
Glossary
- BMI
body mass index
- CI
confidence interval
- HR
hazard ratio
- REGARDS
Reasons for Geographic and Racial Differences in Stroke
Footnotes
Patient Page, page e1728
Author contributions
M. Petrov was responsible for the conception and interpretation of this work, drafting of the work, providing approval of the final version, and agrees to be accountable for all aspects of the work. Dr. Howard was responsible for the design and interpretation of the work, revising it critically for important intellectual content, providing approval of the final version, and agrees to be accountable for all aspects of the work. Drs. Grandner, Kleindorfer, and Molano made substantial contributions to the interpretation of the data for the work, revising it critically for important intellectual content, providing approval of the final version, and agree to be accountable for all aspects of the work. Dr. Howard was responsible for the design and interpretation of the work, revising it critically for important intellectual content, providing approval of the final version, and agrees to be accountable for all aspects of the work.
Study funding
The REGARDS research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurologic Disorders and Stroke, NIH, Department of Health and Human Services. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurologic Disorders and Stroke or the NIH. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis, or interpretation of the data. The authors thank the other investigators, the staff, and the participants of the REGARDS study for their contributions. A full list of participating REGARDS investigators and institutions can be found at regardsstudy.org.
Disclosure
M. Petrov and G. Howard report no disclosures relevant to the manuscript. M. Grandner has performed paid scientific advisory and/or consulting services for Fitbit, Curaegis Technologies, and Natrol. He has received research support from Nexalin Technologies and Kemin Industries. D. Kleindorfer reports no disclosures relevant to the manuscript. J. Molano is on the editorial boards for NEJM Journal Watch Neurology and Neurology Now. V. Howard reports no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
Publication history
Received by Neurology December 28, 2017. Accepted in final form July 9, 2018.
References
- 1.Howard G, Cushman M, Kissela BM, et al. Traditional risk factors as the underlying cause of racial disparities in stroke: lessons from the half full (empty?) glass. Stroke 2011;42:3369–3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leng Y, Cappuccio FP, Wainwright NW, et al. Sleep duration and risk of fatal and nonfatal stroke: a prospective study and meta-analysis. Neurology 2015;84:1072–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yin J, Jin X, Shan Z, et al. Relationship of sleep duration with all-cause mortality and cardiovascular events: a systematic review and dose-response meta-analysis of prospective cohort studies. J Am Heart Assoc 2017;6:e005947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cappuccio FP, Cooper D, D'elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J 2011;32:1484–1492. [DOI] [PubMed] [Google Scholar]
- 5.Li W, Wang D, Cao S, et al. Sleep duration and risk of stroke events and stroke mortality: a systematic review and meta-analysis of prospective cohort studies. Int J Cardiol 2016;223:870–876. [DOI] [PubMed] [Google Scholar]
- 6.Ge B, Guo X. Short and long sleep durations are both associated with increased risk of stroke: a meta-analysis of observational studies. Int J Stroke 2015;10:177–184. [DOI] [PubMed] [Google Scholar]
- 7.Itani O, Jike M, Watanabe N, Kaneita Y. Short sleep duration and health outcomes: a systematic review, meta-analysis, and meta-regression. Sleep Med 2017;32:246–256. [DOI] [PubMed] [Google Scholar]
- 8.Whinnery J, Jackson N, Rattanaumpawan P, Grandner MA. Short and long sleep duration associated with race/ethnicity, sociodemographics, and socioeconomic position. Sleep 2014;37:601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrov ME, Lichstein KL. Differences in sleep between black and white adults: an update and future directions. Sleep Med 2016;18:74–81. [DOI] [PubMed] [Google Scholar]
- 10.Howard VJ, Kleindorfer DO, Judd SE, et al. Disparities in stroke incidence contributing to disparities in stroke mortality. Ann Neurol 2011;69:619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kleindorfer DO, Khoury J, Moomaw CJ, et al. Stroke incidence is decreasing in whites but not in blacks: a population-based estimate of temporal trends in stroke incidence from the Greater Cincinnati/Northern Kentucky Stroke Study. Stroke 2010;41:1326–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cruz-Flores S, Rabinstein A, Biller J, et al. Racial-ethnic disparities in stroke care: the American experience: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:2091–2116. [DOI] [PubMed] [Google Scholar]
- 13.Petrov ME, Letter AJ, Howard VJ, Kleindorfer D. Self-reported sleep duration in relation to incident stroke symptoms: nuances by body mass and race from the REGARDS study. J Stroke Cerebrovasc Dis 2014;23:e123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zizi F, Pandey A, Murrray-Bachmann R, et al. Race/ethnicity, sleep duration, and diabetes mellitus: analysis of the National Health Interview Survey. Am J Med 2012;125:162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knutson KL, Van Cauter E, Rathouz PJ, et al. Association between sleep and blood pressure in midlife: the CARDIA sleep study. Arch Intern Med 2009;169:1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grandner MA, Chakravorty S, Perlis ML, Oliver L, Gurubhagavatula I. Habitual sleep duration associated with self-reported and objectively determined cardiometabolic risk factors. Sleep Med 2014;15:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill AN, Williams NJ, Salifu I, et al. The role of race/ethnicity and gender in the association between inadequate sleep and hypercholesterolemia. J Sleep Disord Ther 2015;4:194. [Google Scholar]
- 18.Ceïde ME, Pandey A, Ravenell J, Donat M, Ogedegbe G, Jean-Louis G. Associations of short sleep and shift work status with hypertension among black and white Americans. Int J Hypertens 2015;2015:697275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vishnu A, Shankar A, Kalidindi S. Examination of the association between insufficient sleep and cardiovascular disease and diabetes by race/ethnicity. Int J Endocrinol 2011;2011:789358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howard VJ, Cushman M, Pulley L, et al. The Reasons for Geographic and Racial Differences in Stroke Study: objectives and design. Neuroepidemiology 2005;25:135–143. [DOI] [PubMed] [Google Scholar]
- 21.Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep 2015;38:843–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirshkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health 2015;1:40–43. [DOI] [PubMed] [Google Scholar]
- 23.Stroke WHO. Recommendations on stroke prevention, diagnosis, and therapy: report of the WHO task force on stroke and other cerebrovascular disorders. Stroke 1989;20:1407–1431. [DOI] [PubMed] [Google Scholar]
- 24.Melchior LA, Huba GJ, Brown VB, et al. A short depression index for women. Educ Psychol Meas 1993;53:1117–1125. [Google Scholar]
- 25.Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Ann Noninvasive Electrocardiol 2001;6:343–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Int Med 1999;131:485–491. [DOI] [PubMed] [Google Scholar]
- 27.Chen JC, Brunner RL, Ren H, et al. Sleep duration and risk of ischemic stroke in postmenopausal women. Stroke 2008;39:3185–3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim Y, Wilkens LR, Schembre SM, Henderson BE, Kolonel LN, Goodman MT. Insufficient and excessive amounts of sleep increase the risk of premature death from cardiovascular and other diseases: the Multiethnic Cohort Study. Prev Med 2013;57:377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qureshi AI, Giles WH, Croft JB, Bliwise DL. Habitual sleep patterns and risk for stroke and coronary heart disease: a 10-year follow-up from NHANES I. Neurology 1997;48:904–910. [DOI] [PubMed] [Google Scholar]
- 30.Akinseye O, Williams SK, Ojike NI, et al. Abstract TP188: Black-white differences in susceptibility to stroke secondary to abnormal sleep duration. Stroke 2016;47:ATP188. [Google Scholar]
- 31.Akinseye OA, Ojike NI, Akinseye LI, Dhandapany PS, Pandi-Perumal SR. Association of sleep duration with stroke in diabetic patients: analysis of the national health interview survey. J Stroke Cerebrovasc Dis 2016;25:650–655. [DOI] [PubMed] [Google Scholar]
- 32.Beihl DA, Liese AD, Haffner SM. Sleep duration as a risk factor for incident type 2 diabetes in a multiethnic cohort. Ann Epidemiol 2009;19:351–357. [DOI] [PubMed] [Google Scholar]
- 33.Seixas AA, Auguste E, Butler M, et al. Differences in short and long sleep durations between blacks and whites attributed to emotional distress: analysis of the National Health Interview Survey in the United States: sleep health. J Natl Sleep Found 2017;3:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lauderdale DS. Survey questions about sleep duration: does asking separately about weekdays and weekends matter? Behav Sleep Med 2014;12:158–168. [DOI] [PubMed] [Google Scholar]
- 35.Knutson KL, Van Cauter E, Rathouz PJ, DeLeire T, Lauderdale DS. Trends in the prevalence of short sleepers in the USA: 1975–2006. Sleep 2010;33:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan X, Chapman CD, Cedernaes J, Benedict C. Association between long sleep duration and increased risk of obesity and type 2 diabetes: a review of possible mechanisms. Sleep Med Rev 2018;40:127–134. [DOI] [PubMed] [Google Scholar]
- 37.Patel SR, Zhu X, Storfer-Isser A, et al. Sleep duration and biomarkers of inflammation. Sleep 2009;32:200–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Our study uses data from the REGARDS cohort. In order to abide by its obligations with NIH/National Institute of Neurologic Disorders and Stroke and the institutional review board of the University of Alabama at Birmingham, REGARDS facilitates data sharing through formal data use agreements. Any investigator is welcome to access the REGARDS data through this process. Requests for data access may be sent to regardsadmin@uab.edu.