Abstract
Background and Aims
Despite the negative consequences associated with caffeine use among children and youth, its use is increasingly widespread among middle school students. Cross-sectional studies reveal links between caffeine and other substance use. The potential for caffeine use to confer increased vulnerability to substance use, however, has not been investigated using prospective designs. We hypothesized that caffeine use at baseline would be associated positively with increased alcohol use, drunkenness, smoking and e-cigarette use.
Design
Prospective cohort study with 12 months separating baseline from follow-up.
Setting
West Virginia, USA.
Participants
Middle school students (6th and 7th grades; n = 3932) in three West Virginia (WV) counties provided data at baseline and follow-up 12 months later.
Measurements
Youth self-reported their use of caffeine from multiple sources (e.g. soda, energy drinks, coffee and tea), cigarette smoking, electronic cigarette use, alcohol use and drunkenness.
Findings
Cross-lagged path models for individual substance use categories provided a good fit to the data. Controlling for demographic variables and other substance use at baseline, caffeine at time 1 (T1) was associated positively with T2 cigarette smoking (β = 0.27, P = 0.001), e-cigarette use (β = 0.21, P = 0.001), alcohol use (β = 0.17, P = 0.001) and drunkenness (β = 0.15, P = 0.001). Conversely, non-significant relations emerged between three of four substances at T1 and caffeine at T2. Positive relations were found between e-cigarette use at T1 and caffeine use at T2 (β = 0.07, P = 0.006). These findings were supported by an omnibus model with all substances included. Specifically, significant relations were observed between caffeine at T1 and all substance use outcomes at T2, whereas no significant relations were observed between substance use and caffeine over time.
Conclusions
Caffeine may promote early use of other types of substances among middle school-aged adolescents.
Keywords: Alcohol use, Appalachia, caffeine, early adolescents, primary prevention, smoking
INTRODUCTION
Caffeine is a psychostimulant possessing arousal, motor activation and reinforcing properties [1]. Stimulant effects are believed to be a product of the competitive blockade of adenosine receptors both centrally and peripherally [2,3]. Similar to other psychoactive substances, regular caffeine consumption leads to physical dependence, evidenced by withdrawal symptoms upon abstinence that include headache, fatigue, difficulty concentrating, mood disturbances and flu-like symptoms [4]. Onset of withdrawal symptoms usually occurs 12–24 hours after abstinence and symptoms may persist for 2–9 days. Abstinence from as little as 100 mg of daily intake can produce withdrawal symptoms [4]. In addition to withdrawal, the Diagnostic and Statistical Manual of Mental Disorders (DSM-5®) [5] contains multiple caffeine-related diagnoses, including caffeine intoxication, caffeine-induced anxiety and caffeine-induced sleep disorders. Despite the potential for negative consequences, however, caffeine is distinguished by being the only psychoactive substance that is readily available to, and even marketed directly to, children and youth [6,7].
Children and adolescents most often consume caffeine via soda (e.g. Mountain Dew, Coca-Cola), and increasingly in the form of ‘energy’ drinks (e.g. Red Bull, Monster). Youth also consume caffeine in coffee, tea and candy [8]. Whereas caffeinated soda typically contains between 18 and 55 mg of caffeine per 12-ounce serving, the caffeine concentration of energy drinks typically varies from 70 to 130 mg per 12 ounces, with the latter level being more characteristic of the caffeine concentration of regular coffee [9,10]. The increasing popularity of energy drinks among youth has coincided with elevated rates of acute complications of caffeine overuse, including seizures, cardiac dysrhythmia and heart failure [11]. In the 3 years from 2006 to 2008, Poison Control Centers in the United States received an average of 5332 calls per year related to caffeine toxicity, 25% of which involved people aged 6–19 years, and 29% required medical intervention [10]. Although rare, youth fatalities have been attributed to energy drink consumption, particularly when combined with physical stress, such as may occur in high-intensity sports or when combined with alcohol [12–15].
Although the acute effects of caffeine overuse on youth are a clear cause for concern, the long-term health consequences of routine caffeine use among adolescents have received less empirical attention. Studies of middle and high school-aged samples have generally found that between 50 and 70% of respondents consume caffeine daily or several times per week [6,10,16–18]. Among children and youth, even moderate consumption has been associated with symptoms including headache, migraine, nausea, drowsiness, tiredness, fatigue, sluggishness, concentration difficulties, mood disturbances and sleeplessness [19–23].
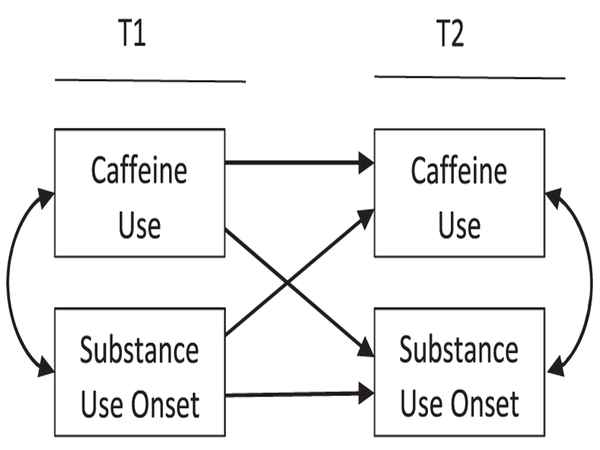
Routine caffeine use may have uniquely detrimental effects on adolescents. The adolescent years are characterized by extensive environmental changes, as well as physical and neurodevelopmental maturation. These changes affect youths’ need for sleep, sleep phase preferences, sensitivity to reward and stress and propensity for risky behavior, including substance use [24–26]. In the context of rapid developmental changes, routine stimulant use in the form of caffeine consumption and cycles of dependence and withdrawal may confer significant vulnerability for adolescent problem behaviors, and may have consequences not observed among less vulnerable adults (Fig. 1).
Figure 1.
Cross-lagged path models (for demonstrative purposes, control variables are implied)
Caffeine effects on the early onset of substance use is of particular concern. Substance use onset in early adolescence (i.e. ages 10–13 years) has prognostic significance for substance use problems in later adolescence and adulthood. Emerging research supports the plausibility of links between caffeine use and early substance use. Studies with older adolescents and young adults link caffeine consumption to smoking, alcohol use, heavy drinking episodes and marijuana use [17,18,27–32]. Evidence on the effects of routine caffeine use on young adolescents’ substance use is scarce. We are aware of only one prospective examination of caffeine use on subsequent substance use among early adolescents [33], and that study was limited to consumption of energy drinks. Results, however, supported the need for additional investigations of caffeine use on substance use onset. Employing a sample of 144 middle school students (age 12 years) in New Jersey, Miyake & Marmorstein [33] found that energy drink use predicted alcohol use 16 months post-baseline.
The present study addresses the need for prospective investigations of the influence of caffeine use on substance use among early adolescents. We address several gaps in the research outlined above. First, extant studies focus primarily on energy drink use. Recent research suggests, however, that total caffeine consumption from all sources plays a significant role in the assessment of caffeine consequences [23,34]. Accordingly, we measure total caffeine consumption using instruments validated in previous studies with adolescents [17,35]. Secondly, we employ a prospective design and analytical methods that consider both the potential of caffeine use to forecast substance use and the reverse hypotheses that substance use promotes caffeine use. The use of cross-lagged path analyses provides important information on the developmental sequencing of substance use vulnerability. Thirdly, we consider the potential influences of caffeine consumption on multiple substance use outcomes including alcohol use, drunkenness, cigarette smoking and e-cigarette use. Finally, we use an amply powered sample drawn from a census of three middle school districts with two waves of data from more than 4000 students.
In summary, despite widespread caffeine use among early adolescents, the potential for caffeine to contribute to early substance use is unclear. We hypothesized that among early adolescents, baseline caffeine consumption would forecast substance use 12 months later. We controlled for covariates identified in previous studies to be linked to caffeine, alcohol and nicotine use; these included gender, family structure, parental education and race/ethnicity. In addition, to increase confidence in the direction of effects, we tested alternative hypotheses that use of various substances is associated with caffeine consumption 12 months later.
METHOD
Sample, procedures and handling of missing data
Data were collected from 15 public schools in three diverse WV counties: Wood County, in the far western part of the State, Berkeley County, in the far eastern part, and McDowell County, the southernmost county of WV. Of 55 counties in WV these rank numbers 9, 32 and 55, respectively, on the latest Robert Wood Johnson County Health Rankings. They represent a wide distribution of family economic status, including communities characterized by extreme rural poverty, working-class families and middle/upper-class families. The study was reviewed and approved by West Virginia University’s Institutional Review Board (IRB). During the fall of 2015 (September–October), students in grades 6 (49.6%) and 7 (50.4%) in the participating counties were invited to participate in the study and again in the fall of 2016. Parents were notified of the study via letter and given the opportunity to exclude their children (parental opt-out rate was < 1%). Upon passive parental consent, survey data were collected by teachers under the supervision of county research coordinators, who operated as a liaison to the research team. Similar data collection procedures have been published elsewhere [36].
At time 1 (T1) 4150 children provided data (response rate = 84.7%) and at T2 4057 provided data (response rate = 84.2%). Survey responses across time were matched using a self-reported student ID number. Data cleaning procedures resulted in 218 inconsistent respondents being omitted from the analyses, with a final sample of 3932 for these analyses. Missing data at T2 were imputed with a Bayesian estimation and 50 iterations using all variables in the analyses file as auxiliary indicators to strengthen estimation.
An analysis into potential group differences between students who did not report an ID at T1 and those who did (within wave) revealed no differences for family structure, grade, parental education level or race/ethnicity or patterns of substance use. There were significant differences in reporting a confidential ID number by gender, with girls more likely than boys to report their student ID number in both survey waves (P < 0.01).
Measures
Caffeine consumption
Total daily caffeine consumption was estimated using an inventory validated previously with a national sample of adolescents [23]. The caffeine measure was designed to assess daily consumption of caffeine from multiple beverages. With reference to ‘the past 30 days’ as a time-frame, respondents were asked: ‘How much, if anything, do you drink of the following drinks on a typical day?’. Participants answered the questions for each of the following beverages: coffee, tea, soda/pop that contains caffeine (Coca-Cola, Pepsi, Dr Pepper, Mountain Dew, etc.), and energy drinks that contain caffeine (e.g. Red Bull, Monster, etc.). Response options ranged from 1 (none) to 7 (six glasses/cups or more). As reported previously [17,34], each type of caffeine beverage was weighted to reflect the differences in caffeine content: coffee (6×), tea (3×), soda/pop (1×) and energy drinks (3×). A composite measure was created by summing the scores of the four weighted caffeine questions to a scale (range = 13–91). Because of high skew and kurtosis values in the composite caffeine measure at T1 and T2 (2.46/7.91 and 2.57/8.69, respectively) the measure was transformed with a natural logarithm which brought its distribution into an ideal range (0.92/0.66 and 1.000/0.95 at T1 and T2, respectively). All statistical models employ the transformed caffeine measures at T1 and T2.
Other substance use
Life-time use of other substances was assessed with the following questions: ‘Have you ever tried cigarette smoking, even just one or two puffs’; ‘Have you ever tried electronic cigarettes (e-cigarettes or vapors), even just one or two puffs’; ‘Have you ever had a drink of alcohol, other than a few sips’; and ‘Have you ever been drunk from drinking alcohol?’. The questions were scored with 1 = ‘yes’ and 0 = ‘no’.
Control variables
Control variables included gender (girls = 1), family structure (0 = lives with both parents, 1 = other living arrangements), race (1 = white, 0 = other) and mother’s education (1 = elementary or middle school or less to 9 = graduated with a Master’s, Doctorate or professional degree). Descriptive statistics for all study variables are shown in Table 1.
Table 1.
Descriptive statistics for all study variables.
| Variable | Range | Mean (SD)/%, time 1 | Mean (SD)/%, time 2 |
|---|---|---|---|
| Coffee | 1–7 | 1.63 (1.14) | 1.58 (1.07) |
| Tea | 1–7 | 2.52 (1.81) | 2.40(1.70) |
| Caffeinated soda/pop | 1–7 | 2.86 (1.83) | 2.72 (1.73) |
| Energy drinks | 1–7 | 1.39 (1.12) | 1.38 (1.09) |
| Caffeine (weighted, non-transformed) | 13–91 | 24.24 (13.00) | 23.38 (12.22) |
| Caffeine (weighted, log-transformed) | 2.56–4.51 | 3.09 (0.42) | 3.06 (0.41) |
| Ever cigarette smoking (%) | 0–1 | 6.4 | 8.1 |
| Ever use of e-cigarettes (%) | 0–1 | 5.9 | 10.3 |
| Ever used alcohol (%) | 0–1 | 8.8 | 14.4 |
| Ever been drunk (%) Control variables |
0–1 | 3.9 | 5.6 |
| Girls (%) | 0–1 | 49.8 | |
| Lives with both parents (%) | 0–1 | 56.8 | |
| White (%) | 0–1 | 77.4 | |
| Mother’s education | 1–9 | 2.91 (2.46) |
SD = standard deviation.
Data analyses
Data analyses were conducted using cross-lagged path models with linear and probit regression coefficients and both continuous and binary outcomes. Cross-lagged models provide information on directions of influences by simultaneously modeling the bidirectional influences of constructs over two time-points, while controlling for stability in each construct over time. All statistical models were run in Mplus [37], with the robust weighted least-squares estimator using a diagonal weight matrix while adjusting standard errors for clustering based on school. The intraclass correlation coefficient was 0.039 (3.9%) and average cluster size was 117.33.
First, we ran separate cross-lagged path models for caffeine and each substance use outcome at T1 and T2. Caffeine at T1 was allowed to correlate with each substance use outcome at T1. Similarly, caffeine at T2 was allowed to correlate with each substance use outcome at T2. Both caffeine and each outcome measure at T1 were also allowed to correlate with the covariates gender, family structure, race and mother’s education. Secondly, we ran an omnibus cross-lagged path model with caffeine and all four outcomes at T1 and T2. As before, caffeine was allowed to correlate with each substance use and the control variables at T1 and caffeine at T2 was allowed to correlate with each substance use at T2. Based on the previous literature and through multiple model comparisons, we specified the best-fitting model by comparing it to the most parsimonious model (, P < 0.01). The final omnibus model included added pathways between e-cigarettes and cigarette smoking [38] and alcohol use and drunkenness [34].
RESULTS
Average caffeine intake was estimated to be 253 mg/day at T1 and 236 mg/day at T2. By way of illustration, these intake levels could be achieved by consuming the approximate equivalent of two cups of coffee, four cups of tea, five to eight glasses of soda/pop or three to four cans of energy drinks. For T1 and T2 combined, an average of 75% of participants consumed soda/pop on a typical day, followed by 62% who consumed tea, 41% coffee and 20% energy drinks. That the sum of these estimated percentages substantially exceeds 100 confirms that participants frequently consumed more than one type of caffeine beverage.
Table 2 summarizes the results of the four cross-lagged path models for each substance. The data demonstrated a good fit to each model, with the comparative fit indices (CFI) ranging from 0.981 to 0.984, the Tucker-Lewis indices (TLI) ranging from 0.956 to 0.963 and root mean square error of approximation (RMSEA) ranging from 0.019 to 0.021. In all models, a significant prospective association was observed between caffeine at T1 and caffeine at T2, with βs ranging from 0.58 to 0.59. In each model, the relation between caffeine at T1 and substance use at T2 was positive and significant, with βs ranging from 0.15 for drunkenness to 0.27 for cigarette smoking (P < 0.001 in all models). Also, in all models each respective substance use at T1 was related positively to the same substance use at T2, with βs ranging from 0.56 for smoking and e-cigarettes to 0.60 for drunkenness (P < 0.001 in all models). In three of four models, the substance use variable at T1 was not related to caffeine at T2. Only e-cigarette use at T1 was related to caffeine at T2 (P = 0.006), demonstrating a small effect (β = 0.07). Variance explained ranged from 35 to 36% for caffeine at T2, and from 33 (drunkenness) to 46% (cigarette smoking) for substance use outcomes.
Table 2.
Results from cross-lagged path models. Caffeine with each individual outcome.
| Beta | Stand. Beta | SE | P | Model fits | |
|---|---|---|---|---|---|
| DVs: ever smoking at T2, caffeine at T2 | |||||
| Caffeine at T1 > caffeine at T2 | 0.57 | 0.59 | 0.022 | 0.001 | CFI = 0.984 |
| Caffeine at T1 > smoking at T2 | 0.63 | 0.27 | 0.079 | 0.001 | TLI = 0.963 |
| Smoking at T1 > smoking at T2 | 2.27 | 0.56 | 0.102 | 0.001 | RMSEA = 0.020 |
| Smoking at T1 > caffeine at T2 | 0.08 | 0.05 | 0.058 | 0.195 | WRMR = 0.855 |
| DVs: ever e-cigarettes at T2, caffeine at T2 | |||||
| Caffeine at T1 > caffeine at T2 | 0.57 | 0.58 | 0.022 | 0.001 | CFI = 0.981 |
| Caffeine at T1 > e-cigarettes at T2 | 0.51 | 0.21 | 0.078 | 0.001 | TLI = 0.956 |
| E-cigarettes at T1 > e-cigarettes at T2 | 20.36 | 0.56 | 0.111 | 0.001 | RMSEA = 0.021 |
| E-cigarettes at T1 > caffeine at T2 | 0.12 | 0.07 | 0.044 | 0.006 | WRMR = 0.889 |
| DVs: ever alcohol at T2, caffeine at T2 | |||||
| Caffeine at T1 > caffeine at T2 | 0.57 | 0.58 | 0.023 | 0.001 | CFI = 0.984 |
| Caffeine at T1 > alcohol at T2 | 0.41 | 0.17 | 0.068 | 0.001 | TLI = 0.962 |
| Alcohol at T1 > alcohol at T2 | 20.03 | 0.57 | 0.096 | 0.001 | RMSEA = 0.020 |
| Alcohol at T1 > caffeine at T2 | 0.08 | 0.06 | 0.044 | 0.066 | WRMR = 0.857 |
| DVs: ever drunk at T2, caffeine at T2 | |||||
| Caffeine at T1 > caffeine at T2 | 0.58 | 0.59 | 0.019 | 0.001 | CFI = 0.982 |
| Caffeine at T1 > drunk at T2 | 0.46 | 0.15 | 0.096 | 0.001 | TLI = 0.959 |
| Drunk at T1 > drunk at T2 | 20.58 | 0.60 | 0.196 | 0.001 | RMSEA = 0.019 |
| Drunk at T > caffeine at T2 | 0.02 | −0.01 | 0.080 | 0.850 | WRMR = 0.864 |
RMSEA = root mean square error of approximation; WRMR = weighted root mean square residual; CFI = Comparative Fit Index; TLI = Tucker–Lewis Index; SE = standard error; DV = Dependent variables.
Table 3 presents the omnibus model including caffeine and each substance at T1 and T2. Overall, the data demonstrated a good fit, with a CLI = 0.980, TLI = 0.941 and RMSEA = 0.034. In this model caffeine at T1 was related positively with caffeine at T2 (β = 0.57, P < 0.001), but all measures of substance use at T1 were not related with caffeine at T2 (P ranging from 0.172 to 0.966, respectively). Conversely, caffeine at T1 was related positively with smoking, e-cigarettes, alcohol use and drunkenness at T2 (P > 0.001 in all models). As before, each substance use at T1 was related positively with the same use at T2 (P < 0.001 in all instances). In addition, e-cigarettes use at T1 was related positively with smoking at T2 (β = 0.18, P < 0.001) and alcohol use at T1 was related positively with drunkenness at T2 (β = 0.27, P < 0.001). The variance explained for outcome variables was 37% for caffeine, 60% for e-cigarettes, 54% for alcohol, 53% for cigarette smoking and 41% for drunkenness.
Table 3.
Results from the final cross-lagged path model; caffeine with all outcomes.
| DVs: caffeine at T2, smoking at T2, e-cigarettes at T2, alcohol at T2, drunk at T2 | Beta | Stand. Beta | SE | P | Model fits |
|---|---|---|---|---|---|
| Caffeine at T1 > caffeine at T2 | 0.57 | 0.58 | 0.022 | 0.001 | CFI = 0.980 |
| Smoking at T1 > caffeine at T2 | 0.00 | 0.00 | 0.075 | 0.966 | TLI = 0.941 |
| E-cigarettes at T1 > caffeine at T2 | 0.10 | 0.05 | 0.075 | 0.184 | RMSEA = 0.034 |
| Alcohol at T1 > caffeine at T2 | 0.09 | 0.06 | 0.067 | 0.172 | WRMR = 10.25 |
| Drunk at T1 > caffeine at T2 | −0.08 | −0.04 | 0.083 | 0.323 | |
| Caffeine at T1 > smoking at T2 | 0.54 | 0.23 | 0.073 | 0.001 | |
| Smoking at T1 > smoking at T2 | 20.12 | 0.52 | 0.166 | 0.001 | |
| E-cigarettes at T1 > smoking at T2 | 0.78 | 0.18 | 0.151 | 0.001 | |
| Caffeine at T1 > e-cigarettes at T2 | 0.46 | 0.19 | 0.080 | 0.001 | |
| E-cigarettes at T1 > e-cigarettes at T2 | 30.10 | 0.70 | 0.201 | 0.001 | |
| Caffeine at T1 > alcohol at T2 | 0.42 | 0.18 | 0.087 | 0.001 | |
| Alcohol at T1 > alcohol at T2 | 20.49 | 0.67 | 0.158 | 0.001 | |
| Caffeine at T1 > drunk at T2 | 0.41 | 0.17 | 0.098 | 0.001 | |
| Drunk at T1 > drunk at T2 | 20.21 | 0.43 | 0.229 | 0.001 | |
| Alcohol at T1 > drunk at T2 | 10.00 | 0.27 | 0.163 | 0.001 |
RMSEA = root mean square error of approximation; WRMR = weighted root mean square residual; CFI = Comparative Fit Index; TLI = Tucker-Lewis Index; SE = standard error; DV = Dependent variables
DISCUSSION
In 2007, accumulated evidence of harm from caffeine o veruse among adolescents led the Institute of Medicine (IOM) to recommend a prohibition on the sale of energy drinks in schools for youth under age 16 years [39]. Despite these concerns, the majority of middle school-aged children ingest caffeine on a daily basis from a range of sources, including sodas, energy drinks, coffee and tea [27,40]. In response to the dearth of research on the influence of caffeine use on early-onset substance use, we conducted a prospective investigation of almost 4000 middle school students. To our knowledge, this is the first study to examine prospectively how daily caffeine consumption is associated with substance use risk in this population. We found that caffeine consumption was associated with increases in life-time use of cigarettes, e-cigarettes and alcohol, and in life-time experience of drunkenness. In particular, cross-lagged path models of individual substances revealed robust associations of caffeine at T1 with cigarette smoking, e-cigarette use, alcohol use and drunkenness at T2, with βs ranging from 0.15 to 0.27 (P < 0.001 in all instances). These effects were apparent after having adjusted for multiple socio-demographic variables and baseline substance use. Only e-cigarette use at T1 was significantly, albeit weakly, related with caffeine use at T2 (β = 0.07, P = 0.006). It is possible that the comparatively large increase in e-cigarette use from T1 to T2 (see Table 1) contributed to the bidirectional association between e-cigarettes and caffeine. A multivariate cross-lagged path model which included all substances at T1 and T2 supported further the potential contribution of caffeine to cigarette smoking, e-cigarette use, alcohol use and drunkenness.
Although research on middle school-aged students is limited, our findings are consistent with cross-sectional studies [27] and findings from longitudinal analyses linking energy drink to alcohol [33]. Similarly, Tucker et al. [41] found a prospective association between alcohol mixed with energy drinks and substance use 3 years later in a sample of high school students in California. The present study builds upon those findings by including a more complete measure of caffeine consumption, multiple substances and by employing a large sample of early adolescents from socio-economically diverse regions. Together, these studies underscore the potential harms of caffeine consumption on youth health and behavior in general, and on their vulnerability to substance use in particular.
The present study is the first prospective analysis to examine reciprocal influences between caffeine and early-onset substance use. In cross-lagged analyses, neither cigarette smoking, alcohol use nor the experience of drunkenness were associated with changes in caffeine use. These findings suggest that caffeine may operate to promote other substance use. As such, identifying potential mechanisms of action by which caffeine use promotes substance use in middle school youth deserves greater consideration. Experimental research and neuroscience perspectives suggest that caffeine’s effects on adenosine receptors may affect striatal pathways associated with the production of dopamine and reward sensitivity. This area of the brain is particularly responsive during early adolescence [25]. To the extent that caffeine is experienced as increasingly rewarding, the use of substances to regulate mood in general may be reinforced. This mechanism is particularly concerning for youth who are experimenting with new substances, as caffeine use may potentiate the rewarding effects of the new substance and lead to escalation and chronic problems [1,42]. In that regard, early caffeine consumption could serve to increase the use of nicotine, alcohol and substances in general [43,44].
Additionally, the caffeine-to-substance-use pathway may reflect an underlying disposition or set of personality traits associated with the use of both caffeine and other substances. In this case, caffeine would not be related causally with the risk of later substance use, but would merely signify an underlying predisposing variable. Research with adults suggests that personality influences the experience of caffeine [45,46]. Existing research into such relations with early adolescents, however, is sparse. Other studies suggest that consumption of caffeinated beverages and alcohol may be influenced by a common factor such as low levels of parental monitoring [47].
Social-developmental perspectives provide another possible explanation for the effects of caffeine on substance use [48]. The transition from elementary to middle school, particularly within large public schools, represents a difficult transition for many youth [49]. Work-loads and demands for passive learning increase and compete with peers for youths’ attention. Simultaneously, many adolescents begin to experience sleep deprivation as a consequence of media use and delayed sleep preferences [23]. The effects of caffeine may become a means for maintaining attention or coping with an increasingly difficult and stressful school day.
Although our study findings reliably implicated caffeine use in downstream substance use onset, the individual substance use models revealed one reciprocal association, between e-cigarette use and caffeine consumption. Accordingly, e-cigarette use at T1 was related positively with caffeine at T2, albeit not at strongly, as caffeine encouraged e-cigarette use. However, this finding was not confirmed in the multivariate model. Nevertheless, additional research is warranted to clarify the causal paths regarding caffeine and e-cigarette use in young adolescents.
Specific limitations and strengths of the present study are noteworthy. Given that our sample was drawn from three counties in West Virginia, findings may not be generalizable to other regions in the United States or to other countries. Our sample is distinctive, however, in that it includes communities that are representative of an area of persistent rural poverty where energy drink and Mountain Dew (a highly caffeinated soda) consumption are elevated among youth [27]. Additional focus on the health needs of this population is needed. Despite employing longitudinal data in the analyses, our findings are correlational in nature, which renders causal inferences inapplicable. The use of self-reported substance and caffeine use in our study is subject to social desirability and recall biases. Test-re-test reliability for similar self-reports of life-time substance use, however, appear to be substantial, with kappas for cigarette smoking and alcohol use items exceeding 0.78 [50]. Our precision in measurement and change over time in caffeine use and substance use is unbalanced: high for caffeine but limited for substance use, given the ‘ever use’ assessment at both T1 and T2. Future studies should ideally employ ordinal measures for substance use variables over time. Such more finely calibrated measures of substance use could help to establish whether caffeine may serve to promote increase in other forms of substance use over time by those who had already initiated their use at T1. Our assessment also lacks information concerning how long caffeine or other substances have been used by participants prior to T1. The data do not include measures on quantity per unit of time (i.e. once per week, once per day, only on Friday nights, etc.), and our attempt to adjust the weights of individual caffeine questions is designed to provide a reasonable approximation of actual use for epidemiological research. Future research would benefit from a longer time-frame of assessment and additional examination of other substances of abuse. Moreover, it should be noted that the focus of our study was total caffeine consumption, rather than the consumption of particular caffeine beverages. As such, our findings, which relate primarily to overall caffeine exposure, may not generalize to individual beverage types. Finally, given that caffeine is being added to an increasingly wide range of foods, our caffeine consumption measure based on beverage use may be an underestimate of total consumption. This limitation, however, suggests that our findings are conservative and that the true effect could be greater than observed.
A number of strengths also characterize this study. Rather than focusing exclusively on energy drinks, we assessed caffeine consumption across a variety of sources. Our sample was the largest to date using prospective data to examine caffeine effects on a number of substance use outcomes with adolescents. Additionally, in order to add to the robustness of the findings, we ran the data both with individual substance use modeled at T1 and T2 as well as an omnibus model with all substances at both T1 and T2.
We conclude that caffeine use in middle school students may increase the risk of cigarette smoking, e-cigarette use, alcohol use and drunkenness during a 12-month period. As such, our findings support IOM recommendations [39,51] intended to limit caffeine consumption by middle school-aged youth.
Acknowledgements
The authors wish to thank the Substance Abuse and Mental Health Services Administration (SAMHSA) (Prime Award # GRTAWD04021500003266) and the Sisters Health Foundation in Parkersburg WV (Grant # f2014-hl-30) for their generous support for this study.
Footnotes
Declaration of interests
None.
References
- 1.Ferré S Caffeine and substance use disorders. J Caffeine Res 2013; 3: 57–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunwiddie TV, Masino SA The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci 2001; 24: 31–55. [DOI] [PubMed] [Google Scholar]
- 3.Luebbe AM, Bell DJ Mountain dew or mountain don’t? A pilot investigation of caffeine use parameters and relations to depression and anxiety symptoms in 5th and 10th grade students. J School Health 2009; 79: 380–7. [DOI] [PubMed] [Google Scholar]
- 4.Juliano LM, Griffiths RR A critical review of caffeine withdrawal: empirical validation of symptoms and signs, incidence, severity, and associated features. Psychopharmacology (Berl) 2004; 176: 1–29. [DOI] [PubMed] [Google Scholar]
- 5.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th edn Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 6.Kristjansson AL, Sigfusdottir ID, Frost SS, James JE Adolescent caffeine consumption and self-reported violence and conduct disorder. J Youth Adolesc 2013; 42: 1053–62. [DOI] [PubMed] [Google Scholar]
- 7.Pomeranz JL Advanced policy options to regulate sugar-sweetened beverages to support public health. J Public Health Policy 2012; 33: 75–88. [DOI] [PubMed] [Google Scholar]
- 8.Temple JL, Dewey AM, Briatico LN Effects of acute caf feine administration on adolescents. Exp Clin Psychopharmacol 2010; 18: 510–20. [DOI] [PubMed] [Google Scholar]
- 9.Seifert SM, Schaechter JL, Hershorin ER, Lipshultz SE Health effects of energy drinks on children, adolescents, and young adults. Pediatrics 2011; 127: 511–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oddy WH, O’Sullivan TA Energy drinks for children and adolescents. BMJ 2009; 339: b5268. [DOI] [PubMed] [Google Scholar]
- 11.Heckman MA, Sherry K, Gonzalez de Mejia E Energy drinks: an assessment of their market size, consumer demographics, ingredient profile, functionality, and regulations in the United States. Compr Rev Food Sci Food Saf 2010; 9: 303–17. [DOI] [PubMed] [Google Scholar]
- 12.Schneider MB, Benjamin HJ, Bhatia JJS, Abrams SA, De Ferranti SD, Silverstein J et al. Clinical report—sport drinks and energy drinks for children and adolescents: are they appropriate? Pediatrics 2011; 127: 1182–9. [DOI] [PubMed] [Google Scholar]
- 13.James JE Dietary caffeine: ‘unnatural’ exposure requiring precaution? J Subst Use 2014; 19: 394–7. [Google Scholar]
- 14.Temple JL Caffeine use in children: what we know, what we have left to learn, and why we should worry. Neurosci Biobehav Rev 2009; 33: 793–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pettit ML, DeBarr KA Perceived stress, energy drink consumption, and academic performance among college students. J Am Coll Health 2011; 59: 335–41. [DOI] [PubMed] [Google Scholar]
- 16.Marczinski CA Combined alcohol and energy drink use: hedonistic motives, adenosine, and alcohol dependence. Alcohol Clin Exp Res 2014; 38: 1822–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.James JE, Kristjansson AL, Sigfusdottir ID A gender-specific analysis of adolescent dietary caffeine, alcohol consumption, anger, and violent behavior. Subst Use Misuse 2015; 50: 257–67. [DOI] [PubMed] [Google Scholar]
- 18.Azagba S, Langille D, Asbridge M An emerging adolescent health risk: caffeinated energy drink consumption patterns among high school students. Prev Med 2014; 62: 54–9. [DOI] [PubMed] [Google Scholar]
- 19.Hering-Hanit R, Gadoth N Caffeine-induced headache in children and adolescents. Cephalalgia 2003; 23: 332–5. [DOI] [PubMed] [Google Scholar]
- 20.Oberstar JV, Bernstein GA, Thuras P. D Caffeine use and dependence in adolescents: one-year follow-up. J Child Adolesc Psychopharmacol 2002; 12: 127–35. [DOI] [PubMed] [Google Scholar]
- 21.Juliano LM, Huntley ED, Harrell PT, Westerman AT Development of the caffeine withdrawal symptom questionnaire: caffeine withdrawal symptoms cluster into 7 factors. Drug Alcohol Depend 2012; 124: 229–34. [DOI] [PubMed] [Google Scholar]
- 22.Kristjansson AL, Sigfusdottir ID, Mann MJ, James JE Caffeinated sugar-sweetened beverages and common physical complaints in Icelandic children aged 10–12 years. Prev Med 2014; 58:40–4. [PubMed] [Google Scholar]
- 23.James JE, Kristjansson AL, Sigfusdottir ID Adolescent substance use, sleep, and academic achievement: evidence of harm due to caffeine. J Adolesc 2011; 34: 665–73. [DOI] [PubMed] [Google Scholar]
- 24.Viner RM, Ozer EM, Denny S, Marmot M, Resnick M, Fatusi A et al. Adolescent Health 2 Adolescence and the social determinants of health. Lancet 2012; 379: 1641–52. [DOI] [PubMed] [Google Scholar]
- 25.Galvan A Adolescent development of the reward system. Front Hum Neurosci 2010; 4: 116–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colrain IM, Baker FC Changes in sleep as a function of adolescent development. Neuropsychol Rev 2011; 21: 5–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mann MJ, Smith ML, Kristjansson AL Energy drink consumption and substance use risk in middle school students. Prev Med Rep 2016; 3: 279–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller KE Energy drinks, race, and problem behaviors among college students. J Adolesc Health 2008; 43: 490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patrick ME, Maggs JL Energy drinks and alcohol: links to alcohol behaviors and consequences across 56 days. J Adolesc Health; 54: 454–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Velazquez CE, Poulos NS, Latimer LA, Pasch KE Associations between energy drink consumption and alcohol use behaviors among college students. Drug Alcohol Depend 2012; 123: 167–72. [DOI] [PubMed] [Google Scholar]
- 31.Arria AM, Caldeira KM, Kasperski SJ, Vincent KB, Griffiths RR, O’Grady KE Energy drink consumption and increased risk for alcohol dependence. Alcohol Clin Exp Res 2011; 35: 365–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson BL, Juliano LM Behavior, sleep and problematic caffeine consumption in a college-aged sample. J Caffeine Res 2012; 2: 38–44. [Google Scholar]
- 33.Miyake ER, Marmorstein NR Energy drink consumption and later alcohol use among early adolescents. Addict Behav 2015; 43: 60–5. [DOI] [PubMed] [Google Scholar]
- 34.Kristjansson AL, Mann MJ, Sigfusdottir ID, James JE Mode of daily caffeine consumption among adolescents and the practice of mixing alcohol with energy drinks: relationships to drunkenness. J Stud Alcohol Drugs 2015; 76: 397–405. [DOI] [PubMed] [Google Scholar]
- 35.Kristjansson AL, Sigfusdottir ID, Allegrante JP, James JE Adolescent caffeine consumption, daytime sleepiness and anger. J Caffeine Res 2011; 1: 75–82. [Google Scholar]
- 36.Kristjansson AL, Sigfusson J, Sigfusdottir ID, Allegrante JP Data collection procedures for school-based surveys among adolescents: the Youth in Europe Study. J School Health 2013; 83: 662–7. [DOI] [PubMed] [Google Scholar]
- 37.Muthén LK, Muthén BO Mplus User’s Guide, 7th edn Los Angeles, CA: Muthén & Muthén; 1998–2015. [Google Scholar]
- 38.Leventhal AM, Strong DR, Kirkpatrick MG, Unger JB, Sussman S, Riggs NR et al. Association of electronic cigarette use with initiation of combustible tobacco product smoking in early adolescence. JAMA 2015; 314: 700–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Institute of Medicine. Nutrition Standards for Foods in Schools: Leading the Way to Healthier Youth. Washington, DC: National Academies Press; 2007. [Google Scholar]
- 40.Terry-McElrath YM, O’Malley P M, Johnston LD Energy drinks, soft drinks, and substance use among US secondary school students. J Addict Med 2014; 8: 6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tucker JS, Troxel WM, Ewing BA, D’Amico EJ Alcohol mixed with energy drinks: associations with risky drinking and functioning in high school. Drug Alcohol Depend 2016; 167: 36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frau L, Simola N, Morelli M Contribution of caffeine to the psychostimulant, neuroinflammatory and neurotoxic effects of amphetamine-related drugs. J Caffeine Res; 3: 79–84. [Google Scholar]
- 43.Cauli O, Morelli M Caffeine and the dopaminergic system. Behav Pharmacol 2005; 16: 63–77. [DOI] [PubMed] [Google Scholar]
- 44.Freedman ND, Park Y, Abnet CC, Hollenbeck AR, Sinha R Association of coffee drinking with total and cause-specific mortality N Engl J Med 2012; 366: 1891–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones HA, Lejuez CW Personality correlates of caffeine dependence: the role of sensation seeking, impulsivity, and risk taking. Exp Clin Psychopharmacol 2005; 13: 259–66. [DOI] [PubMed] [Google Scholar]
- 46.Evans AH, Lawrence AD, Potts J, MacGregor L, Katzenschlager R, Shaw K et al. Relationship between impulsive sensation seeking traits, smoking, alcohol and caffeine intake, and Parkinson’s disease. J Neurol Neurosurg Psychiatry 2006; 77: 317–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marmorstein NR Energy drink and coffee consumption and psychopathology symptoms among early adolescents: cross-sectional and longitudinal associations. J Caffeine Res 2016; 6: 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Y, Lerner RM Trajectories of school engagement during adolescence: implications for grades, depression, delinquency, and substance use. Dev Psychol 2011; 47: 233–47. [DOI] [PubMed] [Google Scholar]
- 49.Rudolph KD, Lambert SF, Clark AG, Kurlakowsky KD Negotiating the transition to middle school: the role of self-regulatory processes. Child Dev 2001; 72: 929–46. [DOI] [PubMed] [Google Scholar]
- 50.Brener ND, Collins JL, Kann L, Warren CW, Williams BI Reliability of the youth risk behavior survey questionnaire. Am J Epidemiol 1995; 141: 575–80. [DOI] [PubMed] [Google Scholar]
- 51.Institute of Medicine. Caffeine in Food and Dietary Supplements: Examining Safety. Washington, DC: National Academies Press; 2014. [Google Scholar]