Abstract
Recent findings point to a role of Checkpoint Inhibitor (CPI) receptors at the tissue level in immune homeostasis. Here we investigated the role of CPI molecules on immune cells in relation to cardiac function. Participants recruited in Chennai, India consisted of HIV+ ART naive viremic (Gp1 n = 102), HIV+ on ART, virologically suppressed (Gp2, n = 172) and HIV negative healthy controls (Gp3, n = 64). A cross-sectional analysis of cardiac function, arterial resistance and immunologic assessment of CPI expressing T cells was performed. Data indicate that ART naive exhibited cardiac function impairment and greater arterial stiffness than the other groups. Frequencies of CD4+ T cells expressing LAG-3 and PD1 were higher in ART naïve while TIGIT and TIM3 were similar among the patient groups. LAG-3+, PD1+ and dual LAG-3+PD1+ CD4 T cells were inversely correlated with cardiac function and arterial elasticity and directly with arterial stiffness in ART naïve participants and with arterial elasticity in virally suppressed group on ART. We conclude that HIV induced upregulation of LAG-3 singly or in combination with PD1 in immune cells may regulate cardiac health and warrant mechanistic investigations. The implications of these findings have bearing for the potential utility of anti-LAG-3 immunotherapy for cardiac dysfunction in chronic HIV infection.
Introduction
Cardiovascular disease (CVD) is a major contributor to mortality and morbidity in HIV infection, and is largely attributed to underlying inflammation and immune activation (IA) which is known to persist, albeit at a lower level following antiretroviral therapy (ART) [1, 2]. Early in the era of ART, the drugs themselves were found to be cardiotoxic, but this issue is now considered of less relevance with newer drugs that have minimal or no cardiac toxicity [3]. Persistent T cell activation in chronic HIV infection leads to a chronic inflammatory environment that has multiple deleterious effects at the tissue level, directly or indirectly inflicting damage to different organ systems, the mechanisms of which are not well understood. Immune activation at the cellular level, that involves CD4 and CD8 T cells results in T cell proliferation and dysfunction [4, 5]. Intrinsic mechanisms that maintain T cell numbers at a constant level do so by balancing immune activation and homeostatic proliferation. These mechanisms include regulation of cell death molecules such as Fas/FasL [6, 7] and immune checkpoint inhibitor (CPI) molecules such as Programmed cell death protein 1 (PD1), Lymphocyte-activation gene 3 (LAG-3), T cell immunoglobulin and mucin domain 3 (TIM3), T cell immunoreceptor with Ig and ITIM domains (TIGIT) and cytotoxic T-lymphocyte-associated protein4 (CTLA-4) [8–10]. In lymphocytes, the CPI have critical roles in the maintenance of immune homeostasis by ensuring contraction of effector T cell responses [11, 12] and protects the host from exuberant anti-microbial responses. The expression of LAG-3, TIGIT and CTLA-4 on T regulatory cells (Tregs) enable the Tregs to suppress effector T cell function [13–17]. In acute HIV infection as well, CPI may serve to protect the host from end organ damage, and may be cardio-protective. In contrast to acute infection, in chronic untreated HIV infection and malignant states however, chronic antigen stimulation can lead to sustained immune activation and inflammation resulting in elevated expression of CPI molecules on effector T cells with dampened immunity manifesting as functional unresponsiveness of the immune system [18, 19] and reduced effector function of CD4 and CD8 T cells [8, 20, 21]. Together these effects may lead to end organ damage that potentially could be rescued by effective ART as shown in the present study.
While all CPI are considered in general terms as having an immunoregulatory role, they may have unique properties and distinct mechanisms of action [8, 22–24]. For example co-inhibitory receptors CTLA-4 and PD1 are primarily responsible for maintaining self tolerance by restricting T cell clonal proliferation in lymphoid organs while LAG-3, TIM3, and TIGIT have been assigned specific roles related to regulation of tissue inflammation [8, 16, 25–31]. Specificity for the regulatory activity of the CPI resides at the tissue level, based on the ligands expressed on tissues that maintain tissue tolerance and inhibit immunopathology. In fact, there is evidence for the expression of the MHC class II, the receptor for LAG-3 in cardiac endothelial cells and endocardial cells in certain disease states in humans and rodents [32–35] as well as in cultured human fetal cardiac myocytes in experimental systems [36].
The present study investigated the expression of CPI molecules on CD4 T cells in HIV+ ART naïve viremic and in virologically suppressed group on ART for their relationship to measures of cardiac function and arterial stiffness. Additionally, healthy individuals were investigated and served as controls.
Materials and methods
Study setting and subjects
This study was conducted at YRG CARE, a tertiary care center located at Chennai in South India, and enrolled 274 male and female subjects with chronic HIV infection (HIV+) and 63 HIV-uninfected healthy controls (HC) in the age range >18 yr-50 yr (Table 1). Among the HIV+ participants, 102 were ART naïve (group 1) and 172 were on ART for >12 months (group 2) with viral suppression as determined by two consecutive plasma viral load values of <40 copies/mL. The 63 HC were categorized as group 3. Patients with pre-exposure or post-exposure ART prophylaxis and pregnant women were excluded. Distribution of participants was matched for age, race, gender, basal metabolic rate, smoking, and diet history. At enrollment, among ART naïve and ART treated virally suppressed HIV+ patients respectively 8% (8/101) and 13% (22/172) were current smokers, 67.5% (68/101) and 57% (98/172) had never smoked; 24.6% (25/101) and 30% (52/172) had smoked in the past. Only 2 in each group were HBV and HCV+. The study was approved by both YRG CARE and University of Miami institutional review boards. Informed consent was obtained from all enrolled participants. A detailed interview was conducted at the time of enrolment to collect demographic information. All participants had a one time blood draw for collection of plasma and peripheral blood mononuclear cells (PBMC). Blood samples were processed within an hour of collection according to guidelines of the AIDS Clinical Trials Group. Plasma was stored in aliquots at -80°C and PBMC were cryopreserved in liquid nitrogen in aliquots of 5 million cells/mL.
Table 1. Characteristics of the study cohort.
| Gp 1, ART Naive |
Gp 2, Virologically Suppressed on ART |
Gp 3 Healthy Controls |
P- value | ||
|---|---|---|---|---|---|
| Number | 102 | 172 | 63 | - | |
| Gender, M/F | 47/55 | 116/56 | 22/41 | ||
| Age (in years) | 36.5 ±5.8 | 38.6 ±5.9 | 36.8 ±7.09 | ||
| Median duration of infection; (in mo.) (range) |
15 (2–42) |
72 (39.5–92) |
NA | <0.001* (Gp1 vs Gp 2) |
|
| Mean Nadir CD4+ T-cell count, cells/ μL; (range) | NA | 312.3 ± 199 (5–1425) |
NA | __ | |
| Mean CD4+ T-cell count at study entry; cells/ μL) ± SD (range) | 421.4 ± 321.6 (21–1897) |
743.4 ± 321.6 (154–1863) |
NA | <0.001* | |
| Median Duration of ART in months; (range) | __ | 43 (22–72) |
NA | __ | |
| Body Mass Index (Kg/m2); Median (range) | 22.7 (20.1–26.3) |
22 (19–25.8) |
24.6 (22.5–27.1) |
0.013* (Gp1vs3) 0.006*(Gp 2vs3) |
|
All descriptive variables are provided as median and interquartile ranges except for age, Nadir CD4 and CD4 counts at entry which are provided as mean ± SD. T-test was used to calculate p-value between the groups for ‘age’ which was normally distributed, while Mann-Whitney U-test was used for other non-normally distributed variables.
* indicates statistical significance.
Measures of cardiac function and arterial stiffness
Pulse rate, stroke volume, stroke volume index, cardiac output, cardiac index and cardiac ejection time were determined to ascertain cardiac functioning. Arterial stiffness was estimated by pulse-wave velocity (PWV) using the HDI/PulseWave CR-2000 (Hypertension Diagnostics, Inc., Eagan, MN), a diagnostic tool that was previously applied in the INSIGHT Strategic Timing of Anti Retroviral Treatment arterial stiffness sub-study [37]. Along with Large Artery Elasticity index (LAE) and Small Artery Elasticity index (SAE) measures, systemic vascular resistance (SVR) and total vascular impedance (TVI) were measured as arterial stiffness parameters.
Flow cytometry for analysis of checkpoint inhibitor molecules and immune activation
Thawed PBMC were rested overnight and 1x106 cells were stained with Live/Dead Aqua followed by staining for surface markers CD3, CD4, CD8, checkpoint inhibitors PD1, TIGIT, TIM3, and LAG-3 and immune activation markers HLA-DR and CD38. Cells were then fixed, and acquired on a Flow cytometer (BD LSRFortessa, San Jose, CA) and analyzed by FlowJo V10 (Treestar, Ashland, OR). Frequencies of CPI molecules either alone or in combinations were analyzed on live (Aqua-) CD3+CD4+ and CD3+CD8+ T cells. Immune activation was measured based on the dual expression of HLA-DR and CD38 on CD4 and CD8 T cells.
Soluble LAG-3 in plasma by ELISA
Plasma levels of soluble LAG-3 (sLAG-3) were measured by ELISA (Abcam, Cambridge, MA) as per the manufacturer’s recommendations using a sample dilution of 1:5. Data are expressed as pg/ml.
Statistical analysis
Descriptive statistics such as percentages, means and standard deviation, and median were used to describe the demographic characteristics of the study population. For unpaired data, Levene’s test was used firstly to check variance heterogeneity followed by Wilcoxon rank-sum test (also called ‘Mann-Whitney’ U test) was performed using R stats package. For Correlation analyses, Shapiro test was used to check if data are normally distributed using R stats package. If Shapiro test showed p>0.05, Pearson correlation coefficient was performed using R stats package; in other instances Spearman's rank correlation coefficient was performed using R stats package. A p value of <0.05 was considered as significant. The data are presented as scatter plots with regression lines and correlation coefficients with P values.
Results
Demographic characteristics of study population
Demographic characteristics of the study population are shown in Table 1. The mean ages were similar among the study groups. Median duration of infection was lower in the ART naïve compared to ART treated virologically suppressed group. Of the patients on ART, 140 patients were on first-line reverse transcriptase inhibitors (RTI; AZT/D4T/TDF+3TC/FTC+EFV/NVP) while 32 were on Protease Inhibitors (RTV-boosted LPV) based second-line therapy at the time of study enrolment. Absolute CD4 counts were significantly lower in the ART naive compared to virologically suppressed while BMI was significantly lower in both HIV+ groups compared to healthy controls.
Checkpoint inhibitor molecule expressing T cells are increased in HIV+ individuals
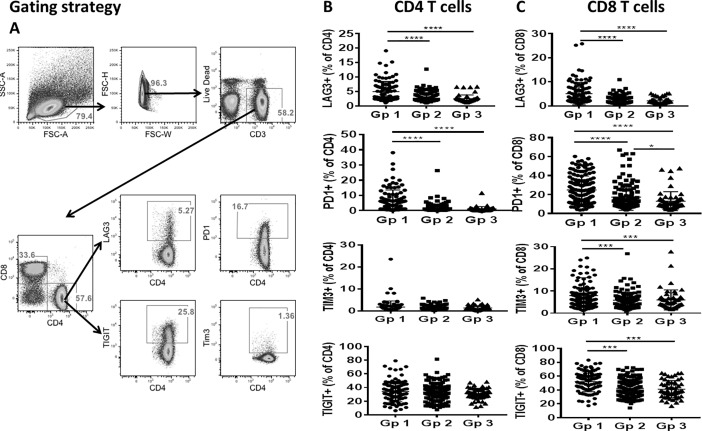
We analyzed the expression of CPI molecules LAG-3, PD1, TIGIT and TIM3 on CD4 and CD8 T cells in the study groups. Flow cytometry gating strategy for analysis of the CPI on CD4 T cells is depicted in Fig 1A. Among CPI, as shown in Fig 1B, frequencies of LAG-3 and PD-1 on CD4 T cells and MFI (not shown) were significantly higher in ART naive compared to virologically suppressed on ART and healthy controls, but the frequencies and MFI (not shown) of TIGIT and TIM3 were not different between the study groups.
Fig 1. Higher frequencies of LAG-3, PD1, TIGIT and TIM3 on CD4 and CD8 T cells in ART naïve group.
Checkpoint inhibitor (CPI) molecules and T cell immune activation markers were analyzed on CD4 and CD8 T cells by flow cytometry in ART naïve (Gp1) and virologically suppressed (Gp2) patients and healthy controls (Gp3). A), Representative flow cytometry dot plots showing gating strategy for the analysis of CPI on CD4 T cells. Frequencies of LAG-3+, PD1+, TIGIT+, and TIM3+ cells are shown in B), for CD4 T cells and C), for CD8 T cells. Line and error bars within the plot indicate the Mean ± SD. Data compared between groups using Wilcoxon rank-sum test. A p value <0.05 was considered significant. *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001.
In the CD8 T cell compartment, both frequencies and MFI (not shown) of LAG-3, PD1, TIM3 and TIGIT were significantly higher in the ART naive compared to virologically suppressed group and healthy control group (Fig 1C). Moreover, ART treated virologically suppressed group also showed higher frequencies of PD1+CD8 T cells compared to healthy control group (Fig 1C).
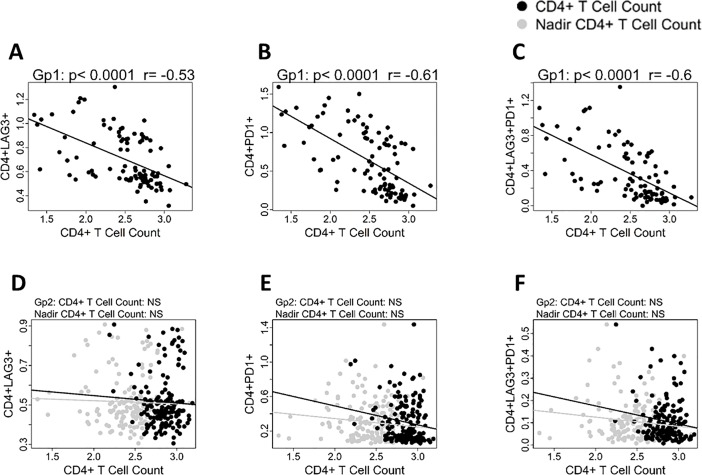
Absolute numbers of CD4 T cells at study entry inversely correlated with LAG-3, PD1 and with dual LAG-3+PD1+ CD4 T cells in ART naïve group (Fig 2A and 2B and C, respectively). In the virologically suppressed group, no correlation was observed of absolute CD4 T cell counts or of nadir CD4 T cell counts with LAG-3 or PD1 or dual LAG-3+PD1+ CD4 T cells (Fig 2D, 2E and 2F, respectively).
Fig 2. Absolute CD4 numbers correlate with CPI molecule expression on CD4 T cells in ART naïve individuals.
A-C: ART naïve Gp1 patients. Linear regression analysis shows correlation between CD4 T cell counts at study entry with A), LAG-3+CD4; B), PD1+CD4 and C), LAG-3+PD1+ CD4 T cells. D-F: Virologically suppressed Gp2 patients. Linear regression analysis shows correlation between CD4 T cells count (black circles) and nadir CD4 T cells (grey circles) with D), LAG-3+CD4; E), PD1+CD4 and F), LAG-3+ PD1+ CD4 T cells. Pearson correlation was performed based on data distribution; a p value of <0.05 was considered as significant.
We also measured soluble form of LAG-3 in plasma from a subset of participants from each of the three groups, (S1 Fig). Levels of sLAG-3 were significantly higher in the ART naïve group as compared to virologically suppressed- and healthy control groups, which were not different from each other.
CD4 and CD8 T cell immune activation in ART naïve group correlates with LAG-3 and PD1 expression on CD4 and CD8 T cells
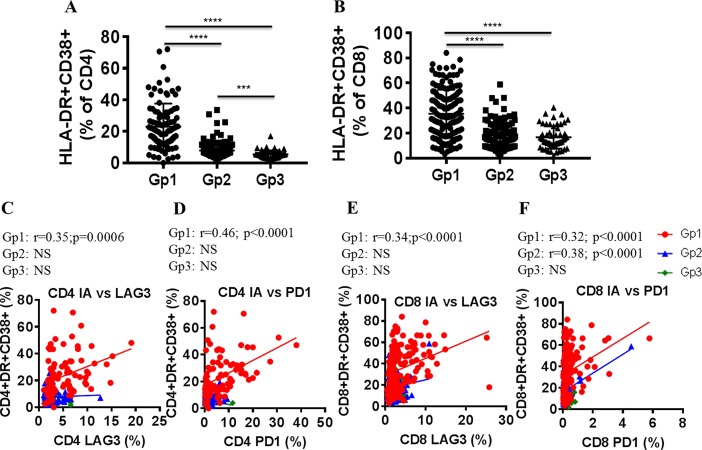
Analysis of CD4 and CD8 T cell immune activation showed higher frequencies of HLA-DR+CD38+ CD4 and CD8 T cells in the ART naïve group compared to ART treated virologically suppressed and healthy control groups (Fig 3A and 3B). In addition, the virologically suppressed group showed higher frequencies of HLA-DR+CD38+ CD4 T cells compared to the healthy control group (Fig 3A). Both CD4 and CD8 T cell immune activation correlated with LAG-3 and PD1 expression on CD4 and CD8 T cells in ART naïve group (Fig 3C–3F). CD8 T cell immune activation in virologically suppressed group on ART also showed direct correlation with PD1+CD8 T cells (Fig 3F).
Fig 3. LAG-3 and PD1 expression on CD4 and CD8 T cells correlate with T cell immune activation in ART naïve groups.
Immune activation on CD4 and CD8 T cells was measured based on the co-expression of HLA-DR and CD38 by flow cytometry. Frequencies of HLA-DR+CD38+ expressing A), CD4 and B), CD8 T cells are shown. Linear regression analysis shows correlation between CD4 T cell immune activation with C), LAG-3+ and D), PD1+ CD4 T cells and of CD8 T cell immune activation with E), LAG-3+ and F), PD1+ CD8 T cells in ART naïve (Gp1, red circles), virologically suppressed (Gp2, blue circles) and healthy control (Gp3, green circles) groups. Pearson correlation was performed based on data distribution; a p value of <0.05 was considered as significant.
Cardiac function and arterial stiffness are impaired in ART naïve group
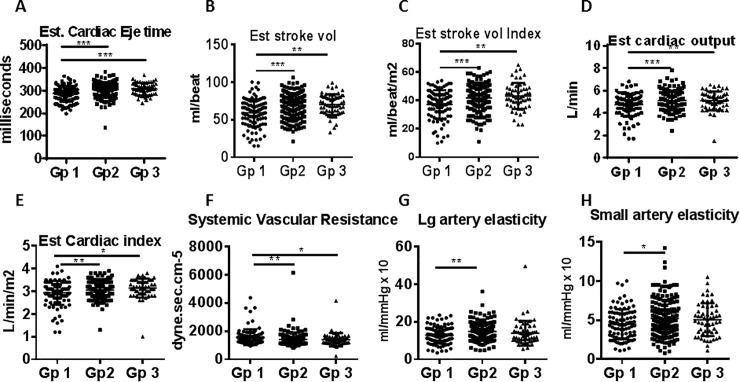
Measures of cardiac function and arterial stiffness in the study groups are shown in Fig 4. The ART naïve had lower cardiac ejection time (Fig 4A), lower stroke volume (Fig 4B), lower stroke volume index (Fig 4C), lower cardiac output (Fig 4D), and lower cardiac index (Fig 4E) compared to virologically suppressed on ART and healthy controls. Measures of arterial stiffness showed higher systemic vascular resistance (Fig 4F) with lower large and small artery elasticity (Fig 4G and 4H respectively) in ART naive compared to virologically suppressed group. Cardiac function and arterial stiffness parameters were not different between virologically suppressed group and healthy controls. These data support an HIV induced effect on multiple cardiac functions and on arterial stiffness in ART naïve HIV+ group. In the virologically suppressed group, these parameters did not differ significantly from healthy controls implying ART- mediated improvement.
Fig 4. Cardiac functions are impaired in HIV+ ART naive group.
Cardiac function and arterial stiffness related measures were compared between ART naïve (Gp1) and virologically suppressed on-ART (Gp2) patients and healthy controls (Gp3). Measures of cardiac functions include A), estimated cardiac ejection time; B), estimated stroke volume; C), estimated stroke volume index; D), estimated cardiac output and E), estimated cardiac index. F-H: Measures of arterial stiffness include F), systemic vascular resistance, G), large artery elasticity index and H), small artery elasticity index. Data between groups are compared using Wilcoxon rank-sum test; a p value of <0.05 was considered significant. *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001.
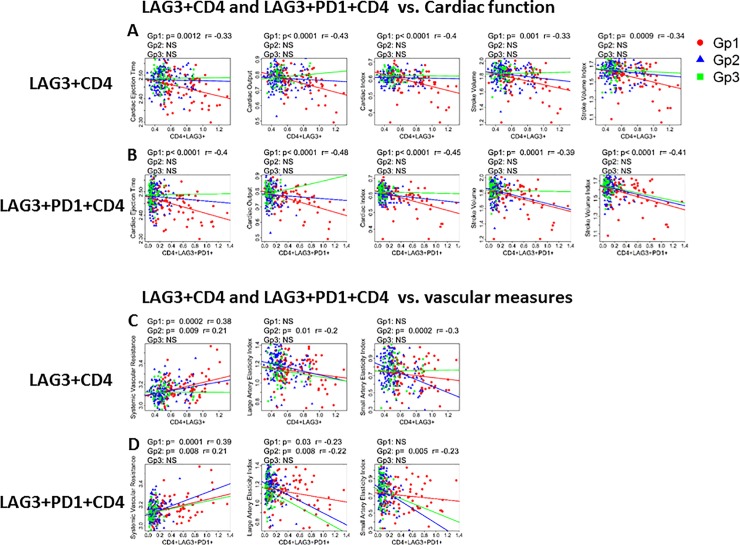
Correlation of cardiac function and arterial stiffness with LAG-3+ CD4 T cells
We investigated the relationship of CPI molecules on measures of cardiac function. Linear regression plots for association of cardiac function measures (Fig 5A and 5B) showed that in ART naïve group, single LAG-3 or LAG-3 plus PD1 expressing CD4 T cell subsets respectively were inversely correlated with measures of cardiac ejection time, cardiac output, cardiac index, stroke volume, and stroke volume index. In the virologically suppressed and healthy control groups, no association of LAG-3 or LAG-3 plus PD1 expressing CD4 T cells with measures of cardiac function was evident (Table 2). In addition to CD4 T cells, LAG-3, PD1, and LAG-3 plus PD1 expressing CD8 T cells, also showed a weak inverse correlation with markers of cardiac function in ART naïve group (Table 3).
Fig 5. Markers of cardiac function and arterial stiffness correlate with CPI expression on CD4 T cells.
Linear regression analysis showing correlation of cardiac function with A), LAG-3+ CD4 T cells; B), LAG-3+PD1+ CD4 T cells. Correlation of arterial stiffness with C), LAG-3+CD4 and D), LAG-3+PD1+ CD4 T cells shown for ART naïve (Gp1, red), Virologically suppressed (Gp2, blue) and healthy controls, (Gp3, green). Pearson correlation was performed based on data distribution; a p value of <0.05 was considered as significant.
Table 2. Correlations between LAG-3, PD1 and LAG-3+PD1+ expressing CD4 T cells and cardiac function.
| Cardiac function | CD4+LAG 3+ (%) | CD4+PD1+ (%) | CD4+LAG 3+ PD1+ (%) | |||
|---|---|---|---|---|---|---|
| Gp1 (ART Naïve) | p value | r value | p value | r value | p value | r value |
| Cardiac ejection time | 0.001 | -0.33 | 0.001 | -0.33 | <0.0001 | -0.4 |
| Cardiac output | <0.0001 | -0.38 | 0.001 | -0.33 | <0.0001 | -0.42 |
| Cardiac index | <0.0001 | -0.4 | <0.0001 | -0.39 | <0.0001 | -0.45 |
| Stroke volume | 0.001 | -0.33 | 0.005 | -0.29 | <0.0001 | -0.39 |
| Stroke volume index | <0.0001 | -0.34 | 0.001 | -0.33 | <0.0001 | -0.41 |
| Systemic vascular-resistance | 0.0002 | 0.38 | 0.0005 | 0.36 | 0.0001 | 0.39 |
| Large artery elasticity | NS | - | NS | - | 0.03 | -0.23 |
| Gp2 (Virologically suppressed) | ||||||
| systemic vascular-resistance | 0.009 | 0.21 | 0.03 | 0.17 | 0.008 | -0.21 |
| Large artery elasticity | 0.009 | -0.21 | 0.01 | -0.21 | <0.0001 | -0.19 |
| Small artery elasticity | 0.001 | -0.26 | NS | - | 0.03 | -0.17 |
Cardiac function and vascular measures were correlated with LA-G3+, PD1+ and LAG-3 plus PD1+ CD4 T cells using Pearson correlation. Data are shown for correlation coefficient (r) and significance (p). A p value of <0.05 was considered significant.
Table 3. Correlations between LAG-3, PD1 and LAG-3+PD1+ expressing CD8 T cells and cardiac function.
| Cardiac function | CD8+LAG 3+ (%) | CD8+PD1+ (%) | CD8+LAG 3+PD1+ (%) | |||
|---|---|---|---|---|---|---|
| Gp1 (ART Naïve) | p value | r value | p value | r value | p value | r value |
| Cardiac ejection time | 0.023 | -0.22 | 0.005 | -0.28 | 0.0003 | -0.36 |
| Cardiac output | 0.002 | -0.30 | 0.017 | -0.24 | 0.002 | -0.31 |
| Cardiac index | 0.004 | -0.28 | 0.013 | -0.24 | 0.002 | -0.31 |
| Stroke volume | 0.013 | -0.24 | 0.007 | -0.27 | 0.0006 | -0.34 |
| Stroke volume index | 0.013 | -0.28 | 0.004 | -0.28 | 0.0005 | -0.35 |
| Systemic vascular-resistance | 0.017 | 0.24 | NS | - | NS | - |
| Large artery elasticity | NS | - | NS | - | 0.004 | -0.3 |
| Gp2 (Virologically suppressed) | ||||||
| systemic vascular-resistance | 0.009 | 0.21 | NS | - | NS | - |
| Large artery elasticity | 0.021 | -0.17 | 0.036 | -0.16 | NS | - |
| Small artery elasticity | 0.001 | -0.25 | NS | - | NS | - |
Cardiac function and vascular measures were correlated with LAG-3+, PD1+ and LAG-3+plus PD1+ CD8 T cells using Pearson correlation. Data are shown for correlation coefficient (r) and significance (p). A p value of <0.05 was considered significant.
Vascular measures of systemic vascular resistance directly correlated with LAG-3 (Fig 5C) or LAG-3+PD1+ CD4 T cells in ART naive and virologically suppressed groups (Fig 5D). Large artery elasticity index inversely correlated with LAG-3+PD1+ CD4 T cells in Gp1 but with LAG-3 alone, or in combination with PD1 in Gp2. Inverse correlations of small artery elasticity index and single LAG-3 or LAG-3 co-expressed with PD1 on CD4 T cells were only found in virologically suppressed group. Single PD1 expressing CD4 T cells followed a similar pattern but were not consistent. A summary of correlations are depicted in a heatmap shown in S2 Fig. No associations were found between arterial stiffness measures and CPI in healthy controls (Fig 5C and 5D). Levels of sLAG-3 did not show a correlation with cardiac markers (data not shown).
Discussion and conclusions
Cardiovascular disease is a major contributor to mortality and morbidity in HIV infection [3, 38–40]. This cross sectional study was aimed at defining relationship of checkpoint inhibitor molecules on T cells and CVD in chronic HIV infection. We conducted the study in ART naïve viremic patients as well as in patients on ART with viral suppression and healthy age-matched HIV uninfected controls. In the ART naïve group, cardiac function was decreased with evidence of increased vascular resistance. We observed that LAG-3, PD1 or LAG-3 plus PD1 expressing CD4 T cells were inversely correlated with cardiac function, while being directly correlated with vascular resistance. Virologically suppressed patients, despite having started ART at CD4 nadirs similar to ART naïve, had little evidence of impaired cardiac function but had residual increased vascular resistance involving large and small vessel elasticity, which was also inversely correlated with LAG-3 expressing CD4 T cells and maximally with cells co-expressing LAG-3 and PD1. These observations, together with the high LAG-3 expression in monocytes from healthy controls (unpublished observations), imply a dominant role of cells expressing LAG-3 alone or in combination with PD1, but without association of TIGIT or TIM3 in regulating cardiac health, particularly in viremic patients with chronic HIV infection who have not started ART. The benefits of ART on CVD and on expression of CPI on CD4 T cells were clearly manifest in this study based on comparisons of ART naïve- with ART treated and healthy control groups.
Recent evidence points to expression of receptors for CPI molecules/receptors at the tissue level for controlling organ immune homeostasis. The receptor for LAG-3 is the major histocompatibility complex Class II molecule. Caforio et at al investigated human cardiac tissue for expression of MHC Class II molecules [32]. These authors found that endothelial and endocardial cells expressed MHC Class II molecules in a majority of patients with dilated cardiomyopathy (DCM), and less frequently in other acquired cardiac diseases, congenital heart disease, or in normal hearts. The expression of MHC II molecule on cardiac endothelial and endocardial cells suggests a possible pathogenic role of these molecules in the initiation and/or perpetuation of DCM [32]. Activated endothelial cells may be involved in the homing and trafficking of lymphocytes [41, 42] in the affected tissue in the early stages of the disease. It is possible that a similar phenomenon occurs in HIV infection with MHC class II expression on cardiac endothelial and endocardial cells and trafficking of CD4+LAG3+ T cells to these sites. Only studies at the tissue level in ART naive and ART treated subjects can elucidate the pathology leading to cardiac dysfunction in HIV subjects.
LAG-3 is a 498-amino acid transmembrane protein identified on activated human NK and T cells [43]. The gene for LAG-3 in humans is located adjacent to CD4 on chromosome 12 and is structurally homologous to CD4 with four extracellular immunoglobulin superfamily like domains D1, D2, D3, and D4 [44–46]. In T cells, LAG-3 mRNA levels increase 10 fold upon cell activation [47] which is highly relevant as LAG-3 function is mediated by modulation of LAG-3 expression at the transcriptional level [46]. Intracellular storage in lysosomal compartments, may also serve to facilitate rapid LAG-3 cell surface expression following T cell activation [11, 12, 48, 49].
Upregulation of LAG-3 on T cells also defines a subpopulation with functional exhaustion that correlates with disease progression in HIV infected individuals (50). LAG-3 expression in T cells was significantly upregulated in HIV infected individuals and was correlated with disease progression. In 28 HIV infected individuals they analysed the relationship of LAG-3 to immune activation markers CD38 and HLA-DR and noted that LAG-3 was largely co-expressed with CD38 on 70% of CD4 T cells and 74% of CD8 T cells, while the percentage of cells co-expressing LAG-3 and HLA-DR was less. In that study, MFI of LAG-3 expression declined in both CD4 and CD8 T cells after more than one year of ART treatment [50]. LAG-3 is a natural high-affinity ligand for MHC class II molecules [8, 44, 45] and has an inhibitory role in regulating T cell immune responses [8, 46] and also acts synergistically with PD1 to regulate T cell function [46, 51]. Our findings are in agreement with these observations as we found that immune activation and LAG-3 and PD1 expressing CD4 T cells were greater in ART naïve viremic subjects than in those virally suppressed on ART where the lower LAG-3 expression is most likely a consequence of ART induced viral suppression and reduction in immune activation. Virologically suppressed participants were similar to healthy controls in both, the measures of CVD and frequency of CPI expressing CD4 T cells, supporting the beneficial effect of ART and virologic suppression on cardiac function. Although CPI molecules are associated with regulating immune activation, the direct association between T cell immune activation and CPI in the presence of viremia merits further investigation.
Risk factors for CVD are under intense investigation and involve both traditional and non-traditional factors. A study by Golden et al [52] found that the lipoprotein scavenger receptor class B type 1 (SCARBI) rs10846744 noncoding variant is significantly associated with atherosclerotic disease independently of traditional cardiovascular risk factors. They identified a connection between rs10846744 with sLAG-3 in plasma and concluded that plasma sLAG-3 is an independent predictor of HDL-cholesterol levels and CVD risk. We did not find a correlation of sLAG-3 with cardiac measures in our study participants, but cannot rule out the possibility that it is a player in mediating tissue damage to the heart. In murine studies, sLAG-3 has not been found to have any biologic function [47, 53]. Soluble LAG-3 is released by cell surface shedding and is cleaved by the metalloproteinases ADAM10 and ADAM17, which cleave a wide range of transmembrane proteins including CD62L, TIM3 and TNFα [54]. The action of ADAM17 becomes evident following cell activation [11, 12, 48, 49], and sLAG-3 has been reported to be elevated in humans in situations of T cell activation, as in infection or autoimmunity [55–57]. In our study sLAG-3 in plasma followed a similar pattern as surface LAG-3 and the levels of sLAG-3 were higher in ART naïve group compared to ART treated or healthy controls. The biologic relevance of sLAG-3 in the context of cardiac disease in HIV is unclear.
It is important to understand the physiological role of individual CPI molecules in terms of their independent effects and dependence upon each other. Although they belong to the same class of receptors, different CPI often act in a tier fashion, with CTLA-4 and PD1 in the first tier for maintaining self-tolerance and LAG-3, TIM3 and TIGIT in a second tier with distinct roles in regulating immune responses particularly at sites of tissue inflammation [8, 16, 25–31]. In a murine transplant tumor model, it was observed that PD1 and LAG-3 were coexpressed on tumor-infiltrating CD4 and CD8 T cells at the tissue level and also in cancer patients [49, 58–60]. In our study PD1 and LAG-3 coexpression on CD4 T cells was highly correlated with cardiac disease measures and significance was often greater than that of independent molecules, implicating their involvement in regulation of organ systems at tissue levels.
The inference about the potential involvement of PD1 on CD4 T cells in influencing cardiac function was based on its higher expression in ART naive patients compared to other groups and data of the correlation analyses with cardiac measures. PD1 is an inhibitory receptor expressed by activated T, B and myeloid cells that was discovered by the Nobel laureate Tasuku Honjo in 1992 [61], who later with Freeman et al discovered its ligand PD-L1 as a member of the B7 gene family [62]. Engagement of PD1 by PD-L1 leads to the inhibition of T cell receptor-mediated lymphocyte proliferation and cytokine secretion [62]. Nishimura et al demonstrated the occurrence of autoimmune dilated cardiomyopathy and cardiac dysfunction in PD1 receptor deficient mice with diffuse deposition of IgG on the surface of cardiomyocytes and high titer circulating Ig-G autoantibodies [63]. Cardiomyocytes were found to be a major source of inflammatory cytokine generation in experimental systems using isolated ischemic-reperfused hearts [64–66]. Importantly, PD1 and PD-L1 were found to be expressed on cardiomyocytes and on different populations of cardiac cells. Upregulation of both PD1 and PD-L1 has been demonstrated in an acute cardiac injury model of ischemic-reperfused and cryoinjured hearts [67]. In this study PD1/PD-L1 pathway has been postulated to play an important role in cardiac injury by increasing GADD153, a regulator of the inflammatory response, leading to pro-inflammatory changes in the ischemic refused hearts with a marked increase of IL-17+ cells and only a mild increase in IL-10+ cells. Furthermore, PD-L1 blocking antibody treatment reduced cardiac GADD153 expression with reduction in expression of inflammatory cytokines [67]. It is possible that LAG-3 and PD1 contribute towards immune dysfunction at the local tissue level within the heart muscle microenvironment as ligands for both can be expressed on cardiac myocardium endothelial cells and LAG-3 plus PD1 coexpressing immune cells may inflict maximal cardiac damage.
The impact of CPI on immune cells in tissues is most likely influenced by state of virological control and severity of HIV disease status. Our data indicate that ART induced virus suppression in chronic HIV infection may lead to better cardiac function and reduced arterial stiffness together with decrease in immune activation as demonstrated by fewer CD38+HLA-DR+ CD4 T cells compared to the untreated group. In situations of chronic treated HIV, the presence of CD4 T cells expressing CPI molecules such as LAG-3 and PD1 may act to curb immune activation. In the ART naive viremic group on the other hand the CPI are not cardioprotective and immune activation is associated with increase in soluble markers of inflammation in plasma. Increased TNFRI, TNFRII, IL-6, IL-8, TNFα, LPS, and sCD14 were associated with lower cardiac function (data not shown).
Our study is limited by the fact that it is cross-sectional and we cannot provide direct evidence for increased LAG-3 or LAG-3 plus PD1 expressing CD4 T cells in the heart tissue leading to low cardiac function. In addition, we cannot formally show whether the increase in cells with these CPI molecules precedes the development of impaired cardiac function. Although we tried to adjust for all known risk factors, some other confounders may have existed among the study groups. Longitudinal studies are necessary to further explore and confirm the relationship of CPI LAG-3 and PD1 on cardiac function in HIV infected individuals on ART.
In conclusion, we found a novel association of LAG-3 expressing CD4 T cells either alone or in combination with PD1 with cardiac dysfunction in ART naïve viremic HIV+ patients, and with cardiac artery stiffness in both treatment naive and virally suppressed patients on ART. Key questions to investigate are 1) mechanism of action of LAG-3 for affecting the cardiac function and arterial structure, and 2) the basis that underlies synergies of LAG-3 with PD1 in regulating cardiac function. This understanding could provide insight into potential role of LAG-3 immunotherapy (which is in early clinical trials in cancer) in prevention or treatment of cardiac dysfunction in HIV.
Supporting information
sLAG3 in plasma were measured by ELISA in ART naive (Gp 1, n = 42), Virologically suppressed on ART Gp 2, (n = 21) and healthy controls (Gp 3, n = 21). Data compared between groups using Wilcoxon rank-sum test. A p value <0.05 was considered significant. ***p<0.001; ****p<0.0001.
(TIF)
Results shown for ART naïve (group 1,G1), and virologically suppressed on ART (group 2,G2) patients. Colored boxes represent significant (P < 0.05) correlation between analytes. Scale indicates the correlation coefficient with red color indicating the inverse correlations. For correlation analyses, Pearson correlation was performed based on data distribution. A p value of <0.05 was considered as significant as indicated by asterisk (*).
(TIF)
Acknowledgments
We are grateful to the clinical and laboratory staff at YRG Centre for AIDS Research and Education, VHS, Chennai, India, for assistance with the study and the Laboratory Sciences Core at the Miami CFAR as well as the Flow Cytometry Core facility at the University of Miami Sylvester Comprehensive Cancer Center. We thank Margaret Roach and Maria Pallin for technical assistance and the study participants who consented for this study.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by National Institutes of Health (R21AI106373) to SP, the Indian Council for Medical Research, New Delhi, India (ECD/NTF/17/2013-14) to NK and the Miami Center for AIDS Research (P30AI073961) to SP. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Grome HN, Barnett L, Hagar CC, Harrison DG, Kalams SA, Koethe JR. Association of T Cell and Macrophage Activation with Arterial Vascular Health in HIV. AIDS research and human retroviruses. 2017;33(2):181–6. 10.1089/AID.2016.0113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Longenecker CT, Sullivan C, Baker JV. Immune activation and cardiovascular disease in chronic HIV infection. Current opinion in HIV and AIDS. 2016;11(2):216–25. 10.1097/COH.0000000000000227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinto DSM, da Silva M. Cardiovascular Complications of Human Immunodeficiency Virus Infection. Current cardiology reviews. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hileman CO, Funderburg NT. Inflammation, Immune Activation, and Antiretroviral Therapy in HIV. Current HIV/AIDS reports. 2017;14(3):93–100. 10.1007/s11904-017-0356-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Utay NS, Hunt PW. Role of immune activation in progression to AIDS. Current opinion in HIV and AIDS. 2016;11(2):131–7. 10.1097/COH.0000000000000242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dyrhol-Riise AM, Stent G, Rosok BI, Voltersvik P, Olofsson J, Asjo B. The Fas/FasL system and T cell apoptosis in HIV-1-infected lymphoid tissue during highly active antiretroviral therapy. Clinical immunology (Orlando, Fla). 2001;101(2):169–79. [DOI] [PubMed] [Google Scholar]
- 7.Yagi T, Sugimoto A, Tanaka M, Nagata S, Yasuda S, Yagita H, et al. Fas/FasL interaction is not involved in apoptosis of activated CD4+ T cells upon HIV-1 infection in vitro. Journal of acquired immune deficiency syndromes and human retrovirology: official publication of the International Retrovirology Association. 1998;18(4):307–15. [DOI] [PubMed] [Google Scholar]
- 8.Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity. 2016;44(5):989–1004. 10.1016/j.immuni.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burton BR, Britton GJ, Fang H, Verhagen J, Smithers B, Sabatos-Peyton CA, et al. Sequential transcriptional changes dictate safe and effective antigen-specific immunotherapy. Nat Commun. 2014;5:4741 10.1038/ncomms5741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wykes MN, Lewin SR. Immune checkpoint blockade in infectious diseases. Nature reviews Immunology. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Workman CJ, Cauley LS, Kim IJ, Blackman MA, Woodland DL, Vignali DA. Lymphocyte activation gene-3 (CD223) regulates the size of the expanding T cell population following antigen activation in vivo. Journal of immunology (Baltimore, Md: 1950). 2004;172(9):5450–5. [DOI] [PubMed] [Google Scholar]
- 12.Workman CJ, Vignali DA. Negative regulation of T cell homeostasis by lymphocyte activation gene-3 (CD223). Journal of immunology (Baltimore, Md: 1950). 2005;174(2):688–95. [DOI] [PubMed] [Google Scholar]
- 13.Camisaschi C, Casati C, Rini F, Perego M, De Filippo A, Triebel F, et al. LAG-3 expression defines a subset of CD4(+)CD25(high)Foxp3(+) regulatory T cells that are expanded at tumor sites. Journal of immunology (Baltimore, Md: 1950). 2010;184(11):6545–51. [DOI] [PubMed] [Google Scholar]
- 14.Durham NM, Nirschl CJ, Jackson CM, Elias J, Kochel CM, Anders RA, et al. Lymphocyte Activation Gene 3 (LAG-3) modulates the ability of CD4 T-cells to be suppressed in vivo. PLoS One. 2014;9(11):e109080 10.1371/journal.pone.0109080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang CT, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, et al. Role of LAG-3 in regulatory T cells. Immunity. 2004;21(4):503–13. 10.1016/j.immuni.2004.08.010 [DOI] [PubMed] [Google Scholar]
- 16.Kurtulus S, Sakuishi K, Ngiow SF, Joller N, Tan DJ, Teng MW, et al. TIGIT predominantly regulates the immune response via regulatory T cells. The Journal of clinical investigation. 2015;125(11):4053–62. 10.1172/JCI81187 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Liang B, Workman C, Lee J, Chew C, Dale BM, Colonna L, et al. Regulatory T cells inhibit dendritic cells by lymphocyte activation gene-3 engagement of MHC class II. Journal of immunology (Baltimore, Md: 1950). 2008;180(9):5916–26. [DOI] [PubMed] [Google Scholar]
- 18.Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell. 2009;138(1):30–50. 10.1016/j.cell.2009.06.036 [DOI] [PubMed] [Google Scholar]
- 19.Zarour HM. Reversing T-cell Dysfunction and Exhaustion in Cancer. Clin Cancer Res. 2016;22(8):1856–64. 10.1158/1078-0432.CCR-15-1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baumeister SH, Freeman GJ, Dranoff G, Sharpe AH. Coinhibitory Pathways in Immunotherapy for Cancer. Annu Rev Immunol. 2016;34:539–73. 10.1146/annurev-immunol-032414-112049 [DOI] [PubMed] [Google Scholar]
- 21.Turnis ME, Andrews LP, Vignali DA. Inhibitory receptors as targets for cancer immunotherapy. European journal of immunology. 2015;45(7):1892–905. 10.1002/eji.201344413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Attanasio J, Wherry EJ. Costimulatory and Coinhibitory Receptor Pathways in Infectious Disease. Immunity. 2016;44(5):1052–68. 10.1016/j.immuni.2016.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Callahan MK, Postow MA, Wolchok JD. Targeting T Cell Co-receptors for Cancer Therapy. Immunity. 2016;44(5):1069–78. 10.1016/j.immuni.2016.04.023 [DOI] [PubMed] [Google Scholar]
- 24.Huard B, Prigent P, Tournier M, Bruniquel D, Triebel F. CD4/major histocompatibility complex class II interaction analyzed with CD4- and lymphocyte activation gene-3 (LAG-3)-Ig fusion proteins. European journal of immunology. 1995;25(9):2718–21. 10.1002/eji.1830250949 [DOI] [PubMed] [Google Scholar]
- 25.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine. 2010;363(8):711–23. 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joller N, Hafler JP, Brynedal B, Kassam N, Spoerl S, Levin SD, et al. Cutting edge: TIGIT has T cell-intrinsic inhibitory functions. Journal of immunology (Baltimore, Md: 1950). 2011;186(3):1338–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415(6871):536–41. 10.1038/415536a [DOI] [PubMed] [Google Scholar]
- 28.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. The New England journal of medicine. 2015;372(26):2521–32. 10.1056/NEJMoa1503093 [DOI] [PubMed] [Google Scholar]
- 29.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. The Journal of experimental medicine. 2010;207(10):2187–94. 10.1084/jem.20100643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3(5):541–7. [DOI] [PubMed] [Google Scholar]
- 31.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science (New York, NY). 1995;270(5238):985–8. [DOI] [PubMed] [Google Scholar]
- 32.Caforio AL, Stewart JT, Bonifacio E, Burke M, Davies MJ, McKenna WJ, et al. Inappropriate major histocompatibility complex expression on cardiac tissue in dilated cardiomyopathy. Relevance for autoimmunity? Journal of autoimmunity. 1990;3(2):187–200. [DOI] [PubMed] [Google Scholar]
- 33.Hufnagel G, Maisch B. Expression of MHC class I and II antigens and the Il-2 receptor in rejection, myocarditis and dilated cardiomyopathy. European heart journal. 1991;12 Suppl D:137–40. [DOI] [PubMed] [Google Scholar]
- 34.Thelemann C, Haller S, Blyszczuk P, Kania G, Rosa M, Eriksson U, et al. Absence of nonhematopoietic MHC class II expression protects mice from experimental autoimmune myocarditis. European journal of immunology. 2016;46(3):656–64. 10.1002/eji.201545945 [DOI] [PubMed] [Google Scholar]
- 35.Ahmed-Ansari A, Tadros TS, Knopf WD, Murphy DA, Hertzler G, Feighan J, et al. Major histocompatibility complex class I and class II expression by myocytes in cardiac biopsies posttransplantation. Transplantation. 1988;45(5):972–8. [DOI] [PubMed] [Google Scholar]
- 36.Wang YC, Herskowitz A, Gu LB, Kanter K, Lattouf O, Sell KW, et al. Influence of cytokines and immunosuppressive drugs on major histocompatibility complex class I/II expression by human cardiac myocytes in vitro. Human immunology. 1991;31(2):123–33. [DOI] [PubMed] [Google Scholar]
- 37.Baker JV, Engen NW, Huppler Hullsiek K, Stephan C, Jain MK, Munderi P, et al. Assessment of arterial elasticity among HIV-positive participants with high CD4 cell counts: a substudy of the INSIGHT Strategic Timing of AntiRetroviral Treatment (START) trial. HIV medicine. 2015;16 Suppl 1:109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ballocca F, D'Ascenzo F, Gili S, Grosso Marra W, Gaita F. Cardiovascular disease in patients with HIV. Trends in cardiovascular medicine. 2017;27(8):558–63. 10.1016/j.tcm.2017.06.005 [DOI] [PubMed] [Google Scholar]
- 39.Glesby MJ. Cardiovascular Complications of HIV Infection. Topics in antiviral medicine. 2017;24(4):127–31. [PMC free article] [PubMed] [Google Scholar]
- 40.Holloway CJ, Boccara F. HIV-related cardiovascular disease: closing the gap in mortality. Current opinion in HIV and AIDS. 2017;12(6):509–12. 10.1097/COH.0000000000000420 [DOI] [PubMed] [Google Scholar]
- 41.Mantovani A, Dejana E. Cytokines as communication signals between leukocytes and endothelial cells. Immunology today. 1989;10(11):370–5. 10.1016/0167-5699(89)90270-3 [DOI] [PubMed] [Google Scholar]
- 42.Jalkanen S, Steere AC, Fox RI, Butcher EC. A distinct endothelial cell recognition system that controls lymphocyte traffic into inflamed synovium. Science (New York, NY). 1986;233(4763):556–8. [DOI] [PubMed] [Google Scholar]
- 43.Triebel F, Jitsukawa S, Baixeras E, Roman-Roman S, Genevee C, Viegas-Pequignot E, et al. LAG-3, a novel lymphocyte activation gene closely related to CD4. The Journal of experimental medicine. 1990;171(5):1393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huard B, Mastrangeli R, Prigent P, Bruniquel D, Donini S, El-Tayar N, et al. Characterization of the major histocompatibility complex class II binding site on LAG-3 protein. Proc Natl Acad Sci U S A. 1997;94(11):5744–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang JH, Meijers R, Xiong Y, Liu JH, Sakihama T, Zhang R, et al. Crystal structure of the human CD4 N-terminal two-domain fragment complexed to a class II MHC molecule. Proc Natl Acad Sci U S A. 2001;98(19):10799–804. 10.1073/pnas.191124098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andrews LP, Marciscano AE, Drake CG, Vignali DA. LAG3 (CD223) as a cancer immunotherapy target. Immunol Rev. 2017;276(1):80–96. 10.1111/imr.12519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li N, Wang Y, Forbes K, Vignali KM, Heale BS, Saftig P, et al. Metalloproteases regulate T-cell proliferation and effector function via LAG-3. The EMBO journal. 2007;26(2):494–504. 10.1038/sj.emboj.7601520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bae J, Lee SJ, Park CG, Lee YS, Chun T. Trafficking of LAG-3 to the surface on activated T cells via its cytoplasmic domain and protein kinase C signaling. Journal of immunology (Baltimore, Md: 1950). 2014;193(6):3101–12. [DOI] [PubMed] [Google Scholar]
- 49.Woo SR, Li N, Bruno TC, Forbes K, Brown S, Workman C, et al. Differential subcellular localization of the regulatory T-cell protein LAG-3 and the coreceptor CD4. European journal of immunology. 2010;40(6):1768–77. 10.1002/eji.200939874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tian X, Zhang A, Qiu C, Wang W, Yang Y, Qiu C, et al. The upregulation of LAG-3 on T cells defines a subpopulation with functional exhaustion and correlates with disease progression in HIV-infected subjects. Journal of immunology (Baltimore, Md: 1950). 2015;194(8):3873–82. [DOI] [PubMed] [Google Scholar]
- 51.Huang RY, Eppolito C, Lele S, Shrikant P, Matsuzaki J, Odunsi K. LAG3 and PD1 co-inhibitory molecules collaborate to limit CD8+ T cell signaling and dampen antitumor immunity in a murine ovarian cancer model. Oncotarget. 2015;6(29):27359–77. doi: 10.18632/oncotarget.4751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Golden D, Kolmakova A, Sura S, Vella AT, Manichaikul A, Wang XQ, et al. Lymphocyte activation gene 3 and coronary artery disease. JCI Insight. 2016;1(17):e88628 10.1172/jci.insight.88628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li N, Workman CJ, Martin SM, Vignali DA. Biochemical analysis of the regulatory T cell protein lymphocyte activation gene-3 (LAG-3; CD223). Journal of immunology (Baltimore, Md: 1950). 2004;173(11):6806–12. [DOI] [PubMed] [Google Scholar]
- 54.Clayton KL, Douglas-Vail MB, Nur-ur Rahman AK, Medcalf KE, Xie IY, Chew GM, et al. Soluble T cell immunoglobulin mucin domain 3 is shed from CD8+ T cells by the sheddase ADAM10, is increased in plasma during untreated HIV infection, and correlates with HIV disease progression. J Virol. 2015;89(7):3723–36. 10.1128/JVI.00006-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lienhardt C, Azzurri A, Amedei A, Fielding K, Sillah J, Sow OY, et al. Active tuberculosis in Africa is associated with reduced Th1 and increased Th2 activity in vivo. European journal of immunology. 2002;32(6):1605–13. [DOI] [PubMed] [Google Scholar]
- 56.Annunziato F, Manetti R, Cosmi L, Galli G, Heusser CH, Romagnani S, et al. Opposite role for interleukin-4 and interferon-gamma on CD30 and lymphocyte activation gene-3 (LAG-3) expression by activated naive T cells. European journal of immunology. 1997;27(9):2239–44. 10.1002/eji.1830270918 [DOI] [PubMed] [Google Scholar]
- 57.Scala E, Carbonari M, Del Porto P, Cibati M, Tedesco T, Mazzone AM, et al. Lymphocyte activation gene-3 (LAG-3) expression and IFN-gamma production are variably coregulated in different human T lymphocyte subpopulations. Journal of immunology (Baltimore, Md: 1950). 1998;161(1):489–93. [PubMed] [Google Scholar]
- 58.Grosso JF, Kelleher CC, Harris TJ, Maris CH, Hipkiss EL, De Marzo A, et al. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. The Journal of clinical investigation. 2007;117(11):3383–92. 10.1172/JCI31184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Waugh KA, Leach SM, Moore BL, Bruno TC, Buhrman JD, Slansky JE. Molecular Profile of Tumor-Specific CD8+ T Cell Hypofunction in a Transplantable Murine Cancer Model. Journal of immunology (Baltimore, Md: 1950). 2016;197(4):1477–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72(4):917–27. 10.1158/0008-5472.CAN-11-1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. The EMBO journal. 1992;11(11):3887–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. The Journal of experimental medicine. 2000;192(7):1027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science (New York, NY). 2001;291(5502):319–22. [DOI] [PubMed] [Google Scholar]
- 64.Baban B, Liu JY, Mozaffari MS. Pressure overload regulates expression of cytokines, gammaH2AX, and growth arrest- and DNA-damage inducible protein 153 via glycogen synthase kinase-3beta in ischemic-reperfused hearts. Hypertension. 2013;61(1):95–104. 10.1161/HYPERTENSIONAHA.111.00028 [DOI] [PubMed] [Google Scholar]
- 65.Baban B, Liu JY, Mozaffari MS. SGK-1 regulates inflammation and cell death in the ischemic-reperfused heart: pressure-related effects. Am J Hypertens. 2014;27(6):846–56. 10.1093/ajh/hpt269 [DOI] [PubMed] [Google Scholar]
- 66.Mozaffari MS, Liu JY, Abebe W, Baban B. Mechanisms of load dependency of myocardial ischemia reperfusion injury. Am J Cardiovasc Dis. 2013;3(4):180–96. [PMC free article] [PubMed] [Google Scholar]
- 67.Baban B, Liu JY, Qin X, Weintraub NL, Mozaffari MS. Upregulation of Programmed Death-1 and Its Ligand in Cardiac Injury Models: Interaction with GADD153. PLoS One. 2015;10(4):e0124059 10.1371/journal.pone.0124059 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
sLAG3 in plasma were measured by ELISA in ART naive (Gp 1, n = 42), Virologically suppressed on ART Gp 2, (n = 21) and healthy controls (Gp 3, n = 21). Data compared between groups using Wilcoxon rank-sum test. A p value <0.05 was considered significant. ***p<0.001; ****p<0.0001.
(TIF)
Results shown for ART naïve (group 1,G1), and virologically suppressed on ART (group 2,G2) patients. Colored boxes represent significant (P < 0.05) correlation between analytes. Scale indicates the correlation coefficient with red color indicating the inverse correlations. For correlation analyses, Pearson correlation was performed based on data distribution. A p value of <0.05 was considered as significant as indicated by asterisk (*).
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.