Abstract
A variety of cytokines play a role in the response to an inflammatory stimulus. The interleukin-6 (IL-6)-type cytokines are released in response to tissue injury or an inflammatory stimulus. They act locally and systemically to generate a variety of physiologic responses, principal among them is the acute phase response. The IL-6 type cytokines demonstrate pleiotropy and redundancy of actions. This is made possible by the distinctive characteristics of the IL-6 receptor complex, which contains an ubiquitous subunit that is shared by most IL-6-type cytokines, as well as a cytokine-specific subunit.
Keywords: IL-6, cytokine, interleukin, critical care, ARDS, laparoscopy, sepsis, trauma
In the coordination of the response to tissue injury, infection, and pathogen exposure, many cytokines are involved. These mediators function through autocrine, paracrine, and endocrine mechanisms. Several members of the interleukin-6 (IL-6)-type cytokine family play a particularly prominent role in determining the local and systemic inflammatory response. In part I of this review, we examine the basic science of IL-6-type cytokines as it relates to surgery, trauma, and critical care. In part II, we apply these principles to elucidate their role in these clinicopathological conditions.
Interleukin-6 Type Cytokine Biologic Activities
Interleukin-6 and its family members have a variety of effects on a multitude of cell types. In turn, elevated systemic concentrations of IL-6 in the postoperative period or in critically ill patients not only indicate the magnitude of the inflammatory response, but also provide an understanding of the mechanisms responsible for an exaggerated inflammatory response and adverse outcome. This review focuses primarily on IL-6; however, other IL-6 family members are discussed because they provide redundancy for IL-6 actions.
Interleukin-6
Interleukin-6 targets multiple cell types and induces a broad array of responses (Table 1). These responses are often, simplistically, classified as pro- or anti-inflammatory in nature. A key function of IL-6, which was demonstrated in the IL-6 knockout mouse, is mediation of the acute phase response.1,2 The acute phase response occurs when an inflammatory stimulus is severe enough to generate a large number of accompanying systemic changes that reset normal homeostatic mechanisms.3 Specifically, tissue injury incites a local reaction that includes activation of leukocytes, endothelial cells, and fibroblasts. This activation results in the release of cytokines that induce a systemic response characterized by fever, leukocytosis, and the release of acute phase proteins (APPs).4,5 There are at least 40 plasma proteins that are classified as such because their concentrations change by at least 25% after an inflammatory stimulus.6 Interestingly, the speed of protein concentration change parallels the magnitude of the inflammatory stimulus.3 Induced proteins include clotting proteins, complement components, antiproteases, and proteins used for transport.6 Although the acute phase response is classically described as a pro-inflammatory phenomenon, many of the acute-phase reactants have inhibitory effects on the immune system.1,2,7 For example, the APP haptoglobin inhibits the respiratory burst in neutrophils, inhibits lipopolysaccharide (LPS)-induced tumor necrosis factor-α (TNFα) production in monocytes, and inhibits LPS-induced proliferation of lymphocytes.7 C-reactive protein (CRP), another APP, has a variety of important pro-inflammatory functions, suchas promoting opsonization, enhancing phagocytosis, activating complement, and stimulating cytokine release and adhesion molecule expression.6 However, CRP also has anti-inflammatory effects, such as inhibiting the neutrophil respiratory burst and degranulation, and thereby limiting tissue injury.8
Table 1.
Overview of IL-6 Actions
| Acute phase response |
| Hypothalamic-pituitary-adrenal axis |
| Fever |
| Hormone release |
| Immune cell maturation and activation |
| Immunoglobulin production by B cells |
| Proliferation and differentiation of T cells |
| Hemostasis |
| Platelet production from megakaryocytes |
| Hematopoesis |
| Endothelial cell activation |
| Neuronal cell differentiation |
| Keratinocyte proliferation |
| Cardiac muscle hypertrophy |
| Osteoclast activation |
Abbreviation: IL-6, interleukin-6.
Interleukin-6 has a variety of effects on the immune system. It plays an important role in immune cell maturation. It induces immunoglobulin production by B cells and differentiation of T cells.9 Although IL-6 is believed to be essential for antibody production by B cells, it does not affect proliferation of activated B cells.10 Interleukin-6 activates mitogen-stimulated T cells by inducing IL-2 production and IL-2 receptor expression. It acts synergistically with IL-2 in propelling T cell differentiation into cytotoxic lymphocytes.10 Interleukin-6 activates endothelial cells and induces chemokine production as well as adhesion molecule expression, leading to the recruitment of leukocytes to sites of inflammation.11 Additional pro-inflammatory effects of IL-6 include inducing expression of phospholipase A2 (PLA2).1 In turn, PLA2 actions generate leukotrienes, prostaglandins, and platelet-activating factor (PAF).1 Platelet activating factor also acts synergistically with IL-6 to prime polymorphonuclear cells (PMNs).12 In vitro anti-inflammatory effects of IL-6 include inhibition of TNFα production and IL-1 inhibitor release, as well as induction of tissue inhibitor of matrix metalloproteinase (TIMP).1,4,9,13,14
Interleukin-6 also stimulates hemostasis. It stimulates platelet production by megakaryocytes.9,15 In vitro experiments on human platelets have demonstrated morphologic alterations as well as platelet activation as measured by adenosine triphosphate, P-selectin, and dense granule concentrations.15 In vivo experiments in dogs have demonstrated that administration of IL-6 reduces the concentration of thrombin required to activate platelets and enhances their responsiveness to PAF.15 Furthermore, IL-6 induces tissue factor expression in monocytes; in turn, the binding of tissue factor to factor VIIa eventually leads to thrombin and fibrin generation.16
Interleukin-6 plays an important role in the neuroendocrine system. Interleukin-6 binds to the hypothalamus and induces fever.17 Interleukin-6 is a “robust” stimulant of the hypothalamic-pituitary-adrenal axis, both centrally and at the adrenal gland. It stimulates the release of corticotrophin-releasing factor from the central nervous system (CNS), adrenocorticotrophic hormone (ACTH) release from the pituitary gland, and cortisol release from the adrenal gland.18–20 Conversely, cortisol inhibits IL-6 production.20 Interleukin-6 also stimulates vasopressin and growth hormone secretion by the pituitary gland but inhibits thyroid-stimulating hormone secretion.20 Several IL-6 family members, including IL-6, IL-11, ciliary neurotropic factor (CNTF), and oncostatin M (OSM), also mediate the “immuno-neuroendocrine interface.”21 Interleukin-6 induces hyperglycemia by releasing glucose from hepatic glycogen stores; increased serum concentrations of IL-6 are associated with insulin resistance.22 Conversely, hyperglycemia increases IL-6 serum concentrations by augmenting IL-6 production by monocytes.22 In vitro experiments suggest that insulin resistance in a variety of disease states, including infection, may be mediated by IL-6.23 Interleukin-6 has been shown to inhibit insulin signaling in hepatocytes; this in turn may be mediated by induction of suppressor of cytokine signaling-3 (SOCS-3) proteins (vide infra).23
Interleukin-6 is also expressed and secreted by osteoblasts and osteoclasts upon stimulation with parathyroid hormone, Vitamin D, or IL-8; it also activates these cells.9,14,24,25 Finally, IL-6 stimulates multilineage blast cell colonyformation in hematopoietic stem cells, G0 to G1 cell-cycle progression, differentiation of neural cells, and proliferation of keratinocytes.9,10,20
Knowledge of this myriad of actions is essential to understanding the multiple roles that IL-6 plays in surgery, trauma, and critical illness. Serum IL-6 concentrations increase in surgical patients, in proportion to the magnitude of the surgical stress.19,26,27 Similarly, IL-6 concentrations increase in sepsis, in proportion to the severity of illness and correlate with adverse outcome.28,29
Other IL-6 Family Members
The actions of IL-6 are redundant with other members of its family. The IL-6 cytokine family also includes IL-11, IL-27, IL-31, OSM, leukemia inhibitory factor (LIF), cardiotrophin-1 (CT-1), cardiotrophin-like cytokine (CLC), neuropoietin/cardiotrophin 2 (NP), and CNTF (Table 2). Although these cytokines provide redundancy for IL-6 actions, such as the acute phase response, they also have highly specific actions. For example, LIF, named for its antiproliferative effects in a mouse leukemia cell line, plays important roles in embryogenesis, hematopoiesis, bone metabolism, and inflammation, including the acute phase response.30–32 Additionally, LIF has protective effects on neurons and peripheral tissues.21 Elevated LIF concentrations have been noted with sepsis and correlated with adverse outcome.33,34
Table 2.
Overview of Actions of Several Members of the IL-6 Cytokine Familya
| IL-11 | OSM | LIF |
| Acute phase response | Acute phase response | Acute phase response |
| Neurogenesis | Megakaryocyte differentiation | Neuronal survival factor |
| Hematopoiesis | Adhesion molecule expression | Hematopoiesis |
| Immune cell development | Mediates wound healing | Bone metabolism |
| Bone metabolism | ||
| CNTF | CT-I | CLC |
| Acute phase response | Acute phase response | Neuronal support |
| Activates acetylcholine transferase in motor neurons | Neuronal survival factor | Embryogenesis |
| Increases platelet and erythrocyte counts | ||
| Anti-apoptotic effects after nerve injury | Cardiac myocyte hypertrophy | |
| IL-27 | IL-31 | NP |
| Pro- and anti-inflammatory activities on white blood cells | Role in skin inflammation | Nervous system development in rodents |
| Role in bronchopulmonary inflammation |
Abbreviations: IL, interleukin; CNTF, ciliary neurotrophic factor; OSM, Oncostatin M; CT-1, cardiotrophin-1; LIF, leukemia inhibitory factor; CLC, cardiotrophin-like cytokine; NP, neuropoetin/cardiotrophin 2.
Modified from Biochem J. 1998; 334(pt 2): 297–314.30
Oncostatin M is also a pleiotropic cytokine that is produced by activated T cells and macrophages; it induces acute phase protein and IL-6 production.35 Oncostatin M appears to be more closely related to LIF, as opposed to IL-11.36 Indeed, OSM and LIF are believed to have arisen via a gene duplication event. They compete for binding to the LIF receptor.36 Oncostatin M effectively recruits stromal, endothelial, and epithelial cells at local concentrations.37 Its functions include promotion of megakaryocyte differentiation/thrombocytosis, induction of neurotrophic peptides, mediation of wound healing, and inhibition of tumor cell growth.35 In addition, it stimulates plasminogen activator and induces TIMP-1.36 Meanwhile, it also plays a role in limiting inflammation, as suggested by its delayed temporal appearance in the inflammatory response, (indirect) suppression of TNFα, and suppression of certain IL-1 functions.35 Furthermore, in vitro, elevated concentrations of OSM have been demonstrated to inhibit IL-6-IL-6 receptor complex formation.38 Systemic OSM concentrations are elevated in patients with septic shock.35
Cardiotrophin-1 is cardioprotective and neuroprotective. It induces cardiac myocyte hypertrophy and the acute phase response.39,40 Experimental data indicates that CT-1 induces atrial natriuretic peptide and brain natriuretic peptide secretion. Cardiotrophin-1 elicits hypotensive effects via a nitric oxide-dependent mechanism.41 It also increases platelet and red blood cell counts.30 Like other IL-6 cytokine family members, CT-1 also has anti-inflammatory effects. In animal models, CT-1 has been shown to inhibit TNFα-induced LPS production, and CT-1 administration inhibits both endotoxin-induced neutrophil accumulation in the lung and lung edema.42 Cardiotrophin-1 expression in the heart is found in myocytes as well as nonmyocytes.41 Significant concentrations of CT-1 have also been demonstrated in the kidneys, lung, aorta, and skeletal muscle.41
Cardiotrophin-like cytokine plays a role in embryogenesis and neuronal support. Neuropoetin plays a role in nervous system development in rodents.43 Ciliary neurotropic factor is a survival factor for neuronal cells that prevents degeneration of motor axons after axotomy.40,43CNTF also regulates the acute phase response and down-regulates pro-inflammatory cytokines such as IL-1 and IL-8.30 Elevated systemic concentrations of CNTF, LIF, and OSM have been noted in patients with septic shock, but these cytokines were undetectable in normal patients.44
Interleukin-11 is produced bya variety of stromal cells, including fibroblasts, epithelial cells, and osteoblasts.45 Interleukin-11 stimulates acute phase protein production, immune cell development, erythropoesis, and megakaryopoesis.14,40 It also plays an important role in bone metabolism, tissue remodeling, and protection of mucosal surfaces.46 It limits tissue injury via protection of clonogenic stem cells, regulates epithelial cell proliferation, inhibits apoptosis, and modulates cytokine production by macrophages.45,46 Like IL-6, OSM, and LIF, it upregulates TIMP-1.30 Furthermore, IL-11 has anti-inflammatory functions similar to Th2 cytokines.47 Specifically, it inhibits IL-12 and interferon-γ (IFN-γ) production.46 Significant elevations of IL-11 have been demonstrated in trauma.45,46 Although IL-11 concentration elevations did not appear to correlate with injury severity in a study of 216 patients by Schinkel et al,45 IL-11 concentrations were significantly higher in nonsurvivors.
Interleukin-27 is produced mainly by macrophages and dendritic cells; it has pro- and anti-inflammatory activities with white blood cells, including T cells.48,49 Finally, IL-31 has been shown to play a role in skin inflammation and bronchopulmonary inflammation.50–54
Interleukin-6 Type Cytokine and Receptor Pathophysiology
Interleukin-6 is produced by a wide variety of immune and nonimmune cell types, especially macrophages, dendritic cells, lymphocytes, endothelial cells, and fibroblasts.10,14,20,55,56 More recently, muscle cells were shown to produce IL-6, giving IL-6 the designation “myokine.”57 Indeed, virtually every tissue studied has cells that can produce IL-6.58 Besides the immune cells that play an important role in the inflammatory response that accompanies surgery and critical illness, stromal cells would also be expected to play an important role, not only in the production of IL-6, but also in responding to it.
Interleukin-6 Synthesis, Regulation, and Measurement
The IL-6 type cytokines belong to the long-chain 4α-helix hematopoietic cytokine family. Interleukin-6 is a 212 amino acid cytokine; its gene maps to chromosome 7p21 and contains 4 introns and 5 exons.10,14,20,55 It is observed as a 22- to 28-kDA phosphorylated and variably glycosylated protein.30 The IL-6 gene contains a variety of single nucleotide polymorphisms (SNPs); the role of these SNPs in generating physiologically relevant differences in IL-6 expression in inflammation and outcome is under intense investigation. One commonly evaluated SNP is the G to C transition at position – 174 base pair (bp) of the IL-6 promoter; it has been variably associated with outcome in critical illness.59 In some reports, patterns of several SNPs that are in linkage disequilibrium (haplotypes) were investigated. Evolutionarily related haplo-type groups comprise haplotype clades.59 Various IL-6 haplo-type clades have been associated with increased mortality in critical illness.59
The IL-6 gene has glucocorticoid-responsive elements; cortisol suppresses IL-6 production.10,20 Conceivably, these glucocorticoid-responsive elements and the effects of IL-6 on the HPA axis could begin to explain the adverse effects of relative adrenal insufficiency in patients with septic shock and consequently, the reported benefits of corticosteroid administration in this circumstance.60 The IL-6 gene is not constitutively expressed, but its expression can be induced by a myriad of stimuli, including viral infections, LPS, IL-1β, TNFα, platelet-derived growth factor (PDGF), and IFN-γ.10 Catecholamines also stimulate IL-6 production, whereas the sex steroids suppress its production.20
A key component of IL-6 production during the inflammatory response is the recognition of structures that are shared among infectious agents, that is, pathogen-associated molecular patterns (PAMPs), by Toll-like (TLRs) receptors.61 In humans, greater than 10 TLRs have been described. They are expressed primarily on monocytes/macrophages, dendritic cells, and neutrophils.61–63 They are also expressed on B cells.64 Recognition of PAMPs by TLRs activates dendritic cells and represents a key step in the innate and adaptive immune response. Although TLR2 signaling is elicited by gram-positive bacterial products, TLR4 signaling is induced by LPS and the lipid A component of gram-negative bacteria.62,64 Toll-like receptors 4 and 8 stimulate IL-6 production; TLR2 also appears to contribute to IL-6 production.62,64
Interleukin-6 is a normally circulating plasma protein.58 However, measurement of IL-6 levels is problematic. Although large (nanomolar) quantities are present in the blood in healthy individuals, it may be undetectable by various assays, including bioassays and enzyme-linked immunosorbent assays (ELISAs).58 Part of the difficulty in IL-6 measurement arises because IL-6 exists as monomer, dimers, and multimers. Furthermore, although IL-6 is present in blood at concentrations sufficient to elicit the whole gamut of IL-6 effects in the healthy individual, it remains relatively inert. This suggests the presence of chaperone proteins which modify or limit its biologic activity and camouflage it from assay systems. Interleukin-6 can exist in high-molecular-weight complexes with various proteins such as CRP, complement components, and the soluble IL-6 receptor. Finally, some individuals may also have IL-6 autoantibodies, which may also function as chaperones. The various assays may have trouble detecting IL-6 in these multiple forms.38,58,65 Because of these chaperones, depending on the assay used, concentrations may differ by more than 10-fold.38 These findings suggest that free IL-6 may have only a transient presence in blood.65
Interleukin-6-Type Cytokine Receptors
The IL-6 type cytokines engage receptors that share overall structural organization characteristics with the long-chain type I hematopoietic receptor family. Generally, the IL-6-type cytokines are recognized by ligand-specific binding receptor subunits (α subunits) that associate with a common receptor (β) subunit to transduce signal (vide infra).37 Similar to other hematopoietic cytokines, the IL-6 cytokines are recognized by receptor systems based on structural motifs formed by loop and helical elements (termed sites I, II, and III). These cytokines coordinate the formation of a complex signaling competent unit, by forming complementary interfacing contacts with sites within the cytokine recognition site of the extracellular domain of the receptor subunits.66
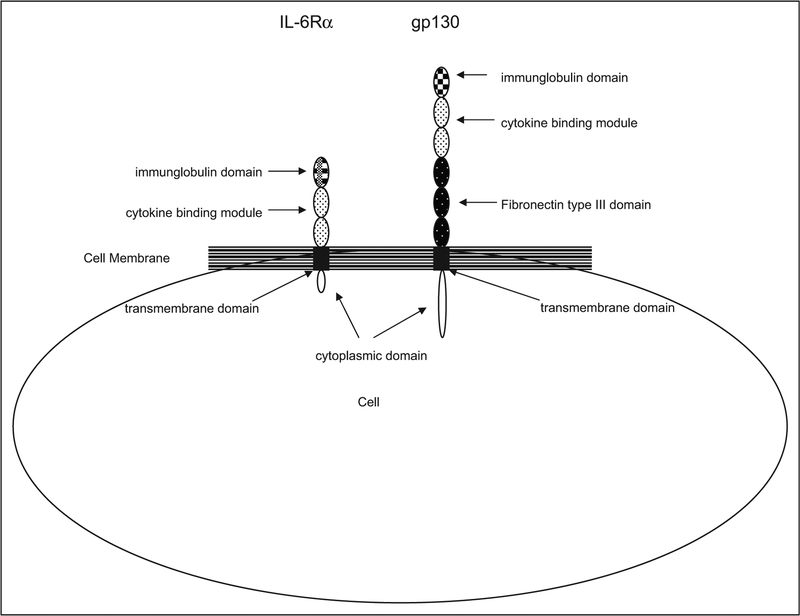
The α and β subunits share a common cytokine homology domain (cytokine binding module) that is formed from 2 fibronectin type III domains (Figure 1). Note that a domain is defined as an element of protein structure that stabilizes itself and often folds independently of the remaining portions of the protein.68 These domains have conserved β sheet structures that are stabilized by 2 pairs of cysteine residues at their N-terminus and have a tryptophan-serine-x-tryptophan-serine (WSXWS) sequence motif at their C-terminus. Additional conserved structural modules in the extracellular receptor portion include an immunoglobulin-like domain, a single transmembrane domain, and an intracellular domain that lacks intrinsic enzymatic activity. This intracellular domain is longer in β subunits. All signaling-capable (ie, β) subunits have 3 additional fibronectin type III domains that separate the cytokine homology domain from the transmembrane region, thereby providing a larger cytokine interaction site to the extracellular milieu.30 Although the tertiary structure of the IL-6 cytokines and their engagement of shared receptor subunits results in functional overlap, there is also considerable specificity. Factors responsible for this specificity include the different spatial and temporal expression of the cytokines as well as tissue localization of their receptors.69
Figure 1.
A schematic of membrane-bound IL-6 receptors: IL-6Rα and gp130. These receptors engage with IL-6 in multimeric complexes to transduce signal. Both have a cytokine-binding module which has 2 pairs of conserved cysteine residues at the N-terminus as well as the WSXWS motif in the extracellular domain, at the C-terminus. This cytokine-binding module is composed of 2 fibronectin type III domains. Additionally, both receptors have an immunoglobulin-like domain, a single transmembrane domain, and an intracellular domain that lacks intrinsic enzymatic activity. The intracellular domain is longer in gp 130. The gp130 subunit also has 3 additional fibronectin type III domains. Adapted from Biochem J. 1998; 334 (pt 2): 297–31430 and Clin Rev Allergy Immunol. 2005; 28(3): 177–18667. IL indicates interleukin; gp, glycoprotein; WSXWS, tryptrophanserine-x-tryptophan-serine.
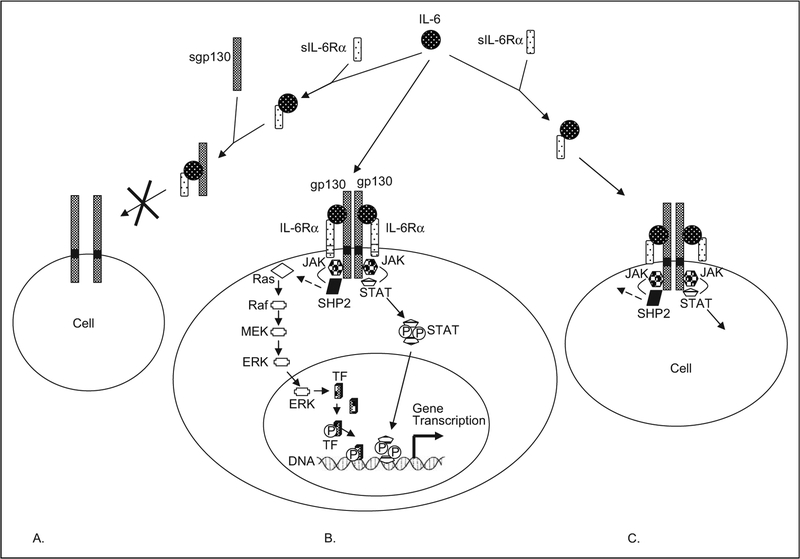
The receptor subunits may be present in membrane-bound or soluble forms. The soluble forms are generated via alternative mRNA splicing or proteolysis of fully processed and cell-surface exposed receptor subunits. This latter mode of production is often observed as a consequence of tissue injury and inflammation. Soluble forms of receptor subunits have been reported for many different receptor systems including growth factors, hormones, and cytokines. Depending upon the requirement for forming a signaling-competent receptor complex on cell surface, in particular the recruitment of subunits with cytoplasmic domains that contribute to signal-transduction reactions, the soluble receptors in combination with cytokines can assume agonistic or antagonistic actions (Figure 2).
Figure 2.
A schematic of IL-6 and IL-6 receptor interactions. A. Soluble gp130, an antagonist of IL-6 activity. Serum IL-6 binds with sIL-6Rα. This complex in turn binds to sgp130. Soluble gp130 thereby competitively antagonizes the binding of the IL-6-sIL-6Rα complex to membrane-bound gp130, and thereby inhibits IL-6 effects on this cell. B. Membrane-bound gp130 and membrane-bound IL-6Rα. Membrane-bound IL-6Rα is restricted in location and is found in only select cell types, whereas gp130 is ubiquitous. In this case, serum IL-6 complexes with membrane-bound IL-6Rα and then associates with membrane-bound gp130, to generate a signaling-capable hexameric complex. As classically described, signaling is mediated proximally by the Jak kinases and STAT proteins. Phosphorylation of STAT proteins by the Jak kinases and their subsequent translocation to the nucleus induces gene transcription. The Jak kinases, with the help of tyrosine phosphatase, SHP2, also activate a series of additional kinases (Ras, Raf, Mek, and ERK), which in turn eventually phosphorylate a transcription factor (TF) and thereby induce gene transcription. A third pathway, not illustrated involves PI3K (see text for details). C. Soluble IL-6Rα, an agonist for IL-6 activities. Soluble IL-6Rα binds to serum IL-6. This complex then engages membrane-bound gp130, which is found on most cells, to transduce signal in those cells that lack membrane-bound IL-6Rα. This phenomenon, termed “trans-signaling,” allows IL-6 to target multiple cell types. Adapted from Arthritis Res. 2002; 4(suppl 3): S233-S242,9 Biochem J. 2003; 374(pt 1):1–20,70 Crit Care Med. 2005; 33(8):1839–1844,71 and Acta Biochim Pol. 2003; 50(3):603–611.72 IL indicates interleukin; gp, glycoprotein; sIL-6Rα, soluble IL-6Rα STAT, signal transducer and activator of transcription.
α Subunits.
The alpha subunits are the nonsignaling components of the IL-6 receptor complex. They include IL-6Rα, IL-11Rα, CT-1Rα, and CNTFRα. These components exhibit tissue-specific expression and therefore contribute to specificity of cytokine action.69 Interleukin-6Rα (gp80, CD126) has a molecular weight of 80 kDa, maps to chromosome 1, and is primarily expressed by hepatocytes and lymphocytes but is also detectable in low concentrations (and thus low activity) in other cells such as mesenchymal, endothelial, epithelial, and neural cells.38,55,73,74 Interleukin-6Rα exists in membrane-bound and soluble forms.
Soluble IL-6Rα serves as an IL-6-binding component; it cannot transduce signal on its own.25 Soluble IL-6Rα (sIL-6Rα) is generated via proteolysis or alternative mRNA splicing.14,19 Various stimuli can induce shedding of IL-6Rα from the cell surface including CRP, f-met-leu-phe (fMLP), phorbol myristate acetate (PMA), bacterial pore-forming toxins, and neutrophil degranulation.25,73,75 Soluble IL-6Rα complexes with IL-6 and gp130 with an affinity that is similar to membrane-bound IL-6Rα. This complex in combination with the membrane-bound form of gp130 can induce signaling in cells that do not express IL-6Rα, that is, “trans-signaling.”73 The complex of IL-6 bound to soluble IL-6Rα acts as a potent agonist on any cell type that expresses gp130. Considering that essentially every cell type in the body expresses gp130, but not always the membrane from of IL-6Rα, the IL-6-sIL-6Rα complex assumes a very potent signaling function.66,73,76,77 Trans-signaling helps explain the myriad of previously described IL-6-mediated functions noted in multiple cell types. For example, IL-6 facilitates erythropoiesis and it also stimulates chemokine production in endothelial cells.38
Soluble IL-6Rα prolongs the half-life of IL-6 and makes detection of IL-6 easier as the antibodies used to measure IL-6 concentrations in clinical studies often detect both free and bound IL-6.78 Responses that are generated by the IL-6-sIL-6Rα complex can in part be mimicked by other IL-6-type cytokines, such as CT-1, IL-11, LIF, and OSM.78 Soluble IL-6Rα acts as an agonist for IL-6 activities, in contrast to soluble gp130 or other receptor subunits for LIF or OSM, which act as antagonists (vide infra).70,78 Soluble forms of IL-6Rα and gp130 can be detected in the circulation of healthy persons.25 Decreased circulating concentrations of sIL-6Rα have been reported in sepsis, along with an alteration of the IL-6-IL-6 receptor “axis.”25,71,79 In contrast, serum sIL-6Rα concentrations have been reported to be elevated in several inflammatory diseases, but its levels did not change after stroke.78,80
β Subunits.
The signaling-capable β subunits for the IL-6-type cytokines include gp130, LIFR, and OSMRβ. Interleukin-31Rα, although often classified as a β subunit, can only transduce signal when combined with another β subunit.52 The β subunits may combine with another β subunit or with the α subunits of selected IL-6 cytokines to form dimeric or multi-meric complexes.70,81 The shared subunit for the IL-6 cytokines, with the exception of IL-31, is gp130.30,70,82 The gp130 subunit (molecular weight 130 kDa) maps to chromosome 5q11 and a pseudogene maps to chromosome 17; it is expressed by nearly all cells in the body.38,70,74,83 It is a transmembrane protein, whose cytoplasmic domains can engage STAT proteins and Jak kinases to transduce signal.66 Again, the ubiquitous expression of gp130 as well as its shared use by nearly all members of the IL-6 family helps account for the pleiotropic effects and redundancy of action of the IL-6 type cytokines.30,69,82
Like IL-6Rα, gp130 can also exist in a soluble form (sgp130).72,73 However, unlike sIL-6Rα, sgp130 is a biologic response inhibitor. Soluble gp130 binds only to sIL-6Rα-IL-6 complexes and inhibits their biologic response. Similarly, it inhibits the actions of LIF and OSM. It does not bind to free IL-6 or to membrane-bound IL-6/IL-6Rα complexes, that is, it inhibits “trans-signaling,” but not classical signaling via membrane-bound IL-6Rα.14,72,73 Concentrations of sgp130 vary in various clinical conditions, for example stroke and cardiac surgery are associated with decreased sgp130 concentrations.80,84
Interleukin-6 – IL-6 receptor component “axis.”.
Variations in the concentration of IL-6 and its receptors suggest that tissue injury and inflammation are associated with perturbations of the IL-6-IL-6 receptor component axis. Alterations in this axis have been demonstrated in experimental endotoxemia. Marsik et al55 in 2005 measured serum cytokine concentrations in 9 healthy male volunteers following LPS injection in a randomized, double-blinded, placebo-controlled trial. They found that LPS infusion significantly increased plasma IL-6 concentrations and significantly increased the percentage of gp130-positive neutrophils. However, monocyte and neutro-phil IL-6Rα concentrations were not significantly altered in low-grade endotoxemia. Dekkers et al,25 in 2000, measured cell-surface and systemic cytokine concentrations in 8 healthy participants before and after LPS infusion. Plasma sIL-6Rα and sgp130 were detected at baseline in healthy volunteers and were not significantly influenced by LPS injection. At baseline, IL-6Rα, gp130, and LIFR were detectable on the surface of peripheral blood monocytes and granulocytes. However, LPS infusion significantly reduced IL-6Rα expression on both cell types, whereas gp130 and LIFR expression were unchanged. Interleukin-6 concentrations, while undetectable at baseline, peaked at 3 hours postinjection at 5.99 pg/mL. It should be noted that this degree of IL-6 elevation is below detection threshold by some IL-6 enzyme-linked-immunosorbent serologic assays (ELISAs) and could be considered as background noise, suggesting that the concentration of LPS infused had minimal systemic effects, perhaps intentionally.
Interleukin-6-IL-6 Receptor Interactions and Signaling
To generate a signal, site I on IL-6 binds to the cytokine binding domain of IL-6Rα. Then, IL-6 site II binds to the cytokine binding module of gp130. Intekeukin-6 site III binds to the immunoglobulin-like domain of a second gp130 receptor subunit that is recruited, and thereby generates a functional complex.14,66 Stoichiometric experiments suggest that the functional complex is hexameric, composed of 2 units each of IL-6, IL-6Rα, and gp130. Analogous, although subunit- specific, combinations are coordinated between other members of the IL-6 family and their receptors.66,70 Whereas signaling involves the combination of an α subunit with a β subunit for several IL-6 cytokines, 2 β subunits may combine to transduce signal also, for example, OSM can signal via OSMRβ/gp130 or LIFR/gp130.70 Leukemia inhibitory factor signals via LIFR/gp130. Interleukin-31 signals via IL-31Rα/OSMRβ.52 Finally, CNTF and CLC signal via the CNTFRa/LIFR/gp130 complex.70
Ligand-binding by the receptor leads to activation of cytoplasmic Janus tyrosine kinases (Jak). Specifically, IL-6 activates Jak1, Jak2, and Tyk2. 56,85,86 Of these, Jak1 is believed to play a key role.66 Activation of Jak kinases leads to phosphorylation of signal transducer and activator of transcription (STAT) proteins (Figure 2). The IL-6 type cytokines, via gp130, activate STAT3 potently and to a lesser extent, STAT1.66 In animal models, STAT3 activation is believed to be an important regulator of neutrophil trafficking in inflammation.87 LIFR and OSMR can also activate STAT5.64 As classically described, phosphorylation of STATs induces their dimerization and translocation into the nucleus and thereby results in activation of gene transcription. However, it should be noted that much of the information about the STATs is in a state of flux, where the manner of existence of these proteins, importance of dimerization in nuclear translocation, and a variety of other fundamental observations about them is being challenged.88 It appears that rather than simply existing as monomers or dimers, STAT proteins associate with a variety of cytoplasmic proteins including chaperones, regulatory proteins, and trafficking proteins.88 Various STAT proteins, Jak kinases, and IL-6 receptor components at least partially exist in caveolin-1 containing, cholesterol-rich, detergent-resistant plasma membrane raft (microdomains) fractions, which limit their bioavailability.89,90 These raft microdomains represent the unit of function for signaling.89
Interleukin-6 receptor complexes also activate the mitogen-activated protein kinase/extracellular signal-regulated kinase (ERK) pathway, with the assistance of the tyrosine phosphatase, Src homology phosphatase (SHP2; Figure 2).66,86,87 Specifically, ERK 1/2 is activated.56 This in turn leads to activation of transcription factors and gene expression. Finally, IL-6 can also transduce signal via the PI3K/Akt pathway.70
Interleukin-6 signaling also has a negative feedback system that is mediated by phosphatases, namely, the inducible suppressors of cytokine signaling (SOCS) system and the constitutively active protein inhibitors of activated STATs (PIAS) system.10,70 It appears that the SOCS proteins play an important role in inhibiting IL-6 signaling, mediated by TNFα.70
Conclusions
Expression of membrane receptors for IL-6 determines IL-6 actions on target cells. The relative number of receptor molecules defines the limits of signaling. Cells that lack membrane-bound IL-6Rα can be rendered sensitive to IL-6 via trans-signaling. The coexistence of sIL-6Rα and sgp130 determines the extent to which trans-signaling can occur. The production and action of all components of the IL-6-IL-6 receptor axis is strongly regulated by trauma and inflammation.91 Elevated concentrations of IL-6 are found in nearly every case that involves infectious, traumatic, and inflammatory states, rising within minutes of an insult and remaining elevated for days. In general, greater tissue trauma is associated with greater mediator production and inflammatory response.92 In turn, the systemic dissemination of circulating IL-6 promotes the widespread development of focal areas of inflammation. The temporal change in IL-6 concentrations renders certain predictions possible regarding the regulation of cell responses. Consequences of this systemic action include the induced expression of C-reactive protein, which has been used as the assay par excellence for grading inflammation severity in patients.91,93 In considering the functions of IL-6-regulated genes in the progression of trauma and/or septic response and recovery, an understanding of a cause-effect relationship of tissue damage, IL-6 expression, and the inflammatory response provides important diagnostic and predictive information in surgery, trauma and critical care, as discussed in part II of this review.
Acknowledgments
Funding
Research in Dr. Heinz Baumann’s laboratory was supported by NCI grant (CA085580) and RPCI grant (016056).
Footnotes
Declaration of Conflicting Interests
The author(s) declared no conflicts of interest with respect to the authorship and/or publication of this article.
References
- 1.Tilg H, Dinarello CA, Mier JW. IL-6 and APPs: anti-inflammatory and immunosuppressive mediators. Immunol Today. 1997;18(9):428–432. [DOI] [PubMed] [Google Scholar]
- 2.Kopf M, Baumann H, Freer G, et al. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994; 368(6469):339–342. [DOI] [PubMed] [Google Scholar]
- 3.Kushner I, Rzewnicki DL. The acute phase response: general aspects. Baillieres Clin Rheumatol. 1994;8(3):513–530. [DOI] [PubMed] [Google Scholar]
- 4.Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265(3):621–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kushner I C-reactive protein in rheumatology. Arthritis Rheum. 1991;34(8):1065–1068. [DOI] [PubMed] [Google Scholar]
- 6.Black S, Kushner I, Samols D. C-reactive Protein. J Biol Chem. 2004;279(47):48487–48490. [DOI] [PubMed] [Google Scholar]
- 7.Arredouani MS, Kasran A, Vanoirbeek JA, Berger FG, Baumann H, Ceuppens JL. Haptoglobin dampens endotoxin-induced inflammatory effects both. in vitro. and in vivo. Immunology. 2005;114(2):263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mortensen RF, Zhong W. Regulation of phagocytic leukocyte activities by C-reactive protein. J Leukoc Biol. 2000;67(4): 495–500. [DOI] [PubMed] [Google Scholar]
- 9.Naka T, Nishimoto N, Kishimoto T. The paradigm of IL-6: from basic science to medicine. Arthritis Res. 2002;4(3):S233–S242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park JY, Pillinger MH. Interleukin-6 in the pathogenesis of rheumatoid arthritis. Bull NYU Hosp Jt Dis. 2007;65(1):S4–S10. [PubMed] [Google Scholar]
- 11.Cronstein BN. Interleukin-6–a key mediator of systemic and local symptoms in rheumatoid arthritis. Bull NYU Hosp Jt Dis. 2007; 65(1):S11–S15. [PubMed] [Google Scholar]
- 12.Biffl WL, Moore EE, Moore FA, Carl VS, Kim FJ, Franciose RJ. Interleukin-6 potentiates neutrophil priming with platelet-activating factor. Arch Surg. 1994;129(11):1131–1136. [DOI] [PubMed] [Google Scholar]
- 13.Nijsten MW, Hack CE, Helle M, ten Duis HJ, Klasen HJ, Aarden LA. Interleukin-6 and its relation to the humoral immune response and clinical parameters in burned patients. Surgery. 1991;109(6):761–767. [PubMed] [Google Scholar]
- 14.Scheller J, Rose-John S. Interleukin-6 and its receptor: from bench to bedside. Med Microbiol Immunol. 2006;195(4):173–183. [DOI] [PubMed] [Google Scholar]
- 15.Peng J, Friese P, George JN, Dale GL, Burstein SA. Alteration of platelet function in dogs mediated by interleukin-6. Blood. 1994; 83(2):398–403. [PubMed] [Google Scholar]
- 16.Grignani G, Maiolo A. Cytokines and hemostasis. Haematologica. 2000;85(9):967–972. [PubMed] [Google Scholar]
- 17.Ahad A Baumann H, Elias J, et al. Interleukin-6-type cytokines in diagnostics and therapeutics: roundtable discussion. Ann N Y Acad Sci. 1995;762:375–387. [Google Scholar]
- 18.Dimopoulou I, Tsagarakis S, Kouyialis AT, et al. Hypothalamic-pituitary-adrenal axis dysfunction in critically ill patients with traumatic brain injury: incidence, pathophysiology, and relationship to vasopressor dependence and peripheral interleukin-6 concentrations. Crit Care Med. 2004;32(2):404–408. [DOI] [PubMed] [Google Scholar]
- 19.Kashiwabara M, Miyashita M, Nomura T, et al. Surgical trauma-induced adrenal insufficiency is associated with postoperative inflammatory responses. J Nippon Med Sch. 2007;74(4):274–283. [DOI] [PubMed] [Google Scholar]
- 20.Papanicolaou DA, Wilder RL, Manolagas SC, Chrousos GP. The pathophysiologic roles of interleukin-6 in human disease. Ann Intern Med. 1998;128(2):127–137. [DOI] [PubMed] [Google Scholar]
- 21.Chesnokova V, Melmed S. Minireview: neuro-immunoendocrine modulation of the hypothalamic-pituitary-adrenal (HPA) axis by gp130 signaling molecules. Endocrinology. 2002;143(5):1571–1574. [DOI] [PubMed] [Google Scholar]
- 22.Wasmuth HE, Kunz D, Graf J, et al. Hyperglycemia at admission to the intensive care unit is associated with elevated serum concentrations of interleukin-6 and reduced ex vivo secretion of tumor necrosis factor-alpha. Crit Care Med. 2004;32(5): 1109–1114. [DOI] [PubMed] [Google Scholar]
- 23.Senn JJ, Klover PJ, Nowak IA, et al. Suppressor of cytokine signaling-3 (SOCS-3), a potential mediator of interleukin-6-dependent insulin resistance in hepatocytes. J Biol Chem. 2003; 278(16):13740–13746. [DOI] [PubMed] [Google Scholar]
- 24.Beeton CA, Chatfield D, Brooks RA, Rushton N. Circulating levels of interleukin-6 and its soluble receptor in patients with head injury and fracture. J Bone Joint Surg Br. 2004;86(6): 912–917. [DOI] [PubMed] [Google Scholar]
- 25.Dekkers PE, Juffermans NP, ten Hove T, de Jonge E, van Deventer SJ, van der Poll T. Endotoxin down-regulates monocyte and granulocyte interleukin-6 receptors without influencing gp130 expression in humans. J Infect Dis. 2000;181(3):1055–1061. [DOI] [PubMed] [Google Scholar]
- 26.Yahara N, Abe T, Morita K, Tangoku A, Oka M. Comparison of interleukin-6, interleukin-8, and granulocyte colony-stimulating factor production by the peritoneum in laparoscopic and open surgery. Surg Endosc. 2002;16(11):1615–1619. [DOI] [PubMed] [Google Scholar]
- 27.Ohzato H, Yoshizaki K, Nishimoto N, et al. Interleukin-6 as a new indicator of inflammatory status: detection of serum levels of interleukin-6 and C-reactive protein after surgery. Surgery. 1992;111(2):201–209. [PubMed] [Google Scholar]
- 28.Damas P, Ledoux D, Nys M, et al. Cytokine serum level during severe sepsis in human IL-6 as a marker of severity. Ann Surg. 1992;215(4):356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hack CE, De Groot ER, Felt-Bersma RJ, et al. Increased plasma levels of interleukin-6 in sepsis. Blood. 1989;74(5):1704–1710. [PubMed] [Google Scholar]
- 30.Heinrich PC, Behrmann I, Müller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334(2):297–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Auernhammer CJ, Melmed S. Leukemia-inhibitory factor-neuroimmune modulator of endocrine function. Endocr Rev. 2000;21(3):313–345. [DOI] [PubMed] [Google Scholar]
- 32.Metcalf D The unsolved enigmas of leukemia inhibitory factor. Stem Cells. 2003;21(1):5–14. [DOI] [PubMed] [Google Scholar]
- 33.Ranieri VM, Suter PM, Tortorella C, et al. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1999;282(1):54–61. [DOI] [PubMed] [Google Scholar]
- 34.Pan W, Yu C, Hsuchou H, Zhang Y, Kastin AJ. Neuroinflammation facilitates LIF entry into brain: role of TNF. Am J Physiol Cell Physiol. 2008;294(6):C1436–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wahl AF, Wallace PM. Oncostatin M in the anti-inflammatory response. Rev Physiol Biochem Pharmacol. 2003;149:39–52.12811586 [Google Scholar]
- 36.Wallace P, MacMaster J, Rillema J, et al. In vivo properties of Oncostatin M. Ann N Y Acad Sci. 1995;762(1):42–54. [DOI] [PubMed] [Google Scholar]
- 37.Gómez-Lechón MJ. Oncostatin M: signal transduction and biological activity. Life Sci. 1999;65(20):2019–2030. [DOI] [PubMed] [Google Scholar]
- 38.Pignatti P, Ciapponi L, Galle P, et al. High circulating levels of biologically inactive IL-6/SIL-6 receptor complexes in systemic juvenile idiopathic arthritis: evidence for serum factors interfering with the binding to gp130. Clin Exp Immunol. 2003;131(2):355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Latchman DS. Cardiotrophin-1: a novel cytokine and its effects in the heart and other tissues. Pharmacol Ther. 2000;85(1):29–37. [DOI] [PubMed] [Google Scholar]
- 40.Huising MO, Kruiswijk CP, Flik G. Phylogeny and evolution of class-I helical cytokines. J Endocrinol. 2006;189(1):1–25. [DOI] [PubMed] [Google Scholar]
- 41.Hamanaka I, Saito Y, Nishikimi T, et al. Effects of cardiotrophin-1 on hemodynamics and endoc1rine function of the heart. Am J Physiol Heart Circ Physiol. 2000;279(1):H388–H396. [DOI] [PubMed] [Google Scholar]
- 42.Pulido EJ, Shames BD, Pennica D, et al. Cardiotrophin-1 attenuates endotoxin-induced acute lung injury. J Surg Res. 1999;84(2): 240–246. [DOI] [PubMed] [Google Scholar]
- 43.Derouet D, Rousseau F, Alfonsi F, et al. Neuropoietin, a new IL-6-related cytokine signaling through the ciliary neurotrophic factor receptor. Proc Natl Acad Sci USA. 2004;101(14):4827–4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guillet C, Fourcin M, Chevalier S, Pouplard A, Gascan H. ELISA detection of circulating levels of LIF, OSM, and CNTF in septic shock. Ann N Y Acad Sci. 1995;762:407–409. [DOI] [PubMed] [Google Scholar]
- 45.Schinkel C, Wick M, Muhr G, Köller M. Analysis of systemic interleukin-11 after major trauma. Shock. 2005;23(1):30–34. [DOI] [PubMed] [Google Scholar]
- 46.Heizmann O, Koeller M, Muhr G, Oertli D, Schinkel C. Th1- and Th2-type cytokines in plasma after major trauma. J Trauma. 2008;65(6):1374–1378. [DOI] [PubMed] [Google Scholar]
- 47.Opal S, Keith J Jr, Jhung J, et al. Orally administered recombinant human interleukin-11 is protective in experimental neutropenic sepsis. J Infect Dis. 2003;187(1):70–76. [DOI] [PubMed] [Google Scholar]
- 48.Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–242. [DOI] [PubMed] [Google Scholar]
- 49.Villarino AV, Hunter CA. Biology of recently discovered cytokines: discerning the pro- and anti-inflammatory properties of interleukin-27. Arthritis Res Ther. 2004;6(5):225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takaoka A, Arai I, Sugimoto M, Yamaguchi A, Tanaka M, Nakaike S. Expression of IL-31 gene transcripts in NC/Nga mice with atopic dermatitis. Eur J Pharmacol. 2005;516(2):180–181. [DOI] [PubMed] [Google Scholar]
- 51.Dillon SR, Sprecher C, Hammond A, et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat Immunol. 2004;5(7):752–60. Erratum in Nat Immunol. 2005;6(1):114. [DOI] [PubMed] [Google Scholar]
- 52.Jawa R, Chattopadhyay S, Tracy E, et al. Regulated expression of the IL-31 receptor in bronchial and alveolar epithelial cells, pulmonary fibroblasts, and pulmonary macrophages. J Interferon Cytokine Res. 2008;28(4):207–219. [DOI] [PubMed] [Google Scholar]
- 53.Ip WK, Wong CK, Li ML, et al. Interleukin-31 induces cytokine and chemokine production from human bronchial epithelial cells through activation of mitogen-activated protein kinase signalling pathways: implications for the allergic response. Immunology. 2007;122(4):532–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perrigoue JG, Li J, Zaph C, et al. IL-31-IL-31R interactions negatively regulate type 2 inflammation in the lung. J Exp Med. 2007; 204(3):481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marsik C, Halama T, Cardona F, Schlifke I, Mittermayer F, Jilma B. Endotoxemia enhances expression of the signaling receptor (GP130) on protein and molecular level. Clin Immunol. 2005;114(3):293–298. [DOI] [PubMed] [Google Scholar]
- 56.Yu X, Kennedy RH, Liu SJ. JAK2/STAT3, not ERK1/2, mediates interleukin-6-induced activation of inducible nitric-oxide synthase and decrease in contractility of adult ventricular myocytes. J Biol Chem. 2002;278(18):16304–16309. [DOI] [PubMed] [Google Scholar]
- 57.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98(4):1154–1162. [DOI] [PubMed] [Google Scholar]
- 58.May L, Ndubisi M, Patel K, Garcia D. Interleukin-6 chaperones in blood. Ann N Y Acad Sci. 1995;762:120–128. [PubMed] [Google Scholar]
- 59.Sutherland AM, Walley KR, Manocha S, Russell JA. The association of interleukin-6 haplotype clades with mortality in critically ill adults. Arch Intern Med. 2005;165(1):75–82. [DOI] [PubMed] [Google Scholar]
- 60.Annane D, Sébille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288(7):862–871. Erratum in JAMA. 2008;300(14):1652. [DOI] [PubMed] [Google Scholar]
- 61.Sochorova K, Horvath R, Rozkova D, et al. Impaired Toll-like receptor 8-mediated IL-6 and TNF-alpha production in antigen presenting cells from patients with X-linked agammaglobulinemia. Blood. 2007;109(6):2553–2556. [DOI] [PubMed] [Google Scholar]
- 62.Krishnan J, Selvarajoo K, Tsuchiya M, Lee G, Choi S. Toll-like receptor signal transduction. Exp Mol Med. 2007;39(4):421–438. [DOI] [PubMed] [Google Scholar]
- 63.Schilling D, Thomas K, Nixdorff K, Vogel SN, Fenton MJ. Toll-like receptor 4 and Toll-IL-1 receptor domain-containing adapter protein (TIRAP)/myeloid differentiation protein 88 adapter-like (Mal) contribute to maximal IL-6 expression in macrophages. J Immunol. 2002;169(10):5874–5880. [DOI] [PubMed] [Google Scholar]
- 64.Dissanayake S, Amith RS, Shahin A. Taenia crassiceps carbohydrates stimulate IL-6 expression in naϊve murine macrophages via Toll-like receptors (TLRs). Mol Immunol. 2004;41(4):391–398. [DOI] [PubMed] [Google Scholar]
- 65.Ndubuisi MI, Patel K, Rayanade RJ, Mittelman A, May LT, Sehgal PB. Distinct classes of chaperoned IL-6 in human blood: differential immunological and biological availability. J Immunol. 1998;160(1):494–501. [PubMed] [Google Scholar]
- 66.Bravo J, Heath JK. Receptor recognition by gp130 cytokines. EMBO J. 2000;19(11):2399–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kishimoto T IL-6: from laboratory to bedside. Clin Rev Allergy Immunol. 2005;28(3):177–186. [DOI] [PubMed] [Google Scholar]
- 68.http://www.wikipedia.com. Accessed October 14, 2009.
- 69.Zvonic S, Baugh JE Jr, Arbour-Reily P, Mynatt RL, Stephens JM. Cross-talk among gp130 cytokines in adipocytes. J Biol Chem. 2005;280(40):33856–33863. [DOI] [PubMed] [Google Scholar]
- 70.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Müller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signaling and its regulation. Biochem J. 2003;374(pt 1): 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pathan N, Williams EJ, Oragui EE, Stephens AC, Levin M. Changes in the interleukin-6/soluble interleukin-6 receptor axis in meningococcal septic shock. Crit Care Med. 2005;33(8):1839–1844. [DOI] [PubMed] [Google Scholar]
- 72.Rose-John S Interleukin-6 biology is coordinated by membrane bound and soluble receptors. Acta Biochim Pol. 2003;50(3): 603–611. [PubMed] [Google Scholar]
- 73.Rose-John S, Scheller J, Elson G, Jones SA. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J Leukoc Biol. 2006;80(2):227–236. [DOI] [PubMed] [Google Scholar]
- 74.Fitzgerald K, O’Neill L, Gearing A, Callard R. Interleukin-6. The cytokine factsbook. London, UK: Academic Press; 2001:69–72. [Google Scholar]
- 75.Walev I, Vollmer P, Palmer M, Bhakdi S, Rose-John S. Pore-forming toxins trigger shedding of receptors for interleukin-6 and lipopolysaccharide. Proc Natl Acad Sci USA. 1996;93(15): 7882–7887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Taga T, Hibi M, Hirata Y, et al. Interleukin-6 triggers the association of its receptor with a possible signal transducer, gp130. Cell. 1989;58(3):573–581. [DOI] [PubMed] [Google Scholar]
- 77.Nakajima T, Yamamoto S, Cheng M, et al. Soluble interleukin-6 receptor is released from receptor-bearing cell lines in vitro. Jpn J Cancer Res. 1992;83(4):373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jones SA, Horiuchi S, Topley N, Yamamoto N, Fuller GM. The soluble interleukin-6 receptor: mechanisms of production and implications in disease. FASEB J. 2001;15(1):43–58. [DOI] [PubMed] [Google Scholar]
- 79.Campo S, Serlupi-Crescenzi O, Arseni B, et al. Comparative activity of Sant7 and anti-IL-6, IL-6R monoclonal antibodies in a murine model of B-cell lymphoma. Cytokine. 2005;31(5): 368–374. [DOI] [PubMed] [Google Scholar]
- 80.Acalovschi D, Wiest T, Hartmann M, et al. Multiple levels of regulation of the interleukin-6 system in stroke. Stroke. 2003;34(8): 1864–1869. [DOI] [PubMed] [Google Scholar]
- 81.Boulanger MJ, Chow DC, Brevnova EE, Garcia KC. Hexameric structure and assembly of the interleukin-6/IL-6 alpha-receptor/gp130 complex. Science. 2003;300(5628):2101–2104. Erratum in Science. 2003;301:918.. [DOI] [PubMed] [Google Scholar]
- 82.Diveu C, Lak-Hal AH, Froger J, et al. Predominant expression of the long isoform of GP130-like (GPL) receptor is required for interleukin-31 signaling. Eur Cytokine Netw. 2004;15(4):291–302. [PubMed] [Google Scholar]
- 83.http://www.ncbi.nlm.nih.gov/gene. Accessed September 14, 2010.
- 84.Corbi P, Rahmati M, Delwail A, et al. Circulating soluble gp130, soluble IL-6R, and IL-6 in patients undergoing cardiac surgery, with or without extracorporeal circulation. Eur J Cardiothorac Surg. 2000;18(1):98–103. [DOI] [PubMed] [Google Scholar]
- 85.Andrejko KM, Raj NR, Kim PK, Cereda M, Deutschman CS. IL-6 modulates sepsis-induced decreases in transcription of hepatic organic anion and bile acid transporters. Shock. 2008;29(4): 490–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hirano T, Ishihara K, Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene. 2000;19(21): 2548–2556. [DOI] [PubMed] [Google Scholar]
- 87.Fielding CA, McLoughlin RM, McLeod L, et al. IL-6 regulates neutrophil trafficking during acute inflammation via STAT3. J Immunol. 2008;181(3):2189–2195. [DOI] [PubMed] [Google Scholar]
- 88.Sehgal PB. Paradigm shifts in the cell biology of STAT signaling. Semin Cell Dev Biol. 2008;19(4):329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sehgal PB. Plasma membrane rafts and chaperones in cytokine/STAT signaling. Acta Biochim Pol. 2003;50(3):583–594. [PubMed] [Google Scholar]
- 90.Shah M, Patel K, Fried VA, Sehgal PB. Interactions of STAT3 with caveolin-1 and heat shock protein 90 in plasma membrane raft and cytosolic complexes. Preservation of cytokine signaling during fever. J Biol Chem. 2002;277(47):45662–45669. [DOI] [PubMed] [Google Scholar]
- 91.Kushner I, Rzewnicki D, Samols D. What does minor elevation of C-reactive protein signify? Am J Med. 2006;119(2):166.e17–166.e28. [DOI] [PubMed] [Google Scholar]
- 92.Billiar TR, Curran RD, Williams DL, Kispert PH. Liver nonparenchymal cells are stimulated to provide interleukin-6 for induction of the hepatic acute-phase response in endotoxemia but not in remote localized inflammation. Arch Surg. 1992;127(1): 31–36. [DOI] [PubMed] [Google Scholar]
- 93.Derhaschnig U, Bergmair D, Marsik C, Schlifke I, Wijdenes J, Jilma B. Effect of interleukin-6 blockade on tissue factor-induced coagulation in human endotoxemia. Crit Care Med. 2004;32(5): 1136–1140. Erratum in Crit Care Med. 2004;32(8): 1813. [DOI] [PubMed] [Google Scholar]