Abstract
Objective:
To evaluate elvitegravir and cobicistat pharmacokinetics during pregnancy compared to postpartum and in infant washout samples after delivery.
Design:
Nonrandomized, open-label, parallel-group, multi-center phase-IV prospective study of antiretroviral pharmacokinetics in HIV-infected pregnant women and their children in the U.S.
Methods:
Intensive steady-state 24 hour pharmacokinetic profiles after 150 mg of elvitegravir and 150 mg of cobicistat given orally in fixed dose combination once-daily were performed during the second trimester, third trimester, and postpartum. Infant washout samples were collected after birth. Elvitegravir and cobicistat were measured in plasma by a validated LC-MS/MS assay with a lower quantitation limit of 10 ng/mL. A two-tailed Wilcoxon signed-rank test (α = 0.10) was employed for paired within-participant comparisons.
Results:
Thirty pregnant women taking elvitegravir and cobicistat once-daily enrolled in the study. Compared to paired postpartum data, elvitegravir AUC0–24 was 24% lower in the second trimester (n=14, P=0.058, GMR=0.76, 90% CI 0.57–1.0) and 44% lower in the third trimester (n=24, P=0.0001, GMR=0.56, 90% CI 0.42–0.73), while cobicistat AUC0–24 was 44% lower in the second trimester (n=14, P=0.0085, GMR=0.56, 90% CI 0.37 – 0.85) and 59% lower in the third trimester (n=24, p<.0001, GMR=0.41, 90% CI 0.30 – 0.57). Median cord blood elvitegravir concentration was 540.6 ng/mL and the median ratio of cord blood to maternal plasma elvitegravir concentrations was 0.91.
Conclusions:
Standard elvitegravir and cobicistat dosing during pregnancy results in significantly lower exposure which may increase the risk of virologic failure and mother-to-child transmission. Additional studies are needed to optimize elvitegravir and cobicistat dosing regimens in pregnant women.
Keywords: Elvitegravir, cobicistat, integrase inhibitor, HIV infection, pharmacokinetics, perinatal transmission, pregnancy
Introduction
Integrase strand transfer inhibitors (INSTI) are a potent class of antiretrovirals which target the HIV integrase enzyme and block incorporation of viral HIV-1 DNA into the host cell genome. INSTIs are currently recommended as first-line treatment for antiretroviral-naïve adults and children in the U.S. living with HIV.[1, 2] While safe and effective antiretroviral treatment options continue to increase, data on newer agents for use in pregnant women remain sparse.
Antiretroviral treatment is widely used for HIV-infected pregnant women both as primary treatment of maternal HIV infection and to prevent mother-to-child HIV transmission. Physiological changes during pregnancy have a substantial impact on drug disposition which may affect exposure to antiretrovirals and subsequently dosing requirements.[3, 4] For example, drugs metabolized by the cytochrome P450 3A (CYP3A) enzyme family, such as protease inhibitors, often show decreased exposure during pregnancy.[5–8] Exposure to other antiretrovirals during pregnancy is generally decreased to a lesser extent, with the exception of etravirine exposure which is increased during pregnancy.[9] Lower antiretroviral exposure increases the risk of inadequate maternal suppression of HIV replication and transmission of HIV to the infant, while increased drug exposure may subject mother and child to increased risk of drug toxicities. [10]
Elvitegravir is a second generation INSTI indicated for HIV-1 infection in combination with other antiretroviral drugs. Elvitegravir is primarily metabolized by CYP3A-mediated metabolism with additional glucuronidation of the hydroxylated metabolite by UGT1A1 and UGT1A3.[11] In clinical practice, elvitegravir is coadministered with the pharmacokinetic enhancer cobicistat, a potent mechanism-based inhibitor of CYP3A, in order to increase elvitegravir exposure and facilitate once-daily dosing. Cobicistat is primarily metabolized by CYP3A and to a minor extent by CYP2D6.[12] Elvitegravir is available in two fixed-dose combination (FDC) tablets containing elvitegravir 150 mg, cobicistat 150 mg, emtricitabine 200 mg, and either tenofovir disoproxil fumarate (TDF) 300 mg or tenofovir alafenamide (TAF) 10 mg.
Two prior case reports have described elvitegravir and cobicistat pharmacokinetics during pregnancy and postpartum in individual patients. In both cases, evitegravir and cobicistat exposure were considerably lower during pregnancy than reference values in non-pregnant patients.[13, 14] A separate study in pregnant women taking elvitegravir as part of a FDC showed detectable elvitegravir concentrations in cord blood and placental cells suggesting transfer to the fetal circulation.[15] The Panel on Treatment of Pregnant Women with HIV Infection and Prevention of Perinatal Transmission, convened by the Office of AIDS Research Advisory Committee (OARAC) and supported by the Health Resources and Services Administration (HRSA) and the National Institutes of Health (NIH), currently classifies elvitegravir/cobicistat as “Not Recommended for Initial Use in Pregnancy” based upon preliminary data showing inadequate levels of both drugs during the 2nd and 3rd trimester as well as viral breakthroughs.[10] The primary objective of this study was to characterize the pharmacokinetics of elvitegravir and cobicistat during pregnancy and postpartum in HIV-1 infected women.
Methods
Study population and design
IMPAACT P1026s “Pharmacokinetic Properties of Antiretroviral and Related Drugs during Pregnancy and Postpartum” (ClinicalTrials.gov NCT00042289), is an ongoing non-randomized, open-label, parallel-group, multi-center, phase IV prospective study. The study recruited pregnant HIV-infected women ≥ 20 weeks gestation receiving elvitegravir and cobicistat in combination with emtricitabine and tenofovir disoproxil fumarate (Stribild®, Gilead Sciences, Inc.) or tenofovir alafenamide (Genvoya ®, Gilead Sciences, Inc.) once daily prescribed for clinical care. All participants were from the United States. Participants had to be stable on their antiretroviral regimen for two weeks and intend to continue the same regimen through 6–12 weeks postpartum. Maternal exclusion criteria were multiple gestation, a clinical or laboratory toxicity necessitating a medication change during the study, and the use of specific medications known to interact with elvitegravir or cobicistat.
Each study site received ethical and local institutional review board approval. All participants gave informed consent prior to study participation. Medications were prescribed by each participant’s clinical care provider. Pharmacokinetic sampling was performed during the second trimester, third trimester, and postpartum. Collected samples were assayed in real time and results reported to each study participant and her clinician.
Infant enrollment occurred immediately after maternal enrollment with maternal consent, with eligibility confirmed at birth. Infant inclusion criteria were birth weight >1,000g, singleton delivery and maternal enrollment in P1026s. Infant exclusion criteria were a severe congenital malformation or medical condition that would interfere with study participation as deemed by site clinicians and use of specific medications known to interfere with elvitegravir disposition.
Clinical and laboratory monitoring
Each study visit included monitoring of HIV-1 RNA, CD4+ lymphocyte cell count, hematology, and serum biochemistry. The lower limit of detection for HIV-1 RNA assays performed locally ranged from 50–400 copies/mL. All infants received physical examinations after birth and laboratory evaluations were performed if clinically indicated. Adverse events were reported at each study visit and management was determined by each participant’s clinician. The National Institute of Allergy and Infectious Diseases (NIAID), Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events was used to grade adverse event severity.
Sample collection
Intensive 24-hour pharmacokinetic evaluations were performed during the second trimester (20–26 weeks gestation), third trimester (30–38 weeks gestation) and postpartum (6–12 weeks following delivery). Requirements prior to pharmacokinetic sampling were self-reported elvitegravir and cobicistat adherence for two weeks and consistent dosing times for the last three days. Elvitegravir and cobicistat were dosed with food. On sampling days the pre-dose sample was drawn and study medications were administered under observation. Post-dose samples were drawn at 1, 2, 4, 6, 8, 12 and 24 hours. At delivery, cord blood and maternal plasma samples were collected when possible. In infants, four plasma samples were collected at 2–10 hours, 18–28 hours, 36–72 hours and 5–9 days after birth.
Elvitegravir and cobicistat plasma concentration measurements
Plasma samples were analyzed at the IMPAACT Pharmacology Support Laboratory at the University of Alabama at Birmingham. The laboratory adheres to Clinical Laboratory Improvement Amendments (CLIA) and performs standardized inter- laboratory testing through the AIDS Clinical Trial Group (ACTG) clinical pharmacology quality assurance and quality control program. Quantitative determination of elvitegravir and cobicistat in human plasma was accomplished by the use of protein precipitation and high-performance liquid chromatography with tandem mass spectrometry detection (LC-MS/MS). Labeled elvitegravir (2H6) and cobicistat (2H8) are used as the internal standards (IS), respectively. Elvitegravir, cobicistat and IS are extracted from 20 μL of human plasma using protein precipitation with acetonitrile. Extracts are analyzed by reverse-phase chromatography using a Waters xBridge C18 column under isocratic conditions at a flow rate of 400 μL/minute. The column temperature is maintained at 30°C. The mobile phase A consists of 5 mM ammonium bicarbonate in water and mobile phase B is acetonitrile. A triple quadrupole mass spectrometer (AB Sciex 5500) equipped with TurboV IonSpray® operating in positive-ion mode is used. Column effluents are analyzed by multiple reaction monitoring (MRM). The precursor/product transitions are 449.1→345.0 m/z for elvitegravir, 455.2→351.3 m/z for elvitegravir IS, 777.2→98.0 m/z for cobicistat and 784.5→614.3 m/z for cobicistat IS. The calibration curve is fit using weighted (1/x2) linear regression analysis of the elvitegravir/IS or cobicistat/IS peak area ratio versus the respective elvitegravir or cobicistat concentration from 10.00–5,000 ng/mL. Concentrations of incurred and quality control samples are calculated with the same regression analysis.
Pharmacokinetic analyses
Elvitegravir and cobicistat maximum, minimum, and last plasma concentrations (Cmax, Cmin, C24) along with corresponding time points (Tmax, Tmin) were observed directly. Steady-state area under the concentration versus time curve from time 0 to 24 hours post-dose (AUC0–24) was estimated with the trapezoidal rule. Half-life (t1⁄2) was calculated as 0.693/λz where λz is the elimination rate constant derived from the terminal slope of the log concentration versus time curve. For participants with pre-dose concentrations below the assay quantification limit, single-dose AUC from time 0 to infinity was estimated as AUC0–24 plus the C24 divided by λz. Apparent oral clearance (CL/F) was calculated as dose divided by AUC0–24. Undetectable concentrations were set at half the lower limit of quantification to calculate summary statistics. Absorption lags were defined as 1-hour post-dose concentrations that were lower than observed pre-dose concentrations. The minimum exposure target for elvitegravir was the 10th percentile AUC0–24 in non-pregnant HIV infected patients (16100 ng*hr/mL), which was estimated from published pharmacokinetic parameters.[16]
Statistical analyses
The target sample size was 25 women with evaluable third trimester pharmacokinetic data, with at least 12 who had evaluable second trimester pharmacokinetic data. Each individual woman’s elvitegravir exposure during pregnancy was determined in real time, compared with the 50th and 10th percentile AUCs estimated for non-pregnant adult historical controls, and reported to each participant’s care provider.
Descriptive statistics were calculated for pharmacokinetic parameters during each study period. Pharmacokinetic parameters during the second trimester versus postpartum and during the third trimester versus postpartum were compared at the within-participant level using the Wilcoxon signed-rank test, with a two-sided P≤0.10 considered statistically significant. Within-participant geometric mean ratios (GMR) and 90% confidence intervals (CI) for pharmacokinetic parameters in the pregnant versus non-pregnant conditions were calculated for elvitegravir and cobicisistat to estimate the range of percentage changes between the two conditions that would be consistent with the observed data and assess clinical importance, to inform dosing recommendations. The body weights of participants who met or did not meet the elvitegravir AUC0–24 target in each study period were compared using the Mann-Whitney U test. Elvitegravir AUC0–24 and C24 in participants with or without viral suppression, defined in this study as HIV-1 RNA ≤ 50 copies/mL, were also compared using the Mann-Whitney U test.
Results
Participant Characteristics
Thirty pregnant women taking elvitegravir and cobicistat once-daily enrolled in the study. Paired pregnancy and postpartum data were available for 14 of 17 women who had second trimester visits and for 24 of 26 women who had third trimester visits. Two mothers withdrew from the study before delivery and follow up is available for 28 infants. Maternal and infant clinical characteristics are summarized in Table 1.
Table 1.
Clinical Characteristics
| N (%) or Median (Range) | |
|---|---|
| Maternal Demographics (n = 30) | |
| Age at Delivery (years) | 32.3 (19.5 – 47.8) |
| Weight at Delivery (kg) | 86.3 (57.9 – 131.8) |
| Race/Ethnicity - White; Black; Hispanic; Asian, Pacific Islander | 3 (10%); 20 (67%); 6 (20%); 1 (3%) |
| Concomitant ARVs 2T PK visit: FTC; ZDV; MVC; TDF; TAF | 17 (100%); 2 (12%); 1 (6%); 16 (94%); 1 (6%) |
| Concomitant ARVs 3T PK visit: FTC; ZDV; MVC; TDF; TAF | 26 (100%); 3 (12%); 1 (4%); 26 (100%); 0 (0%) |
| Country: United States | 30 (100%) |
| 2T: HIV-1 RNA ≤ 50 copies/mL | 13/17 (77%) |
| 2T: CD4 (cells/mm3) | 678 (253 – 1267) |
| 3T: HIV-1 RNA ≤ 50 copies/mL | 24/26 (92%) |
| 3T: CD4 (cells/mm3) | 719 (145 – 1285) |
| Delivery: HIV-1 RNA ≤ 50 copies/mL | 19/25 (76%) |
| Delivery: CD4 (cells/mm3) | 616.5 (129 – 1590) |
| PP: HIV-1 RNA ≤ 50 copies/mL | 19/25 (76%) |
| PP: CD4 (cells/mm3) | 945 (245 – 1829) |
| Infant Demographics (n=28)* | |
| Gestational Age (weeks) | 38.8 (34.6 – 41.3) |
| Birth Weight (grams) | 3060.5 (1885 – 4050) |
| Infection Status: Uninfected; Indeterminate; Pending |
25 (89%); 2 (7%); 1 (4%) |
Two mothers went off study before delivery; 2T, second trimester; 3T, third trimester; PP, postpartum
Elvitegravir Pharmacokinetics
Median (IQR) elvitegravir AUC0–24 in the second trimester, third trimester and postpartum periods were 15283 ng*hr/mL (11939 – 19038), 14004 ng*hr/mL (9119– 18798) and 21039 ng*hr/mL (13532 –32788), respectively. Compared to paired postpartum data, elvitegravir AUC0–24 was 24% lower in the second trimester (n=14, P=0.06, GMR=0.76, 90% CI 0.57–1.0) and 44% lower in the third trimester (n=24, P=0.0001, GMR=0.56, 90% CI 0.42–0.73) (Table 2, Figure 1). The frequency of participants meeting the target AUC0–24 of 16100 ng*hr/mL was 8/17 (47%) in the second trimester, 10/26 (38%) in the third trimester, and 18/25 (72%) postpartum. The median (IQR) body weight of participants who met the target AUC0–24 was 72.2 kg (66.3 – 77.8) in the second trimester, 76.8 kg (73.8 – 84.4) in the third trimester, and 72.9 kg (66.9 – 85.3) postpartum. In those participants who did not meet the target AUC0–24, the median (IQR) body weight was 82.1 kg (76.0 – 100.2) in the second trimester (P=0.21 compared to second trimester body weight of participants who met the target AUC0–24), 90.2 kg (79.0 – 104.8) in the third trimester (P=0.04 compared to third trimester body weight of participants who met the target AUC0–24), and 85.3 kg (74.0 – 91.8) postpartum (P=0.30 compared to postpartum body weight of participants who met the target AUC0–24).
Table 2.
Maternal Elvitegravir Pharmacokinetic Parameters, Median (IQR)
| Parameter | Second Trimester | Third Trimester | Postpartum | Non-pregnant HIV-Infected Adult | GMR2 (90% CI) | GMR2 (90% CI) |
|---|---|---|---|---|---|---|
| n = 17 | n = 26 | n = 25 | Subjects1 | 2T/PP, n=14 | 3T/PP, n=24 | |
| AUC0–24 (ng*hr/mL) | 15283 (11939 – 19038) | 14004 (9119– 18798) | 21039 (13532 –32788) | 23000 (7500) | 0.76 (0.57–1.0)* | 0.56 (0.42–0.73)* |
| C0 (ng/mL) | 18 (11.6 – 188.3) | 25.1 (5.0 – 69.7) | 228.5 (85.9 – 847.9) | - | 0.2 (0.10–0.41)* | 0.14 (0.08–0.26)* |
| Cmax (ng/mL) | 1447.1 (1133.6 – 1579.0) | 1432.8 (705.7– 1570.4) | 1713.1 (955.7– 2284.6) | 1700 (400) | 0.92 (0.71–1.2) | 0.72 (0.55–0.93)* |
| Tmax (hr) | 4 (2 – 4) | 4 (2 – 6) | 4 (2 – 6) | - | - | - |
| C24 (ng/mL) | 25.8 (17.9 – 67.2) | 48.7 (14.0– 75.1) | 377.1 (228.5– 568.8) | - | 0.19 (0.10–0.35)* | 0.11 (0.081–0.15)* |
| Cmin (ng/mL) | 16.8 (10.9 – 21.8) | 18.3 (5.0 – 61.3) | 185.6 (48.5– 377.1) | 450 (260) | 0.18 (0.098–0.33)* | 0.14 (0.09–0.23)* |
| CL/F (L/hr) | 9.8 (7.9 – 12.6) | 10.7 (8.0 – 16.4) | 7.1 (4.6 – 11.1) | - | 1.3 (0.99–1.8) | 1.8 (1.4–2.4)* |
| T1/2 (hr) | 3.1 (2.6 – 3.9) | 3.4 (2.7 – 4.7) | 8.8 (7.0 – 13.2) | - | 0.38 (0.26–0.54)* | 0.38 (0.30–0.48)* |
p<0.10 compared to postpartum
Historical data from STRIBILD® (elvitegravir, cobicistat, emtricitabine, tenofovir disoproxil fumarate) package insert, represented as geometric mean (S.D.)
Paired comparisons
2T, second trimester; 3T, third trimester; PP, postpartum
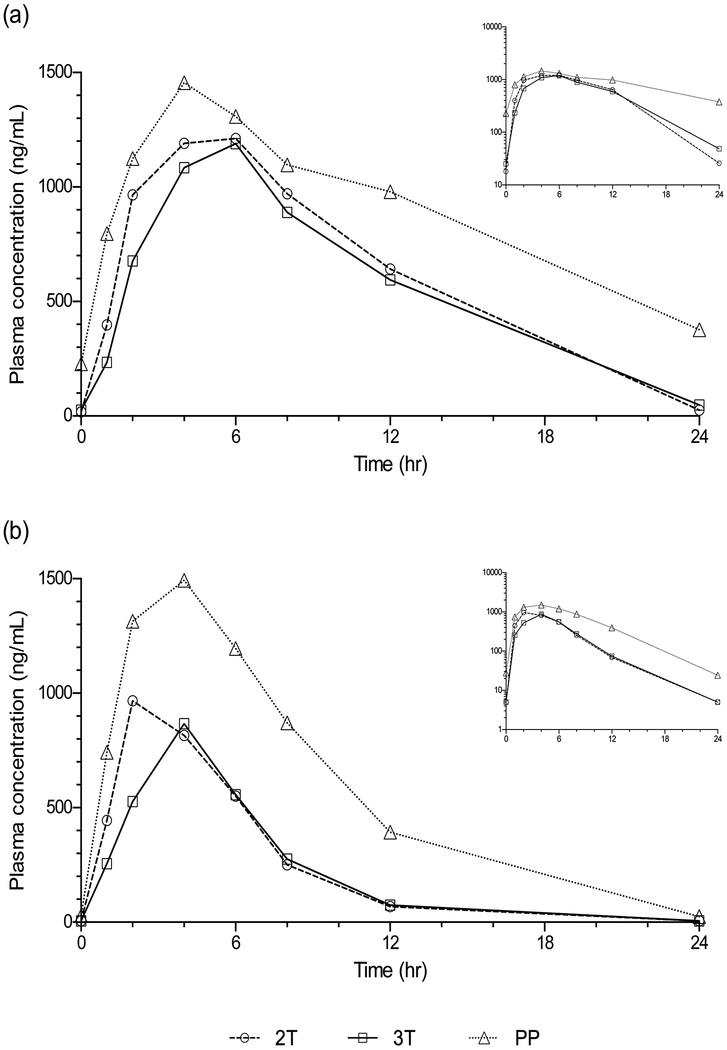
Figure 1.
Maternal median plasma concentration versus time profiles for (a) elvitegravir and (b) cobicistat. Inset displays data plotted on a semilog scale.
Elvitegravir Cmax was not significantly different in the second trimester compared to paired postpartum data but was 28% lower in the third trimester (P=0.36 and 0.02, respectively). Elvitegravir C24 was 81% lower in the second trimester and 89% lower in the third trimester compared to paired postpartum data (P=0.009 and P=0.0001, respectively). Lags in absorption were seen in 4/17 (24%), 3/26 (12%) and 7/25 (28%) women in the second trimester, third trimester, and postpartum, respectively.
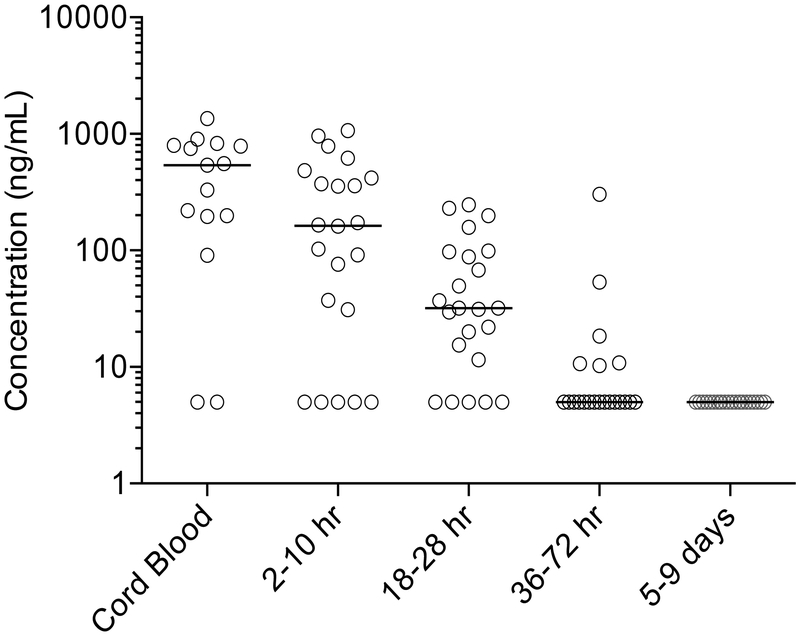
Median (IQR) concentrations of elvitegravir in cord blood and maternal plasma at delivery were 540.6 ng/mL (197.1–792.4, n=15) and 543.0 ng/mL (66.95–976.35, n=15, respectively. The ratio of cord blood to maternal plasma was 0.91 (0.65–1.03). In infants after birth, the median (IQR) maximum observed plasma concentration was 161 ng/mL (31.0 – 373.5). Infant elvitegravir plasma concentrations post-delivery are displayed in Figure 2. No plasma samples had measurable elvitegravir concentrations (>10 ng/mL) at the final washout sample (between 5–9 days of life). The median (IQR) elvitegravir half-life in infants was 7.6 (6.3 – 10.2) hours.
Figure 2.
Scatter plot of individual infant elvitegravir plasma concentrations in cord blood and post-delivery. The horizontal line represents the median concentration at each time point. No plasma samples had quantifiable elvitegravir concentrations at the final washout sample (between 5–9 days of life). Samples below the lower limit of quantitation of the assay (LLOQ; 10 ng/mL) are shown as 1/2 LLOQ (5 ng/mL).
Cobicistat Pharmacokinetics
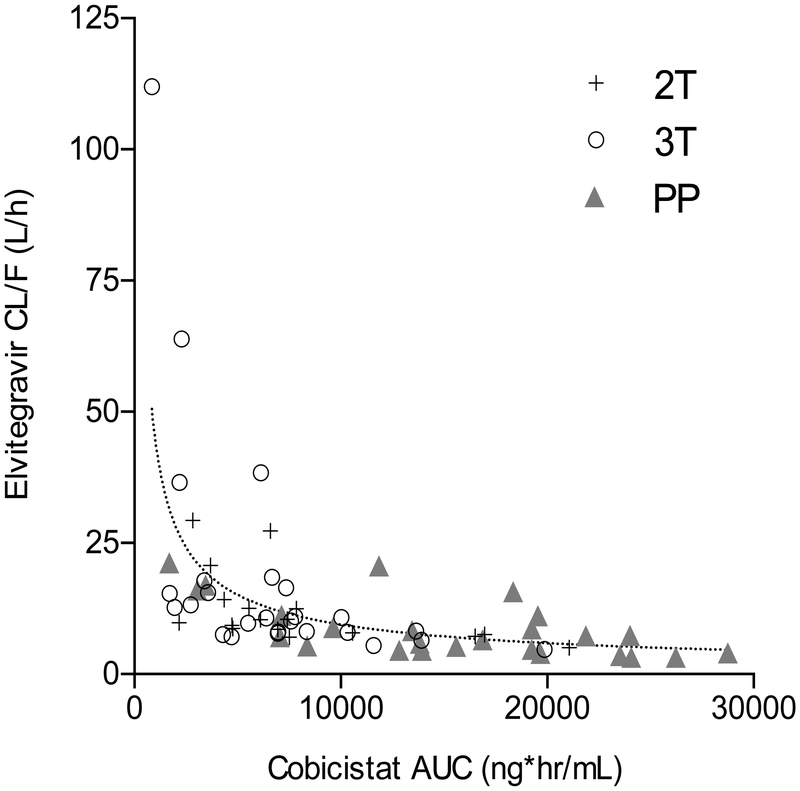
Median (IQR) cobicistat AUC0–24 in the second trimester, third trimester and postpartum periods were 6599 ng*hr/mL (4725 – 7840), 6530 ng*hr/mL (3399– 8357) and 15593 ng*hr/mL (9638 –19667), respectively. Compared to paired postpartum data, cobicistat AUC0–24 was 44% lower in the second trimester (n=14, P=0.009, GMR=0.56, 90% CI 0.37 – 0.85) and 59% lower in the third trimester (n=24, p<.0001, GMR=0.41, 90% CI 0.30 – 0.57) (Table 3, Figure 1). Cobicistat AUC was negatively associated with elvitegravir apparent oral clearance (CL/F) (Figure 3).
Table 3.
Maternal Cobicistat Pharmacokinetic Parameters, Median (IQR)
| Parameter | Second Trimester | Third Trimester | Postpartum | Non-pregnant HIV-Infected Adult | GMR2 (90% CI) | GMR2 (90% CI) |
|---|---|---|---|---|---|---|
| n = 17 | n = 26 | n = 25 | Subjects1 | 2T/PP, n=14 | 3T/PP, n=24 | |
| AUC0–24 (ng*hr/mL) | 6599 (4725 – 7840) | 6530 (3399– 8357) | 15593 (9638 –19667) | 8300 (3800) | 0.56 (0.37–0.85)* | 0.41 (0.30–0.57)* |
| C0 (ng/mL) | <10 (<10 – 19.2) | <10 (<10 – 10.7) | 27.8 (10.3– 121.6) | - | 0.34 (0.17–0.69)* | 0.20 (0.12–0.35)* |
| Cmax (ng/mL) | 967.3 (861.0– 1578.1) | 1150.0 (666.1– 1483.0) | 1784.4 (1195.9 – 2612.8) | 1100 (400) | 0.72 (0.49–1.1)* | 0.62 (0.46–0.84)* |
| Tmax (hr) | 2 (2 – 4) | 2 (1 – 4) | 2 (2 – 4) | - | - | - |
| C24 (ng/mL) | <10 (<10 – <10) | <10 (<10 – <10) | 24.3 (12.4– 57.0) | - | 0.4 (0.22–0.73)* | 0.24 (0.17–0.34)* |
| Cmin (ng/mL) | <10 (<10 – <10) | <10 (<10 – <10) | 15.6 (<10 – 44.5) | 50 (130) | 0.39 (0.21–0.7)* | 0.33 (0.23–0.47)* |
| CL/F (L/hr) | 22.7 (19.1 – 31.7) | 23.0 (17.9 – 44.1) | 9.6 (7.6 – 15.6) | - | 1.8 (1.2–2.7)* | 2.4 (1.8–3.3)* |
| T1/2 (hr) | 2.0 (1.8 – 2.5) | 2.6 (1.8 – 3.2) | 3.0 (2.8 – 3.9) | - | 0.65 (0.56–0.76)* | 0.79 (0.66–0.94)* |
p<0.10 compared to postpartum
Historical data from STRIBILD® (elvitegravir, cobicistat, emtricitabine, tenofovir disoproxil fumarate) package insert, represented as geometric mean (S.D.)
Paired comparisons
2T, second trimester; 3T, third trimester; PP, postpartum
Figure 3.
Elvitegravir apparent oral clearance (CL/F) versus cobicistat AUC. The dotted line represents the combined data fit to a non-linear power model. 2T, second trimester; 3T, third trimester; PP, postpartum.
Cobicistat Cmax was 28% lower in the second trimester and 38% lower in the third trimester compared to paired postpartum data (p=0.08 and p=0.02, respectively). Cobicistat C24 was 60% lower in the second trimester (n=14, GMR=0.401, 90% CI 0.22 – 0.73) and 76% lower in the third trimester compared to paired postpartum data (n=24, GMR=0.24, 90% CI 0.17– 0.34) (p=0.05 and p<0.0001, respectively). Lags in absorption were seen in 1/17 (6%), 2/26 (8%) and 3/25 (12%) women in the second trimester, third trimester, and postpartum. Pre-dose concentrations below the quantitation limit were observed in 11/17 (65%), 19/26 (73%) and 6/25 (24%) of women in the second trimester, third trimester, and postpartum.
Cord blood samples were obtained from 15 women at delivery. Of those, cobicistat concentrations were below the quantitation limit in 8 samples. Of the remaining samples, cobicistat concentrations in cord blood ranged between 11.1 and 110.6 ng/mL with a median of 35.3 ng/mL. The median concentration of cobicistat in maternal plasma at delivery was 172.4 ng/mL. A total of 7 women had cord blood and maternal plasma at delivery obtained with cobicistat concentrations above the limit of quantitation in each. In those participants, the median ratio (IQR) of cord blood to maternal plasma was 0.09 (0.05–0.12). Cobicistat was not detected in any neonatal plasma samples after birth.
Maternal and Infant Outcomes
Preterm labor, classified as possibly treatment related, occurred in one woman.
Congenital anomalies that were classified as possibly treatment related occurred in 2/26 infants: one infant with amniotic band syndrome, microcephaly, and intrauterine growth restriction and one infant with ulnar postaxial polydactyly.
The percentage of women with suppression of HIV replication (viral suppression, defined in this study as HIV-1 RNA ≤ 50 copies/mL), at the second trimester, third trimester, delivery, and postpartum was 76.5%, 92.3%, 76%, and 76%, respectively. No correlation was observed between viral suppression and elvitegravir exposure.
Discussion
Increasing use of INSTIs as first-line treatment regimens for adults living with HIV, particularly in resource-rich settings, will lead to more pregnancies occurring in women on elvitegravir and cobicistat-containing regimens. This study is the first to analyze the pharmacokinetics of elvitegravir boosted with cobicistat in a large population of pregnant HIV-infected women receiving elvitegravir and cobicistat in combination with emtricitabine and tenofovir disoproxil fumarate (STRIBILD®, Gilead Sciences, Inc.) or tenofovir alafenamide (GENVOYA ®, Gilead Sciences, Inc.). Tenofovir alafenamide has not been extensively studied during pregnancy and is not currently recommended for initiation in pregnant women in the current guidelines by the Panel on Treatment of Pregnant Women with HIV Infection and Prevention of Perinatal Transmission.[10] Although women in this study may have received either tenofovir disoproxil fumarate or tenofovir alafenamide, neither drug is expected to alter the pharmacokinetics of elvitegravir or cobicistat.[17, 18]
Compared to paired postpartum data, elvitegravir AUC0–24 was 24% lower in the second trimester and 44% lower in the third trimester, while C24 was 81% lower in the second trimester and 89% lower in the third trimester. Postpartum elvitegravir exposure at 6–12 weeks following delivery was comparable to that of non-pregnant adults (Table 2). Subtherapeutic antiretroviral exposure during pregnancy may increase the risk of virologic failure in the mother and mother-to-child HIV transmission. In this study, sub-therapeutic elvitegravir exposure was defined as less than the 10th percentile elvitegravir AUC0–24 (16100 ng*hr/mL) of non-pregnant adults.[16] Fewer participants met this minimum threshold during pregnancy (47% in second trimester; 38% in third trimester) compared to postpartum (72%).
Prior analyses of the relationship between elvitegravir exposure and virologic outcome have been performed using data from Phase III trials conducted during drug development. In 373 treatment-naïve adult participants administered elvitregravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate fixed-dose combination, a relatively “flat” relationship was observed between elvitegravir exposure (C0 and AUC0–24) and the percentage of participants achieving virologic success (viral load < 50 HIV-1 RNA copies/mL).[19] However, the median C0 in the lowest decile (156 ng/mL) was well above the C0 observed in this study during the second trimester (18 ng/mL) and third trimester (25.1 ng/mL) of pregnancy.
Although FDA bioequivalence criteria typically include evaluation of only AUC and Cmax, maintaining high trough concentrations is critically important for INSTIs as these agents display antiviral activity only when their plasma concentrations are continually maintained above a minimum threshold. Elvitegravir does not exhibit intracellular persistence associated with NRTIs such as tenofovir and protects cells from viral rechallenge only when concentrations are maintained above the concentration which inhibits viral suppression by 95% (EC95).[11] These in vitro findings are translatable to clinical pharmacokinetic-pharmacodynamic relationships, where elvitegravir Cmin values are more closely correlated with antiviral activity than Cmax or AUC. In exposure-response analyses, Cmin values but not Cmax or AUC were capable of fitting an Emax model, where effect is defined as the log10 maximum change in HIV-1 RNA.[20] Thus, the virologic potency of elvitegravir as a boosted ARV is in part due to the substantially higher Cmin achieved with ritonavir or cobicistat relative to unboosted elvitegravir. Notably, elvitegravir median Cmin concentrations at both the second and third trimesters were below the protein binding-adjusted EC95 of 45 ng/mL.[21] Postpartum AUC and Cmax in this study were similar to previously reported values in non-pregnant adults.
Physiologic changes in pregnancy likely contribute to the observed altered pharmacokinetics of elvitegravir and cobicistat. Increases in blood volume, total body water and body mass can have a dilutional effect on drug concentrations and plasma proteins. Higher production of several hormones, such as progesterone, induce metabolic enzymes, including CYP3A. Changes in GI tract function, including gastric pH and hepatic plasma flow, can affect drug absorption, distribution, metabolism and excretion. The metabolism of both elvitegravir and cobicistat is primarily by CYP3A which is localized in the liver, intestine, uterus, placenta, and elsewhere.[22, 23] Hormones such as placental growth hormone, estrogen, cortisol and progesterone induce a two-fold increase in CYP3A activity.[24] Increases in CYP3A metabolism may contribute to decreased exposure to both drugs during pregnancy. Further, cobicistat is used as a pharmacokinetic enhancer to inhibit CYP3A-mediated metabolism of elvitegravir, thereby increasing elvitegravir systemic exposure. In this study, cobicistat exposure was 44% lower during the second trimester and 59% lower during the third trimester relative to paired postpartum data. The reduction in elvitegravir exposure during pregnancy may result from pregnancy related changes in elvitegravir drug disposition (reduced absorption, increased volume of distribution or increased metabolism) or less cobicistat boosting from lower plasma cobicistat concentrations, or both.
Elvitegravir apparent oral clearance (CL/F) was negatively correlated with cobicistat AUC and this effect was more prounouced in the second trimester and third trimester, where cobicistat AUC0–24 was 44% lower and 59% lower compared to paired postpartum data, respectively. No obvious relationship was seen between elvitegravir half-life and cobicistat exposure, suggesting that the higher CL/F in patients with low cobicistat AUC may be driven in large part by reduced elvitegravir oral bioavailability. Additionally, a prior population pharmacokinetic study of elvitegravir and cobicistat in 144 non-pregnant HIV-1-infected patients estimated that a reduction in cobicistat AUC of 25%, 50% and 75% would reduce elvitegravir exposure by 38.0%, 117.3% and 372.4%, respectively.[25]
Elvitegravir is highly protein-bound in plasma (98–99%), primarily to albumin.[26] The concentration of albumin is decreased in pregnancy. In addition, drug binding to albumin may be displaced by increased hormone binding to this protein during pregnancy. Although the unbound fraction is responsible for pharmacologic activity, unbound concentrations were not measured in this study. Although lower elvitegravir exposure was observed during pregnancy, the therapeutic unbound free fraction during pregnancy is unknown.
This study determined the washout kinetics of elvitegravir transferred in utero across the placenta in infants born to mothers receiving elvitegravir during pregnancy. The median ratio of the elvitegravir concentration in cord blood/maternal plasma at delivery was 0.91, suggesting high placental transfer. The median elimination half-life in 23 infants was 7.54 hours which is similar to that of non-pregnant adults. Cobicistat was not detected above the quantitation limit of the assay in any neonatal plasma samples. A longer elimination half-life would be expected if elvitegravir were to be administered to infants with cobicistat.
Adherence was not directly measured in the study. Pre-dose elvitegravir trough levels (C0) below the lower limit of quantitation (LLOQ) of the assay (10 ng/mL) may be suggestive of non-adherence. In the second trimester, 3/17 women (17.6%) had pre-dose trough concentrations below the LLOQ. In the third trimester and postpartum, 7/26 women (26.9%) and 3/25 women (12%), respectively, had pre-dose trough levels below the LLOQ. However, C24 concentrations below or near the LLOQ were also noted in some women following an observed elvitegravir dose. Overall, the number of study participants who had a pre-dose trough concentration below the LLOQ and a C24 at least twice the LLOQ was 3/17 in the second trimester, 3/26 in the third trimester, and 3/25 postpartum. These results suggest that the observed changes in elvitegravir pharmacokinetics in this study are true pregnancy-related effects and do not represent non-adherence.
A limitation of this study is that recruitment of women already taking elvitegravir and cobicistat may result in a study population that is more likely to respond to the regimen without treatment-limiting adverse effects. This selection bias may overestimate positive outcomes and underestimate adverse events. Another limitation is that meals at the time of dosing were not standardized in terms of size and fat content. Prior studies have shown that, relative to fasting, a light meal (~373 kcal, 20% fat) results in 34% higher elvitegravir exposure (90% CI: 19% – 51%) while a heavy meal (~800 kcal, 50% fat) results in 87% higher elvitegravir exposure (90% CI : 66% – 110%).[11] It is also possible that some participants may not have received their study doses with food. In addition, we do not have data on when participants took their doses relative to prenatal vitamin doses, which contain minerals that may also impair elvitegravir/cobicistat absorption.[11] Finally, infant washout analysis included wide sampling windows with sparse time points.
In light of the reduced elvitegravir exposure observed in this study, higher or more frequent elvitegravir dosing may be required during pregnancy. However, elvitegravir is primarily used as a component of a fixed dose combination tablet which does not provide dosing flexibility. Elvitegravir alone (Vitekta®) was approved by the U.S. Food and Drug Administration (FDA) in 2012 as 85 mg and 150 mg tablets, but the manufacture of these formulations was permanently discontinued in 2016. Another approach to overcoming low elvitegravir exposure would be to increase the cobicistat dose. This option may be preferable as inhibition of CYP3A activity is more likely to maintain higher elvitegravir Cmin concentrations which are important to antiviral activity. Cobicistat is also used primarily as a component of fixed dose combinations yet it is available in an FDA-approved single agent formulation (Tybost®).
To conclude, standard elvitegravir/cobicistat dosing during pregnancy results in significantly lower exposure during pregnancy which may increase the risk of virologic failure and mother-to-child transmission. Additional studies are needed to optimize elvitegravir/cobicistat dosing regimens in pregnant women.
Table 4.
Elvitegravir and Cobicistat Exposure at Delivery and in Infants, Median (IQR)
| At Delivery (n=15) | ||
|---|---|---|
| Elvitegravir | Cobicistat | |
| Cord Blood (ng/mL) | 540.6 (197.1 – 792.4) | <10 (<10 – 29.2) |
| Maternal Plasma (ng/mL) | 543.0 (66.95 – 976.35) | 172.4 (<10 – 319.5) |
| Cord Blood/Maternal Plasma Ratio | 0.91 (0.65 –1.03)1 | 0.09 (0.05 – 0.12)2 |
| Elvitegravir Infant Washout Samples after Delivery (n=27)3,4 | ||
| Maximum observed concentration (ng/mL) | 161.0 (31.0, 373.5) | |
| Concentration (2 – 10 hours, ng/mL) | 131.9 (24.4, 396.0) | |
| Concentration (18 – 28 hours, ng/mL) | 31.9 (11.5, 87.9) | |
| Concentration (36 – 72 hours, ng/mL) | <10 (<10 – 10.5) | |
| Concentration (5 – 9 days, ng/mL) | <10 (<10 – <10) | |
| T1/2 (hr) | 7.6 (6.3, 10.2) | |
Calculated in 12 patients for whom both cord blood and maternal plasma samples were obtained at time of delivery with quantifiable elvitegravir concentrations (> 10 ng/mL).
Calculated in 7 patients for whom both cord blood and maternal plasma samples were obtained at time of delivery with quantifiable cobicistat concentrations (> 10 ng/mL).
Washout samples were obtained from a total of 27 infants. The numbers of infants used to describe the maximum observed concentration and concentrations at 2 – 10 hours, 18 – 28 hours, 36 – 72 hours, and 5 – 9 days were 25, 25, 24, and 23. The elimination haf-life was calculated from concentration data from 17 infants.
Cobicistat was below the limit of quantitation (10 ng/mL) in all infant washout samples after delivery.
Acknowledgements
We thank the study participants and their families. Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH). Additional support was provided by Gilead Sciences, Inc. The content is solely the responsibility of the authors and does not necessarily represent the views of the NIH or Gilead, Sciences, Inc.
Support:
Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH). Additional support was provided by Gilead Sciences, Inc. The content is solely the responsibility of the authors and does not necessarily represent the views of the NIH or Gilead, Sciences, Inc.
Appendix
In addition to the authors, members of the IMPAACT P1026s protocol team include Francesca Aweeka, Michael Basar, Mark Byroads, Nantasak Chotivanich, Diane Costello, Kayla Denson, Lisa M. Frenkel, Kathleen George, Adriane Hernandez, Benjamin Johnston, Rita Patel, Christina Reding, Kittipong Rungruengthanakit and Pra-ornsuda Sukrakanchana.
We thank the participating staff and sites: Texas Children’s Hospital (Mary Paul, MD; Chivon McMullen-Jackson, RN, BSN; Norma Cooper, MA, BSN, ACRN); University of Miami Pediatric Perinatal HIV/AIDS (Charles D. Mitchell, MD; Grace A. Alvarez A, M, MPH; Ernesto Ruiz Valdes, MD); University of California San Diego Mother-Child-Adolescent Program (Andrew D. Hull, MD FRCOG FACOG; Mary Caffery, RN, MSN; Stephen A. Spector, MD); Emory University (Martina L. Badell, MD; Alexis Ahonen, APRN, NP; LaTeshia Thomas-Seaton, APRN, NP); SUNY Stony Brook (Cristina Witzke, MPH; Jennifer Griffin, CNM, NP, MSN; Erin Infanzon); University of Southern California School of Medicine– Los Angeles County Maternal-Child-Adolescent Program (Françoise Kamer, MD; LaShonda Spencer, MD; James Homans, MD); University of Colorado Denver (Jenna Wallace, MSW, CCRP; Torri D. Metz, MD, MS; Kay Kinzie, MSN, FNP); Rush University Cook County Hospital Chicago (Julie Schmidt, MD; Maureen McNichols, RN, MSN, CCRC; Mariam Aziz, MD); Johns Hopkins University (Allison Agwu, MD, ScM, FAAP, FIDSA; Aleisha Collinson-Streng, RN, ACRN; Thuy Anderson, RN, BSN); David Geffen School of Medicine at UCLA (Jaime G. Deville, MD; Carla Janzen, MD, PhD; Michele F. Carter, BS, RN); Bronx-Lebanon Hospital Center (Jenny Gutierrez, MD; Martha Cavallo, NP; Karen Beckerman, MD)
References
- 1.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf.
- 2.Panel on Antiretroviral Therapy and Medical Management of HIV-Infected Children. Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection . Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/pediatricguidelines.pdf. In.
- 3.Costantine MM. Physiologic and pharmacokinetic changes in pregnancy. Front Pharmacol 2014; 5:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol 2015; 39(7):512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mirochnick M, Capparelli E. Pharmacokinetics of antiretrovirals in pregnant women. Clin Pharmacokinet 2004; 43(15):1071–1087. [DOI] [PubMed] [Google Scholar]
- 6.Stek A, Best BM, Wang J, Capparelli EV, Burchett SK, Kreitchmann R, et al. Pharmacokinetics of Once Versus Twice Daily Darunavir in Pregnant HIV-Infected Women. J Acquir Immune Defic Syndr 2015; 70(1):33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stek AM, Mirochnick M, Capparelli E, Best BM, Hu C, Burchett SK, et al. Reduced lopinavir exposure during pregnancy. AIDS 2006; 20(15):1931–1939. [DOI] [PubMed] [Google Scholar]
- 8.Acosta EP, Bardeguez A, Zorrilla CD, Van Dyke R, Hughes MD, Huang S, et al. Pharmacokinetics of saquinavir plus low-dose ritonavir in human immunodeficiency virus-infected pregnant women. Antimicrob Agents Chemother 2004; 48(2):430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mulligan N, Schalkwijk S, Best BM, Colbers A, Wang J, Capparelli EV, et al. Etravirine Pharmacokinetics in HIV-Infected Pregnant Women. Front Pharmacol 2016; 7:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panel on Treatment of Pregnant Women with HIV Infection and Prevention of Perinatal Transmission. Recommendations for Use of Antiretroviral Drugs in Transmission in the United States. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf. Accessed May 24, 2018. [Google Scholar]
- 11.Ramanathan S, Mathias AA, German P, Kearney BP. Clinical pharmacokinetic and pharmacodynamic profile of the HIV integrase inhibitor elvitegravir. Clin Pharmacokinet 2011; 50(4):229–244. [DOI] [PubMed] [Google Scholar]
- 12.Deeks ED. Cobicistat: a review of its use as a pharmacokinetic enhancer of atazanavir and darunavir in patients with HIV-1 infection. Drugs 2014; 74(2):195–206. [DOI] [PubMed] [Google Scholar]
- 13.Marzolini C, Decosterd L, Winterfeld U, Tissot F, Francini K, Buclin T, et al. Free and total plasma concentrations of elvitegravir/cobicistat during pregnancy and postpartum: a case report. Br J Clin Pharmacol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schalkwijk S, Colbers A, Konopnicki D, Greupink R, Russel FG, Burger D, et al. First reported use of elvitegravir and cobicistat during pregnancy. AIDS 2016; 30(5):807–808. [DOI] [PubMed] [Google Scholar]
- 15.Rimawi BH, Johnson E, Rajakumar A, Tao S, Jiang Y, Gillespie S, et al. Pharmacokinetics and Placental Transfer of Elvitegravir, Dolutegravir, and Other Antiretrovirals during Pregnancy. Antimicrob Agents Chemother 2017; 61(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.STRIBILD [package insert]. Foster City, CA:Gilead Sciences, Inc.; 2016. [Google Scholar]
- 17.Lyseng-Williamson KA, Reynolds NA, Plosker GL. Tenofovir disoproxil fumarate: a review of its use in the management of HIV infection. Drugs 2005; 65(3):413–432. [DOI] [PubMed] [Google Scholar]
- 18.U.S. Food and Drug Administration, Clinical Pharmacology Review of Emtricitabine/tenofovir alafenamide. Available at https://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/UCM504953.pdf Accessed May 24, 2018. [Google Scholar]
- 19.U.S. Food and Drug Administration. Center for Drug Evaluation and Research. STRIBILD Clinical Pharmacology and Biopharmaceutics Review. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/203100Orig1s000ClinPharmR.pdf). In. [Google Scholar]
- 20.DeJesus E, Berger D, Markowitz M, Cohen C, Hawkins T, Ruane P, et al. Antiviral activity, pharmacokinetics, and dose response of the HIV-1 integrase inhibitor GS-9137 (JTK-303) in treatment-naive and treatment-experienced patients. J Acquir Immune Defic Syndr 2006; 43(1):1–5. [DOI] [PubMed] [Google Scholar]
- 21.Podany AT, Scarsi KK, Fletcher CV. Comparative Clinical Pharmacokinetics and Pharmacodynamics of HIV-1 Integrase Strand Transfer Inhibitors. Clin Pharmacokinet 2017; 56(1):25–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hakkola J, Raunio H, Purkunen R, Pelkonen O, Saarikoski S, Cresteil T, et al. Detection of cytochrome P450 gene expression in human placenta in first trimester of pregnancy. Biochem Pharmacol 1996; 52(2):379–383. [DOI] [PubMed] [Google Scholar]
- 23.Sarkar MA, Vadlamuri V, Ghosh S, Glover DD. Expression and cyclic variability of CYP3A4 and CYP3A7 isoforms in human endometrium and cervix during the menstrual cycle. Drug Metab Dispos 2003; 31(1):1–6. [DOI] [PubMed] [Google Scholar]
- 24.Papageorgiou I, Grepper S, Unadkat JD. Induction of hepatic CYP3A enzymes by pregnancy-related hormones: studies in human hepatocytes and hepatic cell lines. Drug Metab Dispos 2013; 41(2):281–290. [DOI] [PubMed] [Google Scholar]
- 25.Barcelo C, Gaspar F, Aouri M, Panchaud A, Rotger M, Guidi M, et al. Population pharmacokinetic analysis of elvitegravir and cobicistat in HIV-1-infected individuals. J Antimicrob Chemother 2016; 71(7):1933–1942. [DOI] [PubMed] [Google Scholar]
- 26.Custodio JM, Rhee M, Shen G, Ling KH, Kearney BP, Ramanathan S. Pharmacokinetics and safety of boosted elvitegravir in subjects with hepatic impairment. Antimicrob Agents Chemother 2014; 58(5):2564–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]