Abstract
Introduction
To analyze the appropriate treatment methods or timing to use epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) and brain radiation treatment (RT) for symptomatic and asymptomatic brain metastases (BM) in patients with EGFR mutation non-small cell lung cancer (NSCLC).
Material and methods
We retrospectively studied patients diagnosed with EGFR gene mutated NSCLC who developed brain metastasis between June 2006 and December 2015 at Zhejiang Cancer Hospital. Treatment data were assessed in 181 patients with 49 symptomatic BM and 132 asymptomatic BM retrospectively.
Results
In 49 symptomatic BM patients, the median OS of the stereotactic radiosurgery (SRS)-treated group was longer than in the whole brain radiotherapy (WBRT) group (37.7 vs. 21.1 months) (p = 0.194). In the group of 132 asymptomatic brain metastasis patients, the median overall survival (mOS) was longer in upfront brain radiotherapy compared with the upfront TKI group (24.9 vs. 17.4 months) (p = 0.035). In further analysis regarding the timing of using radiotherapy, out of all 74 patients, 33 underwent concurrent TKI and brain radiation, 13 received TKI after first-line RT treatment and 28 patients received radiotherapy after TKI failure. The intracranial progression free survival (iPFS) of the three groups was 11.1 months, 11.3 months and 8.1 months (p = 0.032), respectively. The mOS of the three groups was 21.9 months, 26.2 months and 17.1 months, respectively (p = 0.085).
Conclusions
Our research indicated that delayed brain RT may result in inferior iPFS in EGFR mutated NSCLC patients with asymptomatic brain metastases, but no OS benefit was obtained. In addition, our study revealed that patients treated with SRS had a significantly longer OS for symptomatic BM. Future prospective study of the optimal management strategy with WBRT or SRS and TKI for this patient cohort is urgently needed.
Keywords: brain metastases, epidermal growth factor recptor tyrosine kinase inhibitors, non-small cell lung cancer, radiotherapy
Introduction
Lung cancer is the most common causes of cancer death throughout the world [1, 2]. Brain metastases (BM) are manifest in 10–30% of non-small cell lung cancer (NSCLC) patients at diagnosis [3, 4]. Approximately 30–50% of NSCLC patients will develop BM during their disease course [5]. Patients with brain metastases commonly have poor prognoses and untreated patients have a median survival of just 2 months [6]. Whole brain radiotherapy (WBRT) improves median survival to 4–6 months [7]. Along with the finding of the epidermal growth factor receptor (EGFR) gene, some studies further demonstrated that patients with activating EGFR mutated had higher risk than EGFR wild type brain metastasis patients [8, 9]. Also, EGFR mutation positive patients with BM tend to do significantly better as compared to those with wild type EGFR, when treated with EGFR tyrosine kinase inhibitors (TKIs) and brain irradiation [10, 11]. Current cranial radiotherapy treatment options include stereotactic radiosurgery (SRS) and whole brain radiation therapy (WBRT) depending on the characteristics of patients’ brain metastases [12]. The SRS is a newer treatment option, applied in selected conditions of relatively limited brain disease [13, 14].
EGFR TKIs have been approved as first-line therapy in EGFR gene mutated patients of advanced NSCLC [15]. Compared with standard chemotherapies used for advanced NSCLC patients, EGFR-TKIs are much more effective at crossing the blood brain barrier [16]. In this context, the combination of brain radiotherapy and targeted therapies seems relevant. Brain metastases of NSCLC patients will manifest either symptomatically or not; the management will depend on the circumstances of diagnosis. Some studies indicated that EGFR TKIs plus brain radiotherapy (RT) showed greater efficacy than radiotherapy plus chemotherapy treatment or brain radiotherapy alone [17–19]. Other studies suggested that TKIs combined with brain RT treatment showed no superiority in intracranial progression-free survival or overall survival [20]. However, in some studies, it was demonstrated that EGFR TKIs plus radiotherapy would have a negative impact on survival time and many more side reactions [21]. Moreover, among patients with asymptomatic brain metastasis who do not require symptom relief immediately, the appropriate therapy program is still controversial. On the other hand, the time of using brain irradiation, such as upfront TKI, combination with TKI or after TKI resistance, is not well established.
Therefore, we retrospectively evaluated the efficacy of EGFR-TKI plus irradiation or TKI alone to treat NSCLC brain metastasis EGFR-mutant patients. We also assessed the efficacy of upfront EGFR-TKI, combined with TKI and deferral of RT, in asymptomatic brain metastasis patients.
Material and methods
Patient characteristics
482 patients were diagnosed with lung cancer with EGFR mutated between June 2006 and December 2015 at Zhejiang Cancer Hospital. It retrospectively enrolled and analyzed 181 patients with brain metastases at preliminary diagnosis. At the time of diagnosis, every patient underwent laboratory tests and imaging examinations. Patient information included computed tomography (CT) scans of chest and upper abdomen, emission computed tomography (ECT), and magnetic resonance imaging (MRI) of the brain. Because the primary purpose of the research was to evaluate TKI-naive patients with newly diagnosed BM, we excluded all patients who developed BM after taking an EGFR-TKI, or did not receive EGFR-TKI after SRS or WBRT. Patients who underwent surgical resection at the time of initial BM were excluded to remove a potentially confounding variable. Finally, those with insufficient information in the medical records and less than 6 months of follow-up were also excluded.
Clinical information included age, gender, smoking history, histopathology, EGFR mutation status, number of brain metastatic lesions, extracranial metastases situation, prior chemotherapy before TKIs, EGFR-TKIs drug, brain radiotherapy methods, data on 4 graded prognostic assessment (GPA), Eastern Cooperative Oncology Group (ECOG) performance status.
Treatment response assessment
A total of 181 patients all received EGFR-TKI oral treatment (icotinib 125 mg/day, tid; gefitinib 250 mg qd; erlotinib 150 mg qd). The total dose of WBRT was 30 Gy administered in 10 fractions (3 Gy fractions once a day, 5 days a week). All metastatic tumors in the brain were treated with SRS using Leksell Gamma Knife model C (Elekta, Stockholm, Sweden). To irradiate the tumor margins, the median peripheral dose of 18.2 Gy (range: 15.5–20.5 Gy) was prescribed at the median 45.9% isodose line (range: 40–70%). All patients underwent imaging examinations after two courses of chemotherapy or every 4 ±1 week for the first 2 months of EGFR-TKI treatment. Patients underwent chest, abdomen, and pelvic CT and brain MRI every 3 months until disease progression. The tumor response was assessed by the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1, including complete remission (CR), partial remission (PR), stable disease (SD), and progression of disease (PD).
Follow-up and statistical analysis
The last follow-up date was 30 April, 2016. No patient evaluated for OS was lost to follow-up. Intracranial progression-free survival was defined as the time from using RT or EGFR-TKI until intracranial progression (iPFS). Overall survival (OS) time was calculated from the day of diagnosis NSCLC with brain metastases to the date of death or the last follow-up. Survival analysis was conducted with a Kaplan-Meier analysis. The impact of the potential variables affecting PFS and OS was assessed by univariate analysis with the log-rank test. Multivariate testing was done by the Cox regression analyses. Statistical analysis was performed using SPSS software, version 19.0 (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
The baseline characteristics are shown in Table I. The study included 181 EGFR mutation patients with brain metastases at the initial diagnosis of advanced NSCLC, with 99 females and 82 males. 74 patients had an EGFR exon 19 deletion and 58 had EGFR exon 21. The median age of patients was 59.0 years (range: 31–77 years). In patients with the initial diagnosis of brain metastases, 49 patients showed symptomatic brain metastasis and the remaining patients (n = 132) were asymptomatic. After diagnosis and general evaluation, 181 patients received either chemotherapy or a TKI as first line systemic treatment. The regimens included pemetrexed, paclitaxel, gemcitabine, docetaxel or vinorelbine combined with cisplatin or carboplatin. Ninety-six patients received TKI as first-line therapy when the diagnosis was BM. The remaining 85 patients were administered a TKI as second-line or more therapy.
Table I.
Clinical and molecular characteristics of patients (n = 181)
| Characteristics | All patients (n = 181) | Symptomatic brain metastasis (n = 49) | Asymptomatic brain metastasis (n = 132) |
|---|---|---|---|
| Gender: | |||
| Male | 82 (45.3%) | 22 (44.9%) | 60 (73.2%) |
| Female | 99 (54.7%) | 27 (55.1%) | 72 (54.5%) |
| Age [years]: | |||
| < 65 | 127 (70.2%) | 36 (73.5%) | 91 (70.2%) |
| ≥ 65 | 54 (29.8%) | 13 (26.5%) | 41 (29.8%) |
| ECOG PS: | |||
| 0–1 | 141 (77.9%) | 25 (51.0%) | 116 (87.9%) |
| 2 | 40 (22.1%) | 24 (49.0%) | 16 (12.1%) |
| Smoking status: | |||
| Yes | 60 (33.1%) | 19 (38.8%) | 41 (31.1%) |
| No | 121 (66.9%) | 30 (61.2%) | 91 (68.9%) |
| Pathological type: | |||
| Adenocarcinomas | 174 (96.1%) | 44 (89.8%) | 130 (98.5%) |
| Non-adenocarcinomas | 7 (3.9%) | 5 (10.2%) | 2 (1.5%) |
| Number of intracranial lesions: | |||
| 1–3 | 95 (52.5%) | 24 (49.0%) | 71 (53.8%) |
| ≥ 3 | 86 (47.5%) | 25 (51.0%) | 61 (46.2%) |
| RTOG GPA: | |||
| 0–1 | 50 (27.6%) | 15 (30.6%) | 35 (26.5%) |
| 1.5–2.5 | 104 (57.5%) | 27 (55.1%) | 77 (58.3%) |
| 3.0 | 17 (9.4%) | 4 (8.2%) | 13 (9.8%) |
| 3.5–4.0 | 10 (5.5%) | 3 (6.1%) | 7 (5.3%) |
| EGFR mutation: | |||
| Exon 19 | 99 (54.7%) | 29 (59.2%) | 70 (53.0%) |
| Exon 21L858R | 75 (41.4%) | 17 (34.7%) | 58 (43.9%) |
| Others | 7 (3.9%) | 3 (6.1%) | 4 (3.1%) |
| Type of EGFR-TKIs: | |||
| Icotinib | 153 (84.5%) | 37 (75.5%) | 116 (87.9%) |
| Gefitinib | 19 (10.5%) | 9 (18.4%) | 10 (7.6%) |
| Erlotinib | 9 (5.0%) | 3 (6.1%) | 6 (4.5%) |
| Line of treatment of EGFR-TKI: | |||
| First line | 96 (53.0%) | 25 (51.0%) | 71 (53.8%) |
| Second line or more | 85 (47.0%) | 24 (49.0%) | 61 (46.2%) |
| Brain treatment: | |||
| WBRT | 103 (56.9%) | 40 (81.6%) | 63 (47.7%) |
| SRS | 16 (8.8%) | 5 (10.2%) | 11 (8.3%) |
| No | 62 (34.3%) | 4 (8.2%) | 58 (44.0%) |
| Extracranial metastases: | |||
| Yes | 90 (49.7%) | 16 (32.7%) | 74 (56.1%) |
| No | 91 (50.3%) | 33 (67.3%) | 58 (43.9%) |
Treatment characteristics
One hundred and nineteen received radiation therapy (102 received WBRT and 17 SRS). In all 49 symptomatic BM patients received radiotherapy, except 4 patients who refused treatment. There were 45 patients who had brain radiotherapy, 39 received WBRT and 6 SRS. Among 132 asymptomatic brain metastasis patients, 74 received radiotherapy (63 WBRT and 11 SRS). 26 patients were still stable after TKI alone by the follow-up time. Twenty-two patients treated with TKI alone did not get information about brain RT after intracranial progression until the last follow-up. Ten patients refused brain RT treatment. Among 74 patients, 33 underwent concurrent TKI and radiation therapy. Thirteen were given TKI after failure of radiotherapy. Twenty-eight patients received radiotherapy when they developed progression of disease after TKI.
Response rates
The response of treated brain lesions was evaluated at 4–8 weeks after initiation of therapy using RECIST criteria. All of 181 patients, 91 patients received brain radiotherapy before or concurrently with TKI. The remaining 90 patients did not receive radiotherapy before TKI therapy during the whole course of treatment including those who received it after the failure of TKI. Although the objective response rate (CR and PR) was slightly higher in the upfront RT group, the difference was not statistically significant (62.6% vs. 55.6%; p = 0.333).
Survival outcomes
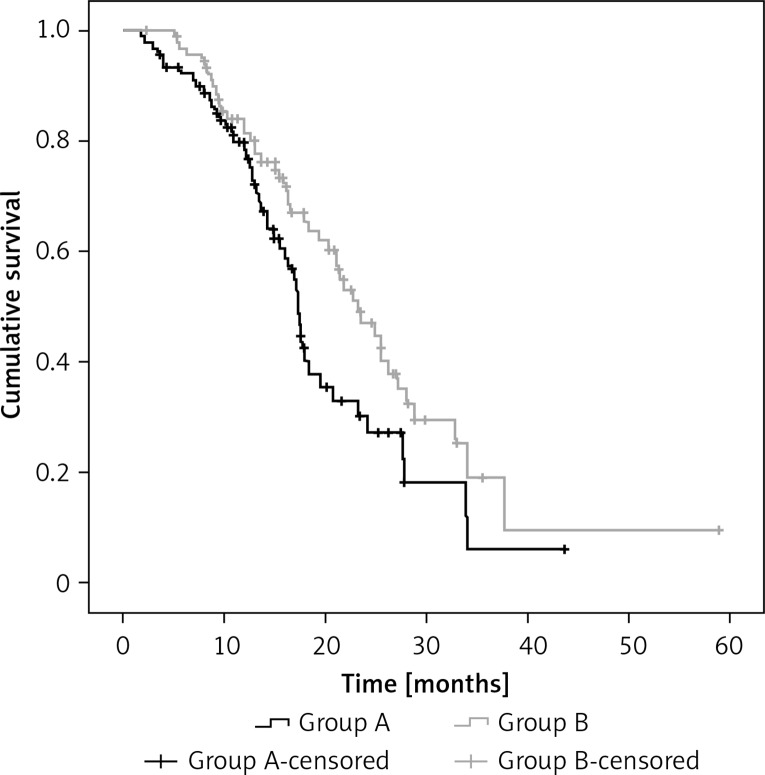
The median follow-up for all patients was 16.8 months (range: 3.6–63.4 months), and the median OS from used TKI was 20.3 months (95% confidence interval (CI): 17.3–23.4 months). The median progression-free time to iPFS for the entire cohort was 10.63 months (95% CI: 9.3–11.8 months). The iPFS in the before or concurrent RT (group B) and the upfront TKI (group A) group were 11.7 months and 9.7 months (p = 0.037), respectively. Overall survival was significantly longer in the before or concurrent RT group compared with the upfront TKI group (23.2 vs. 17.4 months, p = 0.042; Figure 1).
Figure 1.
The iPFS of all 181 patients in the before or concurrent RT (group B) and the upfront TKI (group A) group was 11.7 months and 9.7 month (p = 0.037), respectively
In all 49 symptomatic BM patients, 45 received RT including 39 WBRT and 6 SRS. Among 6 SRS recipients, only 1 patient received WBRT after intracranial progression. The iPFS for patients treated with SRS and WBRT was 12.4 and 9.5 months (p = 0.895), respectively. Although median OS in the SRS group was also greater than in those treated with WBRT (37.7 vs. 21.1 months), this finding was not statistically significant (p = 0.194).
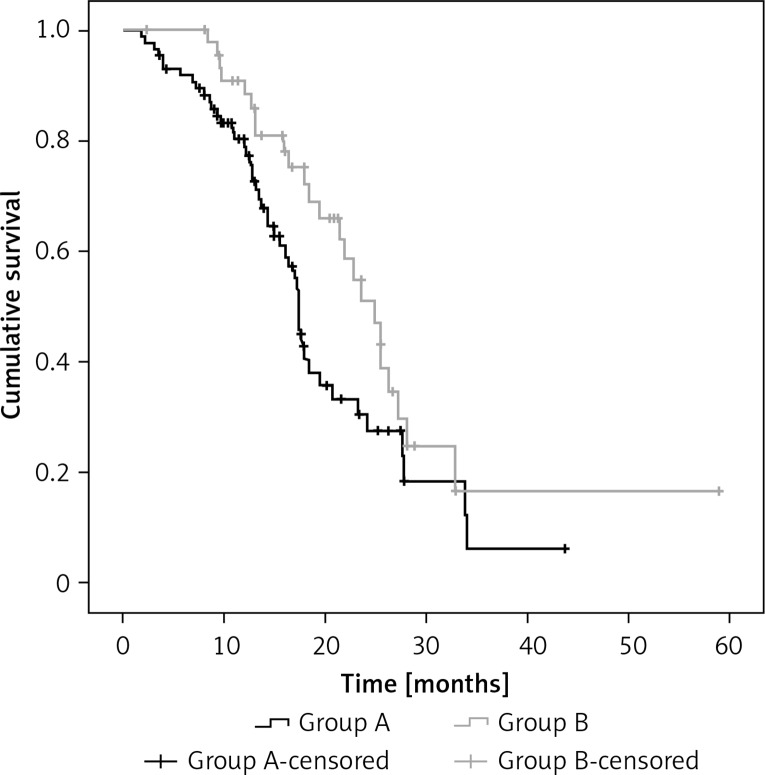
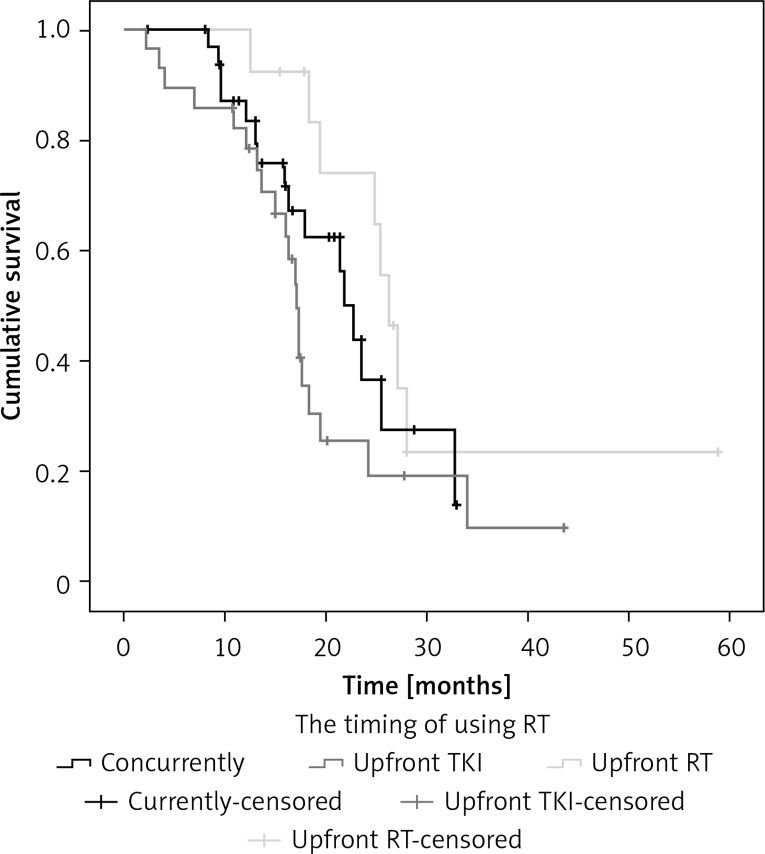
In the group of 132 asymptomatic brain metastasis patients, 74 patients received radiotherapy (63 WBRT and 11 SRS) and the median OS was 18.3 months in WBRT and 24.1 months in SRS (p = 0.696). There were 86 patients who did not receive brain radiotherapy before TKI (group A) and 46 received RT whether upfront or concurrent TKI (group B). Patient and treatment characteristics are presented in Table II. Group B had more patients with 1–3 BM than group A (p = 0.035). The iPFS in group A and group B was 9.6 months and 11.3 months (p = 0.172), respectively. The median OS in group B was also longer than in group A (24.9 vs. 17.4 months); this finding was statistically significant (p = 0.035) (Figure 2). By univariate and multivariate analysis, PS (p < 0.001) and whether RT was before TKI (p = 0.026) were independent prognostic factors (Table III). In further analysis with subgroups according to the timing of using radiotherapy, among the 74 patients, 33 underwent concurrent TKI and radiation therapy, 13 were given TKI after failure of first-line radiotherapy plus chemotherapy and 28 patients received radiotherapy after TKI. The iPFS of the three groups was 11.1 months, 11.3 months and 8.1 months (p = 0.032), respectively. The mOS of the three groups was 21.9 months, 26.2 months and 17.1 months, respectively (p = 0.085) (Figure 3). The univariate and multivariate analysis both showed that PS (p < 0.001) was the only prognostic significant factor with OS of these patients.
Table II.
Clinical and molecular characteristics of all asymptomatic patients (n = 132)
| Characteristics | Upfront or concurrent RT (n = 46) | No RT before TKI treatment (n = 86) | P-value |
|---|---|---|---|
| Gender: | 0.484 | ||
| Male | 19 (41.3%) | 41 (47.7%) | |
| Female | 27 (58.7%) | 45 (52.3%) | |
| Age [years]: | 0.091 | ||
| < 65 | 36 (78.3%) | 31 (36.0%) | |
| ≥ 65 | 10 (21.7%) | 55 (64.0%) | |
| ECOG PS: | 0.747 | ||
| 0–1 | 41 (89.1%) | 75 (87.2%) | |
| 2 | 5 (10.9%) | 11 (12.8%) | |
| Smoking status: | 0.910 | ||
| Yes | 14 (30.4%) | 27 (31.4%) | |
| No | 32 (69.6%) | 59 (68.6%) | |
| Pathological type: | 0.297 | ||
| Adenocarcinomas | 46 (100.0%) | 84 (97.7%) | |
| Non-adenocarcinomas | 0 (0) | 2 (2.3%) | |
| Number of intracranial lesions: | 0.035 | ||
| 1–3 | 19 (41.3%) | 52 (73.2%) | |
| ≥ 3 | 27 (58.7%) | 34 (39.5%) | |
| RTOG GPA: | 0.960 | ||
| 0–1 | 12 (26.1%) | 23 (26.7%) | |
| 1.5–2.5 | 28 (60.9%) | 49 (57.0%) | |
| 3.0 | 4 (8.7%) | 9 (69.2%) | |
| 3.5–4.0 | 2 (4.3%) | 5 (5.8%) | |
| EGFR mutation: | 0.416 | ||
| Exon 19 | 28 (60.9%) | 46 (53.5%) | |
| Exon 21L858R | 18 (39.1%) | 40 (46.5%) | |
| Line of treatment of EGFR-TKI: | 0.170 | ||
| First line | 21 (45.7%) | 50 (58.1%) | |
| Second line or more | 25 (54.3%) | 36 (41.9%) | |
| Extracranial metastases: | 0.511 | ||
| Yes | 24 (52.2%) | 50 (58.1%) | |
| No | 22 (47.8%) | 36 (41.9%) | |
Figure 2.
The median OS of 132 asymptomatic BM patients in the before or concurrent RT (group B) was also longer than in the upfront TKI (group A) (24.9 vs. 17.4 months) (p = 0.035)
Table III.
Univariate predictors of overall survival in 132 patients with asymptomatic BM from non-small cell lung cancer
| Variables | P-value | |
|---|---|---|
| Univariate | Multivariate | |
| Gender | 0.558 | 0.811 |
| Age | 0.051 | 0.131 |
| ECOG PS | < 0.001 | < 0.001 |
| Smoking status | 0.591 | 0.796 |
| Number of intracranial lesions | 0.651 | 0.418 |
| RTOG GPA | 0.421 | 0.595 |
| EGFR mutation | 0.078 | 0.106 |
| Line of treatment of EGFR-TKI | 0.569 | 0.531 |
| Extracranial metastases | 0.451 | 0.630 |
| Whether RT before TKI | 0.035 | 0.026 |
Figure 3.
The mOS of 74 asymptomatic BM patients with RT in three groups (underwent concurrent TKI and radiation therapy vs. upfront radiotherapy vs. upfront TKI) was 21.9 months, 26.2 months and 17.1 months (p = 0.085), respectively
Discussion
In recent years, researchers have utilized EGFR-TKIs to treat EGFR mutated patients with brain metastasis (BM) and EGFR mutated patients have a better prognosis than wild type [22, 23]. Currently, WBRT is still considered as a standard therapy option in patients with BMs from NSCLC [24]. And in patients with oligometastatic NSCLC, SRS and surgical resection are now approved by the National Comprehensive Cancer Network (NCCN) guidelines. In the EGFR-TKI era, TKIs such as gefitinib, erlotinib and icotinib have the possibility of penetrating the blood brain barrier (BBB) [25–27]. Some studies have demonstrated the efficacy of EGFR-TKIs alone for BM patients [28–30]. Therefore, the treatment strategy of either TKI alone or combination of TKI and radiotherapy remains a significant clinical controversy. Some studies have suggested that EGFR-TKIs can provide enough radio-sensitization to the brain to improve their antitumor efficacy [31, 32]. Radiotherapy plus EGFR TKIs seems to have a promising anticancer effect. However, choosing which method of brain radiotherapy (WBRT or SRS) and the timing of radiotherapy remain questionable. Therefore we respectively analyzed the efficacy in the timing of using brain radiotherapy for EGFR mutated NSCLC patients who developed brain metastasis.
Nowadays, WBRT is a standard treatment option for patients with brain metastases [33, 34]. However, some trials indicated that WBRT reduces intracranial relapse but does not prolong survival and may increase the risk of cognitive function impairment [35, 36]. In order to enhance local tumor control and minimize radiation toxicity, employing definitive SRS is a modern movement to treat a finite number of brain lesions. Xue et al. [37] found that WBRT resulted in a higher incidence of radiation-related toxicities than SRS. In other words, SRS alone may provide an applicable approach in accordance with a limited number and size of metastases. Currently, the simultaneous presence of BMs and EGFR mutations is common [38, 39], and the clinical safety and efficacy of the additional optimal therapy of SRS or WBRT with TKI in NSCLC patients remains unclear. Sperduto et al. [40] carried out a prospective study which showed that the addition of erlotinib to WBRT + SRS in 126 NSCLC patients with 1 to 3 brain metastases did not improve survival and may even have increased toxicity. The median survival times for WBRT + SRS and WBRT + SRS + TKI were qualitatively different (13.4 and 6.1 months, respectively), although the differences were not statistically significant. And the N0574 study [41] showed that adjuvant WBRT to SRS did not improve OS despite better brain control in 213 patients with 1-3 brain metastases and each lesion < 3 cm. The median OS was 10.4 for SRS alone versus 7.4 months for SRS + WBRT respectively (p = 0.92). There was more deterioration in the WBRT + SRS arm in immediate recall (30% vs. 8%, p = 0.0043), delayed recall (51% vs. 20%, p = 0.0009), and verbal fluency (19% vs. 2%, p = 0.0098). In addition, for multiple metastases, Yamamoto et al. [42] suggest that stereotactic radiosurgery might be a suitable alternative for patients with up to ten brain metastases (largest tumor < 10 ml in volume and < 3 cm in longest diameter; total cumulative volume ≤ 15 ml). Median overall survival after stereotactic radiosurgery was 13.9 months in the 455 patients with one tumor, 10.8 months in the 531 patients with two to four tumors, and 10.8 months in the 208 patients with five to ten tumors. In our study the median OS of the SRS group was longer than the WBRT group, but this finding was not statistically significant in either symptomatic BMs (37.7 vs. 21.1 months, p = 0.329), or asymptomatic BMs (24.1 vs. 18.3 months, p = 0.696). Cai et al. [43] reported that 7 patients with symptomatic BMs and EGFR mutated NSCLC underwent SRS concurrent gefitinib therapy. The median PFS was 10 months and median OS was 16 months. The study showed that improvement of KPS and survival was reliable by SRS with concurrent TKI therapy. Therefore, we surmised that treating with SRS had a longer OS than with WBRT in clinical practice. In the era of EGFR-TKI, selecting SRS combined with TKIs for symptomatic BMs on the basis of limited metastases and tumor volume would prolong survival and decrease the risk of neurocognitive problems. Hence initial treatment with SRS and close monitoring is recommended to better preserve cognitive function in patients with newly diagnosed brain metastases that are amenable to SRS.
In addition, for asymptomatic brain metastases, the relative benefits of radiation therapy (RT) and EGFR-TKI in EGFR-mutated patients have not been determined. Because brain RT is associated with potential neurocognitive long-term toxicities, it is of significant clinical interest whether EGFR-TKI therapy is sufficient to manage BM in this population. Liu et al. [44] demonstrated that brain RT as first line therapy failed to prolong survival time in 96 TKI-naive EGFR gene mutated patients with asymptomatic BM. And univariate analysis revealed that the timing of brain RT was not significantly related to OS (p = 0.246). Jiang et al. [45] carried out a study to compare the therapeutic effect of WBRT plus EGFR TKIs (51 patients) versus EGFR TKI alone (116 patients) in 167 NSCLC patients with EGFR mutation and BM. The results showed the iPFS was similar in the two groups (6.9 vs. 7.4 months, p = 0.232). The OS was worse in patients who received EGFR TKIs plus WBRT than in those who received EGFR TKIs alone (21.6 vs. 26.4 months, p = 0.049). However, this study included asymptomatic and symptomatic brain metastases. In contrast, Chen et al. [46] analyzed 132 EGFR mutated patients with BM (73.5% showed multiple intracranial lesions, and 50.8% had asymptomatic BM) to compare TKI alone with TKI plus WBRT. The results showed that the median intracranial time to progression (TTP) in patients who received WBRT was significantly longer than that in those who received EGFR-TKI alone (24.7 months vs. 18.2 months, p = 0.004). There was no significant difference in overall survival (OS) between WBRT and EGFR-TKI alone groups (median: 48.0 vs. 41.1 months; p = 0.740). Likewise, Magnuson et al. [47] conducted a retrospective analysis of 50 patients with EGFR-mutant lung adenocarcinoma who developed BM in evaluating the efficacy of upfront EGFR-TKIs and upfront radiation therapy (RT). The median OS was longer in the upfront RT group compared with the upfront EGFR-TKI group (34.1 vs. 19.4 months; p = 0.01). On further analysis, the SRS group had significant longer OS than the upfront EGFR-TKI group (58.4 vs. 19.4 months; p = 0.01), but the WBRT group did not (29.9 vs. 19.4 months; p = 0.09). It was found that patients who received upfront RT were far more likely to be symptomatic (54% vs. 6%, p < 0.001). It also did not distinguish symptomatic brain metastases from asymptomatic BM. In our study, the upfront or concurrent RT group had 4 or more intracranial lesions (57.8% vs. 39.8%, p = 0.035). In our research, we found that asymptomatic BM patients who received upfront or concurrent brain radiotherapy followed by TKI had longer median OS (24.9 months) than the upfront TKI group (17.4 months); this finding was statistically significant (p = 0.035). These findings suggest that the use of upfront or concurrent radiotherapy could improve survival time, particularly in multiple asymptomatic BM. We think it is controversial for this group of patients and it needs a prospective trial. To further address this issue, the randomized trial CTONG 1201 BRAIN is an ongoing study to compare icotinib alone with icotinib or chemotherapy plus WBRT for patients with BM and EGFR mutated patients. We look forward to its results.
According to our subset analysis regarding the timing of using RT in association with the efficacy of TKI agents, we found that 33 underwent concurrent TKI and radiation therapy, 13 were given TKI after failure of first-line brain radiotherapy plus chemotherapy and 28 patients received radiotherapy after TKI. The iPFS of the three groups was 11.1 months, 11.3 months and 8.1 months (p = 0.032), respectively. Median OS was 21.9 months, 26.2 months and 17.1 months, respectively (p = 0.085). The univariate and multivariate analysis both showed that PS (p < 0.001) was the only prognostic significant factor with median OS. These findings demonstrate that concurrent TKI and brain RT could improve survival time in good performance status patients.
Although our results are significant, we recognize that there are limitations to the study. First, our study’s major limitation was being retrospective. Second, the statistical power is diminished secondary to a small sampling of using SRS. Sixty-one patients were administered various chemotherapy regimens as first-line systemic therapy. And a number of the patients received chemotherapy and TKI crossover drugs treatment. However, there are now no randomized prospective clinical studies about the survival time of EGFR-TKI combined with or without RT treatment for asymptomatic BM.
In conclusion, this study revealed that patients treated with SRS might improve significantly overall survival time for symptomatic BMs. On the other hand, this study demonstrates that the deferral of brain RT may result in inferior OS in patients harboring EGFR activating mutations for asymptomatic brain metastases. For now, the standard-of-care treatment for newly diagnosed BM whether symptomatic or asymptomatic brain metastases should remain combination RT with EGFR-TKI therapy. Therefore, a prospective, randomized trial of EGFR-TKI with brain radiotherapy at intracranial progression and overall survival versus TKI alone is urgently needed in asymptomatic and symptomatic brain metastases respectively, and particularly in distinguishing treatment methods (WBRT or SRS) based on brain metastasis numbers, size, and site of lesions.
Acknowledgments
The study was approved by the Ethics Committee of Zhejiang Cancer Hospital.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22:2865–72. doi: 10.1200/JCO.2004.12.149. [DOI] [PubMed] [Google Scholar]
- 4.Berger LA, Riesenberg H, Bokemeyer C, Atanackovic D. CNS metastases in non-small-cell lung cancer: current role of EGFR-TKI therapy and future perspectives. Lung Cancer. 2013;80:242–48. doi: 10.1016/j.lungcan.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Lombardi G, Di Stefano AL, Farina P, Zagonel V, Tabouret E. Systemic treatments for brain metastases from breast cancer, non-small cell lung cancer, melanoma and renal cell carcinoma: an overview of the literature. Cancer Treat Rev. 2014;40:951–9. doi: 10.1016/j.ctrv.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Owonikoko TK, Arbiser J, Zelnak A, et al. Current approaches to the treatment of metastatic brain tumours. Nat Rev Clin Oncol. 2014;11:203–22. doi: 10.1038/nrclinonc.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta MP, Rodrigus P, Terhaard CH, et al. Survival and neurologic outcomes in a randomized trial of motexafin gadolinium and whole brain radiation therapy in brain metastases. J Clin Oncol. 2003;21:2529–36. doi: 10.1200/JCO.2003.12.122. [DOI] [PubMed] [Google Scholar]
- 8.Sekine A, Kato T, Hagiwara E, et al. Metastatic brain tumors from non-small cell lung cancer with EGFR mutations: distinguishing influence of exon 19 deletion on radiographic features. Lung Cancer. 2012;77:64–9. doi: 10.1016/j.lungcan.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 9.Shin DY, Na II, Kim CH, Park SH, Baek HJ, Yang SH. EGFR mutation and brain metastasis in pulmonary adenocarcinomas. J Thorac Oncol. 2014;9:195–9. doi: 10.1097/JTO.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 10.Eichler AF, Chung E, Kodack DP, et al. The biology of brain metastases-translation to new therapies. Nat Rev Clin Oncol. 2011;8:344–56. doi: 10.1038/nrclinonc.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Porta R, Sánchez-Torres JM, Paz-Ares L, et al. Brain metastases from lung cancer responding to erlotinib: the importance of EGFR mutation. Eur Respir J. 2011;37:624–31. doi: 10.1183/09031936.00195609. [DOI] [PubMed] [Google Scholar]
- 12.Scoccianti S, Ricardi U. Treatment of brain metastases: review of phase III randomized controlled trials. Radiother Oncol. 2012;102:168–79. doi: 10.1016/j.radonc.2011.08.041. [DOI] [PubMed] [Google Scholar]
- 13.Lim SH, Lee JY, Lee MY, et al. A randomized phase III trial of stereotactic radiosurgery (SRS) versus observation for patients with asymptomatic cerebral oligo-metastases in non-small lung cancer. Ann Oncol. 2015;26:762–8. doi: 10.1093/annonc/mdu584. [DOI] [PubMed] [Google Scholar]
- 14.Bowden G, Kano H, Caparosa E, et al. Gamma knife radiosurgery for the management of cerebral metastases from non-small cell lung cancer. J Neurosurg. 2015;122:766–72. doi: 10.3171/2014.12.JNS141111. [DOI] [PubMed] [Google Scholar]
- 15.Ettinger DS, Wood DE, Akerley W, et al. NCCN Guidelines insights: non-small cell lung cancer, version 4.2016. J Natl Compr Canc Netw. 2016;14:255–64. doi: 10.6004/jnccn.2016.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heon S, Yeap BY, Lindeman NI, et al. The impact of initial gefitinib or erlotinib versus chemotherapy on central nervous system progression in advanced non-small cell lung cancer with EGFR mutations. Clin Cancer Res. 2012;18:4406–14. doi: 10.1158/1078-0432.CCR-12-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai L, Zhu JF, Zhang XW, et al. A comparative analysis of EGFR mutation status in association with the efficacy of TKI in combination with WBRT/SRS/surgery plus chemotherapy in brain metastasis from non-small cell lung cancer. J Neurooncol. 2014;120:423–30. doi: 10.1007/s11060-014-1570-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang F, Ning F, Liu C, et al. Comparison of gefitinib versus VMP in the combination with radiotherapy for multiple brain metastases from non-small cell lung cancer. Cell Biochem Biophys. 2015;71:1261–5. doi: 10.1007/s12013-014-0286-9. 2015. [DOI] [PubMed] [Google Scholar]
- 19.Zhuang HZ, Yuan JW, Zhao L, Pang Q, Wang P. Phase II study of whole brain radiotherapy with or without erlotinib in patients with multiple brain metastases from lung adenocarcinoma. Drug Des Devel Ther. 2013;7:1179–86. doi: 10.2147/DDDT.S53011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee SM, Lewanski CR, N Counsell C, et al. Randomized trial of erlotinib plus whole-brain radiotherapy for NSCLC patients with multiple brain metastases. J Natl Cancer Inst. 2014;106:pii. doi: 10.1093/jnci/dju151. dju151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sperduto PW, Wang HI, Robins MC, et al. A phase 3 trial of whole brain radiation therapy and stereotactic radiosurgery alone versus WBRT and SRS with temozolomide or erlotinib for non-small cell lung cancer and 1 to 3 brain metastases: Radiation Therapy Oncology Group 0320. Int J Radiat Oncol Biol Phys. 2013;85:1312–8. doi: 10.1016/j.ijrobp.2012.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eichler AF, Kahle KT, Wang DL, et al. EGFR mutation status and survival after diagnosis of brain metastasis in non small cell lung cancer. Neuro Oncol. 2010;12:1193–9. doi: 10.1093/neuonc/noq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gow CH, Chien CR, Chang YL, et al. Radiotherapy in lung adenocarcinoma with brain metastases: effects of activating epidermal growth factor receptor mutations on clinical response. Clin Cancer Res. 2008;14:162–8. doi: 10.1158/1078-0432.CCR-07-1468. [DOI] [PubMed] [Google Scholar]
- 24.Khuntia D, Brown P, Li J, Mehta MP. Whole-brain radiotherapy in the management of brain metastasis. J Clin Oncol. 2006;24:1295–304. doi: 10.1200/JCO.2005.04.6185. [DOI] [PubMed] [Google Scholar]
- 25.Togashi Y, Masago K, Fukudo M, et al. Cerebrospinal fluid concentration of erlotinib and its active metabolite OSI-420 in patients with central nervous system metastases of non-small cell lung cancer. J Thorac Oncol. 2010;5:950–5. doi: 10.1097/JTO.0b013e3181e2138b. [DOI] [PubMed] [Google Scholar]
- 26.Togashi Y, Masago K, Fukudo M, et al. Efficacy of increased dose erlotinib for central nervous system metastases in non-small cell lung cancer patients with epidermal growth factor receptor mutation. Cancer Chemother Pharmacol. 2011;68:1089–92. doi: 10.1007/s00280-011-1691-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan Y, Huang Z, Fang L, et al. A phase II study of icotinib and whole-brain radiotherapy in Chinese patients with brain metastases from non-small cell lung cancer. Cancer Chemother Pharmacol. 2015;76:517–23. doi: 10.1007/s00280-015-2760-5. [DOI] [PubMed] [Google Scholar]
- 28.Iuchi T, Shingyoji M, Sakaida T, et al. Phase II trial of gefitinib alone without radiation therapy for Japanese patients with brain metastases from EGFR-mutant lung adenocarcinoma. Lung Cancer. 2013;82:282–7. doi: 10.1016/j.lungcan.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 29.Wu YL, Zhou C, Cheng Y, et al. Erlotinib as second-line treatment in patients with advanced non-small-cell lung cancer and asymptomatic brain metastases: a phase II study (CTONG-0803) Ann Oncol. 2013;24:993–9. doi: 10.1093/annonc/mds529. [DOI] [PubMed] [Google Scholar]
- 30.Ceresoli GL, Cappuzzo F, Gregorc V, et al. Gefitinib in patients with brain metastases from non-small-cell lung cancer: a prospective trial. Ann Oncol. 2004;15:1042–7. doi: 10.1093/annonc/mdh276. [DOI] [PubMed] [Google Scholar]
- 31.Porta R, Sanchez-Torres JM, Paz-Ares L, et al. Brain metastases from lung cancer responding to erlotinib: the importance of EGFR mutation. Eur Respir J. 2011;37:624–31. doi: 10.1183/09031936.00195609. [DOI] [PubMed] [Google Scholar]
- 32.Weber B, Winterdahl M, Memon A, et al. Erlotinib accumulation in brain metastases from non-small cell lung cancer: visualization by positron emission tomography in a patient harboring a mutation in the epidermal growth factor receptor. J Thorac Oncol. 2011;6:1287–9. doi: 10.1097/JTO.0b013e318219ab87. [DOI] [PubMed] [Google Scholar]
- 33.Tsao MN, Lloyd N, Wong RK, et al. Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases. Cochrane Database Syst Rev. 2012;18:CD003869. doi: 10.1002/14651858.CD003869.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gaspar LE, Mehta MP, Patchell RA, et al. The role of whole brain radiation therapy in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96:17–32. doi: 10.1007/s11060-009-0060-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10:1037–44. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 36.Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29:134–41. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xue J, Kubicek GJ, Grimm J, et al. Biological implications of whole-brain radiotherapy versus stereotactic radiosurgery of multiple brain metastases. J Neurosurg. 2014;121(Suppl):60–8. doi: 10.3171/2014.7.GKS141229. [DOI] [PubMed] [Google Scholar]
- 38.Matsumoto S, Takahashi K, Iwakawa R, et al. Frequent EGFR mutations in brain metastases of lung adenocarcinoma. Int J Cancer. 2006;119:1491–4. doi: 10.1002/ijc.21940. [DOI] [PubMed] [Google Scholar]
- 39.Lee DW, Shin DY, Kim JW, et al. Additional prognostic role of EGFR activating mutations in lung adenocarcinoma patients with brain metastasis: integrating with lung specific GPA score. Lung Cancer. 2014;86:363–8. doi: 10.1016/j.lungcan.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 40.Sperduto PM, Wang M, Robins HI, et al. A phase 3 trial of whole brain radiation therapy and stereotactic radiosurgery alone versus WBRT & SRS with temozolomide or erlotinib for non-small cell lung cancer and 1-3 brain metastases: Radiation Therapy Oncology Group 0320. Int J Radiat Oncol Biol Phys. 2013;85:1312–8. doi: 10.1016/j.ijrobp.2012.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown P, Asher A, Ballman K, et al. BMET-05. NCCTG N0574 (ALLIANCE): a phase III randomized trial of WBRT in addition to SRS in patients with 1 to 3 brain metastases. Neuro-Oncology. 2015;17:45–54. [Google Scholar]
- 42.Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 2014;15:387–95. doi: 10.1016/S1470-2045(14)70061-0. [DOI] [PubMed] [Google Scholar]
- 43.Cai L, Qiu X, Yang H, et al. Evaluation on efficacy and safety of the addition of X-knife therapy to gefitinib in NSCLC patients with symptomatic brain metastases. Oncotarget. 2017;8:57470–6. doi: 10.18632/oncotarget.10420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu S, Qiu B, Chen L, et al. Radiotherapy for asymptomatic brain metastasis in epidermal growth factor receptor mutant non-small cell lung cancer without prior tyrosine kinase inhibitors treatment: a retrospective clinical study. Radiat Oncol. 2015;10:118. doi: 10.1186/s13014-015-0421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang T, Su C, Li X, et al. EGFR TKIs plus WBRT demonstrated no survival benefit other than that of TKIs alone in patients with NSCLC and EGFR mutation and brain metastases. J Thorac Oncol. 2016;11:1718–28. doi: 10.1016/j.jtho.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 46.Chen Y, Yang J, Li X, et al. First-line EGFR-TKI alone or with whole-brain radiotherapy for brain metastases in EGFR-mutated lung adenocarcinoma patients. Cancer Sci. 2016;107:1800–5. doi: 10.1111/cas.13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Magnuson WJ, Yeung JT, Guillod PD, Gettinger SN, Yu JB, Chiang VL. Impact of deferring radiation therapy in patients with epidermal growth factor receptor-mutant non-small cell lung cancer who develop brain metastases. Int J Radiat Oncol Biol Phys. 2016;95:673–9. doi: 10.1016/j.ijrobp.2016.01.037. [DOI] [PubMed] [Google Scholar]