Abstract
Copper is used as an alternative to antibiotics for growth promotion and disease prevention. However, bacteria developed tolerance mechanisms for elevated copper concentrations, including those encoded by the pco operon in Gram-negative bacteria. Using cohorts of weaned piglets, this study showed that the supplementation of feed with copper concentrations as used in the field did not result in a significant short-term increase in the proportion of pco-positive fecal Escherichia coli. The pco and sil (silver resistance) operons were found concurrently in all screened isolates, and whole-genome sequencing showed that they were distributed among a diversity of unrelated E. coli strains. The presence of pco/sil in E. coli was not associated with elevated copper minimal inhibitory concentrations (MICs) under a variety of conditions. As found in previous studies, the pco/sil operons were part of a Tn7-like structure found both on the chromosome or on plasmids in the E. coli strains investigated. Transfer of a pco/sil IncHI2 plasmid from E. coli to Salmonella enterica resulted in elevated copper MICs in the latter. Escherichia coli may represent a reservoir of pco/sil genes transferable to other organisms such as S. enterica, for which it may represent an advantage in the presence of copper. This, in turn, has the potential for co-selection of resistance to antibiotics.
Keywords: copper, resistance, swine, Escherichia coli
1. Introduction
As restrictions on the use of antimicrobial agents for the purpose of growth promotion and disease prevention in farm animals are increasing, alternatives to these agents are becoming more popular. Feed supplementation with copper is one of the most frequently used, particularly in the swine industry [1]. The copper concentrations used in swine feed for growth promotion are relatively high and usually in the range of 100 to 250 ppm [2].
Bacteria developed mechanisms to cope with high concentrations of copper. In Gram-positive bacteria, the most well-known mechanism is the tcrB gene [3,4], which provides a selective advantage to intestinal enterococci in swine and cattle [5,6,7]. It also seems to be involved in the co-selection of bacteria resistant to antimicrobial agents of importance for both veterinary and human medicine [6,8]. Several tolerance and homeostasis mechanisms were described in Gram-negative bacteria and in Enterobacteriaceae in particular (for a review, see, for instance, References [9,10,11]). Although most are chromosomally encoded and present in the majority of bacteria from the species in which they reside, one of them initially found in Escherichia coli was shown to be plasmid-borne and not present in every isolate of the species [12]. The pco gene cluster associated with this system was later characterized in more detail [13] and shown to consist of seven genes (pcoA, B, C, D, R, S, and E [14,15,16]). This cluster was found in a variety of Enterobacteriaceae species and, depending on bacterial species and strain, the associated copper tolerance phenotype was variable, both in terms of copper minimal inhibitory concentration and inducibility [13]. Since then, several studies showed that pco genes are not always plasmid-borne but can also regularly be found on the chromosome of Enterobacteriaceae species, including Salmonella enterica and E. coli [17,18,19]. This spread and mobility may be related to the location of the pco genes on a Tn7-like transposon [17]. This Tn7-like element frequently carries both the pco gene cluster and the sil gene cluster [17] associated with silver tolerance [20]. Investigations on silver and copper tolerance in S. enterica isolates from Portugal showed a clear association between the presence of sil genes and copper tolerance, while the presence of pco genes did not seem to show any evident correlation with this phenotype [18,21]. Similarly, recent experimental studies on the effect of feed supplementation with copper on fecal E. coli and on the fecal metagenome of swine did not demonstrate any clear or systematic selective effect for pco genes [2,22]. These results suggest that either the concentrations of copper used in feed (125 ppm) may have been too low to have such an effect, or the presence/absence of the pco genes did not affect the tolerance of E. coli and other bacteria to elevated copper concentrations under the conditions found in the gut of the animals. However, a negative association was observed between copper supplementation and resistance to antimicrobials, as well as resistance to extended-spectrum cephalosporins in particular [2]. Also, an association between pco genes and the tet(B) tetracycline resistance gene was detected in E. coli, while these two genes were negatively associated with the blaCMY and tet(A) genes encoding for extended-spectrum cephalosporins and tetracycline resistance, respectively [2].
Based on these observations, the objectives of this study were (a) to replicate the previous experiments of Agga and collaborators [2] and reassess the associations between the pco genes and tet(A), tet(B), blaCMY, and blaCTX-M among E. coli from groups of swine subjected to diverse combinations of copper and tetracycline feed supplementation; (b) to use whole-genome sequencing to assess the genetic diversity and clonal relationships of E. coli isolates recovered from these experiments and carrying diverse combinations of these genes; (c) to compare the copper susceptibility and genome sequences of selected isolates with plasmid-borne and chromosomally encoded pco genes; and (d) to transfer E. coli plasmids carrying the pco and sil gene clusters into S. enterica by conjugation, and assess the associated copper susceptibility. These objectives related to the use of copper in feed and its effect on copper tolerance in E. coli were part of a broader study on alternatives to antibiotics [23]. The latter also included the use of zinc and oregano oil, but is not discussed here.
2. Materials and Methods
2.1. Experiment Design
The Kansas State University Institutional Animal Care and Use Committee approved the protocol for this experiment (AUP # 3135). The study was conducted at the university’s Segregated Early Weaning Facility in Manhattan, KS. Each pen (1.22 × 1.22 m) had metal tri-bar flooring, one four-hole self-feeder, and a cup waterer to provide ad libitum access to feed and water. This experiment was also described in a publication by Feldpausch and collaborators [23].
A total of 350 piglets (21 days old) were assigned to one of 70 pens (five piglets per pen), which were then randomly assigned to each of the 10 in-feed treatments arranged in a 2 × 2 × 2 (+2) factorial design. In detail, the ten dietary treatments were (1) a basal swine diet fully meeting National Research Council (NRC) nutritional guidelines, including 16.5 ppm of supplemental copper and 165 ppm of supplemental zinc (control group); (2) a basal diet supplemented with 125 ppm of copper provided by copper sulfate; (3) a basal diet supplemented with zinc at 3000 ppm of zinc provided by zinc oxide; (4) a basal diet supplemented with oregano premix containing 5% oregano oil (Regano 500; Ralco-mix Products, Marshall, MN, USA); (5) a basal diet with both 125 ppm of copper and zinc at 3000 ppm; (6) a basal diet with both 125 ppm of copper and oregano premix; (7) a basal diet with both zinc at 3000 ppm and oregano premix; (8) a basal diet containing copper, zinc, and oregano premix; (9) a basal diet containing a preventive level of chlortetracycline (CTC) (22 mg/kg body weight (BW); High CTC); and (10) a basal diet containing a subtherapeutic level of CTC (4 mg/kg BW; Low CTC). These latter treatment groups (9 and 10) did not interact with other main treatment factors (Zn, Cu, and oregano oil) in the study design, so as to assess the impact of antimicrobial alternatives versus both true negative controls and the “existing standard controls” represented by antimicrobial use groups. The basal diet consisted of corn, soybean meal, vitamins, amino acids, and trace mineral supplements per NRC requirements.
The study lasted 49 days with an initial seven days of acclimation, and 28 days of feeding trial, followed by 14 days of washout phase. Three fresh fecal samples were collected from random pigs in each pen by gentle rectal massage at days 0 and 28 of the feeding trial. Fecal samples were transported to the laboratory for further processing. The fecal samples were thoroughly mixed with 50% glycerol (1:1) and stored at −80 °C. Laboratory personnel were blinded to the treatment groups.
2.2. Selection of Isolates and Detection of pco
A total of 420 samples, 210 from day 0 (for pre-treatment effect) and 210 from day 28 (maximum treatment effect), were subjected to bacteriological culture and quantified for E. coli using standard isolation techniques and spiral plating. Briefly, one gram of 50:50 glycerol and feces were diluted in 9 mL of phosphate-buffered saline (PBS). A 50-μL aliquot of the fecal suspension was spiral-plated onto each of MacConkey agar, MacConkey agar supplemented with 16 mg/L tetracycline, and MacConkey agar supplemented with 4 mg/L ceftriaxone using an Eddy Jet 2 spiral plater (Neu-tec Group Inc., Farmingdale, NY, USA). Crude quantification values were determined by the Flash & Go Automatic Colony Counter (Neu-tec Group Inc.). A single, randomly selected colony was used from a plain MacConkey plate and confirmed as E. coli by lactose fermentation and an indole test; the species identity was also later confirmed with Illumina-based DNA sequencing. Isolates were preserved at −80 °C in protectant CryoBeads™ for further characterization.
Antimicrobial susceptibility testing was conducted by broth microdilution using the Sensititre™ system (TREK, Thermo Scientific Microbiology, Oakwood Village, OH, USA) and Sensititre™ NARMS Gram-negative plates (CMV3AGNF) on 403 E. coli isolates. Escherichia coli ATCC 25922, Escherichia coli ATCC 35218, Pseudomonas aeruginosa ATCC 27853, Staphylococcus aureus ATCC 29213, and Enterococcus faecalis ATCC 29212 were used as quality control strains. Plates were incubated at 37 °C for 18 h and read on a Sensititre OptiRead™ (TREK). The results were interpreted according to Clinical and Laboratory Standards Institute (CLSI) guidelines [24]. Intermediate isolates were interpreted as susceptible for binary statistical analyses.
Detection of pco, tetracycline, and extended-spectrum cephalosporin resistance genes was performed by PCR with the primers described in Table 1. Thermocycling conditions were the same as those defined in the respective references. Amplicons were visualized by horizontal gel electrophoresis and ultraviolet (UV) imaging.
Table 1.
PCR targets and primers used for detection of pco, sil, and antimicrobial resistance genes.
| Target | Primer | Sequence | Amplicon | Reference |
|---|---|---|---|---|
| pco | pcoD-F | CAGGAACGGTGATTGTTGTA | 700 bp | [2] |
| pcoD-R | CCGTAAAATCAAAGGGCTTA | |||
| sil | silA_Fw | GCAAGACCGGTAAAGCAGAG | 936 bp | [21] |
| silA_Rv | CCTGCCAGTACAGGAACCAT | |||
| tet(A) | TetA-L | GGCGGTCTTCTTCATCATGC | 502 bp | [25] |
| TetA-R | CGGCAGGCAGAGCAAGTAGA | |||
| tet(B) | TetBGK-F2 | CGCCCAGTGCTGTTGTTGTC | 173 bp | [25] |
| TetBGK-R2 | CGCGTTGAGAAGCTGAGGTG | |||
| bla CMY | CMYF | GACAGCCTCTTTCTCCACA | 1000 bp | [25] |
| CMYR | TGGACACGAAGGCTACGTA | |||
| bla CTX-M | CTX-M-F | ATGTGCAGYACCAGTAA | 512 bp | [26] |
| CTX-M-R | CCGCTGCCGGTYTTATC |
2.3. Copper Susceptibility
Susceptibility to copper was analyzed by broth microdilution for four randomly selected pco/sil-positive E. coli, and two pco/sil-negative isolates. Isolates were from day 0 (KSC9, 27, 64, and 207) and day 28 (KSC857 and 1031), from animals within the copper treatment group (KSC27, 857, and 1031) and from those without (KSC9, 64, and 207). A stock solution of 400 mM copper(II) sulfate (Sigma-Aldrich, St. Louis, MO, USA) was prepared in double-distilled water (ddH2O), and filter-sterilized. A non-serial dilution range (0, 4, 8, 16, 20, 24, 36, 48, 64, and 100 mM) was prepared in Mueller–Hinton II broth, cation-adjusted (Becton Dickinson, Franklin Lakes, NJ, USA), and each dilution was adjusted to pH 7.2 using 5 M NaOH [11]. Bacterial suspensions of a 0.5 McFarland standard were diluted 1/100, and 50 μL of this suspension was inoculated in a 96-well plate with 50 μL of the copper dilutions, resulting in halving of the initial copper concentrations. Microplates were incubated at 37 °C for 16 h, under both aerobic and anaerobic conditions. Minimum inhibitory concentration (MIC) was defined as the first concentration without visible growth. Minimum bactericidal concentrations (MBCs) were determined by removing 10 μL from wells that showed no visible growth, and plating them on Mueller–Hinton II agar plates for incubation at 37 °C for 16 h. The ATCC 25922 E. coli strain (pco/sil-negative) was used as a negative control for susceptibility testing.
Minimum inhibitory concentrations were also determined using agar plate dilutions of copper, as described by Mourão and collaborators [21]. Briefly, copper dilutions of 0, 0.5, 1, 2, 4, 8, 12, 16, 20, 24, 28, 32, and 36 mM were prepared in Mueller–Hinton II agar, and the pH was adjusted as above. One microliter of an approximate 107 colony forming units (CFU)/mL culture was pipetted onto the surface of each plate. Growth at 37 °C in both aerobic and anaerobic conditions was assessed after 16 h.
2.4. Expression of pco by Complementary DNA Synthesis and Real-Time PCR
Three E. coli isolates were selected randomly (two pco/sil-positive and one negative control, none of which were isolated from copper-treated animals) for determining the expression of pco under aerobic conditions, with and without induction with low concentrations of copper, performed as previously described [15,27]. Briefly, isolates were plated overnight at 37 °C on Luria–Bertani (LB) agar (Becton Dickinson) plates. A single loop of bacteria was inoculated into 1 mL of LB broth, and vortexed; 200 μL of this suspension was inoculated into 20 mL of LB broth supplemented with 0, 1 mM, and 5 mM copper(II) sulfate and incubated at 37 °C for approximately 2.5 h, until optical densities of 0.5 were reached at 600 nm. Broth microdilution MICs were performed again as above under aerobic conditions to observe any effect of this induction on copper tolerance. In parallel, 10 mL of broth was centrifuged, and the resulting pellet was resuspended in 1 mL of RNAlater (QIAGEN Inc., Valencia, CA, USA). Total RNA was extracted using an RNAeasy Mini kit (QIAGEN), according to the manufacturer’s instructions. An additional DNase step was performed to ensure all traces of DNA were removed, and was verified by a pco PCR using 1 μL as a template. RNA was quantified using a BioAnalyzer 2100 instrument (Agilent Technologies, Santa Clara, CA, USA), and 100 ng of each RNA preparation was used for complementary DNA (cDNA) synthesis using an Applied Biosystems High-Capacity cDNA Reverse Transcription kit (Thermo Fisher Scientific, Carlsbad, CA, USA).
PCR was performed to amplify gene fragments to be used for cloning into a plasmid vector, for use as a standard curve for real-time PCR. Amplicons of the pcoA and pcoD genes were produced using primers forward pcoA (pcoA_F), CGGGTATGCAAAGTCATCCT; reverse pcoA (pcoA_R), TTGATCAGCGTGATCCTGAG; and pcoD_F, AAGCGGTGTCAGACATGAAA; pcoD_R, GATGGGTCAGATCGCTCAGT, respectively. As controls, two housekeeping gene amplicons for hcaT (HcaT major facilitator superfamily transporter) and rrsA (16S ribosomal RNA) were amplified using primers hcaT_F, CTGATGCTGGTGATGATTGG; hcaT_R, CAATGCAGAATTTGCACCAC; and rrsA_F, CGGACGGGTGAGTAATGTCT; rrsA_R, GTTAGCCGGTGCTTCTTCTG, respectively. Each amplicon was cloned into a pCR 2.1-TOPO plasmid vector, using an Invitrogen TOPO-TA cloning kit (Thermo Fisher Scientific). Inserted sequences were confirmed by DNA sequencing, and plasmid DNA was prepared using a Plasmid Midi Kit (QIAGEN). Plasmid DNA was quantified using Quant-IT Picogreen dsDNA reagent (Thermo Fisher Scientific) and read using a DTX 880 Multimode detector (Beckman Coulter, Brea, CA, USA). Gene copy numbers were then predicted by the DNA concentration divided by the molecular weight of the plasmid.
Real-time PCR was used to quantify the expression of each pcoA, pcoD, hcaT, and rrsA gene using primers internal to the fragments described above. In triplicate, 1 μL of cDNA or plasmid standards were added to 19 μL of LightCycler 480 SYBR Green I Master (Roche Diagnostics, Indianapolis, IN, USA) containing 250 nM of each primer. Primers used for the quantification of pco expression were RT_pcoAF, TGGTTGATATGCAGGCGATG; RT_pcoAR, TCCGCGTACGTGAGAACCTT; and RT_pcoDF, GTCAGGCTCTGTGCCCTGTT; RT_pcoDR, CCCACTCATCGTCATCAGCA. Housekeeping gene primers used for hcaT and rrsA were those described by Zhou and collaborators [28].
2.5. Next-Generation Sequencing
A subset of 82 isolates was selected to represent suspected extended-spectrum β-lactamase (ESBL)-producing isolates and isolates with elevated ciprofloxacin MICs (based on Sensititre phenotypes and ciprofloxacin MICs of ≥0.05 mg/L; n = 26), isolates carrying the blaCMY gene (n = 26), and a representative sample of isolates with resistance phenotypes determined by Sensititre (n = 30). In addition, all 34 E. coli carrying the pcoD gene were also included. Genomic DNA was prepared for MiSeq sequencing (Illumina, San Diego, CA, USA) for all of these 116 isolates using a QIAamp DNA extraction kit (QIAGEN), and libraries were prepared using a Nextera XT kit (Illumina). Achtman sequence types were determined with the SRST2 plugin for BaseSpace Labs (Illumina) using the MiSeq paired-end reads, where sufficient read quality was obtained. These reads were also used for core-genome multilocus sequence typing (cgMLST) (EnteroBase typing scheme) using the wgMLST application for BioNumerics v7.6 (Sint-Martens-Latem, Belgium). Single-nucleotide polymorphism (SNP) analysis of each pco gene cluster (pcoEABCDRSE) was also performed using the wgSNP analysis tool from BioNumerics.
DNA was also prepared for four of the isolates tested for susceptibility to copper and harboring pco (KSC9, KSC64, KSC207, and KSC1031) using a MasterPure DNA Purification Kit (Epicentre, Madison, WI, USA) for PacBio RS II sequencing (Pacific Biosciences, Menlo Park, CA, USA). Sequencing and assembly of these four isolate genomes were performed at the McGill University and Génome Québec Innovation Centre, Montreal, QC, Canada. PacBio sequencing assembly was completed on chromosome and plasmid assemblies of sheared large inserts (~20 kbp) using the de novo genome assembly pipeline Hierarchical Genome Assembly Process (HGAP); alignments were further polished using the Quiver consensus algorithm. Genomes and/or plasmids (pMRGN207 and pMRGN1031) containing the pco gene cluster were uploaded to GenBank under BioProject PRJNA355857. Annotations were performed using the NCBI Prokaryotic Genome Annotation Pipeline (PGAP) version 4.0.
2.6. Conjugation of a pco/sil Plasmid into Salmonella
Based on the known plasmid-borne location in two of our sequenced E. coli isolates, we attempted to transfer these plasmids into Salmonella recipients to observe any change to copper susceptibility. Six Salmonella isolates were selected at random from a large collection of isolates maintained by the Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS) between 2008 and 2011. Isolates were screened for pco and sil by PCR, and three pco/sil-positive and three pco/sil-negative were used for copper susceptibility testing. Escherichia coli isolates KSC207 and KSC1031 were used as plasmid donor strains, with pco/sil plasmid incompatibility types HI2 and FII, respectively (as determined by DNA sequence analysis), while pco/sil-negative Salmonella isolates SA8197 (serovar Kentucky) and SA82540 (serovar Infantis) were used as recipients. Briefly, 50 µL of donor and 100 µL of recipient overnight broth cultures were mixed, plated on LB agar, and incubated overnight to facilitate conjugation. Growth was then resuspended in LB broth, and plated on Brilliant Green agar to inhibit the growth of donor E. coli, as both plasmids carried a tetracycline resistance determinant—tet(A) and tet(B), respectively. Additionally, 12 µg/mL tetracycline was included in the selective plates. Up to five pink-colored colonies were then sub-cultured for purity, and indole testing and PCR for pco and sil were used to confirm transfer. These transconjugant Salmonella were then used for copper susceptibility testing as before using anaerobic agar dilution, and performed in triplicate.
2.7. Statistics
Descriptive and inferential statistical methods were performed using Stata version 15 (StataCorp, College Station, TX, USA). Categorical data (i.e., resistance phenotypes and presence of genes) were tabulated and cross-tabulated to explore bivariable associations between treatments and the following outcomes: (1) presence of phenotypic resistance to (a) tetracyclines, (b) cefoxitin, for preliminary classification of ESBL (susceptible) versus AmpC (resistant) producing β-lactamases, (c) third-generation cephalosporins, and (d) multidrug resistance (MDR) count (integer count), out of 14 antimicrobials tested on a broth microdilution panel; and (2) presence of resistance genes for (a) tetracyclines (tet(A) and tet(B)), (b) extended-spectrum cephalosporins (blaCMY-2 and blaCTX-M), and (c) metal resistance genes (pco and sil). Likelihood ratio χ2 or Fisher’s exact tests (when zero-cells were abundant) were used in bivariable analyses; statistical significance was determined at p < 0.05. Differences in growth (log10 CFU) of coliforms on plain versus antimicrobial (tetracycline or ceftriaxone) impregnated plates were examined by unpaired t-tests. Multivariable mixed logistic (binary outcomes) and linear models (count and log10 CFU outcomes) using a four-way factorial design (plus two additional indicator variables for low- and high-dose CTC) were built and assessed for each of the binary response (logistic) and quantitative (log10 CFU differences) endpoints. Full factorial models were subjected to reduction, firstly removing non-significant interaction terms and then main effects. Of note, in-feed copper (low versus high), sampling day (0 versus 28), and their interaction were always retained marginal means estimated with p-values representing the post hoc multiple comparisons adjusted using Bonferroni’s correction.
3. Results
3.1. Resistance Determinants
Not all samples (n = 420) yielded lactose- and indole-positive isolates. A total of 403 E. coli isolates were included in this analysis. Thirty-four of the 403 isolates (8.4%) were positive for pco by PCR. Twelve isolates carried a blaCTX-M gene (3.0%), and 60 carried blaCMY (14.9%, all variant CMY-2). All but two isolates (99.5%) were positive for tet(A) or tet(B) (121 (30.0%) and 267 (66.3%), respectively); 13 isolates (3.2%) carried both of these tetracycline resistance genes.
All blaCTX-M sequences encoded the CTX-M-27 variant, and all blaCTX-M-positive isolates were pco-negative, but tet(B)-positive; 11 of these 12 isolates were ST744 and had an ampicillin/ceftriaxone/ciprofloxacin/nalidixic acid/tetracycline resistance phenotype.
3.2. Associations of Copper Treatment Groups with pco Prevalence
Multi-level model-adjusted estimates of the occurrence of the pco gene were initially unstable in the presence of the full factorial specification. A reduced model containing copper (forced into model), day (and its interaction), and low- versus high-dose CTC yielded a model significant at p < 0.03. Copper did not select for the pco gene (p = 0.249); that is, copper supplemented at NRC requirements yielded 0.07 (95% confidence intervals (CIs): 0.04–0.11) of isolates with the gene, versus 0.11 (95% CIs: 0.04–0.17) in the group supplemented with copper beyond nutrient needs. Likewise, copper did not appear to select for any of the additional microbiological endpoints (p > 0.05; data not shown). Low-dose CTC did select (p = 0.001) for increased pco with 0.28 (95% CIs: 0.11–0.46) of the isolates in the low-dose group harboring the gene versus 0.06 (95% CIs: 0.04–0.09) in pigs not receiving any CTC. The difference between the high-dose CTC and each of the other two levels was not significant (0.12; 95% CIs: 0.00–0.24). Of note, though unexplained by the field trial study design, the pco gene was significantly (p = 0.012) associated with a lower MDR count; however, most of this difference was due to a lack of the highest MDR counts (maximum with pco present = 7 versus 11 in pco-negative isolates). No associations were significant (p > 0.05) among the genes tested, with the notable exception of pco/sil for which there was complete agreement (34/34; Fisher’s exact test, p < 0.0001).
3.3. Susceptibility to Copper and Expression of pco
Susceptibility to copper by broth microdilution ranged from 12 to 18 mM in all seven E. coli isolates tested (Table 2). The presence of the pco gene cluster did not appear to have an effect on susceptibility to copper under both aerobic and anaerobic conditions, nor did the use of broth or solid media. All MBC values were identical to their respective MIC results. The ATCC 25922 (pco/sil-negative) isolate had an MIC and MBC of 12 mM. For the Salmonella isolates, no systematic differences were observed when using broth dilution under anaerobic conditions (MICs of 12 to 18 mM), but the MICs of the pco/sil-positive and -negative isolates differed when tested on agar, with values of 24 mM and 4 mM, respectively (Table 2).
Table 2.
Isolates used in this study for copper susceptibility testing. All reported minimum inhibitory concentration (MIC) values were determined under anaerobic conditions. MBC—minimum bactericidal concentration.
| Isolate | Bacteria | Serovar | pco | sil | Location (Similar to) | Broth MIC 1 | Broth MBC 1 | Agar MIC 1 |
|---|---|---|---|---|---|---|---|---|
| KSC9 | Escherichia coli | + | + | chromosome (IAI1) | 18 | 18 | 16 | |
| KSC64 | E. coli | + | + | chromosome (E24377A) | 18 | 18 | 16 | |
| KSC207 | E. coli | + | + | 278 kbp plasmid (pR478) | 18 | 18 | 16 | |
| KSC1031 | E. coli | + | + | 149 kbp plasmid (p1540) | 12 | 12 | 12 | |
| KSC27 | E. coli | − | − | 12 | 12 | 16 | ||
| KSC857 | E. coli | − | − | 12 | 12 | 16 | ||
| ATCC 25922 | E. coli | − | − | 12 | 12 | 16 | ||
| SA10689 | Salmonella | Senftenberg | + | + | unknown | 18 | 18 | 24 |
| SA12224 | Salmonella | Ouakam | + | + | unknown | 18 | 18 | 24 |
| SA13423 | Salmonella | Ouakam | + | + | unknown | 18 | 18 | 24 |
| SA82699 | Salmonella | Kentucky | − | − | 18 | 18 | 4 | |
| SA81917 | Salmonella | Kentucky | − | − | 12 | 18 | 4 | |
| SA82540 | Salmonella | Infantis | − | − | 12 | 18 | 4 | |
| SA81917-TC | Salmonella | Kentucky | + | + | plasmid from E. coli KSC207 | ND 2 | ND | 24 |
| SA82540-TC | Salmonella | Infantis | + | + | plasmid from E. coli KSC207 | ND | ND | 24 |
1 concentration in mM; MICs are averages of three complete biological replicates. 2 ND (not done).
Induction with 1 mM and 5 mM under aerobic conditions appeared to have no effect on the MIC of each isolate tested (data not shown). RNA extraction and cDNA analysis also showed no significant change in pcoA or pcoD transcription, observed by real-time PCR. Using hcaT as the reference gene (expression of rrsA expression was considerably higher than all other genes, and was not used in the analysis), expressions of pcoA and pcoD were measured as the “target” in all samples using relative quantification. The average adjusted crossing point (CP) across all three induction concentrations was 31.9 (±1.7) for pcoA, and 32.1 (±1.9) for pcoD.
3.4. Next-Generation Sequencing and cgMLST
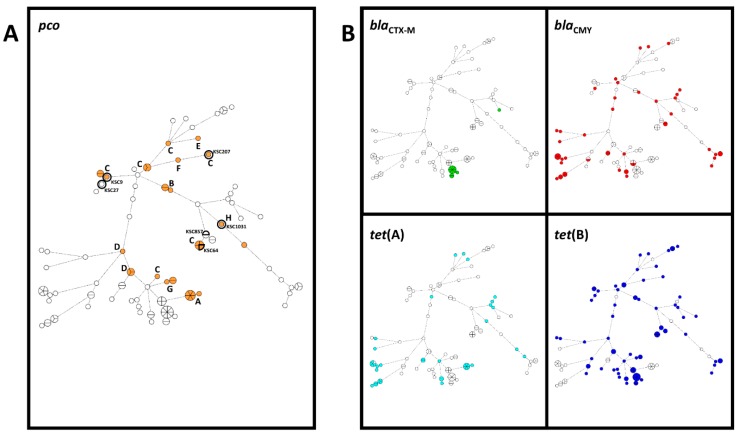
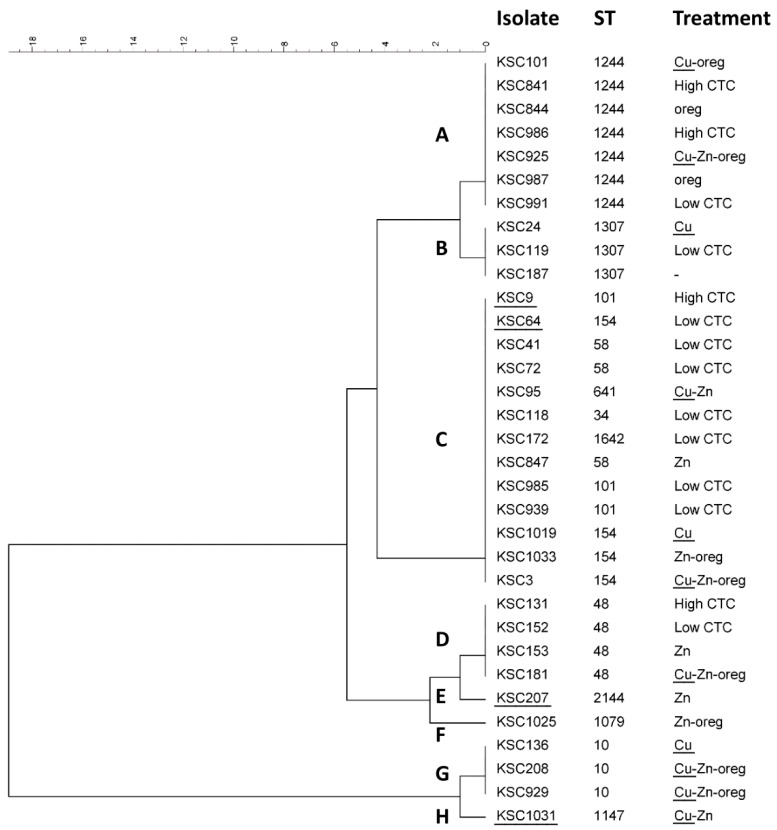
All 116 E. coli isolates selected for MiSeq sequencing were analyzed using the BioNumerics software (Figure 1). All 34 pco-positive isolates recovered during this study also carried the sil gene cluster (determined by reference mapping of MiSeq reads), and none of the 82 pco-negative isolates carried any sil gene. Core-genome MLST analysis of the MiSeq data showed a random distribution of pco/sil among sequence types and no association with a specific clonal lineage (Figure 1). Further pco SNP analysis showed the gene cluster to be highly conserved within a sequence type (ST), but had some variation between most STs (Figure 2).
Figure 1.
Minimum spanning tree of 116 Escherichia coli isolates, using core-genome multilocus sequence typing (MLST) analysis comprising 2513 genes (BioNumerics E. coli/Shigella EnteroBase scheme). A tree with the highest resampling support is shown, using 1000-resampling bootstrapping. (A) Isolates carrying the pco gene cluster are highlighted in orange; (B) isolates carrying the resistance genes blaCTX-M, blaCMY, tet(A), and tet(B) are indicated. Letters in 1A indicated single-nucleotide polymorphism (SNP) types found in Figure 2. Circles containing multiple sections indicate multiple isolates within a core-genome sequence type. Isolates used for minimum inhibitory concentration (MIC) testing are also highlighted and labeled in 1A.
Figure 2.
Phylogenetic analysis of the pcoEABCDRSE gene cluster (5487 bp) of all pco-positive isolates in this study, using a categorical (differences) similarity coefficient and unweighted pair group with arithmetic mean (UPGMA) cluster analysis. Treatment groups, including Cu (copper), Zn (zinc), oreg (oregano oil), and high/low-dose chlortetracycline (CTC) are shown. Letters indicating identical SNP groups are also shown in Figure 1A. Isolates used for MIC testing are underlined. ST: sequence type.
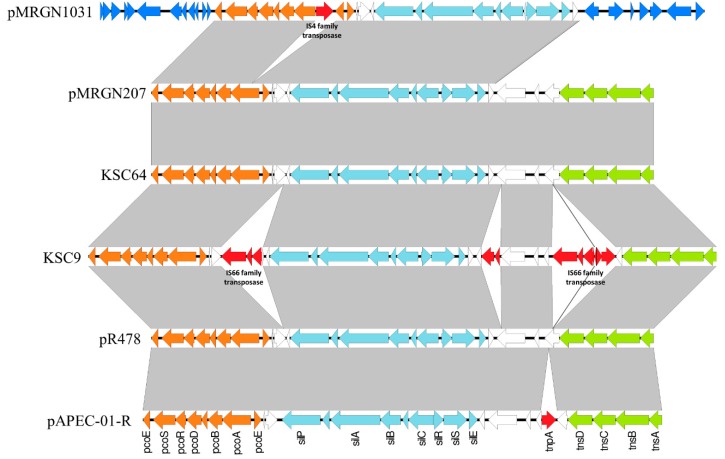
Pacific Biosciences long-read sequencing and assembly showed the pco gene cluster to be both plasmid and chromosomally encoded (Figure 3). The gene cluster was accompanied by the sil gene cluster in all four pco-positive isolates, and was flanked by a Tn7-like transposable element in three of them. Using this pco–sil–tns sequence as a template, short-read Illumina sequences were successfully mapped onto all but one pco-positive isolate (KSC1031) for the complete structure, demonstrating the highly conserved nature of this transposable element across multiple STs and plasmid types found in this study.
Figure 3.
Genetic context of the pco–sil–tns area of four isolates from this study sequenced by PacBio. KSC1031 (plasmid pMRGN1031; GenBank accession number CP019561), KSC207 (plasmid pMRGN207; CP019559), KSC64 (CP018840), and KSC9 (CP018323) were compared to the pR478 (BXX664015) and pAPEC-O1-R (DQ517526) previously published sequences. Colors indicate the pco operon (orange), the sil operon (light blue), transposases (red), Tn7 genes (green), and others (dark blue).
Both of the two chromosomal Tn7-like elements were found in approximately the same position, 300 kbp downstream of the preferential glmS insertion point for Tn7 [29], much farther than previously reported [30,31]. Neither plasmid harboring the pco/sil gene cluster contained any known virulence factors (using Virulence Finder v1.5 [32]). In one instance, the pcoA gene was interrupted by a transposase, while the sil gene cluster was always intact (Figure 3).
3.5. Conjugation of a pco/sil Plasmid into Salmonella
The pco/sil plasmid (IncHI2) from E. coli isolate KSC207 was successfully transferred to both Salmonella recipient isolates, as confirmed by PCR. Using agar dilution under anaerobic conditions, a clear difference was observed between the transconjugants carrying the sil/pco gene clusters (MIC = 24), and the recipient isolates (MIC = 4; Table 2). The IncFII plasmid from E. coli isolate KSC1031 could not be successfully transferred to either Salmonella recipient.
4. Discussion
The plasmid-borne nature of the pco gene cluster [12,13,19] and the recent demonstration of its linkage with important antimicrobial resistance genes [11,17] warrant further investigations on the potential medical and public health implications of copper use in animal feed. A previous set of experiments under controlled conditions failed to demonstrate any significant selection of pco-positive E. coli isolates [2] or increase in pco copy numbers [22] in feces from pigs fed copper after weaning. However, pco-positive isolates were more frequently associated with the tetracycline resistance gene tet(B) than with its tet(A) counterpart [2]. This suggested some possible gene linkage between pco genes and tet(B) on mobile elements or clonal expansion of strains carrying both genes. The replication of these experiments described here showed the same lack of selection of pco-positive E. coli with copper concentrations in feed (125 mM) similar to those used in the field (100–250 mM; [2]). Although this does not exclude some selection in the long term or the selection of other genes, no significant effect could be detected during a single feeding period. Since none of the 82 pco-negative isolates sequenced carried the sil operon (31 of which were from samples of animals receiving copper supplementation), it is also unlikely that the copper treatment would have selected for this latter operon alone. A pco–tet(B) positive association, as well as a negative association between pco and blaCMY, was observed previously by Agga et al. [2]; however, while the direction of association was similar here, the associations were not significant (p = 0.344 and 0.087, respectively). We did see much higher levels of pco among isolates from pigs subjected to low doses of CTC (a dosage regimen for growth promotion purposes not permitted in the US since 1 January 2017), which may suggest that indirect selection of pco and pco-positive strains could occur when using tetracyclines in swine. In the present study, the prevalence of tet(A) was highest in the group receiving high-dose CTC (45.0% versus 22.5%) whereas the prevalence of tet(B) was highest in the group receiving low-dose CTC (77.5% versus 62.5%). This finding may help explain the relationship of tetracycline uses with pco, though such hypotheses are largely based on the previous findings of Agga et al. [2].
Analysis of genomic similarities between pco-positive isolates through cgMLST demonstrates that the pco genes are distributed across a variety of clonal lineages and do not cluster in only a few clear discrete groups of closely related isolates. The most frequent pco single-nucleotide polymorphism (SNP group C in Figure 1A and Figure 2) is also present in several STs and unrelated clonal lineages. Both observations illustrate the active horizontal transfer of the pco gene cluster in E. coli populations. However, the associations between most of the other pco SNP groups and STs (Figure 2) or clonal lineages (Figure 1) suggest that both a combination of short-term or local clonal spread and broader long-term horizontal gene transfer (HGT) play a role in the distribution of this gene cluster in E. coli from the swine population examined. Similar to the pco–sil clusters, the tet(A), tet(B), and blaCMY genes which have been present in Enterobacteriaceae from farm animals in North America for several decades also appeared to be distributed randomly and did not cluster clearly together with pco genes in a discrete number of clonal lineages (Figure 1). Overall, these observations suggest that the positive and negative statistical associations observed between pco and tet(B) or tet(A) and blaCMY, respectively, do not rely on the expansion and contraction of a very limited number of major clonal lineages. This differs from the blaCTX-M-27 gene which was found mainly (11/12) in closely related isolates. CTX-M β-lactamases were reported in food animals much later in North America than in other continents [33] and may have emerged in swine in the US only recently. It may, therefore, be only in the early stages of its spread through HGT in bacteria from swine and still limited to a small number of clonal lineages.
The pco genes were located together with the sil cluster on a Tn7-like transposon structure [17,19,34] in all but one isolate in this study (KSC1031). The high transposition frequency of Tn7 and related elements [29] may be an important reason for the distribution of the pco–sil cluster in a wide diversity of strains illustrated in the present study. Tn7 transposons developed refined strategies to insert preferentially on mobile plasmids [29]. It may, therefore, not appear entirely surprising that the pco plasmid we were able to transfer by conjugation (pMRGN207) carried the full pco/sil/Tn7-like element, while pMRGN1031 missing the Tn7 part of the element was not transferable. Coincidentally, the plasmid we were able to transfer was an IncHI2 plasmid, an incompatibility group already shown by others to carry pco genes in different geographic locations and bacterial species [17,18,35,36]. Tn7 transposons also developed refined strategies to insert preferentially into the same selectively neutral attTn7 chromosomal site located in proximity of the glmS gene [29]. However, the locations of the two chromosomal Tn7-like elements associated with the pco–sil cluster in the closed genome sequences generated with PacBio long reads show that this mobile element does not always insert in the same attTn7 site or in the proximity of glmS. This may warrant further investigations on the transposition mechanisms of this Tn7-like transposable element. Together, these findings further stress the likely important role of IncHI2 plasmids and Tn7-like elements in the spread of the pco–sil gene clusters.
The overall structure of the region encompassing the pco–sil clusters was highly conserved and identical to pR478 [36] in two of the four isolates we investigated in detail (one plasmid-borne and the other chromosomal). This conserved region also included the tns gene cluster of Tn7 and the intervening region between the tns and sil genes. This structure was described by others on several plasmids [17,19]. Insertions were present in the pco–sil clusters for the two other isolates. In one of them (chromosomal), three insertions were present in this region, but all were within open reading frames encoding putative proteins of unknown function, and were not affecting the pco or the sil gene clusters. However, in pMRGN1031, an insertion was disrupting the pcoA gene. This latter insertion would be expected to inactivate the copper resistance if a phenotype were detectable [14].
In addition to the loss of the tns gene cluster and parts of the genes upstream of the sil cluster already mentioned above, SNP analysis also showed that the pco genes in pMRGN1031 are clearly divergent from the majority of those from the other isolates of this study. This strongly supports the hypothesis that the pco–sil gene clusters on this plasmid have a longer or different evolutionary history than those found on other plasmids, and that parts of it may possibly be decaying.
The surprising initial lack of difference in susceptibility to copper between pco/sil-positive and pco/sil-negative isolates that we obtained in broth under aerobic growth conditions triggered further investigations under a variety of other conditions. Previous publications showed that the copper resistance phenotype of pco-positive isolates is inducible and can be triggered by preliminary incubation in subinhibitory concentrations of copper [13,36]. Subjecting our isolates to subinhibitory concentrations of copper similar to those described in these studies did not result in any change in copper MIC, and our isolates did not show any significant change in RNA transcription of the pcoA and pcoD genes after induction. Copper susceptibility of E. coli and S. enterica was tested by others with a variety of methods, including broth [17] and agar dilutions [21,37], as well as under aerobic [17,21] and anaerobic conditions [21,37]. No differences in copper MICs were observed by these authors between pco/sil-positive and -negative isolates under aerobic conditions, neither for E. coli, nor for S. enterica. However, differences were consistently observed for S. enterica when agar dilutions were used under anaerobic conditions [18,21]. Therefore, we also tested our E. coli and a few S. enterica isolates by agar dilution under anaerobic conditions. As expected, an evident dichotomization of MICs was visible under these conditions for S. enterica, but this was not the case for E. coli. These data are in agreement with results from others showing that copper resistance associated with the pco gene cluster is host-dependent [13]. The increase in MIC observed in S. enterica after transfer of a pco/sil plasmid from E. coli clearly confirmed this hypothesis.
Overall, the results from this study strongly suggest that the pco/sil gene clusters may have only a minor effect on copper MICs in typical wild-type intestinal E. coli, and may not represent a major selective advantage in this bacterial species in the gut of swine fed high concentrations of copper. Some of our findings are based on a relatively limited number of isolates, and confirmation on larger numbers of isolates is needed. Escherichia coli may represent a reservoir of mobile copper resistance determinants of potential importance for S. enterica. As illustrated here with pMRGN207 and by other researchers [17,18], transferable pco/sil plasmids concomitantly carry antimicrobial resistance determinants. These antimicrobial resistance determinants may help maintain these mobile plasmids, and indirectly, the pco–sil cluster in E. coli populations. Antimicrobial resistance may, in turn, be maintained and selected in S. enterica harboring these plasmids by the supplementation of feed with copper. Further animal experiments are needed to clarify the latter points. The role of IncHI2 plasmids in this context and the exact mechanisms and dynamics of transposition of Tn7-like transposons associated with the pco–sil gene clusters certainly also warrant further investigations, as do the respective roles and contribution of the pco versus sil genes in the observed copper resistance in S. enterica.
Acknowledgments
The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the USDA or NIFA.
Author Contributions
H.M.S. and P.B. conceived and designed the experiments; H.M.S., R.G.A., T.G.N. and M.D.T. designed and performed the animal study; K.M.R., K.N.N., R.G.A. and G.C. performed the experiments; K.N.N., H.M.S., P.B. and G.C. analyzed the data; G.C., H.M.S. and P.B. wrote the original draft of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the USDA National Institute of Food and Agriculture, AFRI Food Safety Challenge Grant project #2013-68003-21257.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.National Research Council . Nutrient Requirements of Swine. Eleventh Revised Edition. The National Academies Press; Washington, DC, USA: 2012. [Google Scholar]
- 2.Agga G.E., Scott H.M., Amachawadi R.G., Nagaraja T.G., Vinasco J., Bai J., Norby B., Renter D.G., Dritz S.S., Nelssen J.L., et al. Effects of chlortetracycline and copper supplementation on antimicrobial resistance of fecal Escherichia coli from weaned pigs. Prev. Vet. Med. 2014;114:231–246. doi: 10.1016/j.prevetmed.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Hasman H., Aarestrup F.M. tcrB, a gene conferring transferable copper resistance in Enterococcus faecium: occurrence, transferability, and linkage to macrolide and glycopeptide resistance. Antimicrob. Agents Chemother. 2002;46:1410–1416. doi: 10.1128/AAC.46.5.1410-1416.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hasman H. The tcrB gene is part of the tcrYAZB operon conferring copper resistance in Enterococcus faecium and Enterococcus faecalis. Microbiology. 2005;151:3019–3025. doi: 10.1099/mic.0.28109-0. [DOI] [PubMed] [Google Scholar]
- 5.Amachawadi R.G., Scott H.M., Alvarado C.A., Mainini T.R., Vinasco J., Drouillard J.S., Nagaraja T.G. Occurrence of the transferable copper resistance gene tcrB among fecal enterococci of U.S. feedlot cattle fed copper-supplemented diets. Appl. Environ. Microbiol. 2013;79:4369–4375. doi: 10.1128/AEM.00503-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hasman H., Kempf I., Chidaine B., Cariolet R., Ersbøll A.K., Houe H., Hansen H.C.B., Aarestrup F.M. Copper resistance in Enterococcus faecium, mediated by the tcrB gene, is selected by supplementation of pig feed with copper sulfate. Appl. Environ. Microbiol. 2006;72:5784–5789. doi: 10.1128/AEM.02979-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amachawadi R.G., Shelton N.W., Shi X., Vinasco J., Dritz S.S., Tokach M.D., Nelssen J.L., Scott H.M., Nagaraja T.G. Selection of fecal Enterococci exhibiting tcrB-mediated copper resistance in pigs fed diets supplemented with copper. Appl. Environ. Microbiol. 2011;77:5597–5603. doi: 10.1128/AEM.00364-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amachawadi R.G., Scott H.M., Aperce C., Vinasco J., Drouillard J.S., Nagaraja T.G. Effects of in-feed copper and tylosin supplementations on copper and antimicrobial resistance in faecal enterococci of feedlot cattle. J. Appl. Microbiol. 2015;118:1287–1297. doi: 10.1111/jam.12790. [DOI] [PubMed] [Google Scholar]
- 9.Rensing C., Franke S. Copper homeostasis in Escherichia coli and other Enterobacteriaceae. EcoSal Plus. 2007;2 doi: 10.1128/ecosalplus.5.4.4.1. [DOI] [PubMed] [Google Scholar]
- 10.Hao X., Lüthje F.L., Qin Y., McDevitt S.F., Lutay N., Hobman J.L., Asiani K., Soncini F.C., German N., Zhang S., et al. Survival in amoeba—A major selection pressure on the presence of bacterial copper and zinc resistance determinants? Identification of a “copper pathogenicity island”. Appl. Microbiol. Biotechnol. 2015;99:5817–5824. doi: 10.1007/s00253-015-6749-0. [DOI] [PubMed] [Google Scholar]
- 11.Rensing C., Moodley A., Cavaco L.M., McDevitt S.F. Resistance to metals used in agricultural production. In: Schwarz S., Cavaco L., Shen J., editors. Antimicrobial Resistance in Bacteria from Livestock and Companion Animals. ASM Press; Washington, DC, USA: 2018. pp. 83–107. [Google Scholar]
- 12.Tetaz T.J., Luke R.K. Plasmid-controlled resistance to copper in Escherichia coli. J. Bacteriol. 1983;154:1263–1268. doi: 10.1128/jb.154.3.1263-1268.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams J.R., Morgan A.G., Rouch D.A., Brown N.L., Lee B.T. Copper-resistant enteric bacteria from United Kingdom and Australian piggeries. Appl. Environ. Microbiol. 1993;59:2531–2537. doi: 10.1128/aem.59.8.2531-2537.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown N.L., Barrett S.R., Camakaris J., Lee B.T.O., Rouch D.A. Molecular genetics and transport analysis of the copper-resistance determinant (pco) from Escherichia coli plasmid pRJ1004. Mol. Microbiol. 1995;17:1153–1166. doi: 10.1111/j.1365-2958.1995.mmi_17061153.x. [DOI] [PubMed] [Google Scholar]
- 15.Rouch D.A., Brown N.L. Copper-inducible transcriptional regulation at two promoters in the Escherichia coli copper resistance determinant pco. Microbiology. 1997;143:1191–1202. doi: 10.1099/00221287-143-4-1191. [DOI] [PubMed] [Google Scholar]
- 16.Lee S.M., Grass G., Rensing C., Barrett S.R., Yates C.J.D., Stoyanov J.V., Brown N.L. The Pco proteins are involved in periplasmic copper handling in Escherichia coli. Biochem. Biophys. Res. Commun. 2002;295:616–620. doi: 10.1016/S0006-291X(02)00726-X. [DOI] [PubMed] [Google Scholar]
- 17.Fang L., Li X., Li L., Li S., Liao X., Sun J., Liu Y. Co-spread of metal and antibiotic resistance within ST3-IncHI2 plasmids from E. coli isolates of food-producing animals. Sci. Rep. 2016;6:25312. doi: 10.1038/srep25312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mourão J., Marçal S., Ramos P., Campos J., Machado J., Peixe L., Novais C., Antunes P. Tolerance to multiple metal stressors in emerging non-typhoidal MDR Salmonella serotypes: A relevant role for copper in anaerobic conditions. J. Antimicrob. Chemother. 2016;71:2147–2157. doi: 10.1093/jac/dkw120. [DOI] [PubMed] [Google Scholar]
- 19.Staehlin B.M., Gibbons J.G., Rokas A., O’Halloran T.V., Slot J.C. Evolution of a heavy metal homeostasis/resistance island reflects increasing copper stress in Enterobacteria. Genome Biol. Evol. 2016;8:811–826. doi: 10.1093/gbe/evw031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta A., Matsui K., Lo J.-F., Silver S. Molecular basis for resistance to silver cations in Salmonella. Nat. Med. 1999;5:183–188. doi: 10.1038/5545. [DOI] [PubMed] [Google Scholar]
- 21.Mourão J., Novais C., Machado J., Peixe L., Antunes P. Metal tolerance in emerging clinically relevant multidrug-resistant Salmonella enterica serotype 4,[5],12:i:− clones circulating in Europe. Int. J. Antimicrob. Agents. 2015;45:610–616. doi: 10.1016/j.ijantimicag.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 22.Agga G.E., Scott H.M., Vinasco J., Nagaraja T.G., Amachawadi R.G., Bai J., Norby B., Renter D.G., Dritz S.S., Nelssen J.L., et al. Effects of chlortetracycline and copper supplementation on the prevalence, distribution, and quantity of antimicrobial resistance genes in the fecal metagenome of weaned pigs. Prev. Vet. Med. 2015;119:179–189. doi: 10.1016/j.prevetmed.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Feldpausch J.A., Amachawadi R.G., Tokach M.D., Scott H.M., Dritz S.S., Goodband R.D., Woodworth J.C., DeRouchey J.M. Effects of dietary chlortetracycline, Origanum essential oil, and pharmacological Cu and Zn on growth performance of nursery pigs. Trans. Anim. Sci. 2018;2:62–73. doi: 10.1093/tas/txx004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clinical and Laboratory Standards Institute . CLSI Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2013. Second Informational Supplement; VET01-S2. [Google Scholar]
- 25.Kozak G.K., Boerlin P., Janecko N., Reid-Smith R.J., Jardine C. Antimicrobial resistance in Escherichia coli isolates from swine and wild small mammals in the proximity of swine farms and in natural environments in Ontario, Canada. Appl. Environ. Microbiol. 2009;75:559–566. doi: 10.1128/AEM.01821-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cottell J.L., Kanwar N., Castillo-Courtade L., Chalmers G., Scott H.M., Norby B., Loneragan G.H., Boerlin P. blaCTX-M-32 on an IncN plasmid in Escherichia coli from beef cattle in the United States. Antimicrob. Agents Chemother. 2013;57:1096–1097. doi: 10.1128/AAC.01750-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rouch D., Camakaris J., Lee B.T., Luke R.K. Inducible plasmid-mediated copper resistance in Escherichia coli. J. Gen. Microbiol. 1985;131:939–943. doi: 10.1099/00221287-131-4-939. [DOI] [PubMed] [Google Scholar]
- 28.Zhou K., Zhou L., Lim Q.E., Zou R., Stephanopoulos G., Too H.-P. Novel reference genes for quantifying transcriptional responses of Escherichia coli to protein overexpression by quantitative PCR. BMC Mol. Biol. 2011;12:18. doi: 10.1186/1471-2199-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peters J.E., Craig N.L. Tn7: Smarter than we thought. Nat. Rev. Mol. Cell Biol. 2001;2:806–814. doi: 10.1038/35099006. [DOI] [PubMed] [Google Scholar]
- 30.Mitra R., McKenzie G.J., Yi L., Lee C.A., Craig N.L. Characterization of the TnsD-attTn7 complex that promotes site-specific insertion of Tn7. Mob. DNA. 2010;1:18. doi: 10.1186/1759-8753-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chakrabarti A., Desai P., Wickstrom E. Transposon Tn7 protein TnsD binding to Escherichia coli attTn7 DNA and its eukaryotic orthologs. Biochemistry. 2004;43:2941–2946. doi: 10.1021/bi035535u. [DOI] [PubMed] [Google Scholar]
- 32.Joensen K.G., Scheutz F., Lund O., Hasman H., Kaas R.S., Nielsen E.M., Aarestrup F.M. Real-time whole-genome sequencing for routine typing, surveillance, and outbreak detection of verotoxigenic Escherichia coli. J. Clin. Microbiol. 2014;52:1501–1510. doi: 10.1128/JCM.03617-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seiffert S.N., Hilty M., Kronenberg A., Droz S., Perreten V., Endimiani A. Extended-spectrum cephalosporin-resistant Escherichia coli in community, specialized outpatient clinic and hospital settings in Switzerland. J. Antimicrob. Chemother. 2013;68:2249–2254. doi: 10.1093/jac/dkt208. [DOI] [PubMed] [Google Scholar]
- 34.Randall C.P., Gupta A., Jackson N., Busse D., O’Neill A.J. Silver resistance in Gram-negative bacteria: A dissection of endogenous and exogenous mechanisms. J. Antimicrob. Chemother. 2015;70:1037–1046. doi: 10.1093/jac/dku523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Falgenhauer L., Ghosh H., Guerra B., Yao Y., Fritzenwanker M., Fischer J., Helmuth R., Imirzalioglu C., Chakraborty T. Comparative genome analysis of IncHI2 VIM-1 carbapenemase-encoding plasmids of Escherichia coli and Salmonella enterica isolated from a livestock farm in Germany. Vet. Microbiol. 2017;200:114–117. doi: 10.1016/j.vetmic.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 36.Gilmour M.W., Thomson N.R., Sanders M., Parkhill J., Taylor D.E. The complete nucleotide sequence of the resistance plasmid R478: Defining the backbone components of incompatibility group H conjugative plasmids through comparative genomics. Plasmid. 2004;52:182–202. doi: 10.1016/j.plasmid.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 37.Medardus J.J., Molla B.Z., Nicol M., Morrow W.M., Rajala-Schultz P.J., Kazwala R., Gebreyes W.A. In-feed use of heavy metal micronutrients in U.S. swine production systems and its role in persistence of multidrug-resistant salmonellae. Appl. Environ. Microbiol. 2014;80:2317–2325. doi: 10.1128/AEM.04283-13. [DOI] [PMC free article] [PubMed] [Google Scholar]