Abstract
A recent analysis found that exposure to air pollution during specific weeks of pregnancy was negatively associated with risk of autism spectrum disorder (ASD) when mutually adjusted for postnatal air-pollution exposure. In this commentary, we describe 2 possible selection-bias processes that might lead to such results, both related to live-birth bias (i.e., the inevitable restriction of the analyzed sample to live births). The first mechanism is described using a directed acyclic graph and relates to the chance of live birth being a common consequence of both exposure to air pollution and another risk factor of ASD. The second mechanism involves preferential depletion of fetuses susceptible to ASD in the higher air-pollution exposure group. We further discuss the assumptions underlying these processes and their causal structures, their plausibility, and other studies where similar phenomena might have occurred.
Keywords: air pollution, autism spectrum disorder, live-birth bias
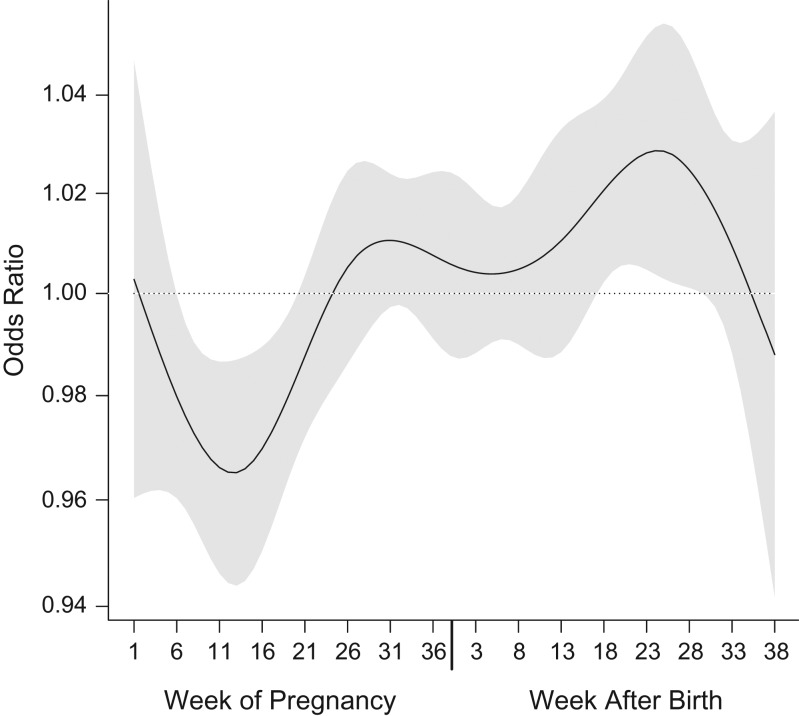
The question of whether exposure to air pollution is a risk factor for development of autism spectrum disorder (ASD) has produced more than 15 published epidemiologic analyses. Yet—and despite published reviews, meta-analyses and commentaries (1–4)—there is still lack of agreement on key specifics among analyses and authors. In a recent publication of ours in the Journal (5), we described a distributed lag model implemented with population-based ASD data and nitrogen dioxide (NO2—a tracer for traffic emissions) models from Israel that are highly resolved in time and space, to shed light on the critical perinatal period for exposure. We found that, when mutually adjusted in a distributed lag model (to avoid bias from correlation among exposure periods) (6), associations vary by week. Postnatal exposures show statistically significant positive associations a few weeks after pregnancy, while prenatal exposures reached a statistically significant negative peak (i.e., an apparent protective association) near the end of the first trimester (Figure 1, reproduced from Raz et al. (5)).
Figure 1.
Results from a distributed-lag model representing polynomial time-dependent associations between weekly nitrogen dioxide exposure and risk of autism spectrum disorder among children born in central coastal Israel during 2005–2009 (reproduced with permission from Raz et al. (5)). The black line represents the time-varying function estimating risk of autism spectrum disorder with weekly exposures during 38 weeks of pregnancy (left) and the first 38 weeks of life (right), and the gray area around it represents its 95% confidence interval. These results are from a nonlinear distributed-lag model with 7 degrees of freedom. A linear association was assumed between the exposure and the outcome at each time point. Results were adjusted for year of birth, calendar month of birth, population group, paternal age, and census poverty index.
One possible explanation for the shape of the curve is that high exposure to traffic air pollution close to the first and second trimesters is indeed protective of ASD in the offspring, through some unknown mechanism. However, we are not aware of a possible biological mechanism for this effect or of other convincing examples of air pollution being beneficial for human or animal health and development. Another possible explanation is that this is a result of a very strong negative correlation between NO2 and some other variable that is a risk factor for ASD. There are not many factors that could vary inversely with NO2 in that way. Season might be one, but the original results were adjusted for season. This adjustment should also rule out other variables that vary seasonally (e.g., pesticide application). Ozone is another possibility because it was not adjusted for and a negative correlation between these 2 gases is expected (7). In this case, exposure to ozone during mid-pregnancy would have to be a risk factor for ASD. In addition, the original results would suggest that ozone would be a protective factor after pregnancy. However, the correlation between weekly levels of NO2 and ozone in the study population is just −0.28, which would require an exceptionally strong association between ozone and ASD to fully explain the observed protective association with NO2 prenatally. Instead, we suggest that the shape of the curve could be the result of live-birth bias (8).
Live-birth bias arises from use of live births as the study population to examine prenatal exposures. The potential problem arises because it is estimated that 30%–40% of fertilized eggs will not result in a live birth (9, 10). Under several assumptions presented below, this inevitable selection of only live births into a given analysis could lead to bias in the observed association compared with the actual causal association. In this commentary, we describe 2 different ways in which live-birth bias could arise in the case of air pollution and ASD.
CONDITIONING ON A COLLIDER
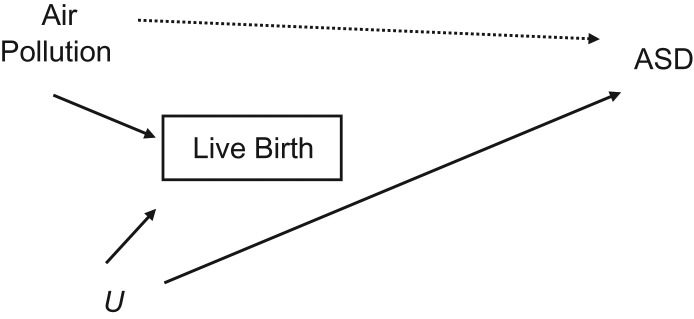
The first bias results from selection processes that might affect the distribution of exposures in the analyzed sample. Specifically, selecting on live births could result in selecting a group less likely to have coexposure to both high air pollution and high levels of other causes of ASD that also reduce the likelihood of live birth. Thus, in the analyzed sample, those with high air-pollution exposure are less likely to have other causes of ASD (and thus a lower percentage of ASD cases) compared with those with low air-pollution exposure. This scenario is described in a directed acyclic graph (DAG) (11) in Figure 2, where “Air Pollution” represents exposure to air pollution during pregnancy; “U” represents an unobserved, unknown, or simply ignored common cause of pregnancy loss and ASD; and the square around “Live Birth” represents (by DAG convention) the restriction of the analysis to those pregnancies that ended in live birth. In our case, this restriction is inevitable because ASD status cannot be defined or assessed in cases of pregnancy loss. This selective analysis could create a statistical association between air pollution and ASD, and this could bias the overall observed association between these 2 variables (for an explanation of collider, backdoor path, and other DAG terms, see Pearl (11)). An intuitive explanation for this bias is that if both air pollution and U increase the likelihood of pregnancy loss, then any live-born child with high air-pollution exposure is less likely to have also had U exposure (than a live-born child with low air-pollution exposure). Thus, live-born children exposed to high levels of air pollution are being compared with live-born children exposed to low levels of air pollution who are more likely to have been exposed to U. If U is also a risk factor for ASD, then the live-born children who had low air-pollution exposure during pregnancy will be more likely to have ASD. (Note that the same bias would occur if U protected against both pregnancy loss and ASD). This bias has the same structure as in the “birth weight paradox,” in which smoking appears protective for neonatal mortality among low-birth-weight babies but not overall (12).
Figure 2.
A directed acyclic graph representing the bias resulting from conditioning on a collider. Conditioning on the collider, live birth, opens the backdoor path: Autism Spectrum Disorder (ASD) ← Unobserved (U) → Live Birth ← Air Pollution, therefore leading to selection bias.
For the bias to act, only 2 assumptions need to hold, as specified in the DAG: 1) Air pollution during pregnancy affects the chance of a live birth; and 2) another variable (U) exists that affects both the chance of a live birth and ASD in the child. In the case where air pollution is a risk factor for pregnancy loss, then—for a protective association with ASD to appear—the U variable must either increase the risk of both pregnancy loss and ASD or decrease the risk of both.
Assumption 1 (Air Pollution → Live Birth) is not established in the literature and has been rarely examined. We believe that the main reason for this is that most events of pregnancy loss are caused by spontaneous abortions, which are not fully documented in medical databases (and losses that occur in very early stages might be from pregnancies that were not even recognized by the pregnant woman herself). Still, several studies support this link: Increased risk of stillbirth with higher exposure to air pollution was found in studies from New Jersey (13) and Ohio (14) in the United States and from Sao Paulo, Brazil (15), although other studies have examined this question and not found significant associations (16, 17). The role for air-pollution exposure in pregnancy loss in earlier stages of pregnancy is harder to study because many of these incidents are not documented, but some evidence from various locations exists (18–23), and these are further supported by mechanistic results from toxicological studies in animals (24, 25).
One example would be enough to verify assumption 2, that a variable U exists that is a common cause of both pregnancy loss and ASD. Such an example might be prenatal stress: Existing literature supports its involvement in increased risk for both pregnancy loss (26–36) and ASD in the offspring (37–45). Thus, prenatal stress is a valid candidate for our U, although the suggested bias mechanism is not limited to this specific factor.
DEPLETION OF SUSCEPTIBLES
The second possible bias results from processes by which the exposure of interest (here air pollution) selects into the analyzed sample (live-born children) those fetuses that are less susceptible to developing ASD later on. Here, we first assume that among all conceptions there is a group that is more susceptible to developing ASD (henceforth: susceptible fetuses) and a group less susceptible (henceforth: nonsusceptible fetuses). If all fetuses survived long enough to be diagnosed with ASD if they had it, then a larger proportion of susceptible fetuses would develop ASD than nonsusceptible fetuses. For a paradoxical protective association to arise with an exposure like air pollution, the key factor is that air pollution must lead to fetal loss (selection), and it must do so preferentially among the susceptible fetuses. In this case, the proportion of susceptible fetuses selected into the analysis sample (live-born children that survive to the age of ASD diagnosis) will vary across air-pollution levels, with a lower relative proportion of susceptible fetuses at higher air-pollution exposures.
Because this form of bias involves effect modification, which cannot be depicted by a DAG, we used a different graphical illustration for it (Web Figure 1, available at https://academic.oup.com/aje). We assumed no association between air pollution and ASD and a binary exposure for simplicity. If air pollution causes the loss of susceptible fetuses, the group of live-born children with higher exposure to air pollution will be skewed to have fewer susceptible fetuses than the lower-exposed group. Thus, the lower-exposed group will appear to have a higher incidence of ASD, and the exposure of interest will appear protective. This bias will generally arise when the exposure of interest (in our example, air pollution) affects the distribution in the analyzed sample (typically through selection into the analyzed sample) of another risk factor of the outcome of interest that is not accounted for in analyses—usually because information is not available. One possibility for this other unaccounted-for risk factor could be some genetic susceptibility to ASD, but it could also be nongenetic factors, such as components of the timing and intensity of the exposure that are not captured by the exposure assessment model. This downward bias can also be present when air pollution is causally related to ASD.
To give a concrete example: Imagine that underlying genetic differences modify cellular processes of oxidative stress, and thus render only a subset of fetuses susceptible to developing ASD (from various factors that might increase oxidative stress). Let us assume that air pollution leads to preferential loss of some of the fetuses with this genetic susceptibility for ASD, possibly because it induces oxidative stress that could lead to fetal loss. In this case, fetuses exposed to relatively high levels of air pollution during pregnancy and who also survived to live birth would be skewed away from susceptible fetuses because of pregnancy loss, and so fewer cases of ASD would occur. On the other hand, fetuses with low exposure to air pollution that survived to live birth would not be skewed to fewer susceptible fetuses by air-pollution-induced pregnancy loss, and so more ASD would occur in those fetuses from other risk factors acting through oxidative-stress mechanisms. As with the first bias described, this bias also requires air pollution to cause fetal loss. In addition, it requires that this loss occur preferentially among fetuses more likely to develop ASD (susceptible fetuses).
CONCLUSION
In summary, we suggest that live-birth bias can lead to a paradoxical reversal of an effect estimate through 2 related but slightly different biases. One possible reason for this is selection of a group for analysis among whom those with high exposure to air pollution have low exposure to other risk factors for ASD (or higher exposure to protective factors). Another possible reason is selection of a group for analysis among whom those with high exposure to air pollution have, on average in the live-born population, lower susceptibility to the effects of other risk factors for ASD than those with lower air-pollution exposure.
As described by Liew et al. (8), live-birth bias might exist in other situations of prenatal exposure and postnatally diagnosed outcomes. In their scenario, the suspicion was that the live-birth bias might have induced a bias towards the null of the true effect of exposure to perfluoroalkyl substances during pregnancy on attention deficit–hyperactivity disorder (8). A more recent study, however, found prenatal exposure to perfluoroalkyl substances to be negatively associated with ASD in the offspring (46). This could suggest a “paradoxical” protective association like the findings discussed above for NO2. Importantly, other developmental outcomes might well be affected by live-birth bias as well.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Braun School of Public Health and Community Medicine, the Hebrew University of Jerusalem, Jerusalem, Israel (Raanan Raz); Department of Environmental Health Sciences, Columbia University Mailman School of Public Health, New York City, New York (Marianthi-Anna Kioumourtzoglou); Department of Environmental Health, Harvard T.H. Chan School of Public Health, Boston, Massachusetts (Marc G. Weisskopf); and Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, Massachusetts (Marc G. Weisskopf).
This study was funded by the National Institutes of Health (grants ES026900, ES000002, and ES009080).
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of any of the authors’ affiliated institutions. The funding sources played no role in the design and conduct of the study; the collection, management, analysis, and interpretation; or the preparation, review, and approval of the manuscript.
Conflict of interest: none declared.
Abbreviations
- ASD
autism spectrum disorder
- DAG
directed acyclic graph
- NO2
nitrogen dioxide
REFERENCES
- 1. Lam J, Sutton P, Kalkbrenner A, et al. . A systematic review and meta-analysis of multiple airborne pollutants and autism spectrum disorder. PLoS One. 2016;11(9):e0161851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yang C, Zhao W, Deng K, et al. . The association between air pollutants and autism spectrum disorders. Environ Sci Pollut Res Int. 2017;24(19):15949–15958. [DOI] [PubMed] [Google Scholar]
- 3. Flores-Pajot MC, Ofner M, Do MT, et al. . Childhood autism spectrum disorders and exposure to nitrogen dioxide, and particulate matter air pollution: a review and meta-analysis. Environ Res. 2016;151:763–776. [DOI] [PubMed] [Google Scholar]
- 4. Weisskopf MG, Kioumourtzoglou MA, Roberts AL. Air pollution and autism spectrum disorders: causal or confounded? Curr Environ Health Rep. 2015;2(4):430–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Raz R, Levine H, Pinto O, et al. . Traffic related air pollution and autism spectrum disorder: a population based nested case-control study in Israel. Am J Epidemiol. 2018;187(4):717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wilson A, Chiu YM, Hsu HL, et al. . Potential for bias when estimating critical windows for air pollution in children’s health. Am J Epidemiol. 2017;186(11):1281–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Seinfeld JH, Pandis SN. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change. New York City, NY: Wiley-VCH; 2006. [Google Scholar]
- 8. Liew Z, Olsen J, Cui X, et al. . Bias from conditioning on live birth in pregnancy cohorts: an illustration based on neurodevelopment in children after prenatal exposure to organic pollutants. Int J Epidemiol. 2015;44(1):345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chard T. 11 Frequency of implantation and early pregnancy loss in natural cycles. Baillieres Clin Obstet Gynaecol. 1991;5(1):179–189. [DOI] [PubMed] [Google Scholar]
- 10. Wilcox AJ, Weinberg CR, O’Connor JF, et al. . Incidence of early loss of pregnancy. N Engl J Med. 1988;319(4):189–194. [DOI] [PubMed] [Google Scholar]
- 11. Pearl J. Causal inference in the health sciences: a conceptual introduction. Health Serv Outcomes Res Methodol. 2001;2(3–4):189–220. [Google Scholar]
- 12. Hernández-Díaz S, Schisterman EF, Hernán MA. The birth weight “paradox” uncovered? Am J Epidemiol. 2006;164(11):1115–1120. [DOI] [PubMed] [Google Scholar]
- 13. Faiz AS, Rhoads GG, Demissie K, et al. . Ambient air pollution and the risk of stillbirth. Am J Epidemiol. 2012;176(4):308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. DeFranco E, Hall E, Hossain M, et al. . Air pollution and stillbirth risk: exposure to airborne particulate matter during pregnancy is associated with fetal death. PLoS One. 2015;10(3):e0120594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pereira LA, Loomis D, Conceição GM, et al. . Association between air pollution and intrauterine mortality in Sao Paulo, brazil. Environ Health Perspect. 1998;106(6):325–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Medeiros AP, Gouveia N, Machado RP, et al. . Traffic-related air pollution and perinatal mortality: a case–control study. Environ Health Perspect. 2009;117(1):127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hwang BF, Lee YL, Jaakkola JJ. Air pollution and stillbirth: a population-based case-control study in Taiwan. Environ Health Perspect. 2011;119(9):1345–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Green RS, Malig B, Windham GC, et al. . Residential exposure to traffic and spontaneous abortion. Environ Health Perspect. 2009;117(12):1939–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Enkhmaa D, Warburton N, Javzandulam B, et al. . Seasonal ambient air pollution correlates strongly with spontaneous abortion in Mongolia. BMC Pregnancy Childbirth. 2014;14:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hou HY, Wang D, Zou XP, et al. . Does ambient air pollutants increase the risk of fetal loss? A case-control study. Arch Gynecol Obstet. 2014;289(2):285–291. [DOI] [PubMed] [Google Scholar]
- 21. Hemminki K, Niemi ML. Community study of spontaneous abortions: relation to occupation and air pollution by sulfur dioxide, hydrogen sulfide, and carbon disulfide. Int Arch Occup Environ Health. 1982;51(1):55–63. [DOI] [PubMed] [Google Scholar]
- 22. Moridi M, Ziaei S. Exposure to ambient air pollutants and spontaneous abortion. J Obstet Gynaecol Res. 2014;40(3):743–748. [DOI] [PubMed] [Google Scholar]
- 23. Ha S, Sundaram R, Buck Louis GM, et al. . Ambient air pollution and the risk of pregnancy loss: a prospective cohort study. Fertil Steril. 2018;109(1):148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Januário DA, Perin PM, Maluf M, et al. . Biological effects and dose-response assessment of diesel exhaust particles on in vitro early embryo development in mice. Toxicol Sci. 2010;117(1):200–208. [DOI] [PubMed] [Google Scholar]
- 25. Veras MM, Damaceno-Rodrigues NR, Guimarães Silva RM, et al. . Chronic exposure to fine particulate matter emitted by traffic affects reproductive and fetal outcomes in mice. Environ Res. 2009;109(5):536–543. [DOI] [PubMed] [Google Scholar]
- 26. Brunton PJ. Effects of maternal exposure to social stress during pregnancy: consequences for mother and offspring. Reproduction. 2013;146(5):R175–R189. [DOI] [PubMed] [Google Scholar]
- 27. Plana-Ripoll O, Parner E, Olsen J, et al. . Severe stress following bereavement during pregnancy and risk of pregnancy loss: results from a population-based cohort study. J Epidemiol Community Health. 2016;70(5):424–429. [DOI] [PubMed] [Google Scholar]
- 28. Coughlan C, Walters S, Ledger W, et al. . A comparison of psychological stress among women with and without reproductive failure. Int J Gynecol Obstet. 2014;124(2):143–147. [DOI] [PubMed] [Google Scholar]
- 29. Dean RG, Dean J, Heller GZ, et al. . A mass shooting at Port Arthur, Tasmania, Australia: a study of its impact on early pregnancy losses using a conception time-based methodology. Hum Reprod. 2015;30(11):2671–2676. [DOI] [PubMed] [Google Scholar]
- 30. Neugebauer R, Kline J, Stein Z, et al. . Association of stressful life events with chromosomally normal spontaneous abortion. Am J Epidemiol. 1996;143(6):588–596. [DOI] [PubMed] [Google Scholar]
- 31. Nepomnaschy PA, Welch KB, McConnell DS, et al. . Cortisol levels and very early pregnancy loss in humans. Proc Natl Acad Sci U S A. 2006;103(10):3938–3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wainstock T, Lerner-Geva L, Glasser S, et al. . Prenatal stress and risk of spontaneous abortion. Psychosom Med. 2013;75(3):228–235. [DOI] [PubMed] [Google Scholar]
- 33. Fenster L, Schaefer C, Mathur A, et al. . Psychologic stress in the workplace and spontaneous abortion. Am J Epidemiol. 1995;142(11):1176–1183. [DOI] [PubMed] [Google Scholar]
- 34. Wisborg K, Barklin A, Hedegaard M, et al. . Psychological stress during pregnancy and stillbirth: prospective study. BJOG. 2008;115(7):882–885. [DOI] [PubMed] [Google Scholar]
- 35. Li W, Newell-Price J, Jones GL, et al. . Relationship between psychological stress and recurrent miscarriage. Reprod Biomed Online. 2012;25(2):180–189. [DOI] [PubMed] [Google Scholar]
- 36. Bruckner TA, Mortensen LH, Catalano RA. Spontaneous pregnancy loss in Denmark following economic downturns. Am J Epidemiol. 2016;183(8):701–708. [DOI] [PubMed] [Google Scholar]
- 37. Class QA, Abel KM, Khashan AS, et al. . Offspring psychopathology following preconception, prenatal and postnatal maternal bereavement stress. Psychol Med. 2014;44(1):71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Babenko O, Kovalchuk I, Metz GA. Stress-induced perinatal and transgenerational epigenetic programming of brain development and mental health. Neurosci Biobehav Rev. 2015;48:70–91. [DOI] [PubMed] [Google Scholar]
- 39. Bercum FM, Rodgers KM, Benison AM, et al. . Maternal stress combined with terbutaline leads to comorbid autistic-like behavior and epilepsy in a rat model. J Neurosci. 2015;35(48):15894–15902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Walder DJ, Laplante DP, Sousa-Pires A, et al. . Prenatal maternal stress predicts autism traits in 6½ year-old children: Project Ice Storm. Psychiatry Res. 2014;219(2):353–360. [DOI] [PubMed] [Google Scholar]
- 41. Roberts AL, Lyall K, Rich-Edwards JW, et al. . Association of maternal exposure to childhood abuse with elevated risk for autism in offspring. JAMA Psychiatry. 2013;70(5):508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Roberts AL, Koenen KC, Lyall K, et al. . Women’s posttraumatic stress symptoms and autism spectrum disorder in their children. Res Autism Spectr Disord. 2014;8(6):608–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Beversdorf DQ, Manning SE, Hillier A, et al. . Timing of prenatal stressors and autism. J Autism Dev Disord. 2005;35(4):471–478. [DOI] [PubMed] [Google Scholar]
- 44. Kinney DK, Munir KM, Crowley DJ, et al. . Prenatal stress and risk for autism. Neurosci Biobehav Rev. 2008;32(8):1519–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kinney DK, Miller AM, Crowley DJ, et al. . Autism prevalence following prenatal exposure to hurricanes and tropical storms in Louisiana. J Autism Dev Disord. 2008;38(3):481–488. [DOI] [PubMed] [Google Scholar]
- 46. Hertz-Picciotto I, Park HY, Dostal M, et al. . Prenatal exposures to persistent and non-persistent organic compounds and effects on immune system development. Basic Clin Pharmacol Toxicol. 2008;102(2):146–154. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.