Abstract
Cadherin-based adherens junctions (AJs) and desmosomes are crucial to couple intercellular adhesion to the actin or intermediate filament cytoskeletons, respectively. As such, these intercellular junctions are essential to provide not only integrity to epithelia and other tissues but also the mechanical machinery necessary to execute complex morphogenetic and homeostatic intercellular rearrangements. Moreover, these spatially defined junctions serve as signaling hubs that integrate mechanical and chemical pathways to coordinate tissue architecture with behavior. This review takes an evolutionary perspective on how the emergence of these two essential intercellular junctions at key points during the evolution of multicellular animals afforded metazoans with new opportunities to integrate adhesion, cytoskeletal dynamics, and signaling. We discuss known literature on cross-talk between the two junctions and, using the skin epidermis as an example, provide a model for how these two junctions function in concert to orchestrate tissue organization and function.
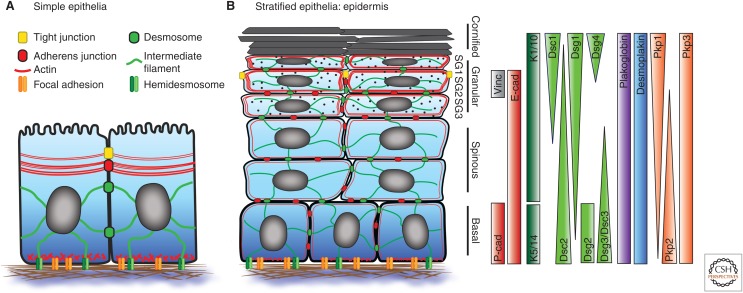
Epithelial barrier formation, homeostasis, renewal, and restoration require cells to integrate different intercellular adhesive cues, cytoskeletal dynamics, and signaling in- and output. Central to the evolution of multicellular metazoans was the ability to connect adhesion at intercellular junctions with the organization of the cytoskeleton to coordinate formation, shape, and function of simple epithelial sheets (Abedin and King 2008; Hulpiau et al. 2013; Miller et al. 2013). This evolution drove the formation of the first intercellular junction, the adherens junction (AJ), in which the plasma membrane served as an organizing platform for adhesive cadherin–catenin complexes. Ultimately, these primitive junctions provided a blueprint for the formation of specialized, spatially and structurally defined junctional adhesive and/or barrier-forming complexes along the basal to apical axis (Rodriguez-Boulan and Macara 2014), essential for managing the functions of simple epithelia (Fig. 1A). These functions include spatial regulation of signaling, vectorial vesicle transport, tissue specific barrier function and, especially, regulation of cytoskeletal dynamics to control cell and tissue mechanics crucial for tissue morphogenesis, homeostasis, and regeneration.
Figure 1.
Apicobasolateral polarization of junctions and the cytoskeleton in epithelia. (A) In simple epithelia and (B) in the epidermis as an example of stratified epithelia. The spatial distribution of adherens junction (AJ) and desmosomal proteins across the different layers of the epidermis is also shown.
Later in evolution, additional epithelial complexity arose in the form of stratified epithelia and their appendages. The most visible example is the epidermis, the first line of defense against water loss, mechanical insults, and pathogens in higher vertebrates (Fuchs 2007; Watt 2014). This constantly regenerating barrier balances proliferation in the basal layer with a tightly controlled differentiation program in which cells move upward while undergoing stepwise transcriptional and cell shape changes to form the distinct suprabasal layers: the stratum spinosum, stratum granolusum and stratum corneum (Fig. 1B). Distribution of chemical signaling that controls proliferation and differentiation must thus be closely coordinated with the adhesive and cytoskeleton machinery that drives the structural cell shape changes associated with formation of this stratified physical barrier.
What evolutionary strategies were used to create such additional tissue complexity required to correctly distribute the chemical and mechanical apparatus in a highly patterned and reproducible 3D fashion? To accommodate this increasing organismal and tissue-specific complexity, junctions and their core components diversified from adhesive actin-linked AJs and barrier-promoting tight and septate junctions to channel-forming gap junctions (GJs) and, in vertebrates, adhesive intermediate filament (IF)-linked desmosomes (Green et al. 2010). Each of these junctions have been studied extensively with respect to composition and their adhesive, mechanical, signaling or barrier properties (see, e.g., Braga 2017; Delmar et al. 2017; Hatzfeld et al. 2017; Mege and Ishiyama 2017; Yap et al. 2017). What is much less appreciated is how cells integrate different junctional mechanical and signaling activities into a higher order network essential to coordinate cell shape and positioning with tissue renewal, differentiation, and regeneration.
In this review, we will highlight the literature that uncovers synergy between cadherin adhesive intercellular junctions in tissue architecture and/or signaling. We will first touch on the evolutionary significance of cadherin-based junctions and their molecular components. We will then briefly introduce the main lessons on junctional interdependence in simple epithelia and nonepithelial tissues and discuss recent literature on AJs and desmosomes in the epidermis that show how these cadherin-based junctions integrate adhesive, mechanical, and kinase-transmitted signals to control cell shape and/or differentiation. Finally, using the mammalian epidermis, we will propose a model by which a highly synergistic and dynamic intercellular junctional network provides a template for organizing tissue structure tailored for tissue-specific functional requirements.
MAMMALIAN CADHERIN-BASED INTERCELLULAR JUNCTIONS: A BRIEF INTRODUCTION
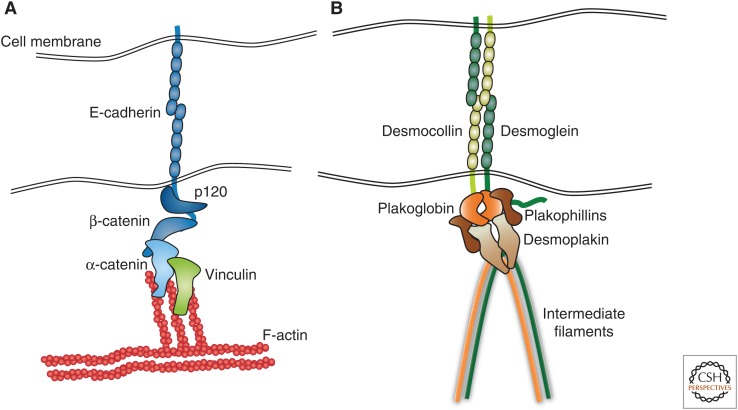
Intercellular junctions allow cells to adhere and communicate with each other while also separating tissues from the external world or from each other. Organisms also adopted these junctions as spatially defined signaling platforms that allowed them to orchestrate cyto-architectural changes with signal communication (see, e.g., Chiasson-MacKenzie and McClatchey 2017). Specialized mammalian junctions include the tight junctions (TJ) that form a paracellular barrier ion- and size barrier (Van Itallie and Anderson 2014; Balda and Matter 2016) and GJ that form small molecule channels to promote intercellular communication (Nielsen et al. 2012). Here, we will focus on cadherin-based intercellular junctions as a paradigm for adhesion complexes found in mammalian epithelia (Fig. 2).
Figure 2.
Schematic representation of core composition of (A) adherens junction and (B) desmosomes.
Adherens Junctions
AJs are multiprotein complexes that mechanically couple cell–cell adhesion to the F-actin cytoskeleton. In addition to AJs at the lateral membrane, simple epithelia have developed a specialized AJ, the apically positioned zonula adherens (ZA). Members of the classical cadherin family of proteins, for example, E-cadherin, N-cadherin, VE-cadherin, and P-cadherin, form the calcium-dependent adhesive backbone of AJs that through homophilic and/or heterophilic interactions connect cells (Niessen et al. 2011). At their cytoplasmic face, this cadherin subfamily interacts with the armadillo repeat proteins p120ctn and β-catenin and through the latter connect via α-catenin with the actin cytoskeleton (Fig. 2A).
Through this core complex, cadherins can interact with a range of other cytoskeletal linker molecules, adaptor proteins, and signaling proteins, as was shown by both targeted biochemical and cell biology analyses as well as recent unbiased proteomic analysis (Padmanabhan et al. 2015). The latter was also referred to as the cadherin adhesome. Cadherins can not only mechanically sense but also respond to extracellular and intracellular mechanical signals to modulate actomyosin connections and thus the mechanical strength of AJs (see, for example, Mege and Ishiyama 2017; Yap et al. 2017). For example, force will stabilize the interaction of the core cadherin–catenin complex with actin through a catch bond (Buckley et al. 2014). One of the best characterized mechanical AJ pathway is the regulated recruitment of vinculin to the AJs, which relies on a force-dependent conformational change in α-catenin that unmasks a vinculin-binding site (Leckband and de Rooij 2014; Ladoux et al. 2015). Several other mechanosensitive interactions at AJs have recently also been identified (Bays et al. 2017; Conway et al. 2017; Hart et al. 2017). Based on the magnitude of the cadherin adhesome (Guo et al. 2014), it is likely that many of the described interactions will be subject to mechanical- and/or signaling-dependent regulation. The challenge will be to identify conditions that reveal the physiological relevance for many of these interactions.
Desmosomes
Like AJs, desmosomes are cadherin-based, multiprotein complexes that couple intercellular adhesion not to the actin cytoskeleton, but instead, to the IF system (Fig. 2B). Desmosomes are mainly found in epithelia and in the heart of vertebrates. The desmosomal cadherin subfamily consists of desmogleins (in humans, Dsg1–4) and desmocollins (in humans, Dsc1–3) that show tissue- and differentiation-specific expression patterns. How desmosomal cadherins promote adhesion is still not well understood but it has been reported that both homophilic and heterophilic interactions between desmogleins and desmocollins can occur. The extent to which one or the other functions in vivo is not known. The desmosomal cadherin cytoplasmic domains bind to the armadillo protein plakoglobin, a β-catenin homolog, and to the plakophilin (PKP) family of armadillo proteins. These armadillo linker proteins then interact with the IF-binding protein desmoplakin (DP), thus connecting desmosomes to the IF cytoskeleton (Fig. 2B). For further details on desmosomal composition and function, we refer to several outstanding reviews (Delva et al. 2009; Thomason et al. 2010; Broussard et al. 2015). Unlike AJs, desmosomes can adopt a hyperadhesive state that is independent of calcium and for example, controlled through protein kinase C (PKC)-dependent posttranslational modifications in desmosomal components (Garrod and Tabernero 2014; Hatzfeld et al. 2017).
As mutations in different desmosomal components result in skin blistering and sudden cardiac arrest syndromes (reviewed in Samuelov and Sprecher 2015), desmosomes are considered essential for providing mechanical strength to tissues. Studies indicate that the desmosome/IF system, like the AJ/actin system, is not only important for withstanding mechanical stress but also instructive in the establishment of the inherent mechanical properties of cells. Keratinocytes deficient for all keratins revealed that keratins are key determinants of cell stiffness to control their migratory behavior (Ramms et al. 2013; Seltmann et al. 2013). A very recent study showed that the IF-binding domain within DP controls intercellular tension and cell stiffness, which in part are mediated through regulation of the actin cytoskeleton (Broussard et al. 2017).
It is becoming increasingly clear that desmosomal components as well as IF, and thus likely desmosomes as a whole, also serve a range of other purposes related to growth, differentiation, and immune homeostasis. Null mutations in the cadherin Dsg1 or DP result in a metabolic wasting syndrome further characterized by inflammation (Samuelov et al. 2013; McAleer et al. 2015). These keratinocytes also show increased expression of inflammatory mediators, thus highlighting the potential relevance of these other functions. Albeit, these phenotypes could also be secondary effects resulting from disturbed epithelial barrier function as a consequence of impaired intercellular cohesion. It is thus essential to consider the desmosome as an integrator of mechanics and signaling that coordinates tissue structure with function when examining the underlying causes of phenotypes observed in desmosomal diseases.
Testifying to the notion that AJs and desmosomes functionally integrate adhesive, cytoskeletal, and signaling responses, it is important to note that several mammalian tissues/organs have molecularly distinct or mixed junctions crucial for tissue function. For example, the majority of intercellular junctions in cardiomyocytes of higher vertebrates are hybrid junctions (Franke et al. 2009; Vite and Radice 2014) that contain components of both AJs and desmosomes. Similarly, AJs in vascular endothelial cells contain desmosomal components like plakoglobin and DP and can connect to the IF cytoskeleton as well (Franke et al. 2009). Thus, in these tissues, the AJ and desmosome systems integrate their function also on a structural level.
EVOLUTIONARY ORIGINS OF CADHERIN-DEPENDENT ADHESION AND CYTOSKELETAL ORGANIZATION
The ability of cells to form multicellular clusters with physical behaviors mimicking a fluid that support intercellular rearrangements is considered a major transition in evolution that drove the appearance of metazoa (King 2004; Newman 2016). Acquisition of these material properties that enable cellular populations to undergo morphogenesis requires coordination and/or coupling of intercellular adhesion and cytoskeletal dynamics to control cell–cell rearrangements and cell shape of the first primitive epithelia. The appearance of classical cadherins in one of the earliest metazoa, sponges, that mediate intercellular adhesion and through their newly evolved cytoplasmic domain engaged catenins to control cell polarity and the cytoskeleton (Oda and Takeichi 2011), was thus a crucial step for such a transition. Interestingly, the two molecular groups necessary for this transition, cadherins and cytoskeletal linker/adaptor molecules, seem in part to have evolved independently from each other.
Evolution of Classical and Desmosomal Cadherin Adhesion Receptors
Members of the cadherin superfamily are typically multidomain proteins that have evolved through duplication, divergence, as well as shuffling of domains between different families (Oda and Takeichi 2011; Hulpiau et al. 2013). The recent identification of a single cadherin in Capsaspora owczarzaki, the unicellular outgroup of choanoflaggelates and metazoan lineages, indicates that cadherins predate the divergence of C. owczarzaki, choanoflagelate, and metazoan lineages (Nichols et al. 2012). Interestingly, the two species of choanoflaggelates thus far analyzed both have >20 types of cadherin molecules that contain cytoplasmic domains unrelated to classical cadherins. These can be further classified in at least three families, two of which are thus far only found in sponges and choanoflaggelates, the lefftry and cohesion families. The lefftry family is characterized by a laminin amino-terminal domain, four epidermal growth factor receptor (EGFR) repeats, and a furin repeat; one or two extracellular fibronectin 3 repeats located close to the transmembrane domain; as well as a cytoplasmic inactive tyrosine phosphatase domain. The sponge Oscarella carmela also contains a lefftry family member but with an active PTP domain. The cohesion family contains a bacterial/archaeal cohesin domain. Hedgling, the third family, is found in choanoflaggelates, sponges, and cnidarians but is absent from bilaterians. This family is characterized by an amino-terminal hedgehog domain connected to a Von Willebrand A domain and many members contain several epidermal growth factor receptor (EGF) repeats (Nichols et al. 2012).
The reasons for this relatively rapid expansion of cadherins in one of the closest relatives of metazoa as well as their function are not entirely clear. The colony-forming variants of choanoflaggelates form cell–cell contacts through cytoplasmic bridges. One theory is that these cadherins are important for capture of bacterial prey and subsequent feeding (Nichols et al. 2012). Additional evidence for such a role comes from the observation that both sponges and choanoflaggelates have a motile flagellum used to generate water flow to capture prey on a surrounding microvillar collar where these bacteria are phagocytosed (Alegado and King 2014). One of the cadherins of Meloe brevicollis has been shown to localize to this collar (Abedin and King 2010; Pizarro-Cerda et al. 2012). In agreement with a potential evolutionary conserved function in bacterial recognition and internalization, human E-cadherin serves as a receptor for listeria that is necessary for its internalization (Pizarro-Cerda et al. 2012).
The last evolutionary addition to the cadherin superfamily is the desmosomal cadherin family, found only in vertrebrates (Broussard et al. 2015). What drove the evolution of desmosomal cadherins is not entirely clear. It is noteworthy, nevertheless, that their appearance coincided with further evolutionary developments such as the expansion of epithelial tissue complexity, including stratified epithelia, primitive hearts consisting of multiple chambers that were connected to an outward flow (Olson 2006), and the arrival of a more complex immune system including the adaptive immune system (Cooper and Alder 2006).
Interestingly, the extracellular domains of vertebrate classical and desmosomocal cadherins no longer combine cadherin extracellular (EC) domains with other extracellular matrix (ECM)-like and/or signaling-like repeats unlike, for example, mammalian CelsR/Flamingo or Fat nonclassical cadherin family members or Drosophila classical cadherins (Oda and Takeichi 2011; Hulpiau et al. 2013). The increasingly complex morphogenetic movements and/or increasing number of epithelial tissues later in metazoan evolution might thus have required structural separation of cadherin repeat function from signaling and ECM-like domain function to allow diversification of signaling and adhesion. Alternatively, domains other than the cadherin repeat domain may have been lost in classical and desmosomal cadherins, as their presence may have hampered increasingly dynamic adhesive interactions required to drive expanding epithelial complexity. This selective pressure might have been less strong in other classical cadherin super family members that are involved in intercellular interactions but not strong adhesion itself.
Cadherin–Cytoskeletal Linker Proteins
Both AJ and desmosomal cadherins directly interact with so-called Armadillo (Arm) repeat proteins, which contain a domain first named after the Drosphila β-catenin homolog armadillo. This Arm repeat is an evolutionarily old motif that is also found in a range of other proteins, such as importins (Gul et al. 2017). Classical cadherins interact with β-catenin/plakoglobin and p120-catenin members of the Arm repeat family, whereas desmosomal cadherins associate with plakoglobin and the plakophilin family that belong to the p120 super family. These interactions are essential to link these adhesive systems to their respective cytoskeletons via either α-catenin (classical cadherins) or DP, respectively (Green et al. 2010). As well, they regulate cell surface stability and/or control of signaling pathways crucial for cytoskeletal dynamics (Kourtidis et al. 2013; Hatzfeld et al. 2014). Interestingly, albeit outside the scope of this review, all these cadherin-interacting Arm repeat proteins can also translocate into the nucleus and interact with transcription factors (e.g., p120) or the translational machinery (e.g., Pkp1) (reviewed in McCrea and Gottardi 2016). The best characterized example is β-catenin that, next to its crucial function in adhesion, is a central player of the Wnt pathway, which evolved also around the time of the first metazoa (Pires-daSilva and Sommer 2003).
The initial evolution of these linker molecules may in part have occurred independently from the cadherins. Approximately 70% of the cadherin adhesome orthologs predate metazoans (Murray and Zaidel-Bar 2014); albeit, it is not entirely clear whether some of these were able to interact with premeatozan cadherin cytoplasmic domains. The best evidence for initially independent evolution of cadherins and the AJ complex members, α- and β-catenins, comes from the amoeba Dictyostelium discoidum. This social amoeba does not have any cadherin-like molecules but contains an α-catenin/vinculin homolog and a β-catenin-like armadillo repeat protein, Aardvark, which can interact with mammalian α-catenin as well, thus indicating functional conservation. Importantly, these two proteins are essential for actin organization and the formation of an epithelial-like cell sheet that surrounds the tip of the stalk during the multicellular stage of Dictyostelium (Grimson et al. 2000; Dickinson et al. 2011, 2012b). Although in Dictyostelium, α- and β-catenin are associated with junctions that recruit actin and ultrastructurally resemble AJs, it is not clear how these proteins associate with the cortex. Moreover, unlike metazoa, the formation of these AJ-like structures occurs independently of either α- or β-catenin (Dickinson et al. 2012a). It will thus be interesting to determine whether in social amoebae the function of α- and β-catenin in cytoskeletal and epithelial organization was independent of adhesion or requires interaction with one of the adhesion molecules found in Dictyostelium, such as the calcium-dependent, noncadherin Cad molecules or the IgG-like adhesion receptors (Siu et al. 2004). Thus, AJ cytoplasmic plaque proteins were one driving evolutionary force for promoting cell sheet formation and epithelial polarization.
In contrast, the p120 super family of proteins may have coevolved with the cadherins. Poriferans (sponges), the most-simple metazoans, already contain both a classical cadherin and a p120ctn armadillo repeat family member that is most closely related to vertebrate δ-catenin (Carnahan et al. 2010). Unlike in vertebrates, in which p120ctn is essential for cadherin function, in lower organisms such as C. elegans and Drosophila, p120ctn binding is not crucial but serves to modulate cadherin complex function (Myster et al. 2003; Pacquelet et al. 2003; Hardin 2015; Bulgakova and Brown 2016). p120ctn super family members control cadherin cell surface stability and regulate the activity of the Rho family of small GTPases (Kourtidis et al. 2013; Hatzfeld et al. 2014). These GTPases are key regulators of actomyosin dynamics, junctions, and cell shape (Garcia-Cattaneo and Braga 2013). Thus, very early on, classical cadherins may have gained the ability to not only interact with the cytoskeleton but through interactions with p120ctn members dynamically regulate surface tension and thus intercellular rearrangements.
In vertebrates, the p120ctn family expanded to 7 members (Carnahan et al. 2010), likely to adapt to the increasingly different morphogenetic demands in tissue complexity as reflected in the expansion of vertebrate classical and desmosomal cadherins. The ubiquitously expressed p120ctn may have coevolved with nonneuronal cadherins whereas δ-catenin became mostly restricted to neuronal lineages, and may have further evolved with predominantly neuronally expressed cadherins (N- and R-cadherin). Moreover, PKP1–3, and the β-catenin homolog plakoglobin, are only found in vertebrates (Zhao et al. 2011) thus coinciding with the arrival of desmosomal cadherins on the evolutionary stage. This similar timing indicates a mutual dependence of function early during vertebrate development. PKP proteins are crucial in recruiting DP, and thus IFs, to desmosomes. In addition, these proteins in part may have similar functions as p120 as these proteins can regulate actin and Rho family activity (Hatzfeld et al. 2014), thus allowing the newly arrived desmosome to communicate with the evolutionary older AJ.
DP, the molecule that links desmosomal cadherins to IFs through plakophilins and plakoglobin, is a member of the plakin family of cytoskeletal linkers. Proteins in this family are characterized by amino- and carboxy-terminal globular domains flanking a central a-helical coiled-coil rod. The amino-terminal plakin domain comprises a series of spectrin repeats and a Src-homogy 3 domain involved in junctional targeting and the carboxy-terminal domain comprises a series of plakin repeat domains (PRDs) that associate with IFs to confer structural integrity and resilience to tissues (Leung et al. 2002). Plakins, first observed in chordates, most likely arose from the spectraplakin family, which was first found in sponges and characterized by their ability to directly engage different cytoskeletal systems, either in a single protein or through expression of splice variants (Huelsmann and Brown 2014). In C. elegans, one splice variant of a spectraplakin, vab10a, is essential to link IFs to hemidesmosomes in epidermal cells, an adhesive structure that connects muscle to the cuticle (Bosher et al. 2003), thus sharing a key feature with DP. DP itself likely coevolved with desmosmal cadherins in vertebrates.
Taken together, then, there were two decisive evolutionary developments for intercellular junctional cadherin complexes:
Convergence of the initially independent evolution of cadherin adhesive domains and the actin-engaging α-, β-catenin module, with the later appearing classical cadherin cytoplasmic domain enabled organisms to couple cell adhesion to cortical tension. The simultaneous arrival of the first p120-like molecule likely allowed these simple early metazoans to dynamically and mechanically control the cadherin–cytoskeletal link to drive intercellular morphogenetic rearrangements. It is worth noting that key signaling receptors necessary for intercellular communication and fate determination, such Wnt and receptor tyrosine kinases also appeared at the same time with the classical cadherin/catenin complex (Pires-daSilva and Sommer 2003). It may thus well be possible that signaling and adhesion-dependent mechanics were integrated early during metazoan evolution.
The simultaneous evolutionary diversification of cadherins, p120, and β-catenin arm families, and plakin family into desmosomal cadherins, PKPs, plakoglobin, and DP, respectively, in vertrebrates enabled the formation of desmosomes to engage IFs. These junctions thus provide mechanical stability and resilience likely necessary to accommodate more complex tissue structures such as a multichambered heart, or stratifying epithelia. These early vertebrates also faced new challenges in the terrestrial environment, such as sunlight, and exposure to different pathogens; desmosomes may then have contributed to newly acquired epidermal barrier functions including integration of the innate immune with the adaptive immune system that arose at the same time.
INTERPLAY BETWEEN JUNCTIONS: LESSONS FROM EARLY DEVELOPMENT, SIMPLE EPITHELIA, AND HEART
Tissue morphogenesis, homeostasis, and regeneration require coordinated cellular rearrangements. Early studies identified AJs as key regulators of epithelial structure and intercellular junction formation. Complete loss of E-cadherin, β-catenin, or α-catenin, in Drosophila, C. elegans, Xenopus, Zebrafish, or mice results in early embryonic lethality with severe defects in epithelial integrity, junction formation, and polarity (reviewed in Harris and Tepass 2010). Moreover, in simple epithelial cell culture models, antibodies to E-cadherin interfere not only with AJs but also desmosome, GJ, and TJ formation (Gumbiner et al. 1988). Although in several simple epithelia (e.g., liver, mammary, or thyroid gland), loss of E-cadherin did not obviously affect intercellular junctions (Boggetti and Niessen 2012), loss of E-cadherin in intestinal epithelial cells impaired junctions and barrier function (Schneider et al. 2010; Bondow et al. 2012). In keratinocytes, loss of E-cadherin is sufficient to interfere with TJs, whereas only combined loss of both classical cadherins, E- and P-cadherin, prevented formation of desmosomes in these cells (Michels et al. 2009). Classical cadherin-dependent AJs may thus serve as master regulators to initiate the formation of intercellular junctions in simple epithelia. In agreement, cell culture and organismal models reveal that AJ formation precedes the assembly of desmosomes, GJs, and TJs (Fleming et al. 1994; Vasioukhin et al. 2001b).
Even though known human diseases arising from mutations related to desmosomal proteins (see below) suggest that desmosomes are only essential in heart and skin, inactivation of the desmosomal cadherins Dsg2 (Eshkind et al. 2002) and Dsc3 (Den et al. 2006) or the cytoplasmic linker proteins DP (Gallicano et al. 1998), are early embryonic lethal in mice. Similarly, loss of zebrafish Dsc and Dsg interferes with epiboly (Goonesinghe et al. 2012), further revealing important roles for these proteins in early morphogenetic cellular rearrangements. The majority of these phenotypes are associated with impaired desmosomal adhesive or IF linkage function, leading to increased tissue fragility. The role of desmosomal proteins in the regulation of other junctions or other functions apart from providing mechanical stability have not been well studied in these early lethal mutants. Mouse knockouts for those desmosomal proteins that did not result in early embryonic lethality (e.g., Dsg3, Dsc1, PG, PKP1) resulted in impaired heart development and/or skin blistering (McCauley and Wehrens 2009; Ganeshan et al. 2010), thus providing direct evidence that the observed mutations in the human gene counterparts or the auto-antibodies against desmosomal cadherins are indeed causal for the associated diseases (Samuelov and Sprecher 2015).
The role of desmosomal proteins in simple epithelia has only been marginally explored. Surprisingly, inactivation of DP in the mouse intestinal epithelium did not obviously affect AJs, TJs, or intercellular adhesion, resulting in viable mice, suggesting that desmosomes are dispensable in this tissue (Sumigray and Lechler 2012). Instead, microvilli structure is shortened, thus providing further in vivo evidence that desmosomes affect actin-based structures, similar to what has been observed in keratinocytes (Vasioukhin et al. 2001b). In contrast, CRISPR/Cas-mediated inactivation of Dsc2 and Dsg2 in colon carcinoma DLD1 cells caused intercellular fragility accompanied by the absence of desmosomes but not AJs and TJs (Fujiwara et al. 2015). Together, these data thus suggest that in simple epithelia desmosomes do not obviously regulate the formation of other junctions. Having said that, in the future it will be essential to examine the function of different desmosomal proteins in other simple epithelia as well as challenge desmosomal-specific knockout mice that may unravel stress-associated functions for desmosomes in intercellular junctional rearrangements and tissue regeneration.
Interestingly, recent studies in the heart have revealed an important role for desmosomes in controlling GJ function in addition to their mechanical contribution at the intercalated disc. Cardiomyocytes are electrically coupled through GJs, essential for cardiac rhythm and contraction. Mice with a cardiomyocyte-specific deletion of DP show loss of connexin-40 and -43, the molecular building blocks of the GJ channels, accompanied by right ventricular conduction defects, leading to AC (Lyon et al. 2014). Similarly, cardiac loss of Dsg2 or cardiac expression of an adhesion-defective Dsg2 mutant results in AC and an altered connexin-43 distribution, which was linked to the adhesive function of Dsg2 (Kant et al. 2015). Mutations in human DP that are linked to AC also result in junctional loss of connexin-43 (Samuelov and Sprecher 2015). The molecular mechanism responsible remains to be elucidated. DP mutations disrupt binding to the microtubule-associated protein end-binding1 (EB1), which regulates trafficking of connexin-43 to GJs (Patel et al. 2014). Desmosomes may thus stabilize microtubules (MTs) through EB1, providing a spatial cue for membrane targeting of connexin-43. Together, these results provide a potential molecular explanation on how desmosomes regulate GJ stability.
POLARITY AND JUNCTIONS IN STRATIFIED EPITHELIA
Many of the insights regarding junctional cooperativity in the formation, maintenance, and restoration of epithelial tissues come from studies performed on the epidermis, a stratified epithelium. Unlike simple epithelia, stratified epithelia, also including epithelia of the esophagus, urinary tract, and oral cavity, constitute two or more layers. These additional layers essentially make it impossible to establish apicobasolateral polarity within one layer, as observed in simple epithelia. Instead, stratified epithelia establish polarity of cytoskeletal organization and intercellular junctions along the basal to apical axis of the entire tissue through as yet poorly understood mechanisms (Fig. 1B; Muroyama and Lechler 2012; Tellkamp et al. 2014). Below, we will briefly introduce the epidermis and address the contribution of AJs and desmosomes in epidermal polarity, structure, and function.
The self-renewing epidermis forms the outermost skin structural and innate immune barrier that protects from dehydration and external challenges, such as UV and microbes (Blanpain and Fuchs 2009). This tissue is a remarkable example of exquisite and robust control of continuous, dynamic cellular rearrangements and cell-shape changes while cells are en route from the basal to the stratum corneum layer, all while retaining an intact barrier. To maintain homeostasis or to restore injury without inflicting disease thus requires integration of signals that regulate barrier architecture with those that govern innate immunity and metabolic activity. Improper development or disturbed maintenance of the epidermal barrier results in a range of skin diseases including common inflammatory skin diseases, such as atopic dermatitis and psoriasis, impaired healing, and skin cancer (Kubo et al. 2012).
The process of stratification that generates this multilayered epithelial barrier requires a spatiotemporally orchestrated set of transcriptional and architectural changes that result in complete remodeling of epidermal cell shape and function (Candi et al. 2005; Simpson et al. 2011). During this process, cell architectural features such as junctions and cytoskeletal proteins are polarized across layers along the basal to apical axis of the stratified epithelium. Perhaps, the best example for epidermal tissue polarization is the restricted localization of the TJs to the second granular layer (SG2) of the epidermis (Fig. 1B). Recent whole-mount analysis of newborn and adult mouse and human epidermis showed that TJs localize apically within these cells (Yokouchi et al. 2016; Rübsam et al. in press). These TJs are essential to prevent unnecessary water loss from the organism (Furuse et al. 2002; Tunggal et al. 2005), an essential feature for terrestrial life. Molecularly, only occludin and claudin-4 are specifically found in the stratum granulosum, whereas ZO-1 and claudin-1 are present in all cell layers, suggesting other functions outside of TJs for these proteins. Although desmosomes and AJs are present in all layers, the molecular components of these junctions differ between the specific layers; basal layers express E- and P-cadherin as well as Dsg3. On initiation of differentiation and movement into the first suprabasal layer, P-cadherin, Dsg2, Dsg3, and Dsc3 are down-regulated whereas Dsg1 is up-regulated with further increasing expression in suprabasal layers (Fig. 1B). Although E-cadherin containing AJs are found in all layers, vinculin, a mechano-sensitive component of AJs, is only highly enriched in the SG2 layer, in which TJs are formed (Rübsam et al. in press). Intriguingly, Dsg4 expression seems to be confined only to the SG1 and the SC. Similarly, PKP1 and 3 show a counter-gradient across layers, whereas plakoglobin, DP, and PKP2 are found in all layers. This layer-specific expression and recruitment to junctions is functionally important, as inappropriate in vivo expression of desmosomal cadherins, E-cadherin, or claudin-6 interferes with epidermal morphogenesis and barrier function (Elias et al. 2001; Henkler et al. 2001; Merritt et al. 2002; Turksen and Troy 2002; Jamora et al. 2003).
The cytoskeletal networks also show different layer-dependent configurations: Although MTs form a centrosomal network in the basal layer, they concentrate at cell–cell junctions in suprabasal layers. Interestingly, this suprabasal redistribution of MTs depends on recruitment of centrosomal proteins to DP at the junctional area (Sumigray et al. 2011). The keratin filament system consists of pairs of type I and type II keratins (see also, Hatzfeld et al. 2017). The basal layer expresses K5/K14 whereas suprabasal layers express K1/K10-based networks. Thus, desmosomes are connected to keratin networks of different composition and likely varying mechanical resilience in basal versus suprabasal layers. Finally, F-actin, like MTs, is differentially organized across layers with the highest cortical F-actin organization in the granular layer (Fig. 1B) (Rübsam et al. in press). Together, these highly specific and differential distributions of adhesion molecules and cytoskeletal organization indicate that the different layers adopt architecturally and mechanically different states likely essential to drive layer-dependent transitions and functions.
JUNCTIONS COORDINATE EPIDERMAL ORGANIZATION, GROWTH, AND DIFFERENTIATION
Evidence indicating that junctional organization of the cytoskeleton is important for tissue organization and mechanics comes from in vivo epidermal loss-of-function mouse models, 3D organotypic culture models, and from human skin diseases associated with mutations in, or antibodies against, junctional components. For example, auto-antibodies against Dsg3 or Dsg1 result in skin blistering diseases with separation either between basal and suprabasal layer (Dsg1 and Dsg3) or upper suprabasal layers (Dsg1). Mutations in desmosomal cadherins, plakoglobin, PKP1, PKP3, or DP result in a range of human diseases characterized by skin blistering and/or AC, the severity of which depends both on the specific gene and type of mutation (Lai-Cheong et al. 2007; Samuelov and Sprecher 2015). As noted earlier, these human phenotypes by and large have been confirmed by the respective knockout mice. In contrast, P-cadherin mutations in humans are associated with a mild ectodermal dysplasia syndrome predominantly characterized by hair loss (Lai-Cheong et al. 2007), whereas mice deficient for P-cadherin showed no obvious skin phenotype (Tinkle et al. 2008). Interestingly, combined loss of P-cadherin and desmoglein-3 results in a much stronger oral blistering phenotype than loss of desmoglein 3 alone (Lenox et al. 2000), showing that synergy between AJs and desmosomes also occurs on the tissue level. As anticipated, loss of E-cadherin alone or in combination with P-cadherin or loss of α-catenin disturbs F-actin organization (Rübsam et al. in press; Vasioukhin et al. 2001a; Tinkle et al. 2008). In agreement E-cadherin both intercellular and cortical tension in keratinocyte cell sheets. However, it was unexpected that loss of E-cadherin disturbed the polarized tissue organization of F-actin, resulting in increased cortical F-actin organization in lower layers (Rübsam et al. in press), suggesting that in these lower layers E-cadherin actively restricts cortical F-actin organization.
Desmosomal Regulation of Actin-Based Junctions
Perturbations of desmosomal components not only disturb mechanical integrity directly linked to desmosomal/keratin-mediated dysfunction but often also result in alterations in cell behaviors known to be regulated by the actin cytoskeleton, such as cell migration. For example, epidermal inactivation of DP is perinatal lethal because of mechanically stress-induced loss of epidermal integrity (Vasioukhin et al. 2001b). Interestingly, DP null keratinocytes not only have reduced desmosomes that no longer are linked to the keratin network but also reduced AJs. These keratinocytes show increased myosin contractility and are unable to properly reorganize the actin cytoskeleton (Vasioukhin et al. 2001b; Sumigray et al. 2014). This regulation likely requires binding of DP to IFs as deletion of this interaction domain altered actin-dependent intercellular tension (Broussard et al. 2017).
Vice versa, either combined loss of the main epidermal classical cadherins, E- and P-cadherin, or loss of α-catenin in keratinocytes is perinatal lethal and strongly impairs both AJ and desmosome assembly (Vasioukhin et al. 2001a; Tinkle et al. 2008; Michels et al. 2009). Along these lines, loss of Dsg1 in 3D human epidermal cultures also results in increased cell size and variability of cell shape (Getsios et al. 2009), characteristics linked to actin organization. Together, these results indicate that AJs and desmosomes act synergistically to promote epidermal integrity while at the same enabling individual cell shape changes during their traverse through the different layers.
There are multiple examples linking desmosomes with upstream and downstream regulators of actin organization (Green et al. 2010; Hatzfeld et al. 2014). One involves PKP2. In both squamous cell lines and atrial cardiomyocytes, decreased PKP2 levels result in a failure of active RhoA to localize to cell–cell contacts and a decrease in cortical actin remodeling after cadherin engagement (Godsel et al. 2010). Moreover, PKP2 loss, at least in part through RhoA, decreases cell spreading, increases stress fiber formation, and induces more stable focal adhesions, thus reducing cell migration rates (Godsel et al. 2010; Koetsier et al. 2014).
Junctional Control of SG2 Tight Junctional Barrier Formation
Unlike simple epithelia, there is clear evidence for a role of desmosomes in the formation and function of epidermal TJs. For example, ectopic expression of Dsg3 in the upper layers of mouse epidermis results in neonatal lethality as a result of increased transepidermal water loss (Elias et al. 2001), suggesting impairment of TJs, although the SC barrier is also altered. In contrast, inactivation of PKP1 in mouse epidermis did not functionally alter the outside-in SC barrier but did impair TJ barrier function, likely as a consequence of impaired intercellular junction formation. Moreover, DP was shown to control claudin expression (Sumigray et al. 2014). Thus, there are many indications that desmosomes not only affect AJs but also TJs, which perhaps is not that surprising taken their role in regulating actin dynamics.
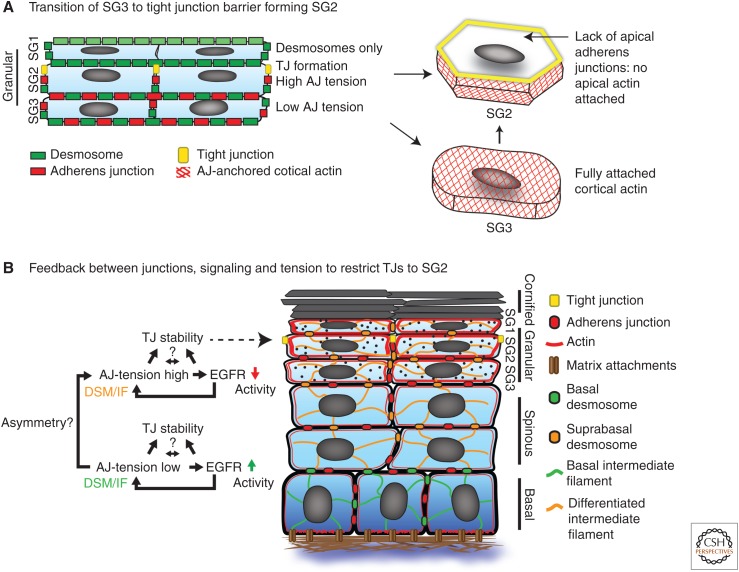
The mechanisms that confine TJs to the SG2 layer are mostly unknown. Very recently, it was shown that E-cadherin is essential to restrict TJs to the SG2. Epidermal loss of E-cadherin or β-catenin resulted in breaches in the SG2 TJ barrier (Tunggal et al. 2005; Ray et al. 2013). Similar to increased cortical F-actin organization, premature spot-like TJ-like structures were observed in lower layers up on loss of E-cadherin. One potential mechanism is that E-cadherin integrates junctional tension and EGFR activity to inhibit premature TJ complex formation in lower layers while promoting increased tension and TJ stability in the SG2 (Fig. 4B) (Rübsam et al. in press).
Figure 4.
Role of cadherin-based junctions and signaling in the formation of the tight junction (TJ)-containing granular layer. (A) Proposed model how asymmetric junction organization in SG2 promotes the formation of tension-high adherens junction, apically positioned tight junctions, and extended F-actin organization and adopt a shape resembling Kelvin’s tetracaidecahedron when SG3 cells become SG2 cells. Whereas in SG3 F-actin is attached through AJs at all cellular interfaces, in SG2 the apical F-actin network is uncoupled from the membrane due to absence of apical AJs. (B) Model integrating the role of adherens junctions (AJs), desmosomes and epidermal growth factor receptor (EGFR) in the regulation of actomyosin-dependent intercellular and cortical tension and TJ stability.
Epidermal Junctions in Signaling and Differentiation
Recent evidence suggests that AJs and desmosomes not only guarantee cohesion and barrier function but also actively coordinate signaling and the differentiation process itself. Dsg1 promotes keratinocyte differentiation by suppressing EGFR and mitogen-activated protein kinase (MAPK) signaling cascades (Getsios et al. 2009). Instead, the Dsg1-mediated effects on differentiation require its interaction with the cytosolic protein Erbin (ErbB2 interacting protein), which results in the disruption of Ras–Raf complexes mediated by the scaffolding protein SHOC2 (Harmon et al. 2013). Interestingly, this mechanism also does not require the adhesive ectodomain of Dsg1. In addition to regulating differentiation through EGFR-related signaling, Dsg1 has been shown to be important for the entry of keratinocytes into the terminal differentiation program through the tyrosine kinase EphA2 (Lin et al. 2010).
Other desmosomal components also have been shown to regulate/be regulated by tyrosine kinase signaling. For example, PKP2, which is widely expressed in simple epithelia and concentrated in the lower layers of stratified epithelia, is a positive regulator of EGFR activation. Loss of PKP2 disrupts EGFR signaling and leads to decreased cancer cell migration and proliferation (Arimoto et al. 2014). PKP3 is present in most simple epithelia and is expressed more uniformly in stratified epithelia like the epidermis. PKP3 is phosphorylated in response to pemphigus IgG, resulting in dissociation from Dsg3 and destabilization of adhesion. These effects are diminished on suppression of Src activity (Cirillo et al. 2014). Finally, uniformly expressed plakoglobin has been shown in both keratinocytes and prostate cancer cell lines to inhibit cell motility through a mechanism that involves the regulation of Src and the extracellular matrix (Todorovic et al. 2010; Franzen et al. 2012).
Aberrant expression of Dsg2 in the upper layers of the epidermis promotes a hyperproliferative phenotype through growth factor signaling cascades that stimulate both cell proliferation and survival (Brennan et al. 2007). In addition, reduced expression of Dsg2 in intestinal epithelia suppresses cell proliferation through a reduction in EGFR signaling (Kamekura et al. 2014). Moreover, there are potential growth factor-mediated signaling feedback loops, as EGFR signaling promotes the endocytic turnover of Dsg2 (Klessner et al. 2009).
As desmosomes and AJs control Rho and actin dynamics, this potentially provides additional pathways to allow these junctions to integrate the regulation of cell shape and balanced renewal versus differentiation in the epidermis. Rho-family dependent modulation of the actin cytoskeleton can regulate cell behaviors through transcription. The polymerization of F-actin, downstream from Rho signaling, drives the transcriptional coactivator MAL into the nucleus where it aids in serum response factor (SRF)-dependent transcription (Posern and Treisman 2006), thus linking actin dynamics to differentiation. In keratinocytes, loss-of-function of the RhoA guanine nucleotide exchange factor (GEF) breakpoint cluster region (Bcr) has been shown to reduce RhoA activity and inhibit an SRF-mediated, prodifferentiation phenotype, and restoring the levels of Dsg1 rescues the Bcr-induced differentiation defects (Dubash et al. 2013). Along similar lines, increasing evidence indicates that AJs control Yap signaling through α-catenin mechanical signals to balance growth and differentiation (Schlegelmilch et al. 2011; Zhang et al. 2011) and that defective cell adhesion results in unrestricted epidermal Yap signaling that promotes epidermal overgrowth (Li et al. 2016; Walko et al. 2017).
INTEGRATIVE MODELS FOR AJS AND DESMOSOMES MECHANICS AND SIGNALING
As discussed, AJs and desmosomes not only bidirectionally control their formation and activity but also determine cell shape and differentiation status. However, whether differentiation-dependent signals and control of cell and tissue architecture are integrated at the level of desmosomes and AJs is less clear. Using the transition from the basal to the first suprabasal layer, as well as the transition from SG1 to SG2 as two examples, we now propose two models for how AJs and desmosomes cooperate to integrate mechanical and chemical signals to robustly promote the transition into a new layer with different mechanical properties and differentiation status (Figs. 3, 4). These models are based on developmental models in which differences in tissue surface tension trigger cell sorting and cell shape changes to drive tissue organization (Amack and Manning 2012; Heisenberg and Bellaiche 2013).
Figure 3.
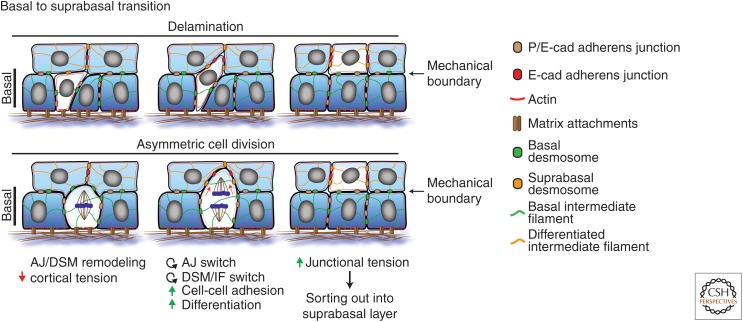
Cadherin-based junctions in basal to suprabasal transition. Proposed model how dynamic reorganization of adherens junctions (AJs) and desmosomes control tension and differentiation to promote the basal to spinous layer transition either when cells delaminate or up on asymmetric cell division resulting in the formation of a mechanical tissue boundary that allows the tissue to couple cell position and differentiation status.
Basal to Suprabasal Transition
At present, two different mechanisms have been proposed to explain how basal cells become suprabasal cells: (1) through asymmetric division coupled to perpendicular spindle orientations that position one of the daughters more suprabasally; and (2) through delamination by loosening cell–matrix contacts. It is well established that changes in cortical tension transmitted through cadherins drive cell–cell segregation and cell sorting to establish tissue boundaries. We propose that initial local remodeling of basal intercellular junctions alters cortical tension. This local tension disequilibrium is necessary to drive (in case of delamination) and/or promote upward movement (in both cases). Subsequent differentiation-induced changes in key AJ and desmosomal adhesion molecules further reinforce these intercellular rearrangements necessary to establish a mechanical boundary between basal and suprabasal cells.
In the case of a delaminating cell (Fig. 4A), we propose that a combined loss of cell–matrix contacts and dynamic AJ and desmosomal rearrangements locally reduce cortical tension compared with its neighbors driving initial upward movement and initiation of differentiation. During this process, the basal cadherins P-cadherin and Dsg3 are transcriptionally down-regulated whereas E-cadherin and Dsg1 expression increase in the delaminating cell, accompanied by a switch from a K5/K14 network to a K1/K10 network, allowing the delaminating cell to more specifically interact with the suprabasal cells, which express E-cadherin and a higher Dsg1:Dsg3 ratio. Strengthening desmosomal interactions through DP engagement of K1/10 will not only provide more resilience but also strengthen E-cadherin AJs (Broussard et al. 2017), thus increasing junctional tension and resulting in integration of the delaminating cell into the first suprabasal layer (Fig. 3).
In the case of an asymmetric mitotic spindle, during late anaphase/telophase the future upper daughter cell membranes are positioned away from the basement membrane and will start to engage E-cadherin and Dsg1 of suprabasal cells. Moreover, cytokinesis itself, through pulling forces of the cytokinetic ring, can locally alter and remodel neighboring junctions, promoting self-organizing actin flows in these cells (Pinheiro et al. 2017) that likely further reinforce proper positioning of the two daughters either basally or suprabasally (Fig. 3). Thus, both delamination and asymmetric division induce remodeling of neighboring cell–cell interactions that spatiotemporally alter local cortical tension, which we propose is essential to position cells suprabasally.
Transition of SG3 into a TJ Barrier Forming SG2
Similarly, the upward movement of individual cells toward the granular layer (Rompolas et al. 2016) requires dynamic rearrangements of intercellular junctions. Whether this involves fast cadherin turnover at the cell surface, local fast exchange of adhesive and/or cytoskeletal contacts, or other mechanisms is not known at present. This transit through the different layers also needs to be coupled to differentiation status. As outlined above (see sections on “Polarity and Junctions” and “Epidermal Organization”), recent results indicate the presence of a mechanical boundary between the SG3 and SG2 granular layer, as only these latter cells have basolateral vinculin-positive AJs, apical barrier forming TJs, and a strong increase in F-actin organization (Rübsam et al. in press). These TJ-containing SG2 cells also adopt a defined shape that resembles a Kelvin’s tetracaidecahedron. Modelling showed that this specific shape enables TJ barrier maintenance when individual SG2 cells rearrange their contacts with their SG2 neighbors to transit toward the SG1 (Yokouchi et al. 2016). The mechanism that controls this mechanical transition and defined shape change are unclear but, as mentioned, in part depend on E-cadherin and EGFR signaling (Rübsam et al. in press). Of note, there is a potential anisotropy of cortical tension in SG2 cells. This is because SG2 cells only form AJs at their basolateral but not at apical SG2–SG1 interfaces, which contrasts with cells in the lower layers, which uniformly connect all intercellular interfaces to the keratin and F-actin cytoskeleton through desmosomes and AJs, respectively. We propose that the differential distribution of AJs and desmosomes in SG2 through synergistic regulation of intercellular and cortical tension, and tyrosine receptor kinase signaling create an asymmetry in junctional tension in SG2. This tension disequilibrium is necessary to promote the formation of tension-high, vinculin-positive AJs and apical positioning of TJs the formation of tension-high AJs, apical TJs, and extended F-actin organization, which then collectively drive the cell shape changes necessary to form tetracaidecahedron-shaped SG2 cells (Fig. 4A).
Integrating Junctional Control of Cell Shape, Positioning, and Differentiation
The above models implicate the synergistic action of cadherin-based junction as major drivers of mechanical transition states in the epidermis. But, do these transitions directly affect the differentiation status? Even though direct evidence is currently lacking, we propose that AJs and desmosomes synergistically coordinate epidermal cell shape, position, and differentiation status through integration of mechanical and chemical signals such as tyrosine kinase receptor signaling (see also Chiasson-MacKenzie and McClatchey 2017). As already discussed, Dsg1 directly promotes differentiation by inhibiting EGFR (Getsios et al. 2009), and several recent observations suggest that this regulation likely involves AJs and cortical actomyosin activity (Erasmus et al. 2015; Broussard et al. 2017). Firstly, E-cadherin has been implicated in regulating keratinocyte differentiation (Hines et al. 1999; Charest et al. 2009) and, like loss of Dsg1, epidermal loss of E-cadherin also promotes EGFR activity (Rübsam et al. in press). Interestingly, differentiation is apparently normal in these mice, perhaps because Dsg1 and P-cadherin are up-regulated in these mice restricting increased EGFR activity only to the basal layer (Tunggal et al. 2005). In agreement, removing all AJ mechanical signaling through the loss of α-catenin promotes hyperproliferation and disturbs tissue architecture associated with an increase in IGF-1R as well as Yap signaling (Vasioukhin et al. 2001a; Li et al. 2016). Moreover, inactivation of myosin IIa in the epidermis not only impairs TJs (Sumigray et al. 2012), but also induces premature differentiation, suggesting that the initial drop in tension observed when cells initiate upward movement simultaneously promotes delamination and differentiation (Le et al. 2016). Finally, cortical actomyosin organization and activity regulates differentiation, TJs (Connelly et al. 2010; Zhou et al. 2013), and EGFR activity status, whereas EGFR activity itself can determine tension states of cells and TJ stability and function (Fig. 4B) (Rübsam et al. in press). Together, these data suggest a model in which AJs and desmosomes cooperatively coordinate intercellular tension states with receptor tyrosine kinase activity, which through reinforcing feedback loops coordinate cell shape, position, barrier function, and differentiation status.
CONCLUDING REMARKS
The evolutionary invention of multicellular sheets undergoing morphogenetic movements required that cells mechanically and chemically interact. Classical cadherin/catenin adhesion complexes were instrumental in facilitating this development as these complexes integrated through the formation of AJs three key properties: control of adhesion, cortical tension, and signaling. The later arrival of desmosomes in vertebrates enabled organisms to not only mechanically reinforce newly developed tissues such as the heart and skin epidermis, but also provided novel regulatory opportunities to integrate mechanical and chemical adhesion, cytoskeleton, and signaling networks necessary to accomplish increasing demands in terrestrial barrier function, immune regulation, and organism/tissue size and complexity. Advances in high-resolution live imaging, optogenetics, and biophysical approaches will greatly facilitate future studies to determine in vivo junctional and tissue tension and monitor signaling and cell fate in mammalian tissues to establish the principles by which cadherin-based junctions coordinate spatiotemporal cell position, shape, and fate.
ACKNOWLEDGMENTS
We thank the Niessen, Green, and Wickström laboratory members for many fruitful and inspiring discussions as well as Alpha S. Yap for being supportive and providing great feedback. The author’s work is supported by DFG SFB 829 A11, the Max Planck Society and Max Planck Foundation (to S.A.W.), National Institutes of Helath (NIH) Grants R01 AR041836, R37 AR43380, R01 CA122151, and the J.L. Mayberry Endowment (to K.J.G.) and German Research Foundation (DFG) Grants SFB 829 A1 and A5, SPP1782-NI1234/6–1 and the German Cancer Aid (to C.M.N.). Dr. Green receives support as Associate Director of Basic Sciences in the Robert H Lurie Comprehensive Cancer Center of Northwestern University (P30 CA60553). K.J.G. and C.M.N. also thank the Alexander von Humboldt Foundation for supporting K.J.G. while she was performing research at the University of Cologne, thus providing the basis for the concepts put forward in this review.
Footnotes
Editors: Carien M. Niessen and Alpha S. Yap
Additional Perspectives on Cell–Cell Junctions available at www.cshperspectives.org
REFERENCES
*Reference is also in this subject collection.
- Abedin M, King N. 2008. The premetazoan ancestry of cadherins. Science 319: 946–948. [DOI] [PubMed] [Google Scholar]
- Abedin M, King N. 2010. Diverse evolutionary paths to cell adhesion. Trends Cell Biol 20: 734–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alegado RA, King N. 2014. Bacterial influences on animal origins. Cold Spring Harb Perspect Biol 6: a016162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amack JD, Manning ML. 2012. Knowing the boundaries: Extending the differential adhesion hypothesis in embryonic cell sorting. Science 338: 212–215. [DOI] [PubMed] [Google Scholar]
- Arimoto K, Burkart C, Yan M, Ran D, Weng S, Zhang DE. 2014. Plakophilin-2 promotes tumor development by enhancing ligand-dependent and -independent epidermal growth factor receptor dimerization and activation. Mol Cell Biol 34: 3843–3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balda MS, Matter K. 2016. Tight junctions as regulators of tissue remodelling. Curr Opin Cell Biol 42: 94–101. [DOI] [PubMed] [Google Scholar]
- Bays JL, Campbell HK, Heidema C, Sebbagh M, DeMali KA. 2017. Linking E-cadherin mechanotransduction to cell metabolism through force-mediated activation of AMPK. Nat Cell Biol 19: 724–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Fuchs E. 2009. Epidermal homeostasis: A balancing act of stem cells in the skin. Nat Rev Mol Cell Biol 10: 207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggetti B, Niessen CM. 2012. Adherens junctions in mammalian development, homeostasis and disease: Lessons from mice. Subcell Biochem 60: 321–355. [DOI] [PubMed] [Google Scholar]
- Bondow BJ, Faber ML, Wojta KJ, Walker EM, Battle MA. 2012. E-cadherin is required for intestinal morphogenesis in the mouse. Dev Biol 371: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosher JM, Hahn BS, Legouis R, Sookhareea S, Weimer RM, Gansmuller A, Chisholm AD, Rose AM, Bessereau JL, Labouesse M. 2003. The Caenorhabditis elegans vab-10 spectraplakin isoforms protect the epidermis against internal and external forces. J Cell Biol 161: 757–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Braga V. 2017. Signaling by small GTPases at cell–cell junctions: Protein interactions building control and networks. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a028746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan D, Hu Y, Joubeh S, Choi YW, Whitaker-Menezes D, O’Brien T, Uitto J, Rodeck U, Mahoney MG. 2007. Suprabasal Dsg2 expression in transgenic mouse skin confers a hyperproliferative and apoptosis-resistant phenotype to keratinocytes. J Cell Sci 120: 758–771. [DOI] [PubMed] [Google Scholar]
- Broussard JA, Getsios S, Green KJ. 2015. Desmosome regulation and signaling in disease. Cell Tissue Res 360: 501–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broussard JA, Yang R, Huang C, Nathamgari SSP, Beese AM, Godsel LM, Hegazy MH, Lee S, Zhou F, Sniadecki NJ, et al. 2017. The desmoplakin/intermediate filament linkage regulates cell mechanics. Mol Biol Cell 10.1091/mbc.E16-07-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley CD, Tan J, Anderson KL, Hanein D, Volkmann N, Weis WI, Nelson WJ, Dunn AR. 2014. Cell adhesion. The minimal cadherin–catenin complex binds to actin filaments under force. Science 346: 1254211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulgakova NA, Brown NH. 2016. Drosophila p120–catenin is crucial for endocytosis of the dynamic E-cadherin–Bazooka complex. J Cell Sci 129: 477–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candi E, Schmidt R, Melino G. 2005. The cornified envelope: A model of cell death in the skin. Nat Rev Mol Cell Biol 6: 328–340. [DOI] [PubMed] [Google Scholar]
- Carnahan RH, Rokas A, Gaucher EA, Reynolds AB. 2010. The molecular evolution of the p120–catenin subfamily and its functional associations. PLoS ONE 5: e15747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charest JL, Jennings JM, King WP, Kowalczyk AP, Garcia AJ. 2009. Cadherin-mediated cell–cell contact regulates keratinocyte differentiation. J Invest Dermatol 129: 564–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Chiasson-MacKenzie C, McClatchey AI. 2017. Cell–cell contact and receptor tyrosine kinase signaling. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a029215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo N, AlShwaimi E, McCullough M, Prime SS. 2014. Pemphigus vulgaris autoimmune globulin induces Src-dependent tyrosine-phosphorylation of plakophilin 3 and its detachment from desmoglein 3. Autoimmunity 47: 134–140. [DOI] [PubMed] [Google Scholar]
- Connelly JT, Gautrot JE, Trappmann B, Tan DW, Donati G, Huck WT, Watt FM. 2010. Actin and serum response factor transduce physical cues from the microenvironment to regulate epidermal stem cell fate decisions. Nat Cell Biol 12: 711–718. [DOI] [PubMed] [Google Scholar]
- Conway DE, Coon BG, Budatha M, Arsenovic PT, Orsenigo F, Wessel F, Zhang J, Zhuang Z, Dejana E, Vestweber D, et al. 2017. VE-cadherin phosphorylation regulates endothelial fluid shear stress responses through the polarity protein LGN. Curr Biol 27: 2219–2225.e2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MD, Alder MN. 2006. The evolution of adaptive immune systems. Cell 124: 815–822. [DOI] [PubMed] [Google Scholar]
- *.Delmar M, Laird DW, Naus CC, Nielsen MS, Versleis VK, White TW. 2017. Connexins and disease. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a029348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delva E, Tucker DK, Kowalczyk AP. 2009. The desmosome. Cold Spring Harb Perspect Biol 1: a002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Z, Cheng X, Merched-Sauvage M, Koch PJ. 2006. Desmocollin 3 is required for pre-implantation development of the mouse embryo. J Cell Sci 119: 482–489. [DOI] [PubMed] [Google Scholar]
- Dickinson DJ, Nelson WJ, Weis WI. 2011. A polarized epithelium organized by β- and α-catenin predates cadherin and metazoan origins. Science 331: 1336–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson DJ, Nelson WJ, Weis WI. 2012a. An epithelial tissue in Dictyostelium challenges the traditional origin of metazoan multicellularity. Bioessays 34: 833–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson DJ, Robinson DN, Nelson WJ, Weis WI. 2012b. α-Catenin and IQGAP regulate myosin localization to control epithelial tube morphogenesis in Dictyostelium. Dev Cell 23: 533–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubash AD, Koetsier JL, Amargo EV, Najor NA, Harmon RM, Green KJ. 2013. The GEF Bcr activates RhoA/MAL signaling to promote keratinocyte differentiation via desmoglein-1. J Cell Biol 202: 653–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias PM, Matsuyoshi N, Wu H, Lin C, Wang ZH, Brown BE, Stanley JR. 2001. Desmoglein isoform distribution affects stratum corneum structure and function. J Cell Biol 153: 243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erasmus JC, Welsh NJ, Braga VM. 2015. Cooperation of distinct Rac-dependent pathways to stabilise E-cadherin adhesion. Cell Signal 27: 1905–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshkind L, Tian Q, Schmidt A, Franke WW, Windoffer R, Leube RE. 2002. Loss of desmoglein 2 suggests essential functions for early embryonic development and proliferation of embryonal stem cells. Eur J Cell Biol 81: 592–598. [DOI] [PubMed] [Google Scholar]
- Fleming TP, Butler L, Lei X, Collins J, Javed Q, Sheth B, Stoddart N, Wild A, Hay M. 1994. Molecular maturation of cell adhesion systems during mouse early development. Histochemistry 101: 1–7. [DOI] [PubMed] [Google Scholar]
- Franke WW, Rickelt S, Barth M, Pieperhoff S. 2009. The junctions that don’t fit the scheme: Special symmetrical cell-cell junctions of their own kind. Cell Tissue Res 338: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzen CA, Todorovic V, Desai BV, Mirzoeva S, Yang XJ, Green KJ, Pelling JC. 2012. The desmosomal armadillo protein plakoglobin regulates prostate cancer cell adhesion and motility through vitronectin-dependent Src signaling. PLoS ONE 7: e42132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E. 2007. Scratching the surface of skin development. Nature 445: 834–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara M, Nagatomo A, Tsuda M, Obata S, Sakuma T, Yamamoto T, Suzuki ST. 2015. Desmocollin-2 alone forms functional desmosomal plaques, with the plaque formation requiring the juxtamembrane region and plakophilins. J Biochem 158: 339–353. [DOI] [PubMed] [Google Scholar]
- Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, Noda T, Kubo A, Tsukita S. 2002. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: A lesson from claudin-1-deficient mice. J Cell Biol 156: 1099–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallicano GI, Kouklis P, Bauer C, Yin M, Vasioukhin V, Degenstein L, Fuchs E. 1998. Desmoplakin is required early in development for assembly of desmosomes and cytoskeletal linkage. J Cell Biol 143: 2009–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganeshan R, Chen J, Koch PJ. 2010. Mouse models for blistering skin disorders. Dermatol Res Pract 2010: 584353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cattaneo A, Braga VM. 2013. Hold on tightly: How to keep the local activation of small GTPases. Cell Adh Migr 7: 283–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrod D, Tabernero L. 2014. Hyper-adhesion: A unique property of desmosomes. Cell Commun Adhes 21: 249–256. [DOI] [PubMed] [Google Scholar]
- Getsios S, Simpson CL, Kojima S, Harmon R, Sheu LJ, Dusek RL, Cornwell M, Green KJ. 2009. Desmoglein 1-dependent suppression of EGFR signaling promotes epidermal differentiation and morphogenesis. J Cell Biol 185: 1243–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godsel LM, Dubash AD, Bass-Zubek AE, Amargo EV, Klessner JL, Hobbs RP, Chen X, Green KJ. 2010. Plakophilin 2 couples actomyosin remodeling to desmosomal plaque assembly via RhoA. Mol Biol Cell 21: 2844–2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goonesinghe A, Luan XM, Hurlstone A, Garrod D. 2012. Desmosomal cadherins in zebrafish epiboly and gastrulation. BMC Dev Biol 12: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KJ, Getsios S, Troyanovsky S, Godsel LM. 2010. Intercellular junction assembly, dynamics, and homeostasis. Cold Spring Harb Perspect Biol 2: a000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimson MJ, Coates JC, Reynolds JP, Shipman M, Blanton RL, Harwood AJ. 2000. Adherens junctions and β-catenin-mediated cell signalling in a non-metazoan organism. Nature 408: 727–731. [DOI] [PubMed] [Google Scholar]
- Gul IS, Hulpiau P, Saeys Y, van Roy F. 2017. Metazoan evolution of the armadillo repeat superfamily. Cell Mol Life Sci 74: 525–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner B, Stevenson B, Grimaldi A. 1988. The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J Cell Biol 107: 1575–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Neilson LJ, Zhong H, Murray PS, Zanivan S, Zaidel-Bar R. 2014. E-cadherin interactome complexity and robustness resolved by quantitative proteomics. Sci Signal 7: rs7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin J. 2015. Getting to the core of cadherin complex function in Caenorhabditis elegans. F1000Res 10.12688/f1000research.6866.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon RM, Simpson CL, Johnson JL, Koetsier JL, Dubash AD, Najor NA, Sarig O, Sprecher E, Green KJ. 2013. Desmoglein-1/erbin interaction suppresses ERK activation to support epidermal differentiation. J Clin Invest 123: 1556–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TJ, Tepass U. 2010. Adherens junctions: From molecules to morphogenesis. Nat Rev Mol Cell Biol 11: 502–514. [DOI] [PubMed] [Google Scholar]
- Hart KC, Tan J, Siemers KA, Sim JY, Pruitt BL, Nelson WJ, Gloerich M. 2017. E-cadherin and LGN align epithelial cell divisions with tissue tension independently of cell shape. Proc Natl Acad Sci 114: E5845–E5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzfeld M, Wolf A, Keil R. 2014. Plakophilins in desmosomal adhesion and signaling. Cell Commun Adhes 21: 25–42. [DOI] [PubMed] [Google Scholar]
- *.Hatzfeld M, Keil R, Magin TM. 2017. Desmosomes and intermediate filaments: Their consequences for tissue mechanics. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a029157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg CP, Bellaiche Y. 2013. Forces in tissue morphogenesis and patterning. Cell 153: 948–962. [DOI] [PubMed] [Google Scholar]
- Henkler F, Strom M, Mathers K, Cordingley H, Sullivan K, King I. 2001. Trangenic misexpression of the differentiation-specific desmocollin isoform 1 in basal keratinocytes. J Invest Dermatol 116: 144–149. [DOI] [PubMed] [Google Scholar]
- Hines MD, Jin HC, Wheelock MJ, Jensen PJ. 1999. Inhibition of cadherin function differentially affects markers of terminal differentiation in cultured human keratinocytes. J Cell Sci 112: 4569–4579. [DOI] [PubMed] [Google Scholar]
- Huelsmann S, Brown NH. 2014. Spectraplakins. Curr Biol 24: R307–R308. [DOI] [PubMed] [Google Scholar]
- Hulpiau P, Gul IS, van Roy F. 2013. New insights into the evolution of metazoan cadherins and catenins. Prog Mol Biol Transl Sci 116: 71–94. [DOI] [PubMed] [Google Scholar]
- Jamora C, DasGupta R, Kocieniewski P, Fuchs E. 2003. Links between signal transduction, transcription and adhesion in epithelial bud development. Nature 422: 317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamekura R, Kolegraff KN, Nava P, Hilgarth RS, Feng M, Parkos CA, Nusrat A. 2014. Loss of the desmosomal cadherin desmoglein-2 suppresses colon cancer cell proliferation through EGFR signaling. Oncogene 33: 4531–4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant S, Holthofer B, Magin TM, Krusche CA, Leube RE. 2015. Desmoglein 2-dependent arrhythmogenic cardiomyopathy is caused by a loss of adhesive function. Circ Cardiovasc Genet 8: 553–563. [DOI] [PubMed] [Google Scholar]
- King N. 2004. The unicellular ancestry of animal development. Dev Cell 7: 313–325. [DOI] [PubMed] [Google Scholar]
- Klessner JL, Desai BV, Amargo EV, Getsios S, Green KJ. 2009. EGFR and ADAMs cooperate to regulate shedding and endocytic trafficking of the desmosomal cadherin desmoglein 2. Mol Biol Cell 20: 328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koetsier JL, Amargo EV, Todorovic V, Green KJ, Godsel LM. 2014. Plakophilin 2 affects cell migration by modulating focal adhesion dynamics and integrin protein expression. J Invest Dermatol 134: 112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtidis A, Ngok SP, Anastasiadis PZ. 2013. p120 catenin: An essential regulator of cadherin stability, adhesion-induced signaling, and cancer progression. Prog Mol Biol Transl Sci 116: 409–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo A, Nagao K, Amagai M. 2012. Epidermal barrier dysfunction and cutaneous sensitization in atopic diseases. J Clin Invest 122: 440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladoux B, Nelson WJ, Yan J, Mege RM. 2015. The mechanotransduction machinery at work at adherens junctions. Integr Biol (Camb) 7: 1109–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai-Cheong JE, Arita K, McGrath JA. 2007. Genetic diseases of junctions. J Invest Dermatol 127: 2713–2725. [DOI] [PubMed] [Google Scholar]
- Le HQ, Ghatak S, Yeung CC, Tellkamp F, Gunschmann C, Dieterich C, Yeroslaviz A, Habermann B, Pombo A, Niessen CM, et al. 2016. Mechanical regulation of transcription controls Polycomb-mediated gene silencing during lineage commitment. Nat Cell Biol 18: 864–875. [DOI] [PubMed] [Google Scholar]
- Leckband DE, de Rooij J. 2014. Cadherin adhesion and mechanotransduction. Annu Rev Cell Dev Biol 30: 291–315. [DOI] [PubMed] [Google Scholar]
- Lenox JM, Koch PJ, Mahoney MG, Lieberman M, Stanley JR, Radice GL. 2000. Postnatal lethality of P-cadherin/desmoglein 3 double knockout mice: Demonstration of a cooperative effect of these cell adhesion molecules in tissue homeostasis of stratified squamous epithelia. J Invest Dermatol 114: 948–952. [DOI] [PubMed] [Google Scholar]
- Leung CL, Green KJ, Liem RK. 2002. Plakins: A family of versatile cytolinker proteins. Trends Cell Biol 12: 37–45. [DOI] [PubMed] [Google Scholar]
- Li P, Silvis MR, Honaker Y, Lien WH, Arron ST, Vasioukhin V. 2016. αE-catenin inhibits a Src–YAP1 oncogenic module that couples tyrosine kinases and the effector of Hippo signaling pathway. Genes Dev 30: 798–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Gordon K, Kaplan N, Getsios S. 2010. Ligand targeting of EphA2 enhances keratinocyte adhesion and differentiation via desmoglein 1. Mol Biol Cell 21: 3902–3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon RC, Mezzano V, Wright AT, Pfeiffer E, Chuang J, Banares K, Castaneda A, Ouyang K, Cui L, Contu R, et al. 2014. Connexin defects underlie arrhythmogenic right ventricular cardiomyopathy in a novel mouse model. Hum Mol Genet 23: 1134–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAleer MA, Pohler E, Smith FJ, Wilson NJ, Cole C, MacGowan S, Koetsier JL, Godsel LM, Harmon RM, Gruber R, et al. 2015. Severe dermatitis, multiple allergies, and metabolic wasting syndrome caused by a novel mutation in the N-terminal plakin domain of desmoplakin. J Allergy Clin Immunol 136: 1268–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley MD, Wehrens XH. 2009. Animal models of arrhythmogenic cardiomyopathy. Dis Model Mech 2: 563–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea PD, Gottardi CJ. 2016. Beyond β-catenin: Prospects for a larger catenin network in the nucleus. Nat Rev Mol Cell Biol 17: 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Mege RM, Ishiyama N. 2017. Integration of cadherin adhesion and cytoskeleton at adherens junctions. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a028738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt AJ, Berika MY, Zhai W, Kirk SE, Ji B, Hardman MJ, Garrod DR. 2002. Suprabasal desmoglein 3 expression in the epidermis of transgenic mice results in hyperproliferation and abnormal differentiation. Mol Cell Biol 22: 5846–5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels C, Buchta T, Bloch W, Krieg T, Niessen CM. 2009. Classical cadherins regulate desmosome formation. J Invest Dermatol 129: 2072–2075. [DOI] [PubMed] [Google Scholar]
- Miller PW, Clarke DN, Weis WI, Lowe CJ, Nelson WJ. 2013. The evolutionary origin of epithelial cell–cell adhesion mechanisms. Curr Top Membr 72: 267–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muroyama A, Lechler T. 2012. Polarity and stratification of the epidermis. Semin Cell Dev Biol 23: 890–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PS, Zaidel-Bar R. 2014. Pre-metazoan origins and evolution of the cadherin adhesome. Biol Open 3: 1183–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myster SH, Cavallo R, Anderson CT, Fox DT, Peifer M. 2003. Drosophila p120catenin plays a supporting role in cell adhesion but is not an essential adherens junction component. J Cell Biol 160: 433–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman SA. 2016. ‘Biogeneric’ developmental processes: Drivers of major transitions in animal evolution. Philos Trans R Soc Lond B Biol Sci 371: 20150443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols SA, Roberts BW, Richter DJ, Fairclough SR, King N. 2012. Origin of metazoan cadherin diversity and the antiquity of the classical cadherin/β–catenin complex. Proc Natl Acad Sci 109: 13046–13051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen MS, Axelsen LN, Sorgen PL, Verma V, Delmar M, Holstein-Rathlou NH. 2012. Gap junctions. Compr Physiol 2: 1981–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessen CM, Leckband D, Yap AS. 2011. Tissue organization by cadherin adhesion molecules: Dynamic molecular and cellular mechanisms of morphogenetic regulation. Physiol Rev 91: 691–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda H, Takeichi M. 2011. Evolution: Structural and functional diversity of cadherin at the adherens junction. J Cell Biol 193: 1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EN. 2006. Gene regulatory networks in the evolution and development of the heart. Science 313: 1922–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacquelet A, Lin L, Rorth P. 2003. Binding site for p120/δ-catenin is not required for Drosophila E-cadherin function in vivo. J Cell Biol 160: 313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan A, Rao MV, Wu Y, Zaidel-Bar R. 2015. Jack of all trades: Functional modularity in the adherens junction. Curr Opin Cell Biol 36: 32–40. [DOI] [PubMed] [Google Scholar]
- Patel DM, Dubash AD, Kreitzer G, Green KJ. 2014. Disease mutations in desmoplakin inhibit Cx43 membrane targeting mediated by desmoplakin–EB1 interactions. J Cell Biol 206: 779–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro D, Hannezo E, Herszterg S, Bosveld F, Gaugue I, Balakireva M, Wang Z, Cristo I, Rigaud SU, Markova O, et al. 2017. Transmission of cytokinesis forces via E-cadherin dilution and actomyosin flows. Nature 545: 103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires-daSilva A, Sommer RJ. 2003. The evolution of signalling pathways in animal development. Nat Rev Genet 4: 39–49. [DOI] [PubMed] [Google Scholar]
- Pizarro-Cerda J, Kuhbacher A, Cossart P. 2012. Entry of Listeria monocytogenes in mammalian epithelial cells: An updated view. Cold Spring Harb Perspect Med 2: a010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posern G, Treisman R. 2006. Actin’ together: Serum response factor, its cofactors and the link to signal transduction. Trends Cell Biol 16: 588–596. [DOI] [PubMed] [Google Scholar]
- Ramms L, Fabris G, Windoffer R, Schwarz N, Springer R, Zhou C, Lazar J, Stiefel S, Hersch N, Schnakenberg U, et al. 2013. Keratins as the main component for the mechanical integrity of keratinocytes. Proc Natl Acad Sci 110: 18513–18518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Foote HP, Lechler T. 2013. β-Catenin protects the epidermis from mechanical stresses. J Cell Biol 202: 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Macara IG. 2014. Organization and execution of the epithelial polarity programme. Nat Rev Mol Cell Biol 15: 225–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompolas P, Mesa KR, Kawaguchi K, Park S, Gonzalez D, Brown S, Boucher J, Klein AM, Greco V. 2016. Spatiotemporal coordination of stem cell commitment during epidermal homeostasis. Science 352: 1471–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rübsam M, Mertz AF, Kubo A, Marg S, Jüngst C, Goranci-Buzhala G, Schauss A, Horsley V, Dufresne ER, Moser M, et al. E-cadherin integrates mechanotransduction and EGFR signaling to control junctional tissue polarization and Tight Junction positioning. Nat Commun. (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelov L, Sprecher E. 2015. Inherited desmosomal disorders. Cell Tissue Res 360: 457–475. [DOI] [PubMed] [Google Scholar]
- Samuelov L, Sarig O, Harmon RM, Rapaport D, Ishida-Yamamoto A, Isakov O, Koetsier JL, Gat A, Goldberg I, Bergman R, et al. 2013. Desmoglein 1 deficiency results in severe dermatitis, multiple allergies and metabolic wasting. Nat Genet 45: 1244–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, Zhou D, Kreger BT, Vasioukhin V, Avruch J, Brummelkamp TR, et al. 2011. Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell 144: 782–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider MR, Dahlhoff M, Horst D, Hirschi B, Trulzsch K, Muller-Hocker J, Vogelmann R, Allgauer M, Gerhard M, Steininger S, et al. 2010. A key role for E-cadherin in intestinal homeostasis and Paneth cell maturation. PLoS ONE 5: e14325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltmann K, Fritsch AW, Kas JA, Magin TM. 2013. Keratins significantly contribute to cell stiffness and impact invasive behavior. Proc Natl Acad Sci 110: 18507–18512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson CL, Patel DM, Green KJ. 2011. Deconstructing the skin: Cytoarchitectural determinants of epidermal morphogenesis. Nat Rev Mol Cell Biol 12: 565–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu CH, Harris TJ, Wang J, Wong E. 2004. Regulation of cell–cell adhesion during Dictyostelium development. Semin Cell Dev Biol 15: 633–641. [DOI] [PubMed] [Google Scholar]
- Sumigray KD, Lechler T. 2012. Desmoplakin controls microvilli length but not cell adhesion or keratin organization in the intestinal epithelium. Mol Biol Cell 23: 792–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumigray KD, Chen H, Lechler T. 2011. Lis1 is essential for cortical microtubule organization and desmosome stability in the epidermis. J Cell Biol 194: 631–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumigray KD, Foote HP, Lechler T. 2012. Noncentrosomal microtubules and type II myosins potentiate epidermal cell adhesion and barrier formation. J Cell Biol 199: 513–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumigray K, Zhou K, Lechler T. 2014. Cell–cell adhesions and cell contractility are upregulated upon desmosome disruption. PLoS ONE 9: e101824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellkamp F, Vorhagen S, Niessen CM. 2014. Epidermal polarity genes in health and disease. Cold Spring Harb Perspect Med 4: a015255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason HA, Scothern A, McHarg S, Garrod DR. 2010. Desmosomes: Adhesive strength and signalling in health and disease. Biochem J 429: 419–433. [DOI] [PubMed] [Google Scholar]
- Tinkle CL, Pasolli HA, Stokes N, Fuchs E. 2008. New insights into cadherin function in epidermal sheet formation and maintenance of tissue integrity. Proc Natl Acad Sci 105: 15405–15410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorovic V, Desai BV, Patterson MJ, Amargo EV, Dubash AD, Yin T, Jones JC, Green KJ. 2010. Plakoglobin regulates cell motility through Rho- and fibronectin-dependent Src signaling. J Cell Sci 123: 3576–3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunggal JA, Helfrich I, Schmitz A, Schwarz H, Gunzel D, Fromm M, Kemler R, Krieg T, Niessen CM. 2005. E-cadherin is essential for in vivo epidermal barrier function by regulating tight junctions. EMBO J 24: 1146–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turksen K, Troy TC. 2002. Permeability barrier dysfunction in transgenic mice overexpressing claudin 6. Development 129: 1775–1784. [DOI] [PubMed] [Google Scholar]
- Van Itallie CM, Anderson JM. 2014. Architecture of tight junctions and principles of molecular composition. Semin Cell Dev Biol 36: 157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasioukhin V, Bauer C, Degenstein L, Wise B, Fuchs E. 2001a. Hyperproliferation and defects in epithelial polarity upon conditional ablation of α-catenin in skin. Cell 104: 605–617. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Bowers E, Bauer C, Degenstein L, Fuchs E. 2001b. Desmoplakin is essential in epidermal sheet formation. Nat Cell Biol 3: 1076–1085. [DOI] [PubMed] [Google Scholar]
- Vite A, Radice GL. 2014. N-cadherin/catenin complex as a master regulator of intercalated disc function. Cell Commun Adhes 21: 169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walko G, Woodhouse S, Pisco AO, Rognoni E, Liakath-Ali K, Lichtenberger BM, Mishra A, Telerman SB, Viswanathan P, Logtenberg M, et al. 2017. A genome-wide screen identifies YAP/WBP2 interplay conferring growth advantage on human epidermal stem cells. Nat Commun 8: 14744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt FM. 2014. Mammalian skin cell biology: At the interface between laboratory and clinic. Science 346: 937–940. [DOI] [PubMed] [Google Scholar]
- *.Yap AS, Duszyc K, Viasnoff V. 2017. Mechanosensing and mechanotransduction at cell–cell junctions. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a028761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokouchi M, Atsugi T, Logtestijn MV, Tanaka RJ, Kajimura M, Suematsu M, Furuse M, Amagai M, Kubo A. 2016. Epidermal cell turnover across tight junctions based on Kelvin’s tetrakaidecahedron cell shape. Elife 5: e19593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Pasolli HA, Fuchs E. 2011. Yes-associated protein (YAP) transcriptional coactivator functions in balancing growth and differentiation in skin. Proc Natl Acad Sci 108: 2270–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZM, Reynolds AB, Gaucher EA. 2011. The evolutionary history of the catenin gene family during metazoan evolution. BMC Evol Biol 11: 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou K, Muroyama A, Underwood J, Leylek R, Ray S, Soderling SH, Lechler T. 2013. Actin-related protein2/3 complex regulates tight junctions and terminal differentiation to promote epidermal barrier formation. Proc Natl Acad Sci 110: E3820–E3829. [DOI] [PMC free article] [PubMed] [Google Scholar]