Abstract
Mesenchymal stem cells are fundamental for bone formation and repair since they respond to microenvironmental stimuli by undergoing osteogenic differentiation. We show that the kinase and cation channel TRPM7 and the magnesium transporter MagT1 have a role in harmonizing the osteogenic differentiation of human mesenchymal stem cells. TRPM7 and MagT1 are upregulated in osteogenic differentiation and silencing either one accelerates osteogenic differentiation, partly through the activation of autophagy. Intriguingly, similar results were obtained when the cells were cultured under magnesium deficient conditions. These results underpin the contribution of magnesium, TRPM7 and MagT1 to autophagy and osteoblastogenesis.
Introduction
The bone is a metabolically active tissue that is continuously remodeled in development and throughout life to repair microdamages and adjust its architecture to changing mechanical needs1. This dynamic process relies on the coordinated and timely balance between bone resorption by osteoclasts and bone formation by osteoblasts. In particular, osteoblasts arise from bone marrow mesenchymal stem cells (MSC), rare pluripotent cells that activate the genetic program leading to osteoblastogenesis in response to specific stimuli from the microenvironment2. There is a growing interest in MSC because of their use in cell-based therapy as a treatment strategy in orthopedics. It is therefore essential to disclose the molecular events involved in their differentiation into osteoblasts. Both chemical and physical cues modulate the fate commitment of bone MSC3. In particular, upon exposure to shear forces MSC exhibit dose- and time-dependent changes in gene expression that lead to the acquisition of an osteogenic phenotype4. Recently, Transient Receptor Potential Melastatin 7 (TRPM7), a dual-function kinase and cation channel, has been shown to mediate the osteogenic differentiation of murine MSC in response to shear stress5. Accordingly, in these cells TRPM7 directly senses membrane tension and is involved in mechanotransduction6. Moreover, TRPM7 is fundamental for murine MSC survival7. While TRPM7 is implicated in the transport of divalent cations, primarily calcium (Ca) and magnesium (Mg)8 both crucial components of the bone, Mg transporter 1 (MagT1), which is expressed in all human tissues, selectively transports Mg across the plasma membrane9. Rather little is known about the expression and the role of MagT1 in bone. Rat MSC cultured on Zn/Mg surfaces, which promote osteogenesis, significantly upregulate MagT1 gene expression10. In rat MSC, silencing MagT1 blunts osteogenic differentiation11. Since both MagT1 and TRPM7 contribute to the maintenance of Mg homeostasis at the cellular level, it should be recalled that Mg, the fourth most abundant metal ion in the body mostly stored in the skeleton12, plays a crucial role in bone metabolism and in the regulation of bone cell functions13. A recent report shows that Mg deprivation as well as mesendogen, an inhibitor of TRPM7, robustly enhance mesoderm and definitive endoderm differentiation of embryonic stem cells14.
On these bases, we investigated the expression and the role of TRPM7 and MagT1 in human MSC (hMSC) induced to differentiate into osteoblasts by exposure to an osteogenic cocktail. We evaluated the expression of some osteogenic differentiation markers. In particular, we focused on Runt-related transcription factor 2 (RUNX2) and collagen type I (COL1A1). RUNX2, the master regulator of osteogenesis15, acts early to commit MSC to the osteochondral lineages and then induces the expression of COL1A1, which is crucial for the osteogenic phenotype. The rational for studying the expression of RUNX2 and COL1A1 resides in our recent findings showing that if the upregulation of RUNX2 is not accompanied by the increase of COL1A1, calcium deposition does not occur and this prevents hMSC full differentiation into osteoblasts16.
A connection exists between osteogenesis and autophagy, an evolutionary conserved self-degradative system that delivers cytoplasmic constituents to the lysosomes17. In vivo, the deletion of FIP200, an essential component of the autophagosome complex, suppresses autophagy and causes osteopenia in mice by inhibiting osteoblast differentiation18. Similarly, knocking down autophagy-related gene ATG5, a component of the autophagosome, reduces bone mineralization19. In vitro, the autophagy proteins ATG7 and beclin 1 are required for mineralization in an osteoblastic cell line19. Therefore, we evaluated the activation and the role of autophagy in hMSC after inhibiting the expression of MagT1 or TRPM7 or culturing the cells under Mg deficient conditions.
Results
TRPM7 and MagT1 are overexpressed in hMSC induced to differentiate into osteoblasts
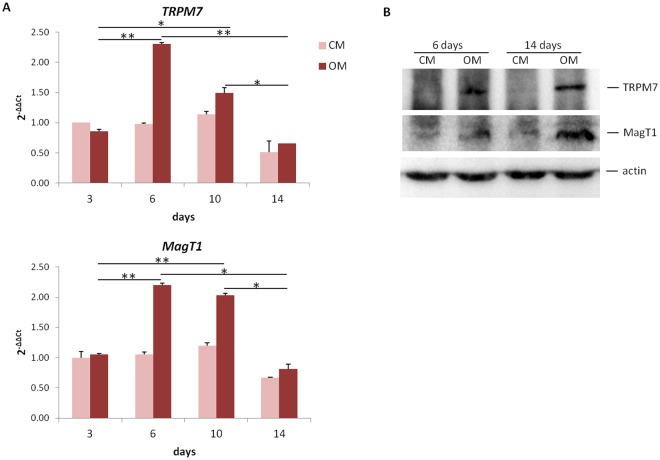
Confluent cells were cultured for 3, 6, 10 and 14 days in an osteogenic medium containing vitamin D (OM) or in their culture medium (CM) as a control. By real-time PCR we demonstrate an overexpression of TRPM7 and MagT1 in cells exposed to OM for 6 and 10 days from the beginning of the experiment (Fig. 1A). Western blot shows that both TRPM7 and MagT1 are upregulated in hMSC exposed to the osteogenic medium for 6 and 14 days (Figs 1B and S1A,B). It is noteworthy that while the expression of TRPM7 and MagT1 drops at day 14, the protein levels remain elevated until the end of the experiment.
Figure 1.
Osteogenic differentiation associates with the upregulation of TRPM7 and MagT1. (A) hMSC were cultured in OM or CM for 3, 6, 10 and 14 days. Real-Time PCR was performed on RNA extracted from hMSC using primers designed on TRPM7 and MagT1 sequence. (B) Western blot was performed on extracts from hMSC cultured in OM or CM for 6 and 14 days using antibodies against TRPM7 or MagT1. Actin was used as a control of loading. A representative blot is shown and quantification is provided in the Supplementary information (Fig. S1A).
siRNAs against TRPM7 or MagT1 boost the expression of osteogenic differentiation markers
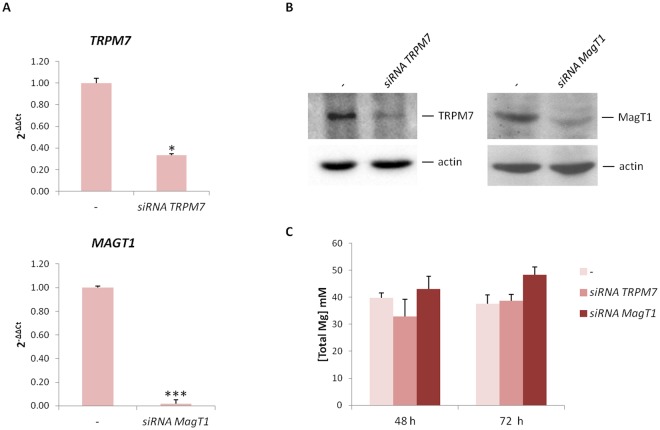
To investigate the role of TRPM7 and MagT1 in the osteogenic differentiation of hMSC, we transiently transfected the cells with specific siRNAs for TRPM7, MagT1 or with a non-silencing siRNA as a control (−) and then cultured hMSC in CM for 3 days. By real-time PCR MagT1 expression resulted completely suppressed and TRPM7 expression appeared dramatically reduced but not totally abrogated (Fig. 2A). Western blot shows the downregulation of the two proteins (Figs 2B and S2A,B). To get an overview about Mg homeostasis, we measured total intracellular Mg 48 and 72 h after transfection without finding any significant difference between cells silencing TRPM7 or MagT1 and relative controls (Fig. 2C).
Figure 2.
Specific siRNAs silence TRPM7 or MagT1 in hMSC. (A) After exposure to siRNA, hMSC were cultured in CM for 3 days. Real-Time PCR was performed on RNA extracted from hMSC using primers designed on the sequence of TRPM7 and MagT1. The controls, indicated as -, were exposed to non-silencing, scrambled sequences. (B) Western blot was performed on extracts from hMSC after 3 days silencing. Antibodies against TRPM7 or MagT1 were used. Actin was used as a control of loading. A representative blot is shown and quantification is provided in the Supplementary information (Fig. S2A). (C) Total Mg was measured using the fluorescent chemosensor DCHQ5 as described.
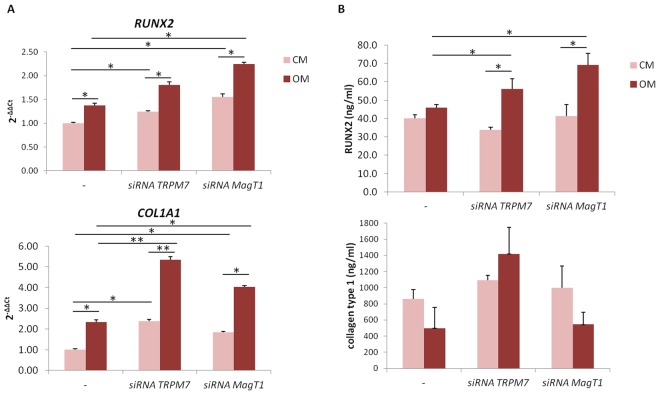
In siRNA transfected cells, we analyzed the expression of RUNX2 and COL1A1 and found it slightly upregulated even in the absence of the osteogenic cocktail (Fig. 3A). Moreover, cells downregulating TRPM7 or MagT1 maintained their sensitivity to the stimulatory effect of the osteogenic cocktail and upregulated both RUNX2 and COL1A1 more than controls (Fig. 3A). By ELISA, we demonstrate the significant increase of RUNX2 in hMSC downregulating TRPM7 or MagT1 after 5 days of osteogenic induction (Fig. 3B), while collagen type 1 is not induced probably because its accumulation represents a late event in osteoblastogenesis15.
Figure 3.
siRNAs targeting TRPM7 or MagT1 enhance osteogenic differentiation. (A) After exposure to siRNA, hMSC were cultured in CM or OM for 3 days. Real-Time PCR was performed on RNA extracted from hMSC using primers designed on RUNX2 and COL1A1 sequence. (B) ELISA for RUNX2 and collagen type 1 was conducted on extracts from hMSC cultured in CM or OM for 5 days. The controls, indicated as -, were exposed to non-silencing, scrambled sequences.
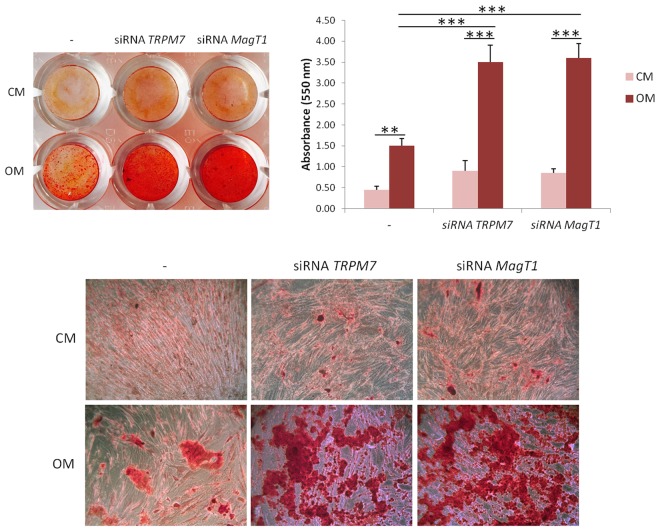
Osteogenic differentiation of hMSC ultimately leads to the deposition of calcium in the extracellular matrix. We therefore evaluated calcium deposition by Alizarin Red S staining16 in silenced cells cultured in OM or CM for 14 days. In support to the results obtained by RT-PCR and ELISA, we detected some calcium deposits in siRNA transfected cells cultured in CM, but not in controls transfected with non silencing siRNAs (Fig. 4). Upon exposure to OM, siRNA transfected hMSC deposited much higher amounts of calcium phosphate crystals than non silenced cells.
Figure 4.
siRNAs targeting MagT1 or TRPM7 induce the deposition of calcium. Alizarin Red S staining was performed after exposure to CM or OM for 14 days. Whole well image (upper left panel) and photographs taken at 10X magnification (lower panel) are shown. After acid extraction the absorbance was measured at 550 nm (upper right panel).
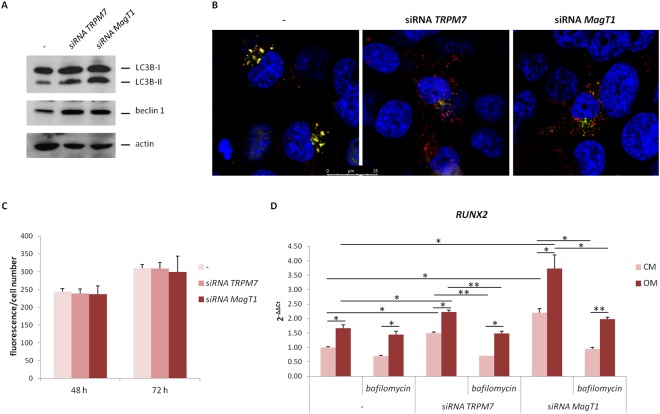
Autophagy is involved in accelerating osteogenesis in hMSC exposed to siRNAs against TRPM7 or MagT1
Autophagy contributes to the osteogenic differentiation of hMSC20. In agreement with these findings, we show the conversion of microtubule-associated protein 1A/1B light-chain phosphatidylethanolamine conjugate (LC3B) to autophagosome-associated LC3B-II, which is the most widely used autophagosome marker21, in hMSC treated with siRNAs targeting MagT1 or TRPM7 for 3 days (Figs 5A and S5A,B). We also evaluated the total amounts of beclin 1, which contributes to the initiation of autophagosome formation by interacting with phosphatidylinositol 3-kinase22. Figures 5A and S5A,B show that beclin 1 is markedly induced in cells silencing TRPM7 or MagT1.
Figure 5.
siRNAs targeting TRPM7 or MagT1 induce autophagy. hMSC were exposed to siRNAs targeting TRPM7 or MagT1, or to non-silencing sequences (−), for 3 days. (A) The cells were lysed and Western blot was performed using antibodies against LC3B and beclin 1. Actin was used as a control of loading. A representative blot is shown and quantification is provided in the Supplementary information (Fig. S5A). (B) Autophagic flux was detected by Tandem fluorescent-tagged LC3 assay as described in the methods. (C) Intracellular free Ca was measured using Fura-2-AM as described. (D) Real-Time PCR was performed on RNA extracted from hMSC in CM or OM, treated or untreated with bafilomycin A1 (10 nM) for 3 days. Primers designed on RUNX2 sequence were used.
To reinforce these results, we performed a Tandem fluorescent-tagged LC3 assay to assess autolysosome function. hMSC were transfected with the plasmid containing the sequence for the fusion protein LC3-GFP-RFP. Briefly, in the lysosome where the pH is low, the fluorescence of GFP is quenched, while that of RFP is stable. Formation of autophagosomes increases the number of GFP-positive/RFP-positive (yellow) vesicles, which become GFP-negative/RFP-positive (red) once fusion with lysosomes occurs. Figure 5B confirms the induction of autophagy in cells silencing TRPM7 or MagT1 as indicated by the increase of the number of vesicles and of autophagolysosomes in silenced cells vs controls.
Considering the complex role of free calcium in autophagy23, it is noteworthy that we did not detect any modulation of intracellular free Ca in silenced cells vs their controls (Fig. 5C).
Next we tested whether bafilomycin A1, which inhibits autophagy by preventing the acidification of endosomes and lysosomes, affects the expression of RUNX2 in hMSC exposed to siRNA targeting MagT1 or TRPM7 for 3 days. Figure 5D shows that bafilomycin A1 prevented the increase of RUNX2 expression in siRNA treated cells, thus indicating that autophagy contributes to accelerating osteogenic differentiation in cells silencing TRPM7 or MagT1.
Low extracellular Mg enhances osteogenic differentiation of hMSC by activating autophagy
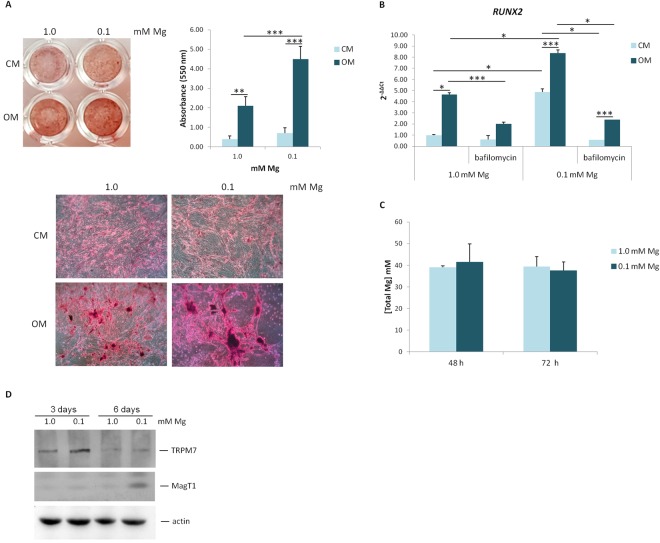
We asked whether Mg deprivation has an effect on hMSC differentiation and found that culture in 0.1 mM Mg for 14 days enhances Ca deposition in the extracellular matrix as detected by Alizarin Red S staining (Fig. 6A). Accordingly, the expression of RUNX2 is higher in Mg deficient cells versus their controls both in the presence and in the absence of osteogenic stimuli (Fig. 6B).
Figure 6.
Mg deficiency accelerates osteogenic differentiation. (A) Alizarin Red S staining was performed on hMSC cultured in 0.1 or 1.0 mM Mg with or without the osteogenic cocktail for 14 days. Whole well image (upper left panel) and photographs taken at 10X magnification (lower panel) are shown. Absorbance was measured at 550 nm after acid extraction (upper right panel). (B) Real-Time PCR was performed on RNA extracted from hMSC cultured in 1.0 or 0.1 mM Mg, additioned or not with the osteogenic cocktail, treated or untreated with bafilomycin A1 (10 nM) for 3 days. Primers designed on RUNX2 sequence were used. (C) Total Mg was measured using the fluorescent chemosensor DCHQ5 as described. (D) Western blot was performed on extracts from hMSC cultured in 1.0 or 0.1 mM Mg for 3 and 6 days using antibodies against TRPM7 or MagT1. Actin was used as a control of loading. A representative blot is shown and quantification is provided in the Supplementary information (Fig. S6A).
No differences were observed in the content of total intracellular Mg in hMSC maintained in Mg deficient medium vs controls (Fig. 6C). Moreover, western blot shows that TRPM7 is upregulated after 3 days and MagT1 after 6 days of culture in medium containing 0.1 mM Mg (Figs 6D and S6A,B).
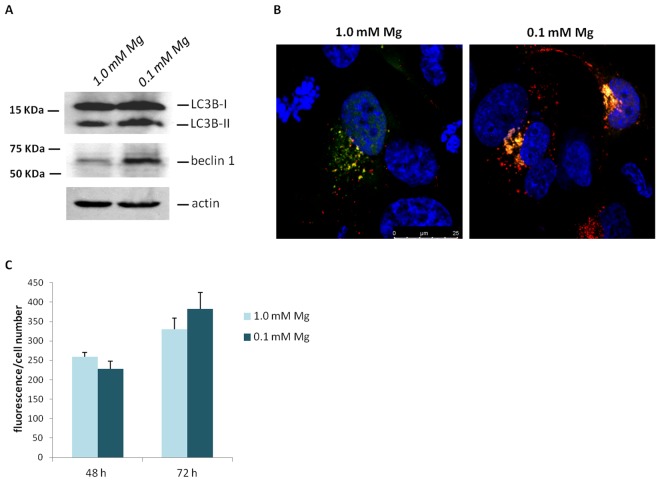
Next, we demonstrate that culture in low extracellular Mg (0.1 mM Mg) stimulates the conversion of LC3B to LC3B-II and upregulates beclin 1 in our experimental model (Figs 7A and S7A,B). These results were confirmed by Tandem fluorescent-tagged LC3 assay described above which shows an increase of the number of vesicles and of autophagolysosomes in Mg deficient hMSC (Fig. 7B). Figure 7C demonstrates no variation of intracellular free Ca between cells cultured in 0.1 or 1.0 mM Mg medium.
Figure 7.
Mg deficiency induces autophagy. (A) hMSC were cultured in 0.1 or 1.0 mM Mg. After 3 days the cells were lysed and Western blot was performed using antibodies against LC3B and beclin 1. Actin was used as a control of loading. A representative blot is shown and quantification is provided in the Supplementary information (Fig. S7A). (B) Autophagic flux was detected by Tandem fluorescent-tagged LC3 assay as described in the methods. (C) Intracellular free Ca was measured using Fura-2-AM as described.
The contribution of autophagy to osteogenic differentiation is supported by the fact that bafilomycin A1 reduced the expression of RUNX2 in cells cultured in low (0.1 mM) or normal (1.0 mM) extracellular Mg for 3 days (Fig. 6B).
To the best of our knowledge this is the first evidence of a link between Mg, its transporters and autophagy.
Discussion
Mg is required for every biological process not only because it is necessary for the function of hundreds of enzymes but also because it maintains the active conformation of macromolecules, regulates second messengers, various transporters and ion channels12. Therefore, it is not surprising that the amounts of intracellular Mg are balanced by a coordinated interplay among channels and transporters mediating Mg influx, exchangers regulating its efflux, and Mg shuffling from organelles to cytosol and viceversa24.
About 60% of total Mg is stored in the bone and a tight control of magnesium homeostasis is crucial for bone health13. A key event in bone formation is the differentiation of MSC into osteoblasts, a process which involves Mg and its transporters5–7,11,25. It has recently been reported that TRPM7, which is important in skeletogenesis26, acts as a mechanotransducer in murine MSC and commits their fate towards osteogenic differentiation in response to mechanical stimulation5. Also MagT111 and the Na/Mg exchanger SLC41A27 contribute to osteoblastogenesis28.
We focused our studies on TRPM7 and MagT1, both contributing to Mg transport into the cells12, in a model of hMSC induced to differentiate into osteoblasts. We show the overexpression of TRPM7 and MagT1 with a peak after 6 days of osteogenic induction and a gradual return to control levels within 14 days. The increase of the transcripts is paralleled by the upregulation of TRPM7 and MagT1 at the protein level, which is retained up to day 14 from osteogenic induction. We hypothesize that post-translational mechanisms might be implicated in maintaining high the amounts of TRPM7 and MagT1 until hMSC reach full differentiation. While further studies are required, it is noteworthy that our results with MagT1 are in agreement with a report showing the increase of MagT1 in rat MSC exposed to an osteogenic medium11.
To unravel the function of TRPM7 and MagT1 in hMSC, we utilized specific siRNAs and demonstrate that downregulating TRPM7 or MagT1 accelerates hMSC osteogenic differentiation. Our observation in hMSC silencing MagT1 is in disagreement with previous results in rat MSC showing that knocking down MagT1 inhibits osteogenic differentiation11. This discordance could be ascribed either to the different behavior of cells of different species or to the different protocols used to induce differentiation. While we used vitamin D to induce osteogenic differentiation, Zheng et al. used dexamethasone which has been shown to modulate TRPM7 in some tissues29.
Since i) MagT1 and TRPM7 are upregulated in hMSC differentiation, and ii) siRNAs targeting TRPM7 or MagT1 hasten the expression of osteogenic markers as well as Ca deposition, we hypothesize that these transporters contribute to coordinate hMSC response to osteogenic stimuli by preventing excessive osteoblastogenesis. In this light, TRPM7 and MagT1 might be considered as sensors-controllers of the extent of hMSC osteogenic differentiation.
Silencing TRPM7 in different cell types, among which colon carcinoma cells, thymocytes and human endothelial cells, does not influence intracellular Mg content30–32, while knocking down MagT1 in mammalian cell lines lowered the levels of intracellular Mg33. We did not detect differences in intracellular Mg content in hMSC silencing TRPM7 or MagT1, suggesting that either TRPM7 or MagT1 is sufficient to maintain intracellular Mg homeostasis in hMSC. Considering that many different Mg transporters have been described and account for Mg balance24, it is feasible to propose that also other proteins contribute to maintain Mg intracellular concentrations.
A connection between bone health and autophagy has been described17–19. It is noteworthy that autophagy is activated in the early phases of hMSC differentiation20. It is also interesting that resting quiescent hMSC accumulate autophagosomes34, which indicates that the cells are ready to react to different stimuli by activating autophagy. Moreover, autophagy is induced in embryonic stem cells early during differentiation35 and in somatic cells reprogrammed to induced pluripotent stem cells36. We suggest that siRNA targeting TRPM7 or MagT1 boosts the expression of markers of osteogenic differentiation, at least in part, by activating autophagy, which removes unnecessary organelles, generates substrates to supply energy and recycles macromolecular blocks to drive cell differentiation. Accordingly, bafilomycin A1, an inhibitor of autophagy, prevents the acceleration of osteogenic differentiation in our experimental model. Ca does not seem to be implicated in triggering autophagy in cells silencing TRPM7 or MagT1 as well as in hMSC cultured in low extracellular Mg. To this purpose, it is noteworthy that early after exposure to a Mg deficient medium, TRPM7 increases, while later, when TRPM7 declines to baseline levels, MagT1 is upregulated. We hypothesize that the sequential upregulation of the two proteins allows to maintain unaltered the concentrations of intracellular Mg. Moreover, Mg deficiency mimics the effects of silencing TRPM7 or MagT1 because it accelerates the osteogenic differentiation of hMSC25 and this happens in association with the trigger of autophagy without any detectable increase of free Ca.
Apart from transporting Mg, TRPM7 and MagT1 serve other functions. MagT1 contributes to the oligosaccharyltransferase complex, thus being involved in the glycosylation of various proteins37. It should be noted that the components of the extracellular matrix are glycosylated and this is important for bone formation and remodeling38. Moreover, it has been proposed that MagT1 might regulate Mg transport by glycosylating Mg transporters24. TRPM7 possesses a kinase domain and we cannot rule out the possibility that silencing TRPM7 might affect the phosphorylation of specific substrates, some of which have been identified24. Of interest, one of the protein phosphorylated by TRPM7 is annexin 1, a Ca-regulated phospholipid binding protein implicated in the regulation of cell growth, apoptosis39,40 and also in osteogenic differentiation41. On these bases, downregulating TRPM7 and/or MagT1 might impact on the post-translational modification of proteins.
In conclusion, we demonstrate that MagT1 and TRPM7 as well as Mg deficiency contribute to the regulation of hMSC osteogenic differentiation partly by modulating autophagy in a Ca-independent fashion.
Methods
Culture of hMSC
hMSC were isolated from adult human bone marrow withdrawn from bilateral punctures of the posterior iliac crests of healthy male volunteers, after obtaining informed consent from all the subjects at the Policlinico in Milano, in compliance with the Helsinki declaration, according to institutional guidelines and regulations of the Ethical Committee of “IRCCS Policlinico” Milano. These cells were used in previous studies16,25.
The cells were cultured at 37 °C and 5% CO2 in Dulbecco’s Modified Eagle’s Medium containing 10% fetal bovine serum and 2 mM glutamine (culture medium, CM). All the reagents for cell culture were from Sigma-Aldrich. The cells were used between passage 2 and 6.
To obtain a transient downregulation of MagT1 and TRPM7, we utilized the stealth siRNAs developed by Qiagen for TRPM7 and Invitrogen for MagT1. siRNAs targeting TRPM7 were transfected using HiPerFect Transfection Reagent (Qiagen) while for siRNAs targeting MagT1 we used Lipofectamine RNAiMAX (Thermo Fisher Scientific). Non-silencing, scrambled sequences were used as controls (−)42. Viable cells were counted using a cell counter (Logos Biosystems).
In some experiments, hMSC were cultured in Mg-free MEM (Invitrogen, Thermo Fisher Scientific) or Mg-free MEM supplemented with MgSO4 (Sigma-Aldrich) to reach the physiological concentration, i.e. 1 mM.
To induce osteogenic differentiation, hMSC were seeded in 6-well or 96-well plates. Once the cells were confluent, an osteogenic induction cocktail was added to the medium (osteogenic medium, OM). The osteogenic cocktail contains 2 × 10−8 M 1α,25-Dihydroxyvitamin D3, 10 mM β-glycerolphosphate and 0.05 mM ascorbic acid (Sigma-Aldrich)16. To analyze Ca deposition by hMSC, the cells were rinsed with PBS, fixed (70% ethanol, 1 h) and stained for 10 min with 2% Alizarin Red S (pH 4.2, Sigma-Aldrich)43. Alizarin Red S staining was released from the cell matrix by incubation in 10% cetylpyridinium chloride in 10 mM sodium phosphate (pH 7.0), for 15 min and the absorbance measured at 550 nm. The experiment was repeated three times in triplicate. Photographs were taken at 10X magnification.
Real-Time PCR
Total RNA was extracted by the PureLink RNA Mini kit (Thermo Fisher Scientific). Single-stranded cDNA was synthesized from 0.2 µg RNA in a 20 µL final volume using High Capacity cDNA Reverse Transcription Kit, with RNase inhibitor (Thermo Fisher Scientific) according to the manufacturer’s instructions. Real-time PCR was performed using the 7500 FAST Real Time PCR System instrument using TaqMan Gene Expression Assays (Life Technologies, Thermo Fisher Scientific): Hs00231692_m1 (RUNX2), Hs00164004_m1 (COL1A1), Hs00918928_g1 (TRPM7), Hs00997540_m1 (MagT1).
The housekeeping gene GAPDH (Hs99999905_m1) was used as an internal reference gene. Relative changes in gene expression were analyzed by the 2−ΔΔCt method16. The experiments were repeated three times in triplicate.
Western blot analysis
Western blot was performed on hMSC induced to differentiate into osteoblasts, exposed to siRNAs targeting TRPM7 and/or MagT1 or cultured in medium containing 0.1 mM Mg. After lysis, samples (80 µg/lane) were separated on SDS-polyacrylamide gel, transferred to nitrocellulose sheets at 150 mA for 16 h, and probed with antibodies against TRPM7 (Bethyl), MagT1 (Abcam), LC3B, beclin 1 (Cell Signalling Technology) and actin (Santa Cruz Biotechnology). Secondary antibodies were labelled with horseradish peroxidase (GE healthcare). The ECL Western Blotting Substrate (Thermo Fisher Scientific) was used to detect immunoreactive proteins. The western blots were repeated at least three times and a representative blot is shown. Densitometry was performed using ImageJ software and results are shown as the mean ± standard deviation.
Tandem fluorescent-tagged LC3 assay
The Tandem fluorescent-tagged LC3 assay, which is based on the different pH stability of two fluorescent proteins, allows to monitor autophagic flux and provides information about the number of vesicles and/or the progression to the late phase of autophagy44. Briefly, hMSC were transfected with a plasmid containing the sequence coding for the LC3 protein fused with two fluorophores, specifically RFP (red) and GFP (green) using lipofectamine 2000 for 4 h. Twenty-four hours post transfection hMSC were silenced for TRPM7 or MagT1 as described above. 48 h later the cells were fixed and analysed by confocal microscopy (LEICA SP8, magnification 40x). The early phase, characterized by phagosome formation, is detectable through the visualization of vesicles containing the two fluorophores (yellow fluorescence). The late phase, characterized by the fusion of the phagosome with the lysosome and the formation of the phagolysosome, is characterized by the cleavage of the GFP fragment, which is sensitive to the acidic pH of the lysosome, so that only RFP signal is detected (red fluorescence).
Quantification of intracellular Mg and Ca
Total Mg content was measured on sonicated hMSC using the fluorescent chemosensor DCHQ5 (kindly donated by Prof. S. Iotti, Università di Bologna) as described31,32. Fluorescence intensities were acquired at 510 nm. Mg concentrations of the samples were obtained by the interpolation of their fluorescence with the standard curve performed using MgSO4.
To quantify free Ca, Fura-2/AM (10 μM) was added to the culture medium for 60 min. Then the cells were washed with a buffer containing NaCl 125 mM, KCl 5 mM, MgSO4 1.2 mM, CaCl2 2 mM, glucose 6 mM, Hepes-NaOH buffer 25 mM (pH 7.4), removed by trypsinization, and suspended in the aforementioned buffer. Fluorescence intensities were acquired using a spectrophotometer with excitation wavelengths of 360 nm and emission at 450 nm45. Results were normalized on cell number.
ELISA
hMSC were exposed to CM or OM for 5 days. For the quantitative determination of RUNX2 and collagen type 1, Cusabio ELISA kit were used according to the manufacturer’s instructions. ELISAs were performed at least three times, and each sample was measured in triplicate. Data are shown as the mean ± standard deviation.
Statistical analysis
Statistical significance was determined using Student’s t test and set as following: *P < 0.05, **P < 0.01, ***P < 0.001.
Electronic supplementary material
Acknowledgements
We thank Dr. Silvia Zecchini for her help in performing the Tandem fluorescent-tagged LC3 assay and Prof. Stefano Iotti and the Bologna team for providing the fluorescent chemosensor DCHQ5. This work was supported by intramural funds.
Author Contributions
S.C. and J.A.M. conceived and designed the experiments. S.C., V.R., L.L. and A.C. performed the experiments. S.C., V.R., L.L., A.C. and J.A.M. analyzed the data. J.A.M. wrote the paper. All authors reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-34324-8.
References
- 1.Crockett JC, Rogers MJ, Coxon FP, Hocking LJ, Helfrich MH. Bone remodelling at a glance. J. Cell. Sci. 2011;124:991–998. doi: 10.1242/jcs.063032. [DOI] [PubMed] [Google Scholar]
- 2.Abdallah BM, Kassem M. Human mesenchymal stem cells: from basic biology to clinical applications. Gene Ther. 2008;15:109–116. doi: 10.1038/sj.gt.3303067. [DOI] [PubMed] [Google Scholar]
- 3.Chen Q, et al. Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell Death Differ. 2016;23:1128–39. doi: 10.1038/cdd.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hung BP, Hutton DL, Warren LG. Mechanical control of tissue-engineered bone. Stem Cell Research & Therapy. 2013;4:10. doi: 10.1186/scrt158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu YS, et al. Mechanosensitive TRPM7 mediates shear stress and modulates osteogenic differentiation of mesenchymal stromal cells through Osterix pathway. Sci. Rep. 2015;5:16522. doi: 10.1038/srep16522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao E, et al. Brief reports: TRPM7 Senses mechanical stimulation inducing osteogenesis in human bone marrow mesenchymal stem cells. Stem Cells. 2015;33:615–621. doi: 10.1002/stem.1858. [DOI] [PubMed] [Google Scholar]
- 7.Cheng H, et al. Transient receptor potential melastatin type 7 channel is critical for the survival of bone marrow derived mesenchymal stem cells. Stem Cells Dev. 2010;19:1393–13403. doi: 10.1089/scd.2009.0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmitz C, et al. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell. 2003;114:191–200. doi: 10.1016/S0092-8674(03)00556-7. [DOI] [PubMed] [Google Scholar]
- 9.Quamme GA. Molecular identification of ancient and modern mammalian magnesium transporters. Am. J. Physiol. Cell. Physiol. 2010;298:C407–429. doi: 10.1152/ajpcell.00124.2009. [DOI] [PubMed] [Google Scholar]
- 10.Yu Y, et al. Multifunctions of dual Zn/Mg ion co-implanted titanium on osteogenesis, angiogenesis and bacteria inhibition for dental implants. Acta Biomater. 2017;49:590–603. doi: 10.1016/j.actbio.2016.11.067. [DOI] [PubMed] [Google Scholar]
- 11.Zheng J, Mao X, Ling J, Chen C, Zhang W. Role of Magnesium Transporter Subtype 1 (MagT1) in the Osteogenic Differentiation of Rat Bone Marrow Stem Cells. Biol. Trace Elem. Res. 2016;171:131–137. doi: 10.1007/s12011-015-0459-4. [DOI] [PubMed] [Google Scholar]
- 12.de Baaij JH, Hoenderop JG, Bindels RJ. Magnesium in man: implications for health and disease. Physiol. Rev. 2015;95:1–46. doi: 10.1152/physrev.00012.2014. [DOI] [PubMed] [Google Scholar]
- 13.Castiglioni S, Cazzaniga A, Albisetti W, Maier JA. Magnesium and osteoporosis: current state of knowledge and future research directions. Nutrients. 2013;5:3022–33. doi: 10.3390/nu5083022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geng Y, Feng B. Mesendogen, a novel inhibitor of TRPM6, promotes mesoderm and definitive endoderm differentiation of human embryonic stem cells through alteration of magnesium homeostasis. Heliyon. 2015;1:e00046. doi: 10.1016/j.heliyon.2015.e00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stein GS, et al. Runx2 control of organization, assembly and activity of the regulatory machinery for skeletal gene expression. Oncogene. 2004;23:4315–4329. doi: 10.1038/sj.onc.1207676. [DOI] [PubMed] [Google Scholar]
- 16.Cazzaniga A, Maier JA, Castiglioni S. Impact of simulated microgravity on human bone stem cells: New hints for space medicine. Biochem. Biophys. Res. Commun. 2016;473:181–186. doi: 10.1016/j.bbrc.2016.03.075. [DOI] [PubMed] [Google Scholar]
- 17.Hocking LJ, Whitehouse C, Helfrich MH. Autophagy: a new player in skeletal maintenance? J. Bone Miner. Res. 2012;27:1439–1447. doi: 10.1002/jbmr.1668. [DOI] [PubMed] [Google Scholar]
- 18.Liu F, et al. Suppression of autophagy by FIP200 deletion leads to osteopenia in mice through the inhibition of osteoblast terminal differentiation. J. Bone Miner. Res. 2013;28:2414–2430. doi: 10.1002/jbmr.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nollet M, et al. Autophagy in osteoblasts is involved in mineralization and bone homeostasis. Autophagy. 2014;10:1965–1977. doi: 10.4161/auto.36182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pantovic A, et al. Coordinated time-dependent modulation of AMPK/Akt/mTOR signaling and autophagy controls osteogenic differentiation of human mesenchymal stem cells. Bone. 2013;52:524–531. doi: 10.1016/j.bone.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 21.Kabeya Y, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKnight NC, Zhenyu Y. Beclin 1, an Essential Component and Master Regulator of PI3K-III in Health and Disease. Curr. Pathobiol. Rep. 2013;1:231–238. doi: 10.1007/s40139-013-0028-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bootman MD, Chehab T, Bultynck G, Parys JB, Rietdorf K. The regulation of autophagy by calcium signals: Do we have a consensus? Cell Calcium. 2018;70:32–46. doi: 10.1016/j.ceca.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Schäffers OJM, Hoenderop JGJ, Bindels RJM, de Baaij JHF. The rise and fall of novel renal magnesium transporters. Am. J. Physiol. Renal Physiol. 2018;314:F1027–F1033. doi: 10.1152/ajprenal.00634.2017. [DOI] [PubMed] [Google Scholar]
- 25.Sargenti Azzurra, Castiglioni Sara, Olivi Elena, Bianchi Francesca, Cazzaniga Alessandra, Farruggia Giovanna, Cappadone Concettina, Merolle Lucia, Malucelli Emil, Ventura Carlo, Maier Jeanette, Iotti Stefano. Magnesium Deprivation Potentiates Human Mesenchymal Stem Cell Transcriptional Remodeling. International Journal of Molecular Sciences. 2018;19(5):1410. doi: 10.3390/ijms19051410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elizondo MR, et al. Defective skeletogenesis with kidney stone formation in dwarf zebrafish mutant for trpm7. Curr. Biol. 2005;15:667–671. doi: 10.1016/j.cub.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 27.Fleig Andrea, Schweigel-Röntgen Monika, Kolisek Martin. Solute carrier family SLC41: what do we really know about it? Wiley Interdisciplinary Reviews: Membrane Transport and Signaling. 2013;2(6):227–239. doi: 10.1002/wmts.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsao YT, Shih YY, Liu YA, Liu YS, Lee OK. Knockdown of SLC41A1 magnesium transporter promotes mineralization and attenuates magnesium inhibition during osteogenesis of mesenchymal stromal cells. Stem Cell. Res. Ther. 2017;8:39. doi: 10.1186/s13287-017-0497-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cuffe JS, Steane S, Moritz KM, Paravicini TM. Differential mRNA expression and glucocorticoid-mediated regulation of TRPM6 and TRPM7 in the heart and kidney throughout murine pregnancy and development. PLoS One. 2015;10:e0117978. doi: 10.1371/journal.pone.0117978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin J, et al. Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science. 2008;322:756–760. doi: 10.1126/science.1163493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castiglioni S, et al. Magnesium homeostasis in colon carcinoma LoVo cells sensitive or resistant to doxorubicin. Sci. Rep. 2015;5:16538. doi: 10.1038/srep16538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malucelli E, et al. Single cell versus large population analysis: cell variability in elemental intracellular concentration and distribution. Anal. Bioanal. Chem. 2018;410:337–348. doi: 10.1007/s00216-017-0725-8. [DOI] [PubMed] [Google Scholar]
- 33.Zhou H, Clapham DE. Mammalian MagT1 and TUSC3 are required for cellular magnesium uptake and vertebrate embryonic development. Proc. Natl. Acad. Sci. USA. 2009;106:15750–5. doi: 10.1073/pnas.0908332106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nuschke A, et al. Human mesenchymal stem cells/multipotent stromal cells consume accumulated autophagosomes early in differentiation. Stem Cell. Res. Ther. 2014;5:140. doi: 10.1186/scrt530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tra T, et al. Autophagy in human embryonic stem cells. PLoS One. 2011;6:e27485. doi: 10.1371/journal.pone.0027485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen T, et al. Rapamycin and other longevity-promoting compounds enhance the generation of mouse induced pluripotent stem cells. Aging Cell. 2011;10:908–911. doi: 10.1111/j.1474-9726.2011.00722.x. [DOI] [PubMed] [Google Scholar]
- 37.Cherepanova NA, Gilmore R. Mammalian cells lacking either the cotranslational or posttranslocational oligosaccharyltransferase complex display substrate-dependent defects in asparagine linked glycosylation. Sci. Rep. 2016;6:20946. doi: 10.1038/srep20946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alberts, B., Johnson, A., Lewis, J., Morgan, D. & Raff, M. Molecular Biology of the cell. (Garland Science, 2014).
- 39.Gerke V, Moss SE. Annexins: from structure to function. Physiol. Rev. 2002;82:331–371. doi: 10.1152/physrev.00030.2001. [DOI] [PubMed] [Google Scholar]
- 40.Arur. S, et al. Annexin I is an endogenous ligand that mediates apoptotic cell engulfment. Dev. Cell. 2003;4:587–598. doi: 10.1016/S1534-5807(03)00090-X. [DOI] [PubMed] [Google Scholar]
- 41.Pan X, Peng L, Yin G. Downregulation of Annexin A1 by short hairpin RNA inhibits the osteogenic differentiation of rat bone marrow-derived mesenchymal stem cells. Int. J. Mol. Med. 2015;36:406–14. doi: 10.3892/ijmm.2015.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cazzaniga A, et al. The different expression of TRPM7 and MagT1 impacts on the proliferation of colon carcinoma cells sensitive or resistant to doxorubicin. Sci. Rep. 2017;7:40538. doi: 10.1038/srep40538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gregory CA, Gunn WG, Peister A, Prockop DJ. An Alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Anal Biochem. 2004;329:77–84. doi: 10.1016/j.ab.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 44.Klionsky DJ, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition) Autophagy. 2016;12:1–222. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Libako P, et al. Blocking the rise of intracellular calcium inhibits the growth of cells cultured in different concentrations of magnesium. Magnes. Res. 2012;25:12–20. doi: 10.1684/mrh.2012.0302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.