Abstract
Aims
Thoracoscopic surgical ablation has evolved into a successful strategy for symptomatic atrial fibrillation (AF) refractory to other therapy. More widespread referral is limited by the lack of information on potential complications. Our aim was to systematically evaluate 30-day complications of totally thoracoscopic surgical ablation.
Methods and results
We retrospectively studied consecutive patients undergoing totally thoracoscopic surgical ablation at a referral centre in the Netherlands (2007–2016). Patients received pulmonary vein isolation, with additional lesion lines as needed, and left atrial appendage exclusion. The primary outcomes were freedom from any complications and freedom from irreversible complications at 30-days. Secondary outcomes included intra- and post-operative complications according to severity. Included were 558 patients with median age 62 years (interquartile range 56–68 years), 70% male and 53% with a previous failed catheter ablation. The cohort consisted of 43% paroxysmal AF, 47% persistent AF, and 10% long-standing persistent AF. Freedom from any 30-day complication was 88.2%, and from complications with life-long affecting consequences 97.5%. The intra-operative complication rate was 2.3% with no strokes or death observed. The median hospital length of stay was 4 days. The percentage of patients with major and minor complications at 30-days was 3.2% and 8.1%, respectively, with one patient dying of an ischaemic stroke. The only patient groups with excess complications were women aged ≥70 years and patients with a history of congestive heart failure.
Conclusions
Totally thoracoscopic ablation is associated with a low complication rate in a referral centre and may be a useful alternative to other rhythm control strategies.
Keywords: Atrial fibrillation, Thoracoscopic, Thoracoscopy, Ablation, Surgery, Complications
What’s new?
This is the first comprehensive assessment of complication rates with thoracoscopic ablation in a large cohort of patients operated across a 9-year interval in a dedicated atrial fibrillation centre.
Overall complication rates were low and mostly procedure-related and manageable.
Uniquely due to the large sample size, we were also able to explore patient factors associated with complication risk. Only older female patients aged 70 years or above, and those with history of congestive heart failure had increased risk.
A scheme for comprehensive complication reporting in thoracoscopic surgical ablation was provided, that could also be adapted for other surgical studies to aid clinical decision making and comparison of safety with different treatment options.
Introduction
Atrial fibrillation (AF) is common, becoming more prevalent,1 and is associated with poor quality of life and adverse prognosis.2 Numerous techniques for rhythm control of AF are available to clinicians, including electrical cardioversion, anti-arrhythmic drug (AAD) therapy, catheter (endocardial) ablation and surgical AF (epicardial) ablation, and hybrid approaches.2 All of these options have potential risks and complications, but direct comparison is difficult due to the heterogeneity of patients referred for each procedure.
As highlighted by the rapid increase in patients undergoing catheter ablation worldwide, rhythm control is a vital component of effective AF management to alleviate the burden of AF symptoms. However, many patients develop recurrent AF after catheter ablation, are unsuitable for the procedure, need left atrial appendage (LAA) closure, or prefer a more durable intervention. Totally thoracoscopic surgical ablation has evolved over a number of years towards a successful, minimally invasive treatment for AF, typically in patients with refractory, symptomatic AF. A randomized trial found that thoracoscopic ablation was superior to catheter ablation in terms of restoration of sinus rhythm,3 and other studies have shown a 2-year success rate of 70–80% for preventing recurrent AF.4 However, the complication rate associated with thoracoscopic ablation is less clear yet would seem to be higher than catheter ablation.4,5
While further randomized trials should be the means for gaining additional information on rhythm efficacy using thoracoscopic ablation, observational and registry data are able to provide information on complications more relevant to clinical patients undergoing these procedures. Our objective was to systematically assess and report the 30-day complication rate associated with totally thoracoscopic surgical ablation independently from rhythm outcome, in order to provide cardiologists and referring physicians with adequate information to inform their choice of rhythm control therapy. Further, we aim to set a standard for future reporting of complications in the field of thoracoscopic AF ablation surgery.
Methods
Patient selection
We included all consecutive patients who underwent totally thoracoscopic epicardial AF ablation in the period from 2007 until January 2016 in a major referral centre in the Netherlands. Patients were discussed in a multi-disciplinary team; those that required other thoracic or cardiac surgery were instead referred for Cox-maze III/IV surgery. The referral unit accepts patients from across the country and is a leading European centre for thoracoscopic and surgical AF ablation. In this period, all procedures were performed by five surgeons. The study was granted approval by the medical ethics committee of the St. Antonius Hospital (reference number W16.011), without the need for informed consent from individual participants.
Data and outcomes
Medical charts and records were collated for all patients, including demographics, operation notes, post-operative data, and details of any potential complications. When patients were referred from other centres, these centres were contacted to confirm complications during the 30-day post-operative period. All complications were critically appraised by an event committee consisting of the lead researcher (L.V.) and two experienced cardiothoracic surgeons (G.G. and B.P.). In cases of disagreement, a consensus decision was recorded by the surgical panel (G.C., B.P., W.B., F.H., and B.M.), with the surgeons blinded to patient details. See the Supplementary material online, Table S1 for details of how each complication was assessed.
The primary outcome for this study was freedom from complications at 30-days, divided into any complications (intra- or post-operative) and those with life-long affecting consequences (defined as death, strokes, venous lung infarction, permanent phrenic nerve paralysis, pacemaker implantation, renal failure necessitating dialysis, atrium-oesophagus fistula, and myocardial infarction). For mortality, all patients were checked with the Dutch civil register. Secondary outcomes were intra-operative complications and major and minor post-operative complications. Intra-operative complications were defined as a conversion to (mini)-sternotomy or (mini)-thoracotomy, or premature abortion of the operation because of bleeding. Post-operative complications (up to 30 days) were classified as major for those likely to result in prolonged hospitalization, long-term disability, or death. These included cerebral adverse events (stroke or transient ischaemic attack), re-interventions (e.g. for haemothorax or tamponade), re-intubation (with or without haemodynamic instability), embolic events (lung or peripheral embolism), pulmonary vein stenosis, atrium-oesophageal fistula, permanent phrenic nerve paralysis, renal failure necessitating dialysis, myocardial infarction, and sepsis. Minor post-operative complications included pericardial effusion necessitating drainage, permanent pacemaker implantation, the need for a temporary thoracostomy drain, culture proven infectious complications (urinary tract or airway, except for wound infections, which were scored when clear signs of infection were present and antibiotic or interventional treatment was necessary) and delirium. Conservatively treated pneumothorax was not considered a complication because it is a direct consequence from collapsing the lung and CO2 pressure used during the operation. Atrial rhythm disturbances were not counted as complications during the 30-day post-operative period, in line with the Heart Rhythm Society consensus statement, which takes a 3-month blanking period into account.5
Surgical procedure
The surgical technique of the totally thoracoscopic surgical ablation has been described by our group previously.6 Briefly, patients were positioned in supine position and operated under general anaesthesia and ventilated with a double-lumen endotracheal tube. A bilateral totally thoracoscopic epicardial ablation approach was applied. The thoracic cavity was inflated with CO2 (8 mmHg, 6 L/min). Pulmonary vein isolation (PVI) was performed with the AtriCure Isolator Synergy ablation clamp (AtriCure Inc, West Chester, OH, USA). With the evolution of the procedure over the years, technical aspects of the procedure evolved, including variation in lesion sets, selected by discretion of the operator. However, all patients received epicardial PVI (including those with prior endocardial PVI from catheter ablation), with additional lesions as described in Table 1. Additional lesions were made with the AtriCure Isolator Transpolar Pen (AtriCure, Inc., West Chester, OH, USA), the Cool rail (Atricure Inc., West Chester, OH, USA), or the multifunctional linear pen (Atricure, Inc., West Chester, OH, USA). A bidirectional block (entry/exit block) of the pulmonary veins was confirmed in all patients. From both sides, entry block was tested by sensing the ipsilateral pulmonary veins with the use of the AtriCure Isolator Transpolar Pen (Atricure Inc, West Chester, OH, USA). Exit block was confirmed by pacing the pulmonary veins after cardioversion in case of AF. In case of creation of an additional posterior box, exit block was tested from the left side, and confirmed when ventricular capture was not observed on ECG monitoring while pacing non-ablated tissue within the box, with a maximum output of 25 mA. In case of ventricular capture, additional ablation was performed until exit block could be confirmed. The LAA was excluded in all patients as a standard procedure for stroke prevention and as part of the ablation lesion set to eliminate any potential pro-arrhythmic triggers from the LAA. Exclusion of the LAA was achieved with the Endo-Gia stapler (Tyco Healthcare Group, North Haven, CT, USA), loop stitches, or more recently the AtriClip (Atricure Inc., West Chester, OH, USA).
Table 1.
Patient characteristics and procedural details
| Characteristics | Total (n = 558) |
|---|---|
| Age (years), median (IQR) | 62 (56–68) |
| Male gender, n (%) | 392 (70.3) |
| Body mass index (kg/m2), median (IQR) | 27.1 (24.8–29.8) |
| Type of AF, n (%) | |
| Paroxysmal | 238 (42.7) |
| Persistent | 265 (47.5) |
| Long-standing persistent | 55 (9.9) |
| Left ventricular ejection fraction, n (%) | |
| ≥50% | 505 (90.5) |
| 40–49% | 36 (6.5) |
| <40% | 17 (3.0) |
| Duration of AF (years), median (IQR) | 5.1 (2.8–9.4) |
| History of ≥1 failed catheter ablation, n (%) | 295 (52.9) |
| Pre-operative pacemaker, n (%) | 34 (6.1) |
| Pre-operative cerebral events, n (%): | |
| Transient ischaemic attack | 32 (5.7) |
| Ischaemic stroke | 30 (5.4) |
| Haemorrhagic stroke | 7 (1.3) |
| Procedural details | |
| Confirmed pulmonary vein isolation, n (%) | 555 (99.5) |
| Additional lesions, n (%) | |
| Roof linea | 64 (11.5) |
| Posterior box | 431 (77.2) |
| Trigone | 277 (49.6) |
| ≥1 other additional lesionb | 76 (13.6) |
| Ganglionic plexus ablation | 365 (65.4) |
| Left atrial appendage exclusion, n (%) | 549 (98.4) |
AF, atrial fibrillation; IQR, interquartile range.
Roofline without floorline.
For example, Bicaval and/or right atrial lesion.
Post-operative care and follow-up
Anti-arrhythmic drugs were post-operatively adjusted according to the individual patient’s heart rate and rhythm. In general, pre-operative rhythm medication was continued after the procedure at least for the length of the blanking period. Patients received oral anticoagulation (OAC) after surgery at least for the length of the blanking period. In case of persisting post-operative AF during admission, at least one attempt to restore sinus rhythm with electrical cardioversion was performed. After discharge, management of AAD and OAC were left to the discretion of the referring cardiologist.
Statistical analysis
Descriptive statistics are given as percentages, medians with interquartile range (IQR), or mean and standard deviation (SD). Continuous variables were analysed with the Mann–Whitney U test and categorical data with the χ2 test, or the Fisher’s exact test for low expected counts (<5). Freedom from complication events were demonstrated using the Kaplan–Meier plots for all patients and according to subgroups of clinical importance [previous catheter ablation, baseline body mass index (BMI), left ventricular ejection fraction, and a history of congestive heart failure]. Multivariate analysis of patient variables associated with freedom from any complications at 30-days was performed with the Cox proportional hazards model, including age, gender, prior stroke/transient ischaemic attack (TIA)/embolus, BMI, duration of AF, type of AF, CHA2DS2-VASc score and history of hypertension, diabetes, heart failure, and previous failed catheter ablation. Three patients had missing data on heart failure status and two patients had missing BMI. Schoenfeld residuals were used to confirm proportionality over time and interactions were assessed in bivariate and multivariate Cox and logistic regression models. Statistical analysis used SPSS (version 22.0; SPSS Inc., Chicago, IL, USA) and Stata (version 14.2; StataCorp LP, College Station, TX, USA). A two-tailed P-value <0.05 was considered statistically significant.
Results
Five hundred and fifty-eight patients were included in our analysis, with median age 62 years (IQR 56–68 years) and 70% male. Patient characteristics and procedural details are shown in Table 1. Paroxysmal AF was present in 43% and 53% of patients had a history of failed previous catheter ablation (mean number of 1.7 procedures). The average duration of AF was 5 years and the mean CHA2DS2-VASc score was 1.4 (SD 1.3; range 0–6). All patients received PVI, with additional lesions applied in the majority of patients (Table 1). The LAA was excluded in all but 12 patients (97.5%); the reasons for not excluding the LAA were prior watchman device implantation (n = 4), adhesions (n = 4), poor exposure (n = 2), request by the patient (n = 1), and an aborted procedure due to bleeding (n = 1). The median hospital length of stay was 4 days (IQR 3–6). During the admission, 30.5% of patients (170/558) developed at least one episode of AF, flutter, or another atrial tachyarrhythmia. At discharge, 12.5% (70/558) were in AF. A proportion of these patients were scheduled for electrical cardioversion in the outpatient setting due to inadequate anticoagulation status during admission, or due to patient preference.
Primary outcome and mortality
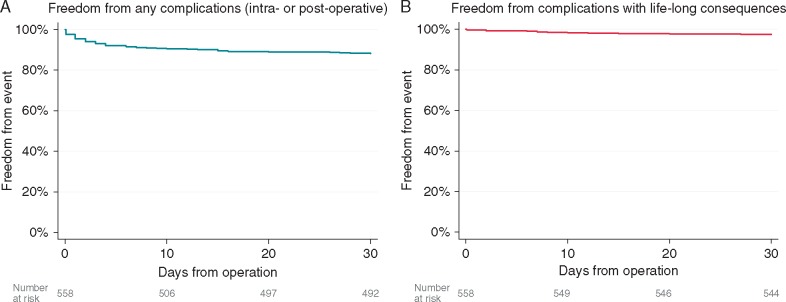
Overall freedom from any 30-day complications was 88.2% (492/558); Figure 1A. Freedom from complications with life-long affecting consequences (defined as death, strokes, venous lung infarction, permanent phrenic nerve paralysis, and pacemaker implantation) was 97.5% (544/558); Figure 1B. No patients died during their operation. One patient died within 30 days; this patient was discharged after 4 days of an uncomplicated hospital stay for PVI and LAA exclusion. Three weeks later this patient was re-admitted because of an ischaemic stroke in sinus rhythm, but with an inadequately regulated international normalized ratio and subsequently died.
Figure 1.
The Kaplan–Meier event curves for (A) freedom from any complications and (B) those with life-long consequences at 30-days.
Intra-operative complications
Operative complications occurred in 2.3% of patients (13/558) related to conversions for bleeding including sternotomy, mini-sternotomy, and mini-thoracotomy (Table 2). In one patient the operation was aborted before any ablation took place, because of bleeding of the pulmonary artery. No intra-operative strokes occurred.
Table 2.
Intra-operative complications
| Complication | n (%) |
|---|---|
| Mortality | 0 (0.0) |
| Stroke | 0 (0.0) |
| Transient ischaemic attack | 0 (0.0) |
| Sternotomy for bleeding | 9 (1.6) |
| Mini-sternotomy for bleeding | 2 (0.4) |
| Mini-thoracotomy for bleeding | 1 (0.2) |
| Bleeding with discontinuation of procedure | 1 (0.2) |
| Total number of intra-operative complications, n (%) | 13/558 (2.3) |
Major post-operative complications
Major complications occurred in 3.2% (n = 18) of the patients (Table 3). Re-interventions were performed in 1.6% of the patients (n = 9) due to haemothorax or pericardial effusion/tamponade, and one patient developed an empyema after pleural fluid drainage. One patient had a TIA, and another patient had an ischaemic stroke (with only mild aphasia once recovered). Sub-segmental lung embolus (subsequently fully recovered) was encountered twice and permanent phrenic nerve paralysis was encountered once. One patient had a venous lung infarction; this patient was re-intubated for respiratory insufficiency. The medical history of this patient included four catheter ablations, so (partial) pulmonary vein stenosis may already have been pre-existent. Two other patients were also re-intubated for reasons of bilateral pneumothorax and haemodynamic instability without known cause and subsequently were discharged without any sequelae.
Table 3.
Post-operative complications
| n (%) | |
|---|---|
| Major complications | |
| Death | 1 (0.2) |
| Reinterventionsa | |
| Haemothorax | 7 (1.3) |
| Pericardial effusion/tamponade | 1 (0.2) |
| Empyema | 1 (0.2) |
| Re-intubation without haemodynamic instability | 1 (0.2) |
| Re-intubation due to haemodynamic instability | 2 (0.4) |
| Venous lung infarction | 1 (0.2) |
| Lung emboli | 2 (0.4) |
| Permanent phrenic nerve paralysis | 1 (0.2) |
| Stroke | 1 (0.2) |
| Transient ischaemic attack | 1 (0.2) |
| Atrium-oesophagus fistula | 0 (0.0) |
| Myocardial infarction | 0 (0.0) |
| Renal failure necessitating dialysis | 0 (0.0) |
| Sepsis | 0 (0.0) |
| Total number of patients with ≥1 major complication | 18 (3.2) |
| Total number of major complications | 19 (3.4) |
| Minor complications | |
| Pericardial fluid necessitating pericardiocentesis | 4 (0.7) |
| Permanent pacemaker implantation | 10 (1.8) |
| Thoracostomy drain for | |
| Pneumothorax | 12 (2.2) |
| Pleural effusion | 6 (1.1) |
| Haemothorax | 2 (0.4) |
| Infections | |
| Airway infection | 7 (1.3) |
| Urinary tract infection | 2 (0.4) |
| Superficial wound infection | 1 (0.2) |
| Delirium | 1 (0.2) |
| Gastrointestinal bleeding | 1 (0.2) |
| Total number of patients with ≥1 minor complication | 45 (8.1) |
| Total number of minor complications | 46 (8.2) |
Including thoracotomy, sternotomy, or video-assisted-thoracoscopic surgery.
Minor post-operative complications
Minor complications occurred in 8.1% (n = 45) of the patients (Table 3), including chest drainage (3.6%, n = 20), pericardiocentesis without clinical signs of tamponade (0.7%, n = 4), and permanent pacemaker implantation (1.8%, n = 10; all due to sick sinus syndrome).
Factors associated with any complication after thoracoscopic ablation
We identified an interaction between age and gender for the development of complications after thoracoscopic surgical ablation. While the complication rate in men was similar across all ages, women had lower complication rates at younger ages and higher rates than men at the age of 70 or above (Figure 2).
Figure 2.
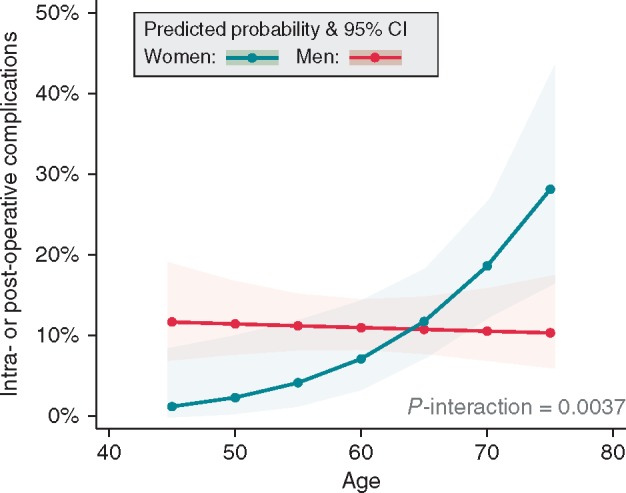
Probability of any complication related to the interaction between age and gender. 95% CI, 95% confidence interval.
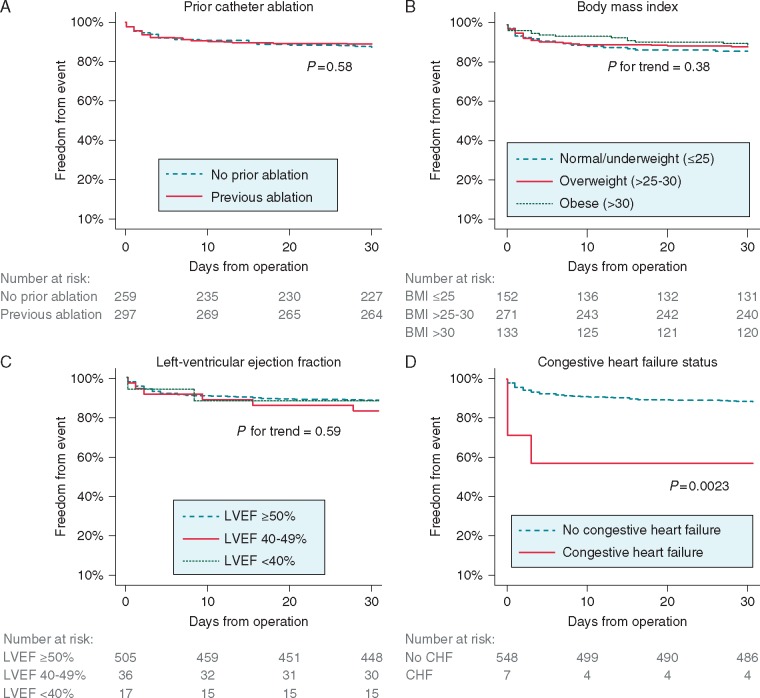
There were no differences in complications in those with or without preceding endocardial catheter ablation (Figure 3A). Similarly, BMI was not significantly associated with complications (Figure 3B); numerically, those with obesity had the lowest complication rate. Left-ventricular ejection fraction was unrelated to complications (Figure 3C). Although only present in a small number of patients and hence exploratory, congestive heart failure was associated with adverse outcomes in the Kaplan–Meier analysis (Figure 3D) and multivariate regression (Supplementary material online, Table S2; hazard ratio 5.08, 95% CI 1.42–18.18; P = 0.012).
Figure 3.
The Kaplan–Meier event curves for freedom from any complications in subgroups of interest: (A) history of prior catheter ablation, (B) BMI categories, (C) baseline LVEF, and (D) CHF. BMI, body mass index; CHF, congestive heart failure; LVEF, left ventricular ejection fraction.
Complications, hospital length of stay, and readmissions
The length of hospital stay was not different for patients with intra-operative complications compared to those with an uneventful intra-operative course [median duration of 5 days (IQR 3–11) compared to 4 days (IQR 3–6) respectively; P = 0.12]. However, patients with at least one post-operative complication had significantly longer hospitalization compared to those without any post-operative complications [8 days (IQR 5–10) vs. 4 days (IQR 3–6), respectively; P < 0.001). Nineteen patients (3.4%) were re-admitted within 30 days of thoracoscopy [median duration of 3 days (IQR 2–7)], with the majority admitted for additional diagnostics (e.g. rhythm observation, lung imaging with chest X-ray, or computed tomography). Four of these patients were classified as having a complication of the thoracoscopic ablation and are included as post-operative complications above.
Learning curve effects
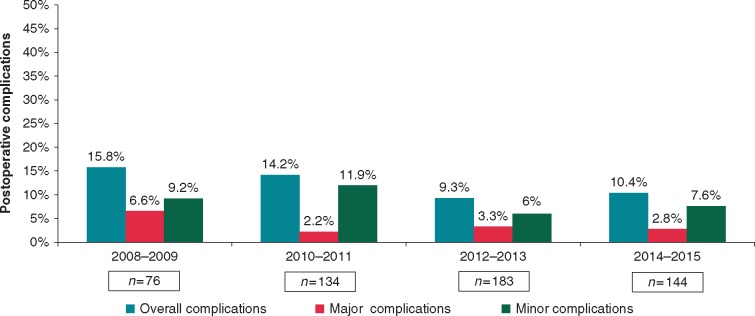
Complications were analysed over time to assess the potential learning curve of totally thoracoscopic surgical ablation. Figure 4 shows the post-operative complications from 2008 to 2015, highlighting a downward trend in the overall post-operative complication rate in our specialist referral centre.
Figure 4.
Post-operative complications over time. Excludes the first year of the program with only a small number of cases (n = 21) and no complications.
Discussion
This is the first comprehensive assessment of totally thoracoscopic epicardial ablation complications, performed with critical appraisal on a case-by-case basis in a large cohort of patients operated across a 9-year interval. Our study showed that 88.2% of our patients were free from any complication at 30 days. The majority of complications that occurred were transient and the freedom from irreversible complications further affecting a patient’s long-term prognosis was 97.5%. No operative mortality or stroke was observed, although one patient with poor INR control suffered a stroke and died during follow-up (mortality rate 0.2%). Post-operative complications were mostly procedure-related and manageable with conventional treatment. Previous thoracoscopic surgical ablation studies have focused on rhythm outcomes and only included complications as a secondary safety outcome. We speculate that this might have resulted in over- and under-estimation of complication rates in some series, which range widely from 0 to 34%.4,5,7–10 This variation in complication rates can further be explained by the small size of patient numbers included in past reports and by the limited amount of operators involved. In contrast, we systematically catalogued complications from all consecutive patients operated on in our referral centre by multiple surgeons without any patient selection, and identified low rates of mortality, stroke and 30-day complications.
Uniquely due to the large sample size, we were also able to explore patient factors associated with complication risk. Neither the type of AF, history of previous catheter ablation, nor ejection fraction was related to complications, and our analysis refuted the commonly held belief that obesity increases complication risk. Only older female patients aged 70 years or above, and those with history of congestive heart failure, had increased risk. With respect to heart failure, these patients should be adequately treated according to the latest evidence prior to undergoing any rhythm control procedure.11,12
Rhythm control is an important part of the management of AF and is targeted to improve symptoms, rather than prognosis. The 2016 AF guidelines from the European Society of Cardiology (ESC) give a Class IIa (B) recommendation for epicardial PVI for patients with symptomatic AF after failed catheter ablation, and IIa (C) for other patients with refractory symptomatic persistent AF.2 Decisions involving surgery should be supported by an AF Heart Team that includes experienced cardiologists, electrophysiologists, and AF surgeons, to advise patients on available options for rhythm control.13 As all decisions on rhythm control must balance the risk of complications with symptomatic benefit, our systematic appraisal of complications in this large series is vital for the AF Heart Team to decide on whether AAD, (repeat) catheter ablation, or thoracoscopic ablation is more appropriate for individual patients. Comparison between thoracoscopic and catheter ablation is limited by the heterogeneity of patients and different techniques (endocardial vs. epicardial approach); however, our data would suggest comparable complication rates. The FAST trial is one of only three prospective randomized trials that have compared catheter with thoracoscopic ablation, reporting a higher success rate but also a higher complication rate after surgical ablation, mainly due to a higher incidence of conservatively treated pneumothorax.3 Catheter ablation is associated with severe complications in 5–7%, and 2–3% will experience life-threatening but usually manageable complications.2 Thoracoscopic surgical ablation is a more invasive procedure with associated surgical complications, but does not result in radiation exposure.14,15 Atrial-oesophageal fistula is an exceptionally rare complication after thoracoscopic ablation surgery that can be avoided with good surgical technique.16
Clinical perspective
Since the first description of thoracoscopic surgical ablation in 2003, this technique has evolved from PVI only towards routine use of extended lesion sets. Although thoracoscopic ablation is recommended as an alternative to catheter ablation in certain categories of patients, the procedure is not widely available at present. However, there is a growing trend towards less invasive surgical access and thoracoscopic approaches in particular. Whilst patients currently have to be referred to dedicated referral centres and have a longer inpatient hospital stay than catheter ablation, it is anticipated that thoracoscopic ablation will become more accessible in the near future because of its effectiveness for long-term rhythm control. Our data confirm a short learning curve with further reduction in complication rates with ongoing experience.
The introduction of thoracoscopic ablation surgery was driven by the need for less invasive strategies that could replace the Cox-maze III/IV operation, which although effective, requires extra-corporal circulation and cardiac arrest. Balancing this, thoracoscopic ablation provides a less complete lesion set than conventional surgery due to limited access to cardiac structures, and hence lower success rates for maintenance of sinus rhythm.17 The Cox-maze III/IV operation can also be performed by a minimally invasive approach in many centres avoiding the need for sternotomy, although reported complication rates are higher than our series.17 No data comparing this approach with thoracoscopic ablation are available, and the Cox-maze III/IV operation is not a viable alternative to catheter ablation for most patients.
Limitations, strengths, and future studies
The main limitations of our analysis were the observational nature and retrospective design. Although we systematically assessed complications after totally thoracoscopic ablation surgery, there is a risk of incomplete data or bias with this approach. We did not consider adverse events relating to AAD therapy and also limited our assessment to 30-day complications. However, we would expect to capture most, if not all operative complications at this point, and the data can be more easily compared with other procedures with 30-day outcomes. We consider the heterogeneity of patients and procedures a strength of this analysis, as the complication rate is more likely to reflect clinical practice than studies with strict inclusion criteria. Thoracoscopic ablation typically uses an array of lesion sets6; although our study is unable to determine whether this approach increases the risk of complications beyond PVI alone, additional lesions may yield incremental value for rhythm control and most patients have already failed conventional endocardial PVI. Our surgeons were clearly not blinded to operative outcomes, although bias was minimized by utilizing an independent research fellow to catalogue complications. Finally, although we collected data from multiple surgeons consecutively, these were all based at a dedicated AF surgical centre with a specialized and experienced team of staff. Therefore, our finding that this procedure can be delivered with a low and acceptable complication rate may not be generalizable to other smaller centres or those with a lower volume of referred patients. Combining data from multiple institutions and generating longer-term data on the maintenance of sinus rhythm are the next steps, after ensuring that the methods of obtaining such data are robust and systematic. Outcome definition and reporting need to be standardized, credible, and accurate, in order to correctly inform patients and referring clinicians. In Supplementary material online, Table S1,18–20 we provide a potential scheme for comprehensive complication reporting in thoracoscopic surgical ablation, which could also be adapted for other surgical studies to encourage consistent reporting of complications.
Conclusion
Totally thoracoscopic epicardial ablation has evolved towards a safe surgical treatment for refractory symptomatic AF and is associated with a low complication rate in an AF surgical centre. The majority of complications relating to this procedure were reversible and not expected to adversely affect patient prognosis. Where suitably trained thoracoscopic surgeons are available, thoracoscopic surgical ablation should be considered a viable alternative or adjunct to catheter ablation to improve symptoms related to recurrent AF.
Funding
D.K. is funded by a National Institute for Health Research (NIHR) Career Development Fellowship (CDF-2015-08-074). The opinions expressed are those of the authors and do not represent the NIHR or the UK Department of Health.
Conflict of interest: Dr van Putte has received speaker fees from Atricure and is a proctor for Atricure. Dr Kotecha has received research grants from Menarini, speaker fees from Atricure and professional development support from Daiichi Sankyo. The other authors have no conflicts of interest to declare.
Supplementary Material
References
- 1. Lane DA, Skjoth F, Lip GYH, Larsen TB, Kotecha D.. Temporal trends in incidence, prevalence, and mortality of atrial fibrillation in primary care. J Am Heart Assoc 2017;6:e005155.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B. et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016;18:1609–78. [DOI] [PubMed] [Google Scholar]
- 3. Boersma LV, Castella M, van Boven W, Berruezo A, Yilmaz A, Nadal M. et al. Atrial fibrillation catheter ablation versus surgical ablation treatment (FAST): a 2-center randomized clinical trial. Circulation 2012;125:23–30. [DOI] [PubMed] [Google Scholar]
- 4. Van Laar C, Kelder J, van Putte BP.. The totally thoracoscopic maze procedure for the treatment of atrial fibrillation. Interact Cardiovasc Thorac Surg 2017;24:102–11. [DOI] [PubMed] [Google Scholar]
- 5. Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA. et al. 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace 2012;14:528–606. [DOI] [PubMed] [Google Scholar]
- 6. Van Laar C, Geuzebroek GSC, Hofman FN, van Putte BP.. The totally thoracoscopic left atrial maze procedure for the treatment of atrial fibrillation. Multimed Man Cardiothorac Surg 2016;2016, doi: 10.1093/mmcts/mmv043. [DOI] [PubMed] [Google Scholar]
- 7. Phan K, Phan S, Thiagalingam A, Medi C, Yan TD.. Thoracoscopic surgical ablation versus catheter ablation for atrial fibrillation. Eur J Cardiothorac Surg 2016;49:1044–51. [DOI] [PubMed] [Google Scholar]
- 8. Krul SPJ, Driessen AHG, Zwinderman AH, van Boven WJ, Wilde AA, de Bakker JM. et al. Navigating the mini-maze: systematic review of the first results and progress of minimally-invasive surgery in the treatment of atrial fibrillation. Int J Cardiol 2013;166:132–40. [DOI] [PubMed] [Google Scholar]
- 9. La Meir M, Gelsomino S, Lucà F, Pison L, Colella A, Lorusso R. et al. Minimal invasive surgery for atrial fibrillation: an updated review. Europace 2013;15:170–82. [DOI] [PubMed] [Google Scholar]
- 10. Geuzebroek GSC, Bentala M, Molhoek SG, Kelder JC, Schaap J, Van Putte BP.. Totally thoracoscopic left atrial Maze: standardized, effective and safe. Interact Cardiovasc Thorac Surg 2016;22:259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kotecha D, Piccini JP.. Atrial fibrillation in heart failure: what should we do? Eur Heart J 2015;36:3250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kotecha D, Lam CS, Van Veldhuisen DJ, Van Gelder IC, Voors AA, Rienstra M.. Heart failure with preserved ejection fraction and atrial fibrillation—vicious twins. J Am Coll Cardiol 2016;68:2217–28. [DOI] [PubMed] [Google Scholar]
- 13. Kotecha D, Breithardt G, Camm AJ, Lip GYH, Schotten U, Ahlsson A. et al. Integrating new approaches to atrial fibrillation management: the 6th AFNET/EHRA Consensus Conference Europace 2018;20:395--407. [DOI] [PubMed] [Google Scholar]
- 14. Gupta A, Perera T, Ganesan A, Sullivan T, Lau DH, Roberts-Thomson KC. et al. Complications of catheter ablation of atrial fibrillation: a systematic review. Circ Arrhythm Electrophysiol 2013;6:1082–8. [DOI] [PubMed] [Google Scholar]
- 15. Haegeli LM, Calkins H.. Catheter ablation of atrial fibrillation: an update. Eur Heart J 2014;35:2454–9. [DOI] [PubMed] [Google Scholar]
- 16. Van Putte BP, Weimar T.. Atrial-esophageal fistula after thoracoscopic maze surgery: the real perspective. Thorac CardioVasc Surg 2017;65:471.. [DOI] [PubMed] [Google Scholar]
- 17. Je HG, Shuman DJ, Ad N.. A systematic review of minimally invasive surgical treatment for atrial fibrillation: a comparison of the Cox-Maze procedure, beating heart epicardial ablation, and the hybrid procedure on safety and efficacy. Eur J Cardiothorac Surg 2015;48:531–41. [DOI] [PubMed] [Google Scholar]
- 18. Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A. et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:2064–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Holmes DR, Monahan KH, Packer D.. Pulmonary vein stenosis complicating ablation for atrial fibrillation. J Am Coll Cardiol Cardiovasc Interv 2009;2:267–76. [DOI] [PubMed] [Google Scholar]
- 20. Pappone C, Vicedomini G, Santinelli V.. Atrio-esophageal fistula after AF ablation: pathophysiology, prevention & treatment. J Atr Fibrillation 2013;6:102–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.