Abstract
The rising prevalence of non-alcoholic fatty liver disease (NAFLD) parallels the global increase in the number of people diagnosed with obesity and metabolic syndrome. The gut-liver axis (GLA) plays an important role in the pathogenesis of NAFLD/non-alcoholic steatohepatitis (NASH). In this review, we discuss the clinical significance and underlying mechanisms of action of gut-derived secretory factors in NAFLD/NASH, focusing on recent human studies. Several studies have identified potential causal associations between gut-derived secretory factors and NAFLD/NASH, as well as the underlying mechanisms. The effects of gut-derived hormone-associated drugs, such as glucagon-like peptide-1 analog and recombinant variant of fibroblast growth factor 19, and other new treatment strategies for NAFLD/NASH have also been reported. A growing body of evidence highlights the role of GLA in the pathogenesis of NAFLD/NASH. Larger and longitudinal studies as well as translational research are expected to provide additional insights into the role of gut-derived secretory factors in the pathogenesis of NAFLD/NASH, possibly providing novel markers and therapeutic targets in patients with NAFLD/NASH.
Keywords: non-alcoholic fatty liver disease, non-alcoholic steatohepatitis, gut-liver axis, resistin like molecule β, glucagon-like peptide-1, glucagon-like peptide-2, fibroblast growth factor 19, neurotensin
1. Introduction
The rising prevalence of non-alcoholic fatty liver disease (NAFLD) parallels the global increase in the number of people diagnosed with obesity and metabolic syndrome. A subset of individuals with NAFLD develops non-alcoholic steatohepatitis (NASH), which is characterized by hepatocellular lipid accumulation along with inflammation and fibrosis of varying severities [1,2,3]. NASH can lead to cirrhosis and even liver cancer. Furthermore, NAFLD is also recognized as an independent risk factor for cardiovascular mortality [4], such that the development of effective therapeutic interventions is eagerly awaited. Despite the very high number of pharmacological interventions currently being proposed, the identification of an effective therapy for NAFLD beyond the widely recommended lifestyle measures remains an open issue and represents a major clinical and research challenge [5].
The strong anatomical and functional interactions between the gastrointestinal tract and the liver underlie the gut-liver axis (GLA) concept. This intimate connection begins in embryogenesis, as the liver and gastrointestinal tract both arise from the ventral foregut endoderm [6]. The GLA is characterized as showing bidirectional traffic. Nutrients and factors derived from the gut lumen reach the liver via the portal circulation; bile acids produced by hepatocytes are then released via the biliary tract into the small intestine. However, this description is overly simple because the GLA has functions beyond its nutritional component. This is a complex axis and alterations, especially in two of its components (intestinal barrier and gut microbiota) appear to play key roles in the onset and progression of liver damage [7].
The GLA plays an important role in the pathogenesis of NAFLD/NASH. Recently, we demonstrated possible causal links between gut-derived resistin like molecule β (RELMβ) and NASH, as well as the underlying mechanisms participating in its pathogenesis [8]. The effects of gut-derived hormone-associated drugs for treating NAFLD/NASH have also been reported. Therefore, gut-derived secretory factors represent a novel marker and potential therapeutic target in patients with NAFLD/NASH.
In this review, we discuss the clinical significance and underlying mechanisms of action of gut-derived secretory factors in NAFLD/NASH.
2. RELMβ
RELMβ, a protein homologous to resistin, is expressed in the secretory granules of intestinal goblet cells, macrophages and bronchi [9,10]. Three resistin-related proteins, designated RELM α, β and γ (encoded by the genes Retnla, Retnlb and Retnlg, respectively) have been identified in mice. Resistin and RELMβ have been identified in humans, while RELMα and γ have not [11,12].
RELMβ is a bactericidal protein secreted apically into the intestinal lumen which directly kills pathogens [13,14]. Colonic RELMβ expression is undetectably low in germ-free mice [9], and the absence of RELMβ influences the microbial composition in the gut [15]. A recent study demonstrated both mouse and human RELMβ to selectively kill gram-negative bacteria by forming size-selective pores which result in permeabilization of the bacterial membrane [14]. RELMβ also contributes to host defense against intestinal nematode infections. RELMβ is induced by intestinal nematode infections through a host Th2-mediated immune responses, and inhibits both chemotaxis and feeding [16,17].
RELMβ also contributes to immune regulation in the gut. RELMβ expression is induced in some animal models of intestinal inflammation [18,19]. RELMβ was considered to be delivered from the lumen into the lamina propria through a disrupted epithelial barrier and to influence macrophage activation resulting in the production of inflammatory cytokines [20]. Indeed, RELMβ knock-out (KO) mice showed suppression of dextran sodium sulfate-induced colitis [20] and infection-induced intestinal inflammation [21]. The RELMβ promoter contains functional binding sites for the hepatocyte nuclear factor 4α (HNF4α), a transcriptional factor expressed in the intestine. HNF4α has two isoforms, P1 and P2, and specific expression of P2-HNF4α in mice promoted RELMβ expression and inflammation in a murine colitis model [22]. RELMβ also reportedly promotes colitis in mucin KO mice with dysbiosis, as evidenced by depleting protective commensal microbiota (Lactobacillus spp.). Interestingly, administration of Lactobacillus reduced RELMβ positive cells in the mouse colon [23]. Thus, it appears that RELMβ and the gut microbiota regulate each other, with both contributing to the maintenance of gut homeostasis, including immune and inflammatory responses.
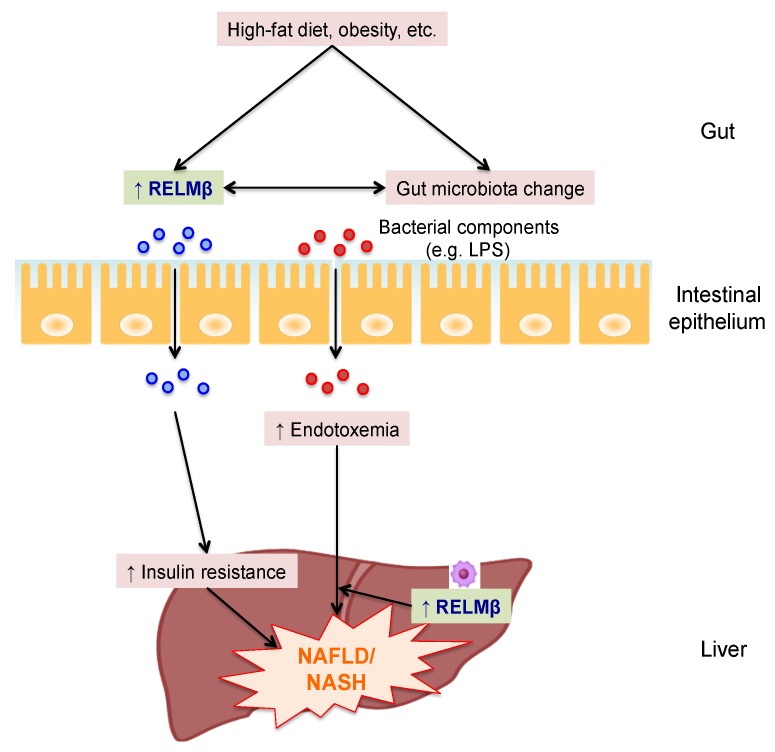
Insulin resistance plays an important role in the pathogenesis of NAFLD [24]. In insulin resistant models such as high fat diet (HFD) fed and db/db mice, the intestinal expression and serum concentration of RELMβ were both increased [11]. Peripheral infusion of RELMβ impaired insulin action in the rat liver [25]. In addition, transgenic mice overexpressing RELMβ in the liver showed insulin resistance and hepatic steatosis as compared to control mice under HFD-fed conditions. RELMβ contributes to metabolic dysfunction by signaling through mitogen-activated protein kinase pathways and suppressing insulin signaling in hepatocytes [26]. Thus, insulin resistance caused by RELMβ might be involved in the pathogenesis of NAFLD. However, RELMβ KO mice are also resistant to NASH with a higher proportion of lactic acid bacteria (Lactobacillus species) in feces and reduced endotoxemia in a methionine choline-deficient (MCD) diet model showing neither obesity nor insulin resistance [8]. The MCD diet was found to increase RELMβ expression in both Kupffer cells (resident macrophages in the liver) and the colon. Interestingly, mouse experiments using radiation chimeras showed wild type and RELMβ KO to be required in both organs for full manifestation of NASH. The RELMβ in Kupffer cells enhanced hepatic inflammation induced by the endotoxin lipopolysaccharide (LPS) entering the liver, since LPS-induced inflammatory cytokine expressions were significantly suppressed in peritoneal macrophages isolated from RELMβ KO mice and a previous report showed RELMβ activated macrophages to express MHC class II and inflammatory cytokines [21]. Regarding the role of RELMβ in the colon, changes in RELMβ-induced gut microbiota might be involved in the impairment of gut permeability and the induction of endotoxemia, since RELMβ KO mice showed a higher proportion of Lactobacillus, which reportedly contributes to the normalization of tight junction proteins and reduced endotoxemia [27,28,29]. Though RELMβ itself might be a key factor impairing gut barrier function independently of the gut microbiota, its impact on barrier function is still debated [20,30]. Thus, RELMβ may raise not only the level of circulating LPS but also activate macrophages to be highly responsive to LPS. The proposed role of RELMβ in the pathogenesis of NAFLD/NASH is shown in Figure 1.
Figure 1.
The proposed role of RELMβ in the pathogenesis of NAFLD/NASH. Intake of a high-fat diet and obesity increase both intestinal expression and circulating levels of resistin like molecule β (RELMβ). Circulating RELMβ elicits insulin resistance, and increased gut-derived RELMβ and gut microbiota appear to regulate each other, thereby increasing translocation of the endotoxin lipopolysaccharide (LPS) from the intestine into the bloodstream and liver, which induces hepatic steatosis and inflammation. In the liver, RELMβ in Kupffer cells exacerbates hepatic inflammation, along with endotoxemia, which further worsens non-alcoholic fatty liver disease (NAFLD)/non-alcoholic steatohepatitis (NASH).
A human study demonstrated plasma levels of RELMβ to be measurable [31]. Though further investigation of the relationships of RELMβ with metabolic parameters in humans is needed, the data accumulated thus far suggest that suppressing gut-derived RELMβ expression by approaches such as gut microbiota modification might be a therapeutic strategy for managing NAFLD/NASH. In addition, RELMβ receptors have not as yet been identified. Further investigation of the receptors and activation mechanisms for RELMβ might lead to a new class of agents that antagonize the pathological effects of RELMβ, and could serve as novel therapies for NAFLD/NASH.
3. Glucagon-Like Peptide-1
Glucagon-like peptide-1 (GLP-1), a 30-amino acid peptide produced from the proglucagon gene in endocrine L-cells of the small intestine, is secreted into the bloodstream in response to nutrients [32,33]. GLP-1 receptor (GLP-1R) is a G protein coupled receptor widely expressed in pancreatic islets, the stomach, duodenum, central nervous system (CNS), heart, lung, kidney and immune cells [34,35]. Whether GLP-1R is expressed in hepatocytes remains controversial [36,37].
GLP-1 reduces blood glucose concentrations by glucose-dependently stimulating insulin secretion and suppressing glucagon secretion, thereby promoting satiety and delaying gastric emptying through central mechanisms, which in turn reduces food intake [38,39]. GLP-1 was also reported to directly reduce steatosis by decreasing lipogenesis and increasing fatty acid oxidation in hepatocytes [36,40,41]. Considering the association of NAFLD/NASH with obesity and type 2 diabetes mellitus (T2DM), these pleiotropic effects make GLP-1 an attractive therapeutic target in patients with NAFLD/NASH.
The half-life of GLP-1 is short, only one to two minutes, due to N-terminal degradation by the enzyme dipeptidyl peptidase-4 (DPP-4). Synthetic GLP-1R agonists (GLP-1RA) which are resistant to degradation by DPP-4, and DPP-4 inhibitors which prevent GLP-1 degradation, thereby increasing the plasma levels of endogenous GLP-1, are now in widespread clinical use for T2DM [42,43]. Interestingly, glucose-induced GLP-1 secretion is reportedly decreased in patients with NAFLD/NASH as compared to healthy control subjects [44]. In a meta-analysis of 6 randomized-controlled trials, the LEAD (Liraglutide Efficacy and Action in Diabetes) trials, daily injection of GLP-1RA liraglutide (1.8 mg per day) produced a significant decrease in plasma alanine aminotransferase (ALT) (a liver injury biomarker) in patients with T2DM, and the effect was dose-dependent (no significant difference vs. placebo with liraglutide 0.6 or 1.2 mg). This effect appeared to be mediated by GLP-1RA actions on weight loss and glycemic control [45]. Another report showed a significant reduction in liver fat in T2DM patients treated with either the GLP-1RA exenatide or liraglutide for 6 months that correlated with improved glycemic control [46]. The LEAN trial (Liraglutide Efficacy and Action in NASH) was the first randomized, placebo-controlled trial to examine the effect of liraglutide on liver histology in patients with NASH. Participants in the Liraglutide (1.8 mg per day) treatment group showed resolution of definite NASH as compared with participants in the placebo group, although no anti-fibrotic effects were seen. The effects of liraglutide were attributed to a combination of direct hepatic effects and an effect on weight loss. Liraglutide was safe, well tolerated and had an adverse effect profile similar to that of the placebo, with the exception of predictable gastrointestinal symptoms including diarrhea, constipation and loss of appetite [47]. Japanese studies (LEAN J study) also reported that liraglutide (0.9 mg per day) decreased liver fat deposition and improved ALT and aspartate aminotransferase (AST) in patients with NASH [48]. The effects of exenatide in NASH have also been explored (NCT01208649). Semaglutide is a GLP-1 agonist that requires only weekly dosing. In the SUSTAIN 1 trial, drug-naive patients with T2DM were randomly assigned to once weekly semaglutide (0.5 mg or 1.0 mg) or a placebo for 30 weeks. HbA1c, body weight, body mass index (BMI) and waist circumstance were significantly reduced in both semaglutide groups as compared to the placebo group [49]. A large multicenter trial designed to compare the efficacy and safety profiles of three doses of once daily subcutaneous semaglutide versus a placebo in patients with biopsy proven NASH is currently underway (NCT02970942). In addition, oral semaglutide is now being examined in clinical trials [50]. Considering the improved acceptance and adherence, oral semaglutide might be a promising treatment for NASH. However, the American Association for the Study of Liver Diseases warns that it is premature to consider using GLP-1RA to specifically treat NASH/NAFLD in the absence of diabetes because clinical evidence of efficacy and safety is still insufficient [51]. Larger long-term randomized-controlled trials are needed to establish the role of GLP-1RA in the management of NAFLD/NASH patients.
In randomized double-blind placebo-controlled studies, the DPP-4 inhibitor sitagliptin (100 mg per day) was no more effective than a placebo for ameliorating hepatic steatosis and fibrosis in patients with NAFLD/NASH [52,53]. Considering that DPP-4 inhibitors increase endogenous GLP-1 to physiological levels (10–25 pmol/L), whereas GLP-1RA reaches higher pharmacological levels (60–90 pmol/L), the difference in elevated GLP-1 concentrations might contribute to the differing efficacies in patients with NAFLD/NASH, though whether tissue distributions are similar for all GLP-1R and what level of GLP-1 exposure produces each effect, are not well known [43]. The clinical trials involving NAFLD/NASH patients employing GLP-1RA or DPP-4 inhibitors are summarized in Table 1.
Table 1.
Summary of clinical trials of GLP-1RA or DPP-4 inhibitors involving NAFLD/NASH patients.
| References | Study Design | Study Subjects | Therapy and Follow-Up Duration | Outcomes |
|---|---|---|---|---|
| Cuthbertson et al. 2012 [46] | SA | 25 (T2DM); NAFLD | Exenatide 20 μg (n = 19) or Liraglutide 1.2 mg (n = 6); 6 months | ↓ALT; ↓Liver fat (1H MRS) |
| Armstrong et al. 2016 [47] | DB, RAND, PLAC | 23; NASH (biopsy proven) |
Liraglutide 1.8 mg vs. placebo; 12 months | Histology (disappearance of ballooning without worsening of fibrosis) improved |
| Eguchi et al. 2015 [48] | SA | 19 (T2DM); NASH (biopsy proven) |
Liraglutide 0.9 mg; 6 months | ↓AST, ALT; ↓Liver fat (CT) |
| Cui et al. 2016 [52] | DB, RAND, PLAC | 24 (prediabetes or early diabetes); NAFLD | Sitagliptin 100 mg; 6 months | Liver fat (MRI) not improved |
| Joy et al. 2017 [53] | DB, RAND, PLAC | 6 (T2DM); NASH (biopsy proven) |
Sitagliptin 100 mg; 6 months | Histology (Fibrosis and NAS) not improved |
Abbreviations: ALT, alanine transaminase; AST, aspartate aminotransferase; DB, double blind; 1H MRS, proton magnetic resonance spectroscopy; NAFLD, non-alcoholic fatty liver disease; NAS, NAFLD activity score; NASH, non-alcoholic steatohepatitis; OAD, oral antidiabetic drug; PLAC; placebo controlled; RAND, randomized; SA, single arm; T2DM, type 2 diabetes mellitus.
A recent study showed HFD-induced dysbiosis to impair GLP-1 signaling involved in the control of insulin secretion and gastric emptying in mice [54]. NAFLD/NASH patients reportedly have dysbiosis [55]. Future studies of GLP-1 resistance in human subjects targeting GLP-1 signaling may reveal whether correcting dysbiosis is a potentially novel strategy for treating NAFLD/NASH.
4. Glucagon-Like Peptide-2
Glucagon-like peptide 2 (GLP-2) is a 33-amino acid proglucagon-derived peptide produced by L-cells in the small and large intestine, as well as in the brain, predominantly the brainstem [56]. Prohormone convertase 1/3 processes proglucagon resulting in GLP-1, GLP-2, intervening peptide-2, oxynthomodulin and glicentin in the gastrointestinal tract and in the brain [57].
GLP-2 is secreted in response to nutrients such as carbohydrates and fats [58]. In humans, GLP-2 has a short half-life of about 7 min due to degradation to inactive GLP-2 (3–33) by DPP-IV [59]. DPP-IV-resistant GLP-2 analogues such as teduglutide, in which alanine is replaced with glycine at the second position from the N-terminus of GLP-2, exhibit greater bioactivity relative to the native molecule, due to their longer circulating half-lives [60]. GLP-2 exerts its action by binding the GLP-2 receptor (GLP-2R), a member of the G protein coupled receptor superfamily. GLP-2R exhibits high homology with GLP-1R, with which it shares several intercellular signaling mediators such as cyclic adenosine monophosphate (cAMP) and protein kinase A. GLP-2R is widely expressed in the gastrointestinal tract, CNS, lung, vagal afferents, heart and liver [61,62,63,64,65,66].
GLP-2 is produced by neurons in the brainstem and fibers that project to the dorsomedial hypothalamic nucleus where GLP-2R is located. Intra-cerebroventricular infusion of GLP-2 inhibits food intake in rats. Thus, GLP-2 is considered to be a neurotransmitter which is involved in the control of feeding behavior [67]. While human studies have not demonstrated a decrease in food intake after peripheral GLP-2 administration, the data obtained to date suggest that circulating GLP-2 at physiological concentrations might not be involved in the control of feeding behavior in humans [68].
GLP-2 has also been shown to induce crypt cell proliferation and to inhibit apoptosis, thereby increasing villus height and expanding the absorptive mucosal surface in both the small and the large intestine [56,69,70]. In addition, in parenterally fed rats with short bowel syndrome (SBS), GLP-2 treatment reversed the associated increases in villus height and the intestinal mucosal surface area [71]. Indeed, a GLP-2R agonist, teduglutide, is under clinical development for patients with SBS [72,73].
Energy absorption in the gastrointestinal tract is promoted by GLP2, via mechanisms involving both non-specific and specific adaptation. GLP2 increases the uptakes of sugars via augmented activities and expressions of transporters [74,75], as well as by enhancing the expressions of various digestive enzymes [76]. GLP2 is also involved in lipid absorption. Indeed, GLP2 reportedly facilitates intestinal absorption of lipids [77] and enhances and regulates chylomicron secretion from the intestine in human subjects [78].
GLP-2 modulates intestinal permeability. GLP-2 treated mice show intestinal epithelial barrier enhancement with increased total mucosal thickness and villus height [79]. GLP-2 was also reported to directly enhance intestinal permeability in human intestinal epithelial cells [80]. Pharmacological GLP-2 treatment improves gut permeability markers, while reducing endotoxemia and systemic and hepatic inflammation in genetically obese (ob/ob) mice [81]. Patients with NAFLD/NASH reportedly show increased gut permeability and elevated plasma endotoxin concentrations, which are considered to be involved in the pathogenesis of NAFLD/NASH [82,83]. GLP-2 enhances epithelial barrier function and ameliorates inflammation [84], thereby possibly contributing to suppression of the development of NAFLD/NASH. Chronic treatment with GLP-2 (3–33), a competitive GLP-2R antagonist [85], also reportedly exacerbates dyslipidemia and hepatic lipid accumulation in HFD-fed mice [86], suggesting that endogenous GLP-2 may have a defensive role acting against lipid imbalance under conditions of obesity.
At present, commercial GLP-2 measurement kits detect both the 1–33 (active) and 3–33 (inactive) forms of GLP-2 [87,88]. Development of a measurement kit that can detect only the active form of GLP-2, such as is the case for GLP-1, might deepen our understanding of the relationship between GLP-2 and NAFLD/NASH in humans. Furthermore, GLP-2R exerts its actions in the liver. Thus, we need to elucidate the direct effects of GLP-2 in order to understand the role of GLP-2 in the pathogenesis of NAFLD/NASH. Though there are concerns about the potential for carcinogenesis with the use of GLP-2 or its analogues [89] and further studies are needed, GLP2 analogues such as teduglutide might be an attractive approach for clinical treatment of NAFLD/NASH through structural and functional restoration of the intestinal epithelium, which would presumably reduce both intestinal permeability and endotoxemia.
5. Fibroblast Growth Factor 19
Fibroblast growth factor (FGF) 15 (in mice)/19 (in humans) is an endocrine gastrointestinal hormone expressed in ileal enterocytes, where it was shown to be induced by the bile acid acting farnesoid X receptor (FXR) [90]. Recent studies have suggested that FGF19 is also regulated by other food-derived components such as fat-soluble vitamins [91] and cholesterol [92].
FGF15/19 binds to its preferred receptor, FGF receptor (FGFR) 4, and co-receptor β-klotho [93]. The liver is the major site of the metabolic actions of FGF19, which are exerted via activation of the FGFR4-β-klotho complex. FGF19 also has biological functions in other tissues such as white adipose tissue, where it binds and activates FGFR1c-β-klotho [93], and in the brain via an as yet unknown FGFR complex [94].
FGF15/19 plays a key role in bile acid- and FXR-mediated CYP7A1 inhibition by binding to the FGFR4-β-klotho complex [90]. Indeed, FGF15, FGFR4 and β-klotho KO animal models all show dysregulated bile acid metabolism, and administration of exogenous FGF19 fails to suppress CYP7A1 in both FGFR4 and β-klotho KO animals [90,95,96,97,98].
In addition to their roles in the regulation of bile acid homeostasis, both FGF15/19 and FGFR4 are involved in the maintenance of glucose and protein metabolism [99]. FGF15 KO mice showed failure of proper blood glucose level maintenance and exhibited reduced hepatic glycogen and glucose intolerance that were corrected by FGF19 administration [100]. The ability of FGF19 to maintain glucose homeostasis also relies on gluconeogenesis inhibition through a pathway involving inhibition of the cAMP response element binding protein—peroxisome proliferator activated receptor-γ co-activator 1α signaling cascade [101]. Conversely, administration or overexpression of FGF19 in mice provided protection from diet-induced obesity and promoted the enhancement of energy expenditure as a result of increased hepatic fatty acid oxidation via suppression of acetyl-CoA carboxylase 2 and stearoyl-CoA desaturase 1, as well as an increased brown adipose tissue mass [94,102].
Recently reported observations have suggested the CNS to play a role in FGF19-mediated glucose homeostasis regulation, based on demonstrations of intra-cerebroventricular infusions of FGF19 improving glycemic status and potentiating peripheral insulin signaling in a murine insulin resistance model [103]. In addition, intra-cerebroventricular infusions of FGF19 also reduced both food intake and body weight [103,104].
Bile acid accumulation within hepatocytes can lead to mitochondrial dysfunction, endoplasmic reticulum stress, and immune cell infiltration, possibly producing inflammation, cell death, and hepatic injury [105,106,107]. In fact, NASH patients reportedly have elevated hepatic and circulating bile acid concentrations [108,109]. Furthermore, the circulating FGF19 concentration is reduced in patients with NAFLD/NASH [110,111], suggesting that dysregulated FGF19 expression might contribute to the pathogenesis of NAFLD/NASH. The aforementioned pleiotropic effects of FGF19 confirm the FGF19 agonism-inducing strategies to be promising therapeutic approaches for the treatment of NASH. However, the therapeutic promise held by FGF19 is blunted, to some degree, by its mitogenic potential which raises the possibility of hepatic tumorigenesis [112,113]. As this tumorigenic activity has been ascribed to FGFR4 [114,115], FGF19 variants specifically designed to eliminate FGFR4 binding have been generated. One engineered variant of FGF19 (NGM282, also known as M70) showed fully retained bile acid regulatory activity, but was devoid of pro-tumoral activity in mouse models [116]. NGM282 exhibited differential signaling pathway activation as compared with FGF19, activating only a subset of signaling pathways downstream from FGFR4. In animal models of NASH, treatment with NGM282 resulted in rapid and profound reductions in the concentrations of ALT and AST, as well as clear improvements in all histological features of NASH with suppression of de novo bile acid synthesis and inhibition of fatty acid synthesis and de novo lipogenesis, presumably through mechanisms that ameliorate hepatic bile acid toxicity and lipotoxicity [117].
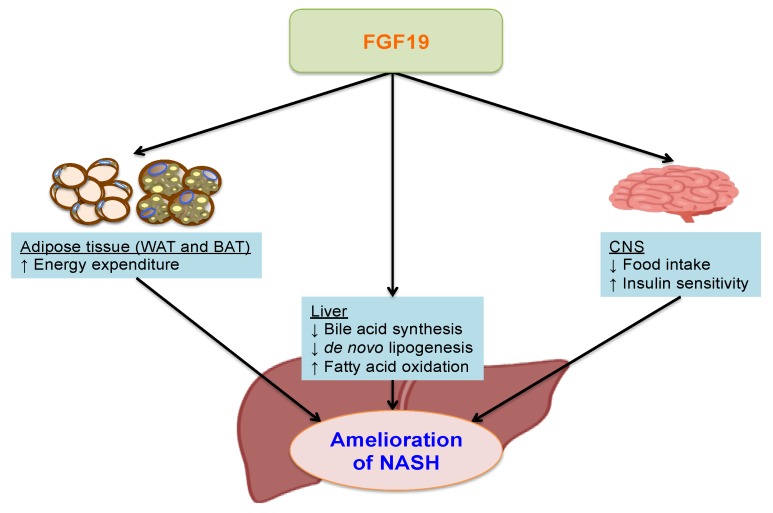
In healthy individuals, NGM282 was safe and well tolerated and reduced serum concentrations of 7α-hydroxy-4-cholesten-3-one (C4), a marker for hepatic CYP7A1 activity [118]. Recently, in a randomized, placebo-controlled, double-blind study, NGM282 3 mg and 6 mg doses were shown to reduce C4 concentrations and significantly and rapidly improve the liver fat content over 12 weeks of treatment. In addition to changes in absolute liver fat content, NGM282 produced significant improvements in ALT, AST, and non-invasive serum fibrosis biomarkers including pro-peptide of type III collagen and the total enhanced liver fibrosis score. Patients in the 6 mg NGM282 group reportedly showed significant reductions in weight and BMI as compared to the placebo group, but the reductions in liver fat content were independent of weight loss and BMI. NGM282 was generally well tolerated at both doses, but adverse events including diarrhea, abdominal pain, and nausea were reported [119]. These results support further exploration of NGM282 for the treatment of NASH. Future studies should aim to elucidate optimal doses and the precise mechanisms of the anti-NASH effects of NGM282 in human subjects. The proposed beneficial effects of FGF19 against the pathogenesis of NASH are presented schematically in Figure 2.
Figure 2.
The putative beneficial effects of FGF19 on the pathogenesis of NASH. In the liver, fibroblast growth factor (FGF) 19 suppresses bile acid synthesis, increases fatty acid oxidation and suppresses de novo lipogenesis. In adipose tissue, elevated FGF19 in brown adipose tissue enhances energy expenditure, and in the central nervous system (CNS), FGF19 reduces food intake and improves insulin sensitivity. Overall, these pleiotropic effects of FGF19 ameliorate hepatic steatosis, bile acid toxicity and lipotoxicity, thereby exerting beneficial effects on the pathogenesis of non-alcoholic steatohepatitis (NASH).
6. Neurotensin
Neurotensin (NT) is a 13-amino acid peptide released from neuroendocrine cells of the small intestine [120]. NT exerts its physiological action by binding three NT receptor (NTR) types, NTR1, NTR2, and NTR3 [121,122]. The release of NT into the circulation is triggered by fat intake [123] and facilitates fatty acid translocation in the rat intestine [124]. In addition, NT increases small intestinal local blood flow [125] and pancreatic exocrine activity [126] promoting absorptive processes in the gut postprandially.
NT also functions as a neurotransmitter in the CNS by regulating pathways associated with ghrelin and leptin that mediate satiety and food ingestion in the lateral portion of the hypothalamus. In experimental mice, loss of the leptin action mediated by NT neurons co-expressing the long form of the receptor for leptin results in excess weight and impairs the ability to respond appropriately to energy deprivation, revealing NT to play a crucial role in mediating the leptin and ghrelin pathways [127]. These effects of NT are suggested to be involved in maintaining energy homeostasis and contributing to fat storage and metabolic disorders.
The fasting pro-NT (a stable NT precursor fragment produced in equimolar amounts relative to NT) concentrations were reported to be associated with the incidence of T2D, cardiovascular disease, breast cancer, and total and cardiovascular mortality [128]. NT deficient mice showed significantly reduced intestinal fat absorption and were protected from developing obesity, hepatic steatosis and insulin resistance associated with HFD feeding. Furthermore, NT was demonstrated to attenuate the activation of AMP-activated protein kinase and to stimulate fatty acid absorption in mice and in cultured intestinal cells. In human subjects, the same study also showed higher plasma pro-NT levels to be associated with obesity and insulin resistance, and doubled the risk of developing obesity later in life in non-obese subjects [129]. This study demonstrated a direct relationship between NT and increased intestinal fat absorption and obesity, and suggested that NT might be a prognostic marker of future obesity and a potential target for treating obesity-related diseases.
Recently, obese subjects with biopsy-proven NAFLD were shown to have significantly higher plasma pro-NT levels than those without NAFLD. Furthermore, the circulating pro-NT levels were shown to correlate positively with the presence and severity of NAFLD [130]. In morbidly obese patients, circulating pro-NT levels were also reported to be positively correlated with the presence and severity of NAFLD, as evaluated by the NAFLD activity score and histological evidence of visceral adipose tissue inflammation that leads to systemic low-grade inflammation, increased circulating fatty acid concentrations, insulin resistance and aberrant fat deposition in the liver [131]. These studies suggest that NT might be partially responsible for the pathogenesis of NAFLD/NASH through increased intestinal fat absorption and the induction of pro-inflammatory conditions in adipose tissue. Another report showed, however, that circulating levels of NT were decreased in women with NAFLD associated with morbid obesity as compared with to those in women of normal weight [132]. This discrepancy might be due to sex differences and variability in blood test results between NT (instable) and pro-NT (stable) [133]. The relationships between circulating NT/pro-NT levels in subjects with NAFLD/NASH are summarized in Table 2.
Table 2.
Summary of relationships between the circulating NT/pro-NT levels in patients with NAFLD/NASH.
| References | Groups | Findings |
|---|---|---|
| Barchetta et al. 2018 [130] | 28 Obesity without NAFLD; 32 Obesity with NAFLD | Obesity with NAFLD vs. Obesity without NAFLD, ↑Plasma pro-NT; Plasma pro-NT correlated positively with NAFLD, presence and severity |
| Barchetta et al. 2018 [131] | 40 MO | Plasma pro-NT correlated positively with NAFLD presence and severity, and VAT inflammation. |
| Auguet et al. 2018 [132] | 20 Normal weight; 18 MO without NAFLD; 33 MO with NAFLD |
MO with NAFLD vs. Normal weight, ↓Plasma NT; MO with NAFLD vs. MO without NAFLD, ↓Plasma NT; No difference in plasma NT between SS and NASH. |
Abbreviations: MO; morbid obesity; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; NT, neurotensin; SS, simple steatosis; VAT, visceral adipose tissue.
NT might be both a clinical parameter of and a therapeutic target for NAFLD/NASH, though larger studies with a longitudinal design are needed to elucidate the possible role of NT in the pathogenesis of NAFLD/NASH.
7. Conclusions
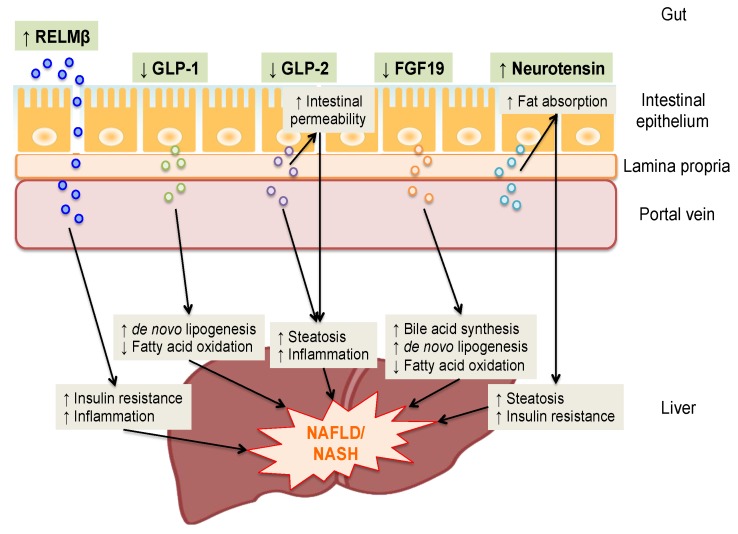
The putative roles of gut-derived secretory factors in the pathogenesis of NAFLD/NASH on the basis of GLA are summarized in Figure 3. A growing body of evidence highlights the role of GLA in the pathogenesis of NAFLD/NASH. Thus, gut-derived secretory factors are considered to be a novel potential therapeutic target, and interventions such as GLP-1 analogues and recombinant variants of FGF19 have been utilized in clinical practice and examined in trials. Furthermore, other novel possibilities are currently being investigated. However, the precise mechanisms of action of gut-derived secretory factors in the pathogenesis of NAFLD/NASH have yet to be fully elucidated in humans. In conducting clinical studies, it is advantageous that secretory factors can be measured by blood test. Larger studies with a longitudinal design, and translational research are anticipated to provide additional insights into gut-derived secretory factors in the pathogenesis of NAFLD/NASH and may offer new biomarkers and therapeutic strategies.
Figure 3.
Summary of the putative roles of gut-derived secretory factors in the pathogenesis of NAFLD/NASH on the basis of the gut-liver axis. Resistin like molecule β (RELMβ) is secreted by goblet cells and delivered from the intestinal lumen into the lamina propria through a disrupted epithelial barrier, and then translocates into the portal vein and reaches the liver. Glucagon-like peptide-1 (GLP-1), glucagon-like peptide-2 (GLP-2), fibroblast growth factor (FGF) 19 and neurotensin are released from enteroendocrine cells into the lamina propria and reach the liver through the portal vein. Increased intestinal secretion and circulating RELMβ and neurotensin levels and decreases in those of GLP-1, GLP-2 and FGF19 could be involved in the pathogenesis of non-alcoholic fatty liver disease (NAFLD)/non-alcoholic steatohepatitis (NASH) in various ways.
Abbreviations
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| BMI | Body mass index |
| CNS | Central nervous system |
| DPP-4 | Dipeptidyl peptidase-4 |
| FXR | Farnesoid X receptor |
| FGF | Fibroblast growth factor |
| GLP-1 | Glucagon-like peptide-1 |
| GLP-2 | Glucagon-like peptide 2 |
| GLA | Gut-liver axis |
| HNF4α | Hepatocyte nuclear factor 4α |
| HFD | High fat diet |
| KO | Knock-out |
| LPS | Lipopolysaccharide |
| MCD | Methionine choline-deficient |
| NT | Neurotensin |
| NAFLD | Non-alcoholic fatty liver disease |
| NASH | Non-alcoholic steatohepatitis |
| RELMβ | Resistin like molecule β |
| SBS | Short bowel syndrome |
| T2DM | Type 2 diabetes mellitus |
Author Contributions
All authors (H.O. (Hirofumi Okubo), A.K., Y.N., T.Y., Y.M., M.F., H.S., H.O. (Haruya Ohno), M.Y., T.A.) took part in writing this manuscript and preparing figures. All authors read and approved the final draft.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- 1.Vernon G., Baranova A., Younossi Z.M. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment. Pharmacol. Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 2.Younossi Z.M., Stepanova M., Afendy M., Fang Y., Younossi Y., Mir H., Srishord M. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin. Gastroenterol. Hepatol. 2011;9:524–530. doi: 10.1016/j.cgh.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 3.Hashimoto E., Taniai M., Tokushige K. Characteristics and diagnosis of NAFLD/NASH. J. Gastroenterol. Hepatol. 2013;28(Suppl. 4):64–70. doi: 10.1111/jgh.12271. [DOI] [PubMed] [Google Scholar]
- 4.Targher G., Day C.P., Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N. Engl. J. Med. 2010;363:1341–1350. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- 5.Brodosi L., Marchignoli F., Petroni M.L., Marchesini G. NASH: A glance at the landscape of pharmacological treatment. Ann. Hepatol. 2016;15:673–681. doi: 10.5604/16652681.1212318. [DOI] [PubMed] [Google Scholar]
- 6.Zorn A.M., Wells J.M. Vertebrate endoderm development and organ formation. Annu. Rev. Cell Dev. Biol. 2009;25:221–251. doi: 10.1146/annurev.cellbio.042308.113344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paolella G., Mandato C., Pierri L., Poeta M., Di Stasi M., Vajro P. Gut-liver axis and probiotics: Their role in non-alcoholic fatty liver disease. World J. Gastroenterol. 2014;20:15518–15531. doi: 10.3748/wjg.v20.i42.15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okubo H., Kushiyama A., Sakoda H., Nakatsu Y., Iizuka M., Taki N., Fujishiro M., Fukushima T., Kamata H., Nagamachi A., et al. Involvement of resistin-like molecule beta in the development of methionine-choline deficient diet-induced non-alcoholic steatohepatitis in mice. Sci. Rep. 2016;6:20157. doi: 10.1038/srep20157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He W., Wang M.L., Jiang H.Q., Steppan C.M., Shin M.E., Thurnheer M.C., Cebra J.J., Lazar M.A., Wu G.D. Bacterial colonization leads to the colonic secretion of RELMbeta/FIZZ2, a novel goblet cell-specific protein. Gastroenterology. 2003;125:1388–1397. doi: 10.1016/j.gastro.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Kushiyama A., Sakoda H., Oue N., Okubo M., Nakatsu Y., Ono H., Fukushima T., Kamata H., Nishimura F., Kikuchi T., et al. Resistin-like molecule beta is abundantly expressed in foam cells and is involved in atherosclerosis development. Arterioscler. Thromb. Vasc. Biol. 2013;33:1986–1993. doi: 10.1161/ATVBAHA.113.301546. [DOI] [PubMed] [Google Scholar]
- 11.Shojima N., Ogihara T., Inukai K., Fujishiro M., Sakoda H., Kushiyama A., Katagiri H., Anai M., Ono H., Fukushima Y., et al. Serum concentrations of resistin-like molecules beta and gamma are elevated in high-fat-fed and obese db/db mice, with increased production in the intestinal tract and bone marrow. Diabetologia. 2005;48:984–992. doi: 10.1007/s00125-005-1735-1. [DOI] [PubMed] [Google Scholar]
- 12.Renigunta A., Hild C., Rose F., Klepetko W., Grimminger F., Seeger W., Hanze J. Human RELMbeta is a mitogenic factor in lung cells and induced in hypoxia. FEBS Lett. 2006;580:900–903. doi: 10.1016/j.febslet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Steppan C.M., Brown E.J., Wright C.M., Bhat S., Banerjee R.R., Dai C.Y., Enders G.H., Silberg D.G., Wen X., Wu G.D., et al. A family of tissue-specific resistin-like molecules. Proc. Natl. Acad. Sci. USA. 2001;98:502–506. doi: 10.1073/pnas.98.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Propheter D.C., Chara A.L., Harris T.A., Ruhn K.A., Hooper L.V. Resistin-like molecule beta is a bactericidal protein that promotes spatial segregation of the microbiota and the colonic epithelium. Proc. Natl. Acad. Sci. USA. 2017;114:11027–11033. doi: 10.1073/pnas.1711395114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hildebrandt M.A., Hoffmann C., Sherrill-Mix S.A., Keilbaugh S.A., Hamady M., Chen Y.Y., Knight R., Ahima R.S., Bushman F., Wu G.D. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology. 2009;137:13596–13600. doi: 10.1053/j.gastro.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Artis D., Wang M.L., Keilbaugh S.A., He W., Brenes M., Swain G.P., Knight P.A., Donaldson D.D., Lazar M.A., Miller H.R., et al. RELMbeta/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proc. Natl. Acad. Sci. USA. 2004;101:13596–13600. doi: 10.1073/pnas.0404034101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herbert D.R., Yang J.Q., Hogan S.P., Groschwitz K., Khodoun M., Munitz A., Orekov T., Perkins C., Wang Q., Brombacher F., et al. Intestinal epithelial cell secretion of RELM-beta protects against gastrointestinal worm infection. J. Exp. Med. 2009;206:2947–2957. doi: 10.1084/jem.20091268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnes S.L., Vidrich A., Wang M.L., Wu G.D., Cominelli F., Rivera-Nieves J., Bamias G., Cohn S.M. Resistin-like molecule beta (RELMbeta/FIZZ2) is highly expressed in the ileum of SAMP1/YitFc mice and is associated with initiation of ileitis. J. Immunol. 2007;179:7012–7020. doi: 10.4049/jimmunol.179.10.7012. [DOI] [PubMed] [Google Scholar]
- 19.Bergstrom K.S., Morampudi V., Chan J.M., Bhinder G., Lau J., Yang H., Ma C., Huang T., Ryz N., Sham H.P., et al. Goblet Cell Derived RELM-beta Recruits CD4+ T Cells during Infectious Colitis to Promote Protective Intestinal Epithelial Cell Proliferation. PLoS Pathog. 2015;11:e1005108. doi: 10.1371/journal.ppat.1005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McVay L.D., Keilbaugh S.A., Wong T.M., Kierstein S., Shin M.E., Lehrke M., Lefterova M.I., Shifflett D.E., Barnes S.L., Cominelli F., et al. Absence of bacterially induced RELMbeta reduces injury in the dextran sodium sulfate model of colitis. J. Clin. Investig. 2006;116:2914–2923. doi: 10.1172/JCI28121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nair M.G., Guild K.J., Du Y., Zaph C., Yancopoulos G.D., Valenzuela D.M., Murphy A., Stevens S., Karow M., Artis D. Goblet cell-derived resistin-like molecule beta augments CD4+ T cell production of IFN-gamma and infection-induced intestinal inflammation. J. Immunol. 2008;181:4709–4715. doi: 10.4049/jimmunol.181.7.4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chellappa K., Deol P., Evans J.R., Vuong L.M., Chen G., Briancon N., Bolotin E., Lytle C., Nair M.G., Sladek F.M. Opposing roles of nuclear receptor HNF4alpha isoforms in colitis and colitis-associated colon cancer. Elife. 2016;5 doi: 10.7554/eLife.10903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morampudi V., Dalwadi U., Bhinder G., Sham H.P., Gill S.K., Chan J., Bergstrom K.S., Huang T., Ma C., Jacobson K., et al. The goblet cell-derived mediator RELM-beta drives spontaneous colitis in Muc2-deficient mice by promoting commensal microbial dysbiosis. Mucosal Immunol. 2016;9:1218–1233. doi: 10.1038/mi.2015.140. [DOI] [PubMed] [Google Scholar]
- 24.Kitade H., Chen G., Ni Y., Ota T. Nonalcoholic Fatty Liver Disease and Insulin Resistance: New Insights and Potential New Treatments. Nutrients. 2017;9:387. doi: 10.3390/nu9040387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajala M.W., Obici S., Scherer P.E., Rossetti L. Adipose-derived resistin and gut-derived resistin-like molecule-beta selectively impair insulin action on glucose production. J. Clin. Investig. 2003;111:225–230. doi: 10.1172/JCI16521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kushiyama A., Shojima N., Ogihara T., Inukai K., Sakoda H., Fujishiro M., Fukushima Y., Anai M., Ono H., Horike N., et al. Resistin-like molecule beta activates MAPKs, suppresses insulin signaling in hepatocytes, and induces diabetes, hyperlipidemia, and fatty liver in transgenic mice on a high fat diet. J. Biol. Chem. 2005;280:42016–42025. doi: 10.1074/jbc.M503065200. [DOI] [PubMed] [Google Scholar]
- 27.Montalto M., Maggiano N., Ricci R., Curigliano V., Santoro L., Di Nicuolo F., Vecchio F.M., Gasbarrini A., Gasbarrini G. Lactobacillus acidophilus protects tight junctions from aspirin damage in HT-29 cells. Digestion. 2004;69:225–228. doi: 10.1159/000079152. [DOI] [PubMed] [Google Scholar]
- 28.Ahrne S., Hagslatt M.L. Effect of lactobacilli on paracellular permeability in the gut. Nutrients. 2011;3:104–117. doi: 10.3390/nu3010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okubo H., Sakoda H., Kushiyama A., Fujishiro M., Nakatsu Y., Fukushima T., Matsunaga Y., Kamata H., Asahara T., Yoshida Y., et al. Lactobacillus casei strain Shirota protects against nonalcoholic steatohepatitis development in a rodent model. Am. J. Physiol. Gastrointest. Liver Physiol. 2013;305:G911–G918. doi: 10.1152/ajpgi.00225.2013. [DOI] [PubMed] [Google Scholar]
- 30.Hogan S.P., Seidu L., Blanchard C., Groschwitz K., Mishra A., Karow M.L., Ahrens R., Artis D., Murphy A.J., Valenzuela D.M., et al. Resistin-like molecule beta regulates innate colonic function: Barrier integrity and inflammation susceptibility. J. Allergy Clin. Immunol. 2006;118:257–268. doi: 10.1016/j.jaci.2006.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neilson A.P., Djuric Z., Land S., Kato I. Plasma levels of resistin-like molecule beta in humans. Cancer Epidemiol. 2011;35:485–489. doi: 10.1016/j.canep.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perry T., Greig N.H. The glucagon-like peptides: A double-edged therapeutic sword? Trends Pharmacol. Sci. 2003;24:377–383. doi: 10.1016/S0165-6147(03)00160-3. [DOI] [PubMed] [Google Scholar]
- 33.Koliaki C., Doupis J. Incretin-based therapy: A powerful and promising weapon in the treatment of type 2 diabetes mellitus. Diabetes Ther. 2011;2:101–121. doi: 10.1007/s13300-011-0002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abu-Hamdah R., Rabiee A., Meneilly G.S., Shannon R.P., Andersen D.K., Elahi D. Clinical review: The extrapancreatic effects of glucagon-like peptide-1 and related peptides. J. Clin. Endocrinol. Metab. 2009;94:1843–1852. doi: 10.1210/jc.2008-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitchell P.D., Salter B.M., Oliveria J.P., El-Gammal A., Tworek D., Smith S.G., Sehmi R., Gauvreau G.M., Butler M., O’Byrne P.M. Glucagon-like peptide-1 receptor expression on human eosinophils and its regulation of eosinophil activation. Clin. Exp. Allergy. 2017;47:331–338. doi: 10.1111/cea.12860. [DOI] [PubMed] [Google Scholar]
- 36.Gupta N.A., Mells J., Dunham R.M., Grakoui A., Handy J., Saxena N.K., Anania F.A. Glucagon-like peptide-1 receptor is present on human hepatocytes and has a direct role in decreasing hepatic steatosis in vitro by modulating elements of the insulin signaling pathway. Hepatology. 2010;51:1584–1592. doi: 10.1002/hep.23569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pyke C., Heller R.S., Kirk R.K., Orskov C., Reedtz-Runge S., Kaastrup P., Hvelplund A., Bardram L., Calatayud D., Knudsen L.B. GLP-1 receptor localization in monkey and human tissue: Novel distribution revealed with extensively validated monoclonal antibody. Endocrinology. 2014;155:1280–1290. doi: 10.1210/en.2013-1934. [DOI] [PubMed] [Google Scholar]
- 38.Drucker D.J., Nauck M.A. The incretin system: Glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 39.Baggio L.L., Drucker D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 40.Ding X., Saxena N.K., Lin S., Gupta N.A., Anania F.A. Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology. 2006;43:173–181. doi: 10.1002/hep.21006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ben-Shlomo S., Zvibel I., Shnell M., Shlomai A., Chepurko E., Halpern Z., Barzilai N., Oren R., Fishman S. Glucagon-like peptide-1 reduces hepatic lipogenesis via activation of AMP-activated protein kinase. J. Hepatol. 2011;54:1214–1223. doi: 10.1016/j.jhep.2010.09.032. [DOI] [PubMed] [Google Scholar]
- 42.Garber A.J. Long-Acting Glucagon-Like Peptide 1 Receptor Agonists A review of their efficacy and tolerability. Diabetes Care. 2011;34:S279–S284. doi: 10.2337/dc11-s231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nauck M. Incretin therapies: Highlighting common features and differences in the modes of action of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes. Metab. 2016;18:203–216. doi: 10.1111/dom.12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bernsmeier C., Meyer-Gerspach A.C., Blaser L.S., Jeker L., Steinert R.E., Heim M.H., Beglinger C. Glucose-induced glucagon-like Peptide 1 secretion is deficient in patients with non-alcoholic fatty liver disease. PLoS ONE. 2014;9:e87488. doi: 10.1371/journal.pone.0087488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Armstrong M.J., Houlihan D.D., Rowe I.A., Clausen W.H., Elbrond B., Gough S.C., Tomlinson J.W., Newsome P.N. Safety and efficacy of liraglutide in patients with type 2 diabetes and elevated liver enzymes: Individual patient data meta-analysis of the LEAD program. Aliment Pharmacol. Ther. 2013;37:234–242. doi: 10.1111/apt.12149. [DOI] [PubMed] [Google Scholar]
- 46.Cuthbertson D.J., Irwin A., Gardner C.J., Daousi C., Purewal T., Furlong N., Goenka N., Thomas E.L., Adams V.L., Pushpakom S.P., et al. Improved glycaemia correlates with liver fat reduction in obese, type 2 diabetes, patients given glucagon-like peptide-1 (GLP-1) receptor agonists. PLoS ONE. 2012;7:e50117. doi: 10.1371/journal.pone.0050117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Armstrong M.J., Gaunt P., Aithal G.P., Barton D., Hull D., Parker R., Hazlehurst J.M., Guo K., Abouda G., Aldersley M.A., et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): A multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679–690. doi: 10.1016/S0140-6736(15)00803-X. [DOI] [PubMed] [Google Scholar]
- 48.Eguchi Y., Kitajima Y., Hyogo H., Takahashi H., Kojima M., Ono M., Araki N., Tanaka K., Yamaguchi M., Matsuda Y., et al. Pilot study of liraglutide effects in non-alcoholic steatohepatitis and non-alcoholic fatty liver disease with glucose intolerance in Japanese patients (LEAN-J) Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2015;45:269–278. doi: 10.1111/hepr.12351. [DOI] [PubMed] [Google Scholar]
- 49.Sorli C., Harashima S., Tsoukas G.M., Unger J., Karsbol J.D., Hansen T., Bain S.C. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): A double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017;5:251–260. doi: 10.1016/S2213-8587(17)30013-X. [DOI] [PubMed] [Google Scholar]
- 50.Davies M., Pieber T.R., Hartoft-Nielsen M.L., Hansen O.K.H., Jabbour S., Rosenstock J. Effect of Oral Semaglutide Compared with Placebo and Subcutaneous Semaglutide on Glycemic Control in Patients with Type 2 Diabetes: A Randomized Clinical Trial. JAMA. 2017;318:1460–1470. doi: 10.1001/jama.2017.14752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chalasani N., Younossi Z., Lavine J.E., Charlton M., Cusi K., Rinella M., Harrison S.A., Brunt E.M., Sanyal A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 52.Cui J., Philo L., Nguyen P., Hofflich H., Hernandez C., Bettencourt R., Richards L., Salotti J., Bhatt A., Hooker J., et al. Sitagliptin vs placebo for non-alcoholic fatty liver disease: A randomized controlled trial. J. Hepatol. 2016;65:369–376. doi: 10.1016/j.jhep.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Joy T.R., McKenzie C.A., Tirona R.G., Summers K., Seney S., Chakrabarti S., Malhotra N., Beaton M.D. Sitagliptin in patients with non-alcoholic steatohepatitis: A randomized, placebo-controlled trial. World J. Gastroenterol. 2017;23:141–150. doi: 10.3748/wjg.v23.i1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grasset E., Puel A., Charpentier J., Collet X., Christensen J.E., Terce F., Burcelin R. A Specific Gut Microbiota Dysbiosis of Type 2 Diabetic Mice Induces GLP-1 Resistance through an Enteric NO-Dependent and Gut-Brain Axis Mechanism. Cell Metab. 2017;25:1075–1090. doi: 10.1016/j.cmet.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 55.Okubo H., Nakatsu Y., Kushiyama A., Yamamotoya T., Matsunaga Y., Inoue M.K., Fujishiro M., Sakoda H., Ohno H., Yoneda M., et al. Gut Microbiota as a Therapeutic Target for Metabolic Disorders. Curr. Med. Chem. 2018;25:984–1001. doi: 10.2174/0929867324666171009121702. [DOI] [PubMed] [Google Scholar]
- 56.Drucker D.J., Yusta B. Physiology and Pharmacology of the Enteroendocrine Hormone Glucagon-Like Peptide-2. Ann. Rev. Physiol. 2014;76:561–583. doi: 10.1146/annurev-physiol-021113-170317. [DOI] [PubMed] [Google Scholar]
- 57.Ugleholdt R., Zhu X., Deacon C.F., Orskov C., Steiner D.F., Holst J.J. Impaired intestinal proglucagon processing in mice lacking prohormone convertase 1. Endocrinology. 2004;145:1349–1355. doi: 10.1210/en.2003-0801. [DOI] [PubMed] [Google Scholar]
- 58.Xiao Q.A., Boushey R.P., Drucker D.J., Brubaker P.L. Secretion of the intestinotropic hormone glucagon-like peptide 2 is differentially regulated by nutrients in humans. Gastroenterology. 1999;117:99–105. doi: 10.1016/S0016-5085(99)70555-X. [DOI] [PubMed] [Google Scholar]
- 59.Hartmann B., Harr M.B., Jeppesen P.B., Wojdemann M., Deacon C.F., Mortensen P.B., Holst J.J. In vivo and in vitro degradation of glucagon-like peptide-2 in humans. J. Clin. Endocr. Metab. 2000;85:2884–2888. doi: 10.1210/jc.85.8.2884. [DOI] [PubMed] [Google Scholar]
- 60.Wallis K., Walters J.R.F., Gabe S. Short bowel syndrome: The role of GLP-2 on improving outcome. Curr. Opin. Clin. Nutr. 2009;12:526–532. doi: 10.1097/MCO.0b013e32832d23cd. [DOI] [PubMed] [Google Scholar]
- 61.Munroe D.G., Gupta A.K., Kooshesh F., Vyas T.B., Rizkalla G., Wang H., Demchyshyn L., Yang Z.J., Kamboj R.K., Chen H.Y., et al. Prototypic G protein-coupled receptor for the intestinotrophic factor glucagon-like peptide 2. Proc. Natl. Acad. Sci. USA. 1999;96:1569–1573. doi: 10.1073/pnas.96.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yusta B., Huang L., Munroe D., Wolff G., Fantaske R., Sharma S., Demchyshyn L., Asa S.L., Drucker D.J. Enteroendocrine localization of GLP-2 receptor expression in humans and rodents. Gastroenterology. 2000;119:744–755. doi: 10.1053/gast.2000.16489. [DOI] [PubMed] [Google Scholar]
- 63.Dube P.E., Brubaker P.L. Frontiers in glucagon-like peptide-2: Multiple actions, multiple mediators. Am. J. Physiol. Endocrinol. Metab. 2007;293:E460–E465. doi: 10.1152/ajpendo.00149.2007. [DOI] [PubMed] [Google Scholar]
- 64.Nelson D.W., Sharp J.W., Brownfield M.S., Raybould H.E., Ney D.M. Localization and activation of glucagon-like peptide-2 receptors on vagal afferents in the rat. Endocrinology. 2007;148:1954–1962. doi: 10.1210/en.2006-1232. [DOI] [PubMed] [Google Scholar]
- 65.Angelone T., Filice E., Quintieri A.M., Imbrogno S., Amodio N., Pasqua T., Pellegrino D., Mule F., Cerra M.C. Receptor identification and physiological characterisation of glucagon-like peptide-2 in the rat heart. Nutr. Metab. Cardiovasc. Dis. NMCD. 2012;22:486–494. doi: 10.1016/j.numecd.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 66.El-Jamal N., Erdual E., Neunlist M., Koriche D., Dubuquoy C., Maggiotto F., Chevalier J., Berrebi D., Dubuquoy L., Boulanger E., et al. Glugacon-like peptide-2: Broad receptor expression, limited therapeutic effect on intestinal inflammation and novel role in liver regeneration. Am. J. Physiol. Gastrointest. Liver Physiol. 2014;307:G274–G285. doi: 10.1152/ajpgi.00389.2012. [DOI] [PubMed] [Google Scholar]
- 67.Tang-Christensen M., Larsen P.J., Thulesen J., Romer J., Vrang N. The proglucagon-derived peptide, glucagon-like peptide-2, is a neurotransmitter involved in the regulation of food intake. Nat. Med. 2000;6:802. doi: 10.1038/77535. [DOI] [PubMed] [Google Scholar]
- 68.Sorensen L.B., Flint A., Raben A., Hartmann B., Holst J.J., Astrup A. No effect of physiological concentrations of glucagon-like peptide-2 on appetite and energy intake in normal weight subjects. Int. J. Obes. Relat. Metab. Disord. 2003;27:450–456. doi: 10.1038/sj.ijo.0802247. [DOI] [PubMed] [Google Scholar]
- 69.Drucker D.J., Ehrlich P., Asa S.L., Brubaker P.L. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc. Natl. Acad. Sci. USA. 1996;93:7911–7916. doi: 10.1073/pnas.93.15.7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tsai C.H., Hill M., Asa S.L., Brubaker P.L., Drucker D.J. Intestinal growth-promoting properties of glucagon-like peptide-2 in mice. Am. J. Physiol. Endocrinol. Metab. 1997;273:E77–E84. doi: 10.1152/ajpendo.1997.273.1.E77. [DOI] [PubMed] [Google Scholar]
- 71.Martin G.R., Wallace L.E., Sigalet D.L. Glucagon-like peptide-2 induces intestinal adaptation in parenterally fed rats with short bowel syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;286:G964–G972. doi: 10.1152/ajpgi.00509.2003. [DOI] [PubMed] [Google Scholar]
- 72.Jeppesen P.B., Gilroy R., Pertkiewicz M., Allard J.P., Messing B., O'Keefe S.J. Randomised placebo-controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut. 2011;60:902–914. doi: 10.1136/gut.2010.218271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jeppesen P.B., Pertkiewicz M., Messing B., Iyer K., Seidner D.L., O’Keefe S.J., Forbes A., Heinze H., Joelsson B. Teduglutide reduces need for parenteral support among patients with short bowel syndrome with intestinal failure. Gastroenterology. 2012;143:1473–1481. doi: 10.1053/j.gastro.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 74.Au A., Gupta A., Schembri P., Cheeseman C.I. Rapid insertion of GLUT2 into the rat jejunal brush-border membrane promoted by glucagon-like peptide 2. Biochem. J. 2002;367:247–254. doi: 10.1042/bj20020393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ramsanahie A.P., Berger U.V., Zinner M.J., Whang E.E., Rhoads D.B., Ashley S.W. Effect of glucagon-like peptide-2 (GLP-2) on diurnal SGLT1 expression. Dig. Dis. Sci. 2004;49:1731–1737. doi: 10.1007/s10620-004-9561-8. [DOI] [PubMed] [Google Scholar]
- 76.Petersen Y.M. Glucagon-Like Peptide 2 Enhances Maltase-Glucoamylase and Sucrase-Isomaltase Gene Expression and Activity in Parenterally Fed Premature Neonatal Piglets. Pediatr. Res. 2002;52:498–503. doi: 10.1203/00006450-200210000-00007. [DOI] [PubMed] [Google Scholar]
- 77.Meier J.J., Nauck M.A., Pott A., Heinze K., Goetze O., Bulut K., Schmidt W.E., Gallwitz B., Holst J.J. Glucagon-like peptide 2 stimulates glucagon secretion, enhances lipid absorption, and inhibits gastric acid secretion in humans. Gastroenterology. 2006;130:44–54. doi: 10.1053/j.gastro.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 78.Dash S., Xiao C., Morgantini C., Connelly P.W., Patterson B.W., Lewis G.F. Glucagon-like peptide-2 regulates release of chylomicrons from the intestine. Gastroenterology. 2014;147:1275–1284. doi: 10.1053/j.gastro.2014.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Benjamin M.A., McKay D.M., Yang P.C., Cameron H., Perdue M.H. Glucagon-like peptide-2 enhances intestinal epithelial barrier function of both transcellular and paracellular pathways in the mouse. Gut. 2000;47:112–119. doi: 10.1136/gut.47.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moran G.W., O'Neill C., McLaughlin J.T. GLP-2 enhances barrier formation and attenuates TNFalpha-induced changes in a Caco-2 cell model of the intestinal barrier. Regul. Pept. 2012;178:95–101. doi: 10.1016/j.regpep.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 81.Cani P.D., Possemiers S., Van de Wiele T., Guiot Y., Everard A., Rottier O., Geurts L., Naslain D., Neyrinck A., Lambert D.M., et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091–1103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miele L., Valenza V., La Torre G., Montalto M., Cammarota G., Ricci R., Masciana R., Forgione A., Gabrieli M.L., Perotti G., et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877–1887. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 83.Verdam F.J., Rensen S.S., Driessen A., Greve J.W., Buurman W.A. Novel evidence for chronic exposure to endotoxin in human nonalcoholic steatohepatitis. J. Clin. Gastroenterol. 2011;45:149–152. doi: 10.1097/MCG.0b013e3181e12c24. [DOI] [PubMed] [Google Scholar]
- 84.Moore B.A., Peffer N., Pirone A., Bassiri A., Sague S., Palmer J.M., Johnson D.L., Nesspor T., Kliwinski C., Hornby P.J. GLP-2 receptor agonism ameliorates inflammation and gastrointestinal stasis in murine postoperative ileus. J. Pharmacol. Exp. Ther. 2010;333:574–583. doi: 10.1124/jpet.109.161497. [DOI] [PubMed] [Google Scholar]
- 85.Baldassano S., Liu S., Qu M.H., Mule F., Wood J.D. Glucagon-like peptide-2 modulates neurally evoked mucosal chloride secretion in guinea pig small intestine in vitro. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;297:G800–G805. doi: 10.1152/ajpgi.00170.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baldassano S., Amato A., Rappa F., Cappello F., Mule F. Influence of endogenous glucagon-like peptide-2 on lipid disorders in mice fed a high-fat diet. Endocr. Res. 2016;41:317–324. doi: 10.3109/07435800.2016.1141950. [DOI] [PubMed] [Google Scholar]
- 87.Valderas J.P., Padilla O., Solari S., Escalona M., Gonzalez G. Feeding and bone turnover in gastric bypass. J. Clin. Endocrinol. Metab. 2014;99:491–497. doi: 10.1210/jc.2013-1308. [DOI] [PubMed] [Google Scholar]
- 88.Lopes L.S., Schwartz R.P., Ferraz-de-Souza B., da Silva M.E., Correa P.H., Nery M. The role of enteric hormone GLP-2 in the response of bone markers to a mixed meal in postmenopausal women with type 2 diabetes mellitus. Diabetol. Metab. Syndr. 2015;7:13. doi: 10.1186/s13098-015-0006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wallis K., Walters J.R., Forbes A. Review article: Glucagon-like peptide 2--current applications and future directions. Aliment Pharmacol. Ther. 2007;25:365–372. doi: 10.1111/j.1365-2036.2006.03193.x. [DOI] [PubMed] [Google Scholar]
- 90.Inagaki T., Choi M., Moschetta A., Peng L., Cummins C.L., McDonald J.G., Luo G., Jones S.A., Goodwin B., Richardson J.A., et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 91.Schmidt D.R., Holmstrom S.R., Fon Tacer K., Bookout A.L., Kliewer S.A., Mangelsdorf D.J. Regulation of bile acid synthesis by fat-soluble vitamins A and D. J. Biol. Chem. 2010;285:14486–14494. doi: 10.1074/jbc.M110.116004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Henkel A.S., Anderson K.A., Dewey A.M., Kavesh M.H., Green R.M. A chronic high-cholesterol diet paradoxically suppresses hepatic CYP7A1 expression in FVB/NJ mice. J. Lipid Res. 2011;52:289–298. doi: 10.1194/jlr.M012781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kurosu H., Choi M., Ogawa Y., Dickson A.S., Goetz R., Eliseenkova A.V., Mohammadi M., Rosenblatt K.P., Kliewer S.A., Kuro-o M. Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J. Biol. Chem. 2007;282:26687–26695. doi: 10.1074/jbc.M704165200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fu L., John L.M., Adams S.H., Yu X.X., Tomlinson E., Renz M., Williams P.M., Soriano R., Corpuz R., Moffat B., et al. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology. 2004;145:2594–2603. doi: 10.1210/en.2003-1671. [DOI] [PubMed] [Google Scholar]
- 95.Yu C.D., Wang F., Kan M., Jin C.L., Jones R.B., Weinstein M., Deng C.X., McKeehan W.L. Elevated cholesterol metabolism and bile acid synthesis in mice lacking membrane tyrosine kinase receptor FGFR4. J. Biol. Chem. 2000;275:15482–15489. doi: 10.1074/jbc.275.20.15482. [DOI] [PubMed] [Google Scholar]
- 96.Ito S., Fujimori T., Furuya A., Satoh J., Nabeshima Y., Nabeshima Y. Impaired negative feedback suppression of bile acid synthesis in mice lacking betaKlotho. J. Clin. Investig. 2005;115:2202–2208. doi: 10.1172/JCI23076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tomiyama K., Maeda R., Urakawa I., Yamazaki Y., Tanaka T., Ito S., Nabeshima Y., Tomita T., Odori S., Hosoda K., et al. Relevant use of Klotho in FGF19 subfamily signaling system in vivo. Proc. Natl. Acad. Sci. USA. 2010;107:1666–1671. doi: 10.1073/pnas.0913986107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu A.L., Coulter S., Liddle C., Wong A., Eastham-Anderson J., French D.M., Peterson A.S., Sonoda J. FGF19 regulates cell proliferation, glucose and bile acid metabolism via FGFR4-dependent and independent pathways. PLoS ONE. 2011;6:e17868. doi: 10.1371/journal.pone.0017868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Potthoff M.J., Kliewer S.A., Mangelsdorf D.J. Endocrine fibroblast growth factors 15/19 and 21: From feast to famine. Genes Dev. 2012;26:312–324. doi: 10.1101/gad.184788.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kir S., Beddow S.A., Samuel V.T., Miller P., Previs S.F., Suino-Powell K., Xu H.E., Shulman G.I., Kliewer S.A., Mangelsdorf D.J. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science. 2011;331:1621–1624. doi: 10.1126/science.1198363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Potthoff M.J., Boney-Montoya J., Choi M., He T., Sunny N.E., Satapati S., Suino-Powell K., Xu H.E., Gerard R.D., Finck B.N., et al. FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1alpha pathway. Cell Metab. 2011;13:729–738. doi: 10.1016/j.cmet.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tomlinson E., Fu L., John L., Hultgren B., Huang X.J., Renz M., Stephan J.P., Tsai S.P., Powell-Braxton L., French D., et al. Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology. 2002;143:1741–1747. doi: 10.1210/endo.143.5.8850. [DOI] [PubMed] [Google Scholar]
- 103.Marcelin G., Jo Y.H., Li X., Schwartz G.J., Zhang Y., Dun N.J., Lyu R.M., Blouet C., Chang J.K., Chua S., Jr. Central action of FGF19 reduces hypothalamic AGRP/NPY neuron activity and improves glucose metabolism. Mol. Metab. 2014;3:19–28. doi: 10.1016/j.molmet.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ryan K.K., Kohli R., Gutierrez-Aguilar R., Gaitonde S.G., Woods S.C., Seeley R.J. Fibroblast growth factor-19 action in the brain reduces food intake and body weight and improves glucose tolerance in male rats. Endocrinology. 2013;154:9–15. doi: 10.1210/en.2012-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sokol R.J., Straka M.S., Dahl R., Devereaux M.W., Yerushalmi B., Gumpricht E., Elkins N., Everson G. Role of oxidant stress in the permeability transition induced in rat hepatic mitochondria by hydrophobic bile acids. Pediatr. Res. 2001;49:519–531. doi: 10.1203/00006450-200104000-00014. [DOI] [PubMed] [Google Scholar]
- 106.Allen K., Jaeschke H., Copple B.L. Bile acids induce inflammatory genes in hepatocytes: A novel mechanism of inflammation during obstructive cholestasis. Am. J. Pathol. 2011;178:175–186. doi: 10.1016/j.ajpath.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Adachi T., Kaminaga T., Yasuda H., Kamiya T., Hara H. The involvement of endoplasmic reticulum stress in bile acid-induced hepatocellular injury. J. Clin. Biochem. Nutr. 2014;54:129–135. doi: 10.3164/jcbn.13-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Aranha M.M., Cortez-Pinto H., Costa A., da Silva I.B., Camilo M.E., de Moura M.C., Rodrigues C.M. Bile acid levels are increased in the liver of patients with steatohepatitis. Eur. J. Gastroenterol. Hepatol. 2008;20:519–525. doi: 10.1097/MEG.0b013e3282f4710a. [DOI] [PubMed] [Google Scholar]
- 109.Ferslew B.C., Xie G., Johnston C.K., Su M., Stewart P.W., Jia W., Brouwer K.L., Barritt A.S. Altered Bile Acid Metabolome in Patients with Nonalcoholic Steatohepatitis. Dig. Dis. Sci. 2015;60:3318–3328. doi: 10.1007/s10620-015-3776-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wojcik M., Janus D., Dolezal-Oltarzewska K., Kalicka-Kasperczyk A., Poplawska K., Drozdz D., Sztefko K., Starzyk J.B. A decrease in fasting FGF19 levels is associated with the development of non-alcoholic fatty liver disease in obese adolescents. J. Pediatr. Endocrinol. Metab. 2012;25:1089–1093. doi: 10.1515/jpem-2012-0253. [DOI] [PubMed] [Google Scholar]
- 111.Alisi A., Ceccarelli S., Panera N., Prono F., Petrini S., De Stefanis C., Pezzullo M., Tozzi A., Villani A., Bedogni G., et al. Association between Serum Atypical Fibroblast Growth Factors 21 and 19 and Pediatric Nonalcoholic Fatty Liver Disease. PLoS ONE. 2013;8:e67160. doi: 10.1371/journal.pone.0067160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nicholes K., Guillet S., Tomlinson E., Hillan K., Wright B., Frantz G.D., Pham T.A., Dillard-Telm L., Tsai S.P., Stephan J.-P., et al. A Mouse Model of Hepatocellular Carcinoma. Am. J. Pathol. 2002;160:2295–2307. doi: 10.1016/S0002-9440(10)61177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Miura S., Mitsuhashi N., Shimizu H., Kimura F., Yoshidome H., Otsuka M., Kato A., Shida T., Okamura D., Miyazaki M. Fibroblast growth factor 19 expression correlates with tumor progression and poorer prognosis of hepatocellular carcinoma. BMC Cancer. 2012;12:56. doi: 10.1186/1471-2407-12-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pai R., Dunlap D., Qing J., Mohtashemi I., Hotzel K., French D.M. Inhibition of fibroblast growth factor 19 reduces tumor growth by modulating beta-catenin signaling. Cancer Res. 2008;68:5086–5095. doi: 10.1158/0008-5472.CAN-07-2325. [DOI] [PubMed] [Google Scholar]
- 115.French D.M., Lin B.C., Wang M., Adams C., Shek T., Hotzel K., Bolon B., Ferrando R., Blackmore C., Schroeder K., et al. Targeting FGFR4 inhibits hepatocellular carcinoma in preclinical mouse models. PLoS ONE. 2012;7:e36713. doi: 10.1371/journal.pone.0036713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhou M., Wang X., Phung V., Lindhout D.A., Mondal K., Hsu J.Y., Yang H., Humphrey M., Ding X., Arora T., et al. Separating Tumorigenicity from Bile Acid Regulatory Activity for Endocrine Hormone FGF19. Cancer Res. 2014;74:3306–3316. doi: 10.1158/0008-5472.CAN-14-0208. [DOI] [PubMed] [Google Scholar]
- 117.Zhou M., Learned R.M., Rossi S.J., DePaoli A.M., Tian H., Ling L. Engineered FGF19 eliminates bile acid toxicity and lipotoxicity leading to resolution of steatohepatitis and fibrosis in mice. Hepatol. Commun. 2017;1:1024–1042. doi: 10.1002/hep4.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Luo J., Ko B., Elliott M., Zhou M., Lindhout D.A., Phung V., To C., Learned R.M., Tian H., DePaoli A.M., et al. A nontumorigenic variant of FGF19 treats cholestatic liver diseases. Sci. Transl. Med. 2014;6:247ra100. doi: 10.1126/scitranslmed.3009098. [DOI] [PubMed] [Google Scholar]
- 119.Harrison S.A., Rinella M.E., Abdelmalek M.F., Trotter J.F., Paredes A.H., Arnold H.L., Kugelmas M., Bashir M.R., Jaros M.J., Ling L., et al. NGM282 for treatment of non-alcoholic steatohepatitis: A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2018;391:1174–1185. doi: 10.1016/S0140-6736(18)30474-4. [DOI] [PubMed] [Google Scholar]
- 120.Polak J.M., Sullivan S.N., Bloom S.R., Buchan A.M., Facer P., Brown M.R., Pearse A.G. Specific localisation of neurotensin to the N cell in human intestine by radioimmunoassay and immunocytochemistry. Nature. 1977;270:183–184. doi: 10.1038/270183a0. [DOI] [PubMed] [Google Scholar]
- 121.Vincent J.P., Mazella J., Kitabgi P. Neurotensin and neurotensin receptors. Trends Pharmacol. Sci. 1999;20:302–309. doi: 10.1016/S0165-6147(99)01357-7. [DOI] [PubMed] [Google Scholar]
- 122.Kalafatakis K., Triantafyllou K. Contribution of neurotensin in the immune and neuroendocrine modulation of normal and abnormal enteric function. Regul. Pept. 2011;170:7–17. doi: 10.1016/j.regpep.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 123.Ferris C.F., Hammer R.A., Leeman S.E. Elevation of plasma neurotensin during lipid perfusion of rat small intestine. Peptides. 1981;2(Suppl. 2):263–266. doi: 10.1016/0196-9781(81)90042-5. [DOI] [PubMed] [Google Scholar]
- 124.Armstrong M.J., Parker M.C., Ferris C.F., Leeman S.E. Neurotensin stimulates [3H]oleic acid translocation across rat small intestine. Am. J. Physiol. 1986;251:G823–G829. doi: 10.1152/ajpgi.1986.251.6.G823. [DOI] [PubMed] [Google Scholar]
- 125.Hammer R.A., Matsumoto B.K., Blei A.T., Pearl G., Ingram H. Local Effect of Neurotensin on Canine Ileal Blood-Flow, and Its Release by Luminal Lipid. Scand. J. Gastroenterol. 1988;23:449–457. doi: 10.3109/00365528809093893. [DOI] [PubMed] [Google Scholar]
- 126.Wood J.G., Hoang H.D., Bussjaeger L.J., Solomon T.E. Effect of Neurotensin on Pancreatic and Gastric-Secretion and Growth in Rats. Pancreas. 1988;3:332–339. doi: 10.1097/00006676-198805000-00015. [DOI] [PubMed] [Google Scholar]
- 127.Brown J.A., Bugescu R., Mayer T.A., Gata-Garcia A., Kurt G., Woodworth H.L., Leinninger G.M. Loss of Action via Neurotensin-Leptin Receptor Neurons Disrupts Leptin and Ghrelin-Mediated Control of Energy Balance. Endocrinology. 2017;158:1271–1288. doi: 10.1210/en.2017-00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Melander O., Maisel A.S., Almgren P., Manjer J., Belting M., Hedblad B., Engstrom G., Kilger U., Nilsson P., Bergmann A., et al. Plasma proneurotensin and incidence of diabetes, cardiovascular disease, breast cancer, and mortality. JAMA. 2012;308:1469–1475. doi: 10.1001/jama.2012.12998. [DOI] [PubMed] [Google Scholar]
- 129.Li J., Song J., Zaytseva Y.Y., Liu Y., Rychahou P., Jiang K., Starr M.E., Kim J.T., Harris J.W., Yiannikouris F.B., et al. An obligatory role for neurotensin in high-fat-diet-induced obesity. Nature. 2016;533:411–415. doi: 10.1038/nature17662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Barchetta I., Cimini F.A., Leonetti F., Capoccia D., Di Cristofano C., Silecchia G., Orho-Melander M., Melander O., Cavallo M.G. Increased Plasma Proneurotensin Levels Identify NAFLD in Adults with and Without Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2018;103:2253–2260. doi: 10.1210/jc.2017-02751. [DOI] [PubMed] [Google Scholar]
- 131.Barchetta I., Cimini F.A., Capoccia D., Bertoccini L., Ceccarelli V., Chiappetta C., Leonetti F., Di Cristofano C., Silecchia G., Orho-Melander M., et al. Neurotensin Is a Lipid-Induced Gastrointestinal Peptide Associated with Visceral Adipose Tissue Inflammation in Obesity. Nutrients. 2018;10:526. doi: 10.3390/nu10040526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Auguet T., Aragones G., Berlanga A., Martinez S., Sabench F., Aguilar C., Villar B., Sirvent J.J., Del Castillo D., Richart C. Low Circulating Levels of Neurotensin in Women with Nonalcoholic Fatty Liver Disease Associated with Severe Obesity. Obesity. 2018;26:274–278. doi: 10.1002/oby.22058. [DOI] [PubMed] [Google Scholar]
- 133.Ernst A., Hellmich S., Bergmann A. Proneurotensin 1-117, a stable neurotensin precursor fragment identified in human circulation. Peptides. 2006;27:1787–1793. doi: 10.1016/j.peptides.2006.01.021. [DOI] [PubMed] [Google Scholar]