Abstract
Purpose:
To compare visual acuity, refractive error, corneal curvature, and the stability of these parameters during the early postoperative period following small-incision lenticule extraction (SMILE) and femtosecond laser-assisted in situ keratomileusis (FS-LASIK) surgery.
Methods:
One hundred and five eyes and 110 eyes were enrolled in SMILE and FS-LASIK group, respectively. Uncorrected and best-corrected distance visual acuity (UCVA and BCVA), manifest refraction, corneal curvature, intraocular pressure, and slit-lamp examinations were performed preoperatively, 1 day, 1 week, 1 month, and 3 months postoperatively.
Results:
No significant differences in postoperative UCVA or BCVA were observed between the SMILE and FS-LASIK groups at any time point. SMILE group had significant better postoperative spherical equivalent (SE) values than FS-LASIK group at 1 day, 1 week, and 1-month follow-up. However, there was no significant difference in postoperative SE values at 3-month follow-up. Significant differences in mean postoperative corneal curvature were observed during all follow-up examinations.
Conclusion:
SMILE surgery was associated with more accurate postoperative refractive correction up to 1 month following surgery. SMILE surgery also resulted in less significant corneal curvature changes than FS-LASIK. Furthermore, FS-LASIK was associated with decreased stability of postoperative refractive error and corneal curvature relative to SMILE.
Keywords: Corneal curvature, femtosecond laser, laser in situ keratomileusis, refractive error, small-incision lenticule extraction
Developed in the 1990s, laser-assisted in situ keratomileusis (LASIK) has become the most popular refractive surgical procedure for the treatment of myopia. LASIK alters the corneal refractive power by sculpting the desired shape via laser ablation after creating an intrastromal flap.[1,2,3] The application of femtosecond laser in LASIK (femtosecond laser-assisted in situ keratomileusis: FS-LASIK) increases the predictability of flap depth, diameter, and hinge width, improving the safety and accuracy of the procedure.[4,5,6]
Femtosecond laser not only produce an individualized flap but also have been associated with improvements in uncorrected distance visual acuity (UCVA), more stable biomechanics, and decreased epithelial injury postoperatively.[7,8,9,10] However, despite high success rates of FS-LASIK, side effects such as dryness, glare, haloes, and flap-related complications remain a significant concern.[11,12]
Recently, a novel form of flap-free surgery known as small-incision lenticule extraction (SMILE) has emerged. During SMILE surgery, femtosecond laser is used to create an intrastromal lenticule, which is then manually extracted through a small arcuate incision, allowing most of the epithelium and Bowman's membrane to remain intact. Several studies have reported that SMILE is safe, effective, and reliable for the correction of myopia.[13,14,15,16,17] However, some researchers have reported conflicting results regarding postoperative visual recovery and corneal stability between SMILE and FS-LASIK.[13,18,19]
To authors’ knowledge, few studies have examined changes in corneal curvature and the stability of refractive error following SMILE and FS-LASIK surgery. Riau et al. reported that the refractive lenticule extraction procedure resulted in minimal topographic changes than LASIK, especially in patients requiring a high degree of refractive correction.[20] Kanellopoulos and Asimellis demonstrated that keratometric stability was within 0.22 D at 12-month follow-up in patients with severe myopia following LASIK.[21] A 2015 study by Gyldenkerne A et al. further demonstrated that after SMILE, the sagittal curvature was constant for central diameters up to 4 mm, whereas a gradual steepening of the curvature was observed with increasing diameter following FS-LASIK.[22] However, no studies have investigated the stability of refractive error and curvature in the early postoperative period. We hypothesize that flapless surgery (SMILE) and FS-LASIK may therefore differ with regard to the stability of refractive error and corneal curvature. The objective of the present clinical study was to compare visual acuity, refractive error, corneal curvature, and the stability of both refractive error and curvature in patients with moderate-to-severe myopia following SMILE and FS-LASIK surgery.
Methods
This is a prospective and comparative clinical study. All patients were recruited between January 2016 and November 2016.
The inclusion criteria were as follows: (1) age ≥18 years, (2) stable refraction over the previous 2 years, (3) use of contact lenses discontinued for at least 2 weeks, (4) spherical equivalent (SE) of −3.00 to −9.00 diopters, (5) minimum corneal center thickness of 500 μm, (6) residual stromal bed at least 280 μm, and (7) attendance at all follow-up visits. Exclusion criteria were systemic or ocular diseases, pregnancy or breast feeding, forme fruste keratoconus diagnosed on corneal topography, corneal scanning, severe dry eye, and collagen vascular diseases. All patients meeting the aforementioned criteria were randomly allocated to either the SMILE or FS-LASIK surgery group.
One hundred and five patients who had undergone SMILE and 110 patients who had undergone FS-LASIK were included.
Preoperative assessment
All patients underwent a complete ophthalmologic examination prior to surgery, consisting of measurements of UCVA, best-corrected distance visual acuity (BCVA), manifest refraction (sphere, cylinder, and SE), intraocular pressure, corneal pachymetry, corneal curvature (ARK 510A; NIDEK, Gamagori, Japan), corneal topography (Pentacam; Oculus Optikgerate GmbH, Wetzlar, Germany), slit-lamp evaluation, and funduscopy. Each eye underwent three corneal curvature and corneal topography assessments. Only the right eye of each patient was included in the analysis.
Surgical procedure
All surgical procedures were performed by an experienced surgeon. Following application of topical anesthesia (oxybuprocaine hydrochloride, Santen Pharmaceutical, Japan), the standard surgical procedures were performed.
In FS-LASIK surgery, the flaps were created using a 60-kHz femtosecond laser (IntraLase Corp, Irvine, CA) with the following parameters: raster pattern; 70° side-cut angle; superior hinge; hinge angle of 50°; flap diameter of 8.3–8.5 mm; and attempted flap depth of 105–110 μm. The excimer laser ablation was then completed using an Allegretto Wave and Eye-Q 400-Hz laser (WaveLight Laser Technologie AG, Germany). The optical zone diameter ranged from 6.0 to 6.6 mm, and the transition zone diameter was 1.0 mm larger than the optical zone diameter.
In SMILE surgery, a VisuMax femtosecond laser (Carl Zeiss Meditec AG, Jena, Germany) with the following parameters was used: frequency of 500 kHz; energy cut index of 27–29; lenticule diameter of 6.5 mm; transition zone of 0.1 mm; cap diameter of 7.5 mm; intended cap thickness of 120–130 μm; and incision length of 2.5 mm.
After surgery, all patients received topical fluorometholone 0.1% (Santen Pharmaceutical, Japan) four times daily for 1 week, followed by a reduced dosage of three times daily for 3 weeks, and levofloxacin 0.3% (Bausch & Lomb Freda Inc., China) three times daily for 1 week. Artificial tears were also administered as needed.
Postoperative assessment
Postoperative examinations were performed 1 day, 1 week, 1 month, and 3 months following each procedure. UCVA and BCVA were recorded in logMAR format. UCVA, BCVA, manifest refraction, corneal curvature, intraocular pressure, and slit-lamp examinations were performed at each visit. Corneal topography was evaluated at 3 months follow-up. Changes in keratometry were characterized based on the ΔK/SE index, which was used to correct the change between preoperative and postoperative values according to the SE, as follows: ΔK/SE= (Kmpost − Kmpre)/SE; where Km = (K1 + K2)/2, K1 represents the keratometry value for the flat meridian, K2 represents the keratometry for the steep meridian, Kmpost represents the postoperative Km, and Kmpre represents the preoperative Km.
Statistical analysis
All statistical analyses were performed using SPSS 24. Visual acuity outcomes in logMAR notation were compared. Independent sample Student's t-tests were used to evaluate differences between the SMILE and FS-LASIK groups. A P value of <0.05 was considered statistically significant for all tests.
Results
A total of 105 eyes of 105 patients who had undergone SMILE and 110 eyes of 110 patients who had undergone FS-LASIK were included in the final analysis. Table 1 shows the baseline characteristics of the participants in each group. There were no significant differences (P > 0.05) in age, preoperative spherical equivalent, UCVA, BCVA, central corneal thickness (pachymetry), or corneal curvature between the two groups.
Table 1.
Baseline characteristics of SMILE and FS-LASIK groups

All surgeries were completed successfully, and all patients completed follow-up visits at 1 day, 1 week, 1 month, and 3 months. All eyes had UCVA of 0.10 (logMAR) or better at the 3-month follow-up. No significant differences in postoperative UCVA and BCVA were observed between the SMILE and FS-LASIK at any follow-up point. At 1 day after surgery, there were 69 and 74% of treated eyes that had 20/20 or better UCVA in SMILE and FS-LASIK groups, respectively. Moreover, there were no difference between the two groups in all follow-up points. Regarding the BCVA at 1 week postoperatively, 77% patients showed no change or gained better in SMILE group, and 82% in FS-LASIK group. No difference was found in the two groups [Table 2].
Table 2.
Postoperative values in the SMILE and FS-LASIK groups

Significant differences were observed between the two groups in mean postoperative sphere values at 1 day, 1 week, and 1 month follow-up. No significant difference was observed at 3 months follow-up, and no significant differences in mean postoperative cylinder values were observed at any time point. All treated eyes were within ±1.00 D (achieved vs. attempted correction); 99 and 99% of eyes were within ±0.50 D in SMILE and FS-LASIK groups at 3 months follow-up. However, significant differences in mean postoperative spherical equivalent values were observed between the two groups at 1 day, 1 week, and 1 month follow-up points [Table 2 and Fig. 1].
Figure 1.
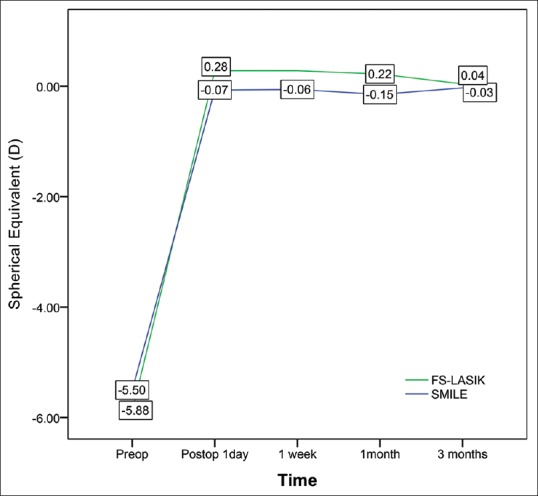
Comparison of preoperative and postoperative spherical equivalent values in the SMILE and FS-LASIK groups
Preoperative corneal curvature values obtained via auto-keratometry and Pentacam (Oculus Optikgerate GmbH, Wetzlar, Germany) were 43.20 ± 1.37 D and 43.27 ± 1.37 D, respectively, indicating a lack of significant difference between the two methods (t = 0.87, P = 0.97). Furthermore, no significant differences in postoperative corneal curvature were observed between auto-keratometry (38.92 ± 1.54 D) and Pentacam assessments (38.85 ± 1.60 D) at 3 months follow-up (t = 0.64, P = 0.40). Significant differences in postoperative corneal curvature values were observed between the two procedures at all follow-up points. [Table 2 and Fig. 2].
Figure 2.
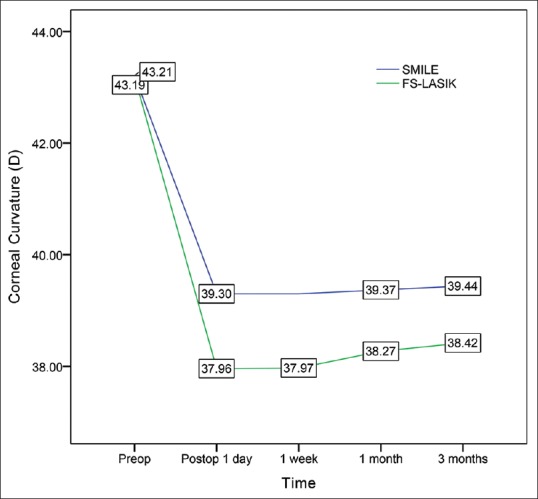
Comparison of preoperative and postoperative corneal curvature values in the SMILE and FS-LASIK groups
A significant difference in ablation depth was observed between the SMILE (109 ± 22.78 μm) and FS-LASIK (85.28 ± 11.98 μm) groups (t = 10.82, P = 0.01). Significant differences in ΔK/SE were also observed between the SMILE group (1 week: 0.71 ± 0.06; 3 months: 0.69 ± 0.06) and the FS-LASIK group (1 week: 0.89 ± 0.07; 3 months: 0.81 ± 0.08) at 1 week and 3 months follow-up (t = 12.67, P = 0.03; and t = 6.49, P = 0.02).
Discussion
In the present study, our findings demonstrated that both the SMILE and FS-LASIK procedures have better efficacy, safety, and predictability in the correction of moderate-to-severe myopia, in accordance with the findings of several previous studies.[13,14,15,17,19] At all follow-up visits, all patients of both groups exhibited improvements in UCVA and BCVA, as well as better postoperative refractive outcome.
Interestingly, although we observed a significant difference in postoperative residual error (sphere and SE) at 1 day, 1 week, and 1 month following surgery, no significant difference was observed at 3 months follow-up.
Patients of the SMILE group exhibited lower postoperative residual error values at 1 day, 1 week, and 1 month follow-up. On postoperative day 1, the postoperative residual error was −0.07 ± 0.32 D in the SMILE group and 0.28 ± 0.39 D in the FS-LASIK group, similar to values reported by Ganesh and Gupta.[16] These authors also observed that the postoperative mean SE was −0.14 ± 0.28 D in the SMILE group, which was significantly better than that observed in the FS-LASIK group (−0.27 ± 0.24 D). Ganesh and Gupta have pointed out that the primary reason for this discrepancy is associated with variations in the hydration status of the corneal stroma.[16] In FS-LASIK, the stroma bed is exposed for a longer period during lifting of the flap and scanning. In contrast, the refractive lenticule is cut by a femtosecond laser in the stroma prior to SMILE surgery, which requires a much shorter period of exposure.
Patients of the SMILE group exhibited increased refractive stability relative to that observed in the FS-LASIK group in early postoperative period. In postoperative 1 day, 1 week, and 1 month, SMILE group has better refractive correction; however, FS-LASIK group has slight overcorrection. But both groups exhibited similar postoperative residual error at 3 months follow-up. These findings suggest that FS-LASIK induces greater changes in postoperative corneal remodeling and myopic shifting in patients with moderate or severe myopia than SMILE. Indeed, previous studies have revealed that myopic shifting and regression may occur following LASIK, especially in patients who have undergone a high degree of myopia correction.[23,24,25]
Previous studies have demonstrated that SMILE results in better corneal curvature stability than LASIK.[21] We observed similar results in the present study. As shown in Fig. 2, the keratometry value was almost unchanged in the SMILE group, whereas mild progressive corneal steepening was observed in the FS-LASIK group. Significant differences in corneal curvature change were also observed between the two groups at all follow-up points, likely due to differences in the procedures and the wound-healing response.[21,26]First, in FS-LASIK, the creation of the flap combined with the inflammation caused by the excimer laser may result in a more dramatic wound healing response, and the higher the degree to correction, the heavier the inflammatory infiltration.[21,26] However, in SMILE, the lenticule shape is based on the attempted correction, and inflammation does not increase with the higher degree to correction.[21] Furthermore, the excimer laser uses an ultraviolet light source to break molecular bonds during treatment,[27] whereas the femtosecond laser is a near-infrared laser that allows for photo-disruption of stromal tissue with less tissue injury.[28]
Second, we speculate that the effect of creating a corneal flap on corneal curvature is similar to that associated with large limbal relaxing incisions. Keratometric flattening after FS-LASIK, which regresses with wound healing, may be associated with excimer laser scanning and flap creation. In the present study, we indeed observed progressive corneal steepening in the early postoperative period following FS-LASIK, in accordance with the findings of several previous studies: Lim et al. reported that healing and remodeling of the cornea occur mainly within the first 10 weeks following limbal relaxing incisions.[29] Budak et al. further demonstrated that limbal relaxing incisions represent a practical approach for the correction of lower degrees of astigmatism, and that most cases of keratometric astigmatism regression occur during the early postoperative/remodeling period (between 1 and 3 months).[30] Finally, the smaller cap diameter and absence of flaps in the SMILE procedure may have resulted in less tissue inflammation and have reduced the time required for remodeling.
Previous studies have also reported that the biomechanical effects are stronger in anterior and peripheral areas of the cornea.[31,32] Some studies have reported that FS-LASIK and SMILE induce similar biomechanical changes in the cornea[33]; however, other studies have reported that greater biomechanical alterations occur following FS-LASIK.[34,35] If SMILE were to induce greater better biomechanical stability, greater improvements in refractive and keratometric stability would be observed following the procedure, relative to those observed following FS-LASIK. In the present study, we observed that ablation depth was significantly greater in the SMILE group than in the FS-LASIK group, although postoperative changes in keratometry were significantly lesser in the SMILE group than in the FS-LASIK group.
A previous study by Maldonado-Bas and Onnis reported keratometric flattening of 4.98 D (11.29%) in patients requiring correction of −3.00 to −6.00 D (−5.12 ± 0.81 D), and of 7.07 D (15.94%) in patients requiring correction of −6.00 to −10.00 D (−8.33 ± 1.24 D).[36] These findings are in contrast with those of the present study. In the SMILE group, we observed keratometric flattening of 3.77 D (8.72%), while keratometric flattening in the FS-LASIK group was 4.77 D (11.04%). Such discrepancies are likely due to differences in surgical procedures and the degree of correction (−3.00 to −9.00 in our study). We also observed that ΔK/SE was 0.71 ± 0.06 and 0.89 ± 0.07 in the SMILE and FS-LASIK groups on postoperative day 1, respectively. Combined use of the ΔK/SE and postoperative refraction parameters may allow for more precise evaluation of over-correction or under-correction following surgery.
Corneal curvature is typically measured using an automatic refractometer in our country, which is fast, convenient, and relatively affordable. We utilized both automatic refractometry and the Pentacam (Oculus Optikgerate GmbH, Wetzlar, Germany) system to decrease error in the present study. No significant differences were observed between measurements obtained using either system.
Based on the findings of previous studies and our clinical experience, the efficacy, safety, and predictability of SMILE and FS-LASIK surgeries remain stable at 3 months postoperatively and beyond.[13,19] However, this short follow-up may be considered a limitation of the present study. We observed instability of curvature and refractive error in the FS-LASIK at the last follow-up (3 months). Thus, future studies with longer follow-up periods and a larger number of cases are required.
Conclusion
In summary, our findings demonstrated that SMILE surgery was associated with more accurate postoperative refractive correction up to 1 month following surgery, although this difference became insignificant at the 3 months follow-up. SMILE surgery also resulted in less significant corneal curvature changes than FS-LASIK. In addition, we observed a slight myopic shift in postoperative refractive error and a steeper shift in corneal curvature in the FS-LASIK group, and that patients of this group exhibited decreased stability of postoperative refractive error and curvature relative to those of the SMILE group.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Sandoval HP, de Castro LE, Vroman DT, Solomon KD. Refractive surgery survey 2004. J Cataract Refract Surg. 2005;31:221–33. doi: 10.1016/j.jcrs.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 2.Yuen LH, Chan WK, Koh J, Mehta JS, Tan DT SingLasik Research Group. A 10-year prospective audit of LASIK outcomes for myopia in 37,932 eyes at a single institution in Asia. Ophthalmology. 2010;117:1236–44. doi: 10.1016/j.ophtha.2009.10.042. [DOI] [PubMed] [Google Scholar]
- 3.Alió JL, Muftuoglu O, Ortiz D, Pérez-Santonja JJ, Artola A, Ayala MJ, et al. Ten-year follow-up of laser in situ keratomileusis for myopia of up to − 10 diopters. Am J Ophthalmol. 2008;145:46–54. doi: 10.1016/j.ajo.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Chen YG, Xia YJ. Comparison of corneal flap morphology using AS-OCT in LASIK with the WaveLight FS200 femtosecond laser versus a mechanical microkeratome. J Refract Surg. 2013;29:320–4. doi: 10.3928/1081597X-20130415-03. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Y, Zhang J, Tian L, Zhai C. Comparison of the Ziemer FEMTO LDV femtosecond laser and Moria M2 mechanical microkeratome. J Refract Surg. 2012;28:189–94. doi: 10.3928/1081597X-20120208-01. [DOI] [PubMed] [Google Scholar]
- 6.Von Jagow B, Kohnen T. Corneal architecture of femtosecond laser and microkeratome flaps imaged by anterior segment optical coherence tomography. J Cataract Refract Surg. 2009;35:35–41. doi: 10.1016/j.jcrs.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Pajic B, Vastardis I, Pajic-Eggspuehler B, Gatzioufas Z, Hafezi F. Femtosecond laser versus mechanical microkeratome-assisted flap creation for LASIK: A prospective, randomized, paired-eye study. Clin Ophthalmol. 2014;22:1883–9. doi: 10.2147/OPTH.S68124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guber I, Moetteli L, Magnin L, Majo F. Moving from a mechanical microkeratome to a femtosecond laser for LASIK to correct astigmatic patients: Clinical outcomes of a retrospective, consecutive, comparative study. Klin Monbl Augenheilkd. 2013;230:337–41. doi: 10.1055/s-0032-1328384. [DOI] [PubMed] [Google Scholar]
- 9.Sun Q, Deng ZZ, Zhou YH, Zhang J, Peng XY. Effect of femtosecond and microkeratome flaps creation on the cornea biomechanics during laser in situ keratomileusis: one year follow-up. Int J Ophthalmol. 2016;18:1409–14. doi: 10.18240/ijo.2016.10.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santhiago MR, Kara-Junior N, Waring GO. Microkeratome versus femtosecond flaps: accuracy and complications. Curr Opin Ophthalmol. 2014;25:270–4. doi: 10.1097/ICU.0000000000000070. [DOI] [PubMed] [Google Scholar]
- 11.Sutton G, Lawless M, Hodge C. Laser in situ keratomileusis in 2012: A review. Clin Exp Optom. 2014;97:18–29. doi: 10.1111/cxo.12075. [DOI] [PubMed] [Google Scholar]
- 12.Moshirfar M, Gardiner JP, Schliesser JA, Espandar L, Feiz V, Mifflin MD, et al. Laser in situ keratomileusis flap complications using mechanical microkeratome versus femtosecond laser: Retrospective comparison. J Cataract Refract Surg. 2010;36:1925–33. doi: 10.1016/j.jcrs.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 13.Shen Z, Shi K, Yu Y, Lin Y, Yao K. Small incision lenticule extraction (SMILE) versus femtosecond laser-assisted in situ keratomileusis (FS-LASIK) for myopia: A systematic review and meta-analysis. PLoS One. 2016;11:e0158176. doi: 10.1371/journal.pone.0158176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chansue E, Tanehsakdi M, Swasdibutra S, McAlinden C. Efficacy, predictability and safety of small incision lenticule extraction (SMILE) Eye Vis (Lond) 2015;2:14. doi: 10.1186/s40662-015-0024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JR, Kim BK, Mun SJ, Chung YT, Kim HS. One-year outcomes of small-incision lenticule extraction (SMILE): Mild to moderate myopia vs. high myopia. BMC Ophthalmol. 2015;15:59. doi: 10.1186/s12886-015-0051-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ganesh S, Gupta R. Comparison of visual and refractive outcomes following femtosecond laser-assisted Lasik with SMILE in patients with myopia or myopic astigmatism. J Refract Surg. 2014;30:590–6. doi: 10.3928/1081597X-20140814-02. [DOI] [PubMed] [Google Scholar]
- 17.Lin F, Xu Y, Yang Y. Comparison of the visual results after SMILE and femtosecond laser-assisted LASIK for myopia. J Refract Surg. 2014;30:248–54. doi: 10.3928/1081597X-20140320-03. [DOI] [PubMed] [Google Scholar]
- 18.Li M, Zhao J, Shen Y, Li T, He L, Xu H, et al. Comparison of dry eye and corneal sensitivity between small incision lenticule extraction and femtosecond LASIK for myopia. PLoS One. 2013;8:e77797. doi: 10.1371/journal.pone.0077797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu M, Chen Y, Wang D, Zhou Y, Zhang X, He J, et al. Clinical outcomes after SMILE and femtosecond laser-assisted LASIK for myopia and myopic astigmatism: A prospective randomized comparative study. Cornea. 2016;35:210–6. doi: 10.1097/ICO.0000000000000707. [DOI] [PubMed] [Google Scholar]
- 20.Riau AK, Angunawela RI, Chaurasia SS, Lee WS, Tan DT, Mehta JS. Early corneal wound healing and inflammatory responses after refractive lenticule extraction (ReLEx) Invest Ophthalmol Vis Sci. 2011;52:6213–21. doi: 10.1167/iovs.11-7439. [DOI] [PubMed] [Google Scholar]
- 21.Kanellopoulos AJ, Asimellis G. Refractive and keratometric stability in high myopic LASIK with high-frequency femtosecond and excimer lasers. J Refract Surg. 2013;29:832–7. doi: 10.3928/1081597X-20130924-02. [DOI] [PubMed] [Google Scholar]
- 22.Gyldenkerne A, Ivarsen A, Hjortdal JØ. Comparison of corneal shape changes and aberrations induced by FS-LASIK and SMILE for myopia. J Refract Surg. 2015;31:223–9. doi: 10.3928/1081597X-20150303-01. [DOI] [PubMed] [Google Scholar]
- 23.Lim SA, Park Y, Cheong YJ, Na KS, Joo C-K. Factors affecting long-term myopic regression after laser in situ keratomileusis and laser-assisted subepithelial keratectomy for moderate myopia. Korean J Ophthalmol. 2016;30:92–100. doi: 10.3341/kjo.2016.30.2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim G, Christiansen SM, Moshirfar M. Change in keratometry after myopic laser in situ keratomileusis and photorefractive keratectomy. J Cataract Refract Surg. 2014;40:564–74. doi: 10.1016/j.jcrs.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 25.Zhao LQ, Zhu H, Li LM. Laser-assisted subepithelial keratectomy versus laser in situ keratomileusis in myopia: A systematic review and meta-analysis. ISRN Ophthalmol. 2014:672146. doi: 10.1155/2014/672146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong Z, Zhou X, Wu J, Zhang Z, Li T, Zhou Z, et al. Small incision lenticule extraction (SMILE) and femtosecond laser LASIK: Comparison of corneal wound healing and inflammation. Br J Ophthalmol. 2014;98:263–9. doi: 10.1136/bjophthalmol-2013-303415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marshall J, Sliney DH. Endoexcimer laser intraocular ablative photodecomposition. Am J Ophthalmol. 1986;101:130–1. doi: 10.1016/0002-9394(86)90489-7. [DOI] [PubMed] [Google Scholar]
- 28.Sugar A. Ultrafast (femtosecond) laser refractive surgery. Curr Opin Ophthalmol. 2002;13:246–9. doi: 10.1097/00055735-200208000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Lim R, Borasio E, Ilari L. Long-term stability of keratometric astigmatism after limbal relaxing incisions. J Cataract Refract Surg. 2014;40:1676–81. doi: 10.1016/j.jcrs.2014.01.045. [DOI] [PubMed] [Google Scholar]
- 30.Budak K, Yilmaz G, Aslan BS, Duman S. Limbal relaxing incisions in congenital astigmatism: 6 month follow-up. J Cataract Refract Surg. 2001;27:715–9. doi: 10.1016/s0886-3350(00)00687-8. [DOI] [PubMed] [Google Scholar]
- 31.Dupps WJ, Jr, Wilson SE. Biomechanics and wound healing in the cornea. Exp Eye Res. 2006;83:709–20. doi: 10.1016/j.exer.2006.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smolek MK, McCarey BE. Interlamellar adhesive strength in human eyebank cornea. Invest Ophthalmol Vis Sci. 1990;31:1087–95. [PubMed] [Google Scholar]
- 33.Zhang J, Zheng L, Zhao X, Xu Y, Chen S. Corneal biomechanics after small-incision lenticule extraction versus Q-value-guided femtosecond laser-assisted in situ keratomileusis. J Curr Ophthalmol. 2016;28:181–7. doi: 10.1016/j.joco.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu D, Wang Y, Zhang L, Wei S, Tang X. Corneal biomechanical effects: Small-incision lenticule extraction versus femtosecond laser-assisted laser in situ keratomileusis. J Cataract Refract Surg. 2014;40:954–62. doi: 10.1016/j.jcrs.2013.07.056. [DOI] [PubMed] [Google Scholar]
- 35.Sinha Roy A, Dupps WJ, Jr, Roberts CJ. Comparison of biomechanical effects of small-incision lenticule extraction and laser in situ keratomileusis: Finite-element analysis. J Cataract Refract Surg. 2014;40:971–80. doi: 10.1016/j.jcrs.2013.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maldonado-Bas A1, Onnis R. Results of laser in situ keratomileusis in different degrees of myopia. Ophthalmology. 1998;105:606–11. doi: 10.1016/S0161-6420(98)94012-X. [DOI] [PubMed] [Google Scholar]


