Abstract
Purpose:
Sequential treatment with targeted therapies can result in complex combinations of resistance mutations in drug targets. This mutational complexity has spurred the development of pan-target inhibitors, i.e. therapies for which no single target mutation can cause resistance. Since the propensity for on- versus off-target resistance varies across cancer types, a deeper understanding of the mutational burden in drug targets could rationalize treatment outcomes, and prioritize pan-target inhibitors for indications where on-target mutations are most likely.
Experimental design:
To measure and model the mutational landscape of a drug target at high resolution, we integrated single-molecule Duplex Sequencing of the ABL1 gene in Philadelphia-positive (Ph+) leukemias with computational simulations.
Results:
A combination of drug target mutational burden and tumor-initiating cell fraction is sufficient to predict that most patients with chronic myeloid leukemia (CML) are unlikely to harbor ABL1 resistance mutations at the time of diagnosis, rationalizing the exceptional success of targeted therapy in this setting. In contrast, our analysis predicts that many patients with Ph+ acute lymphoblastic leukemia (Ph+ ALL) harbor multiple pre-existing resistant cells with single mutants. The emergence of compound mutations can be traced to initial use of an ABL1 inhibitor that is susceptible to resistance from single point mutations.
Conclusion:
These results argue that early use of therapies that achieve pan-inhibition of ABL1 resistance mutants might improve outcomes in Ph+ ALL. Our findings show how a deep understanding of the mutational burden in drug targets can be quantitatively coupled to phenotypic heterogeneity to rationalize clinical phenomena.
Introduction
Molecularly targeted therapy is revolutionizing cancer treatment. However, drug resistance can occur from the selective growth of rare resistant cells that are hidden within the bulk of the population, resulting in treatment failure (1). The continued evolution of drug resistance in cancer has driven major efforts to produce successive generations of therapies that can overcome the evolutionary liabilities of previous efforts. For example, imatinib has improved outcomes in Philadelphia-positive (Ph+) leukemias (2), however imatinib resistance spurred the development of second generation tyrosine kinase inhibitors (TKIs), dasatinib (3) and nilotinib (4). These TKIs retain ABL1 kinase inhibition in the presence of many resistance mutations, but certain variants can still lead to treatment failure. Subsequently, the TKI ponatinib was developed as a “pan-target inhibitor” intended to overcome all known resistance mutations (5,6). While ponatinib appears effective in the setting of all known single resistance mutations, as well as some compound mutations (i.e. two co-existing mutations in the same allele of ABL1), certain compound mutations can lead to resistance to ponatinib (7,8).
The diversity of therapeutic options creates many potential strategies for therapeutic sequencing of TKIs. However, current targeted therapies are often given to patients in the order in which they are discovered, and sequential use of inhibitors for which single point mutations can cause resistance may lead to increasingly complex resistance in the drug target. Two independent mutations within the same allele (compound mutations) have now been found in refractory chronic myeloid leukemia (CML) and Ph+ acute lymphoblastic leukemia (Ph+ ALL) (7), ALK fusion NSCLC (9), and EGFR mutant NSCLC (10), and can result in resistance to all available targeted therapies.
Drug resistance mutations in ABL1 are rare in chronic-phase CML (CP-CML) (11–13), and TKI treatment is likely curative in a subset of patients (14). In stark contrast, while the outcomes of Ph+ ALL have also been improved with TKIs (15), ABL1 drug resistance mutations are common in Ph+ ALL and the disease can be rapidly fatal (16,17). Pre-existing resistance mutations have been reported to be directly detectable in newly diagnosed CP-CML patients (18–23). However, these reports present two important paradoxes. (i) How can therapy be successful in so many CP-CML patients, if they frequently harbor resistance mutations at baseline? (ii) If pre-existing resistance is common in both CP-CML and Ph+ ALL (24), why is clinical and mutational resistance to TKIs somewhat uncommon in CML (11,12), yet frequent in Ph+ ALL (16,17)?
To overcome this paradox and establish the landscape of drug resistance mutations prior to therapy in Ph+ leukemias, we utilized an exceptionally accurate method for mutation detection, Duplex Sequencing (25–27), which is based on separately tagging and sequencing of each of the two strands of individual DNA molecules. True mutations are seen at the same position in both strands, while false mutations arising from technical error occur in only one of the strands. Duplex Sequencing has a calculated background error rate of less than one false mutation per billion nucleotides sequenced (25), and can thus reveal mutational heterogeneity in a drug target with unprecedented accuracy. We applied Duplex Sequencing to Ph+ leukemias to map the landscape of resistance mutations in the ABL1 gene with single-molecule resolution, and utilized computational simulations to determine implications of sub-clonal mutation patterns for targeted cancer therapy. We theorized that understanding mutational patterns in ABL1 would rationalize differences in ABL1-driven drug resistance patterns across Ph+ leukemias and would inform the use of pan-target inhibitors that can suppress all known single resistance mutations.
Materials and Methods
Patient samples
Informed consent was obtained from all patients, and the institutional review boards at the University of Washington and the Fred Hutchinson Cancer Research Center approved the study. The study was conducted in accordance with the Declaration of Helsinki. Sequencing was performed from peripheral blood leukocytes. Details of sample selection and processing are available in the Supplementary Methods.
Duplex Sequencing
Duplex Sequencing of the ABL1 gene from genomic DNA was performed essentially as previously described (26,27). Details are available in Supplementary Methods.
IC50 measurements in BaF3 cells
BaF3 cells from DSMZ were infected with the indicated BCR-ABL1 mutant at a limiting dilution of the virus. Cells were selected by removal of IL-3 and tested for puromycin resistance. Cells were plated at between 3000 and 6000 cells per well in a 96 well plate. Cells were cultured in the presence of drug for 72 hours, and cell density was measured using Cell-Titer Glo. IC50s were calculated via Excel Fit. IC50 values were normalized using a linear model that accounts for batch to batch variation.
Computational simulations
Details of computational simulations are provided in Supplementary Methods.
Results
Duplex Sequencing removes false mutations and provides high resolution measurements of drug target mutation burden
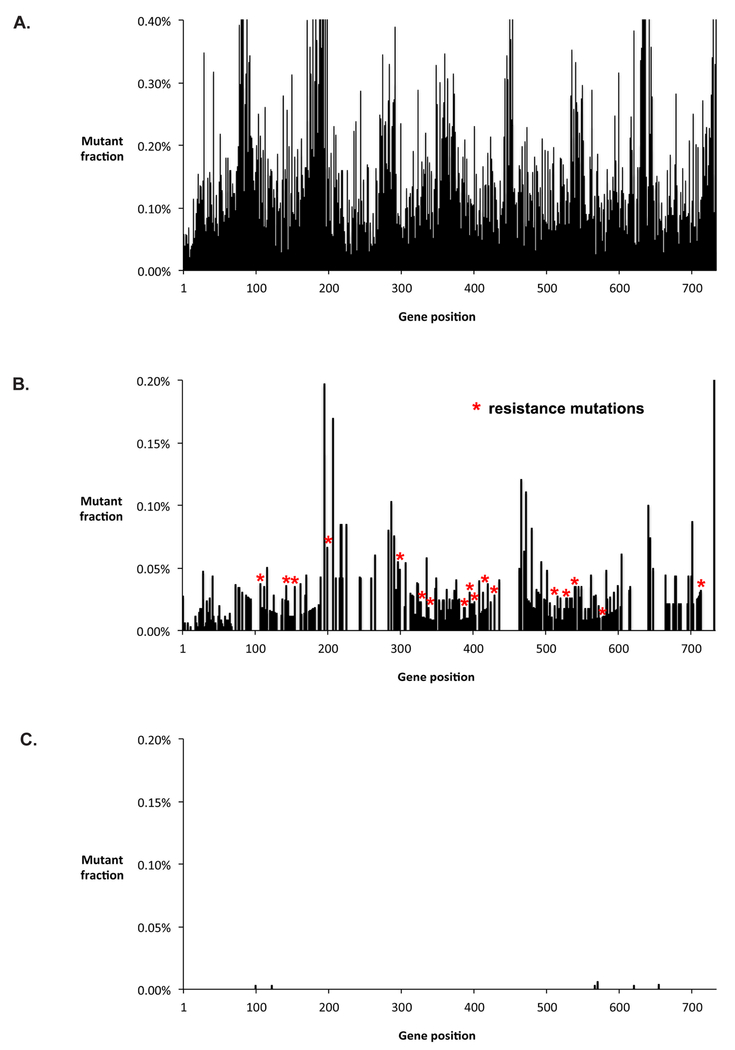
Given the presented paradox, wherein pre-existing ABL1 resistance mutations are suggested to be common in untreated CP-CML patients, but ABL1-driven resistance is rare, we suspected that previous methodologies could introduce false positives. We first explored whether RT-PCR, which has been used in most prior studies of mutations in Ph+ leukemias, introduces a substantial background of artifactual mutations (28–30) that would limit accurate detection of sub-clonal mutations. We subjected a sample from a newly diagnosed CML patient to RT-PCR of the ABL1 gene by a standard protocol and then performed conventional high-throughput sequencing or Duplex Sequencing on the amplified material. In parallel, we performed Duplex Sequencing on genomic DNA without a preceding amplification step. RT-PCR followed by conventional high-throughput sequencing (Figure 1A) results in false mutations at nearly every position in the gene, precluding the detection of bona fide rare variants. Duplex Sequencing removes high-throughput sequencing errors, however performing RT-PCR amplification prior to ligation of Duplex Sequencing adapters leads to hundreds of false mutations from RT-PCR errors, including 17 mutations that have previously been implicated in imatinib resistance (Figure 1B). The RT-PCR error frequency of 5.3×10−5 unique mutations per nucleotide sequenced (see Methods) corresponds to an average of one mutation in every 26 cDNA transcripts. In contrast, performing Duplex Sequencing on genomic DNA without a preceding RT-PCR step removes these background errors (Figure 1C), revealing an extremely low frequency of mutations in the sample.
Figure 1. RT-PCR amplification introduces artefactual mutations.
A. RT-PCR amplification of ABL1 exons 4–8 was performed, followed by conventional high-throughput sequencing. Individual nucleotide positions within ABL1 are shown on the horizontal axis, and the percent of bases mutated at each position is indicated on the vertical axis. Artefactual errors are present at nearly every position due to errors in the sequencing platform, which precludes detection of minority variants. B. RT-PCR amplification was performed, followed by Duplex Sequencing. Duplex Sequencing eliminates sequencing errors, however because RT-PCR was performed prior to attachment of Duplex Sequencing adapters, reverse transcription errors and PCR errors persist. Red asterisks correspond to false resistance mutations that occurred as a result of RT-PCR amplification. C. Duplex Sequencing was performed directly on genomic DNA from the same patient, without preceding RT-PCR. Errors are removed, revealing an extremely low frequency of mutations. Exons 4–10 were analyzed by Duplex Sequencing of genomic DNA, however only the exons amplified by the RT-PCR primer set (exons 4–8, corresponding to codons 199–456) are shown in the figure. The data were generated from patient sample #7 in Supplementary Table 3. See Methods for details.
We found that RT-PCR errors are heavily biased toward transition mutations (Supplementary Figure 1 and Supplementary Table 1). The biased error spectrum results in recurrent errors that persist even if technical replicates are performed (Supplementary Table 2). The “gatekeeper” resistance mutation, T315I, is especially likely to arise as a false mutation due to C➔T errors at the second position of its codon, ACT. Comparison of the mutation spectrum obtained in the presence or absence of RT-PCR reveals that >99% of C➔T mutations are RT-PCR artifacts. Thus, RT-PCR prior to sequencing fundamentally limits accurate sub-clonal mutation detection below a certain threshold.
To assess whether amplification errors might have biased prior analyses of mutations in patients with untreated CML, we compiled the types of mutations identified in six prior studies (Supplementary Table 3). We observed that nearly all previously reported pre-existing resistance mutations in CML correspond to the error signature of RT-PCR amplification (C➔T and T➔C mutations). Our data suggests the possibility for prior studies to have been affected by false mutations from sample amplification errors. Therefore, we utilized Duplex Sequencing of genomic DNA without preceding RT-PCR amplification for subsequent measurements to avoid this source of background error..
Low burden of ABL1 mutations in patients with newly diagnosed CP-CML
To establish the extent of ABL1 mutations in newly diagnosed patients with CP-CML, we performed Duplex Sequencing of ABL1 in 16 patients arbitrarily selected from historic clinical trials of imatinib efficacy (31,32) (patient information is provided in Supplementary Table 4). We found a low burden of mutations in each sample, with an average of 3.7×10−7 (+/− 3.2×10−7) unique mutations per base pair sequenced (mean +/− standard deviation). Peripheral blood was assayed from two healthy individuals, yielding a similarly low mutation frequency of 1.8×10−7 (+/− 2.5×10−7). Only a single hypothetical resistant variant, M472I, was identified in a single DNA molecule from one CML patient, with a mutant fraction of 0.004%. Four of the patients subsequently failed imatinib with development of a resistance mutation. In no case was the mutation detected prior to imatinib therapy (Supplementary Table 5).
Direct detection of resistance variants via Duplex Sequencing was rare or non-existent in the untreated CP-CML patients that we examined. However, some previous reports have indicated the T315I “gatekeeper” variant can be directly detected in a significant proportion of baseline samples. To assess the likelihood of detectable T315I as an unselected variant arising in untreated patients from random mutational events, we extrapolated the mutation burden determined by Duplex Sequencing (see Supplementary Methods for details). This approach revealed that in the absence of a growth advantage, T315I is expected to be present in <0.001% of cells in nearly all patients (Supplementary Figure 2), which is far below the background error rate of RT-PCR amplification. Consequently, in the absence of selective growth, T315I mutations would not be expected to be detectable by any method in newly diagnosed CP-CML. This suggests observations that T315I is present in a significant fraction of newly diagnosed CP-CML and Ph+ ALL patients could potentially have been limited by artifacts of RT-PCR.
Elevated burden of sub-clonal ABL1 mutations in patients with refractory Ph+ leukemias
Next, we explored the extent of sub-clonal ABL1 mutations in patients with advanced-phase and refractory Ph+ leukemias. For these analyses we studied samples from the PACE trial (6), in which patients with refractory Ph+ leukemias were treated with the TKI ponatinib. Most patients in this study had undergone multiple rounds of treatment, with 93% of enrolled patients having failed two or more prior TKIs. We aimed to (i) establish estimates of ABL1 mutation burden across distinct Ph+ leukemias for quantitative modeling, and (ii) determine if ABL1 mutations that arise at the end of TKI treatment are present prior to therapy. We identified a cohort of consented PACE patients who gained a mutation (by Sanger sequencing) by the end of treatment, but for whom Sanger or NGS sequencing could not identify the same mutation at baseline (Supplementary Table 4).
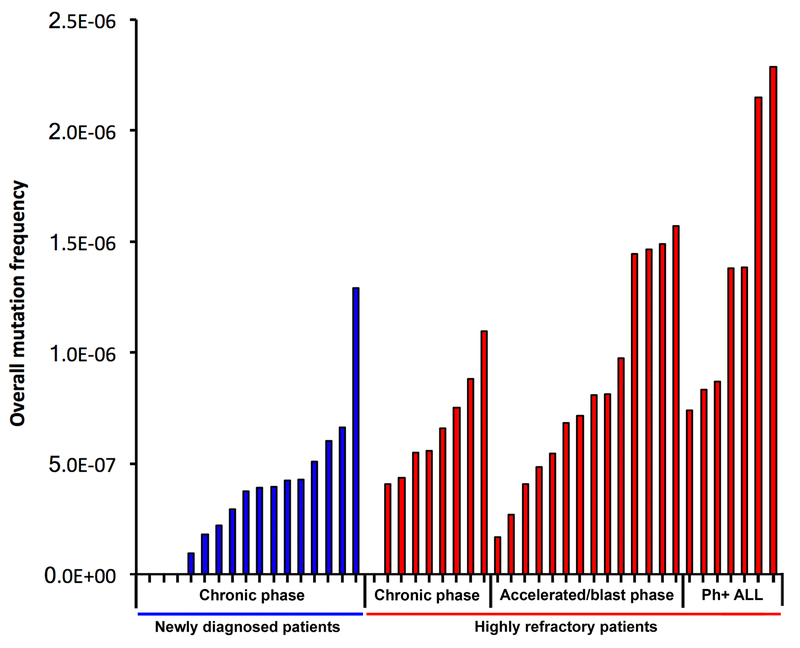
We found an increase in overall mutation burden in patients with advanced-phase disease. However, intergroup heterogeneity was high, and a range of mutation burdens were observed in individuals across disease stages (Figure 2). In 93% of patients, sub-clonal mutations were identified that were not resolved by conventional sequencing approaches (Supplementary Figure 3 and Supplementary Table 6), including multiple sub-clonal TKI resistance mutations that fell below the detection limit of other approaches (Table 1). Many of these failed to grow out during ponatinib treatment, and appear to be the result of drug selection from previous therapy. Even the highest mutation burdens measured, however, remained well below the background error rate of RT-PCR (Supplementary Figure 4).
Figure 2. Overall mutation frequencies in Ph+ leukemia patients, as determined by Duplex Sequencing.
Every bar represents an individual patient. Patients are sorted from lowest to highest mutation frequency within each category. Mutation frequency is calculated as total number of unique mutations identified by Duplex Sequencing, divided by the total number of nucleotides sequenced for that patient. As indicated in the main text, “highly refractory” patients are from the PACE trial.
TABLE 1.
TKI resistance mutations identified by Duplex Sequencing
| Patient # | Genomes sequenced | Resistance mutations identified by Duplex Sequencing* | |
|---|---|---|---|
| initial diagnosis | 1 | 4001 | --- |
| 2 | 26,076 | --- | |
| 3 | 973 | --- | |
| 4 | 4,403 | --- | |
| 5 | 20,805 | --- | |
| 6 | 4,486 | --- | |
| 7 | 27,072 | M472I (0.004%) | |
| 8 | 4,021 | --- | |
| 9 | 4,921 | --- | |
| 10 | 3,436 | --- | |
| 11 | 1,746 | --- | |
| 12 | 2,318 | --- | |
| 13 | 6,249 | --- | |
| 14 | 9,294 | --- | |
| 15 | 9,033 | --- | |
| 16 | 6,319 | --- | |
| highly refractory CP-CML | 25 | 4,074 | C250E (0.021%) |
| 26 | 5,905 | E355A” (39.61%), L248V (0.032%),T315I (0.10%), A397P (0.083%), F486S (0.017%) | |
| 27 | 6,722 | T315la (4.36%). K247R (0.014%), L248V (0.014%), V299L (0.016%) | |
| 28 | 8,065 | T315Ia (4.30%) F317La (28.25%) F359Ca (31.62%) | |
| 29 | 4,868 | --- | |
| 30 | 5,035 | M244Va (1.70%). F359Va (31.68%). Y253H (0.019%). Q250E (0.19%) | |
| 31 | 4,753 | --- | |
| 32 | 4,369 | --- | |
| 33 | 4,844 | V299La (42.45%), F359Va (42.94%), Y253H (0.014%) | |
| highly refractory AP-CML | 34 | 6,201 | F317Lb (37.74%), G250E (0.051%), F317Lc (1.78%) |
| 35 | 4,348 | --- | |
| 36 | 4,370 | G250Eb (2.37%), M351Tb (27.10%), E275K (0.30%), F317L (0.085%) | |
| highly refractory BP-CML | 37 | 3,297 | --- |
| 38 | 7,292 | Y253Hb (53.72%) | |
| 39 | 3,574 | E255Kb (45.66%), Q252H (0.020%), E255V (1.02%), E459K (0.28%) | |
| 40 | 3,681 | G250Eb (17.46%), V299Lb (25.88%) | |
| 41 | 4,833 | F317Lb (25.90%), F359Vb (30.43%), F359I (1.58%) | |
| 42 | 5,179 | L248V (0.015%). T315I (0.020%) | |
| 43 | 3,946 | F359Cb (40.72%), M237V (0.020%), E255V (0.042%) | |
| 44 | 6,509 | F359Cb (27.16%) | |
| 45 | 5,447 | T315lb (20.19%), L387F (0.017%) | |
| 46 | 5,206 | T315lb (30.11%) | |
| 47 | 9,879 | G250Eb (45.89%), T315A (45.91%), H396R 10.010%) | |
| highly refractory Ph+ ALL | 48 | 1,648 | T315lb (2.28%) |
| 49 | 5,125 | F317I (48.54%), V299L (0.026%), V299Lc (1.26%) | |
| 50 | 1,171 | T315lb (37.63%) | |
| 51 | 3,186 | F317b (4.67%) | |
| 52 | 3,595 | T315lb (3.35%) | |
| 53 | 5,136 | G250Eb (27.09%), F317Lb (57.74%), T315I (0.21%), F359V (2.02%), H396P (0.034%) | |
| 54 | 5,094 | T315lb (0.28%), F317Lb (0.073%) | |
notes:
“Highly refractory” patients additionally had mutation detection performed by NGS or Sanger sequencing. Resistance mutations identified by Duplex Sequencing which were not revealed by NGS/Sanger are underlined
Mutation was also identified by conventional NGS
Mutation was also identified by Sanger Sequencing
Same amino acid mutation encoded by a distinct nucleotide substitutionTABLE
We next examined whether the elevation in ABL1 mutation burden is significantly different when considering only silent mutations, which would not be expected to impact drug resistance. We found a significant increase in silent mutations in patients with refractory Ph+ ALL versus refractory CP-CML (OR 6.82, p=0.0009 for CP-CML versus Ph+ ALL, Fisher’s Exact Test), suggesting that Ph+ ALL can harbor an elevated mutation burden in ABL1 relative to CP-CML.
Mutation gain and compound mutations are more frequent in Ph+ ALL than in CP-CML
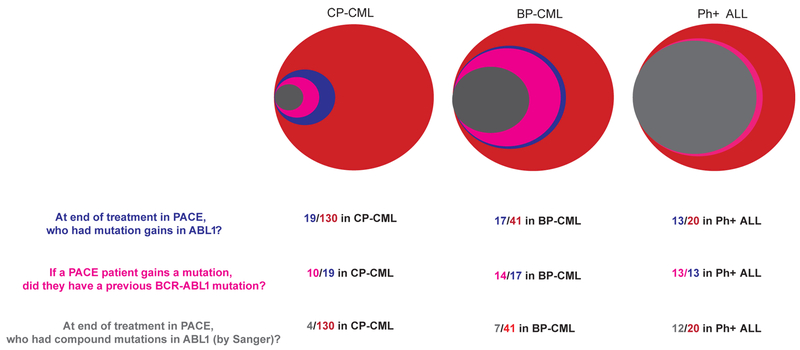
Previous studies suggest that Ph+ ALL patients have a higher rate of on-target resistance mutations in BCR-ABL1 following TKI therapy relative to CP-CML patients (11–13,16,17). However, this observation has not been previously tested in a single trial. Thus, we analyzed patterns of on-target resistance in all evaluable patients from the PACE trial (N=130/267 CP-CML versus N=41/62 BP-CML and N=20/32 Ph+ ALL were evaluable, see Supplementary Methods for details). Note that most un-evaluable CP-CML patients were in deep molecular response (33) as of trial termination, while most un-evaluable BP-CML and Ph+ ALL patients progressed before an end of treatment sample could be collected. End of treatment Sanger sequencing of peripheral blood samples was used to identify patients who had gained mutations during ponatinib treatment. 65% of evaluable Ph+ ALL patients gained mutations in ABL1 versus 15% of CP-CML patients (OR 4.1, p=0.003, Fisher’s exact test) (Figure 3). These mutation gains could have occurred on the background of previous mutations, creating compound mutations (two mutations in the same allele), or alternatively they may have occurred in distinct cells creating a mixed population. To discern which mutations were compound, we combined inference by Sanger sequencing with next-generation sequencing of BCR-ABL1 mRNA (8) (Supplementary Methods). This analysis suggested that 60% of evaluable Ph+ ALL patients had compound mutations at the end of treatment while only 3% of CP-CML patients had compound mutations (OR 10.0, p<1E-5, Fisher’s exact test) (Figure 3). These data demonstrate that Ph+ ALL patients have a higher propensity to acquire on target mutations in ABL1 relative to CP-CML patients.
Figure 3. Patterns of resistance mutations in heavily pre-treated Ph+ leukemia patients differ by disease phase.
Resistance mutations in patients from the PACE cohort are shown for CP-CML, BP-CML, and Ph+ ALL. The area of each circle reflects the number of patients in each category. Red: total number of evaluable patients. Blue: patients with mutation gains in ABL1. Pink: patients with mutation gains who had a previous ABL1 mutation. Gray: patients with ABL1 compound mutations.
Drug resistance mutations likely pre-exist in patients with refractory disease
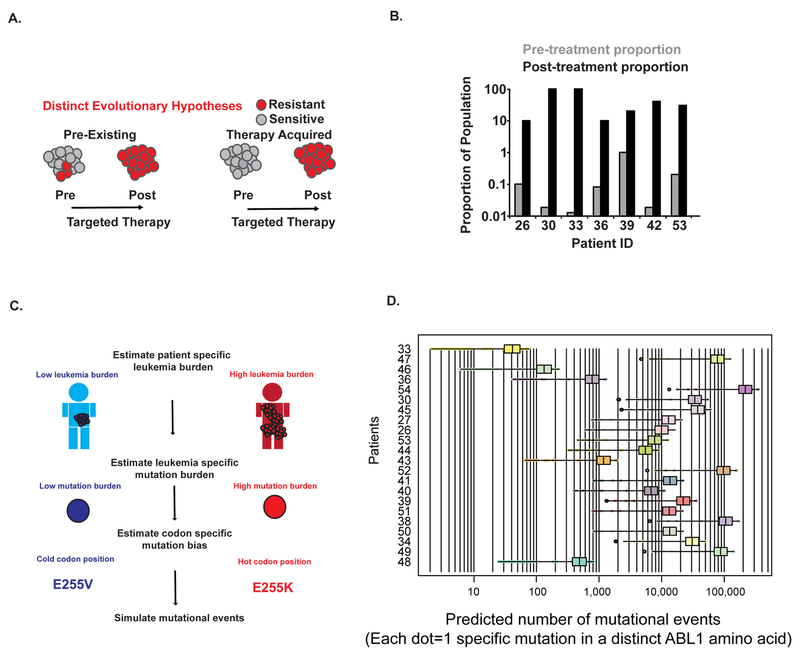
Having established frequent mutation gains in Ph+ ALL (and to a lesser extent in CP-CML), we next investigated whether mutations that apparently arise during ponatinib therapy are present prior to treatment as sub-clonal variants. If drug resistance exists in patients with refractory Ph+ ALL before the initiation of treatment, as opposed to being acquired during ponatinib therapy (Figure 4A), there are important practical and conceptual implications. Practically, the direct detection of specific mutants prior to therapy may guide the choice of TKI, as specific mutations are known to confer resistance to particular TKIs (34). Conceptually, the pre-existence of mutational resistance in refractory populations implies that previous TKI failure pre-determines the outcome of pan-target therapies like ponatinib in later lines of treatment.
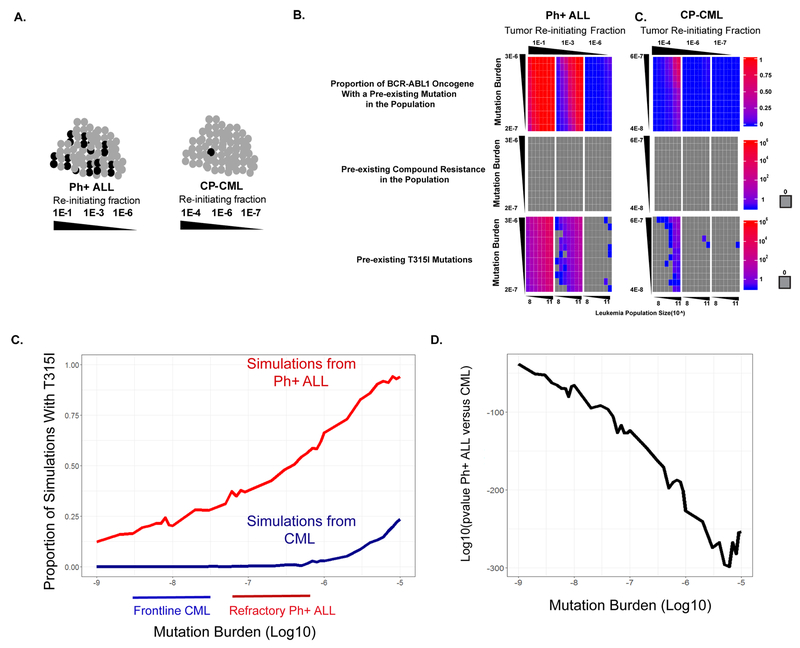
Figure 4. Patients with highly refractory Ph+ leukemias harbor pre-existing sub-clonal resistance mutations.
A. Resistance mutations could pre-exist prior to therapy (left panel), or could arise de novo during therapy (right panel). B. Direct detection of pre-existing resistance mutations in patients from the PACE cohort. All 7 patients had a resistance mutation detected after therapy that was not identified by conventional sequencing approaches prior to treatment (NGS or Sanger sequencing, see Supplementary Table 4). The post-treatment variant allele fraction is shown by a black bar for each variant. The same mutation was directly detected prior to treatment by Duplex Sequencing, with the variant allele fraction shown by gray bars. The following mutations are shown: #26: T315I, #30: Y253H, #33: Y253H, #36: F317L, #39: E255V #42: T315I #53: T315I. C. Methodology for simulation of random mutations in ABL1. In brief, the leukemia burden in individual patients was estimated as described in Supplementary Methods. Mutation burden was determined by Duplex Sequencing. The likelihood of a mutation occurring at each codon position in ABL1 was determined by mapping the spectrum of mutations identified by Duplex Sequencing onto the codon usage of the ABL1 gene. See the Methods section for details. D. Simulations of mutation accumulation in individual patients. The approach depicted in panel C was applied to individual patients, with the ID number of individual patients shown along the vertical axis. The horizontal axis indicates the average predicted number of mutational events in ABL1 from simulations, with each value on the horizontal axis representing the average of all simulations for a specific mutation in the ABL1 kinase domain. Boxplots depict the range of pre-existing mutational events across all residues. Values that fall outside of the interquartile range are shown as dots.
In 7/30 patients, the relapse-associated mutation was directly detectable by Duplex Sequencing prior to the start of ponatinib therapy, with mutant fractions ranging from 0.014% to 1.02% (Figure 4B and Supplementary Table 5). Direct detection of pre-existing mutations by Duplex Sequencing is limited by the sequencing depth and the tiny fraction of the patient’s total leukemia burden being sampled in a blood draw. Thus, we sought other approaches to infer the origin of resistant clones. The change in tumor burden as estimated by the ratio of BCR-ABL1 to ABL1 (see Supplementary Figure 5 for an example) is proportional to the leukemic cell number (35) and has been shown to follow exponential kinetics as resistance grows out (36,37). We simulated a stochastic birth-death process with a population harboring 0 clones at treatment initiation (Supplementary Methods). For all patients, the stochastically simulated molecular response lagged behind the observed response. In fact, in many instances, spontaneously arising resistant clones failed to seed a clinically detectable subpopulation during the simulation. These results suggest that it would be difficult to explain the observed clinical data without invoking pre-existing drug resistant clones (Supplementary Figure 5).
To further evaluate the likelihood of pre-existing resistance mutations, we took a third approach and performed computational simulations of ABL1 mutational events (see Supplementary Methods for details). For this analysis, we took advantage of several direct measurements that we had available. (i) We could estimate patient-specific levels of leukemia burden (Figure 4C top panel, Supplementary Methods). (ii) Duplex Sequencing suggests an estimate of the mutation burden (mutations/nucleotide sequenced) in individual patients. We simulate successes (mutations) using a binomial distribution (Figure 4C middle panel). (iii) The nucleotide substitution bias suggested by Duplex Sequencing and the codon usage of the ABL1 gene defines the probability of individual mutations to occur at specific positions in ABL1(and creates a multinomial distribution) (Figure 4C bottom panel and Supplementary Figure 6). Utilizing these parameters, we simulated the occurrence of specific amino acid resistance mutations in individual patients with refractory Ph+ leukemias (Supplementary Methods).
This approach indicated that most, if not all, resistance mutations are likely to pre-exist in patients with refractory Ph+ ALL prior to ponatinib therapy (Figure 4D). However, this analysis suggests the need for an additional parameter in simulations, as the depth of sub-clonal heterogeneity implied by the result is at odds with clinical experience: refractory patients with CP-CML, BP-CML and Ph+ ALL were all predicted to have many pre-occurring resistance mutations, yet we observed a ~20-fold difference in the rate of acquisition of compound mutations in Ph+ ALL versus CP-CML in the PACE trial. Thus, the difference in ABL1 mutation burden between Ph+ ALL and CP-CML that we observed is not quantitatively sufficient to predict the differences in ABL1 mutation mediated resistance.
Phenotypic heterogeneity explains on target drug resistance patterns in Ph+ ALL versus CP-CML
To address this discrepancy, we turned to an additional parameter. For a mutation to result in clinical drug resistance, it must occur in a cell that is capable of clonally expanding and repopulating a tumor, i.e. a leukemia-initiating cell (38–40). Specifically, leukemia-initiating cells are uncommon in CP-CML, as the bulk of cells typically consist of terminally differentiated granulocytes in which a resistance mutation would be of no clinical consequence. Therefore, we updated our simulations to account for leukemia initiating cell fractions from established literature values for CP-CML and Ph+ ALL (Supplementary Methods). For mutation burden in these simulations, we used parameters that encompass the distribution of mutation burden in refractory patients. Prior studies indicate that the leukemia initiating fraction is very different between CP-CML and Ph+ ALL (Figure 5A), which has the functional effect of lowering the effective population size of the tumor. Our simulations revealed that patients with refractory Ph+ ALL are highly likely to have resistance mutations that occur within a leukemia initiating cell at baseline. Only at the lowest level of leukemia initiating cells (i.e. 1 in 106) are resistance mutations unlikely to pre-occur in a Ph+ ALL of almost any clinically relevant population size (Figure 5B). In contrast, patients with CP-CML are expected to have a low burden of resistance mutations within leukemia initiating cells. In patients with CP-CML, only the largest of the relevant stem cell fractions and the largest initial population sizes predict pre-existing resistance mutations (Figure 5C). To give this analysis a more quantitative grounding we sought to sample leukemia initiating fractions from the distribution of values present in the human population for each disease. To do this we used estimates that were derived using comparable methodology in the same lab (41,42). Simulations were run as before, but instead of examining the landscape of pre-existing resistance across all potentially relevant parameters, we quantitatively examined the probability that resistance will develop in CP-CML and Ph+ ALL across all mutation burdens analyzed, given the distribution of tumor initiating cell burden measured experimentally. These simulations revealed that regardless of the mutation burden, the tumor initiating cell fraction predicts that Ph+ ALL will have a dramatically higher burden of pre-existing T315I mutations (p-val~10−100-10−300)(Figure 5C,D). Thus, simulations incorporating mutational and phenotypic heterogeneity produced results consistent with clinical experience, and rationalize the disparate drug resistance patterns between CP-CML and Ph+ ALL.
Figure 5. Likelihood of pre-existing resistance mutations in Ph+ leukemias is driven by the size of the tumor-initiating population and the mutation burden.
A. Leukemia-initiating cells (black) comprise a subset of the total cells (gray) in patients. Prior studies have shown that leukemia-initiating cells are more prevalent in Ph+ ALL than in CML. B. Top row: Computational simulations of the proportion of codons in ABL1 exons 4–10 expected to have a pre-existing mutation in Ph+ ALL. Simulations were performed as described in the Methods section. Simulations were performed as described in Supplementary Methods across the full range of tumor repopulation fractions obtained from the literature, with the three panels corresponding to the three tumor repopulation fractions that are specified. Each small box represents an average of 10 simulations, utilizing the specified parameters. Each small box is colored with results scaled to the heat map at the right side of the figure. A gray box denotes a count of 0. Vertical axis: sub-clonal mutation burden, corresponding to the random mutation frequencies measured by Duplex Sequencing. Horizontal axis: total number of leukemia cells in a given patient. Middle row: Number of pre-existing compound resistance mutations in the population (i.e. number of cells expected to have two co-occurring resistance mutations in ABL1 exons 4–10 in the same allele; see Supplementary Methods) in Ph+ ALL. Bottom row: Number of pre-existing T315I mutations in the overall population in Ph+ ALL. Right. Analogous simulations were performed, utilizing parameters for CP-CML. C. Simulations to quantitatively assess the difference in preexisting T315I mutations in populations of CP-CML versus Ph+ ALL patients. Across the diversity of mutation burdens measured in this study, we compared the number of simulations (out of 1000) that contained a T315I mutation when the phenotypic heterogeneity of the tumor re-initiating fraction was sampled from an empirical cumulative distribution function (eCDF). This eCDF sampling randomly draws (with replacement) a relevant re-initiating cell fraction from the empirical distribution of initiating cell measurements identified in published studies (41,42). D. p-values for a Fisher’s Exact Test of the events in C. across different mutation burdens.
Compound resistance mutations are expected to arise following clonal expansion of single resistance mutations
Compound resistance mutations (i.e. two simultaneous resistance mutations in the same allele of ABL1) can result in resistance to all available TKI’s, and can frequently lead to treatment failure in pre-treated patients with advanced-phase Ph+ leukemias (Figure 3). We determined the likelihood of compound mutations to pre-exist prior to therapy in computational simulations, and found that while pre-existing single resistance variants can occur in both CP-CML and Ph+ ALL with certain simulation parameters, pre-existing compound resistance mutations are extremely unlikely to occur in either disease, even at the extreme ranges of the parameters that were used in simulations (Figure 5C). This suggests that compound mutations arise because of sequential selection with narrow-spectrum ABL1 inhibitors (i.e. inhibitors for which single point mutations can confer resistance), rather than arising de novo. Consistent with this hypothesis, we further analyzed Sanger sequencing and next-generation sequencing data from the PACE trial, and found that among patients with mutation gains, one of the two mutations comprising the compound mutation was identified prior to ponatinib treatment in 12/13 Ph+ ALL patients.
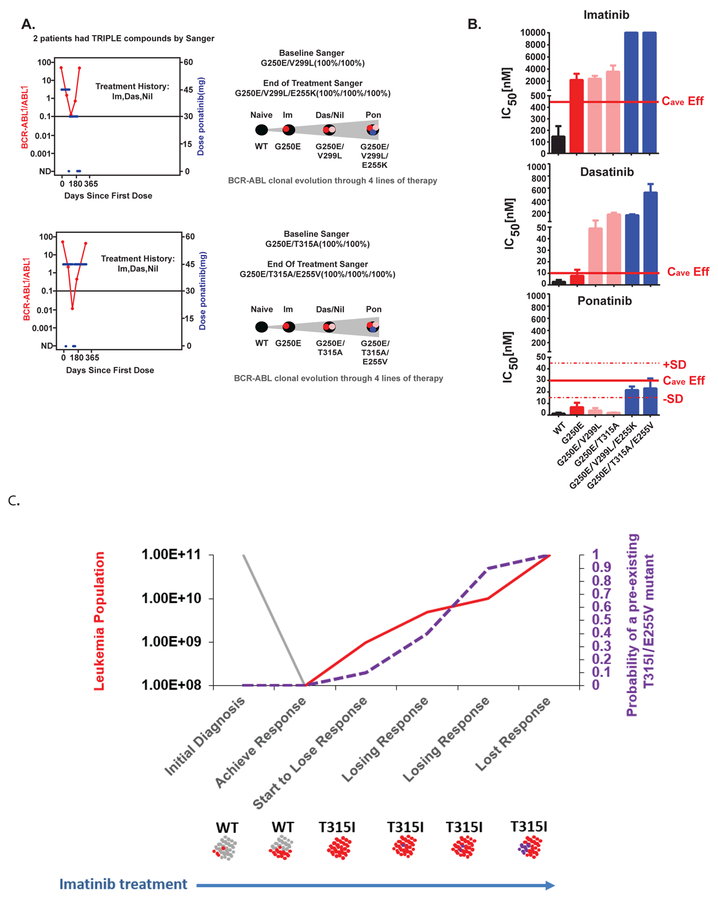
We next investigated whether two mutations in the ABL1 drug target is the maximum that the kinase domain can accumulate and still function as an oncogene. While we are aware of a single prior report of a patient who developed triple compound resistance mutations following sequential TKI failure(43), we hoped to assess whether triple compound resistance can be a frequent occurrence in heavily pre-treated patients in the PACE trial.
We identified three fourth-line patients who indeed acquired compound triple mutations at the end of therapy. All three patients had compound double mutations prior to ponatinib therapy. We experimentally verified the drug resistance phenotype of two of the compound triple mutants using BaF3 cells, and inferred a hypothetical stepwise accumulation of the three resistance mutations during prior lines of TKI therapy, given the patient treatment history (Figures 6A and 6B). This analysis highlights the risk for evolution of highly drug resistant clones following sequential TKI therapy.
Figure 6. Compound resistance mutations are likely to arise from sequential mutation gains .
A. Plots (left side of panel) show BCR-ABL1 kinetics for two patients who developed compound triple mutations. The right side of the panel shows theoretical ordering of sequential mutation gains. We do not have direct sequencing information after each sequential line of therapy prior to enrollment on the PACE trial, and thus mutation gain ordering was inferred from available sequencing data, treatment history, and IC50 values of each therapy. B. Experimental validation of compound triple resistance mutations. The mutations shown in panel C were cloned into PLVX-IRES-PURO and infected into BaF3 cells (see Methods) as single, double, and triple mutants and the extent of drug resistance was determined for imatinib, dasatinib, and ponatinib. The predicted average clinical exposure adjusted for the effects of serum proteins is indicated. This is denoted as Cave-eff (60). C. Conceptual model for acquisition of compound mutations. At the time of initial diagnosis of Ph+ ALL, most cells will harbor wild-type ABL1 (shown in gray), with resistance mutations pre-existing in minority populations. Therapy with a narrow-spectrum inhibitor results in selective expansion of mutant cells (red). As the population of mutant cells expands, additional mutations will accumulate, resulting in cells with compound mutations (purple). Up-front use of a pan-inhibitor which can overcome any single resistance mutation would be expected to circumvent drug resistance, as compound resistance mutations are extremely unlikely to pre-exist prior to therapy.
Finally, to emphasize the risk of compound mutation acquisition in Ph+ ALL, we present a conceptual model (Figure 6C) to suggest that pan-target inhibition in the first line might be particularly relevant to Ph+ ALL. At diagnosis a Ph+ ALL patient might present with a high BCR-ABL1/ABL1 level, and an “intermediate” tumor-initiating cell population size, ABL1 mutation burden and white blood cell count. Using simulation values for this intermediate series of parameters implies that this patient would be likely to have a T315I mutation at baseline, but very unlikely to have a compound mutation. Successful treatment (i.e. a major molecular response) with imatinib (or nilotinib/dasatinib) would then enrich for this T315I population. As this T315I population expands, further mutations inevitably arise on the background of the clonally expanded T315I population, and the chances of creating a compound mutation increase dramatically. By the time relapse is apparent and ponatinib is started, the patient almost certainly harbors a compound resistance mutant that can cause resistance to ponatinib. Thus, using a pan-target inhibitor in the frontline setting could potentially eliminate this route to compound on-target resistance.
Conclusion/Discussion
We applied high-resolution Duplex Sequencing and computational simulations to gain insight into patterns of resistance mutations in a drug target. Our objectives were to understand the evolution of drug resistance, and to explore optimal clinical indications for the use of pan-target therapies. We utilized Ph+ leukemias, the prototypical example of targeted therapy, as a model system. Ph+ leukemias range from relatively indolent (CP-CML) to highly aggressive (BP-CML and Ph+ ALL), have a well-defined resistance mechanism (ABL1 mutation), and multiple approved therapies are available that harbor differential sensitivities to specific resistance mutations. We find that RT-PCR errors substantially limit the resolution of mutation detection in Ph+ leukemias, and overcome this limitation with single-molecule Duplex Sequencing. Measurements of ABL1 mutation burden suggest that a combination of mutational burden and leukemia-initiating cell fraction predicts a low likelihood of pre-existing drug resistance in CP-CML, and rationalizes the exceptional success of targeted therapy in this setting. In contrast, our results also indicate that patients with Ph+ ALL harbor a high burden of pre-occurring ABL1 resistance in leukemia initiating cells, which is consistent with the high rate of drug resistance mutations in Ph+ ALL. Moreover, compound mutations appear to be unlikely to pre-exist at the time of diagnosis in either CP-CML or Ph+ ALL, and rather are predicted to arise from the clonal expansion of a single resistance mutation that occurs following the initial use of a narrow-spectrum targeted inhibitor. This suggests that outcomes in Ph+ ALL could be improved with the up-front use of pan-target therapies. These observations have important implications for optimizing treatment paradigms in Ph+ leukemias, and are also relevant to targeted cancer therapy in other malignancies.
Theoretical modelling suggests that once cancers reach a certain size, essentially every resistance mutation can pre-occur (44). Cell culture models, where pre-existing drug resistant cells can be experimentally identified and tracked (45) also suggest that reistance mutations exist prior to therapy, yet whether these findings recapitulate the diversity of resistance mechanisms in humans is unclear, as expansion and passaging of cells in vitro can create a population structure that is quite distinct from the original sample.. Here we used direct estimates of mutation burden and leukemia cell burden in individuals to address the question of pre-existing mutations across discrete clinical populations. By using relatively simple computational modeling that is particularly suited to Duplex Sequencing measurements, we demonstrate that a combination of both mutational heterogeneity and phenotypic heterogeneity can rationalize clinical experience in Ph+ malignancies. Patients with CP-CML have a lower risk of pre-existing resistance mutations in leukemia-initiating cells, which may explain the remarkable frontline success of ABL1 inhibitors that have multiple single mutation liabilities in the ABL1 kinase domain and the rarity of on-target resistance mutations in CP-CML (11,12). In contrast, in Ph+ ALL, many resistance mutations can pre-exist simultaneously in many leukemia initiating cells, consistent with the high risk of TKI failure and the frequent occurrence of ABL1 resistance mutations in Ph+ ALL (16,17). Given the relatively small number of patients in this study, it is difficult to estimate the probability distribution of mutation burden and stem cell fractions across all patients with Ph+ leukemias. However our results are consistent across a wide range of simulation values. The majority of plausible Ph+ ALL parameters suggest substantial pre-existing resistance, while only a minority of CP-CML parameters appear consistent with pre-existing resistance.
Molecularly targeted therapies have created an evolutionary “arms race” between drug discovery and tumor evolution, and successive generations of targeted therapies have been developed to overcome stepwise resistance in drug targets such as ABL1, ALK, and EGFR that develops during first and second generation therapies. It is widely debated whether drugs that inhibit all single resistance mutations should be saved for late-line therapy of resistant patients, or alternatively if they should be used as early as possible to eliminate the evolutionary selection of resistant variants. Our results are directly applicable to this conundrum: we find that patients with highly refractory Ph+ leukemias can harbor double- and triple- compound mutations, while compound resistance mutations are extremely unlikely to exist before therapy in a wild type background. Because compound mutations are unlikely to pre-exist without a pre-existing single mutant, our results suggest that outcomes in Ph+ ALL could potentially be improved with front-line use of pan-inhibitors.
In Ph+ malignancies, ponatinib is the only approved pan-target inhibitor, though pan-target combinations may emerge in the future (46). Ponatinib has an increased incidence of adverse events relative to other TKIs at the approved dose of 45 mg (47) and is thus often reserved for patients refractory to other TKIs. However, given the high risk of treatment failure from ABL1 mutations in Ph+ ALL and the poor overall prognosis of Ph+ ALL with median 3 year overall survival of 48% (48), up-front use of ponatinib could improve outcomes, as our results indicate that use of ponatinib after failure of other TKI’s can select for compound resistance mutations that confer resistance to ponatinib. Consistent with this prediction, in a phase II study of frontline ponatinib with chemotherapy, impressive outcomes were seen with 3 year overall survival of 83% (49,50). Responses were durable and were associated with improved progression-free survival and overall survival relative to dasatinib (50), which suggests that broad inhibition of the ABL1 kinase with ponatinib does not hasten resistance from alternative, ABL1-independent mechanisms. These data in part reflect why National Comprehensive Cancer Network (NCCN) guidelines now include ponatinib as an option for frontline treatment of Ph+ ALL (51). Simultaneous treatment with multiple TKI’s would be another potential strategy to forestall resistance, although cumulative toxicity may limit the feasibility of combining multiple TKI’s in a single patient.
Guidelines from the NCCN (52) and the European Leukemia Network (53) recommend ABL1 mutation testing for patients with an inadequate response to TKI therapy, as choice of subsequent therapy can be guided based on the specific mutation(s) identified. Assignment of the appropriate TKI therapy is thus dependent on accurate mutation detection. This is of note, as we find that many low-level mutations are missed by the approaches in common clinical use (Sanger sequencing or high throughput sequencing). Prior work indicates that resistance mutations can be highly clinically relevant and predictive of treatment failure, even if present near the limit of detection of higher sensitivity approaches such as mass spectrometry (54). Some of the mutations we identified by Duplex Sequencing were in a small proportion of the overall population, e.g. 0.01% of cells. When considering the total blood volume of a patient, a mutant fraction of 0.01% corresponds to a population of millions of cells, and thus the selective growth of treatment-resistant cells would be expected to result in further expansion of the population. In multiple cases, we found that mutations present at very low levels (<0.02%) indeed expand and become the dominant clone following treatment. Their selective expansion during drug therapy suggests that minor sub-clones are likely to be highly relevant to the emergence of clinical resistance. Prospective clinical trials will be needed to determine if early detection of sub-clonal resistance mutations and a change in therapy based on the results will lead to improved patient outcomes.
Our studies focused on target-driven resistance mutations in ABL1. However most CP-CML patients (and some Ph+ ALL patients) who fail therapy develop TKI resistance in the absence of ABL1 mutations (55). In these cases, mutations elsewhere in the genome are likely to contribute to treatment failure. A high degree of mutational burden in the ABL1 gene in an individual could conceivably reflect elevated mutagenesis elsewhere in the genome, although mutation burden is likely to differ at distinct genomic regions based on multiple factors such as replication timing and gene expression (56). Future studies could investigate if the total burden of sub-clonal mutations, independent of any specific mutation, correlate with clinical parameters such as the likelihood of developing resistance to therapy by on-target or off-target mechanisms.
While Ph+ leukemias represent the prototypical application of targeted cancer therapy, the use of molecularly targeted drugs is rapidly expanding in oncology, and intra-tumor heterogeneity is likely a determinant of treatment failure across multiple diseases (1). For example, chronic lymphocytic leukemia (CLL) treatment is being revolutionized by the BTK-targeting drug ibrutinib, yet mutation of the target gene can result in treatment failure (57). Likewise lung cancer therapy is undergoing a dramatic shift with use of agents targeting EGFR and ALK, yet in both cases, mutation of the drug target causes resistance to therapy (58). Moreover, c-KIT driven GIST has a tremendous propensity to develop on-target mutations in c-KIT, and this drives the simultaneous presence of many sub-clonal resistance mutations (59). Next-line drugs which overcome specific resistance mutations have been developed in these diseases, however sequential use of drugs with increasingly broad abilities to overcome resistance may lead to progressive increases in mutational heterogeneity, compound resistance mutations, and intractable disease. Ultimately, establishing the magnitude of drug target mutation burden in individual diseases, and in individual patients, may allow for optimized application of targeted therapies for maximal patient benefit in multiple indications.
Supplementary Material
Statement of Translational Relevance.
Targeted cancer therapy has greatly improved outcomes in some malignancies, however drug resistance can occur from mutations in the drug target. Pan-inhibitors, defined as therapies that can overcome any individual resistance mutation, are now available in some indications, yet compound resistance (2 co-occurring resistance mutations in the drug target) can still result in treatment failure. This study demonstrates that compound resistance mutations are unlikely to pre-exist prior to therapy of Philadelphia-positive leukemias. Rather, compound resistance is predicted to arise as a consequence of initial therapy with a drug that is susceptible to resistance from single mutations. In the clinic, front-line use of pan-target therapies (i.e. therapies that can overcome any single resistance mutation) could improve outcomes by forestalling the onset of resistance.
Acknowledgements:
This work was supported by National Institutes of Health grants T32HL007093 (M.W.S.), R33 CA181771 (L.A.L.), P01 CA077852 (L.A.L.), R01 CA175215 (J.P.R.), and P01 CA018029 (J.P.R.). We would like to thank Tom Walsh and Ming Lee for assistance with DNA sequencing, and members of the Loeb lab for insightful discussions.
Footnotes
Data availability
All sequencing data from the study have been uploaded to the Sequence Read Archive under BioProject# PRJNA308332.
Conflict of interest statement
M.W.S. and L.A.L. declare stock ownership and consulting roles at TwinStrand Biosciences, Inc. J.P.R. declares consulting roles at ARIAD, Novartis, Bristol-Myers Squibb (BMS), and Pfizer, and has received research support from Novartis and BMS. J.R.P., J.G.H., and V.M.R. declare previous stock ownership at ARIAD Pharmaceuticals, Inc. J.R.P. and V.M.R. declare a consulting role at Takeda Pharmaceuticals.
References and Notes:
- 1.Schmitt MW, Loeb LA, Salk JJ. The influence of subclonal resistance mutations on targeted cancer therapy. Nature Reviews Clinical Oncology. 2016;13:335–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hochhaus A, Larson RA, Guilhot F, Radich JP, Branford S, Hughes TP, et al. Long-Term Outcomes of Imatinib Treatment for Chronic Myeloid Leukemia. The New England Journal of Medicine. 2017;376:917–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Talpaz M, Shah NP, Kantarjian H, Donato N, Nicoll J, Paquette R, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. The New England Journal of Medicine. 2006;354:2531–41. [DOI] [PubMed] [Google Scholar]
- 4.Kantarjian H, Giles F, Wunderle L, Bhalla K, O’Brien S, Wassmann B, et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. The New England Journal of Medicine. 2006;354:2542–51. [DOI] [PubMed] [Google Scholar]
- 5.O’Hare T, Shakespeare WC, Zhu X, Eide CA, Rivera VM, Wang F, et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. 2009;16:401–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre P, Paquette R, Chuah C, et al. A phase 2 trial of ponatinib in Philadelphia chromosome–positive leukemias. The New England Journal of Medicine. 2013;369:1783–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zabriskie MS, Eide CA, Tantravahi SK, Vellore NA, Estrada J, Nicolini FE, et al. BCR-ABL1 compound mutations combining key kinase domain positions confer clinical resistance to ponatinib in Ph chromosome-positive leukemia. Cancer Cell. 2014;26:428–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deininger MW, Hodgson JG, Shah NP, Cortes JE, Kim D-W, Nicolini FE, et al. Compound mutations in BCR-ABL1 are not major drivers of primary or secondary resistance to ponatinib in CP-CML patients. Blood. 2016;127:703–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gainor JF, Dardaei L, Yoda S, Friboulet L, Leshchiner I, Katayama R, et al. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discovery. 2016;1–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnes TA, O’Kane GM, Vincent MD, Leighl NB. Third-generation tyrosine kinase inhibitors targeting epidermal growth factor receptor mutations in non-small cell lung cancer. Frontiers in Oncology. 2017;7:69–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cortes JE, Saglio G, Kantarjian HM, Baccarani M, Mayer J, Boqué C, et al. Final 5-year study results of DASISION: the dasatinib versus imatinib study in treatment-naïve chronic myeloid leukemia patients trial. Journal of Clinical Oncology. 2016;34:2333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hochhaus A, Saglio G, Hughes TP, Larson RA, Kim DW, Issaragrisil S, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30:1044–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hughes TP, Saglio G, s-Cardama AQA, Mauro MJ, Kim DW, Lipton JH, et al. BCR-ABL1 mutation development during first-line treatment with dasatinib or imatinib for chronic myeloid leukemia in chronic phase. Leukemia. 2015;29:1832–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Etienne G, Guilhot J, Rea D, Rigal-Huguet F, Nicolini F, Charbonnier A, et al. Long-Term Follow-Up of the French Stop Imatinib (STIM1) Study in Patients With Chronic Myeloid Leukemia. Journal of Clinical Oncology. 2016;:1–10. [DOI] [PubMed] [Google Scholar]
- 15.Druker BJ, Sawyers CL, Kantarjian H, Resta DJ, Reese SF, Ford JM, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. The New England Journal of Medicine. 2001;344:1038–42. [DOI] [PubMed] [Google Scholar]
- 16.Piccaluga PP, Paolini S, Martinelli G. Tyrosine kinase inhibitors for the treatment of Philadelphia chromosome-positive adult acute lymphoblastic leukemia. Cancer. 2007;110:1178–86. [DOI] [PubMed] [Google Scholar]
- 17.Soverini S, De Benedittis C, Papayannidis C, Paolini S, Venturi C, Iacobucci I, et al. Drug resistance and BCR-ABL kinase domain mutations in Philadelphia chromosome-positive acute lymphoblastic leukemia from the imatinib to the second-generation tyrosine kinase inhibitor era: The main changes are in the type of mutations, but not in the frequency of mutation involvement. Cancer. 2014;120:1002–9. [DOI] [PubMed] [Google Scholar]
- 18.Roche-Lestienne C, Soenen-Cornu V, Grardel-Duflos N, Laï J-L, Philippe N, Facon T, et al. Several types of mutations of the Abl gene can be found in chronic myeloid leukemia patients resistant to STI571, and they can pre-exist to the onset of treatment. Blood. 2002;100:1014–8. [DOI] [PubMed] [Google Scholar]
- 19.Roche-Lestienne C, Laï J-L, Darré S, Facon T, Preudhomme C. A mutation conferring resistance to imatinib at the time of diagnosis of chronic myelogenous leukemia. The New England Journal of Medicine. 2003;348:2265–6. [DOI] [PubMed] [Google Scholar]
- 20.Wongboonma W, Thongnoppakhun W, Auewarakul CU. BCR-ABL kinase domain mutations in tyrosine kinase inhibitors-naïve and -exposed Southeast Asian chronic myeloid leukemia patients. Experimental and Molecular Pathology. 2012;92:259–65. [DOI] [PubMed] [Google Scholar]
- 21.Iqbal Z, Aleem A, Iqbal M, Naqvi MI, Gill A, Taj AS, et al. Sensitive detection of pre-existing BCR-ABL kinase domain mutations in CD34+ cells of newly diagnosed chronic-phase chronic myeloid leukemia patients is associated with imatinib resistance: implications in the post-imatinib era. Ellis SR, editor. PLoS ONE. 2013;8:e55717. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Willis SG, Lange T, Demehri S, Otto S, Crossman L, Niederwieser D, et al. High-sensitivity detection of BCR-ABL kinase domain mutations in imatinib-naive patients: correlation with clonal cytogenetic evolution but not response to therapy. Blood. 2005;106:2128–37. [DOI] [PubMed] [Google Scholar]
- 23.Kreuzer K-A, le Coutre P, Landt O, Na I-K, Schwarz M, Schultheis K, et al. Preexistence and evolution of imatinib mesylate-resistant clones in chronic myelogenous leukemia detected by a PNA-based PCR clamping technique. Annals of Hematology. 2003;82:284–9. [DOI] [PubMed] [Google Scholar]
- 24.Soverini S, Vitale A, Poerio A, Gnani A, Colarossi S, Iacobucci I, et al. Philadelphia-positive acute lymphoblastic leukemia patients already harbor BCR-ABL kinase domain mutations at low levels at the time of diagnosis. Haematologica. 2011;96:552–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmitt MW, Kennedy SR, Salk JJ, Fox EJ, Hiatt JB, Loeb LA. Detection of ultra-rare mutations by next-generation sequencing. Proceedings of the National Academy of Sciences U S A. 2012;109:14508–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kennedy SR, Schmitt MW, Fox EJ, Kohrn BF, Salk JJ, Ahn EH, et al. Detecting ultralow-frequency mutations by Duplex Sequencing. Nature Protocols. 2014;9:2586–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmitt MW, Fox EJ, Prindle MJ, Reid-Bayliss KS, True LD, Radich JP, et al. Sequencing small genomic targets with high efficiency and extreme accuracy. Nature Methods. 2015;12:423–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Svarovskaia ES, Cheslock SR, Zhang W-H, Hu W-S, Pathak VK. Retroviral mutation rates and reverse transcriptase fidelity. Frontiers in Bioscience. 2003;8:d117–34. [DOI] [PubMed] [Google Scholar]
- 29.Eckert KA, Kunkel TA. DNA polymerase fidelity and the polymerase chain reaction. PCR Methods Appl. 1991;1:17–24. [DOI] [PubMed] [Google Scholar]
- 30.Reid-Bayliss KS, Loeb LA. Accurate RNA consensus sequencing for high-fidelity detection of transcriptional mutagenesis-induced epimutations. Proceedings of the National Academy of Sciences U S A. 2017;114:9415–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cortes JE, Kantarjian HM, Goldberg SL, Powell BL, Giles FJ, Wetzler M, et al. High-dose imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: high rates of rapid cytogenetic and molecular responses. Journal of Clinical Oncology. 2009;27:4754–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baccarani M, Druker BJ, Branford S, Kim D-W, Pane F, Mongay L, et al. Long-term response to imatinib is not affected by the initial dose in patients with Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: final update from the Tyrosine Kinase Inhibitor Optimization and Selectivity (TOPS) study. International Journal of Hematology. 2014;99:616–24. [DOI] [PubMed] [Google Scholar]
- 33.Mahon F-X, Etienne G. Deep molecular response in chronic myeloid leukemia: the new goal of therapy? Clinical Cancer Research. 2014;20:310–22. [DOI] [PubMed] [Google Scholar]
- 34.Soverini S, Branford S, Nicolini FE, Talpaz M, Deininger MWN, Martinelli G, et al. Implications of BCR-ABL1 kinase domain-mediated resistance in chronic myeloid leukemia. Leukemia Research. 2014;38:10–20. [DOI] [PubMed] [Google Scholar]
- 35.Saldanha J, Silvy M, Beaufils N, Arlinghaus R, Barbany G, Branford S, et al. Characterization of a reference material for BCR-ABL (M-BCR) mRNA quantitation by real-time amplification assays: towards new standards for gene expression measurements. Leukemia. 2007;21:1481–7. [DOI] [PubMed] [Google Scholar]
- 36.Stein AM, Bottino D, Modur V, Branford S, Kaeda J, Goldman JM, et al. BCR-ABL transcript dynamics support the hypothesis that leukemic stem cells are reduced during imatinib treatment. Clinical Cancer Research. 2011;17:6812–21. [DOI] [PubMed] [Google Scholar]
- 37.Michor F, Hughes TP, Iwasa Y, Branford S, Shah NP, Sawyers CL, et al. Dynamics of chronic myeloid leukaemia. Nature. 2005;435:1267–70. [DOI] [PubMed] [Google Scholar]
- 38.Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell Stem Cell. 2014;14:275–91. [DOI] [PubMed] [Google Scholar]
- 39.Greaves M Evolutionary determinants of cancer. Cancer Discovery. 2015;5:806–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fong CY, Gilan O, Lam EYN, Rubin AF, Ftouni S, Tyler D, et al. BET inhibitor resistance emerges from leukaemia stem cells. Nature. 2015;525:538–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lewis ID, McDiarmid LA, Samels LM, To LB, Hughes TP. Establishment of a reproducible model of chronic-phase chronic myeloid leukemia in NOD/SCID mice using blood-derived mononuclear or CD34+ cells. Blood. 1998;91:630–40. [PubMed] [Google Scholar]
- 42.Notta F, Mullighan CG, Wang JCY, Poeppl A, Doulatov S, Phillips LA, et al. Evolution of human BCR-ABL1 lymphoblastic leukaemia-initiating cells. Nature. 2011;469:362–7. [DOI] [PubMed] [Google Scholar]
- 43.Shah NP, Skaggs BJ, Branford S, Hughes TP, Nicoll JM, Paquette RL, et al. Sequential ABL kinase inhibitor therapy selects for compound drug-resistant BCR-ABL mutations with altered oncogenic potency. Journal of Clinical Investigation. 2007;117:2562–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iwasa Y, Nowak MA, Michor F. Evolution of resistance during clonal expansion. Genetics. 2006;172:2557–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bhang H-EC, Ruddy DA, Krishnamurthy Radhakrishna V, Caushi JX, Zhao R, Hims MM, et al. Studying clonal dynamics in response to cancer therapy using high-complexity barcoding. Nature Medicine. 2015. [DOI] [PubMed] [Google Scholar]
- 46.Wylie AA, Schoepfer J, Jahnke W, Cowan-Jacob SW, Loo A, Furet P, et al. The allosteric inhibitor ABL001 enables dual targeting of BCR-ABL1. Nature. 2017;543:733–7. [DOI] [PubMed] [Google Scholar]
- 47.Dorer DJ, Knickerbocker RK, Baccarani M, Cortes JE, Hochhaus A, Talpaz M, et al. Impact of dose intensity of ponatinib on selected adverse events: Multivariate analyses from a pooled population of clinical trial patients. Leukemia Research. 2016;48:84–91. [DOI] [PubMed] [Google Scholar]
- 48.Daver N, Thomas D, Ravandi F, Cortes J, Garris R, Jabbour E, et al. Final report of a phase II study of imatinib mesylate with hyper-CVAD for the front-line treatment of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Haematologica. 2015;100:653–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jabbour E, Kantarjian H, Ravandi F, Thomas D, Huang X, Faderl S, et al. Combination of hyper-CVAD with ponatinib as first-line therapy for patients with Philadelphia chromosome-positive acute lymphoblastic leukaemia: a single-centre, phase 2 study. The Lancet Oncology. 2015;16:1547–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sasaki K, Jabbour EJ, Ravandi F, Short NJ, Thomas DA, Garcia-Manero G, et al. Hyper-CVAD plus ponatinib versus hyper-CVAD plus dasatinib as frontline therapy for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: A propensity score analysis. Cancer. 2016;122:3650–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown PA, Advani A, Aoun P, Barta SK, Boyer MW, Bryan T, et al. NCCN Clinical Practice Guidelines in Oncology: Acute Lymphoblastic Leukemia, v1.2017 [Internet]. [cited 2017 Aug 9]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/all.pdf
- 52.O’Brien S, Radich JP, Abboud CN, Akhtari M, Altman JK, Berman E, et al. Chronic myelogenous leukemia, version 1.2015. Journal of the National Comprehensive Cancer Network. 2014;12:1590–610. [DOI] [PubMed] [Google Scholar]
- 53.Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parker WT, Lawrence RM, Ho M, Irwin DL, Scott HS, Hughes TP, et al. Sensitive detection of BCR-ABL1 mutations in patients with chronic myeloid leukemia after imatinib resistance is predictive of outcome during subsequent therapy. Journal of Clinical Oncology. 2011;29:4250–9. [DOI] [PubMed] [Google Scholar]
- 55.Eide CA, O’Hare T. Chronic myeloid leukemia: advances in understanding disease biology and mechanisms of resistance to tyrosine kinase inhibitors. Current Hematologic Malignancy Reports. 2015;10:158–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Woyach JA, Furman RR, Liu T-M, Ozer HG, Zapatka M, Ruppert AS, et al. Resistance mechanisms for the Bruton’s tyrosine kinase inhibitor ibrutinib. The New England Journal of Medicine. 2014;370:2286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Romanidou O, Landi L, Cappuzzo F, Califano R. Overcoming resistance to first/second generation epidermal growth factor receptor tyrosine kinase inhibitors and ALK inhibitors in oncogene-addicted advanced non-small cell lung cancer. Therapeutic Advances in Medical Oncology. 2016;8:176–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garner AP, Gozgit JM, Anjum R, Vodala S, Schrock A, Zhou T, et al. Ponatinib inhibits polyclonal drug-resistant KIT oncoproteins and shows therapeutic potential in heavily pretreated gastrointestinal stromal tumor (GIST) patients. Clinical Cancer Research. 2014;20:5745–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gozgit JM, Schrock A, Chen T-H, Clackson T, Rivera VM. Comprehensive Analysis of the in vitro potency of ponatinib, and all other approved BCR-ABL tyrosine kinase inhibitors (TKIs), against a panel of single and compound BCR-ABL mutants [Abstract]. Blood. 2013;122:3992. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.