Abstract
Background
The aim of this systematic review and meta-analysis was to evaluate the benefit of platelet-rich plasma (PRP) in non-surgical orthopaedic procedures.
Material and methods
We searched the Cochrane Wounds Specialized Register, CENTRAL, MEDLINE (through PUBMED), Embase, and SCOPUS. We also searched clinical trials registries for ongoing and unpublished studies and checked reference lists to identify additional studies.
Results
We found 36 randomised controlled trials (2,073 patients) that met our inclusion criteria. The included studies mostly had small numbers of participants (from 20 to 225). Twenty-eight studies included patients with lateral epicondylitis or plantar fasciitis. PRP was compared to local steroids injection (19 studies), saline injection (6 studies), autologous whole blood (4 studies), local anaesthetic injection (3 studies), dry needling injection (3 studies), and to other comparators (4 studies). Primary outcomes were pain and function scores, and adverse events. On average, it is unclear whether or not use of PRP compared to controls reduces pain scores and functional score at short- (up to 3 months) and medium- (4–6 months) term follow-up. The available evidence for all the comparisons was rated as very low quality due to inconsistency, imprecision, and risk of bias in most of the selected studies. There were no serious adverse events related to PRP injection or control treatments.
Conclusions
The results of this meta-analysis, which documents the very marginal effectiveness of PRP compared to controls, does not support the use of PRP as conservative treatment in orthopaedics.
Keywords: platelet-rich plasma, PRP, orthopaedics, treatment
Introduction
Platelet-rich plasma (PRP) is a mixture of highly concentrated platelets and associated growth factors. It is obtained from whole blood through a 2-phase centrifugation process: the first for the separation of blood components, and the second for the final PRP production. There are currently over 40 commercial systems that have been developed to concentrate autologous whole blood into a platelet-rich substance1. Besides platelets, PRP contains some inflammatory cells (i.e. monocytes and polymorphonuclear neutrophils) and large amounts of proteins, including platelet-derived growth factor (PDGF), transforming growth factor beta (TGF-β), vascular endothelial growth factor (VEGF), epithelial growth factor (EGF), and adhesion molecules (i.e. fibrin, fibronectin and vitronectin)2–4. Such growth factors and cells have been shown to promote cell recruitment, proliferation and angiogenesis, which may be implicated in tissue regeneration and healing5–8. Thanks to these biological regenerative properties, a number of investigators have studied the potential clinical benefit of PRP, also from human umbilical cord blood7,8, in a wide array of conditions ranging from dermatological disorders to oromaxillofacial surgery9–11
In addition, a number of randomised controlled clinical trials (RCTs) have evaluated PRP use in the orthopaedic setting, particularly for tendon and ligament injuries, and several systematic reviews and meta-analysis have been subsequently published, although with contrasting results12–20. With the aim of elucidating this controversial issue, we have performed a systematic review and meta-analysis on the efficacy of PRP as conservative treatment in orthopaedics.
Material and methods
This systematic review was conducted according to the recommended PRISMA checklist guidelines21.
Search strategy
A computer-assisted literature search of the MEDLINE (through PUBMED), EMBASE, SCOPUS, OVID and Cochrane Library electronic databases was performed (last search April 30, 2018) to identify RCTs on the conservative non-surgical use of PRP in orthopaedics. A combination of the following text words was used to maximise search specificity and sensitivity: “platelet rich plasma”, “conservative”, “orthopaedics”, “tendon”, “tendinopathy”, “tendinitis”, “ligament”, “randomized clinical controlled trials”, “Achilles tendinopathy”, “plantar fasciitis”, “lateral epicondylitis”, “tennis elbow”, “patellar tendinopathy” and “rotator cuff tendinopathy”. In addition, we checked the reference lists of the most relevant items (original studies and reviews) in order to identify potentially eligible studies not captured by the initial literature search.
Study selection and inclusion criteria
Study selection was performed independently by two reviewers (MF and MC), with disagreements resolved through discussion and on the basis of the opinion of a third reviewer (CM). Eligibility assessment was based on the title or abstract and on the full text if required. Articles were eligible if they reported either in the title or in the abstract the use of PRP in orthopaedics. Only RCTs published in full in English were included in this systematic review and meta-analysis. Studies enrolling less than 10 patients were excluded, along with RCTs evaluating platelet-poor plasma and autologous conditioned plasma.
For the purpose of this systematic review, trials evaluating the role of PRP in surgical orthopaedic procedures were excluded. We selected five groups of disorders:
- lateral epicondylitis;
- Achilles tendinopathy;
- plantar fasciitis;
- patellar tendinopathy;
- rotator cuff tendinopathy.
Types of interventions
Trials evaluating platelet-poor plasma and autologous conditioned plasma were excluded. We compared intralesional, injected PRP preparation with:
- local steroids injection;
- placebo injection;
- whole blood injection;
- local anaesthetic injection;
- exercise and other physical therapies (e.g. low-dose radiation therapy, eccentric loading programme);
- any other medications given locally or systemically aimed at treating pain; and
- combinations of the active interventions listed above.
Outcomes
Primary outcomes included pain as measured by standard validated pain scale, e.g. Visual Analogue Score (VAS) is a continuous scale comprised of a horizontal (HVAS) or vertical (VVAS) line, usually 10 centimetres (100 millimetres, mm) in length, anchored by 2 verbal descriptors, one for each symptom extreme (higher scores = worse pain). In order to compare the results of the studies, the different scales used were converted into mm. Functional measurement by any standard validated scale, such as the American Orthopedic Foot and Ankle Society Score (AOFAS), and Disabilities of the Arm, Shoulder and Hand (DASH) score were also included. With functional scale, a higher scale indicated better function. Serious adverse events (e.g. infection at the injection site, heel fat pad atrophy, and plantar fascia rupture) were also evaluated. Secondary outcomes included tendon thickness in mm evaluated by ultrasounds.
The outcome measures were sub-grouped into two-time periods: short-term (within 3 months from the intervention) and medium-term (from 4 to 6 months). A long-term period (12 months) was not evaluated because few studies reported it and a pooled analysis of data was not possible. If multiple time points were reported within our time frames, we extracted the latest time point (e.g. if data were reported at four weeks, five weeks, three months and six months, we extracted outcomes at three and six months).
Data collection and analysis
For each RCT included in the systematic review, the following data were extracted by two reviewers (MF and MC) independently: first author, year of publication, orthopaedic disease, details of intervention in study and control group, sample size, mean age and male:female ratio (PRP and control groups), outcome measurements, follow-up period, and main results. Measures of treatment effect were mean differences (MD) together with 95% confidence intervals (CI) for continuous outcome measures (e.g. pain scores and functional improvement). For this measure, the score had to be reported as mean and standard deviation (SD); when studies reported other dispersion measures such as standard error (SE) of the mean or 95% Confidence Interval (CI) of the mean, we calculated the SD in order to perform the relevant meta-analytical pooling. We used final scores in preference to change in scores or cumulative incidence such as reduction of pain score reaching a predetermined size (for example ≥25% or ≥50%, indicated as “success”). Disagreement was resolved by consensus and by the opinion of a third reviewer (CM), if necessary.
The study weight was calculated using the Mantel-Haenszel method. We assessed statistical heterogeneity using t2, Cochran’s Q and I2 statistics. The I2 statistic describes the percentage of total variation across trials that is due to heterogeneity rather than sampling error. In the case of no heterogeneity (I2=0), studies were pooled using a fixed-effects model. Where values of I2 were >0, a random-effects analysis was undertaken. All calculations were made using Stata 15.1, R version 3.4.3, and REVMAN 522.
We undertook subgroup analysis for duration of follow-up (short-term and medium-term, as defined above) and, where appropriate, for type of control intervention (e.g. PRP vs local steroids injection).
Assessment of risk of bias in included studies
Two review Authors (MF, MC) independently assessed the risk of bias of each included study following the domain-based evaluation described in the Cochrane Handbook for Systematic Reviews of Interventions23. They discussed any discrepancies and achieved consensus on the final assessment. The Cochrane “Risk of bias” tool addresses six specific domains: sequence generation, allocation concealment, blinding, incomplete data, selective outcome reporting, and other issues relating to bias. For the selective reporting domain, we added an item for the outcome “adverse events” because reporting was inadequate only for this outcome. We have presented our assessment of risk of bias using two “Risk of bias” summary figures: 1) a summary of bias for each item across all studies; and 2) a cross-tabulation of each trial by all of the “Risk of bias” items.
Summary of findings tables
We used the principles of the GRADE system to assess the quality of the body of evidence associated with specific outcomes and constructed a summary of findings table using REVMAN 524. These tables present key information concerning the certainty of the evidence, the magnitude of the effects of the interventions examined, and the sum of available data for the main outcomes25. The summary of findings tables also includes an overall grading of the evidence related to each of the main outcomes using the GRADE approach, which defines the certainty of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The certainty of a body of evidence involves consideration of within-trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates, and risk of publication bias27.
When evaluating the “Risk of bias” domain, we down-graded the GRADE assessment when we classified a study as being at high risk of bias for one or more of the following domains: selection, attrition, performance, detection, reporting, and other bias; or when the “Risk of bias” assessment for selection bias was unclear (this was classified as unclear for either the generation of the randomisation sequence or the allocation concealment domain). For the outcomes VAS, AOFAS and DASH, we down-graded for high risk of bias in performance and detection domains, since we judged that these outcomes, self-reported by patients or collected by physicians to help standardise the assessments of patients with these disorders, are likely to be influenced by lack of blinding. The following outcomes have been presented in the summary of findings table: i) pain outcomes: VAS at 3 and 6 months of follow-up; ii) functional outcome: AOFAS at 3 and 6 months of follow-up; iii) serious adverse events (0–24 months).
Results
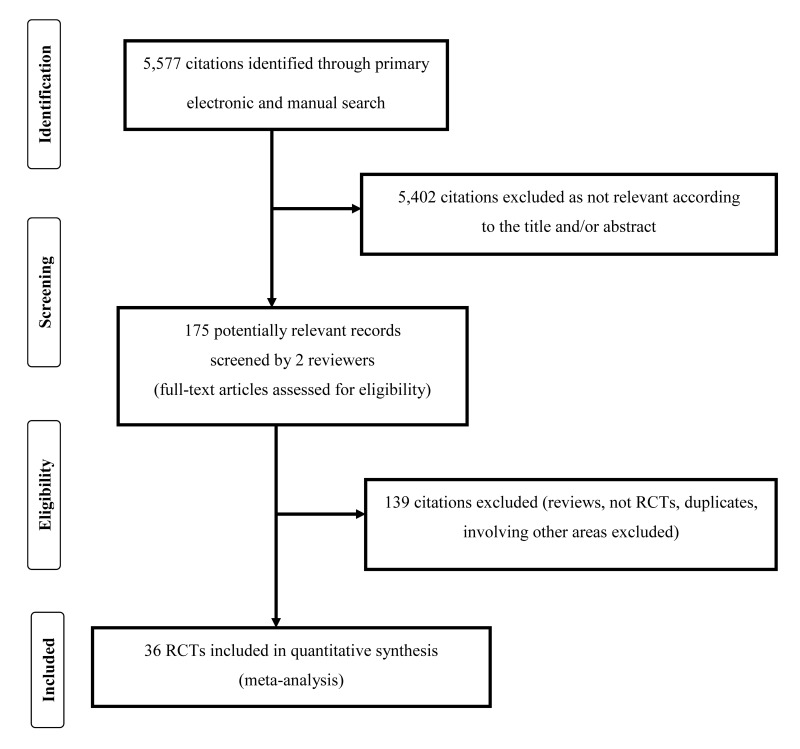
A total of 5,577 articles were identified after the initial electronic and manual search (Figure 1). Of these, 5,402 were excluded because they focused on other topics. Thus, 175 potentially relevant articles were selected and the next screening led to the exclusion of 139 additional studies (reviews, protocols of RCTs, non-randomised studies, duplicates, studies containing no informative data). Among RCTs reporting different follow-up of the same trial27–30, we included only the last published update28,30. Thirty-six randomised studies28,30–64 were included in the systematic review (see Table I for main characteristics and results of the included studies). Overall, 2,337 patients were enrolled in the 36 RCTs selected for the review. Of the 36 studies included in the systematic review, 11 were conducted in patients with lateral epicondylitis28,31,32,37,40,41,45,47–49,51, 14 in patients with plantar fasciitis38,43,44 46,50,52–55,58,60,62–64, 4 in patients with Achilles tendinopathy30,36,56,61, 3 in patients with rotator cuff tendinopathy34,39,57, 2 in patients with patellar tendinopathy35,42, and one58 in patients with shoulder impingement syndrome; one of these studies33 included both elbow tendinopathy and plantar fasciitis patients. In the 36 studies, PRP was compared to local steroids injection (19 studies)28,33,37,38,44,46–54,57,60,61,64, to saline injection (6 studies)30,34,37,56,61,62, to autologous whole blood (4 studies)31,32,40,58, to local anaesthetic injection (3 studies)41,43,45, to dry needling injection (3 studies)39,42,63, and others comparators (4 studies)35,36,55,59 (Table I). The outcomes more commonly reported were: VAS, AOFAS, DASH, a miscellanea of other scores (see Table I).
Figure 1.
Flow chart of the inclusion of the studies.
RCT: randomised controlled clinical trials.
Table I.
Characteristics and main results of the included studies on the non-surgical use of platelet-rich plasma (PRP) in orthopaedics.
| Study (year)ref | Disease | Intervention | Cases/controls (number) | Cases/controls (mean age) | Cases/controls (M:F ratio) | Outcome measurement | Follow-up (months) | Results | |
|---|---|---|---|---|---|---|---|---|---|
| PRP | Control | ||||||||
| De Jonge et al. (2011)30 | Achilles tendinopathy | Single injection 4 mL | Saline single injection 4 mL | 27/27 | 49/50 | 13:14/13:14 | VISA-A score, US | 12 | No superiority of PRP vs. placebo |
|
| |||||||||
| Thanasas et al. (2011)31 | Lateral epicondylitis | Single injection 3 mL | AWB single injection 3 mL | 14/14 | 36.6/35.9 | 4:10/3:11 | VAS, Liverpool Elbow score | 6 | Significant VAS improvement in PRP group at 6 weeks |
|
| |||||||||
| Gosens et al. (2011)28 | Lateral epicondylitis | Single injection 3 mL | Corticosteroid single injection 3 mL | 51/49 | 46.8/47.3 | 23:24/23:24 | VAS, DASH | 24 | Significant pain reduction and function improvement in PRP group at 2 years |
|
| |||||||||
| Creaney et al. (2011)32 | Lateral epicondylitis | Two injections 1.5 mL | AWB two injections 1.5 mL | 80/70 | 48/53 | 46:34/39:31 | PRTEE | 6 | PRP was not superior to AWB |
|
| |||||||||
| Omar et al. (2012)33 | Lateral epicondylitis | Single injection | Single steroid injection | 15/15 | 40.5/37.5 | 6:9/5:10 | VAS, DASH | 1.5 | No significant differences between groups |
|
| |||||||||
| Plantar fasciitis | Single injection | Single steroid injection | 15/15 | 42.5/44.5 | 0:15/0:15 | VAS, FHSQ | 1.5 | PRP was superior to steroid injection | |
|
| |||||||||
| Kesikburun et al. (2013)34 | Rotator cuff tendinopathy | Single injection 5 mL | Saline single injection 4 mL | 20/20 | 45.5/51.4 | 7:13/6:14 | VAS, WORC, SPADI score | 12 | No superiority of PRP vs. placebo |
|
| |||||||||
| Vetrano et al (2013)35 | Patellar tendinopathy | Two injections 2 mL | ESWT three sessions | 23/23 | 26.9/26.8 | 20:3/17:6 | VAS, VISA-P, Blazina scale | 12 | PRP was superior to ESWT at 12 months |
|
| |||||||||
| Kearney et al. (2013)36 | Achilles tendinopathy | Single injection 3–5 mL | Eccentric loading programme | 10/10 | 47.8/49.9 | 4:6/3:7 | VISA-A, EQ-5D | 6 | No statistically significant difference between groups |
|
| |||||||||
| Krogh et al. (2013)37 | Lateral epicondylitis | Single injection 3–3.5 mL | Single injection 3 mL steroid or saline | 20/40 | 47.6/43.9 | 9:11/11:9 | PRTEE | 3 | No differences between groups at 3 months |
|
| |||||||||
| Tiwari and Barghava (2013)38 | Plantar fasciitis | Single injection 5 mL | Single injection 40 mg/mL steroid | 30/30 | NR | NR | VAS | 6 | PRP was superior to steroid at 6 months |
|
| |||||||||
| Rha et al. (2013)39 | Rotator cuff tendinopathy | Single injection 3 mL | Single dry needling injection | 20/19 | 52.2/53.9 | 9:11/8:11 | SPADI score, US | 6 | PRP was superior to dry needling at 6 months |
|
| |||||||||
| Raeissadat et al. (2014)40 | Lateral epicondylitis | Single injection 2 mL | AWB single injection 2 mL | 31/30 | 47.2/45.3 | 8:23/6:24 | VAS, Mayo score, PTT | 2 | PRP was not superior to AWB |
|
| |||||||||
| Mishra et al. (2014)41 | Lateral epicondylitis | Single injection 2–3 mL | Single injection 2–3 mL bupivacaine | 112/113 | 48.4/47.4 | NR | VAS | 6 | Significant VAS improvement in PRP group at 6 months |
|
| |||||||||
| Dragoo et al. (2014)42 | Patellar tendinopathy | Single injection 6 mL | Single dry needling injection | 9/12 | 28/40 | 8:1/12:0 | VAS, VISA-P, Tegner activity scale, Lysholm knee scale, SF-12 | 6 | PRP was not superior to dry needling at 6 months |
|
| |||||||||
| Kim and Lee (2014)43 | Plantar fasciitis | Two injections 2 mL | Two injections 2 mL dextrose/lidocaine | 10/11 | 36.2/37.8 | 4:6/7:4 | FFI | 6 | No statistically significant difference between groups |
|
| |||||||||
| Monto (2014)44 | Plantar fasciitis | Single injection 3 mL | Single injection 40 mg steroid | 20/20 | 51/59 | 8:12/9:11 | AOFAS score | 24 | PRP was more effective and durable than steroid |
|
| |||||||||
| Behera et al. (2015)45 | Lateral epicondylitis | Single injection 3.5 mL | Single injection 3.5 mL bupivacaine | 15/10 | 38/37 | 3:12/5:4 | VAS, MMCPIE, Nirschl score | 12 | PRP was superior to bupivacaine |
|
| |||||||||
| Jain et al (2015)46 | Plantar fasciitis | Single injection 2.5 mL | Single injection 40 mg steroid | 24/22 | 56 | 8:16/8:14 | VAS, AOFAS, RM score | 12 | PRP was significantly more effective than steroid |
|
| |||||||||
| Yadav et al (2015)47 | Lateral epicondylitis | Single injection 1 mL | Single injection 40 mg (1 mL) steroid | 30/30 | 36.6/36.7 | 10:20/7:23 | VAS, DASH | 3 | All parameters improved more significantly in PRP group at 3 months |
|
| |||||||||
| Khaliq et al. (2015)48 | Lateral epicondylitis | Single injection 3 mL | Single injection 3 mL steroid | 51/51 | 33.6/34.2 | 21:30/24:27 | VAS | 3 weeks | PRP was more effective than steroid |
|
| |||||||||
| Gautam et al. (2015)49 | Lateral epicondylitis | Single injection 2 mL | Single injection 2 mL (40 mg/mL) steroid | 15/15 | NR | NR | VAS, DASH | 6 | PRP was superior to steroid at 6 months |
|
| |||||||||
| Sherpy et al. (2016)50 | Plantar fasciitis | Single injection 3 mL | Single injection 2 mL (40 mg/mL) steroid | 25/25 | 37.5/38.5 | 2:23/0:25 | VAS, FHSQ, US | 3 | No significant difference between groups |
|
| |||||||||
| Palacio et al. (2016)51 | Lateral epicondylitis | Single injection 3 mL | Single injection 3 mL steroid | 20/20 | 46.6/46.2 | NR | DASH, PRTEE | 6 | No significant difference between groups |
|
| |||||||||
| Mahindra et al. (2016)52 | Plantar fasciitis | Single injection 2.5–3 mL | Single injection 2 mL (40 mg) steroid | 25/25 | 30.7/33.9 | 8:17/12:13 | VAS, AOFAS | 3 | Better AOFAS outcome in PRP group at 3 months |
|
| |||||||||
| Vahdatpour et al. (2016)53 | Plantar fasciitis | Single injection 3 mL | Single injection 2 mL steroid | 16/16 | 45.4/47.12 | 4:12/5:11 | VAS, RMS | 6 | Significant improvement with PRP |
|
| |||||||||
| Homayouni et al (2016)54 | Plantar fasciitis | Single injection 2 mL | Single injection 2 mL steroid | 15/15 | 44.7/43.6 | 7:8/6:9 | VAS, FAAM | 2 | No significant differences between groups at 2 months |
|
| |||||||||
| Gogna et al. (2016)55 | Plantar fasciitis | Single injection 3 mL | Low-dose radiation (total 3.0 Gy) | 20/20 | 28.6/26.5 | 14:6/12:8 | VAS, AOFAS, US | 6 | No significant differences between groups |
|
| |||||||||
| Krogh et al. (2016)56 | Achilles tendinopathy | Single injection 6 mL | Single injection 6 mL saline | 12/12 | 46.7/51.8 | 7:5/6:6 | VISA-A | 3 | PRP was not superior to placebo |
|
| |||||||||
| Shams et al. (2016)57 | Rotator cuff tendinopathy | Single injection 2–2.5 mL | Single injection 5 mL (40 mg) steroid | 20/20 | 52/50 | 10:10/11:9 | ASES, VAS, CMS, SST | 6 | No significant difference between groups at 6 months |
|
| |||||||||
| Vahdatpour et al. (2016)58 | Plantar fasciitis | Single injection 3 mL | AWB single injection | 17/17 | 45.5/47.5 | 4:13/5:12 | RMS, US | 3 | No significant differences between groups |
|
| |||||||||
| Nejati et al. (2017)59 | Shoulder impingement syndrome | Single injection 6 mL | Exercise therapy | 22/20 | 52.5/54 | 9:13/6:14 | VAS, ROM, DASH, WORC | 6 | PRP was not superior to exercise therapy |
|
| |||||||||
| Acosta-Olivo et al. (2017)60 | Plantar fasciitis | Single injection 3 mL | Single injection steroid | 16/16 | NR | NR | VAS, FADI, AOFAS | 4 | No significant difference between groups |
|
| |||||||||
| Boesen et al. (2017)61 | Achilles tendinopathy | Four injections | Single injection steroid or saline | 19/38 | 43.1/40.9 | NR | VISA-A, VAS | 6 | Steroid was more effective than PRP at 6 months |
|
| |||||||||
| Shekhar et al. (2017)62 | Plantar fasciitis | Single injection 4 mL | Single injection 4 mL saline | 60/60 | 42.0/46.8 | 24:36/27:33 | VAS, AOFAS Foot Scale, US | 6 | PRP was superior to placebo at 6 months |
|
| |||||||||
| El Mallah et al. (2017)63 | Plantar fasciitis | Single injection 3 mL | Dry needling protocol | 15/15 | 43.0/45.0 | 5:10/4:11 | FFI, US | 3 | PRP was more effective than dry needling at 3 months |
|
| |||||||||
| Tank et al. (2017)64 | Plantar fasciitis | Single injection 3 mL | Single injection steroid | 30/50 | 40.9/37.8 | 11:19/19:31 | VISA, FAAM, US | 6 | PRP was more effective than steroid at 6 months |
PRP: platelet-rich plasma; AWB: autologous whole blood; M: male; F: female; VAS: visual analog scale; PTT: pressure pain threshold; VISA-A: Victorian Institute of Sport Assessment-Achilles; VISA-P: Victorian Institute of Sport Assessment-Patella; US: ultrasound; NR: not reported; WORC: Western Ontario Rotator Cuff Index; SPADI: Shoulder PAIN And Disability Index; DASH: Disability of the Arm, Shoulder and Hand score; PRTEE: Patient-Related Tennis Elbow Evaluation; SF-12: 12 Item Short Form Health Survey; ESWT: Extracorporeal Shock Wave Therapy; FHSQ: Foot Health Status Questionnaire; ROM: range of motion; FFI: Foot Function Index; AOFAS: American Orthopedic Foot and Ankle Society; RM: Roles-Maudsley; EQ-5D: EuroQuol 5-Dimension questionnaire; FADI: Fot and Ankle Disability Index; RMS: Roles and Maudsley score; FAAM: Foot and Ankle Ability Measurement; ADL: activity of daily living; IHOT-33: International Hip Outcome Tool-33; MHHS: Modified Harris Hip Score; ASES: American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form; CMS: Constant-Murley Score; SST: Simple Shoulder Test.
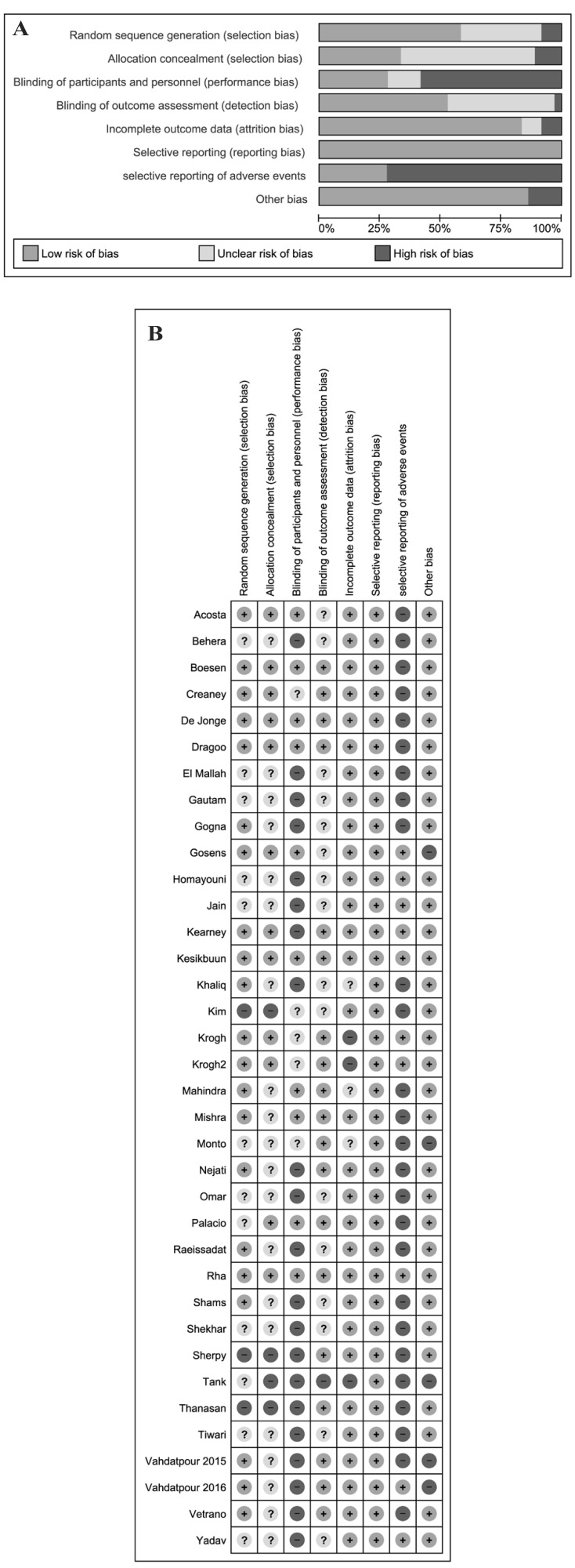
Risk of bias in included studies
Thirty-four studies (94%) were at high risk of bias for one or more domains, and 28 studies (77%) were at unclear risk of bias for one or more domains; 2 studies34,39 were judged at low risk of bias in all the domains (Figure 2A and B).
Figure 2.
A) Risk of bias graph and (B) summary.
PRP: Platelet-rich plasma; SD: standard deviation; IV: intravenous; CI: Confidence Interval.
Allocation
We assessed 3 studies as being at high risk of selection bias, as randomisation was by alternating the 2 treatments, so the intervention allocations could have been foreseen in advance31,43,50. The reports of another 22 studies were unclear as far as random sequence generation and/or allocation concealment were concerned, while 11 studies were at low risk of selection biases.
Blinding
Twenty-one studies (58%) reported as open label, and these were graded as high risk of performance bias (blinding of participants and personnel). Five studies were graded at unclear risk of detection bias due to the fact that they did not provide information to permit judgement about “high” or “low” risk of bias related to the blinding of participants and personnel31,36,42,43,55. Ten studies were reported as double blind28,30,34 39,41,42,51,52,60,61. Nineteen studies were graded at low risk of detection bias due to the fact that the assessor was blinded to treatment allocation; 16 studies were graded at unclear risk of detection bias due to the fact that they did not provide information to permit judgement about “high” or “low” risk of bias related to the blinding of outcome assessors; one study65 was graded at “high risk” of bias.
Incomplete outcome data
Three studies36,55,64 were judged at high risk of attrition bias because there was a high proportion of enrolled subjects who left the study due to unsatisfactory effect of the initial treatment. Another 3 studies43,47,51 were judged at unclear risk of bias. The remaining studies were judged at low risk of bias.
Selective reporting
Selective reporting was low in all included studies for all the outcomes except adverse events. For the outcome adverse events, 26 out of 36 trials (72%) were judged at high risk of bias. The reporting of adverse events was generally inadequate, and 14 trials did not mention complications of treatment at all. Where adverse events were reported, these often consisted of short statements of the absence of adverse events in the study results or discussion without any indication of systematic recording.
Other potential sources of bias
We judged five studies to be at high risk for other sources of bias: four because of unbalance at baseline28,44,53,54 and one64 because it did not provide information on the randomisation process despite enrolling 30 patients in the experimental group and 50 in the control group.
Effects of interventions
For the summary of findings for the main comparison, see Table II, Figures 3 and 4, and Online supplementary content (Figures S1–S10).
Table II.
Platelet rich plasma (PRP) compared with control intervention for tendinopathies: summary of findings§.
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | N. of participants (studies) | Quality of evidence (GRADE) | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Control | PRP | |||||
| PAIN score: Visual Analogue Score (VAS) | Various controls, including local steroids injection | VAS 0–100 (higher scores = worse pain) | ||||
| VAS - short-term follow-up (1–3 months) - Elbow tendinopathy |
Mean VAS score across control groups ranged from 17 to 45.5 in control groups | Mean VAS score in the intervention groups was 2.86 lower (8.57 lower to 2.85 higher) | MD −2.86 (95% CI: −8.57 to 2.85) | 6 studies (328 participants) | ⊕⊖⊖⊖ very low1, 2, 3 |
On average, it is unclear whether or not use of PRP compared to controls reduces pain score at short-term follow up. The between group differences were small and unlikely to be clinically important |
| VAS - medium-term follow up (4–6 months). - Elbow tendinopathy |
Mean VAS score across control groups ranged from 25 to 55.8 in control groups | Mean VAS score in the intervention groups was 12.97 lower (5.34 to 20.61 lower) | MD −12.97 (95% CI: −20.61 to −5.34) | 3 studies (158 participants) | ⊕⊖⊖⊖ very low1,2 |
Marginal clinical benefit of PRP at medium-term follow up. The between group differences were small and unlikely to be clinically important |
| VAS - short-term follow up (1–3 months) - Plantar fasciitis |
Mean VAS score ranged across control groups from 5 to 65 in controls groups | Mean VAS score in the intervention groups was 2.86 lower (8.57 lower to 2.85 higher) | MD −2.86 (95% CI: −8.57 to 2.85) | 8 studies (420 participants) | ⊕⊖⊖⊖ very low 1,2, 3 |
On average, it is unclear whether or not use of PRP compared to controls reduces pain score at short-term follow up. The between group differences were small and unlikely to be clinically important |
| VAS - medium-term follow up (4–6 months) -Plantar fasciitis |
Mean VAS score across control groups ranged from 5 to 48 in controls groups | Mean VAS score in the intervention groups was 7.87 lower (14.9 lower to 0.85 lower) | MD −7.87 (95% CI: −14.9 to −0.85) | 6 studies (300 participants) | ⊕⊖⊖⊖ very low1, 2 |
Marginal clinical benefit of PRP at medium-term follow up. The between group differences were small and unlikely to be clinically important |
| Serious adverse events (0–6 months) - Elbow tendinopathy, plantar fasciitis, Achilles tendinopathy, rotator cuff tendinopathy |
0 events | 0 events | Not estimable | 22 studies (1,265 participants) | ⊕⊖⊖⊖ very low1, 2, 3 |
There were no reports of serious adverse events (e.g. injection site infection, plantar fascia rupture) during the follow-up period (1.5–24 months) of 22 trials |
| Function score: American Orthopedic Foot and Ankle Society Score (AOFAS) | Controls were represented only by local steroids injection | AOFAS 0–100 (higher score=better function) | ||||
| AOFAS - short-term follow up (1–3 months) - Plantar fasciitis |
Mean AOFAS score across control groups ranged from 81 to 96.8 in control groups (steroids) | Mean AOFAS score in the steroids groups was 4.26 higher (3.96 lower to 12.47 higher) than in intervention group | MD 4.26 (95% CI: −3.96 to 12.47) | 4 studies (188 participants) | ⊕⊖⊖⊖ very low1, 2, 3 |
On average, it seems that the use of PRP compared to local steroids injection does not increase function score at short-term follow-up |
| AOFAS - medium-term follow up (4–6 months) - Plantar fasciitis |
Mean VAS score across control groups ranged from 74 to 97.2 in controls groups (steroids) | Mean AOFAS score in the steroids groups was 4.25 higher (5.92 lower to 14.42 higher) than in intervention group | MD 4.25 (95% CI: −5.92 to 14.42) | 5 studies (218 participants) | ⊕⊖⊖⊖ very low1,2, 3 |
On average, it seems that the use of PRP compared to local steroids injection does not increase function score at medium-term follow-up |
Patient/population: adults with elbow tendinopathy or plantar fasciitis; Settings: outpatient; Intervention: PRP; Comparison: various controls, including local steroids injection, placebo, autologous whole blood, anaesthetic.
The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95%CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95%CI). GRADE: Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate.
Down-graded once for inconsistency, due to substantial heterogeneity (I2>80%).
Down-graded twice because of high risk of bias or unclear risk of selection bias, and at high risk of other bias (unbalance at baseline between groups) in several of the selected studies.
Down-graded once for imprecision (95%CI includes line of no effect).
Down-graded once due to serious risk of bias (especially reporting bias) and twice for very serious imprecision (no events). CI: confidence interval; MD: mean difference.
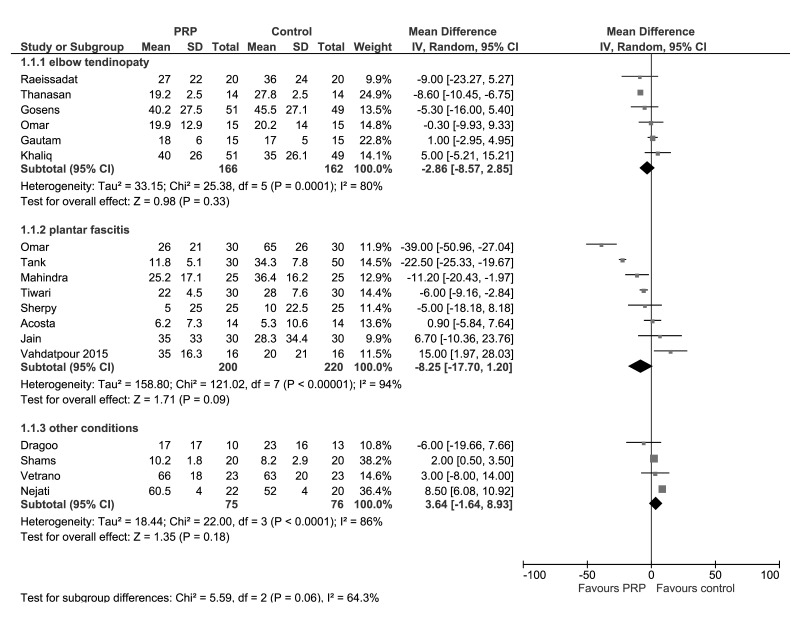
Figure 3.
Forest plot of Visual Analogue Score (VAS) at 3 months..
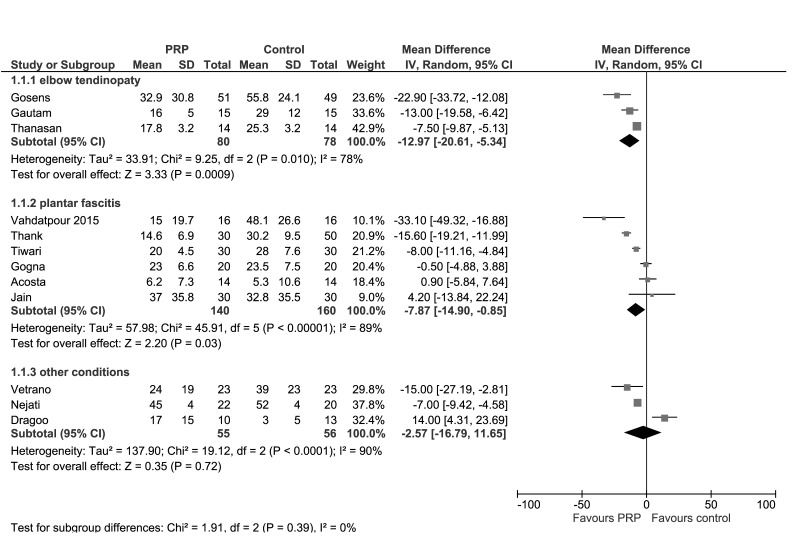
Figure 4.
Forest plot of Visual Analogue Score (VAS) at 6 months.
Lateral epicondylitis
Data from seven studies investigating PRP for lateral epicondylitis reported mean and SD for pain and/or functional measure scales28,31,33,40,48,49,51. The results for VAS at 3 and 6 months in PRP and any control groups are presented in Figures 3 and 4. At 3 months, pooled data from 6 trials (328 patients) showed no clear between group differences in VAS (MD −2.86; 95% CI: −8.57/2.85; I2=80%); very low-quality evidence down-graded for serious risk of bias (particularly selection and performance bias), for inconsistency (due to substantial heterogeneity), and for imprecision (95% CIs include line of no effect). (See summary of findings in Table II). At 6 months, pooled data from 3 trials (158 patients) showed slightly better pain scores of PRP compared to control (MD −12.97; 95% CI: −20.61/−5.34; I2=78%); very low-quality evidence, down-graded twice for serious risk of bias (selection, performance and other bias), once for inconsistency. The results were much the same in subgroup analyses of studies with steroids as control (Online supplementary content, Figures S1 and S2). At 3 months, pooled data from four trials (260 patients) showed no clear between-group differences in VAS (MD 0.67; 95% CI: −2.61/3.95; I2=0%); very low-quality evidence, down-graded for serious risk of biases and serious imprecision. At 6 months, pooled data from 2 trials (130 patients) showed slightly better pain scores of PRP compared to control (MD −16.98; 95% CI: −26.50/−7.47; I2=57%); very low-quality evidence was down-graded (for serious risk of biases and for inconsistency).
Elbow pain was also reported as DASH score in 4 studies (200 patients)28,33,49,51. At 3 months, DASH did not change significantly between groups (Online supplementary content, Figures S3 and S4). At 6 months, PRP showed slightly better pain scores compared to any control (MD −7.53; 95% CI: −9.11/−5.95) and in the subgroup analysis versus steroids (MD −8.17; 95% CI: −10.03/−6.31) (Online supplementary content, Figures S5 and S6). All these comparisons were graded as very low-quality evidence due to serious risk of bias (selection, performance and other bias), imprecision (at 3 months), and inconsistency.
Plantar fasciitis
Data from 15 studies investigating PRP for plantar fasciitis reported mean and SD for pain and/or functional measure scales33,38,43,44,46,50,52–55,58,60,62–64. The results for VAS at 3 months (8 studies, 420 patients) and 6 months (6 studies, 300 patients) in PRP and any control groups are presented in Figures 3 and 4. Pooled data showed slightly better pain scores in PRP treated group at 6 months (MD −7.87; 95% CI: −14.90/−0.85; I2=89%), but not at 3 months (MD −8.25; 95% CI: −17.70/1.20; I2=94%); very low-quality evidence down-graded for serious risk of biases, inconsistency and serious imprecision at 3 months (Table II). Likewise, in subgroup analyses of studies with steroids as control (Online supplementary content, Figures S1 and 2S), pooled data showed slightly better pain scores in PRP treated group both at 6 months (5 trials, 260 patients; MD −9.47; 95% CI: −17.98/−0.97; I2=92%;) but not at 3 months (8 studies, 420 patients; MD −8.95; 95% CI: −17.70/1.20; I2=94%); very low-quality evidence, down-graded for serious risk of biases, inconsistency and serious imprecision at 3 months.
The most commonly reported function measure was AOFAS; all these studies were conducted in plantar fasciitis patients and had local steroids injection as control group. Both at 3 months (4 studies, 178 patients)44,46,52,60 and at 6 months (5 studies, 218 patients)44,46,52,55,60, AOFAS did not change significantly between the PRP and steroids group (MD, 4.26; 95% CI: −5.96/12.47; and 4.25; 95% CI: −5.92/14.42, respectively) (Online supplementary content, Figures S7 and S8). All these comparisons were graded as very low-quality evidence due to risk of bias, imprecision and inconsistency. As shown in Table I, there were other functional measurements included as outcome measures reported in the included studies, e.g. Foot Health Status Questionnaire (FHSQ), Mayo clinic performance index for elbow (MCPIE), maximum grip strength (MGS), and others, but because few (1 or 2) studies reported them, we decided not to conduct a quantitative synthesis for these outcomes. Four studies reported plantar fascia thickness measured by ultrasounds50,55,58,63. The results at 3 months (3 studies, 112 patients) and 6 months (4 studies, 152 patients) showed no clear between-group differences (Online supplementary content, Figures S9 and S10). All these comparisons were graded as very low-quality evidence due to risk of bias and serious imprecision (112 patients from 3 trials).
Other tendinopathies
Data from 4 studies (151 patients) investigating PRP for a variety of tendinopathies (patellar tendinopathy42, jumper’s knee35, subacromial impingement syndrome59, and rotator cuff tears57) reported mean and SD for pain measure scales. The results at 3 and 6 months showed no clear between-group differences in VAS in the cumulative analysis (Figures 3 and 4).
Adverse events
In 22 studies (1,265 participants), no participant was reported to have developed any serious events (e.g. application site infections, heel fat pad atrophy, and plantar fascia rupture) in the follow-up period (from 1.5 to 24 months) in either PRP or control groups. Most trials did not describe monitoring processes for identifying or recording complications; and usually limited the reporting to a single statement regarding their absence. This comparison was graded as very low-quality evidence, and was down-graded once due to serious risk of bias (especially reporting bias) and twice for very serious imprecision (no events), reflecting the fact that numbers were not sufficient to detect rare events.
Other less serious, short-term adverse events, mostly post-injection pain, were reported in 6 trials. In one study, comparing PRP to dextrose prolotherapy for the treatment of chronic recalcitrant plantar fasciitis44, it was reported that most patients in both groups reported local pain or discomfort that started on the day of injection and subsided gradually afterwards. Likewise, an initial worsening of pain because of the activation of the inflammation cycle was observed in patients with lateral epicondylitis receiving PRP28; this usually lasted 1–2 weeks. Local pain or discomfort were also reported from another trial in most of the patients receiving PRP for lateral epicondylitis31. Pain at the site of injection was also reported in a small proportion of patients receiving PRP or controls in 3 studies35,37,41.
Discussion
Since its first development in the 1980s, PRP therapy has been gaining popularity, and orthopaedics immediately seemed to be the ideal sector in which to test the regenerative potential of this technology3. Since then, PRP has been used in the clinic to promote healing in a wide array of musculoskeletal disorders18. However, in spite of this extensive experience, relatively few studies have been conducted on the use of PRP as conservative treatment in orthopaedics. A recently published meta-analysis which evaluated the clinical impact of PRP on tendinopathy compared to placebo or dry needling injections did not demonstrate a significantly greater clinical benefit for PRP at a 6-month follow-up65. In addition, a 2014 meta-analysis found no evidence that PRP was effective in chronic lateral epicondylitis66.
In the present meta-analysis, the largest published so far on this issue, we found a very low quality of evidence that PRP injection may not result in lower pain and function scores in the short- (1–3 months) and medium- (4–6 months) term follow-up, although a marginal benefit at medium-term follow-up (4–6 months) for the VAS outcome was observed. In most of the comparisons, the 95% CI crossed the line of no benefit, and at best indicates the possibility of a very marginal clinical benefit. Difference in pain is a measure often derived from a 100 mm VAS. The minimal clinically important difference (MCID) between pre- and post-intervention is taken as 8 mm for average pain and 19 mm for first step pain67. Our findings show that the mean VAS score in the PRP group was 2.86 mm lower than in the control group on short-term follow-up, and 12.97 mm lower at medium-term follow-up in lateral epicondylitis, and 8.25 mm lower at short-term follow-up, and 7.87 mm lower at medium-term follow-up in plantar fasciitis; these are differences that can be regarded as clinically marginal.
The quantitative analysis conducted in this systematic review has, however, several limitations which do not allow us to draw definite conclusions on the PRP efficacy in this setting. The first limitation is certainly the heterogeneity of the studies evaluated, particularly in the efficacy outcomes. Another important limitation of this meta-analysis is that we were not able to determine the long-term (>12 months) effect of PRP due to the lack of enough time points in the studies evaluated. It is indeed possible, as claimed by some investigators68, that the best clinical benefit of PRP application in orthopaedics can occur in the long-term period. Finally, we would like to outline the lack of standardisation for PRP production among the different studies, which makes the PRP products heterogeneous and qualitatively very different from each other, and this limits the validity of an inter-studies comparison.
Further, adequately powered, randomised trials are needed to better define potential indications, long-term benefit, and optimal treatment protocols of PRP as conservative treatment in orthopaedics. These studies should also perform an adequate cost-benefit analysis of PRP therapy compared with other standard, less expensive, treatments.
Supplementary Information
Footnotes
Disclosure of conflicts of interest
GML is the Editor-in-Chief of Blood Transfusion and this manuscript has undergone additional external review as a result. The other Authors declare no conflicts of interest.
References
- 1.Hsu WK, Mishra A, Rodeo SR, et al. Platelet-rich plasma in orthopaedic applications: evidence-based recommendations for treatment. J Am Acad Orthop Surg. 2013;21:739–48. doi: 10.5435/JAAOS-21-12-739. [DOI] [PubMed] [Google Scholar]
- 2.Wasterlain AS, Braun HJ, Harris AH, Kim HJ, Dragoo JL. The systemic effects of platelet-rich plasma injection. Am J Sports Med. 2013;41:186–93. doi: 10.1177/0363546512466383. [DOI] [PubMed] [Google Scholar]
- 3.Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62:489–96. doi: 10.1016/j.joms.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Hussain N, Johal H, Bhandari M. An evidence-based evaluation on the use of platelet rich plasma in orthopedics - a review of the literature. SICOT J. 2017;3:57. doi: 10.1051/sicotj/2017036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez CE, Smith PC, Palma Alvarado VA. The influence of platelet-derived products on angiogenesis and tissue repair: a concise update. Fron Physiol. 2015;6:290. doi: 10.3389/fphys.2015.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foster TE, Puskas BL, Mandelbaum BR, et al. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37:2259–72. doi: 10.1177/0363546509349921. [DOI] [PubMed] [Google Scholar]
- 7.Ferri AL, Ceserani V, Greppi N, et al. Osteogenic differentiation of adipose tissue-derived mesenchymal stem cells cultured on a scaffold made of silk fibroin and cord blood platelet gel. Blood Transfus. 2016;14:206–11. doi: 10.2450/2016.0209-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rebulla P, Pupella S, Santodirocco M, et al. Italian Cord Blood Platelet Gel Study Group. Multicentre standardisation of a clinical grade procedure for the preparation of allogeneic platelet concentrates from umbilical cord blood. Blood Transfus. 2016;14:73–9. doi: 10.2450/2015.0122-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piccin A, Di Pierro AM, Canzian L, et al. Platelet gel: a new therapeutic tool with great potential. Blood Transfus. 2017;15:333–40. doi: 10.2450/2016.0038-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirmani BH, Jones SG, Datta S, et al. A meta-analysis of platelet gel for prevention of sternal wound infections following cardiac surgery. Blood Transfus. 2017;15:57–65. doi: 10.2450/2016.0231-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez-Zapata MJ, Orozco L, Balius R, et al. PRP-RICE group. Efficacy of autologous platelet-rich plasma for the treatment of muscle rupture with haematoma: a multicentre, randomised, double-blind, placebo-controlled clinical trial. Blood Transfus. 2016;14:245–54. doi: 10.2450/2015.0099-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andia I, Latorre PM, Gomez MC, Burgos-Alonso N, et al. Platelet-rich plasma in the conservative treatment of painful tendinopathy: a systematic review and meta-analysis of controlled studies. Br Med Bull. 2014;110:99–115. doi: 10.1093/bmb/ldu007. [DOI] [PubMed] [Google Scholar]
- 13.Sheth U, Simunovic N, Klein G, et al. Efficacy of autologous platelet rich plasma use for orthopaedic indications: a meta-analysis. J Bone Joint Surg Am. 2012;94:298–307. doi: 10.2106/JBJS.K.00154. [DOI] [PubMed] [Google Scholar]
- 14.Yuan T, Zhang CQ, Wang JH. Augmenting tendon and ligament repair with platelet-rich plasma (PRP) Muscles Ligaments Tendons J. 2013;3:139–49. [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao JG, Zhao L, Jiang Y-X, et al. Platelet rich plasma in arthroscopic rotator cuff repair: a meta-analysis of randomized controlled trials. Arthroscopy. 2015;31:125–135. doi: 10.1016/j.arthro.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Chen X, Jones IA, Park C, Vangsness CT., Jr The efficacy of platelet-rich plasma on tendon and ligament healing: a systematic review and meta-analysis with bias assessment. Am J Sports Med. 2018;46:2020–32. doi: 10.1177/0363546517743746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohn CS, Lockhart E. Autologous platelet-rich plasma: evidence for clinical use. Curr Opin Hematol. 2015;22:527–32. doi: 10.1097/MOH.0000000000000183. [DOI] [PubMed] [Google Scholar]
- 18.Cohn CS, Lockhart E, McCullough JJ. The use of autologous platelet-rich plasma in the orthopedic setting. Transfusion. 2015;55:1812–20. doi: 10.1111/trf.13005. [DOI] [PubMed] [Google Scholar]
- 19.Fitzpatrick J, Bulsara M, Zheng MH. The effectiveness of platelet-rich plasma in the treatment of tendinopathy: a meta-analysis of randomized controlled clinical trials. Am J Sports Med. 2017;45:226–33. doi: 10.1177/0363546516643716. [DOI] [PubMed] [Google Scholar]
- 20.Taylor DW, Petrera M, Hendry M, Theodoropoulos JS. A systematic review of the use of platelet-rich plasma in sports medicine as a new treatment for tendon and ligament injuries. Clin J Sport Med. 2011;21:344–52. doi: 10.1097/JSM.0b013e31821d0f65. [DOI] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions - Version 5.1.0. The Cochrane Collaboration; [Accessed on: 31/05/2018]. [updated March 2011] Available at: http://www.cochranehandbook.org. [Google Scholar]
- 24.Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings’ tables. In: Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. [Accessed on: 31/05/2018]. (updated March 2011) Available at: www.handbook.cochrane.org. [Google Scholar]
- 25.Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. [Accessed on: 31/05/2018]. (updated March 2011) Available at: www.handbook.cochrane.org. [Google Scholar]
- 26.Guyatt GH, Oxman AD, Kunz R, et al. What is ‘quality of evidence’ and why is it important to clinicians? BMJ. 2008;336:995–8. doi: 10.1136/bmj.39490.551019.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peerbooms JC, Sluimer J, Bruijn DJ, Gosens T. Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: Platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med. 2010;38:255–62. doi: 10.1177/0363546509355445. [DOI] [PubMed] [Google Scholar]
- 28.Gosens T, Peerbooms JC, van Laar W, Oudsten den BL. Ongoing positive effect of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with 2-year follow-up. Am J Sports Med. 2011;39:1200–8. doi: 10.1177/0363546510397173. [DOI] [PubMed] [Google Scholar]
- 29.de Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: A randomized controlled trial. JAMA. 2010;303:144–9. doi: 10.1001/jama.2009.1986. [DOI] [PubMed] [Google Scholar]
- 30.de Jonge S, de Vos RJ, Weir A, et al. One-year follow-up of platelet-rich plasma treatment in chronic Achilles tendinopathy: a double-blind randomized placebo-controlled trial. Am J Sports Med. 2011;39:1623–9. doi: 10.1177/0363546511404877. [DOI] [PubMed] [Google Scholar]
- 31.Thanasas C, Papadimitriou G, Charalambidis C, et al. Platelet-rich plasma versus autologous whole blood for the treatment of chronic lateral elbow epicondylitis: a randomized controlled clinical trial. Am J Sports Med. 2011;39:2130–4. doi: 10.1177/0363546511417113. [DOI] [PubMed] [Google Scholar]
- 32.Creaney L, Wallace A, Curtis M, Connell D. Growth factor-based therapies provide additional benefit beyond physical therapy in resistant elbow tendinopathy: a prospective, single-blind, randomised trial of autologous blood injections versus platelet-rich plasma injections. Br J Sports Med. 2011;45:966–71. doi: 10.1136/bjsm.2010.082503. [DOI] [PubMed] [Google Scholar]
- 33.Omar AS, Ibrahim ME, Ahmed AS, et al. Local injection of autologous platelet rich plasma and corticosteroid in treatment of lateral epicondylitis and plantar fasciitis: Randomized clinical trial. Egypt Rheumatol. 2012;34:43–9. [Google Scholar]
- 34.Kesikburun S, Tan AK, Yilmaz B, et al. Platelet-rich plasma injections in the treatment of chronic rotator cuff tendinopathy: a randomized controlled trial with 1-year follow-up. Am J Sports Med. 2013;41:2609–16. doi: 10.1177/0363546513496542. [DOI] [PubMed] [Google Scholar]
- 35.Vetrano M, Castorina A, Vulpiani MC, et al. Platelet-rich plasma versus focused shock waves in the treatment of jumper’s knee in athletes. Am J Sports Med. 2013;41:795–803. doi: 10.1177/0363546513475345. [DOI] [PubMed] [Google Scholar]
- 36.Kearney RS, Parsons N, Costa ML. Achilles tendinopathy management: A pilot randomised controlled trial comparing platelet-richplasma injection with an eccentric loading programme. Bone Joint Res. 2013;2:227–32. doi: 10.1302/2046-3758.210.2000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krogh TP, Fredberg U, Stengaard-Pedersen K, et al. Treatment of lateral epicondylitis with platelet-rich plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. Am J Sports Med. 2013;41:625–35. doi: 10.1177/0363546512472975. [DOI] [PubMed] [Google Scholar]
- 38.Tiwari M, Bhargava R. Platelet rich plasma therapy: a comparative effective therapy with promising results in plantar fasciitis. J Clin Orthop Trauma. 2013;4:31–5. doi: 10.1016/j.jcot.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rha DW, Park GY, Kim YK, et al. Comparison of the therapeutic effects of ultrasound-guided platelet-rich plasma injection and dry needling in rotator cuff disease: a randomized controlled trial. Clin Rehabil. 2013;27:113–22. doi: 10.1177/0269215512448388. [DOI] [PubMed] [Google Scholar]
- 40.Raeissadat SA, Rayegani SM, Hassanabadi H, et al. Is platelet-rich plasma superior to whole blood in the management of chronic tennis elbow: one year randomized clinical trial. BMC Sports Sci Med Rehabil. 2014;6:12. doi: 10.1186/2052-1847-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mishra AK, Skrepnik NV, Edwards SG, et al. Efficacy of platelet-rich plasma for chronic tennis elbow: a double-blind, prospective, multicenter, randomized controlled trial of 230 patients. Am J Sports Med. 2014;42:463–71. doi: 10.1177/0363546513494359. [DOI] [PubMed] [Google Scholar]
- 42.Dragoo JL, Wasterlain AS, Braun HJ, Nead KT. Platelet-rich plasma as a treatment for patellar tendinopathy: a double-blind, randomized controlled trial. Am J Sports Med. 2014;42:610–8. doi: 10.1177/0363546513518416. [DOI] [PubMed] [Google Scholar]
- 43.Kim E, Lee JH. Autologous platelet-rich plasma versus dextrose prolotherapy for the treatment of chronic recalcitrant plantar fasciitis. PM R. 2014;6:152–8. doi: 10.1016/j.pmrj.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 44.Monto RR. Platelet-rich plasma efficacy versus corticosteroid injection treatment for chronic severe plantar fasciitis. Foot Ankle Int. 2014;35:313–8. doi: 10.1177/1071100713519778. [DOI] [PubMed] [Google Scholar]
- 45.Behera P, Dhillon M, Aggarwal S, et al. Leukocyte-poor platelet-rich plasma versus bupivacaine for recalcitrant lateral epicondylar tendinopathy. J Orthop Surg (Hong Kong) 2015;23:6–10. doi: 10.1177/230949901502300102. [DOI] [PubMed] [Google Scholar]
- 46.Jain K, Murphy PN, Clough TM. Platelet rich plasma versus corticosteroid injection for plantar fasciitis: A comparative study. Foot (Edinb) 2015;25:235–7. doi: 10.1016/j.foot.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 47.Yadav R, Kothari SY, Borah D. Comparison of local injection of platelet rich plasma and corticosteroids in the treatment of lateral epicondylitis of humerus. J Clin Diagn Res. 2015;9:RC05–7. doi: 10.7860/JCDR/2015/14087.6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khaliq A, Khan I, Inam M, et al. Effectiveness of platelets rich plasma versus corticosteroids in lateral epicondylitis. J Pak Med Assoc. 2015;65(11 Suppl 3):S100–4. [PubMed] [Google Scholar]
- 49.Gautam VK, Verma S, Batra S, et al. Platelet-rich plasma versus corticosteroid injection for recalcitrant lateral epicondylitis: clinical and ultrasonographic evaluation. J Orthop Surg (Hong Kong) 2015;23:1–5. doi: 10.1177/230949901502300101. [DOI] [PubMed] [Google Scholar]
- 50.Sherpy NA, Hammad MA, Hagrass HA, et al. Local injection of autologous platelet rich plasma compared to corticosteroid treatment of chronic plantar fasciitis patients: A clinical and ultrasonographic follow-up study. Egypt Rheumatol. 2016;38:247–52. [Google Scholar]
- 51.Palacio EP, Schiavetti RR, Kanematsu M, et al. Effects of platelet-rich plasma on lateral epicondylitis of the elbow: prospective randomized controlled trial. Rev Bras Ortop. 2016;51:90–5. doi: 10.1016/j.rboe.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mahindra P, Yamin M, Selhi HS, et al. Chronic plantar fasciitis: effect of platelet-rich plasma, corticosteroid, and placebo. Orthopedics. 2016;39:e285–9. doi: 10.3928/01477447-20160222-01. [DOI] [PubMed] [Google Scholar]
- 53.Vahdatpour B, Kianimehr L, Moradi A, et al. Beneficial effects of platelet rich plasma on improvement of pain severity and physical disability in patients with plantar fasciitis: a randomized trial. Adv Biomed Res. 2015;5:179. doi: 10.4103/2277-9175.192731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Homayouni K, Karimian H, Golkar HR, Jalati N. Treatment of chronic plantar fasciitis with ultrasound-guided injection of platelet-rich plasma. J Arch Mild Med. 2016;4:e42332. [Google Scholar]
- 55.Gogna P, Gaba S, Mukhopadhyay R, et al. Plantar fasciitis: A randomized comparative study of platelet rich plasma and low dose radiation in sportspersons. Foot (Edinb) 2016;28:16–9. doi: 10.1016/j.foot.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 56.Krogh TP, Ellingsen T, Christensen R, et al. Ultrasound-guided injection therapy of Achilles tendinopathy with platelet-rich plasma or saline: a randomized, blinded, placebo-controlled trial. Am J Sports Med. 2016;44:1990–7. doi: 10.1177/0363546516647958. [DOI] [PubMed] [Google Scholar]
- 57.Shams A, El-Sayed M, Gamal O, Ewes W. Subacromial injection of autologous platelet-rich plasma versus corticosteroid for the treatment of symptomatic partial rotator cuff tears. Eur J Orthop Surg Traumatol. 2016;26:837–842. doi: 10.1007/s00590-016-1826-3. [DOI] [PubMed] [Google Scholar]
- 58.Vahdatpour B, Kianimehr L, Ahrar MH. Autologous platelet-rich plasma compared with whole blood for the treatment of chronic plantar fasciitis; a comparative clinical trial. Adv Biomed Res. 2016;5:84. doi: 10.4103/2277-9175.182215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nejati P, Ghahremaninia A, Naderi F, et al. Treatment of subacromial impingement syndrome: platelet-rich plasma or exercise therapy? A randomized controlled trial. Orthop J Sports Med. 2017;5 doi: 10.1177/2325967117702366. 2325967117702366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Acosta-Olivo C, Elizondo-Rodriguez J, Lopez-Cavazos R, et al. Plantar fasciitis. A comparison of treatment with intralesional steroids versus platelet-rich plasma (PRP). A randomized, blinded study. J Am Podiatr Med Assoc. 2017;107:490–6. doi: 10.7547/15-125. [DOI] [PubMed] [Google Scholar]
- 61.Boesen AP, Hansen R, Boesen MI, et al. Effect of high-volume injection, platelet-rich plasma, and sham treatment in chronic midportion Achilles tendinopathy: a randomized double-blinded prospective study. Am J Sports Med. 2017;45:2034–43. doi: 10.1177/0363546517702862. [DOI] [PubMed] [Google Scholar]
- 62.Shekhar C, Jhan A, Singh G, et al. Role of leukocyte free platelet rich plasma in planter fasciitis: a prospective study. Int J Res Med Sci. 2017;5:5432–9. [Google Scholar]
- 63.El Mallah R, Elattar EA, Zidan HF. Platelet-rich plasma versus dry needling of myofascial meridian trigger points in the treatment of plantar fasciitis. Egypt Rheumatol Rehabil. 2017;44:58–68. [Google Scholar]
- 64.Tank G, Gupta R, Gupta A, Rohila R. Comaparative study of platelet-rich plasma and corticosteroid injection in the treatment of plantar fasciitis. J Foot Ankle Surg. 2017;4:84–9. [Google Scholar]
- 65.Tsikopoulos K, Tsikopoulos I, Simeonidis E, et al. The clinical impact of platelet-rich plasma on tendinopathy compared to placebo or dry needling injections: A meta-analysis. Phys Ther Sport. 2016;17:87–94. doi: 10.1016/j.ptsp.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 66.de Vos RJ, Windt J, Weir A. Strong evidence against platelet-rich plasma injections for chronic lateral epicondylar tendinopathy: a systematic review. Br J Sports Med. 2014;48:952–6. doi: 10.1136/bjsports-2013-093281. [DOI] [PubMed] [Google Scholar]
- 67.Landorf KB, Radford JA, Hudson S. Minimal important difference (MID) of two commonly used outcome measures for foot problems. J Foot Ankle Res. 2010;3:7. doi: 10.1186/1757-1146-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miller LE, Parrish WR, Roides B, Bhattacharyya S. Efficacy of platelet-rich plasma injections for symptomatic tendinopathy: systematic review and meta-analysis of randomised injection-controlled trials. BMJ Open Sport Exerc Med. 2017;3:e000237. doi: 10.1136/bmjsem-2017-000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.