Abstract
Bone loss is a common complication in individuals with sickle cell disease (SCD). The mechanism(s) of bone loss in SCD subjects has not been fully investigated, and there are no targeted therapies to prevent or treat compromised bone health in this population. Recent studies showed that depletion of gut microbiota with antibiotics significantly reduced the number of aged neutrophils, thereby dramatically improved the inflammation-related organ damages in SCD mice. Since neutrophils, abundantly present in bone marrow (BM), regulate bone cells, and BM neutrophils, induced by inflammatory cytokines, are associated with a low number of osteoblasts (OBs), we hypothesize that neutrophil aging in the BM of SCD mice impairs OB function. Flow cytometry analysis showed BM neutrophil aging was significantly increased in SCD mice that was reduced with antibiotic treatment. In vitro co-culture of calvarial OBs from control (Ctrl) mice with BM neutrophils from Ctrl or SCD mice showed that BM neutrophils from SCD mice inhibit OB function but was rescued when neutrophils were from antibiotic-treated SCD mice. In summary, there is an accumulation of aged neutrophils in BM from SCD mice that may contribute to impaired OB function, and antibiotic treatment is able to partially rescue impaired OB function by decreasing neutrophil aging in the BM of SCD mice.
Keywords: Sickle cell disease, Bone marrow, Neutrophil aging, Osteoblasts, Antibiotics
Highlights
-
•
There is increased neutrophil ageing in bone marrow of sickle cell disease mice.
-
•
Bone marrow neutrophils in sickle cell disease mice impair osteoblast function.
-
•
Antibiotics rescue the osteoblast dysfunctions by decreasing aged neutrophils.
1. Introduction
Sickle cell disease (SCD) is the most common genetic disorder worldwide. Osteoporosis and osteopenia are common bone complications in both children and adults with SCD [1], Eighty percent of adults with SCD have low BMD and develop osteopenia and osteoporosis that predispose them to increased fractures and vertebral collapse and bone pain [2]. Adults with SCD have low bone mineral density (BMD) that is independent of usual risk factors such as age, gender and menopausal status. This suggests the etiology of bone loss in SCD differs from the general population. However, the mechanism(s) of bone loss in SCD subjects has not been fully investigated, and there are no targeted therapies to prevent or treat compromised bone health in this population.
Studies showed that SCD patients have higher numbers of baseline-activated neutrophils compared to healthy controls [3], [4]. Leukocytosis, in the absence of infection, is common in SCD patients and predicts for overall mortality [5]. SCD patients receiving penicillin for prophylaxis have fewer circulating activated neutrophils [6]. Recent studies showed an increased number of circulating aged neutrophils, a subset of neutrophils that is regulated by the intestinal microbiota and is overly activated in SCD mice [6]. Treatment of SCD mice with antibiotics (Abxs) significantly reduced the number of circulating aged neutrophil and dramatically improved the inflammation-related liver and spleen damages in SCD mice [6]. Neutrophils are abundantly present in bone marrow (BM), where aged neutrophils home for clearance [8], [9]. They can also adhere to bone cells [7] and regulate bone cells. BM neutrophilia induced by inflammatory cytokine, interleukin-6 (IL-6), was shown to be associated with a low number of osteoblast (OBs) [10], [11]. Recently, using SCD mouse models, we published that there were reduced OB terminal differentiation marker genes that could contribute to reduced bone formation in SCD mice [12]. In the current study, we wanted to use this SCD murine model to examine whether there was accumulation of aged neutrophils in BM from SCD mice and whether BM neutrophil aging contributes to impaired OB function in SCD mice.
In this study we treated control (Ctrl) and SCD mice with either H2O or Abx (to inhibit neutrophil aging by depleting gut microbiota) and analyzed the BM aged neutrophils. We co-cultured calvarial OBs from Ctrl mice with BM neutrophils isolated from Ctrl and SCD mice treated with H2O or Abx to examine their effect on OB functions. We demonstrated significantly increased aged neutrophils in BMs from SCD mice that were associated with impaired OB function in co-cultures. Treatment of SCD mice with Abx significantly reduced the number of BM aged neutrophils, and dramatically improved the OB function defect observed in OBs co-cultured with BM neutrophils from H2O-treated SCD mice.
2. Materials and methods
2.1. Animals
Townes sickle cell mice [13] on a mixed C57BL/6 and 129 genetic backgrounds were purchased from Jackson Laboratory (Stock number: 013071, Bar Harbor, Maine, USA). Sickle cell trait (SCT) x SCT breeding pairs are housed in the Center for Comparative Medicine at the UConn Health to generate Ctrl and SCD littermates used in this study. The UConn Health Institutional Animal Care and Use Committee approved all animal protocols.
2.2. Abx treatment
Mice were housed by genotype after weaning. At 4 months of age Ctrl and SCD male mice were singly housed to avoid cage effects and were randomly assigned to H2O or Abx treatment groups. Abx treatment protocol was based on the Nature paper by Dr. Frenette's group that has been shown to decrease neutrophil aging in blood by depleting gut microbiota [6]. For Abx treatment, mice were treated with ampicillin (1 g/L), neomycin (1 g/L), metronidazole (1 g/L) and vancomycin (1 g/L) in drinking water. Drinking water containing Abx was changed every 3–4 days. Mice were treated for 2 weeks and euthanized utilizing the approved CO2 method for BM neutrophil analysis and isolation. Abxs were purchased from Sigma (St. Louis, MO).
2.3. Flow cytometry analysis
Flow cytometry analysis was performed on BM cellularity and leukocyte subset analysis from 4-month-old Ctrl and SCD male mice treated with H2O or Abx. BMs were surface-stained in staining buffer consisting of PBS supplemented with 1% BSA for 20 min on ice, fixed in 2% PFA for 5 min, then kept in staining buffer for flow cytometry analysis. Multiparametric flow cytometric analyses were performed on an LSRII (BD Biosciences, San Jose, CA). Neutrophils were gated by Gr-1hi CD115lo SSChi; T cells, B cells and monocyte were gated by CD3+, B220 + and CD115hi, aged neutrophils were gated by CD62Llo CXCR4hi within the neutrophil population [6], [14]. Unstained BMs were used as gating control. The following fluorophore-conjugated antibodies were used: FITC anti-mouse CD62L, Pacific Blue anti-mouse Ly-6 G/Ly-6C (Gr-1), PE anti-mouse CD115 (CSF-1R), APC anti-mouse CD184 (CXCR4), APC anti-mouse CD3, FITC anti-mouse B220 were from BioLegend (San Diego, CA).
2.4. Ex vivo BM neutrophil senescence assay
To confirm BM neutrophil aging in SCD mice, we performed an ex vivo cellular aging assay using a 96-Well Cellular Senescence Assay Kit (CBA-231, Cell Biolabs). BM neutrophils from genotype-housed 5-month-old Ctrl and SCD female mice were isolated with MajoSort Mouse Neutrophil Isolation Kit (BioLegend) following manufacture's protocol and cultured for 2 h. Then the cells were lysed, and then the cell lysate was incubated with senescence-associated beta-galactosidase (SA-βGal) at 37||C for 1 h. The fluorescence signal was detected in a TECAN multiplate reader at excitation 360 nm and emission 465 nm. The SA-βGal activity was normalized to total protein concentration.
2.5. Co-culture of calvarial OBs with BM neutrophils from Ctrl and SCD mice
Co-culture of calvarial OBs from Ctrl mice and BM neutrophils from Ctrl and SCD mice treated with H2O or Abx were used to assess the direct effect of neutrophils on OB functions. Calvarial OBs were isolated from 3-day-old Ctrl mice. Briefly, neonatal mice were sacrificed utilizing the approved decapitation method of euthanasia. Calvarias were digested five times with collagenase type 2 (250 U/ml) and trypsin (0.05%) plus EDTA (0.02%) in the PBS [15]. The cells released from digests 2–5 were collected as primary calvarial OB plated on a 6-well plate in proliferation media consisting of alpha modified eagles media (αMEM) supplemented with 10% heat inactivated bovine serum (HIFBS), 100 U/ml penicillin-streptomycin (P/S). BM neutrophils from Ctrl and SCD male mice treated with H2O or Abx were isolated from BM using MajoSort Mouse Neutrophil Isolation Kit (BioLegend), then fixed in 2% PFA for 5 min. After thorough washing, fixed neutrophils at 6 × 106 cell/well were plated on top of confluence OBs in osteogenic medium consisting of αMEM supplemented with 10% HIFBS, 100 U/ml P/S, 50 μM ascorbic acid, 4 mM β-glycerophosphate. Media were changed every other day without disturbing neutrophil cell layers. At 7 days of co-culture, after vigorous washing with PBS, OBs were solubilized with 1% Triton X-100 in 0.9% NaCl, and assayed for ALP activity [16]. Briefly, 130ul of Alkaline Phosphatase Yellow Liquid Substrate (Sigma) was combined with 5 μg protein samples, then the kinetics of p-nitrophenol formation were followed for 30 min at 405 nm at 37 °C. At 14 days of co-culture, OBs were fixed, then Alizarin Red was added for 20 min. After washing with H2O, the dishes were scanned, and then Alizarin Red was extracted by incubating cells with 20% methanol and 10% acetic acid in water. After 15 min, liquid was transferred to a 96-well plate and the quantity of Alizarin Red was read on the spectrophotometer at a wavelength of 450 nm [17]. Parallel dishes were used for RNA extraction.
For transwell study, calvarial OBs were plated on the bottom of 6.5-mm Transwell (Corning, Tewksbury, MA). Purified then fixed BM neutrophils from 4-month-old male Ctrl and SCD mice were plated on 0.4 µm pore polycarbonate membrane inserts. Cells were cultured in osteogenic media. ALP activity of OBs cultured on the bottom of the Transwell was measured on day 7 of culture.
2.6. RNA isolation and quantitative real-time PCR (qPCR) analysis
Total RNA was extracted from cultured cells using Trizol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA). For quantitative reverse transcription real-time polymerase chain reaction analysis, RNA was reverse-transcribed by the Super-Script™ First-Strand Synthesis System. qPCR was carried out using the QuantiTect™ SYBR Green PCR kit (Qiagen) on a MyiQ™ instrument (BIO-RAD Laboratories Inc. Hercules, CA). β-actin was used as an internal reference for each sample. mRNA was normalized to the β-actin mRNA level and expressed as the fold-change relative to the first sample for each experimental group. Relative mRNA expression was calculated using a formula reported previously [18]. Mouse specific primers used were as follows: β-actin forward: 5′-ATCTGGCACCACCCTTCTACAA-3′, β-actin reverse: 5′-ATG GCT GGG GTG TTG AAG GT-3′; osteocalcin (Ocn) forward: 5′-GAG GGC AAT AAG GTA GTG AAC AGA-3′, Ocn reverse: 5′-AAG CCA TAC TGG TTT GAT AGC TCG-3′; Il1β forward: 5′-GCAACTGTTCCTGAACTCAACT-3′, Il1β reverse: 5′-ATCTTTTGGGGTCCGTCAACT-3′.
2.7. Bacterial genomic DNA extraction from feces and real-time PCR
After 2 weeks of treatment, fecal samples were collected. Bacterial genomic DNA was extracted from 100 mg of fecal material, using the GenElute Stool DNA Isolation Kit (Sigma-Aldrich) according to the manufacturer's instructions. The abundance of commensal bacteria was quantified by real-time PCR, using the universally conserved 16 S rRNA primer pair, eubacteria forward: ACTCCTACGGGAGGCAGCAGT, eubacteria reverse: ATTACCGCGGCTGCTGGC.
2.8. Statistics
Experimental values are reported as mean ± standard error (SE). ANOVA followed by Least Significant Difference (LSD) for Post Hoc Multiple Comparisons or T-test was used. SPSS software was used for statistical analysis, and the results were considered significantly different at p < 0.05.
3. Results
3.1. Abx treatment rescued increased cellularity and neutrophils in BM of SCD mice
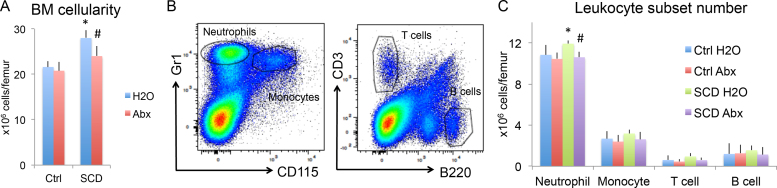
Since studies showed that depletion of gut microbiota with broad spectrum Abx led to selective reduction of neutrophil numbers in circulation of SCD mice [6], we examined for similar changes in BM from 4-month-old Ctrl and SCD male mice treated with H2O or Abx. As shown in Fig. 1, BM cellularity (Fig. 1A) and neutrophil number in BM (Fig. 1B&C) were increased in SCD-H2O group compared with Ctrl-H2O. Abx treatment partially rescued the increased BM cellularity and neutrophil number observed in SCD mice. There were no significant differences in numbers of monocytes, T cells or B cells between groups, suggesting Abx treatment selectively reduces neutrophils in BM of SCD mice.
Fig. 1.
Flow cytometry analysis of BM cellularity and leukocyte subsets from Ctrl and SCD mice treated with H2O or Abx. Male mice at 4 months of age were singly housed and treated with H2O or Abx for 2 weeks. BMs were collected from femur. Flow cytometry analysis of (A) BM cellularity. (B) Representative flow cytometry images showing gating for neutrophils, monocytes, T cells, and B cells. (C) Neutrophil, monocyte, T cell, and B cell counts in BM. n = 6 mice/group. * : Compared with Ctrl-H2O group p < 0.05; #: compared with corresponding H2O group p < 0.05 by two-way ANOVA.
3.2. Abx treatment rescued increased aged neutrophils in BM from SCD mice
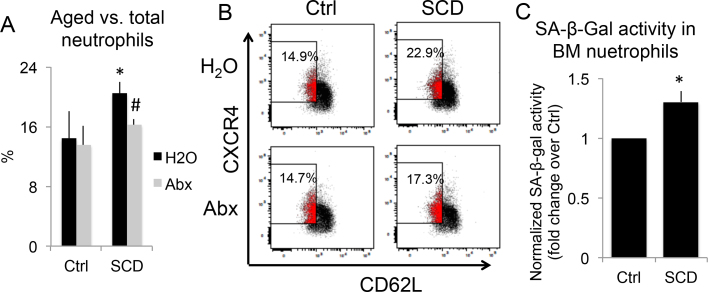
To examine possible aged neutrophil accumulation in the BM of SCD mice and to examine the effect of microbiota depletion with Abx on BM neutrophil aging, we performed flow cytometry on BM neutrophils from 4-month-old Ctrl and SCD male mice treated with H2O or Abx. As shown in Fig. 2A&B, the percentage of aged neutrophils in total neutrophils was significantly increased in SCD-H2O group compared with Ctrl-H2O group, which was rescued with Abx treatment.
Fig. 2.
Evaluation of BM neutrophil aging in Ctrl and SCD mice treated with H2O or Abx. (A&B) Single-housed 4-month-old male mice were treated with H2O or Abx for 2 weeks. BMs were stained for flow cytometry analysis. (A) Percentage of aged neutrophils vs. total neutrophils in BMs. n = 3 mice/group. (B) Representative flow cytometry image of BM aged neutrophils of each group. (C) BM neutrophils from genotype-housed 5-month-old Ctrl and SCD female mice were purified and cultured for 2 h. Cellular aging was measured using the Cellular Senescence Assay Kit, in which the SA-βGal activity was normalized to total protein concentration. n = 3 mice/group. * p < 0.05, compared with Ctrl-H2O group; #p < 0.05 compared with corresponding H2O group; two-way ANOVA (A); T-test (C).
Since senescence is one of the hallmarks of aging [19] and SA-βGal is the most widely used biomarker for senescent and aging cells, we also performed an ex vivo cellular aging assay using a Cellular Senescence Assay Kit. As shown in Fig. 2C, SA-βGal activity was significantly higher in BM neutrophils from 5-month-old SCD female mice compared with Ctrl, suggesting increased age.
3.3. Abx treatment rescued OB dysfunction from co-culture with BM neutrophils of SCD mice
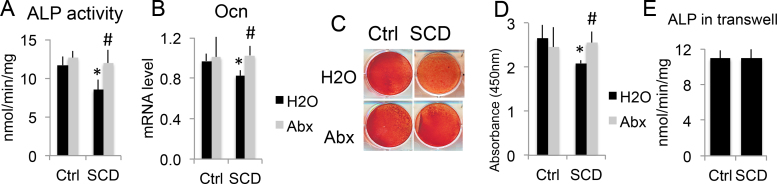
To examine whether BM neutrophils in SCD mice play a direct role in impairing OB function, a co-culture system of calvarial OBs with BM neutrophils was used. As shown in Fig. 3A, on day 7 of co-culture, ALP activity (OB activity biochemical marker) was significantly decreased in OBs co-cultured with BM neutrophils from SCD-H2O mice compared with neutrophils from Ctrl-H2O mice, but ALP activity was rescued when OBs were co-cultured with BM neutrophils from Abx-treated SCD mice. On day 14 of co-culture, Ocn mRNA expression was decreased in OBs co-cultured with BM neutrophils from SCD mice, but Ocn mRNA expression was rescued when OBs were co-cultured with BM neutrophils from Abx-treated SCD mice (Fig. 3B). Alizarin red staining and quantification (Fig. 3C&D) showed decreased mineralization in OBs co-cultured with BM neutrophils from SCD-H2O mice compared with neutrophils from Ctrl-H2O mice, but decreased mineralization was rescued when co-cultured with BM neutrophil from Abx-treated SCD mice. These findings suggest that BM neutrophils from SCD mice impaired OB function and can be rescued with Abx treatment. Interestingly, when fixed neutrophils from Ctrl and SCD mice were plated on 0.4 µm pore polycarbonate membrane inserts, ALP activity of OBs on the bottom of transwells (molecule transport, but no cell contact) was similar between groups (Fig. 3E). This suggests direct neutrophil-to-OB interaction is involved in SCD neutrophil-mediated OB functional defects.
Fig. 3.
Abx treatment rescued OB dysfunction co-cultured with BM neutrophils from SCD mice. Calvarial OBs were digested from 3-day-old Ctrl mice. BM neutrophils were isolated using MojoSort Mouse Neutrophil Isolation Kit from single-housed 4-month-old Ctrl and SCD male mice treated with H2O or Abx. (A-D) Fixed neutrophils were plated on top of confluence OBs in osteogenic medium. (A) ALP activity at day 7 of co-culture. (B) Ocn mRNA expression at day 14 of co-culture. (C) Representative images and (D) quantitation of Alizarin Red staining at day 14 of co-culture. (E) OBs were plated on the bottom of Transwells. Fixed BM neutrophils were plated on 0.4 µm pore polycarbonate membrane inserts in osteogenic media. ALP activity was measured on day 7 of culture. n = 4 mice/group. * p < 0.05 compared with Ctrl-H2O; #p < 0.05 compared with corresponding H2O group; two-way ANOVA (A, B,D); T-test (E).
4. Discussion
Our research revealed BM neutrophil aging in SCD mice contribute to impaired OB functions that can be rescued by normalizing the number of aged neutrophils with Abx treatment.
Studies suggest that there is a chronic translocation of bacteria and/or bacterial products across the intestinal wall causing inflammation and leukocytosis but not overt infection in SCD. This is supported by a recent study in SCD mice showing that the intestinal microbiome regulates the number of aged neutrophils in blood [6]. Neutrophil aging is driven by the microbiome via toll-like receptors (TLRs) and myeloid differentiation factor 88-mediated (Myd88) signaling pathway [6]. Consistent with this, we found that SCD mice exhibited expansion of neutrophil subset compared to Ctrl mice, and the percentage of aged neutrophils was expanded in BM of SCD mice. SCD mice exhibited significant expansion of BM cellularity that is consistent with findings in SCD subjects that were attributed to marrow hyperplasia [20]. Microbiota-derived signals regulate neutrophil aging and activity, and depletion of gut microbiota with broad spectrum Abxs significantly reduced the number of circulating aged neutrophils [6]; therefore, we used the same Abx treatment regiment to decrease neutrophil aging in BM. Treating mice with Abx for 2 weeks led to highly efficient elimination of the gut microbiota in both genotypes (Supplemental Fig. 1). Abx treatment led to a significant and selective reduction of neutrophils, but not other leukocyte populations. Notably, the expansion of the percentage of aged neutrophils in SCD mice was completely abrogated by Abx treatment.
Aged neutrophils die within a short period of time by spontaneous apoptosis under healthy conditions, in order to maintain homeostatic cell numbers. In vivo, cytokines and growth factors are major regulators of neutrophil survival. Inflammatory cytokines such as INF-γ, IL-8 and IL-1β inhibit apoptosis, which may lead to enhanced neutrophil survival during inflammation [21]. Consistent with this we found significantly increased IL-1β mRNA expression in whole tibiae of 6-month-old female SCD mice compared with Ctrl (Supplemental Fig. 2), which may be involved in the increased BM neutrophil aging observed in SCD mice.
There have been a few publications that identify a correlation between neutrophils, OBs, and OB functions, but to date, the significance of this has not been defined. For instance, community-dwelling older men with declining hip BMD have higher peripheral neutrophil count [22], and BM mesenchymal stem/progenitor cells and OB number are inversely correlated with granulocyte-colony stimulating factor-driven BM neutrophil expansion [23]. Moreover, neutrophils can affect OB function in children on chronic glucocorticoid therapy [25], and inflammatory microcrystals promote a functional adhesion of neutrophils to OBs that contribute to decreased bone formation and increased bone resorption [26], [27]. In addition, increased BM neutrophil suppressed OB function in IL-6 transgenic mice [11]. Studies showed neutrophil-neutralizing antiserum administered systemically in rats increased bony trabeculae within injury site and increased expression of osteoblastic differentiation transcription factor, Runx2, and bone matrix protein, osteocalcin, in the injured growth plate, suggesting neutrophil-mediated inflammatory response suppresses mesenchymal cell osteoblastic differentiation [24]. Previous studies have shown that neutrophil adhesion to OB that can impact OB function [17] and aged neutrophils represent an overly active subset [6], which suggest that our observed accumulation of BM aged neutrophils may contribute to OB dysfunction in SCD mice.
Since neutrophils normally have a very short life span (6–7 h in blood) and readily undergo spontaneous apoptosis [28], we used fixed neutrophils [7], instead of living neutrophils for co-culture. Our data showed that fixed neutrophils from SCD mice are still able to inhibit OB function, and this was abrogated when neutrophils were plated in inserts that do not directly contact OB. This indicates that cell-to-cell interactions are important to neutrophil-mediated OB functions. Studies showed that neutrophil adhesion to OB impact OB function via integrin signaling [17]. OBs express integrins with potential counter-receptors for neutrophil integrins and adhesion molecules [29], [30], [31]. Future studies are warranted to determine whether adhesion molecules play a role in BM neutrophil-mediated OB defect in SCD mice.
Although neutrophils are short-lived cells with limited synthetic capacity, activated neutrophils have been shown to synthesize considerable amounts of proteins and lipids that participate in the inflammatory process [32], [33]. Soluble factors of neutrophils may also play a role in mediating OB function. Further studies are needed to culture OBs with supernatants of cultured neutrophils in order to examine the effect of soluble factors of neutrophils on OB functions.
To our knowledge, this is the first report that BM neutrophils from SCD mice impair OB functions that is associated with increased BM neutrophil aging. The impaired OB functions can be rescued after normalization of the increased BM neutrophil aging by Abx treatment. Future in vivo studies are needed to address whether Abx are able to rescue impaired bone formation in SCD mice. This may open up new avenues for developing novel therapeutic approaches to treat the destructive bone loss endured by individuals with SCD.
Acknowledgments
We would like to thank Dr. Evan R Jellison from the Flow Cytometry Facility at UConn Health for his support in flow cytometry analysis.
Acknowledgments
Funding
This research was supported by the Department of Medicine Scholars Award and by a curriculum developed by the Connecticut Institute for Clinical and Translational Sciences (CICATS) at the University of Connecticut. The content is solely the responsibility of the authors and does not necessarily represent the official views of CICATS.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2018.10.009.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2018.10.009.
Appendix A. Transparency document
Supplementary material.
Appendix A. Supplementary material
Supplementary material.
References
- 1.Ballas S.K., Kesen M.R., Goldberg M.F., Lutty G.A., Dampier C., Osunkwo I., Wang W.C., Hoppe C., Hagar W., Darbari D.S., Malik P. Beyond the definitions of the phenotypic complications of sickle cell disease: an update on management. ScientificWorldJournal. 2012;2012:949535. doi: 10.1100/2012/949535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osunkwo I. An update on the recent literature on sickle cell bone disease. Curr. Opin. Endocrinol. Diabetes Obes. 2013;20:539–546. doi: 10.1097/01.med.0000436192.25846.0b. [DOI] [PubMed] [Google Scholar]
- 3.Wun T. The role of inflammation and leukocytes in the pathogenesis of sickle cell disease. Hematology. 2000;5:403–412. doi: 10.1080/10245332.2000.11746536. [DOI] [PubMed] [Google Scholar]
- 4.West M.S., Wethers D., Smith J., Steinberg M. Laboratory profile of sickle cell disease: a cross-sectional analysis. The cooperative study of sickle cell disease. J. Clin. Epidemiol. 1992;45:893–909. doi: 10.1016/0895-4356(92)90073-v. [DOI] [PubMed] [Google Scholar]
- 5.Wun T. The Role of Inflammation and Leukocytes in the Pathogenesis of Sickle Cell Disease; Haemoglobinopathy. Hematology. 2001;5:403–412. [PubMed] [Google Scholar]
- 6.Zhang D., Chen G., Manwani D., Mortha A., Xu C., Faith J.J., Burk R.D., Kunisaki Y., Jang J.E., Scheiermann C., Merad M., Frenette P.S. Neutrophil ageing is regulated by the microbiome. Nature. 2015;525:528–532. doi: 10.1038/nature15367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chakravarti A., Raquil M.A., Tessier P., Poubelle P.E. Surface RANKL of Toll-like receptor 4-stimulated human neutrophils activates osteoclastic bone resorption. Blood. 2009;114:1633–1644. doi: 10.1182/blood-2008-09-178301. [DOI] [PubMed] [Google Scholar]
- 8.Strydom N., Rankin S.M. Regulation of circulating neutrophil numbers under homeostasis and in disease. J. Innate Immun. 2013;5:304–314. doi: 10.1159/000350282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furze R.C., Rankin S.M. The role of the bone marrow in neutrophil clearance under homeostatic conditions in the mouse. FASEB J. 2008;22:3111–3119. doi: 10.1096/fj.08-109876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girasole G., Jilka R.L., Passeri G., Boswell S., Boder G., Williams D.C., Manolagas S.C. 17 beta-estradiol inhibits interleukin-6 production by bone marrow-derived stromal cells and osteoblasts in vitro: a potential mechanism for the antiosteoporotic effect of estrogens. J. Clin. Invest. 1992;89:883–891. doi: 10.1172/JCI115668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitamura H., Kawata H., Takahashi F., Higuchi Y., Furuichi T., Ohkawa H. Bone marrow neutrophilia and suppressed bone turnover in human interleukin-6 transgenic mice. A cellular relationship among hematopoietic cells, osteoblasts, and osteoclasts mediated by stromal cells in bone marrow. Am. J. Pathol. 1995;147:1682–1692. [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao L., Andemariam B., Taxel P., Adams D.J., Zempsky W.T., Dorcelus V., Hurley M.M. Loss of Bone in Sickle Cell Trait and Sickle Cell Disease Female Mice Is Associated With Reduced IGF-1 in Bone and Serum. Endocrinology. 2016;157:3036–3046. doi: 10.1210/en.2015-2001. [DOI] [PubMed] [Google Scholar]
- 13.Ryan T.M., Ciavatta D.J., Townes T.M. Knockout-transgenic mouse model of sickle cell disease. Science. 1997;278:873–876. doi: 10.1126/science.278.5339.873. [DOI] [PubMed] [Google Scholar]
- 14.Casanova-Acebes M., Pitaval C., Weiss L.A., Nombela-Arrieta C., Chevre R., N A.G., Kunisaki Y., Zhang D., van Rooijen N., Silberstein L.E., Weber C., Nagasawa T., Frenette P.S., Castrillo A., Hidalgo A. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell. 2013;153:1025–1035. doi: 10.1016/j.cell.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sabbieti M.G., Agas D., Marchetti L., Santoni G., Amantini C., Xiao L., Menghi G., Hurley M.M. Signaling pathways implicated in PGF2alpha effects on Fgf2+/+ and Fgf2-/- OBs. J. Cell Physiol. 2010;224:465–474. doi: 10.1002/jcp.22143. [DOI] [PubMed] [Google Scholar]
- 16.Xiao L., Liu P., Li X., Doetschman T., Coffin J.D., Drissi H., Hurley M.M. Exported 18-kDa isoform of fibroblast growth factor-2 is a critical determinant of bone mass in mice. J. Biol. Chem. 2009;284:3170–3182. doi: 10.1074/jbc.M804900200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allaeys I., Rusu D., Picard S., Pouliot M., Borgeat P., Poubelle P.E. Osteoblast retraction induced by adherent neutrophils promotes osteoclast bone resorption: implication for altered bone remodeling in chronic gout. Lab Invest. 2011;91:905–920. doi: 10.1038/labinvest.2011.46. [DOI] [PubMed] [Google Scholar]
- 18.Pfaffl M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez-Otin C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mauriello A., Giacobbi E., Saggini A., Isgro A., Facchetti S., Anemona L. Histological features of bone marrow in paediatric patients during the asymptomatic phase of early-stage Black African sickle cell anaemia. Pathology. 2017;49:297–303. doi: 10.1016/j.pathol.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 21.Maianski N.A., Maianski A.N., Kuijpers T.W., Roos D. Apoptosis of neutrophils. Acta Haematol. 2004;111:56–66. doi: 10.1159/000074486. [DOI] [PubMed] [Google Scholar]
- 22.Valderrabano R.J., Lui L.Y., Lee J., Cummings S.R., Orwoll E.S., Hoffman A.R., Wu J.Y., Osteoporotic G. Fractures in Men Study Research, Bone Density Loss Is Associated With Blood Cell Counts. J. Bone Miner. Res. 2017;32:212–220. doi: 10.1002/jbmr.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh P., Hu P., Hoggatt J., Moh A., Pelus L.M. Expansion of bone marrow neutrophils following G-CSF administration in mice results in osteolineage cell apoptosis and mobilization of hematopoietic stem and progenitor cells. Leukemia. 2012;26:2375–2383. doi: 10.1038/leu.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung R., Cool J.C., Scherer M.A., Foster B.K., Xian C.J. Roles of neutrophil-mediated inflammatory response in the bony repair of injured growth plate cartilage in young rats. J. Leukoc. Biol. 2006;80:1272–1280. doi: 10.1189/jlb.0606365. [DOI] [PubMed] [Google Scholar]
- 25.Brunetti G., Faienza M.F., Piacente L., Ventura A., Oranger A., Carbone C., Benedetto A.D., Colaianni G., Gigante M., Mori G., Gesualdo L., Colucci S., Cavallo L., Grano M. High dickkopf-1 levels in sera and leukocytes from children with 21-hydroxylase deficiency on chronic glucocorticoid treatment. Am. J. Physiol. Endocrinol. Metab. 2013;304:E546–E554. doi: 10.1152/ajpendo.00535.2012. [DOI] [PubMed] [Google Scholar]
- 26.Bouchard L., de Medicis R., Lussier A., Naccache P.H., Poubelle P.E. Inflammatory microcrystals alter the functional phenotype of human osteoblast-like cells in vitro: synergism with IL-1 to overexpress cyclooxygenase-2. J. Immunol. 2002;168:5310–5317. doi: 10.4049/jimmunol.168.10.5310. [DOI] [PubMed] [Google Scholar]
- 27.Bouchard L., Naccache P.H., Poubelle P.E. Promotion of neutrophil adherence to human osteoblasts by microcrystals and f-Met-Leu-Phe. Biochem Biophys. Res Commun. 2002;296:759–764. doi: 10.1016/s0006-291x(02)00911-7. [DOI] [PubMed] [Google Scholar]
- 28.Luo H.R., Loison F. Constitutive neutrophil apoptosis: mechanisms and regulation. Am. J. Hematol. 2008;83:288–295. doi: 10.1002/ajh.21078. [DOI] [PubMed] [Google Scholar]
- 29.Gronthos S., Stewart K., Graves S.E., Hay S., Simmons P.J. Integrin expression and function on human osteoblast-like cells. J. Bone Miner. Res. 1997;12:1189–1197. doi: 10.1359/jbmr.1997.12.8.1189. [DOI] [PubMed] [Google Scholar]
- 30.Albelda S.M., Buck C.A. Integrins and other cell adhesion molecules. FASEB J. 1990;4:2868–2880. [PubMed] [Google Scholar]
- 31.Barczyk M., Carracedo S., Gullberg D. Integrins. Cell Tissue Res. 2010;339:269–280. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen M., Lam B.K., Kanaoka Y., Nigrovic P.A., Audoly L.P., Austen K.F., Lee D.M. Neutrophil-derived leukotriene B4 is required for inflammatory arthritis. J. Exp. Med. 2006;203:837–842. doi: 10.1084/jem.20052371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edwards S.W., Hallett M.B. Seeing the wood for the trees: the forgotten role of neutrophils in rheumatoid arthritis. Immunol. Today. 1997;18:320–324. doi: 10.1016/s0167-5699(97)01087-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.
Supplementary material.