Abstract
Mesenchymal stem cell (MSC) aging seriously affects its function in stem cell transplantation for treatment. Extensive studies have focused on how to inhibit senescence in MSCs. However, the mechanism of senescence in MSC was not clear. In this study, we used d-galactose to induce MSC aging. Then we found that the number of aging cells was increased compared with untreated MSCs. We discovered that ascorbic acid could inhibit the production of reactive oxygen species (ROS) and activation of AKT/mTOR signaling in MSCs caused by d-galactose. Especially, when treated together with a ROS scavenger or AKT inhibitor, the senescent cells were obviously decreased in d-galactose-induced MSCs. Taken together, we identify that ascorbic acid owns the potential to inhibit the senescence of MSCs through ROS and Akt/mTOR signaling. Together, our data supports that ascorbic acid can be used to prevent MSCs from senescence, which can enhance the efficiency of stem cell transplantation in the clinic.
Electronic supplementary material
The online version of this article (10.1007/s10616-018-0220-x) contains supplementary material, which is available to authorized users.
Keywords: Senescence, MSCs, ROS, d-Galactose
Introduction
Mesenchymal stem cells (MSCs) are important in self-renewal and tissue-specific regeneration with advancing age. They have been an essential cellular source for stem cell treatment for a long time and are so far being tested in many ongoing clinical trials (Tsuchiya et al. 2017). MSCs have been proven to own immunomodulatory effects on many activated immune cells, such as T cells, natural killer cells and B cells (Nauta and Fibbe 2007). Moreover, because of their low immunogenicity and accompanying absence of expression of co-stimulatory molecules, MSCs have the ability to escape alloantigen recognition (Sun et al. 2007). Such properties make MSCs promising seeding cells for protecting against the rejection of certain organ transplants.
However, recent studies have found that MSC’s function declines as the individuals age, thus leading to the dysfunction of MSC which influences the effects of autologous MSC transplantation in those individuals (Patel et al. 2013). In recent studies it was also found that the MSC aging can be induced by old rat serum in recent studies (Mayack et al. 2010; Villeda et al. 2011, 2014). So it is important to find an efficient method to delay MSC senescence in order to maintain the function of MSC transplantation.
As we all know, there are a variety of factors included in the process of cellular senescence. It is a complicated pathophysiological process, for instance the oxidative stress, shortening of telomere length, abatement of histone deacetylase and DNA methyltransferase and so on (Zhang et al. 2014). It is critical that the balance between prooxidants and antioxidants can affect the survival of MSCs. ROS are not only the endogenous outcome of metabolism, but also the fundamental second messages in cellular signaling including cell cycle arrest and cell death. More and more evidence has accumulated to explain that intracellular ROS is the main factor resulting in cellular aging. Excessive ROS accumulation could harm cellular proteins and DNA which could result in cellular senescence and even the death of cell. Studies have been done to prove that excessive activation of Wnt/β-catenin signaling has something to do with MSC aging induced by ROS (Wang and Tan 2013; Zhang et al. 2013). It is believed that inhibiting the senescence of cell can recover cell viability and MSC function. So the most important thing to do is to seek safe and effective antioxidants that inhibit the oxidative damage and cellular senescence induced by ROS.
Ascorbic acid works as a cofactor in many biological processes. At particular concentration, ascorbic acid works as a growth promoter to promote proliferation when supplied to the culture medium (Choi et al. 2008). However, when at elevated concentration elevated ascorbic acid can be cytotoxic resulting in apoptosis and proliferation inhibition. Ascorbic acid is a naturally effective antioxidant. It can clean up ROS effectively. AKT and mammalian target of rapamycin (mTOR) signaling pathways have been found to be associated with stem cell aging. We also found that there are connections between Akt/mTOR signaling and ROS. Both of the pathways can impact each other (Maiese et al. 2012). Increasing ROS generation correlates with the high levels of mTOR. Thus, we need to clarify whether ascorbic acid can retard MSC senescence through AKT/mTOR pathway.
Although ascorbic acid has been found to accelerate the growth of MSC, the molecular mechanisms and main effect by which ascorbic acid modulates stem cell aging are still unknown. In this article, for the first time we clarify that ascorbic acid can retard the senescence of MSC through AKT/mTOR signaling pathway.
Materials and methods
Isolation and culture of BMSCs
Seven-day-old Sprague–Dawley (SD) rats were obtained from the experimental animal center of Shanghai Jiao Tong University. The investigation was allowed by the law of the People’s Republic of China on the protection of wildlife and the protocol was approved by Shanghai Medical Academy of Science, China. SD rats’ femurs and tibias were removed and the bone marrow was flushed out with blank medium. The cells were centrifuged at 1000 rpm for 3 min. The cell was resuspended in 5 ml Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Gaithersburg, MD, USA) supplemented with 10% fetal bovine serum (FBS, Gibco) and penicillin (100 U/ml)/streptomycin (100 U/ml) at 37 °C in humid air with 5% CO2 and then plated in a 25 cm2 plastic flask (Corning, USA) to let the MSCs adhere. Three days later, the medium was changed and the nonadherent cells were discarded. Fresh medium was changed every 3 days. After about 7–10 days, the adherent cells were released from the flask with 0.25% trypsin (Gibco) and seeded into new fresh culture flasks. They were marked as passage 1 and cells were continued to be cultured until passage 3. All experiments described below were performed using cells of the 3rd passage.
Cell counting Kit-8 assay
BMSCs plated in 96-well plates were treated with d-gal at different doses and time points. Cell viability was assessed by the Cell Counting Kit-8 assay (CCK-8; Dojindo, Kumamoto, Japan) following the manufacturer’s protocol.
Cell culture and d-gal treatment
BM-MSCs were cultured in growth medium at 37 °C with 5% CO2 in 175 cm2 cell culture flasks. Medium was changed every 3 days. Cells were dissociated by 0.25% trypsin–EDTA and reseeded into 6 cm plates for the next stage of the experiments. In order to induce premature senescence, BM-MSCs at approximately 70% confluence were exposed to 1, 10, and 100 g/l of d-gal (Gibco) for 48 h.
Treatments with ascorbic acid
BM-MSCs were pretreated with 200 μmol/l ascorbic acid (Gibco) for 2 h followed by co-exposure to 10 g/l d-gal and ascorbic acid for the next 48 h.
SA-β-gal staining
The expression of senescence-associated β-galactosidase (SA-β-gal) in BMSCs has been used as a typical biomarker of premature senescence. SA-β-gal staining was performed by using a SA-β-gal staining kit (Cell Signaling Technology, Beverly, MA, USA) following the manufacturer’s instructions. The cells were incubated at 37 °C without CO2 overnight. The number of positive cells was counted using a phase-contrast microscope and the experiment was repeated five times in each group.
Detection of ROS level
Intracellular ROS staining was performed using a DCFH-DA staining kit (Genmed, Plymouth, MN, USA), according to the manufacturer’s protocol. BMSCs were treated with fluorescent probes DCFH-DA (1:1000) and then incubated at 37∘C for 20 min after washing with serum-free medium. The cells were observed using a fluorescence microscope (Eclipse 50I, Nikon, Tokyo, Japan) under 488 nm excitation wavelength and 525 nm emission wavelength in order to quantify the ROS level. Experiments were repeated five times.
Western blot analysis
Cells samples were lysed in ice-cold RIPA including protease inhibitor cocktail (Sigma-Aldrich) for 30 min. Then the lysates were centrifuged at 12,500g for 15 min 4 °C and the supernatant was collected. The protein concentration was quantified with a Pierce BCA protein assay kit (Thermo Scientific, Fremont, CA, USA), according to the manufacturer’s instruction. Equivalent amounts of protein were loaded and separated by SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membrane (Millipore, Billerica, MA, USA). Membranes were incubated with horseradish peroxidase-(HRP)-conjugated anti-rabbit or anti-mouse secondary antibodies (1:2000, Dako, Carpinteria, CA, USA), and proteins were detected by enhanced chemiluminescence (ECL) (Amersham Biosciences Corp., Pittsburgh, PA, USA).
Statistical analysis
Data are presented as mean ± SD. Significance testing was performed using one-way analysis of variance (ANOVA) to compare data from different experimental groups. Statistical values were calculated using the SPSS 19.0 software and illustrated using the GraphPad Prism 5.0. Differences with a value of P < 0.05 were regarded as statistically significant.
Results
d-Gal induced cellular senescence in BMSCs in vitro
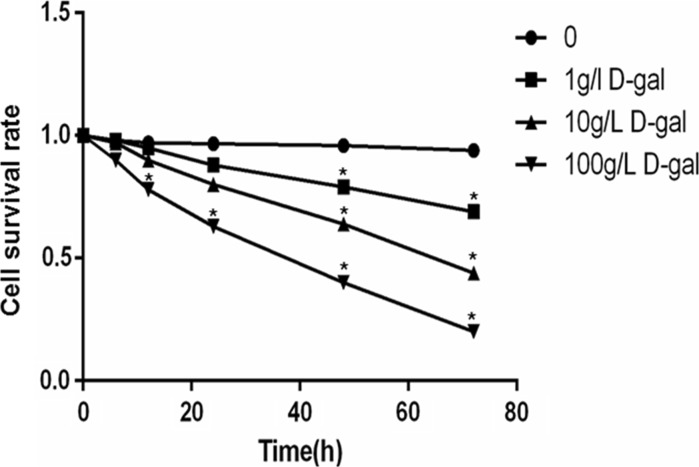
In order to detect the particular dose and action time of d-gal, the cell survival rate was measured by CCK-8. BMSCs were treated with d-gal at different doses (1, 10, 100 g/l) and time points (6, 12, 24, 48, 72 h). The relative cell number was measured to evaluate the cell growth. We found that cell survival rate was inhibited by d-gal in a time- and dose-dependent manner. According to the survival curve (Fig. 1), according to the IC50 and the obvious downward trend, induction by 10 g/l d-gal for 48 h could be applied in the follow-up experiments.
Fig. 1.
BMSCs are incubated with d-gal at different doses and time points. Cell survival rate was assayed by using CCK8 to evaluate cell viability (*P < 0.05, vs. control at the respective time points). Data are expressed as mean ± SE. Results represent three independent experiments
Ascorbic acid retards d-gal-induced MSCs senescence
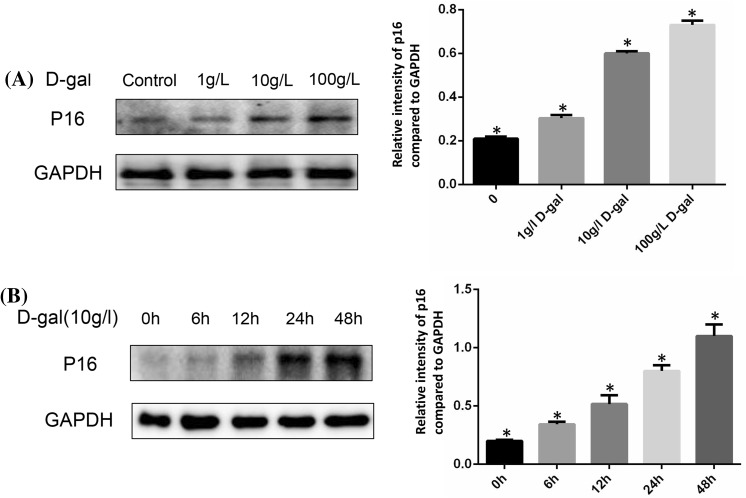
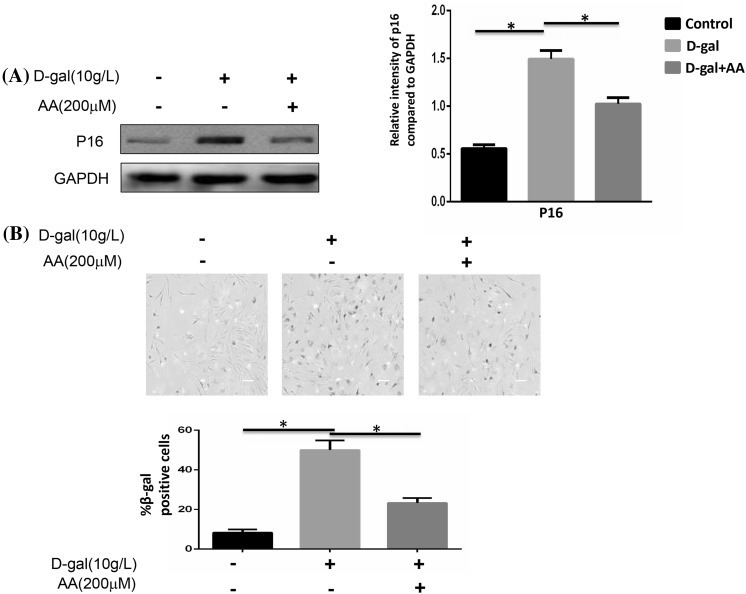
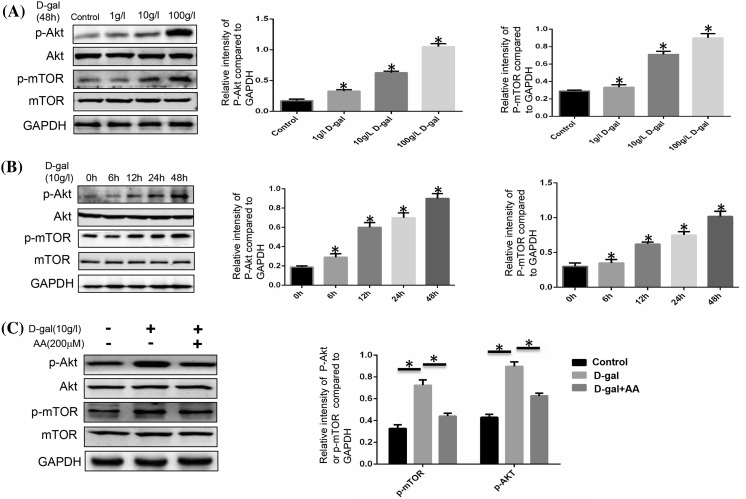
Two major signaling pathways are involved in senescence: the p53/p21 and the p16INK4a/CDK/pRb pathways (Kailong et al. 2007). Here, we simply examined p16INK4a protein expression to evaluate cellular senescence. The expression of p16 was gradually increased as the concentration raised (Fig. 2a). Comparison with the control group was clear enough. We next chose different time point to confirm the effect of d-gal to induce the aging of BMSCs. The expression of p16 was time dependent (Fig. 2b). However, the increased expression of p16 induced by d-gal could be reduced when treated with ascorbic acid (AA) (Fig. 3a). The technique of SA-β-gal staining was performed to examine aging cells. We found that SA-β-gal positive cells ratio was obviously increased by d-gal stimulation. After adding AA, this ratio could be decreased compared to d-gal only group (Fig. 3b).
Fig. 2.
Cellular senescence induced by d-gal stimulation. a BMSCs were stimulated with different doses of d-gal for 48 h, protein expressions of p16 were detected by Western blotting. GAPDH was used as internal control. The expression of p16 was gradually increased as the dose increased (*P < 0.05). b BMSCs were stimulated by 10 g/l d-gal for different time durations and protein expressions of p16 were detected by Western blotting. As time increased, the expression of p16 increased (*P < 0.05). Results represent at least three independent experiments
Fig. 3.
Cellular senescence was affected by d-gal and ascorbic acid. BMSCs were stimulated with 10 g/l d-gal and 200 μM ascorbic acid for 48 h. a The protein expressions of p16 were detected by Western blotting. The higher expression of p16 induced by d-gal was reduced when ascorbic acid was added (*P < 0.05). b Quantification of SA-β-gal-positive cells. The total number of SA-β-gal-positive cells among 100 random cells was counted using phase-contrast microscopy. Scale bar = 25 μm. The number of SA-β-gal-positive cells was obviously decreased compared with that in the d-gal treated group (*P < 0.05). Results represent at least three independent experiments
The role of ROS/mTOR signaling pathway and ascorbic acid in d-gal-induced senescence
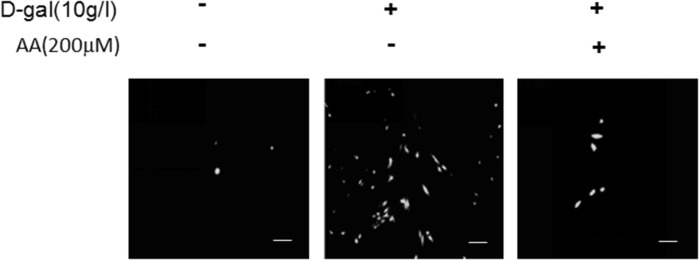
ROS and AKT/mTOR signaling pathways are involved in a variety of physiological processes, including cellular senescence (Schieber and Chandel 2014). In this study, we intend to determine the significance of ROS/mTOR signaling pathway in cellular senescence and the correlation between each other in this process. ROS can be induced by d-gal and d-gal has been used in many animal models in order to make aging models (Dong et al. 2017). So we used d-gal to stimulate BMSCs and check the change of ROS and the activation of AKT/mTOR signaling pathway. As we forecasted before, ROS fluorescence was enhanced by d-gal, and AA could weaken the ROS change (Fig. 4). The expressions of p-AKT and p-mTOR were promoted by d-gal in a time- and dose-dependent manner (Fig. 5a, b). Activation of AKT/mTOR signaling pathway was increased in the d-gal group, and weakened when AA was added (Fig. 5c). As we discussed earlier, d-gal can induce cellular senescence, and AA was able to block the progress. The trend of ROS generation and Akt/mTOR signaling pathway change matches with cellular senescence stimulated by d-gal and AA. We have the reason to say that ROS and AKT/mTOR signaling pathway play important roles in d-gal-induced cellular senescence.
Fig. 4.
ROS fluorescence in BMSCs. BMSCs were stimulated with 10 g/l d-gal and ascorbic acid (200 μM) for 48 h, and stained with ROS fluorescence. Results represent three independent experiments
Fig. 5.
Expressions of AKT, p-AKT, mTOR, p-mTOR in BMSCs. a Western blot analysis. BMSCs were stimulated with different doses of d-gal for 48 h. The p-AKT and p-mTOR expression level was evidently higher as the dose increased (*P < 0.05). b Western blot analysis. BMSCs were stimulated with 10 g/l d-gal for different time. The p-AKT and p-mTOR expression level was evidently higher as time increased (*P < 0.05). c BMSCs were stimulated with 10 g/l d-gal and 200 μM ascorbic acid for 48 h. The higher expression of p-AKT and p-mTOR induced by d-gal was reduced when ascorbic acid was added (*P < 0.05). Results represent at least three independent experiments
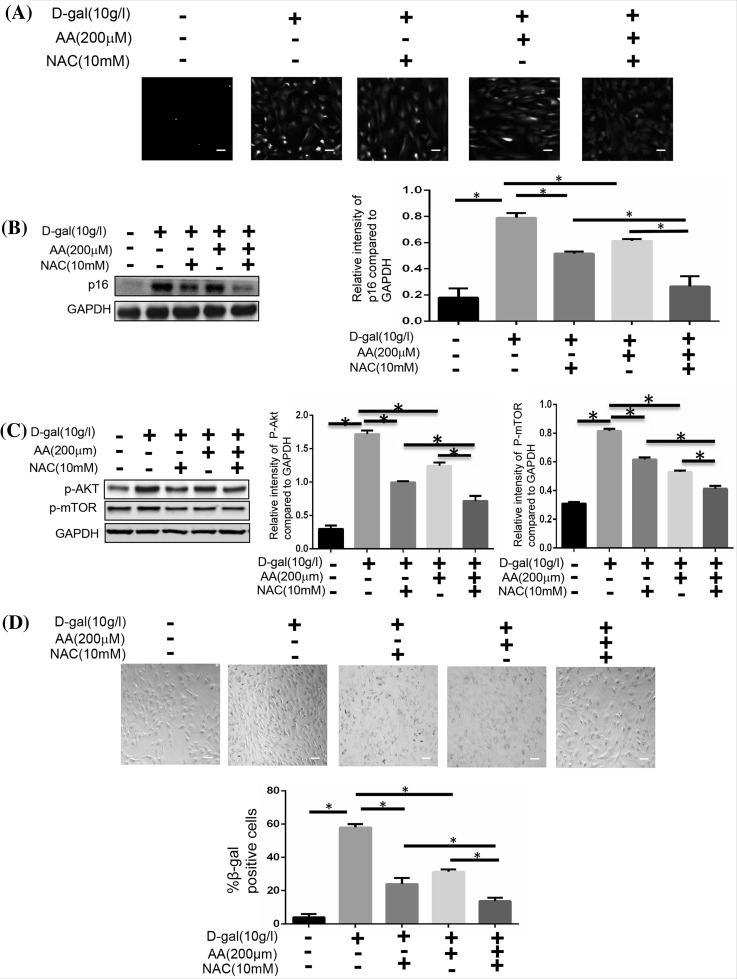
Blocking ROS/mTOR signaling pathway can retard d-gal-induced cellular senescence in BMSCs
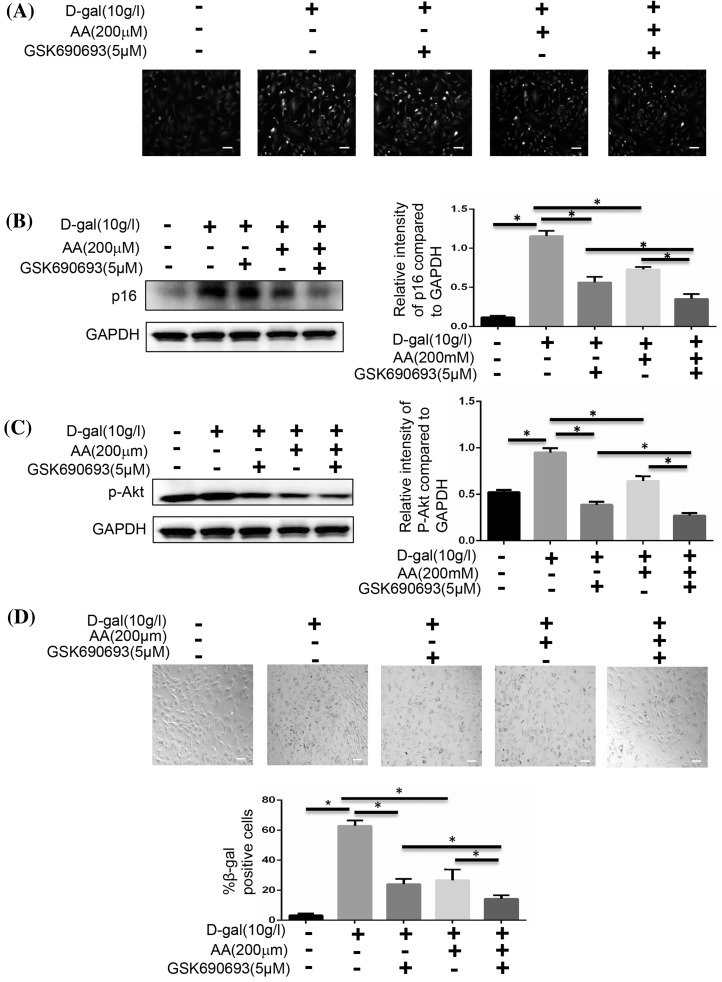
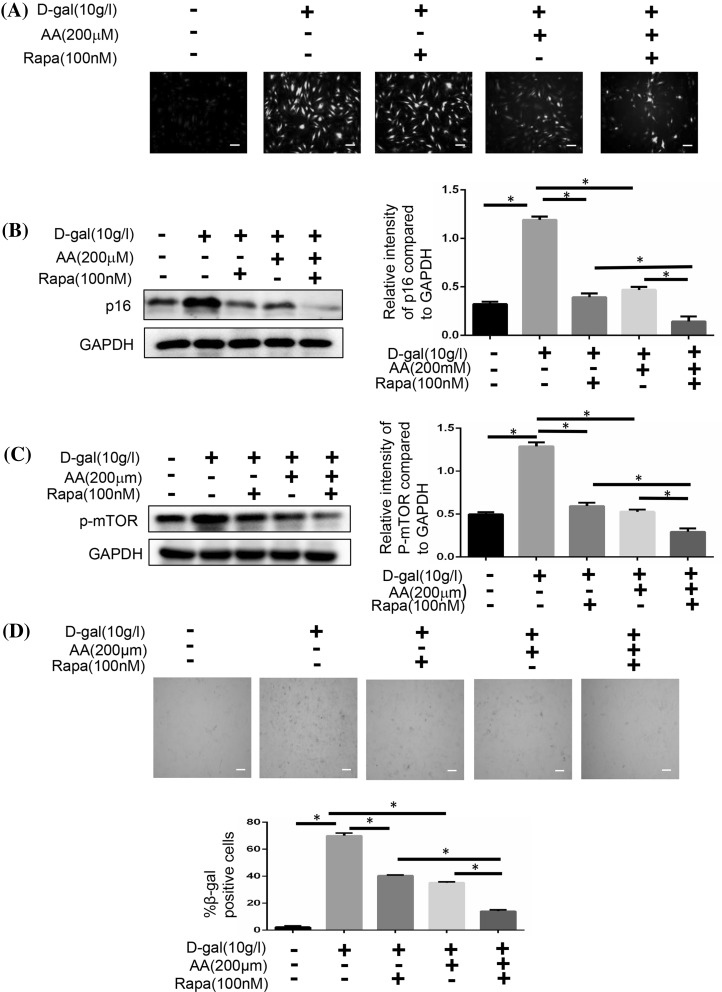
We analysed the expression of p16 and the SA-β-gal of BMSCs to confirm these findings. We used N-acetylcysteine to inhibit ROS signaling pathway, and found that the generation of ROS was decreased along with the reduced number of cells (Fig. 6a, b, d). According to the expression of p-AKT and p-mTOR, we found that the activation of mTOR signaling pathway was decreased, too (Fig. 6c). We next inhibited the AKT signaling pathway with GSK690693. The cellular aging induced by d-gal was also decreased, just like the N-acetylcysteine did (Fig. 7b, d). Meanwhile, activation of mTOR signaling pathway was weakened without the change of ROS generation (Fig. 7a, c). Then we inhibited the mTOR signaling pathway with Rapamycin. We also could find that cellular aging induced by d-gal was decreased without the change of ROS generation (Fig. 8).
Fig. 6.
Blocking ROS affects the cellular senescence and AKT/mTOR pathway activation. BMSCs were induced with 10 g/l d-gal, 200 μM ascorbic acid or 10 mM NAC. a BMSCs were stained with ROS fluorescence. Scale bar = 25 μm. b The protein expressions of p16 were measured by Western blotting (*P < 0.05). c The protein expressions of p-AKT and p-mTOR were measured by Western blotting (*P < 0.05). d The senescent cells were examined by SA-β-gal staining (*P < 0.05). Scale bar = 25 μm. Results represent at least three independent experiments
Fig. 7.
Blocking AKT affects cellular senescence. BMSCs were induced with 10 g/l d-gal, 200 μM ascorbic acid or 5 μM GSK690693. a BMSCs were stained with ROS fluorescence. Scale bar = 25 μm. b The protein expressions of p16 were measured by Western blotting (*P < 0.05). c The protein expression of p-AKT was measured by Western blotting (*P < 0.05). d The senescent cells were examined by SA-β-gal staining (*P < 0.05). Scale bar = 25 μm. Results represent at least three independent experiments
Fig. 8.
Blocking mTOR affects cellular senescence. BMSCs were induced with 10 g/l d-gal, 200 μM ascorbic acid or 100 nM Rapamycin. a BMSCs were stained with ROS fluorescence. Scale bar = 25 μm. b The protein expressions of p16 were measured by Western blotting (*P < 0.05). c The protein expression of p-mTOR was measured by Western blotting (*P < 0.05). d The senescent cells were examined by SA-β-gal staining (*P < 0.05). Scale bar = 25 μm. Results represent at least three independent experiments
Discussion
In the present study, we examined the effects and underlying mechanisms of AA on d-gal-induced premature senescence in MSCs. Our results demonstrated that treatment with AA can reverse cellular senescence and that AA-mediated anti-senescence effects involve the activation of the ROS and Akt/mTOR pathway.
MSCs possess a self-renewal ability and can differentiate into cells of multiple lineages (Goerke et al. 2012; Wakao et al. 2012), which seem to be a large therapeutic promise in clinical cell transplantation therapy (Abdallah and Kassem 2008; Mohammadian et al. 2013). However, many studies have shown that senescence of MSCs influences the effect of their clinical application (Raggi and Berardi 2012; Yu and Kang 2013; Zhang et al. 2011). Although the exact mechanisms of cellular senescence are still indeterminate, “the free radical theory of aging” has been widely accepted (Harman 2006). In MSCs, excess ROS can impair self-renewal, differentiation capacity, and proliferation (Denu and Hematti 2016; Mohtasebi et al. 2014; Tan and Suda 2017). The increasing accumulation of free radicals can harm most of the biomolecules, including DNA, protein, and lipids. High ROS levels may cause cellular damage and dysfunction, leading to the senescence of the cell (D’Apolito et al. 2017; Salazar et al. 2017). Many researches have proven that d-gal-induced mouse can be used as an experimental model for researching aging (Li et al. 2014; Xie et al. 2012). Especially, there were studies having proven that the mechanism of d-gal induced MSC aging was mainly through promoting ROS generation (Hu et al. 2015). In our experiment, we have found that the number of SA-β-gal-positive MSCs in 10 g/l d-gal group was obviously increased.
Ascorbic acid (AA) is a powerful antioxidant that mediates several beneficial effects on redox oxidative pathways (Gegotek et al. 2017). Several recent studies have shown that AA owns an important protective ability to protect cells from apoptosis, such as in skin and hair, chondrocytes and fibroblasts (Chang et al. 2015; Wakame et al. 2017). However, it is still unclear if AA plays a role in the senescence of stem cells. This is the first time we have discovered that AA can delay the senescence of mesenchymal stem cells through ROS and AKT/mTOR signaling.
The present study found that the main mechanism of the protective effects of AA on cellular aging was that it could decrease the oxidative stress. Yet, ROS is connected with many intracellular pathways (Maiese et al. 2012; Wang et al. 2014). Some of these signaling pathways are involved in the cellular senescence (Bishayee et al. 2013). The AKT/mTOR pathway is one of the pathways which plays an important part in the aging of the cells (Castilho et al. 2009; Li et al. 2013), and in cancer cells this pathway has been proven that it has relation to ROS pathway (Shin et al. 2013). Also, there is evidence that inhibition of this pathway may extend the lifespan of many species (Harrison et al. 2009). Researcher showed that active AKT inhibits the transcriptional activity of FOXO3a and down-regulates MnSOD, resulting in an increased level of ROS that induced cellular senescence (Xu et al. 2014). Therefore, we hypothesized that AKT/mTOR signaling inactivation was the reason why AA inhibited MSC senescence induced by d-gal.
We measured the ROS and the protein expressions of p-AKT and p-mTOR to ensure the activation of ROS and AKT/mTOR signaling pathway. In this process, we found that there was the same trend between the activation of ROS/AKT/mTOR signaling pathway and the increased senescence. As the senescence cells increased, we found the ROS increased and AKT/mTOR signaling pathway activated. Then we used the inhibitor to block the ROS or AKT/mTOR signaling pathway, we found the number of senescent cells was decreased, too. Thus we confirmed that the ROS and AKT/mTOR signaling pathway had something to do with the cellular aging. We also detected that the number of senescent cells and the activation of mTOR signaling pathway were clearly different between the d-gal group and the d-gal + AA group after blocking ROS; also there were important differences between the d-gal group and the d-gal + AA group in senescence after blocking AKT.
The mechanism of d-gal which causes cellular senescence is mostly the excessive ROS production. In this experiment, we confirm that d-gal can affect the production of ROS. At the same time, we investigated the connection between d-gal and AKT/mTOR signaling pathway. By testing the p-AKT and p-mTOR, we found that d-gal could affect the activation of mTOR signaling pathway at the same time. However, the AKT inhibitor can reduce the cellular senescence induced by d-gal. We confirm that the AKT/mTOR signaling pathway is involved in the mechanism of d-gal-induced senescence.
Our study has found the effect of ascorbic acid on senescence. However, the specific mechanism is not explicit. Da Silva et al. (2017) found the inhibition effect of Ascorbic acid on oxidative stress. Kashino et al. reported that AA had protective effects against cellular senescence via scavenging ROS (Kashino et al. 2003). In our study, we found that ascorbic acid could affect ROS, which was in line with previous studies. Meanwhile, we also studied the link between AA and AKT/mTOR signaling pathway. By detecting the change of p-AKT and p-mTOR, we found that AA could affect the activation of mTOR signaling pathway. By blocking AKT, the function of AA can be affected. Thus the effect of antisenescence of ascorbic acid may have close association with AKT/mTOR signaling pathway.
Although ROS and AKT/mTOR signaling pathways have an important effect on d-dal-induced aging, there were no explicit quantified standards for us to explore the particular points d-gal and AA worked on. Thus, more studies need to be performed.
Conclusion
In conclusion, d-gal can induce the MSC aging through promoting ROS generation. Ascorbic acid can obviously decrease the number of SA-β-gal-positive cells and the expression of p16 in d-gal-treated MSCs. Ascorbic acid may be an effective protection agent of stem cell senescence induced by ROS. The AKT/mTOR signaling may be the main mediator of ascorbic acid inhibiting MSC senescence. Further deeper studies of the mechanisms of ascorbic acid involved in stem cell aging will help extend the clinical applications and improve the clinical value of ascorbic acid.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1 The MSC surface markers and the multipotent differentiation potential have been verified in our experiment. (A) The surface markers of cells were measured by flow cytometry, the expression of CD31 and CD45 was negative and the expression of CD29 and CD90 was high, which was in line with the characteristics of mesenchymal stem cells. (B) The Rat Mesenchymal Stem Cell Functional Identification Kit is designed for the identification of rat BMSCs based on their ability to differentiate into multiple mesenchymal lineages. After 14-21 days’ osteogenesis culture, we use immunocytochemistry to put into evidence the change of cells. Blue fluorescence represents the nucleus and green fluorescence represents the osteocalcin. The cells could be induced to osteogenic differentiation. (C) After 7-21 days’ adipogenesis culture, we use mmunocytochemistry to put into evidence the change of cells. Blue fluorescence represents the nucleus and red fluorescence represents the FABP4. The cells could be induced to adipocytes differentiation. (TIFF 274 kb)
Acknowledgements
This work was supported by the National Natural Science Fund of China (Nos. 81371979 and 30700853).
References
- Abdallah BM, Kassem M. Human mesenchymal stem cells: from basic biology to clinical applications. Gene Ther. 2008;15:109–116. doi: 10.1038/sj.gt.3303067. [DOI] [PubMed] [Google Scholar]
- Bishayee K, Paul A, Ghosh S, Sikdar S, Mukherjee A, Biswas R, Boujedaini N, Khuda-Bukhsh AR. Condurango-glycoside-A fraction of Gonolobus condurango induces DNA damage associated senescence and apoptosis via ROS-dependent P53 signalling pathway in hela cells. Mol Cell Biochem. 2013;382:173–183. doi: 10.1007/s11010-013-1732-5. [DOI] [PubMed] [Google Scholar]
- Castilho RM, Squarize CH, Chodosh LA, Williams BO, Gutkind JS. mTOR mediates Wnt-induced epidermal stem cell exhaustion and aging. Cell Stem Cell. 2009;5:279–289. doi: 10.1016/j.stem.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Z, Huo L, Li P, Wu Y, Zhang P. Ascorbic acid provides protection for human chondrocytes against oxidative stress. Mol Med Rep. 2015;12:7086–7092. doi: 10.3892/mmr.2015.4231. [DOI] [PubMed] [Google Scholar]
- Choi KM, Seo YK, Yoon HH, Song KY, Kwon SY, Lee HS, Park JK. Effect of ascorbic acid on bone marrow-derived mesenchymal stem cell proliferation and differentiation. J Biosci Bioeng. 2008;105:586–594. doi: 10.1263/jbb.105.586. [DOI] [PubMed] [Google Scholar]
- Da Silva LM, Maria-Ferreira D, Beltrame OC, da Silva-Santos JE, de Paula Werner MF. Vitamin C improves gastroparesis in diabetic rats: effects on gastric contractile responses and oxidative stress. Dig Dis Sci. 2017;62:2338–2347. doi: 10.1007/s10620-017-4632-9. [DOI] [PubMed] [Google Scholar]
- D’Apolito M, Colia AL, Lasalvia M, Capozzi V, Falcone MP, Pettoello-Mantovani M, Brownlee M, Maffione AB, Giardino I. Urea-induced ROS accelerate senescence in endothelial progenitor cells. Atherosclerosis. 2017;263:127–136. doi: 10.1016/j.atherosclerosis.2017.06.028. [DOI] [PubMed] [Google Scholar]
- Denu RA, Hematti P. Effects of oxidative stress on mesenchymal stem cell biology. Oxid Med Cell Longev. 2016;2016:2989076. doi: 10.1155/2016/2989076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z, Xu M, Huang J, Chen L, Xia J, Chen X, Jiang R, Wang L, Wang Y. The protective effect of Ginsenoside Rg1 on aging mouse pancreas damage induced by d-galactose. Exp Ther Med. 2017;14:616–622. doi: 10.3892/etm.2017.4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegotek A, Bielawska K, Biernacki M, Zareba I, Surazynski A, Skrzydlewska E. Comparison of protective effect of ascorbic acid on redox and endocannabinoid systems interactions in in vitro cultured human skin fibroblasts exposed to UV radiation and hydrogen peroxide. Arch Dermatol Res. 2017;309:285–303. doi: 10.1007/s00403-017-1729-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goerke SM, Plaha J, Hager S, Strassburg S, Torio-Padron N, Stark GB, Finkenzeller G. Human endothelial progenitor cells induce extracellular signal-regulated kinase-dependent differentiation of mesenchymal stem cells into smooth muscle cells upon cocultivation. Tissue Eng Part A. 2012;18:2395–2405. doi: 10.1089/ten.tea.2012.0147. [DOI] [PubMed] [Google Scholar]
- Harman D. Free radical theory of aging: an update: increasing the functional life span. Ann N Y Acad Sci. 2006;1067:10–21. doi: 10.1196/annals.1354.003. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Jing P, Wang L, Zhang Y, Yong J, Wang Y. The positive effects of Ginsenoside Rg1 upon the hematopoietic microenvironment in a d-galactose-induced aged rat model. BMC Complement Altern Med. 2015;15:119. doi: 10.1186/s12906-015-0642-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kailong L, Du X, Yani H, Lin Z, Jvrong Y, Ruihua S, Lin C. P53-Rb signaling pathway is involved in tubular cell senescence in renal ischemia/reperfusion injury. Biocell. 2007;31:213–223. [PubMed] [Google Scholar]
- Kashino G, Kodama S, Nakayama Y, Suzuki K, Fukase K, Goto M, Watanabe M. Relief of oxidative stress by ascorbic acid delays cellular senescence of normal human and Werner syndrome fibroblast cells. Free Radic Biol Med. 2003;35:438–443. doi: 10.1016/S0891-5849(03)00326-5. [DOI] [PubMed] [Google Scholar]
- Li J, Yu L, Zhao Y, Fu G, Zhou B. Thymosin Beta4 reduces senescence of endothelial progenitor cells via the Pi3 k/Akt/Enos signal transduction pathway. Mol Med Rep. 2013;7:598–602. doi: 10.3892/mmr.2012.1180. [DOI] [PubMed] [Google Scholar]
- Li YN, Guo Y, Xi MM, Yang P, Zhou XY, Yin S, Hai CX, Li JG, Qin XJ. Saponins from Aralia taibaiensis attenuate d-galactose-induced aging in rats by activating Foxo3a and Nrf2 pathways. Oxid Med Cell Longev. 2014;2014:320513. doi: 10.1155/2014/320513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Wang S, Shang YC. Oxidant stress and signal transduction in the nervous system with the Pi 3-K, Akt, and mTOR cascade. Int J Mol Sci. 2012;13:13830–13866. doi: 10.3390/ijms131113830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayack SR, Shadrach JL, Kim FS, Wagers AJ. Systemic signals regulate ageing and rejuvenation of blood stem cell niches. Nature. 2010;463:495–500. doi: 10.1038/nature08749. [DOI] [PubMed] [Google Scholar]
- Mohammadian M, Shamsasenjan K, Lotfi Nezhad P, Talebi M, Jahedi M, Nickkhah H, Minayi N, Movassaghpour A. Mesenchymal stem cells: new aspect in cell-based regenerative therapy. Adv Pharm Bull. 2013;3:433–437. doi: 10.5681/apb.2013.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohtasebi MS, Nasri F, Kamali Sarvestani E. Effect of DiD carbocyanine dye labeling on immunoregulatory function and differentiation of mice mesenchymal stem cells. Stem Cells Int. 2014;2014:457614. doi: 10.1155/2014/457614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- Patel DM, Shah J, Srivastava AS. Therapeutic potential of mesenchymal stem cells in regenerative medicine. Stem Cells Int. 2013;2013:496218. doi: 10.1155/2013/496218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raggi C, Berardi AC. Mesenchymal stem cells, aging and regenerative medicine. Muscles Ligaments Tendons J. 2012;2:239–242. [PMC free article] [PubMed] [Google Scholar]
- Salazar G, Huang J, Feresin RG, Zhao Y, Griendling KK. Zinc regulates Nox1 expression through a NF-κB and mitochondrial ROS dependent mechanism to induce senescence of vascular smooth muscle cells. Free Radic Biol Med. 2017;108:225–235. doi: 10.1016/j.freeradbiomed.2017.03.032. [DOI] [PubMed] [Google Scholar]
- Schieber M, Chandel NS. Ros function in redox signaling and oxidative stress. Curr Biol. 2014;24:R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S, Jing K, Jeong S, Kim N, Song KS, Heo JY, Park JH, Seo KS, Han J, Park JI, Kweon GR, Park SK, Wu T, Hwang BD, Lim K. The Omega-3 polyunsaturated fatty acid DHA induces simultaneous apoptosis and autophagy via mitochondrial ROS-mediated Akt–mTOR signaling in prostate cancer cells expressing mutant P53. Biomed Res Int. 2013;2013:568671. doi: 10.1155/2013/568671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun LY, Zhang HY, Feng XB, Hou YY, Lu LW, Fan LM. Abnormality of bone marrow-derived mesenchymal stem cells in patients with systemic lupus erythematosus. Lupus. 2007;16:121–128. doi: 10.1177/0961203306075793. [DOI] [PubMed] [Google Scholar]
- Tan DQ, Suda T. Reactive oxygen species and mitochondrial homeostasis as regulators of stem cell fate and function. Antioxid Redox Signal. 2017 doi: 10.1089/ars.2017.7273. [DOI] [PubMed] [Google Scholar]
- Tsuchiya A, Kojima Y, Ikarashi S, Seino S, Watanabe Y, Kawata Y, Terai S. Clinical trials using mesenchymal stem cells in liver diseases and inflammatory bowel diseases. Inflamm Regen. 2017;37:16. doi: 10.1186/s41232-017-0045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, Lucin KM, Czirr E, Park JS, Couillard-Despres S, Aigner L, Li G, Peskind ER, Kaye JA, Quinn JF, Galasko DR, Xie XS, Rando TA, Wyss-Coray T. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeda SA, Plambeck KE, Middeldorp J, Castellano JM, Mosher KI, Luo J. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat Med. 2014;20:659–663. doi: 10.1038/nm.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakame K, Komatsu KI, Nakata A, Sato K, Takaguri A, Masutomi H, Nagashima T, Uchiyama H. Transcriptome analysis of skin from SMP30/GNL knockout mice reveals the effect of ascorbic acid deficiency on skin and hair. Vivo. 2017;31:599–607. doi: 10.21873/invivo.11100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakao S, Kuroda Y, Ogura F, Shigemoto T, Dezawa M. Regenerative effects of mesenchymal stem cells: contribution of muse cells, a novel pluripotent stem cell type that resides in mesenchymal cells. Cells. 2012;1:1045–1060. doi: 10.3390/cells1041045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HJ, Tan YZ. Methods for assessing effects of Wnt/β-catenin signaling in senescence of mesenchymal stem cells. Methods Mol Biol. 2013;976:111–130. doi: 10.1007/978-1-62703-317-6_9. [DOI] [PubMed] [Google Scholar]
- Wang H, Wang L, Li NL, Li JT, Yu F, Zhao YL, Wang L, Yi J, Wang L, Bian JF, Chen JH, Yuan SF, Wang T, Lv YG, Liu NN, Zhu XS, Ling R, Yun J. Subanesthetic isoflurane reduces zymosan-induced inflammation in murine kupffer cells by inhibiting ROS-activated P38 MAPK/NF-κB signaling. Oxid Med Cell Longev. 2014;2014:851692. doi: 10.1155/2014/851692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Zhang H, Gao P, Wang L, Zhang A, Xie S, Li J. Overexpression of Sirt6 in porcine fetal fibroblasts attenuates cytotoxicity and premature senescence caused by d-galactose and tert-butylhydroperoxide. DNA Cell Biol. 2012;31:745–752. doi: 10.1089/dna.2011.1435. [DOI] [PubMed] [Google Scholar]
- Xu Y, Li N, Xiang R, Sun P. Emerging roles of the P38 MAPK and PI3 K/AKT/mTOR pathways in oncogene-induced senescence. Trends Biochem Sci. 2014;39:268–276. doi: 10.1016/j.tibs.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu KR, Kang KS. Aging-related genes in mesenchymal stem cells: a mini-review. Gerontology. 2013;59:557–563. doi: 10.1159/000353857. [DOI] [PubMed] [Google Scholar]
- Zhang DY, Wang HJ, Tan YZ. Wnt/β-catenin signaling induces the aging of mesenchymal stem cells through the DNA damage response and the P53/P21 pathway. PLoS ONE. 2011;6:e21397. doi: 10.1371/journal.pone.0021397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DY, Pan Y, Zhang C, Yan BX, Yu SS, Wu DL, Shi MM, Shi K, Cai XX, Zhou SS, Wang JB, Pan JP, Zhang LH. Wnt/β-catenin signaling induces the aging of mesenchymal stem cells through promoting the ROS production. Mol Cell Biochem. 2013;374:13–20. doi: 10.1007/s11010-012-1498-1. [DOI] [PubMed] [Google Scholar]
- Zhang W, Hui R, Yang S. Telomeres, cardiovascular aging, and potential intervention for cellular senescence. Sci China Life Sci. 2014;57:858–862. doi: 10.1007/s11427-014-4700-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1 The MSC surface markers and the multipotent differentiation potential have been verified in our experiment. (A) The surface markers of cells were measured by flow cytometry, the expression of CD31 and CD45 was negative and the expression of CD29 and CD90 was high, which was in line with the characteristics of mesenchymal stem cells. (B) The Rat Mesenchymal Stem Cell Functional Identification Kit is designed for the identification of rat BMSCs based on their ability to differentiate into multiple mesenchymal lineages. After 14-21 days’ osteogenesis culture, we use immunocytochemistry to put into evidence the change of cells. Blue fluorescence represents the nucleus and green fluorescence represents the osteocalcin. The cells could be induced to osteogenic differentiation. (C) After 7-21 days’ adipogenesis culture, we use mmunocytochemistry to put into evidence the change of cells. Blue fluorescence represents the nucleus and red fluorescence represents the FABP4. The cells could be induced to adipocytes differentiation. (TIFF 274 kb)