Abstract
Adrenocortical carcinoma (ACC) is a rare and often fatal cancer, affecting ~1 person per million per year worldwide. Approximately 75% of patients with ACC eventually develop metastases and progress on the few available standard-of-care medical therapies, highlighting an incredible need for an improved understanding of the molecular biology of this disease. Although it has long been known that ACC is characterized by certain histological and genetic features (e.g., high mitotic activity, chromosomal instability, and overexpression of IGF2), only in the last two decades of genomics has the molecular landscape of ACC been more thoroughly characterized. In this review, we describe the findings of historical genetics and recent genomics studies on ACC and discuss how underlying concepts emerging from these studies contribute to the current model of critical pathways for adrenocortical carcinogenesis. Integrative synthesis across these studies reveals that ACC consists of three distinct molecular subtypes with divergent clinical outcomes and implicates differential regulation of Wnt signaling, cell cycle, DNA methylation, immune biology, and steroidogenesis in ACC biology. These cellular programs are pharmacologically targetable and may enable the development of therapeutic strategies to improve outcomes for patients facing this devastating disease.
Keywords: adrenocortical carcinoma, adrenal, genomics, targeted therapy
The adrenal glands are paired endocrine organs that reside above each kidney and produce a variety of hormones critical for life. Each gland consists of an outer cortex and inner medulla, which produce steroid hormones and catecholamines, respectively. Adrenocortical carcinoma (ACC) is a rare cancer of the adrenal cortex affecting 0.5 to 2 people per million per year worldwide [1–3]. Though rare, ACC is often aggressive, with only ~35% of patients surviving 5 years after diagnosis [4]. To date, the only curative therapy for ACC is surgical resection of the primary tumor [5]. However, 50% of patients with ACC present with disseminated metastases, and approximately one-third of patients with localized or locoregional disease at diagnosis develop postoperative metastases [6]. For these patients, ACC-specific US Food and Drug Administration–approved medical therapies are limited to the adrenolytic agent mitotane [op’-DDD; 1,1-dichloro-2-(o-chlorophenyl)-2-(p-chlorophenyl) ethane] [4].
Mitotane is widely used in the medical management of ACC at experienced centers; however, evidence supporting mitotane therapy in the adjuvant setting or for patients with disseminated disease is limited. The two largest studies on adjuvant mitotane are retrospective, and they suggest it prolongs time to recurrence with no impact on overall survival [7, 8]. Few nonrandomized studies support mitotane use for patients with metastatic ACC; these include a recently published large retrospective study on patients with advanced ACC by Megerle et al. [9], in which investigators identified that patients with low tumor burden or late diagnosis of advanced ACC may benefit from mitotane monotherapy. Historically, ≤30% of patients respond to mitotane therapy with reduction in tumor burden, and ≤70% respond with control of hormonal symptoms [4]. However, mitotane efficacy is ultimately limited by its high lipophilicity, poor pharmacokinetic properties, and dose-limiting toxicities. Indeed, achieving therapeutic serum levels of mitotane (≥14 mg/mL) typically takes several months of drug administration [10] and may require patients to initially consume ≥12 pills daily. Such high initiation dosing can often be reduced over time during maintenance therapy.
Recent studies have evaluated the use of cytotoxic antineoplastic agents in the treatment of metastatic ACC. Reports of efficacy for combined therapies consisting of etoposide, doxorubicin, cisplatin, and mitotane (EDP-M) or streptozotocin and mitotane (Sz-M) for metastatic ACC [11, 12] gave rise to the First International Randomized Trial in Locally Advanced and Metastatic Adrenocortical Carcinoma Treatment (FIRM-ACT; NCT00094497). The study evaluated EDP-M and Sz-M in a randomized controlled phase III clinical trial enrolling patients with metastatic ACC and no prior cytotoxic chemotherapy, and it demonstrated that EDP-M was a superior treatment regimen [13]. One-fifth of patients responded to first-line EDP-M therapy, leading to a median progression-free survival interval of 5.0 months, compared with 2.2 months for patients on an Sz-M regimen [13]. Current clinical consensus is to give EDP-M to all patients with disseminated ACC and to consider the use of cytotoxic agents in patients with localized but histologically aggressive disease (Ki67 > 10% or >20 mitoses per 50 high-powered fields) [5, 14, 15]. Additional insights about ACC management are elaborated in the recent clinical practice guidelines from the European Network for the Study of Adrenal Tumors; this work represents the first clinical practice guidelines for ACC based on comprehensive literature review [16].
Despite these recent advances in medical management of ACC, only a minority of patients receive therapeutic benefit, which is often short lived. These heterogeneous responses demonstrate a strong need for improved personalized medical therapies for this disease, which provide durable therapeutic benefit and target core molecular programs driving most (if not all) ACC tumors. Development of these therapies necessarily relies on a deep understanding of the genetic, transcriptional, and epigenetic programs driving adrenocortical carcinogenesis. In this review, we will discuss genetics and genomics studies that have informed the current understanding of the underlying molecular programs driving ACC, from the genetics of familial cancer syndromes to comprehensive genomics studies such as those by Assié et al. [17] and Juhlin et al. [18] and The Cancer Genome Atlas (TCGA) study on adrenocortical carcinoma (ACC-TCGA) [19]. We will then describe the implications of this work for molecular stratification and targeted therapies and relevant translational, preclinical, and clinical studies. Importantly, although we will discuss pediatric forms of ACC in the context of familial syndromes, the scope of this review is restricted to adult ACC.
1. Methods
We identified literature to incorporate in this review by searching the National Institutes of Health/National Center for Biotechnology Information PubMed database on 19 July 2018, for the following search terms: “adrenocortical carcinoma” (3304 results), “adrenocortical carcinoma genomic” (289 results), “adrenocortical carcinoma transcriptome” (29 results), “adrenocortical carcinoma methylation” (39 results), “adrenocortical carcinoma microarray” (36 results), “adrenocortical carcinoma genomics” (42 results), “adrenocortical carcinoma preclinical” (53 results), “adrenocortical carcinoma clinical trial” (99 results), and “adrenocortical carcinoma profiling” (106 results). We identified additional resources in D. Therapeutics by searching PubMed for the relevant molecular program. Search results were manually curated.
2. Results
A. Molecular Lessons From Early Genetics and Familial Syndromes
It has long been known that malignant tumors of the adrenal cortex are distinguished by a high degree of mitotic activity, atypical mitoses, and aneuploidy [20–22]. Early molecular studies that used comparative genomic hybridization characterized these qualitative observations with higher resolution, illustrating that rampant chromosomal instability and widespread, heterogeneous patterns of chromosomal abnormalities characterize ACC. Although many ACC exhibited complex genomic patterns of gains and losses, select copy number alterations were remarkably recurrent across tumors, including gains in 9q34 and 5p, which respectively encompass NR5A1 (encoding steroidogenic factor 1, the master regulator of steroidogenesis) and oncogene TERT; and losses in 11q, 13q, and 17p, which respectively encompass known tumor suppressor genes MEN1, RB1, and TP53 [23–27]. Taken together, these findings suggested that this hallmark chromosomal instability contributes to adrenocortical carcinogenesis by facilitating upregulation of adrenocortical oncogenes and downregulation of critical tumor suppressors.
Additional clues illuminating signaling pathways core to adrenocortical carcinogenesis came from families with classic neoplasia syndromes in which ACC or adrenal hyperplasia is a feature. Patients with Beckwith-Wiedemann syndrome or Li-Fraumeni syndrome have a significantly higher likelihood of developing ACC than the general population [28–32]. Patients with multiple endocrine neoplasia 1 or familial adenomatous polyposis often develop bilateral adrenal hyperplasia, which may evolve to ACC [33–35]. These clinical observations informed targeted approaches in the pregenomics era evaluating the prevalence of somatic alterations in loci encompassing IGF2, TP53, MEN1, APC, and CTNNB1. Indeed, such studies demonstrated that loss of heterozygosity (LOH) of 11p15, leading to biallelic expression of IGF2 and silencing of CDKN1C, is a hallmark feature of ≤90% of ACC [36, 37]. Additional studies also demonstrated that somatic alterations leading to inactivation of TP53 are present in ~30% of ACC [38–42], and LOH of regions of 11q13 encompassing the MEN1 locus is present in >60% of ACC [43–46]. Finally, targeted sequencing approaches identified that somatic APC mutations are somewhat rare [33], but mutations in CTNNB1 are common, present in ≤30% of ACC [47, 48].
B. Early Omics
B-1. Transcriptome
Global gene expression profiling (transcriptome) studies enable the identification of transcriptional programs and gene expression patterns unique to individual tissues or disease states. Global characterization of transcriptional programs recurrently altered in ACC compared with adrenocortical adenomas (ACA) and physiological adrenocortical tissue began with studies that used microarrays. Such studies identified that the transcriptomes of ACC diverge substantially from physiological adrenocortical tissue and ACA [49, 50]. They also demonstrated that ACC are distinguished from ACA by high expression of activators of cellular proliferation and components of growth factor signaling, including MKI67, CCNE1, TOP2A, BUB1B, and IGF2, and lower expression of negative cell cycle regulators and steroidogenic machinery such as CDKN1C, RB1, CYP11B1, and HSD3B2 [50–52]. Supporting earlier genetic studies identifying recurrent chromosomal alterations in ACC, Giordano et al. [49] identified that ACC are characterized by transcriptional repression of 11q, 17p, and 1p and transcriptional upregulation of 5q and 12q.
The dramatic differences in the transcriptomes of benign and malignant adrenocortical lesions suggested that transcriptional profiling may illuminate molecular subtypes of ACC with distinct clinical outcomes. Giordano et al. [49] identified that patients with ACC tumors featuring the highest expression of proliferation machinery and genes related to “functional aneuploidy” have poor survival outcomes compared with tumors with gene expression profiles that more closely resemble benign lesions. In a landmark study, de Reyniès et al. [53] demonstrated that unsupervised clustering identifies two unique transcriptional subtypes of ACC tumors, called C1A and C1B. Like Giordano et al., this group identified that tumors of the more aggressive C1A subtype bore higher expression of mitotic cell cycle genes, whereas those of the less aggressive C1B subtype bore higher expression of cell metabolism, apoptosis, and cell differentiation genes. This group extended these findings further to identify a two-gene predictor of survival and identified that a BUB1B-PINK1 score could discriminate between ACA-like ACC tumors of good prognosis (survival fraction >90%) and other ACC [53]. This BUB1B-PINK1 score has also been validated in an independent retrospective cohort study of adult ACC [54]. Finally, in a precursor to recent integrated molecular studies, Ragazzon et al. [55] demonstrated that C1A tumors are enriched for somatic mutations in CTNNB1 or TP53, supported by immunohistochemical evidence of nuclear accumulation of p53 or nuclear localization of β-catenin. Together, these studies illustrate that molecular subtypes of ACC are driven by distinct transcriptional programs, which may be associated with differences in disease aggressiveness.
B-2. Methylome
Methylation of DNA is a covalent epigenetic modification, often serving as a mechanism of transcriptional regulation. DNA methylation often involves methylation of cytosines located in CpG motifs, which may be individual or in contiguous regions (islands, shores, shelves, open seas) scattered throughout the promoter, coding, and noncoding regions of the genome. The emergence of array technology to query genomewide CpG methylation (methylome) enabled studies characterizing the methylation profiles of adrenocortical tumors. Fonseca et al. [56] identified that a subset of ACC genomes is characterized by hypermethylation directed to CpG islands, compared with ACA and physiological adrenal tissue. Select differentially methylated genes in ACC included transcriptionally silenced tumor suppressors, such as CDKN2A, GATA4, and PYCARD, giving rise to the hypothesis that dysregulated epigenetic programming in ACC promotes tumorigenesis partially through tumor suppressor silencing [56]. A subsequent genomic methylation study supported these findings, identifying that hypermethylated CpGs in ACC are largely restricted to CpG islands, whereas hypomethylated CpGs are present in open sea regions [57]. Resolving the methylation landscape of ACC further, Barreau et al. [58] demonstrated that ACC consists of three CpG island methylator phenotype (CIMP) signatures with low, intermediate, and high levels of CpG island methylation compared with ACA. This group identified that promoter CpG island methylation was often but not always associated with decreased gene expression and that patients with CIMP-high tumors had the worst overall survival [58]. Together, these studies suggested DNA methylation contributes to ACC tumorigenesis and highlighted a potential prognostic role for DNA methylation in ACC.
B-3. miRNome
miRNAs are endogenous, single-stranded noncoding RNA molecules ranging from 19 to 25 nucleotides in length that posttranscriptionally regulate target mRNA expression. A single miRNA may regulate hundreds of mRNA targets involved in a variety of biological programs through miRNA/mRNA sequence specificity. Large-scale miRNA profiling studies characterizing the miRNome of adrenocortical tumors have also illuminated that miRNA networks are uniquely dysregulated in ACC [59, 60]. Tömböl et al. [60] identified that miRNAs differentially expressed in ACC may regulate cell cycle progression via DNA damage checkpoint regulation and proposed that the difference in expression between miR-511 (upregulated in ACC vs cortisol-secreting ACA) and miR-503 (downregulated in ACC vs cortisol-secreting ACA) could distinguish ACC and ACA. Soon et al. [59] also demonstrated that miRNAs are dysregulated in ACC compared with ACA and showed that downregulation of miR-195 and upregulation of miR-483-5p identify a subgroup of ACC with worse outcomes. These studies suggest that miRNA regulatory networks may be dysregulated in ACC to control transcription of genes that regulate cell cycle dynamics and tumor aggressiveness.
B-4. Broad conclusions from early omics
Taken together, early omics illustrated that ACC and ACA are unique molecular entities in terms of gene expression, methylation, and posttranscriptional regulation, suggesting that these entities are unlikely to exist on a continuum. Supporting this model but not discussed in this review, the mutational spectra of ACC also diverge from ACA [61]. Early omics studies confirmed initial genetic studies identifying recurrent chromosomal abnormalities and LOH leading to biallelic expression of IGF2, demonstrating that upregulation of IGF2 and growth factor signaling programs are hallmarks of ACC. These studies illuminated that ACC consists of distinct molecular classes that are also associated with differences in survival, highlighting that targeted assessment of mRNA, miRNA, and DNA methylation may serve as useful biomarkers for risk stratification in ACC.
C. Comprehensive and Integrated Omics
Although early genetics and omics studies provided essential insight into genetic, epigenetic, and transcriptional programs dysregulated in adrenocortical carcinogenesis, lack of integration across platforms limited the understanding of the interplay between recurrent somatic alterations and consequent molecular programs. Somatic mutational profiling was restricted to candidate gene approaches, failing to identify additional genetic events (not linked to familial syndromes) that may be recurrent across ACC. Additionally, given the rarity of these lesions and the obvious molecular heterogeneity of ACC, multi-institutional studies with larger cohort sizes became essential to illustrate the comprehensive landscape of molecular alterations contributing to ACC biology.
In the first integrated genomics study on ACC, Assié et al. [17] confirmed previous findings but uncovered several novel features of ACC. The authors performed multiplatform molecular profiling of germline and tumor exomes, copy number, gene expression, DNA methylation, and miRNAs. Supporting historical literature and prior single nucleotide polymorphism array profiling studies [62], the authors identified that somatic copy number alterations (gains and losses) are common in ACC, with LOH throughout the genome. Confirming previously identified alterations in CTNNB1, TP53, CDKN2A, RB1, and MEN1, the authors also identified novel somatic alterations in ZNRF3, DAXX, TERT, and MED12. The gene most frequently targeted for somatic alteration (biallelic deletion or loss of function mutation) was ZNRF3, altered in 21% of ACC and mutually exclusive with mutations in CTNNB1. This finding was of particular interest because ZNRF3 had been recently characterized as a negative regulator of Wnt signaling that promotes turnover of cell surface Wnt receptors (e.g., Frizzled receptors) [63]. Selection for this alteration suggests that a subset of ACC may be fueled by secreted Wnt ligands.
The authors also identified that a unique miRNA signature associated with an imprinted DLK1-MEG3 cluster located on 14q32.2 is downregulated in a subset of C1B ACC. Finally, the authors demonstrated that C1A ACC have higher mutation rate and higher incidence of recurrent mutations, and a subset of C1A exclusively bears intermediate or high levels of CpG island methylation. Importantly, there was also a subset of tumors (approximately one-third, encompassing a variety of miRNA, mRNA, and DNA methylation subclasses) in which no recurrent somatic alteration was identified [17]. Shortly after this publication, Assié et al.’s findings were validated by Juhlin et al. [18], who also identified frequent ZNRF3 deletions.
In 2016, a landmark multiplatform study was published as part of the largest consortium of genomic cancer studies to date: The Cancer Genome Atlas project on adrenocortical carcinoma (ACC-TCGA) [19]. This was one of the first TCGA studies of a rare malignancy, enabling the incorporation of ACC into pan-cancer analyses. Using improved, often next-generation sequencing-based multiplatform approaches, ACC-TCGA confirmed several findings from Assié et al. but also identified numerous previously undiscovered molecular programs dysregulated in ACC. ACC-TCGA identified additional recurrent somatic alterations in PRKAR1A, RPL22, TERF2, and CCNE1 and somatic alterations in epigenetic modifiers including MLL family members, SETD2, TET1, and SMARCA4. The identification of recurrent loss of function alterations in PRKAR1A, the regulatory subunit that mitigates protein kinase A activity, implicated protein kinase A signaling for the first time in sporadic ACC. Importantly, as in previous studies, in a subset of ACC (~20%) no recurrent somatic alteration was identified. To summarize, ~45% of ACC bore somatic alterations leading to cell cycle activation, ~40% of ACC bore somatic alterations leading to Wnt pathway activation, and ~20% of ACC bore somatic alterations in epigenetic modifiers [19].
ACC-TCGA comprehensively characterized the somatic copy number alteration landscape, identifying three recurrent profiles: quiet (tumor genome is diploid, no focal gains and losses), chromosomal (tumor genome is characterized by frequent whole chromosome LOH and hypodiploidy in a subset of tumors), and noisy (frequent focal, arm-level gains and losses painting the picture of a “shattered” tumor genome). Intriguingly, a subset of tumors in noisy and chromosomal classes also exhibited a whole genome doubling (WGD) phenomenon associated with overexpression of TERT. Chromosomal tumors with WGD and noisy signatures were associated with worse prognosis [19].
ACC-TCGA identified differential regulation of six miRNA signatures across ACC. ACC-TCGA also identified that, although nearly all ACC have overexpression of IGF2, ACC is driven largely by three transcriptional programs: steroid-low/immune-high (low expression of steroidogenic enzymes, high expression of immune cell-specific markers), steroid-high, and steroid-high + proliferative (high expression of steroidogenic machinery coupled with cell cycle activation). Steroid-low/immune-high captured tumors assigned to the previously identified C1B signature, whereas steroid-high and steroid-high + proliferative signatures together comprised tumors assigned to C1A. Confirming published DNA methylome findings from other cohorts, ACC-TCGA identified three subgroups: CIMP-low, CIMP-intermediate, and CIMP-high [19].
Finally, ACC-TCGA’s integrative analysis identified that ACC consists of three distinct molecular subtypes, referred to as COC1, COC2, and COC3. COC1 tumors have a lower frequency of somatic alterations and are genomically quiet or chromosomal ± WGD, steroid-low/immune-high, and CIMP-low. COC2 and COC3 tumors have a higher frequency of somatic alterations leading to activation of the Wnt pathway. COC2 tumors are steroid-high, genomically quiet or chromosomal ± WGD, and CIMP-intermediate. Finally, COC3 tumors have a higher frequency of somatic alterations leading to cell cycle activation and are steroid-high + proliferative, genomically noisy ± WGD, and CIMP-high. Astonishingly, COC status was associated with clinical outcome; patients with COC1 tumors had best prognosis, patients with COC2 tumors had intermediate prognosis, and patients with COC3 tumors had dismal prognosis, with uniform disease progression and poor overall survival. Other remarkable findings of this study included the revelation that ACC is relatively pure (e.g., comprising cancer cells with little stromal infiltrate) compared with other malignancies and that the degree of immune infiltration is inversely proportional to steroidogenic capacity, measured by key steroidogenic enzymes [19].
Taken together, multiplatform, comprehensive, integrated omics studies have confirmed findings from single platform studies and provided insights into ACC biology. These studies have highlighted the importance of pathways coordinating Wnt signaling, cell cycle, DNA methylation, chromosome architecture, and steroidogenesis in adrenocortical carcinogenesis. ACC-TCGA particularly demonstrated that these programs are coordinated in specific ways in three unique molecular subtypes of ACC and illuminated a role for immune infiltration in a subtype of ACC with low steroidogenic activity [19]. The identification of three molecular ACC subtypes reinforces the importance of developing biomarkers that permit prospective stratification in accordance with molecular programs. Finally, these differentially regulated molecular programs are largely pharmaceutically targetable, warranting study of currently available pharmaceutical compounds and therapies under development to guide future research and clinical trials on ACC.
D. Therapeutics
D-1. Inhibition of IGF2/IGF1R signaling
Given the ~90% prevalence of IGF2 overexpression in ACC, the first studies evaluating targeted therapies for the disease focused on pharmacological inhibitors targeting IGF2 signaling via the IGF1R receptor. Preclinical studies including xenograft models of ACC were promising, suggesting that inhibition of IGF2/IGF1R signaling may indeed be therapeutically efficacious in ACC [64]. A subsequent phase I study demonstrated that IGF2/IGF1R inhibition with figitumumab was well tolerated, affording some patients with pretreated, metastatic ACC clinical benefit [65]. However, subsequent phase II and III studies with cixutumumab + mitotane vs. mitotane [66] or linsitinib vs. placebo [67] demonstrated that IGF2/IGF1R inhibitors fail most patients with advanced ACC. Importantly, few patients exhibited clinically relevant responses, including disease stabilization, tumor shrinkage, and long-term ACC regression. Indeed, 3% to 5% of patients with refractory metastatic ACC randomly assigned to linsitinib had confirmed, long-term ACC regression by RECIST version 1.1, and nearly one-fifth of linsitinib-treated patients had disease stabilization at 4 months [67]. Though disappointing for most patients, the demonstration that a subset of patients with ACC respond to IGF2/IGF1R therapy suggests that acquired genetic and molecular alterations secondary to LOH of the IGF2 locus may restrict tumor response to therapy. This possibility is supported not only by the complex landscape of molecular alterations identified in ACC-TCGA and other studies [17, 19] but also by in vivo work demonstrating that overexpression of IGF2 is insufficient to initiate adrenocortical tumorigenesis but probably collaborates with other events, such as activation of β-catenin signaling [68]. Although this would need to be evaluated clinically, it is possible that patients who respond to IGF2/IGF1R therapy may indeed be among the ~20% of patients with tumors bearing no recurrent somatic alterations who are a subset of ACC-TCGA COC1 [19].
D-2. Wnt pathway inhibition
Activation of β-catenin signaling is a hallmark of ~40% of ACC, largely encompassing COC2 and COC3 tumors in ACC-TCGA [19]. In addition to recurrent somatic alterations in Wnt/β-catenin signaling components CTNNB1, ZNRF3, and rarely APC, it is well known that ACC often express nuclear, transcriptionally active β-catenin [48, 55]. The classic in vitro model of ACC is the NCI-H295R cell line, which also expresses constitutively active β-catenin (encoded by p.S45P CTNNB1) [48]. Studies using the NCI-H295R model have demonstrated that inhibition of β-catenin–dependent transcription impairs proliferation and steroidogenesis and promotes apoptosis [69–71], supporting the evaluation of such therapeutic approaches in preclinical and clinical studies. Together, these findings suggest that pharmacological inhibition of β-catenin–dependent transcription or of autocrine/paracrine Wnt signaling in tumors with ZNRF3 alterations may be therapeutically efficacious. However, inhibition of Wnt/β-catenin signaling remains clinically challenging.
β-catenin is a critical regulator of development and homeostasis of numerous tissues including the adrenal cortex [72]; not surprisingly, many inhibitors of β-catenin–dependent transcription cause on-target toxicity in Wnt-dependent tissues such as the intestine [73]. New ligand-based therapies are under development, including inhibitors of Porcupine (an acyltransferase enzyme whose activity is essential for the secretion of all Wnt ligands) and Frizzled proteins, which may be more effective in potentially ligand-fueled malignancies such as ACC with somatic alterations in ZNRF3 [73–75]. However, even these approaches fail to totally evade the challenges of therapeutic tissue selectivity [76]. In the absence of pharmacological agents targeting tissue-specific Wnt signaling machinery, it is unlikely that these approaches would yield sufficient therapeutic benefit as monotherapy. Targeting Wnt/β-catenin signaling is therefore more likely to be efficacious with limited toxicity in combination therapy regimens [73, 77].
D-3. Cell cycle inhibition
Classic observations that ACC with higher proliferation indices tend to be associated with worse prognosis [22, 78] fortify more recent genomics studies establishing a role for cell cycle activation in aggressive or rapidly recurrent ACC [19, 49–52]. These and additional studies have also highlighted that pharmacologically targetable DNA replication mechanisms such as TOP2A are overexpressed on mRNA and protein levels in aggressive ACC tumors [50, 52, 79, 80]. Indeed, the standard-of-care combination therapy for patients with metastatic or high-risk ACC includes etoposide (EDP-M) and is efficacious in approximately one-fifth of patients with metastatic disease [5, 13–15]. Clinical studies have also suggested that therapies including antimetabolite cytotoxic agents such as 5-fluorouracil, capecitabine, or gemcitabine may be efficacious in a subset of tumors [81, 82]. Importantly, with all cytotoxic treatment regimens, extratumoral toxicity is often dose-limiting [83]; this drawback may be overcome with the implementation of metronomic or combination dosing schema with these or other cytotoxic agents [82, 84]. Recent translational and preclinical studies have also demonstrated that ACC may be sensitive to newer cell cycle inhibitors targeting kinases with well-established roles in cell cycle progression, such as CDK4/6 [85, 86]. PLK1 [87], Aurora kinases [88], and MELK [89]. These therapeutic approaches have had moderate success in preclinical models of other solid tumors, and the recent advances in design of more selective kinase inhibitors have improved toxicity profiles, enabling assessment of therapeutic efficacy in clinical trials [90].
Given the ACC-TCGA finding that COC3 ACC are a rapidly recurrent group of tumors with a chromosomally noisy genomic landscape in the setting of increased cell cycle activation [18], it is likely that COC3 tumors will be most responsive to cell cycle inhibitors. The rapid recurrence pattern of this subgroup suggests that these patients may even benefit from neoadjuvant/adjuvant cytotoxic chemotherapy, warranting further study. However, there is an additional compelling potential therapeutic avenue for COC3 ACC that remains unexplored. Although the etiology of the hallmark chromosomal noisiness of this tumor type is completely unknown, the high expression of cell cycle machinery (including DNA replication machinery) in the setting of profound genomic instability suggests that this subset of ACC may be exquisitely reliant on efficacious and COC3-specific DNA damage response mechanisms. Indeed, a recent pan-TCGA analysis demonstrated that a subset of ACC exhibits a high level of homologous recombination deficiency, associated with rapidly progressive disease [91]. This finding, coupled with the success of poly(ADP-ribose) polymerase (PARP) inhibitors in tumors with homologous recombination deficiency [92] suggests that these agents may have efficacy for this subset of ACC. Of course, application of this (or any) therapy to this subtype requires reliable biomarkers that enable accurate, prospective identification of patients with COC3 tumors.
D-4. Immunotherapy
ACC-TCGA demonstrated that ACC is largely immune poor [19], and this finding is supported by recent pan-cancer analyses demonstrating that ACC (in bulk) has lowest expression of immune-related genes of nearly all TCGA cancers [93]. However, COC1 tumors with lower expression of steroidogenic machinery exhibited a transcriptional program consistent with immune infiltration [19]. Immunotherapy is a promising therapeutic avenue for many solid tumors including ACC, particularly in light of the US Food and Drug Administration’s recent accelerated approval of anti–PD-1 therapy for treatment of mismatch repair-deficient tumors. Approximately 30% of ACC from ACC-TCGA bore somatic events leading to silencing of mismatch repair genes [91], and ACC has been recently recognized as a Lynch syndrome–associated cancer [94]. It is therefore likely that several patients with ACC will be candidates for anti–PD-1 therapy. However, success of anti–PD-1 therapy relies on several factors, including tumor immune infiltration, which is characteristically low in tumors resistant to anti–PD-1 therapy [95]. It will be essential to consider rational application of this therapy in patients with mismatch repair deficiency and prominent immune infiltrate. The recent development of a preclinical model of ACC from a patient with Lynch syndrome [96] represents a meaningful bench-to-bedside tool that will enable the evaluation of immunotherapy in different ACC subtypes through the use of humanized, patient-derived xenograft–bearing mice [97].
D-5. Combination therapies
It is evident that the landscape of molecular alterations in ACC is complex and tumor subtype specific [17, 19]. ACC-TCGA demonstrated that COC2-3 tumors, characterized by profound dysregulation of machinery coordinating cellular programs such as steroidogenesis and DNA methylation, are often driven by oncogenic programs leading to constitutive activation of Wnt/β-catenin signaling or cell cycle [19]. It is well known that ACC cells are sensitive in vitro to inhibition of cholesterol homeostasis and steroidogenesis by numerous agents, including mitotane and ATR-101 [98, 99], but it is less clear that monotherapy with inhibitors of steroidogenesis will be durably efficacious for most patients with COC2-3 ACC. Similarly, although ACC are apparently sensitive to DNA demethylation in vitro [56, 100, 101], there is no evidence supporting a clinical trial for patients with COC2-3 ACC that uses DNA demethylation monotherapy, especially given the additional molecular events that are common to these tumors. Tumor reliance on these biological programs presents a unique opportunity for combined therapies with DNA demethylating agents or inhibitors of steroidogenesis with inhibitors of Wnt/β-catenin signaling or cell cycle. Indeed, mitotane is known to synergize with other pharmaceutical agents to induce toxicity in ACC cells [102, 103]. In other solid tumors, pharmacological modulation of epigenetic programs has improved efficacy of standard-of-care cytotoxic agents and may be a promising therapeutic strategy for ACC [104, 105].
3. Conclusions
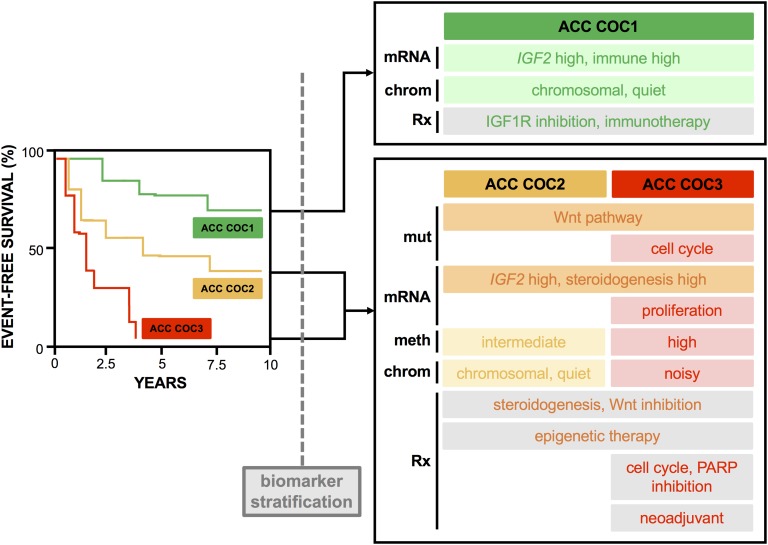
ACC is a rare, aggressive, and often fatal cancer. Most patients develop metastatic disease, which is largely incurable with currently available systemic agents; these statistics highlight a critical need for a deeper understanding of the molecular biology of ACC to fuel the development of more advanced medical therapies. Early genetics and genomics studies on ACC have resulted in enormous advances in the understanding of key pathways contributing to adrenocortical carcinogenesis. Integrative, comprehensive omics studies demonstrate that ACC consists of three distinct molecular subtypes with divergent clinical outcomes, and they implicate differential regulation of Wnt signaling, cell cycle, DNA methylation, immune biology, and steroidogenesis in ACC biology. These findings have illuminated a spectrum of currently available targeted therapies that may have efficacy as monotherapy or combination therapy in distinct subtypes of ACC, as described in this review. Importantly, individual therapeutic approaches are unlikely to be efficacious in all ACC subtypes. As we consider directing these targeted therapies to patients with ACC, it will be critical to accurately stratify patients according to molecular subtype by using molecular biomarkers that permit prospective assessment (Fig. 1).
Figure 1.
Comprehensive omics studies reveal ACC consists of three molecular subtypes with distinct clinical outcomes and therapeutic targets. Comprehensive, integrated omics studies have transformed the understanding of molecular programs driving adrenocortical carcinogenesis. In particular, ACC-TCGA [19] demonstrated that ACC consists of three molecular subtypes with distinct clinical outcomes (COC1, good prognosis; COC2, intermediate prognosis; and COC3, poor prognosis). Each ACC subtype is predicted to be driven by distinct genetic, transcriptional, and epigenetic programs that are largely pharmacologically targetable. The unique clinical features of each group are captured with a hypothetical event-free survival curve (left). The unique molecular features of each group (largely informed by ACC-TCGA [19]) are captured below each category; mut refers to subtype-specific somatic alteration profile, mRNA refers to subtype-specific transcriptional program, meth refers to subtype-specific DNA methylation/CIMP signature, chrom refers to subtype-specific somatic copy number alteration signature, and Rx refers to proposed relevant therapeutic strategy. Importantly, application of these therapies to ACC will require the development of prospective biomarkers that accurately stratify patients into each molecular subtype. Survival curve and terminology of this figure (e.g., “COC1,” “COC2,” and “COC3”) are adapted from ACC-TCGA [19].
Acknowledgments
Financial Support: This work is supported by the University of Michigan Rogel Cancer Center (TACR Program Funds to G.D.H.). D.R.M. is supported by the University of Michigan Medical Scientist Training Program (grants 5 T32 GM 7863-35 and 5 T32 GM 7863-37), the University of Michigan Doctoral Program in Cancer Biology, and the University of Michigan Rogel Cancer Center.
Disclosure Summary: G.D.H. is cofounder of, has equity interest in, and is a consultant for Millendo Therapeutics. The remaining authors have nothing to disclose.
Glossary
Abbreviations:
- ACA
adrenocortical adenoma
- ACC
adrenocortical carcinoma
- ACC-TCGA
The Cancer Genome Atlas study on adrenocortical carcinoma
- CIMP
CpG island methylator phenotype
- EDP-M
etoposide, doxorubicin, cisplatin, and mitotane
- LOH
loss of heterozygosity
- Sz-M
streptozotocin and mitotane
- TCGA
The Cancer Genome Atlas
- WGD
whole genome doubling
References and Notes
- 1. Kerkhofs TM, Verhoeven RH, Van der Zwan JM, Dieleman J, Kerstens MN, Links TP, Van de Poll-Franse LV, Haak HR. Adrenocortical carcinoma: a population-based study on incidence and survival in the Netherlands since 1993. Eur J Cancer. 2013;49(11):2579–2586. [DOI] [PubMed] [Google Scholar]
- 2. Wajchenberg BL, Albergaria Pereira MA, Medonca BB, Latronico AC, Campos Carneiro P, Alves VA, Zerbini MC, Liberman B, Carlos Gomes G, Kirschner MA. Adrenocortical carcinoma: clinical and laboratory observations. Cancer. 2000;88(4):711–736. [PubMed] [Google Scholar]
- 3. Wooten MD, King DK. Adrenal cortical carcinoma. Epidemiology and treatment with mitotane and a review of the literature. Cancer. 1993;72(11):3145–3155. [DOI] [PubMed] [Google Scholar]
- 4. Else T, Kim AC, Sabolch A, Raymond VM, Kandathil A, Caoili EM, Jolly S, Miller BS, Giordano TJ, Hammer GD. Adrenocortical carcinoma. Endocr Rev. 2014;35(2):282–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fassnacht M, Kroiss M, Allolio B. Update in adrenocortical carcinoma. J Clin Endocrinol Metab. 2013;98(12):4551–4564. [DOI] [PubMed] [Google Scholar]
- 6. Else T, Williams AR, Sabolch A, Jolly S, Miller BS, Hammer GD. Adjuvant therapies and patient and tumor characteristics associated with survival of adult patients with adrenocortical carcinoma. J Clin Endocrinol Metab. 2014;99(2):455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Berruti A, Grisanti S, Pulzer A, Claps M, Daffara F, Loli P, Mannelli M, Boscaro M, Arvat E, Tiberio G, Hahner S, Zaggia B, Porpiglia F, Volante M, Fassnacht M, Terzolo M. Long-term outcomes of adjuvant mitotane therapy in patients with radically resected adrenocortical carcinoma. J Clin Endocrinol Metab. 2017;102(4):1358–1365. [DOI] [PubMed] [Google Scholar]
- 8. Terzolo M, Angeli A, Fassnacht M, Daffara F, Tauchmanova L, Conton PA, Rossetto R, Buci L, Sperone P, Grossrubatscher E, Reimondo G, Bollito E, Papotti M, Saeger W, Hahner S, Koschker AC, Arvat E, Ambrosi B, Loli P, Lombardi G, Mannelli M, Bruzzi P, Mantero F, Allolio B, Dogliotti L, Berruti A. Adjuvant mitotane treatment for adrenocortical carcinoma. N Engl J Med. 2007;356(23):2372–2380. [DOI] [PubMed] [Google Scholar]
- 9. Megerle F, Herrmann W, Schloetelburg W, Ronchi CL, Pulzer A, Quinkler M, Beuschlein F, Hahner S, Kroiss M, Fassnacht M; German ACC Study Group . Mitotane monotherapy in patients with advanced adrenocortical carcinoma. J Clin Endocrinol Metab. 2018;103(4):1686–1695. [DOI] [PubMed] [Google Scholar]
- 10. Terzolo M, Baudin AE, Ardito A, Kroiss M, Leboulleux S, Daffara F, Perotti P, Feelders RA, deVries JH, Zaggia B, De Francia S, Volante M, Haak HR, Allolio B, Al Ghuzlan A, Fassnacht M, Berruti A. Mitotane levels predict the outcome of patients with adrenocortical carcinoma treated adjuvantly following radical resection. Eur J Endocrinol. 2013;169(3):263–270. [DOI] [PubMed] [Google Scholar]
- 11. Berruti A, Terzolo M, Pia A, Angeli A, Dogliotti L; Italian Group for the Study of Adrenal Cancer . Mitotane associated with etoposide, doxorubicin, and cisplatin in the treatment of advanced adrenocortical carcinoma. Cancer. 1998;83(10):2194–2200. [PubMed] [Google Scholar]
- 12. Khan TS, Imam H, Juhlin C, Skogseid B, Gröndal S, Tibblin S, Wilander E, Oberg K, Eriksson B. Streptozocin and o,p’DDD in the treatment of adrenocortical cancer patients: long-term survival in its adjuvant use. Ann Oncol. 2000;11(10):1281–1287. [DOI] [PubMed] [Google Scholar]
- 13. Fassnacht M, Terzolo M, Allolio B, Baudin E, Haak H, Berruti A, Welin S, Schade-Brittinger C, Lacroix A, Jarzab B, Sorbye H, Torpy DJ, Stepan V, Schteingart DE, Arlt W, Kroiss M, Leboulleux S, Sperone P, Sundin A, Hermsen I, Hahner S, Willenberg HS, Tabarin A, Quinkler M, de la Fouchardière C, Schlumberger M, Mantero F, Weismann D, Beuschlein F, Gelderblom H, Wilmink H, Sender M, Edgerly M, Kenn W, Fojo T, Müller HH, Skogseid B; FIRM-ACT Study Group . Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med. 2012;366(23):2189–2197. [DOI] [PubMed] [Google Scholar]
- 14. Fassnacht M, Libé R, Kroiss M, Allolio B. Adrenocortical carcinoma: a clinician’s update. Nat Rev Endocrinol. 2011;7(6):323–335. [DOI] [PubMed] [Google Scholar]
- 15. Varghese J, Habra MA. Update on adrenocortical carcinoma management and future directions. Curr Opin Endocrinol Diabetes Obes. 2017;24(3):208–214. [DOI] [PubMed] [Google Scholar]
- 16. PMID:30299884.
- 17. Assié G, Letouzé E, Fassnacht M, Jouinot A, Luscap W, Barreau O, Omeiri H, Rodriguez S, Perlemoine K, René-Corail F, Elarouci N, Sbiera S, Kroiss M, Allolio B, Waldmann J, Quinkler M, Mannelli M, Mantero F, Papathomas T, De Krijger R, Tabarin A, Kerlan V, Baudin E, Tissier F, Dousset B, Groussin L, Amar L, Clauser E, Bertagna X, Ragazzon B, Beuschlein F, Libé R, de Reyniès A, Bertherat J. Integrated genomic characterization of adrenocortical carcinoma. Nat Genet. 2014;46(6):607–612. [DOI] [PubMed] [Google Scholar]
- 18. Juhlin CC, Goh G, Healy JM, Fonseca AL, Scholl UI, Stenman A, Kunstman JW, Brown TC, Overton JD, Mane SM, Nelson-Williams C, Bäckdahl M, Suttorp AC, Haase M, Choi M, Schlessinger J, Rimm DL, Höög A, Prasad ML, Korah R, Larsson C, Lifton RP, Carling T. Whole-exome sequencing characterizes the landscape of somatic mutations and copy number alterations in adrenocortical carcinoma. J Clin Endocrinol Metab. 2015;100(3):E493–E502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zheng S, Cherniack AD, Dewal N, Moffitt RA, Danilova L, Murray BA, Lerario AM, Else T, Knijnenburg TA, Ciriello G, Kim S, Assié G, Morozova O, Akbani R, Shih J, Hoadley KA, Choueiri TK, Waldmann J, Mete O, Robertson AG, Wu HT, Raphael BJ, Shao L, Meyerson M, Demeure MJ, Beuschlein F, Gill AJ, Sidhu SB, Almeida MQ, Fragoso MCBV, Cope LM, Kebebew E, Habra MA, Whitsett TG, Bussey KJ, Rainey WE, Asa SL, Bertherat J, Fassnacht M, Wheeler DA, Hammer GD, Giordano TJ, Verhaak RGW; Cancer Genome Atlas Research Network . Comprehensive pan-genomic characterization of adrenocortical carcinoma [published correction appears in Cancer Cell. 2016;30(2):P363] Cancer Cell. 2016;29(5):723–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Suzuki T, Sasano H, Nisikawa T, Rhame J, Wilkinson DS, Nagura H. Discerning malignancy in human adrenocortical neoplasms: utility of DNA flow cytometry and immunohistochemistry. Mod Pathol. 1992;5(3):224–231. [PubMed] [Google Scholar]
- 21. Weiss LM. Comparative histologic study of 43 metastasizing and nonmetastasizing adrenocortical tumors. Am J Surg Pathol. 1984;8(3):163–169. [DOI] [PubMed] [Google Scholar]
- 22. Weiss LM, Medeiros LJ, Vickery AL Jr. Pathologic features of prognostic significance in adrenocortical carcinoma. Am J Surg Pathol. 1989;13(3):202–206. [DOI] [PubMed] [Google Scholar]
- 23. Dohna M, Reincke M, Mincheva A, Allolio B, Solinas-Toldo S, Lichter P. Adrenocortical carcinoma is characterized by a high frequency of chromosomal gains and high-level amplifications. Genes Chromosomes Cancer. 2000;28(2):145–152. [PubMed] [Google Scholar]
- 24. Figueiredo BC, Stratakis CA, Sandrini R, DeLacerda L, Pianovsky MA, Giatzakis C, Young HM, Haddad BR. Comparative genomic hybridization analysis of adrenocortical tumors of childhood. J Clin Endocrinol Metab. 1999;84(3):1116–1121. [DOI] [PubMed] [Google Scholar]
- 25. Kjellman M, Kallioniemi OP, Karhu R, Höög A, Farnebo LO, Auer G, Larsson C, Bäckdahl M. Genetic aberrations in adrenocortical tumors detected using comparative genomic hybridization correlate with tumor size and malignancy. Cancer Res. 1996;56(18):4219–4223. [PubMed] [Google Scholar]
- 26. Sidhu S, Marsh DJ, Theodosopoulos G, Philips J, Bambach CP, Campbell P, Magarey CJ, Russell CF, Schulte KM, Röher HD, Delbridge L, Robinson BG. Comparative genomic hybridization analysis of adrenocortical tumors. J Clin Endocrinol Metab. 2002;87(7):3467–3474. [DOI] [PubMed] [Google Scholar]
- 27. Zhao J, Roth J, Bode-Lesniewska B, Pfaltz M, Heitz PU, Komminoth P. Combined comparative genomic hybridization and genomic microarray for detection of gene amplifications in pulmonary artery intimal sarcomas and adrenocortical tumors. Genes Chromosomes Cancer. 2002;34(1):48–57. [DOI] [PubMed] [Google Scholar]
- 28. Kleihues P, Schäuble B, zur Hausen A, Estève J, Ohgaki H. Tumors associated with p53 germline mutations: a synopsis of 91 families. Am J Pathol. 1997;150(1):1–13. [PMC free article] [PubMed] [Google Scholar]
- 29. Lapunzina P. Risk of tumorigenesis in overgrowth syndromes: a comprehensive review. Am J Med Genet C Semin Med Genet. 2005;137C(1):53–71. [DOI] [PubMed] [Google Scholar]
- 30. Wagner J, Portwine C, Rabin K, Leclerc JM, Narod SA, Malkin D. High frequency of germline p53 mutations in childhood adrenocortical cancer. J Natl Cancer Inst. 1994;86(22):1707–1710. [DOI] [PubMed] [Google Scholar]
- 31. Wasserman JD, Novokmet A, Eichler-Jonsson C, Ribeiro RC, Rodriguez-Galindo C, Zambetti GP, Malkin D. Prevalence and functional consequence of TP53 mutations in pediatric adrenocortical carcinoma: a Children’s Oncology Group study. J Clin Oncol. 2015;33(6):602–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Weksberg R, Shuman C, Beckwith JB. Beckwith-Wiedemann syndrome. Eur J Hum Genet. 2010;18(1):8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gaujoux S, Pinson S, Gimenez-Roqueplo AP, Amar L, Ragazzon B, Launay P, Meatchi T, Libé R, Bertagna X, Audebourg A, Zucman-Rossi J, Tissier F, Bertherat J. Inactivation of the APC gene is constant in adrenocortical tumors from patients with familial adenomatous polyposis but not frequent in sporadic adrenocortical cancers. Clin Cancer Res. 2010;16(21):5133–5141. [DOI] [PubMed] [Google Scholar]
- 34. Griniatsos JE, Dimitriou N, Zilos A, Sakellariou S, Evangelou K, Kamakari S, Korkolopoulou P, Kaltsas G. Bilateral adrenocortical carcinoma in a patient with multiple endocrine neoplasia type 1 (MEN1) and a novel mutation in the MEN1 gene. World J Surg Oncol. 2011;9(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Skogseid B, Larsson C, Lindgren PG, Kvanta E, Rastad J, Theodorsson E, Wide L, Wilander E, Oberg K. Clinical and genetic features of adrenocortical lesions in multiple endocrine neoplasia type 1. J Clin Endocrinol Metab. 1992;75(1):76–81. [DOI] [PubMed] [Google Scholar]
- 36. Gicquel C, Leblond-Francillard M, Bertagna X, Louvel A, Chapuis Y, Luton JP, Girard F, Le Bouc Y. Clonal analysis of human adrenocortical carcinomas and secreting adenomas. Clin Endocrinol (Oxf). 1994;40(4):465–477. [DOI] [PubMed] [Google Scholar]
- 37. Gicquel C, Raffin-Sanson ML, Gaston V, Bertagna X, Plouin PF, Schlumberger M, Louvel A, Luton JP, Le Bouc Y. Structural and functional abnormalities at 11p15 are associated with the malignant phenotype in sporadic adrenocortical tumors: study on a series of 82 tumors. J Clin Endocrinol Metab. 1997;82(8):2559–2565. [DOI] [PubMed] [Google Scholar]
- 38. Barzon L, Chilosi M, Fallo F, Martignoni G, Montagna L, Palù G, Boscaro M. Molecular analysis of CDKN1C and TP53 in sporadic adrenal tumors. Eur J Endocrinol. 2001;145(2):207–212. [DOI] [PubMed] [Google Scholar]
- 39. Libè R, Groussin L, Tissier F, Elie C, René-Corail F, Fratticci A, Jullian E, Beck-Peccoz P, Bertagna X, Gicquel C, Bertherat J. Somatic TP53 mutations are relatively rare among adrenocortical cancers with the frequent 17p13 loss of heterozygosity. Clin Cancer Res. 2007;13(3):844–850. [DOI] [PubMed] [Google Scholar]
- 40. Lin SR, Lee YJ, Tsai JH. Mutations of the p53 gene in human functional adrenal neoplasms. J Clin Endocrinol Metab. 1994;78(2):483–491. [DOI] [PubMed] [Google Scholar]
- 41. Ohgaki H, Kleihues P, Heitz PU. p53 mutations in sporadic adrenocortical tumors. Int J Cancer. 1993;54(3):408–410. [DOI] [PubMed] [Google Scholar]
- 42. Reincke M, Karl M, Travis WH, Mastorakos G, Allolio B, Linehan HM, Chrousos GP. p53 mutations in human adrenocortical neoplasms: immunohistochemical and molecular studies. J Clin Endocrinol Metab. 1994;78(3):790–794. [DOI] [PubMed] [Google Scholar]
- 43. Görtz B, Roth J, Speel EJ, Krähenmann A, De Krijger RR, Matias-Guiu X, Muletta-Feurer S, Rütmann K, Saremaslani P, Heitz PU, Komminoth P. MEN1 gene mutation analysis of sporadic adrenocortical lesions. Int J Cancer. 1999;80(3):373–379. [DOI] [PubMed] [Google Scholar]
- 44. Heppner C, Reincke M, Agarwal SK, Mora P, Allolio B, Burns AL, Spiegel AM, Marx SJ. MEN1 gene analysis in sporadic adrenocortical neoplasms. J Clin Endocrinol Metab. 1999;84(1):216–219. [DOI] [PubMed] [Google Scholar]
- 45. Kjellman M, Roshani L, Teh BT, Kallioniemi OP, Höög A, Gray S, Farnebo LO, Holst M, Bäckdahl M, Larsson C. Genotyping of adrenocortical tumors: very frequent deletions of the MEN1 locus in 11q13 and of a 1-centimorgan region in 2p16. J Clin Endocrinol Metab. 1999;84(2):730–735. [DOI] [PubMed] [Google Scholar]
- 46. Yano T, Linehan M, Anglard P, Lerman MI, Daniel LN, Stein CA, Robertson CN, LaRocca R, Zbar B. Genetic changes in human adrenocortical carcinomas. J Natl Cancer Inst. 1989;81(7):518–523. [DOI] [PubMed] [Google Scholar]
- 47. Gaujoux S, Grabar S, Fassnacht M, Ragazzon B, Launay P, Libé R, Chokri I, Audebourg A, Royer B, Sbiera S, Vacher-Lavenu MC, Dousset B, Bertagna X, Allolio B, Bertherat J, Tissier F. β-catenin activation is associated with specific clinical and pathologic characteristics and a poor outcome in adrenocortical carcinoma. Clin Cancer Res. 2011;17(2):328–336. [DOI] [PubMed] [Google Scholar]
- 48. Tissier F, Cavard C, Groussin L, Perlemoine K, Fumey G, Hagneré AM, René-Corail F, Jullian E, Gicquel C, Bertagna X, Vacher-Lavenu MC, Perret C, Bertherat J. Mutations of beta-catenin in adrenocortical tumors: activation of the Wnt signaling pathway is a frequent event in both benign and malignant adrenocortical tumors. Cancer Res. 2005;65(17):7622–7627. [DOI] [PubMed] [Google Scholar]
- 49. Giordano TJ, Kuick R, Else T, Gauger PG, Vinco M, Bauersfeld J, Sanders D, Thomas DG, Doherty G, Hammer G. Molecular classification and prognostication of adrenocortical tumors by transcriptome profiling. Clin Cancer Res. 2009;15(2):668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Giordano TJ, Thomas DG, Kuick R, Lizyness M, Misek DE, Smith AL, Sanders D, Aljundi RT, Gauger PG, Thompson NW, Taylor JM, Hanash SM. Distinct transcriptional profiles of adrenocortical tumors uncovered by DNA microarray analysis. Am J Pathol. 2003;162(2):521–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. de Fraipont F, El Atifi M, Cherradi N, Le Moigne G, Defaye G, Houlgatte R, Bertherat J, Bertagna X, Plouin PF, Baudin E, Berger F, Gicquel C, Chabre O, Feige JJ. Gene expression profiling of human adrenocortical tumors using complementary deoxyribonucleic acid microarrays identifies several candidate genes as markers of malignancy. J Clin Endocrinol Metab. 2005;90(3):1819–1829. [DOI] [PubMed] [Google Scholar]
- 52. Fernandez-Ranvier GG, Weng J, Yeh RF, Khanafshar E, Suh I, Barker C, Duh QY, Clark OH, Kebebew E. Identification of biomarkers of adrenocortical carcinoma using genomewide gene expression profiling. Arch Surg. 2008;143(9):841–846, discussion 846. [DOI] [PubMed] [Google Scholar]
- 53. de Reyniès A, Assié G, Rickman DS, Tissier F, Groussin L, René-Corail F, Dousset B, Bertagna X, Clauser E, Bertherat J. Gene expression profiling reveals a new classification of adrenocortical tumors and identifies molecular predictors of malignancy and survival. J Clin Oncol. 2009;27(7):1108–1115. [DOI] [PubMed] [Google Scholar]
- 54. Fragoso MC, Almeida MQ, Mazzuco TL, Mariani BM, Brito LP, Gonçalves TC, Alencar GA, Lima LO, Faria AM, Bourdeau I, Lucon AM, Freire DS, Latronico AC, Mendonca BB, Lacroix A, Lerario AM. Combined expression of BUB1B, DLGAP5, and PINK1 as predictors of poor outcome in adrenocortical tumors: validation in a Brazilian cohort of adult and pediatric patients. Eur J Endocrinol. 2012;166(1):61–67. [DOI] [PubMed] [Google Scholar]
- 55. Ragazzon B, Libé R, Gaujoux S, Assié G, Fratticci A, Launay P, Clauser E, Bertagna X, Tissier F, de Reyniès A, Bertherat J. Transcriptome analysis reveals that p53 and beta-catenin alterations occur in a group of aggressive adrenocortical cancers. Cancer Res. 2010;70(21):8276–8281. [DOI] [PubMed] [Google Scholar]
- 56. Fonseca AL, Kugelberg J, Starker LF, Scholl U, Choi M, Hellman P, Åkerström G, Westin G, Lifton RP, Björklund P, Carling T. Comprehensive DNA methylation analysis of benign and malignant adrenocortical tumors. Genes Chromosomes Cancer. 2012;51(10):949–960. [DOI] [PubMed] [Google Scholar]
- 57. Rechache NS, Wang Y, Stevenson HS, Killian JK, Edelman DC, Merino M, Zhang L, Nilubol N, Stratakis CA, Meltzer PS, Kebebew E. DNA methylation profiling identifies global methylation differences and markers of adrenocortical tumors. J Clin Endocrinol Metab. 2012;97(6):E1004–E1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Barreau O, Assié G, Wilmot-Roussel H, Ragazzon B, Baudry C, Perlemoine K, René-Corail F, Bertagna X, Dousset B, Hamzaoui N, Tissier F, de Reynies A, Bertherat J. Identification of a CpG island methylator phenotype in adrenocortical carcinomas. J Clin Endocrinol Metab. 2013;98(1):E174–E184. [DOI] [PubMed] [Google Scholar]
- 59. Soon PS, Tacon LJ, Gill AJ, Bambach CP, Sywak MS, Campbell PR, Yeh MW, Wong SG, Clifton-Bligh RJ, Robinson BG, Sidhu SB. miR-195 and miR-483-5p identified as predictors of poor prognosis in adrenocortical cancer. Clin Cancer Res. 2009;15(24):7684–7692. [DOI] [PubMed] [Google Scholar]
- 60. Tömböl Z, Szabó PM, Molnár V, Wiener Z, Tölgyesi G, Horányi J, Riesz P, Reismann P, Patócs A, Likó I, Gaillard RC, Falus A, Rácz K, Igaz P. Integrative molecular bioinformatics study of human adrenocortical tumors: microRNA, tissue-specific target prediction, and pathway analysis. Endocr Relat Cancer. 2009;16(3):895–906. [DOI] [PubMed] [Google Scholar]
- 61. Lerario AM, Moraitis A, Hammer GD. Genetics and epigenetics of adrenocortical tumors. Mol Cell Endocrinol. 2014;386(1–2):67–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ronchi CL, Sbiera S, Leich E, Henzel K, Rosenwald A, Allolio B, Fassnacht M. Single nucleotide polymorphism array profiling of adrenocortical tumors--evidence for an adenoma carcinoma sequence? PLoS One. 2013;8(9):e73959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hao HX, Xie Y, Zhang Y, Charlat O, Oster E, Avello M, Lei H, Mickanin C, Liu D, Ruffner H, Mao X, Ma Q, Zamponi R, Bouwmeester T, Finan PM, Kirschner MW, Porter JA, Serluca FC, Cong F. ZNRF3 promotes Wnt receptor turnover in an R-spondin–sensitive manner. Nature. 2012;485(7397):195–200. [DOI] [PubMed] [Google Scholar]
- 64. Barlaskar FM, Spalding AC, Heaton JH, Kuick R, Kim AC, Thomas DG, Giordano TJ, Ben-Josef E, Hammer GD. Preclinical targeting of the type I insulin-like growth factor receptor in adrenocortical carcinoma. J Clin Endocrinol Metab. 2009;94(1):204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Haluska P, Worden F, Olmos D, Yin D, Schteingart D, Batzel GN, Paccagnella ML, de Bono JS, Gualberto A, Hammer GD. Safety, tolerability, and pharmacokinetics of the anti-IGF-1R monoclonal antibody figitumumab in patients with refractory adrenocortical carcinoma. Cancer Chemother Pharmacol. 2010;65(4):765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lerario AM, Worden FP, Ramm CA, Hesseltine EA, Stadler WM, Else T, Shah MH, Agamah E, Rao K, Hammer GD. The combination of insulin-like growth factor receptor 1 (IGF1R) antibody cixutumumab and mitotane as a first-line therapy for patients with recurrent/metastatic adrenocortical carcinoma: a multi-institutional NCI-sponsored trial [published correction appears in Horm Cancer. 2014;5(6):424] Horm Cancer. 2014;5(4):232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fassnacht M, Berruti A, Baudin E, Demeure MJ, Gilbert J, Haak H, Kroiss M, Quinn DI, Hesseltine E, Ronchi CL, Terzolo M, Choueiri TK, Poondru S, Fleege T, Rorig R, Chen J, Stephens AW, Worden F, Hammer GD. Linsitinib (OSI-906) versus placebo for patients with locally advanced or metastatic adrenocortical carcinoma: a double-blind, randomised, phase 3 study. Lancet Oncol. 2015;16(4):426–435. [DOI] [PubMed] [Google Scholar]
- 68. Heaton JH, Wood MA, Kim AC, Lima LO, Barlaskar FM, Almeida MQ, Fragoso MC, Kuick R, Lerario AM, Simon DP, Soares IC, Starnes E, Thomas DG, Latronico AC, Giordano TJ, Hammer GD. Progression to adrenocortical tumorigenesis in mice and humans through insulin-like growth factor 2 and β-catenin. Am J Pathol. 2012;181(3):1017–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Doghman M, Cazareth J, Lalli E. The T cell factor/beta-catenin antagonist PKF115-584 inhibits proliferation of adrenocortical carcinoma cells. J Clin Endocrinol Metab. 2008;93(8):3222–3225. [DOI] [PubMed] [Google Scholar]
- 70. Leal LF, Bueno AC, Gomes DC, Abduch R, de Castro M, Antonini SR. Inhibition of the Tcf/beta-catenin complex increases apoptosis and impairs adrenocortical tumor cell proliferation and adrenal steroidogenesis. Oncotarget. 2015;6(40):43016–43032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gaujoux S, Hantel C, Launay P, Bonnet S, Perlemoine K, Lefèvre L, Guillaud-Bataille M, Beuschlein F, Tissier F, Bertherat J, Rizk-Rabin M, Ragazzon B. Silencing mutated β-catenin inhibits cell proliferation and stimulates apoptosis in the adrenocortical cancer cell line H295R. PLoS One. 2013;8(2):e55743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kim AC, Reuter AL, Zubair M, Else T, Serecky K, Bingham NC, Lavery GG, Parker KL, Hammer GD. Targeted disruption of beta-catenin in Sf1-expressing cells impairs development and maintenance of the adrenal cortex. Development. 2008;135(15):2593–2602. [DOI] [PubMed] [Google Scholar]
- 73. Kahn M. Can we safely target the WNT pathway? Nat Rev Drug Discov. 2014;13(7):513–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Jiang X, Hao HX, Growney JD, Woolfenden S, Bottiglio C, Ng N, Lu B, Hsieh MH, Bagdasarian L, Meyer R, Smith TR, Avello M, Charlat O, Xie Y, Porter JA, Pan S, Liu J, McLaughlin ME, Cong F. Inactivating mutations of RNF43 confer Wnt dependency in pancreatic ductal adenocarcinoma. Proc Natl Acad Sci USA. 2013;110(31):12649–12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Koo BK, van Es JH, van den Born M, Clevers H. Porcupine inhibitor suppresses paracrine Wnt-driven growth of Rnf43;Znrf3-mutant neoplasia. Proc Natl Acad Sci USA. 2015;112(24):7548–7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Funck-Brentano T, Nilsson KH, Brommage R, Henning P, Lerner UH, Koskela A, Tuukkanen J, Cohen-Solal M, Movérare-Skrtic S, Ohlsson C. Porcupine inhibitors impair trabecular and cortical bone mass and strength in mice. J Endocrinol. 2018;238(1):13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Boone JD, Arend RC, Johnston BE, Cooper SJ, Gilchrist SA, Oelschlager DK, Grizzle WE, McGwin G Jr, Gangrade A, Straughn JM Jr, Buchsbaum DJ. Targeting the Wnt/β-catenin pathway in primary ovarian cancer with the porcupine inhibitor WNT974. Lab Invest. 2016;96(2):249–259. [DOI] [PubMed] [Google Scholar]
- 78. Beuschlein F, Weigel J, Saeger W, Kroiss M, Wild V, Daffara F, Libé R, Ardito A, Al Ghuzlan A, Quinkler M, Oßwald A, Ronchi CL, de Krijger R, Feelders RA, Waldmann J, Willenberg HS, Deutschbein T, Stell A, Reincke M, Papotti M, Baudin E, Tissier F, Haak HR, Loli P, Terzolo M, Allolio B, Müller HH, Fassnacht M. Major prognostic role of Ki67 in localized adrenocortical carcinoma after complete resection. J Clin Endocrinol Metab. 2015;100(3):841–849. [DOI] [PubMed] [Google Scholar]
- 79. Ip JC, Pang TC, Glover AR, Soon P, Zhao JT, Clarke S, Robinson BG, Gill AJ, Sidhu SB. Immunohistochemical validation of overexpressed genes identified by global expression microarrays in adrenocortical carcinoma reveals potential predictive and prognostic biomarkers. Oncologist. 2015;20(3):247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Roca E, Berruti A, Sbiera S, Rapa I, Oneda E, Sperone P, Ronchi CL, Ferrari L, Grisanti S, Germano A, Zaggia B, Scagliotti GV, Fassnacht M, Volante M, Terzolo M, Papotti M. Topoisomerase 2α and thymidylate synthase expression in adrenocortical cancer. Endocr Relat Cancer. 2017;24(7):299–307. [DOI] [PubMed] [Google Scholar]
- 81. Henning JEK, Deutschbein T, Altieri B, Steinhauer S, Kircher S, Sbiera S, Wild V, Schlötelburg W, Kroiss M, Perotti P, Rosenwald A, Berruti A, Fassnacht M, Ronchi CL. Gemcitabine-based chemotherapy in adrenocortical carcinoma: a multicenter study of efficacy and predictive factors. J Clin Endocrinol Metab. 2017;102(11):4323–4332. [DOI] [PubMed] [Google Scholar]
- 82. Sperone P, Ferrero A, Daffara F, Priola A, Zaggia B, Volante M, Santini D, Vincenzi B, Badalamenti G, Intrivici C, Del Buono S, De Francia S, Kalomirakis E, Ratti R, Angeli A, Dogliotti L, Papotti M, Terzolo M, Berruti A. Gemcitabine plus metronomic 5-fluorouracil or capecitabine as a second-/third-line chemotherapy in advanced adrenocortical carcinoma: a multicenter phase II study. Endocr Relat Cancer. 2010;17(2):445–453. [DOI] [PubMed] [Google Scholar]
- 83. Dominguez-Brauer C, Thu KL, Mason JM, Blaser H, Bray MR, Mak TW. Targeting mitosis in cancer: emerging strategies. Mol Cell. 2015;60(4):524–536. [DOI] [PubMed] [Google Scholar]
- 84. Ferrero A, Sperone P, Ardito A, Rossi G, Del Buono S, Priola AM, Bracarda S, Taberna E, Terzolo M, Berruti A. Metronomic chemotherapy may be active in heavily pre-treated patients with metastatic adreno-cortical carcinoma. J Endocrinol Invest. 2013;36(3):148–152. [DOI] [PubMed] [Google Scholar]
- 85. Fiorentini C, Fragni M, Tiberio GAM, Galli D, Roca E, Salvi V, Bosisio D, Missale C, Terzolo M, Memo M, Berruti A, Sigala S. Palbociclib inhibits proliferation of human adrenocortical tumor cells. Endocrine. 2018;59(1):213–217. [DOI] [PubMed] [Google Scholar]
- 86. Hadjadj D, Kim SJ, Denecker T, Ben Driss L, Cadoret JC, Maric C, Baldacci G, Fauchereau F. A hypothesis-driven approach identifies CDK4 and CDK6 inhibitors as candidate drugs for treatments of adrenocortical carcinomas. Aging (Albany NY). 2017;9(12):2695–2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bussey KJ, Bapat A, Linnehan C, Wandoloski M, Dastrup E, Rogers E, Gonzales P, Demeure MJ. Targeting polo-like kinase 1, a regulator of p53, in the treatment of adrenocortical carcinoma. Clin Transl Med. 2016;5(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Borges KS, Andrade AF, Silveira VS, Marco Antonio DS, Vasconcelos EJR, Antonini SRR, Tone LG, Scrideli CA. The aurora kinase inhibitor AMG 900 increases apoptosis and induces chemosensitivity to anticancer drugs in the NCI-H295 adrenocortical carcinoma cell line. Anticancer Drugs. 2017;28(6):634–644. [DOI] [PubMed] [Google Scholar]
- 89. Kiseljak-Vassiliades K, Zhang Y, Kar A, Razzaghi R, Xu M, Gowan K, Raeburn CD, Albuja-Cruz M, Jones KL, Somerset H, Fishbein L, Leong S, Wierman ME. Elucidating the role of the maternal embryonic leucine zipper kinase in adrenocortical carcinoma. Endocrinology. 2018;159(7):2532–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Otto T, Sicinski P. Cell cycle proteins as promising targets in cancer therapy. Nat Rev Cancer. 2017;17(2):93–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Knijnenburg TA, Wang L, Zimmermann MT, et al. Genomic and molecular landscape of DNA damage repair deficiency across The Cancer Genome Atlas. Cell Rep. 2018;23(1):239–254 e236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Mateo J, Carreira S, Sandhu S, Miranda S, Mossop H, Perez-Lopez R, Nava Rodrigues D, Robinson D, Omlin A, Tunariu N, Boysen G, Porta N, Flohr P, Gillman A, Figueiredo I, Paulding C, Seed G, Jain S, Ralph C, Protheroe A, Hussain S, Jones R, Elliott T, McGovern U, Bianchini D, Goodall J, Zafeiriou Z, Williamson CT, Ferraldeschi R, Riisnaes R, Ebbs B, Fowler G, Roda D, Yuan W, Wu YM, Cao X, Brough R, Pemberton H, A’Hern R, Swain A, Kunju LP, Eeles R, Attard G, Lord CJ, Ashworth A, Rubin MA, Knudsen KE, Feng FY, Chinnaiyan AM, Hall E, de Bono JS. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373(18):1697–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Thorsson V, Gibbs DL, Brown SD, et al. The immune landscape of cancer. Immunity. 2018;48(4):812–830 e814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Raymond VM, Everett JN, Furtado LV, Gustafson SL, Jungbluth CR, Gruber SB, Hammer GD, Stoffel EM, Greenson JK, Giordano TJ, Else T. Adrenocortical carcinoma is a lynch syndrome-associated cancer. J Clin Oncol. 2013;31(24):3012–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20(19):5064–5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kiseljak-Vassiliades K, Zhang Y, Bagby SM, Kar A, Pozdeyev N, Xu M, Gowan K, Sharma V, Raeburn CD, Albuja-Cruz M, Jones KL, Fishbein L, Schweppe RE, Somerset H, Pitts TM, Leong S, Wierman ME. Development of new preclinical models to advance adrenocortical carcinoma research. Endocr Relat Cancer. 2018;25(4):437–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zumwalde NA, Gumperz JE. Modeling human antitumor responses in vivo using umbilical cord blood-engrafted mice. Front Immunol. 2018;9:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sbiera S, Leich E, Liebisch G, Sbiera I, Schirbel A, Wiemer L, Matysik S, Eckhardt C, Gardill F, Gehl A, Kendl S, Weigand I, Bala M, Ronchi CL, Deutschbein T, Schmitz G, Rosenwald A, Allolio B, Fassnacht M, Kroiss M. Mitotane inhibits sterol-O-acyl transferase 1 triggering lipid-mediated endoplasmic reticulum stress and apoptosis in adrenocortical carcinoma cells. Endocrinology. 2015;156(11):3895–3908. [DOI] [PubMed] [Google Scholar]
- 99. LaPensee CR, Mann JE, Rainey WE, Crudo V, Hunt SW III, Hammer GD. ATR-101, a selective and potent inhibitor of acyl-CoA acyltransferase 1, induces apoptosis in H295R adrenocortical cells and in the adrenal cortex of dogs. Endocrinology. 2016;157(5):1775–1788. [DOI] [PubMed] [Google Scholar]
- 100. Liu J, Li XD, Vaheri A, Voutilainen R. DNA methylation affects cell proliferation, cortisol secretion and steroidogenic gene expression in human adrenocortical NCI-H295R cells. J Mol Endocrinol. 2004;33(3):651–662. [DOI] [PubMed] [Google Scholar]
- 101. Suh I, Weng J, Fernandez-Ranvier G, Shen WT, Duh QY, Clark OH, Kebebew E. Antineoplastic effects of decitabine, an inhibitor of DNA promoter methylation, in adrenocortical carcinoma cells. Arch Surg. 2010;145(3):226–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ruggiero C, Doghman-Bouguerra M, Ronco C, Benhida R, Rocchi S, Lalli E. The GRP78/BiP inhibitor HA15 synergizes with mitotane action against adrenocortical carcinoma cells through convergent activation of ER stress pathways. Mol Cell Endocrinol. 2018;474:57–64. [DOI] [PubMed] [Google Scholar]
- 103. Kroiss M, Sbiera S, Kendl S, Kurlbaum M, Fassnacht M. Drug synergism of proteasome inhibitors and mitotane by complementary activation of ER stress in adrenocortical carcinoma cells. Horm Cancer. 2016;7(5–6):345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Matei D, Fang F, Shen C, Schilder J, Arnold A, Zeng Y, Berry WA, Huang T, Nephew KP. Epigenetic resensitization to platinum in ovarian cancer. Cancer Res. 2012;72(9):2197–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Azad N, Zahnow CA, Rudin CM, Baylin SB. The future of epigenetic therapy in solid tumours--lessons from the past. Nat Rev Clin Oncol. 2013;10(5):256–266. [DOI] [PMC free article] [PubMed] [Google Scholar]