Abstract
Aging is typically associated with substantial declines in motor functioning as well as robust changes in the functional organization of brain networks. Previous research has investigated the link between these 2 age-varying factors but examinations were predominantly limited to the functional organization within motor-related brain networks. Little is known about the relationship between age-related behavioral impairments and changes in functional organization at the whole brain (i.e., multiple network) level. This knowledge gap is surprising given that the decreased segregation of brain networks (i.e., increased internetwork connectivity) can be considered a hallmark of the aging process. Accordingly, we investigated the association between declines in motor performance across the adult lifespan (20–75 years) and age-related modulations of functional connectivity within and between resting state networks. Results indicated that stronger internetwork resting state connectivity observed as a function of age was significantly related to worse motor performance. Moreover, performance had a significantly stronger association with the strength of internetwork as compared with intranetwork connectivity, including connectivity within motor networks. These findings suggest that age-related declines in motor performance may be attributed to a breakdown in the functional organization of large-scale brain networks rather than simply age-related connectivity changes within motor-related networks.
Keywords: aging, bimanual, functional connectivity, motor performance, resting state
Introduction
A substantial impediment to healthy living in older adults is the compromised functioning of the motor system (for reviews, see Seidler et al. 2010; King et al. 2013; Maes et al. 2017). These age-related impairments in motor behavior may be attributed to changes at the neural level, including decreases in cortical excitability, gray matter atrophy, changes in white matter microstructure as well as alterations in the functional organization of brain networks (Raz and Rodrigue 2006; Seidler et al. 2010; Goh 2011; Tomasi and Volkow 2012; Ferreira and Busatto 2013; Bhandari et al. 2016). These investigations into the neural underpinnings of age-related declines in motor functioning are critical for our efforts to minimize these movement difficulties in an ever-increasing aging society.
The overarching objective of this research was to examine the relationship between age-related modulations of the functional organization of large-scale brain networks, as assessed by fMRI resting state connectivity, and age-associated declines in motor behavior. It is well established that brain activity at rest in young adults is organized into predominantly segregated functional networks; regions within these different networks demonstrate spontaneous yet correlated fluctuations in activity that are interpreted to be functionally connected (Biswal et al. 1995; Fox et al. 2005; Damoiseaux et al. 2006; Fox and Raichle 2007; van den Heuvel and Hulshoff Pol 2010). These networks are known to change substantially with age, with a decrease in connectivity within the default mode network being the most commonly reported finding (Andrews-Hanna et al. 2007; Damoiseaux et al. 2008; Tomasi and Volkow 2012; Ferreira and Busatto 2013; Geerligs et al. 2015; Ferreira et al. 2016). Investigations into age-related changes within motor resting state networks have revealed both increased and decreased connectivity, heterogeneous results that appear to be at least partially attributed to the specific brain regions included as part of the networks of interest (Wu, Zang, Wang, Long, Hallett, et al. 2007; Wu, Zang, Wang, Long, Li, et al. 2007; Tomasi and Volkow 2012; Bo et al. 2014; Solesio-Jofre et al. 2014; Song et al. 2014; Geerligs et al. 2015; Seidler et al. 2015).
To examine the link between age-related changes in resting state connectivity and motor functioning, the majority of previous research has limited analyses to various motor-related networks or seed regions (Wu, Zang, Wang, Long, Hallett, et al. 2007; Wu, Zang, Wang, Long, Li, et al. 2007; Langan et al. 2010; Fling et al. 2012; Solesio-Jofre et al. 2014; Seidler et al. 2015; Mary et al. 2016, 2017). For example, age-related increases in resting state connectivity between interhemispheric dorsal and ventral premotor cortices were significantly related to worse bimanual coordination performance (Solesio-Jofre et al. 2014). Little is known about the relationship between age-related declines in motor behavior and changes in functional organization at the multiple network level, and the connectivity between functional networks in particular. This knowledge gap is surprising given that: (1) a hallmark of aging is decreased segregation of (i.e., increased connectivity between) functional brain networks during both task performance and at rest (Antonenko and Flöel 2014; Betzel et al. 2014; Cao et al. 2014; Chan et al. 2014; Geerligs et al. 2014, 2015; Song et al. 2014; Archer et al. 2016; Ng et al. 2016; Siman-Tov et al. 2016; Spreng et al. 2016; Ferreira et al. 2016; Grady et al. 2016; Damoiseaux 2017); and (2) many motor tasks that exhibit age-related declines are not pure motor tasks per se, but rather depend on multiple brain networks that presumably interact to ensure successful task completion (e.g., regions thought to be involved in feedback processing or cognitive functioning in addition to motor-related areas) (see, Heuninckx et al. 2005; Goble et al. 2010, for examples).
In this study, we investigated the association between age-related modulations of connectivity within and between resting state brain networks and declines in motor performance across the healthy adult lifespan (20–75 years). Examinations into resting state, as opposed to task-related, functional connectivity offered the advantage of avoiding task-related confounds, such as age-related differences in motor performance or movement speed (Ferreira and Busatto 2013). To assess age-related differences in motor behavior, we used a bimanual coordination task (BCT) analogous to that employed in our previous research (Goble et al. 2010; Heitger et al. 2013; Solesio-Jofre et al. 2014; Beets et al. 2015; Santos Monteiro et al. 2017). It was hypothesized that age-related declines in motor performance would not only be associated with age-related modulations of within-motor network connectivity, but also with modulations of connectivity between functional resting state brain networks. Moreover, given that successful motor performance is dependent on multiple brain networks, we investigated whether age-related differences in performance were more strongly associated with internetwork resting state connectivity than connectivity within functional networks, including within motor-related networks.
Materials and Methods
Participants
A total of 106 healthy, right-handed (Oldfield 1971) participants between the ages of 18 and 80 years were recruited from Leuven and the surrounding area to serve as participants. To be eligible for the experimental protocol, subjects had normal or corrected-to-normal vision, were free of psychoactive (e.g., anti-depressant or -anxiety) medications, reported no known psychological, psychiatric or neurological disorders, and had no magnetic resonance imaging (MRI) contra-indications. Of these 106 participants, 10 were excluded from analyses: 2 voluntarily withdrew from the study before completion of the protocol, 1 reported a change in medication/health status in the middle of the experiment, 2 were excluded for acquisition issues with the resting state fMRI data, 3 were removed for excessive movement during the resting state MRI acquisition (>2.5 mm or degrees), and 2 subjects were considered statistical outliers (defined as >2 SD from the average score of participants greater than 50 years of age) on the Montreal Cognitive Assessment (MoCA) (Nasreddine et al. 2005). For the majority of analyses, participants were divided into 4 distinct age groups (20–35, 35–50, 50–65, and 65–75 years). However, age was used as a continuous variable to examine age-related modulations of resting state functional connectivity. This analytic choice was made in order to be consistent with an analogous analysis examining the association between functional connectivity and motor performance, in which performance on the motor task was input as a continuous variable. Characteristics for the 96 participants included in analyses are detailed in Table 1.
Table 1.
Participant characteristics
| Age group | ||||
|---|---|---|---|---|
| 20–35 years | 35–50 years | 50–65 years | 65–75 years | |
| n | 28 | 23 | 21 | 24 |
| Gender | 13 F | 12 F | 8 F | 10 F |
| Mean age ± SD (y) | 25.7 ± 4.4 | 43.2 ± 3.4 | 57.1 ± 5.0 | 69.4 ± 2.8 |
| Mean MoCA ± SD | 28.3 ± 1.1 | 28.3 ± 1.4 | 27.8 ± 1.5 | 27.3 ± 1.8 |
Characteristics for the 96 participants included in statistical analyses. Although participants between 18 and 80 years of age were recruited, the age range for those included was 20.1–74.4 years. Age of the participants was specified as a decimal (in years) and the precise cutoffs for the 4 experimental groups were 20.00–34.99, 35.00–49.99, 50.00–64.99, and ≥65.00 years. For simplicity, the groups are simply referred to by the integer cutoffs shown in the table above. Note that the Montreal Cognitive Assessment (MoCA) means and SD for the 20–35- and 35–50-year-old groups were computed from 23 and 21 participants, respectively, due to missing data.
Written informed consent was obtained before testing. The local ethics committee for biomedical research approved all experimental procedures.
Overview of Experimental Design Procedures
The experimental protocol consisted of 3 sessions. The first session was used for screening/familiarization purposes, during which participants were informed about the experiment, completed screening-related questionnaires and assessments (i.e., health history, MoCA, etc.) and executed familiarization blocks of practice on the BCT while positioned supine in a mock MRI scanner (see below for BCT details). The subsequent 2 sessions consisted of MRI scanning and were completed at the University Hospital of KU Leuven. The first scanning session consisted of a standard scanning protocol (90 min in total), including the acquisition of a high-resolution T1-weighted structural image and functional resting state data (see below for scan acquisition details). The resting state scan was acquired towards the end of this 90-min protocol and participants were not asked to complete any cognitive or motor task for the scanning period preceding this acquisition sequence, thus minimizing the influence of immediate experiences on resting state connectivity. During the resting state scan, the screen visible to the participants was turned to black and subjects were instructed to keep their eyes open and to not think of anything in particular. The second scanning session was completed approximately one week following the first and consisted of 9 runs of the BCT while functional MRI images were obtained (task-related imaging data not presented in this article).
Bimanual Coordination Task
Task Setup and Procedures
While lying supine either in the mock (Session 1) or real (Session 3) MRI scanner, participants were asked to complete a BCT analogous to that employed in our previous research (Sisti et al. 2011; Solesio-Jofre et al. 2014; Beets et al. 2015). A customized nonferromagnetic apparatus was positioned above the participants’ laps. The device contained 2 dials (5 cm diameter) to be rotated by the 2 hands in order to control the movement of a cursor. The left and right hands controlled movements along the vertical and horizontal axes, respectively. When the left-hand dial was rotated clockwise (CW), the cursor moved up, whereas the cursor moved down when the left-hand dial was rotated counterclockwise (CCW). CW and CCW movements of the right-hand dial resulted in movements to the right and left, respectively. Angular displacements of the dials were registered with nonferromagnetic high precision optical shaft encoders (HP, 2048 pulses per revolution, sampling frequency of 100 Hz), which were fixed to the movement axes of both dials. This enabled registration of kinematics as well as the display of online visual feedback. Visual information depicting task stimuli and feedback were shown on a LCD projector, visible via a mirror placed in front of the eyes.
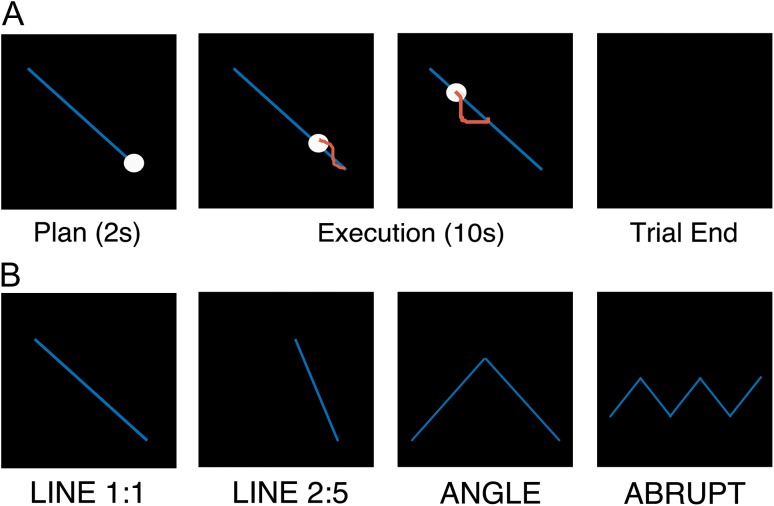
The goal of the BCT was to track a visual target presented on the screen by simultaneously rotating the 2 dials with the 2 hands. Each trial started by depicting a desired behavioral trajectory (blue line), a target dot (white circle) as well as the cursor to be moved by the participants (Fig. 1A). This cursor representing the participants’ position was automatically moved to the appropriate starting point (i.e., equivalent location as the target dot) prior to each trial. Following a planning period of 2 s, the target dot moved along the target trajectory at a constant speed for a duration of 10 s. Participants were instructed to rotate the dials in order to have the cursor depicting their movements “match” the moving target as accurately as possible. During execution, online visual feedback of the participants’ performance was provided via a red cursor, which depicted current position as well as the positions corresponding to the previous second. After the 10 s execution period, the screen turned black, regardless of the participants’ location and the next target appeared following an interval of 3 s.
Figure 1.
Bimanual coordination task (BCT). (A) Timeline of an exemplar trial. Blue line depicts the desired movement trajectory, white circle shows the target that moves along the trajectory at a constant speed for a 10 s duration and red cursor provides visual feedback of the participants’ performance. (B) Four different movement trajectories (see main text for details).
Four different movement trajectories were included in order to modulate task complexity (Fig. 1B). The first 2 trajectories required participants to follow a diagonal line on the screen but differed in terms of the slope of the line and thus the relative velocities (i.e., frequency ratios) the 2 hands had to rotate in order to appropriately perform the task. Participants moved the hands either at a 1:1 or 2:5 velocity ratio. Thus, the 2 hands needed to rotate at the same velocity for the Line 1:1 condition (with the direction of movement either from the screen’s bottom-right to top-left or vice versa; equal number of trials per block). Conversely, for the Line 2:5 condition, the left hand had to rotate 2 units for every 5 units the right hand rotated (or vice versa; equal number of trials per block). The third condition required the 2 hands to move at a 1:1 frequency ratio, but the trajectory followed a V- or inverted-V shaped pattern (equal number of trials per block) in which participants had to change the direction or angle of their movement (herein referred to as the condition Angle). Last, participants again moved at a 1:1 frequency ratio, but the trajectory abruptly altered directions in a zig-zag pattern (herein referred to as condition Abrupt). This zig-zag pattern was either oriented horizontally (Fig. 1B) or vertically (equal number of trials per block).
Each BCT session contained 8 blocks, with each block consisting of 24 trials (6 per movement trajectory) and lasting approximately 6 min. The 2 different sessions of the BCT were identical with the following exceptions. First, the initial session was completed in the mock scanner in order to familiarize and train the participants to ensure they could perform the task appropriately during the subsequent experimental session completed in the real scanner. Accordingly, performance on the initial familiarization phase was not included in data analyses. Second, between blocks 4 and 5 for the session completed in the actual MRI scanner, a control/rest block was completed in which participants did not move their hands but watched a visual presentation depicting exemplar BCT performance. This run not only allowed participants to rest, but also served as a control condition for task-based fMRI analyses (not presented in this article).
Behavioral Analyses
The x and y positions of both the target circle and the participants’ cursor were sampled at 100 Hz and recorded for subsequent offline processing conducted in Matlab R2016b (The Mathworks). The primary dependent measure, labeled as movement accuracy, reflected the percentage of the target line that was “covered” by the participants’ movements. For each sample within a trial (i.e., every 10 ms), a line with the shortest distance between the participant’s cursor and the ideal movement trajectory was projected. The projection point on the target line was marked as covered and then movement accuracy was quantified as the percentage of the ideal movement trajectory that was covered by the participant. As a consequence of this computation, if a participant was moving away from the target line, for example, the line with the shortest distance between the participant’s cursor and the ideal trajectory would repeatedly project to the ideal trajectory at the same location (i.e., covering the same segment of the desired trajectory). This ultimately would result in a smaller proportion of the desired trajectory being covered and thus a lower accuracy score. Analogously, moving too slow or too fast or cutting corners (on the Angle and Abrupt conditions) would also result in lower accuracy scores. This particular measure was chosen, as compared with the average Euclidean distance within a trial, as it better captures performance for the irregular movement trajectories (e.g., Angle and Abrupt) used in this study (see Supplemental Fig. S1). No trials were labeled as statistical outliers for the accuracy dependent measure, defined as scores greater than 2SD from the mean for that particular individual, block and movement trajectory. Outlier analyses were completed separately for each block to account for performance changes as a function of practice. All behavioral statistical analyses were conducted in R (Version 3.3.1) with a significance threshold set to 0.05. Accuracy was analyzed with a 4 (age group: 20–35, 35–50, 50–65, and 65–75 years) × 4 (trajectory: Line11, Line25, Angle and Abrupt) × 8 (block) ANOVA, with block and trajectory as within-subject factors and age group as the between-subjects factor. Appropriate follow-up comparisons were conducted with Holm–Bonferroni (HB) correction for multiple comparisons.
Brain Imaging
Acquisition Parameters
A Philips Achieva 3.0T MRI system and a 32-channel head coil were used for image acquisition. Functional resting state data were acquired with an ascending gradient echo-planar imaging (EPI) pulse sequence for T2*-weighted images (repetition time = 2500 ms; echo time = 30 ms; flip angle=90°; 45 transverse slices; slice thickness = 2.5 mm; interslice gap = 0.25 mm; voxel size = 2.5 × 2.56 × 2.5 mm3; field of view = 200 × 200 × 123.5 mm3; matrix=80 × 78; 162 dynamic scans plus 4 dummy scans discarded at the beginning of the sequence). A 3D high-resolution T1-weighted structural image was acquired with a magnetization-prepared rapid-acquisition gradient-echo (MPRAGE) sequence (TR/TE = 9.6/4.6 ms; voxel size = 1 × 1 × 1.2 mm3; field of view = 250 × 250 × 192 mm3; 160 coronal slices).
Preprocessing
Functional volumes were preprocessed using SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/; Welcome Department of Neuroimaging Neuroscience, London, UK) implemented in Matlab. Each participant’s functional volumes were realigned to the first volume of the session using rigid body transformations and then slice time corrected to the middle slice (reference slice = 22). Functional images were co-registered to the high-resolution T1-weighted anatomical image using a rigid body transformation optimized to maximize the normalized mutual information between the 2 images. The structural image was segmented into gray matter, white matter, cerebrospinal fluid (CSF), bone, soft tissue, and background. An average subject-based template was created using DARTEL in SPM12, and registered to the Montreal Neurological Institute (MNI) space. All functional and anatomical images were then normalized to the resulting template.
As a result of the functional realignment preprocessing step above, head motion in 6 dimensions (i.e., rotations and linear translations in 3 planes of movement) was quantified for each subject during the resting state scan. For the participants included in the analyses, the average ± SD of the maximum displacements across all volumes and 3 planes of movement were: 0.56 ± 0.41 and −0.43 ± 0.35 mm for linear translations in the positive and negative directions, respectively, and 0.63° ± 0.58° and −0.50° ± 0.40° for rotations in the positive and negative directions, respectively. Critically, these movement parameters did not differ significantly among the 4 age groups (all P > 0.3; see Supplemental Table S1 for details).
Defining Regions of Interest
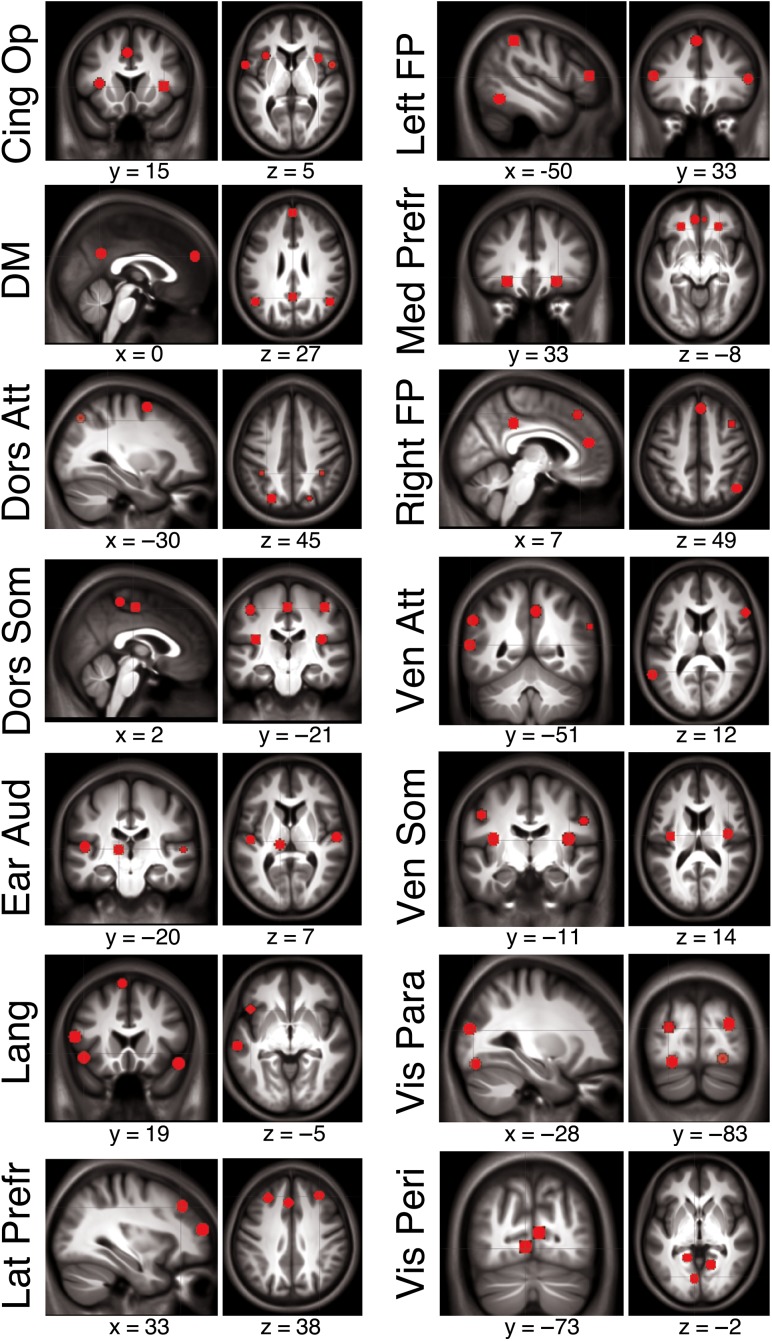
To investigate age- and motor performance-related modulations of functional connectivity within and between established resting state networks, our analyses were based on the 14 networks identified via independent component analysis (ICA) in an independent sample of participants in our previous research (Mantini et al. 2013). These networks were labeled as cingulo-operculum, default mode, dorsal and ventral attention, dorsal and ventral somatomotor, early auditory, language, lateral and medial prefrontal, left and right frontoparietal, visual parafoveal, and visual peripheral. Using the connectivity maps from Mantini et al. (2013) and for each of the 14 networks of interest, we extracted the MNI coordinates of the local (connectivity) maximum for each cluster of voxels that exhibited significant within-network connectivity. For each local maximum, a regions of interest (ROI) with a 6 mm radius sphere centered on the peak voxel was built with the MarsBAR toolbox in Matlab. In total, 84 seed regions from the 14 networks were extracted and used in subsequent functional connectivity analyses (Fig. 2; see Supplemental Table S2 for coordinates and peak Z-scores of each seed). Extracting seed regions from within these resting state networks allowed us to quantify connectivity among seeds within the same network (i.e., within-network connectivity) as well as connectivity among seeds across networks (i.e., between-network connectivity).
Figure 2.
Seed regions from the 14 resting state networks of interest identified in Mantini et al. (2013) overlaid on the mean structural image from the 96 participants included in analyses. Note that slices from 2 different planes were selected in order to offer a representative depiction of each network and thus not every single seed is visible in the images shown. See Supplemental Table S2 for MNI coordinates for each seed. Cing Op = cingulo-operculum; DM = default mode; Dors Att = dorsal attention; Dors Som = dorsal somatomotor; Ear Aud = early auditory; Lat Prefr = lateral prefrontal; Left FP = left frontoparietal; Med Prefr = medial prefrontal; Right FP = right frontoparietal; Ven Att = ventral attention; Ven Som = ventral somatomotor; Vis Para = visual parafoveal; Vis Peri = visual peripheral.
Functional Connectivity Analyses
The analysis pipeline was conducted in Matlab and was similar to that employed in our previous research (Solesio-Jofre et al. 2014). Specifically, prior to running the connectivity analyses, additional preprocessing steps were completed to remove variance from spurious sources. First, to minimize the impact of motion on the correlations between ROIs, volumes in which the scan-to-scan displacement exceeded 0.5 mm were discarded. Remaining volumes were high-pass filtered with a cutoff of 0.01 Hz. Masks of white matter and CSF were used to quantify the average signals in these regions. Regression analyses were performed on the fMRI time-series, including the white matter and CSF signals as well as the 6-dimensional head motion realignment parameters, as well as the realignment parameters squared, their derivatives, and the squared of the derivatives, as regressors. The resulting residuals were then low-pass filtered with a cutoff of 0.08 Hz. Data filtering served to minimize high-frequency noise that may be the result of cardiac and respiratory factors (Fox et al. 2005; Fox and Raichle 2007). Data were spatially smoothed with a Gaussian kernel of FWHM (6 mm).
At the individual level, the time-series across all voxels within each of the 84 ROIs were averaged and Pearson correlation coefficients among all ROIs were computed. Each correlation coefficient r was converted to z-values using the formula z = arctanh(r). This formula is commonly referred to as Fishers r-to-z transformation. To ensure normality, statistical analyses of the correlation data were performed on these z-values.
Our research questions were focused on connectivity at the network level, and not the individual seed level. For example, we were interested in connectivity between cingulo-operculum and dorsal attention networks and not connectivity between cingulo-operculum seed 2 and dorsal attention seed 5. Accordingly, for the results presented here in the main text, we averaged the Z-transformed connectivity values across the seeds within and between networks. For instance, the cingulo-operculum and dorsal attention networks had 5 and 6 seed regions, respectively. To compute the connectivity between these 2 networks, we averaged across the 30 seed pairs. Similarly, to quantify connectivity within the cingulo-operculum network, we first computed the connectivity between the 10 unique pairs of seeds (i.e., [5 seeds × (5–1) seeds]/2) and then averaged across these values. Thus, intranetwork connectivity reflects the averaged connectivity between the different seeds within the same network. It should be noted that this is in contrast to a subset of previous research in which the signal is first averaged across all seeds within a network and then correlated to itself (as well as other networks), effectively producing a within-network, nontransformed correlation value of 1. By averaging the Z-transformed connectivity values across the seeds within and between networks, the pairwise connectivity matrices were reduced from dimensions of 84 × 84 to 14 × 14, which aided in the visualization and interpretation of the data. For completeness, plots depicting seed-level connectivity (i.e., 84 × 84 matrices) are provided in Supplemental Figs S2–4.
To assess resting state functional connectivity within each age group, t-statistics were computed for each of the unique network pairs and subsequent one-sample t-tests were conducted. To examine how intra- and internetwork connectivity varied as a function of age or motor performance, single-subject correlations between network pairs were correlated with the participants’ age and performance on the Abrupt condition of the BCT, respectively. Tests of statistical significance were based on comparisons of the correlation coefficients to a correlation value of 0. Performance on the Abrupt condition was used to conduct correlational analyses with the functional connectivity data as this trajectory can be considered the most complex and thus demonstrated the most robust age-related differences. (Please note, however, that Supplementary Fig. S6 depicts correlations with performance on the Line 1:1 condition for comparison purposes.) For all connectivity analyses, statistical probabilities associated with each family of hypothesis tests were considered significant if surviving the false discovery rate (FDR) method for multiple comparisons. The desired FDR value was set to 0.05; the critical P-value for each family of hypothesis tests then varied based on the original uncorrected P-values. The critical P-value for each family of tests is explicitly provided in the Results. For completeness, figures indicate significance at thresholds of both P(FDR) < 0.05 as well as P(uncorrected) < 0.05.
Results
Bimanual Coordination Task
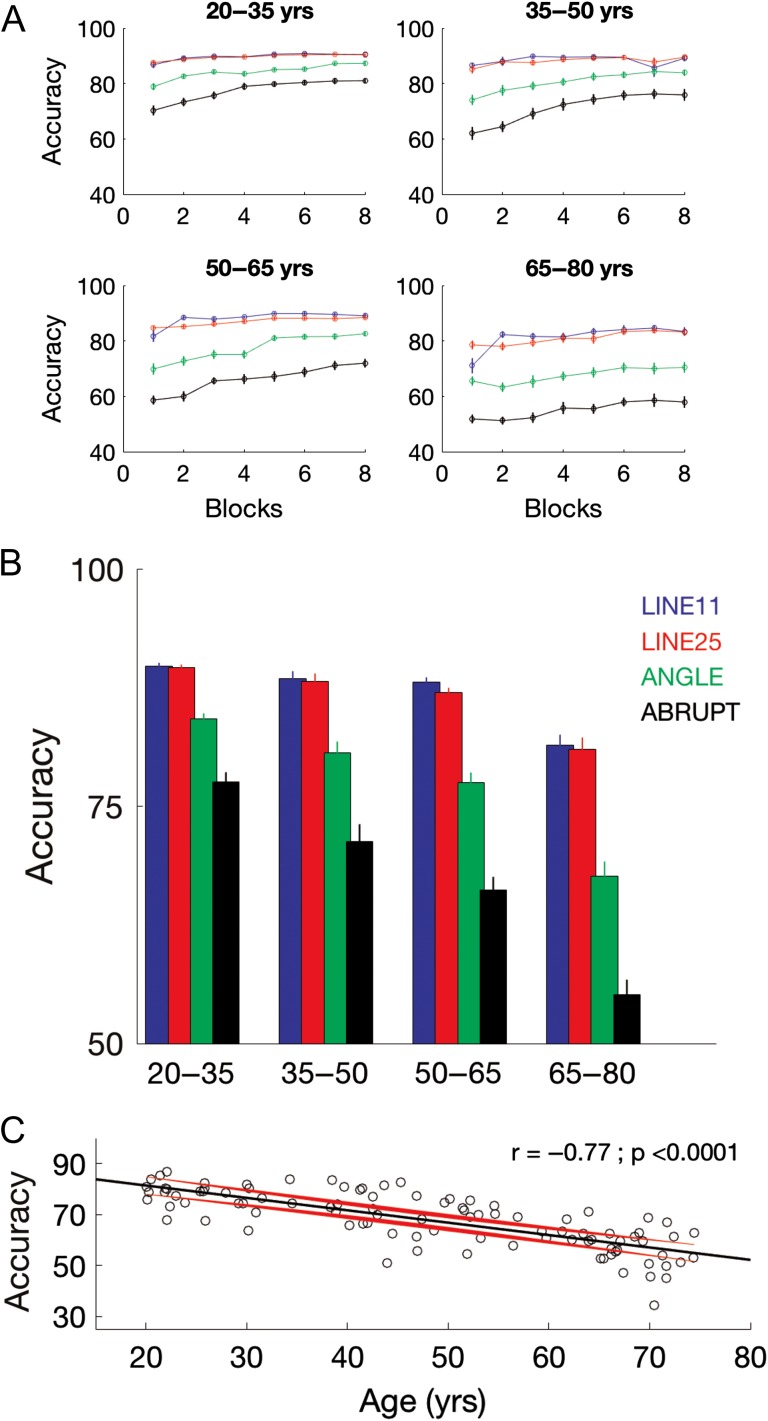
Table 2 contains results from the 4 (age group) × 4 (movement trajectory) × 8 (block) ANOVA conducted on the dependent variable accuracy. There was a significant age group x block × trajectory interaction (Fig. 3A; P < 0.0001), indicating that the rate of improvement across blocks of practice differed by age and movement trajectory. This significant three-way interaction is decomposed in Supplemental Tables S3 and S4. Since our primary research questions of interest were not concerned with practice-dependent improvements, the remaining results presented in the main text will collapse across the factor block.
Table 2.
Summary of ANOVA results on BCT performance
| Effect | df | F | P-value |
|---|---|---|---|
| Age group | 3, 92 | 47.2 | <0.0001 |
| Block | 7, 644 | 119.4 | <0.0001 |
| Trajectory | 3, 276 | 683.5 | <0.0001 |
| Age group × block | 21, 644 | 1.6 | 0.044 |
| Age group × trajectory | 9, 276 | 19.4 | <0.0001 |
| Block × trajectory | 21, 1932 | 12.7 | <0.0001 |
| Age group × block × trajectory | 63, 1932 | 3.1 | <0.0001 |
df = Degrees of freedom. Numbers represent df for the effect of interest (numerator) and the residuals (denominator).
Figure 3.
BCT performance. (A) Accuracy as a function of practice block for the 4 age groups and movement trajectories. (B) Accuracy averaged across blocks. Error bars in (A and B) depict SE. (C) Accuracy for the Abrupt condition plotted as a function of age. Black and red trajectories depict linear fit and corresponding 95% prediction intervals, respectively.
Main effects of age group (P < 0.0001) and trajectory (P < 0.0001) were both significant (Fig. 3B). Follow-up comparisons indicated that BCT accuracy was worse in the older participants as well as in the more complex movement trajectory conditions (i.e., Angle and Abrupt). Specifically, t-tests with HB correction for multiple comparisons (Holm 1979) revealed that all age groups performed significantly different from one another except the 35–50 and 50–65 year olds (20–35 vs. 35–50: t = 2.9, P[HB] = 0.023; 20–35 vs. 50–65: t = 6.9, P[HB] < 0.0001; 20–35 vs. 65–75: t = 11.4; P[HB] < 0.0001; 35–50 vs. 65–75: t = 6.7; P[HB] < 0.0001; 50–65 vs. 65–75: t = 5.83; P[HB] < 0.0001). Paired t-tests with HB correction following the significant main effect of trajectory indicated that all combinations of movement conditions were significantly different from one another except for the Line11 and Line25 trajectories (Line11 vs. Angle: t = 17.3, P[HB] < 0.0001; Line11 vs. Abrupt: t = 22.9; P[HB] < 0.0001; Line25 vs. Angle: t = 16.3; P[HB] < 0.0001; Line25 vs. Abrupt: t = 23.7; P[HB] < 0.0001; Angle vs. Abrupt: t = 20.0; P[HB] < 0.0001).
Results from the ANOVA detailed in Table 2 also indicated a significant age group × trajectory interaction (P < 0.0001), as the older age groups performed worse in the more complex conditions (i.e., Angle and Abrupt) as compared with the younger age groups (see Supplemental Tables S3 and S4 for additional follow-up contrasts). Figure 3C depicts performance for the most difficult movement trajectory, Abrupt, as a function of age. As age-related impairments were most pronounced in this condition, these scores served as the primary motor performance-related regressor of interest in the connectivity analyses presented in the subsequent section.
Behavioral results collectively demonstrated substantial age-related declines in motor performance; moreover, these impairments increased with task complexity. These findings are consistent with numerous earlier studies indicating age-related degradations in motor functioning, including on BCTs similar to that employed in the current study (Heuninckx et al. 2008; Solesio-Jofre et al. 2014; Serbruyns et al. 2015) (see, Maes et al. 2017, for review).
Resting State Connectivity
Resting State Connectivity Within Each Age Group
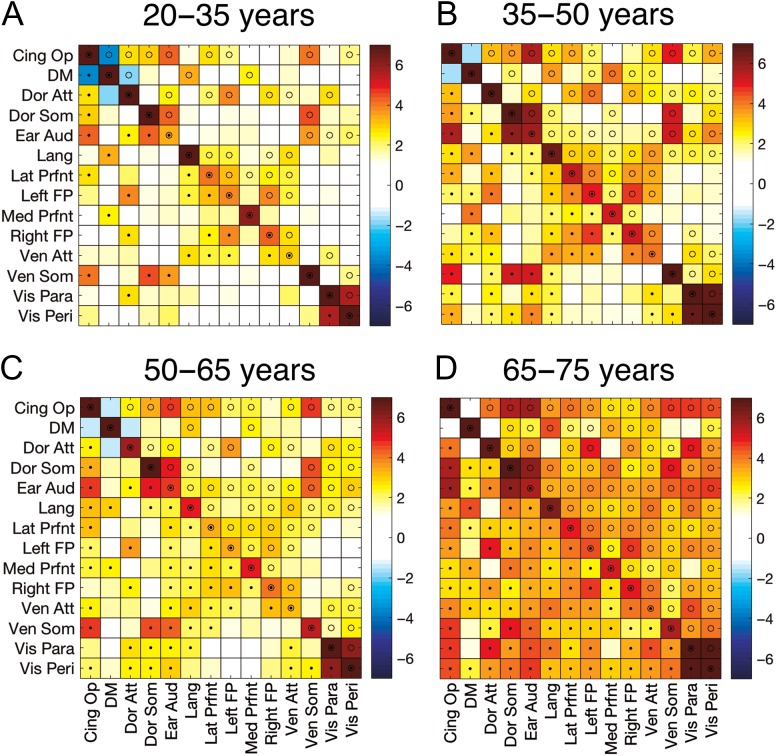
Functional connectivity values for the 20–35-year-old participants are depicted in Figure 4A in which each square represents the t-statistic based on the average of the Z-transformed correlations from the various seeds within (along the diagonal) and between (off-diagonal) the 14 networks. Activity within each of the 14 networks was highly and positively correlated, with each network demonstrating significant connectivity after FDR correction for multiple comparisons. This also confirms that our extracted seeds were indeed part of a functionally connected network in our sample of participants. The significant within-network connectivity can be contrasted with the relatively small correlations, and thus few significant pairs, between the 14 networks (off diagonal), indicative of network segregation. For comparison purposes, (B–D) in Figure 4 depict t-statistics for the 35–50, 50–65, and 65–75-year-old age groups, respectively. Intranetwork connectivity was high and significant in all 3 groups. Notably, internetwork connectivity appeared to be stronger in the older age groups.
Figure 4.
Resting state connectivity for the 4 age groups. Values represent t-statistics based on the averaged z-transformed correlations among seeds within (diagonal) and between (off-diagonal) the 14 networks of interest. Tests of statistical significance were based on one-sample t-tests corrected for multiple comparisons with a false discovery rate (FDR) threshold set to 0.05. Corrected P-value thresholds for the 4 age groups (from youngest to oldest) were 0.0182, 0.0303, 0.0297, and 0.0215. • = P(FDR) < 0.05 (below diagonal); o = P(uncorrected) < 0.05 (above the diagonal).
Age-Related Modulations of Resting State Connectivity
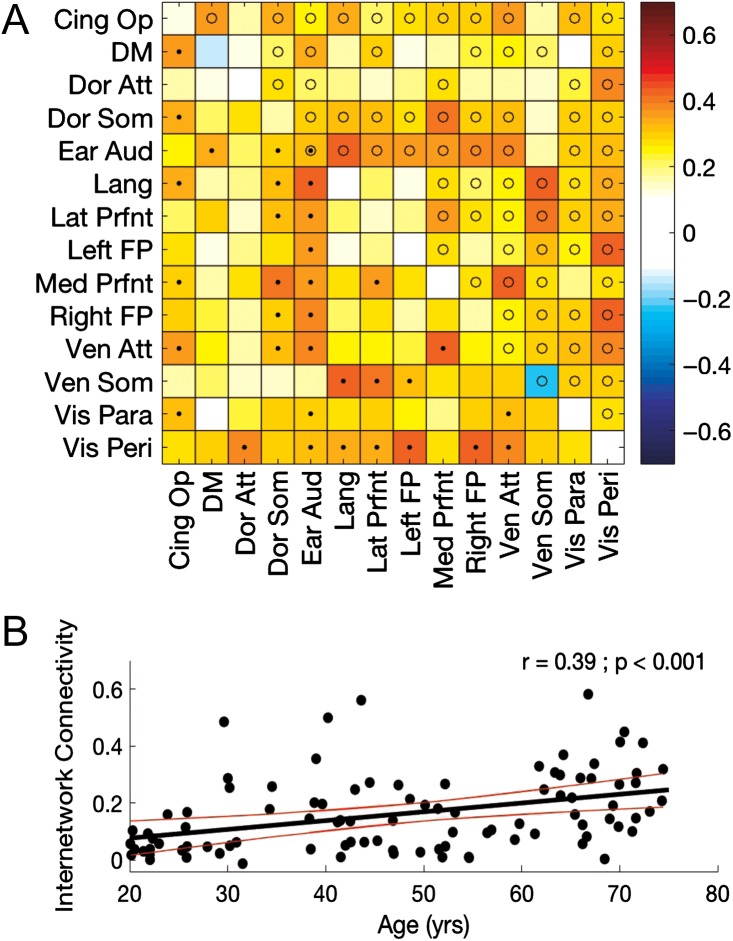
To examine modulations of intra- and internetwork connectivity across the healthy adult lifespan, we correlated the participants’ age with the Z-transformed connectivity values (Fig. 5A; for completeness, Supplemental Fig. S5 depicts pairwise comparisons between each age group). Age-related modulations of intranetwork connectivity were relatively minimal, as only connectivity within the early auditory network demonstrated a significant age-associated modulation (i.e., stronger connectivity as a function of age) after correction for multiple comparisons. In contrast, stronger internetwork connectivity was consistently observed with older age; and, over 30% of the network pairs showed significant positive associations between connectivity and age after FDR correction. To further highlight this age-related effect, we computed the mean internetwork connectivity across all network pairs—independent of whether the connectivity between the pair of networks was significantly correlated with age—for each participant. There was a significant and positive relationship between age and mean internetwork connectivity (r = 0.39; P < 0.001; Fig. 5B), indicative of a decrease in resting state network segregation as a function of age.
Figure 5.
(A) Age-related modulations of resting state connectivity across the full sample of 20–75-year-old participants. Values represent correlations between age and intranetwork (diagonal) and internetwork (off-diagonal) connectivity. Tests of statistical significance were based on comparisons of the correlation coefficients to a correlation of 0 and corrected for multiple comparisons with a false discovery rate (FDR) threshold set to 0.05. Corrected P-value threshold was 0.0028. • = P(FDR) < 0.05 (below diagonal); o = P(uncorrected) < 0.05 (above the diagonal). (B) Mean internetwork connectivity plotted as a function of age. Black and red trajectories depict linear fit and corresponding 95% prediction intervals, respectively.
Motor Performance-Related Modulations of Resting State Connectivity
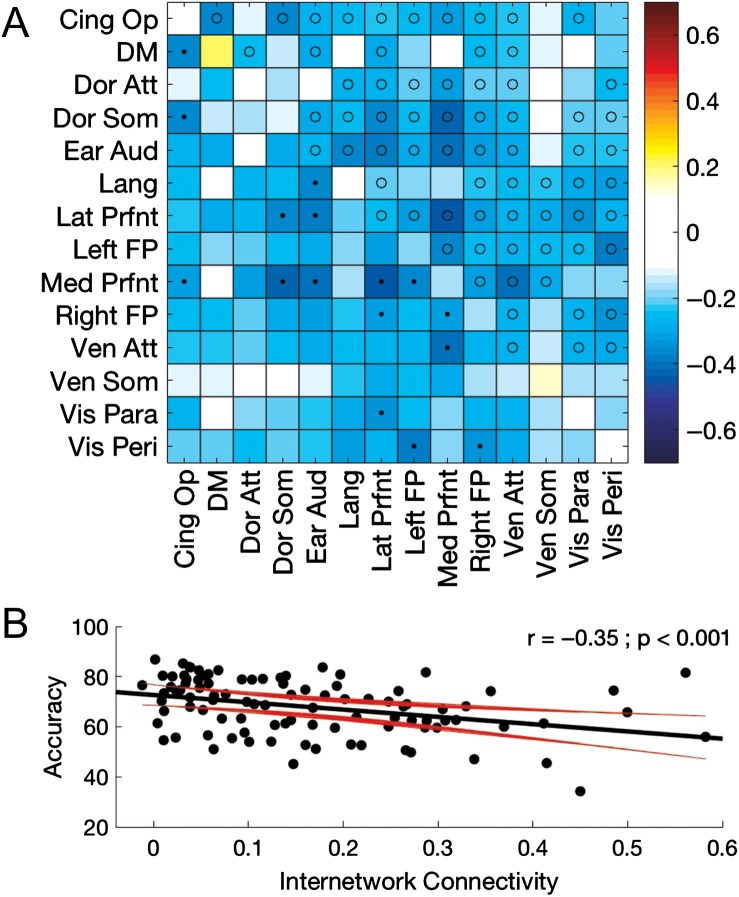
To investigate the link between resting state connectivity and motor performance, we correlated accuracy scores on the Abrupt condition of our BCT task with the Z-transformed connectivity values (Fig. 6A; but see Supplemental Fig. S6 for correlations between connectivity and performance on the Line11 condition). After correction for multiple comparisons, there were no significant relationships between connectivity within any of the 14 networks and motor performance. Conversely, internetwork connectivity between multiple network pairs was significantly and negatively related to performance. Indeed, the negative correlation between movement accuracy and mean internetwork connectivity computed across all network pairs was highly significant (r = −0.35; P < 0.001; Fig. 6B), indicating that decreased segregation of the networks observed in older adults was associated with worse motor performance.
Figure 6.
(A) Motor performance-related (abrupt condition) modulations of resting state connectivity across the full sample of 20–75-year-old participants. Values represent correlations between performance and both intranetwork (diagonal) and internetwork (off-diagonal) connectivity. Negative values indicate increased connectivity was associated with decreased performance (accuracy). Tests of statistical significance were based on comparisons of the correlation coefficients to a correlation of 0 and corrected for multiple comparisons with a false discovery rate (FDR) threshold set to 0.05. Corrected p-value threshold was 0.0011. • = P(FDR) < 0.05 (below diagonal); o = P(uncorrected) < 0.05 (above the diagonal). (B) Accuracy on the abrupt condition plotted as a function of mean internetwork connectivity. Black and red trajectories depict linear fit and corresponding 95% prediction intervals, respectively.
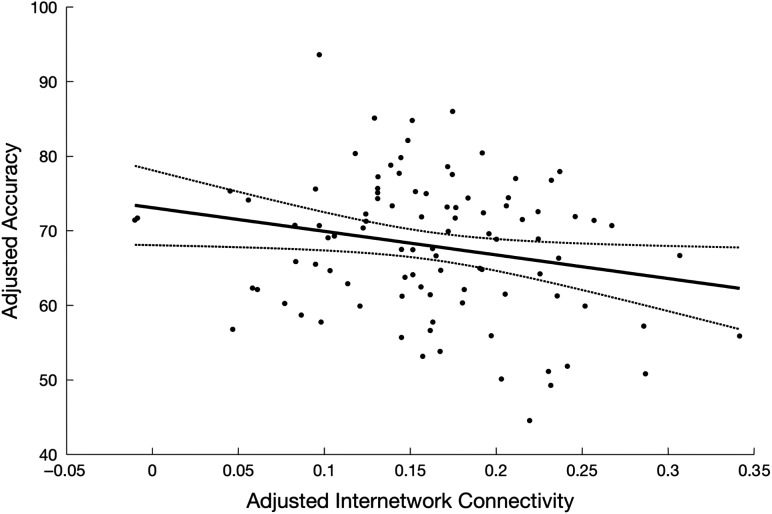
The results presented above suggest that internetwork, but not intranetwork, connectivity was significantly related to motor performance. To further assess this statistically, we completed a hierarchical regression where intranetwork connectivity from each of the 14 resting state networks were first input into a regression model in order to explain variance in performance on the motor task. Importantly, all intranetwork connectivity measures were kept in the model and were not added or removed based on a particular selection method (i.e., forward/backward). After adjusting for the contributions of connectivity within these 14 networks, including connectivity within the 2 motor-related resting state networks, we added internetwork connectivity to the model and the negative relationship between internetwork connectivity and motor performance remained significant (P = 0.047; Fig. 7).
Figure 7.
Relationship between internetwork connectivity and accuracy on the abrupt BCT condition remained significant (P = 0.047) after adjusting for the variance explained by connectivity within each of the 14 resting state networks, including the 2 motor-related networks. Trajectories depict linear fit and corresponding 95% prediction intervals.
The preceding section demonstrated that internetwork connectivity, even above and beyond the effects of intramotor network connectivity, was significantly associated with motor performance. It could be argued, however, that the relationship between motor performance and resting state connectivity within motor networks was relatively minimal due to the specific networks investigated (i.e., the particular motor networks extracted from Mantini et al. (2013) are not critical for the motor task investigated). To assess this possibility, we conducted a secondary analysis in which we related motor performance to resting state connectivity within a network of regions involved in the execution of the BCT used here. Specifically, ROIs were extracted from a separate BCT-related fMRI data set (Beets et al. 2015) and also used as part of resting state connectivity analyses in a previous study (Solesio-Jofre et al. 2014) (see Supplemental Table S5 and Supplemental Fig. S7 for details on this network and corresponding analyses). Results were consistent with the primary analysis detailed above. The relationship between motor performance and connectivity within this BCT-specific network was minimal (r = −0.14; P = 0.19). More importantly, after adjusting for the connectivity within this BCT network and all nonmotor networks from Mantini et al. (2013) with a similar hierarchical regression as detailed above, the negative relationship between internetwork connectivity and motor performance remained significant (P = 0.033). This additional analysis provides further evidence for a significant link between internetwork connectivity, even above and beyond the contributions of connectivity within a motor network known to be involved in the specific task investigated, and impairments in motor functioning observed as a result of aging.
Discussion
The results from the current study demonstrate that: (1) aging is associated with impairments in motor performance; and, these declines are more robust with increases in task complexity; (2) aging is associated with stronger connectivity between established large-scale resting state networks (i.e., decreased network segregation); (3) stronger internetwork resting state connectivity observed as a function of age is related to worse motor performance; and (4) motor performance has a significantly stronger association with the strength of internetwork connectivity than intranetwork connectivity, including connectivity within motor networks.
Age-Related Modulations of Inter- and Intranetwork Resting State Connectivity
Brain activity at rest in young adults is organized into predominantly segregated functional networks, where regions within these different networks demonstrate spontaneous and correlated fluctuations in activity (Biswal et al. 1995; Fox et al. 2005; Damoiseaux et al. 2006; Fox and Raichle 2007; van den Heuvel and Hulshoff Pol 2010). Critically, this organization of predominantly segregated networks is thought to be critical for efficient information processing (Bullmore and Sporns 2012; Sporns 2013). Our results revealed a substantial age-related modulation in the organization of these large-scale brain networks; specifically, stronger internetwork resting state connectivity was observed with older age. This finding adds to recent studies that also showed decreased segregation of functional networks in older adults (Antonenko and Flöel 2014; Betzel et al. 2014; Cao et al. 2014; Chan et al. 2014; Geerligs et al. 2014, 2015; Song et al. 2014; Archer et al. 2016; Ng et al. 2016; Siman-Tov et al. 2016; Spreng et al. 2016; Ferreira et al. 2016; Grady et al. 2016; Damoiseaux 2017). Our results are novel in that this stronger internetwork connectivity observed as a function of age was significantly associated with declines in motor performance, a finding that will be discussed in more detail in the subsequent section.
The age-related decrease in network segregation reported in this study is consistent with the dedifferentiation hypothesis, which is based on the collection of studies demonstrating that aging results in decreased functional specialization (i.e., less selective recruitment) of brain areas during task performance (Logan et al. 2002; Heuninckx et al. 2005, 2008; Dennis and Cabeza 2011; Geerligs et al. 2014) (for reviews, see Cabeza 2002; Goh 2011; Grady 2012; Antonenko and Flöel 2014). For example, during performance of a hand–foot coordination task, older adults exhibited greater activation across a diffuse set of areas, including those typically involved in sensory processing, action monitoring, and movement inhibition (Heuninckx et al. 2005). This greater activation was in addition to the recruitment of common motor-related regions that were also activated in healthy young adults (Heuninckx et al. 2005). This greater task-related activation observed in older adults can simply reflect dedifferentiation of the aging brain or may be indicative of compensatory mechanisms if it is thought to serve a beneficial role by minimizing age-related declines in motor performance (Heuninckx et al. 2008; Goble et al. 2010; Dennis and Cabeza 2011) (see, Reuter-Lorenz 2002; Reuter-Lorenz and Lustig 2005; Grady 2008, 2012, for reviews). Our data cannot speak to the compensation versus dedifferentiation issue directly as our connectivity data was acquired independent of the task. Nonetheless, our results and those in the available literature collectively indicate that aging is associated with decreased specialization of functional networks not only during both task performance, but also at rest.
The strongest associations between internetwork connectivity and age appeared to involve the cingulo-operculum, dorsal and ventral somatomotor, early auditory, and visual peripheral networks (Fig. 5A). Although there is considerable variability in the available literature with respect to the precise networks and seed regions investigated, our results are consistent with those of Geerligs et al. (2015) who reported age-related increases in connectivity between the visual, cingulo-operculum and somatomotor networks. We are, however, hesitant to put too much emphasis on any specific pairs of networks, as the age-related modulations of internetwork connectivity were fairly consistent across most network pairs. Indeed, connectivity between many networks was simply slightly below the threshold for statistical significance after correction for multiple comparisons.
An age-related decrease in connectivity within the default mode network has been one of the most consistently reported resting state functional connectivity findings (Andrews-Hanna et al. 2007; Damoiseaux et al. 2008; Tomasi and Volkow 2012; Ferreira and Busatto 2013; Solesio-Jofre et al. 2014; Geerligs et al. 2015; Ferreira et al. 2016). Our results also revealed an age-related decrease, although the strength of this relationship was not statistically significant. It is worth noting, however, that the correlation between connectivity within the default mode network and age in our study was only marginally less than in previous research that also used a large cross-sectional design with a similar age range (e.g., r = −0.15 as compared with −0.18 in Tomasi and Volkow (2012)). Moreover, when comparing our youngest (20–35 years) and oldest (65–75 years) age groups, there was a trend for a significant difference in connectivity within the DMN (P = 0.07; see Supplemental Fig. S5); thus, our results are relatively in line with the available literature.
With respect to motor-related networks, stronger connectivity, albeit nonsignificant, was observed as a function of age within the dorsal somatomotor network whereas weaker connectivity was observed with older age within the ventral somatomotor network (P-uncorrected < 0.05 but did not survive FDR correction). This suggests that age-related modulations of connectivity within motor-related networks are dependent on the specific regions included as part of a motor network and thus help explain the heterogeneity in the available literature (Wu, Zang, Wang, Long, Hallett, et al. 2007; Wu, Zang, Wang, Long, Li, et al. 2007; Tomasi and Volkow 2012; Bo et al. 2014; Solesio-Jofre et al. 2014; Song et al. 2014; Geerligs et al. 2015; Seidler et al. 2015).
Association Between Motor Performance and Age-Related Modulations of Resting State Functional Connectivity
The primary finding of the current study was that the stronger connectivity between functional resting state brain networks observed with age was associated with worse motor performance. Moreover, these age-related modulations of internetwork connectivity that extended to nonmotor networks even had a stronger association with performance than connectivity within motor-related networks. These results collectively suggest that the decreased segregation of functional brain networks in older adults, even above and beyond changes in connectivity within motor networks, contribute to the robust age-related declines in motor performance reported in the available literature.
A recent investigation examined how resting state connectivity, as assessed by magnetoencephalography (MEG), between the sensorimotor cortex and the rest of the brain was related to motor behavior (specifically, sequence learning) in both young and older adults (Mary et al. 2016). In older individuals, learning was negatively related to connectivity between sensorimotor cortex and dorsolateral prefrontal cortex, angular gyrus, anterior cingulate, and the precuneus (i.e., regions linked to the default mode and dorsal attention networks) (Mary et al. 2016), suggesting that stronger connectivity between motor and nonmotor networks in older adults hinders the ability to learn a sequence of finger movements. This previous study focused on connectivity with a single sensorimotor seed. Our research is thus the first, to our knowledge, to demonstrate that age-related modulations of the functional organization of large-scale brain networks, and even between nonmotor-related networks, are associated with age-related declines in motor performance.
Similar to the age-related modulations of connectivity discussed in the preceding section, the associations between motor performance and internetwork connectivity were fairly consistent across the various networks. Indeed, the vast majority of network pairs demonstrated a negative relationship between performance and internetwork connectivity, although many did not survive correction for multiple comparisons. Nonetheless, the strongest associations between connectivity and performance appeared to involve the lateral and medial resting state prefrontal networks (Fig. 6A). Interestingly, older adults also exhibit widespread cortical hyperactivation during task performance, including in prefrontal regions (Mattay et al. 2002; Ward and Frackowiak 2003; Heuninckx et al. 2005, 2008; Goble et al. 2010). This task-related prefrontal hyperactivation in older adults, however, was linked to better motor performance (Heuninckx et al. 2008), whereas stronger resting state connectivity between prefrontal regions and other functional networks were associated with worse motor performance in the current study. It is difficult to draw too many conclusions across these studies given the differences in approach (i.e., task-related activity vs. resting state connectivity), but these results collectively speak to the modulatory role of prefrontal regions in the motor functioning of older adults.
Surprisingly, there were no significant relationships between motor performance and connectivity within any of the 14 resting state networks, including the 2 motor-related networks. This lack of an effect is in contrast to previous research in which a relationship between intramotor-network connectivity and motor performance was revealed in older adults (Fling et al. 2012; Solesio-Jofre et al. 2014; Seidler et al. 2015). For example, age-related increases in resting state motor network connectivity, and between interhemispheric dorsal and ventral premotor cortices in particular, were significantly related to worse bimanual coordination performance (Solesio-Jofre et al. 2014). These differing results do not appear to be related to the specific motor networks investigated. We employed a secondary analysis with seed regions from a motor network recruited during performance of a nearly identical BCT and used in earlier resting state connectivity analyses (Solesio-Jofre et al. 2014) (see Supplementary Fig. S7A and Table S5). These findings indicated that the averaged resting state connectivity within this BCT-specific network was not related to performance on the version of the BCT employed in the current research. Moreover, even after accounting for variance explained by connectivity within this BCT-specific network, the relationship between internetwork connectivity and motor performance remained significant. Thus, and most importantly, our results indicating that age-related decrements in motor performance have a significantly stronger association with internetwork connectivity than intramotor-network connectivity are consistent across multiple resting state motor networks.
It has been suggested that the execution of relatively simple tasks is more strongly related to communication within a single relevant network whereas more complex processes are linked to communication across multiple networks (Cohen and D’Esposito 2016). Extending this explanation to the current study, it could be speculated that the association between internetwork connectivity and motor performance is modulated by task complexity or difficulty. We assessed this possibility by comparing the associations between internetwork connectivity and motor performance on the Line11 and Abrupt BCT conditions (see Supplemental Fig. S6A). There were no internetwork pairs that demonstrated a significant relationship between connectivity and performance on the Line11 condition, conceptualized as the simpler movement trajectory. This was in contrast to what was observed for the Abrupt task condition (Fig. 6 in the main text). Statistically comparing the relationships between internetwork connectivity and performance on the 2 task conditions revealed a trend for a significant difference (P = 0.06; Supplemental Fig. S6B), indicating that internetwork connectivity had a marginally stronger association to performance on the more complex as compared with the simpler bimanual condition. This notion that task complexity modulates the association between resting state internetwork connectivity and motor performance, however, certainly warrants further attention in future research. It also should be considered that the Line11 movement trajectory employed in the current study may not be the best example of a “simpler” motor task, as the successful completion of this particular condition still necessitates the execution of a precise coordination pattern between the 2 hands. Thus, future research investigating this specific question may opt to employ an even simpler motor task as a reference.
Limitations, Methodological Considerations, and Future Directions
It is important to acknowledge that our results are based on correlations among age, behavior and brain connectivity metrics and thus we are restricted to discussing the associations among these variables as opposed to inferring causality of the effects. Moreover, as all 3 of these variables are inter-related, it is difficult to decipher the precise relationship between any 2 of the factors independent of the influence of the third variable (see, Damoiseaux 2017, for more detailed discussion). A fairly common approach to address this issue is to relate, for example, behavioral and connectivity measures after factoring out the influence of age (Chan et al. 2014). Although additional insights may certainly be gained, the subsequent interpretations following the output of this approach certainly have limitations (Lindenberger and Pötter 1998). In the context of the results of the current study, the correlation between age and the behavior of interest (i.e., movement accuracy on the Abrupt condition of the BCT) was equal to −0.77 (Fig. 3C). Thus, as age already explained nearly 60% of the variance in behavior, statistically demonstrating a significant relationship between connectivity and motor performance above and beyond the influence of age would have been difficult given the inter-relatedness of all 3 variables. This limitation notwithstanding, our results do suggest that internetwork functional connectivity, as opposed to solely connectivity within motor networks, may be a primary contributor to the age-related declines in motor performance reported in the available literature.
It is worth explicitly acknowledging that we opted to not regress out the influence of the global signal in our functional connectivity analyses (see, Fox et al. 2009; Jo et al. 2013, for a more detailed discussion on this issue). Although the global signal regression approach certainly has its utility, it does impose correlational structure to the data and often induces negative (i.e., anti-) correlations between some networks. Our results will then have less between-network anticorrelations than previous research that employed global signal regression.
It is likely that the decreased segregation of resting state functional networks reported both in the current study and elsewhere are simply manifestations of other age-dependent degenerative processes. For example, alterations in resting state connectivity may be a byproduct of age-related changes in the concentration of specific neurochemicals, gray matter atrophy or alterations in white matter microstructure. Future research should aim to disentangle the relationships among the plethora of age-related changes at the neural level and their influence on motor functioning.
It would also be interesting for future research to examine the interindividual variability in internetwork functional connectivity. For example, a visual inspection of Figure 5B depicts 7 individuals of 30–45-year-old who exhibited high internetwork connectivity relative to their age-similar peers. Interestingly, one of these subjects reported a family history of dementia and a second subject had a MOCA score that was more than 2SD below the mean of subjects 30–45 years of age. There are certainly too few subjects to speculate about the potential relationship between predisposition to neurodegenerative disease and resting state functional connectivity, but this is a line of research that warrants further attention.
Conclusions
Our results demonstrated that the stronger connectivity between functional resting state brain networks observed as a function of age was significantly associated with declines in motor performance across the adult lifespan. This relationship between motor behavior and internetwork connectivity remained significant even after accounting for the contributions of connectivity within each of the 14 resting state networks that were examined, including 2 motor-related networks. These findings suggest that age-related impairments in motor performance may be attributed to degradations in the functional organization of large-scale brain networks. It would be advantageous for future research investigating the relationship between age-related declines in motor functioning and brain connectivity to extend analyses beyond strictly motor-related networks, as connectivity between networks appears to have a significantly stronger association with motor behavior.
Supplementary Material
Funding
KU Leuven Special Research Fund (grant C16/15/070 awarded to S.P.S. and D.M.) as well as from grants from the Research Foundation—Flanders (FWO; G.0708.14) and the Francqui Foundation. European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement (No. 703490) and a postdoctoral fellowship from the Research Foundation—Flanders (FWO; No 132635) to B.R.K. Wellcome Trust (grant 101253/A/13/Z to D.M.).
Notes
Conflict of Interest: None declared.
References
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. 2007. Disruption of large-scale brain systems in advanced aging. Neuron. 56:924–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonenko D, Flöel A. 2014. Healthy aging by staying selectively connected: a mini-review. Gerontology. 60:3–9. [DOI] [PubMed] [Google Scholar]
- Archer JA, Lee A, Qiu A, Chen S-HA. 2016. A comprehensive analysis of connectivity and aging over the adult life span. Brain Connect. 6:169–185. [DOI] [PubMed] [Google Scholar]
- Beets IAM, Gooijers J, Boisgontier MP, Pauwels L, Coxon JP, Wittenberg G, Swinnen SP. 2015. Reduced neural differentiation between feedback conditions after bimanual coordination training with and without augmented visual feedback. Cereb Cortex. 25:1958–1969. [DOI] [PubMed] [Google Scholar]
- Betzel RF, Byrge L, He Y, Goñi J, Zuo X-N, Sporns O. 2014. Changes in structural and functional connectivity among resting-state networks across the human lifespan. Neuroimage. 102(Pt 2):345–357. [DOI] [PubMed] [Google Scholar]
- Bhandari A, Radhu N, Farzan F, Mulsant BH, Rajji TK, Daskalakis ZJ, Blumberger DM. 2016. A meta-analysis of the effects of aging on motor cortex neurophysiology assessed by transcranial magnetic stimulation. Clin Neurophysiol. 127:2834–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. 1995. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 34:537–541. [DOI] [PubMed] [Google Scholar]
- Bo J, Lee C-M, Kwak Y, Peltier SJ, Bernard JA, Buschkuehl M, Jaeggi SM, Wiggins JL, Jonides J, Monk CS, et al. . 2014. Lifespan differences in cortico-striatal resting state connectivity. Brain Connect. 4:166–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. 2012. The economy of brain network organization. Nat Rev Neurosci. 13:336–349. [DOI] [PubMed] [Google Scholar]
- Cabeza R. 2002. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 17:85–100. [DOI] [PubMed] [Google Scholar]
- Cao M, Wang J-H, Dai Z-J, Cao X-Y, Jiang L-L, Fan F-M, Song X-W, Xia M-R, Shu N, Dong Q, et al. . 2014. Topological organization of the human brain functional connectome across the lifespan. Dev Cogn Neurosci. 7:76–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan MY, Park DC, Savalia NK, Petersen SE, Wig GS. 2014. Decreased segregation of brain systems across the healthy adult lifespan. Proc Natl Acad Sci USA. 111:E4997–E5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JR, D’Esposito M. 2016. The segregation and integration of distinct brain networks and their relationship to cognition. J Neurosci. 36:12083–12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS. 2017. Effects of aging on functional and structural brain connectivity. Neuroimage, 10.1016/j.neuroimage.2017.01.077. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJS, Barkhof F, Scheltens P, Stam CJ, Smith SM, Rombouts SARB. 2008. Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex. 18:1856–1864. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SARB, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. 2006. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci USA. 103:13848–13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Cabeza R. 2011. Age-related dedifferentiation of learning systems: an fMRI study of implicit and explicit learning. Neurobiol Aging. 32:2318.e17–e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira LK, Busatto GF. 2013. Resting-state functional connectivity in normal brain aging. Neurosci Biobehav Rev. 37:384–400. [DOI] [PubMed] [Google Scholar]
- Ferreira LK, Regina ACB, Kovacevic N, Martin M da GM, Santos PP, Carneiro Cde G, Kerr DS, Amaro E, McIntosh AR, Busatto GF. 2016. Aging effects on whole-brain functional connectivity in adults free of cognitive and psychiatric disorders. Cereb Cortex. 26:3851–3865. [DOI] [PubMed] [Google Scholar]
- Fling BW, Kwak Y, Peltier SJ, Seidler RD. 2012. Differential relationships between transcallosal structural and functional connectivity in young and older adults. Neurobiol Aging. 33:2521–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. 2007. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 8:700–711. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. 2005. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. 2009. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 101:3270–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerligs L, Maurits NM, Renken RJ, Lorist MM. 2014. Reduced specificity of functional connectivity in the aging brain during task performance. Hum Brain Mapp. 35:319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerligs L, Renken RJ, Saliasi E, Maurits NM, Lorist MM. 2015. A brain-wide study of age-related changes in functional connectivity. Cereb Cortex. 25:1987–1999. [DOI] [PubMed] [Google Scholar]
- Goble DJ, Coxon JP, Van Impe A, De Vos J, Wenderoth N, Swinnen SP. 2010. The neural control of bimanual movements in the elderly: brain regions exhibiting age-related increases in activity, frequency-induced neural modulation, and task-specific compensatory recruitment. Hum Brain Mapp. 31:1281–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh JOS. 2011. Functional dedifferentiation and altered connectivity in older adults: neural accounts of cognitive aging. Aging Dis. 2:30–48. [PMC free article] [PubMed] [Google Scholar]
- Grady C. 2012. The cognitive neuroscience of ageing. Nat Rev Neurosci. 13:491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady C, Sarraf S, Saverino C, Campbell K. 2016. Age differences in the functional interactions among the default, frontoparietal control, and dorsal attention networks. Neurobiol Aging. 41:159–172. [DOI] [PubMed] [Google Scholar]
- Grady CL. 2008. Cognitive neuroscience of aging. Ann NY Acad Sci. 1124:127–144. [DOI] [PubMed] [Google Scholar]
- Heitger MH, Goble DJ, Dhollander T, Dupont P, Caeyenberghs K, Leemans A, Sunaert S, Swinnen SP. 2013. Bimanual motor coordination in older adults is associated with increased functional brain connectivity—a graph-theoretical analysis. PLoS One. 8:e62133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuninckx S, Wenderoth N, Debaere F, Peeters R, Swinnen SP. 2005. Neural basis of aging: the penetration of cognition into action control. J Neurosci. 25:6787–6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuninckx S, Wenderoth N, Swinnen SP. 2008. Systems neuroplasticity in the aging brain: recruiting additional neural resources for successful motor performance in elderly persons. J Neurosci. 28:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S. 1979. A simple sequentially rejective multiple test procedure. Scand J Stat. 6:65–70. [Google Scholar]
- Jo HJ, Gotts SJ, Reynolds RC, Bandettini PA, Martin A, Cox RW, Saad ZS. 2013. Effective preprocessing procedures virtually eliminate distance-dependent motion artifacts in resting state fMRI. J Appl Math. 2013:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BR, Fogel SM, Albouy G, Doyon J. 2013. Neural correlates of the age-related changes in motor sequence learning and motor adaptation in older adults. Front Hum Neurosci. 7:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langan J, Peltier SJ, Bo J, Fling BW, Welsh RC, Seidler RD. 2010. Functional implications of age differences in motor system connectivity. Front Syst Neurosci. 4:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberger U, Pötter U. 1998. The complex nature of unique and shared effects in hierarchical linear regression: implications for developmental psychology. Psychol Methods. 3:218–230. [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. 2002. Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron. 33:827–840. [DOI] [PubMed] [Google Scholar]
- Maes C, Gooijers J, Orban de Xivry J-J, Swinnen SP, Boisgontier MP. 2017. Two hands, one brain, and aging. Neurosci Biobehav Rev. 75:234–256. [DOI] [PubMed] [Google Scholar]
- Mantini D, Corbetta M, Romani GL, Orban GA, Vanduffel W. 2013. Evolutionarily novel functional networks in the human brain? J Neurosci. 33:3259–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mary A, Wens V, Op de Beeck M, Leproult R, De Tiège X, Peigneux P. 2016. Resting-state functional connectivity is an age-dependent predictor of motor learning abilities. Cereb Cortex. 27:4923–4932. [DOI] [PubMed] [Google Scholar]
- Mary A, Wens V, Op de Beeck M, Leproult R, De Tiège X, Peigneux P. 2017. Age-related differences in practice-dependent resting-state functional connectivity related to motor sequence learning. Hum Brain Mapp. 38:923–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hariri AR, Das S, Callicott JH, Weinberger DR. 2002. Neurophysiological correlates of age-related changes in human motor function. Neurology. 58:630–635. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. 2005. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 53:695–699. [DOI] [PubMed] [Google Scholar]
- Ng KK, Lo JC, Lim JKW, Chee MWL, Zhou J. 2016. Reduced functional segregation between the default mode network and the executive control network in healthy older adults: a longitudinal study. Neuroimage. 133:321–330. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. 1971. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 9:97–113. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. 2006. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev. 30:730–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA. 2002. New visions of the aging mind and brain. Trends Cogn Sci. 6:394–400. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Lustig C. 2005. Brain aging: reorganizing discoveries about the aging mind. Curr Opin Neurobiol. 15:245–251. [DOI] [PubMed] [Google Scholar]
- Santos Monteiro T, Beets IAM, Boisgontier MP, Gooijers J, Pauwels L, Chalavi S, King B, Albouy G, Swinnen SP. 2017. Relative cortico-subcortical shift in brain activity but preserved training-induced neural modulation in older adults during bimanual motor learning. Neurobiol Aging. 58:54–67. [DOI] [PubMed] [Google Scholar]
- Seidler R, Erdeniz B, Koppelmans V, Hirsiger S, Mérillat S, Jäncke L. 2015. Associations between age, motor function, and resting state sensorimotor network connectivity in healthy older adults. Neuroimage. 108:47–59. [DOI] [PubMed] [Google Scholar]
- Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, Kwak Y, Lipps DB. 2010. Motor control and aging:links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev. 34:721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbruyns L, Gooijers J, Caeyenberghs K, Meesen RL, Cuypers K, Sisti HM, Leemans A, Swinnen SP. 2015. Bimanual motor deficits in older adults predicted by diffusion tensor imaging metrics of corpus callosum subregions. Brain Struct Funct. 220:273–290. [DOI] [PubMed] [Google Scholar]
- Siman-Tov T, Bosak N, Sprecher E, Paz R, Eran A, Aharon-Peretz J, Kahn I. 2016. Early age-related functional connectivity decline in high-order cognitive networks. Front Aging Neurosci. 8:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisti HM, Geurts M, Clerckx R, Gooijers J, Coxon JP, Heitger MH, Caeyenberghs K, Beets IAM, Serbruyns L, Swinnen SP. 2011. Testing multiple coordination constraints with a novel bimanual visuomotor task. PLoS One. 6:e23619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solesio-Jofre E, Serbruyns L, Woolley DG, Mantini D, Beets IAM, Swinnen SP. 2014. Aging effects on the resting state motor network and interlimb coordination. Hum Brain Mapp. 35:3945–3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Birn RM, Boly M, Meier TB, Nair VA, Meyerand ME, Prabhakaran V. 2014. Age-related reorganizational changes in modularity and functional connectivity of human brain networks. Brain Connect. 4:662–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O. 2013. Network attributes for segregation and integration in the human brain. Curr Opin Neurobiol. 23:162–171. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Viviano JD, Schacter DL. 2016. Attenuated anticorrelation between the default and dorsal attention networks with aging: evidence from task and rest. Neurobiol Aging. 45:149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. 2012. Aging and functional brain networks. Mol Psychiatry. 17(471):549–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Hulshoff Pol HE. 2010. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol. 20:519–534. [DOI] [PubMed] [Google Scholar]
- Ward NS, Frackowiak RSJ. 2003. Age-related changes in the neural correlates of motor performance. Brain. 126:873–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Zang Y, Wang L, Long X, Hallett M, Chen Y, Li K, Chan P. 2007. Aging influence on functional connectivity of the motor network in the resting state. Neurosci Lett. 422:164–168. [DOI] [PubMed] [Google Scholar]
- Wu T, Zang Y, Wang L, Long X, Li K, Chan P. 2007. Normal aging decreases regional homogeneity of the motor areas in the resting state. Neurosci Lett. 423:189–193. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.