Abstract
Drug delivery to the brain has been a major challenge due to the presence of the blood brain barrier (BBB), which limits the uptake of most chemotherapeutics into brain. We developed a dual-functionalized liposomal delivery system, conjugating cell penetrating peptide penetratin to transferrin-liposomes (Tf-Pen-conjugated liposomes) to enhance the transport of an anticancer chemotherapeutic drug, 5-fluorouracil (5-FU), across the BBB into the tumor cells. The in vitro cellular uptake study showed that the dual-functionalized liposomes are capable of higher cellular uptake in glioblastoma (U87) and brain endothelial (bEnd.3) cells monolayer. In addition, dual-functionalized liposomes demonstrated significantly higher apoptosis in U87 cells. The liposomal nanoparticles showed excellent blood compatibility and in vitro cell viability, as studied by hemolysis and MTT assay, respectively. The 5-FU loaded dual-functionalized liposomes demonstrated higher transport across the brain endothelial barrier and delivered 5-FU to tumor cells inside PLGA-chitosan scaffold (an in vitro brain tumor model), resulting in significant tumor regression.
Keywords: blood brain barrier, liposomes, nanomedicine, biocompatibility, cancer chemotherapy, targeted drug delivery
Introduction
Glioblastoma multiforme (GBM) is a tumor of the central nervous system of grade IV histological malignancy and arises from astrocytes. They are highly malignant and life threatening because the tumor cells reproduce quickly and infiltrate rapidly which results in disruption of normal tissue. Complete surgical removal and/or radiotherapy of these tumors are impossible due to invasion and growth within the normal brain tissue.1 In addition, chemotherapy is another common method for treatment of glioblastoma. However, even after intravenous administration, the chemotherapeutic agents do not reach the brain tissues because of the presence of the blood brain barrier (BBB)2 and thereby invalidate the effectiveness of systemically administered chemotherapeutic agents. Therefore, there is a need to develop a delivery system which can not only penetrate the BBB but also effectively deliver chemotherapeutic agents to the core of tumor cells as well as migratory cells in the infiltration zone.3
There have been many attempts to develop a delivery vector which can successfully translocate across the BBB, however, the transport of water soluble chemotherapeutics into the brain still remains a challenge. Several receptors present on the surface of BBB that facilitate the transport of amino acids, glucose or nucleic acids into the brain can be exploited to transport drug cargo across the BBB into the brain tissues.4,5 In recent decades, nanocarriers such as amphiphilic molecules forming liposomes are attracting increased attention for the delivery of therapeutics across the BBB.6 Liposomes are artificially prepared phospholipid bilayer vesicles that can be surface engineered for receptor targeting, hence can be utilized as a carrier for targeted drug delivery.7–9 Transferrin receptors (TfRs) are overexpressed on the surface of both brain endothelial cells as well as glioblastoma cells, which can be used for delivery of therapeutic moieties.10,11 Several studies have demonstrated that intravenous administration of transferrin (Tf) receptor targeted sterically stabilized carriers lead to enhanced translocation of the carriers across the brain.4,12,13 However, drug delivery through receptor mediated targeting is restricted by the saturation of receptors.5,14 In addition, the endocytic uptake of drug loaded nanocarriers result in endosomal entrapment that decrease their intracellular delivery. Moreover, there are several reports showing positive results but it is difficult to compare the ligand’s effectiveness for brain delivery based on the results. There are several factors which are important for targeting, such as particle type, ligand density, receptor saturation, endosomal entrapment etc.5,14,15 Therefore, the concept of single ligand targeted drug delivery vectors has long been studied and there is a need for improving delivery of targeted vectors by surface modification of such nanoparticles with other types of ligands, such as cell penetrating peptides (CPPs)16,17 that are well known for their ability to enhance cell penetration. The rationale for using two ligands in one carrier is to use different mechanisms for translocation of delivery vector across the BBB and into the brain, thereby reducing the possibility of receptor saturation.5,18 CPPs are amphipathic cationic peptides that enable the internalization of exogenous cargo such as liposomes or nanoparticles.19–21 The significance of this study lies in the design and formulation of dual functionalized liposomal nanoparticles as an effective drug carrier to the brain tumor site. In the present study, we evaluated the anti-tumor efficacy of CPP (Penetratin) coupled Tf-conjugated liposomes (Tf-Pen-conjugated liposomes) to transport an anticancer chemotherapeutic drug, 5-fluorouracil (5-FU), across the BBB and into tumor cells using an in vitro brain tumor model.
The brain tumor pathology is very complex and it is difficult to develop an in vitro brain tumor model. Although several models have been developed, none addresses the complex pathology of the brain tumor and the developed models do not provide any information about whether the human glioma spheroids were juxtaposed with non-neoplastic spheroids or the tumor cells were cultured on organotypic brain slices.22,23 In addition, these models alone do not take into account the presence of the BBB which restricts the transport of therapeutic agents into the brain. In the present study, we overcome the aforementioned issues and designed an in vitro brain tumor model using a biodegradable PLGA-chitosan scaffold.18 A 3-dimensional glioblastoma tumor was grown inside the scaffold and the transport of liposomes across brain endothelial barrier into the 3-d tumor was determined. The advantage of this model is that we mimicked in vivo like conditions where the systemically administered delivery carrier needs to translocate across the BBB first before reaching target tumor sites. In addition, the co-culture brain endothelial model (brain endothelial and glial cells) form a tight barrier, thereby demonstrating the improvement of junctional proteins as well as physical strength provided by glial cells. By using this model, we evaluated the efficacy of dual functionalized liposomal nanoparticles in translocating across co-culture brain endothelial barrier and efficiently delivering 5-FU to the U87 tumor cells inside PLGA-chitosan scaffold, thereby killing the tumor cells.
5-Fluorouracil (5-FU) is a potent antimetabolite used for chemotherapy of glioblastoma.24,25 However, due to its hydrophilic nature, the drug does not easily cross BBB which prevents its accumulation in glioblastoma tumors and limits its efficacy. Penetratin (Pen) is a 16 amino acid cationic peptide, derived from naturally occurring protein, Antennapedia homeodomain and is capable of inducing internalization of a large variety of exogenous cargo.26,27
In this study, we developed dual-functionalized liposomes, surface modified with Tf for receptor targeting and Pen for enhanced cell penetration across the BBB. Transport across BBB would be followed by endocytosis into tumor cells present inside PLGA-chitosan scaffold, resulting in increased accumulation of 5-FU in tumor, thereby improving anti-tumor efficacy (illustrated in figure 1). We performed detailed investigation to determine the targeting and penetration ability of the nanocarriers by evaluating their cellular uptake and spheroids penetration capability. in vitro cytotoxicity and hemolysis studies were performed to evaluate the biocompatibility for In vivo administration. We also investigated the transport and anti-tumor efficacy of dual functionalized, Tf-Pen liposomes across in vitro brain tumor model.
Figure 1.
Schematic showing the transport of 5-FU encapsulated dual-functionalized liposomes across the BBB, followed by endocytosis into tumor cells.
Materials and Methods
Materials:
1,2-dioleoyl-3-trimethylammonium-propane chloride (DOTAP), 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[carboxy(polyethylene glycol)-2000] (DSPE-PEG-COOH), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl) (DOPE-lissamine rhodamine), and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) were procured from Avanti Polar Limited (Birmingham, Alabama). 5-Fluorouracil (5-FU) was purchased from AK scientific (Union city, California). Coumarin-6 was purchased from Tokyo chemical industry Co. Ltd. (Portland, Oregon). Transferrin, Cholesteryl hemi succinate (CHS) and Chitosan (50 kDa) were obtained from Sigma-Aldrich Company (St. Louis, Missouri). Penetratin (Pen) was purchased from Anaspec Inc. (Fremont, California). Fetal bovine serum (FBS) was obtained from Omega scientific Inc. (Tarzana, California). Dulbecco’s modified eagle medium (DMEM) and Dulbecco’s phosphate buffered saline (DPBS) were procured from Mediatech Inc. (Manassas, Virginia). N-hydroxysuccinimide (NHS) and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC.HCl), and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were obtained from Alfa Aesar (Ward Hill, Massachusetts) and Creosalus Inc. (Louisville, Kentucky), respectively. Polyethylene terephthalate (PET) thincerts, cell culture inserts were purchased from Greiner Bio-One International (Monroe, North Carolina). FITC Annexin V Apoptosis detection kit was obtained from BD Biosciences (San Diego, California). Poly (D, L-lactide-co-glycolide) 50:50 was purchased from Polyscitech (West Lafayette, Indiana). Tissue Tek® OCT compound was procured from Sakura Finetek (Torrance, California). All the chemicals used were of analytical grade. Brain endothelial cells (bEnd.3) were procured from American Type Culture Collection (ATCC, Rockville, Maryland).
Synthesis of DSPE-PEG(2000)-Pen and DSPE-PEG(2000)-Tf:
Coupling Of Pen to DSPE-PEG(2000)-COOH:
Penetratin was coupled through the reaction of carboxyl group of DSPE-PEG(2000)-COOH and the amine group of penetratin in the presence of EDC/NHS. Briefly, DSPE-PEG(2000)-COOH (0.64 μmole) was suspended in HEPES buffered saline pH 5.0 to form micelles. This suspension was treated for 60 mins with 360 ml of both 0.5 M EDC and 0.5 M NHS per 10 μl of DSPE-PEG(2000)-COOH to activate carboxyl group of DSPE-PEG(2000)-COOH. Excess EDC/NHS were removed using dialysis membrane MWCO 1,000 Da. Furthermore, the pH was adjusted to 7.4 using 0.1 N sodium hydroxide. Penetratin (0.213 μmole) was added to the suspension in the molar ratio 1:3 (Pen: DSPE-PEG) and stirred overnight at room temperature. Uncoupled penetratin was removed through dialysis membrane MWCO 3.5 kDa. The coupling efficiency was determined by micro bicinchoninic acid (BCA) assay in accordance with manufacturer’s protocol. Penetratin was used as a standard and DSPE-PEG(2000)-COOH was used as a control for the studies.
Coupling Of Tf to DSPE-PEG(2000)-COOH:
The coupling of transferrin to DSPE-PEG(2000)-COOH was performed through EDC/NHS reaction, as previously described.5,28 Tf (125 μg /μ mole of the lipid) was added to the activated DSPE-PEG(2000)-COOH and stirred for 8 h at room temperature. The unbound transferrin was separated using sephadex G-100 column. The coupling efficiency was determined using micro BCA assay. Briefly, 100 μl of liposome suspension was mixed with 400 μl of methanol and this mixture was vortexed and centrifuged at 9,000 × g for 30 s. Further, 200 μl of chloroform was added to this mixture and vortexed and centrifuged at 9,000 × g for 30 s. The phase was separated by adding 300 μl of water to this mixture. Thereafter, the mixture was vortexed and centrifuged at 9,000 × g for 1 min. The upper phase was discarded and 300 μl of methanol was added to the interphase between chloroform and protein precipitate, that was followed by centrifugation at 9,000 × g for 2 mins. The supernatant layer was removed and precipitated protein pellet was air dried. The pellet was dissolved in 100 μl of phosphate buffered saline (PBS), pH 7.4 and analyzed for coupling efficiency using micro BCA assay in accordance with manufacturer’s protocol.28,29
Preparation Of Dual-Functionalized Liposomes:
The dual-functionalized liposomes were prepared using post-insertion technique.5 Briefly, Pen-PEG(2000))-DSPE and other phospholipids, in the following molar ratio: DOTAP/DOPE/CHS/Pen-PEG(2000))-DSPE (43.5:43.5:5:4 mole %) were dissolved in chloroform: methanol (2:1; v/v). The solvent of the mixture was evaporated using rotavapor (Buchi Rotavapor RII, New Castle, DE) to form a thin lipid film. Pen coupled liposomes were prepared by hydrating the dried lipid thin film with HEPES buffered saline, pH 7.4. Furthermore, the Pen-liposomes were stirred overnight with DSPE-PEG(2000))-Tf micelles at room temperature to form dual functionalized liposomes (Tf-Pen-conjugated liposomes). The free DSPE-PEG(2000)-Tf micelles were removed from Tf-Pen-liposomes by passing the liposomes through sephadex G-100 column.
For preparation of lissamine-rhodamine labeled liposomes, 0.5 mole % of lissamine rhodamine coupled DOPE was dissolved as a liposomal membrane marker along with other lipids in chloroform: methanol (2:1; v/v) and evaporated to form a thin lipid film. For coumarin-6 labeled liposomes, 0.5 mole % (28 μg) of coumarin6 was added to the lipid mixture prior to the formation of thin lipid film. The average particle size and zeta potential of the liposomes were determined by Zetasizer Nano ZS 90 (Malvern Instruments, Worcestershire, UK) at 25°C.
5-Fluorouracil loading:
5-FU was loaded into liposomes using pH gradient method as previously reported.30 Briefly, the thin film was hydrated using 300 mM sodium carbonate pH 9.6, to form Pen-conjugated liposomes, followed by stirring with DSPE-PEG(2000)-Tf micelles. To create pH gradient, the formulations were passed through sephadex G-100 pre-equilibrated with HEPES buffered saline pH 7.4. The drug 5-FU was added to the liposomal formulation and incubated at 50°C for 1 h. The 5-FU loaded liposomes were then cooled down to room temperature and passed through sephedex G-100 column to separate unentrapped drug. Entrapment efficiency was quantified using high performance liquid chromatography (HPLC) (Agilent Technologies 1120 Compact LC). Briefly, 100 μl of liposomal formulation before and after passing through the column was dispersed in 200 μl of D.I. water with 100 μl of methanol and 100 μl of 0.5% triton X-100. The analysis of 5-FU was determined at a wavelength of 264 nm using C-18 column (Thermoscientific Hypersil BDS, 5 μm, 250 × 4.6 mm) and mobile phase containing 0.2 M potassium phosphate monobasic: acetonitrile (98:2) with a flow rate of 0.750 mL/min at room temperature.
In Vitro Release Of 5-FU:
In vitro release studies of 5-FU were performed by diluting liposomes in PBS, pH 7.4 with 10% FBS. Briefly, 5-FU encapsulated liposomes were placed inside tightly sealed dialysis tube (MWCO 6,000-8,000 Da) and immersed in 50 ml of PBS (pH 7.4) and incubated at 37 ±0.5 ° with mild oscillation of 50 rpm. At predetermined time points, 1 ml of samples were taken and replaced with 1 ml of fresh PBS (pH 7.4). The samples were analyzed using HPLC, as described previously for evaluation of 5-FU loading.
In Vitro Cytotoxicity Assay:
Cytotoxicity study is an important in vitro parameter to determine the biocompatibility of the liposomal formulations. High cationic charge of penetratin may cause cytotoxicity in cells due to its membrane disruptive potential.31 In this study, MTT assay was used to determine the cytotoxic potential of liposomes made with different concentrations of phospholipids in glioblastoma U87 and brain endothelial bEnd.3 cells. For the study, cells were seeded in 96 well plates at a density of 1,000 cells per well in 200 μl of DMEM supplemented with 10% FBS and 1% penicillin-streptomycin and incubated at 37°C under 5% CO2 atmosphere, 24 h before treatment. Cells were subsequently treated with different phospholipid concentrations of liposomes (plain, Tf, Pen, and Tf-Pen-conjugated liposomes) for 2 h in serum free media. After treatment, the formulation containing media was removed and replaced with fresh media supplemented with serum and cells were incubated for 48 h at 37 °C under 5% CO2 atmosphere. MTT assay was performed to evaluate the cell viability. The control group consisted of untreated cells, incubated at similar conditions for same duration.
In vitro Cellular Uptake Study:
Cellular uptake study provides an insight about how much drug is taken up by the cells. Each well of a 6 well plate was seeded with 6 × 105 cells, 24 h prior to the uptake study. Cells were incubated with different 5-FU loaded liposomes (200 nM of phospholipid concentration in each well) and free 5-FU (5 μg/mL) and cellular uptake was studied for 2 h. Subsequently, the liposomal formulations were removed from wells and cells were rinsed with DPBS, pH 7.4, 3 times to remove the formulation on the surface of cells. Thereafter cell nuclei was stained with 1 mL of 1 μg/mL Hoechst 33342 and fluorescence of the cells were observed using Leica DMi8 fluorescence microscope (Leica Microsystems Inc., Buffalo Grove, IL). The quantitative uptake of 5-FU was determined by lysis of cells by triton X-100 followed by extraction in methanol. The cell lysate was centrifuged at 10,000 rpm for 15 mins at 4°C and the supernatant was quantified by HPLC to calculate the amount of 5-FU taken up by cells. The analysis was performed as mentioned previously for determination of 5-FU entrapment.
Apoptosis Study:
Apoptosis induction by liposomal formulations in U87 cells was quantified by FITC-annexin-V/PI double staining assay. Briefly, U87 cells were seeded in 6 well plates at a density of 1 × 106 cells per well. 24 h after seeding, cells were treated with different 5-FU loaded liposomes (200 nM of phospholipid concentration in each well) and free 5-FU (5μg/mL) for 5 hours, followed by replacement of formulations with fresh complete medium and incubated for an additional 24 h at 37°C. Subsequently, the cells were trypsinized, collected, and stained with Annexin V-FITC apoptosis detection kit. The cells were assessed using BD Accuri C6 flow cytometer (BD Biosciences Accuri cytometers, San Jose, CA) in accordance with manufacturer’s protocol.
Penetration Ability into U87 Spheroids:
U87 spheroids were grown using liquid overlay system to study the penetration ability of dual-functionalized liposomes.32 Briefly, low melting point agarose was added into DPBS and heated to 80°C for 30 mins to form 2% (w/v) solution. Each well of 24 well plate was coated with 150 μl of the prepared agarose. After cooling to ambient temperature, 1 × 103 U87 cells were seeded into each well of the 24 well plate. The plates were allowed to agitate for 5 mins and incubated at 37 ° C, followed by supplementation with DMEM containing 10% FBS and 1% penicillin-streptomycin for 6 days. The media was changed every other day. U87 spheroids were treated with different coumarin-6 loaded liposomal formulations (200 nM of phospholipid concentration) at 37 °C for 12 h. Subsequently, the spheroids were washed with cold DPBS and subjected to imaging using FV300 confocal laser scanning microscope (Olympus, Melville, NY). The z-stage images were obtained by scanning the different layers of the spheroids from the top to the equatorial plane.
Hemolysis Study:
Hemolysis study provides an insight about interaction between negatively charged erythrocytes membrane and CPP coupled liposomes which may cause hemolysis. For the study, blood from an adult rat was collected into EDTA containing tubes and centrifuged at 2,000 rpm for 10 mins. The obtained pellet was washed thrice with PBS, pH 7.4, counted and pre-determined number of erythrocytes were incubated with different phospholipid concentrations of liposomes (plain, Tf, Pen, and Tf-Pen-conjugated liposomes) for 60 mins at 37 °C. Subsequently, the samples were centrifuged at 2,000 rpm for 10 mins and the absorbance of the supernatant was noted at 540 nm using a spectrophotometer (SpectraMax® M5, Molecular devices, Sunnyvale, CA). Erythrocytes treated with triton X-100 and PBS were taken as controls for 100% and 0% hemolysis, respectively. Percent hemolysis less than 10% was considered non-toxic33,34.
Design of In Vitro Endothelial Barrier:
The in vitro endothelial barrier was constructed by combination of bEnd.3 cells on luminal side and primary glial cells on abluminal side of the culture insert. Briefly, primary glial cells (15,000 cells/cm2) in DMEM with 20% FBS were seeded on the bottom side of the culture insert overnight to allow adherence of the cells to the culture insert’s membrane.5 Following day, brain endothelial cells (37,500 cells/cm2) were seeded on the inside of culture inserts, placed in a 24 well plate and were cultured for 6 days to form a tight barrier.35 The media was replaced every 2 days and cells were checked for confluence. The integrity of the barrier was determined by measuring the flux of sodium fluorescein (Na-F) across the endothelial barrier layer and transendothelial electrical resistance (TEER) using EVOM2 with STX2 (World Precision Instruments, Sarasota, FL), as previously reported.5,35 Briefly, the culture inserts with both bEnd.3 and glial cells (co-culture) or only endothelial cells (monolayer) were placed in 24 well plates with 1 ml of DPBS in the lower compartment. In the upper compartment of the culture inserts, the medium was replaced with 500 μl containing 10 μg/ml Na-F. The samples were taken at specific time points by transferring the culture inserts to new wells containing 1 ml of DPBS. The paracellular transport was evaluated by measuring the fluorescence intensity of Na-F from the upper and lower compartment using spectrophotometer microplate reader at excitation/emission wavelengths of 485/535 nm respectively (SpectraMax® M5, Molecular devices, Sunnyvale, CA). The endothelial permeability coefficient (Pe) was calculated by measuring the flux across cell free inserts for both models, as per previously published reports.5,36,37
Preparation Of PLGA-Chitosan Scaffold:
Emulsion freeze drying technique was used to prepare porous PLGA-chitosan scaffold.18,37 Briefly, poly(D,L-lactide-co-glycolide) (50:50) (PLGA) at a concentration of 0.2 g/ml was dissolved in dichloromethane. Separately, 500 mg of chitosan and 150 mg of polyvinyl alcohol (PVA) were dissolved in 10 ml of acetic acid buffer pH 4.5. To this mixture, previously prepared PLGA solution was added at a rate of 2 ml/min with constant stirring to form an emulsified paste. 500 μl of 0.1% w/v collagen solution in 0.1 M acetic acid was added to the paste. Further, the paste was poured into rod-shaped mold and freeze dried. Then, this was cut into 2mm thick circular discs.
Growth of Tumor Cells Inside PLGA-Chitosan Scaffold:
To provide strength, the scaffolds were treated with 5 M sodium hydroxide and washed thrice with DPBS to remove excess sodium hydroxide. The scaffolds were then treated with 70% ethanol and washed with sterile DPBS pH 7.4. Thereafter, the scaffolds were soaked in DMEM supplemented with 30% FBS overnight before seeding tumor cells on them. The surface of the scaffold was seeded with 5 × 105 U87 cells and incubated for 6 h, followed by subsequent addition of fresh media containing 30% FBS. The cells in the scaffold were cultured for 21 days to form 3D tumors on the scaffold. At different time points, the sections of the scaffold containing U87 tumor cells were embedded in OCT and frozen. The frozen scaffold was subsequently sectioned using cryostat and mounted on polylysine coated slides and the cell growth was monitored using hematoxylin eosin staining. The seeding efficiency was determined by MTT assay, as per previously published report.38
Liposomal Transport Across In vitro Brain Tumor Model:
The in vitro brain tumor model was prepared by placing the culture insert (seeded with bEnd.3 on upper side and primary glial cells on the bottom of the insert membrane) above the glioblastoma tumor grown scaffold on day 14 and the entire unit was further cultured for 7 days. This model mimics in vivo conditions, where the liposomal formulation prior to reaching the target U87 tumor inside the scaffold must cross the endothelial barrier. The transport of coumarin-6 loaded liposomes (plain, Tf, Pen, and Tf-Pen) was quantified across the in vitro brain tumor model by placing them in sterile DPBS containing 10% FBS to mimic the in vivo environment. The culture insert with bEnd.3 and glial cells were placed on U87 tumor grown scaffold in 24 well plates containing 1 ml DPBS supplemented with 10% FBS in the lower compartment. In the upper compartment of inserts, the medium was replaced with the coumarin-6 loaded liposomal suspension (200 nM) in 500 μl of fresh serum containing buffer. Samples were taken at specific time points of 1, 2, 4, 6, 12 and 24 h by transferring the culture inserts to new wells containing 1 ml DPBS with serum. Following transport, the scaffolds were rinsed with DPBS and tumor cells on scaffold were lyzed by adding 50 μl of 0.5% triton X-100 followed by addition of 450 μl of methanol to extract coumarin-6. The lysate was evaluated by quantifying the fluorescence intensity of coumarin-6 from the upper and lower compartments using fluorescence SpectraMax® M5 spectrophometer microplate reader (excitation/emission wavelengths 465/502 nm respectively) to calculate the amount of coumarin-6 transport across the endothelial barrier.
Evaluation of Efficacy of Liposomes on Tumor Regression:
The anti-tumor efficacy of liposomes was evaluated by adding different 5-FU encapsulated liposomes to the culture inserts seeded with brain endothelial and glial cells and placed on tumor scaffold. The media surrounding the scaffold was replaced with 1 ml DPBS pH 7.4 containing serum. In the upper compartment of inserts, the medium was replaced with 5-FU encapsulated liposomal suspension (200 nM) and free 5-FU (5 μg) in 500 μl of fresh serum containing DPBS. The in vitro brain tumor model was treated for 24 h and the scaffolds were thereafter incubated for 6 more days in fresh DMEM with 30% FBS at 37°C under 5% CO2 atmosphere. The media of the scaffolds was changed every other day. The percent tumor cell viability was determined by using MTT assay. For fluorescence imaging, the scaffolds were stained using viability/cytotoxicity assay kit (Biotum Inc., Fremont, CA) according to manufacturer’s protocol. Following that, the scaffolds were embedded in OCT and frozen. Subsequently, the frozen scaffolds were sectioned using cryostat and mounted on polylysine coated slides and stained with hematoxylin-eosin. The fluorescence images of scaffolds were assessed using Leica DMi8 fluorescence microscope (Leica Microsystems Inc., Buffalo Grove, IL).
Data analysis:
All statistical analysis were performed using Graphpad Prism 5.0 for Windows (GraphPad Software, Inc., La Jolla, CA). All the data were expressed as mean ± standard deviation (S.D.). Student’s t-test and one or two-way ANOVA were used to determine statistical significance among groups. Significant difference was considered at p < 0.05. All the experiments were repeated in quadruplicates.
Results and Discussion
Characterization Of Liposomes:
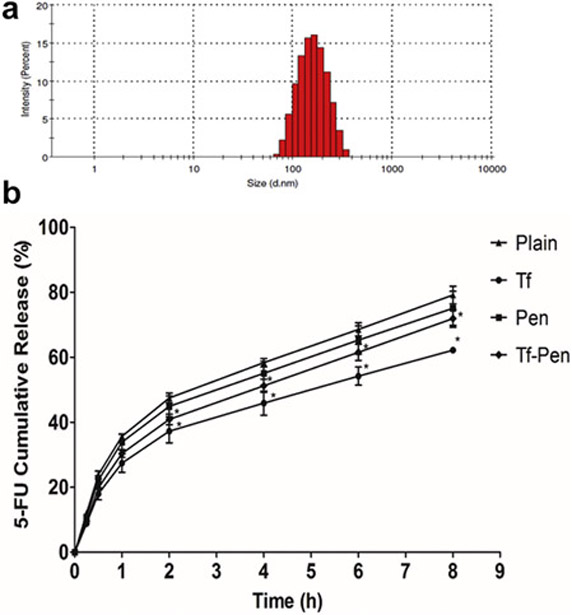
The dual-functionalized liposomes were prepared by post-insertion technique, which efficiently inserts active ligands into preformed liposomes, thereby eliminates the probability of degradation of encapsulated agents by reacting with coupling agents.39,40 In addition, the post-insertion technique is a spontaneous process, where hydrophobic part of lipid membrane interacts with hydrophobic part of PEG derivatives.41 The post-insertion technique is majorly used for the insertion of large targeting protein to the liposomes which may eliminate the chances of protein conformational changes and decrease in targeting efficiency.42,43 The incorporation of Pen-PEG(2000))-DSPE with other lipids directly in the formation of liposomes, gives accuracy and reproducibility in production of stable liposomes as well as helps in controlling the amount of penetratin in the formulation.43,44 As listed in Table 1, the mean particle size and zeta potential of Tf-Pen-conjugated liposomes were less than 200 nm and ~ 3 mV, respectively. The average size of plain liposomes and Tf-Pen-conjugated liposomes was 172.52 ± 6.71 and 178.12 ± 4.67, respectively. Therefore, the coupling of transferrin and penetratin to the liposomes showed no significant difference (p > 0.05) in the particle size of the liposomes. A typical size distribution graph of Tf-pen-conjugated liposomes is shown in figure 2A. In addition, the zeta potential of plain and Tf-Pen-conjugated liposomes was observed to be 4.86 ± 2.15 mV and 2.12 ± 1.56 mV, respectively. The results showed that coupling of transferrin protein to the liposomes changed the zeta potential to a net negative value, which is because of presence of negative charges on transferrin. However, the Tf-Penconjugated liposomes carry near neutral zeta potential (0-15 mV), which could be attributed to counter balancing of the negative charge of transferrin with the positive charge of penetratin. The near neutral charge of dual functionalized liposomes is anticipated to prevent elimination by macrophage system.45,46 The coupling efficiency of transferrin and penetratin as determined by micro BCA assay, was 56.1 ± 2.83 % and 62.81 ± 5.29%, respectively.
Table 1.
Particle size distribution and zeta potential of various liposomal formulations
| Liposomes | Particle size (nm) | PDIa | Zeta Potential (mV) |
|---|---|---|---|
| Plain | 172.52 ± 6.71 | 0.195 ± 0.012 | 4.86 ± 2.15 |
| Tf | 176.42 ± 5.86 | 0.267 ± 0.080 | −8.11 ± 3.68 |
| Pen | 176.80 ± 1.84 | 0.253 ± 0.043 | 7.66 ± 1.23 |
| Tf-Pen | 178.12 ± 4.67 | 0.274 ± 0.085 | 2.12 ± 1.56 |
Polydispersity index (PDI). The data represented as mean ± SD, (n=4).
Figure 2.
Size distribution of (A) Tf-Pen liposomes as obtained from dynamic light scattering. (B) Percent cumulative 5-FU release from plain, Tf, Pen, and Tf-Pen liposomes. Statistically significant (p < 0.05) differences is shown as (*) with plain liposomes. Data represented as mean ± S.D. (n=4).
pH gradient method was used to ensure high entrapment of 5-FU.30 The use of 300 mM sodium carbonate pH 9.6 is to protonate 5-FU intraliposomally, which leads to further diffusion of the neutral unionized 5-FU from outside, according to concentration gradient. In order to achieve high entrapment, the core of the liposomes must be highly buffered to sustain the pH gradient to accumulate more 5-FU. The 5-FU encapsulation efficiencies of plain, Tf, Pen, and Tf-Pen liposomes were quantified as 25.08 ± 2.33%, 25.67 ± 1.47%, 24.98 ± 1.59%, and 25.61 ± 1.13%, respectively and showed no significant difference (p > 0.05) amongst the formulations. This might be because 5-FU is a small, membrane permeable and highly water soluble drug molecule. The retention of such a small and water soluble molecule in the hydrophilic core of liposomes is therefore quite difficult. In addition, no interference was seen in the entrapment of 5-FU due to presence of penetratin and transferrin on the liposomal surface. The in vitro release of 5-FU from liposomes was studied to examine the drug release property of liposomes in 10% FBS. The results showed that the percent cumulative release was 79.16 ± 2.69%, 62.16 ± 0.97%, 75.05 ± 5.22%, and 71.90 ± 2.71% from plain, Tf, Pen, and Tf-Pen liposomes, respectively over a period of 8 h (figure 2B). The plain liposomes showed higher percent cumulative release of 5-FU as compared to Tf, and Tf-Pen-conjugated liposomes. This can be explained by the presence of negatively charged transferrin, thereby reducing the interaction with serum proteins. However, positively charged penetratin showed greater interactions leading to a greater release of 5-FU from plain liposomes compared to Tf-liposomes.18
In Vitro Biocompatibility Of Liposomes:
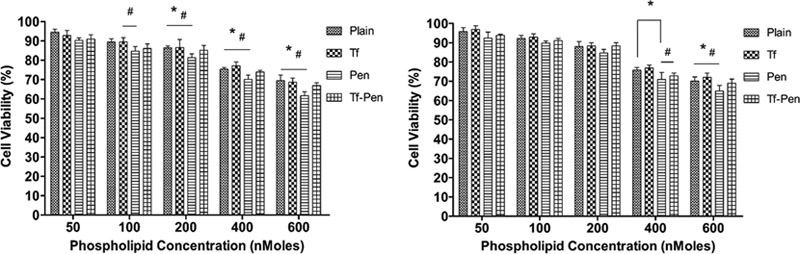
The cytotoxicity was determined using MTT assay in glioblastoma (U87) and brain endothelial (bEnd.3) cells. It is important for a delivery carrier to be biocompatible at a desired concentration and should also have the ability to deliver the encapsulated agent. The cytotoxicity results revealed cell viability of more than 85%, relative to the untreated control group (p<0.05), after exposure to Tf-Pen-conjugated liposomes up to a phospholipid concentration of 200 nM, which demonstrates that liposomes are nontoxic and biocompatible with glioblastoma and brain endothelial cells (figure 3). However, the cytotoxicity increased at higher phospholipid concentrations, which can be attributed to the presence of cationic peptide at the surface of liposomes. As depicted in Figure 3, the cell viabilities at 600 nM lipid concentration were found to be 66.83 ± 1.56% and 68.96 ± 2.15% in U87 and bEnd.3 cells, respectively. The cell viability of positively charged pen-conjugated liposomes was lower compared to the negatively charge Tf-conjugated liposomes, Tf-pen liposomes, and plain liposomes, irrespective of the type of cells. However, Tf-conjugated liposomes demonstrated greater cell viability as compared to Tf-Pen liposomes because of the negative charge of transferrin.
Figure 3.
In vitro cell viabilities of (A) U87and (B) bEnd.3 cells after exposure to different phospholipid concentrations of plain, Tf, Pen, and Tf-Pen liposomes. Statistically significant (p < 0.05) differences is shown as (*) with plain liposomes and (#) with Tf-liposomes. Data represented as mean ± S.D., (n=4).
Cellular Uptake Evaluation:
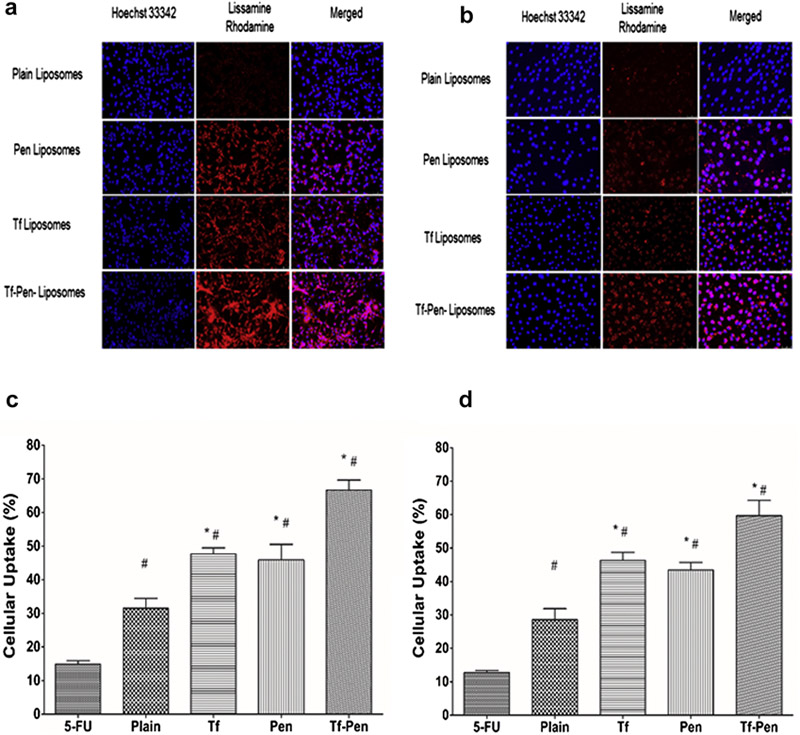
The uptake of surface modified liposomes was studied quantitatively as well as qualitatively in U87 and bEnd.3 cells. Tf-Pen-conjugated liposomes labeled with lissamine rhodamine demonstrated maximum uptake as observed from the strong pattern of fluorescence throughout the cytoplasm as well in the nucleus, compared to plain liposomes that showed lowest uptake (Figures 4A and B). Moreover, Tf-conjugated liposomes showed higher uptake than Pen-conjugated liposomes and plain liposomes. The quantitative estimation of 5-FU uptake by the cells also showed higher uptake of dual functionalized liposomes, which confirmed the higher effectiveness of dual functionalized liposomes on cellular uptake over single ligand or plain liposomes (Figures 4C and D). The uptake of 5-FU loaded Tf-Pen-conjugated liposomes was found to be 66.64 ± 3.00% and 59.68 ± 4.55% in U87 and bEnd.3 cells, respectively, which was significantly higher than single ligand (47.66 ± 1.78 % and 46.28 ± 2.39 % respectively for Tf and 45.91 ± 4.62 and 43.42 ± 2.31 respectively for Pen) or plain liposomes (31.51 ± 2.92% and 28.56 ± 3.29% respectively). This can be explained by the presence of cationic charge on penetratin which facilitated the binding and internalization of Tf-Pen-conjugated liposomes. It is reported that electrostatic interaction of positively charged penetratin with negatively charged heparin sulfate proteoglycans on the cell surface enables the internalization of penetratin through endocytic transcytosis.5 This electrostatic interaction is postulated to facilitate cellular uptake of Pen-conjugated liposomes. However, the cellular uptake of Tf-conjugated liposomes was not significantly higher as compared to Pen-conjugated liposomes, which shows greater uptake via receptor-mediated transcytosis. Therefore, the cellular uptake of Tf-pen-conjugated liposomes is believed to be a combination effect of both initial binding of penetratin followed by Tf receptor mediated transcytosis that resulted in enhanced uptake of liposomes. The U87 cells showed higher cellular uptake of Tf-Pen-conjugated liposomes compared to bEnd.3 cells, illustrating a cell-type dependent liposomal uptake.18 In summary, the results from the uptake study assessed the importance of dual targeting mechanism over single mechanism of receptor targeting or cell penetration.
Figure 4.
Fluorescence microscopic images (10× magnification) showed uptake of lissamine rhodamine labeled liposomes (excitation/emission wavelengths: 560/583 nm) in A) U87 and B) bEnd.3 cells after 2 h incubation. The nuclei of the cells were stained with Hoechst 33342 (excitation/emission wavelengths: 350/461 nm). The images show overlap of lissamine rhodamine labeled liposomes (red) and nuclei of the cells (blue). Graphs represent cellular uptake of 5-FU encapsulated liposomes in C) U87 and D) bEnd.3 cells after 2 h incubation. Data represented as mean ± SD, (n=4). Statistically significant (p < 0.05) differences is shown as (*) with plain liposomes and (#) with 5-FU.
Apoptosis Assessment:
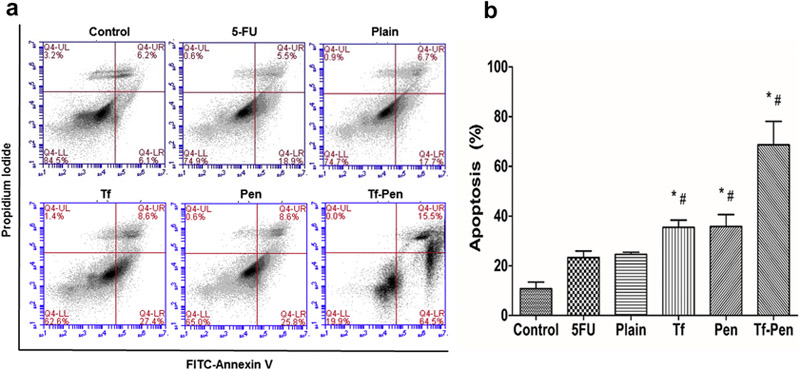
The induction of apoptosis in U87 cells is demonstrated in Figure 5. The total percentage of apoptosis in U87 cells was found to be 68.7 ± 9.36%, 35.42 ± 2.92%, 35.82 ± 4.76%, 24.55 ± 0.86%, 23.3 ± 2.62%, and 10.82 ± 2.56% for Tf-Pen, Tf, Pen, plain liposomes, free 5-FU, and control, respectively. The in vitro cytotoxicity study revealed the apoptotic effects of 5-FU encapsulated liposomes via induction of apoptosis leading to cell death, therefore proving the anticancer potency of 5-FU liposomes in U87 cells. The higher uptake of Tf-Pen liposomes showed greater apoptotic effects as compared to single ligand or plain liposomes of the cells induced by the various formulations.
Figure 5.
(A) Apoptosis study on U87 cells after treatment with 5-FU liposomes and free 5-FU for 5 h. (B) Graph shows the proportion of apoptosis and necrosis in U87. Data represented as mean ± S.D. (n=4). Statistically significant (p < 0.05) differences is shown as (*) with plain liposomes and (#) with free 5-FU.
Penetration Ability into U87 Tumor Spheroids:
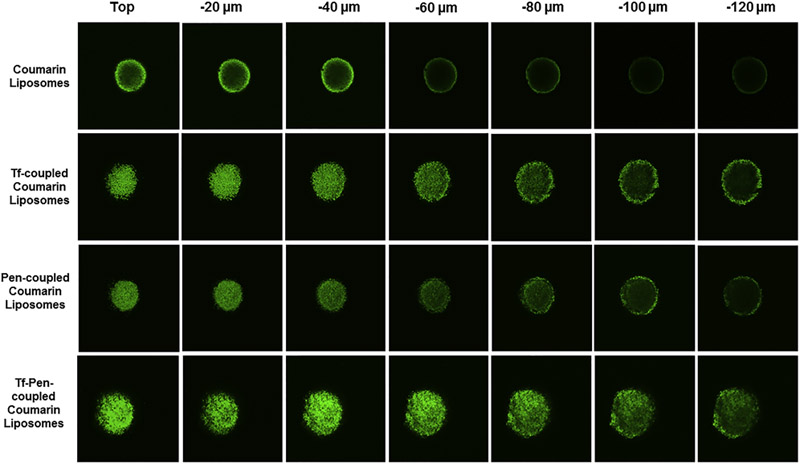
U87 tumor spheroids that show characteristics of glioblastoma tumor in vivo, was used as a model to exhibit the penetration ability of liposomes, as displayed in Figure 6. After incubation with various coumarin-6 loaded liposomal formulations, images were taken at different layers from the top to the equatorial plane of a spheroid using confocal laser scanning microscope (CLSM). Results showed that Tf-Pen liposomes displayed strongest fluorescence intensity compared to single ligand or plain liposomes. In addition, the images demonstrated the strong ability of Tf-Pen liposomes to penetrate much deeper into the core of U87 tumor spheroids as compared to single ligand or plain liposomes.
Figure 6.
Penetration ability of coumarin-6 labeled liposomes into U87 tumor spheroids after 12 h. The CLSM images (10× magnification) of U87 tumor spheroids penetration of liposomes in different planes.
Hemolysis Assay:
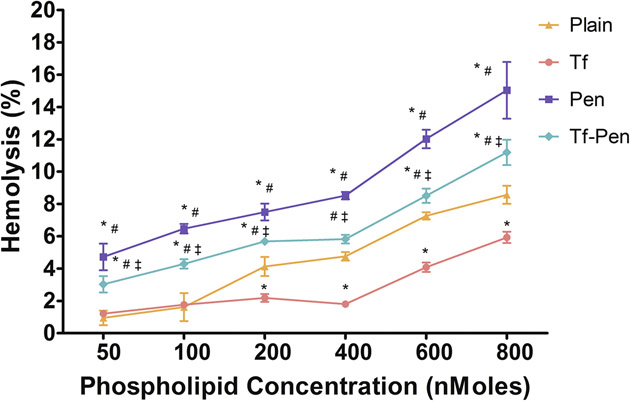
The Tf-Pen-conjugated liposomes were designed to be administered into systemic circulation and therefore it is essential to determine the biocompatibility of liposomes for in vivo administration. The cationic charge on liposomes may initiate non-specific interactions between erythrocytes and liposomes and cause lysis of cells and a subsequent release of hemoglobin. Such interactions may lead to significant decrease in halflife, reproducibility of medication, embolization, trigger thrombosis and hemolysis, in vivo.47–50 The hemocompatibility of the liposomes was determined by quantifying the release of hemoglobin from erythrocytes by spectrophotometer after treatment with different concentrations of phospholipids. Results demonstrated higher release of hemoglobin with increasing phospholipid concentration. Both Tf-Pen and Tf-conjugated liposomes were observed to be non-toxic and biocompatible to up to 600 nM phospholipid concentration (Figure 7). However, Pen-conjugated liposomes showed significantly higher hemolysis at the same phospholipid concentration. This can be explained by the presence of cationic charges on these liposomes, which leads to high interaction with erythrocyte membrane. On the other hand, Tf-conjugated liposomes showed minimal hemolysis due to presence of negative charges on the liposomes, thereby lesser interactions.
Figure 7.
Hemolytic activity (%) of various liposomes. Red blood cells were exposed to different liposomes at varying concentrations. PBS and triton X-100 were used as positive and negative controls, respectively. Up to 10% hemolysis was considered non-toxic. Statistically significant (p < 0.05) differences is shown as (*) with plain liposomes, (#) with Tf-liposomes, and (‡) with Pen-liposomes. The data is represented as mean ± S.D. (n=4).
Endothelial Barrier Layer Integrity:
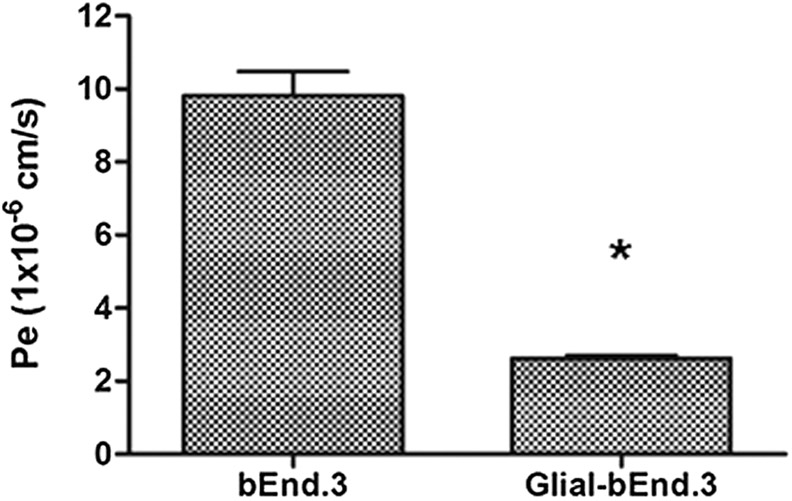
In the present study, we designed an in vitro brain tumor model to evaluate the ability of Tf-Pen liposomes to cross the endothelial barrier. The endothelial barrier was prepared by seeding glial cells and brain endothelial cells on the bottom and upper side of Polyethylene terephthalate (PET) membrane of culture inserts, respectively. Lower number of glial cells was used in comparison to endothelial cells to mimic in vivo barrier conditions as well as to avoid unnecessary entrapment of liposomes. However, before initiation of the study, it was crucial to determine the integrity of the endothelial barrier. The intactness of the coculture model (endothelial and glial cells) and monolayer model (only endothelial cells) was determined by measuring the flux of sodium fluorescein (Na-F) across the barrier layer. The paracellular permeability coefficient (Pe) is correlated with the tightness of the endothelial barrier. Lower the value of permeability coefficient, the more tightly packed are the barrier cells. The paracellullar transport of Na-F across the in vitro BBB model constructed by co-culture of brain endothelial cells and glial cells was observed to be 2.63 × 10−6 cm/s, which was significantly less compared to brain endothelial cell monolayer (Pe = 9.8 × 10−6 cm/s) and confirmed the intactness of the coculture model (Figure 8). Similarly, the TEER values for co-culture model (173.82 ± 11.31 Ω cm2) was found to be significantly (p < 0.05) higher compared to the monolayer BBB model (115.41 ± 9.37 Ω cm2). This can be explained by the improvement of junctional proteins as well as physical strength provided by glial cells, thereby forming a tight barrier. 51 The results were in agreement with the previously published studies demonstrating the importance of using glial cells in the formation and maintenance of BBB.5,18,52,53 Hence, the co-culture model was used to study the transport of liposomes into brain tumor cells in vitro.
Figure 8.
Endothelial cell permeability coefficient (Pe, expressed in 10−6 cm/s) for sodium fluorescein (Na–F) of co-culture model (endothelial and glial cells) and endothelial monolayer only. Data represented as mean ± S.D. (n=4). The Pe values for co-culture model was observed to be significantly (p<0.05) lesser than endothelial monolayer (*).
Development Of In Vitro Brain Tumor Model:
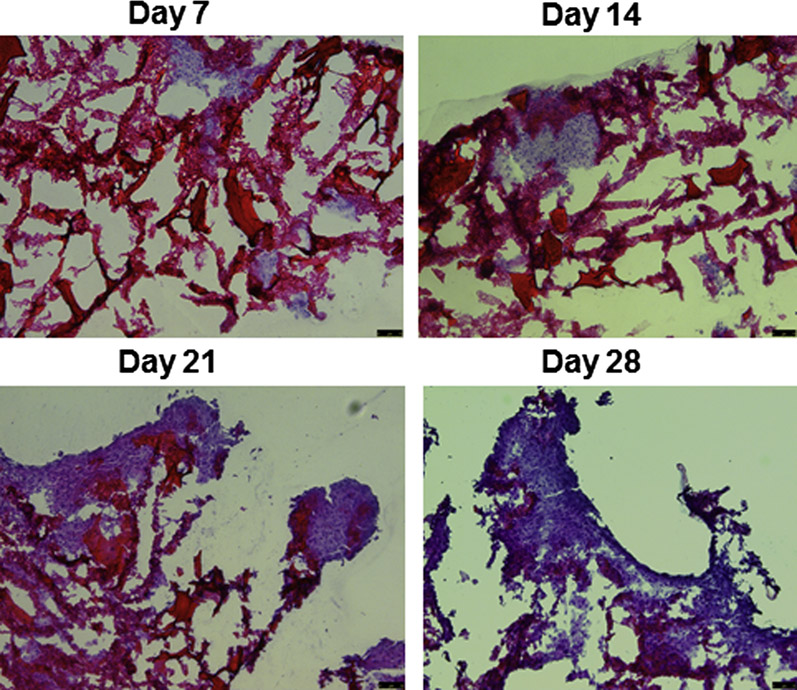
Microscopic images confirmed the gradual growth of tumor cells in scaffold as monitored by hematoxylin-eosin staining (Figure 9). The scaffold displayed cellular biocompatibility, which enhanced the formation of tumor spheroids inside the scaffold. The histological image of 20 μm thick sections of the scaffold showed dense growth of tumor cells on day 21. The percent seeding efficiency of U87 cells on porous scaffold was 31 ± 3.2 %.
Figure 9.
Histological evaluation of tumor cell proliferation in PLGA-chitosan scaffold at different time points. The images show hematoxylin-eosin staining of scaffold sections with tumor cells growth (10× magnification).
Transport of Liposomes Across In Vitro Brain Tumor Model:
The transport of liposomes across in vitro brain tumor model was evaluated through lysis of tumor cells and quantification of coumarin-6 in presence of 10% serum, which eliminates the possibility of liposomes entrapped in the endothelial barrier and thereby mimics in vivo conditions. The transport of coumarin-6 encapsulated Tf-Pen-conjugated liposomes was significantly higher across the in vitro brain tumor model compared to single ligand or plain liposomes. The percent liposomal transport of Tf-Pen in 24 h was about 17.84 ± 0.37% while that for Tf-conjugated liposomes and Pen-conjugated liposomes were 10.02 ± 0.15% and 9.26 ± 0.50%, respectively (Figure 10B). As depicted in figure 10A, Tf-Pen-conjugated liposomes (Pe = 4.97 × 10−6 cm/s) showed significantly (p < 0.05) higher permeability across endothelial co-culture barrier layer compared to single ligand (2.82 × 10−6 cm/s for Tf and 2.58 × 10−6 cm/s for Pen) or plain liposomes (1.16 × 10−6 cm/s), which was in accordance with previously published report.18 The advantage of using in vitro brain tumor model over in vitro BBB model was that a 3-dimensional glioblastoma tumor was grown inside the scaffold, which mimicked the complex pathology of brain tumor and we studied the transport of liposomes across this brain endothelial barrier into the 3-d tumor. Overall, Tf-Pen-conjugated liposomes demonstrated higher transport as well as maximum permeability across barrier layer. However, Tf-conjugated liposomes showed higher cellular uptake, permeability and transport in comparison to Pen-conjugated liposomes, thereby demonstrating the significance of receptor mediated transcytosis over cell penetration. The electrostatic binding of cationic Pen-conjugated liposomes with negative charges on the cell membrane is postulated to facilitate cellular uptake. Moreover, a study showed that Tf-Pen and Tf-conjugated liposomes undergo clathrin-mediated uptake as the major pathway of transport, while Pen-conjugated liposomes demonstrate transport through macropinocytes and clathrin coated vesicles.18 However, the presence of serum proteins can interfere with initial binding of the Pen-conjugated liposomes. On the contrary, Tf-conjuagted liposomes specifically bind to Tf receptor, which eliminates the non-specific interaction with serum proteins. The results showed that the surface modified liposomes with Tf and Pen displayed enhanced transport across the in vitro brain tumor model as well as higher permeability across endothelial co-culture model. Hence, the dual functionalized liposomes emphasized the significance of dual mechanism of transport through receptor facilitated targeting as well as enhanced cell penetration.
Figure 10.
(A) Endothelial cell permeability coefficient (Pe, expressed in 10−6 cm/s) for different liposomes encapsulated with coumarin-6, across endothelial co-culture barrier model. (B) Graph shows the percent transport of different liposomes encapsulated with coumarin-6, across in vitro brain tumor model. Data represented as mean ± SD, (n=4). Significantly higher (p < 0.05) permeability coefficient and transport of Tf-pen liposomes in comparison to plain liposomes was observed (*).
Anti-Tumor Efficacy Of Liposomes:
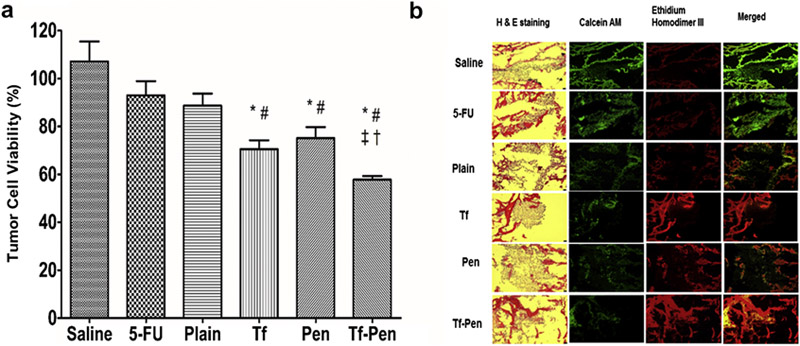
A robust and simple in vitro brain tumor model was designed to evaluate the efficacy of Tf-Pen-conjugated liposomes by determining the regression of glioblastoma tumor housed inside a PLGA-chitosan scaffold. The model was constructed by placing the culture inserts carrying a tightly packed barrier of brain endothelial and glial cells on glioblastoma tumor grown inside PLGA-chitosan scaffold to mimic in vivo tumor environment. The porous scaffold enabled the tumor cells to grow inside them in a 3-dimensional environment, attaching to the scaffold fibers and pores inside scaffold to form a 3-D tumor.54–56 Antitumor efficacies of various liposomal formulations were determined by quantifying the percent tumor cell viability in the scaffold using MTT assay. After 24 h treatment, the percent tumor cell viability in the in vitro tumor model decreased to 88.66 ± 5.05% and 57.78 ± 1.51% for 5-FU encapsulated plain liposomes and Tf-Pen liposomes, respectively. Figure 11A shows that 5-FU encapsulated Tf-Pen-conjugated liposomes significantly decreased the percent tumor cell viability as compared to single ligand or plain liposomes. We believe that Tf-Pen-conjugated liposomes efficiently crossed the endothelial barrier via dual mechanisms of Tf receptor mediated transcytosis and enhanced cell penetration, subsequently reaching the tumor cells inside the scaffold and delivering the encapsulated 5-FU to the tumor cells. The anti-tumor efficacy of Tf-Pen-conjugated liposomes was also confirmed by fluorescence images of the treated scaffold sections. As depicted in the figure 11B, the tumor cells inside the scaffold subjected to Tf-Pen treatment were mostly dead, which confirmed the superior anti-tumor efficacy of these liposomes. Based on these results, it can be concluded that Tf-Pen-conjugated liposomes efficiently translocate across the brain endothelial barrier and endocytoses into the tumor cells present inside the scaffold, thereby increasing the concentration of 5-FU in the tumor cells, thus demonstrating excellent anti-tumor efficacy.
Figure 11.
(A) Graph shows the percent tumor cell viability 24 h after treatment with different 5-FU encapsulated liposomes using an in vitro brain tumor model. Data represented as mean ± SD, (n=4). Statistically significant (p < 0.05) differences with plain liposomes (*), free 5-FU (#), (‡) Tf liposomes, and (†) Pen liposomes was observed. (B) The fluorescence images show tumor cell death in scaffold after treatment.
Conclusions
The dual-functionalized liposomes were successfully designed to enhance the transport of chemotherapeutic agents across a co-cultured endothelial barrier into a scaffold housing 3-d glioblastoma tumor. Incorporation of Pen to Tf-conjugated liposomes showed better biocompatibility, high cellular uptake, and efficient transport of 5-fluorouracil across brain endothelial barrier and into the tumor present inside the scaffold, which resulted in regression of the glioblastoma tumor in the scaffold. Based on the promising results obtained from Tf-Pen in vitro studies, we plan to perform in vivo biodistribution, anti-tumor efficacy in glioblastoma bearing mice and biocompatibility studies using Tf-Pen-conjugated liposomes in the future. We predict that this dual functionalized liposomes will contribute to development of an efficient delivery system to the brain by enhancing translocation across the BBB and subsequent cellular uptake and endocytosis into brain tumor cells.
Supplementary Material
Acknowledgements
This work was supported by National Institutes of Health (NIH) grant RO1 AG051574. We thank Dr. Amrita Banerjee, Department of Pharmaceutical Sciences, North Dakota State University, for helping in preparation of manuscript. The authors declare no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yan H, Wang J, Yi P, et al. Imaging brain tumor by dendrimer-based optical/paramagnetic nanoprobe across the blood-brain barrier. Chem Commun 2011;47(28):8130–8132. doi: 10.1039/C1CC12007G [DOI] [PubMed] [Google Scholar]
- 2.Donahue MJ, Blakeley JO, Zhou J, Pomper MG, Laterra J, van Zijl PCM. Evaluation of human brain tumor heterogeneity using multiple T1-based MRI signal weighting approaches. Magn Reson Med 2008;59(2):336–344. doi: 10.1002/mrm.21467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agarwal S, Sane R, Oberoi R, Ohlfest JR, Elmquist WF. Delivery of molecularly targeted therapy to malignant glioma, a disease of the whole brain. Expert Rev Mol Med 2011;13:e17. doi: 10.1017/S1462399411001888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qian ZM, Li H, Sun H, Ho K. Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacol Rev 2002;54(4):561–587. [DOI] [PubMed] [Google Scholar]
- 5.Sharma G, Modgil A, Sun C, Singh J. Grafting of cell-penetrating peptide to receptor-targeted liposomes improves their transfection efficiency and transport across blood-brain barrier model. J Pharm Sci 2012;101(7):2468–2478. doi: 10.1002/jps.23152 [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Garcia E, Andrieux K, Gil S, Couvreur P. Colloidal carriers and blood-brain barrier (BBB) translocation: a way to deliver drugs to the brain? Int J Pharm 2005;298(2):274–292. doi: 10.1016/j.ijpharm.2005.03.031 [DOI] [PubMed] [Google Scholar]
- 7.Amstad E, Kohlbrecher J, Müller E, Schweizer T, Textor M, Reimhult E. Triggered release from liposomes through magnetic actuation of iron oxide nanoparticle containing membranes. Nano Lett 2011;11(4):1664–1670. doi: 10.1021/nl2001499 [DOI] [PubMed] [Google Scholar]
- 8.Obata Y, Tajima S, Takeoka S. Evaluation of pH-responsive liposomes containing amino acid-based zwitterionic lipids for improving intracellular drug delivery in vitro and in vivo. J Control Release. 2010;142(2):267–276. doi: 10.1016/j.jconrel.2009.10.023 [DOI] [PubMed] [Google Scholar]
- 9.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol 2007;2(12):751–760. doi: 10.1038/nnano.2007.387 [DOI] [PubMed] [Google Scholar]
- 10.Skarlatos S, Yoshikawa T, Pardridge WM. Transport of [125I]transferrin through the rat blood-brain barrier. Brain Res 1995;683(2):164–171. [DOI] [PubMed] [Google Scholar]
- 11.Prabhakar K, Afzal SM, Kumar PU, Rajanna A, Kishan V. Brain delivery of transferrin coupled indinavir submicron lipid emulsions-Pharmacokinetics and tissue distribution. Colloids Surfaces B Biointerfaces. 2011;86(2):305–313. doi: 10.1016/j.colsurfb.2011.04.013 [DOI] [PubMed] [Google Scholar]
- 12.Cheng Y, Zak O, Aisen P, Harrison SC, Walz T. Structure of the Human Transferrin Receptor-Transferrin Complex. Cell. 2004;116(4):565–576. doi: 10.1016/S0092-8674(04)00130-8 [DOI] [PubMed] [Google Scholar]
- 13.Pardridge WM, Eisenberg J, Yang J. Human blood-brain barrier transferrin receptor. Metabolism. 1987;36(9):892–895. doi: 10.1016/0026-0495(87)90099-0 [DOI] [PubMed] [Google Scholar]
- 14.Kibria G, Hatakeyama H, Ohga N, Hida K, Harashima H. Dual-ligand modification of PEGylated liposomes shows better cell selectivity and efficient gene delivery. J Control Release. 2011;153(2):141–148. doi: 10.1016/j.jconrel.2011.03.012 [DOI] [PubMed] [Google Scholar]
- 15.Chen C, Duan Z, Yuan Y, et al. Peptide-22 and Cyclic RGD Functionalized Liposomes for Glioma Targeting Drug Delivery Overcoming BBB and BBTB. ACS Appl Mater Interfaces. 2017;9(7):5864–5873. doi: 10.1021/acsami.6b15831 [DOI] [PubMed] [Google Scholar]
- 16.Zong T, Mei L, Gao H, et al. Synergistic dual-ligand doxorubicin liposomes improve targeting and therapeutic efficacy of brain glioma in animals. Mol Pharm 2014;11(7):2346–2357. doi: 10.1021/mp500057n [DOI] [PubMed] [Google Scholar]
- 17.Bolhassani A Potential efficacy of cell-penetrating peptides for nucleic acid and drug delivery in cancer. Biochim Biophys Acta - Rev Cancer. 2011. ;1816(2):232–246. doi: 10.1016/j.bbcan.2011.07.006 [DOI] [PubMed] [Google Scholar]
- 18.Sharma G, Modgil A, Zhong T, Sun C, Singh J. Influence of short-chain cell-penetrating peptides on transport of doxorubicin encapsulating receptor-targeted liposomes across brain endothelial barrier. Pharm Res 2014;31(5):1194–1209. doi: 10.1007/s11095-013-1242-x [DOI] [PubMed] [Google Scholar]
- 19.Morris MC, Depollier J, Mery J, Heitz F, Divita G. A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nat Biotechnol 2001;19(12):1173–1176. doi: 10.1038/nbt1201-1173 [DOI] [PubMed] [Google Scholar]
- 20.Furuhata M, Izumisawa T, Kawakami H, Toma K, Hattori Y, Maitani Y. Decaarginine-PEG-liposome enhanced transfection efficiency and function of arginine length and PEG. Int J Pharm 2009;371(1-2):40–46. doi: 10.1016/j.ijpharm.2008.12.011 [DOI] [PubMed] [Google Scholar]
- 21.Yoon DJ, Chu DSH, Ng CW, et al. Genetically engineering transferrin to improve its in vitro ability to deliver cytotoxins. J Control Release. 2009;133(3):178–184. doi: 10.1016/j.jconrel.2008.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray S, H R, R S, Pilkington G. A novel three-dimensional “all human” in vitro brain tumor invasion model. Neuro Oncol 2007;7(307–308). [Google Scholar]
- 23.Meng W, Kallinteri P, Walker D a, Parker TL, Garnett MC. Evaluation of poly (glycerol-adipate) nanoparticle uptake in an in vitro 3-D brain tumor co-culture model. Exp Biol Med (Maywood). 2007;232(8):1100–1108. doi: 10.3181/0612-RM-301 [DOI] [PubMed] [Google Scholar]
- 24.Miller CR, Williams CR, Buchsbaum DJ, Gillespie GY. Intratumoral 5-fluorouracil produced by cytosine deaminase/5-fluorocytosine gene therapy is effective for experimental human glioblastomas. Cancer Res 2002;62(3):773–780. [PubMed] [Google Scholar]
- 25.Menei P, Capelle L, Guyotat J, et al. Local and sustained delivery of 5-fluorouracil from biodegradable microspheres for the radiosensitization of malignant glioma: A randomized phase II trial. Neurosurgery. 2005;56(2):242–247. doi: 10.1227/01.NEU.0000144982.82068.A2 [DOI] [PubMed] [Google Scholar]
- 26.Sharma G, Lakkadwala S, Modgil A, Singh J. The Role of Cell-Penetrating Peptide and Transferrin on Enhanced Delivery of Drug to Brain. Int J Mol Sci 2016;17(6):806. doi: 10.3390/ijms17060806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bárány-Wallje E, Keller S, Serowy S, et al. A Critical Reassessment of Penetratin Translocation Across Lipid Membranes. Biophys J 2005;89(4):2513–2521. doi: 10.1529/biophysj.105.067694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X, Ding L, Xu Y, Wang Y, Ping Q. Targeted delivery of doxorubicin using stealth liposomes modified with transferrin. Int J Pharm 2009;373(1-2):116–123. doi: 10.1016/j.ijpharm.2009.01.023 [DOI] [PubMed] [Google Scholar]
- 29.Anabousi S, Laue M, Lehr C-M, Bakowsky U, Ehrhardt C. Assessing transferrin modification of liposomes by atomic force microscopy and transmission electron microscopy. Eur J Pharm Biopharm 2005;60(2):295–303. doi: 10.1016/j.ejpb.2004.12.009 [DOI] [PubMed] [Google Scholar]
- 30.Mayer L, Bally MB, Cullis PR, Ginsberg RS, Mitilenes GN. High drug:lipid formulations of liposomal-antineoplastic agents. September 1988. https://www.google.com/patents/WO1988006442A1?cl=en.
- 31.Saar K, Lindgren M, Hansen M, et al. Cell-penetrating peptides: A comparative membrane toxicity study. Anal Biochem 2005;345(1):55–65. doi: 10.1016/j.ab.2005.07.033 [DOI] [PubMed] [Google Scholar]
- 32.Ying X, Wen H, Lu W-L, et al. Dual-targeting daunorubicin liposomes improve the therapeutic efficacy of brain glioma in animals. J Control Release. 2010;141(2):183–192. doi: 10.1016/j.jconrel.2009.09.020 [DOI] [PubMed] [Google Scholar]
- 33.Lee D-W, Powers K, Baney R. Physicochemical properties and blood compatibility of acylated chitosan nanoparticles. Carbohydr Polym 2004;58(4):371–377. doi: 10.1016/j.carbpol.2004.06.033 [DOI] [Google Scholar]
- 34.Fischer D, Li Y, Ahlemeyer B, Krieglstein J, Kissel T. In vitro cytotoxicity testing of polycations: Influence of polymer structure on cell viability and hemolysis. Biomaterials. 2003;24(7):1121–1131. doi: 10.1016/S0142-9612(02)00445-3 [DOI] [PubMed] [Google Scholar]
- 35.Ying X, Wen H, Lu WL, et al. Dual-targeting daunorubicin liposomes improve the therapeutic efficacy of brain glioma in animals. J Control Release. 2010;141 (2):183–192. doi: 10.1016/j.jconrel.2009.09.020 [DOI] [PubMed] [Google Scholar]
- 36.Xie Y, Ye L, Zhang X, et al. Transport of nerve growth factor encapsulated into liposomes across the blood-brain barrier: in vitro and in vivo studies. J Control Release. 2005;105(1-2):106–119. doi: 10.1016/j.jconrel.2005.03.005 [DOI] [PubMed] [Google Scholar]
- 37.Franke H, Galla H-J, Beuckmann CT. Primary cultures of brain microvessel endothelial cells: a valid and flexible model to study drug transport through the blood-brain barrier in vitro. Brain Res Protoc 2000;5(3):248–256. doi: 10.1016/S1385-299X(00)00020-9 [DOI] [PubMed] [Google Scholar]
- 38.Thevenot P, Nair A, Dey J, Yang J, Tang L. Method to Analyze Three-Dimensional Cell Distribution and Infiltration in Degradable Scaffolds. Tissue Eng Part C Methods. 2008;14(4):319–331. doi: 10.1089/ten.tec.2008.0221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iden DL, Allen TM. In vitro and in vivo comparison of immunoliposomes made by conventional coupling techniques with those made by a new post-insertion approach. Biochim Biophys Acta 2001;1513(2):207–216. [DOI] [PubMed] [Google Scholar]
- 40.Moreira JN, Ishida T, Gaspar R, Allen TM. Use of the post-insertion technique to insert peptide ligands into pre-formed stealth liposomes with retention of binding activity and cytotoxicity. Pharm Res 2002;19(3):265–269. [DOI] [PubMed] [Google Scholar]
- 41.Nag KO, Awasthi V. Surface Engineering of Liposomes for Stealth Behavior. Pharm 2013;5(4). doi: 10.3390/pharmaceutics5040542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Torchilin VP, Khaw BA, Smirnov VN, Haber E. Preservation of antimyosin antibody activity after covalent coupling to liposomes. Biochem Biophys Res Commun 1979;89(4):1114–1119. doi: 10.1016/0006-291X(79)92123-5 [DOI] [PubMed] [Google Scholar]
- 43.Marqués-Gallego P, De Kroon AIPM. Ligation strategies for targeting liposomal nanocarriers. Biomed Res Int 2014;2014:12. doi: 10.1155/2014/129458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee S-H, Sato Y, Hyodo M, Harashima H. Topology of Surface Ligands on Liposomes: Characterization Based on the Terms, Incorporation Ratio, Surface Anchor Density, and Reaction Yield. Biol Pharm Bull 2016;39(12):1983–1994. doi: 10.1248/bpb.b16-00462 [DOI] [PubMed] [Google Scholar]
- 45.Chen Y, Liu L. Modern methods for delivery of drugs across the blood-brain barrier. Adv Drug Deliv Rev 2012;64(7):640–665. doi: 10.1016/j.addr.2011.11.010 [DOI] [PubMed] [Google Scholar]
- 46.Roney C, Kulkarni P, Arora V, et al. Targeted nanoparticles for drug delivery through the blood-brain barrier for Alzheimer’s disease. J Control Release. 2005;108(2-3):193–214. doi: 10.1016/j.jconrel.2005.07.024 [DOI] [PubMed] [Google Scholar]
- 47.Sharma G, Modgil A, Layek B, et al. Cell penetrating peptide tethered bi-ligand liposomes for delivery to brain in vivo: Biodistribution and transfection. J Control Release. 2013;167(1):1–10. doi: 10.1016/j.jconrel.2013.01.016 [DOI] [PubMed] [Google Scholar]
- 48.Antohi S, Brumfeld V. Polycation-cell surface interactions and plasma membrane compartments in mammals. Interference of oligocation with polycationic condensation. Zeitschrift fur Naturforschung Sect C, Biosci 1984;39(7-8):767–775. [DOI] [PubMed] [Google Scholar]
- 49.Zhu S, Qian F, Zhang Y, Tang C, Yin C. Synthesis and characterization of PEG modified N-trimethylaminoethylmethacrylate chitosan nanoparticles. Eur Polym J 2007;43(6):2244–2253. doi: 10.1016/j.eurpolymj.2007.03.042 [DOI] [Google Scholar]
- 50.Kainthan RK, Gnanamani M, Ganguli M, et al. Blood compatibility of novel water soluble hyperbranched polyglycerol-based multivalent cationic polymers and their interaction with DNA. Biomaterials. 2006;27(31):5377–5390. doi: 10.1016/j.biomaterials.2006.06.021 [DOI] [PubMed] [Google Scholar]
- 51.Ishihara H, Kubota H, Lindberg RLP, et al. Endothelial cell barrier impairment induced by glioblastomas and transforming growth factor beta2 involves matrix metalloproteinases and tight junction proteins. J Neuropathol Exp Neurol 2008;67(5):435–448. doi: 10.1097/NEN.0b013e31816fd622 [DOI] [PubMed] [Google Scholar]
- 52.Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987;325(6101):253–257. doi: 10.1038/325253a0 [DOI] [PubMed] [Google Scholar]
- 53.Arthur FE, Shivers RR, Bowman PD. Astrocyte-mediated induction of tight junctions in brain capillary endothelium: an efficient in vitro model. Dev Brain Res 1987;36(1):155–159. doi: 10.1016/0165-3806(87)90075-7 [DOI] [PubMed] [Google Scholar]
- 54.Bell E. Strategy for the selection of scaffolds for tissue engineering. Tissue Eng 1995;1 (2):163–179. doi: 10.1089/ten.1995.1.163 [DOI] [PubMed] [Google Scholar]
- 55.Sourla A, Doillon C, Koutsilieris M. Three-dimensional type I collagen gel system containing MG-63 osteoblasts-like cells as a model for studying local bone reaction caused by metastatic cancer cells. Anticancer Res 1996;16(5 A):2773–2780. [PubMed] [Google Scholar]
- 56.Kim J Bin. Three-dimensional tissue culture models in cancer biology. Semin Cancer Biol 2005;15(5):365–377. doi: 10.1016/j.semcancer.2005.05.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.