Summary
Treatment of medical device-related infections is challenging, and recurrence is common. The main reason for this is that microorganisms adhere to the surfaces of medical devices, and enter into a biofilm state in which they display distinct growth rates, structural features, and protection from antimicrobial agents and host immune mechanisms compared with their planktonic counterparts. This article reviews how microorganisms form biofilms and mechanisms of protection against antimicrobial agents and the host immune system provided by biofilms. Also discussed are innovative strategies for diagnosis of biofilm-associated infection, and novel approaches to treatment and prevention of medical device-associated infections.
Keywords: Biofilm, extracellular polymeric substance, tolerance, medical device-associated infection, surface-coating or eluting substrate, physical–mechanical approach, extracellular polymeric substance targeting therapy
Introduction
Device-associated infections are one of the most common and feared complications in medical practice. Treatment of medical device-related infections is notoriously challenging, and recurrence is common.1 The main reason for this is that microorganisms adhere to surfaces of medical devices and enter into a biofilm state in which they display distinct structural features, growth rates, and microenvironments, when compared with planktonic organisms.2,3 Decreased susceptibility to antimicrobial agents and the host immune system is observed in microorganisms in biofilms compared with planktonically-grown organisms.4 The biofilm structure itself, decreased growth rate, antimicrobial-destroying enzymes within the matrix, upregulation of stress-response genes and horizontal transfer of antimicrobial resistance genes are involved in reduced antimicrobial susceptibility.3,4 Microorganisms in mature biofilms over 7 days old are 500–5000 times less susceptible to killing by antimicrobial agents compared to planktonic organisms.5
Many controversies and uncertainties exist in the diagnosis, prevention and treatment of biofilm-associated infection.6 Once a biofilm develops on a medical device, eradication of microorganisms becomes extremely challenging and cost can be substantial due to the frequent need for prolonged hospitalization, surgery, and long-term antimicrobial treatment.7 Bacterial biofilms are associated with approximately 1.7 million hospital-acquired infections annually in the United States, incurring an annual economic burden of approximately 11 billion dollars.8 Biofilm formation is now accepted as one of the most important virulence factors in medical device-associated infections.2
This article reviews the means by which microorganisms develop biofilms and their defense mechanisms against the host immune system and antimicrobial agents. Also discussed are innovative concepts for the diagnosis of biofilm-associated infection and novel approaches to treatment and prevention of medical device-associated infections.
Body of Text
Understanding of Biofilms
Definition and structure of biofilms
Biofilms appear very early in the fossil record and can be formed by a diverse range of microorganisms; they are widespread in natural, industrial and hospital settings.9 A biofilm is generally defined as “an aggregate of microorganisms adherent to a biotic or abiotic surface, embedded within a matrix of extracellular polymeric substance (EPS) (Figure 1).10 Interestingly, free-floating cells can also self-aggregate and form a biofilm, which can display features similar to those of a medical device-associated biofilm.10,11 A major feature of biofilms is their self-produced EPS which consists of polysaccharides, nucleic acids and/or proteins.3 The EPS matrix advances microbial attachment to surfaces and cell-to-cell adhesion and aggregation, and functions as a three-dimensional barrier to protect cells against from external threats, including host defense mechanisms and antimicrobial treatment.12 Moreover, the EPS matrix can create harsh environments by modulating chemical and nutrient gradients, and contribute to important virulence attributes.12 Host-derived components, including fibrin, platelets and immunoglobulins, may also be components of biofilms in complex host environments. A description of biofilms as ‘aggregated, microbial cells surrounded by a polymeric self-produced matrix, which may contain host components’ was suggested at the 5th ASM Biofilm Conference.10 Microorganisms can attach to almost all types of medical devices and also biotic surfaces (e.g., skin, bone, airway, connective tissue, intestinal mucosa, vascular endothelium).9 Therefore, biofilms may be associated with various types of tissue-associated chronic infections, in addition to their association with medical devices (Table 1). Medical device-associated infections are most commonly caused by Staphylococcus epidermidis and Staphylococcus aureus, but a long list of species of bacteria and fungi can cause these infections.13,14 While some authors suggest that S. epidermidis accounts for approximately 80% of the bacteria causing medical device-related infections,15 in the hospital setting, multidrug-resistant Gram-negative bacteria such as Escherichia coli, Klebsiella pneumoniae, Acinetobacter baumannii and Pseudomonas aeruginosa, have emerged as serious concerns, especially in catheter-associated urinary tract infection (CAUTI).16
Figure 1. Scanning electron microscopy of Staphylococcus epidermidis biofilm.
S. epidermidis was grown in the laboratory on a Teflon surface.
Table 1.
Biofilm-associated infections.
| Medical devices associated with infections | Tissues associated with infections |
|---|---|
| Cardiovascular implantable electronic devices | Biliary tract |
| Catheters, shunts and stents | Internal ear (chronic otitis media) |
| Cochlear implants | Tonsils (chronic tonsillitis) |
| Contact lenses | Sinuses (chronic sinusitis) |
| Deep brain stimulators | Wounds |
| Endotracheal tubes | Teeth (dental caries) |
| Dental implants | Heart valves (endocarditis) |
| Orthopedic implants | Kidney stones |
| Tissue fillers, including breast implants | Lung (cystic fibrosis patients) |
| Sutures and surgical meshes | Bone (osteomyelitis) |
| Vascular grafts |
(Data from Hoiby N, Bjarnsholt T, Moser C, et al. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin Microbiol Infect. 2015;21 Suppl 1:S1–25.)
Stages of biofilms
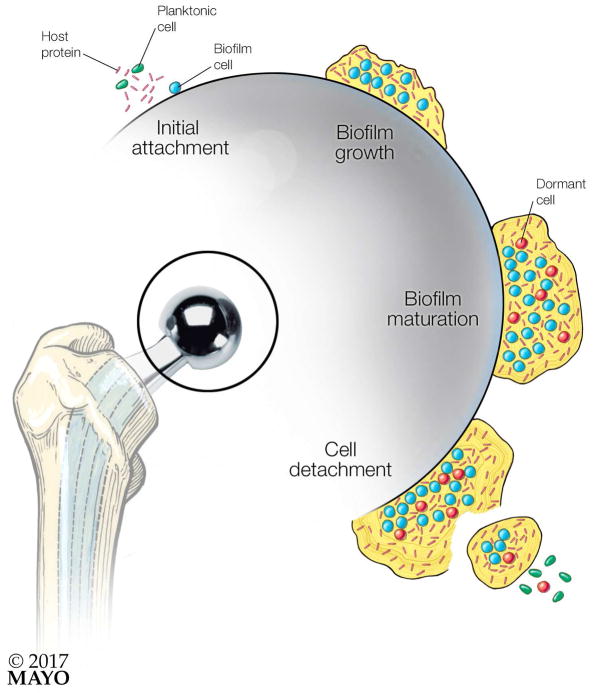
The initial step of biofilm formation is initiated by complex interactions between surfaces and the microorganism (or microorganisms). Biofilm formation consists of several stages, beginning with attachment and progressing to detachment (Figure 2).2 At the stage of initial attachment, surface characteristics such as hydrophobicity, charge, topography, and exposure time influence attachment of microorganisms to the surface of medical devices.17 Adherence of microorganisms to medical devices has been reported to occur through cell surface proteins, such as biofilm-associated protein - a fimbria-like polymer -, and the protein autolysin of S. epidermidis, and the capsular polysaccharide/adhesion of S. epidermidis and other coagulase-negative staphylococci.18–21 Host-derived proteins, such as fibronectin, fibrinogen, and vitronectin, released to aid in healing, are absorbed onto the surfaces of medical devices, producing a conditioning film, which enhances microbial colonization through interactions between microbial and host proteins.17,18 At the stage of biofilm growth, microorganisms proliferate, and cell-to-cell adhesion on the colonized surface is enhanced. These organized structures are then surrounded by a self-produced EPS.2,3,8 As biofilms mature, they become a structured multicellular community providing protection against from external threats, including host defense mechanisms and antimicrobial treatment. Microorganisms in biofilms release autoregulators and have altered gene expression that stimulates production of virulence factors, enhancing their own survival.22 At the stage of cell detachment, planktonic cells may be released from the surface, potentially resulting in distant metastatic infections and/or further regional biofilm formation. Dispersed microorganisms revert to an active state, comparable to that of their planktonic counterparts, making them more susceptible to antimicrobial agents.23 In addition, dispersed biofilm cells lose the protective effects granted by the biofilm community and its structured organization. The cyclic di-GMP (c-di-GMP) second messenger reported in E. coli, P. aeruginosa, and Salmonella enterica,24 is an example of a molecule responsible for biofilm dispersal.25
Figure 2. Steps in biofilm formation on an orthopedic prosthesis.
Biofilm formation has distinct stages: Initial Attachment, in which microorganism attaches to an orthopedic implant through interactions between the microorganism and host molecules on the foreign body surface as well as the foreign body surface itself; Biofilm Growth, in which the microorganism begins to proliferate, and individual cells adhere to one another, and become surrounded by a self-produced extracellular polymeric substance; Biofilm Maturation, whereby the biofilm develops a structured multicellular community protecting its members against from external threats, including host defense mechanisms and antimicrobial treatments; and finally Cell Detachment, whereby planktonic cells may be released from the surface of large biofilms, causing distant metastatic infections and further regional biofilm establishment.
Tolerance and resistance of microorganisms in biofilms
Biofilm-associated infections are particularly challenging to treat. Several mechanisms account for protection against the host immune system and antimicrobial agents, compared with microorganisms in the planktonic state. This type of resistance is not mainly due to the genetic antimicrobial resistance that occurs by mutation or horizontal gene transfer but is rather better described as a reversible tolerance to antimicrobial agents.4,26 Tolerance can be the result of entrapment or inactivation of antimicrobials, and/or of the slow growth that is characteristic of biofilms.4,26 Restricted penetration of antimicrobial agents into the depth of a mature biofilm due to the EPS matrix of the biofilm itself can contribute to the antimicrobial tolerance.4,26 EPS matrix has also been shown to inactivate antimicrobial substances by harboring enzymes secreted into it.4,26 Another mechanism involves slow-growing or non-growing microorganisms due to nutrient and oxygen depletion within biofilms, particularly with regard to resistance to killing by growth-dependent antimicrobial agents.9 This phenomenon can be amplified by the presence of phenotypic variants or “persisters”.27 Persister cells are thought to be tolerant to antimicrobial agents because they are in a particularly dormant state.28 Importantly, dispersed planktonic microorganisms can lose tolerance and restore their susceptibility to antimicrobial agents; thus, targeting dispersal mechanisms is a potential adjuvant strategy to render conventional antimicrobial agents active against biofilms.29 Growth within a biofilm may facilitate acquisition of genetic changes such as mutations and gene transfer.30,31 One study showed that plasmid conjugation was up to 700–fold more efficient in biofilms compared with free-living microorganisms.32 Similarly, hypermutability may occur in biofilms, with mutation rates for S. aureus and S. epidermidis being 4- and 60-fold higher, respectively, in biofilms than that under planktonic conditions.31 Together, increased gene transfer and hypermutability can increase selection of genetic antimicrobial resistance.
Diagnosis of medical device-associated infections
Bearing in mind that most medical device-associated infection are associated with biofilms on the surfaces of the devices, diagnostic strategies that approach the surface of the device are preferred. Sampling surfaces of medical devices may require invasive procedures such as aspiration, biopsy, or extirpation of medical devices. However, device removal is not necessarily required for diagnosis in all situations. For central-line associated bloodstream infections (CLABSI), diagnostic methods based on qualitative (or quantitative) blood cultures, including differential time to positivity, may be used.33–35 Swab cultures are not recommended because of the small volume of sample available for culture; negative results do not necessarily correlate with the absence of infection.36 Nevertheless, for some medical device-associate infections, microorganisms may not be identified until the medical device is removed. Moreover, culture is not always positive even when the device is removed. Slow growth rates in biofilms can lead to the ‘viable-but-nonculturable’ state (VBNC state) of microorganisms.37 S. aureus, for example, can enter the VBNC state in biofilms, rendering it undetectable using standard growth media;38 daptomycin and vancomycin are particularly noteworthy for inducing a VBNC state in S. aureus biofilms.39 The emergence of small colony variants (SCVs) can also render successful diagnosis difficult.40,41 SCVs are slow growing subpopulations of microorganisms that differ from normal microorganisms in their small colonial size and biochemical characteristics.41,42 S. aureus SCVs have increased intracellular persistence.41
Sonication may improve culture positivity of large device-related infections.43 One study, focusing on prosthetic hip and knee infection, demonstrated that sonicate fluid cultures had a sensitivity of 79% compared with 61% for periprosthetic tissue cultures.43 Sonication is not, however, recommended for all devices; the use of a quantitative sonication technique to detect catheter colonization has been shown to be no better than the easier-to-perform semiquantitative roll-plate culture method.44 Combinations of sonication with certain nucleic acid amplification tests further enhance the sensitivity to diagnose infection.45,46 Several studies show differences between findings using culture and molecular diagnostic methods; molecular methods may identify additional organisms (i.e., increased diversity) compared to culture and/or may detect microorganisms in culture-negative cases.47,48 In culture-negative endocarditis, for example, identification of the causative bacterium by broad-range bacterial (e.g., 16S ribosomal RNA gene) PCR of heart valve tissues can be useful.49 Some infections may be missed or their microbiology not defined because of a high rate of false negative microbiological results with conventional culture methods; an example is arthroplasty failure.50, 51,52 As mentioned above, implant sonication can improve diagnosis. Alternatively, DL-dithiothreitol has been used as for detection of biofilms on orthopedic implants.53 Disclosing agents have been suggested as an intra-operative strategy to visualize biofilms, but the sensitivity of this approach is not defined.54 Confocal laser scanning microscopy (CLSM) and scanning electron microscopy (SEM) are advanced options to visualize biofilms in resected specimens,55,56 but are not typically used in direct patient care.
Treatment and prevention of medical device-associated infections
Basic principles of infection prevention should be applied to prevent microbial contamination of implanted devices because, as mentioned, this can readily lead to biofilm formation. Device implantation and handling must be performed as outlined in current guidelines.57 Appropriate perioperative antibiotic prophylaxis should be administered to cases of surgically implanted devices. And of course, the need for the indwelling medical device itself must be justifiable at any time.
Current preventive (and to some extent therapeutic) approaches can be divided into two broad categories, surface-coating or elution, and physical/mechanical/electrical/biological approaches. Surface modification of medical devices using antibiotics and silver has been the focus of much research to reduce microbial colonization and biofilm formation.1,2,6,58 Minocycline-rifampin catheters, which are commercially-available, have been associated with reductions in microbial colonization and CLABSI.59 Although impregnated and standard catheters have similar CLABSI risks over first 10 days after placement; a cost-effectiveness analysis suggested minocycline-rifampin catheters to be most attractive if the catheter is anticipated to be in place for eight or more days.60 Chlorhexidine-silver sulfadiazine catheters also decrease microbial colonization of surfaces.61 Antibiotic impregnated materials may reduce the incidence of orthopedic foreign body-associated infections.62 A silver impregnated endotracheal tube (ETT) showed a maximal effect during the first 10 days of intubation and reduced mortality in patients with ventilator-associated pneumonia (VAP).63 Water sprays and jets have been used as physical–mechanical approaches for biofilm removal (e.g., debridement of surgical-site, exudates or dental biofilms).64 In addition, the use of dedicated devices to mechanically remove ETT biofilm is supported by a limited number of studies.65 Electrical and electrochemical strategies are being investigated as strategies to prevent biofilm formation on device surfaces.66
When confronted with therapeutic difficulties, removal of the indwelling medical device is a definitive option for curing a medical device-associated infection. However, removal of a medical device may not always be feasible or desirable and the removal procedure itself may be prone to complications and associated with substantial cost. Microorganisms in biofilms show a wide degree of tolerance to different antimicrobial agents. Antibiotics such as rifampin, for the staphylococci in particular, and the fluoroquinolones, may exhibit activity.67,68 Conversely, antibiotics that inhibit cell wall synthesis (e.g., β-lactams) may be less active because microorganisms in biofilms display slow growth rates.69 When using rifampin-based therapy, combination with another antimicrobial agent, rather than monotherapy, must be employed to minimize the emergence of rifampin resistance. Glycopeptides or linezolid when combined with rifampin showed enhanced effects against staphylococcal biofilms.67,70 In a cage-associated methicillin-resistant S. aureus (MRSA) infection model in guinea pigs, the combination of levofloxacin or daptomycin with rifampin had higher activity than the combination of vancomycin or linezolid with rifampin.71 Rifampin and fosfomycin or tedizolid also showed enhanced effects in treating medical device infections caused by MRSA biofilms in vivo.72,73 Dalbavancin alone has recently been shown to have in vitro activity against staphylococcal and enterococcal biofilms, potentially providing an option to treat dalbavancin-susceptible staphylococcal and enterococcal biofilm-associated infections.74,75 Oritavancin also demonstrates activity against staphylococcal biofilms.76 Age of the biofilm and biofilm species composition are important variables that impact susceptibility microorganisms in biofilms. Age of S. epidermidis biofilms was shown to be related with activity of erythromycin, clindamycin, cephalothin, teicoplanin, and vancomycin.77 With respect to biofilm species composition, susceptibility of Streptococcus pneumoniae to β-lactam antibiotics was reduced by the co-presence of β-lactamase producing Moraxella catarrhalis in the biofilm.78 In a biofilm composed of Candida albicans and S. epidermidis combined, the staphylococcal EPS inhibited azole penetration into the biofilm, and C. albicans appeared to protect S. epidermidis against vancomycin.79 High dosages of antibiotics and prolonged duration of treatment are also important when treating medical device-associated infections. The application of catheter lock solutions is a strategy to eradicate established biofilm in the catheter lumen; using this approach, antimicrobial agents dwell at supratherapeutic concentrations (but concentrations sufficient to exhibit anti-biofilm activity) in the catheter lumen for a prolonged time. Antimicrobial lock solutions have been shown to decrease the risk of CLABSI in immunocompromised hematologic patients and those undergoing hemodialysis.80,81 Antibiotic lock therapy is used in conjunction with systemic antibiotics in the treatment of patients with uncomplicated CLABSI.82
Recent advances in surface technologies and materials have ushered in development of defined surface patterns of chemistry and topography that can impact biofilm formation without adding antimicrobial agents.58 Novel materials such as zinconium oxide and electropolished stainless steel reduce bacterial adhesion.27 Incorporation of the Sharklet micropattern (motivated by shark skin) on the surface of medical devices may reduce microbial colonization and biofilm formation.83 Several studies show the feasibility and efficacy of surface modification of medical devices with antifouling polyurethanes and hydrogels to reduce microbial colonization.1,27 Bacteriophages are viruses that propagate in their bacterial host, and can kill their host and/or produce anti-biofilm substances.84 Pretreating hydrogel-coated catheters with a single S. epidermidis bacteriophage or a cocktail of P. aeruginosa bacteriophages mitigated biofilm formation by relevant bacteria in vitro.85–87
Recent advances in understanding the complexity of biofilm biology has informed the development of novel biofilm-targeting therapeutic strategies.1,2,27 EPS-degrading enzymes are a new strategy that may enhance efficacy of antimicrobial agents against biofilms.88,89 Enzymes such as deoxyribonuclease 1 (DNase 1) and dispersin B (DspB) may be useful adjuvants in this regard.88,89 Both DNase 1 and DspB are being investigated as promising options for biomedical coatings.90 Another antibiofilm strategy is the use of lysins - bacteriophage-encoded peptidoglycan hydrolases.91 Small molecules such as mannosides or peptides impede bacterial adhesins binding to host surfaces, thereby preventing biofilm formation.92–94 The widespread use of quorum sensing systems of bacteria for controlling virulence and biofilm formation constitutes another target tactic for the development of novel therapeutics.95 Nanoparticles provide yet 96 another exciting area of development of new biofilm-targeting methodologies. Nanoparticles are currently in the spotlight mainly for their intrinsic antimicrobial activity and strong anti-biofilm potential together with relatively low toxicity to the host.97 Nanoparticles can be used for targeted delivery of antibacterial and antibiofilm agents.98 Liposomes are widely used as representative organic nanoparticles for delivery agents for antimicrobial agents. 99–103 Recently, nanomodified ETTs have been shown to have decreased bacterial colonization compared to unmodified ETTs.104,105 Nano- and chemical engineering approaches can be used to develop improved materials for prevention of biofilm formation. Finally, electrical and electrochemical strategies are being developed for their anti-biofilm activities.96,106–115
Summary
Medical device-associated infections are biofilm-associated infections related to organized communities of microorganisms embedded within a matrix of EPS of microbial and host origin. Because of the entrapment or inactivation of antimicrobial agents, and of the slow growth in biofilms, microorganisms in biofilms display tolerance to a wide range of antimicrobial agents. The VBNC state and emergence of SCVs in biofilms can make successful diagnosis difficult using standard microbiological assays. New diagnostic techniques such as sonication of large implants and molecular diagnostic methods may improve not only identification of pathogens but also reveal greater microbial diversity than previously appreciated. Although most currently available antibiotics have poor activity against microorganisms in biofilms, some, notably rifampin against staphylococci, have been shown to be active against biofilms. Surface-coating or eluting substrates and physical/mechanical/chemical/electrical/biological approaches aimed at inhibition of initial attachment and biofilm removal are two main current biofilm-targeting approaches. Recent advances in surface technologies and materials have ushered in development of material optimization and surface modification with antifouling polyurethanes, hydrogels, and bacteriophages. Recent insights into the biofilm matrix have accelerated novel biofilm-targeting therapeutic strategies such as extracellular polymeric substance-degrading enzymes, small molecules targeting host–extracellular polymeric substance interactions, and quorum sensing systems involved in biofilm formation and dispersal. Electrical and electrochemical strategies are under development.
Box 1. Clinical features of medical device-associated infections.
Presence of an indwelling medical device
Clinical findings suggestive of infection, often with low-grade inflammation
Infection lasting more than one week
Failure of antibiotic treatment without planktonic genetic antimicrobial resistance
Recurrence of infection (particularly if same microorganism is detected over multiple time points, and clinical findings improve/resolve with antibiotic therapy, only to recur after therapy has ceased)
(Data from Hoiby N, Bjarnsholt T, Moser C, et al. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin Microbiol Infect. 2015;21 Suppl 1:S1–25.)
Key Points.
Treatment of medical device-related infections is challenging because microorganisms adhere to and accumulate on the surfaces of medical devices producing biofilms. Microorganisms in biofilms display tolerance to a wide range of antimicrobial agents.
The ‘viable-but-non-culturable’ state, alongside emergence of small colony variants, can render successful diagnosis of biofilm-associated infections difficult using standard microbiological assays. Sonication of infected implants and molecular diagnostic methods may improve not only detection and identification of pathogens but also reveal greater microbial diversity than traditionally recognized.
Surface-coating or eluting substrates and physical/mechanical/chemical/electrical/biological approaches targeted at inhibition of initial attachment and bacterial removal are two biofilm-targeting approaches in use and/or under development.
Recent advances in surface technologies and materials have ushered in development of material optimization and surface modification with antifouling polyurethanes, hydrogels, and bacteriophages.
Recent insights into the biofilm matrix have accelerated novel biofilm-targeting therapeutic strategies such as extracellular polymeric substance-degrading enzymes, small molecules targeting host–extracellular polymeric substance interactions, and interventions targeting quorum sensing systems involved in biofilm formation and dispersal.
Acknowledgments
This publication was supported by the National Institutes of Health under awards number R01 AR056647, R01 AI091594 and R21 AI125870. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Financial Disclosures: Dr. Patel reports grants from CD Diagnostics, BioFire, Curetis, Merck, Hutchison Biofilm Medical Solutions, Accelerate Diagnostics, Allergan, and The Medicines Company. Dr. Patel is or has been a consultant to Curetis, Qvella, St. Jude, Beckman Coulter, Morgan Stanley, Heraeus Medical GmbH, CORMATRIX, Specific Technologies, Diaxonit, Selux Dx, GenMark Diagnostics, LBT Innovations Ltd, PathoQuest and Genentech; monies are paid to Mayo Clinic. In addition, Dr. Patel has a patent on Bordetella pertussis/parapertussis PCR issued, a patent on a device/method for sonication with royalties paid by Samsung to Mayo Clinic, and a patent on an anti-biofilm substance issued. Dr. Patel has served on an Actelion data monitoring board. Dr. Patel receives travel reimbursement from ASM and IDSA and an editor’s stipend from ASM and IDSA, and honoraria from the NBME, Up-to-Date and the Infectious Diseases Board Review Course. Dr Wi has nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Percival SL, Suleman L, Vuotto C, Donelli G. Healthcare-associated infections, medical devices and biofilms: risk, tolerance and control. J Med Microbiol. 2015;64(Pt 4):323–334. doi: 10.1099/jmm.0.000032. [DOI] [PubMed] [Google Scholar]
- 2.Koo H, Allan RN, Howlin RP, Stoodley P, Hall-Stoodley L. Targeting microbial biofilms: current and prospective therapeutic strategies. Nat Rev Microbiol. 2017 doi: 10.1038/nrmicro.2017.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flemming HC, Wingender J, Szewzyk U, Steinberg P, Rice SA, Kjelleberg S. Biofilms: an emergent form of bacterial life. Nat Rev Microbiol. 2016;14(9):563–575. doi: 10.1038/nrmicro.2016.94. [DOI] [PubMed] [Google Scholar]
- 4.Brauner A, Fridman O, Gefen O, Balaban NQ. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat Rev Microbiol. 2016;14(5):320–330. doi: 10.1038/nrmicro.2016.34. [DOI] [PubMed] [Google Scholar]
- 5.Khoury AE, Lam K, Ellis B, Costerton JW. Prevention and control of bacterial infections associated with medical devices. Asaio j. 1992;38(3):M174–178. doi: 10.1097/00002480-199207000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Hoiby N, Bjarnsholt T, Moser C, et al. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin Microbiol Infect. 2015;21(Suppl 1):S1–25. doi: 10.1016/j.cmi.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 7.Crowe JF, Sculco TP, Kahn B. Revision total hip arthroplasty: hospital cost and reimbursement analysis. Clin Orthop Relat Res. 2003;(413):175–182. doi: 10.1097/01.blo.0000072469.32680.b6. [DOI] [PubMed] [Google Scholar]
- 8.Romling U, Kjelleberg S, Normark S, Nyman L, Uhlin BE, Akerlund B. Microbial biofilm formation: a need to act. J Intern Med. 2014;276(2):98–110. doi: 10.1111/joim.12242. [DOI] [PubMed] [Google Scholar]
- 9.Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2(2):95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 10.Hall-Stoodley L, Stoodley P, Kathju S, et al. Towards diagnostic guidelines for biofilm-associated infections. FEMS Immunol Med Microbiol. 2012;65(2):127–145. doi: 10.1111/j.1574-695X.2012.00968.x. [DOI] [PubMed] [Google Scholar]
- 11.Perez K, Patel R. Biofilm-like aggregation of Staphylococcus epidermidis in synovial fluid. J Infect Dis. 2015;212(2):335–336. doi: 10.1093/infdis/jiv096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hobley L, Harkins C, MacPhee CE, Stanley-Wall NR. Giving structure to the biofilm matrix: an overview of individual strategies and emerging common themes. FEMS Microbiol Rev. 2015;39(5):649–669. doi: 10.1093/femsre/fuv015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Becker K, Heilmann C, Peters G. Coagulase-negative staphylococci. Clin Microbiol Rev. 2014;27(4):870–926. doi: 10.1128/CMR.00109-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gotz F. Staphylococcus and biofilms. Mol Microbiol. 2002;43(6):1367–1378. doi: 10.1046/j.1365-2958.2002.02827.x. [DOI] [PubMed] [Google Scholar]
- 15.von Eiff C, Heilmann C, Peters G. New aspects in the molecular basis of polymer-associated infections due to staphylococci. Eur J Clin Microbiol Infect Dis. 1999;18(12):843–846. doi: 10.1007/s100960050417. [DOI] [PubMed] [Google Scholar]
- 16.Niveditha S, Pramodhini S, Umadevi S, Kumar S, Stephen S. The isolation and the biofilm formation of uropathogens in the patients with catheter associated urinary tract infections (UTIs) J Clin Diagn Res. 2012;6(9):1478–1482. doi: 10.7860/JCDR/2012/4367.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rochford ET, Richards RG, Moriarty TF. Influence of material on the development of device-associated infections. Clin Microbiol Infect. 2012;18(12):1162–1167. doi: 10.1111/j.1469-0691.2012.04002.x. [DOI] [PubMed] [Google Scholar]
- 18.Heilmann C, Hussain M, Peters G, Gotz F. Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface. Mol Microbiol. 1997;24(5):1013–1024. doi: 10.1046/j.1365-2958.1997.4101774.x. [DOI] [PubMed] [Google Scholar]
- 19.Hagihara M, Crandon JL, Nicolau DP. The efficacy and safety of antibiotic combination therapy for infections caused by Gram-positive and Gram-negative organisms. Expert Opin Drug Saf. 2012;11(2):221–233. doi: 10.1517/14740338.2012.632631. [DOI] [PubMed] [Google Scholar]
- 20.Muller E, Hubner J, Gutierrez N, Takeda S, Goldmann DA, Pier GB. Isolation and characterization of transposon mutants of Staphylococcus epidermidis deficient in capsular polysaccharide/adhesin and slime. Infect Immun. 1993;61(2):551–558. doi: 10.1128/iai.61.2.551-558.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veenstra GJ, Cremers FF, van Dijk H, Fleer A. Ultrastructural organization and regulation of a biomaterial adhesin of Staphylococcus epidermidis. J Bacteriol. 1996;178(2):537–541. doi: 10.1128/jb.178.2.537-541.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mangwani N, Dash HR, Chauhan A, Das S. Bacterial quorum sensing: functional features and potential applications in biotechnology. J Mol Microbiol Biotechnol. 2012;22(4):215–227. doi: 10.1159/000341847. [DOI] [PubMed] [Google Scholar]
- 23.McDougald D, Rice SA, Barraud N, Steinberg PD, Kjelleberg S. Should we stay or should we go: mechanisms and ecological consequences for biofilm dispersal. Nat Rev Microbiol. 2011;10(1):39–50. doi: 10.1038/nrmicro2695. [DOI] [PubMed] [Google Scholar]
- 24.Valentini M, Filloux A. Biofilms and Cyclic di-GMP (c-di-GMP) Signaling: Lessons from Pseudomonas aeruginosa and other bacteria. The Journal of biological chemistry. 2016;291(24):12547–12555. doi: 10.1074/jbc.R115.711507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karatan E, Watnick P. Signals, regulatory networks, and materials that build and break bacterial biofilms. Microbiol Mol Biol Rev. 2009;73(2):310–347. doi: 10.1128/MMBR.00041-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olsen I. Biofilm-specific antibiotic tolerance and resistance. Eur J Clin Microbiol Infect Dis. 2015;34(5):877–886. doi: 10.1007/s10096-015-2323-z. [DOI] [PubMed] [Google Scholar]
- 27.Lebeaux D, Ghigo JM, Beloin C. Biofilm-related infections: bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol Mol Biol Rev. 2014;78(3):510–543. doi: 10.1128/MMBR.00013-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conlon BP, Rowe SE, Lewis K. Persister cells in biofilm associated infections. Adv Exp Med Biol. 2015;831:1–9. doi: 10.1007/978-3-319-09782-4_1. [DOI] [PubMed] [Google Scholar]
- 29.Thuptimdang P, Limpiyakorn T, McEvoy J, Pruss BM, Khan E. Effect of silver nanoparticles on Pseudomonas putida biofilms at different stages of maturity. J Hazard Mater. 2015;290:127–133. doi: 10.1016/j.jhazmat.2015.02.073. [DOI] [PubMed] [Google Scholar]
- 30.Hausner M, Wuertz S. High rates of conjugation in bacterial biofilms as determined by quantitative in situ analysis. Appl Environ Microbiol. 1999;65(8):3710–3713. doi: 10.1128/aem.65.8.3710-3713.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryder VJ, Chopra I, O’Neill AJ. Increased mutability of staphylococci in biofilms as a consequence of oxidative stress. PloS one. 2012;7(10):e47695. doi: 10.1371/journal.pone.0047695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krol JE, Wojtowicz AJ, Rogers LM, et al. Invasion of E. coli biofilms by antibiotic resistance plasmids. Plasmid. 2013;70(1):110–119. doi: 10.1016/j.plasmid.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Safdar N, Fine JP, Maki DG. Meta-analysis: methods for diagnosing intravascular device-related bloodstream infection. Ann Intern Med. 2005;142(6):451–466. doi: 10.7326/0003-4819-142-6-200503150-00011. [DOI] [PubMed] [Google Scholar]
- 34.Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(1):1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raad I, Hanna HA, Alakech B, Chatzinikolaou I, Johnson MM, Tarrand J. Differential time to positivity: a useful method for diagnosing catheter-related bloodstream infections. Annals of internal medicine. 2004;140(1):18–25. doi: 10.7326/0003-4819-140-1-200401060-00007. [DOI] [PubMed] [Google Scholar]
- 36.Lindsay D, von Holy A. Bacterial biofilms within the clinical setting: what healthcare professionals should know. J Hosp Infect. 2006;64(4):313–325. doi: 10.1016/j.jhin.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 37.Li L, Mendis N, Trigui H, Oliver JD, Faucher SP. The importance of the viable but non-culturable state in human bacterial pathogens. Front Microbiol. 2014;5:258. doi: 10.3389/fmicb.2014.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zandri G, Pasquaroli S, Vignaroli C, et al. Detection of viable but non-culturable staphylococci in biofilms from central venous catheters negative on standard microbiological assays. Clin Microbiol Infect. 2012;18(7):E259–261. doi: 10.1111/j.1469-0691.2012.03893.x. [DOI] [PubMed] [Google Scholar]
- 39.Pasquaroli S, Zandri G, Vignaroli C, Vuotto C, Donelli G, Biavasco F. Antibiotic pressure can induce the viable but non-culturable state in Staphylococcus aureus growing in biofilms. J Antimicrob Chemother. 2013;68(8):1812–1817. doi: 10.1093/jac/dkt086. [DOI] [PubMed] [Google Scholar]
- 40.Maduka-Ezeh AN, Greenwood-Quaintance KE, Karau MJ, et al. Antimicrobial susceptibility and biofilm formation of Staphylococcus epidermidis small colony variants associated with prosthetic joint infection. Diagn Microbiol Infect Dis. 2012;74(3):224–229. doi: 10.1016/j.diagmicrobio.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 41.Tuchscherr L, Heitmann V, Hussain M, et al. Staphylococcus aureus small-colony variants are adapted phenotypes for intracellular persistence. J Infect Dis. 2010;202(7):1031–1040. doi: 10.1086/656047. [DOI] [PubMed] [Google Scholar]
- 42.Perez K, Patel R. Staphylococcus epidermidis small-colony variants are induced by low pH and their frequency reduced by lysosomal alkalinization. J Infect Dis. 2017;215(3):488–490. doi: 10.1093/infdis/jiw503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trampuz A, Piper KE, Jacobson MJ, et al. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med. 2007;357(7):654–663. doi: 10.1056/NEJMoa061588. [DOI] [PubMed] [Google Scholar]
- 44.Erb S, Frei R, Schregenberger K, Dangel M, Nogarth D, Widmer AF. Sonication for diagnosis of catheter-related infection is not better than traditional roll-plate culture: a prospective cohort study with 975 central venous catheters. Clin Infect Dis. 2014;59(4):541–544. doi: 10.1093/cid/ciu352. [DOI] [PubMed] [Google Scholar]
- 45.Bereza PL, Ekiel A, Augusciak-Duma A, et al. Identification of silent prosthetic joint infection: preliminary report of a prospective controlled study. Int Orthop. 2013;37(10):2037–2043. doi: 10.1007/s00264-013-1955-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gomez E, Cazanave C, Cunningham SA, et al. Prosthetic joint infection diagnosis using broad-range PCR of biofilms dislodged from knee and hip arthroplasty surfaces using sonication. J Clin Microbiol. 2012;50(11):3501–3508. doi: 10.1128/JCM.00834-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Larsen MK, Thomsen TR, Moser C, Hoiby N, Nielsen PH. Use of cultivation-dependent and -independent techniques to assess contamination of central venous catheters: a pilot study. BMC Clin Pathol. 2008;8:10. doi: 10.1186/1472-6890-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hall-Stoodley L, Hu FZ, Gieseke A, et al. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. Jama. 2006;296(2):202–211. doi: 10.1001/jama.296.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liesman RM, Pritt BS, Maleszewski JJ, Patel R. Laboratory diagnosis of infective endocarditis. J Clin Microbiol. 2017;55(9):2599–2608. doi: 10.1128/JCM.00635-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tunney MM, Patrick S, Gorman SP, et al. Improved detection of infection in hip replacements. A currently underestimated problem. J Bone Joint Surg Br. 1998;80(4):568–572. doi: 10.1302/0301-620x.80b4.8473. [DOI] [PubMed] [Google Scholar]
- 51.Costerton JW, Post JC, Ehrlich GD, et al. New methods for the detection of orthopedic and other biofilm infections. FEMS Immunol Med Microbiol. 2011;61(2):133–140. doi: 10.1111/j.1574-695X.2010.00766.x. [DOI] [PubMed] [Google Scholar]
- 52.Stoodley P, Conti SF, DeMeo PJ, et al. Characterization of a mixed MRSA/MRSE biofilm in an explanted total ankle arthroplasty. FEMS Immunol Med Microbiol. 2011;62(1):66–74. doi: 10.1111/j.1574-695X.2011.00793.x. [DOI] [PubMed] [Google Scholar]
- 53.De Vecchi E, Bottagisio M, Bortolin M, Toscano M, Lovati AB, Drago L. Improving the bacterial recovery by using dithiothreitol with aerobic and anaerobic broth in biofilm-related prosthetic and joint infections. Adv Exp Med Biol. 2017;973:31–39. doi: 10.1007/5584_2016_51. [DOI] [PubMed] [Google Scholar]
- 54.Parry JA, Karau MJ, Kakar S, Hanssen AD, Patel R, Abdel MP. Disclosing agents for the intraoperative identification of biofilms on orthopedic implants. J Arthroplasty. 2017;32(8):2501–2504. doi: 10.1016/j.arth.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 55.Stoodley P, Nistico L, Johnson S, et al. Direct demonstration of viable Staphylococcus aureus biofilms in an infected total joint arthroplasty. A case report. J Bone Joint Surg Am. 2008;90(8):1751–1758. doi: 10.2106/JBJS.G.00838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walker JT, Verran J, Boyd RD, Percival S. Microscopy methods to investigate structure of potable water biofilms. Methods Enzymol. 2001;337:243–255. doi: 10.1016/s0076-6879(01)37018-0. [DOI] [PubMed] [Google Scholar]
- 57.O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52(9):e162–193. doi: 10.1093/cid/cir257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Swartjes JJ, Sharma PK, van Kooten TG, et al. Current developments in antimicrobial surface coatings for biomedical applications. Curr Med Chem. 2015;22(18):2116–2129. doi: 10.2174/0929867321666140916121355. [DOI] [PubMed] [Google Scholar]
- 59.Darouiche RO, Raad II, Heard SO, et al. A comparison of two antimicrobial-impregnated central venous catheters. Catheter Study Group. N Engl J Med. 1999;340(1):1–8. doi: 10.1056/NEJM199901073400101. [DOI] [PubMed] [Google Scholar]
- 60.Marciante KD, Veenstra DL, Lipsky BA, Saint S. Which antimicrobial impregnated central venous catheter should we use? Modeling the costs and outcomes of antimicrobial catheter use. Am J Infect Control. 2003;31(1):1–8. doi: 10.1067/mic.2003.35. [DOI] [PubMed] [Google Scholar]
- 61.Rupp ME, Lisco SJ, Lipsett PA, et al. Effect of a second-generation venous catheter impregnated with chlorhexidine and silver sulfadiazine on central catheter-related infections: a randomized, controlled trial. Ann Intern Med. 2005;143(8):570–580. doi: 10.7326/0003-4819-143-8-200510180-00007. [DOI] [PubMed] [Google Scholar]
- 62.Marschall J, Lane MA, Beekmann SE, Polgreen PM, Babcock HM. Current management of prosthetic joint infections in adults: results of an Emerging Infections Network survey. Int J Antimicrob Agents. 2013;41(3):272–277. doi: 10.1016/j.ijantimicag.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kollef MH, Afessa B, Anzueto A, et al. Silver-coated endotracheal tubes and incidence of ventilator-associated pneumonia: the NASCENT randomized trial. Jama. 2008;300(7):805–813. doi: 10.1001/jama.300.7.805. [DOI] [PubMed] [Google Scholar]
- 64.Fabbri S, Johnston DA, Rmaile A, et al. Streptococcus mutans biofilm transient viscoelastic fluid behaviour during high-velocity microsprays. J Mech Behav Biomed Mater. 2016;59:197–206. doi: 10.1016/j.jmbbm.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 65.Berra L, Coppadoro A, Bittner EA, et al. A clinical assessment of the Mucus Shaver: a device to keep the endotracheal tube free from secretions. Crit Care Med. 2012;40(1):119–124. doi: 10.1097/CCM.0b013e31822e9fe3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Del Pozo JL, Rouse MS, Euba G, et al. Prevention of Staphylococcus epidermidis biofilm formation using electrical current. Journal of applied biomaterials & functional materials. 2014;12(2):81–83. doi: 10.5301/jabfm.5000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vergidis P, Rouse MS, Euba G, et al. Treatment with linezolid or vancomycin in combination with rifampin is effective in an animal model of methicillin-resistant Staphylococcus aureus foreign body osteomyelitis. Antimicrobial agents and chemotherapy. 2011;55(3):1182–1186. doi: 10.1128/AAC.00740-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abdi-Ali A, Mohammadi-Mehr M, Agha Alaei Y. Bactericidal activity of various antibiotics against biofilm-producing Pseudomonas aeruginosa. Int J Antimicrob Agents. 2006;27(3):196–200. doi: 10.1016/j.ijantimicag.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 69.Curtin J, Cormican M, Fleming G, Keelehan J, Colleran E. Linezolid compared with eperezolid, vancomycin, and gentamicin in an in vitro model of antimicrobial lock therapy for Staphylococcus epidermidis central venous catheter-related biofilm infections. Antimicrobial agents and chemotherapy. 2003;47(10):3145–3148. doi: 10.1128/AAC.47.10.3145-3148.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vergidis P, Schmidt-Malan SM, Mandrekar JN, Steckelberg JM, Patel R. Comparative activities of vancomycin, tigecycline and rifampin in a rat model of methicillin-resistant Staphylococcus aureus osteomyelitis. The Journal of infection. 2015;70(6):609–615. doi: 10.1016/j.jinf.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 71.John AK, Baldoni D, Haschke M, et al. Efficacy of daptomycin in implant-associated infection due to methicillin-resistant Staphylococcus aureus: importance of combination with rifampin. Antimicrobial agents and chemotherapy. 2009;53(7):2719–2724. doi: 10.1128/AAC.00047-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mihailescu R, Furustrand Tafin U, Corvec S, et al. High activity of Fosfomycin and Rifampin against methicillin-resistant Staphylococcus aureus biofilm in vitro and in an experimental foreign-body infection model. Antimicrobial agents and chemotherapy. 2014;58(5):2547–2553. doi: 10.1128/AAC.02420-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Park KH, Greenwood-Quaintance KE, Mandrekar J, Patel R. Activity of tedizolid in methicillin-resistant Staphylococcus aureus experimental foreign body-associated osteomyelitis. Antimicrobial agents and chemotherapy. 2016;60(11):6568–6572. doi: 10.1128/AAC.01248-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fernandez J, Greenwood-Quaintance KE, Patel R. in vitro activity of dalbavancin against biofilms of staphylococci isolated from prosthetic joint infections. Diagn Microbiol Infect Dis. 2016;85(4):449–451. doi: 10.1016/j.diagmicrobio.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 75.Neudorfer K, Schmidt-Malan SM, Patel R. Dalbavancin is active in vitro against biofilms formed by dalbavancin-susceptible enterococci. Diagnostic Microbiology and Infectious Disease. doi: 10.1016/j.diagmicrobio.2017.09.015. In press. [DOI] [PubMed] [Google Scholar]
- 76.Yan Qun, Karau MJ, Patel Robin. in vitro activity of oritavancin against biofilms of staphylococci isolated from prosthetic joint infection. Abstract presented at ASM Microbe; 2018. [DOI] [PubMed] [Google Scholar]
- 77.Monzon M, Oteiza C, Leiva J, Lamata M, Amorena B. Biofilm testing of Staphylococcus epidermidis clinical isolates: low performance of vancomycin in relation to other antibiotics. Diagn Microbiol Infect Dis. 2002;44(4):319–324. doi: 10.1016/s0732-8893(02)00464-9. [DOI] [PubMed] [Google Scholar]
- 78.Budhani RK, Struthers JK. Interaction of Streptococcus pneumoniae and Moraxella catarrhalis: investigation of the indirect pathogenic role of beta-lactamase-producing moraxellae by use of a continuous-culture biofilm system. Antimicrobial agents and chemotherapy. 1998;42(10):2521–2526. doi: 10.1128/aac.42.10.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Adam B, Baillie GS, Douglas LJ. Mixed species biofilms of Candida albicans and Staphylococcus epidermidis. J Med Microbiol. 2002;51(4):344–349. doi: 10.1099/0022-1317-51-4-344. [DOI] [PubMed] [Google Scholar]
- 80.Sanders J, Pithie A, Ganly P, et al. A prospective double-blind randomized trial comparing intraluminal ethanol with heparinized saline for the prevention of catheter-associated bloodstream infection in immunosuppressed haematology patients. J Antimicrob Chemother. 2008;62(4):809–815. doi: 10.1093/jac/dkn284. [DOI] [PubMed] [Google Scholar]
- 81.Yahav D, Rozen-Zvi B, Gafter-Gvili A, Leibovici L, Gafter U, Paul M. Antimicrobial lock solutions for the prevention of infections associated with intravascular catheters in patients undergoing hemodialysis: systematic review and meta-analysis of randomized, controlled trials. Clin Infect Dis. 2008;47(1):83–93. doi: 10.1086/588667. [DOI] [PubMed] [Google Scholar]
- 82.Fortun J, Grill F, Martin-Davila P, et al. Treatment of long-term intravascular catheter-related bacteraemia with antibiotic-lock therapy. J Antimicrob Chemother. 2006;58(4):816–821. doi: 10.1093/jac/dkl318. [DOI] [PubMed] [Google Scholar]
- 83.May RM, Magin CM, Mann EE, et al. An engineered micropattern to reduce bacterial colonization, platelet adhesion and fibrin sheath formation for improved biocompatibility of central venous catheters. Clin Transl Med. 2015;4:9. doi: 10.1186/s40169-015-0050-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Donlan RM. Preventing biofilms of clinically relevant organisms using bacteriophage. Trends Microbiol. 2009;17(2):66–72. doi: 10.1016/j.tim.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 85.Curtin JJ, Donlan RM. Using bacteriophages to reduce formation of catheter-associated biofilms by Staphylococcus epidermidis. Antimicrobial agents and chemotherapy. 2006;50(4):1268–1275. doi: 10.1128/AAC.50.4.1268-1275.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fu W, Forster T, Mayer O, Curtin JJ, Lehman SM, Donlan RM. Bacteriophage cocktail for the prevention of biofilm formation by Pseudomonas aeruginosa on catheters in an in vitro model system. Antimicrobial agents and chemotherapy. 2010;54(1):397–404. doi: 10.1128/AAC.00669-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Akanda ZZ, Taha M, Abdelbary H. Current review - The rise of bacteriophage as a unique therapeutic platform in treating peri-prosthetic joint infections. J Orthop Res. 2017 doi: 10.1002/jor.23755. [DOI] [PubMed] [Google Scholar]
- 88.Kaplan JB. Biofilm matrix-degrading enzymes. Methods Mol Biol. 2014;1147:203–213. doi: 10.1007/978-1-4939-0467-9_14. [DOI] [PubMed] [Google Scholar]
- 89.Okshevsky M, Regina VR, Meyer RL. Extracellular DNA as a target for biofilm control. Curr Opin Biotechnol. 2015;33:73–80. doi: 10.1016/j.copbio.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 90.Darouiche RO, Mansouri MD, Gawande PV, Madhyastha S. Antimicrobial and antibiofilm efficacy of triclosan and dispersinB combination. J Antimicrob Chemother. 2009;64(1):88–93. doi: 10.1093/jac/dkp158. [DOI] [PubMed] [Google Scholar]
- 91.Schuch R, Khan BK, Raz A, Rotolo JA, Wittekind M. Bacteriophage lysin CF-301, a potent antistaphylococcal biofilm agent. Antimicrobial agents and chemotherapy. 2017;61(7) doi: 10.1128/AAC.02666-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nett JE, Cabezas-Olcoz J, Marchillo K, Mosher DF, Andes DR. Targeting fibronectin to disrupt in vivo Candida albicans biofilms. Antimicrobial agents and chemotherapy. 2016;60(5):3152–3155. doi: 10.1128/AAC.03094-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Spaulding CN, Klein RD, Ruer S, et al. Selective depletion of uropathogenic E. coli from the gut by a FimH antagonist. Nature. 2017;546(7659):528–532. doi: 10.1038/nature22972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Totsika M, Kostakioti M, Hannan TJ, et al. A FimH inhibitor prevents acute bladder infection and treats chronic cystitis caused by multidrug-resistant uropathogenic Escherichia coli ST131. J Infect Dis. 2013;208(6):921–928. doi: 10.1093/infdis/jit245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bjarnsholt T, Givskov M. Quorum sensing inhibitory drugs as next generation antimicrobials: worth the effort? Curr Infect Dis Rep. 2008;10(1):22–28. doi: 10.1007/s11908-008-0006-y. [DOI] [PubMed] [Google Scholar]
- 96.Sultana ST, Call DR, Beyenal H. Eradication of Pseudomonas aeruginosa biofilms and persister cells using an electrochemical scaffold and enhanced antibiotic susceptibility. NPJ biofilms and microbiomes. 2016;2:2. doi: 10.1038/s41522-016-0003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gallo J, Panacek A, Prucek R, et al. Silver nanocoating technology in the prevention of prosthetic joint infection. Materials (Basel, Switzerland) 2016;9(5) doi: 10.3390/ma9050337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schutz CA, Juillerat-Jeanneret L, Mueller H, Lynch I, Riediker M. Therapeutic nanoparticles in clinics and under clinical evaluation. Nanomedicine (Lond) 2013;8(3):449–467. doi: 10.2217/nnm.13.8. [DOI] [PubMed] [Google Scholar]
- 99.Wang LS, Gupta A, Rotello VM. Nanomaterials for the treatment of bacterial biofilms. ACS infectious diseases. 2016;2(1):3–4. doi: 10.1021/acsinfecdis.5b00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Duncan B, Li X, Landis RF, et al. Nanoparticle-stabilized capsules for the treatment of bacterial biofilms. ACS nano. 2015;9(8):7775–7782. doi: 10.1021/acsnano.5b01696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Giri K, Yepes LR, Duncan B, et al. Targeting bacterial biofilms via surface engineering of gold nanoparticles. RSC advances. 2015;5(128):105551–105559. doi: 10.1039/C5RA16305F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Forier K, Raemdonck K, De Smedt SC, Demeester J, Coenye T, Braeckmans K. Lipid and polymer nanoparticles for drug delivery to bacterial biofilms. J Control Release. 2014;190:607–623. doi: 10.1016/j.jconrel.2014.03.055. [DOI] [PubMed] [Google Scholar]
- 103.Rukavina Z, Vanic Z. Current trends in development of liposomes for targeting bacterial biofilms. Pharmaceutics. 2016;8(2) doi: 10.3390/pharmaceutics8020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Machado MC, Tarquinio KM, Webster TJ. Decreased Staphylococcus aureus biofilm formation on nanomodified endotracheal tubes: a dynamic airway model. Int J Nanomedicine. 2012;7:3741–3750. doi: 10.2147/IJN.S28191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Machado MC, Webster TJ. Decreased Pseudomonas aeruginosa biofilm formation on nanomodified endotracheal tubes: a dynamic lung model. Int J Nanomedicine. 2016;11:3825–3831. doi: 10.2147/IJN.S108253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schmidt-Malan SM, Karau MJ, Cede J, et al. Antibiofilm activity of low-amperage continuous and intermittent direct electrical current. Antimicrobial agents and chemotherapy. 2015;59(8):4610–4615. doi: 10.1128/AAC.00483-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ruiz-Ruigomez M, Badiola J, Schmidt-Malan SM, et al. Direct electrical current reduces bacterial and yeast biofilm formation. Int J Bacteriol. 2016;2016:9727810. doi: 10.1155/2016/9727810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brinkman CL, Schmidt-Malan SM, Karau MJ, et al. Exposure of bacterial biofilms to electrical current leads to cell death mediated in part by reactive oxygen species. PloS one. 2016;11(12):e0168595. doi: 10.1371/journal.pone.0168595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Del Pozo JL, Rouse MS, Euba G, et al. The electricidal effect is active in an experimental model of Staphylococcus epidermidis chronic foreign body osteomyelitis. Antimicrobial agents and chemotherapy. 2009;53(10):4064–4068. doi: 10.1128/AAC.00432-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schmidt-Malan SM, Brinkman CL, Greenwood-Quaintance KE, Karau MJ, Mandrekar JN, Patel R. Activity of electrical current in experimental Propionibacterium acnes foreign-body osteomyelitis. Antimicrobial agents and chemotherapy. 2017;61(2) doi: 10.1128/AAC.01863-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Voegele P, Badiola J, Schmidt-Malan SM, et al. Antibiofilm activity of electrical current in a catheter Model. Antimicrobial agents and chemotherapy. 2015;60(3):1476–1480. doi: 10.1128/AAC.01628-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.del Pozo JL, Rouse MS, Mandrekar JN, Steckelberg JM, Patel R. The electricidal effect: reduction of Staphylococcus and Pseudomonas biofilms by prolonged exposure to low-intensity electrical current. Antimicrobial agents and chemotherapy. 2009;53(1):41–45. doi: 10.1128/AAC.00680-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.del Pozo JL, Rouse MS, Mandrekar JN, Sampedro MF, Steckelberg JM, Patel R. Effect of electrical current on the activities of antimicrobial agents against Pseudomonas aeruginosa, Staphylococcus aureus, and Staphylococcus epidermidis biofilms. Antimicrobial agents and chemotherapy. 2009;53(1):35–40. doi: 10.1128/AAC.00237-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sultana ST, Atci E, Babauta JT, et al. Electrochemical scaffold generates localized, low concentration of hydrogen peroxide that inhibits bacterial pathogens and biofilms. Scientific reports. 2015;5:14908. doi: 10.1038/srep14908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sultana ST, Call DR, Beyenal H. Eradication of Pseudomonas aeruginosa biofilms and persister cells using an electrochemical scaffold and enhanced antibiotic susceptibility. NPJ Biofilms Microbiomes. 2017;2:2. doi: 10.1038/s41522-016-0003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]