Abstract
Neoadjuvant chemotherapy (NAC) is a significant modality in breast cancer therapy. We sought to characterize prognostic factors in patients scheduled for NAC who had a pretreatment positron-emission tomography paired with diagnostic quality contrast-enhanced computed tomography (CT) (positron-emission tomography/CT [PET/CT]). A total of 118 breast cancer patients were analyzed through chart review who underwent pretreatment PET/CT imaging and received NAC from 2008 to 2014. We collected information on molecular markers, PET/CT, pathologic complete response (pCR), survival, and disease status. Pretreatment standard uptake value (SUV) max of the primary breast tumor showed no relationship to pCR; however, there was a statistically significant relationship with relapse-free survival (RFS) using univariate cox regression (P = 0.03, odds ratio (OR) = 1.06 [1.01–1.12]) with comparable findings observed with overall survival (OS). Multivariate analysis revealed SUV max to be significantly correlated with shortened OS (P = 0.022, OR = 1.08 [1.01–1.16]), with a similar trend reported for RFS. By pathological subtype, this correlation was the strongest within hormone receptor (HR+)/human epidermal growth factor receptor 2 (HER2−) tumors. In addition, Kaplan–Meier estimates demonstrated a significant difference between the RFS of triple-negative tumors and HER2 positive tumors (P = 0.001). Interestingly, within this cohort, multivariate Cox regression analysis showed HER2 positivity to be associated with favorable outcome (P = 0.04, HR = 0.22 [0.05–0.94]). These findings demonstrate a significant association between SUV max of HR+/HER2−− tumors and relapse-free and OS. Furthermore, highlighted here is the favorable survival in the once classically aggressive HER2+ breast cancer subgroup.
Keywords: Breast cancer, human epidermal growth factor receptor 2, neoadjuvant chemotherapy, positron-emission tomography, prognostic factors, standard uptake value
INTRODUCTION
Classic clinicopathologic features such as tumor stage and grade are vital in determining both treatment and prognosis of cancer patients. In addition, advances in modern imaging and disease understanding have identified other factors such as histopathological treatment response and positron-emission tomography (PET) avidity to further characterize the patient disease.[1] Unlike tumor stage and grade, these methods can provide in-treatment correlates to therapeutic efficacy; however, additional research is needed to describe the relationship between these correlates, histopathologic markers, and long-term outcome measures.
PET avidity is described by the uptake of 18F-fluoro-2-deoxy -D-glucose (FDG) in a target of interest normalized to the background signal within the liver or mediastinum. FDG uptake is numerically defined as the standard uptake value (SUV), with elevated SUVs generally suggestive of malignancy.[2] FDG uptake or PET avidity is used for the detection of distant metastasis in a variety of cancers, including breast, lung, colorectal, and head-and-neck.[3,4,5,6] Moreover, PET with diagnostic quality contrast-enhanced computed tomography (CT) (positron emission tomography/CT [PET/CT]) is reliable for identifying regional nodal disease involvement within breast cancer as well.[7]
In addition to staging purposes, PET avidity has been shown to have prognostic utility within a variety of malignancies, such as lymphoma, head-and-neck squamous cell carcinoma, gastric carcinoma, nonsmall cell lung cancer, and breast cancer.[8,9,10,11,12] Furthermore, researchers have associated the SUV of tumors with several clinicopathologic features. Previous reports demonstrated SUV max of the primary to be associated with adverse histopathologic observations in nonsmall cell lung cancer.[12] While SUV max had no prognostic utility in the head-and-neck squamous cell carcinoma, volumetric analysis of FDG uptake correlated with poor outcome.[9] Moreover, interval PET/CT scanning can be invaluable for evaluating treatment response and by extension survival-based outcome in rectal, esophageal, and head-and-neck cancers.[8,10,11,13] Similar findings were observed in breast cancer, where PET positivity following neoadjuvant chemotherapy (NAC) in preoperative breast cancer patients was inversely correlated with disease-free survival (DFS). In fact, preliminary studies demonstrated the pretreatment SUV max to be associated with shortened DFS within the molecular and pathological subtypes of breast cancer.[14,15,16,17] Nevertheless, further work is needed on the association of pretreatment FDG uptake and therapeutic response, as well as other clinically relevant endpoints.
One histopathological method to assess therapeutic efficacy is pathologic complete response (pCR). Esserman et al., described pCR in breast cancer as the absence of pathologically-detected malignant cells from the surgically resected primary site and relevant lymph nodes following NAC.[1] Patients that achieve pCR have significantly improved survival-based outcomes compared to patients with a residual tumor on resection. A similar relationship between pCR and prognosis has been observed in multiple cancers, including rectal and esophageal cancers.[13,18,19] Moreover, pCR is currently being used as a surrogate endpoint for evaluating potential new therapies within breast cancer.[1] Paradoxically, it is typical to observe higher pCR rates within intrinsically aggressive cancer subtypes, including high-grade tumors, triple-negative breast cancer (TNBC), and human epidermal growth factor receptor 2 (HER2) positive breast cancer.[20] By comparison, patients with hormone-receptor-positive (i.e., estrogen receptor and/or progesterone receptor positive) (hormone receptor [HR]) and HER2 negative breast cancer are less likely to achieve pCR despite classically being more indolent. As FDG uptake reflects tumor characteristics such as metabolic activity, it is possible PET avidity informs the pCR rate of breast cancer. Several studies have examined the relationship between FDG uptake and treatment response in a variety of malignancies, most commonly by comparing pretreatment PET/CT imaging to interval scans during cycles of chemotherapy, then ultimately to tissue pathology at resection.[21,22,23] In this setting, reductions in PET avidity during treatment were correlated with lower burdens of residual disease, or in some cases pCR. In the current study, we investigated the prognostic utility of pretreatment SUV max in breast cancer and how it correlates with the pCR rates. In addition, we also evaluated survival-based outcome measures within the pathological subtypes of breast cancer.
METHODS
Positron-emission tomography/computed tomography
Patients were fasting for at least 4 h before the procedure. Between 370 MBq and 425 MBq intravenous FDG was given and imaging was performed 1 h later. Images were obtained from the base of skull to thigh. SUV max was calculated using Syngo.via (Siemens)
Pathological examination
Following NAC, patients underwent either a modified radical mastectomy or lumpectomy, followed by an axillary lymph node dissection or sentinel node biopsy when appropriate. Nottingham criterion determined the overall histologic grade. Patients were clinically staged according to the American Joint Committee on Cancer 8th edition (AJCC). Pathologic complete response was defined as the absence of microscopically detected malignant cells in the resection bed of the primary lesion as well as the resected lymph nodes, ypT0/ypN0. Estrogen receptor or progesterone receptor positivity was defined as ≥1% of positive cells by immunohistochemistry. HER2 positivity was defined as 3+ by immunohistochemistry or fluorescence in situ hybridization ratio >2.2. Relapse was defined as pathologically confirmed recurrent disease or findings on advanced imaging within reasonable clinical suspicion if biopsy could not be performed.
Statistical analysis
Data analyses were performed using IBM SPSS Statistics version 21 IBM Analytics (Armonk, New York, USA) along with Graphpad Prism 5.0 for figure construction. For survival analysis, univariate Cox regression and multivariate Cox regression were performed. Associations between SUV max and pCR was determined using Spearman's correlation. Log-rank test with post hoc pairwise comparisons was performed to evaluate outcome measures between the pathological subtypes. Significance was determined using the value of P < 0.05.
RESULTS
We retrospectively evaluated 927 female breast cancer patients, ultimately analyzing 118 who had pretreatment PET/CT imaging and received NAC from 2008 to 2014 [Figure 1 and Table 1]. Within this cohort, the mean and median age was 51 years (range 23–82) and mean follow-up was 50.25 months, median was 44 months (range 7.3–101.5); nearly 47% were AJCC Stage II, 53% Stage III; 6% were Grade 1, 42% Grade 2, and 49% Grade 3. Tumor grade was not reported in three patients. The pathological subtypes of these breast cancers were 52% HR+/HER2−, 31% HER2 positive, and 17% TNBC. About 92.5% with HER2 positive tumors received NAC containing at least one HER2 targeted agent, with a full description of NAC regimens in Supplemental Table 1 (2.1MB, tif) . Of these patients, 18% relapsed and 11% died over this period.
Figure 1.
Flow diagram for patient selection. Charts of 927 patients were reviewed. 128 were scheduled for neoadjuvant chemotherapy and had pretreatment positron emission tomography/computed tomography imaging. Of these patients, 10 with Stage IV disease were excluded
Table 1.
Description of patient cohort

Neoadjuvant chemotherapy regimens
The SUV max of 109 primary breast tumors was analyzed with a mean of 8.9 ± 6.8. The SUV max from nine primary tumors could not be obtained. SUV max of the primary breast tumor was significantly associated with relapse-free survival (RFS) by univariate cox regression (P = 0.03, odds ratio (OR) = 1.06 (1.01–1.12)) with similar findings observed with overall survival (OS) (P = 0.009, OR = 1.07 [1.02–1.13]) [Figure 2]. Moreover, multivariate analysis, including grade and stage of disease found SUV max to be significantly correlated with shortened OS (P = 0.022, OR = 1.08 [1.01–1.16]), with a similar trend reported for RFS (P = 0.057, OR = 1.06 [0.99–1.1]) [Figure 2]. In patients with HR+/HER2− tumors, SUV max demonstrated a significant relationship with both RFS (P = 0.019, OR = 1.10 [1.02–1.19]) and OS (P = 0.003, OR = 1.14 [1.04–1.24]) through univariate regression [Figure 2]. Multivariate cox regression, which included SUV max, tumor stage, and grade, found significant associations between SUV max of HR+/HER2− tumors and both outcome measures (RFS: P = 0.046, OR = 1.00–1.19); OS: (P = 0.02 OR = 1.12 [1.02–1.23]) [Figure 2]. No significant associations between SUV max and outcome were observed for TNBC or HER2+ disease.
Figure 2.
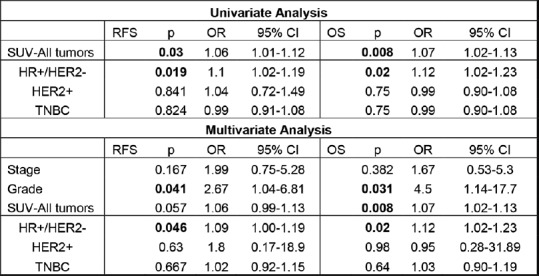
Association between pretreatment standard uptake value max and outcome. Univariate cox regression was performed comparing standard uptake value as continuous data with relapse-free survival or overall survival. Multivariate cox regression was performed similarly, including tumor stage and grade within the analysis. Significance was determined by P < 0.05
Overall pCR rate, defined as ypT0/ypN0, was 19.5%, with rates of 15%, 8.2%, and 40.5% observed in the TNBC, HR+/HER2−, and HER2+ groups, respectively [Table 2]. Consistent with prior studies, pCR was associated with the favorable outcome overall and all three subtypes [Supplemental Figure 1 (2.1MB, tif) ]. FDG uptake had no significant association with pathologic complete response by Spearman's correlation (R = 0.128, P = 0.186).
Table 2.
Standard uptake value and pathologic complete response

Association between pCR and survival
Log-rank analysis with post hoc pairwise comparisons showed a significant difference between the RFS of triple-negative tumors and HER2 positive tumors (P = 0.001), while the comparison between HR-positive/HER2 negative and HER2 positive was not statistically significant (P = 0.12) [Figure 3]. Similar findings were observed with OS [Figure 3]. Multivariate cox regression analysis including grade and stage of tumors showed HER2 positivity to be significantly associated with a lengthened RFS (P = 0.04, HR = 0.22 [0.05–0.94]); however, no statistically significant association with OS was observed (P = 0.182, OR = 0.42 [0.03–1.94]). Of note, tumor grade was significantly related to RFS (P = 0.039, OR = 2.60 [1.05–6.46]) and OS (P = 0.028, OR = 5.28 [1.19–23.2]), whereas clinical stage was not predictive in this context.
Figure 3.

A comparative analysis between survival and pathologic subtype of patients. (a) Log-rank test with post hoc pairwise comparisons between the pathologic subtypes for relapse-free survival. (b) Log-rank test with post hoc pairwise comparisons between the pathologic subtypes for overall survival. Significance was determined by P < 0.05
DISCUSSION
Advancing technology and understanding of cancer have ushered in new ways to complement traditional clinicopathologic classifications, thus aiding in the characterization of human disease. Previous reports have found numerous approaches in which FDG avidity can be incorporated into the prognostic portrait of a patient.[8,9,10,11,12,13] Indeed, in this cohort primary SUV, max was significantly associated with poor outcome [Figure 2]. However, given the weak OR of this finding, it is unlikely to be of any real prognostic utility. Therefore, in the setting of all breast cancers, it is unlikely that primary SUV max could substantially contribute to the prognostic portrait of the patient. Previous studies have reported the prognostic value of SUV max within the molecular and pathological subtypes of breast cancer, particularly HR+/HER2− and TNBC.[14,17] In the agreement, in our data, the association between primary SUV max and survival was differentially strongest within the HR+/HER2− subtype [Figure 1], with no statistically significant relationships, observed within the HER2+ or TNBC groups for either endpoint.[24] Patients with HR+/HER2− generally do very well within the short-term. However the propensity of these tumors to reoccur distally, often many years past diagnosis and treatment, can make providing long-term prognostic outlooks challenging.[25] Therefore, incorporating SUV max of the primary into the clinical picture is most attractive for patients with HR+/HER2− disease and may further enhance outcome predictions within this subset of patients.
The relationship between FDG uptake and pCR has primarily been studied in the context of comparing pre- and post-treatment avidity. Researchers have used the change in SUV-based metrics to determine the future efficacy of a chemotherapeutic regimen as well as predicting the presence (or absence) of residual disease on surgical resection.[26,27,28,29] In the current study, pretreatment SUV max alone had no predictive value for pCR, suggesting that baseline metabolic characteristics are not closely associated with chemosensitivity in this population.
Prior reports observed that pCR can achieved within the HER2+ positive tumors treated with trastuzumab in 30%–50% of patients, whereas generally HR+/HER2− and TNBC tumors respond completely in up to 8%–16% and 33% of patients, respectively.[20] Consistent with prior findings, pCR rates in our study within the HER2+ and HR+/HER2− tumors was 40.5% and 8.2%, respectively. However, only 15% of patients with TNBC tumors achieved pCR in this report. The reason for this discrepancy is unclear, likely in part impacted by the relatively low number of TNBC patients in the cohort.
By comparison, the long-term outcomes of patients within this cohort were better than expected. A majority of patients studied presented with Stage III disease (53%), yet there were only 21 (18%) relapses and 13 (11%) deaths. In particular, patients with HER2+ disease fared, especially well with only two observed relapses and one death during the follow-up period, with an estimated RFS of 96% at 3 years, 91% at 5 years. Other studies which compared regimens with or without trastuzumab for patients with HER2 positive disease demonstrated a 3-year event-free survival (EFS) of 88% within the trastuzumab-containing arm.[30] In addition, the NeoALTTO trial which examined three different anti-HER2 regimens reported EFS at 3 years to be 76%–84%.[31]
CONCLUSION
Limitations of this study include biases inherent to all retrospective studies, and the fact data were collected from a single institution, subsequently restricting a broader application for these findings. Nevertheless, this report has documented several interesting conclusions. We have examined the prognostic utility of pretreatment SUVs, revealing a potential role in patients with HR+/HER2− tumors. Furthermore, there was no apparent connection between pretreatment FDG uptake and pCR following NAC. Finally, the survival measures for patients with HER2+ breast cancer were markedly improved as compared to previous data, exemplifying the efficacy of targeted therapy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Esserman LJ, Berry DA, DeMichele A, Carey L, Davis SE, Buxton M, et al. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: Results from the I-SPY 1 TRIAL – CALGB 150007/150012, ACRIN 6657. J Clin Oncol. 2012;30:3242–9. doi: 10.1200/JCO.2011.39.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ziai P, Hayeri MR, Salei A, Salavati A, Houshmand S, Alavi A, et al. Role of optimal quantification of FDG PET imaging in the clinical practice of radiology. Radiographics. 2016;36:481–96. doi: 10.1148/rg.2016150102. [DOI] [PubMed] [Google Scholar]
- 3.Gong Y, Wang Q, Dong L, Jia Y, Hua C, Mi F, et al. Different imaging techniques for the detection of pelvic lymph nodes metastasis from gynecological malignancies: A systematic review and meta-analysis. Oncotarget. 2017;8:14107–25. doi: 10.18632/oncotarget.12959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lei L, Wang X, Chen Z. PET/CT imaging for monitoring recurrence and evaluating response to treatment in breast cancer. Adv Clin Exp Med. 2016;25:377–82. doi: 10.17219/acem/29853. [DOI] [PubMed] [Google Scholar]
- 5.Maffione AM, Lopci E, Bluemel C, Giammarile F, Herrmann K, Rubello D, et al. Diagnostic accuracy and impact on management of (18) F-FDG PET and PET/CT in colorectal liver metastasis: A meta-analysis and systematic review. Eur J Nucl Med Mol Imaging. 2015;42:152–63. doi: 10.1007/s00259-014-2930-4. [DOI] [PubMed] [Google Scholar]
- 6.Taghipour M, Sheikhbahaei S, Marashdeh W, Solnes L, Kiess A, Subramaniam RM, et al. Use of 18F-fludeoxyglucose-positron emission tomography/Computed tomography for patient management and outcome in oropharyngeal squamous cell carcinoma: A Review. JAMA Otolaryngol Head Neck Surg. 2016;142:79–85. doi: 10.1001/jamaoto.2015.2607. [DOI] [PubMed] [Google Scholar]
- 7.Heusner TA, Kuemmel S, Hahn S, Koeninger A, Otterbach F, Hamami ME, et al. Diagnostic value of full-dose FDG PET/CT for axillary lymph node staging in breast cancer patients. Eur J Nucl Med Mol Imaging. 2009;36:1543–50. doi: 10.1007/s00259-009-1145-6. [DOI] [PubMed] [Google Scholar]
- 8.Maffione AM, Marzola MC, Capirci C, Colletti PM, Rubello D. Value of (18)F-FDG PET for predicting response to neoadjuvant therapy in rectal cancer: Systematic review and meta-analysis. AJR Am J Roentgenol. 2015;204:1261–8. doi: 10.2214/AJR.14.13210. [DOI] [PubMed] [Google Scholar]
- 9.Romesser PB, Qureshi MM, Shah BA, Chatburn LT, Jalisi S, Devaiah AK, et al. Superior prognostic utility of gross and metabolic tumor volume compared to standardized uptake value using PET/CT in head and neck squamous cell carcinoma patients treated with intensity-modulated radiotherapy. Ann Nucl Med. 2012;26:527–34. doi: 10.1007/s12149-012-0604-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kikuchi M, Nakamoto Y, Shinohara S, Fujiwara K, Yamazaki H, Kanazawa Y, et al. Early evaluation of neoadjuvant chemotherapy response using FDG-PET/CT predicts survival prognosis in patients with head and neck squamous cell carcinoma. Int J Clin Oncol. 2013;18:402–10. doi: 10.1007/s10147-012-0393-9. [DOI] [PubMed] [Google Scholar]
- 11.Zhu W, Xing L, Yue J, Sun X, Sun X, Zhao H, et al. Prognostic significance of SUV on PET/CT in patients with localised oesophagogastric junction cancer receiving neoadjuvant chemotherapy/chemoradiation: A systematic review and meta-analysis. Br J Radiol. 2012;85:e694–701. doi: 10.1259/bjr/29946900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Sarraf N, Gately K, Lucey J, Aziz R, Doddakula K, Wilson L, et al. Clinical implication and prognostic significance of standardised uptake value of primary non-small cell lung cancer on positron emission tomography: Analysis of 176 cases. Eur J Cardiothorac Surg. 2008;34:892–7. doi: 10.1016/j.ejcts.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 13.Tulchinsky H, Shmueli E, Figer A, Klausner JM, Rabau M. An interval >7 weeks between neoadjuvant therapy and surgery improves pathologic complete response and disease-free survival in patients with locally advanced rectal cancer? Ann Surg Oncol. 2008;15(10):2661–7. doi: 10.1245/s10434-008-9892-3. doi: 10.1245/s10434-008-9892-3. PubMed PMID: 18389322. [DOI] [PubMed] [Google Scholar]
- 14.Groheux D, Martineau A, Teixeira L, Espié M, de Cremoux P, Bertheau P, et al. 18FDG-PET/CT for predicting the outcome in ER+/HER2- breast cancer patients: Comparison of clinicopathological parameters and PET image-derived indices including tumor texture analysis. Breast Cancer Res. 2017;19:3. doi: 10.1186/s13058-016-0793-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pahk K, Kim S, Choe JG. Early prediction of pathological complete response in luminal B type neoadjuvant chemotherapy-treated breast cancer patients: Comparison between interim 18F-FDG PET/CT and MRI. Nucl Med Commun. 2015;36:887–91. doi: 10.1097/MNM.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 16.Groheux D, Majdoub M, Sanna A, de Cremoux P, Hindié E, Giacchetti S, et al. Early metabolic response to neoadjuvant treatment: FDG PET/CT criteria according to breast cancer subtype. Radiology. 2015;277:358–71. doi: 10.1148/radiol.2015141638. [DOI] [PubMed] [Google Scholar]
- 17.Groheux D, Hindié E, Giacchetti S, Hamy AS, Berger F, Merlet P, et al. Early assessment with 18F-fluorodeoxyglucose positron emission tomography/computed tomography can help predict the outcome of neoadjuvant chemotherapy in triple negative breast cancer. Eur J Cancer. 2014;50:1864–71. doi: 10.1016/j.ejca.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 18.Adham M, Baulieux J, Mornex F, de La Roche de Bransat E, Ducerf C, Souquet JC, et al. Combined chemotherapy and radiotherapy followed by surgery in the treatment of patients with squamous cell carcinoma of the esophagus. Cancer. 2000;89:946–54. [PubMed] [Google Scholar]
- 19.García-Aguilar J, Hernandez de Anda E, Sirivongs P, Lee SH, Madoff RD, Rothenberger DA, et al. A pathologic complete response to preoperative chemoradiation is associated with lower local recurrence and improved survival in rectal cancer patients treated by mesorectal excision. Dis Colon Rectum. 2003;46:298–304. doi: 10.1007/s10350-004-6545-x. [DOI] [PubMed] [Google Scholar]
- 20.Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet. 2014;384:164–72. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 21.Humbert O, Riedinger JM, Vrigneaud JM, Kanoun S, Dygai-Cochet I, Berriolo-Riedinger A, et al. 18F-FDG PET-derived tumor blood flow changes after 1 cycle of neoadjuvant chemotherapy predicts outcome in triple-negative breast cancer. J Nucl Med. 2016;57:1707–12. doi: 10.2967/jnumed.116.172759. [DOI] [PubMed] [Google Scholar]
- 22.Beukinga RJ, Hulshoff JB, van Dijk LV, Muijs CT, Burgerhof JGM, Kats-Ugurlu G, et al. Predicting response to neoadjuvant chemoradiotherapy in esophageal cancer with textural features derived from pretreatment 18F-FDG PET/CT imaging. J Nucl Med. 2017;58:723–9. doi: 10.2967/jnumed.116.180299. [DOI] [PubMed] [Google Scholar]
- 23.Koo PJ, Kim SJ, Chang S, Kwak JJ. Interim fluorine-18 fluorodeoxyglucose positron emission tomography/Computed tomography to predict pathologic response to preoperative chemoradiotherapy and prognosis in patients with locally advanced rectal cancer. Clin Colorectal Cancer. 2016;15:e213–e219. doi: 10.1016/j.clcc.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Ahn SG, Lee M, Jeon TJ, Han K, Lee HM, Lee SA, et al. [18F]-fluorodeoxyglucose positron emission tomography can contribute to discriminate patients with poor prognosis in hormone receptor-positive breast cancer. PLoS One. 2014;9:e105905. doi: 10.1371/journal.pone.0105905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colleoni M, Sun Z, Price KN, Karlsson P, Forbes JF, Thürlimann B, et al. Annual hazard rates of recurrence for breast cancer during 24 years of follow-up: Results from the international breast cancer study group trials I to V. J Clin Oncol. 2016;34:927–35. doi: 10.1200/JCO.2015.62.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berriolo-Riedinger A, Touzery C, Riedinger JM, Toubeau M, Coudert B, Arnould L, et al. [18F]FDG-PET predicts complete pathological response of breast cancer to neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging. 2007;34:1915–24. doi: 10.1007/s00259-007-0459-5. [DOI] [PubMed] [Google Scholar]
- 27.Duch J, Fuster D, Muñoz M, Fernández PL, Paredes P, Fontanillas M, et al. 18F-FDG PET/CT for early prediction of response to neoadjuvant chemotherapy in breast cancer. Eur J Nucl Med Mol Imaging. 2009;36:1551–7. doi: 10.1007/s00259-009-1116-y. [DOI] [PubMed] [Google Scholar]
- 28.Groheux D, Hindié E, Giacchetti S, Delord M, Hamy AS, de Roquancourt A, et al. Triple-negative breast cancer: Early assessment with 18F-FDG PET/CT during neoadjuvant chemotherapy identifies patients who are unlikely to achieve a pathologic complete response and are at a high risk of early relapse. J Nucl Med. 2012;53:249–54. doi: 10.2967/jnumed.111.094045. [DOI] [PubMed] [Google Scholar]
- 29.Rousseau C, Devillers A, Sagan C, Ferrer L, Bridji B, Campion L, et al. Monitoring of early response to neoadjuvant chemotherapy in Stage II and III breast cancer by [18F]fluorodeoxyglucose positron emission tomography. J Clin Oncol. 2006;24:5366–72. doi: 10.1200/JCO.2006.05.7406. [DOI] [PubMed] [Google Scholar]
- 30.Perez EA, Romond EH, Suman VJ, Jeong JH, Davidson NE, Geyer CE, Jr, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: Joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol. 2011;29:3366–73. doi: 10.1200/JCO.2011.35.0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Azambuja E, Holmes AP, Piccart-Gebhart M, Holmes E, Di Cosimo S, Swaby RF, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): Survival outcomes of a randomised, open-label, multicentre, phase 3 trial and their association with pathological complete response. Lancet Oncol. 2014;15:1137–46. doi: 10.1016/S1470-2045(14)70320-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Neoadjuvant chemotherapy regimens
Association between pCR and survival


