Significance
At fertilization, repetitive calcium signals are induced in mammalian eggs that are essential for activating embryo development. These signals are supported by calcium influx from the surrounding medium. Two ion channels supporting calcium influx into eggs, CaV3.2 and TRPV3, were identified previously. Here we find that a third channel, TRPM7, is essential for this function. In the absence of both TRPM7 and CaV3.2, the calcium signals induced by the fertilizing sperm stop prematurely. Female mice carrying eggs lacking TRPM7 and CaV3.2 are subfertile. Because TRPM7 channel function is modulated by ions in culture medium, these findings highlight the importance of exact culture medium composition used during laboratory procedures for human assisted reproduction therapies.
Keywords: oocyte, calcium, fertilization, TRPM7, CaV3.2
Abstract
The success of mammalian development following fertilization depends on a series of transient increases in egg cytoplasmic Ca2+, referred to as Ca2+ oscillations. Maintenance of these oscillations requires Ca2+ influx across the plasma membrane, which is mediated in part by T-type, CaV3.2 channels. Here we show using genetic mouse models that TRPM7 channels are required to support this Ca2+ influx. Eggs lacking both TRPM7 and CaV3.2 stop oscillating prematurely, indicating that together they are responsible for the majority of Ca2+ influx immediately following fertilization. Fertilized eggs lacking both channels also frequently display delayed resumption of Ca2+ oscillations, which appears to require sperm–egg fusion. TRPM7 and CaV3.2 channels almost completely account for Ca2+ influx observed following store depletion, a process previously attributed to canonical store-operated Ca2+ entry mediated by STIM/ORAI interactions. TRPM7 serves as a membrane sensor of extracellular Mg2+ and Ca2+ concentrations and mediates the effects of these ions on Ca2+ oscillation frequency. When bred to wild-type males, female mice carrying eggs lacking TRPM7 and CaV3.2 are subfertile, and their offspring have increased variance in postnatal weight. These in vivo findings confirm previous observations linking in vitro experimental alterations in Ca2+ oscillatory patterns with developmental potential and offspring growth. The identification of TRPM7 and CaV3.2 as key mediators of Ca2+ influx following fertilization provides a mechanistic basis for the rational design of culture media that optimize developmental potential in research animals, domestic animals, and humans.
All animal species utilize a large rise in intracellular cytoplasmic Ca2+ {[Ca2+]i} levels to trigger development of the fertilized egg (1). In mammals, prophase I, germinal vesicle (GV) stage oocytes prepare for fertilization during maturation by accumulating Ca2+ into endoplasmic reticulum (ER) stores (2, 3). This change accompanies nuclear maturation into a metaphase II (MII)-arrested oocyte, henceforth referred to as an “egg.” Introduction of PLCζ by the fertilizing sperm triggers production of inositol 1,4,5-trisphosphate (IP3), which causes Ca2+ release from the egg ER stores by activating its cognate receptor (4–6). [Ca2+]i typically remains elevated for several minutes before returning to baseline as a consequence of Ca2+ reuptake into ER stores and Ca2+ extrusion out of the egg. Repetitive Ca2+ release events follow that cause persistent transient increases in [Ca2+]i, known as Ca2+ oscillations, which continue for several hours following fertilization and are essential for activating successful embryo development (7). However, persistence of these Ca2+ oscillations depends on the continued presence of Ca2+ in the extracellular medium to provide a source to replenish ER stores (8, 9). Hence, Ca2+ influx is essential both during oocyte maturation to generate proper ER Ca2+ stores and following fertilization to support persistent Ca2+ oscillations.
Several recent papers have contributed to our understanding of the ion channels that support Ca2+ influx in maturing oocytes and in eggs. CaV3.2 is a voltage-activated T-type Ca2+ channel, whose pore-forming alpha subunit is encoded by the Cacna1h gene. CaV3.2 supports Ca2+ influx during maturation and following fertilization but is not solely responsible for this function (10). Store-operated Ca2+ entry (SOCE), mediated by the Ca2+ sensor STIM proteins and plasma membrane ORAI channels, is a common mechanism for cells to trigger Ca2+ reuptake in response to loss of Ca2+ from ER stores. However, studies using knockout mouse lines demonstrated that SOCE is not required during mouse oocyte maturation or following fertilization (11). Members of a large family of cation-permeable channels known as transient receptor potential (TRP) channels are widely expressed in both excitable and nonexcitable cell types (12). One TRP family member, TRPV3, is present on the mouse egg plasma membrane and mediates Ca2+ influx but does not have a requisite role at fertilization (13). A channel with characteristics of transient receptor potential cation channel subfamily M, member 7 (TRPM7) is also expressed on the mouse egg surface (14). TRPM7 is a ubiquitously expressed, constitutively active channel permeable to many divalent cations, including Ca2+ and Mg2+, though it is inhibited by low millimolar concentrations of Mg2+ (15, 16). The TRPM7 global knockout mouse has an embryonic lethal phenotype, indicating that it has an essential function in development (17). Interestingly, TRPM7 has both ion channel and kinase activity and is therefore known as a “chanzyme.” Pharmacological modulators of TRPM7 function were used to demonstrate that a TRPM7-like channel can support Ca2+ influx into mouse eggs and preimplantation embryos; a role for this channel at fertilization was not tested in this study (14). However, many ion channel agonists and inhibitors have off-target effects, so there are caveats to the interpretation of these experiments. The Mg2+ sensitivity of TRPM7 makes it an intriguing candidate to have functional importance at fertilization because it was recently demonstrated that Ca2+ oscillation frequency, which depends on Ca2+ influx, is inversely related to Mg2+ concentration in the extracellular medium (18).
Here we show using a conditional knockout approach that TRPM7 has an essential role in supporting Ca2+ influx in mouse oocytes and eggs. We also demonstrate that Ca2+ influx previously attributed to SOCE is to a large extent mediated by the nearly constitutive activity of TRPM7. Additional experiments reveal that TRPM7 is involved in modulation of Ca2+ oscillation frequency in response to changing concentrations of extracellular divalent cations. Finally, we show using a Trpm7/Cacna1h double knockout model that these two channels are the principal mediators of Ca2+ influx that supports sustained Ca2+ oscillations following fertilization in mice.
Results
Genetic Deletion of Trpm7 Causes Loss of Classical TRPM7 Current.
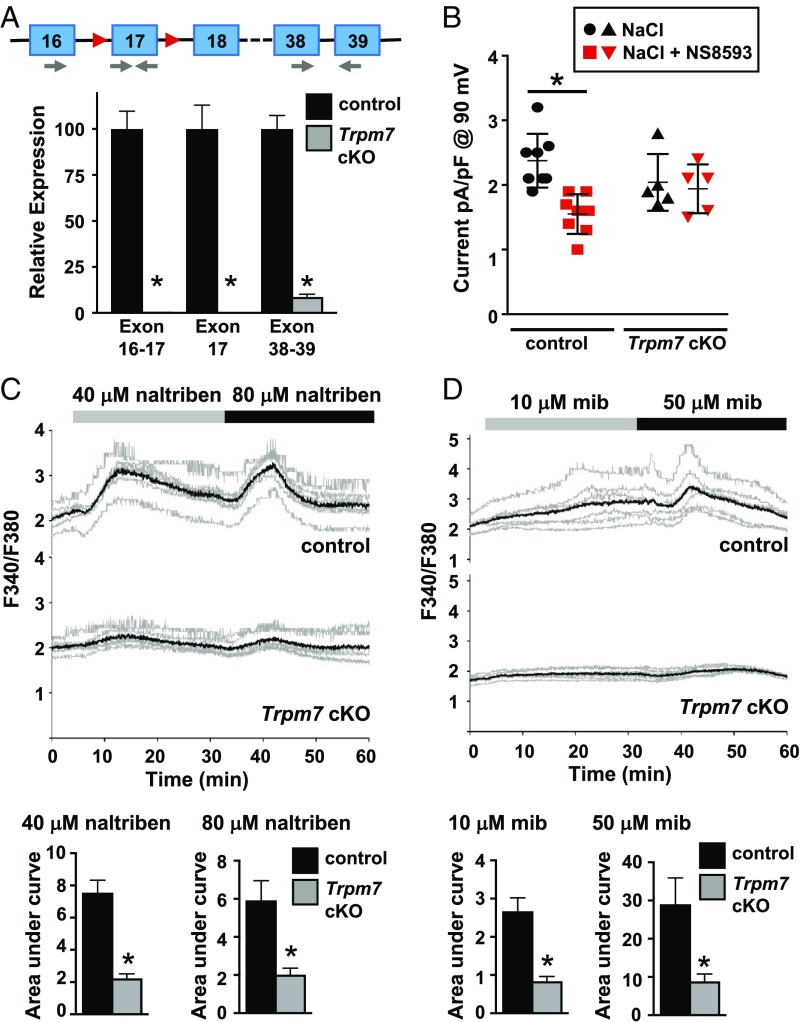
Oocyte-specific deletion of Trpm7 was achieved by mating mice carrying a Trpm7 allele modified to contain LoxP sites flanking exon 17 (Trpm7f) with a Gdf9-cre transgenic line (17, 19). Depletion of Trpm7 transcripts in cre-positive Trpm7 conditional knockout (cKO) oocytes was confirmed by real-time RT-PCR. Transcripts containing exon 17 were nearly undetectable, and levels of Trpm7 mRNA with intact 3′ exons were greatly reduced in Trpm7 cKO MII eggs compared with Trpm7f/f controls (Fig. 1A). Whole-cell patch clamp was performed to determine whether functional TRPM7 channels could be detected in Trpm7 cKO eggs. A voltage ramp (−100 to +100 mV) elicited an outward current in Trpm7f/f eggs; a summary of the current at +90 mV is shown (Fig. 1B). When the TRPM7-selective inhibitor NS8593 was applied to control eggs, a significant reduction in outward current was observed at 90 mV following a voltage ramp, as described previously (14, 20). NS8593 had no effect on this current in Trpm7 cKO eggs, indicating an absence of functional NS8593-sensitive TRPM7 channels. Ca2+ imaging using fura-2 ascertained the effect of two TRPM7 channel activators, naltriben and mibefradil (also a T-type channel inhibitor), on control and Trpm7 cKO eggs (14, 21, 22). An increase in [Ca2+]i was observed when control eggs were exposed to either substance, but very little response was evident for Trpm7 cKO eggs under the same conditions (Fig. 1 C and D), further supporting the functional loss of TRPM7 channels in Trpm7 cKO oocytes.
Fig. 1.
Genetic deletion of Trpm7 causes loss of classical TRPM7 current. (A) Real-time RT-PCR of total RNA from Trpm7f/f (control) or Trpm7f/f;Gdf9-cre (Trpm7 cKO) eggs. Schematic shows partial genomic structure of Trpm7-floxed allele. Exons, blue boxes; loxP sites, red triangles; primer locations, gray arrows. Graph shows expression relative to that in control eggs, mean ± SEM. Eggs were collected from n = 5 individual control and n = 3 Trpm7 cKO mice. *P < 0.05 relative to control with same primer pair, t test. (B) Whole-cell patch-clamp recordings from eggs of control or Trpm7 cKO mice in response to a ramp (−100 to +100 mV). Graph shows current amplitude from individual eggs at +90 mV before (black symbols) and after (red symbols) addition of NS8593; bars indicate mean ± SEM. Control eggs, n = 8; Trpm7 cKO eggs, n = 5. *P < 0.05, paired t test. (C) Ca2+ response of control and Trpm7 cKO oocytes to the indicated concentrations of naltriben. Upper graphs show F340/F380 ratio in individual oocytes (gray) and the mean ratio (black) from both groups in one representative experiment. Lower graphs show mean area under the curve ± SEM for the indicated treatment. n = 3 biological replicates, a total of 18 control and 18 Trpm7 cKO oocytes were imaged. *P < 0.05, Mann–Whitney U test (40 μM naltriben); *P < 0.05, t test with Welch’s correction (80 μM naltriben). (D) Ca2+ response of control and Trpm7 cKO oocytes to the indicated concentrations of mibefradil (mib). Upper graphs in this panel and in all subsequent calcium traces show F340/F380 ratio in individual oocytes (gray) and the mean ratio (black) from both groups in one representative experiment. Lower graphs show mean area under the curve ± SEM for the indicated treatment. n = 2 biological replicates, a total of 23 control and 23 Trpm7 cKO oocytes were imaged. *P < 0.05, t test with Welch’s correction.
TRPM7 Is the Primary Channel Responsible for Spontaneous and Store Depletion-Induced Ca2+ Influx in Oocytes and Eggs.
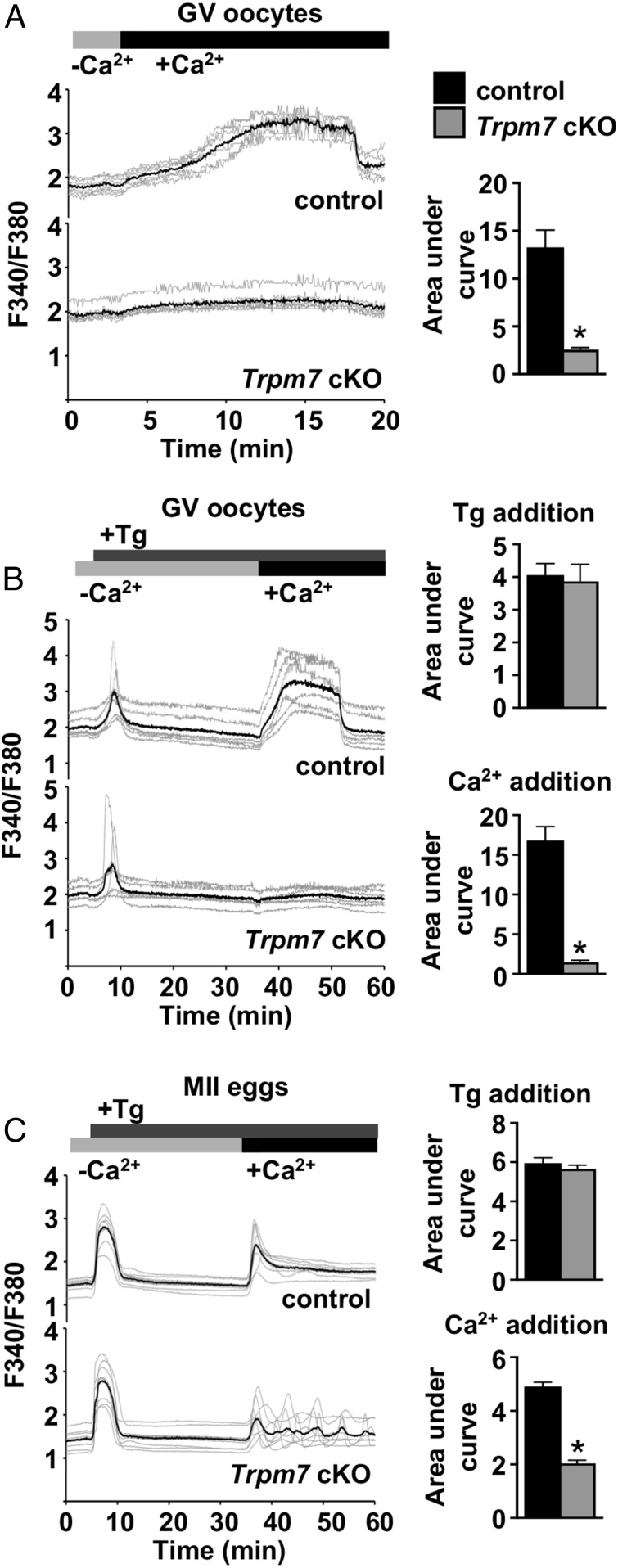
To test whether TRPM7 supports Ca2+ influx in GV oocytes, we placed control or Trpm7 cKO oocytes in nominally Ca2+-free medium and then added Ca2+ to the extracellular medium while monitoring [Ca2+]i. Of note, extended time in Ca2+-free medium was associated with an unhealthy appearance and late lysis in a significant number of both control and Trpm7 cKO oocytes; therefore, only oocytes that had a normal appearance following completion of imaging were included in this analysis. Control GV oocytes had a large increase in [Ca2+]i starting ∼2–3 min following Ca2+ addition, whereas [Ca2+]i in Trpm7 cKO oocytes changed minimally (Fig. 2A). We next tested whether spontaneous Ca2+ entry via TRPM7 contributed to Ca2+ influx that has previously been attributed to SOCE by performing a standard SOCE assay on control and Trpm7 cKO oocytes. GV oocytes were placed in nominally Ca2+-free medium and then thapsigargin was added to cause ER Ca2+ store depletion. The anticipated temporary increase in [Ca2+]i was observed, and was not different between control and Trpm7 cKO GV oocytes (Fig. 2B). However, when Ca2+ was then added to the extracellular medium, control GV oocytes displayed a large increase in [Ca2+]i, whereas Trpm7 cKO oocytes had a barely detectable increase. When a similar assay was performed on control and Trpm7 cKO eggs, [Ca2+]i responses occurred in both control and Trpm7 cKO eggs, but overall response was lower for the cKO (Fig. 2C). Together, these findings indicate that thapsigargin-releasable Ca2+ stores in Trpm7 cKO oocytes and eggs do not differ from controls. However, Ca2+ influx observed after store depletion and Ca2+ readdition is greatly reduced in Trpm7 cKO GV oocytes and partially reduced in Trpm7 cKO eggs, indicating that TRPM7 is a major mediator of this influx.
Fig. 2.
TRPM7 is primarily responsible for spontaneous and store depletion-induced Ca2+ influx in oocytes and eggs. (A) Spontaneous Ca2+ influx into control and Trpm7 cKO GV oocytes. (Left) Representative traces; (Right) mean area under the curve ± SEM. n = 6 biological replicates, a total of 60 control and 59 Trpm7 cKO oocytes were imaged. *P < 0.05, Mann–Whitney U test. (B and C) Measurements of ER Ca2+ stores and Ca2+ influx after thapsigargin (Tg)-induced Ca2+ store depletion in control and Trpm7 cKO GV oocytes (B) or MII eggs (C). (Left) Representative traces; (Right) mean area under the curve ± SEM following Tg addition and following Ca2+ addition. Six biological replicates were performed for GV oocytes, and three for MII eggs, and the total number of cells in each group was: control GV oocytes, 46; Trpm7 cKO GV oocytes, 45; control MII eggs, 33; and Trpm7 cKO MII eggs, 35. *P < 0.05, Mann–Whitney U test (B), t test with Welch’s correction (C).
Loss of TRPM7 Alters Ca2+ Oscillations and Causes Abnormal Offspring Growth.
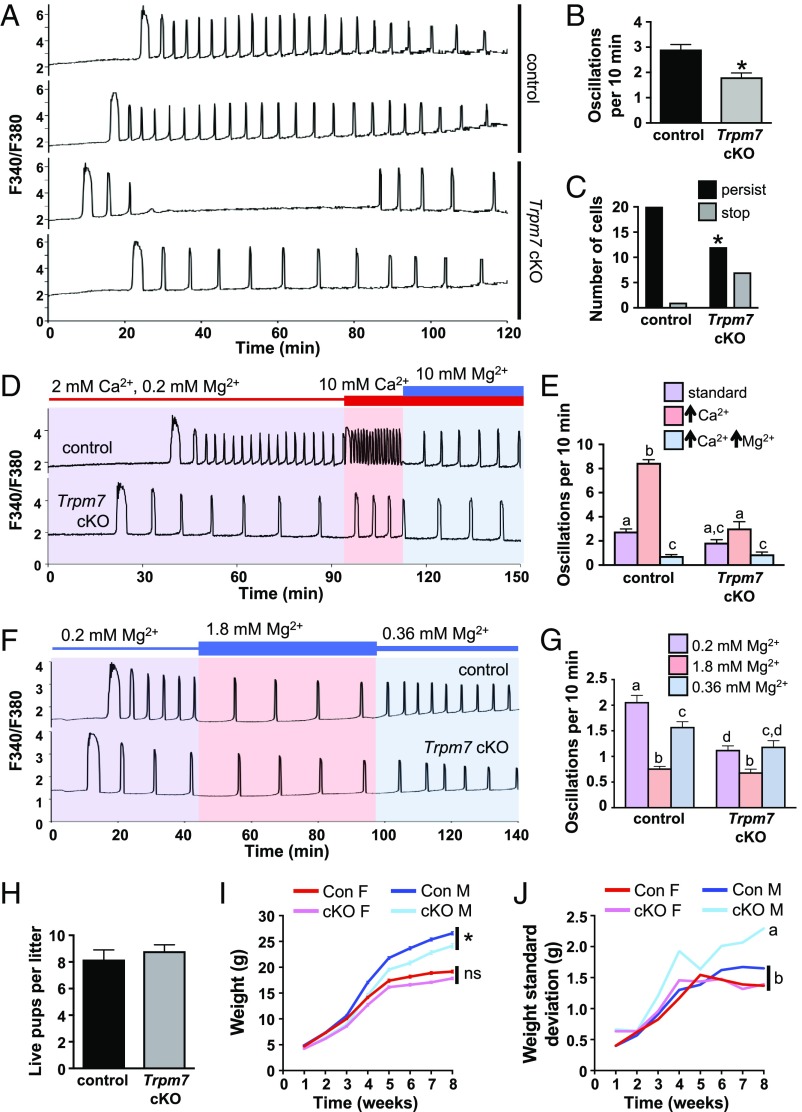
To determine whether TRPM7 mediates Ca2+ influx following fertilization, control and Trpm7 cKO eggs were fertilized while monitoring [Ca2+]i (Fig. 3A). Consistent with the similar Ca2+ stores, there was no difference in the duration of the first Ca2+ transient. However, the oscillation frequency was significantly reduced in the Trpm7 cKO eggs, and the number of eggs that had persistent Ca2+ oscillations for more than 60 min was also reduced (Fig. 3 B and C). Eggs respond to increases in extracellular Ca2+ by increasing their Ca2+ oscillation frequency (8, 23). To determine whether this phenomenon was mediated by TRPM7, we fertilized eggs in the presence of 2 mM Ca2+ and then increased extracellular Ca2+ to 10 mM. In response, control eggs dramatically increased their oscillation frequency, whereas Trpm7 cKO eggs had only a subtle increase (Fig. 3 D and E). Addition of extracellular Mg2+ reduced oscillation frequency, consistent with the inhibitory action of Mg2+ on TRPM7 channel function. To further assess the role of TRPM7 in mediating the Mg2+ response, Mg2+ concentrations were varied in the presence of 2 mM Ca2+. As expected, increasing Mg2+ to 1.8 mM significantly slowed oscillation frequency, whereas diluting Mg2+ to 0.36 mM increased frequency in control eggs; however, these responses were substantially blunted in eggs lacking TRPM7 (Fig. 3 F and G and SI Appendix, Fig. S1). These findings indicate that TRPM7 is a key regulator of Ca2+ influx in response to alterations in extracellular Ca2+ and Mg2+ ion concentrations, although other channels may also participate. To test whether loss of TRPM7 impacted fertility, we performed a 6-mo breeding trial by mating wild-type males to female mice carrying either control or Trpm7 cKO oocytes. There was no difference in the number of live pups per litter (Fig. 3H); however, male pups derived from Trpm7 cKO oocytes gained less weight after weaning than control males (Fig. 3I). Furthermore, the SD of the weight measurements was significantly increased for the males from Trpm7 cKO oocytes (Fig. 3J). Because TRPM7 activity was required during a developmental period when mouse embryos are undergoing epigenetic reprogramming, we tested whether these weight differences could be related to epigenetic alterations in the offspring, in particular at imprinted genes, which have a role in regulating growth. We measured DNA methylation in liver tissue from the offspring at 3 wk of age. There was no difference in either methylation at repetitive elements measured using the luminometric methylation assay (LUMA) (24) or in methylation of imprinted genes at differentially methylated regions that control their expression (SI Appendix, Fig. S2A). These findings suggest that the Ca2+ oscillatory pattern immediately following fertilization has a long-term impact on the growth trajectory of male offspring, but that it is not caused by alterations in DNA methylation.
Fig. 3.
Loss of TRPM7 reduces Ca2+ oscillation frequency and persistence in mouse eggs and causes abnormal growth trajectory in male offspring. (A–C) Ratiometric Ca2+ imaging of n = 22 control and 19 Trpm7 cKO eggs was performed during IVF in three independent experiments. (A) Representative traces. (B) Oscillation frequency, mean ± SEM, *P < 0.05, t test. (C) Number of eggs with persistence of Ca2+ oscillations after 60 min. *P < 0.05, Fisher’s exact test. (D and E) Response of n = 18 control and 13 Trpm7 cKO eggs to the indicated changes in Ca2+ and Mg2+ concentration in three independent experiments. (D) Representative traces. (E) Graph of oscillation frequency during incubation in the indicated conditions, mean ± SEM. Different letters above columns indicate significant difference, P < 0.05, two-way ANOVA with Tukey’s multiple comparisons test. (F and G) Response of n = 29 control and 33 Trpm7 cKO eggs to the indicated changes in Mg2+ concentration in three independent experiments. (F) Representative traces. (G) Graph of oscillation frequency during incubation in the indicated conditions, mean ± SEM. Different letters above columns indicate significant difference, P < 0.05, two-way ANOVA with Tukey’s multiple comparisons test. (H–J) Breeding trial of wild-type males bred for 6 mo to females carrying either control or Trpm7 cKO oocytes; seven breeding pairs per group. (H) Average number of live pups per litter, mean ± SEM. (I) Weight of all male and female offspring over time, mean ± SEM, *P < 0.05, mixed-model ANOVA. ns, not significant. N = 24 female pups, 28 male pups from control dams; 32 female pups, 30 male pups from cKO dams. (J) SD of offspring weight over time. Different letters indicate statistically different SDs between groups (mixed-model ANOVA).
Combined Loss of TRPM7 and CaV3.2 Dramatically Alters Ca2+ Signals and Impairs Fertility.
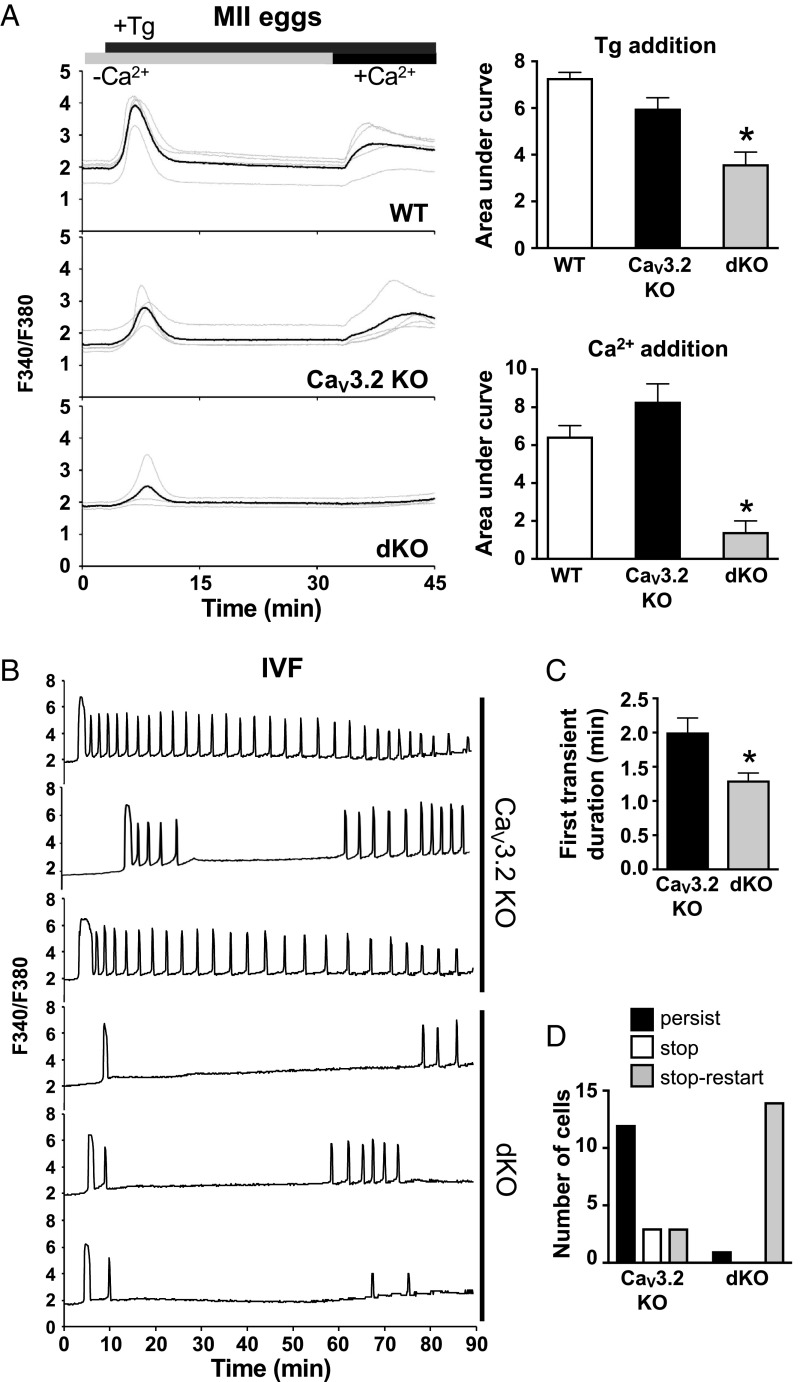
We previously demonstrated that CaV3.2 contributes to ER Ca2+ stores and Ca2+ influx following fertilization (10), so it was possible that CaV3.2 was partially compensating for TRPM7 function in the Trpm7 cKO oocytes. To test this idea, we generated female mice carrying double knockout (dKO) eggs lacking both CaV3.2 and TRPM7 (Cacna1h−/−;Trpm7f/f;Gdf9-cre). For comparison, we used two sets of controls: wild-type eggs from C57BL/6J females and CaV3.2 knockout eggs from Cacna1h−/−;Trpm7f/f females (CaV3.2 KO), which have the same mixed genetic background (129S4/SvJae and C57BL/6J) as the dKO eggs. Thapsigargin-sensitive Ca2+ stores in CaV3.2 KO eggs averaged slightly lower than in wild-type eggs, but this decrease did not reach significance in the three-way comparison (Fig. 4A). Ca2+ influx following store depletion was also not different between these two groups. In contrast, dKO eggs had significantly lower Ca2+ stores and minimal Ca2+ influx following store depletion relative to that in both other egg types (Fig. 4A). The minimal Ca2+ influx in the dKO eggs (Fig. 4A) could be explained by the presence of functional TRPV3 channels (13). These findings indicate that in eggs, both CaV3.2 and TRPM7, and possibly TRPV3, support a “SOCE-like” Ca2+ influx following store depletion that could be misinterpreted as being mediated by STIM/ORAI interactions.
Fig. 4.
Combined loss of TRPM7 and CaV3.2 severely impacts ER Ca2+ store accrual and alters Ca2+ oscillations following IVF. (A) Measurements of ER Ca2+ stores and Ca2+ influx after Tg-induced Ca2+ store depletion in wild-type, CaV3.2 KO, and dKO MII eggs. (Left) Representative traces; (Right) mean area under the curve ± SEM following Tg addition and following Ca2+ addition. n = 3 biological replicates; the total number of cells in each group was: wild-type eggs, 27; CaV3.2 KO eggs, 29; and dKO eggs, 29. *P < 0.05, one-way ANOVA with Tukey’s multiple comparisons test (for Tg addition), *P < 0.05, Kruskal–Wallis test with Dunn multiple comparisons test (for Ca2+ addition). (B–D) Ratiometric Ca2+ imaging of 18 CaV3.2 KO and 15 dKO eggs was performed during IVF in two independent experiments. (B) Representative traces. (C) First transient duration, mean ± SEM, *P < 0.05, Mann–Whitney U test. (D) Number of eggs with indicated patterns of Ca2+ oscillations. Significant difference between patterns in the two groups, P < 0.05, χ2 test.
We next determined Ca2+ oscillatory patterns following in vitro fertilization (IVF). For this experiment, we used CaV3.2 KO eggs as controls to avoid misinterpretation of results that could be related to mouse strain differences. Monitoring of [Ca2+]i during IVF revealed that, consistent with the decrease in Ca2+ stores, the duration of the first Ca2+ transient following IVF was significantly shorter in the dKO eggs relative to eggs lacking only CaV3.2 (Fig. 4 B and C). However, the most dramatic difference between the CaV3.2 KO and dKO eggs was that there were only one or two Ca2+ transients in the dKO eggs before they stopped oscillating (1.71 transients ± 0.16 SEM, n = 14), which is a pattern highly reminiscent of the oscillatory behavior of fertilized wild-type eggs cultured in nominally Ca2+-free medium (9, 23). Interestingly, most of the dKO eggs began oscillating again almost 1 h after the first Ca2+ transient (52 min ± 3.7 SEM) (Fig. 4D). These findings are consistent with the idea that together, CaV3.2 and TRPM7 support the vast majority of Ca2+ influx into eggs during the first hour following fertilization, but that another Ca2+ channel or transporter must subsequently either appear on the plasma membrane or be activated to allow for the Ca2+ oscillation “restart.”
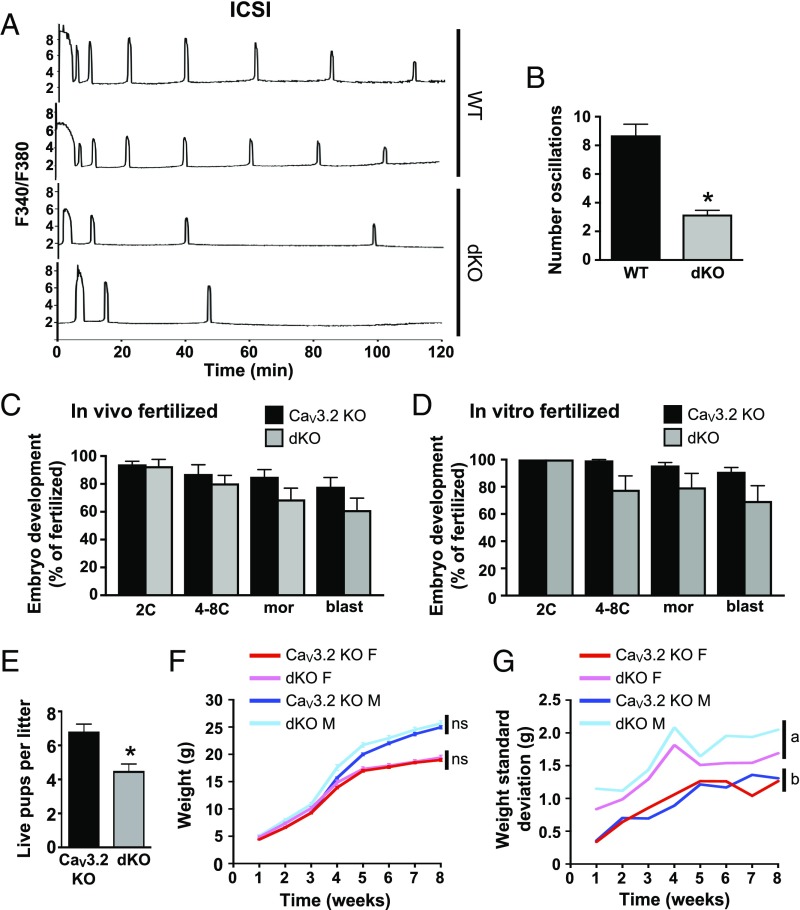
To determine whether sperm–egg membrane fusion was required for the restart oscillation feature in the dKO eggs, we next tested whether restarting occurred in dKO eggs following fertilization by intracytoplasmic sperm injection (ICSI). As a positive control for the quality of the sperm preparation, we used wild-type eggs fertilized by ICSI; these eggs displayed Ca2+ oscillations that had a regular frequency and persisted for over 2 h (Fig. 5A). In contrast, dKO eggs generally had only two initial Ca2+ transients followed by one or two transients at a very low frequency (Fig. 5B), but never displayed a restarting pattern similar to that observed following IVF. These findings suggest that there is either a unique signaling event or membrane composition change that accompanies sperm–egg fusion, but not ICSI, and can support Ca2+ influx into the fertilized egg in the absence of both CaV3.2 and TRPM7.
Fig. 5.
Combined loss of TRPM7 and CaV3.2 reduces Ca2+ oscillation frequency following ICSI, reduces litter sizes, and alters offspring growth variance. (A and B) Ratiometric Ca2+ imaging of 10 wild-type and 23 dKO eggs was performed immediately following ICSI. (A) Representative traces. (B) Number of Ca2+ oscillations during the 120-min period following ICSI, mean ± SEM, *P < 0.05, t test with Welch’s correction. (C and D) Preimplantation development of pronuclear-stage embryos derived from wild-type sperm and eggs of the indicated genotypes following in vivo fertilization and zygote collection (C) or in vitro fertilization (D); n = 6–9 females per group. 2C, two-cell stage; 4–8C, 4- to 8-cell stage; blast, blastocyst; mor, morula. (E–G) Breeding trial of wild-type males bred for 6 mo to females carrying either CaV3.2 KO or dKO oocytes; seven breeding pairs per group. (E) Average number of live pups per litter, mean ± SEM, *P < 0.05, Mann–Whitney U test. (F) Weight of all male and female offspring over time, mean ± SEM, mixed-model ANOVA. ns, not significant. N = 22 female pups, 20 male pups from CaV3.2 KO dams; 15 female pups, 16 male pups from dKO dams. (G) SD of offspring weight over time. Different letters indicate statistically different SDs between groups (mixed-model ANOVA).
To test whether the abnormal Ca2+ oscillatory pattern in dKO eggs affected embryo development, we collected zygotes from CaV3.2 KO and dKO females mated to wild-type males and then monitored preimplantation embryo development in vitro. There was no significant difference in the number of zygotes collected or in the success or timing of development to the blastocyst stage (Fig. 5C). To determine whether a combination of impaired Ca2+ influx and exposure to an in vitro environment during fertilization might further hamper development, eggs from CaV3.2 KO and dKO females were fertilized in vitro and monitored for development. Again, development to the blastocyst stage did not differ significantly between the two groups (Fig. 5D). However, a 6-mo breeding trial using wild-type males mated to females carrying either CaV3.2 KO or dKO oocytes revealed a significant reduction in the number of pups per litter derived from dKO oocytes (Fig. 5E). Together, these findings indicate that implantation and/or postimplantation development are impaired. Both male and female surviving pups from dKO oocytes had an average weight and growth trajectory similar to those from CaV3.2 KO oocytes (Fig. 5F). However, the dKO offspring had a significant increase in variability in their weight from 1 through 8 wk of age (Fig. 5G). This difference could not be explained by alterations in DNA methylation at repetitive elements or methylation of imprinted genes in liver tissue of the offspring (SI Appendix, Fig. S2B). Taken together, these findings suggest that significant abnormalities in Ca2+ signals immediately following fertilization in vivo can lead to postimplantation development failure and aberrations in fetal growth.
Discussion
It was shown previously that three types of ion channels that support Ca2+ influx are present and functional on the mouse oocyte and/or egg plasma membrane: CaV3.2, TRPV3, and a TRPM7-like channel (10, 13, 14). Each of these channels can participate in the accrual of ER Ca2+ stores during oocyte maturation, but only CaV3.2 has been shown to support Ca2+ influx required for a normal Ca2+ oscillatory pattern following fertilization (10). The experiments reported here build on these findings to detail the contributions of TRPM7 and CaV3.2 to Ca2+ homeostasis and Ca2+ signaling before and after fertilization. Using a conditional knockout approach, we demonstrate a pivotal role for TRPM7 channels in regulating Ca2+ oscillation frequency following fertilization. We also show that together, CaV3.2 and TRPM7 channels support the majority of the Ca2+ influx responsible for ER Ca2+ store accrual during oocyte maturation and are critical for establishment of an appropriate postfertilization Ca2+ oscillation pattern. Fertilized eggs lacking both CaV3.2 and TRPM7 frequently display a second wave of Ca2+ oscillations following a delay, suggesting activation of an alternate Ca2+ influx mechanism during the later stages of egg activation. Finally, we provide evidence that disruption of the initial pattern of Ca2+ oscillations following fertilization is associated with postimplantation development failure and alterations in postnatal growth.
TRPM7 is a key mediator of store-depletion–induced Ca2+ influx in GV oocytes, and together, CaV3.2 and TRPM7 are required for the same process in eggs. This Ca2+ influx was previously presumed to occur through a store-operated Ca2+ entry mechanism mediated by STIM/ORAI interactions. However, the data presented here and prior studies showing that Stim1, Stim2, and Orai1 are dispensable for normal Ca2+ homeostasis and postfertilization Ca2+ signaling in mouse oocytes (11, 25, 26) implicate TRPM7 as a primary mediator of this Ca2+ influx and CaV3.2 as a contributor. There are notable species differences regarding reliance of oocyte Ca2+ signaling on SOCE components, with evidence for STIM1 functionality in porcine oocytes (27); thus, the relative importance of TRPM7 in oocytes of other species remains to be addressed. In somatic cells, TRPM7 is also capable of mediating SOCE-like Ca2+ influx independently of STIM and ORAI proteins. Both macrophages and dendritic cells undergo differentiation and have completely normal function when lacking both STIM1 and STIM2 (28). It turns out that macrophages utilize TRPM7-mediated Ca2+ influx, rather than SOCE, to support signaling pathways downstream of toll-like receptor 4 activation by lipopolysaccharide (29). Complicating this issue, recent studies in cultured B lymphocytes indicate that while TRPM7 ion channel activity directly contributes to the maintenance of Ca2+ stores, TRPM7 kinase activity appears to modulate canonical SOCE by an unknown mechanism (30, 31). Despite these findings of an interaction between TRPM7 and canonical SOCE in B lymphocytes, the expendability of Stim1, Stim2, and Orai1 in mouse oocytes indicates that TRPM7 functions independently of canonical SOCE in these cells. Although our data indicate that TRPM7 contributes significantly to the Ca2+ influx to maintain intracellular stores in mouse oocytes, there is not sufficient evidence to claim that the channel is activated by store depletion rather than simply functioning as a Ca2+ conduit through its constitutive activity.
An unusual “stop–restart” pattern of Ca2+ oscillations was observed following IVF of dKO eggs. Importantly, this pattern was not observed following ICSI, which accomplishes fertilization without sperm–egg membrane fusion. In similar fashion, the plasma membrane block to polyspermy does not occur following ICSI or parthenogenetic activation, but instead requires some additional signal induced by sperm–egg fusion for its establishment (32–37). This result is also consistent with the recent observation that fertilization by sperm lacking PLCζ fails to initiate the typical robust pattern of Ca2+ oscillations but prompts a small number of Ca2+ transients that begin following a delay (6). This secondary Ca2+ signal requires sperm–egg fusion and is proposed to reflect a “primitive” intrinsic activation signal (6); it is possible that the second wave of oscillations in dKO eggs is related to this recently discovered PLCζ-independent activation mechanism. The ability of the dKO egg to restart oscillations could be a consequence of the appearance on the egg plasma membrane of sufficient Ca2+-permeable channels to allow refilling of the ER Ca2+ stores. The source of these new channels is not clear. One possibility is that an existing channel(s) at the plasma membrane that is somehow inactive in MII eggs is activated as a consequence of sperm–egg fusion. TRPV3 channels are present on the mouse egg plasma membrane (13) and could be the target of such stimulation in the dKO eggs. Another possibility is that new channels are added to the plasma membrane after sperm–egg fusion, although their source is presently unknown. It is unlikely that new channels are derived from cortical granule membranes, because cortical granule exocytosis occurs normally following ICSI (34, 35); yet ICSI eggs do not display the stop–restart Ca2+oscillation pattern. A possible source, however, is the sperm plasma membrane, which has several types of Ca2+-permeable channels that could function in the newly formed zygote; this phenomenon occurs in Caenorhabditis elegans (38). Lastly, the new channels could come from fusion of egg intracellular vesicles other than cortical granules, with the caveat that this fusion event must be dependent on a signal that originates as a result of sperm–egg fusion. It is also possible that differences in PLCζ accessibility and distribution between IVF and ICSI could influence the generation of the initial and secondary Ca2+ signals. These ideas will be interesting to explore in future studies.
It has been known for decades that there is a direct relationship between extracellular Ca2+ concentration and oscillation frequency following fertilization in mammals (8, 23). How extracellular Mg2+ impacts Ca2+ oscillation frequency was only recently described in an elegant series of experiments using mouse embryos (18). In this report, the authors found that the ratio of extracellular Mg2+ to Ca2+ was a critical factor regulating the frequency and persistence of Ca2+ oscillations in mouse embryos fertilized by ICSI, with a lower Mg2+/Ca2+ ratio correlating with higher oscillation frequency and persistence. Because Mg2+ at the levels used in this report can inhibit TRPM7 ion channel activity (15), and extracellular Ca2+ modulates oscillation frequency via TRPM7 (Fig. 3 D and E), this finding is consistent with our identification of TRPM7 as a critical mediator of Ca2+ influx following fertilization. Furthermore, it suggests that TRPM7 serves as an egg plasma membrane sensor of the extracellular Mg2+/Ca2+ ratio.
Important findings of our study were the altered growth trajectory and/or greater weight variability of offspring derived from TRPM7 cKO or dKO eggs and wild-type sperm. These findings are not surprising because previous studies have documented the importance of the Ca2+ oscillation pattern for promoting robust preimplantation development, postimplantation development to term, and postnatal growth rate (18, 39–41). The exact mechanisms connecting Ca2+ oscillations to fetal growth are not known but could be related to alterations in gene expression during preimplantation development, which have been documented in blastocyst-stage embryos generated following experimental alteration of postfertilization Ca2+ oscillation patterns (40). Another factor contributing to the altered growth patterns could be differences in embryo redox potential. Periodic rises in cytoplasmic Ca2+ levels following fertilization stimulate mitochondrial oxidative phosphorylation, which provides ATP necessary to sustain the Ca2+ oscillations and impacts the cellular redox state (42, 43). Importantly, experimental alterations in redox potential of pronuclear-stage mouse embryos is associated with alterations in postnatal weight variation and growth trajectories (44). These findings are consistent with our observation of an increase in weight variability and altered growth trajectories in heterozygous offspring derived from eggs that likely had an abnormal pattern of Ca2+ oscillations following fertilization.
Although the current studies were performed in the mouse, TRPM7, CACNA1H, and TRPV3 are all expressed in human oocytes and TRPM7 and TRPV3 are expressed in macaque oocytes as indicated by RNA sequencing (45–48). Furthermore, there are numerous missense or loss of function (LoF) mutations identified in human TRPM7 (480 missense, 27 LoF), CACNA1H (1,045 missense, 10 LoF), and TRPV3 (312 missense, 23 LoF) (see Exome Aggregation Consortium browser at exac.broadinstitute.org; ref. 49). These findings support the ideas that (i) TRPM7, CaV3.2, and TRPV3 could also regulate Ca2+ signals following fertilization in human and (ii) functional abnormalities in these channels could contribute to infertility or abnormal growth outcomes in children. Assisted reproduction procedures are responsible for the births of millions of children worldwide. Because the composition of most clinically used media is proprietary, limited information on embryo culture media composition is accessible (50–52) and Ca2+ and Mg2+ content of stand-alone fertilization media is not available at all. Embryo culture media formulations have an extremely wide range of Mg2+/Ca2+ ratios (51, 52) that could dramatically alter Ca2+ influx during embryo development by affecting TRPM7 function. Evidence is mounting that exposure to varying levels of Ca2+ and Mg2+ during fertilization and embryo culture can impact developmental outcomes (18, 53). Culture media formulations also have significant differences in energy substrates including those that can influence redox potential, which also impacts development (18, 44). Studies in human or nonhuman primate oocytes to determine the impact of media composition on Ca2+ oscillatory patterns and redox potential would provide the basis for rational design of appropriate media that optimize reproductive outcomes and offspring health.
Materials and Methods
All animal work was performed in accordance with National Institutes of Health and National Institute of Environmental Health Sciences guidelines under approved animal care and use protocols. A detailed description of mice, gamete and embryo collection and culture, fertilization, Ca2+ imaging, electrophysiology, real-time RT-PCR, DNA methylation analyses, and statistical analyses is provided in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Duy Nguyen (University of Pennsylvania) for assistance with DNA methylation assays; Brian Papas [National Institute of Environmental Health Sciences (NIEHS)] for assistance with published RNA-seq datasets; and Jim Putney, Gary Bird, and Jurrien Dean for critical reading and helpful discussion of this manuscript. This work was supported by the Intramural Research Program of the NIH, NIEHS, Grant 1ZIAES102985 (to C.J.W.) and Grant 1ZIAES103126 (to D.M.U.), by the NIH/National Institute of General Medical Sciences Grant R37GM051279 (to M.S.B.), and by NIH/National Institute of Child Health and Human Development Grants HD51872 and HD092499 (to R.A.F.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1810422115/-/DCSupplemental.
References
- 1.Stricker SA. Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev Biol. 1999;211:157–176. doi: 10.1006/dbio.1999.9340. [DOI] [PubMed] [Google Scholar]
- 2.Cheon B, Lee HC, Wakai T, Fissore RA. Ca2+ influx and the store-operated Ca2+ entry pathway undergo regulation during mouse oocyte maturation. Mol Biol Cell. 2013;24:1396–1410. doi: 10.1091/mbc.E13-01-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tombes RM, Simerly C, Borisy GG, Schatten G. Meiosis, egg activation, and nuclear envelope breakdown are differentially reliant on Ca2+, whereas germinal vesicle breakdown is Ca2+ independent in the mouse oocyte. J Cell Biol. 1992;117:799–811. doi: 10.1083/jcb.117.4.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyazaki S, et al. Block of Ca2+ wave and Ca2+ oscillation by antibody to the inositol 1,4,5-trisphosphate receptor in fertilized hamster eggs. Science. 1992;257:251–255. doi: 10.1126/science.1321497. [DOI] [PubMed] [Google Scholar]
- 5.Hachem A, et al. PLCζ is the physiological trigger of the Ca2+ oscillations that induce embryogenesis in mammals but conception can occur in its absence. Development. 2017;144:2914–2924. doi: 10.1242/dev.150227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nozawa K, Satouh Y, Fujimoto T, Oji A, Ikawa M. Sperm-borne phospholipase C zeta-1 ensures monospermic fertilization in mice. Sci Rep. 2018;8:1315. doi: 10.1038/s41598-018-19497-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ducibella T, Schultz RM, Ozil JP. Role of calcium signals in early development. Semin Cell Dev Biol. 2006;17:324–332. doi: 10.1016/j.semcdb.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Igusa Y, Miyazaki S. Effects of altered extracellular and intracellular calcium concentration on hyperpolarizing responses of the hamster egg. J Physiol. 1983;340:611–632. doi: 10.1113/jphysiol.1983.sp014783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kline D, Kline JT. Thapsigargin activates a calcium influx pathway in the unfertilized mouse egg and suppresses repetitive calcium transients in the fertilized egg. J Biol Chem. 1992;267:17624–17630. [PubMed] [Google Scholar]
- 10.Bernhardt ML, et al. CaV3.2 T-type channels mediate Ca2+ entry during oocyte maturation and following fertilization. J Cell Sci. 2015;128:4442–4452. doi: 10.1242/jcs.180026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernhardt ML, Padilla-Banks E, Stein P, Zhang Y, Williams CJ. Store-operated Ca2+ entry is not required for fertilization-induced Ca2+ signaling in mouse eggs. Cell Calcium. 2017;65:63–72. doi: 10.1016/j.ceca.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carvacho I, Lee HC, Fissore RA, Clapham DE. TRPV3 channels mediate strontium-induced mouse-egg activation. Cell Rep. 2013;5:1375–1386. doi: 10.1016/j.celrep.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carvacho I, et al. TRPM7-like channels are functionally expressed in oocytes and modulate post-fertilization embryo development in mouse. Sci Rep. 2016;6:34236. doi: 10.1038/srep34236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nadler MJ, et al. LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature. 2001;411:590–595. doi: 10.1038/35079092. [DOI] [PubMed] [Google Scholar]
- 16.Prakriya M, Lewis RS. CRAC channels: Activation, permeation, and the search for a molecular identity. Cell Calcium. 2003;33:311–321. doi: 10.1016/s0143-4160(03)00045-9. [DOI] [PubMed] [Google Scholar]
- 17.Jin J, et al. Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science. 2008;322:756–760. doi: 10.1126/science.1163493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozil JP, Sainte-Beuve T, Banrezes B. [Mg2+]o/[Ca2+]o determines Ca2+ response at fertilization: Tuning of adult phenotype? Reproduction. 2017;154:675–693. doi: 10.1530/REP-16-0057. [DOI] [PubMed] [Google Scholar]
- 19.Lan ZJ, Xu X, Cooney AJ. Differential oocyte-specific expression of Cre recombinase activity in GDF-9-iCre, Zp3cre, and Msx2Cre transgenic mice. Biol Reprod. 2004;71:1469–1474. doi: 10.1095/biolreprod.104.031757. [DOI] [PubMed] [Google Scholar]
- 20.Chubanov V, et al. Natural and synthetic modulators of SK (K(ca)2) potassium channels inhibit magnesium-dependent activity of the kinase-coupled cation channel TRPM7. Br J Pharmacol. 2012;166:1357–1376. doi: 10.1111/j.1476-5381.2012.01855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hofmann T, et al. Activation of TRPM7 channels by small molecules under physiological conditions. Pflugers Arch. 2014;466:2177–2189. doi: 10.1007/s00424-014-1488-0. [DOI] [PubMed] [Google Scholar]
- 22.Schäfer S, et al. Mibefradil represents a new class of benzimidazole TRPM7 channel agonists. Pflugers Arch. 2016;468:623–634. doi: 10.1007/s00424-015-1772-7. [DOI] [PubMed] [Google Scholar]
- 23.Miyazaki S. Repetitive calcium transients in hamster oocytes. Cell Calcium. 1991;12:205–216. doi: 10.1016/0143-4160(91)90021-6. [DOI] [PubMed] [Google Scholar]
- 24.Karimi M, et al. LUMA (LUminometric Methylation Assay)–A high throughput method to the analysis of genomic DNA methylation. Exp Cell Res. 2006;312:1989–1995. doi: 10.1016/j.yexcr.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Miao YL, Stein P, Jefferson WN, Padilla-Banks E, Williams CJ. Calcium influx-mediated signaling is required for complete mouse egg activation. Proc Natl Acad Sci USA. 2012;109:4169–4174. doi: 10.1073/pnas.1112333109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi T, Kikuchi T, Kidokoro Y, Shirakawa H. Ca2+ influx-dependent refilling of intracellular Ca2+ stores determines the frequency of Ca2+ oscillations in fertilized mouse eggs. Biochem Biophys Res Commun. 2013;430:60–65. doi: 10.1016/j.bbrc.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 27.Lee K, Wang C, Machaty Z. STIM1 is required for Ca2+ signaling during mammalian fertilization. Dev Biol. 2012;367:154–162. doi: 10.1016/j.ydbio.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 28.Vaeth M, et al. Ca2+ signaling but not store-operated Ca2+ entry is required for the function of macrophages and dendritic cells. J Immunol. 2015;195:1202–1217. doi: 10.4049/jimmunol.1403013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schappe MS, et al. Chanzyme TRPM7 mediates the Ca2+ influx essential for lipopolysaccharide-induced toll-like receptor 4 endocytosis and macrophage activation. Immunity. 2018;48:59–74.e5. doi: 10.1016/j.immuni.2017.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beesetty P, et al. Inactivation of TRPM7 kinase in mice results in enlarged spleens, reduced T-cell proliferation and diminished store-operated calcium entry. Sci Rep. 2018;8:3023. doi: 10.1038/s41598-018-21004-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Faouzi M, Kilch T, Horgen FD, Fleig A, Penner R. The TRPM7 channel kinase regulates store-operated calcium entry. J Physiol. 2017;595:3165–3180. doi: 10.1113/JP274006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gardner AJ, Williams CJ, Evans JP. Establishment of the mammalian membrane block to polyspermy: Evidence for calcium-dependent and -independent regulation. Reproduction. 2007;133:383–393. doi: 10.1530/REP-06-0304. [DOI] [PubMed] [Google Scholar]
- 33.Horvath PM, Kellom T, Caulfield J, Boldt J. Mechanistic studies of the plasma membrane block to polyspermy in mouse eggs. Mol Reprod Dev. 1993;34:65–72. doi: 10.1002/mrd.1080340111. [DOI] [PubMed] [Google Scholar]
- 34.Maleszewski M, Kimura Y, Yanagimachi R. Sperm membrane incorporation into oolemma contributes to the oolemma block to sperm penetration: Evidence based on intracytoplasmic sperm injection experiments in the mouse. Mol Reprod Dev. 1996;44:256–259. doi: 10.1002/(SICI)1098-2795(199606)44:2<256::AID-MRD16>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 35.Sengoku K, et al. Requirement of sperm-oocyte plasma membrane fusion for establishment of the plasma membrane block to polyspermy in human pronuclear oocytes. Mol Reprod Dev. 1999;52:183–188. doi: 10.1002/(SICI)1098-2795(199902)52:2<183::AID-MRD9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 36.Wolf DP, Nicosia SV, Hamada M. Premature cortical granule loss does not prevent sperm penetration of mouse eggs. Dev Biol. 1979;71:22–32. doi: 10.1016/0012-1606(79)90079-4. [DOI] [PubMed] [Google Scholar]
- 37.Wortzman-Show GB, Kurokawa M, Fissore RA, Evans JP. Calcium and sperm components in the establishment of the membrane block to polyspermy: Studies of ICSI and activation with sperm factor. Mol Hum Reprod. 2007;13:557–565. doi: 10.1093/molehr/gam042. [DOI] [PubMed] [Google Scholar]
- 38.Takayama J, Onami S. The sperm TRP-3 channel mediates the onset of a Ca(2+) wave in the fertilized C. elegans oocyte. Cell Rep. 2016;15:625–637. doi: 10.1016/j.celrep.2016.03.040. [DOI] [PubMed] [Google Scholar]
- 39.Ducibella T, et al. Egg-to-embryo transition is driven by differential responses to Ca(2+) oscillation number. Dev Biol. 2002;250:280–291. [PubMed] [Google Scholar]
- 40.Ozil JP, Banrezes B, Tóth S, Pan H, Schultz RM. Ca2+ oscillatory pattern in fertilized mouse eggs affects gene expression and development to term. Dev Biol. 2006;300:534–544. doi: 10.1016/j.ydbio.2006.08.041. [DOI] [PubMed] [Google Scholar]
- 41.Ozil JP, et al. Egg activation events are regulated by the duration of a sustained [Ca2+]cyt signal in the mouse. Dev Biol. 2005;282:39–54. doi: 10.1016/j.ydbio.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 42.Dumollard R, Carroll J, Duchen MR, Campbell K, Swann K. Mitochondrial function and redox state in mammalian embryos. Semin Cell Dev Biol. 2009;20:346–353. doi: 10.1016/j.semcdb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 43.Dumollard R, et al. Sperm-triggered [Ca2+] oscillations and Ca2+ homeostasis in the mouse egg have an absolute requirement for mitochondrial ATP production. Development. 2004;131:3057–3067. doi: 10.1242/dev.01181. [DOI] [PubMed] [Google Scholar]
- 44.Banrezes B, et al. Adult body weight is programmed by a redox-regulated and energy-dependent process during the pronuclear stage in mouse. PLoS One. 2011;6:e29388. doi: 10.1371/journal.pone.0029388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chitwood JL, Burruel VR, Halstead MM, Meyers SA, Ross PJ. Transcriptome profiling of individual rhesus macaque oocytes and preimplantation embryos. Biol Reprod. 2017;97:353–364. doi: 10.1093/biolre/iox114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reyes JM, et al. Differing molecular response of young and advanced maternal age human oocytes to IVM. Hum Reprod. 2017;32:2199–2208. doi: 10.1093/humrep/dex284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yan L, et al. Single-cell RNA-Seq profiling of human preimplantation embryos and embryonic stem cells. Nat Struct Mol Biol. 2013;20:1131–1139. doi: 10.1038/nsmb.2660. [DOI] [PubMed] [Google Scholar]
- 48.Yanez LZ, Han J, Behr BB, Reijo Pera RA, Camarillo DB. Human oocyte developmental potential is predicted by mechanical properties within hours after fertilization. Nat Commun. 2016;7:10809. doi: 10.1038/ncomms10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lek M, et al. Exome Aggregation Consortium Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chronopoulou E, Harper JC. IVF culture media: Past, present and future. Hum Reprod Update. 2015;21:39–55. doi: 10.1093/humupd/dmu040. [DOI] [PubMed] [Google Scholar]
- 51.Morbeck DE, Baumann NA, Oglesbee D. Composition of single-step media used for human embryo culture. Fertil Steril. 2017;107:1055–1060.e1. doi: 10.1016/j.fertnstert.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 52.Morbeck DE, et al. Composition of commercial media used for human embryo culture. Fertil Steril. 2014;102:759–766.e9. doi: 10.1016/j.fertnstert.2014.05.043. [DOI] [PubMed] [Google Scholar]
- 53.Herrick JR, et al. The beneficial effects of reduced magnesium during the oocyte-to-embryo transition are conserved in mice, domestic cats and humans. Reprod Fertil Dev. 2015;27:323–331. doi: 10.1071/RD13268. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.