Abstract
Background
ART adherence is critical for successful HIV treatment outcomes. Once-daily dosing could improve adherence. Plasma concentrations of once- vs twice-daily abacavir+lamivudine are bioequivalent in children, but no randomised trial has compared virological outcomes.
Methods
Children taking abacavir+lamivudine-containing first-line regimens twice-daily for >36 weeks in the ARROW trial (NCT02028676,ISRCTN24791884) were randomised to continue twice-daily versus move to once-daily abacavir+lamivudine (open-label). Co-primary outcomes were viral load (VL) suppression at week-48 (12% non-inferiority margin, measured retrospectively) and lamivudine or abacavir-related grade 3/4 adverse events (AEs).
Results
669 children (median 5 years, range 1-16) were randomised to twice-daily (n=333) vs once-daily (n=336) after median 1.8 years on twice-daily abacavir+lamivudine-containing first-line ART. Children were followed for median 114 weeks. At week-48, 242/331 (73%) twice-daily vs 236/330 (72%) once-daily had VL<80c/ml (difference -1.6% [95% CI -8.4%,+5.2%] p=0.65); 79% twice-daily vs 78% once-daily had VL<400c/ml (p=0.76) (week-96 results similar). One grade 3/4 AE was judged uncertainly related to abacavir+lamivudine (hepatitis; once-daily). At week-48, 9% twice-daily vs 10% once-daily reported missing one or more ART pills in the last 4 weeks (p=0.74), and 8% vs 8% at week-96 (p=0.90). Carers strongly preferred once-daily dosing. There was no difference between randomised groups in post-baseline drug-resistance mutations or drug-susceptibility; WHO 3/4 events; ART-modifying, grade 3/4 or serious AEs; CD4% or weight/height-for-age (all p>0.15).
Conclusions
Once-daily abacavir+lamivudine was non-inferior to twice-daily in VL suppression, with similar resistance, adherence, clinical, immunological and safety outcomes. Abacavir+lamivudine provides the first once-daily nucleoside backbone across childhood that can be used to simplify ART.
Introduction
In 2014, 740,000 HIV-infected children were receiving antiretroviral therapy (ART), the vast majority in Sub-Saharan Africa[1]. However, this was only 32% of those living with HIV and approximately 220,000 children became newly HIV-infected in 2014. HIV-infected children need life-long treatment, which requires optimal adherence[2]. Once-daily medication is one strategy for promoting this[3]. The World Health Organisation (WHO) preferred first-line ART is two nucleoside-reverse-transcriptase-inhibitors (NRTIs), abacavir and lamivudine, and a third drug, either a non-nucleoside-reverse-transcriptase-inhibitor (NNRTI) in those >3 years or a boosted-protease-inhibitor (bPI) in those <3 years[4]. The once-daily lamivudine+abacavir fixed-dose-combination was licensed in adults and adolescents >12 years in 2005 based on three large randomised efficacy trials[5–7], but regulators did not licence paediatric once-daily dosing. This was despite pharmacokinetic bioequivalence among African children aged 3-12 years[8], with similar results in European children aged 3-<36months[9] and 2-13 years[10], and despite carers reporting high acceptability and strong preference for once-daily dosing[11]. Lamivudine and abacavir therefore largely continued to be used twice-daily for children <12 years.
Non-randomised studies among children in Europe[10, 12, 13], and randomised and non-randomised studies in adults[3, 6, 14–17], have reported favourable clinical, immunological, virological, safety, adherence and acceptability outcomes over 24–48 weeks for regimens containing once-daily abacavir and/or lamivudine[3, 6, 9, 10, 13–15, 17]. We therefore randomised Ugandan/Zimbabwean children enrolled in the ARROW trial[18] to once versus twice-daily lamivudine+abacavir and compared treatment outcomes and adherence over 96 weeks.
Methods
This was an open randomized parallel-group trial within the ARROW trial (NCT02028676,ISCRTN24791884)[18] conducted at three centres in Uganda and one in Zimbabwe. The main trial recruited previously untreated HIV-infected children/adolescents who were randomised to initiate ART using standard lamivudine+abacavir+NNRTI or a 36-week induction-maintenance approach (Supplementary Figure 1). Children were also randomised factorially to routine laboratory plus clinical monitoring (LCM) or clinically driven monitoring (CDM). This trial co-enrolled ARROW children on twice-daily lamivudine+abacavir-containing first-line ART for >36 weeks and expected to stay on this regimen for at least 12 weeks. Participants were randomized 1:1 to continue twice-daily versus move to once-daily lamivudine+abacavir. The hypothesis was that once-daily dosing would result in similar outcomes to twice-daily in children on long-term ART (non-inferiority). Caregivers and older children (≥18 years) gave written consent; those 7-18 years gave assent (depending on knowledge of HIV status). The trial was approved by Research Ethics Committees in Uganda, Zimbabwe and the UK.
Abacavir and lamivudine were dosed following WHO guidelines, and taken as single drugs (tablets or solutions) or co-formulated as Kivexa (tablets), depending on other drugs in the regimen (efavirenz, nevirapine or zidovudine following original ARROW factorial randomisation to lamivudine+abacavir+NNRTI continuously (Arm-A); induction-maintenance with 4-drug lamivudine+abacavir+NNRTI+zidovudine for 36 weeks, followed by lamivudine+abacavir+NNRTI (Arm-B) or lamivudine+abacavir+zidovudine (Arm-C; 3NRTI)). The NNRTI (nevirapine/efavirenz) was chosen by clinicians according to local availability (varying by country) and age. Throughout “abacavir+lamivudine” denotes either combined single tablets or the fixed-dose-combination.
Randomization was stratified by centre, and the two factorial randomizations within the main ARROW trial. The computer-generated sequentially numbered randomization list (variable block sizes) was pre-prepared by the Trial Statistician and incorporated securely into the trial database at each centre, concealed from local staff. Allocation was made after eligibility was confirmed by local centre staff who then performed the randomisation.
Children were reviewed at nurse visits every 6 weeks using a standard symptom checklist, with ART and cotrimoxazole adherence assessed by self-reported questions about when doses were last missed. They saw a doctor and had full blood count, CD4, liver and renal function tests (bilirubin, urea, creatinine, AST, ALT) at randomization and then every 12 weeks. All laboratory test results were routinely returned for children randomized to LCM, but no CD4s were returned for children randomised to CDM, and haematology/biochemistry were returned only if needed for clinical management. Toxicity substitutions and/or switch to bPI-containing second-line regimens were at the treating physician’s discretion, following WHO guidelines. Viral loads (VL) were not measured in real-time and not used for management. Participants continued follow-up until ARROW trial closure (16 March 2012).
VL was assayed retrospectively on stored plasma samples at 0, 48 and 96 weeks after randomisation using the Abbott m2000rt with 2:1 dilution as many samples had small volumes, leading to a lower limit of detection of 80 c/ml (rather than 40 c/ml). Samples >1000 copies/ml were genotyped using in house primers (Supplementary Table 1) at the Joint Clinical Research Centre using an automated ABI 3730xl sequencer; where original samples had been exhausted or failed repeatedly, replacements up to 24 weeks before baseline, or within ±18 weeks of weeks 48/96 were assayed. VL assays and genotyping was performed blinded to randomization. Subtype was predicted using REGA v3.0, drug-resistance mutations defined using IAS-USA 2013[19], and drug susceptibility predicted using Stanford v7, using the full sequence data[20].
Carers of children randomised to once-daily completed an acceptability questionnaire about giving twice-daily medication and their views about changing to once-daily administration immediately after randomisation. An equivalent questionnaire asking about actual experiences of once-daily administration was completed 12 and 48 weeks later (see Table 2 for specific questions).
Table 2. Acceptability of move from twice-daily to once-daily lamivudine+abacavir.
| Week 0 (randomisation) | Week 12 | Week 48 | P 0vs12* | P 0vs48* | |
|---|---|---|---|---|---|
| Acceptability form completed (N=336) | 312 (93%) | 274 (82%) | 277 (82%) | - | - |
| Number of people giving medicines to the child, median (IQR) | 2 (1-2) | 2 (1-2) | 2 (1-2) | - | - |
| Timing of twice-daily (week 0) or once-daily (week 12/48) medication: sometimes or always a problem | 50 (16%) | 11 (4%) | 8 (3%) | <0.001 | <0.001 |
| Number of twice-daily (week 0) or once-daily (week 12/48) medicines: sometimes or always a problem | 14 (5%) | 5 (2%) | 5 (2%) | 0.29 | 0.03 |
| Taste of twice-daily (week 0) or once-daily (week 12/48) medication: sometimes or always a problem | 25 (8%) | 7 (3%) | 9 (3%) | 0.003 | 0.002 |
| How switching to once-daily will be (week 0)/was (week 12/48) for the carer | 0.004 | 0.64 | |||
| A lot easier | 187 (60%) | 172 (63%) | 147 (53%) | ||
| A little easier | 55 (18%) | 55 (20%) | 77 (28%) | ||
| No difference | 65 (21%) | 44 (16%) | 49 (18%) | ||
| A little more difficult | 4 (1%) | 2 (1%) | 3 (1%) | ||
| How switching to once-daily will be (week 0)/was (week 12/48) for the child | 0.16 | 0.44 | |||
| A lot easier | 139 (45%) | 99 (37%) | 92 (34%) | ||
| A little easier | 61 (20%) | 72 (27%) | 83 (31%) | ||
| No difference | 104 (34%) | 94 (35%) | 94 (35%) | ||
| A little more difficult | 4 (1%) | 5 (2%) | 3 (1%) | ||
| A lot more difficult | 2 (1%) | 1 (0.4%) | 0 | ||
| Do you think it will be (week 0)/is (week 12/48) easier to give all medicines once daily | 0.22 | 0.34 | |||
| No | 4 (1%) | 6 (2%) | 3 (1%) | ||
| Yes | 285 (93%) | 253 (93%) | 250 (91%) | ||
| Not sure | 19 (6%) | 13 (5%) | 23 (8%) | ||
| Overall, which do you think (week 0)/do you (week 12/48) prefer? | 0.74 | 0.13 | |||
| Once-daily | 305 (98%) | 268 (98%) | 265 (96%) | ||
| Twice-daily | 6 (2%) | 6 (2%) | 11 (4%) | ||
| What time will you (week 0)/do you (week 12/48) give once-daily medicines | 0.46 | 0.72 | |||
| Morning | 148 (48%) | 122 (45%) | 137 (50%) | ||
| Evening | 163 (52%) | 151 (55%) | 139 (50%) | ||
| Ever reported going back to giving medicines twice-daily? | - | 6 (2%) | 21 (8%) | - | - |
signrank test comparing 257 carers with questionnaires at weeks 0 and 12, and 0 and 48.
Note: at week0 (randomisation) carers were asked about their views of giving medicines twice daily (grey shading) and how they thought things would change on once-daily. 12 and 48 weeks after moving to once-daily they were asked how they found the move. Questions about timing, number and taste of medications had three options Often, Sometimes or Never. A small number of responses to specific questions were missing, n (%) are of those with available data.
Co-primary endpoints were VL at week-48 (pre-specified 12% non-inferiority margin for suppression regardless of threshold) and lamivudine or abacavir-related grade 3/4 adverse events (AEs). Secondary endpoints included: VL at week-96; resistance; change in absolute and percentage CD4; WHO stage 3/4 event/death; WHO stage 4 event/death; mortality; hospitalisations; weight- and height-for-age; grade 3/4 AEs; serious adverse events (SAEs); ART-modifying AEs; switch to second-line ART; and ART adherence (self-reporting any missed ART doses in the last 4 weeks). Clinical events and SAEs were reviewed by an Endpoint Review Committee with independent chair and members, blinded to randomization.
631 children provided 90% power to establish non-inferiority of once-daily vs twice-daily lamivudine+abacavir, defined as the upper 95% confidence limit for the difference in suppression (once- minus twice-daily) of no more than 12% (recommended by Food and Drug Administration), assuming 70% suppression on twice-daily and 15% missing VLs due to missing samples, missed visits, death, or loss to follow-up. Interim data were reviewed annually by an independent Data Monitoring Committee.
Statistical Analysis
All comparisons are intention-to-treat; no per-protocol analyses were pre-specified and none were performed given the high compliance with randomised allocation. p-values presented test the null hypothesis of no difference (superiority) between randomized groups; absolute unadjusted differences in percentages (with exact 95% CI) were estimated using Poisson regression to address the original non-inferiority hypothesis. Subgroup analyses of VL <80 c/ml and <400 c/ml were conducted by key baseline characteristics including sex, age, centre, CD4-for-age, weight-for-age, year of randomisation, CD4 monitoring randomisation, ART regimen and formulation using Poisson regression (all pre-specified in the Statistical Analysis Plan except for formulation which was exploratory). Secondary outcomes were compared between randomized groups using log-rank tests for time-to-event outcomes and generalised estimating equations with independent working correlation for global tests of repeated measures (normal distribution for CD4 and weight/height-for-age; logistic distribution for missing doses in the last 4 weeks (adherence)). Self-reported responses to acceptability questions were compared across timepoints using matched-pairs sign-rank tests.
Results
Between 19 August 2009 and 29 June 2010, 732 eligible children were approached; 669 (91%) consented and were randomised to continue twice-daily (n=333) or move to once-daily (n=336) lamivudine+abacavir (Supplementary Figure 2). Main reasons for not consenting were reluctance to change from current twice-daily ART (n=21) and worries about forgetting doses on once-daily regimens (n=20). Median age was 5.5 years (range 1.8–16.9) and 52% were girls; participants had spent a median 1.8 years (range 0.9–3.0) on twice-daily abacavir+lamivudine containing first-line ART, which they were receiving with nevirapine (48%), efavirenz (18%) or zidovudine (34%). Baseline characteristics were similar in twice-daily and once-daily groups (Table 1).
Table 1. Characteristics at randomisation to once-daily vs twice-daily lamivudine+abacavir.
| Twice daily (n=333) |
Once daily (n=336) |
Total (n=669) |
|
|---|---|---|---|
| Centre Entebbe, Uganda | 65 (20%) | 65 (18%) | 130 (19%) |
| Joint Clinical Research Centre, Uganda | 74 (22%) | 77 (23%) | 151 (23%) |
| Baylor-Mulago, Uganda | 87 (26%) | 87 (24%) | 174 (26%) |
| Harare, Zimbabwe | 107 (32%) | 107 (32%) | 214 (32%) |
| Girls | 172 (52%) | 173 (51%) | 345 (52%) |
| Age (years) | 5.1 (3.6-8.3) | 5.9 (2.8-8.6) | 5.5 (3.7-8.5) |
| Pre-ART CD4% | 12.5 (8.5-18.0) | 13.0 (8.5-18.9) | 13.0 (8.5-18.0) |
| Pre-ART CD4 if >5 years at ART initiation (cells/mm3) | 263 (124-404) | 301 (136-416) | 278 (136-410) |
| Years since ART initiation | 1.8 (1.4-2.3) | 1.8 (1.4-2.1) | 1.8 (1.4-2.1) |
| Current CD4% | 33 (27-39) | 33 (28-39) | 33 (28-39) |
| Current CD4 if >5 years at ART initiation (cells/mm3) | 836 (558-1131) | 760 (543-1136) | 812 (557-1134) |
| Current weight-for-age Z-score | -1.3 (-2.0,-0.6) | -1.4 (-2.0,-0.7) | -1.4 (-2.0,-0.7) |
| Current WHO stage (worst ever): | |||
| 1/2 | 96 (29%) | 73 (22%) | 169 (25%) |
| 3 | 185 (56%) | 209 (62%) | 394 (59%) |
| 4 | 52 (16%) | 54 (16%) | 106 (16%) |
| Current VL *: | |||
| <80 c/ml | 250 (76%) | 237 (71%) | 487 (73%) |
| <400 c/ml | 272 (82%) | 266 (79%) | 538 (81%) |
| <1000 c/ml | 281 (85%) | 271 (81%) | 552 (83%) |
| ART regimen | |||
| Lamivudine+abacavir+nevirapine† | 171 (51%) | 148 (44%) | 319 (48%) |
| Lamivudine+abacavir+efavirenz | 49 (15%) | 73 (22%) | 122 (18%) |
| Lamivudine+abacavir+zidovudine† ** | 112 (34%) | 115 (34%) | 227 (34%) |
| Lamivudine+abacavir+stavudine† ** | 1 (0.3%) | 0 | 1 (0.1%) |
| ART formulation | |||
| Any syrup | 26 (8%) | 30 (9%) | 56 (8%) |
| All tablets | 307 (92%) | 306 (91%) | 613 (92%) |
| CD4 monitoring (LCM) | 159 (48%) | 163 (49%) | 322 (48%) |
| No CD4 monitoring (CDM) | 174 (52%) | 173 (51%) | 347 (52%) |
Tested retrospectively; missing 2 twice-daily, 1 once-daily (repeated assay failure)
3NRTI maintenance following 4-drug induction as part of main ARROW factorial randomisation.
nevirapine, zidovudine, and stavudine continued to be given twice-daily.
Note: values are n (%)or median (IQR).
Median follow-up was 114 weeks (IQR 106-125; range 48-134). 7 children were lost (last seen before March 2012; 3 twice-daily, 4 once-daily), of whom 4 formally withdrew consent (Supplementary Figure 2). A further 5 children died, all >48 weeks after randomisation (none drug-related; 4 twice-daily (presumptive pulmonary tuberculosis, pneumonia, cor pulmonale, cause unknown), 1 once-daily (lung-related, specific condition unknown)). After randomisation, 98% vs 97% child-time was spent on twice-daily vs once-daily abacavir+lamivudine in the two groups respectively. 29(4%) children (11 twice-daily, 18 once-daily) ever moved off their allocated dosing strategy, of whom 13 (6 twice-daily, 7 once-daily) switched to bPI-containing second-line ART. Five carers in the once-daily group requested a return to twice-daily, and two carers in the twice-daily group requested once-daily (Supplementary Figure 2).
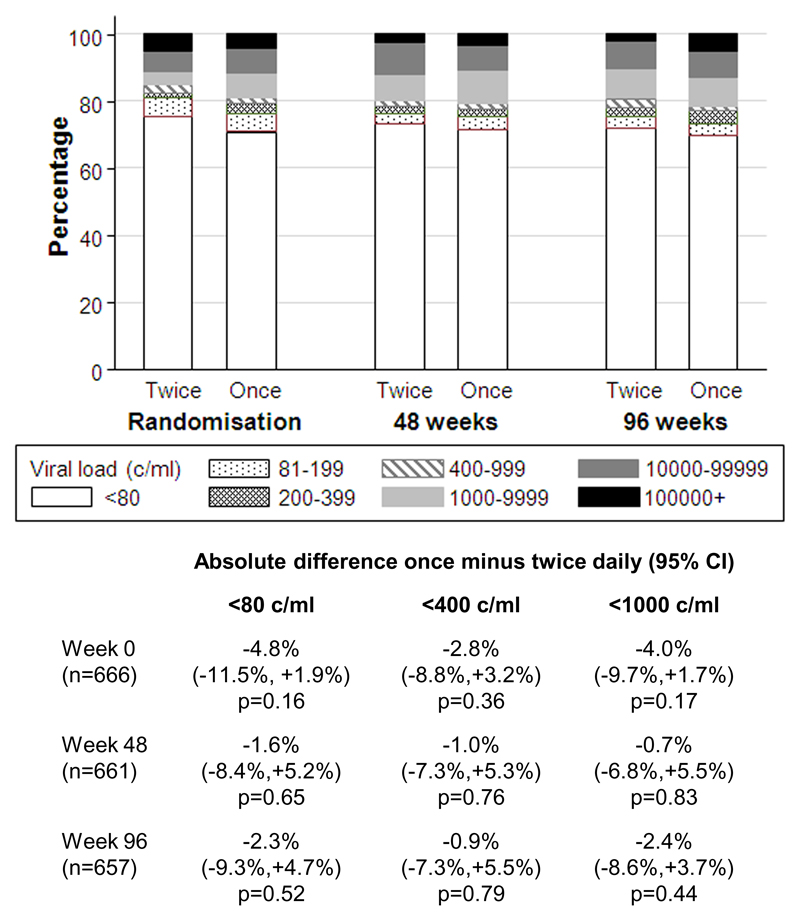
Not all children were virologically suppressed at randomisation (Table 1) because VLs were only tested retrospectively at trial closure. By chance, at randomisation (week 0) there was a small non-significant excess of 3-5% in the percentages >80, >400 and >1000 c/ml in the once-daily group (Figure 1): this had attenuated by week 48 and 96 when VL suppression at all thresholds was very similar in twice-daily and once-daily groups and within ±10% (and thus well within the 12% non-inferiority margin). In particular, 242/331 (73%) twice-daily vs 236/330 (72%) once-daily participants were <80 c/ml at 48 weeks (difference -1.6% [95% CI -8.4%,+5.2%] p=0.65), and 234/326 (72%) vs 230/331 (69%) respectively at 96 weeks (difference -2.3% [-9.3%,+4.7%] p=0.52).
Figure 1. Virologic suppression on twice-daily vs once-daily lamivudine+abacavir.
Note: excluding missing VLs at week-0 (2 twice-daily, 1 once-daily), week-48 (2 twice-daily, 6 once-daily), and week-96 (7 twice-daily, 5 once-daily) due to assay failure or died/lost before week 96.
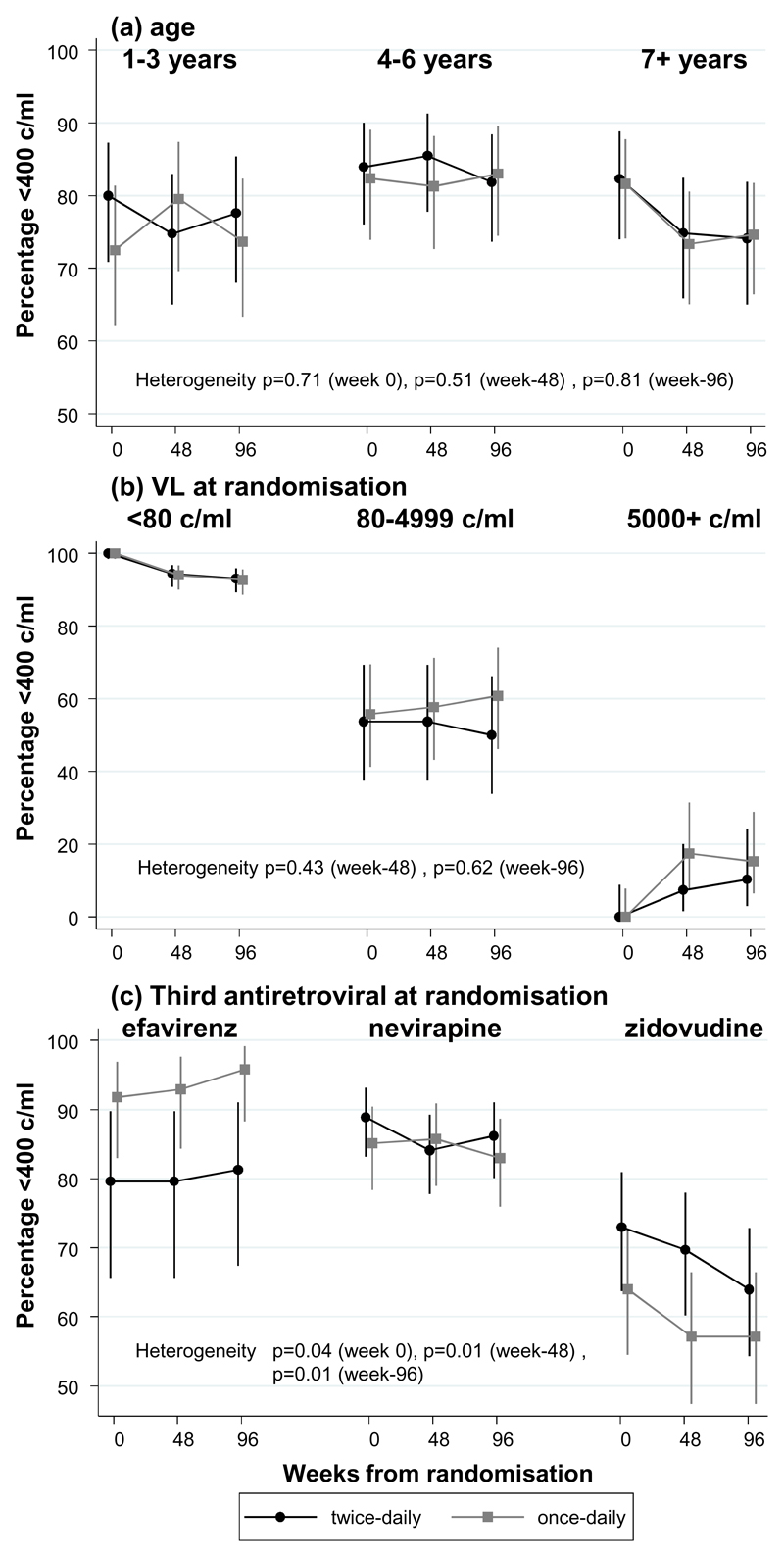
There was no evidence of heterogeneity in the difference between once-daily vs twice-daily administration in suppression <80 c/ml or <400 c/ml at 48 or 96 weeks according to CD4 monitoring (LCM/CDM), sex, age (Figure 2(a)), VL at randomisation (Figure 2(b)) or formulation (p>0.12). By chance, at randomisation more children on lamivudine+abacavir+efavirenz randomised to once-daily, and fewer children on 3NRTI randomised to once-daily, were suppressed (<400 c/ml heterogeneity p=0.04). These differences persisted through 48 and 96 weeks (both heterogeneity p=0.01; Figure 2(c); similar results for <80 c/ml). Overall 86% of children on the current WHO-preferred first-line regimen of once-daily/twice-daily lamivudine+abacavir+NNRTI were <400 c/ml at 48 weeks.
Figure 2. <400 c/ml on twice-daily vs once-daily lamivudine+ abacavir by (a) age (b) baseline VL and (c) third antiretroviral.
Note: Each panel shows VL suppression <400 c/ml in children randomised to twice-daily (black circles) vs once-daily (gray squares), according to different subgroups of age (a), VL at randomisation (b) and third drug (c). In each subgroup, VL suppression at randomisation (week 0), and 48 and 96 weeks later are connected by lines. Similar responses to once- and twice-daily lamivudine+abacavir are reflected in parallel lines. Heterogeneity (interaction) in response to once- and twice-daily lamivudine+abacavir is reflected by different relative positions of black and gray lines in the different subgroups. Overall effect of the subgroup factor is reflected by different average suppression levels in the different subgroups
There was no evidence that the relative effect of once-daily vs twice-daily administration on VL suppression varied by age (Figure 2(a), p>0.5), but univariably suppression appeared lower in younger and older children in both groups. Multivariable logistic models for VL <400 c/ml at 48 weeks including all factors considered in subgroup analyses and centre found that this was partly due to confounding, with a trend towards poorer VL suppression with syrups than tablets (adjusted odds ratio (OR) (tablets:syrups)=2.55 [95% CI 0.89-6.39] p=0.08). Adjusting for formulation, suppression was then significantly poorer only in older children (OR per year older=0.79 [0.70,0.88] p<0.0001), as well as those not receiving efavirenz (OR(3NRTI:efavirenz)=0.20 [0.07,0.29] p=0.001, OR(nevirapine:efavirenz)=0.29 [0.10,0.81] p=0.02), and those with higher baseline VL (p<0.001).
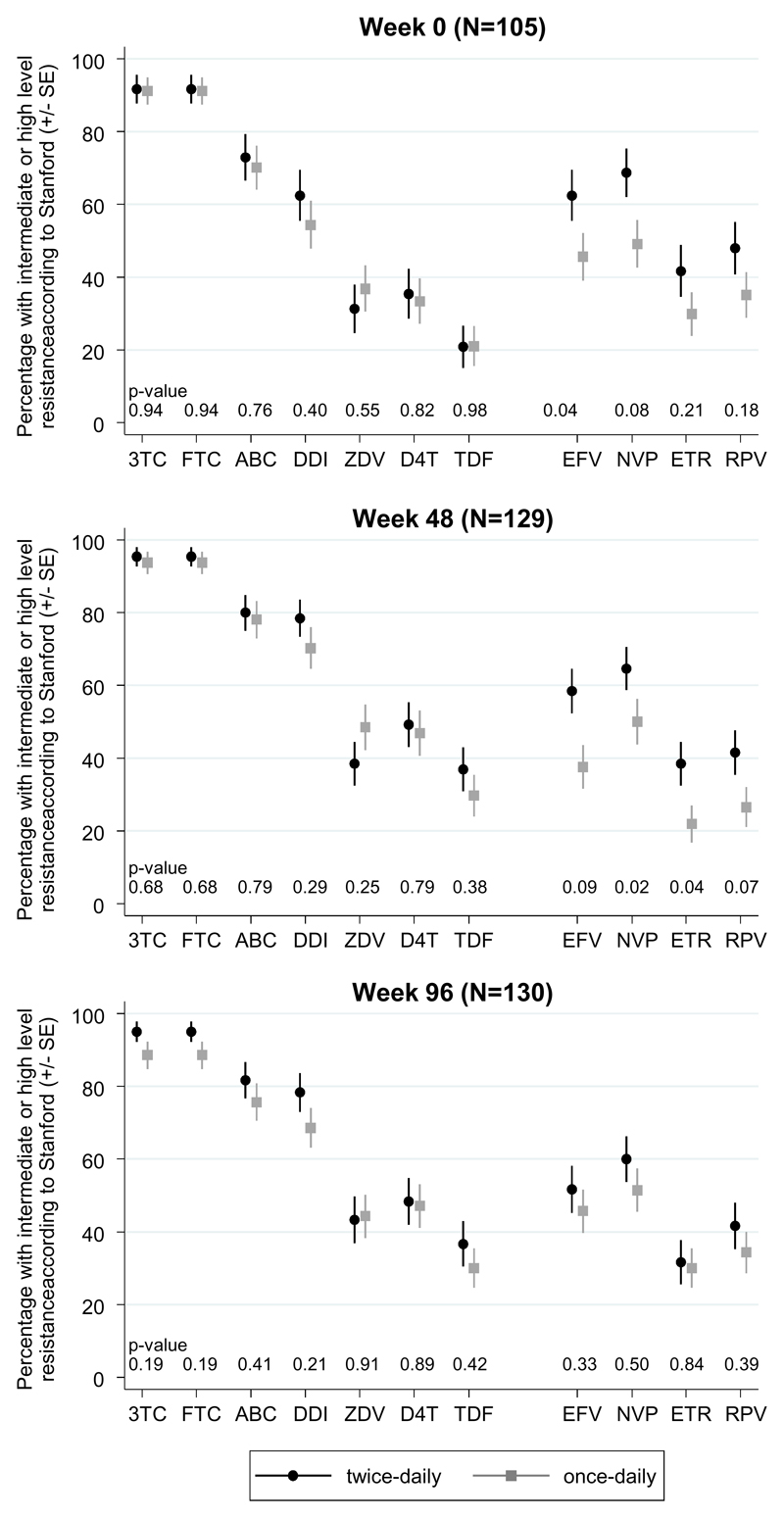
Of 114, 134 and 135 children with VL >1000 c/ml at weeks 0, 48 and 96 respectively, genotypes were obtained in 105 (92%), 129 (96%) and 130 (96%) (p>0.3 comparing twice- vs once-daily). Overall 179 (49%) genotypes were subtype-A, 90 (25%) subtype-C (including all Zimbabwean children) and 72 (20%) subtype-D. Only 6 (6%), 4 (3%) and 4 (3%) children had at most low-level resistance to all NRTIs and NNRTIs at weeks 0, 48 and 96 respectively, suggesting non-adherence. There was no evidence of differences between twice-daily and once-daily groups in intermediate/high-level NRTI resistance, or specific NRTI mutations, at any timepoint (p>0.15, Figure 3, Supplementary Figure 3). Modest differences between randomised groups in intermediate/high-level NNRTI resistance at week 48, but not week 96, appeared to persist from baseline (Figure 3). In the subgroup of children receiving the current WHO preferred regimen of abacavir+lamivudine+NNRTIs (twice- or once-daily), in whom second-line NRTI options would be zidovudine or tenofovir, intermediate/high-level resistance 0/48/96 weeks after randomisation was 15%/16%/8% for tenofovir and 0%/4%/2% for zidovudine, compared to 75%/84%/79% for abacavir (N≥48). In the subgroup of children receiving 3NRTIs, intermediate/high-level resistance was similar to those on abacavir+lamivudine+NNRTIs for abacavir (71%/77%/79% 0/48/96 weeks after randomisation respectively, p>0.3 vs abacavir+lamivudine+NNRTIs), greater for tenofovir (28%/50%/54% respectively; p<0.001 at weeks 48 and 96) and greater for zidovudine (64%/74%/73% respectively, p<0.001) (N≥58).
Figure 3. Predicted drug susceptibility.
Note: Approximately one-half of children with genotypes were receiving triple NRTI (no NNRTI). 3TC=lamivudine, FTC=emtricitabine, ABC=abacavir, DDI=didanosine, ZDV=zidovudine, D4T=stavudine, TDF=tenofovir, EFV=efavirenz, NVP=nevirapine, ETR=etravirine, RPV=rilpivirine.
As expected, M184V/I mutations were common in both groups (Supplementary Figure 3). In the subgroup of children receiving abacavir+lamivudine+NNRTIs (twice- or once-daily) 74V and 115F mutations were observed in 56%/64%/70% and 56%/62%/68% respectively at weeks 0/48/96; but these were much less frequent in children receiving 3NRTIs (3%/3%/3% and 16%/20%/19% respectively; all p<0.001 versus abacavir+lamivudine+NNRTIs). As expected, thymidine-analogue mutations (TAMs) were rarely seen in children on abacavir+lamivudine+NNRTIs. Children had a median (IQR) 3 (2-3), 3 (2-4) and 3 (2-4) NRTI mutations at weeks 0, 48, and 96 respectively suggesting slow accumulation of additional mutations (p>0.3 comparing twice- vs once-daily): only 6 (6%), 8 (6%) and 6 (5%) children had K65R mutations respectively (6/7/3 on abacavir+lamivudine+NNRTI, 0/1/3 on 3NRTI). Q151M was seen in one child on once-daily 3NRTIs at both week 48 and 96 (both also with K65R). There was no evidence of difference (p>0.05) between twice-daily vs once-daily in the change in NRTI Stanford score in 87 children with paired genotypes at weeks 0 and 48 (p>0.1; >62% had no change in score), in 80 children with paired genotypes at weeks 0 and 96 (p>0.1; >56% had no change in score), or in 101 children with paired genotypes at weeks 48 and 96 (p>0.1;>69% had no change in score) with the exception of abacavir, where if anything, Stanford score increased more in the twice-daily group (p=0.045; 87% had no change in score).
Self-reported adherence was similar in the two groups: at 48 weeks, 29/330 (9%) twice-daily vs 32/336 (10%) once-daily reported missing one or more ART pills in the last 4 weeks (p=0.74), and 25/309 (8%) vs 26/311 (8%) respectively at 96 weeks (p=0.90) (global p=0.93 across 6-120 weeks, Supplementary Figure 4). There was no evidence that differences in self-reported adherence between twice-daily and once-daily randomised groups varied across the different third antiretrovirals (heterogeneity p=0.38).
Acceptability questionnaires were completed by 312 (93%), 274 (82%) and 277 (82%) carers 0, 12 and 48 weeks after randomisation to once-daily lamivudine+abacavir (Table 2). At randomisation, 16% of carers reported that timing of twice-daily medication had sometimes/always been a problem: this reduced significantly to 4% after 12 weeks on once-daily (signrank p<0.001). Problems with taste on twice-daily dosing also reduced significantly following move to once daily (8% vs 3% respectively, signrank p=0.003). 78% of carers anticipated that moving to once-daily would make things a lot/little easier for them, expectations that were largely realised (83% and 81% at weeks 12 and 48). Fewer (65%) thought moving to once-daily would make things a lot/little easier for the child, but again these expectations were realised. Many who felt moving to once-daily lamivudine+abacavir had made no difference commented this was because other drugs were still taken twice daily: however, others commented that even giving some drugs once-daily improved administration because the second dose was smaller. Overall carers expressed strong preferences for once-daily administration (98%, 98% and 96% preferring this at weeks 0, 12 and 48 respectively). In contrast to high-income countries, approximately 50% of carers gave once-daily medication in the morning. Comments related to the flexibility of once-daily dosing fitting around work schedules and different places where the child stayed (median two individuals gave drugs to the child); and also to administering medication taking less total time out of the day. Several carers of children on once-daily medication noted that both they and the child now took their drugs together helping them to remember.
There was no difference between groups in all grade 3/4 or serious AEs, WHO 3/4 events, weight-for-age, height-for-age, CD4% or CD4 in those >5 years (all p>0.15). 54 (16%) twice-daily vs 57 (17%) once-daily had one or more grade 3/4 AEs (p=0.82): most commonly asymptomatic neutropenia (n=38) and malaria (n=32). 37 (11%) twice-daily vs 30 (9%) once-daily had one or more SAEs (p=0.31). 14 (4%) twice-daily vs 18 (5%) once-daily had one or more grade 4 AEs (p=0.48). 7 (2%) twice-daily vs 3 (1%) once-daily had new WHO 4 stage events or died (p=0.20), and 12 (4%) vs 9 (3%) respectively had new WHO 3 or 4 stage events or died (p=0.51). One grade 3/4 adverse event (hepatitis) was judged uncertain whether related to abacavir+lamivudine (once-daily group) (no events judged definitely/probably related). There were no AEs directly leading to abacavir or lamivudine modification. There were no reported hypersensitivity reactions. Increases in CD4% in twice-daily vs once-daily were +1.3% vs +0.9% at 48 weeks (p=0.39), and +2.5% vs +1.6% at 96 weeks (p=0.12).
Discussion
Among a large group of HIV-infected children in the ARROW trial[18], over a median 2.2 years of follow-up, we observed comparable viral suppression between children randomised to once- daily versus twice-daily dosing of lamivudine+abacavir, with no difference in all grade 3/4 or serious adverse events. Our findings strengthen the evidence from previous smaller and shorter pharmacokinetic studies among children in Europe[9, 10], and formed the basis for recent US and European licencing of lamivudine and abacavir once-daily for children >3 months of age. This is particularly important now that lamivudine+abacavir is the preferred NRTI backbone in the WHO 2013 and 2015 consolidated ART guidelines[4], with several countries in Sub-Saharan Africa adopting the recommendation, enabling the first once-daily 2NRTI+NNRTI regimen for children.
Whilst we did not observe differences in adherence between once- and twice-daily dosing in this randomised trial, either overall or in subgroups defined by the ART regimen (Supplementary Figure 4), the majority of children were taking lamivudine+abacavir with nevirapine or zidovudine, meaning they were still taking ART twice daily. Power to detect improvements in adherence with once-daily ART was low within the smallest subgroup taking once-daily lamivudine+abacavir+efavirenz. Simplifying ART regimens with completely once-daily dosing would be expected to lead to improved adherence with potentially favourable treatment outcomes[3], that are particularly important in children who need to take ART lifelong. Acceptability questionnaires demonstrated strong preferences from carers for once-daily dosing as a mechanism to improve adherence. Once-daily single tablet regimens (STR) which minimise the number of pills and daily doses have been associated with improved adherence and better quality of life in adults[21]. The most widely used STR among adults is a combination of tenofovir, emtricitabine or lamivudine, and efavirenz; although tenofovir is licensed in children over 2 years[22], it is not recommended as the preferred agent in children under 10 years by WHO. However, abacavir has been safely used among African children, with very low rates of hypersensitivity (0.3%)[18, 23]. Thus an abacavir-based paediatric STR may be more favourable in Sub-Saharan Africa and other low/middle-income settings, potentially combined with the NNRTIs efavirenz or rilpivirine; integrase inhibitors (cobicistat-boosted elvitegravir or dolutegravir); and protease inhibitors (cobicistat-boosted darunavir)[24].
The major concern about once-daily dosing, which had not been demonstrated in adult trials from high-income countries[5–7], was that it could be more “fragile”; namely, if a child missed one dose when taking lamivudine+abacavir twice-daily they still got some drug, whereas if they missed one dose when taking lamivudine+abacavir once-daily they missed drugs for the whole day, potentially leading to lower VL suppression and/or greater accumulation of drug-resistance mutations. As well as not observing any difference in VL suppression between twice-daily and once-daily dosing, we also observed no differences in drug-resistance mutations, predicted drug susceptibility or accumulation of resistance among 364 sequences. Whilst the fact that most children were taking their third antiretroviral twice-daily could theoretically have protected them from viral breakthrough, once replicating virus was present any increased fragility of once-daily lamivudine+abacavir should have been apparent. The fact that this was not observed is therefore re-assuring, particularly given the limited virological monitoring available in many low-income countries.
The main trial limitations relate to its opportunistic incorporation within an ongoing trial, although this also provided substantial efficiency gains. The trial was open-label, although the primary endpoint (VL suppression) was assayed retrospectively blinded to randomisation. Children received different third antiretrovirals with lamivudine+abacavir; although this increased generalisability, different third drugs (zidovudine (in a triple NRTI regimen) vs NNRTI) exerted very different selective pressures, necessitating subgroup analysis of resistance with smaller numbers than overall. Some children also remained on a twice-daily regimen because of other drugs, meaning that potential improvements in VL suppression with completely once-daily regimens could be masked. Also the same formulation could not be used for all age groups. However, this allowed us to identify that the younger children taking syrups were less likely to have virological suppression, similarly to the trial as a whole[23], potentially explained by increased complexity of dosing with syrups, lower bioavailability of lamivudine syrup[25, 26], and a general preference for tablets by the younger children and their caregivers[27]. Randomisation did not occur at ART initiation, but after children had received a median 1.8 years first-line ART; recommending once-daily administration from ART initiation is therefore an extrapolation, although there was no evidence that children who happened to not be virologically suppressed at randomisation did worse on once- vs twice daily.
In summary, once-daily dosing of the WHO-preferred NRTI backbone lamivudine+abacavir was non-inferior to twice-daily dosing in terms of virological suppression, resistance and adherence, as well as clinical, immunological, and safety outcomes. It was strongly preferred by almost all carers. Lamivudine+abacavir is therefore the first dual NRTI regimen which can be used once-daily across the entire age-range to improve acceptability and long-term adherence among HIV-infected children, and also provides the potential for several different paediatric single-tablet once-daily regimens in future.
Supplementary Material
Acknowledgements
We thank the children, carers and staff from all the centers participating in the ARROW trial.
Clinical and Trial Centres
MRC/UVRI Uganda Research Unit on AIDS, Entebbe, Uganda: P Munderi, P Nahirya-Ntege, R Katuramu, J Lutaakome,, F Nankya, G Nabulime, I Ssekamatte, J Kyarimpa, A Ruberantwari, R Sebukyu, G Tushabe, D Wangi, L Matama M Aber, M Musinguzi, D Nakitto-Kesi, SNassimbwa, M Nabankema. Joint Clinical Research Centre, Kampala, Uganda: P Mugyenyi, V Musiime, R Keishanyu, VD Afayo, J Bwomezi, J Byaruhanga, P Erimu, C Karungi, H Kizito, WS Namala, J Namusanje, R Nandugwa, TK Najjuko, E Natukunda, M Ndigendawani, SO Nsiyona, R Kibenge, B Bainomuhwezi, D Sseremba, J Tezikyabbiri, CS Tumusiime, A Balaba, A Mugumya, F Nghania, D Mwebesa, M Mutumba, E Bagurukira, F Odongo, S Mubokyi, M ssenyonga, M Kasango, E Lutalo, P Oronon, I Nankya, E Ndashimye, E Nabulime, L Mugarura, O Senfuma, E D Williams, R Lwalanda, D Odoch, S Abunyang, D Mulima. University of Zimbabwe, Harare, Zimbabwe: KJ Nathoo, MF Bwakura-Dangarembizi, F Mapinge, E Chidziva, T Mhute, T Vhembo, R Mandidewa, M Chipiti, R Dzapasi, C Katanda D Nyoni, GC Tinago, J Bhiri, S Mudzingwa, D Muchabaiwa, M Phiri, V Masore, CC Marozva, SJ Maturure, S Tsikirayi, L Munetsi, KM Rashirai, J Steamer, R Nhema, W Bikwa, B Tambawoga, E Mufuka, K Mataruka, P Kurira, T Chivasa. Baylor College of Medicine Children’s Foundation Uganda, Mulago Hospital Uganda: A Kekitiinwa, P Musoke, S Bakeera-Kitaka, R Namuddu, P Kasirye, A Babirye, J Asello, S Nakalanzi, NC Ssemambo, J Nakafeero, J Tikabibamu, G Musoba, J Ssanyu, M Kisekka MRC Clinical Trials Unit, London, UK: DM Gibb, MJ Thomason, AS Walker, AD Cook, B Naidoo-James, MJ Spyer, C Male, AJ Glabay, LK Kendall, J Crawley, AJ Prendergast
Independent ARROW Trial Monitors: I Machingura, S Ssenyonjo. Trial Steering Committee: I Weller (Chair), E Luyirika, H Lyall, E Malianga, C Mwansambo, M Nyathi, F Miiro, DM Gibb, A Kekitiinwa, P Mugyenyi, P Munderi, KJ Nathoo; Observers S Kinn, M McNeil, M Roberts, W Snowden. Data and Safety Monitoring Committee: A Breckenridge (Chair), A Pozniak, C Hill, J Matenga, J Tumwine, AS Walker. Endpoint Review Committee (independent members): G Tudor-Williams (Chair), H Barigye, HA Mujuru, G Ndeezi; (observers): S Bakeera-Kitaka, MF Bwakura-Dangarembizi, J Crawley, V Musiime, P Nahirya-Ntege, AJ Prendergast, M Spyer. ARROW Economics Group: P Revill, T Mabugu, F Mirimo, S Walker, MJ Sculpher. ARROW Immunology Group: N Klein, B Kampmann, A Gomo, G Pimundu.
Funding
ARROW was funded by the UK Medical Research Council [G0300400] and the UK Department for International Development (DFID). ViiV Healthcare/GlaxoSmithKline donated first-line drugs for ARROW and provided funding for VL assays and genotyping.
Role of Authors
VM, PN-N, MJT, DMG and ASW designed the trial in collaboration with other members of the ARROW trial team. VM, PK, BN-J, PN-N, TM conducted the trial in collaboration with other staff at the ARROW clinical sites. MJS organised sample testing. LM and MM conducted VL assays. EN and IN conducted HIV genotyping. AC and ASW analysed the data. WS and NT supported the study on behalf of ViiV. VM, ASW and DMG wrote the first draft. All authors contributed to interpretation of results and manuscript review, and have approved the final version.
Footnotes
Conflict of Interest
WS and NT are employees of ViiV Healthcare (formally GlaxoSmithKline) with associated renumeration (salary stock). ViiV Healthcare/GlaxoSmithKline donated first-line drugs for ARROW and provided funding for VL assays and genotyping. There are no other conflicts of interest.
References
- 1.UNAIDS. 2014 progress report on the Global Plan towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive. 2014.
- 2.Nachega JB, Hislop M, Dowdy DW, Chaisson RE, Regensberg L, Maartens G. Adherence to nonnucleoside reverse transcriptase inhibitor-based HIV therapy and virologic outcomes. Ann Intern Med. 2007;146:564–573. doi: 10.7326/0003-4819-146-8-200704170-00007. [DOI] [PubMed] [Google Scholar]
- 3.Boyle BA, Jayaweera D, Witt MD, Grimm K, Maa JF, Seekins DW. Randomization to once-daily stavudine extended release/lamivudine/efavirenz versus a more frequent regimen improves adherence while maintaining viral suppression. HIV Clin Trials. 2008;9:164–176. doi: 10.1310/hct0903-164. [DOI] [PubMed] [Google Scholar]
- 4.WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations fora public health approach. 2013. [PubMed]
- 5.Lamarca A, Clumeck N, Plettenberg A, Domingo P, Fu K, Craig C, et al. Efficacy and safety of a once-daily fixed-dose combination of abacavir/lamivudine compared with abacavir twice daily and lamivudine once daily as separate entities in antiretroviral-experienced HIV-1-infected patients (CAL30001 Study) J Acquir Immune Defic Syndr. 2006;41:598–606. doi: 10.1097/01.qai.0000214821.33905.5c. [DOI] [PubMed] [Google Scholar]
- 6.Moyle GJ, DeJesus E, Cahn P, Castillo SA, Zhao H, Gordon DN, et al. Abacavir once or twice daily combined with once-daily lamivudine and efavirenz for the treatment of antiretroviral-naive HIV-infected adults: results of the Ziagen Once Daily in Antiretroviral Combination Study. J Acquir Immune Defic Syndr. 2005;38:417–425. doi: 10.1097/01.qai.0000147521.34369.c9. [DOI] [PubMed] [Google Scholar]
- 7.Sosa N, Hill-Zabala C, Dejesus E, Herrera G, Florance A, Watson M, et al. Abacavir and lamivudine fixed-dose combination tablet once daily compared with abacavir and lamivudine twice daily in HIV-infected patients over 48 weeks (ESS30008, SEAL) J Acquir Immune Defic Syndr. 2005;40:422–427. doi: 10.1097/01.qai.0000184859.24071.bd. [DOI] [PubMed] [Google Scholar]
- 8.Musiime V, Kendall L, Bakeera-Kitaka S, Snowden WB, Odongo F, Thomason M, et al. Pharmacokinetics and acceptability of once- versus twice-daily lamivudine and abacavir in HIV type-1-infected Ugandan children in the ARROW Trial. Antivir Ther. 15:1115–1124. doi: 10.3851/IMP1695. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach. World Health Organization; Geneva, Switzerland: 2013. ( http://www.who.int/hiv/pub/guidelines/arv2013/download/en/). [PubMed] [Google Scholar]
- 10.Bergshoeff A, Burger D, Verweij C, Farrelly L, Flynn J, Le Prevost M, et al. Plasma pharmacokinetics of once- versus twice-daily lamivudine and abacavir: simplification of combination treatment in HIV-1-infected children (PENTA-13) Antivir Ther. 2005;10:239–246. [PubMed] [Google Scholar]
- 11.Musiime V, Fillekes Q, Kekitiinwa A, Kendall L, Keishanyu R, Namuddu R, et al. The pharmacokinetics and acceptability of lopinavir/ritonavir minitab sprinkles, tablets, and syrups in african HIV-infected children. J Acquir Immune Defic Syndr. 2014;66:148–154. doi: 10.1097/QAI.0000000000000135. [DOI] [PubMed] [Google Scholar]
- 12.Pharmacokinetic study of once-daily versus twice-daily abacavir and lamivudine in HIV type-1-infected children aged 3-<36 months. Antivir Ther. 15:297–305. doi: 10.3851/IMP1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LePrevost M, Green H, Flynn J, Head S, Clapson M, Lyall H, et al. Adherence and acceptability of once daily Lamivudine and abacavir in human immunodeficiency virus type-1 infected children. Pediatr Infect Dis J. 2006;25:533–537. doi: 10.1097/01.inf.0000222415.40563.d4. [DOI] [PubMed] [Google Scholar]
- 14.Cohen CJ, Kubota M, Brachman PS, Harley WB, Schneider S, Williams VC, et al. Short-term safety and tolerability of a once-daily fixed-dose abacavir-lamivudine combination versus twice-daily dosing of abacavir and lamivudine as separate components: findings from the ALOHA study. Pharmacotherapy. 2008;28:314–322. doi: 10.1592/phco.28.3.314. [DOI] [PubMed] [Google Scholar]
- 15.DeJesus E, McCarty D, Farthing CF, Shortino DD, Grinsztejn B, Thomas DA, et al. Once-daily versus twice-daily lamivudine, in combination with zidovudine and efavirenz, for the treatment of antiretroviral-naive adults with HIV infection: a randomized equivalence trial. Clin Infect Dis. 2004;39:411–418. doi: 10.1086/422143. [DOI] [PubMed] [Google Scholar]
- 16.Maitland D, Jackson A, Osorio J, Mandalia S, Gazzard BG, Moyle GJ. Switching from twice-daily abacavir and lamivudine to the once-daily fixed-dose combination tablet of abacavir and lamivudine improves patient adherence and satisfaction with therapy. HIV Med. 2008;9:667–672. doi: 10.1111/j.1468-1293.2008.00618.x. [DOI] [PubMed] [Google Scholar]
- 17.Sension MG, Bellos NC, Johnson J, Sepulveda GE, DeJesus E, Santana JL, et al. Lamivudine 300 mg QD versus continued lamivudine 150 mg BID with stavudine and a protease inhibitor in suppressed patients. HIV Clin Trials. 2002;3:361–370. doi: 10.1310/7c8q-qujl-ek1c-n238. [DOI] [PubMed] [Google Scholar]
- 18.Kekitiinwa A, Cook A, Nathoo K, Mugyenyi P, Nahirya-Ntege P, Bakeera-Kitaka S, et al. Routine versus clinically driven laboratory monitoring and first-line antiretroviral therapy strategies in African children with HIV (ARROW): a 5-year open-label randomised factorial trial. Lancet. 381:1391–1403. doi: 10.1016/S0140-6736(12)62198-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson VA, Calvez V, Gunthard HF, Paredes R, Pillay D, Shafer RW et al. Update of the drug resistance mutations in HIV-1: March 2013. Top Antivir Med. 21:6–14. [PMC free article] [PubMed] [Google Scholar]
- 20.Database SUHDR. In; 2014
- 21.Aldir I, Horta A, Serrado M. Single-tablet regimens in HIV: does it really make a difference? Curr Med Res Opin. 30:89–97. doi: 10.1185/03007995.2013.844685. [DOI] [PubMed] [Google Scholar]
- 22.AIDSinfo. Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection. 2014.
- 23.Musoke P, Szubert AJ, Musiime V, Nathoo K, Nahirya-Ntege P, Mutasa K, et al. Single-dose nevirapine exposure does not affect response to antiretroviral therapy in HIV-infected African children aged below 3 years. AIDS. 29:1623–1632. doi: 10.1097/QAD.0000000000000749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Group ISS, Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. 2015;373:795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kasirye P, Kendall L, Adkison KK, Tumusiime C, Ssenyonga M, Bakeera-Kitaka S, et al. Pharmacokinetics of antiretroviral drug varies with formulation in the target population of children with HIV-1. Clin Pharmacol Ther. 2012;91:272–280. doi: 10.1038/clpt.2011.225. [DOI] [PubMed] [Google Scholar]
- 26.Chokephaibulkit K, Cressey TR, Capparelli E, Sirisanthana V, Muresan P, Hongsiriwon S, et al. Pharmacokinetics and safety of a new paediatric fixed-dose combination of zidovudine/lamivudine/nevirapine in HIV-infected children. Antivir Ther. 2011;16:1287–1295. doi: 10.3851/IMP1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nahirya-Ntege P, Cook A, Vhembo T, Opilo W, Namuddu R, Katuramu R, et al. Young HIV-infected children and their adult caregivers prefer tablets to syrup antiretroviral medications in Africa. PLoS One. 7:e36186. doi: 10.1371/journal.pone.0036186. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.