Abstract
Background
Distinguishing between urinary tract infection (UTI) and colonization (UTC) in patients with neurogenic bladders who require clean intermittent catheterization (CIC) is difficult. Urinary neutrophil gelatinase-associated lipocalin concentrations (uNGAL) are increased in UTIs. Our objective was to determine the predictive accuracy of uNGAL for UTI in CIC-dependent children.
Methods
Cross-sectional study of CIC-dependent patients from August, 2015 to November, 2016. UTI was defined as (1) growth of ≥ 50,000 cfu/mL of a uropathogen, (2) > 10 urinary white blood cells/hpf, and (3) ≥ 2 of the following: temperature > 38 °C, abdominal pain, back pain, worsened incontinence, pain with catheterization, or malodorous/cloudy urine. Positive urine cultures that did not meet these criteria were grouped as UTC, and negative cultures were grouped as no growth.
Results
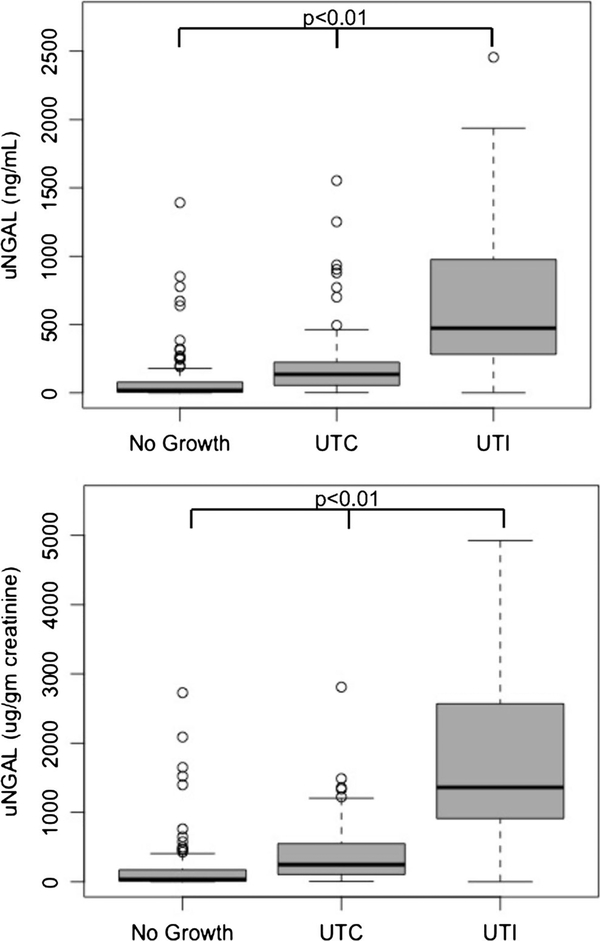
Two hundred one patients were included (no growth = 100, UTC = 77, UTI = 24). Median (interquartile range) uNGAL was higher in the UTI group (UTI 1361 (931, 2516) μg/g creatinine, UTC 246 (106, 548) μg/g creatinine, no growth 36 (11, 179) μg/g creatinine, p < 0.01 for all comparisons). The area under the ROC curve for uNGAL for UTI versus no UTI was 0.89, 95% CI (0.80–0.98).
Conclusion
uNGAL is elevated in CIC-dependent children with UTI compared to those with negative cultures and those with UTC.
Keywords: Urinary tract infection, Neurogenic bladder, Clean intermittent catheterization, Pediatrics
Introduction
Children with neurogenic bladders who require clean intermittent catheterization (CIC) frequently have bacteriuria, which may be the result of a urinary tract infection (UTI) or bacterial colonization of the urinary tract (UTC) [1]. Distinguishing between these entities is important since UTI treatment prevents the spread of infection and resultant bladder and kidney damage, while unnecessary antibiotic exposure can lead to the development of antibiotic-resistant bacteria. However, establishing the diagnosis of UTI in children with neurogenic bladder who require CIC is difficult, as there are no standardized criteria to distinguish UTI from UTC. Due to this diagnostic uncertainty, and because of the risk for morbidity and mortality associated with an untreated infection, patients on CIC receive many courses of antibiotics for bacteriuria. As a result, the prevalence of antibiotic-resistant bacteria in this population is high, with multidrug resistant bacteria isolated from > 15% of urine cultures from CIC-dependent children, compared with 5% of urine cultures from children not receiving CIC [2, 3]. A method to identify patients who are at low risk for UTI, and therefore do not require antibiotics, is needed to prevent further increases in the rate of antibiotic resistance in this population.
Neutrophil gelatinase-associated lipocalin (NGAL) is a 24 kDa protein expressed and secreted by the genitourinary epithelium in response to a variety of injurious stimuli, including infection [4]. NGAL, which functions as part of the innate immune system in the genitourinary tract, is present in low levels in urine from healthy children and is increased in the setting of UTI [5, 6]. Therefore, we sought to measure urinary NGAL (uNGAL) concentrations in children with neurogenic bladders who require CIC, determine if uNGAL could discriminate between UTI and UTC in this patient population, and test the hypothesis that uNGAL levels could identify patients who are at low risk for UTI.
Methods
Patients
All patients with neurogenic bladder with CIC requirement followed at Cincinnati Children’s Hospital Medical Center who had a urine culture sent as part of clinical care from August 1, 2015 through November 1, 2016 were eligible for inclusion in this cross-sectional study. Patients were identified in the electronic health record (EPIC™, Verona, WI) using the following International Classification of Diseases, 9th revision (ICD9), codes: neurogenic bladder (ICD9 596.54), spina bifida (ICD 741), paraplegia (ICD9 344.1), or quadriplegia (ICD9 344). The medical records of these patients were then manually reviewed to ensure that patients were actively performing CIC. Eligible patients were flagged in VigiLanz™ (VigiLanz Corporation, Minneapolis, Minnesota), a real-time lab monitoring software, which alerted the research team when a specified patient had a urinalysis or urine culture sent. Patients were excluded if there was evidence of acute kidney injury, defined per the KDIGO criteria [7], or sepsis [8] at the time of presentation, as both acute kidney injury and sepsis can increase NGAL concentrations [9], or if they had dialysis-dependent end-stage renal disease.
The study was approved by the Cincinnati Children’s Hospital Medical Center Institutional Review Board with a waiver of informed consent as the urine samples were discarded specimens.
Definitions
Patients were included in the UTI group if they met all three of the following criteria: (1) growth of greater than or equal to 50,000 colony forming units per milliliter (cfu/mL) of a single, known uropathogen from urine culture, (2) more than 10 urinary white blood cells/high-powered field in a spun urine specimen, and (3) two or more of the following signs and symptoms: fever greater than 38 °C, abdominal pain, new back pain, new or worsened incontinence, pain with catheterization, or malodorous or cloudy urine. This definition of UTI is adapted from that published by Madden-Fuentes et al., with a modified colony-count of 50,000 cfu/mL for UTI rather than 100,000 cfu/mL included in the initial definition [10]. Patients who did not meet these criteria, but had a positive urine culture, were included in the UTC group. Positive cultures without a concurrent urinalysis were excluded due to inability to categorize the culture. Patients with negative urine cultures were included in the no growth group. Cultures that grew fungi or an unidentified mixture of organisms were excluded from analysis.
Clinical data
All clinical data were collected through manual chart review. The presence or absence of symptoms was taken from notes written by the primary clinician on day of sample collection. Symptoms for all patients with a positive culture were double-entered to ensure accuracy.
Biomarker measurement
Residual urine that was sent to the laboratory as part of clinical care was obtained for processing within 10 hours of initial collection, centrifuged at 12,000 RPM for 10 min, and frozen at – 80 °C. The urine was refrigerated for a maximum of 10 hours between sample collection and processing. There were no freeze/thaw cycles prior to biomarker analysis. uNGAL was measured by ELISA (Bioporto, Grusbakken, Denmark), and urine creatinine by assay (Siemens Dimension RXL Max Clinical Analyzer, Munich, Germany) in the Division of Nephrology Biomarker Laboratory at Cincinnati Children’s Hospital. Samples were frozen no more than 6 months prior to uNGAL and urine creatinine measurement. Analysis was completed with both the normalized (by urine creatinine) and non-normalized uNGAL values.
Statistical analysis
Continuous variables were assessed for normal distribution, and nonparametric statistics were used for those with non-normal distributions. Normally distributed continuous variables (e.g., age) were compared using ANOVA with post-hoc Tukey for multiple comparisons. Urine NGAL was non-normally distributed, and therefore nonparametric testing was used to compare uNGAL concentrations between groups. The Mann-Whitney test was used when comparing two groups, and the Kruskal-Wallis test, with a post-hoc Dunn test, was used for multiple groups. Categorical variables were compared with the chi-square test, or Fisher’s exact as warranted. The area under the receiver operator characteristic (ROC) curve for identifying UTI was calculated using logistic regression models for uNGAL. The performance of uNGAL discriminating between UTI and UTC, and UTI and no UTI (UTC and no growth combined) was analyzed by the ROC curves. Each outcome (UTI vs UTC, UTI vs no growth, UTI vs no UTI) and each marker (normalized and non-normalized uNGAL) were modeled separately. The optimal uNGAL cut-point for the diagnosis of UTI, as well as the associated sensitivity and specificity, was determined using Youden’s Index. Positive and negative predictive values, and positive and negative likelihood ratios, were calculated. Given the potential confounding effect associated with bladder augmentation in a subset of the enrolled patients, the analysis was completed both with and without patients who underwent bladder augmentation included in the cohort. All statistical analysis was done using R (version 3.2.5) [11] with package pROC [12]. A p value of < 0.05 was considered statistically significant. The datasets generated during the current study are available from the corresponding author on reasonable request.
We performed several subanalyses of this data given the lack of a gold-standard of UTI, which may have resulted in miscategorization of some cultures in this work. The first subanalysis used a stricter definition of UTI: we added the presence of nitrites (in the event of a gram-negative infection) to the definition of UTIs. In the second subanalysis, we excluded patients with positive cultures, positive nitrites, and no pyuria from the UTC group. In the third subanalysis, we excluded patients with positive cultures and positive nitrites from the UTC group.
Results
Two hundred eighty-six cultures were reviewed, of which 85 (29.7%) were excluded (Fig. 1). Of the 201 cultures that were included, 100 were no growth, 77 were categorized as UTC, and 24 as UTI. Patient demographics and urinalysis comparisons are listed in Table 1. Patients in the UTC group were older and had a higher proportion of females compared with the no growth group. There was a higher proportion of patients with UTIs with anorectal malformation compared to those with no growth, but there were no other differences in the etiology of neurogenic bladder between groups.
Fig. 1.
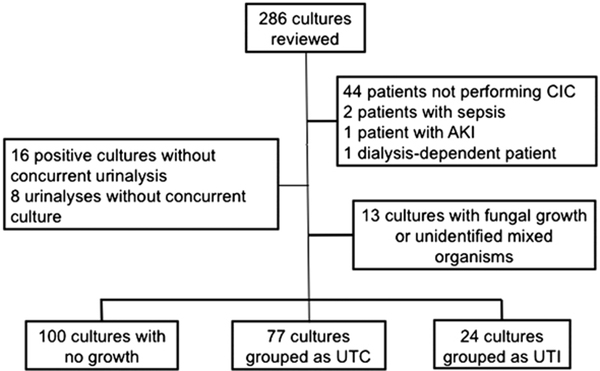
Patient flow diagram
Table 1.
Demographics, urinalysis results, and uropathogens in patients with no growth, UTC, and UTI
| No growth (n = 100) | UTC (n = 77) | UTI (n = 24) | |
|---|---|---|---|
| Age (years), mean (SD) | 9.0 (6.4) | 11.6 (7.1)a | 8.6 (6.4) |
| Female | 35 (35) | 51 (66)a | 13 (54) |
| Black | 10 (10) | 7 (9) | 1 (8) |
| Hispanic | 2 (2) | 3 (4) | 2 (8) |
| Fever on presentation | 30 (30) | 18 (23) | 13 (54)a |
| Mitrofanoff | 16 (16) | 35 (46)a | 7 (29) |
| Bladder augmentation | 5 (5) | 14 (18) | 3 (13) |
| Chronic kidney disease | 20 (20) | 7 (9) | 5 (21) |
| Etiology of neurogenic bladder | |||
| Myelomeningocele | 44 (44) | 31 (41) | 8 (33) |
| Anorectal malformation | 9 (9) | 12 (16) | 5 (21) |
| Tethered cord | 11 (11) | 10 (13) | 4 (17) |
| Urinalysis parameters | |||
| Nitrites present | 2 (3) | 22 (29)a | 10 (42)a |
| Absent LE | 61 (75) | 25 (32)a,b | 0 (0)a |
| Trace LE | 6 (7) | 13 (17)a | 1 (4) |
| Small LE | 4 (5) | 8 (10)a | 3 (13)a |
| Moderate LE | 4 (5) | 13 (17)a | 3 (13)a |
| Large LE | 6 (7) | 18 (23)a,b | 17 (71)a |
| 1–10 urinary WBCs | 30 (56) | 25 (35)a,b | 0 (0)a |
| 11–20 urinary WBCs | 8 (15) | 10 (13) | 5 (21) |
| 21–30 urinary WBCs | 4 (7) | 9 (12) | 2 (8) |
| 31–50 urinary WBCs | 0 (0) | 4 (6) | 4 (13)a |
| > 50 urinary WBCs | 4 (7) | 17 (22)a,b | 13 (54)a |
| Uropathogens | |||
| E. coli | 36 (47) | 13 (54) | |
| Klebsiella pneumoniae | 11 (14) | 6 (25) | |
| Enterococcus spp. | 6 (8) | 0 (0) | |
| Enterobacter spp. | 3 (4) | 0 (0) | |
| Pseudomonas aeruginosa | 4 (5) | 1 (4) | |
| Serratia spp. | 3 (4) | 1 (4) | |
| Proteus mirabilis | 1 (1) | 1 (4) | |
| Symptoms | |||
| Abdominal pain | 7 (7) | 10 (13)b | 10 (42)a |
| Fever | 6 (6) | 13 (17)a,b | 13 (54)a |
| Flank or back pain | 3 (3) | 6 (8)b | 9 (38)a |
| New or worse incontinence | 12 (12) | 17 (22) | 10 (42)a |
| Pain with CIC | 8 (8) | 4 (5) | 2 (8) |
| Malodorous or cloudy urine | 10 (10) | 8 (10)b | 10 (42)a |
All data presented as n(%) unless otherwise stated
uNGAL urine neutrophil gelatinase-associated lipocalin, UTC urinary tract colonization, UTI urinary tract infection, LE leukocyte esterase, WBC white blood cell, CIC clean intermittent catheterization
p < 0.05 compared with no growth
p < 0.05 compared with UTI
Patients with UTI and UTC had a higher proportion of leukocyte esterase at all levels compared to patients with no growth, and there was a greater proportion of patients with greater than 50 urinary white blood cells in the UTI group compared with both UTC and no growth. The most common uropathogens in patients with UTC were E. coli, Klebsiella pneumoniae, and Enterococcus, while E. coli and Klebsiella pneumoniae were most common in patients with UTI. There was a higher proportion of patients in the UTI group who reported symptoms of abdominal pain, fever, flank or back pain, new or worsened incontinence, and cloudy or malodorous urine compared to those with no growth. There were more patients with abdominal pain, fever, flank, or back pain, and malodorous or cloudy urine in the UTI group compared to the UTC group. There was no difference in pain with CIC between all three groups (Table 1).
uNGAL normalized by urine creatinine (uNGAL/Cr) concentrations were higher in the UTI group (median, 1361 μg/g creatinine; interquartile range (IQR) 931, 2516) than the UTC (246 μg/g creatinine; IQR 106, 548) and no growth groups (36 μg/g creatinine; IQR 11, 179, p < 0.01 for all comparisons). Non-normalized uNGAL was also higher for those with UTI (434 ng/mL; IQR: 307, 969) than for those with UTC (135 ng/mL; IQR: 54, 224) or no growth (18 ng/mL; IQR 5, 78) (p < 0.01 for all comparisons) (Fig. 2). The area under the ROC curve (AUC) for uNGAL/Cr for the diagnosis of UTI as opposed to no UTI was 0.89 (95% confidence interval 0.80–0.97). The AUC for non-normalized uNGAL for the diagnosis of UTI was 0.88 (95% CI 0.80–0.97). The AUCs for UTI using the stricter definition with the inclusion of nitrites were lower, although not significantly, than the AUCs for the definition without nitrites for all comparisons (Table 2). There were 22 patients with augmented bladders in this cohort (no growth = 5, UTC = 14, UTI = 3). After exclusion of these patients, there was an increase in the sensitivity (from 83 to 86%) and increase in the specificity (from 86 to 89%) of non-normalized uNGAL and associated changes in the likelihood ratios (Table 2). There were only minor differences in the other markers of predictive accuracy.
Fig. 2.
Boxplots of normalized and non-normalized uNGAL concentrations in “no growth,” “UTC,” and “UTI” groups. Normalized (top graph) and non-normalized (bottom graph) and uNGAL concentrations in patients with no growth, UTC, and UTI. Significant differences exist between all groups for both the normalized and non-normalized uNGAL concentrations. uNGAL urine neutrophil gelatinase-associated lipocalin, UTC urinary tract colonization, UTI urinary tract infection
Table 2.
Area under the ROC curve (AUC) for the diagnosis of UTI normalized and non-normalized uNGAL, clinical opinion, and nitrites, for all patients and all patients without augmented bladders
| Predictor | AUC | Cut-off value | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) | Positive likelihood ratio | Negative likelihood ratic | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Patients with augmented bladders included | ||||||||||
| Diagnosis of UTI versus no UTI | ||||||||||
| UTI definition does not include nitrites (n with UTI = 24) | uNGAL (pg/g crt) | 0.89 (0.80–0.98) | 817 | 83 | 91 | 58 | 97 | 9.2 | 0.2 | |
| uNGAL (ng/mL) | 0.88 (0.80–0.97) | 229 | 83 | 86 | 47 | 97 | 5.9 | 0.2 | ||
| UTI definition does include nitrites (n with UTI = 11) | uNGAL (pg/g crt) | 0.82 (0.65–1.0) | 817 | 82 | 85 | 26 | 81 | 5.5 | 0.2 | |
| uNGAL (ng/mL) | 0.84 (0.67–0.99) | 228 | 91 | 81 | 23 | 99 | 4.8 | 0.1 | ||
| Diagnosis of UTI versus UTC | ||||||||||
| UTI definition does not include nitrites (n with UTI = 24) | uNGAL (pg/g crt) | 0.84 (0.73–0.95) | 817 | 80 | 83 | 67 | 91 | 4.7 | 0.2 | |
| uNGAL (ng/mL) | 0.83 (0.63–0.94) | 229 | 80 | 83 | 67 | 91 | 4.7 | 0.2 | ||
| UTI definition does include nitrites (nwith UTI =11) | uNGAL (pg/g crt) | 0.76 (0.57–0.94) | 817 | 82 | 70 | 29 | 96 | 2.7 | 0.3 | |
| uNGAL (ng/mL) | 0.79 (0.62–0.97) | 229 | 91 | 72 | 33 | 98 | 3.3 | 0.1 | ||
| Patients with augmented bladders excluded | ||||||||||
| Diagnosis of UTI versus no UTI | ||||||||||
| UTI definition does not include nitrites (n with UTI = 21) | uNGAL (pg/g crt) | 0.90 (0.80–1.0) | 856 | 86 | 92 | 62 | 98 | 10.8 | 0.2 | |
| uNGAL (ng/mL) | 0.89 (0.80–0.99) | 229 | 86 | 89 | 54 | 98 | 7.8 | 0.2 | ||
| UTI definition does include nitrites (nwith UTI =10) | uNGAL (pg/g crt) | 0.82 (0.63–1.0) | 856 | 80 | 86 | 27 | 99 | 5.7 | 0.2 | |
| uNGAL (ng/mL) | 0.84 (0.65–1.0) | 229 | 90 | 84 | 26 | 99 | 5.6 | 0.1 | ||
| Diagnosis of UTI versus UTC | ||||||||||
| UTI definition does not include nitrites (n with UTI = 21) | uNGAL (pg/g crt) | 0.85 (0.74–0.97) | 856 | 81 | 85 | 49 | 96 | 5.4 | 0.2 | |
| uNGAL (ng/mL) | 0.85 (0.74–0.96) | 229 | 82 | 85 | 49 | 96 | 5.5 | 0.2 | ||
| UTI definition does include nitrites (nwith UTI = 10) | uNGAL (pg/g crt) | 0.75 (0.55–0.95) | 1035 | 70 | 81 | 39 | 94 | 3.7 | 0.4 | |
| uNGAL (ng/mL) | 0.78 (0.59–0.97) | 229 | 90 | 72 | 36 | 98 | 3.2 | 0.1 | ||
A UC area under the curve, ROC receiver operator characteristic, uNGAL urine neutrophil gelatinase-associated lipocalin, UTC urinary tract colonization, UTI urinary tract infection
Using the definition without nitrites, in the entire cohort, including patients with augmented bladder, the cut-off for uNGAL/Cr was 817 μg/g creatinine, with a sensitivity of 83%, specificity of 91%, positive predictive value of 58%, and negative predictive value of 97%. The cut-off for nonnormalized uNGAL was 229 ng/ml, with a sensitivity of 83%, specificity of 86%, positive predictive value of 47%, and negative predictive value of 97% (Table 2). Of the 163 samples with uNGAL/Cr less than the cut-off of 817 μg/g creatinine, four (2.5%) were from patients with UTI. Similarly, there were only four patients (2.5%) with UTIs with non-normalized uNGAL less than the cut-off value of 229 ng/ mL. The results using the stricter definition of UTI, with the addition of nitrites, have higher sensitivity and lower specificity, with lower AUCs compared to the definition without nitrites (Table 2). When patients with UTC with positive nitrites are removed from analysis, as these patients may represent UTIs miscategorized as UTC, there are no major differences in the markers of predictive accuracy (Table 3).
Table 3.
ROC Analysis with removal of UTC patients with positive nitrites and pyuria
| Predictor | AUC | Cut-off value | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) | Positive likelihood ratio | Negative likelihood ratio | |
|---|---|---|---|---|---|---|---|---|---|
| Diagnosis of UTI versus no UTI | |||||||||
| UTC with nitrites and no pyuria removed | uNGAL (pg/g crt) | 0.92 (0.86–0.97) | 817 | 86 | 89 | 49 | 98 | 7.8 | 0.2 |
| uNGAL (ng/mL) | 0.92 (0.87–0.96) | 229 | 91 | 84 | 41 | 99 | 5.7 | 0.1 | |
| All UTC with nitrites removed | uNGAL (pg/g crt) | 0.92 (0.87–0.97) | 816 | 86 | 90 | 56 | 98 | 8.6 | 0.2 |
| uNGAL (ng/mL) | 0.94 (0.81–0.97) | 229 | 91 | 87 | 51 | 98 | 7.0 | 0.1 | |
| Diagnosis of UTI versus UTC | |||||||||
| UTC with nitrites and no pyuria removed | uNGAL (pg/g crt) | 0.87 (0.79–0.96) | 817 | 86 | 81 | 57 | 95 | 4.5 | 0.3 |
| uNGAL (ng/mL) | 0.87 (0.79–0.95) | 229 | 91 | 78 | 55 | 97 | 4.1 | 0.1 | |
| All UTC with nitrites removed | uNGAL (pg/g crt) | 0.88 (0.80–0.97) | 816 | 86 | 82 | 67 | 93 | 4.8 | 0.2 |
| uNGAL (ng/rnl) | 0.92 (0.85–0.98) | 229 | 91 | 84 | 71 | 96 | 5.7 | 0.1 | |
AUC area under the curve, ROC receiver operator characteristic, uNGAL urine neutrophil gelatinase-associated lipocalin
Of the 101 total positive cultures, 14 grew gram-positive organisms, which had a wide range of uNGAL values. The one patient with a gram-positive UTI in our cohort had uNGAL values above both the normalized and nonnormalized cut-offs. At the cut-off value of 817 μg/g creatinine, 12 of the 13 patients with gram-positive UTC had uNGALs below the cut-off. Conversely, at the cut-off value of 229 ng/ml, 12 of the 13 patients with gram-positive bacteriuria had uNGAL values below the cut-off.
Discussion
In this study, we demonstrate that uNGAL is elevated in patients with neurogenic bladders who have UTI compared to those who have either UTC or no growth. Further, we show that uNGAL has a high negative predictive value for UTI in this population, which suggests that the potential use of this biomarker is to rule out UTI.
The most clinically relevant use of uNGAL in this population is to identify patients who are at low risk for UTI. Clinicians rarely need a marker to decide whether an illappearing patient with a likely source of infection requires antibiotics. However, a marker that can identify a patient as low risk for UTI may have utility whena febrile, but otherwise well-appearing, patient has bacteriuria. This is especially relevant in the outpatient setting, where clinicians are not able to easily observe patients off antibiotic therapy, which may influence the decision to initiate antibiotic treatment. Indeed, recent data demonstrates an increased prevalence of inappropriate antibiotic prescription in outpatient settings [13]. While patients with neurogenic bladders were not included in that prior study, the trend of inappropriate antibiotic prescription in the outpatient setting is likely also seen in patients with neurogenic bladder who present with concern for UTI. Our data suggests that uNGAL may be able to identify patients who are low risk for UTI who can be safely observed off of antibiotics.
The ability to determine when bacteriuria represents a UTI as opposed to UTC in children with neurogenic bladders at the point of care has important implications for this patient population. The frequency of UTIs with multidrug-resistant organisms seen in CIC-dependent children has been rising [2]. This increase in the prevalence of resistance is a result of multiple factors, including frequent exposure to antibiotics for either prevention or treatment of UTI [14]. Due to the difficulty in determining when bacteriuria represents UTC as opposed to UTI, as well as the consequences of an untreated UTI, these patients often receive antibiotics. Of the 51 patients in our cohort who received antibiotics at the point of care for presumed UTI, only 20 were categorized as UTI; the remaining 63% had no growth (n = 2) or UTC (n = 30). Of the four UTI patients who did not receive antibiotics at the point of care, three received antibiotics within 1 week following the culture. Of the 30 UTC patients who were given antibiotics (11 of whom had positive nitrites on urinalysis), the majority (90%) did not meet our definition of UTI based on lack of symptoms. The remaining 10% did not meet our definition due to lack of pyuria. Ten of these 30 patients were seen in an acute setting (e.g., Emergency Department) with concern for UTI, indicating that these patients may have been sicker at presentation, thus warranting antibiotics. The remaining 20 had urine cultures sent as part of routine urodynamics. Of these 20, ten were treated as the uropathogens were ureaseproducing organisms, and the remaining ten were treated due to presence of vesicoureteral reflux. Our data suggests that the use of normalized uNGAL at the point of care in for these patients may have avoided the prescription of antibiotics in 23 patients, suggesting that up to one half of antibiotics given at the point of care for presumed UTIs in CIC-dependent children may be unnecessary. The use of uNGAL at the pointof care has potential to decrease the development of antibioticresistant infections in this population.
The utility of various parameters of the urinalysis to distinguish UTI from UTC is limited in patients who require CIC. The presence of pyuria, which is frequently used as a marker of UTI in the general pediatric population [15], lacks the same specificity in the neurogenic bladder population: CICdependent patients often have chronic inflammation of the genitourinary tract as a result of frequent catheterization [16], which confounds the utility of urinary white blood cells in this population. Further, bladder biopsy samples from children with myelomeningocele show abnormal epithelium with evidence of chronic inflammation [17], further limiting the utility of urinary white blood cells in the neurogenic bladder population. Similarly, leukocyte esterase, another component of the urinalysis used clinically to diagnose UTI [18], is directly related to the presence of urinary WBCs, and therefore also has limited utility.
Nitrites are also an imperfect marker of UTI as the presence of nitrites is only indicative of the presence of specific organisms [19]. At baseline, the urine of patients with neurogenic bladder is home to bacteria that are classically considered to be pathogenic [20]. Therefore, the presence of nitrites on urinalysis only confirms the presence of a uropathogen, but is not indicative of an infection. Further, as it takes approximately 4 hours for bacteria to convert nitrates to nitrites, the amount of time that the urine is in the bladder also impacts the presence of nitrites on urinalysis [21]. Despite this, nitrites have good utility in predicting of bacteriuria in children with neurogenic bladder [22], and therefore are frequently used clinically in this situation. As 29% of patients in the UTC group had positive nitrites, we repeated the ROC curve analysis after excluding these patients as they may represent misclassified UTI patients. The measures of the predictive accuracy after the removal of the samples with nitrites have only minor differences in the normalized form of uNGAL. There are more notable differences in the non-normalized form, but the overall trends continue to have a high negative predictive value. Thus, while some misclassification may have occurred, as evidenced by the minor changes in the analysis when UTC patients with nitrites are removed, we believe that uNGAL remains a potentially useful approach to identifying patients in whom antibiotics may be safely withheld.
We report both the normalized and non-normalized uNGAL values in this work. Traditionally, uNGAL values have been normalized in research studies by urine creatinine to account for urinary concentration [9]. However, urine creatinine is an imperfect method of standardization for uNGAL in the setting of UTI. Urine creatinine comes from the filtrate at the level ofthe glomerulus, whereas uNGAL is produced by the collecting ducts [23], and is essentially free from glomerular filtration [24]. Similar to acute kidney injury, we show that uNGAL levels are increased in UTI regardless of whether or not NGAL is normalized [9], although the nonnormalized form of uNGAL has a greater sensitivity compared with the normalized form, which is more specific. Both forms have high negative predictive values, suggesting that the greatest utility of either form of uNGAL is to advise clinicians when antibiotics can be safely avoided. Further, uNGAL can be easily measured in a clinical laboratory without the need for creatinine normalization. In our institution, uNGAL results are reported within 2 h of receipt of the sample in our laboratory.
There has been limited work in the literature focusing on uNGAL in UTI. Several studies have shown that uNGAL is increased in UTI in the general pediatric population [6, 25], but to our knowledge this is the first work to describe the ability of uNGAL to distinguish between colonization and UTI in CIC-dependent children. While results from the general pediatric population are not directly comparable to those presented in this work due to the underlying differences in the genitourinary epithelium that is seen within the neurogenic bladder population, our results also show increased uNGAL concentrations in UTI.
There are several potential confounders to uNGAL concentrations in this cohort that are related to the complex physiology of this patient population. In addition to the genitourinary epithelium, NGAL is also expressed by the gastrointestinal epithelium [26], and therefore patients with bladder augmentations may potentially have higher uNGAL levels than those without. However, our analysis shows that there are minimal differences in the predictive accuracy of uNGAL for UTI when patients with augmented bladders are included and excluded for the cohort, suggesting that the gastrointestinal epithelium is not a large contributor of uNGAL in this clinical setting. Other potential confounders include the possibility of obstruction, as uNGAL concentrations are elevated in the setting of acute urinary tract obstruction [27]. However, the increase in uNGAL concentrations in acute obstruction is likely due to acute kidney injury, which is not present in our cohort. Conversely, renal injury due to chronically increased bladder pressure results in chronic renal injury, and often occurs in conjunction with renal scarring due to associated vesicoureteral reflux [28]. Renal scarring has a doseresponse relationship with uNGAL concentrations, although the degree of increase is modest [29]. Therefore, while this may be confounding our results, the effect is limited, and likely does not affect the overall trends shown here. Finally, while patients with neurogenic bladders can develop increased pressure in the upper genitourinary tract, it is unclear which, if any, of the included patients had increased pressure and, if so, whether there were systematic differences among the groups. While much of the renal deterioration in this population begins in adolescence [30], our cohort was relatively young, suggesting that significant deterioration in renal function was less likely. Future work will focus on examining the relationship between biomarkers and changes in urodynamics in children with neurogenic bladder.
The other potential confounder is chronic kidney disease (CKD). While rapidly progressive forms of CKD are associated with significant increases in uNGAL concentrations, this is not seenin morequiescent forms of CKD that are classically seen in this population [31]. While CKD is present in a subset of our patients in this cohort, there is no difference in the proportion of patients with CKD between groups, suggesting that this is not a large confounder in our results. Finally, we have excluded patients with end-stage renal disease, as the effects of renal-replacement therapy on uNGAL concentrations is not well-described. Therefore, while CKD is present within a subset of patients in our cohort, it is unlikely to be significantly biasing these results.
Limitations of this work include the lack of a gold-standard method to diagnose UTI. As a result of the heterogeneity in criteria used to diagnose UTI, a standardized definition of UTI in the neurogenic bladder population was proposed for use in research [10]. We adapted this definition but chose to use a colony-count of 50,000 cfu/mL rather than the 100,000 cfu/ mL in the initial definition, based on the American Academy of Pediatrics definition of UTI in which 50,000 cfu/mL is considered a clinically significant bacterial burden [15]. Further, we acknowledge that this is an imperfect definition, with inclusion of symptoms that not all clinicians would agree suggest UTI (e.g., cloudy or foul-smelling urine), chosen for consistency in this area of research. However, only 1 of our 30 UTI patients met criteria for UTI based on the symptoms of foul-smelling urine and fever; the remainder had additional symptomatology that allowed them to be classified as UTI. Therefore, while imperfect, this definition is acceptable for use in this study. A more sensitive definition of UTI should include an indication of clinical improvement following antibiotic treatment, or lack of progression of symptoms in patients who are not treated. Additionally, while reports ofsymptoms were obtained through chart review, it is possible that pertinent positives were not recorded in the chart, thus leading to misclassification. This misclassification, which likely leads to the inaccurate classification of patients with UTI as UTC, may be responsible for the increased uNGAL concentration in the UTC group, compared to patients with no growth. Other limitations of this work include the lack of matching between groups. However, the differences in uNGAL levels between the genders and various ages is minimal, and thuslikely would not affect our results [32].Further, due to the small sample size of patients with UTI, we were unable to complete a sub-group analysis based on specific uropathogens.
Conclusion
Here, we provide data suggesting that uNGAL has utility in discriminating between UTI and UTC in children with neurogenic bladders who require CIC. Urinary NGAL has a high negative predictive value for UTI, suggesting that a low concentration of uNGAL at the point of care may identify patients at low risk for the diagnosis of UTI in whom antibiotics can be safely avoided.
Acknowledgements
We are grateful for the assistance of both Tara Terrell, MS, and Theresa Mottes, MSN, RN, from the Center for Acute Care Nephrology at Cincinnati Children’s Hospital Medical Center, for their assistance with sample collection. Tara Terrell does not have any conflicts of interest, industry relationships, or funding sources to report. Theresa Mottes is on the speaker’s bureau for Baxter.
Funding information CF received research training support from the National Institutes of Health through a National Research Service Award Institutional Training Grant, T32 HRSA 09-046 CFDA No. 93.186.
Footnotes
Compliance with ethical standards
Conflicts of interest Stuart Goldstein receives consulting fees and grant funding from Bioporto, Inc., which owns an assay for NGAL. No support from Bioporto, Inc., was used for the work reported in this manuscript.
The remainder of the authors do not have any conflicts of interest to disclose.
The study was approved by the Cincinnati Children’s Hospital Medical Center Institutional Review Board with a waiver of informed consent as the urine samples were discarded specimens.
References
- 1.Dedeić-Ljubović A, Hukić M (2009) Catheter-related urinary tract infection in patients suffering from spinal cord injuries. Bosn J Basic Med Sci 9:2–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forster CS, Courter J, Jackson E, Mortensen J, Haslam D (2017) Frequency of multidrug-resistant organisms cultured from urine in children on clean intermittent catheterization. J Pediatric Infect Dis Soc 6:332–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin J-N, Chen Y-H, Chang L-L, Lai C-H, Lin H-L, Lin H-H (2011) Clinical characteristics and outcomes of patients with extendedspectrum β-lactamase-producing bacteremias in the emergency department. Intern Emerg Med 6:547–555 [DOI] [PubMed] [Google Scholar]
- 4.Schmidt-Ott KM, Mori K, Li JY, Kalandadze A, Cohen DJ, Devarajan P, Barasch J (2007) Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol 18:407–413 [DOI] [PubMed] [Google Scholar]
- 5.Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, Akira S, Aderem A (2004) Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 432: 917–921 [DOI] [PubMed] [Google Scholar]
- 6.Hatipoglu S, Sevketoglu E, Gedikbasi A, Yilmaz A, Kiyak A, Mulazimoglu M, Aydogan G, Ozpacaci T (2011) Urinary MMP9/NGAL complex in children with acute cystitis. Pediatr Nephrol 26:1263–1268 [DOI] [PubMed] [Google Scholar]
- 7.KDIGO AKI Work Group (2012) KDIGO clinical practice guidelines for acute kidney injury. Kidney Int Suppl 2:1–138 [Google Scholar]
- 8.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ (1992) Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee American College of Chest Physicians/Society of Critical Care Medicine Chest 101:1644–1655 [DOI] [PubMed] [Google Scholar]
- 9.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarjan P (2005) Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 365: 1231–1238 [DOI] [PubMed] [Google Scholar]
- 10.Madden-Fuentes R, McNamara E (2013) Variation in definitions of urinary tract infections in spina bifida patients: a systematic review. Pediatrics 132:132–139 [DOI] [PubMed] [Google Scholar]
- 11.R: A language and environment for statistical computing (2013) Available from https://www.r-project.org/
- 12.Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, Muller M (2011) pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleming-Dutra KE, Hersh AL, Shapiro DJ, Bartoces M, Enns EA, File TM, Finkelstein JA, Gerber JS, Hyun DY, Linder JA, Lynfield R, Margolis DJ, May LS, Merenstein D, Metlay JP, Newlang JG, Piccirillo JF, Roberts RM, Sanchex GV, Suda KJ, Thomas A, Woo TM, Zetts RM, Hicks LA (2016) Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA 315:1864. [DOI] [PubMed] [Google Scholar]
- 14.Dotis J, Printza N, Marneri A, Gidaris D, Papachristou F (2013) Urinary tract infections caused by extended-spectrum betalactamase-producing bacteria in children : a matched case- control study. Turk J Paediatr 55:571–574 [PubMed] [Google Scholar]
- 15.Subcommittee on Urinary Tract Infection, Steering Committee on Quality Improvement and Management, Roberts KB (2011) Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics 128:595–610 [DOI] [PubMed] [Google Scholar]
- 16.Vaidyanathan S, Soni BM, Dundas S, Krishnan KR (1994) Urethral cytology in spinal cord injury patients performing intermittent catheterisation. Paraplegia 32:493–500 [DOI] [PubMed] [Google Scholar]
- 17.Schlager TA, Grady R, Mills SE, Hendley JO (2004) Bladder epithelium is abnormal in patients with neurogenic bladder due to myelomeningocele. Spinal Cord 42:163–168 [DOI] [PubMed] [Google Scholar]
- 18.Bachur R, Harper MB (2001) Reliability of the urinalysis for predicting urinary tract infections in young febrile children. Arch Pediatr Adolesc Med 155:60–65 [DOI] [PubMed] [Google Scholar]
- 19.Branson D (1966) Evaluaion of a rapid nitrate screening test for bacteriuria. Tech Bull Regist Med Technol 36:288–291 [PubMed] [Google Scholar]
- 20.Fouts DE, Pieper R, Szpakowski S, Pohl H, Knoblach S, Suh MJ, Huang ST, Sprague BM, Lucas SK, Torralba M, Nelson KE, Groah SL (2012) Integrated next-generation sequencing of 16S rDNA and metaproteomics differentiate the healthy urine microbiome from asymptomatic bacteriuria in neuropathic bladder associated with spinal cord injury. J Transl Med 10:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powell H, McCredie D, Ritchie M (1987) Urinary nitrite in symptomatic and asymptomatic urinary infection. Arch Dis Child 62: 138–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlager TA, Dilks S, Lohr J, Hayden GF, Kopco J, Hendley JW (1992) Periurethral colonization and urinary leukocytes as markers for bacteriuria in children with neurogenic bladder. Urol Res 20: 361–363 [DOI] [PubMed] [Google Scholar]
- 23.Paragas N, Kulkarni R, Werth M, Schmidt-ott KM, Forster C, Deng R, Zhang Q, Singer E, Klose AD, Shen TH, Francis KP, Ray S, Vijayakumar S, Seward S, Bovino ME, Xu K, Takabe Y, Amaral FE, Mohan S, Wax R, Corbin K, Sanna-Cherchi S, Mori K, Johnson L, Nickolas T, D’Agati V, Lin CS, Qui A, Al-Awqati Q, Ratner AJ, Barasch J (2014) The alpha-intercalated cell defends the urinary system from bacterial infection by excreting H+ and LNC2 in mouse and human urine. J Clin Invest 124:2963–2976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mori K, Lee HT, Rapoport D, Drexler IR, Foster K, Yang J, Schmidt-Ott KM, Chen X, Li JY, Weiss S, Mishra J, Cheema FH, Markowitz G, Suganami T, Sawai K, Mukoyama M, Kunis C, D’Agati V, Devarajan P, Barasch J (2005) Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest 115:610–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yilmaz A, Sevketoglu E, Gedikbasi A, Karyagar S, Kiyak A, Mulazimoglu M, Aydogan G, Ozpacaci T, Hatipoglu S (2009) Early prediction of urinary tract infection with urinary neutrophil gelatinase associated lipocalin. Pediatr Nephrol 24:2387–2392 [DOI] [PubMed] [Google Scholar]
- 26.Nielsen BS, Borregaard N, Bundgaard JR, Timshel S, Sehested M, Kjeldsen L (1996) Induction of NGAL synthesis in epithelial cells of human colorectal neoplasia and inflammatory bowel diseases. Gut 38:414–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sise ME, Forster C, Singer E, Sola-Del Valle D, Hahn B, SchmidtOtt KM, Barasch J, Nickolas TL (2011) Urine neutrophil gelatinase-associated lipocalin identifies unilateral and bilateral urinary tract obstruction. Nephrol Dial Transplant 26:4132–4135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shiroyanagi Y, Suzuki M, Matsuno D, Yamazaki Y (2009) The significance of 99m technetium dimercapto-succinic acidrenal scan in children with spina bifida during long-term follow up. J Urol 181:2262–2266 [DOI] [PubMed] [Google Scholar]
- 29.Parmaks℩z G, Noyan A, Dursun H, İnce E, Anarat R, Cengiz N (2016) Role of new biomarkers for predicting renal scarring in vesicoureteral reflux: NGAL, KIM-1, and L-FABP. Pediatr Nephrol 31:97–103 [DOI] [PubMed] [Google Scholar]
- 30.González Celedón C, Bitsori M, Tullus K (2007) Progression of chronic renal failure in children with dysplastic kidneys. Pediatr Nephrol 22:1014–1020 [DOI] [PubMed] [Google Scholar]
- 31.Nickolas TL, Forster CS, Sise ME, Barasch N, Valle DS-D, Viltard M, Buchen C, Kupferman S, Carnevali BM, Mattei S, Bovino A, Argentiero L, Magnano A, Devarajan P, Mori K, ErdjumentBromage H, Tempst P, Allegri L, Barasch J (2012) NGAL (Lcn2) monomer is associated with tubulointerstitial damage in chronic kidney disease. Kidney Int 82:718–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bennett MR, Nehus E, Haffner C, Ma Q, Devarajan P (2014) Pediatric reference ranges for acute kidney injury biomarkers. Pediatr Nephrol 30:677–685 [DOI] [PMC free article] [PubMed] [Google Scholar]