Abstract
Many evidences support that species from the Human Oral Microbiome Database such as Fusobacterium nucleatum or Bacteroides, linked previously to periodontitis and appendicitis, play a role in colorectal cancer (CRC), including metastasis. These typically oral species are invasive anaerobes that form biofilms in their virulent state.
Aspirin (a NSAID) has been recently included into routine CRC prevention rationale. NSAIDs can prevent the growth of neoplastic lesions by inhibiting COX enzymes and another set of recently identified COX-independent targets, which include the WNT, AMPK and MTOR signaling pathways, the crosstalk between nucleoli and NF-κB transcriptional activity in apoptosis, and the biochemistry of platelets. These are signaling pathways related to tumor-promoting inflammation. In this process, pathogens or simple deregulation of the microbiota play an important role in CRC. Aspirin and other NSAIDs are efficient inhibitors of biofilm formation and able to control periodontitis development preventing inflammation related to the microbiota of the gingival tissue, so its seems plausible to include this pathway in the mechanisms that aspirin uses to prevent CRC.
We propose arguments suggesting that current oral hygiene methods and other future developments against periodontitis might prevent CRC and probably other cancers, alone or in combination with other options; and that the multidisciplinary studies needed to prove this hypothesis might be relevant for cancer prevention.
Keywords: Microbiology, Cancer research, Evidence-based medicine, Dentistry
1. Introduction
1.1. Inflammation and immunity in cancer
Accumulation of mutations in genes encoding either homeostatic regulators or DNA mismatch-repair factors, predominantly in stem cells becoming CSCs (cancer stem cells) is the leading event in carcinogenesis [1, 2]. The crucial role of the immune system in this process, however, didn't came into worldwide attention until Hanahan & Weinberg's update of their “Hallmarks of Cancer” in 2011 [3]. These Hallmarks focused on immunosuppression, a better-known mechanism, on one hand but also in inflammation as an enabling characteristic of tumor growth [4].
Inflammation and immune system are activated to eliminate pathogens and non-self molecules, or to control at least their dissemination or systemic colonization of the organism, but the same physiological responses induce repair mechanisms that recover the function and integrity of tissues in an organ with tumor [5]. Moreover, the inflammation and immunitary cellular infiltrates responsible for tumor initiation and early progression are the same that can destroy the tumors before immunosuppression [6, 7]. Therefore, both hallmarks are two sides of the same coin.
In cancer, there are many possible origins for the inflammatory response. The inflammation is called intrinsic when the mechanisms driving the cell transformation and cancer initiation, overexpression of oncogenes (such as Ras, Myc, Src, or RET) and microRNAs (such as mi-R155), damage and mutation of DNA, and reactive oxygen species (ROS) production in mitochondria, also activate a pro-inflammatory program (the production of chemokines, interferons (IFNs) and tissue rearranging enzymes) in the proper altered cell [4, 5, 6]. Extrinsic inflammation is mediated, by contrast, by infiltrating immune cells [4, 5, 6]. Again DNA or mitochondrial damage, oncogene activation, or endoplasmic reticulum (ER) stress and oxidative damage of the altered cell, in addition to the many signals of cellular stress generated by the damage of the malignant cell-surrounding tissue (because of mechanical, chemical or irradiation causes, or genetic syndromes, hypoxia, the excessive temperature, or nutrients, or by senescence), serve to activate that infiltration.
Chronic inflammation affects all phases of carcinogenesis. In a feedback loop, by the generation of mutations, modifications of functional proteins or epigenetic mechanisms, it drives cell transformation, particularly when a stem cell is affected and become CSCs [3, 4, 8, 9]. At the same time, more hematopoietic inflammatory cells are recruited on the developing cancer. The leukocyte infiltrate includes neutrophils, dendritic cells, macrophages and lymphocytes, all of them capable of secreting those cytokines, ROS, membrane perforating agents, growth factors and cellular products that contribute to the inflammatory milieu present in the environment of a developing tumor, favoring the tumor growth and, later, dissemination of the transformed cells [5, 10, 11].
1.2. Molecular mechanism of inflammation
The immune cells recruited to the tumor express multiple specialized families of pattern recognition receptors (PRRs) located at the plasma membrane, within intracellular vesicles, and in the cytosol. The most known are the Toll-like receptors (TLRs), NLRs (from Nod, nucleotide binding oligomerization domain, -like receptors), PYHIN (pyrin and HIN domain) receptors, C-type lectin receptors, RLRs (RIG-I, retinoic acid inducible gene-I, -like receptors), the oligoadenylate synthase (OAS) -like receptors, the related protein cyclic GMP-AMP synthase (cGAS), and DNA sensors such as STING (stimulator of interferon gene).
Many of them assemble to form signaling complexes, including inflammasomes initiated by NLR and PYHIN receptors and related to cellular oxidative stress, the myddosomes initiated by TLRs and the Interleukin-1 receptor (IL-1R) involving among others the MyD88 protein, and the mitochondrial antiviral signaling protein (MAVS) CARD filament initiated by RIG-1 [10,12].
To note, these “innate receptors”, at least TLR 2 and 4 and NLRP3, have been recently identified also in the proper tumorigenic altered cells or in metabolic tissues, adipocytes, myocytes, liver or pancreas (see below how systemic metabolic alterations such as obesity or diabetes also cause inflammation) [13, 14] in what may represent feedback loops.
These receptors are very promiscuous by definition, i.e. one particular or a very similar ligand may bind to different receptors in the same or in a different cell. Therefore, they identify DAMPS (damage-associated molecular patterns) as those endogenous components released from cells damaged by molecular stress [10, 11, 12], but they were first identified as receptors of PAMPs (pathogen-associated molecular patterns) or receptors that identify components from microbial pathogens, this fact of particular interest for later content of the review. Recent evidence indicate that the major DAMP driving host antitumor immune responses is tumor-derived DNA, sensed by the STING pathway in a certain population of intratumoral DCs, driving the production of type I IFNs and the resulting downstream T cell response (reviewed in [15]).
The same signal transduction pathway, adaptor protein MyD88, is shared by many of these receptors. Although in rodent models, MyD88 has shown a protective role in the development of colonic tumors, MyD88 innate immune signaling and specific microRNAs mechanistically control CRC chemoresistance (see next subheading). Likewise, the same inflammatory mediators induce the transcription factors nuclear factor-κB (NF-κB) and signal transducer and activator of transcription-3 (STAT-3) [[1], [8], [9], [10], [11], [12], [13], [14], [15], [16]].
The first one seems particularly relevant to gastrointestinal cancer development [5] and, in fact, NF-κB signaling pathway has been identified as a COX-independent target of NSAIDs (as reviewed later). STAT-3 of tumor, stromal and immune cells is activated by many of factors released by the tumor and tumor stroma, for example VEGF, interleukin-6 (IL-6), IL-10, IL-11, or IL-23, are transcriptionally regulated by STAT-3 regulates transcriptionally several of these cytokines generating a positive feedback loop [17]. In addition to the carcinogenesis mentioned above, they also allow the tumor to progress and metastasize by establishing a tumoral microenvironment (for example, angiogenesis), including the immunosuppressive mechanisms that prevent the effective immune surveillance.
Finally, it must be emphasized that all these inflammatory mechanisms also affect the response to therapy [1, 15, 18].
1.3. Gut microbiota and CRC
As IBD patients —with Crohn's disease or ulcerative colitis— have a several-fold increased susceptibility to CRC principally, they were considered typical examples of colitis-associated cancer (CAC), the first inflammation-related cancer [19, 20]. In this cancer, the intestinal epithelial barrier already includes in the healthy individual complex molecular and cellular interactions of commensal microbiota with the epithelial, mesenchymal and immune cells to achieve intestinal homeostasis, regeneration, and healing (reviewed in [21]). The gut microbiota composition can be altered by the presence of a specific bacterial species or modulated by an environmental change. The presence of different natural mutagens and carcinogens, of a metabolism provided by the commensal flora that requires or can be regulated by a specific enzymatic activity, or the straightforward interaction of the bacteria or their products with those innate receptors or sensors described above, all can generate either pro- or anti-inflammatory effects (reviewed in [11, 18]). At the beginning of the millennium the first experimental CAC models mimicking human CRC in rodents clearly established that immune cells, cytokines and the STAT-3 and NF-κB transduction pathways all participate in the induction of inflammatory colitis (revs [8, 10, 11, 12]).
The IBD patients also show increased susceptibility to lymphomas/leukemias and hepatocarcinomas among other tumors, which suggests that the pathological intestinal inflammation triggered by the commensal microbiota is responsible for the local tumor-promoting effects but also, through the immune system, to have additional deep systemic effects [11, 18, 21]. In addition, later on the text, reviewed data suggest new roles for the microbiota, for example in CRC metastasis.
Likewise, most of the known main risk factors for CRC, obesity, lack of exercise, fat-rich diets, use of alcohol and tobacco [8, 22], are related with nutrition, being environmental agents that can alter the commensal microbiota [7, 11, 13, 14, 15, 16]. As the wide geographical variation in the incidence of CRC are lost in migrating populations within one generation, it is likely that these risk factors are responsible of CRC in the host populations [22, 23].
Two enterobacterial species, Klebsiella pneumoniae and Proteus mirabilis, participated in the development of colitis and cancer in TRUC (immunodeficient Tbet−/− and Rag2−/− ulcerative colitis) mice [24, 25], however, they didn't induce colitis alone but modify either the healthy gut microbiota composition or its physiology, acting in synergy to activate colon inflammation.
Actually, another fact suggesting the role of the commensal flora in CRC is that germfree animals [26], or treated with mildly colitogenic bacterium Bacteroides vulgatus (the azoxymethane (AOM)-treated Il10−/− model) [27], do not develop tumors. Helicobacter hepaticus infection of the gut enhanced small intestine and colon cancer in APCmin/+ mice, but also mammary adenocarcinoma [28] or hepatocarcinomas [29], thus showing systemic tumor-promoting effects. Likewise, infection with Helicobater pylori in humans is an example that a single bacterial species can trigger carcinogenesis, in this case gastric cancer [30].
Other pathogenic species identified while elucidating molecular mechanisms affected by the microbiota were the enteropathogenic Bacteroides fragilis (activated WNT and NF-κB signalling pathways on the carcinogenic cells) and Escherichia coli, also associated with increased risk of CRC (through actions that down-regulate mismatch-repair genes, at least in vitro [11, 12, 13, 14, 15, 16, 17, 31, 32]). This enteric microbiota also influences the T helper 17 (Th17) response [33, 34] and APC, in particular dendritic cells (DCs), activation [24].
More recently in Nlrp6−/− mice the expansion of other anaerobic genus of bacterial phyla Bacteroidetes (like Bacteroides), Prevotellaceae (traditionally related to periodontal disease), and TM7 (Saccharibacteria, interestingly, recently cultured from the human oral cavity) in the fecal microbiota correlated with susceptibility to colitis [35, 36]. Therefore, at least the NLRP6 receptor of the inflammasome in the innate system [10, 12] also regulates colonic microbial ecology.
However, although IBDs are the main risk factor for CRC, the relative increased risk for colorectal cancer in these patients is only threefold with respect to the control population, the tumors are generated after many years of intestinal pathology, and the cumulative lifetime risk is 18% [19, 20], i.e. most CRCs are not related to any pre-existing obvious intestinal inflammatory disease.
2. Main text
2.1. Gut microbiota of oral origin and biofilms in CRC
In parallel, high-throughput DNA sequencing developments ignited the identification of microbiota that affected the progression of CRC, not only in the experimental animal models mentioned above [37] but also in humans (in biopsies or stool). The marked over-representation of Fusobacterium nucleatum sequences in CRC tumors relative to control specimens was a striking discovery: Fusobacteria are rare constituents of the fecal microbiota, but had been cultured previously from biopsies of inflamed gut mucosa [21, 38], and F. nucleatum in particular is an invasive anaerobe that had been linked previously to periodontitis and appendicitis, but not to cancer.
This and the other anaerobic species of the same or similar genera including Bacteroides, or typically oral species such as some Selenomonas, Prevotella, and Parvimonas micra and Peptostreptococcus stomatis, some mentioned in the previous subheadings, are species included in the Human Oral Microbiome Database and also found in the gut microbiota in a significant percentage of CRC, supporting a role for the aggregate of them in CRC [39, 40, 41], although no microbial feature was universally present in the tumors. This story is very similar to the role that bacteria are playing in the pathogenesis of autoimmune rheumatoid arthritis, opening the door to the study of auto-antibodies against these bacteria both for diagnostic uses and mechanistical studies [42].
Interestingly, these are bacteria capable of forming biofilms. Biofilm structures are a consistent feature of right-sided (proximal) (although not left-sided, distal) CRC. They are constituted by symbionts of Bacteroides fragilis and other mentioned oral pathogens, having tumorigenesis capacity in the mucus layer of the gut [41] by disrupting the normal mucosal barrier, changing the tissue homeostasis, and inducing inflammation [43, 44]. In addition, these structures contribute to the polyamine metabolite pool altering the cancer metabolome and inducing colon cancer growth and progression [43, 45]. However, bacteria originally studied in oral biofilms were also detected in healthy individuals. It was found that a high abundance of Lachnospiraceae avoided the colonization of colonic tissue by oral-like bacterial networks, suggesting that certain microbiota types can protect against CRC, possibly by conferring colonization resistance. Interestingly, these protective types might be dependent of a healthy diet [39].
Some people expected a future similar to that of Helicobacter pylori and stomach cancer, and recently a mouse model of CRC showed that the antibiotic metronidazole partially impaired colorectal tumor growth [30]. Metronidazole is used for some genitourinary infections, but the target in that study was Fusobacterium nucleatum [30]. One concern of this study, apart of the lack of a F. nucleatum specific antibiotic is that other players are also involved.
A novel study in humans has shown that the abundance of F. nucleatum in feces is strongly associated with the presence of CRC but not with the presence of advanced adenomas or non-advanced adenomas, and no association with dietary or lifestyle habits was found [46]. In other study [47], bacterial biofilms found in patients with familial adenomatous polyposis showed increased interleukin-17 and DNA damage in colonic epithelium and faster tumor onset and greater mortality when transplanted to mice. However, their predominant species are Escherichia coli and Bacteroides fragilis [47]. These observations support the hypothesis that Fusobacterium is a passenger that multiplies when the tumor environment is favorable rather than a causal factor in tumorigenesis. Recent reviews [48, 49] discuss this issue, concluding that both initiation and progression of CRC require the organization of these higher-order structures of bacterial communities termed biofilms [50]. Flynn et al have proposed a model in which initial changes in the normal gut microbiota allow oral bacteria such as F. nucleatum to colonize the gut mucosa disrupting the epithelial barrier, and to form biofilms with early and late-colonizing microbes; and the persistent state of inflammation in the colon sustaining both the biofilm and the tumorigenesis [50]. Interestingly, although biofilm formation has been explained as a cooperative enterprise of strains and species for a common goal, biofilm formation should be understood as a response to ecological competition [51].
Strikingly, the presence of F. nucleatum in CRC has been related to the process of metastasis, at least to the liver [30] and lymph node [38]. Although the mechanism is unknown, these bacteria might interact with the metastatic cells [30] and not only be present in the mucosal microbiota where they can adhere to epithelial tumor cells or be found inside the cells [30, 52]. Very recently it has been suggested that F. nucleatum orchestrates on tumor cells a molecular network of the TLR4 and MyD88 innate immune signaling as well as specific microRNAs that activate the autophagy pathway and mechanistically control CRC chemoresistance [52].
Likewise, there are another examples of biofilms associated with cancer, such as Salmonella typhi and development of gallbladder cancer [53], and others should be expected in the multiple organ systems spanning these microbial networks [54].
In this sense, the use of probiotics should also be tested for a hypothetical long-term effect on metastasis or recurrence in patients with these CRC-related microbiota [51, 55]. Emerging human microbiome intervention strategies have been recently reviewed [18, 56, 57]. In addition, conventional chemotherapeutic regimens might be affected by this CRC microbiota and/or will modify it.
2.2. Obesity, metabolic syndrome, cancer cachexia, stress and microbiota
Recent studies have confirmed the association between obesity and cancer susceptibility and survival after detection, including CRC [58, 59] despite the so-called “obesity-paradox” [60]. There are still many challenges exploring the association of cancer with overweight and obesity, but consensus exists about that long-term efforts in prevention should remain focused not only on further reduction of smoking but also engaging in healthy lifestyles, balanced diets and regular physical exercise [58, 61, 62].
Metabolic syndrome is a controversial concept associated with abdominal obesity, blood lipid disorders, inflammation, insulin resistance or full-blown diabetes, and increased risk of developing cardiovascular disease. Abdominal obesity is the most prevalent manifestation of metabolic syndrome and a marker of ‘dysfunctional adipose tissue’ [63] or metaflammation [64]. This metabolic inflammatory state is chronic and low-grade, and has been found not only in adipocytes but also in stromal and immune cells of metabolic tissues that include liver, muscle, pancreas or brain, in response to an excess of nutrients and energy [64]. Decades of research have identified the complex signaling networks between immune response and metabolism, and the cellular and molecular events that take place in situations of altered nutrient or energy flows [7, 13, 14, 15, 16, 65, 66]. Maintaining this delicate balance is crucial for health.
How risk factors in general interact with commensal flora in colorectal carcinogenesis has been reviewed [7, 10, 21]. A recent review exposes how obesity alters the microenvironment of adipose tissue affecting the adipokine secretome, with actions on remote tissues [67]. Moreover, adipocytes, like tumor cells and macrophages, also express TLR 2 and 4. These receptors in adipose tissues can be reached not only by nonbacterial agonists such as diet saturated fatty acids, but also by the commensal flora or their bacterial products such as LPS and lipopeptides, and activate them (rev in [11, 13, 21, 68, 69]).
Obesity is also associated with changes in the composition of the gut microbiota. Overall, bacterial diversity of obese individuals is reduced, in the rate between phylum (an increase in Firmicutes and a corresponding decrease in Bacteroidetes), and in the representation of genes and metabolic pathways that favor energy harvest in bacteria [70].
Intestinal microbiota imbalance (dysbiosis or dismicrobism) has been suggested as the link between IBD, CRC and Type 2 diabetes (T2D) [22, 40, 41, 42, 71]. However, the arrival of the concept of enterotype suggests that this knowledge will be enhanced soon [72, 73]. Although we did not find any report linking gut Fusobacterium nucleatum with obesity, it has been recently reported a relationship of obesity with the subgingival microbiota in periodontal disease [74].
Inflammation and energy metabolism are linked in the wasting syndrome, also called cancer cachexia, present in many patients of certain types of cancer at advanced stages [75, 76, 77]. For example, liver metabolism in cachexia leads to suppression of antitumor immunity mainly through IL-6 [78]. Importantly, this axe of interactive metabolic pathways and immune system can be targeted by psychological stress, particularly those pathways mediated by the sympathetic nervous system [79]. For a general review of the tight coordination between the immune and nervous systems see the review by Talbot S et al [80].
The central nervous system also influences the commensal microbiota through several pathways of the gastrointestinal physiology, for example neurotransmitters affecting epithelial functions such as cell permeability and their production of mucus and antibacterial peptides. Conversely, gut microbiota is interacting with local and systemic inflammatory and immune cells and activating the production of inflammatory metabolites such as cytokines that may reach the brain and affect behavior [81, 82, 83]. This bidirectional axe nervous system-intestinal microbiota can also be, therefore, targeted by stress. Stress is likely to alter the innate immune responses to pathogens generating dysbiosis. Alteration of the gut enterotypes can modify the inflammatory environment and, with the consequent feedback loop, affect the energy metabolism, not only in the tumor but also systemically.
2.3. CRC prevention by nonsteroidal anti-inflammatory drugs
After many studies had shown that aspirin and other non-steroidal anti-inflammatory drugs (NSAIDs) used in a daily basis for extended periods (at least 5 years of aspirin use) reduced the risk of CRC or polyp recurrence, and a recommendation against its use for the prevention of CRC by the U.S. Preventive Services Task Force (USPSTF) in 2007 [84] because of the considerable damage induced to stomach and intestinal lining, or even brain bleeding [85], the USPSTF reversed that position in 2015 [86,87]. Low-dose aspirin use among certain subgroups of adults distinguished aspirin as the first pharmacologic agent for chemoprevention of cancer in a population not characterized as high risk [88, 89]. Additional research into the effect of long-term aspirin use on the overall incidence of cancer according to a range of doses and by subgroups, including age, sex, baseline cancer risk, or comorbid conditions [90, 91], as well as on the additional impact of aspirin use in the setting of CRC screening, including colonoscopy already associated with a significantly lower risk of CRC [89, 92], was addressed in a 2016 study by two large prospective U.S. cohort studies [93]. Regular aspirin use could prevent many thousands of gastrointestinal tumors per year only in the USA, taking into account that only 58% of the eligible population had undergone an accepted screening option [94, 95]. Noteworthy, the regular aspirin use could also prevent CRC among the adults aged 50–75 that underwent CRC screening, as the risk of developing distant metastasis (for overall cancer) is also reduced [93, 96, 97].
Mechanistically, typical NSAIDs aspirin and ibuprofen inhibit cyclooxygenases COX-1 and COX-2. Both enzymes catalyze the production of prostaglandins from fatty acids. COX-2 is up-regulated in tumors at early stages of most cancer types [98, 99, 100, 101, 102, 103]. Inhibition of COX induces apoptosis and prevents the growth of neoplastic lesions (rev in [104]).
However, the effects of COX-2 and prostaglandins are complex because affect also the inflammatory microenvironment and not only the transformed epithelial cells (reviewed in [101, 102, 103]). Also, the tumor growth inhibitory properties of NSAIDs cannot be reversed by addition of prostaglandins, and NSAID-related metabolites that do not inhibit apparently the enzymatic activity of COXs retain their anti-tumor properties. Consequently, there are other COX-independent targets of NSAIDs; the identified so far include signaling pathways such as WNT, AMPK and MTOR (reviewed in [105]) and, in the last years, the nucleoli-dependent regulation of NF-κB transcriptional activity that participates in apoptosis [104].
It has been also proposed that aspirin acetylates COX-1 in platelets consequently inactivating their function, and that this is the only mechanism that can explain the anti-tumor properties of aspirin when taken at low dose, but COX-1-independent effects of aspirin in platelets have also been observed (revs [106, 107]).
Efforts to overcome the limitations of side effects in the initial trials included the use of drug combinations and repurposed or chemically modified NSAIDs such as indole acetic acids, N-phenylanthranilates and aryl propionic acids, and derivatives of sulindac, nitro-NSAIDs and phospho- NSAIDs (second generation NSAIDs) that would retain the anti-inflammatory activities of traditional NSAID without blunting the gastrointestinal cytoprotection sustained by COX1-derived products such as PGE2 (reviewed in [108, 109, 110]). Overall, the clinical trials using NSAIDs for cancer prevention strongly supports the notion that inflammation is an underlying cause of cancer.
However, as minimal gastrointestinal damage and cardiovascular toxicity has not yet achieved [111], other anti-inflammatory drugs targeting different pathways, or in a different way, are being studied. Among the numerous in vitro and in vivo studies that have demonstrated phytochemicals strong antioxidant, anti-inflammatory and anti-cancer properties by regulating specific signaling pathways and molecular markers, the role of sulforaphane, curcumin and resveratrol on prevention and inhibition of CRC development should be noted in particular [112, 113]. The latter review summarizes the antiproliferative actions of resveratrol and its derivative 2,3,5,4′-tetrahydroxystilbene-2-O-β-glucoside (THSG).
2.4. Oral biofilms and periodontitis prevention by NSAIDs
Severe chronic periodontitis affects around 11% of the global adult population [114]. Periodontal disease is caused when biofilms replace the commensal microflora on the dental plaque surface and promote the chronic inflammatory destruction of periodontal tissue. In dentistry, the effects of biofilms on caries and periodontal diseases, and also in oral cancer, are well known, and approaches to fight biofilms are multiple (reviewed in [115, 116, 117, 118, 119]). A recent review have summarized current understanding of the metabolism of the oral microbiome: Saccharolytic bacteria –for example Streptococcus, Actinomyces, and Lactobacillus species- convert carbohydrates into organic acids, which are responsible of dental caries. Proteolytic/amino acid-degrading bacteria, including Prevotella and Porphyromonas species, by deamination finally produce short-chain fatty acids, ammonia, sulfur compounds, and indole/skatole, all of them virulent factors in periodontitis and oral malodor and that can cause oral cancer [120].
Fusobacterium nucleatum is also an important periodontal pathogen present in the bacterial complexity involved in the development of the biofilms [54, 121] and in the locally orchestrated immune responses of the gingival fibroblasts, the first line of defense against oral microorganisms [121]. Likewise, in infected oral epithelial cells, Porphyromonas gingivalis activates NFκB and MAPK pathways [122] and the growth and adherence of Staphylococcus aureus are enhanced through the PGE2 produced by the activated COX-2/PGE2 axe [123]. S. aureus, as well as other strains previously thought to be non-producers of biofilms, are always associated with biofilm producer bacteria in this type of polymicrobial colonization [124]. Interestingly, aspirin and other NSAIDs are efficient inhibitors of biofilm formation [125, 126, 127, 128, 129] and may control periodontitis though the inhibition of the gingival tissue microbioma-related inflammation [74, 130, 131], as in the examples cited above [122, 123]. Resveratrol and its glycosylated derivative THSG [113] have been, intriguingly, proposed for possible complementary treatments for periodontitis through their inhibition of Porphyromonas gingivalis-induced inflammatory responses in human gingival fibroblasts [132].
Recent studies demonstrated a functional relationship between platelets and tumors (reviewed in [105, 106]). Consequently, chemopreventive properties of aspirin may also depend, in part, from its direct modulation of platelet biology and biochemistry [105, 106].
Intriguingly, platelets enhance biofilm formation [133], pointing to the idea that another effect of aspirin on antitumor biology might be platelet-biofilm related.
2.5. The role of platelets in biofilm formation
Platelets, the anucleated circulating blood cells that are key players in haemostasis, tissue integrity and thrombosis by limiting blood loss and promoting wound healing, store different granules -rich in a plethora of proteins, lipids and other factors- including exosomes rich in genetic material, that they selectively release upon stimulation [134].
As mentioned, emerging studies are elaborating further on platelets as active players in all steps of tumorigenesis including tumor growth and tumor cell extravasation and metastasis. In crosstalk between platelets and cancer cells, the latter trigger the platelet granule and extracellular vesicle release when they form heterotypic aggregates, changing the platelet behaviour and phenotype and RNA profiles, plus enhancing thrombopoiesis [135]. In reciprocity, through the effect of those large amounts of factors released from platelet microparticles and exosomes with antiapoptotic plus proliferation signals and angiogenic factors, platelets reinforce tumor growth [135]. These factors additionally inhibit the immune system function that prevents tumor establishment and growth [136, 137], and induce phenotypic changes, via epithelial-mesenchymal transition, of the tumor cells, which acquire invasiveness and thus enhance their metastatic potential [134].
In addition, the heterotypic aggregates facilitate tumor cell survival in circulation, tumor cell arrest at the endothelium, and extravasation [138]. Likewise, platelets can aggregate with inflammatory cells perhaps providing an immune-evasive advantage [136].
These findings suggested that targeting the platelet may represent one of the preventive effects of low-dose aspirin, but also that this could be a novel strategy to treat cancer [134, 135, 137], together with the notion that immunotherapy might likely to benefit from the inclusion of platelet inhibitors, not only aspirin [136].
It has been recently emphasized [137] that apart of cyclooxygenase-dependent effects in platelets, recent developments of cyclooxygenase-independent effects include many protein targets of aspirin-mediated acetylation.
However, the existence of bacteria-platelet aggregates hasn't yet been contemplated in the field of oncology. But this reality is well known in other fields and should be taken into account as can be exemplified with several periodontitis-related bacteria. S. aureus causes, among other infections, infective endocarditis, a disease characterized by “vegetations”, a structure composed of platelets, bacteria, host factors such as fibrin, and bacterial products such as the β-toxin, a virulence factor with biofilm ligase enzymatic activity that also modulates platelet function [139]. In fact, a number of Streptococcus species have been found to interact with platelets and form thrombus (clots) in the blood [140]. S. gordonii, also amongst the common pathogens isolated from subjects with infective endocarditis, is ubiquitous within the human oral cavity. Two of their cell surface proteins mediate adherence and activation of platelets, binding to cellular fibronectin and vitronectin, and also promote formation of biofilms [141, 142]. Platelet aggregation was promoted by biofilms of oral bacteria in vitro, including P. gingivalis [142, 143, 144]. Interestingly, some commercially known antiseptic mouth rinses inhibited the aggregation [142]. Other biofilm-associated and platelet-binding bacteria described are Aerococcus urinae and Enterococcus faecalis [145, 146].
Another place where anaerobic bacteria/platelets aggregate is the storage devices of platelet concentrates that are used in transfusions [147, 148]. Bacterial contaminants have been implicated in adverse transfusion reactions worldwide because biofilms are a chief virulence mechanism in these hospital-acquired chronic infections [149, 150], and how to prevent the biofilm formation is being investigated [150].
2.6. Platelets and dissemination of biofilms
The formation of heterotypic aggregates is related to the two present hypotheses that try to explain how biofilms disseminate from the oral cavity to the colon, which remains to be elucidated [50]. One is a systemic dissemination from the ulcerated gingival pockets into the bloodstream that allows P. gingivalis or F. nucleatum access to the colon. In this context a biofilm-platelet complex make sense [133, 139, 140, 141, 142, 143, 144, 145, 146]. These species have been implicated in other diseases such as adverse pregnancy outcomes including stillbirth, liver cirrhosis, and rheumatoid arthritis [151]. Interestingly, the role of P. gingivalis in the breaking of tolerance and development of autoimmunity in rheumatoid arthritis has been extensively studied [42, 114]. A similar route may be via lymphatic drainage [152].
Other alternative route of infection [151] that cannot be ruled out is via swallowing of oral bacteria that reach the colon and alter the state of the microbiota in some of the explained ways [51].
3. Conclusions
Microbiota dysbiosis plays a role in carcinogenesis at organism-external environment interfaces such as the gut and the upper gastrointestinal tract but also the oral and bronchioalveolar mucosas or skin [48, 123]. Microbiota associated to periodontal disease has been implicated in CRC carcinogenesis through different not well-understood mechanisms. These typically oral anaerobic species form biofilms, a known virulence factor.
Periodontal treatments, such as supragingival scaling or root planing, have been already shown to have an impact in rheumatoid arthritis [153, 154]. As aspirin and other NSAIDs are efficient inhibitors of biofilm formation [125, 126, 127, 128, 129, 130, 131, 132], this might be another role of the anti-tumor effects of aspirin, through inhibition of inflammation in the oral mucosa and/or avoiding gut colonization by biofilms or biofilm/platelet aggregates. Aspirin or related NSAIDs might be included in the daily tooth cleaning as well as local antibiotic therapies. However, the developments to inhibit dental plaque biofilms in humans are in pre-clinical phases.
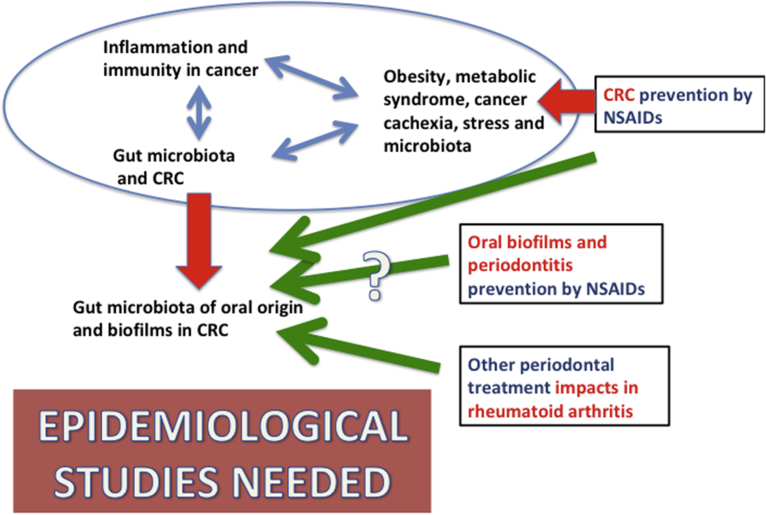
Finally, future multidisciplinary studies ought to have in mind the inclusion of patients' data about routine professional cleaning of dental plaques in epidemiological databases (Fig. 1). To our knowledge, only two epidemiological studies of this type have suggested [155] or showed [156] this direct relationship between periodontitis and cancer. If that relationship could be confirmed, oral hygiene might be a safer and easier approach to prevent CRC and probably other cancers and diseases, as it has been already suggested [157].
Fig. 1.
The red arrows point to demonstrated events in CRC: the role of biofilms and oral microbioma in inflammation-driven tumorigenesis and the preventive role of aspirin in CRC. The green arrows hypothesize that a) one of the pathways affected by aspirin might be the formation of biofilms in the gut; b) advances in periodontitis prevention, by NSAIDs or dental plaque cleaning might impact in cancer prevention; c) the latter has shown an impact on rheumatoid arthritis. However, only two epidemiological studies so far have suggested this relationship between periodontitis prevention and cancer.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This work was supported by the FEDER and Xunta de Galicia [grant number ED431D 2017/23 to the Galician Network for Colorectal Cancer Research (REGICC)].
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Oskarsson T., Batlle E., Massagué J. Metastatic stem cells: sources, niches, and vital pathways. Cell. Stem. Cell. 2014;14:306–321. doi: 10.1016/j.stem.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Melo F.S., Kurtova A.V., Harnoss J.M., Kljavin N., Hoeck J.D., Hung J. A distinct role for Lgr5+ stem cells in primary and metastatic colon cancer. Nature. 2017;543:676–680. doi: 10.1038/nature21713. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Fouad Y.A., Aanei C. Revisiting the hallmarks of cancer. Am. J. Cancer Res. 2017;7:1016–1036. [PMC free article] [PubMed] [Google Scholar]
- 5.Trinchieri G. Cancer and inflammation: an old intuition with rapidly evolving new concepts. Annu. Rev. Immunol. 2012;30:677–706. doi: 10.1146/annurev-immunol-020711-075008. [DOI] [PubMed] [Google Scholar]
- 6.Schreiber R.D., Old L.J., Smyth M.J. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 7.Smyth M.J., Ngiow S.F., Ribas A., Teng M.W. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat. Rev. Clin. Oncol. 2016;13:143–158. doi: 10.1038/nrclinonc.2015.209. [DOI] [PubMed] [Google Scholar]
- 8.Schetter A.J., Okayama H., Harris C.C. The role of microRNAs in colorectal cancer. Cancer J. 2012;18:244–252. doi: 10.1097/PPO.0b013e318258b78f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooks T., Harris C.C., Oren M. Caught in the cross fire: p53 in inflammation. Carcinogenesis. 2014;35:1680–1690. doi: 10.1093/carcin/bgu134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brubaker S.W., Bonham K.S., Zanoni I., Kagan J.C. Innate immune pattern recognition: a cell biological perspective. Annu. Rev. Immunol. 2015;33:257–290. doi: 10.1146/annurev-immunol-032414-112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dzutsev A., Badger J.H., Perez-Chanona E., Roy S., Salcedo R., Smith C.K., Trinchieri G. Microbes and cancer. Annu. Rev. Immunol. 2017;35:199–228. doi: 10.1146/annurev-immunol-051116-052133. [DOI] [PubMed] [Google Scholar]
- 12.Vajjhala P.R., Ve T., Bentham A., Stacey K.J., Kobe B. The molecular mechanisms of signaling by cooperative assembly formation in innate immunity pathways. Mol. Immunol. 2017;86:23–37. doi: 10.1016/j.molimm.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Kirwan A.M., Lenighan Y.M., O'Reilly M.E., McGillicuddy F.C., Roche H.M. Nutritional modulation of metabolic inflammation. Biochem. Soc. Trans. 2017;45:979–985. doi: 10.1042/BST20160465. [DOI] [PubMed] [Google Scholar]
- 14.Ralston J.C., Lyons C.L., Kennedy E.B., Kirwan A.M., Roche H.M. Fatty acids and NLRP3 inflammasome-mediated inflammation in metabolic tissues. Annu. Rev. Nutr. 2017;37:77–102. doi: 10.1146/annurev-nutr-071816-064836. [DOI] [PubMed] [Google Scholar]
- 15.Corrales L., Matson V., Flood B., Spranger S., Gajewski T.F. Innate immune signaling and regulation in cancer immunotherapy. Cell Res. 2017;27:96–108. doi: 10.1038/cr.2016.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marelli G., Sica A., Vannucci L., Allavena P. Inflammation as target in cancer therapy. Curr. Opin. Pharmacol. 2017;35:57–65. doi: 10.1016/j.coph.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Yu H., Lee H., Herrmann A., Buettner R., Jove R. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat. Rev. Cancer. 2014;14:736–746. doi: 10.1038/nrc3818. [DOI] [PubMed] [Google Scholar]
- 18.Roy S., Trinchieri G. Microbiota: a key orchestrator of cancer therapy. Nat. Rev. Cancer. 2017;17:271–285. doi: 10.1038/nrc.2017.13. [DOI] [PubMed] [Google Scholar]
- 19.Bernstein C.N., Blanchard J.F., Rawsthorne P., Yu N. The prevalence of extraintestinal diseases in inflammatory bowel disease: a population-based study. Am. J. Gastroenterol. 2001;96:1116–1122. doi: 10.1111/j.1572-0241.2001.03756.x. [DOI] [PubMed] [Google Scholar]
- 20.Pedersen N., Duricova D., Elkjaer M., Gamborg M., Munkholm P., Jess T. Risk of extra-intestinal cancer in inflammatory bowel disease: meta-analysis of population-based cohort studies. Am. J. Gastroenterol. 2010;105:1480–1487. doi: 10.1038/ajg.2009.760. [DOI] [PubMed] [Google Scholar]
- 21.Kurashima Y., Kiyono H. Mucosal ecological network of epithelium and immune cells for gut homeostasis and tissue healing. Annu. Rev. Immunol. 2017;35:119–147. doi: 10.1146/annurev-immunol-051116-052424. [DOI] [PubMed] [Google Scholar]
- 22.Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc. Nutr. Soc. 2008;67:253–256. doi: 10.1017/S002966510800712X. [DOI] [PubMed] [Google Scholar]
- 23.Kamangar F., Dores G.M., Anderson W.F. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J. Clin. Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 24.Garrett W.S., Punit S., Gallini C.A., Michaud M., Zhang D., Sigrist K.S. Colitis-associated colorectal cancer driven by T-bet deficiency in dendritic cells. Cancer Cell. 2009;16:208–219. doi: 10.1016/j.ccr.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garrett W.S., Gallini C.A., Yatsunenko T., Michaud M., DuBois A., Delaney M.L. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host Microbe. 2010;8:292–300. doi: 10.1016/j.chom.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vannucci L., Stepankova R., Kozakova H., Fiserova A., Rossmann P., Tlaskalova-Hogenova H. Colorectal carcinogenesis in germ-free and conventionally reared rats: different intestinal environments affect the systemic immunity. Int. J. Oncol. 2008;32:609–617. [PubMed] [Google Scholar]
- 27.Uronis J.M., Muhlbauer M., Herfarth H.H., Rubinas T.C., Jones G.S., Jobin C. Modulation of the intestinal microbiota alters colitis-associated colorectal cancer susceptibility. PLoS One. 2009;4 doi: 10.1371/journal.pone.0006026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rao V.P., Poutahidis T., Ge Z., Nambiar P.R., Boussahmain C., Wang Y.Y. Innate immune inflammatory response against enteric bacteria Helicobacter hepaticus induces mammary adenocarcinoma in mice. Cancer Res. 2006;66:7395–7400. doi: 10.1158/0008-5472.CAN-06-0558. [DOI] [PubMed] [Google Scholar]
- 29.Fox J.G., Feng Y., Theve E.J., Raczynski A.R., Fiala J.L., Doernte A.L. Gut microbes define liver cancer risk in mice exposed to chemical and viral transgenic hepatocarcinogens. Gut. 2010;59:88–97. doi: 10.1136/gut.2009.183749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bullman S., Pedamallu C.S., Sicinska E., Clancy T.E., Zhang X., Cai D. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science. 2017;358:1443–1448. doi: 10.1126/science.aal5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mármol I., Sánchez-de-Diego C., Pradilla Dieste A., Cerrada E., Rodriguez Yoldi M.J. Colorectal carcinoma: a general overview and future perspectives in colorectal cancer. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18010197. pii: E197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gagnaire A., Nadel B., Raoult D., Neefjes J., Gorvel J.P. Collateral damage: insights into bacterial mechanisms that predispose host cells to cancer. Nat. Rev. Microbiol. 2017;15:109–128. doi: 10.1038/nrmicro.2016.171. [DOI] [PubMed] [Google Scholar]
- 33.Wu S., Rhee K.J., Albesiano E., Rabizadeh S., Wu X., Yen H.R. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat. Med. 2009;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sears C.L. Enterotoxigenic Bacteroides fragilis: a rogue among symbiotes. Clin. Microbiol. Rev. 2009;22:349–369. doi: 10.1128/CMR.00053-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elinav E., Strowig T., Kau A.L., Henao-Mejia J., Thaiss C.A., Booth C.J. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145:745–757. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arthur J.C., Perez-Chanona E., Mühlbauer M., Tomkovich S., Uronis J.M., Fan T.J. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120–123. doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kostic A.D., Gevers D., Pedamallu C.S., Michaud M., Duke F., Earl A.M. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012;22:292–298. doi: 10.1101/gr.126573.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castellarin M., Warren R.L., Freeman J.D., Dreolini L., Krzywinski M., Strauss J. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012;22:299–306. doi: 10.1101/gr.126516.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flemer B., Warren R.D., Barrett M.P., Cisek K., Das A., Jeffery I.B. The oral microbiota in colorectal cancer is distinctive and predictive. Gut. 2018;67:1454–1463. doi: 10.1136/gutjnl-2017-314814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eklöf V., Löfgren-Burström A., Zingmark C., Edin S., Larsson P., Karling P. Cancer-associated fecal microbial markers in colorectal cancer detection. Int. J. Cancer. 2017;141:2528–2536. doi: 10.1002/ijc.31011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Drewes J.L., White J.R., Dejea C.M., Fathi P., Iyadorai T., Vadivelu J. High-resolution bacterial 16S rRNA gene profile meta-analysis and biofilm status reveal common colorectal cancer consortia. NPJ Biofilms Microbiomes. 2017;3:34. doi: 10.1038/s41522-017-0040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cordero O.J., Varela-Calviño R., López-González T., Grujic M., Juranic Z., Mouriño C. Anti-CD26 autoantibodies are involved in rheumatoid arthritis and show potential clinical interest. Clin. Biochem. 2017;50:903–910. doi: 10.1016/j.clinbiochem.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 43.Johnson C.H., Dejea C.M., Edler D., Hoang L.T., Santidrian A.F., Felding B.H. Metabolism links bacterial biofilms and colon carcinogenesis. Cell Metab. 2015;21:891–897. doi: 10.1016/j.cmet.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raskov H., Burcharth J., Pommergaard H.C. Linking gut microbiota to colorectal cancer. J Cancer. 2017;8:3378–3395. doi: 10.7150/jca.20497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guthrie L., Gupta S., Daily J., Kelly L. Human microbiome signatures of differential colorectal cancer drug metabolism. NPJ Biofilms Microbiomes. 2017;3:27. doi: 10.1038/s41522-017-0034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amitay E.L., Werner S., Vital M., Pieper D.H., Höfler D., Gierse I.J. Fusobacterium and colorectal cancer: causal factor or passenger? Results from a large colorectal cancer screening study. Carcinogenesis. 2017;38:781–788. doi: 10.1093/carcin/bgx053. [DOI] [PubMed] [Google Scholar]
- 47.Dejea C.M., Fathi P., Craig J.M., Boleij A., Taddese R., Geis A.L. Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science. 2018;359:592–597. doi: 10.1126/science.aah3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li S., Konstantinov S.R., Smits R., Peppelenbosch M.P. Bacterial biofilms in colorectal cancer initiation and progression. Trends Mol. Med. 2017;23:18–30. doi: 10.1016/j.molmed.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 49.Drewes J.L., Housseau F., Sears C.L. Sporadic colorectal cancer: microbial contributors to disease prevention, development and therapy. Br. J. Cancer. 2016;115:273–280. doi: 10.1038/bjc.2016.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Flynn K.J., Baxter N.T., Schloss P.D. Metabolic and community synergy of oral bacteria in colorectal cancer. mSphere. 2016;1 doi: 10.1128/mSphere.00102-16. pii: e00102-e00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oliveira N.M., Martinez-Garcia E., Xavier J., Durham W.M., Kolter R., Kim W., Foster K.R. Biofilm formation as a response to ecological competition. PLoS Biol. 2015;13(7) doi: 10.1371/journal.pbio.1002191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu T., Guo F., Yu Y., Sun T., Ma D., Han J. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell. 2017;170 doi: 10.1016/j.cell.2017.07.008. 548-563.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Di Domenico E.G., Cavallo I., Pontone M., Toma L., Ensoli F. Biofilm producing Salmonella typhi: chronic colonization and development of gallbladder cancer. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18091887. pii: E1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kodukula K., Faller D.V., Harpp D.N., Kanara I., Pernokas J., Pernokas M. Gut microbiota and salivary diagnostics: the mouth is salivating to tell us something. Biores. Open Access. 2017;6:123–132. doi: 10.1089/biores.2017.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hibberd A.A., Lyra A., Ouwehand A.C., Rolny P., Lindegren H., Cedgård L., Wettergren Y. Intestinal microbiota is altered in patients with colon cancer and modified by probiotic intervention. BMJ Open Gastroenterol. 2017;4(1) doi: 10.1136/bmjgast-2017-000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kundu P., Blacher E., Elinav E., Pettersson S. Our gut microbiome: the evolving inner self. Cell. 2017;171:1481–1493. doi: 10.1016/j.cell.2017.11.024. [DOI] [PubMed] [Google Scholar]
- 57.Van Raay T., Allen-Vercoe E. Microbial interactions and interventions in colorectal cancer. Microbiol. Spectr. 2017;5 doi: 10.1128/microbiolspec.bad-0004-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Campbell P.T., Newton C.C., Freedman N.D., Koshiol J., Alavanja M.C., Beane Freeman L.E. Body mass index, waist circumference, diabetes, and risk of liver cancer for U.S. Adults. Cancer Res. 2016;76:6076–6083. doi: 10.1158/0008-5472.CAN-16-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arnold M., Karim-Kos H.E., Coebergh J.W., Byrnes G., Antilla A., Ferlay J. Recent trends in incidence of five common cancers in 26 European countries since 1988: analysis of the European Cancer Observatory. Eur. J. Cancer. 2015;51:1164–1187. doi: 10.1016/j.ejca.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 60.Lennon H., Sperrin M., Badrick E., Renehan A.G. The obesity paradox in cancer: a review. Curr. Oncol. Rep. 2016;18:56. doi: 10.1007/s11912-016-0539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Renehan A.G., Zwahlen M., Egger M. Adiposity and cancer risk: new mechanistic insights from epidemiology. Nat. Rev. Cancer. 2015;15:484–498. doi: 10.1038/nrc3967. [DOI] [PubMed] [Google Scholar]
- 62.Arnold M., Leitzmann M., Freisling H., Bray F., Romieu I., Renehan A., Soerjomataram I. Obesity and cancer: an update of the global impact. Cancer Epidemiol. 2016;41:8–15. doi: 10.1016/j.canep.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 63.Despres J.P., Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 64.Gregor M.F., Hotamisligil G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 65.Hotamisligil G.S. Foundations of immunometabolism and implications for metabolic health and disease. Immunity. 2017;47:406–420. doi: 10.1016/j.immuni.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hotamisligil G.S. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542:177–185. doi: 10.1038/nature21363. [DOI] [PubMed] [Google Scholar]
- 67.Fuster J.J., Ouchi N., Gokce N., Walsh K. Obesity-induced changes in adipose tissue microenvironment and their impact on cardiovascular disease. Circ. Res. 2016;118:1786–1807. doi: 10.1161/CIRCRESAHA.115.306885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Engin A. The pathogenesis of obesity-associated adipose tissue inflammation. Adv. Exp. Med. Biol. 2017;960:221–245. doi: 10.1007/978-3-319-48382-5_9. [DOI] [PubMed] [Google Scholar]
- 69.Engin A.B. Adipocyte-macrophage cross-talk in obesity. Adv. Exp. Med. Biol. 2017;960:327–343. doi: 10.1007/978-3-319-48382-5_14. [DOI] [PubMed] [Google Scholar]
- 70.Heiss C.N., Olofsson L.E. Gut microbiota-dependent modulation of energy metabolism. J. Innate Immun. 2018;10:163–171. doi: 10.1159/000481519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jurjus A., Eid A., Al Kattar S., Zeenny M.N., Gerges-Geagea A., Haydar H. Inflammatory bowel disease, colorectal cancer and type 2 diabetes mellitus: the links. BBA Clin. 2015;5:16–24. doi: 10.1016/j.bbacli.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Costea P.I., Hildebrand F., Manimozhiyan A., Bäckhed F., Blaser M.J., Bushman F.D. Enterotypes in the landscape of gut microbial community composition. Nat. Microbiol. 2018;3:8–16. doi: 10.1038/s41564-017-0072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johnson E.L., Heaver S.L., Walters W.A., Ley R.E. Microbiome and metabolic disease: revisiting the bacterial phylum Bacteroidetes. J. Mol. Med. 2017;95:1–8. doi: 10.1007/s00109-016-1492-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maciel S.S., Feres M., Gonçalves T.E., Zimmermann G.S., da Silva H.D., Figueiredo L.C., Duarte P.M. Does obesity influence the subgingival microbiota composition in periodontal health and disease? J. Clin. Periodontol. 2016;43:1003–1012. doi: 10.1111/jcpe.12634. [DOI] [PubMed] [Google Scholar]
- 75.Donohoe C.L., Ryan A.M., Reynolds J.V. Cancer cachexia: mechanisms and clinical implications. Gastroenterol Res. Pract. 2011;2011:601434. doi: 10.1155/2011/601434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shyh-Chang N. Metabolic changes during cancer cachexia pathogenesis. Adv. Exp. Med. Biol. 2017;1026:233–249. doi: 10.1007/978-981-10-6020-5_11. [DOI] [PubMed] [Google Scholar]
- 77.Dong M., Lin J., Lim W., Jin W., Lee H.J. Role of brown adipose tissue in metabolic syndrome, aging, and cancer cachexia. Front. Med. 2018;12:130–138. doi: 10.1007/s11684-017-0555-2. [DOI] [PubMed] [Google Scholar]
- 78.Flint T.R., Fearon D.T., Janowitz T. Connecting the metabolic and immune responses to cancer. Trends Mol. Med. 2017;23:451–464. doi: 10.1016/j.molmed.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 79.Repasky E.A., Eng J., Hylander B.L. Stress, metabolism and cancer: integrated pathways contributing to immune suppression. Cancer J. 2015;21:97–103. doi: 10.1097/PPO.0000000000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Talbot S., Foster S.L., Woolf C.J. Neuroimmunity: physiology and pathology. Annu. Rev. Immunol. 2016;34:421–447. doi: 10.1146/annurev-immunol-041015-055340. [DOI] [PubMed] [Google Scholar]
- 81.Bercik P., Collins S.M. The effects of inflammation, infection and antibiotics on the microbiota-gut-brain axis. Adv. Exp. Med. Biol. 2014;817:279–289. doi: 10.1007/978-1-4939-0897-4_13. [DOI] [PubMed] [Google Scholar]
- 82.De Palma G., Lynch M.D., Lu J., Dang V.T., Deng Y., Jury J. Transplantation of fecal microbiota from patients with irritable bowel syndrome alters gut function and behavior in recipient mice. Sci. Transl. Med. 2017;9(379) doi: 10.1126/scitranslmed.aaf6397. pii: eaaf6397. [DOI] [PubMed] [Google Scholar]
- 83.Collins S.M. The intestinal microbiota in the irritable bowel syndrome. Int. Rev. Neurobiol. 2016;131:247–261. doi: 10.1016/bs.irn.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 84.USPSTF Routine aspirin or nonsteroidal anti-inflammatory drugs for the primary prevention of colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2007;146:361–364. [PubMed] [Google Scholar]
- 85.Rothwell P.M., Fowkes F.G., Belch J.F., Ogawa H., Warlow C.P., Meade T.W. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377:31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 86.Chubak J., Whitlock E.P., Williams S.B., Kamineni A., Burda B.U., Buist D.S., Anderson M.L. Aspirin for the prevention of cancer incidence and mortality: systematic evidence reviews for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2016;164:814–825. doi: 10.7326/M15-2117. [DOI] [PubMed] [Google Scholar]
- 87.Dehmer S.P., Maciosek M.V., Flottemesch T.J. Agency for Healthcare Research and Quality (US); Rockville (MD): 2015. Aspirin Use to Prevent Cardiovascular Disease and Colorectal Cancer: a Decision Analysis: Technical Report. 2015 Sep. Report No: 15-05229-EF-1. USPSTF Evidence Syntheses. [PubMed] [Google Scholar]
- 88.Draft Recommendation Statement: Aspirin to Prevent Cardiovascular Disease and Cancer. U.S. Preventive Services Task Force; http://www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement/aspirin-to-prevent-cardiovascular-disease-and-cancer.
- 89.Chan A.T., Ladabaum U. Where do we stand with aspirin for the prevention of colorectal cancer? The USPSTF recommendations. Gastroenterology. 2015;150:14–18. doi: 10.1053/j.gastro.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 90.Whitlock E.P., Williams S.B., Burda B.U., Feightner A., Beil T. Agency for Healthcare Research and Quality (US); Rockville (MD): 2015 Sep. Aspirin Use in Adults: Cancer, All-cause Mortality, and Harms: a Systematic Evidence Review for the U.S. Preventive Services Task Force. Report No.: 13-05193-EF-1. USPSTF Evidence Syntheses. [PubMed] [Google Scholar]
- 91.Sutcliffe P., Connock M., Gurung T., Freeman K., Johnson S., Kandala N.B. Aspirin for prophylactic use in the primary prevention of cardiovascular disease and cancer: a systematic review and overview of reviews. Health Technol. Assess. 2013;17:1–253. doi: 10.3310/hta17430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nishihara R., Wu K., Lochhead P., Morikawa T., Liao X., Qian Z.R. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N. Engl. J. Med. 2013;369:1095–1105. doi: 10.1056/NEJMoa1301969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cao Y., Nishihara R., Wu K., Wang M., Ogino S., Willett W.C. Population-wide impact of long-term use of aspirin and the risk for cancer. JAMA Oncol. 2016;1:762–769. doi: 10.1001/jamaoncol.2015.6396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Edwards B.K., Ward E., Kohler B.A., Eheman C., Zauber A.G., Anderson R.N. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.National Center for Health Statistics (US) National Center for Health Statistics (US); Hyattsville, MD: 2015. Health, United States, 2014: with Special Feature on Adults Aged 55–64. 2015 May. Report No.: 2015-1232. [PubMed] [Google Scholar]
- 96.Cuzick J., Thorat M.A., Bosetti C., Brown P.H., Burn J., Cook N.R. Estimates of benefits and harms of prophylactic use of aspirin in the general population. Ann. Oncol. 2015;26:47–57. doi: 10.1093/annonc/mdu225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cook N.R., Lee I.M., Zhang S.M., Moorthy M.V., Buring J.E. Alternate-day, low-dose aspirin and cancer risk: long-term observational follow-up of a randomized trial. Ann. Intern. Med. 2013;159:77–85. doi: 10.7326/0003-4819-159-2-201307160-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang D., Dubois R.N. Eicosanoids and cancer. Nat. Rev. Cancer. 2010;10:181–193. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sheehan K.M., Sheahan K., O'Donoghue D.P., MacSweeney F., Conroy R.M., Fitzgerald D.J., Murray F.E. The relationship between cyclooxygenase-2 expression and colorectal cancer. JAMA. 1999;282:1254–1257. doi: 10.1001/jama.282.13.1254. [DOI] [PubMed] [Google Scholar]
- 100.Oshima M., Dinchuk J.E., Kargman S.L., Oshima H., Hancock B., Kwong E. Suppression of intestinal polyposis in ApcΔ716 knockout mice by inhibition of cyclooxygenase 2 (COX-2) Cell. 1996;87:803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- 101.Su C.W., Zhang Y., Zhu Y.T. Stromal COX-2 signaling are correlated with colorectal cancer: a review. Crit. Rev. Oncol. Hematol. 2016;107:33–38. doi: 10.1016/j.critrevonc.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 102.Wang D., DuBois R.N. PPARδ and PGE(2) signaling pathways communicate and connect inflammation to colorectal cancer. Inflamm. Cell Signal. 2014;1 doi: 10.14800/ics.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang D., DuBois R.N. An inflammatory mediator, prostaglandin E2, in colorectal cancer. Cancer J. 2013;19:502–510. doi: 10.1097/PPO.0000000000000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen J., Stark L.A. Aspirin prevention of colorectal cancer: focus on NF-κB signalling and the nucleolus. Biomedicines. 2017;5 doi: 10.3390/biomedicines5030043. pii: E43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Drew D.A., Cao Y., Chan A.T. Aspirin and colorectal cancer: the promise of precision chemoprevention. Nat. Rev. Cancer. 2016;16:173–186. doi: 10.1038/nrc.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lasry A., Zinger A., Ben-Neriah Y. Inflammatory networks underlying colorectal cancer. Nat. Immunol. 2016;17:230–240. doi: 10.1038/ni.3384. [DOI] [PubMed] [Google Scholar]
- 107.Tsioulias G.J., Go M.F., Rigas B. NSAIDs and colorectal cancer control: promise and challenges. Curr. Pharmacol. Rep. 2015;1:295–301. doi: 10.1007/s40495-015-0042-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cuzick J. Preventive therapy for cancer. Lancet Oncol. 2017;18:e472–e482. doi: 10.1016/S1470-2045(17)30536-3. [DOI] [PubMed] [Google Scholar]
- 109.Penning T.M. Aldo-Keto Reductase (AKR) 1C3 inhibitors: a patent review. Expert Opin. Ther. Pat. 2017;27:1329–1340. doi: 10.1080/13543776.2017.1379503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rostom A., Dubé C., Lewin G., Tsertsvadze A., Barrowman N., Code C. Nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors for primary prevention of colorectal cancer: a systematic review prepared for the US Preventive Services Task Force. Ann. Intern. Med. 2007;146:376–389. doi: 10.7326/0003-4819-146-5-200703060-00010. [DOI] [PubMed] [Google Scholar]
- 111.Carnevali S., Buccellati C., Bolego C., Bertinaria M., Rovati G.E., Sala A. Nonsteroidal anti-inflammatory drugs: exploiting bivalent COXIB/TP antagonists for the control of cardiovascular risk. Curr. Med. Chem. 2017;24:3218–3230. doi: 10.2174/0929867324666170602083428. [DOI] [PubMed] [Google Scholar]
- 112.Yin T.F., Wang M., Qing Y., Lin Y.M., Wu D. Research progress on chemopreventive effects of phytochemicals on colorectal cancer and their mechanisms. World J. Gastroenterol. 2016;22:7058–7068. doi: 10.3748/wjg.v22.i31.7058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lin H.Y., Hsieh M.T., Cheng G.Y., Lai H.Y., Chin Y.T., Shih Y.J. Mechanisms of action of nonpeptide hormones on resveratrol-induced antiproliferation of cancer cells. Ann. N. Y. Acad. Sci. 2017;1403:92–100. doi: 10.1111/nyas.13423. [DOI] [PubMed] [Google Scholar]
- 114.Potempa J., Mydel P., Koziel J. The case for periodontitis in the pathogenesis of rheumatoid arthritis. Nat. Rev. Rheumatol. 2017;13:606–620. doi: 10.1038/nrrheum.2017.132. [DOI] [PubMed] [Google Scholar]
- 115.Chanda W., Joseph T.P., Wang W., Padhiar A.A., Zhong M. The potential management of oral candidiasis using anti-biofilm therapies. Med. Hypotheses. 2017;106:15–18. doi: 10.1016/j.mehy.2017.06.029. [DOI] [PubMed] [Google Scholar]
- 116.Noronha V.T., Paula A.J., Durán G., Galembeck A., Cogo-Müller K., Franz-Montan M., Durán N. Silver nanoparticles in dentistry. Dent. Mater. 2017;33:1110–1126. doi: 10.1016/j.dental.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 117.Slobodníková L., Fialová S., Rendeková K., Kováč J., Mučaji P. Antibiofilm activity of plant polyphenols. Molecules. 2016;21(12) doi: 10.3390/molecules21121717. pii: E1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gordya N., Yakovlev A., Kruglikova A., Tulin D., Potolitsina E., Suborova T. Natural antimicrobial peptide complexes in the fighting of antibiotic resistant biofilms: Calliphora vicina medicinal maggots. PLoS One. 2017;12(3) doi: 10.1371/journal.pone.0173559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Scorciapino M.A., Serra I., Manzo G., Rinaldi A.C. Antimicrobial dendrimeric peptides: structure, activity and new therapeutic applications. Int. J. Mol. Sci. 2017;18(3) doi: 10.3390/ijms18030542. pii: E542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Takahashi N. Oral microbiome metabolism: from “who are they?” to “what are they doing?”. J. Dent. Res. 2015;94:1628–1637. doi: 10.1177/0022034515606045. [DOI] [PubMed] [Google Scholar]
- 121.Ahn S.H., Chun S.M., Park C., Lee J.H., Lee S.W., Lee T.H. Transcriptome profiling analysis of senescent gingival fibroblasts in response to Fusobacterium nucleatum infection. PLoS One. 2017;12(11) doi: 10.1371/journal.pone.0188755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Groeger S., Jarzina F., Domann E., Meyle J. Porphyromonas gingivalis activates NFκB and MAPK pathways in human oral epithelial cells. BMC Immunol. 2017;18(1):1. doi: 10.1186/s12865-016-0185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang Y., Ren B., Zhou X., Liu S., Zhou Y., Li B. Growth and adherence of Staphylococcus aureus were enhanced through the PGE2 produced by the activated COX-2/PGE2 pathway of infected oral epithelial cells. PLoS One. 2017;12(5) doi: 10.1371/journal.pone.0177166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Di Domenico E.G., Farulla I., Prignano G., Gallo M.T., Vespaziani M., Cavallo I. Biofilm is a major virulence determinant in bacterial colonization of chronic skin ulcers independently from the multidrug resistant phenotype. Int. J. Mol. Sci. 2017;18(5) doi: 10.3390/ijms18051077. pii: E1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Madariaga-Venegas F., Fernández-Soto R., Duarte L.F., Suarez N., Delgadillo D., Jara J.A. Characterization of a novel antibiofilm effect of nitric oxide-releasing aspirin (NCX-4040) on Candida albicans isolates from denture stomatitis patients. PLoS One. 2017;12(5) doi: 10.1371/journal.pone.0176755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Marvasi M., Durie I.A., Henríquez T., Satkute A., Matuszewska M., Prado R.C. Dispersal of human and plant pathogens biofilms via nitric oxide donors at 4 °C. AMB Express. 2016;6(1):49. doi: 10.1186/s13568-016-0220-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rosato A., Catalano A., Carocci A., Carrieri A., Carone A., Caggiano G. In vitro interactions between anidulafungin and nonsteroidal anti-inflammatory drugs on biofilms of Candida spp. Bioorg. Med. Chem. 2016;24:1002–1005. doi: 10.1016/j.bmc.2016.01.026. [DOI] [PubMed] [Google Scholar]
- 128.El-Mowafy S.A., Abd El Galil K.H., El-Messery S.M., Shaaban M.I. Aspirin is an efficient inhibitor of quorum sensing, virulence and toxins in Pseudomonas aeruginosa. Microb. Pathog. 2014;74:25–32. doi: 10.1016/j.micpath.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 129.Abdelmegeed E., Shaaban M.I. Cyclooxygenase inhibitors reduce biofilm formation and yeast-hypha conversion of fluconazole resistant Candida albicans. J. Microbiol. 2013;51:598–604. doi: 10.1007/s12275-013-3052-6. [DOI] [PubMed] [Google Scholar]
- 130.Naqvi A.Z., Mu L., Hasturk H., Van Dyke T.E., Mukamal K.J., Goodson J.M. Impact of docosahexaenoic acid therapy on subgingival plaque microbiota. J. Periodontol. 2017;88:887–895. doi: 10.1902/jop.2017.160398. [DOI] [PubMed] [Google Scholar]
- 131.Van Dyke T.E. Control of inflammation and periodontitis. Periodontol. 2000. 2007;45:158–166. doi: 10.1111/j.1600-0757.2007.00229.x. [DOI] [PubMed] [Google Scholar]
- 132.Chin Y.T., Cheng G.Y., Shih Y.J., Lin C.Y., Lin S.J., Lai H.Y. Therapeutic applications of resveratrol and its derivatives on periodontitis. Ann. N. Y. Acad. Sci. 2017;1403:101–108. doi: 10.1111/nyas.13433. [DOI] [PubMed] [Google Scholar]
- 133.Jung C.J., Yeh C.Y., Shun C.T., Hsu R.B., Cheng H.W., Lin C.S., Chia J.S. Platelets enhance biofilm formation and resistance of endocarditis-inducing streptococci on the injured heart valve. J. Infect. Dis. 2012;205:1066–1075. doi: 10.1093/infdis/jis021. [DOI] [PubMed] [Google Scholar]
- 134.Xu X.R., Yousef G.M., Ni H. Cancer and platelet crosstalk: opportunities and challenges for aspirin and other antiplatelet agents. Blood. 2018 19;131:1777–1789. doi: 10.1182/blood-2017-05-743187. [DOI] [PubMed] [Google Scholar]
- 135.Contursi A., Sacco A., Grande R., Dovizio M., Patrignani P. Platelets as crucial partners for tumor metastasis: from mechanistic aspects to pharmacological targeting. Cell. Mol. Life Sci. 2017;74:3491–3507. doi: 10.1007/s00018-017-2536-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kanikarla-Marie P., Lam M., Sorokin A.V., Overman M.J., Kopetz S., Menter D.G. Platelet metabolism and other targeted drugs; potential impact on immunotherapy. Front. Oncol. 2018;8:107. doi: 10.3389/fonc.2018.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Haemmerle M., Stone R.L., Menter D.G., Afshar-Kharghan V., Sood A.K. The platelet lifeline to cancer: challenges and opportunities. Cancer Cell. 2018;33:965–983. doi: 10.1016/j.ccell.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ornelas A., Zacharias-Millward N., Menter D.G., Davis J.S., Lichtenberger L., Hawke D., Hawk E., Vilar E., Bhattacharya P., Millward S. Beyond COX-1: the effects of aspirin on platelet biology and potential mechanisms of chemoprevention. Cancer Metastasis Rev. 2017;36:289–303. doi: 10.1007/s10555-017-9675-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Herrera A., Kulhankova K., Sonkar V.K., Dayal S., Klingelhutz A.J., Salgado-Pabón W., Schlievert P.M. Staphylococcal β-toxin modulates human aortic endothelial cell and platelet function through sphingomyelinase and biofilm ligase activities. mBio. 2017;8(2) doi: 10.1128/mBio.00273-17. pii: e00273-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.George N.P., Ymele-Leki P., Konstantopoulos K., Ross J.M. Differential binding of biofilm-derived and suspension-grown Staphylococcus aureus to immobilized platelets in shear flow. J. Infect. Dis. 2009;199:633–640. doi: 10.1086/596316. [DOI] [PubMed] [Google Scholar]
- 141.Haworth J.A., Jenkinson H.F., Petersen H.J., Back C.R., Brittan J.L., Kerrigan S.W., Nobbs A.H. Concerted functions of Streptococcus gordonii surface proteins PadA and Hsa mediate activation of human platelets and interactions with extracellular matrix. Cell Microbiol. 2017;19(1) doi: 10.1111/cmi.12667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tu Y., Chen Y., Zheng C., Chen H. Platelet aggregation promoted by biofilms of oral bacteria and the effect of mouth rinses in vitro. J. Infect. Dev. Ctries. 2016;10:704–711. doi: 10.3855/jidc.6639. [DOI] [PubMed] [Google Scholar]
- 143.Tu Y., Huang W., Pan Z., Hu H., Chen H. Effect of Streptococcus sanguinis/Porphyromonas gingivalis single and combined biofilms upon platelet aggregation. Oral Dis. 2012;18:586–594. doi: 10.1111/j.1601-0825.2012.01913.x. [DOI] [PubMed] [Google Scholar]
- 144.Ali H., Greco-Stewart V.S., Jacobs M.R., Yomtovian R.A., Rood I.G., de Korte D., Ramírez-Arcos S.M. Characterization of the growth dynamics and biofilm formation of Staphylococcus epidermidis strains isolated from contaminated platelet units. J. Med. Microbiol. 2014;63:884–891. doi: 10.1099/jmm.0.071449-0. [DOI] [PubMed] [Google Scholar]
- 145.Shannon O., Mörgelin M., Rasmussen M. Platelet activation and biofilm formation by Aerococcus urinae, an endocarditis-causing pathogen. Infect. Immun. 2010;78:4268–4275. doi: 10.1128/IAI.00469-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Maddox S.M., Coburn P.S., Shankar N., Conway T. Transcriptional regulator PerA influences biofilm-associated, platelet binding, and metabolic gene expression in Enterococcus faecalis. PLoS One. 2012;7 doi: 10.1371/journal.pone.0034398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gregory M.A., Hadley G.P. The evolution of biofilms in venous access devices implanted in children with Wilms' tumour. Pediatr. Surg. Int. 1998;13:400–405. doi: 10.1007/s003830050349. [DOI] [PubMed] [Google Scholar]
- 148.Kumaran D., Kalab M., Rood I.G., de Korte D., Ramirez-Arcos S. Adhesion of anaerobic bacteria to platelet containers. Vox Sang. 2014;107:188–191. doi: 10.1111/vox.12141. [DOI] [PubMed] [Google Scholar]
- 149.Hodgson S.D., Greco-Stewart V., Jimenez C.S., Sifri C.D., Brassinga A.K., Ramirez-ArcosS Enhanced pathogenicity of biofilm-negative Staphylococcus epidermidis isolated from platelet preparations. Transfusion. 2014;54:461–470. doi: 10.1111/trf.12308. [DOI] [PubMed] [Google Scholar]
- 150.Greco C.A., Maurer-Spurej E., Scott M.D., Kalab M., Nakane N., Ramírez-Arcos S.M. PEGylation prevents bacteria-induced platelet activation and biofilm formation in platelet concentrates. Vox Sang. 2011;100:336–339. doi: 10.1111/j.1423-0410.2010.01419.x. [DOI] [PubMed] [Google Scholar]
- 151.Han Y.W., Wang X. Mobile microbiome: oral bacteria in extra-oral infections and inflammation. J. Dent. Res. 2013;92:485–491. doi: 10.1177/0022034513487559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Amodini Rajakaruna G., Umeda M., Uchida K., Furukawa A., Yuan B., Suzuki Y., Noriko E., Izumi Y., Eishi Y. Possible translocation of periodontal pathogens into the lymph nodes draining the oral cavity. J. Microbiol. 2012;50:827–836. doi: 10.1007/s12275-012-2030-8. [DOI] [PubMed] [Google Scholar]
- 153.Okada M., Kobayashi T., Ito S., Yokoyama T., Abe A., Murasawa A. Periodontal treatment decreases levels of antibodies to Porphyromonas gingivalis and citrulline in patients with rheumatoid arthritis and periodontitis. J. Periodontol. 2013;84:e74–e84. doi: 10.1902/jop.2013.130079. [DOI] [PubMed] [Google Scholar]
- 154.Cosgarea R., Tristiu R., Dumitru R.B., Arweiler N.B., Rednic S., Sirbu C.I., Lascu L., Sculean A., Eick S. Effects of non-surgical periodontal therapy on periodontal laboratory and clinical data as well as on disease activity in patients with rheumatoid arthritis. Clin. Oral Invest. 2018:1–11. doi: 10.1007/s00784-018-2420-3. [DOI] [PubMed] [Google Scholar]
- 155.Mai X., Genco R.J., LaMonte M.J., Hovey K.M., Freudenheim J.L., Andrews C.A., Wactawski-Wende J. Periodontal pathogens and risk of incident cancer in postmenopausal females: the buffalo OsteoPerio study. J. Periodontol. 2016;87:257–267. doi: 10.1902/jop.2015.150433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ahn J., Segers S., Hayes R.B. Periodontal disease, Porphyromonas gingivalis serum antibody levels and orodigestive cancer mortality. Carcinogenesis. 2012;33:1055–1058. doi: 10.1093/carcin/bgs112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gao S., Li S., Ma Z., Liang S., Shan T., Zhang M., Zhu X., Zhang P., Liu G., Zhou F., Yuan X., Jia R., Potempa J., Scott D.A., Lamont R.J., Wang H., Feng X. Presence of Porphyromonas gingivalis in esophagus and its association with the clinicopathological characteristics and survival in patients with esophageal cancer. Infect. Agents Cancer. 2016;11:3. doi: 10.1186/s13027-016-0049-x. [DOI] [PMC free article] [PubMed] [Google Scholar]