Abstract
A lower risk of prostate cancer has been reported in men with spinal cord injury (SCI) as compared to that observed in able-bodied subjects. As injury-related consequences can have opposite effects on prostate pathophysiology, this meta-analysis aimed to (1) establish the existence/quantify the extent of decreased prostate cancer risk following SCI and (2) find out if there is any statistically significant difference in prostate-specific antigen (PSA) levels between SCI and able-bodied subjects. MEDLINE, Cochrane Library, Scopus, CINAHL, and ScienceDirect databases were used. Only studies reporting a prostate cancer diagnosis and/or PSA levels following SCI and in able-bodied controls were included. Five studies provided information about prostate cancer on 35 293 subjects with SCI and 158 140 controls. Six studies were included in PSA analysis which reported information on 391 men with SCI and 1921 controls. Pooled estimates indicated that SCI reduced the prostate cancer risk by approximately 50% as compared to controls, whereas differences in PSA levels were not statistically significant. Funnel plots suggested the presence of publication bias only in PSA analysis. Between-study heterogeneity was established and when, according to meta-regression models, analysis was restricted to studies including men with mean age over 55 years, prostate cancer risk in SCI decreased up to 65.0% than that in controls with no heterogeneity (P = 0.33, I2 = 9%). In conclusion, in men over 55 years old, SCI decreases the prostate cancer risk up to 65.0% than that in controls. The large between-study heterogeneity on PSA confirms its poor reliability as a screening tool for prostate cancer in SCI.
Keywords: paraplegia, prostate cancer, prostate-specific antigen, quadriplegia, spinal cord injury
INTRODUCTION
Every year, spinal cord injury (SCI) occurs in 17 000 individuals in the United States and approximately 80% of these individuals are males.1 Yet, with advancements in technology and routine checkups, the life expectancy for these patients has improved substantially and approached to that of the general population,2 with the result that most patients with SCI are likely to go through age-related problems. Age, in particular, represents a well-known risk factor for prostate cancer, which is the most frequently diagnosed cancer among males aged 65–74 years; it is also the third leading cause of cancer-related death in the United States.3 Therefore, it is expected that a larger number of patients with SCI may develop prostate cancer later in their lives. Moreover, the techniques of management of neurogenic bladder, including catheterization, predispose the patients to recurrent urinary tract infections following SCI. The resultant chronic inflammation of the prostate may increase the risk of developing prostate cancer in these subjects, which can be concluded on the basis of an established association between prostatitis and prostate cancer, as documented by population-based case–control studies.4 Nevertheless, a lower prevalence of prostate cancer has been reported among individuals with SCI as compared to able-bodied age-matched individuals. The low levels of circulating androgens,5,6,7,8,9 along with the loss of neurogenic stimulation of prostate growth and activity,10,11,12 peculiar to individuals with SCI, could prove to be protective to some extent, thus contributing to explaining a lower risk of prostate cancer, which, for all practical purposes, usually discourages screening programs in this population. In reality, the utility of enrolling men with SCI in screening programs is not clear because of the uncertainty in the efficiency of the use of prostate-specific antigen (PSA) as a screening tool for prostate cancer in these subjects. On the one hand, a low prostate volume, peculiar to males with chronic SCI,13,14,15,16,17 would be associated with lower levels of PSA when compared to age-specific reference ranges; on the other hand, chronic prostate inflammation, catheterization, and repeated manual bowel evacuation are expected to yield falsely elevated PSA levels. As a result, several case–control studies have produced conflicting results, reporting either lower, similar or higher serum PSA levels in males with SCI as compared to that in able-bodied age-matched subjects.10 In this scenario, where the actual level of the decreased prevalence of prostate cancer in SCI has not yet been determined, the limited use of screening programs could be the reason why prostate cancer would be diagnosed at an advanced stage and grade in this population.18
In order to assess the relationship of SCI with prostate cancer and PSA levels comprehensively, we carried out a meta-analysis of the available case–control studies, aiming to answer the following questions: (1) “Does, and to what extent, the presence of SCI decrease the risk of prostate cancer with respect to the general population?” and (2) “Is there any statistically significant difference in PSA levels between males with SCI and their able-bodied age-matched controls?”
MATERIALS AND METHODS
The study was conducted according to the Cochrane Collaboration and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement;19 it also complies with the guidelines for Meta-Analyses and Systematic Reviews of Observational Studies (MOOSE).20 PRISMA and MOOSE Checklists have been presented as Supplementary Table 1 (1.7MB, tif) and 2 (1.5MB, tif) . The International prospective register of systematic reviews (PROSPERO) registration number is CRD42017057672.
PRISMA Checklist
MOOSE Checklist for Meta-analyses of Observational Studies
Systematic search strategy
We conducted a systematic search in MEDLINE, Cochrane Library, Scopus, CINAHL, and ScienceDirect to identify all relevant studies in English language with the terms: (“spinal cord injur*” OR “spine injur*” OR “spinal injur*” OR paraplegia OR tetraplegia OR quadriplegia) AND (prostate OR “prostate specific antigen” OR PSA OR “prostate tumour” OR “prostate neoplasm” OR “prostate adenocarcinoma”). If it was not clear from title and abstract whether the paper contained relevant data, the full paper was retrieved. The references cited in all full-text articles were also hand-searched in an effort to obtain additional studies for inclusion.
Inclusion and exclusion criteria
The primary outcome of interest was the relationship between SCI and prostate cancer. The secondary outcome was the difference in serum PSA levels between males with SCI and able-bodied subjects. The eligibility criteria used for the study were: (1) observational case–control studies, enrolling individuals aged 18 years or older having SCI and an able-bodied control group; (2) availability of odds ratios (ORs) for prostate cancer or data for its calculation and/or mean ± standard deviation (s.d.) of PSA levels in both groups. Two independent reviewers (AB and SDA) assessed the eligibility of each selected paper; any disagreement was resolved via discussion involving a third reviewer (FF).
Data extraction
Data were extracted from the selected articles by including the first author, publication year, geographic region, and mean age of participants. From the studies evaluating prostate cancer, we extracted the number of events (prostate cancer) and the total number of participants in cases (males with SCI) and controls (age-matched able-bodied subjects); from the studies reporting PSA levels, we obtained mean ± s.d. of PSA measurement along with the total number of participants in cases and controls. Where data were missing or inconsistent, the authors were contacted to obtain the necessary information.
Quality assessment
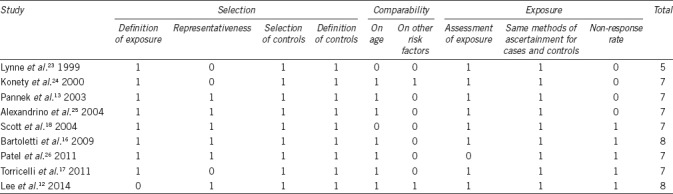
The quality of studies was assessed through the “star system” of the Newcastle–Ottawa Quality Assessment Scale (NOS).21 The minimum number of stars was 0 and the maximum that could be awarded was 9 stars. Those getting scores ≥7 were regarded as high-quality studies. The quality assessment was performed by two reviewers (AB and FF). Any disagreement was resolved by a third author (SF) who reevaluated the original study.
Statistical analysis
The relationship between SCI and prostate cancer was assessed using OR and 95% confidence interval (95% CI) as well as by Mantel–Haenszel estimates. The differences in PSA levels were assessed by calculating the standardized mean difference (SMD). In the presence of significant heterogeneity, data were combined using random effects models, which assumed that the included studies have varying effect sizes, thus providing a conservative estimate of the overall effect. For nonsignificant heterogeneity, the results were pooled in a fixed effects model.
The Cochran's Chi-square (Cochran's Q) test and the I2 test were carried out to analyze statistical heterogeneity between the results of different studies. I2< 25% was considered as no heterogeneity, whereas I2 >50% and/or P < 0.05 indicated substantial heterogeneity. In order to illustrate heterogeneity that may not be fully conveyed by 95% CI, 95% prediction intervals were also included in the summary results of random effects models, as previously reported.22
Meta-regression and subgroup analyses were conducted to investigate between-study heterogeneity, by taking into account covariates that could affect the estimates. In particular, predictors which might be potential sources of heterogeneity, such as publication year, geographic region, NOS quality score of the studies, sample size, and mean age of the participants, were included in linear meta-regression models where the regression coefficient (β) described how the outcome variable (the estimate) changed with a unit increase in the explanatory variable (the potential effect modifier). Publication bias was graphically identified using funnel plots.
The extracted data were analyzed using the R statistical software (version 3.0.3; R Foundation for Statistical Computing, Vienna, Austria) and the Review Manager (RevMan) of the Cochrane Library (version 5.3, 2014; The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark).
RESULTS
Study selection and quality assessment
The electronic search yielded 1159 studies. Three articles were retrieved from the manual search. After removal of duplicate, 839 studies were left, 802 of which were excluded as they were irrelevant on the basis of titles and abstracts. Hence, as shown in Figure 1, a total of 37 studies were identified; however, only 9 of them met the inclusion criteria.12,13,16,17,18,23,24,25,26 The details of the selected articles are reported in Table 1.
Figure 1.
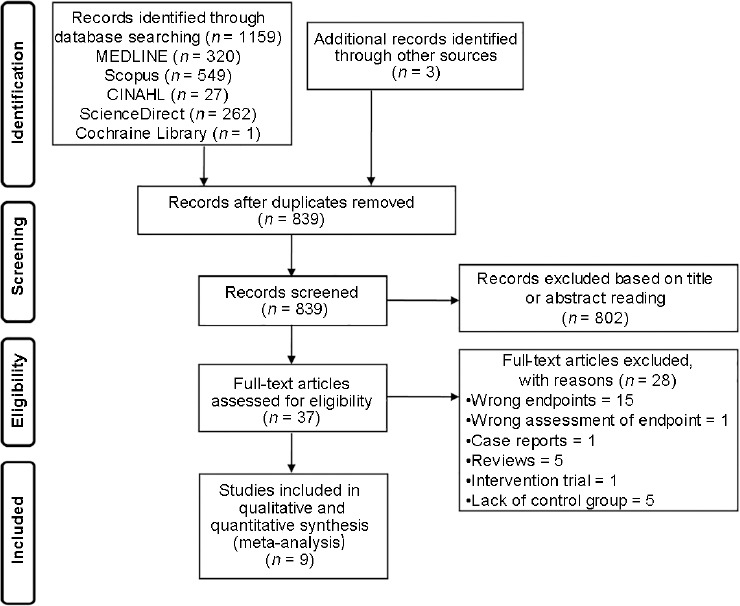
Flow diagram showing an overview of the study selection process.
Table 1.
Characteristics of the nine studies included
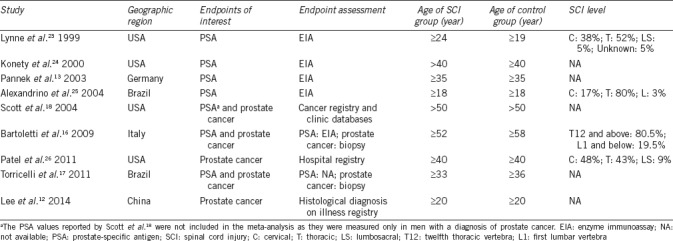
Five studies evaluated the difference in the prevalence of prostate cancer between males with SCI and able-bodied controls.12,16,17,18,26 In all the studies, the included males were diagnosed with prostate cancer after they sustained SCI. Six studies reported serum PSA levels between the two groups of subjects.13,16,17,23,24,25
Quality rating of the studies, based on the NOS score, is outlined in Table 2. Quality scores ranged from 5 to 8. Eight articles were considered to be of high quality,12,13,16,17,18,24,25,26 scoring ≥7; one article was assessed to be moderate.23 In the study by Lynne et al.23 a selection bias could not be ruled out since that study enrolled only volunteers and comparability could not be ensured by adjusting either on age or other variables.
Table 2.
Newcastle–Ottawa Quality Assessment Scale for case–control studies
Synthesis of results
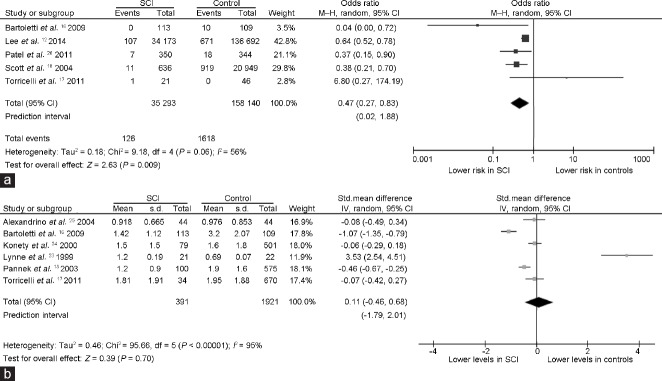
Overall, five studies evaluating the risk of prostate cancer12,16,17,18,26 provided information on 35 293 males with SCI and 158 140 able-bodied controls. As shown in Figure 2a, pooled OR suggested that the presence of SCI reduces the risk of prostate cancer by almost one-half as compared to that in able-bodied males (OR = 0.47, 95% CI: 0.27 to 0.83, P = 0.009). Between-study heterogeneity was revealed by I2 value >50% (Pfor heterogeneity= 0.06, I2 = 56%).
Figure 2.
Forest plots depicting (a) the odds ratios for prostate cancer in men with SCI and able-bodied controls, and (b) standardized mean difference in the serum levels of PSA between men with SCI and able-bodied controls. Diamonds indicate the overall summary estimates for the analysis (the width of the diamonds represents the 95% CI); boxes indicate the weight of the individual study in the pooled analysis. The serum PSA levels are reported with ng ml−1. CI: confidence interval; df: degrees of freedom; IV: inverse variance; M–H: Mantel–Haenszel; s.d.: standard deviation; std.: standardized; PSA: prostate-specific antigen; SCI: spinal cord injury.
The six studies that reported PSA levels13,16,17,23,24,25 provided information on 391 males with SCI and 1921 able-bodied controls. As shown in Figure 2b, the overall difference between the two groups was not found to be statistically significant (pooled SMD: 0.11, 95% CI: −0.46 to 0.68, P = 0.7; Pfor heterogeneity< 0.00001, I2 = 95%).
Publication bias and heterogeneity evaluation
As shown in Figure 3a, the reasonably symmetrical shape of funnel plot suggested the absence of obvious publication bias among studies investigating the risk of prostate cancer. On the contrary, a clear asymmetry was observed in the funnel plot of studies analyzing PSA levels (Figure 3b).
Figure 3.
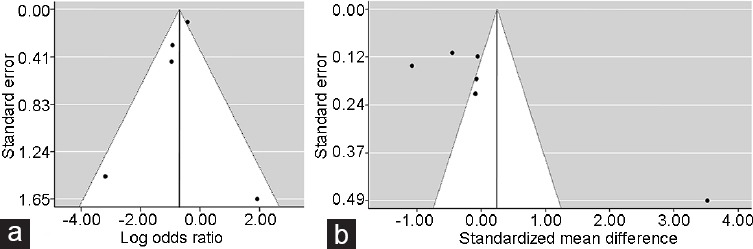
Funnel plots of an overall analysis of the relationship of spinal cord injury with (a) prostate cancer risk and (b) serum prostate-specific antigen (PSA) levels.
As between-study heterogeneity in the pooled analyses was found (Figure 2), linear meta-regression analyses were performed in order to detect possible covariates responsible for this variability.
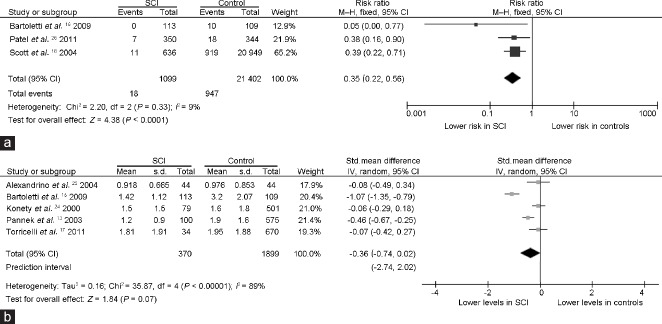
It was observed that only the mean age of study population contributed significantly to the source of heterogeneity in the analysis of prostate cancer: the older age of the participants was significantly associated with a higher decrease in OR for prostate cancer in SCI as compared to the controls (β= −0.10; 95% CI: −0.19 to −0.01; P = 0.02). Accordingly, in a subgroup analysis (Figure 4a), where we excluded the study by Torricelli et al.17 and the largest study by Lee et al.12 both reporting a mean age of participants to be <55 years, the pooled OR for the association between SCI and prostate cancer further decreased up to 0.35 (95% CI: 0.22 to 0.56, P < 0.0001) with no heterogeneity (Pfor heterogeneity= 0.33, I2 = 9%).
Figure 4.
Forest plots depicting the results of the subgroup analyses on the relationship of SCI with (a) prostate cancer risk and (b) serum prostate-specific antigen (PSA) levels. After evaluation of heterogeneity, only studies carried out on samples with mean age >55 years were included in (a) the analysis of prostate cancer risk and the study from Lynne et al.23 exhibiting both the lowest quality score at the Newcastle–Ottawa scale and the smallest sample size was excluded from (b) the analysis of serum PSA levels. The PSA levels are reported in ng ml−1. CI: confidence interval; df: degrees of freedom; IV: inverse variance; M–H: Mantel–Haenszel; s.d.: standard deviation; std.: standardized; SCI: spinal cord injury.
As far as PSA levels were concerned, in the linear meta-regression models, both sample size and NOS quality score of the studies contributed significantly to heterogeneity: lower values of SMD, indicative of lower PSA levels in SCI as compared to that in controls, were associated with a higher NOS quality score (β= −1.48; 95% CI: −1.92 to −1.04; P < 0.0001) and a larger sample size of the study population (β= −0.03; 95% CI: −0.06 to −0.001; P = 0.03). In a subgroup analysis (Figure 4b), where we excluded the study by Lynne et al.23 exhibiting both the lowest NOS score (Table 1) and the smallest sample size, the pooled SMD indicated lower PSA levels in SCI, which, however, were not statistically significant (SMD: −0.36; 95% CI: -0.74 to 0.02, P = 0.07) and associated with the persistence of large heterogeneity (Pfor heterogeneity < 0.00001, I2 = 89%).
DISCUSSION
Although a lower risk of prostate cancer has been reported among males with SCI as compared to the able-bodied age-matched individuals, the actual decrease in its prevalence within this population has not yet been determined. The results from the present meta-analysis of five carefully selected observational case–control studies12,16,17,18,26 suggest that the presence of SCI reduces the risk of prostate cancer by almost one-half as compared to their able-bodied age-matched subjects. Indeed, according to the results of the meta-regression models, when the analysis was restricted to males of mean age over 55 years, the risk decreased up to 65% than that in the controls, with a true population effect size between 44% and 78% (P < 0.0001), indicating that, as the prevalence of prostate cancer increases with an increase in the age of the general population, the “protective” effect of SCI becomes more evident.
The interruption of neural pathways to the prostate has been suggested to be a possible mechanism underlying the lower risk of prostate cancer in individuals with SCI.10 In rats, where autonomic nervous system plays a key role in the growth of the prostate gland,27 prostate denervation promotes changes in the cellular morphology, growth, and functions of the gland.28 However, in humans, the influence of neurologic factors on the physiology/pathophysiology of the prostate remains largely unknown. Nevertheless, an increased risk of prostate cancer in paraplegic male subjects as compared to that in tetraplegic patients has been reported in two studies,12,29 which supports the role of denervation in lowering the incidence of prostate cancer after SCI. A further and even more relevant role would be played by androgen deficiency, peculiar to this population. High rates of biochemical androgen deficiency, ranging from approximately 33% to 46%, have been reported in males having chronic SCI.5,6,7,8,9 Whether and to what extent it can result in clinical hypogonadism is still unclear, because in the presence of SCI, the sexual symptoms, along with other putative clinical features of hypogonadism (e.g., changes in body composition, osteoporosis, anemia, and mood disorders), overlap with direct or indirect consequences of neurological damage and disability. However, regardless of other putative clinical reflections, androgen deficiency could prove to be protective to some extent, resulting in a lower incidence of prostate cancer. Evidence shows that both the occurrence and progression of prostate cancer are influenced by androgens as (1) prolonged administration of high dosages of testosterone induces prostate cancer in rats, (2) malignancies of the prostate rarely occur in dogs and men castrated prior to puberty, and (3) androgen-ablative therapy inhibits the growth of prostate tumor.30 In keeping with the notion of a major role of androgens in prostate cancer, meta-analyses indicate that shorter polymorphic cytosine-adenine-guanine (CAG) repeat sequences in the androgen receptor gene, which promote both a higher receptor binding affinity for androgens and a higher transactivation activity, are associated with an increased risk of prostate cancer.31,32
In the present analysis, a poor value of serum PSA levels as a screening tool for prostate cancer following SCI resulted from the large heterogeneity between the included studies that reported its circulating levels in males with SCI and able-bodied controls (Figure 2b). In the crude analysis, although the overall difference between the two groups was not statistically significant, PSA levels were higher in SCI group. On the contrary, when, according to the results of the meta-regression models, the subgroup analysis was restricted to studies exhibiting both the highest NOS quality score and the largest sample size, the pooled estimate indicated lower PSA levels in SCI, which, however, were not statistically significant and showed persistent large heterogeneity (Figure 4b). Lower PSA levels in males with SCI would be in line with the low prostate volume, which has repeatedly been reported in this population.13,14,15,16,17 Possibly, androgen deficiency and/or prostate denervation counteract the opposite impact of chronic inflammation, catheterization, and repeated manual bowel evacuation on prostate physiology/pathophysiology.
Some limitations of this meta-analysis have been recognized. Firstly, the inclusion of a limited number of studies. This, however, resulted from a strict screening and selection of the literature. Although only five studies were included in the quantitative analysis of prostate cancer, as a whole, they provided information on a very large number of males with SCI and their age-matched able-bodied controls. The study by Lee et al.12 accounted for the largest proportion of the global study population; nevertheless, when this study, where the mean age was below 55 years, was excluded, according to the results of the meta-regression models, the pooled estimate remained statistically significant, with a further decrease (up to 65%) in the risk of prostate cancer in males with SCI as compared to that in their controls. Secondly, the availability of a limited number of studies refrained the authors from performing multivariable meta-regression analyses that would have helped detect possible independent associations of the different covariates with the estimates. A further limitation of this meta-analysis is that in the selected studies, information on the key factors having a potential impact on the pathophysiology of the prostate was largely incomplete. In particular, the lack of data on variables which can exert opposite effects on prostate growth and PSA levels in SCI (on the one hand, testosterone levels and the level of the lesion; on the other hand, chronic prostate inflammation, catheterization, and repeated manual bowel evacuation) did not let the authors explain, by meta-regression analyses, their significance in contributing to the between-study heterogeneity in PSA levels. For instance, only the study by Bartoletti et al.16 reported testosterone levels in 113 males with SCI and 109 age-matched able-bodied subjects; although the SCI group showed significantly lower testosterone levels, PSA values, prostate sizes, and prostate cancer prevalence, the independent association of testosterone with prostate endpoints was not investigated by multivariable regression models.
On the basis of the present study, it can be concluded that, in older males, chronic SCI represents a condition which reduces the risk of prostate cancer up to 65% as compared to that in able-bodied controls. The large between-study heterogeneity on serum PSA levels makes this marker a poor reliable screening tool for prostate cancer following SCI. This was mainly due to the interaction of opposite effects exerted by clinical variables peculiar to this population. These findings should be considered in defining more appropriate screening strategies for prostate cancer in males with SCI.
AUTHOR CONTRIBUTIONS
AB conceived the study, performed the systematic search to identify all relevant studies, assessed the eligibility of each selected study, assessed the quality of the studies, and performed data extraction and statistical analysis; SDA performed the systematic search to identify all relevant studies, assessed the eligibility of the full-text papers of the selected studies, and performed data extraction; AM participated in the systematic search and helped draft the manuscript; GF helped draft the manuscript; SF assessed the quality of the studies and critically revised the manuscript; FF conceived the study, evaluated for eligibility of the full text of the selected studies, assessed the quality of the studies, and wrote the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
ACKNOWLEDGMENTS
We are grateful to the English teacher of the University of L’Aquila, Marta Fiorenza, for the help in language editing.
Supplementary Information is linked to the online version of the paper on Asian Journal of Andrology website.
REFERENCES
- 1.Birmingham: University of Alabama at Birmingham; 2016. National Spinal Cord Injury Statistical Center. Spinal Cord Injury (SCI) facts and figures at a glance; pp. p1–2. [Google Scholar]
- 2.Strauss DJ, Devivo MJ, Paculdo DR, Shavelle RM. Trends in life expectancy after spinal cord injury. Arch Phys Med Rehabil. 2006;87:1079–85. doi: 10.1016/j.apmr.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 4.Dennis LK, Lynch CF, Torner JC. Epidemiologic association between prostatitis and prostate cancer. Urology. 2002;60:78–83. doi: 10.1016/s0090-4295(02)01637-0. [DOI] [PubMed] [Google Scholar]
- 5.Durga A, Sepahpanah F, Regozzi M, Hastings J, Crane DA. Prevalence of testosterone deficiency after spinal cord injury. PM R. 2011;3:929–32. doi: 10.1016/j.pmrj.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Bauman WA, Fountaine MF, Spungen AM. Age-related prevalence of low testosterone in men with spinal cord injury. J Spinal Cord Med. 2014;37:32–9. doi: 10.1179/2045772313Y.0000000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbonetti A, Vassallo MR, Pacca F, Cavallo F, Costanzo M, et al. Correlates of low testosterone in men with chronic spinal cord injury. Andrology. 2014;2:721–8. doi: 10.1111/j.2047-2927.2014.00235.x. [DOI] [PubMed] [Google Scholar]
- 8.Barbonetti A, Caterina Vassallo MR, Cotugno M, Felzani G, Francavilla S, et al. Low testosterone and non-alcoholic fatty liver disease: evidence for their independent association in men with chronic spinal cord injury. J Spinal Cord Med. 2016;39:443–9. doi: 10.1179/2045772314Y.0000000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barbonetti A, Vassallo MR, Felzani G, Francavilla S, Francavilla F. Association between 25(OH)-vitamin D and testosterone levels: evidence from men with chronic spinal cord injury. J Spinal Cord Med. 2016;39:246–52. doi: 10.1179/2045772315Y.0000000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shim HB, Jung TY, Lee JK, Ku JH. Prostate activity and prostate cancer in spinal cord injury. Prostate Cancer Prostatic Dis. 2006;9:115–20. doi: 10.1038/sj.pcan.4500865. [DOI] [PubMed] [Google Scholar]
- 11.Pannek J, Bartel P, Göcking K, Frotzler A. Prostate volume in male patients with spinal cord injury: a question of nerves? BJU Int. 2013;112:495–500. doi: 10.1111/bju.12027. [DOI] [PubMed] [Google Scholar]
- 12.Lee WY, Sun LM, Lin CL, Liang JA, Chang YJ, et al. Risk of prostate and bladder cancers in patients with spinal cord injury: a population-based cohort study. Urol Oncol. 2014;32:51.e1–7. doi: 10.1016/j.urolonc.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 13.Pannek J, Berges RR, Cubick G, Meindl R, Senge T. Prostate size and PSA serum levels in male patients with spinal cord injury. Urology. 2003;62:845–8. doi: 10.1016/s0090-4295(03)00654-x. [DOI] [PubMed] [Google Scholar]
- 14.Frisbie JH, Kumar S, Aguilera EJ, Yalla S. Prostate atrophy and spinal cord lesions. Spinal Cord. 2006;44:24–7. doi: 10.1038/sj.sc.3101804. [DOI] [PubMed] [Google Scholar]
- 15.Hvarness H, Jakobsen H, Biering-Sørensen F. Men with spinal cord injury have a smaller prostate than men without. Scand J Urol Nephrol. 2007;41:120–3. doi: 10.1080/00365590600918048. [DOI] [PubMed] [Google Scholar]
- 16.Bartoletti R, Gavazzi A, Cai T, Mondaini N, Morelli A, et al. Prostate growth and prevalence of prostate diseases in early onset spinal cord injuries. Eur Urol. 2009;56:142–8. doi: 10.1016/j.eururo.2008.01.088. [DOI] [PubMed] [Google Scholar]
- 17.Torricelli FC, Lucon M, Vicentini F, Gomes CM, Srougi M, et al. PSA levels in men with spinal cord injury and under intermittent catheterization. Neurourol Urodyn. 2011;30:1522–4. doi: 10.1002/nau.21119. [DOI] [PubMed] [Google Scholar]
- 18.Scott PA, Sr, Perkash I, Mode D, Wolfe VA, Terris MK. Prostate cancer diagnosed in spinal cord-injured patients is more commonly advanced stage than in able-bodied patients. Urology. 2004;63:509–12. doi: 10.1016/j.urology.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 21.Deeks JJ, Dinnes J, D’Amico R, Sowden AJ, Sakarovitch C, et al. Evaluating non-randomised intervention studies. Health Technol Assess. 2003;7:iii–x. doi: 10.3310/hta7270. 1–173. [DOI] [PubMed] [Google Scholar]
- 22.Riley RD, Higgins JPT, Deeks JJ. Research methods and reporting: interpretation of random effects meta-analyses. BMJ. 2011;342:d549. doi: 10.1136/bmj.d549. [DOI] [PubMed] [Google Scholar]
- 23.Lynne CM, Aballa TC, Wang TJ, Rittenhouse HG, Ferrell SM, et al. Serum and semen prostate specific antigen concentrations are different in young spinal cord injured men compared to normal controls. J Urol. 1999;162:89–91. doi: 10.1097/00005392-199907000-00022. [DOI] [PubMed] [Google Scholar]
- 24.Konety BR, Nguyen TT, Brenes G, Lewis N, Saul M, et al. Evaluation of the effect of spinal cord injury on serum PSA levels. Urology. 2000;56:82–6. doi: 10.1016/s0090-4295(00)00548-3. [DOI] [PubMed] [Google Scholar]
- 25.Alexandrino AP, Rodrigues MA, Matsuo T. Evaluation of serum and seminal levels of prostate specific antigen in men with spinal cord injury. J Urol. 2004;171:2230–2. doi: 10.1097/01.ju.0000125241.77517.10. [DOI] [PubMed] [Google Scholar]
- 26.Patel N, Ngo K, Hastings J, Ketchum N, Sepahpanah F. Prevalence of prostate cancer in patients with chronic spinal cord injury. PM R. 2011;3:633–6. doi: 10.1016/j.pmrj.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 27.McVary KT, Razzaq A, Lee C, Venegas MF, Rademaker A, et al. Growth of the rat prostate gland is facilitated by the autonomic nervous system. Biol Reprod. 1994;51:99–107. doi: 10.1095/biolreprod51.1.99. [DOI] [PubMed] [Google Scholar]
- 28.Wang JM, McKenna KE, McVary KT, Lee C. Requirement of innervations for maintenance of structural and functional integrity in the rat prostate. Biol Reprod. 1991;44:1171–6. doi: 10.1095/biolreprod44.6.1171. [DOI] [PubMed] [Google Scholar]
- 29.Frisbie JH. Cancer of the prostate in myelopathy patients: lower risk with higher levels of paralysis. J Spinal Cord Med. 2001;24:92–4. doi: 10.1080/10790268.2001.11753561. [DOI] [PubMed] [Google Scholar]
- 30.Wilding G. The importance of steroid hormones in prostate cancer. Cancer Surv. 1992;14:113–30. [PubMed] [Google Scholar]
- 31.Nelson KA, Witte JS. Androgen receptor CAG repeats and prostate cancer. Am J Epidemiol. 2002;155:883–90. doi: 10.1093/aje/155.10.883. [DOI] [PubMed] [Google Scholar]
- 32.Zeegers MP, Kiemeney LA, Nieder AM, Ostrer H. How strong is the association between CAG and GGN repeat length polymorphisms in the androgen receptor gene and prostate cancer risk? Cancer Epidemiol Biomarkers Prev. 2004;13:1765–71. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA Checklist
MOOSE Checklist for Meta-analyses of Observational Studies