Abstract
Obstructive Sleep Apnea (OSA) is a heterogeneous sleep disorder with many pathophysiological pathways to disease. Currently, the diagnosis and classification of OSA is based on the apnea-hypopnea index, which poorly correlates to underlying pathology and clinical consequences. A large number of in-laboratory sleep studies are performed around the world every year, already collecting an enormous amount of physiological data within an individual. Clinically, we have not yet fully taken advantage of this data, but combined with existing analytical approaches, we have the potential to transform the way OSA is managed within an individual patient. Currently, respiratory signals are used to count apneas and hypopneas, but patterns such as inspiratory flow signals can be used to predict optimal OSA treatment. Electrocardiographic data can reveal arrhythmias, but patterns such as heart rate variability can also be used to detect and classify OSA. Electroencephalography is used to score sleep stages and arousals, but specific patterns such as the odds-ratio product can be used to classify how OSA patients responds differently to arousals. In this review, we examine these and many other existing computer-aided polysomnography signal processing algorithms and how they can reflect an individual’s manifestation of OSA. Together with current technological advance, it is only a matter of time before advanced automatic signal processing and analysis is widely applied to precision medicine of OSA in the clinical setting.
Keywords: obstructive sleep apnea, physiological signals, polysomnography, precision medicine
1. Introduction
The momentum for Precision Medicine comes from advances in the collection and storage of big data, including clinical, behavioral, anthropometric, molecular, imaging and physiological data, as well as advances in software that can process this large amount of data using model-free unbiased predictive analytics. The use of averages and summary-based analyses limit the application of scientific knowledge gained from clinical trials because it inherently discounts the effect within the individual. Currently, a paradigm shift is underway: the accumulation of high-resolution biological data and the utilization of computational tools developed decades ago has led to Predict-Prevent-Personalize-Participate medicine or “P4 medicine” (Lim et al 2017, Pack 2016). However, large-scale individual-oriented data has yet to be implemented, including that for Sleep Medicine.
Obstructive Sleep Apnea (OSA) is a prevalent sleep disorder characterized by the partial (hypopnea) or complete (apnea) cessation of airflow as a consequence of upper airway obstruction that causes repetitive respiratory pauses during sleep and arousals. Subsequently, this affects sleep architecture and whole body oxygenation, which leads to molecular, immunological, physiological and clinical consequences (Cowie 2017). OSA is a highly heterogeneous disorder in that there are many pathways that to cause OSA. These pathways include varying degrees and combinations of restrictive craniofacial structures, soft tissue fat deposition related to obesity, rostral fluid shift during sleep, airway collapsibility, arousal threshold, negative pressure reflex response and overall loop gain of the ventilatory control system (Pack 2016, Eckert et al 2013). Different pathways may result in one OSA patient having a respiratory event associated with a low arousal threshold, sleep fragmentation and excessive daytime sleepiness, while another patient has a long respiratory event with significant desaturations. Although patients with OSA are often combined into a single group when assessing associations to clinical outcomes, a more accurate assessment might be done if OSA patients were subtyped into groups based on distinct clinical (Ye et al 2014) or polysomnographic manifestations (Zinchuk et al 2018). The lack of more accurately characterizing the heterogeneity of OSA could explain the lack of reproducibility when assessing genetic associations, outcomes and treatment response, as well as finding biomarkers. Hence, a more accurate classification and utilization of OSA subtypes using symptoms, polysomnographic characteristics, biological and other clinical information (Ye et al 2014, Zinchuk et al 2018) will help clarify underlying pathophysiology of OSA within an individual.
For almost 20 years, the AASM manual has maintained standards for signal collection and updated definitions based on evidence and new technology (American Academy of Sleep Medicine 1999). In scoring polysomnographic data for OSA, we have largely used a single metric, the apnea-hypopnea index (AHI), to diagnose and classify severity. Although other parameters such as the oxygen desaturation index (ODI), percentage time below 90% oxygen saturation, cardiorespiratory coupling, respiratory arousal threshold, arousal index, and wake after sleep onset have often been better associated with outcomes of interest (e.g. excessive sleepiness, hypertension and cardiovascular disease) (Ren et al 2016, Martynowicz et al 2017, Beaudin et al 2017, Trimer et al 2014) they are not routinely utilized in clinical practice nor have been integrated into disease classification (American Academy of Sleep Medicine 1999). Despite the weak correlation between AHI and clinical presentation, clinical consequences, and response to treatment, we continue to diagnose and classify OSA severity based solely on the AHI. While computer-aided signal-processing algorithms of PSG physiological signals have been helpful to inform an individual’s phenotype and pathway to OSA (Eckert et al 2013), they have not been widely accepted, integrated or commercialized for clinical use. This suggests that there is a disconnect between what is technically feasible today (i.e. modern applications of signal processing algorithms) and clinical utility.
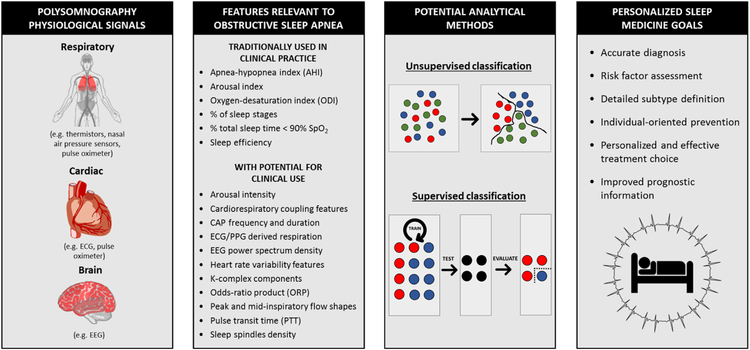
In this review, we will assess the broad range of physiological PSG signals that are routinely being collected and its potential to help us understand the heterogeneity of OSA subtypes in leading to a more personalized approach to sleep medicine (Figure 1). We will also discuss how a more detailed examination of physiological information can lead to a more accurate description of OSA diagnosis, prognosis, clinical consequence, and response to different treatments (Table 1). Finally, we will discuss algorithms and analytical strategies developed for these physiological signals, as well as proven data-driven approaches that could identify OSA subgroups.
Figure 1:
Summary of polysomnography physiological signal features relevant to obstructive sleep apnea, potential analytical approaches and the goals of understanding these data towards personalized medicine for this disorder. Abbreviations: CAP: cyclic alternating pattern; ECG: electrocardiogram; EEG: electroencephalogram; SpO2: oxygen saturation; PPG: photoplethysmography. Some illustrations used with permission and adapted from (Petryszak et al 2016)
Table 1:
Detailed summary of polysomnographic physiological features that can be applied to better characterize obstructive sleep apnea subtypes.
| Feature | Main PSG sensor | Description | Effect on OSA | References | |
|---|---|---|---|---|---|
| Traditionally used in clinical practice | AHI | Thermistors, nasal air pressure, and pulse oximeter | Average number of apnea and hypopnea events per hour of TST. | Current index used to classify OSA severity; increased in more severe patients. | (American Academy of Sleep Medicine 1999, Ho et al 2015) |
| Arousal index | EEG | Number of identified arousals per hour of sleep, as defined by AASM. Can be a measure of sleep fragmentation. | Increased in more severe OSA. | (American Sleep Disorders Association 1992) | |
| ODI | Pulse oximeter | Average number of desaturation episodes per hour of total sleep time. Desaturation usually defined as 3 or 4% decreases from baseline. | Alternative metric associated with OSA severity; it is increased in more severe patients. | (Svanborg et al 1990, Dawson et al 2015) | |
| Percentage of sleep stages | EEG | Proportion of total sleep time spent on sleep stage N1, N2, N3 and REM. | More severe OSA patients spend more time in REM than slow wave sleep and in light sleep than awake. | (Iber et al 2007, Ratnavadivel et al 2009, Bianchi et al 2010) | |
| Percentage of TST with SpO2 <90% | Pulse oximeter | Measure of time with low levels of peripheral oxygen saturation (<90%). Can be a measure of degree of hypoxia and desaturation. | Direct relationship with the duration and severity of hypoxia in patients with OSA. | (Bostanci et al 2015) | |
| Sleep efficiency | EEG | Ratio of total sleep time to time in bed. Indicates how much of the recording time spent on bed was scored as sleep. | Usually decreased in more severe OSA, but with controversial results. | (Ng and Guan 2012, Iber et al 2007, Ratnavadivel et al 2009, Bianchi et al 2010) | |
| With potential for clinical use | Arousal intensity | EEG | Intensity of EEG arousal detected and quantified using discrete wavelet transform. | Associated with increased heart rate response to arousal and respiratory control instability in OSA. | (Azarbarzin et al 2014, Amatoury et al 2016) |
| Cardiorespiratory coupling features | ECG, respiratory inductance plethysmography | Influence of respiration on heart rate. Include % of synchronized time per unit of sleep time and average duration of synchronization. | Percentage of synchronization decreases and average duration increases in severe OSA. | (Sola-Soler et al 2015) | |
| CAP phases frequency and duration | EEG | Number/duration of CAP A-phases subtypes (A1, A2, A3) per unit of sleep time. CAP represents a marker of NREM sleep instability. | Associated with inspiratory flow limitation, poor response to CPAP therapy, fatigue and sleepiness. | (Guilleminault et al 2007, Bosi et al 2018, Gupta and Shukla 2018) | |
| ECG/PPG-derived respiration features | ECG and pulse oximeter | Mean respiratory rate, respiratory frequency and respiratory power spectrum density. Used for apnea detection and ECG-derived AHI calculation. | Mostly used to help automatic classification of respiratory events in patients with OSA. | (Pallás-Areny et al 1989, Penzel et al 2002, de Chazal et al 2009) | |
| HRV features | ECG | Linear, nonlinear, spectral and wavelet characteristics of ECG. These include mean RR interval, SDNN, RMSSD, SDSD, NN50, pNN50, DFA alpha1, DFA alpha2, entropy, ULF, VLF, LF, HF, Allan factor. | Patients with OSA have changes in specific features of HRV that indicate autonomic dysfunction. | (European Society of Cardiology and the North American Society of Pacing and Electrophysiology 1996, Aeschbacher et al 2016) | |
| K-complex components and distribution | EEG | Signal characteristics and frequency of K-complexes per unit of sleep time. | Mild airflow limitation, such as in OSA, increases K-complex frequency and amplitude. | (Nguyen et al 2016) | |
| ORP | EEG | Continuous measure of sleep-wakefulness state based on power spectrum patterns of EEG. | ORP in the immediate 9 seconds following arousals (ORP-9) was associated with sleep instability in OSA. | (Younes et al 2015a, Younes and Hanly 2016) | |
| Peak and mid-inspiratory flow shape features | Nasal air pressure | Indicate aspects of pharyngeal narrowing and site of collapse. Features include negative effort dependence (NED, percent reduction in inspiratory flow between peak and plateau). | Lower NED indicates tongue-related obstruction and severe indicate epiglottis collapse. Can guide therapy choice. | (Genta et al 2017, Azarbarzin et al 2017a, 2017b) | |
| PTT | Pulse oximeter | PPG-derived index that reflects peripheral vascular resistance and intrathoracic pressure. Can estimate blood pressure, respiratory effort and arousal index. | Severe OSA patients have more periods of ≥ 10 mmHg increases in baseline systolic blood pressure. | (Gehring et al 2018, Pépin et al 2009, Schwartz 2005) | |
| Sleep EEG power spectrum density | EEG | Power density and ratios of discretely defined EEG frequencies (e.g. delta, theta, alpha, beta), calculated from short sleep EEG epochs. | Increased delta and theta power in more severe OSA. Increased beta power in NREM and delta power in REM sleep, and correlation with worse driving performance. | (Xiromeritis et al 2011, Vakulin et al 2016) | |
| Sleep spindles density/frequency | EEG | Number of sleep spindles (high-frequency and short EEG oscillations emerging mostly during NREM sleep) per unit of sleep time | Reduced with more severe OSA and increased after CPAP treatment | (Himanen et al 2003, Carvalho et al 2014, Yetkin and Aydogan 2017) | |
AASM: American Academy of Sleep Medicine; AHI: apnea-hypopnea index; CAP: cyclic alternating patterns; CPAP: continuous positive airway pressure; ECG: electrocardiogram; EEG: electroencephalogram; HF: high frequency; HRV: heart rate variability; LF: low frequency; NED: negative effort dependence; NN50: Number of pairs of adjacent NN intervals differing by more than 50 ms; NREM: non-rapid eye-movement; ODI: oxygen desaturation index; ORP: odds-ratio product; OSA: obstructive sleep apnea; pNN50: NN50 count divided by the total number of all NN intervals; PPG: photoplethysmography; PSG: polysomnography; PTT: pulse transit time; REM: rapid eye-movement; RMSSD: square root of the mean squared differences of successive NN intervals; SDNN: standard deviation of NN intervals; SDSD: standard deviation of differences between adjacent NN intervals; SpO2: peripheral oxygen saturation; TST: total sleep time; ULF: ultra low frequency; VLF: very low frequency
2. Overview of Polysomnographic Physiological Signals in OSA
Polysomnography (PSG) is the main tool for diagnosing a broad range of sleep disorders. It is a multi-sensor method characterized by the simultaneous recording of airflow (thermistors, nasal air pressure sensors), blood oxygen levels (pulse oximeter), respiratory effort (respiratory inductance plethysmography), electrical activity of the heart (electrocardiogram [ECG]), brain (electroencephalogram [EEG]), eyes (electrooculogram [EOG]), and skeletal muscle (electromyogram [EMG]). Other sensors usually include body position, and on occasion video and audio monitoring. The PSG performed in a sleep laboratory has been used for decades and is the gold-standard for diagnosing OSA (Lyons et al 2017). In addition, by standardizing signal acquisition and processing, laboratories are able to build on top of each other’s knowledge to describe normal and disturbed sleep physiology, especially in OSA. The AASM Scoring Manual Version 2.4 recommends technical specifications, terminology and rules for scoring sleep and respiratory events in polysomnography recordings (Berry et al 2017). Thus, today, a large repository of standardized physiological signal data has been stored and could potentially undergo modern signal processing techniques to better characterize OSA. In the next three sections, we describe in more detail the technical aspects of respiratory, cardiac and brain PSG signals and the clinical relevance of applying modern signal processing techniques to these signals. In the last section, we describe novel applications and analytical approaches that have been developed with the intent to use PSG signals to better characterize OSA subtypes and severity.
3. Relevance of PSG respiratory signals in OSA
Respiratory events during sleep are a partial or complete absence in airflow or air pressure detected by oronasal thermal sensor and nasal pressure sensor. These events are usually associated with drops in peripheral oxygen saturation levels measured by a pulse oximeter (Allen 2007). While a 3-4% drop in oxygen saturation is the minimum desaturation to define a hypopnea, it is common for severe OSA patients to have larger drops. The origin of respiratory events (central or obstructive) can be determined by measuring respiratory effort using respiratory inductance plethysmography. A number of physiological features are known to occur in response to respiratory events, such as drops in oxygenation, changes in heart rate variability, and arousals (assessed by abrupt changes in the EEG (Roebuck et al 2014)). Although the immediate consequences of these repetitive and intermittent drops in oxygen saturation throughout the night is not yet clear (Ayappa et al 2005), they have been associated with carotid wall thickening and plaque occurrence (Baguet et al 2005), excessive daytime sleepiness (Jacobsen et al 2013), cancer progression (Cao et al 2015), and neurobehavioral and autonomic alterations (Idiaquez et al 2014). This suggests that in addition to duration of the respiratory event, other physiological parameters such as the magnitude of oxygen desaturation may contribute to OSA subtypes, severity and consequences.
Currently, the diagnosis and severity of OSA is determined primarily by the number of respiratory events detected during the sleep study, as measured by the AHI (Berry et al 2017). However, this metric poorly correlates to clinical consequences and may oversimplify the complexity of the disorder (Zinchuk et al 2017). Therefore, the current definition of flow limitation and duration of apnea and hypopnea events may not fully characterize the physiological consequences of respiratory events. Incorporation of other important features that reflect the respiratory event such as respiratory event duration, magnitude of the oxygen desaturation, arousal threshold, sleep fragmentation and sympathetic activation may better characterize physiological consequences of a respiratory event. In addition, the comprehensive characterization of the obstructive breathing, combined with its associated outcomes (i.e. arousals and oxygen desaturations) might improve the understanding of the pathophysiological role of the respiratory event (Arnardottir and Gislason 2016). While it would be time-consuming and inaccurate to score all of these features visually, with the assistance of computer auto-scoring, we propose that a combination of many respiratory physiological signals might better inform OSA diagnosis and treatment and provide better projective information.
OSA is in part due to craniofacial skeletal and/or soft tissue abnormalities such that the pharyngeal airway is compromised and collapses (Dempsey et al 2002). OSA treatment has mostly relied on Continuous Positive Airway Pressure (CPAP), which keeps the entire upper airway open (Edwards et al 2016) or a specific site of collapse (Ng et al 2006). Although CPAP has high efficacy, it is limited by patient adherence and therefore other individualized treatment options are desirable (Carberry et al 2017). Although there are a number of longstanding and emerging alternative treatment options for OSA, such as mandibular advancement devices (Kuhn et al 2017) and hypoglossal nerve stimulation (Mwenge et al 2015), they are usually not universally efficacious like CPAP. It is possible that information from the PSG respiratory signals could better define who will benefit from specific alternative therapies.
For example, a study that measured upper airway collapsibility using pharyngeal critical closing pressure (Pcrit) found that measures of peak and mid-inspiratory flow were useful predictors of active Pcrit (with dilator muscle influence) (Azarbarzin et al 2017b). This study also demonstrated a potential use of airflow measurements on PSG to glean additional information about upper airway collapsibility in a noninvasive way. Although the study uses pneumotachography to measure airflow, a follow up study suggested that it can be feasible to adapt the algorithm for nasal pressure signals from the PSG and hence for use in routine clinical studies (Azarbarzin et al 2017a).
Another example is using PSG inspiratory flow signals to predict OSA treatment. One study identified seven unique inspiratory shapes that were different between two groups of OSA patients (postmenopausal women and post-uvulopalatopharyngoplasty men) and a control group (Aittokallio et al 2001). Separately, another study associated inspiratory flow shapes with site of pharyngeal collapse confirmed by endoscopy (e.g. soft palate vs epiglottis) (Genta et al 2017). This could be helpful in identifying who might benefit from hypoglossal nerve stimulation (Strollo et al 2014) treatment. Together, these data suggest that inspiratory flow shapes could be used as a tool to predict which OSA treatments (e.g. oral appliances, specific surgeries) is the most effective to target specific regions of upper airway collapse within an individual with OSA.
Currently, in-laboratory studies with complicated instrumentation are required to obtain physiological information to tailor alternative OSA therapies(Azarbarzin et al 2017b, Aittokallio et al 2001, Genta et al 2017). However, obtaining physiological information from readily available PSG signals are emerging (Azarbarzin et al 2017a). These can be based to tailor therapy for specific individuals with OSA. For example, sedatives can be helpful in individuals with a low arousal threshold to maintain stable breathing during sleep (Eckert et al 2011, Carter et al 2016). While traditional methods of determining arousal threshold are mostly used in a research setting, a low arousal threshold has been correctly predicted in 84% of patients based on 3 variables from clinical sleep studies (AHI <30, nadir oxygen saturation >82.5%, and fraction of hypopneas >58.3% (Edwards et al 2014). This also led to methodological advancements in the prediction of arousal threshold using only PSG signals, and suggesting its use in a clinical setting (Sands et al 2018). Another example is that supplemental oxygen can blunt the ventilatory response in individuals with high loop gain during sleep (Wellman et al 2008). Again, traditional methods of determining loop gain is used only in research settings, but algorithms have been developed to derive loop gain from PSG signals (Terrill et al 2015). Further studies that relate physiological signals to clinical responses will help clinicians tailor treatment in the individual with OSA.
3.1. Automated processing of respiratory signals.
In designing automatic signal processing algorithms to detect respiratory events most of the time one or more of the PSG sensor signals is employed to identify patterns within a defined epoch. A typical epoch length is 30-60 seconds, which is a tradeoff between longer epochs favoring better detection rates (e.g. more signal is available), and shorter epochs allowing better time resolution of the events. As the epoch is generally longer than the minimum length of an event (10 seconds), epoch based processing of events do not necessarily identify all events individually. However, automated detection of respiratory events can still form an estimated AHI by using regression to establish a relationship between the epochs per hour and the AHI. Research groups have designed many automatic algorithms for processing PSG signals to identify respiratory events (refer Roebuck et al., 2014 (Roebuck et al 2014) for a comprehensive review). Table 1 represents a list with the best validated respiratory signal features that have been used to characterize OSA to date.
Electrocardiography (ECG) derived respiratory signals.
First considered in 1984 (Guilleminault et al 1984), the ECG has been used to identify apneic epochs (Penzel et al 2002, Moody et al 2000). With optimal signal processing, respiration can be extracted out of the ECG signals with a reasonable degree of reliability and this can be valuable information when studying patients with OSA (Langley et al 2010). Extracting out respiration from the ECG signal is possible by three mechanisms. First, the physical effect of respiration causes displacement of the ECG electrodes on the chest relative to the heart resulting in a change of the cardiac vector thereby altering the amplitude of the ECG (Pall*ás-Areny et al 1989). Second, ventilation changes the volume of air within the lung, which alters the electrical impedance of the thorax and the amplitude of the ECG signal (Hahn et al 1995). Third, respiration causes heart rate variability (HRV) such that the R-R interval on an ECG is shortened during inspiration and prolonged during expiration (Eckberg 2003). Algorithms that detect sleep apnea from the ECG use heart rate variability and ECG derived respiration, can thus successfully estimate AHI (de Chazal et al 2003). Another algorithm combined the ECG signal with pulse oximetry and further improved the detection of respiratory events (de Chazal et al 2009).
Photoplethysmography (PPG) derived respiratory signals.
The PPG signal is derived from the optical pulse oximeter signal using time-varying measurements of blood volume in the tissue at the measurement location. Thus, the PPG waveform reflects the heart pumping blood to the periphery (fingers), which is influenced by breathing. During inspiration, the pressure in the thorax becomes more negative thereby decompressing the heart in the process, resulting in a decreased stroke volume and decreased blood volume to the periphery. During expiration, the pressure in the thorax becomes less negative thereby compressing the heart in the process, resulting in an increased stroke volume and increased blood volume to the periphery. Collectively, PPG baseline and amplitude signal fluctuates in the low frequency region, and this corresponds to the breathing rate (Meredith et al 2012, Nitzan et al 1994, 1996). Due to the relative low cost of a pulse oximeter, PPG has become an interesting alternative tool for OSA screening (Romem et al 2014) that is easily implementable in mobile applications (Garde et al 2015, Behar et al 2015) or portable devices (Bilgin et al 2016). We anticipate that the widespread use of these applications with other PSG-derived physiological signal data might inform diagnosis, prognosis and personalized clinical management of OSA patients, as further elaborated in the following sections.
Microphone and Video.
Audio recordings are inexpensive and non-invasive tools used to identify snoring and other disordered breathing events by using sound analysis methods. A recent study supports the use of audio signals to provide insights about the level of airway obstruction, by discriminating normal breathing, apneas and hypopneas (Halevi et al 2016). Video recordings supplements PSG to confirm sleep behavior, but may also be used to gain information about respiration during sleep. Thirty-minute video recordings can be useful to screen children at home for OSA, with an 84% agreement with PSG and 94% sensitivity to diagnose OSA (Sivan et al 1996). A limitation of traditional videography is that blankets, bedclothes and a dark sleep environment may obscure relevant information and severely limit visual analysis. Therefore, thermal/infrared cameras have been used to assess sleep and respiration rate (Sivan et al 1996, Bennett et al 2015). One thermal method is the use of pixel color around the nostril and using post-processing video magnification to ascertain variations in breathing rates (Bennett et al 2015). This promising thermal method was validated against respiratory inductance plethysmography and could be used for apnea detection (Bennett et al 2015). Another thermal method used whole body motion to classify normal breathing, apneic events, and deep breathing using thresholds and had a high (94%) accuracy to detect apnea when validated against the PSG (Wang et al 2014). Therefore, by combining audio and video analysis with other physiological signals in the PSG, we expect to significantly improve OSA detection. Combined with readily available mobile health devices, tools can be developed for large-scale application and potentially widespread OSA screening in populations with poor access to care.
4. Relevance of PSG cardiac signals in OSA
Although the AASM Scoring Manual Version 2.4 (Berry et al 2017) only recommends a single modified electrocardiograph (Lead II) to score cardiac events, it is a rich source of data to assess underlying autonomic activity and cardiovascular physiology in OSA subtypes especially when combined with PPG (Gesche et al 2012). Known cardiac consequences of OSA include hypertension (Peppard et al 2000, Beaudin et al 2017), myocardial ischemia (Morra and Roubille 2017) and atrial fibrillation (Gami et al 2007), making the investigation of cardiac signals critical to the work up of OSA. Although OSA is a well-established risk factor for cardiovascular disease and death (Punjabi et al 2009, Shah et al 2010, Yaggi et al 2005), this association is largely based on the AHI as a single metric of OSA severity (Zinchuk et al 2018). But the AHI does not reflect cardiac pathophysiological effects, this is another opportunity to more accurately assess associations between OSA and cardiovascular outcomes.
4.1. Automated processing of cardiac signals.
OSA patients have marked changes in cardiovascular and respiratory regulation in response to respiratory events and this is the basis of automatic cardiac signal processing techniques to identify cardiac pathology. While automated processing of cardiac signals has not yet been applied clinically, we anticipate that when combined with other physiological signals, will be able identify patients at higher risk of cardiovascular morbidity and mortality. For example, we can quantify cardiac and autonomic function in subjects with OSA by measuring different aspects of heart rate variability and cardiorespiratory coupling to classify cardiovascular consequences. Table 1 represents a list with the best validated ECG signal features that have been investigated in OSA to date.
Heart rate variability (HRV).
While HRV is a normal physiological event in response to the sympathetic and parasympathetic nervous system, as well as the sleep-wake cycle, (European Society of Cardiology and the North American Society of Pacing and Electrophysiology 1996) an abnormal HRV occurs when the heart is no longer able to respond appropriately due to cardiac pathology. In OSA, HRV has a remarkable and characteristic pattern described as cyclical variation of heart rate (Guilleminault et al 1984). During an obstructive apnea event, cyclical variation of the heart rate is bradycardic during a respiratory event and tachycardic during recovery breaths (Guilleminault et al 1984). Consequently, methods to automatically identify apnea events from the ECG signal can be developed (Penzel et al 2016). A recently developed algorithm used only ECG recordings during sleep and extracted features from HRV and ECG-derived respiratory signals and correlated them to respiratory events (Atri and Mohebbi 2015). Specifically, respiratory events were mapped using image recognition techniques of heart rate and R-wave amplitude based time-frequency maps, and respiratory events were most visible at very low frequencies (VLF) around 0.03 Hz (Atri and Mohebbi 2015).
Pulse Transit Time.
Although blood pressure is a very important cardiac measurement to more directly reflect cardiovascular risk in response to arousals and respiratory events, technological limitations prevent this at present from being obtained continuously and routinely. Recently, surrogate measures of blood pressure were derived from the PPG waveform obtained from pulse oximeters (Gesche et al 2012). Pulse transit time (PTT) is an index that reflects variations in sympathetic activity during sleep, peripheral vascular resistance and intrathoracic pressure (Schwartz 2005), and studies have demonstrated that with appropriate calibration, PTT can track relative changes in blood pressure over the course of the night (Gehring et al 2018) as well as arousals and respiratory events to characterize sleep structure and fragmentation (Pépin et al 2009). In the study by Gehring et al, patients with severe OSA presented periods of extremely high systolic blood pressure (superposition) and very low oxygen saturation, suggesting additional evidence for high risk of cardiovascular events during the night (Gehring et al 2018).
Nonlinear analysis of inter-beat intervals.
Non-linear detrended fluctuation analysis of heart rate dynamics results in parameters relevant for OSA detection (Bunde et al 2000, Penzel et al 2003). For example, during slow wave sleep, heart rate shows a strongly short-term correlated behavior whereas during REM sleep it shows a strong long-term correlated behavior, expressed by the parameters alpha-1 and alpha-2. Changes in these parameters can be used to improve detection of sleep apnea severity, as well as estimates of sleep stages (Bunde et al 2000) and ECG-derived AHI (Penzel et al 2003).
Cardiorespiratory coupling.
Coupling between ECG and respiratory signals reflects an important aspect of cardiorespiratory interaction (Kabir et al 2010), where respiration has a strong influence on the cardiovascular system (Dick et al 2014). Aspects of cardiorespiratory coupling have been investigated in healthy subjects during sleep (Bartsch et al 2007), and studies support a tight relationship between high frequency cardiopulmonary coupling and delta power in the EEG (slow wave sleep) (Thomas et al 2014), suggesting that it can be used to measure sleep quality. Patients with severe OSA showed a significant reduction in phase-coupling (Kabir et al 2010) (may be an early marker of OSA severity (Sola-Soler et al 2015)), and CPAP therapy reverses this abnormal cardiorespiratory coupling (Chang et al 2013). Evidence for short-term consequences of cardiovascular decoupling in patients with OSA, specifically between heart rate and systolic blood pressure, has also been reported in a study that evaluated the effect of CPAP therapy for 3 months on daytime cardiovascular regulation in normotensive and hypertensive patients (Penzel et al 2012). These results indicated an effect of CPAP therapy on the baroreflex response in hypertensive patients, while on normotensive patients there were changes in the influence of the systolic blood pressure on the heart rate, from pathological patterns to adaptive mechanisms (Penzel et al 2012). However, it is still unclear whether changes in cardiovascular and cardiopulmonary coupling result in long-term effects on cardiovascular outcomes, and studies aimed to address this are warranted. Thus, the relationship between cardiac signals and the pathophysiology of OSA supports the use of analytical tools to better characterize the connection between OSA and cardiovascular consequences.
5. Relevance of PSG EEG signals in OSA
EEG recordings are widely used in sleep medicine because of its ability to detect variations in cortical activity and differentiate wake from different stages of sleep. Guidelines for scoring wake and sleep stages standardize and improve upon previous ad-hoc methods (Rechtschaffen and Kales 1968, Iber et al 2007). Because sleep is currently assessed using a visual scoring system, some properties and features of the EEG signal are not used in clinical practice, even though it is routinely collected. Therefore, efforts to further process these stored data will be helpful in better characterizing sleep physiology in patients with OSA.
In OSA patients, respiratory events can terminate with an arousal on EEG, which reflects the respiratory threshold to arouse from sleep (Younes and Hanly 2016). Frequent arousals on EEG have been associated with increased sympathetic activity that extends to daytime, and may explain why some patients with severe OSA have increased daytime sleepiness and hypertension (Slater and Steier 2012, Ren et al 2016). Patients with OSA also have more respiratory-induced arousals than controls, and these cause more frequent changes in blood pressure (Bartels et al 2016). In addition, it is common for OSA patients to have significant changes in their sleep architecture (e.g. sleep fragmentation, decreased rapid eye movement sleep and slow wave sleep), which is also associated with excessive daytime sleepiness and serious health consequences (Ayas et al 2014). Today we have the ability to measure specific EEG features of cortical activity that provide information about clinically relevant OSA subtypes. These novel signal processing techniques provide more details of sleep stages and clinical consequences of OSA such as sleepiness, chronic cognitive changes, metabolic changes and cardiovascular consequences (D’Rozario et al 2017).
5.1. Automated processing of EEG signals.
EEG activity during normal sleep remains relatively stable and may be amendable to visual-analog analysis of scoring sleep stages. However, during disrupted sleep, visual-analog analysis may be inadequate to identify and quantify the disruptions without the aid of a computer. To date, there are several digital processing techniques of EEG activity that improve sleep scoring reliability and increases sensitivity to different clinical status (Kubicki and Herrmann 1996, Penzel et al 2007). The use of automated EEG analyses on shorter segments of EEG activity are not constrained by arbitrary definitions of epoch length but at the same time, correlates well to definitions recommended by the task force and can better approximate the effect of OSA on sleep physiology (Malhotra et al 2013, Anderer et al 2010, Park et al 2015, Younes 2017). Automated EEG analyses include the application of spectral analysis, wavelet transformation and combined approaches. Table 1 summarizes the best EEG-derived signals and features relevant to OSA described to date.
Spectral Analysis.
Unlike visual review, EEG spectral analysis relies on computerized identification of discretely defined EEG frequencies. These frequencies correlate with underlying neurophysiological activities known to be associated with sleep, such as slow wave sleep and sleep spindles. Benefits of spectral analysis include the ability to analyze shorter EEG segments, allowing more granular analysis of sleep architecture. The most commonly used applications are the Power Spectral Density (PSD) and the Period-Amplitude Analysis (PAA). While these techniques have been clinically applied in other neurophysiological techniques, they have been largely studied as research tools in PSG, but now should be considered as part of the routine evaluation of sleep disorders in clinical practice, especially for OSA. In the PSD method, the Discrete Fourier Transform (DFT) of a finite epoch is calculated and then power density over that time segment derived. Since sleep stages are defined, in part, by EEG activities of given frequencies, this allows for the quantification of parameters related to sleep stages (Uchida et al 1999). The PAA uses a relatively straightforward methodology of measuring waves of varying frequencies based on their periodicity. The ‘half-period’ of a given sinusoidal wave would cross the ‘Zero’ amplitude of an analog EEG recording and could thus be measured in time frequency (Uchida et al 1999). Several studies have compared the two techniques in sleep EEG recording (Tan et al 2000, Uchida et al 1999) and have demonstrated similar results although the PAA requires some additional processing in order to be useful across the typical sleep EEG frequency spectrum. In OSA studies, spectral analysis has been more widely applied to clinical research. Although with limited evidence, severe OSA patients show specific changes in EEG power density when compared to controls (Vakulin et al 2016, Xiromeritis et al 2011), and these changes have been correlated with worse driving performance in these patients (Xiromeritis et al 2011). Thus, this quantitative EEG technique has the potential to provide complementary clinical information to traditional EEG analysis.
Wavelet Transformation.
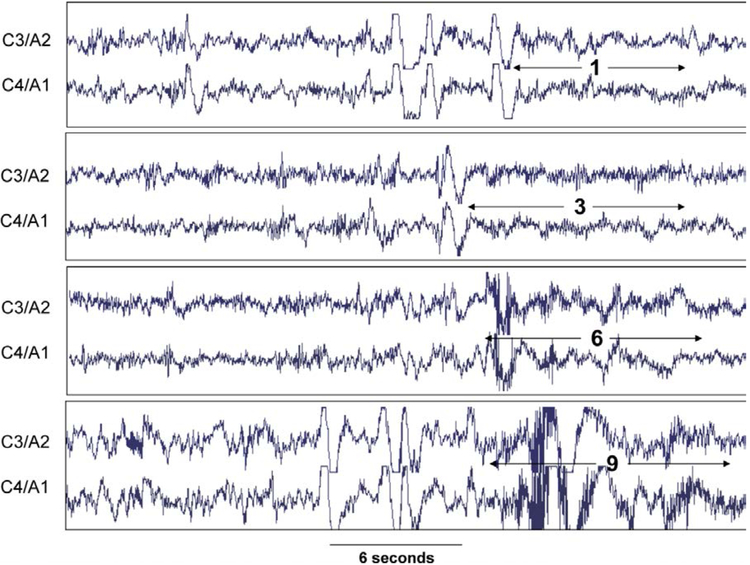
While PSD can resolve the different frequencies in a given segment reliably, it cannot specify the time location of the frequency within that segment (Ebrahimi et al 2008). The Wavelet transformation provides controllable time/frequency trade-off and hence can provide more precise time information particularly for high frequency EEG events. It shows reasonable sleep stage scoring reliability (77.6 to 94.4%) when compared to human scoring (Ebrahimi et al 2008, Oropesa et al 1999). Additionally, recent studies have demonstrated the use of wavelet transformation to estimate arousal intensity (Azarbarzin et al 2014). Figure 2 represents 4 examples of arousals with varying intensities using a scale from 0 (less intense) to 9 (more intense), according to the visual scoring of an expert. The arousal intensity is calculated based on the extraction of wavelet features of arousals that showed the best discrimination between a visually scored arousal, in a training set of 271. By using the pooled output of different classifiers, any EEG arousal in the PSG could then be classified according to its intensity. This index metric was validated by showing a strong relationship between arousal-related changes and heart rate (Azarbarzin et al 2014). Furthermore, these changes in heart rate response to arousal demonstrated a remarkable difference between individuals and have been demonstrated to be heritable in a recent twin study (Gao et al 2017). Collectively, these studies support the application of arousal intensity and heart rate response to arousal to further characterize meaningful physiological responses to OSA, as discussed further below.
Figure 2.
Examples of arousal with different intensity scales (0-least intense to 9-most intense) in the same patient. C3/A2 and C4/A1 are central electroencephalograms. Figure used with permission (Azarbarzin et al 2014)
Cyclic alternating pattern, K-complexes and sleep spindles.
Several other EEG features have been associated with OSA such as cyclic alternating pattern (CAP) (Terzano et al 1985), K-complexes (Nguyen et al 2016) and sleep spindles (Saunamäki et al 2017). Phasic events such as k-complexes, vertex waves, delta-like EEG bursts and short arousals are commonly seen in sleep EEG (Smerieri et al 2007). These NREM phasic events have been described to follow a peculiar time organization described as CAP, or sequences of transient electro-cortical events that are distinct from background EEG activity at up to 1-minute intervals (Terzano et al 1985). Changes in the CAP rate has been associated with fatigue and sleepiness in adults deemed to have the upper airway resistance syndrome (Guilleminault et al 2007). Also, CAP has been reported to improve the accuracy of detecting flow limitation events compared to respiratory-event related arousal in OSA patients (Milioli et al 2015), while another study suggested that increases in CPAP pressure should be avoided in non-CAP NREM sleep (Thomas 2002). Larger studies are needed to determine how NREM sleep instability, as represented by CAP, will have clinical utility in the diagnosis and treatment of patients with OSA, and approaches integrating these parameters with other features of EEG are yet to be investigated.
Automated system approaches and novel features.
The use of combined measures to determine sleep physiology is also an area of active research. A combined approach has the benefit of using more advanced processing algorithms as well as utilizing all to better inform about sleep physiology. Since muscle activity, eye movement activity and others signals have a significant relationship to sleep stages, automatic scoring of sleep stages can be performed using chin EMG, EOG and a limited EEG array (Malhotra et al 2013). In terms of technical scoring and efficiency, there are several published studies that have demonstrated similar sleep stage and respiratory event scoring outcomes between the combined automated system approach and traditional technologist scoring (Younes et al 2016, Malhotra et al 2013, Younes et al 2015b), suggesting that the field should now move to automated approaches. Recently, this has also been applied to generate and evaluate other novel physiological sleep traits of increasing interest in the field (Amatoury et al 2016, Younes and Hanly 2016, Younes et al 2015a). Younes et al have defined a continuous measure of sleep-wakefulness state based on the power spectrum patterns of EEG in 3-second epochs and the probability of each pattern occurring in 30-second epochs scored as awake, standardized by the percentage of epochs scored as awake. This metric of sleep-wake state, termed the odds-ratio product (ORP), when calculated in 30-second epochs, was highly correlated to the likelihood of visually scored arousals or awakenings in the following 30-second epoch (Younes et al 2015a). Thus, the ORP reflects a continuous measurement of sleep-wakefulness state. In a follow up study, the ORP in the immediate 9 seconds following arousals/awakening (termed ORP-9) was used to calculate post arousal dynamics of sleep and might better inform how different patients respond to arousal. The ORP-9 metric was associated with sleep depth in patients with OSA as well as the arousal index and AHI (Younes and Hanly 2016). Furthermore, scoring arousal intensity on a scale from 0 to 9, as calculated using wavelet transforms, was associated with respiratory control instability and is being suggested as a distinct pathophysiological trait in OSA (Amatoury et al 2016). This suggests that novel quantitative indices of sleep physiology will better represent specific aspects of sleep in OSA and could be leveraged as part of the heterogeneity of this disorder and potentially other sleep disorders (refer to Younes (Younes 2017) for further discussion on these novel indices) Therefore, having valid automated systems with standardized measures will be helpful to discern biologically meaningful subtypes of OSA that are biologically homogenous sharing a similar pathway to disease and treatment.
6. Novel analytical approaches of physiological signals to identify OSA subtypes and severity classification
A substantial amount of data is already being collected during sleep studies and novel analytical tools have already been developed to utilize these data to further describe specific aspects of OSA (Table 1). To date, these novel analytical approaches have largely been reported as secondary outcome variables in observational studies to lend support to specific physiological characteristics of the disease. We argue that these well-studied novel approaches that use widely accepted computational methods should be systematically utilized as primary outcome variables to reveal OSA subtypes, provide a more clinically relevant measure of OSA severity, and help clinicians move towards a more personalized approach to OSA management. Currently, there is an opportunity to explore these already available clinical and physiological data, and evaluate how the development of computational algorithms improves the advance of personalized approaches to manage OSA. Several datasets were made publicly available, mainly through the National Sleep Research Resource (https://sleepdata.org) (Dean et al 2016, Zhang et al 2018), a National Heart Lung and Blood-funded resource established to improve access to sleep data, including information from overnight physiological signals. Therefore, the combined expertise of clinicians, physiologists, engineers and computer scientists can offer the field all the components to advance Precision Sleep Medicine. This final section describes initial studies that used novel approaches to identify OSA subtypes, and include a brief description of the computational methods that were utilized in these investigations.
While initial efforts to describe clinically meaningful OSA subtypes were done using symptoms assessment, anthropometric and demographic data (Ye et al 2014, Keenan et al 2018, Kim et al 2018), the use of physiological signals from the PSG is still in its infancy. Two general types of analytical methods could facilitate the integration of these different sources of data in OSA: unsupervised and supervised. Unsupervised models can identify patterns in data that could potentially discover clinical insights not previously recognized. Methods like latent class analysis, K-means clustering and principal component analysis have been extensively used in the medical fields, and have provided support for the existence of different OSA subtypes (Zinchuk et al 2018, Ye et al 2014, Keenan et al 2018, Kim et al 2018). A recent retrospective study using conventional summary indices from the PSG and other demographic data found that OSA patients were grouped into seven different clusters by using unsupervised methods (Zinchuk et al 2018). These clusters, named ‘mild’, ‘periodic limb movements of sleep’, ‘NREM and arousal’, ‘REM and hypoxia’, ‘hypopnea and hypoxia’, ‘arousal and poor sleep’ and ‘combined severe’ were distinguished by a number of polysomnographic features and were associated with a combined outcome of cardiovascular risk, while conventional AHI measured severity were not. Interestingly, the ‘periodic limb movements of sleep’, ‘hypopnea and hypoxia’ and ‘combined severe’ clusters showed benefit of CPAP therapy on subsequent cardiovascular events compared to non-users while other groups did not exhibit such benefits (Zinchuk et al 2018) This study supports the notion that combining several different physiological aspects are more likely to identify OSA subtypes that exhibit cardiovascular benefits from CPAP therapy.
Supervised classification machine learning methods require labeled data to train predictive models that are further tested on an independent set of data. These approaches include logistic regression, support vector machine, classification and regression trees, and random forests. Usually, a number of metrics for classification performance are calculated and evaluated, so the researcher can assess the accuracy of the predictive model. Recently, these methods are becoming more popular to help predict OSA diagnosis and severity using different types of clinical and anthropometric features (Liu et al 2017, Bozkurt et al 2017, Lee et al 2009). An important issue when applying supervised machine learning methods is the balance between accuracy (i.e. how well the model performs) and model generalizability (i.e. how the model is applicable in a dataset that was not used to build the model). One factor that substantially affects these parameters is the feature selection strategy, or the way variables are selected as input in the prediction models. One study assessed different feature selection and classification methods to spectral and nonlinear traits extracted from oxygen saturation and PSG recordings, applied to OSA diagnosis (Alvarez et al 2013). The authors found that the combination of forward stepwise feature selection with logistic regression had the best performance using four features (83.2% validation accuracy), and the combination of a genetic algorithm with support vector machine had the best generalizability using 7 features (84.2% validation accuracy). Other methods evaluation studies have been conducted (Al-Angari and Sahakian 2012, Nemati et al 2014) and suggest that further optimization of feature selection algorithms might improve classification of OSA diagnosis and severity.
While these methods have been applied to inform OSA risk and severity, the studies are very heterogeneous in terms of sample size, input variables, model selection and definition of outcome and severity. To our knowledge, there are no studies attempting to combine more than one set of input variables (e.g. physiological and craniofacial) efficiently. Also, other factors such as ethnicity and genetic background are still not incorporated into OSA risk and severity. Based on the existing literature, we need more studies to explore OSA classification based on all aspects of the disease to increase our ability to better characterize the heterogeneity of this disorder.
7. Conclusion and future directions
This review highlights the extensive amount of available physiological PSG data that has yet to be incorporated into clinical practice. Use of the AHI limits our ability to advance precision medicine of OSA, as it does not reflect the heterogeneity of OSA. Advanced signal-processing methods and data analysis has been used to help subtype OSA, but only in the research setting. In the near future, respiratory, cardiac and EEG signals will undergo automated analyses, further standardizing PSG scoring, and provide, in addition, a rich set of reliable features that can more accurately describe OSA pathophysiology within an individual patient. Large, international consortia provide an ideal platform to collect standardized clinical, behavioral, anthropometric, molecular, imaging and physiological data on an ethnically diverse group of OSA patients. This, combined with advanced signal-processing methods and modern data analysis algorithms, have the promise to transform the way we manage OSA patients.
Acknowledgments
The authors would like to express their acknowledgments to the Sleep Apnea Global Interdisciplinary Consortium (SAGIC) and all its members across the globe supporting and standardizing research on obstructive sleep apnea. We also acknowledge the following funding agency: Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPq, Grant number 401569/2016-0, for author Lia Bittencourt) and National Institutes of Health grant P01 HL094307 (to Allan I. Pack).
Abbreviations List
- AASM
American Academy of Sleep Medicine
- AHI
apnea-hypopnea index
- CAP
cyclic alternating patterns
- CPAP
continuous positive airway pressure
- DFA
detrended fluctuation analysis
- ECG
electrocardiogram
- EEG
electroencephalogram
- EMG
electromyogram
- EOG
electrooculogram
- FFT
fast Fourier transformation
- HF
high frequency
- HRV
heart rate variability
- LF
low frequency
- NED
negative effort dependence
- NN50
Number of pairs of adjacent NN intervals differing by more than 50 ms
- NREM
non-rapid eye-movement
- ODI
oxygen desaturation index
- ORP
odds-ratio product
- OSA
obstructive sleep apnea
- PAA:
period-amplitude analysis
- Pcrit
pharyngeal critical closing pressure
- pNN50
NN50 count divided by the total number of all NN intervals
- PPG
photoplethysmography
- PSG
polysomnography
- PTT
pulse transit time
- REM
rapid eye-movement
- RMSSD
square root of the mean squared differences of successive NN intervals
- SDNN
standard deviation of NN intervals
- SDSD
standard deviation of differences between adjacent NN intervals
- SpO2
peripheral oxygen saturation
- TST
total sleep time
- ULF
ultra low frequency
- VLF
very low frequency
References
- Aeschbacher S, Bossard M, Schoen T, Schmidlin D, Muff C, Maseli A, Leuppi JD, Miedinger D, Probst-Hensch NM, Schmidt-Trucksäss A, Risch M, Risch L and Conen D 2016. Heart Rate Variability and Sleep-Related Breathing Disorders in the General Population Am. J. Cardiol 118 912–7 [DOI] [PubMed] [Google Scholar]
- Aittokallio T, Saaresranta T, Polo-Kantola P, Nevalainen O and Polo O 2001. Analysis of inspiratory flow shapes in patients with partial upper-airway obstruction during sleep Chest 119 37–44 [DOI] [PubMed] [Google Scholar]
- Al-Angari HM and Sahakian AV 2012. Automated recognition of obstructive sleep apnea syndrome using support vector machine classifier IEEE Trans. Inf. Technol. Biomed. Publ. IEEE Eng. Med. Biol. Soc 16 463–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J 2007. Photoplethysmography and its application in clinical physiological measurement Physiol. Meas 28 R1–39 [DOI] [PubMed] [Google Scholar]
- Alvarez D, Hornero R, Marcos JV, Wessel N, Penzel T, Glos M and Del Campo F 2013. Assessment of feature selection and classification approaches to enhance information from overnight oximetry in the context of apnea diagnosis Int. J. Neural Syst 23 1350020. [DOI] [PubMed] [Google Scholar]
- Amatoury J, Azarbarzin A, Younes M, Jordan AS, Wellman A and Eckert DJ 2016. Arousal Intensity is a Distinct Pathophysiological Trait in Obstructive Sleep Apnea Sleep 39 2091–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Sleep Medicine 1999. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force Sleep 22 667–89 [PubMed] [Google Scholar]
- American Sleep Disorders Association 1992. EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association Sleep 15 173–84 [PubMed] [Google Scholar]
- Anderer P, Moreau A, Woertz M, Ross M, Gruber G, Parapatics S, Loretz E, Heller E, Schmidt A, Boeck M, Moser D, Kloesch G, Saletu B, Saletu-Zyhlarz GM, Danker-Hopfe H, Zeitlhofer J and Dorffner G 2010. Computer-Assisted Sleep Classification according to the Standard of the American Academy of Sleep Medicine: Validation Study of the AASM Version of the Somnolyzer 24 × 7 Neuropsychobiology 62 250–64 [DOI] [PubMed] [Google Scholar]
- Arnardottir ES and Gislason T 2016. Quantifying Airflow Limitation and Snoring During Sleep Sleep Med. Clin 11 421–34 [DOI] [PubMed] [Google Scholar]
- Atri R and Mohebbi M 2015. Obstructive sleep apnea detection using spectrum and bispectrum analysis of single-lead ECG signal Physiol. Meas 36 1963–80 [DOI] [PubMed] [Google Scholar]
- Ayappa I, Rapaport BS, Norman RG and Rapoport DM 2005. Immediate consequences of respiratory events in sleep disordered breathing Sleep Med 6 123–30 [DOI] [PubMed] [Google Scholar]
- Ayas NT, Hirsch AAJ, Laher I, Bradley TD, Malhotra A, Polotsky VY and Tasali E 2014. New frontiers in obstructive sleep apnoea Clin. Sci. Lond. Engl. 1979 127 209–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarbarzin A, Marques M, Sands SA, de Beeck SO, Genta PR, Taranto-Montemurro L, de Melo CM, Messineo L, Vanderveken OM, White DP and Wellman A 2017a. Predicting epiglottic collapse in patients with obstructive sleep apnoea Eur. Respir. J 50 1700345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarbarzin A, Ostrowski M, Hanly P and Younes M 2014. Relationship between arousal intensity and heart rate response to arousal Sleep 37 645–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarbarzin A, Sands SA, Taranto-Montemurro L, Oliveira Marques MD, Genta P R, Edwards BA, Butler J, White DP and Wellman A 2017b. Estimation of Pharyngeal Collapsibility During Sleep by Peak Inspiratory Airflow Sleep 40 Online: https://academic.oup.com/sleep/sleep/article/2666705/Estimation [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baguet J-P, Hammer L, Lévy P, Pierre H, Launois S, Mallion J-M and Pépin J-L 2005. The severity of oxygen desaturation is predictive of carotid wall thickening and plaque occurrence Chest 128 3407–12 [DOI] [PubMed] [Google Scholar]
- Bartels W, Buck D, Glos M, Fietze I and Penzel T 2016. Definition and Importance of Autonomic Arousal in Patients with Sleep Disordered Breathing Sleep Med. Clin 11 435–44 [DOI] [PubMed] [Google Scholar]
- Bartsch R, Kantelhardt JW, Penzel T and Havlin S 2007. Experimental evidence for phase synchronization transitions in the human cardiorespiratory system Phys. Rev. Lett 98 054102. [DOI] [PubMed] [Google Scholar]
- Beaudin AE, Waltz X, Hanly PJ and Poulin MJ 2017. Impact of obstructive sleep apnoea and intermittent hypoxia on cardiovascular and cerebrovascular regulation Exp. Physiol 102 743–63 [DOI] [PubMed] [Google Scholar]
- Behar J, Roebuck A, Shahid M, Daly J, Hallack A, Palmius N, Stradling J and Clifford GD 2015. SleepAp: an automated obstructive sleep apnoea screening application for smartphones IEEE J. Biomed. Health Inform 19 325–31 [DOI] [PubMed] [Google Scholar]
- Bennett SL, Goubran R and Knoefel F 2015. The detection of breathing behavior using Eulerian-enhanced thermal video Conf. Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. IEEE Eng. Med. Biol. Soc. Annu. Conf 2015 7474–7 [DOI] [PubMed] [Google Scholar]
- Berry RB, Brooks R, Gamaldo C, Harding SM, Lloyd RM, Quan SF, Troester MT and Vaughn BV 2017. AASM Scoring Manual Updates for 2017 (Version 2.4) J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med 13 665–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi MT, Cash SS, Mietus J, Peng C-K and Thomas R 2010. Obstructive sleep apnea alters sleep stage transition dynamics PloS One 5 e11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilgin C, Erkorkmaz U, Ucar MK, Akin N, Nalbant A and Annakkaya AN 2016. Use of a portable monitoring device (Somnocheck Micro) for the investigation and diagnosis of obstructive sleep apnoea in comparison with polysomnography Pak. J. Med. Sci 32 471–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosi M, Milioli G, Riccardi S, Melpignano A, Vaudano A E, Cortelli P, Poletti V and Parrino L 2018. Arousal responses to respiratory events during sleep: the role of pulse wave amplitude J. Sleep Res 27 259–67 [DOI] [PubMed] [Google Scholar]
- Bostanci A, Turhan M and Bozkurt S 2015. Factors influencing sleep time with oxygen saturation below 90% in sleep-disordered breathing The Laryngoscope 125 1008–12 [DOI] [PubMed] [Google Scholar]
- Bozkurt S, Bostanci A and Turhan M 2017. Can Statistical Machine Learning Algorithms Help for Classification of Obstructive Sleep Apnea Severity to Optimal Utilization of Polysomnography Resources? Methods Inf. Med 56 308–18 [DOI] [PubMed] [Google Scholar]
- Bunde A, Havlin S, Kantelhardt JW, Penzel T, Peter JH and Voigt K 2000. Correlated and uncorrelated regions in heart-rate fluctuations during sleep Phys. Rev. Lett 85 3736–9 [DOI] [PubMed] [Google Scholar]
- Cao J, Feng J, Li L and Chen B 2015. Obstructive sleep apnea promotes cancer development and progression: a concise review Sleep Breath. Schlaf Atm 19 453–7 [DOI] [PubMed] [Google Scholar]
- Carberry JC, Amatoury J and Eckert DJ 2017. Personalized Management Approach for OSA Chest [DOI] [PubMed] [Google Scholar]
- Carter SG, Berger MS, Carberry JC, Bilston LE, Butler JE, Tong BKY, Martins RT, Fisher LP, McKenzie DK, Grunstein RR and Eckert D J 2016. Zopiclone Increases the Arousal Threshold without Impairing Genioglossus Activity in Obstructive Sleep Apnea Sleep 39 757–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho DZ, Gerhardt GJL, Dellagustin G, de Santa-Helena EL, Lemke N, Segal AZ and Schönwald SV 2014. Loss of sleep spindle frequency deceleration in Obstructive Sleep Apnea Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol 125 306–12 [DOI] [PubMed] [Google Scholar]
- Chang JS, Lee SD, Ju G, Kim J-W, Ha K and Yoon I-Y 2013. Enhanced cardiorespiratory coupling in patients with obstructive sleep apnea following continuous positive airway pressure treatment Sleep Med 14 1132–8 [DOI] [PubMed] [Google Scholar]
- de Chazal P, Heneghan C and McNicholas WT 2009. Multimodal detection of sleep apnoea using electrocardiogram and oximetry signals Philos. Transact. A Math. Phys. Eng. Sci 367 369–89 [DOI] [PubMed] [Google Scholar]
- de Chazal P, Heneghan C, Sheridan E, Reilly R, Nolan P and O’Malley M 2003. Automated processing of the single-lead electrocardiogram for the detection of obstructive sleep apnoea IEEE Trans. Biomed. Eng 50 686–96 [DOI] [PubMed] [Google Scholar]
- Cowie MR 2017. Sleep apnea: State of the art Trends Cardiovasc. Med 27 280–9 [DOI] [PubMed] [Google Scholar]
- Dawson A, Loving RT, Gordon RM, Abel SL, Loewy D, Kripke DF and Kline LE 2015. Type III home sleep testing versus pulse oximetry: is the respiratory disturbance index better than the oxygen desaturation index to predict the apnoea-hypopnoea index measured during laboratory polysomnography? BMJ Open 5 e007956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DA, Goldberger AL, Mueller R, Kim M, Rueschman M, Mobley D, Sahoo SS, Jayapandian CP, Cui L, Morrical MG, Surovec S, Zhang G-Q and Redline S 2016. Scaling Up Scientific Discovery in Sleep Medicine: The National Sleep Research Resource Sleep 39 1151–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey JA, Skatrud JB, Jacques AJ, Ewanowski SJ, Woodson BT, Hanson PR and Goodman B 2002. Anatomic determinants of sleep-disordered breathing across the spectrum of clinical and nonclinical male subjects Chest 122 840–51 [DOI] [PubMed] [Google Scholar]
- Dick TE, Mims JR, Hsieh Y-H, Morris KF and Wehrwein EA 2014. Increased cardio-respiratory coupling evoked by slow deep breathing can persist in normal humans Respir. Physiol. Neurobiol 204 99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Rozario AL, Cross NE, Vakulin A, Bartlett DJ, Wong KKH, Wang D and Grunstein RR 2017. Quantitative electroencephalogram measures in adult obstructive sleep apnea - Potential biomarkers of neurobehavioural functioning Sleep Med. Rev 36 29–42 [DOI] [PubMed] [Google Scholar]
- Ebrahimi F, Mikaeili M, Estrada E and Nazeran H 2008. Automatic sleep stage classification based on EEG signals by using neural networks and wavelet packet coefficients Conf. Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. IEEE Eng. Med. Biol. Soc. Annu. Conf 2008 1151–4 [DOI] [PubMed] [Google Scholar]
- Eckberg D L 2003. The human respiratory gate J. Physiol 548 339–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert DJ, Owens RL, Kehlmann GB, Wellman A, Rahangdale S, Yim-Yeh S, White DP and Malhotra A 2011. Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold Clin. Sci. Lond. Engl. 1979 120 505–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert DJ, White DP, Jordan AS, Malhotra A and Wellman A 2013. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets Am. J. Respir. Crit. Care Med 188 996–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards BA, Andara C, Landry S, Sands SA, Joosten SA, Owens RL, White DP, Hamilton GS and Wellman A 2016. Upper-Airway Collapsibility and Loop Gain Predict the Response to Oral Appliance Therapy in Patients with Obstructive Sleep Apnea Am. J. Respir. Crit. Care Med 194 1413–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards BA, Eckert DJ, McSharry DG, Sands SA, Desai A, Kehlmann G, Bakker JP, Genta PR, Owens RL, White DP, Wellman A and Malhotra A 2014. Clinical predictors of the respiratory arousal threshold in patients with obstructive sleep apnea Am. J. Respir. Crit. Care Med 190 1293–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Society of Cardiology and the North American Society of Pacing and Electrophysiology 1996. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Circulation 93 1043–65 [PubMed] [Google Scholar]
- Gami AS, Hodge DO, Herges RM, Olson EJ, Nykodym J, Kara T and Somers VK 2007. Obstructive Sleep Apnea, Obesity, and the Risk of Incident Atrial Fibrillation J. Am. Coll. Cardiol 49 565–71 [DOI] [PubMed] [Google Scholar]
- Gao X, Azarbarzin A, Keenan BT, Ostrowski M, Pack FM, Staley B, Maislin G, Pack AI, Younes M and Kuna ST 2017. Heritability of Heart Rate Response to Arousals in Twins Sleep 40 Online: https://academic.oup.com/sleep/article-lookup/doi/10.1093/sleep/zsx055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garde A, Dehkordi P, Wensley D, Ansermino JM and Dumont GA 2015. Pulse oximetry recorded from the Phone Oximeter for detection of obstructive sleep apnea events with and without oxygen desaturation in children Conf. Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. IEEE Eng. Med. Biol. Soc. Annu. Conf 2015 7692–5 [DOI] [PubMed] [Google Scholar]
- Gehring J, Gesche H, Drewniok G, Küchler G and Patzak A 2018. Nocturnal blood pressure fluctuations measured by using pulse transit time in patients with severe obstructive sleep apnea syndrome Sleep Breath. Schlaf Atm 22 337–43 [DOI] [PubMed] [Google Scholar]
- Genta PR, Sands SA, Butler JP, Loring SH, Katz ES, Demko BG, Kezirian EJ, White DP and Wellman A 2017. Airflow Shape Is Associated With the Pharyngeal Structure Causing OSA Chest 152 537–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesche H, Grosskurth D, Küchler G and Patzak A 2012. Continuous blood pressure measurement by using the pulse transit time: comparison to a cuff-based method Eur. J. Appl. Physiol 112 309–15 [DOI] [PubMed] [Google Scholar]
- Guilleminault C, Connolly S, Winkle R, Melvin K and Tilkian A 1984 Cyclical variation of the heart rate in sleep apnoea syndrome. Mechanisms, and usefulness of 24 h electrocardiography as a screening technique Lancet Lond. Engl 1 126–31 [DOI] [PubMed] [Google Scholar]
- Guilleminault C, Lopes MC, Hagen CC and da Rosa A 2007. The cyclic alternating pattern demonstrates increased sleep instability and correlates with fatigue and sleepiness in adults with upper airway resistance syndrome Sleep 30 641–7 [DOI] [PubMed] [Google Scholar]
- Gupta A and Shukla G 2018. Polysomnographic determinants of requirement for advanced positive pressure therapeutic options for obstructive sleep apnea Sleep Breath. Schlaf Atm 22 401–9 [DOI] [PubMed] [Google Scholar]
- Hahn G, Sipinková I, Baisch F and Hellige G 1995. Changes in the thoracic impedance distribution under different ventilatory conditions Physiol. Meas 16 A161–173 [DOI] [PubMed] [Google Scholar]
- Halevi M, Dafna E, Tarasiuk A and Zigel Y 2016. Can we discriminate between apnea and hypopnea using audio signals? Conf. Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. IEEE Eng. Med. Biol. Soc. Annu. Conf 2014 3211–4 [DOI] [PubMed] [Google Scholar]
- Himanen S-L, Virkkala J, Huupponen E and Hasan J 2003. Spindle frequency remains slow in sleep apnea patients throughout the night Sleep Med 4 229–34 [DOI] [PubMed] [Google Scholar]
- Ho V, Crainiceanu CM, Punjabi NM, Redline S and Gottlieb DJ 2015. Calibration Model for Apnea-Hypopnea Indices: Impact of Alternative Criteria for Hypopneas Sleep 38 1887–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson A and Quan S 2007. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications ed American Academy of Sleep Medicine (Westchester: American Academy of Sleep Medicine; ) [Google Scholar]
- Idiaquez J, Santos I, Santin J, Del Rio R and Iturriaga R 2014. Neurobehavioral and autonomic alterations in adults with obstructive sleep apnea Sleep Med 15 1319–23 [DOI] [PubMed] [Google Scholar]
- Jacobsen JH, Shi L and Mokhlesi B 2013. Factors associated with excessive daytime sleepiness in patients with severe obstructive sleep apnea Sleep Breath. Schlaf Atm 17 629–35 [DOI] [PubMed] [Google Scholar]
- Kabir MM, Dimitri H, Sanders P, Antic R, Nalivaiko E, Abbott D and Baumert M 2010. Cardiorespiratory phase-coupling is reduced in patients with obstructive sleep apnea PloS One 5 e10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan BT, Kim J, Singh B, Bittencourt L, Chen N-H, Cistulli PA, Magalang UJ, McArdle N, Mindel JW, Benediktsdottir B, Amardottir ES, Prochnow LK, Penzel T, Sanner B, Schwab RJ, Shin C, Sutherland K, Tufik S, Maislin G, Gislason T and Pack AI 2018. Recognizable clinical subtypes of obstructive sleep apnea across international sleep centers: a cluster analysis Sleep 41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Keenan BT, Lim DC, Lee SK, Pack AI and Shin C 2018. Symptom-Based Subgroups of Koreans With Obstructive Sleep Apnea J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med 14 437–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki S and Herrmann WM 1996. The future of computer-assisted investigation of the polysomnogram: sleep microstructure J. Clin. Neurophysiol. Off. Publ. Am. Electroencephalogr. Soc 13 285–94 [DOI] [PubMed] [Google Scholar]
- Kuhn E, Schwarz EI, Bratton DJ, Rossi VA and Kohler M 2017. Effects of CPAP and Mandibular Advancement Devices on Health-Related Quality of Life in OSA: A Systematic Review and Meta-analysis Chest 151 786–94 [DOI] [PubMed] [Google Scholar]
- Langley P, Bowers EJ and Murray A 2010. Principal component analysis as a tool for analyzing beat-to-beat changes in ECG features: application to ECG-derived respiration IEEE Trans. Biomed. Eng 57 821–9 [DOI] [PubMed] [Google Scholar]
- Lee RWW, Petocz P, Prvan T, Chan ASL, Grunstein RR and Cistulli PA 2009. Prediction of obstructive sleep apnea with craniofacial photographic analysis Sleep 32 46–52 [PMC free article] [PubMed] [Google Scholar]
- Lim DC, Sutherland K, Cistulli PA and Pack A1 2017. P4 medicine approach to obstructive sleep apnoea Respirol. Carlton Vic 22 849–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W-T, Wu H-T, Juang J-N, Wisniewski A, Lee H-C, Wu D and Lo Y-L 2017. Prediction of the severity of obstructive sleep apnea by anthropometric features via support vector machine PloS One 12 e0176991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons MM, Keenan BT, Li J, Khan T, Elkassabany N, Walsh CM, Williams NN, Pack AIand Gurubhagavatula I 2017. Symptomless Multi-Variable Apnea Prediction Index Assesses Obstructive Sleep Apnea Risk and Adverse Outcomes in Elective Surgery Sleep 40 Online: https://academic.oup.com/sleep/article/doi/10.1093/sleep/zsw081/2845959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra A, Younes M, Kuna ST, Benca R, Kushida CA, Walsh J, Hanlon A, Staley B, Pack AI and Pien GW 2013. Performance of an automated polysomnography scoring system versus computer-assisted manual scoring Sleep 36 573–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martynowicz H, Skomro R, Gać P, Mazur G, Porębska I, Bryłka A, Nowak W, Zieliński M, Wojakowska A and Poręba R 2017. The influence of hypertension on daytime sleepiness in obstructive sleep apnea J. Am. Soc. Hypertens. JASH 11 295–302 [DOI] [PubMed] [Google Scholar]
- Meredith DJ, Clifton D, Charlton P, Brooks J, Pugh CW and Tarassenko L 2012. Photoplethysmographic derivation of respiratory rate: a review of relevant physiology J. Med. Eng. Technol 36 1–7 [DOI] [PubMed] [Google Scholar]
- Milioli G, Bosi M, Grassi A, Riccardi S, Terzano MG, Cortelli P, Poletti V and Parrino L 2015. Can sleep microstructure improve diagnosis of OSAS? Integrative information from CAP parameters Arch. Ital. Biol 153 194–203 [DOI] [PubMed] [Google Scholar]
- Moody GB, Mark RG, Goldberger A and Penzel T 2000. Stimulating rapid research advances via focused competition: the Computers in Cardiology Challenge 2000 (IEEE) pp 207–10 Online: http://ieeexplore.ieee.org/document/898493/ [Google Scholar]
- Morra S and Roubille F 2017. Obstructive sleep apnoea: from respiratory events to coronary microvascular dysfunction Acta Cardiol 1–6 [DOI] [PubMed] [Google Scholar]
- Mwenge GB, Rombaux P, Lengele B and Rodenstein D 2015. Hypoglossal Nerve Stimulation for Obstructive Sleep Apnea Prog. Neurol. Surg 29 94–105 [DOI] [PubMed] [Google Scholar]
- Nemati S, Orr J and Malhotra A 2014. Data-driven phenotyping : graphical models for model-based phenotyping of sleep apnea IEEE Pulse 5 45–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng AK and Guan C 2012. Impact of obstructive sleep apnea on sleep-wake stage ratio. Conf. Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. IEEE Eng. Med. Biol. Soc. Annu. Conf 2012 4660–3 [DOI] [PubMed] [Google Scholar]
- Ng AT, Qian J and Cistulli PA 2006. Oropharyngeal collapse predicts treatment response with oral appliance therapy in obstructive sleep apnea Sleep 29 666–71 [PubMed] [Google Scholar]
- Nguyen CD, Wellman A, Jordan AS and Eckert DJ 2016. Mild Airflow Limitation during N2 Sleep Increases K- complex Frequency and Slows Electroencephalographic Activity Sleep 39 541–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitzan M, de Boer H, Turivnenko S, Babchenko A and Sapoznikov D 1994. Power spectrum analysis of spontaneous fluctuations in the photoplethysmographic signal J. Basic Clin. Physiol. Pharmacol 5 269–76 [DOI] [PubMed] [Google Scholar]
- Nitzan M, Turivnenko S, Milston A, Babchenko A and Mahler Y 1996. Low-frequency variability in the blood volume and in the blood volume pulse measured by photoplethysmography J. Biomed. Opt 1 223–9 [DOI] [PubMed] [Google Scholar]
- Oropesa E, Cycon HL and Jobert M 1999. Sleep stage classification using wavelet transform and neural network Int. Comput. Sci. Inst [Google Scholar]
- Pack AI 2016. Application of Personalized, Predictive, Preventative, and Participatory (P4) Medicine to Obstructive Sleep Apnea. A Roadmap for Improving Care? Ann. Am. Thorac. Soc 13 1456–67 [DOI] [PubMed] [Google Scholar]
- Pallás-Areny R, Colominas-Balagué J and Rosell FJ 1989. The effect of respiration-induced heart movements on the ECG IEEE Trans. Biomed. Eng 36 585–90 [DOI] [PubMed] [Google Scholar]
- Park DY, Kim HJ, Kim C-H, Kim YS, Choi JH, Hong SY, Jung JJ, Lee KI and Lee HS 2015. Reliability and validity testing of automated scoring in obstructive sleep apnea diagnosis with the Embletta X100: Automated Scoring With the Embletta X100 The Laryngoscope 125 493–7 [DOI] [PubMed] [Google Scholar]
- Penzel T, Hirshkowitz M, Harsh J, Chervin RD, Butkov N, Kryger M, Malow B, Vitiello MV, Silber MH, Kushida C A and Chesson A L 2007. Digital analysis and technical specifications J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med 3 109–20 [PubMed] [Google Scholar]
- Penzel T, Kantelhardt JW, Bartsch RP, Riedl M, Kraemer JF, Wessel N, Garcia C, Glos M, Fietze I and Schöbel C 2016. Modulations of Heart Rate, ECG, and Cardio-Respiratory Coupling Observed in Polysomnography Front. Physiol 7 460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzel T, Kantelhardt JW, Grote L, Peter J-H and Bunde A 2003. Comparison of detrended fluctuation analysis and spectral analysis for heart rate variability in sleep and sleep apnea IEEE Trans. Biomed. Eng 50 1143–51 [DOI] [PubMed] [Google Scholar]
- Penzel T, McNames J, Murray A, de Chazal P, Moody G and Raymond B 2002. Systematic comparison of different algorithms for apnoea detection based on electrocardiogram recordings Med. Biol. Eng. Comput 40 402–7 [DOI] [PubMed] [Google Scholar]
- Penzel T, Riedl M, Gapelyuk A, Suhrbier A, Bretthauer G, Malberg H, Schöbel C, Fietze I, Heitmann J, Kurths J and Wessel N 2012. Effect of CPAP therapy on daytime cardiovascular regulations in patients with obstructive sleep apnea Comput. Biol. Med 42 328–34 [DOI] [PubMed] [Google Scholar]
- Pépin J-L, Tamisier R, Borel J-C, Baguet J-P and Lávy P 2009. A critical review of peripheral arterial tone and pulse transit time as indirect diagnostic methods for detecting sleep disordered breathing and characterizing sleep structure Curr. Opin. Pulm. Med 15 550–8 [DOI] [PubMed] [Google Scholar]
- Peppard PE, Young T, Palta M and Skatrud J 2000. Prospective study of the association between sleep-disordered breathing and hypertension N. Engl. J. Med 342 1378–84 [DOI] [PubMed] [Google Scholar]
- Petryszak R, Keays M, Tang YA, Fonseca NA, Barrera E, Burdett T, Füllgrabe A, Fuentes AM-P, Jupp S, Koskinen S, Mannion O, Huerta L, Megy K, Snow C, Williams E, Barzine M, Hastings E, Weisser H, Wright J, Jaiswal P, Huber W, Choudhary J, Parkinson HE and Brazma A 2016. Expression Atlas update--an integrated database of gene and protein expression in humans, animals and plants Nucleic Acids Res 44 D746–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O’Connor GT, Rapoport DM, Redline S, Resnick HE, Robbins JA, Shahar E, Unruh ML and Samet JM 2009. Sleep-disordered breathing and mortality: a prospective cohort study PLoSMed. 6 e1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnavadivel R, Chau N, Stadler D, Yeo A, McEvoy RD and Catcheside PG 2009. Marked reduction in obstructive sleep apnea severity in slow wave sleep J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med 5 519–24 [PMC free article] [PubMed] [Google Scholar]
- Rechtschaffen A and Kales A 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects (Washington, D.C.: Public Health Service, U.S. Government Printing Office; ) [Google Scholar]
- Ren R, Li Y, Zhang J, Zhou J, Sun Y, Tan L, Li T, Wing Y-K and Tang X 2016. Obstructive Sleep Apnea With Objective Daytime Sleepiness Is Associated With Hypertension Hypertens. Dallas Tex 1979 68 1264–70 [DOI] [PubMed] [Google Scholar]
- Roebuck A, Monasterio V, Gederi E, Osipov M, Behar J, Malhotra A, Penzel T and Clifford GD 2014. A review of signals used in sleep analysis Physiol. Meas 35 R1–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romem A, Romem A, Koldobskiy D and Scharf SM 2014. Diagnosis of obstructive sleep apnea using pulse oximeter derived photoplethysmographic signals J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med 10 285–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands SA, Terrill PI, Edwards BA, Taranto Montemurro L, Azarbarzin A, Marques M, de Melo CM, Loring SH, Butler JP, White DP and Wellman A 2018. Quantifying the Arousal Threshold Using Polysomnography in Obstructive Sleep Apnea Sleep 41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunamäki T, Huupponen E, Loponen J and Himanen S-L 2017. CPAP Treatment Partly Normalizes Sleep Spindle Features in Obstructive Sleep Apnea Sleep Disord 2017 2962479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz DJ 2005. The pulse transit time arousal index in obstructive sleep apnea before and after CPAP Sleep Med 6 199–203 [DOI] [PubMed] [Google Scholar]
- Shah NA, Yaggi HK, Concato J and Mohsenin V 2010. Obstructive sleep apnea as a risk factor for coronary events or cardiovascular death Sleep Breath. Schlaf Atm 14 131–6 [DOI] [PubMed] [Google Scholar]
- Sivan Y, Kornecki A and Schonfeld T 1996. Screening obstructive sleep apnoea syndrome by home videotape recording in children Eur. Respir. J 9 2127–31 [DOI] [PubMed] [Google Scholar]
- Slater G and Steier J 2012. Excessive daytime sleepiness in sleep disorders J. Thorac. Dis 4 608–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smerieri A, Parrino L, Agosti M, Ferri R and Terzano MG 2007. Cyclic alternating pattern sequences and non-cyclic alternating pattern periods in human sleep Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol 118 2305–13 [DOI] [PubMed] [Google Scholar]
- Sola-Soler J, Giraldo BF, Fiz JA and Jane R 2015. Cardiorespiratory Phase Synchronization in OSA subjects during wake and sleep states Conf. Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. IEEE Eng. Med. Biol. Soc. Annu. Conf 2015 7708–11 [DOI] [PubMed] [Google Scholar]
- Strollo PJ, Soose RJ, Maurer JT, de Vries N, Cornelius J, Froymovich O, Hanson RD, Padhya TA, Steward DL, Gillespie MB, Woodson BT, Van de Heyning PH, Goetting MG, Vanderveken OM, Feldman N, Knaack L, Strohl KP and STAR Trial Group 2014. Upper-airway stimulation for obstructive sleep apnea N. Engl. J. Med 370 139–49 [DOI] [PubMed] [Google Scholar]
- Svanborg E, Larsson H, Carlsson-Nordlander B and Pirskanen R 1990. A limited diagnostic investigation for obstructive sleep apnea syndrome. Oximetry and static charge sensitive bed Chest 98 1341–5 [DOI] [PubMed] [Google Scholar]
- Tan X, Campbell IG, Palagini L and Feinberg I 2000. High internight reliability of computer-measured NREM delta, sigma, and beta: biological implications Biol. Psychiatry 48 1010–9 [DOI] [PubMed] [Google Scholar]
- Terrill PI, Edwards BA, Nemati S, Butler JP, Owens RL, Eckert DJ, White DP, Malhotra A, Wellman A and Sands SA 2015. Quantifying the ventilatory control contribution to sleep apnoea using polysomnography Eur. Respir. J 45 408–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzano MG, Mancia D, Salati MR, Costani G, Decembrino A and Parrino L 1985. The cyclic alternating pattern as a physiologic component of normal NREM sleep Sleep 8 137–45 [DOI] [PubMed] [Google Scholar]
- Thomas RJ 2002. Cyclic alternating pattern and positive airway pressure titration Sleep Med 3 315–22 [DOI] [PubMed] [Google Scholar]
- Thomas RJ, Mietus JE, Peng C-K, Guo D, Gozal D, Montgomery-Downs H, Gottlieb DJ, Wang C-Y and Goldberger AL 2014. Relationship between delta power and the electrocardiogram-derived cardiopulmonary spectrogram: possible implications for assessing the effectiveness of sleep Sleep Med. 15 125–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimer R, Cabidu R, Sampaio LLM, Stirbulov R, Poiares D, Guizilini S, Bianchi AM, Costa FSM, Mendes RG, Delfino A, Arena R and Borghi-Silva A 2014. Heart rate variability and cardiorespiratory coupling in obstructive sleep apnea: elderly compared with young Sleep Med. 15 1324–31 [DOI] [PubMed] [Google Scholar]
- Uchida S, Feinberg I, March JD, Atsumi Y and Maloney T 1999. A comparison of period amplitude analysis and FFT power spectral analysis of all-night human sleep EEG Physiol. Behav 67 121–31 [DOI] [PubMed] [Google Scholar]
- Vakulin A, D’Rozario A, Kim J-W, Watson B, Cross N, Wang D, Coeytaux A, Bartlett D, Wong K and Grunstein R 2016. Quantitative sleep EEG and polysomnographic predictors of driving simulator performance in obstructive sleep apnea Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol 127 1428–35 [DOI] [PubMed] [Google Scholar]
- Wang C-W, Hunter A, Gravill N and Matusiewicz S 2014. Unconstrained video monitoring of breathing behavior and application to diagnosis of sleep apnea IEEE Trans. Biomed. Eng 61 396–404 [DOI] [PubMed] [Google Scholar]
- Wellman A, Edwards BA, Sands SA, Owens RL, Nemati S, Butler J, Passaglia CL, Jackson AC, Malhotra A and White DP 2013. A simplified method for determining phenotypic traits in patients with obstructive sleep apnea J. Appl. Physiol. Bethesda Md 1985 114 911–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman A, Malhotra A, Jordan AS, Stevenson KE, Gautam S and White DP 2008. Effect of oxygen in obstructive sleep apnea: role of loop gain Respir. Physiol. Neurobiol 162 144–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiromeritis AG, Hatziefthimiou AA, Hadjigeorgiou GM, Gourgoulianis KI, Anagnostopoulou DN and Angelopoulos NV 2011. Quantitative spectral analysis of vigilance EEG in patients with obstructive sleep apnoea syndrome: EEG mapping in OSAS patients Sleep Breath. Schlaf Atm 15 121–8 [DOI] [PubMed] [Google Scholar]
- Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM and Mohsenin V 2005. Obstructive sleep apnea as a risk factor for stroke and death N. Engl. J. Med 353 2034–41 [DOI] [PubMed] [Google Scholar]
- Ye L, Pien GW, Ratcliffe SJ, Björnsdottir E, Amardottir ES, Pack AI, Benediktsdottir B and Gislason T 2014. The different clinical faces of obstructive sleep apnoea: a cluster analysis Eur. Respir. J 44 1600–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yetkin O and Aydogan D 2017. Effect of CPAP on sleep spindles in patients with OSA Respir. Physiol. Neurobiol 247 71–3 [DOI] [PubMed] [Google Scholar]
- Younes M 2017. The case for using digital EEG analysis in clinical sleep medicine Sleep Sci. Pract 1 Online: http://sleep.biomedcentral.com/articles/10.1186/s41606-016-0005-0 [Google Scholar]
- Younes M and Hanly PJ 2016. Immediate postarousal sleep dynamics: an important determinant of sleep stability in obstructive sleep apnea J. Appl. Physiol. Bethesda Md 1985 120 801–8 [DOI] [PubMed] [Google Scholar]
- Younes M, Ostrowski M, Soiferman M, Younes H, Younes M, Raneri J and Hanly P 2015a. Odds ratio product of sleep EEG as a continuous measure of sleep state Sleep 38 641–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younes M, Thompson W, Leslie C, Egan T and Giannouli E 2015b. Utility of Technologist Editing of Polysomnography Scoring Performed by a Validated Automatic System Ann. Am. Thorac. Soc 12 1206–18 [DOI] [PubMed] [Google Scholar]
- Younes M, Younes M and Giannouli E 2016. Accuracy of Automatic Polysomnography Scoring Using Frontal Electrodes J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med 12 735–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G-Q, Cui L, Mueller R, Tao S, Kim M, Rueschman M, Mariani S, Mobley D and Redline S 2018. The National Sleep Research Resource: towards a sleep data commons J. Am. Med. Inform. Assoc Online: https://academic.oup.com/jamia/advance-article/doi/10.1093/jamia/ocy064/5026200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinchuk AV, Gentry MJ, Concato J and Yaggi HK 2017. Phenotypes in obstructive sleep apnea: A definition, examples and evolution of approaches Sleep Med. Rev 35 113–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinchuk AV, Jeon S, Koo BB, Yan X, Bravata DM, Qin L, Selim BJ, Strohl KP, Redeker NS, Concato J and Yaggi HK 2018. Polysomnographic phenotypes and their cardiovascular implications in obstructive sleep apnoea Thorax 73 472–80 [DOI] [PMC free article] [PubMed] [Google Scholar]