Abstract
Aim:
Triple-negative breast cancer (TNBC) is an aggressive breast cancer subtype. Since no targeted therapy is available, gene-directed enzyme prodrug therapy (GDEPT) could be an attractive strategy for treating TNBC.
Materials & methods:
Polyethylene glycol (PEG)ylated-poly(lactic-co-glycolic acid)/polyethyleneimine nanoparticles (PLGA/PEI NPs) were synthesized and complexed with TK–NTR fusion gene. Ultrasound (US) and microbubble (MB) mediated sonoporation was used for efficient delivery of the TK–NTR–DNA–NP complex to TNBC tumor in vivo for cancer therapy. Therapeutic effect was evaluated by treating TNBC cells in vitro and tumor xenograft in vivo by using prodrugs ganciclovir (GCV) and CB1954.
Results:
TNBC cells treated with GCV/CB1954 prodrugs after transfection of TK–NTR–DNA by PEGylated-PLGA/PEI NP resulted in high apoptotic-index. US–MB image-guided delivery of TK–NTR–DNA–NP complex displayed significant expression level of TK–NTR protein and showed tumor reduction when treated with GCV/CB1954 prodrugs in TNBC xenograft in vivo.
Conclusion:
US–MB image-guided delivery of TK–NTR gene by PEGylated-PLGA/PEI NPs could be a potential prodrug therapy for TNBC in the clinic.
Keywords: : gene delivery, gene-directed enzyme prodrug therapy (GDEPT), nanoparticles, NTR, PEG, PEI, PLGA, thymidine kinase (TK), triple-negative breast cancer (TNBC)
Triple-negative breast cancer (TNBC) is an aggressive breast cancer phenotype in humans, and has been considered one of the leading causes of cancer-related deaths in women [1]. TNBC is accounted for about 20% of all breast cancer patients. TNBC cells lack the expression for human ERα, PR and HER-2. Hence, TNBC cannot be treated using currently available targeted therapies such as anti-estrogens targeting ER or monoclonal antibodies targeting HER2 [2]. Combinations of chemotherapies are the only currently recommended treatment options, but the overall response rate is only 16–35% [2]. Therefore, novel treatment strategies for TNBC are critically needed.
Gene-directed enzyme prodrug therapy (GDEPT), also called suicide gene therapy, is an emerging strategy involving tumor-targeted delivery of an exogenous gene that expresses an enzyme which is capable of converting a nontoxic prodrug into an activated cytotoxic agent [3–6]. An additional benefit of GDEPT is the ‘bystander effect’, whereby neighboring tumor cells that elude gene transfer are killed following cell–cell diffusion of the activated cytotoxic metabolites [7]. However, GDEPT suffers from various limitations such as, poor DNA delivery associated low-level expression of therapeutic transgene in tumor cells; absence of selective tumor specific delivery system for efficient GDEPT gene transfer to tumor cells; and host immune response associated clearance of delivery vehicles. Thus, new multilevel in vivo delivery agents with efficient therapeutic gene(s) in combination with low toxic prodrugs are needed for successfully improving the efficiency of cancer gene therapy.
One of the successful GDEPT strategies are a combination of herpes simplex virus-1 thymidine kinase and prodrug ganciclovir (HSV1–TK–GCV) whose active metabolite inhibits DNA synthesis and kills actively proliferating cancer cells [8]. Bacterial NTR enzyme is another important gene therapy system used for cancer therapy where expression of NTR in tumor cells enzymatically reduces the nontoxic prodrug CB1954 (5-(aziridin-1-yl)-2,4-dinitrobenzamide) to its activated cytotoxic form (5-(aziridin-1-yl)-4-hydroxylamino-2-nitrobenzamide: a DNA inter-strand cross-linking agent), which kills cancer cells expressing the enzyme [9]. In contrast to the HSV1-TK/GCV strategy, NTR/CB1954 is expected to demonstrate superior therapeutic efficacy, as it kills both actively and slowly dividing cancer cells, exhibits potent bystander effect and lacks resistance to common chemotherapeutic agents. As NTR does not naturally express in eukaryotes, a significant advantage of this system is tumor-restricted delivery of therapeutic prodrug for therapy. We have previously shown that combining TK and NTR as a fusion gene (TK–NTR) can substantially increase the therapeutic efficiency compared with each of these genes delivered independently [10]. Also, higher therapeutic efficiencies could be achieved at even low gene expression levels.
We have recently shown that biodegradable poly(lactic-co-glycolic acid)/-polyethylene glycol nanoparticles (PLGA-PEG NPs) are efficient vehicles for the therapeutic delivery of microRNAs [11–14] and ultrasound (US) and microbubble (MB) mediated delivery of PLGA-PEG NPs further improve the delivery of microRNAs into the tumors [15,16]. In the current study, we report our results on the development of biodegradable PEGylated PLGA/polyethyleneimine (PEI) NPs for the delivery of plasmid vectors expressing HSV1-sr39TK-NTR (TK–NTR) fusion under a tumor specific survivin promoter at more clinically relevant delivery approach for TNBC therapy.
Materials & methods
Materials
Poly (D,L-lactide-co-gslycolide) acid terminated (Mw 7000–17,000) and linear polyethyleneimine (LPEI, Mw: 10,000) and dextrose were purchased from Sigma–Aldrich (MO, USA). Poly(ethylene glycol) bis(amine) (Mw 3400) was purchased from Laysan Bio, Inc. (Arab, Al, USA). Lipofectamine 2000, fetal bovine serum (FBS), Cell culture media, streptomycin and penicillin (PS) were purchased from Thermo Fisher Scientific (MA, USA). The DNA gel extraction and plasmid extraction kits were procured from Qiagen (CA, USA). CytoCy5S was obtained from GE Healthcare. Clinical grade lipid-shelled perfluorobutane and nitrogen-filled MBs (BR38) obtained from Bracco Research, Geneva, Switzerland.
Formulation of PEGylated PLGA/PEI NPs
Amino-terminated PEGylated PLGA/PEI NPs was prepared using modified w/o/w double emulsion method. In a 20 ml scintillation vial amino-terminated PEGylated PLGA (6 mg/ml) was dissolved in dichloromethane. To this organic solution, 0.25 ml of ultrapure water was added drop wife with slight stirring at room temperature and sonicated at 40% amplitude for 1 min in an ice bath, which forms first emulsion. This first emulsion was drop wise added to the 4 ml of 1% PVA (w/v) aqueous solution containing polyethyleneimine (PEI, 0.5 mg/ml) and sonicated (40% amplitude for 1 min) to form second emulsion. This NPs mixture was further stirred for 3 h at RT to stabilize the NPs. The stabilized NPs were sterile filtered using 0.45 syringe filters and washed with ultrapure water for three-times using 100 kDa centrifugal filters (EMD-Millipore, USA). The concentrated NPs washed diluted to known volume and stored at 4°C for further use. The NPs was lyophilized with 10% sucrose for longer-term storage, and the resulted freeze-dried NP powder was stored at -20°C.
PEGylated PLGA/PEI NPs complex with plasmid-DNA
Plasmid-DNA was complexed with cationic PEGylated-PLGA/PEI NPs at RT in an autoclaved microfuge tube. Dextrose (10%, w/v) was used as an additive to neutralize the toxicity associated with PEI. In brief, plasmid-DNA (10 μg) was suspended in 50 μl of ultrapure water, then 50 μl of 10% Dextrose and 10 μl of PEGylated-PLGA/PEI NPs was added. The complex was gently mixed with pipette without forming any air bubbles and incubated at RT for 30 min. The formed plasmid-DNA-PEGylated-PLGA/PEI NPs complex was characterized by agarose gel electrophoresis and immediately used for in vitro and in vivo delivery studies.
ζ-Potential & particle size measurements
ζ-Potential and sizes of NPs were taken using a Zetasizer-90 (Malvern Instruments, MA, USA). The ζ-potential measurement was executed using Smoluchowski model in an aqueous dip cell in an automatic mode. The size measurement was executed at 90 degrees scattering angle at 25°C. Cumulant analysis was used for the determination of mean hydrodynamic diameter of the NPs.
US device & set-up for MB mediated PEGylated-PLGA/PEI-NP:DNA complex delivery in vivo
We have used our previously optimized US apparatus and US-MB mediated delivery methods for NP:DNA complex delivery [15,16]. Lyophilized MBs (BR38, Bracco, size: 1.6 ± 0.4 μm, ζ potential: -0.3 ± 0.3 mV) was suspended in sterile 0.9% saline prior to the use. A total of 350 μl MBs containing 330 μg of NP and 100 μg of DNA was injected in five cycles (70 μl each cycles). The US treatment consisted of five repetitive cycles of 70 μl MBs administrations combined with US exposures. US pulses of 1.8 MHz produced by an array transducer (P4–1, Philips Healthcare, MA, USA) connected to a research platform (V1, Verasonics, WA, USA) was used for the study. A needle hydrophone (HNR-0500, Onda, CA, USA) was used for calibrating the US pulses in degassed water. The full width at half maximum (FWHM) beam widths on the pressure profile were calibrated to be 1.4, 10.1 and 12.6 mm in the transducer's X, Y and Z directions, respectively. Successful MB delivery to tumor vessels following intravenous injection was confirmed using contrast mode US imaging using a 21-MHz transducer (MS250, VisualSonics, Toronto, Ontario, Canada) connected to a small animal US system (Vevo 2100, VisualSonics; lateral and axial resolution, 165 and 75 μm, respectively).
Results
Synthesis & optimization of PEGylated PLGA/PEI-NPs
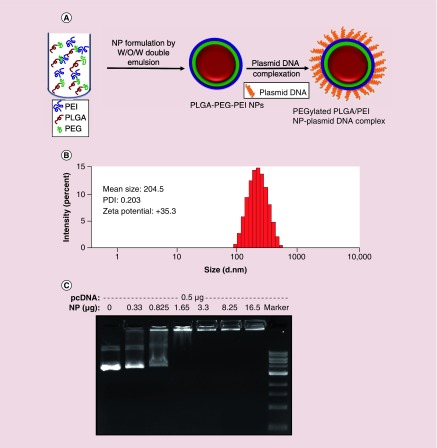
Plasmid-DNA is typically higher in molecular weight compared with polymeric delivery vehicles. Hence it is difficult to efficiently load high molecular weight DNA into low molecular weight polymer NPs. However, cationic polymer NPs has been successfully used for the delivery of plasmid DNA by creating complexes through their electrostatic interactions. Therefore, we have optimized the formulation of highly cationic amino-terminated PEGylated PLGA/PEI NPs using a modified water-in-oil-in-water (w/o/w) double emulsion method (Figure 1A & Table 1). We have used 10 mg of PLGA-PEG-NH2 for optimization of NPs by varying the concentrations of linear PEI with different surfactants under different emulsification conditions. We have conducted the initial experiment using w/o/w double emulsion method with PEI of two different concentrations (7 and 2 mg) in W1 phase and 1% polyvinyl alcohol (PVA) in W2 phase, which resulted in relatively large particle sizes with high polydispersity index (PDI) and positive ζ potential (Table 1, entries 1 & 2). Reducing PEI to 1 mg showed reduction in particle size (161 nm) with relatively higher PDI (0.31) and ζ potential (+44.2) (Table 1, entry 3). NPs prepared by the emulsion-diffusion-evaporation method showed particle size of 245 nm with high PDI (0.286) and positive ζ potential (+55.5) (Table 1, entry 4). NPs prepared without surfactants and by addition of varying concentrations of PEI in W1 or W2 phases displayed high PDI (Table 1, entries 5–7), which indicate that surfactants are necessary for stability of NPs. However, the addition of 2 mg (0.5 mg/ml) of PEI with 1% PVA in W2 phase (4 ml) resulted in lower sizes and lower PDI (Table 1, entry 8). We have also synthesized Cy5-conjugated anti-miR-21 loaded PEGylated PLGA/PEI-NPs for cell uptake studies, which showed similar particle sizes and PDI as we prepared the one without microRNA loading (Table 1, entry 9). However, we observed Cy5-conjugated anti-miR-21 loaded NPs showed lower positive ζ potential compared with the one without microRNA (+35.3 compared with + 25.5), this is due to the neutralization of positive charge by highly anionic anti-microRNA-21 (Table 1, entries 8 & 9). We have used the optimized method (Table 1, entry 8) for synthesizing NPs for all other studies including in vivo delivery performed in this manuscript. Overall, these optimized NPs showed ∼200–250 nm in diameter with lower PDI and positive ζ potential as measured by dynamic light scattering (Figure 1B).
Figure 1. . Formulation and characterization of PEGylated-poly(lactic-co-glycolic acid)/polyethyleneimine nanoparticle for its physicochemical and DNA binding properties.
(A) Schematic illustration of the formulation of PEGylated poly(lactic-co-glycolic acid)/polyethyleneimine nanoparticle and evaluation of its binding efficiency to plasmid DNA; (B) Particle size, ζ potential and polydispersity index evaluation by dynamic light scattering; (C) DNA binding properties of nanoparticles evaluated by agarose gel electrophoresis.
PEG: Polyethylene glycol; PEI: Polyethyleneimine; PDI: Polydispersity index; PLGA: Poly(lactic-co-glycolic acid); NP: Nanoparticles; W/O/W: Water-oil-water.
Table 1. . Synthesis, optimization and characterization of PEGylated poly(lactic-co-glycolic acid)/polyethyleneimine nanoparticles.
| Sl No | PLGA-PEG Weight (mg) | PEI weight (mg) | Method | Surfactant | Organic solvent | Size | PDI | (ζ)-Potential (mV) |
|---|---|---|---|---|---|---|---|---|
| 1 | 10 | 7* | W/O/W | 1% PVA | DCM | 560.3 | 0.530 | +62.1 |
| 2 | ‘’ | 2* | ‘’ | ‘’ | ‘’ | 537.6 | 0.420 | +60.5 |
| 3 | ‘’ | 1* | ‘’ | ‘’ | ‘’ | 161.3 | 0.311 | +44.2 |
| 4 | ‘’ | 2* | EDE | 2% PVA | Ethyl acetate | 245.6 | 0.286 | +52.5 |
| 5 | ‘’ | 2* | ‘’ | No surfactants | ‘’ | 277.7 | 0.447 | +40.2 |
| 6 | ‘’ | 1* | W/O/W | ‘’ | DCM | 426.9 | 0.348 | +66.6 |
| 7 | ‘’ | 2‡ | ‘’ | ‘’ | ‘’ | 436.7 | 0.461 | +63.2 |
| 8 | ‘’ | 2‡ | ‘’ | 1% PVA | ‘’ | 204.5 | 0.203 | +35.3 |
| 9§ | ‘’ | 2‡ | ‘’ | ‘’ | ‘’ | 211.7 | 0.194 | +25.5 |
*Linear PEI was added in W1 phase;
‡Linear PEI was added in W2 phase;
§10 nmol of anti-miR-21 (10% Cy5-cojugated anti-miR-21) loaded to the NPs with 50.4% encapsulation efficiency.
DCM: Dichloromethane; EDE: Emulsion-diffusion-evaporation; NP: Nanoparticle; PDI: Polydispersity index; PEG: Polyethylene glycol; PEI: Polyethyleneimine nanoparticle; PLGA: Poly(lactic-co-glycolic acid); PVA: Polyvinyl alcohol; W/O/W: Water-oil-water.
Evaluation of DNA-binding efficiency of PEGylated PLGA/PEI-NPs & identifying optimal NP to DNA ratio required for efficient complex formation & delivery in cells
The optimized NP was complexed with highly anionic pcDNA-HSV1-sr39TK-NTR plasmid, which expresses TK-NTR therapeutic fusion protein in pcDNA3.1 (+) vector backbone under a survivin promoter (7.4 kb length). The efficiency of pcDNA-HSV1-sr39TK-NTR DNA in forming complexes with NP was studied using agarose gel electrophoresis. We used increasing concentration of NPs with 0.5 μg of DNA (Figure 1C & Supplementary Figure 1). The results of this DNA binding studies indicated that 0.5 μl (1.65 μg PLGA polymer equivalent) of NPs is sufficient to bind to 0.5 μg of plasmid-DNA at a saturated level.
Examination of DNA transfection efficiency of PEGylated PLGA/PEI-NPs formulated by different methods using plasmid vectors expressing TK-NTR & Fluc-eGFP fusion proteins by imaging
After successfully evaluating the DNA binding efficiency (Figure 1C & Supplementary Figure 1), PEGylated PLGA/PEI-NPs prepared using various methods were complexed with pcDNA-HSV1-sr39TK-NTR plasmid DNA and the resulted DNA:NP complexes were transfected in MDA-MB-231 TNBC cells (12-well plates [60,000 cells/well, DMEM medium, 10% FBS, 100 U/ml P&S] and incubated at 37°C for 24 h). Subsequently, cells were treated with CytoCy5S (a nonfluorescent nitro-substituted cyanine dye, functions as a substrate for NTR enzyme) [17] as imaging agent. After incubation of CytoCy5S with cells at 37°C for 2 h, NTR enzyme reduced the nitro group in the CytoCy5S to hydroxylamine, which is highly fluorescent. We imaged the cells for converted fluorescent-signal from CytoCy5S by fluorescent microscopy. The imaging results indicated that pcDNA-TK-NTR transfected with our optimized method (Table 1, entry 10) showed highest fluorescent signal which was comparable to cells transfected with similar concentration of DNA using lipofectamine-2000 transfection reagent (Supplementary Figure 2: v, vi).
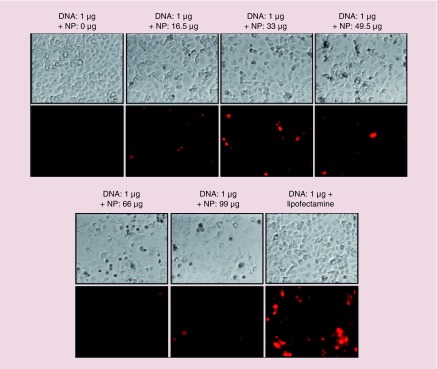
We also independently optimized the NP to DNA ratio needed to transfect TK-NTR plasmid. We have transfected the pcDNA-TK-NTR using our PEGyated PLGA/PEI-NPs in MDA-MB-231 cells with increasing concentrations of NPs to DNA ratio, and incubated the cells at 37°C for 24 h. Lipid based in vitro transfection agent (Lipofectamine 2000) was used as a positive control for observing the transfection efficiency. After 24 h, the cells were incubated with CytoCy5S for 2 h and imaged for converted Cy5 signal using fluorescence microscopy (Figure 2). The microscopic images indicated that the NPs efficiently delivered the plasmid-DNA into the cells with the observed Cy5-signal. The Cy5 signal intensity was increased with increasing concentration of NPs with the maximum intensity observed at 33 μg (10 μl) of NPs. However further higher concentrations of NPs (49.5–99 μg) showed lower Cy5 signal because of lower cell viability due to NP mediated toxicity (Figure 2). These results indicated that NPs efficiently transfected the plasmid-DNA into TNBC cells. We also performed cellular uptake studies using Cy5-anti-miR-21 loaded PEGylated PLGA/PEI NPs in MDA-MB-231 cell. Results showed dose- and time-dependent uptake of NPs in MDA-MB-231 cells as measured by Cy5 fluorescent signal (Supplementary Figure 3).
Figure 2. . Evaluation of DNA transfection efficiency of PEGylated-poly(lactic-co-glycolic acid)/polyethyleneimine nanoparticles in MDA-MB-231 triple negative breast cancer cells.
Various concentration of PEGylated-poly(lactic-co-glycolic acid)/polyethyleneimine nanoparticles was complexed with 1 μg of pcDNA-TK-NR plasmid DNA and transfected to MDA-MB-231 cells for 24 h. The cells were evaluated for nitroreductase enzyme activity using CytoCy5S substrate; Top row: bright filed images of respective condition; Bottom row: Cy5 fluorescence images of cells at respective condition. Images were taken at 20× magnification and Cy5 fluorescence was adjusted to same intensity scale.
NP: Nanoparticles.
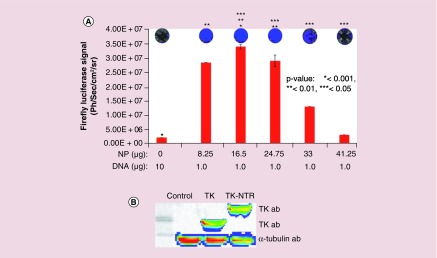
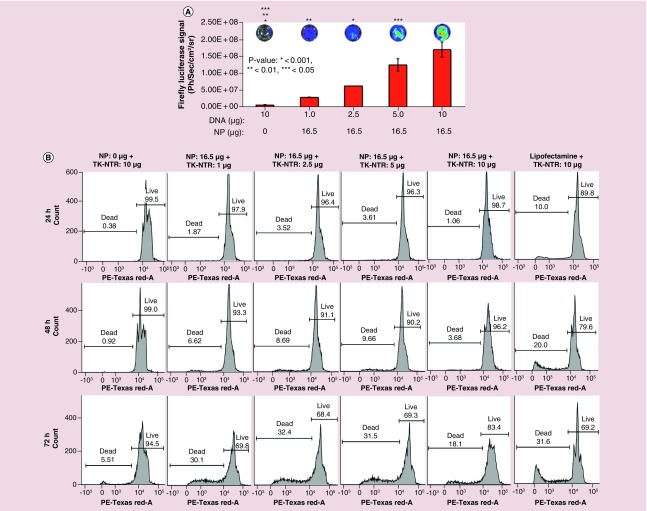
We further evaluated the transfection of plasmid DNA expressing Fluc–eGFP fusion protein under an ubiquitin promoter to check the generalizability of transfection by PEGylated PLGA/PEI NPs. We used 1 μg of DNA for transfection with increasing concentrations of NPs in MDA-MB-231 cells. The transfection efficiency was quantitatively measured by Fluc based bioluminescence imaging (Figure 3A). MDA-MB-231 cells plated in 12 well plates (60,000 cells/well in DMEM medium supplemented with 10% FBS, 1% P&S) were transfected with pcDNA–Fluc–eGFP plasmid (1 μg each) with different concentrations of NPs (8.25 μg [2.5 μl] 16.5 μg [5 μl], 24.75 μg [7.5 μl], 33 μg [10 μl] and 41.25 μg [12.5 μl]). The cells were subsequently assessed for bioluminescence using substrate D-Luciferin (150 μg/ml) and imaged for bioluminescence signal. Results indicated that no detectable bioluminescence signal was observed in cell treated with naked pcDNA-Fluc-eGFP plasmid, whereas cells treated DNA–NP complex showed an NP concentration-dependent luminescence signal with a highest bioluminescence signal observed at 16.5 μg (5 μl) of NPs. However, above 16.5 μg NP concentrations found to be toxic to the cells as indicated by low transfection efficiency (Figure 3A).
Figure 3. . Evaluation of DNA transfection efficiency of PEGylated-poly(lactic-co-glycolic acid)/polyethyleneimine nanoparticles in MDA-MB-231 triple negative breast cancer cells by bioluminescence imaging.
pcDNA-Fluc-eGFP DNA (1 μg) transfection using different concentration of PEGylated poly(lactic-co-glycolic acid)/polyethyleneimine nanoparticles in MDA-MB-231 cells and transfection analysis by bioluminescence imaging. (A) Graph showing the quantitative bioluminescence signal measured (p/s/cm2/sr) from cells treated with respective transfection condition (*p < 0.001, **p < 0.01, ***p < 0.05). Top: Bioluminescence images of cells acquired by optical CCD camera. (B) Immunoblot analysis of TK and TK-NTR proteins in MD-MB-231 cells transfected with respective plasmid DNA using poly(lactic-co-glycolic acid)/polyethyleneimine nanoparticles.
Immunoblot analysis further measures the expression of TK-NTR therapeutic fusion proteins in cells transfected with pcDNA-TK-NTR plasmid by PEGylated PLGA-/PEI-NPs
To evaluate whether transfection of cells with TK-NTR plasmid using PEGylated PLGA/PEI-NPs is able to produce significant amount of protein to exert functional therapeutic effects, MDA-MB-231 cells were transfected with pcDNA-TK (1 μg) and pcDNA-TK-NTR (1 μg) plasmids using PEGylated PLGA/PEI-NPs at 16.5 μg of polymer equivalent. After 24 h of transfection the cells were collected and isolated proteins for immunoblot analysis. The isolated proteins were resolved in SDS-PAGE and transferred to nitrocellulose membrane to test for TK and TK-NTR proteins using TK-specific antibody. The results indicated significant expression of TK and TK-NTR proteins in cells transfected with pcDNA-TK and pcDNA-TK-NTR plasmids by NP, whereas no detectable band was observed in cells transfected with pcDNA3.1 (+) control plasmid (Figure 3B).
NP-mediated cytotoxicity analysis by flow cytometry
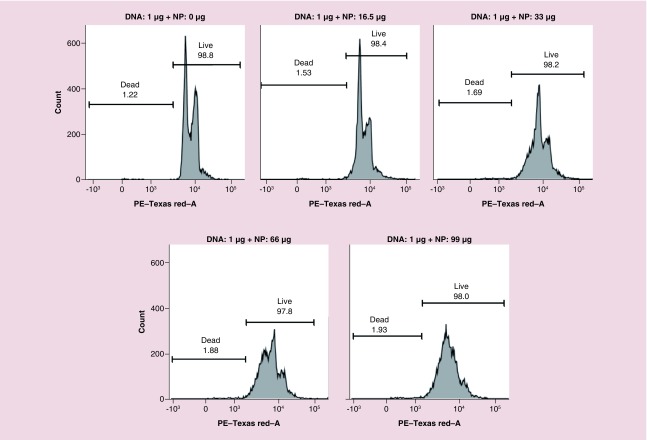
PEI demonstrates a highly positive charge when protonated, and is a second promising candidate nonviral vector for the delivery of nucleic acids for in vitro and in vivo applications [18]. However, PEI is extremely cytotoxic to the cells when it is used independently [19]. The transfection efficiency of PEI, along with its cytotoxicity increases with the increasing molecular weight of PEI [20]. Thus, we have optimized our PEGylated PLGA/PEI-NPs to achieve minimal toxicity with maximum transfection efficiency to cells. We evaluated cytotoxicity of the developed optimal PEGylated PLGA/PEI-NPs formulation in MDA-MB-231 cells by flow cytometry analysis. We transfected the plasmid-DNA using increasing concentration of PEGylated PLGA/PEI NPs in MDA-MB-231 and SUM159 TNBC cells in 12-well plates (DMEM medium with 10% FBS, 1 ml) for 24 h and collected cells were fixed in 70% ethanol and stained with propidium iodide (PI) and assessed by flow cytometry (FACS) analysis using PE-Texas Red-A channel. Results indicated that MDA-MB-231 cells treated with naked plasmid-DNA and NPs at 16.5 μg of NP showed no toxicity. However further increasing the concentration of NPs reduced the separation between G0-G1 and G2/M phase with reduction in the S-phase, indicating that at higher concentrations NPs inhibits cell growth (Figure 4A). Similarly, naked plasmid-DNA and up to 13.2 μg of NPs treated cells showed no toxicity in SUM159 cells (Supplementary Figure 4). However using 1:1 ratio (μl:μg) of NP to DNA complex even at higher concentrations of both NPs and DNA showed no toxicity (Supplementary Figure 5). These results clearly indicate that the DNA complexed with NPs at an optimal ratio is important for reducing the NP-mediated toxicity, and that only free NPs at higher concentrations are toxic to the cells. At the same time it is also important to leave some PEI to display on the NPs surface for efficient transfection.
Figure 4. . Flow-cytometry (FACS) analysis of MDA-MB-231 cells transfected with 1 μg of pcDNA-thymidine kinase-nitroreductase plasmid DNA using different concentration of PEGylated-poly(lactic-co-glycolic acid)/polyethyleneimine nanoparticles for nanoparticle mediated cytotoxicity by propidium iodide staining.
Gene directed enzyme prodrug therapy promoted by PEGylated PLGA/PEI-NPs in TNBC cells
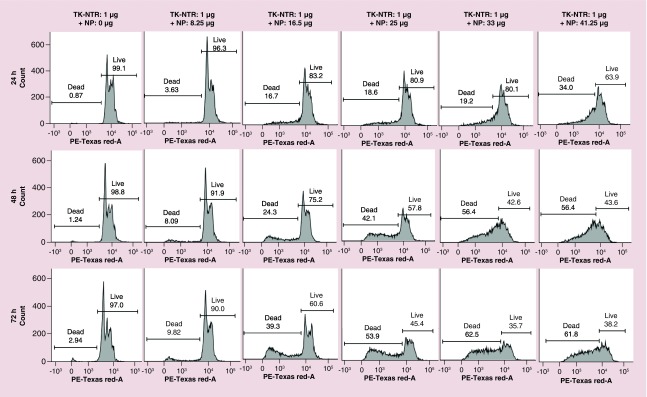
We studied the GDEPT effect after transfection of pcDNA-TK-NTR therapeutic plasmid (1 μg) with different concentration of NPs in MDA-MB-231 cells (Figure 5). We transfected cells with pcDNA-TK-NTR plasmid by PEGylated PLGA/PEI-NPs for 24 h. After 24 h, the medium was replaced with fresh medium (DMEM supplemented with 2% FBS, 1% P&S) and cotreated GCV (1 μg/ml) and CB1954 (30 μM). The cells were further incubated for different time points (24, 48 and 72 h). At each time point the cells were collected by trypsinization and fixed in ice cold 70% ethanol. The fixed cells were washed and stained with PI for flow cytometry analysis. The results showed prodrugs treatment time-dependent increase in apoptotic cell populations of MDA-MB-231 cells (17, 24 and 39% at 24, 48 and 72 h, respectively) after using 1 μg DNA: 16.5–25 μg of NPs (Figure 5). However, higher apoptotic population was observed when using higher than 25 μg of NPs with 1 μg of pcDNA-TK-NTR DNA for transfection, which is mainly due to NP-mediated toxicity to the cells.
Figure 5. . Therapeutic evaluation of thymidine kinase-nitroreductase gene directed enzyme prodrug therapy system in MDA-MB-231 cells transfected using PEGylated poly(lactic-co-glycolic acid)/polyethyleneimine nanoparticles.
The cells transfected with 1 μg of pcDNA-thymidine kinase-nitroreductase plasmid DNA complexed with different concentration (0–41.25 μg) of PEGylated-poly(lactic-co-glycolic acid)/polyethyleneimine nanoparticles for 24 h was evaluated for gene directed enzyme prodrug therapy mediated apoptotic induction at different time points (24, 48 and 78 h) post-treatment with prodrug ganciclovir and CB1954 combination by propidium iodide staining based FACS analysis. Figure showing the histogram of cells analyzed at each condition and marked for dead and live cell populations.
After optimizing the DNA to NP ratio for optimal transfection without toxicity to cells, we have used this optimized condition to study DNA dose-dependent transfection and the associated therapeutic effect in MDA-MB-231 and SUM159 TNBC cells. We first evaluated different concentration of pcDNA-Fluc-eGFP for transfection using 16.5 μg of NPs in MDA-MB-231 cells (Figure 6A). Varying concentrations of pcDNA-Fluc-eGFP (1–10 μg) were complexed with NPs and transfected the MDA-MB-231 cells for 72 h. pcDNA-Fluc-eGFP (10 μg) alone without NP was added as negative control. The cells were washed with PBS and treated with D-Luciferin and imaged for bioluminescence. The results showed DNA concentration-dependent increase in the bioluminescence signal, indicating that our PEGylated PLGA/PEI-NPs was efficient for transfection of higher concentrations of DNA (Figure 6A).
Figure 6. . Evaluation of PLGA/PEI mediated transfection and therapeutic efficiency in MDA-MB-231 cancer cells.
(A) Evaluation of DNA (pcDNA-Fluc-eGFP) dose-dependent transfection efficiency by PEGylated poly(lactic-co-glycolic acid)/polyethyleneimine nanoparticles (PLGA/PEI NPs) in MDA-MB-231 TNBC cells. Varying concentrations of pcDNA-Fluc-eGFP- complexed with a fixed concentration of PEGylated PLGA/PEI NPs (16.5 μg) was used for evaluating transfection efficiency in MDA-MB-231 cells by bioluminescence imaging (*p < 0.001, **p < 0.01, ***p < 0.05). (B ) DNA dose-dependent therapeutic evaluation of thymidine kinase-nitroreductase gene directed enzyme prodrug therapysystem in MDA-MB-231 cells delivered by PEGylated PLGA/PEI NPs. The cells transfected with 1–10 μg of pcDNA-thymidine kinase-nitroreductase plasmid DNA complexed with 16.5 μg of PEGylated PLGA/PEI NP for 24 h was evaluated for gene directed enzyme prodrug therapy mediated apoptotic induction at different time points (24, 48 and 78 h) post-treatment with prodrug ganciclovir and CB1954 combination by propidium iodide staining based FACS analysis. Figure showing the histogram of cells analyzed at each condition and marked for dead and live cell populations.
Furthermore, we also studied DNA dose-dependent GDEPT effect in a parallel experiment in MDA-MB-231 cells. After transfection of varying concentrations pcDNA-TK-NTR plasmid expressing TK-NTR fusion (1–10 μg) with NPs (16.5 μg), we cotreated cells with GCV (1 μg) and CB1954 (30 μM) and incubated for 24 to 72 h. At each time point the cells were collected and stained with PI for flow cytometry analysis as stated above (Figure 6B). Results showed 2, 7 and 30% apoptotic populations in cells transfected with 1 μg-DNA and assessed at 24, 48 and 72 h, respectively. Furthermore, 2.5 and 5.0 μg TK-NTR transfection showed similar level of apoptotic induction as we have seen with 1 μg DNA. However, 10 μg of DNA transfection showed much lower level of apoptotic population (1, 4 and 18%). This indicated the lower transfection efficiency of NPs using 10 μg DNA, which is due to the excess of DNA surrounding the NPs inhibiting NPs transfection property by neutralizing the entire positive charge on the PEI surface.
To test the generalizability of transfection efficiency and effect of GDEPT in another TNBC cells we used SUM159 cells for the study. We have identified 13.2 μg of NPs as optimal dose (Supplementary Figure 4) for this particular cell line. We have transfected the SUM159 cells with 1–8 μg of DNA with 13.2 μg of NPs, and the next day cells were cotreated GCV (1 μg/ml) and CB1954 (30 μM). The results indicated that the best apoptotic cell populations (9, 27 and 46% at 24, 48 and 72 h, respectively) were obtained using at 1 μg of DNA. However, 2 μg DNA also showed similar results, but further increase in concentrations of DNA showed lower apoptotic populations owing to poor DNA transfection efficiency by NPs, which is due to complete neutralization of PEI surface by negatively charged DNA (Supplementary Figure 6). This result clearly supports the fact that the toxicity is different for each cells and the DNA to NP ratio is important for efficient transfection and the associated treatment effect in cells.
US-MB guided delivery of pcDNA-TK-NTR plasmid DNA by PEGylated PLGA/PEI NPs & subsequent GDEPT evaluation in mice
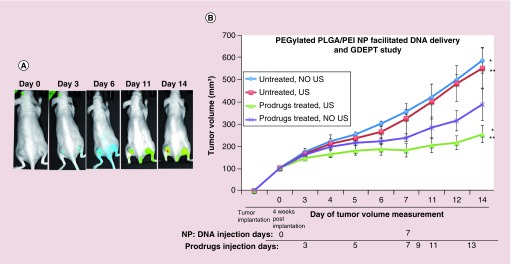
After successfully studying the transfection and subsequent GDEPT evaluation in two different TNBC cell lines in vitro, we studied the delivery of our therapeutic DNA and GDEPT in subcutaneous TNBC tumor xenografts in nude mice. We used our previously developed method for US-MB guided delivery of our NP–DNA complex [16]. To establish subcutaneous TNBC tumor xenografts, 5 × 106 MDA-MB-231 cells were implanted on both side of the lower flanks of nude mice (Nu/Nu, n = 12) and tumor sizes were monitored over time. When the tumor size reached ∼100–300 mm3, approximately after 4 weeks, mice were divided into four groups. The first group of mice was used as untreated control without administration of NP–DNA complex as well as US-MB and prodrugs GCV/CB1954 (Group 1, untreated NO US; n = 3). In the second group of mice NP–DNA complex (330 μg NP and 100 μg DNA; n = 3) was intravenously injected via tail veins and treated with US but without administration of prodrugs (Group 2, No prodrug, US-MB delivery of DNA; n = 3). The third group of mice was intravenously injected with NP–DNA complex and treated with US and MB as well as intraperitoneal administration of prodrugs (Group 3, prodrugs treated, US-MB; n = 3). In the fourth group of mice NP–DNA complex (330 μg NP-100 μg DNA; n = 3) was intravenously administered along with intraperitoneal prodrugs but US was not applied in the tumors (Group 4, prodrugs treated, DNA-NP injected, No US-MB treated; n = 3). The mice were intravenously administered with CytoCy5S (10 μg) right after NP-DNA administration. After 48 h to allow delivery and protein expression in tumors, prodrugs were administered and administration continued every 48 h until completion of experiment on Day 14. The delivered TK-NTR gene expresses the fusion protein, which activates the delivered nonfluorescent CytoCy5S substrate into a highly fluorescent metabolite. We have imaged the mice for converted Cy5 signal on Day 0, Day 3, Day 6, Day 11 and Day 14. Significant amount of Cy5 signal was observed on Day 3 and continued until completion of experiment (Figure 7A). A second dose of US-MB guided NP–DNA complex was administered to the same mice on day 7 using the same dosing and administration protocol as performed in first treatment and prodrug treatment was continued for an additional week. Tumor dimensions were measured by digital caliper (Thermo Fisher Scientific, MA, USA) and volume was calculated using the equation, V = (π/6) × (long) × (short)2, where long and short are the long-axis and perpendicular short-axis dimensions, respectively. Tumor volumes of all mice were calculated and used for assessing the treatment effects (Figure 7B). On day 14 mice were euthanized and tissues were collected for ex vivo analysis. Untreated-control mice (group 1) and untreated-US mice (group 2) showed similar level of tumor growth. However, significant reduction in tumor growth was observed in mice treated with NP-DNA-US-MB and prodrugs (group 3) compared with untreated mice (group 1, 2.3-fold) as well as NP-DNA and prodrugs treated mice without US (group 4, 1.5-fold) (Figure 7B). The ex vivo analysis of spleen, liver and kidney tissues by H&E staining showed no significant toxicity between different groups (Supplementary Figure 7). We did not observe any reduction in animal body weight by different groups. H&E staining of tumor tissues of animals treated with prodrugs after delivering DNA by US-MB showed significant dead cell population compared with control (Supplementary Figure 8).
Figure 7. . Ultrasound-microbubble guided delivery of pcDNA-thymidine kinase-nitroreductase DNA by PEGylated poly(lactic-co-glycolic acid)/polyethyleneimine nanoparticle in triple negative breast cancer tumor xenograft in vivo for prodrug therapy.
(A) Cy5-fluorescence imaging of mice bearing MDA-MB-231 triple negative breast cancer tumors that are treated with CytoCy5S substrate of nitroreductase enzyme followed by ganciclovir and CB1954 prodrugs at different time points after the delivery of NP:DNA complex in mice. (B) Tumor growth analysis of control and mice treated with prodrugs after ultrasound-microbubble mediated delivery of thymidine kinase-nitroreductase fusion gene by PEGylated poly(lactic-co-glycolic acid)/polyethyleneimine nanoparticle (*p < 0.02, **p < 0.05).
Discussion
Viral vectors are established as potent gene delivery vehicle for therapeutic gene therapy in vivo [21,22]. However, development of nonviral gene delivery systems with improved gene transfer ability are required to overcome limitations, such as virus mediated carcinogenesis, complexity in the production of viruses and immunogenicity associated with the viral vectors [21–23]. Several nonviral gene delivery methods have been reported for the delivery of DNA into cancer cells [23–29]. Nonviral delivery systems are superior due to their low immunogenicity, and ease of preparation for further scale-up and clinical translations [23]. PLGA/PEI NPs prepared with one-step process has been previously reported to show positive ζ potential with smaller size. These PLGA/PEI NPs efficiently bind with peGFP and pβ-gal plasmid DNA and the formed PLGA/PEI/DNA complexes displayed higher transfection efficiency with lower toxicity compared with PEI polyplexes [24]. Benita et al. reported the synthesis of PLGA-PEI-NPs with highly positive ζ potential for the delivery of V1Jns plasmid DNA to pulmonary epithelium cells [25]. Branched-PEI-coated gold NPs (AU-PEI-NPs) displayed effective DNA delivery properties in cells with caveolae mediated internalization pathway and showed superior transfection efficiency and considerably low toxicity than free PEI and lipofectamine transfection agent [26]. PLGA/PEI and poly(L-lactic acid) (PLA)/PEI-NPs formulated by a diafiltration method showed positive ζ-potentials and a high DNA adsorption capacity, which also displayed good in vitro transfection efficiency in HEK293T cells [27].
We have previously shown that HSV-1 thymidine kinase (HSV1-sr39TK) and NTR enzymes expressed as fusion protein (HSV1-sr39TK-NTR) can efficiently kill tumor cells in subcutaneous and metastatic xenografts of human TNBC tumors in mice [10,30]. This HSV1-sr39TK-NTR dual reporters system improves cellular distribution of therapeutic enzymes and target two different cellular mechanisms to kill cancer cells and demonstrated much more efficient than each of these systems used individually [30]. This fusion gene can achieve therapeutic efficiency even if only 20% of tumor cells show expression compared with individual genes with their respective prodrugs (GCV/CB1954) [10,30]. Hence, in this study we focused on developing a low toxic, biodegradable NPs for efficient packaging of plasmid DNA coding for TK-NTR fusion gene in combination with clinically translatable US and MB mediated drug delivery platform that we previously optimized for efficient TNBC therapy in vivo [15,16].
Sonoporation induced by US in the presence of MB has been shown to improve therapeutic delivery of genes into the targeted sites [15,16,31–39]. In this strategy, after intravenous delivery of NPs and gas-filled MBs, upon localized US induction on the tumor creates a transient opening in the blood vessels which enables the release of NP–DNA complex to the tumor bed through a process called ‘sonoporation’ [15,16]. Sonoporation is highly connected with the US-induced cavitation, which includes enlargement, contraction and collapse of gas-filled MBs. The US-MB activities induce very high shear stress, which is enough to create opening in the endothelial lining of blood vessels [15]. The advantage of this strategy is that it is an easily translatable noninvasive technique for the delivery of therapeutics for the clinical treatment of various cancers. Chumakova et al. reported the formulation of PLGA and PEI/DNA NPs with positive ζ-potential for US-assisted gene delivery in solid tumors in vivo. Combination of PLGA and PEI/DNA NPs with US significantly increased tumor cell transfection in vivo. [40] Ethylenediamine-conjugated cationic gelatin showed efficient complex formation with plasmid DNA. In vitro transfection efficiency of this cationic gelatin-DNA complex was enhanced by US compared with one without US in rat gastric mucosal-1 cells [41]. These results also suggested that US exposure is an efficient method for the enhancement of plasmid DNA transfection. Polystyrene NPs in combination with US enhanced the delivery of chemotherapeutics and substantially reduced the tumor volume [42]. US enhanced the transfection of PEI/DNA complex and increased the expression of PKC-δ protein, which altered intracellular calcium level in cells [36].
PLGA is a well-known biocompatible and biodegradable polymer, used in FDA-approved therapeutic vehicles [43] and has been extensively used for anticancer drug and gene delivery [11–14,44,45]. PEG is a well-recognized biocompatible polymer that has been shown to increase circulation half-life of NPs, and has also been used in many US FDA approved therapeutics [46]. Thus, PLGA-PEG block co-polymer has attracted great interest for various drugs and small silencing RNAs delivery methods [13]. PEI is a familiar cationic polymer that has been shown to condense anionic nucleic acids by forming positive charged complexes [47].
In this study, we developed an efficient nanoformulation using PGEylated PLGA/PEI copolymers. PEGylated PLGA NPs are well known drug delivery vehicles for cancer therapy [12,13]. We synthesized and optimized PEGylated PLGA/PEI NPs using w/o/w double emulation method. These NPs showed higher positive ζ potential for delivering highly anionic plasmid DNA. These PEGylated PLGA/PEI copolymer NPs efficiently complexed with pcDNA-TK-NTR plasmid DNA expresses TK-NTR dual therapeutic reporter genes when US-MB mediated sonoporation is applied to TNBC tumors in small animals. The results showed significant improvement in therapeutic efficiency when we used the US-MB drug delivery technique. Our DNA binding studies showed low doses (1.65 μg) of PEGylated PLGA/PEI NPs are enough to strongly bind to 0.5 μg of pcDNA-TK-NTR plasmid DNA (Figure 1C). These low doses of NPs showed good transfection efficiency (Figure 2) in MDA-MB-231 and SUM159 TNBC cells. We used CytoCy5S (GE Healthcare), an initial nonfluorescent substrate which become fluorescent when reduced by NTR enzyme, as an imaging agent for indirectly monitoring the transfection efficiency [10,48]. We also used pcDNA-Fluc-eGFP DNA for evaluating the transfection efficiency of these NPs in TNBC cells by bioluminescence imaging. Our results indicated highest transfection efficiency when 1 μg of DNA was complexed with a nontoxic dose of 16.5 μg of PEGylated PLGA/PEI NPs (Figure 3A). The transfection efficiency of NPs was comparable to cationic liposomal transfection agent (Lipofectamine). The low doses of NPs were also found to be nontoxic to the cells as indicated by Flow cytometry analysis (Figure 4), however, at higher doses these NPs showed high toxicity to the cells similar to lipofectamine and other liposomes, especially when not complexed with DNA [49,50].
GDEPT approach can improve the cancer therapy index in comparison to traditional approaches because it involves the genetic modifications of cells that convert the nontoxic prodrugs into cytotoxic drugs, which kill the targeted cancer cells and also kills the neighboring cancer cells through bystander effect without affecting normal tissues [5]. However, lack of selective tumor specific delivery of therapeutic genes to tumor cells in vivo limits the efficiency of GDEPT system. Therefore, new multifunctional in vivo delivery methods with improved therapeutic gene combinations are needed for accomplishing effective cancer gene therapy for TNBC. To address these issues, we have developed PEGylated-PLGA/PEI-NPs in combination with HSV1-sr39TK-NTR dual therapeutic gene fusion for improving GDEPT in vitro and in vivo. Our in vitro transfection of TK-NTR DNA using NPs in cells followed by prodrugs treatment produced efficient therapeutic effect as indicated by apoptotic cell populations (Figure 5). Also, our results are comparable to the cells transfected with lipofectamine (Figure 6). We also efficiently delivered the PEGylated PLGA/PEI-NP-TK-NTR-DNA complexes to the subcutaneous tumor xenografts in mice. We used our previously developed clinically translatable US-MB mediated sonoporation method for improving the delivery of NP:DNA complexes to the tumors [15]. We observed the CytoCy5S signal after delivery of NP:DNA to the tumors which indicated the delivery of our TK-NTR DNA into the tumors (Figure 7A). The reduced growth of tumors treated with prodrugs after US-MB treatment indicated the effectiveness of our delivery system (Figure 7B). Overall, our in vitro and in vivo results clearly indicated that PEGylated PLGA/PEI NPs are efficient gene delivery vehicles for GDEPT in vivo and can be potentially used for clinical applications. The targeted GDEPT approach evaluated in this study improves TNBC therapy by overcoming various nonspecific toxicities associated with the current chemotherapy in the clinic. Although the developed approach is clinically feasible, specific, minimally toxic to nontarget tissues and US-MB delivery system is easily available in the clinics for drug delivery, this system also posses some limitations such as, it cannot be applied for cancers of brain, bone and other organs that are difficult for delivering US energy for drug delivery.
Conclusion
In summary, we have established a method for delivering GDEPT genes using PEGylated PLGA/PEI-NPs and US-MB. The treatment with prodrugs GCV/CB1954 in TNBC cells in vitro after PEGylated PLGA/PEI-NP-DNA delivery showed effective cell killing. In vivo analysis indicated that PEGylated PLGA/PEG-NPs can successfully deliver the GDEPT gene into tumors in mice as indicated by CytoCy5S imaging. The animals delivered with GDEPT genes and prodrugs showed significant reduction in tumor size (2.3-fold) compared with untreated control mice. US-MB guided biodegradable PEGylated PLGA/PEI-NPs as a delivery vehicle for GDEPT genes could represent a potential and clinically translatable therapeutic option for TNBC in patients. It also open up options for: localized delivery of genes into tumors by catheter-based tumor bed injection and the following US-MB treatment, and loading prodrugs and DNA together in NPs as a single agent for local delivery to further improve therapeutic effect while minimizing toxicity.
Future perspective
Overall cancer gene therapy is a procedure developed to eradicate cancers of any subtypes independent of their phenotypes. In this study we showed that therapeutic genes in fusion targeting two different cellular mechanisms can improve therapeutic efficiency several folds than each of these genes in their independent form. In addition, the use of polymer based nanoparticles further warrant the clinical nature of the delivery system. With the development of novel gene therapy system targeting multiple molecular mechanism of cancer cells in combination with clinically translatable nanoparticle based gene delivery system is expected to change the current scenario of cancer gene therapy. In addition, cancer gene therapy can also evolve in to a new dimension by working along with immunotherapy where merging these two as a combination therapy is expected to completely eradicate cancers of any phenotypes, independent of their clinical stages.
Summary points.
The main aim of this study is to develop a successful therapy for treating triple negative breast cancer.
We developed a dual (TK-NTR) cancer gene therapy system targeting multiple cellular mechanisms for efficient killing of cancer cells.
Gene-directed enzyme prodrug therapy with better bystander effect was developed for improved efficiency in cancer therapy.
Developed optimal poly(lactic-co-glycolic acid)-polyethylene glycol-polyethyleneimine nanoparticles for improved delivery of therapeutic genes to cancer cells in vivo.
Targeted delivery of therapeutic gene coated poly(lactic-co-glycolic acid)-polyethylene glycol-polyethyleneimine nanoparticles to tumor bed was achieved by using clinically translatable ultrasound-microbubble mediated delivery system.
Developed a low toxic therapeutic platform with improved efficiency for triple-negative breast cancer treatment.
Developed a nonviral delivery system without immunogenicity for in vivo gene delivery.
Supplementary Material
Acknowledgements
The authors would like to thank Bracco for providing BR38 microbubbles, the Stanford Center for Innovation in In vivo Imaging for help with ultrasound imaging and tumor volume measurements.
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/suppl/10.2217/nnm-2017-0328
Financial & competing interests disclosure
This research was supported by NIH grants: R01CA209888, R21EB022298. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
- 1.Brewster AM, Chavez-Macgregor M, Brown P. Epidemiology, biology, and treatment of triple-negative breast cancer in women of African ancestry. Lancet Oncol. 2014;15(13):E625–E634. doi: 10.1016/S1470-2045(14)70364-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalimutho M, Parsons K, Mittal D, Lopez JA, Srihari S, Khanna KK. Targeted therapies for triple-negative breast cancer: combating a stubborn disease. Trends Pharmacol. Sci. 2015;36(12):822–846. doi: 10.1016/j.tips.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Anderson WF. Gene therapy scores against cancer. Nat. Med. 2000;6(8):862–863. doi: 10.1038/78610. [DOI] [PubMed] [Google Scholar]
- 4.Greco O, Dachs GU. Gene directed enzyme/prodrug therapy of cancer: historical appraisal and future prospectives. J. Cell. Physiol. 2001;187(1):22–36. doi: 10.1002/1097-4652(2001)9999:9999<::AID-JCP1060>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Kale V, Chen M. Gene-directed enzyme prodrug therapy. AAPS J. 2015;17(1):102–110. doi: 10.1208/s12248-014-9675-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denny WA. Prodrugs for gene-directed enzyme-prodrug therapy (suicide gene therapy) J. Biomed. Biotechnol. 2003;2003(1):48–70. doi: 10.1155/S1110724303209098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dachs GU, Hunt MA, Syddall S, Singleton DC, Patterson AV. Bystander or no bystander for gene directed enzyme prodrug therapy. Molecules (Basel, Switzerland) 2009;14(11):4517–4545. doi: 10.3390/molecules14114517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sangro B, Mazzolini G, Ruiz M, et al. A Phase I clinical trial of thymidine kinase-based gene therapy in advanced hepatocellular carcinoma. Cancer Gene Ther. 2010;17(12):837–843. doi: 10.1038/cgt.2010.40. [DOI] [PubMed] [Google Scholar]
- 9.Bridgewater JA, Springer CJ, Knox RJ, Minton NP, Michael NP, Collins MK. Expression of the bacterial nitroreductase enzyme in mammalian cells renders them selectively sensitive to killing by the prodrug CB1954. Eur. J. Cancer. 1995;31A(13–14):2362–2370. doi: 10.1016/0959-8049(95)00436-x. [DOI] [PubMed] [Google Scholar]
- 10.Sekar TV, Foygel K, Willmann JK, Paulmurugan R. Dual-therapeutic reporter genes fusion for enhanced cancer gene therapy and imaging. Gene Ther. 2013;20(5):529–537. doi: 10.1038/gt.2012.66. [DOI] [PubMed] [Google Scholar]
- 11.Devulapally R, Sekar TV, Paulmurugan R. Formulation of anti-miR-21 and 4-hydroxytamoxifen co-loaded biodegradable polymer nanoparticles and their antiproliferative effect on breast cancer cells. Mol. Pharm. 2015;12(6):2080–2092. doi: 10.1021/mp500852s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devulapally R, Sekar NM, Sekar TV, et al. Polymer nanoparticles mediated codelivery of antimiR-10b and antimiR-21 for achieving triple negative breast cancer therapy. ACS Nano. 2015;9(3):2290–2302. doi: 10.1021/nn507465d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devulapally R, Paulmurugan R. Polymer nanoparticles for drug and small silencing RNA delivery to treat cancers of different phenotypes. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014;6(1):40–60. doi: 10.1002/wnan.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devulapally R, Foygel K, Sekar TV, Willmann JK, Paulmurugan R. Gemcitabine and antisense-microRNA co-encapsulated PLGA-PEG polymer nanoparticles for hepatocellular carcinoma therapy. ACS Appl. Mater. Interfaces. 2016;8(49):33412–33422. doi: 10.1021/acsami.6b08153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang TY, Choe JW, Pu K, et al. Ultrasound-guided delivery of microRNA loaded nanoparticles into cancer. J. Control. Rel. 2015;203:99–108. doi: 10.1016/j.jconrel.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mullick Chowdhury S, Wang TY, Bachawal S, et al. Ultrasound-guided therapeutic modulation of hepatocellular carcinoma using complementary microRNAs. J. Control. Rel. 2016;238:272–280. doi: 10.1016/j.jconrel.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhaumik S, Sekar TV, Depuy J, Klimash J, Paulmurugan R. Noninvasive optical imaging of nitroreductase gene-directed enzyme prodrug therapy system in living animals. Gene Ther. 2012;19(3):295–302. doi: 10.1038/gt.2011.101. [DOI] [PubMed] [Google Scholar]
- 18.Boussif O, Lezoualc'h F, Zanta MA, et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Natl Acad. Sci. USA. 1995;92(16):7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khansarizadeh M, Mokhtarzadeh A, Rashedinia M, et al. Identification of possible cytotoxicity mechanism of polyethylenimine by proteomics analysis. Hum. Exp. Toxicol. 2016;35(4):377–387. doi: 10.1177/0960327115591371. [DOI] [PubMed] [Google Scholar]
- 20.Kirtane AR, Panyam J. Polymer nanoparticles: weighing up gene delivery. Nat. Nanotechnol. 2013;8(11):805–806. doi: 10.1038/nnano.2013.234. [DOI] [PubMed] [Google Scholar]
- 21.Gardlik R, Palffy R, Hodosy J, Lukacs J, Turna J, Celec P. Vectors and delivery systems in gene therapy. Med. Sci. Monit. 2005;11(4):RA110–RA121. [PubMed] [Google Scholar]
- 22.Nayak S, Herzog RW. Progress and prospects: immune responses to viral vectors. Gene Ther. 2010;17(3):295–304. doi: 10.1038/gt.2009.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 2014;15(8):541–555. doi: 10.1038/nrg3763. [DOI] [PubMed] [Google Scholar]
- 24.Shau MD, Shih MF, Lin CC, et al. A one-step process in preparation of cationic nanoparticles with poly(lactide-co-glycolide)-containing polyethylenimine gives efficient gene delivery. Eur. J. Pharm. Sci. 2012;46(5):522–529. doi: 10.1016/j.ejps.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Bivas-Benita M, Romeijn S, Junginger HE, Borchard G. PLGA-PEI nanoparticles for gene delivery to pulmonary epithelium. Eur. J. Pharm. Biopharm. 2004;58(1):1–6. doi: 10.1016/j.ejpb.2004.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ortega-Munoz M, Giron-Gonzalez MD, Salto-Gonzalez R, et al. Polyethyleneimine-coated gold nanoparticles: straightforward preparation of efficient DNA delivery nanocarriers. Chem. Asian J. 2016;11(23):3365–3375. doi: 10.1002/asia.201600951. [DOI] [PubMed] [Google Scholar]
- 27.Kim IS, Lee SK, Park YM, et al. Physicochemical characterization of poly(L-lactic acid) and poly(D,L-lactide-co-glycolide) nanoparticles with polyethylenimine as gene delivery carrier. Int. J. Pharm. 2005;298(1):255–262. doi: 10.1016/j.ijpharm.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 28.Mellott AJ, Forrest ML, Detamore MS. Physical non-viral gene delivery methods for tissue engineering. Ann. Biomed. Eng. 2013;41(3):446–468. doi: 10.1007/s10439-012-0678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramamoorth M, Narvekar A. Non viral vectors in gene therapy – an overview. J. Clin. Diagn. Res. 2015;9(1):GE01–GE06. doi: 10.7860/JCDR/2015/10443.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sekar TV, Foygel K, Ilovich O, Paulmurugan R. Noninvasive theranostic imaging of HSV1-sr39TK-NTR/GCV-CB1954 dual-prodrug therapy in metastatic lung lesions of MDA-MB-231 triple negative breast cancer in mice. Theranostics. 2014;4(5):460–474. doi: 10.7150/thno.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Figueiredo M, Esenaliev R. PLGA nanoparticles for ultrasound-mediated gene delivery to solid tumors. J. Drug Deliv. 2012;2012:767839. doi: 10.1155/2012/767839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Panje CM, Wang DS, Willmann JK. Ultrasound and microbubble-mediated gene delivery in cancer: progress and perspectives. Invest. Radiol. 2013;48(11):755–769. doi: 10.1097/RLI.0b013e3182982cc1. [DOI] [PubMed] [Google Scholar]
- 33.Tzu-Yin W, Wilson KE, Machtaler S, Willmann JK. Ultrasound and microbubble guided drug delivery: mechanistic understanding and clinical implications. Curr. Pharm. Biotechnol. 2013;14(8):743–752. doi: 10.2174/1389201014666131226114611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larina IV, Evers BM, Esenaliev RO. Optimal drug and gene delivery in cancer cells by ultrasound-induced cavitation. Anticancer Res. 2005;25(1A):149–156. [PubMed] [Google Scholar]
- 35.Hernot S, Klibanov AL. Microbubbles in ultrasound-triggered drug and gene delivery. Adv. Drug Deliv. Rev. 2008;60(10):1153–1166. doi: 10.1016/j.addr.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JL, Lo CW, Inserra C, Bera JC, Chen WS. Ultrasound enhanced PEI-mediated gene delivery through increasing the intracellular calcium level and PKC-delta protein expression. Pharm. Res. 2014;31(9):2354–2366. doi: 10.1007/s11095-014-1332-4. [DOI] [PubMed] [Google Scholar]
- 37.Panje CM, Wang DS, Pysz MA, et al. Ultrasound-mediated gene delivery with cationic versus neutral microbubbles: effect of DNA and microbubble dose on in vivo transfection efficiency. Theranostics. 2012;2(11):1078–1091. doi: 10.7150/thno.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liao WH, Hsiao MY, Lo CW, et al. Intracellular triggered release of DNA-quaternary ammonium polyplex by ultrasound. Ultrason. Sonochem. 2017;36:70–77. doi: 10.1016/j.ultsonch.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 39.Chen S, Grayburn PA. Ultrasound-targeted microbubble destruction for cardiac gene delivery. In: Ishikawa K, editor. Cardiac Gene Therapy: Methods and Protocols. Springer; New York, NY, USA: 2017. pp. 205–218. [DOI] [PubMed] [Google Scholar]
- 40.Chumakova OV, Liopo AV, Andreev VG, et al. Composition of PLGA and PEI/DNA nanoparticles improves ultrasound-mediated gene delivery in solid tumors in vivo . Cancer Lett. 2008;261(2):215–225. doi: 10.1016/j.canlet.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 41.Hosseinkhani H, Aoyama T, Ogawa O, Tabata Y. Ultrasound enhancement of in vitro transfection of plasmid DNA by a cationized gelatin. J. Drug Target. 2002;10(3):193–204. doi: 10.1080/10611860290022624. [DOI] [PubMed] [Google Scholar]
- 42.Larina IV, Evers BM, Ashitkov TV, Bartels C, Larin KV, Esenaliev RO. Enhancement of drug delivery in tumors by using interaction of nanoparticles with ultrasound radiation. Technol. Cancer Res. Treat. 2005;4(2):217–226. doi: 10.1177/153303460500400211. [DOI] [PubMed] [Google Scholar]
- 43.Makadia HK, Siegel SJ. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers (Basel) 2011;3(3):1377–1397. doi: 10.3390/polym3031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Acharya S, Sahoo SK. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv. Drug Deliv. Rev. 2011;63(3):170–183. doi: 10.1016/j.addr.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 45.Bhargava-Shah A, Foygel K, Devulapally R, Paulmurugan R. Orlistat and antisense-miRNA-loaded PLGA-PEG nanoparticles for enhanced triple negative breast cancer therapy. Nanomedicine (Lond.) 2016;11(3):235–247. doi: 10.2217/nnm.15.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2003;2(3):214–221. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 47.Baker A, Saltik M, Lehrmann H, et al. Polyethylenimine (PEI) is a simple, inexpensive and effective reagent for condensing and linking plasmid DNA to adenovirus for gene delivery. Gene Ther. 1997;4(8):773–782. doi: 10.1038/sj.gt.3300471. [DOI] [PubMed] [Google Scholar]
- 48.Mccormack E, Silden E, West RM, et al. Nitroreductase, a near-infrared reporter platform for in vivo time-domain optical imaging of metastatic cancer. Cancer Res. 2013;73(4):1276–1286. doi: 10.1158/0008-5472.CAN-12-2649. [DOI] [PubMed] [Google Scholar]
- 49.Bauer M, Kristensen BW, Meyer M, et al. Toxic effects of lipid-mediated gene transfer in ventral mesencephalic explant cultures. Basic Clin. Pharmacol. Toxicol. 2006;98(4):395–400. doi: 10.1111/j.1742-7843.2006.pto_310.x. [DOI] [PubMed] [Google Scholar]
- 50.Knudsen KB, Northeved H, Kumar PE, et al. In vivo toxicity of cationic micelles and liposomes. Nanomedicine. 2015;11(2):467–477. doi: 10.1016/j.nano.2014.08.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.