Dear Editor
Caspase-2 is the most conserved member of the caspase family, but its physiological function(s) remains a matter of some debate [1]. Recent work suggests that caspase-2 deficiency in mice makes animals more susceptible to tumorigenesis in various models leading to the concept that caspase-2 is a tumor suppressor [1–3]. One of the key characteristics of caspase-2 knockout (KO) MEFs in culture and cells from caspase-2-deficient tumors is a high degree of chromosomal instability (CIN), suggesting that caspase-2 prevents accumulation of aneuploid cells and CIN [1–4]. Real time imaging of KO cells following prolonged spindle-assembly checkpoint arrest suggests that caspase-2 is required for the deletion of cells carrying mitotic aberrations and this requires the catalytic function of caspase-2 [5]. Another model that may explain increased CIN is suggested by Fava et al. [6]. Their data suggest that supernumerary centrosomes trigger PIDDosome (a caspase-2 activating platform containing Pidd and Raidd)-dependent caspase-2 activation, which then cleaves MDM2, resulting in p53 stabilization and p21-dependent cell cycle arrest [6]. Thus PIDDosome-mediated caspase-2 activation is predicted to be required to restrain polyploidization, preventing CIN, and therefore cancer. The main caveat with such a model is that, unlike Casp2 KO animals, Pidd and Raidd KO mice are not susceptible to enhanced tumourigenesis indicating that PIDDosome is not responsible for the tumor suppressor function of caspase-2 [7, 8].
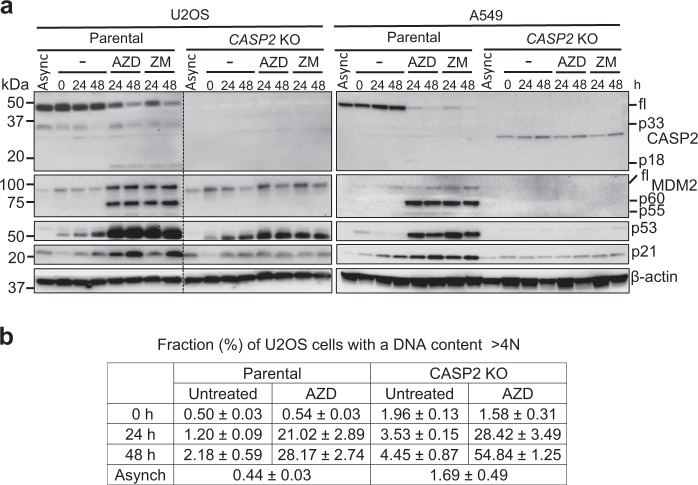
Most of the studies by Fava et al. used A549 lung adenocarcinoma cells, likely because it is a good in vitro model for cell cycle studies. To examine this pathway in other cell types, we generated CASP2 KO U2OS (human osteosarcoma with wild-type p53) cells and compared them with A549 cells following treatment with the Aurora kinase B (AURKB) inhibitors, ZM447439 (as used by Fava et al.) or AZD1152-HQPA, to induce cytokinesis failure (Suppl. Fig. S1a). AZD1152-HQPA is >3000 times more specific toward AURKB compared with AURKA, while ZM447439 used by Fava et al. in most of their studies has lower selectivity [9]. The results confirm some of the observations reported by Fava et al., including MDM2 cleavage and increased levels of p53 and p21 in parental cells treated with the inhibitors (Fig. 1a). We did not observe any difference between the two AURKB inhibitors. Fava et al. [6] found that CASP2 KO in A549 cells results in complete abrogation of MDM2 cleavage, p53 accumulation, and cell cycle arrest of tetraploid cells following cytokinesis failure. Our data are also consistent with these findings in CASP2 KO A549 cells (Fig. 1a). However, we observed that CASP2 KO U2OS cells, still showed increased p53 levels that appear to be independent of MDM2 cleavage in response to the AURKB inhibitors (Fig. 1a). The p53 response was not as robust as in the parental U2OS cells, as indicated by reduced p21 levels in the absence of caspase-2, and this has previously been documented [1, 4]. Interestingly, we also observed that MDM2 cleavage increases at 24 h and then decreases at 48 h following treatment in the non-synchronized A549, but not in U2OS cells (Suppl. Fig. S1b), which is consistent with previous data [6]. The data here, suggest different responses to AURKB inhibition in different cell types. From a functional perspective, DNA content analysis of CASP2KO U2OS cells treated with AZD1152-HQPA showed increased accumulation of polyploid (>4N) cells when compared to parental U2OS cells. However, these results are not as dramatic as reported for A549 cells. In particular, only marginal differences were observed at 24 h following treatment with AZD1152-HQPA (Fig. 1b). We found that U2OS cells are efficiently blocked in cytokinesis by AZD1152-HQPA, ruling out any possibility that the observations are affected by asynchronous cell populations (Suppl. Fig. S1c). Our data indicate that cytokinesis failure can trigger p53-mediated cell cycle arrest in the absence of caspase-2. This suggests the presence of alternative pathways and cell-type specific differences may drive caspase-2 responses to prevent polyploidization.
Fig. 1.
Aurora kinase B inhibition results in p53 accumulation in both parental and CASP2 KO U2OS cells. U2OS and A459 cells were synchronized in G1/S by treatment with 2 mM thymidine for 24 h. After 3 h release, the cells were treated with DMSO or AURKB inhibitors (400 nM AZD1152-HQPA (AZD) or 2 µM ZM447439 (ZM)) for 0, 24, and 48 h, followed by immunoblot and fluorescence-activated cell sorting (FACS) analyses. a Representative immunoblots of cell lysates from treated parental and CASP2 KO cells. Antibodies used for immunoblotting are as indicated. b Percentage of U2OS parental and CASP2 KO cells with polyploid (>4N) DNA content following AZD treatment. Async, asynchronous
As AURKB has various functions in mitosis including mitotic condensation, spindle-assembly checkpoint and cytokinesis [10], inhibiting AURKB by drugs such as AZD1152-HQPA can cause not only cytokinesis failure but also prolonged mitosis [11] and DNA damage, that can induce a p53 response and lead to cell cycle arrest. This, in turn may also contribute to differential responses to these drugs in different cell types [12].
In conclusion, we found that MDM2 cleavage in response to cytokinesis failure is not essential for cell cycle arrest and p53 accumulation in all cell types. Importantly, p53-mediated cell cycle arrest can still occur in the complete absence of caspase-2.
Electronic supplementary material
Acknowledgments
The work in our laboratory is supported by the National Health and Medical Research Council (NHMRC) of Australia project grants 1021456 and 1043057 and a NHMRC Senior Principal Research Fellowship (1103006). We thank Andreas Villunger for providing A549 cells.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Yoon Lim, Email: Yoon.Lim@unisa.edu.au.
Loretta Dorstyn, Email: Loretta.Dorstyn@unisa.edu.au.
Sharad Kumar, Email: Sharad.Kumar@unisa.edu.au.
Electronic supplementary material
The online version of this article (10.1038/s41418-018-0161-0) contains supplementary material, which is available to authorized users.
References
- 1.Miles MA, Kitevska-Ilioski T, Hawkins CJ. Old and novel functions of caspase-2. Int Rev Cell Mol Biol. 2017;332:155–212. doi: 10.1016/bs.ircmb.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Puccini J, Dorstyn L, Kumar S. Caspase-2 as a tumour suppressor. Cell Death Differ. 2013;20:1133–9. doi: 10.1038/cdd.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shalini S, Nikolic A, Wilson CH, Puccini J, Sladojevic N, Finnie J, et al. Caspase-2 deficiency accelerates chemically induced liver cancer in mice. Cell Death Differ. 2016;23:1727–36. doi: 10.1038/cdd.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dorstyn L, Puccini J, Wilson CH, Shalini S, Nicola M, Moore S, et al. Caspase-2 deficiency promotes aberrant DNA-damage response and genetic instability. Cell Death Differ. 2012;19:1288–98. doi: 10.1038/cdd.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dawar S, Lim Y, Puccini J, White M, Thomas P, Bouchier-Hayes L, et al. Caspase-2-mediated cell death is required for deleting aneuploid cells. Oncogene. 2017;36:2704–14. doi: 10.1038/onc.2016.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fava LL, Schuler F, Sladky V, Haschka MD, Soratroi C, Eiterer L, et al. The PIDDosome activates p53 in response to supernumerary centrosomes. Genes Dev. 2017;31:34–45. doi: 10.1101/gad.289728.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manzl C, Peintner L, Krumschnabel G, Bock F, Labi V, Drach M, et al. PIDDosome-independent tumor suppression by caspase-2. Cell Death Differ. 2012;19:1722–32. doi: 10.1038/cdd.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peintner L, Dorstyn L, Kumar S, Aneichyk T, Villunger A, Manzl C. The tumor-modulatory effects of caspase-2 and Pidd1 do not require the scaffold protein Raidd. Cell Death Differ. 2015;22:1803–11. doi: 10.1038/cdd.2015.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marugan C, Torres R, Lallena MJ. Phenotypic screening approaches to develop aurora kinase inhibitors: drug discovery perspectives. Front Oncol. 2015;5:299. doi: 10.3389/fonc.2015.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldenson B, Crispino JD. The aurora kinases in cell cycle and leukemia. Oncogene. 2015;34:537–45. doi: 10.1038/onc.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsuda Y, Iimori M, Nakashima Y, Nakanishi R, Ando K, Ohgaki K, et al. Mitotic slippage and the subsequent cell fates after inhibition of Aurora B during tubulin-binding agent-induced mitotic arrest. Sci Rep. 2017;7:16762. doi: 10.1038/s41598-017-17002-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, Hawkins OE, Su Y, Vilgelm AE, Sobolik T, Thu YM, et al. Targeting aurora kinases limits tumour growth through DNA damage-mediated senescence and blockade of NF-kappaB impairs this drug-induced senescence. EMBO Mol Med. 2013;5:149–66. doi: 10.1002/emmm.201201378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.