Abstract
Herbal dietary supplement (HDS)-induced hepato- and cardiotoxicity is an emerging clinical problem. In this study, we investigated the liver and heart toxicity of HDS OxyELITEPRO™ New Formula (OEP-NF), a dietary supplement marketed for weight loss and performance enhancement that was recently withdrawn from the market. Using a novel NZO/HlLtJ obese mouse model, we demonstrated that administration of clinically relevant mouse equivalent doses (MED) of OEP-NF produced cardio- and hepatotoxic risks following both short- and long-term administration schedules. Specifically, gavaging female NZO/HlLtJ with up to 2X MED of OEP-NF resulted in 40% mortality within two weeks. Feeding mice with either 1X or 3X MED of OEP-NF for eight weeks, while not exhibiting significant effects on body weights, significantly altered hepatic gene expression, increased the number of apoptotic and mast cells in the heart and affected cardiac function. The degree of toxicity in NZO/HlLtJ mice was higher than that observed previously in non-obese CD-1 and B6C3F1 strains, suggesting that an overweight/obese condition can sensitize mice to OEP-NF. Adverse health effects linked to OEP-NF, together with a number of other hepato- and cardiotoxicity cases associated with HDS ingestion, argue strongly for introduction of quality standards and pre-marketing safety assessments for multi-ingredient HDS.
Keywords: cardiotoxicity, herbal-induced liver injury, hepatotoxicity, new dietary ingredient, OXYElite Pro, phytochemicals
1. Introduction
It has been estimated that 50 to 70% of the United States adult population consume dietary supplements, 20% of which comprise botanicals or herbal dietary supplements (HDS) (Brown, 2016; Ronis et al., 2018). Although quite rare, adverse effects associated with ingestion of HDS are not without precedent, with weight loss supplements, and products marketed for exercise or sexual performance enhancement being the three most problematic categories. Hepatotoxicity, cardiovascular toxicity, and nephrotoxicity are the most commonly observed adverse effects, with multi-ingredient products containing caffeine and yohimbe being the most commonly reported suspected causative agents (Brown, 2016; Brown, 2017a; Brown, 2017b; Brown, 2017c).
In 2013, a dietary supplement marketed for weight loss and exercise performance enhancement, OxyELITE Pro™ – New Formula (OEP-NF), was linked to a spate of severe liver injuries, including cases requiring liver transplantation and even death (Heidemann et al., 2016; Johnston et al., 2016; Klontz et al., 2015; Navarro et al., 2017; Roytman et al., 2014). This multi-component formulation contained, among others, synthetic caffeine, yohimbine, and aegeline, the latter being an ingredient for which a New Dietary Ingredient (NDI) notification was not filed with the FDA. An NDI notification had to be submitted because botanicals containing aegeline were not marketed in the U.S. prior to 1994, the year the Dietary Supplement Health and Education Act (DSHEA) was enacted. In total, from January 2011 to February 2014, the FDA received 114 adverse event reports, of which 55 were classified as “liver disease likely due to OEP” (Klontz et al., 2015). In an earlier study using outbred and inbred mouse models, we demonstrated that OEP-NF could modulate a host of biochemical and molecular pathways associated with hepatotoxicity (Miousse et al., 2017a).
Interestingly, the majority of patients hospitalized with OEP-NF-associated liver injury were overweight or obese. This is especially important because of the increased incidence of obesity in the United States, where approximately one third of the adult population is considered obese (Ogden et al., 2012). Obesity, in turn, is a risk factor for the development of metabolic syndrome and metabolic syndrome-associated diseases, such as diabetes and heart disease (Kopelman, 2000). Therefore, in order to examine the role obesity plays in OEP-NF-mediated hepatotoxicity, we employed the NZO/HlLtJ obese mouse model in the present study.
At present, a number of obese mouse models are available for use; however, most either require feeding mice with a high-fat diet or are the result of gene editing technologies (i.e., transgenic models). While these models are invaluable for mechanistic studies, they cannot be utilized for safety assessment since both the diet and genetic modification may influence the toxicity of the substance in question. Consequently, we selected the recently developed NZO/HlLtJ mouse model for use in this study. This non-transgenic mouse strain is characterized by marked obesity involving both visceral and subcutaneous fat depots independent of dietary fat intake (The Jackson Laboratory). Both male and female members of the NZO/Hl substrain exhibit impaired glucose tolerance; however, the subsequent development of type 2 diabetes is limited to males only.
A phytochemical analysis of the OEP-NF formula indicated that this dietary supplement consisted mostly of the alkaloids caffeine, aegeline, coclaurine, higenamine (also known as norcoclaurine), and yohimbine (Miousse et al., 2017a). In addition to the hepatotoxic potential of this alkaloid mixture (Miousse et al., 2017a), each individual alkaloid has been reported to affect the cardiovascular system via their sympathomimetic properties. For example, caffeine induces the release of norepinephrine and dopamine (Nehlig et al., 1992). Higenamine, a β2 receptor agonist (Wu et al., 2016), alters action potential in cardiac cells (Yu et al., 1985), has a positive inotropic effect on isolated guinea pig papillary muscle (Kimura et al., 1989), and induces endothelium-dependent relaxation in isolated rat aorta (Wong et al., 1997). Yohimbine is an indole alkaloid with α2-adrenergic blocking activity (Zaretsky et al., 2015), and aegeline may act as a β3 agonist (Narender et al., 2007). While a short-term exposure to such agents can have protective properties in cardiac injury (Lee et al., 2006; Wang et al., 2013), prolonged exposure can lead to increased blood pressure and cardiac rhythm disturbances (Temple et al., 2017) as well as cardiac toxicities in subjects with poor cardiovascular health (Venhuis et al., 2014). Moreover, when combined, these alkaloids may exacerbate each other’s pharmacodynamic effects (Calvert et al., 2015; Kimura et al., 1989). Such additive or synergistic effects may produce unexpected cardiovascular toxicities, as was demonstrated for caffeine and ephedrine (Brown et al., 2012; Dunnick et al., 2007), and caffeine and 1,3-dimethylamylamine (DMAA) (Farney et al., 2012). Studies in small cohorts of human subjects have shown that short-term administration of caffeine, higenamine and yohimbine to healthy subjects elevate heart rate and systolic blood pressure (Bloomer et al., 2015; Lee et al., 2013). Furthermore, an earlier OEP formulation containing caffeine and DMAA was removed from the market due to suspected adverse cardiovascular effects (Forrester, 2013). Therefore, the purpose of the present study was to determine whether OEP-NF can produce hepato- and/or cardiotoxicity in the NZO/HlLtJ obese mouse model.
2. Materials and Methods
2.1. Chemicals and phytochemical analysis
For the purposes of OEP-NF analysis, the pure compounds, aegeline and higenamine, were synthesized at the National Center for Natural Product Research (NCNPR) (University of Mississippi, University, MS, USA). The racemic aegeline was synthesized from readily available 2-amino-4’-methoxyacetophenone as reported earlier (Avula et al., 2016). Whereas, norcoclaurine was synthesized by heating of 2-(3,4-dimethoxy)ethylamine with 4-methoxyphenyl acetic acid to 180 °C for 4h, Bischler–Napieralski reaction of the resulting amide with POCl3 followed by sodium borohydride reduction in MeOH resulted cocularine in 78% yield. Demethylation with HBr in acetic acid, neutralizing with HCl gas resulted higenamine (demethylcoclaurine or norcoclaurine) hydrochloride in 66% yield. The spectral data of each compound is in full agreement with reported [Somanathan et al., 1983 (for aegeline), Koshiyama et al., 1970 (for demethylcoclaurine)] data and the purity (>95%) was established on the basis of UPLC analysis. Caffeine and yohimbine were purchased from Sigma (St. Louis, MO, USA). Acetonitrile and formic acid were of HPLC grade and these, along with dimethylsulfoxide, were purchased from Fisher Scientific (Fair Lawn, NJ, USA). Water for the HPLC mobile phase was purified in a Milli-Q system (EMD Millipore, Temecula, CA, USA).
Phytochemical content of OEP-NF capsules was determined at the NCNPR via ultra-high performance liquid chromatography (UPLC) with photodiode array detection as described previously (Miousse et al., 2017a). Additional OEP-NF extractions were performed at the University of Arkansas for Medical Sciences (UAMS) College of Pharmacy using both water and dimethylsulfoxide (DMSO) as extraction solvents. The contents of twelve OEP-NF capsules were transferred into six separate 20 mL round bottom, glass screw-cap tubes (12 capsules per tube) and extracted overnight with either 10 mL of water or 10 mL of DMSO using a rotational mixer (12 revolutions per min.). Tubes were centrifuged at 3300 rpm (Fisher Model 225 Centrific™ centrifuge, Fisher Scientific, Waltham, MA, USA) for one hour and supernatants were transferred to sterile 50 mL polypropylene tubes (Corning 430790, Corning, NY, USA). One mL aliquots of both water and DMSO extractions were sent to the NCNPR for phytochemical analysis. Combinations of the water/DMSO extractions were used for acute gavage administrations.
2.2. Animals
NZO/HlLtJ mice were purchased from Jackson Laboratories (Bar Harbor, MA, USA) and were further bred at the UAMS Division of the Laboratory Animal Medicine (DLAM) facility. Because females are more prone to HDS-induced liver injury (Cho et al., 2017; Medina-Caliz et al., 2018; Navarro et al., 2014) and because similar patterns of higher incidence of OEP-NF-associated liver injury were observed in female consumers (Heidemann et al., 2016; Roytman et al., 2014) as well as in the previous mouse study of OEP-NF (Miousse et al., 2017a), only female mice were considered for this study.
Animal experiments were conducted in two stages. The first stage was based on gavaging mice with OEP-NF for two weeks. This stage was performed in order to address the short-term toxicity of OEP-NF when delivered as a daily bolus (mimics ingestion of a single maximum recommended dose in humans). During the second stage, mice were fed with diets containing OEP-NF for eight weeks. The duration of this stage was based upon the maximum period of uninterrupted OEP-NF consumption suggested by the product label. OEP-NF was delivered with chow in order to avoid any physiological stresses that 8 weeks of uninterrupted gavaging would impart.
To avoid potential fasting-exacerbated toxicity, food and water were provided ad libitum. For the gavage study, eleven control and fifteen gavaged with OEP-NF mice were used. Initially mice were scheduled to be gavaged with 2X MED OEP-NF. This dose was selected based on our previous study on OEP-NF toxicity where 3X MED resulted in high mortality rates. Initial gavaging of NZO/HlLtJ mice with 2X MED of OEP-NF caused overt toxicity described in details in section 3.2. Therefore, the initial dose was reconsidered and mice were gavaged with 1X MED for the remaining 9 days of the study. For the feeding study, four mice per each experimental group were used. Each animal was individually identified with an ear tag. Animal body weights were measured and recorded weekly. All procedures complied with the National Institutes of Health guide for the care and use of Laboratory animals, and were approved by the UAMS Institutional Animal Care and Use Committee at UAMS (protocol number: AUP #3701).
2.3. Animal experiments: gavage dosing and diets
The maximum label-recommended human dose of OEP-NF is three capsules daily. Phytochemical analysis revealed that each capsule contained 209.45 mg of total plant alkaloids (i.e., caffeine, aegeline, higenamine, yohimbine, coclaurine in 14.2: 47.2: 26.5: 4.42: 1 w/w ratios). Based upon these findings, the maximum dose of total OEP-NF alkaloids for a 70-kg adult human is 8.98 mg/kg/day. Allometric scaling for mouse equivalent doses of total OEP-NF alkaloids were determined per the recommendation of Wojcikowski and Gobe (2014) which, in turn, is based upon the FDA Industry Guidance for Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Volunteers (Nair and Jacob, 2016; US Food and Drug Administration 2004). A scaling factor of 12.3 is commonly used for mice of normal weight, however, for those mice considered obese the FDA Guidance suggests a different conversion factor based upon the following equation: (Wt.(kg)human/Wt.(kg)mouse)0.33 (US Food and Drug Administration 2004). Because the mean weights of the mice generally fell into two groups (~50 g and ~70 g), the conversion factors relative to a 70 kg adult human were 10.9 and 9.8, respectively. Thus, the mouse equivalent dose (MED) of total OEP-NF alkaloids was calculated to be 98 mg/kg/day for the 50 g group and 88 mg/kg/day for the 70 g group. Gavage doses were assessed at 1X and 2X MED. This range of doses was well within the 100-fold uncertainty factor commonly recommended when designing HDS safety studies.
Because aegeline is poorly extracted in water but readily recovered in DMSO, 1 mL each of the water and DMSO extractions were combined and then diluted with appropriate volumes of 50% DMSO/water such that a 300 μL gavage volume would deliver either the 1X or 2X MED of total OEP-NF alkaloids. Although pure DMSO may exhibit mild toxicity when administered for prolonged periods, it is generally considered non-toxic and has been used extensively as a vehicle in toxicological studies, including our previous study on OEP-NF (Damiano et al., 2018; Hucker et al., 1966; Miousse et al., 2017a). Gavage doses were prepared daily, 1–2 hours prior to administration. Gavage solutions were vortex-mixed immediately prior to administration in each mouse.
Custom-made diets were purchased from Envigo (former Teklad Diets) (Madison, WI, USA). The following diets were used: Diet #8640 (vehicle); OEP-NF – 1X and OEP-NF – 3X. These doses were based upon the product (lot# 421131) label recommendation (no more than 3 capsules daily) coupled with the total alkaloid (i.e., caffeine, aegeline, higenamine, yohimbine, coclaurine) content per capsule (209.5 mg) as determined by the NCNPR and calculated as described above for gavage dosing. Original OEP-NF product from the same lot (# 421131) was used to prepare the diets. The components of the diet for the previously reported study (Miousse et al. 2017a) and this study were prepared by Envigo simultaneously and both studies were run concurrently at the same animal facility. Diet components were independently verified by Envigo before they were shipped to UAMS. Diets were stored in a cool dry place and a portion of the diet was provided to mice daily.
2.4. High-resolution ultrasonography
High-resolution ultrasonography was used to assess in vivo cardiac function after two and eight weeks of feeding mice with OEP-NF. The day before ultrasonography, mice were anesthetized with 3% isoflurane, and hair was removed from the thorax and abdomen using a depilatory cream. On the day of ultrasonography, mice were anesthetized with 1.5–2% isoflurane and placed supine on a heated platform that monitors the animal’s respiration rate and electrocardiogram. The mice were scanned using a Vevo® 2100 imaging system (VisualSonics, Toronto, ON, Canada) with a MS400 (18–38 MHz) transducer. Echocardiographs were obtained in the short axis M-mode at the mid-left ventricular level. Pulsed-wave Doppler was used to determine mitral valve E and A velocities in a four-chamber view of the heart.
The Vevo® 2100 software version 1.6.0 cardiac package was used to obtain echocardiographic parameters such as stroke volume and ejection fraction from three M-mode scans per animal. The vascular package was used to assess mitral valve E and A velocities from three consecutive wave patterns per Pulsed-wave Doppler scan, in up to three scans per animal. Both ultrasonography and subsequent data analysis were performed in a blind fashion.
2.5. Blood sampling
To measure the effects of OEP-NF on liver enzymes (e.g., ALT, AST) characteristic for drug-induced liver injury, blood was collected at the end of each stage of the experiments described above. Blood was collected by trained DLAM personnel from the vena cava into a 1.1 mL Z-gel microtube (Sarstedt, Newton, USA). Tubes were kept on ice and centrifuged at 10,000 rpm for 20 min and the serum was immediately aliquoted and flash-frozen.
2.6. Histopathological assessment
Livers were excised and a 1 mm section was obtained from the left lateral lobe and another from the right medial lobe. For the heart tissue, 5 μm longitudinal sections were obtained. The sections were fixed in 4% formalin for 24 h, then briefly rinsed in PBS and stored in 70% ethanol for 24 h. Livers and hearts were then processed at the UAMS Pathology Core Facility, stained with hematoxylin eosin and shipped to the Oklahoma Health Sciences Center (OHSC) for veterinary pathology assessment.
For histologic evaluation purposes, each liver was represented by two sections obtained from different locations within the liver. Each section was initially evaluated at magnifications of X40 and X100. The sections were then evaluated at X200 and X400 to better determine if significant changes were present and to check for the presence of mitotic figures and apoptotic bodies.
For determination of mast cell numbers, 5 μm longitudinal sections of heart were incubated in 0.5% Toluidine Blue in 0.5 N HCl for 3 days at room temperature, followed by 0.7 N HCl for 10 minutes. Eosin was used as a counterstain. Stained sections were examined with an Axioskop transmitted light microscope (Carl Zeiss, Oberkochen, Germany), and mast cells were counted. Sections were also scanned with a ScanScope CS2 slide scanner to measure the area of cardiac tissue in each section using ImageScope 12 software (Leica Biosystems). Mast cell numbers were corrected for tissue area in each section.
Cardiac apoptotic nuclei were assessed with a TUNEL-based assay (CardioTACS®, Bio-Techne Corporation, Minneapolis, NE), following the manufacturer’s instructions. Sections were also scanned with a ScanScope CS2 slide scanner (Leica Biosystems, Wetzlar, Germany) to measure the area of cardiac tissue in each section using ImageScope 12 software (Leica Biosystems). The number of apoptotic nuclei was corrected for tissue area in each section.
2.7. Clinical biochemistry
For the gavage studies, serum ALT and AST levels were analyzed at Antech Diagnostics (Morrisville, NC, USA). For the feeding studies, serum ALT and AST were detected in house using activity colorimetric assays and quantitated by a standard curve. The assay kits were purchased from BioVision (Milpitas, CA, USA) (Cat. # K752–100 and K753–100 for ALT and AST, respectively). Serum samples from treatment and control groups were diluted in the assay buffer (1:2 or 2:5). Twenty microliter samples and standards were added to a 96-well plate. 100 μL reaction mix composed of 86 μL ALT (AST) Assay Buffer, 2 μL reconstituted ALT (AST) Enzyme mix, 2 μL OxiRed probe, and 10 μL reconstituted ALT (AST) substrate were added to each well as well as the standards and positive controls. The plate was shaken for 3 minutes to mix the reagents. The absorbance (OD) was read at 570 nm (T1) and then again at T2 after the plate incubation at 37 °C for 30–45 min and protected from light. ALT (AST) activity in serum was calculated using a standard curve and formula below:
Where B is the glutamate amount calculated from the standard curve (in nmol); T1 is the time of the first reading (in min); T2 is the time of the second reading (in min); V is the original sample volume added into the reaction well (in mL).
2.8. Gene expression array
Total RNA was extracted from flash frozen liver tissue using the RNeasy Mini Kit (Qiagen, Germantown, MD, USA). Following purification, 1000 ng were reverse transcribed with the High Capacity cDNA Reverse Transcription Kit (ThermoFisher, Waltham, MA, USA). The cDNA was diluted to 5 ng/μL and 105 μL was mixed with an equal volume of 2X TaqMan® Fast Advanced Master Mix. For real-time PCR, 100 μL of the mix was applied to each of two channels on a TaqMan Low Density Hepatotoxicity Array (TLDA) (ThermoFisher, Waltham, MA, USA). Four biological samples were loaded on each array with five samples per each group analyzed. Analysis was performed using the ExpressionSuite Software v1.1 (ThermoFisher, Waltham, MA, USA).
2.9. miRNA expression array
Expression of a panel of miRNAs previously reported altered in response to hepatotoxicant exposure was used (Lin et al., 2017; Miousse et al., 2017a; Miousse et al., 2017b). miRNAs were extracted from flash-frozen livers using the miRNeasy Kit (Qiagen, Germantown, MD, USA). RNA concentration was assessed by Nanodrop 2000 (Thermo Scientific, Waltham, MA, USA) and 1000 ng of RNA containing miRNAs was used for reverse transcription with the miScript reverse transcriptase and reverse transcription primers specific for nine miRNA targets and the internal reference U6). Transcript abundance was measured on a ViiA-7 instrument (Applied Biosystems, Foster City, CA, USA) and results calculated using the ΔΔ Ct method (Livak and Schmittgen 2001).
2.10. Immunoblot analysis
To determine protein expression, immunoblot analysis was performed on frozen heart tissue samples. Tissue samples were homogenized in a 1% Triton-X100 RIPA buffer containing protease inhibitors (Sigma-Aldrich, St Louis, MO, USA; 1:100) and phosphatase inhibitors (Sigma-Aldrich; 1:100) using a Potter-Elvehjem mechanical compact stirrer (BDC2002, Caframo LabSolutions, Georgian Bluffs, ON, Canada). Protein concentration was determined using a BCA protein assay (Bio-Rad, Hercules, CA), and 25 μg protein was added to a 2x Laemmli buffer containing β-mercaptoethanol (5%). Gel electrophoresis was performed, and proteins were transferred to a PVDF membrane. Membranes were first incubated in TBS containing 0.05% Tween-20 and 5% non-fat dry milk to reduce non-specific antibody binding, and then incubated overnight at 4°C with one of the primary antibodies diluted in TBS containing 0.1% Tween-20 and 5% non-fat dry milk listed in Supplementary Table 1. After incubation with horseradish peroxidase-conjugated secondary antibodies (mouse anti-rabbit (Cell Signaling Technology, Danvers, MA, USA) or goat anti-mouse (Jackson Immunoresearch, West Grove, PA, USA), 1:20,000), membranes were covered in ECL Plus Western Blotting Detection Reagent (GE Healthcare Life Sciences, Chicago, IL, USA) and placed on CL-Xposure Film (Thermo Scientific, Waltham, MA, USA). Films were developed and imaged with an AlphaImager® gel documentation system (ProteinSimple, San Jose, CA, USA). Density of protein bands was determined with the publicly available ImageJ software and expressed relative to Glyceraldehyde 3-phosphate dehydrogenase (Gapdh).
2.11. Statistical analyses
All statistical analyses were performed with the Graphpad Prism 6 software (Graphpad Software. San Diego, CA). Treatment groups were compared with their respective untreated group using ANOVA followed by Dunnett’s multiple comparison test. In cases where the data was not normally distributed, a Dunn’s test was used instead.
3. Results
3.1. Analytical chemistry data
Phytochemical analysis of OEP-NF (lot #421131) capsules were reported in our previous study (Miousse et al., 2017a). Briefly, analyzed capsules contained caffeine (134.2 mg/cap.), aegeline (44.9 mg/cap.), higenamine (25.2 mg/cap), yohimbine (4.2 mg/cap), coclarurine (0.95 mg/cap.) and trace amounts of tannins (i.e., gallic acid and gallic acid derivatives). No characteristic marker phytochemicals were noted for extracts of either Bauhinia purpurea (e.g., isoquercetin, quercetin-3,4’-diglucoside, isorhamnetin-3,7-diglucoside, pelargonidin-3-triglucoside, quercetin 3-rutinoside-7-glucoside, kaempferol 3,4’-diglucoside) or Hemerocalis fulva (e.g., fulvanines A-E, kwansonine B-C). Besides yohimbine, no other characteristic marker phytochemicals for Pausinystalia johimbe extract (e.g., rauwolscine, ß-yohimbine, corynanthine, dihydrositsirikine, pseudoyohimbine, yohimbic acid, alloyohimbine, hydroxyyohimbine) were detected. These findings indicate that none of these botanical extracts were components of the formulation, despite label claims for each, and that OEP-NF was a collection of synthetic alkaloids.
3.2. Gavage study
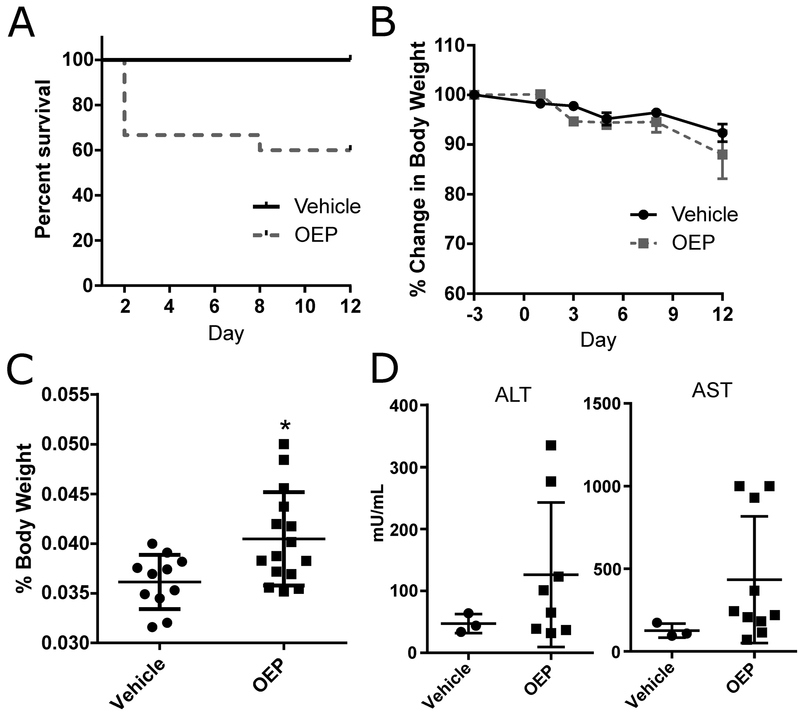
Initially, mice were scheduled to be gavaged with 2X MED OEP-NF for two weeks (Mon-Fri schedule). This dose was selected based upon our previous study, where gavaging of 10X and 3X MED OEP-NF resulted in high mortality rates in CD-1 mice, but only sporadic mortality was observed in B6C3F1 mice with 2X MED OEP-NF (Miousse et al., 2017a). Mice were monitored hourly during the first 8 h after gavage. At 8 h, most of the OEP-NF-gavaged mice exhibited patterns of lethargic behavior (e.g., slow locomotion, closed eyes, and reduced responses to exogenous stressors); however, by 12 h these effects had resolved. At the 23-h time-point, during the morning check-up, three mice gavaged with 2X MED OEP-NF were found dead in their cages. Examination of esophagus and stomach did not identify any signs of damage due to the gavage procedure. Due to this finding, the 2X MED OEP-NF was reduced to 1X MED. Nevertheless, two more mice were found dead within hours after gavaging with 1X MED and another died on Day 7, resulting in 40% OEP-NF-associated mortality (Fig. 1A). Postmortem examination revealed no evidence of damage from gavage needle insertion.
Fig. 1.
Analysis of OEP-NF toxicity in a two week gavage study. A) Survival of female NZO/HlLtJ mice gavaged with 2X MED (Day 1) and 1X MED (Day 2–12); B) Body weight dynamics; C) Liver to body weight ratio; D) Serum plasma concentrations of alanine (ALT) and asparate (AST) aminotransferases. Data are presented as means ± SEM. Asterisks (*) denote significant (p < 0.05) difference from control (n=11 for Vehicle group, n=15 for OEP group, except for ALT and AST where n=3 for vehicle and n=10 for OEP). OEP – OxyELITE Pro™ – New Formula.
3.2.1. Physiological, histopathological and clinical biochemistry evaluation
Gavaging mice with OEP-NF for two weeks did not result in significant changes in body weights compared to control mice (Fig. 1B). No histopathological abnormalities were observed in the livers of mice; however, significant increase in the liver/body weight ratio was observed (Fig. 1C). Furthermore, while no significant differences in mean plasma levels of ALT and AST were noted between the control and OEP-NF-gavaged mice, several mice exhibited extremely high (over 10-fold from control) individual levels of ALT and AST (Fig. 1D).
Gavaging OEP-NF had no significant effect on heart weight. Histological analysis revealed no significant changes in the number of apoptotic cells or the number of mast cells in the heart of either control or gavaged mice (data not shown).
3.2.2. Molecular changes in the livers and hearts
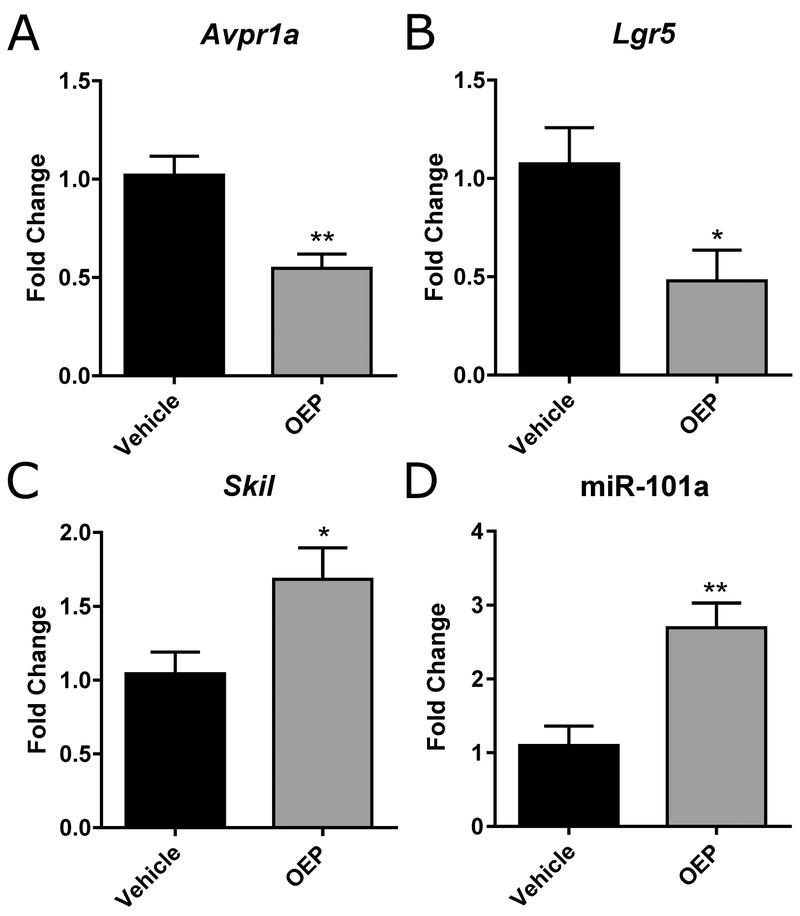
A small subset of genes in the liver was affected by gavaging mice with OEP-NF for two weeks. Specifically, only 3 out 84 gene-markers of hepatotoxicity were significantly deregulated in the livers of mice receiving OEP-NF, compared to controls (Fig. 2). It should be noted, however, that no molecular analysis was performed on the livers of those mice found dead subsequent to dosing because of rapid RNA degradation. We also observed a significant up-regulation of miR-101a in the livers of OEP-NF-gavaged mice (2.7-fold, p<0.01) (Fig. 2).
Fig. 2.
Molecular alterations in the livers of NZO/HlLtJ mice gavaged with OEP-NF for two weeks. A-C) mRNA levels of Avpr1a, Lgr5, and Skil; D) Levels of miR-101a as measured by RT qPCR. Data are presented as means ± SEM. Asterisks (*) denote significant (p < 0.05) and (**) denote significant (p < 0.01) differences from control (n=5 for Vehicle group, n=5 for OEP group). OEP – OxyELITE ProTM – New Formula.
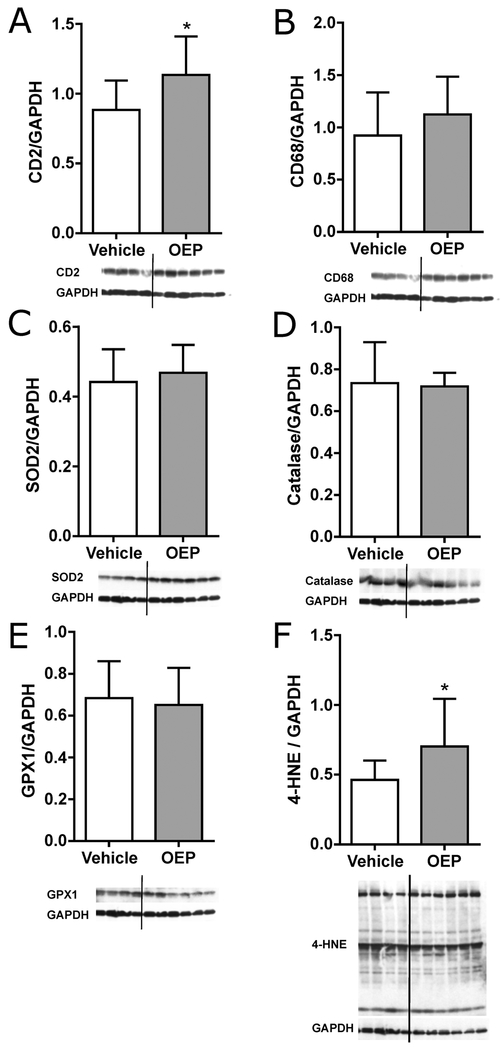
Immunoblot analysis was used to assess cardiac protein levels of inflammatory cell markers, anti-oxidant enzymes, and overall protein 4-hydroxynonenal (4-HNE) adducts. Statistically significant increases in the expression of superoxide dismutase 2 (SOD2) and total protein 4-HNE adducts were observed in OEP-NF-gavaged mice. (Fig. 3). Histological analysis revealed no significant changes in the number of apoptotic cells or the number of mast cells in the hearts of experimental mice (data not shown).
Fig. 3.
Immunoblot analysis of cardiac protein levels of inflammatory cell markers, anti-oxidant enzymes, and overall protein 4-hydroxynonenal (4-HNE) adducts in NZO/HlLtJ mice gavaged with OEP-NF for two weeks. Density of protein bands was determined with the ImageJ software and expressed relative to Glyceraldehyde 3-phosphate dehydrogenase (Gapdh). Data are presented as means ± SEM (n=4 for Vehicle group, n=6 for OEP group). Asterisks (*) denote significant (p < 0.05) difference from control. OEP – OxyELITE ProTM – New Formula.
3.3. Feeding study
3.3.1. Physiological, histopathological and clinical biochemistry evaluation
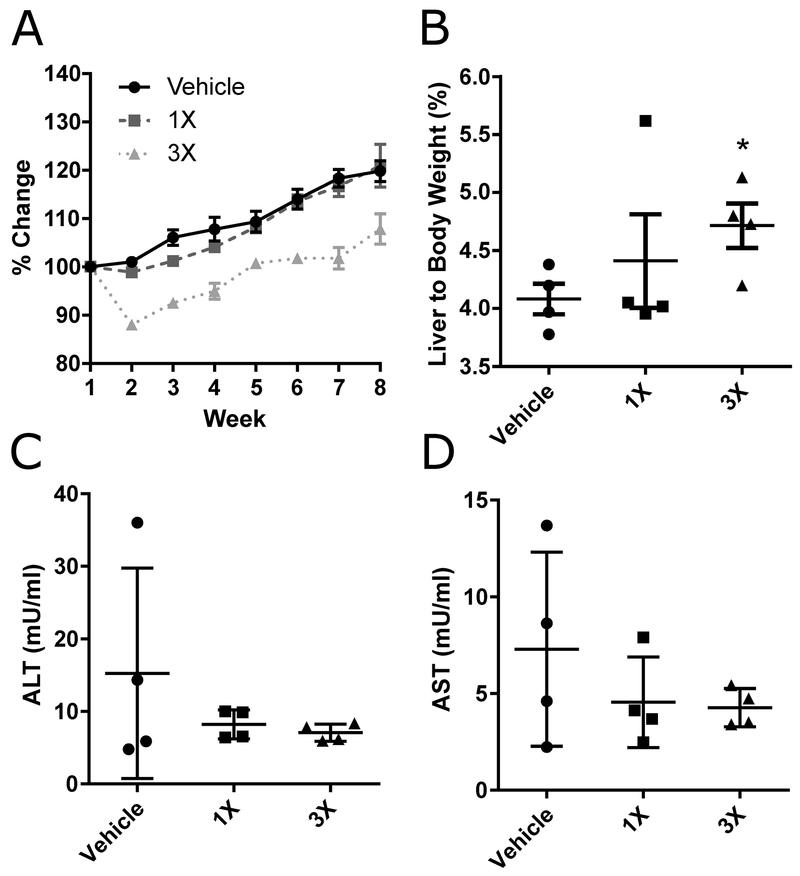
Feeding OEP-NF for eight weeks did not result in mortality in any of the experimental groups. Differences in mortality observed between gavage and ad libitum feeding can likely be explained by the acute aspect of gavaging OEP-NF (i.e., rapid absorption of alkaloids resulting in high blood concentrations), while ingestion via the diet manifested in slower, more sustained alkaloid absorption throughout the day. Feeding mice with 1X MED OEP-NF for 8 weeks did not result in significant differences in body weight compared to mice fed the control diet (Fig. 4a). The 3X MED diet resulted in a 12% body weight loss after week one, although by week 2 mice began re-gaining weight. By the end of week 8, control mice exhibited a 17% increase in mean body weight, whereas mice fed 1X MED and 3X OEP-NF displayed mean weight gains of 18% and 8%, respectively (Fig. 4A). These results, together with those of the gavage study, clearly indicate that OEP-NF does not promote long-term weight loss nor aid in weight management.
Fig. 4.
Analysis of OEP-NF toxicity in an eight week feeding study of female NZO/HlLtJ mice. A) Body weight dynamics; B) Liver to body weight ratio; C-D) Serum plasma concentrations of alanine (ALT) and asparate (AST) aminotransferases. Data are presented as means ± SEM (n=4 for each experimental group). Asterisks (*) denote significant (p < 0.05) difference from control. OEP – OxyELITE ProTM – New Formula.
Similar to the gavage study, feeding mice with OEP-NF resulted in increased liver/body weight ratios. Importantly, the observed increase was dose-dependent (Fig 4B). No histopathological abnormalities or significant changes in the plasma levels of ALT and AST were observed (Fig 4C).
3.3.2. Molecular changes in the livers
We identified 19 genes that were significantly dysregulated in mouse livers as a result of OEP-NF ingestion for 8 weeks (Table 1). Among them, 2 were dysregulated in mice fed 1X MED OEP-NF (1 up-regulated, 1 down-regulated) and 19 in mice fed 3X MED (16 up-regulated and 3 down-regulated). The expression of 9 genes was 3-fold higher in mice fed the OEP-NF-containing diet compared to those fed the control diet. Of particular interest were fatty acid desaturase 1 (Fads1) and carbohydrate-responsive element-binding protein (Chrebp, also known as MLX-interacting protein-like, Mlxipl) genes, where the expression of each was over 5-fold higher in mice fed OEP-NF compared to controls. Another interesting finding was the unexpected down-regulation of Cd36, a gene up-regulated as a result of feeding OEP-NF in two non-obese mouse strains – CD-1 and B6C3F1 (Miousse et al., 2017a).
Table 1.
Gene expression levels of a panel of markers of hepatotoxicity (n=4 for each experimental group).
| Control | IX | 3X | |
|---|---|---|---|
| Abcb4 | 1.00 (±0.28) | 1.70 (±0.27) | 2.24* (±0.21) |
| Abcc3 | 1.00 (±0.33) | 2.07 (±0.52) | 3.24** (±0.40) |
| Aldoa | 1.00 (±0.37) | 1.97 (±0.36) | 4.18** (±0.86) |
| Ccng1 | 1.00 (±0.13) | 0.69 (±0.10) | 0.50* (±0.05) |
| Cd36 | 1.00 (±0.13) | 0.98 (±0.15) | 0.44* (±0.05) |
| Cyp1a2 | 1.00 (±0.22) | 1.70 (±0.35) | 3.66# (±0.81) |
| Fabp1 | 1.00 (±0.19) | 0.43# (±0.06) | 0.56 (±0.04) |
| Fads1 | 1.00 (±0.42) | 3.46 (±1.22) | 5.13* (±1.07) |
| Fasn | 1.00 (±0.38) | 2.49 (±0.69) | 3.35* (±0.64) |
| Fmo1 | 1.00 (±0.13) | 1.41 (±0.24) | 2.78* (±0.65) |
| Gclc | 1.00 (±0.15) | 1.49 (±0.19) | 1.69* (±0.12) |
| Gsr | 1.00 (±0.36) | 1.81 (±0.39) | 2.87* (±0.57) |
| Lss | 1.00 (±0.36) | 1.94 (±0.47) | 3.36** (±0.14) |
| Chrebp | 1.00 (±0.45) | 3.22 (±0.99) | 5.56* (±1.51) |
| Mrps18b | 1.00 (±0.33) | 1.35 (±0.26) | 2.12* (±0.26) |
| Pla2g12a | 1.00 (±0.06) | 1.93* (±0.26) | 2.11* (±0.23) |
| Ppara | 1.00 (±0.26) | 1.82 (±0.45) | 3.08* (±0.60) |
| Scd1 | 1.00 (±0.46) | 2.66 (±0.88) | 5.15* (±0.91) |
| Srebf1 | 1.00 (±0.11) | 1.57 (±0.45) | 2.19* (±0.11) |
Asterisks (*) denote significant (p < 0.05) and (**) denote significant (p < 0.01) differences from control for parametric analyses. Pound signs indicate that a non-parametric analyses was performed on non-normal distributions.
The observed molecular changes were also paralleled by a number of dysregulated miRNAs, with miR-125b and miR-483 being down-regulated at both 1X and 3X MED (Supplemental Figure 1).
3.3.3. Effects of OEP-NF on the mouse heart
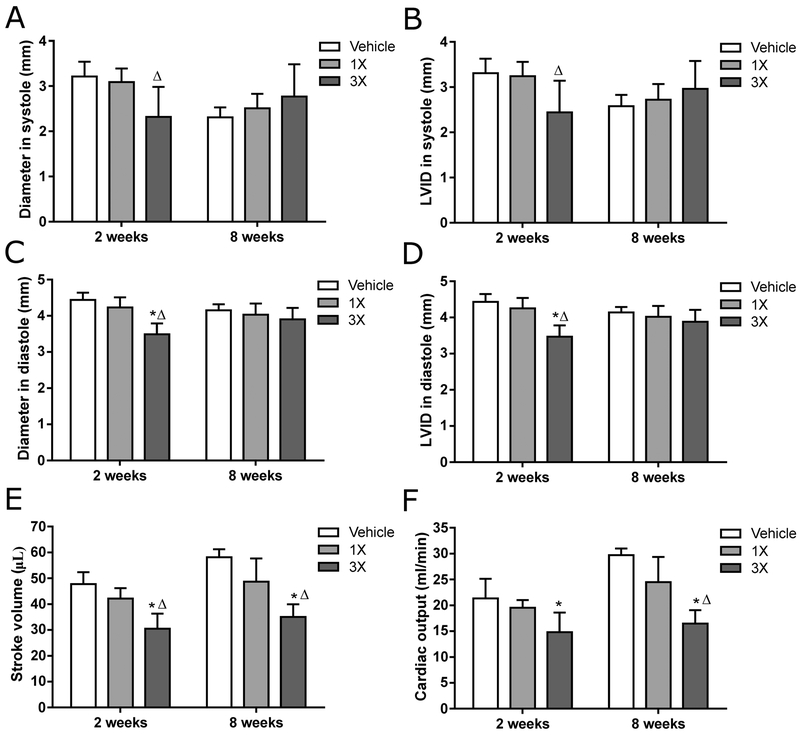
Ultrasonography was used to assess cardiac function after 2 and 8 weeks of feeding OEPNF in the same cohort of mice used for the liver toxicity studies. After 2 weeks, heart rate under anesthesia was not different among the groups (Supplemental Figure 2). Power-Doppler analysis of mitral valve flow revealed a significant decrease in mitral valve A velocity in the 3X MED group compared to the 1X MED group, but the E/A ratios were not affected (Supplemental Figure 2). Results of M-mode analysis are shown in Fig. 5. Both diameter and left ventricular inner diameter (LVID) in systole were significantly lower in the 3X MED mice compared to the 1X MED mice. In diastole, diameter, volume, and LVID were significantly lower in the 3X MED group compared to the 1X MED group and controls. Lastly, both stroke volume and cardiac output, calculated as stroke volume x heart rate, were significantly reduced in the 3X MED group compared to controls.
Fig. 5.
In vivo cardiac structure and function after two and eight weeks of feeding of OEP-NF assessed via ultrasonography. Data are presented as means ± SEM. Asterisks (*) denote significant (p < 0.05) difference from control. Delta (Δ) denotes significant (p<0.05) difference from the low-dose group (n=4 for each experimental group). OEP – OxyELITE ProTM – New Formula.
The mice described above were tested again after 8 weeks of feeding OEP-NF. At that time, heart rate under anesthesia was significantly reduced in the 3X MED group compared to both 1X MED and controls (Supplemental Figure 2). Both mitral valve E and A velocities were significantly decreased, but E/A did not show a significant change (Supplemental Figure 2). In addition, stroke volume and cardiac output were significantly reduced in the 3X MED group compared to both 1X MED and controls (Fig. 5, Supplemental Figure 3, Supplemental Video 1–2).
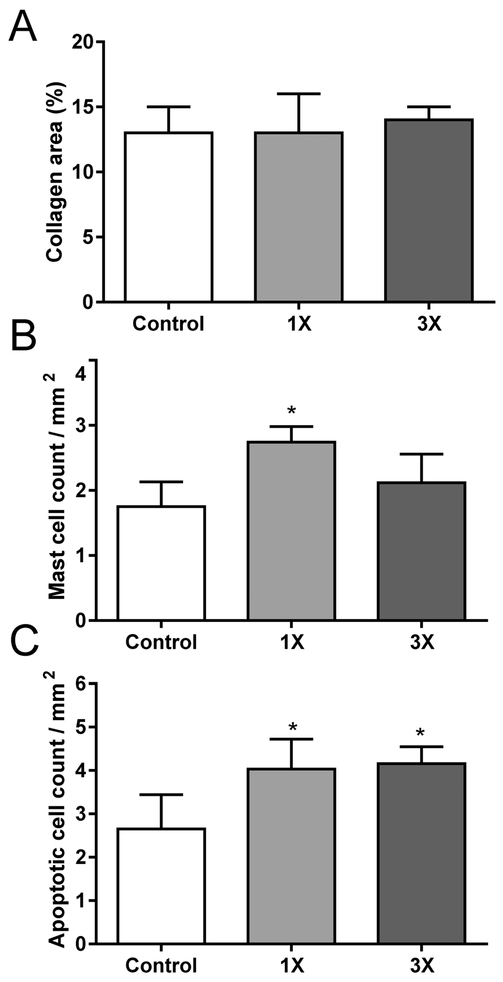
After ultrasonography at the 8-week time-point, cardiac tissue was collected for further analysis. There was no significant effect on heart weight. None of the hearts showed signs of inflammation, myofiber necrosis, interstitial fibrosis, microvascular damage, or epicardial fibrosis. In addition, the percentage of cardiac tissue area occupied by collagens as measured with computerized analysis of Sirius Red staining was not different among the groups. On the other hand, an increase was seen in the number of mast cells per tissue area in the 1X MED group, and an increase in the number of apoptotic cells per area in both dose groups (Fig. 6). Immunoblot analysis revealed no significant changes in left ventricular protein levels of CD2, CD68, SOD2, catalase, gluthathione peroxidase 1 (Gpx-1), or 4-HNE adducts (Supplemental Figure 4).
Fig. 6.
Histological analysis of the heart after eight weeks of feeding of OEP-NF. A) Cardiac area occupied by collagens; B) Cardiac mast cell density; C) Density of apoptotic cells in the heart. Data are presented as means ± SEM (N=4 for each experimental group). Asterisks (*) denote significant (p < 0.05) difference from control. OEP – OxyELITE ProTM – New Formula.
4. Discussion
Despite the growing number of HDS on the U.S. market and their increased usage by consumers, HDS safety assessment remains a significant challenge. Multi-ingredient HDS are complex mixtures of phytochemicals whose combined pharmacological and toxicological effects are often unknown or can differ markedly when compared to their ingestion as single entities. This may result in unanticipated adverse effects, usually not caused by consumption of individual constituents alone. Contributing to the safety challenges of elaborate HDS formulations are a number of quality concerns, including brand-to-brand and lot-to-lot variability in phytochemical content as well as adulteration with prescription medications and contamination with heavy metals or pathogenic microbes (de Boer and Sherker, 2017; Klontz et al., 2015; Navarro et al., 2017; Ronis et al., 2018).
Supplements marketed under the name OEP showcase one of the most recent outbreaks of dietary supplement-associated cardiac and liver toxicity. The first OEP formulation containing DMAA was removed from the market after a number of reports of cardiovascular events as well as acute liver injury in U.S. active duty service members (Foley et al., 2014). However, shortly after introduction of the new formula in which DMAA was substituted with aegeline, an outbreak of acute liver injury cases was reported in Hawaii, followed by numerous cases across the continental U.S. (Heidemann et al., 2016; Johnston et al., 2016; Klontz et al., 2015; Ronis et al., 2018; Roytman et al., 2014). Consequently, OEP-NF was removed from the market on the basis that aegeline was not recognized by the FDA as an NDI. Knowledge regarding the adverse effects caused by aegeline is very limited, although a few studies report hepatotoxicity in rats and cytotoxicity in vitro (Arseculeratne et al., 1985; Mohammed et al., 2016). However, the sequence of events characterized by the toxicity of both OEP formulations suggest that the entire formulation rather than a single ingredient is the likely culprit. Indeed, besides aegeline, a number of other constituents in OEP-NF raises serious safety concerns. Accumulating evidence indicates that caffeine and yohimbine are the two most frequent components of HDS linked to adverse health effects (Brown, 2016). For instance, both were present in Lipokinetix™, a multi-ingredient HDS marketed for weight loss, suspected of causing acute hepatitis and liver failure and ultimately banned by the U.S. FDA (Favreau et al., 2002; Durazo et al., 2004). Although usnic acid present in Lipokinetix™ was believed to be the causative agent, the contributions of caffeine, yohimbine, and other phytochemicals in exacerbating liver injury cannot be ruled out. This is particularly important because caffeine and yohimbine were present in Hydroxycut™, another weight loss product removed from the market following reports of liver injury (Avigan et al., 2006; Fong et al., 2010). In fact, the California Poison Control System identified HDS formulations containing both caffeine and yohimbine as being associated with a greater proportion of adverse health effects (Kearney et al., 2010). Furthermore, the incorporation of yohimbe alkaloids into HDS formulas is prohibited by a number of European countries (Bovee et al., 2016).
It is recognized that obesity can exacerbate the hepatotoxicity of a number of drugs, including methotrexate (Shetty et al., 2017), rosiglitazone (Pan et al., 2005; Ratziu et al., 2010; Rull et al., 2014; Watkins et al., 2002), tamoxifen (Bruno et al., 2005; Elefsiniotis et al., 2004; Saphner et al., 2009), halothane (Eghtesadi-Araghi et al., 2008; Moult et al., 1975), isoflurane (Brunt et al., 1991; Gunaratnam et al., 1995), and benzbromarone (Sun et al., 2018), to name a few. Both clinical and experimental evidence suggests a higher frequency and greater severity of acetaminophen (APAP)-induced liver injury in obese individuals (Aubert et al., 2012; Michaut et al., 2014). Furthermore, overweight and obese conditions are independent risk factors for acute liver injury in acute APAP overdose (Chomchai and Chomchai, 2017). This is especially important because obese consumers frequently turn to HDS that are marketed for weight loss or weight management (Ogden et al., 2012). Moreover, obesity is a risk factor for the development of diabetes, non-alcoholic fatty liver disease (NAFLD) and cardiovascular diseases, each of which can further exacerbate xenobiotic toxicity (Kopelman, 2000; Massart et al., 2017). In the case of OEP-NF, an analysis of the available literature indicates that the majority of consumers who developed severe liver injury were either overweight or obese females (Klontz et al., 2015; Roytman et al., 2014). Therefore, in this study we addressed cardiovascular and liver toxicity in female mice using the obese NZO/HlLtJ model.
We demonstrate that OEP-NF produces significant toxicity in obese NZO/HlLtJ mice. This toxicity was evident at the level of allometrically scaled mouse equivalents to human daily recommended doses. These doses were lower than those at which mortality was observed in other mouse strains (Miousse et al., 2017a). While the lack of significant histomorphological changes in the livers precludes death due to hepatotoxicity, significant increase in the liver/body weight ratio as well as over 5-fold increases in ALT and AST serum levels in a number of mice suggests that this multi-ingredient formulation negatively impacts the liver. In the absence of physical damage to the internal organs due to the gavage or sepsis, the most plausible cause of death could be associated with cardiac arrest or stroke. In this regard, death due to a cumulative overdose of caffeine and/or other alkaloids can be ruled out. Caffeine and other alkaloids typically reach peak blood concentrations within 1–2 hrs after ingestion and the effects, including that characteristic of alkaloid overdose seizures, would be noted at this time-point. In our study, profound lethargy was observed at 8 hrs after the first gavage and no seizures were observed in any animals at any time. Future studies are needed in order to delineate the cause of death in NZO/HlLtJ mice administered OEP-NF.
In this study, we also examined the expression of 84 select genes in NZO/HlLtJ mice that survived OEP-NF administration. Of these genes, only 3 exhibited altered expression (Avpr1a, Lgr5 [reduced] and Skil [increased]) when mice were fed OEP-NF for 2 weeks. Interestingly, expression of these genes was not altered in the sub-chronic (8-week) study nor were they affected by sub-acute and sub-chronic OEP-NF exposure in CD-1 and B6C3F1 mice (Miousse et al., 2017a). This suggests a difference between early and delayed liver responses to OEP-NF. In both mice and rats, lack of the Avpr1a gene is directly linked to increased ethanol intake (Sanbe et al., 2008; Rigter and Crabbe, 1985). In humans, a single nucleotide polymorphism in the AVPR1A gene is associated with severe acetaminophen liver injury (Randesi et al., 2017). Reduced levels of Lgr5, a stem/progenitor cell marker, are observed in necrotizing enterocolitis-induced liver injury (Miyake et al., 2018). Furthermore, knockdown of Lgr5 in mice exacerbates carbon tetrachloride-induced liver injury and worsens fibrosis in mice (Lin et al., 2017b). Therefore, down-regulation of Lgr5 in mice gavaged with OEP-NF is suggestive of a reduced hepatic capacity to respond to injurious agents via stem cell-mediated liver regeneration.
Changes in gene expression among NZO/HlLtJ mice after 8 weeks of OEP-NF ingestion resembled a number of similar trends observed in our previous study with CD-1 and B6C3F1 mice. Specifically, 13 genes were found to be commonly up-regulated in all the 3 strains of mice suggesting a generalized liver response to OEP-NF. Importantly, the magnitude of changes in gene expression among NZO/HlLtJ mice was much higher compared to responses observed in CD-1 and B6C3F1 mice. Specifically, the expression of 9 genes was 3- or more fold higher in mice fed an OEP-NF-containing diet compared to those fed the control diet. For comparison, only 2 genes were similarly dysregulated in CD-1 and B6C3F1 mice, suggesting that obesity imparts a higher responsiveness to OEP-NF. We also identified 6 genes whose dysregulation was unique for NZO/HlLtJ mice – Fabp1, Fads1, Fmo1, Mrps18b, Ppara, and Srebf1.
Several genes affected by OEP-NF exposure, such as Chrebp, Fads1, Fasn, and Srebf1, are involved in lipid metabolism. Chrebp, a transcription factor which plays a central role in glucose metabolism, is a potent inducer of hepatic de novo lipogenesis (Abdul-Wahed et al., 2017). In addition, Chrebp overexpression substantially worsens hepatic steatosis in mice (Benhamed et al., 2012), while inhibition of Chrebp in obese ob/ob mice can reverse hepatic steatosis (Dentin et al., 2006; Iizuka et al., 2006). Coincident with Chrebp, another promoter of hepatic de novo lipogenesis, Fads1, was also found substantially up-regulated in the livers of NZO/HlLtJ mice fed OEP-NF. Recent studies have demonstrated that knocking down Fads1 suppresses hepatic de novo lipogenesis and reduces adiposity (Gromovsky et al., 2018; Powell et al., 2016). These findings clearly indicate a central role for Chrebp and Fads1 in non-alcoholic liver fatty disease and raise serious concerns regarding the ingestion of OEP-NF among overweight/obese consumers whose condition is usually attended with fatty liver. Furthermore, activation of Chrebp appears to be associated with initiation of protective hepatic adaptive responses following exposure to hepatotoxicants (Zhang et al., 2017). Of particular interest is down-regulation of another lipogenic gene, Cd36. This gene was substantially up-regulated by OEP-NF in CD-1 and B6C3F1 mice (Miousse et al., 2017a), and the loss of its expression in NZO/HlLtJ mice suggests that OEP-NF may elicit differential responses in obese individuals.
This study also found that gavaging female NZO/HlLtJ mice with OEP-NF for 2 weeks caused an increase in cardiac protein levels of CD2, an inflammatory cell surface marker. In addition, these animals showed an increase in cardiac levels of proteins with one or more 4-HNE adducts. Although the increase in 4-HNE adducts did not coincide with differences in protein levels of anti-oxidant enzymes SOD2, catalase, or Gpx-1, we cannot exclude that there were changes in the activities of these enzymes.
After 8 weeks, the increase in 4-HNE adducts was no longer seen. On the other hand, ultrasonography denoted a persistent decrease in stroke volume and cardiac output after feeding mice with 3X MED OEP-NF. Similarly, a 10-day administration of caffeine in combination with ephedrine and yohimbine caused a decrease in ejection fraction in obese human subjects (Waluga et al., 1998). A persisting decrease in ejection fraction is one of the main indications of several cardiovascular diseases including heart failure (Hsu et al., 2017).
At 8 weeks, an increase in the number of apoptotic cardiac cells was also observed, independent of the OEP-NF dose. An increase in cardiac cell apoptosis is commonly found in response to prolonged stimulation of the sympathetic nervous system (Dünser et al., 2009; Cetrullo et al., 2011). Although 8 weeks of OEP-NF administration did not cause a change in total cardiac collagen deposition, increases in cardiac mast cell number can be indicative of active extracellular matrix remodeling in the heart (Boerma et al., 2004). Taken together, these studies indicate that prolonged intake of the alkaloid combination present in OEP-NF can have adverse effects on cardiac structure and function in an obese mouse model.
Similar to our earlier study in CD-1 and B6C3F1 mice, no significantly lasting weight loss effect was observed in NZO/HlLtJ mice receiving daily doses of OEP-NF allometrically equivalent to those recommended for humans. While slower dynamics of body weight gain compared to controls was observed in mice fed 3X MED OEP-NF, the physiological nature of this effect is questionable since it was associated with both cardio- and hepatotoxicity.
In conclusion, our study demonstrates that OEP-NF possesses significant hepato- and cardiotoxicity in NZO/HlLtJ mouse model. The degree of toxicity observed in this obese mouse strain was higher than that observed in non-obese strains, suggesting that an overweight/obese condition can sensitize mice to OEP-NF. Taken together, these findings plus those noted earlier in CD-1 and B6C3F1 mice lend credence to the FDA’s removal of OEP-NF from the US market. Moreover, recent adverse health effects linked to OEP-NF and other HDS marketed for weight loss and/or exercise performance enhancement argue strongly for introduction of quality standards and pre-marketing safety assessments for multi-ingredient HDS.
Supplementary Material
Highlights.
Dietary supplement OxyELITE Pro™ - New Formula (OEP-NF) was linked to severe liver injuries in the United States
Toxicity of OEP-NF was assessed using a novel NZO/HlLtJ obese mouse model
OEP-NF produced histological, biochemical and molecular alterations that are characteristic to liver and cardiac injury
OEP-NF-induced weight loss was transitory, followed by body weight gain in all experimental groups
These findings bolster concerns for the safety and efficacy of OEP-NF, and similar products, in humans
Acknowledgements and Disclaimer
This work was supported by the United States Department of Justice [contract # 6L-CIV02–0850], National Institute of General Medical Sciences [grant # P20 GM109005], and Arkansas Bioscience Institute. The authors are thankful to Dr. Christy Simecka, Robin Mulkey, and Bridgette Engi for excellent animal care at the UAMS Animal Facility and to Dr. Stanley Kosanke at Heartland Veterinary Pathology Services PLLC (Edmond, OK) for histopathological evaluation. The authors are also thankful to Dr. Ramesh Khanal of Envigo for his invaluable help and expertise in diet preparation, and to Julia Tobacyk for technical assistance. M.B., B.J.G and I.K. (UAMS) are serving as expert witnesses for the United States Department of Justice.
Abbreviations:
- ALT
alanine aminotransferase
- APAP
acetaminophen
- AST
aspartate aminotransferase
- DILI
drug-induced liver injury
- DILIN
Drug-Induced Liver Injury Network
- DMAA
1,3-dimethylamylamine
- DMSO
dimethyl sulfoxide
- HDS
herbal and dietary supplements
- HILI
herbal-induced liver injury
- MED
mouse equivalent dose
- miRNA
microRNA
- NDI
New Dietary Ingredient
- OEP-NF
OxyELITE Pro New Formula
- qRTPCR
quantitative real-time polymerase chain reaction
- TLDA
TaqMan Low Density Arrays
- UPLC
ultra-high performance liquid chromatography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdul-Wahed A, Guilmeau S, Postic C. Sweet Sixteenth for ChREBP: Established Roles and Future Goals. Cell Metab. 2017. August 1;26(2):324–341. doi: 10.1016/j.cmet.2017.07.004 [DOI] [PubMed] [Google Scholar]
- Arseculeratne SN, Gunatilaka AA, Panabokke RG. Studies of medicinal plants of Sri Lanka. Part 14: Toxicity of some traditional medicinal herbs. J Ethnopharmacol. 1985. July;13(3):323–35. [DOI] [PubMed] [Google Scholar]
- Aubert J, Begriche K, Delannoy M, Morel I, Pajaud J, Ribault C, Lepage S, McGill MR, Lucas-Clerc C, Turlin B, Robin MA, Jaeschke H, Fromenty B. Differences in early acetaminophen hepatotoxicity between obese ob/ob and db/db mice. J Pharmacol Exp Ther. 2012. September;342(3):676–87. doi: 10.1124/jpet.112.193813. [DOI] [PubMed] [Google Scholar]
- Avigan MI, Mozersky RP, Seeff LB. Scientific and Regulatory Perspectives in Herbal and Dietary Supplement Associated Hepatotoxicity in the United States. Int J Mol Sci 2016. March 3;17(3):331. doi: 10.3390/ijms17030331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avula B, Chittiboyina AG, Wang YH, Sagi S, Raman V, Wang M, Khan IA. Simultaneous Determination of Aegeline and Six Coumarins from Different Parts of the Plant Aegle marmelos Using UHPLC-PDA-MS and Chiral Separation of Aegeline Enantiomers Using HPLC-ToF-MS. Planta Med. 2016. April;82(6):580–8. doi: 10.1055/s-0042-103160. [DOI] [PubMed] [Google Scholar]
- Benhamed F, Denechaud PD, Lemoine M, Robichon C, Moldes M, Bertrand-Michel J, Ratziu V, Serfaty L, Housset C, Capeau J, Girard J, Guillou H, Postic C. The lipogenic transcription factor ChREBP dissociates hepatic steatosis from insulin resistance in mice and humans. J Clin Invest. 2012. June;122(6):2176–94. doi: 10.1172/JCI41636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomer RJ, Schriefer JM, Gunnels TA. Clinical safety assessment of oral higenamine supplementation in healthy, young men. Hum Exp Toxicol 2015. October;34(10):935–45. doi: 10.1177/0960327114565490. [DOI] [PubMed] [Google Scholar]
- Boerma M, Zurcher C, Esveldt I, Schutte-Bart CI, Wondergem J. Histopathology of ventricles, coronary arteries and mast cell accumulation in transverse and longitudinal sections of the rat heart after irradiation. Oncol Rep. 2004. August;12(2):213–9. [DOI] [PubMed] [Google Scholar]
- Bovee TF, Mol HG, Bienenmann-Ploum ME, Heskamp HH, Van Bruchem GD, Van Ginkel LA, Kooijman M, Lasaroms JJ, Van Dam R, Hoogenboom RL. Dietary supplement for energy and reduced appetite containing the β-agonist isopropyloctopamine leads to heart problems and hospitalisations. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2016. May;33(5):749–59. doi: 10.1080/19440049.2016.1167965. [DOI] [PubMed] [Google Scholar]
- Brown CE, Trauth SE, Grippo RS, Gurley BJ, Grippo AA. Combined Effects of Ephedrine-Containing Dietary Supplements, Caffeine, and Nicotine on Morphology and Ultrastructure of Rat Hearts. J Caffeine Res 2012. September;2(3):123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AC. An overview of herb and dietary supplement efficacy, safety and government regulations in the United States with suggested improvements. Part 1 of 5 series. Food Chem Toxicol. 2017. September;107(Pt A):449–471. doi: 10.1016/j.fct.2016.11.001. [DOI] [PubMed] [Google Scholar]
- Brown AC. Liver toxicity related to herbs and dietary supplements: Online table of case reports. Part 2 of 5 series. Food Chem Toxicol. 2017a. September;107(Pt A):472–501. doi: 10.1016/j.fct.2016.07.001. [DOI] [PubMed] [Google Scholar]
- Brown AC. Kidney toxicity related to herbs and dietary supplements: Online table of case reports. Part 3 of 5 series. Food Chem Toxicol. 2017b. September;107(Pt A):502–519. doi: 10.1016/j.fct.2016.07.024. [DOI] [PubMed] [Google Scholar]
- Brown AC. Heart Toxicity Related to Herbs and Dietary Supplements: Online Table of Case Reports. Part 4 of 5. J Diet Suppl 2017c. October 5:1–40. doi: 10.1080/19390211.2017.1356418. [DOI] [PubMed] [Google Scholar]
- Bruno S, Maisonneuve P, Castellana P, Rotmensz N, Rossi S, Maggioni M, Persico M, Colombo A, Monasterolo F, Casadei-Giunchi D, Desiderio F, Stroffolini T, Sacchini V, Decensi A, Veronesi U. Incidence and risk factors for non-alcoholic steatohepatitis: prospective study of 5408 women enrolled in Italian tamoxifen chemoprevention trial. BMJ. 2005. April 23;330(7497):932. doi: 10.1136/bmj.38391.663287.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunt EM, White H, Marsh JW, Holtmann B, Peters MG. Fulminant hepatic failure after repeated exposure to isoflurane anesthesia: a case report. Hepatology. 1991. June;13(6):1017–21. [PubMed] [Google Scholar]
- Calvert R, Vohra S, Ferguson M, Wiesenfeld P. A beating heart cell model to predict cardiotoxicity: effects of the dietary supplement ingredients higenamine, phenylethylamine, ephedrine and caffeine. Food Chem Toxicol. 2015. April;78:207–13. doi: 10.1016/j.fct.2015.01.022. [DOI] [PubMed] [Google Scholar]
- Cetrullo S, Tantini B, Facchini A, Pignatti C, Stefanelli C, Caldarera CM, Flamigni F. A pro-survival effect of polyamine depletion on norepinephrine-mediated apoptosis in cardiac cells: role of signaling enzymes. Amino Acids. 2011. April;40(4):1127–37. doi: 10.1007/s00726-010-0736-7. [DOI] [PubMed] [Google Scholar]
- Cho JH, Oh DS, Hong SH, Ko H, Lee NH, Park SE, Han CW, Kim SM, Kim YC, Kim KS, Choi CW, Shin SM, Kim KT, Choi HS, Lee JH, Kim JY, Kang JY, Lee DS, Ahn YC, Son CG. A nationwide study of the incidence rate of herb-induced liver injury in Korea. Arch Toxicol. 2017. December;91(12):4009–4015. doi: 10.1007/s00204-017-2007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomchai S, Chomchai C. Being overweight or obese as a risk factor for acute liver injury secondary to acute acetaminophen overdose. Pharmacoepidemiol Drug Saf. 2018. January;27(1):19–24. doi: 10.1002/pds.4339. [DOI] [PubMed] [Google Scholar]
- Damiano S, Puzio MV, Squillacioti C, Mirabella N, Zona E, Mancini A, Borrelli A, Astarita C, Boffo S, Giordano A, Avallone L, Florio S, Ciarcia R. Effect of rMnSOD on Sodium Reabsorption in Renal Proximal Tubule in Ochratoxin A-Treated Rats. J Cell Biochem. 2018. January;119(1):424–430. doi: 10.1002/jcb.26197. Epub 2017 Jul 7. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- de Boer YS, Sherker AH. Herbal and Dietary Supplement-Induced Liver Injury. Clin Liver Dis. 2017. February;21(1):135–149. doi: 10.1016/j.cld.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dentin R, Benhamed F, Hainault I, Fauveau V, Foufelle F, Dyck JR, Girard J, Postic C. Liver-specific inhibition of ChREBP improves hepatic steatosis and insulin resistance in ob/ob mice. Diabetes. 2006. August;55(8):2159–70. doi: 10.2337/db06-0200 [DOI] [PubMed] [Google Scholar]
- Dunnick JK, Kissling G, Gerken DK, Vallant MA, Nyska A. Cardiotoxicity of Ma Huang/caffeine or ephedrine/caffeine in a rodent model system. Toxicol Pathol 2007. August;35(5):657–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dünser MW, Hasibeder WR. Sympathetic overstimulation during critical illness: adverse effects of adrenergic stress. J Intensive Care Med 2009. Sep-Oct;24(5):293–316. doi: 10.1177/0885066609340519. [DOI] [PubMed] [Google Scholar]
- Durazo FA, Lassman C, Han SH, Saab S, Lee NP, Kawano M, Saggi B, Gordon S, Farmer DG, Yersiz H, Goldstein RL, Ghobrial M, Busuttil RW. Fulminant liver failure due to usnic acid for weight loss. Am J Gastroenterol. 2004. May;99(5):950–2. doi: 10.1111/j.1572-0241.2004.04165.x [DOI] [PubMed] [Google Scholar]
- Eghtesadi-Araghi P, Sohrabpour A, Vahedi H, Saberi-Firoozi M. Halothane hepatitis in Iran: a review of 59 cases. World J Gastroenterol. 2008. September 14;14(34):5322–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elefsiniotis IS, Pantazis KD, Ilias A, Pallis L, Mariolis A, Glynou I, Kada H, Moulakakis A. Tamoxifen induced hepatotoxicity in breast cancer patients with pre-existing liver steatosis: the role of glucose intolerance. Eur J Gastroenterol Hepatol. 2004. June;16(6):593–8. [DOI] [PubMed] [Google Scholar]
- Farney TM, McCarthy CG, Canale RE, Allman RJ Jr, Bloomer RJ. Hemodynamic and hematologic profile of healthy adults ingesting dietary supplements containing 1,3-dimethylamylamine and caffeine. Nutr Metab Insights 2011. December 6;5:1–12. doi: 10.4137/NMI.S8568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favreau JT, Ryu ML, Braunstein G, Orshansky G, Park SS, Coody GL, Love LA, Fong TL. Severe hepatotoxicity associated with the dietary supplement LipoKinetix. Ann Intern Med. 2002. April 16;136(8):590–5. [DOI] [PubMed] [Google Scholar]
- Foley S, Butlin E, Shields W, Lacey B. Experience with OxyELITE pro and acute liver injury in active duty service members. Dig Dis Sci. 2014. December;59(12):3117–21. doi: 10.1007/s10620-014-3221-4. [DOI] [PubMed] [Google Scholar]
- Fong TL, Klontz KC, Canas-Coto A, Casper SJ, Durazo FA, Davern TJ 2nd, Hayashi P, Lee WM, Seeff LB. Hepatotoxicity due to hydroxycut: a case series. Am J Gastroenterol. 2010. July;105(7):1561–6. doi: 10.1038/ajg.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester M Exposures to 1,3-dimethylamylamine-containing products reported to Texas poison centers. Hum Exp Toxicol 2013. January;32(1):18–23. doi: 10.1177/0960327112454895. [DOI] [PubMed] [Google Scholar]
- Gromovsky AD, Schugar RC, Brown AL, Helsley RN, Burrows AC, Ferguson D, Zhang R, Sansbury BE, Lee RG, Morton RE, Allende DS, Parks JS, Spite M, Brown JM. Δ−5 Fatty Acid Desaturase FADS1 Impacts Metabolic Disease by Balancing Proinflammatory and Proresolving Lipid Mediators. Arterioscler Thromb Vasc Biol. 2018. January;38(1):218–231. doi: 10.1161/ATVBAHA.117.309660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaratnam NT, Benson J, Gandolfi AJ, Chen M. Suspected isoflurane hepatitis in an obese patient with a history of halothane hepatitis. Anesthesiology. 1995. December;83(6):1361–4. [DOI] [PubMed] [Google Scholar]
- Johnston DI, Chang A, Viray M, Chatham-Stephens K, He H, Taylor E, Wong LL, Schier J, Martin C, Fabricant D, Salter M, Lewis L, Park SY. Hepatotoxicity associated with the dietary supplement OxyELITE Pro™ - Hawaii, 2013. Drug Test Anal 2016. Mar-Apr;8(3–4):319–27. doi: 10.1002/dta.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidemann LA, Navarro VJ, Ahmad J, Hayashi PH, Stolz A, Kleiner DE, Fontana RJ. Severe Acute Hepatocellular Injury Attributed to OxyELITE Pro: A Case Series. Dig Dis Sci. 2016. September;61(9):2741–8. doi: 10.1007/s10620-016-4181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu JJ, Ziaeian B, Fonarow GC. Heart Failure With Mid-Range (Borderline) Ejection Fraction: Clinical Implications and Future Directions. JACC Heart Fail. 2017. November;5(11):763–771. doi: 10.1016/j.jchf.2017.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hucker HB, Ahmad PM, Miller EA, Brobyn R. Metabolism of dimethyl sulphoxide to dimethyl sulphone in the rat and man. Nature. 1966. February 5;209(5023):619–20. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- Iizuka K, Miller B, Uyeda K. Deficiency of carbohydrate-activated transcription factor ChREBP prevents obesity and improves plasma glucose control in leptin-deficient (ob/ob) mice. Am J Physiol Endocrinol Metab. 2006. August;291(2):E358–64. doi: 10.1152/ajpendo.00027.2006 [DOI] [PubMed] [Google Scholar]
- Kearney T, Tu N, Haller C. Adverse drug events associated with yohimbine-containing products: a retrospective review of the California Poison Control System reported cases. Ann Pharmacother. 2010. June;44(6):1022–9. doi: 10.1345/aph.1P060. [DOI] [PubMed] [Google Scholar]
- Kimura I, Chui LH, Fujitani K, Kikuchi T, Kimura M. Inotropic effects of (+/−)-higenamine and its chemically related components, (+)-R-coclaurine and (+)-S-reticuline, contained in the traditional sino-Japanese medicines “bushi” and “shin-i” in isolated guinea pig papillary muscle. Jpn J Pharmacol. 1989. May;50(1):75–8. [DOI] [PubMed] [Google Scholar]
- Klontz KC, DeBeck HJ, LeBlanc P, Mogen KM, Wolpert BJ, Sabo JL, Salter M, Seelman SL, Lance SE, Monahan C, Steigman DS, Gensheimer K. The Role of Adverse Event Reporting in the FDA Response to a Multistate Outbreak of Liver Disease Associated with a Dietary Supplement. Public Health Rep. 2015. Sep-Oct;130(5):526–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiyama H, Ohkuma H, Kawaguchi H, Hsu HY, Chen YP. Isolation of 1-(p-hydroxybenzyl)-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline (demethylcoclaurine), an active alkaloid from Nelumbo nucifera. Chem Pharm Bull. 1970, 18 (12), 2564–8. [Google Scholar]
- Kopelman PG. Obesity as a medical problem. Nature. 2000. April 6;404(6778):635–43. [DOI] [PubMed] [Google Scholar]
- Lee YS, Kang YJ, Kim HJ, Park MK, Seo HG, Lee JH, Yun-Choi HS, Chang KC. Higenamine reduces apoptotic cell death by induction of heme oxygenase-1 in rat myocardial ischemiareperfusion injury. Apoptosis. 2006. July;11(7):1091–100. [DOI] [PubMed] [Google Scholar]
- Lee SR, Schriefer JM, Gunnels TA, Harvey IC, Bloomer RJ. Acute oral intake of a higenamine-based dietary supplement increases circulating free fatty acids and energy expenditure in human subjects. Lipids Health Dis. 2013. October 21;12:148. doi: 10.1186/1476-511X-12-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Ewing LE, Koturbash I, Gurley BJ, Miousse IR. MicroRNAs as biomarkers for liver injury: Current knowledge, challenges and future prospects. Food Chem Toxicol. 2017b. December;110:229–239. doi: 10.1016/j.fct.2017.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Fang ZP, Liu HJ, Wang LJ, Cheng Z, Tang N, Li T, Liu T, Han HX, Cao G, Liang L, Ding YQ, Zhou WJ. HGF/R-spondin1 rescues liver dysfunction through the induction of Lgr5(+) liver stem cells. Nat Commun. 2017b. October 27;8(1):1175. doi: 10.1038/s41467-017-01341-6. PubMed PMID: ; PubMed Central PMCID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001. December;25(4):402–8. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Massart J, Begriche K, Moreau C, Fromenty B. Role of nonalcoholic fatty liver disease as risk factor for drug-induced hepatotoxicity. J Clin Transl Res. 2017. February;3(Suppl 1):212–232. doi: 10.18053/jctres.03.2017S1.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Caliz I, Garcia-Cortes M, Gonzalez-Jimenez A, Cabello MR, Robles-Diaz M, Sanabria-Cabrera J, Sanjuan-Jimenez R, Ortega-Alonso A, García-Muñoz B, Moreno I, Jimenez-Perez M, Fernandez MC, Ginés P, Prieto M, Conde I, Hallal H, Soriano G, Roman E, Castiella A, Blanco-Reina E, Montes MR, Quiros-Cano M, Martin-Reyes F, Lucena MI, Andrade RJ; Spanish DILI Registry. Herbal and Dietary Supplement-induced Liver Injuries in the Spanish DILI Registry. Clin Gastroenterol Hepatol. 2018. January 4 pii: S1542–3565(18)30010–7. doi: 10.1016/j.cgh.2017.12.051. [DOI] [PubMed] [Google Scholar]
- Michaut A, Moreau C, Robin MA, Fromenty B. Acetaminophen-induced liver injury in obesity and nonalcoholic fatty liver disease. Liver Int. 2014. August;34(7):e171–9. doi: 10.1111/liv.12514. [DOI] [PubMed] [Google Scholar]
- Miousse IR, Skinner CM, Lin H, Ewing LE, Kosanke SD, Williams DK, Avula B, Khan IA, ElSohly MA, Gurley BJ, Koturbash I. Safety assessment of the dietary supplement OxyELITE™ Pro (New Formula) in inbred and outbred mouse strains. Food Chem Toxicol. 2017a. November;109(Pt 1):194–209. doi: 10.1016/j.fct.2017.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miousse IR, Murphy LA, Lin H, Schisler MR, Sun J, Chalbot MG, Sura R, Johnson K, LeBaron MJ, Kavouras IG, Schnackenberg LK, Beger RD, Rasoulpour RJ, Koturbash I. Dose-response analysis of epigenetic, metabolic, and apical endpoints after short-term exposure to experimental hepatotoxicants. Food Chem Toxicol. 2017b. November;109(Pt 1):690–702. doi: 10.1016/j.fct.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake H, Li B, Lee C, Koike Y, Chen Y, Seo S, Pierro A. Liver damage, proliferation, and progenitor cell markers in experimental necrotizing enterocolitis. J Pediatr Surg. 2018. February 7 pii: S0022–3468(18)30060–5. doi: 10.1016/j.jpedsurg.2018.02.006. [DOI] [PubMed] [Google Scholar]
- Mohammed MM, Ibrahim NA, El-Sakhawy FS, Mohamed KM, Deabes DA. Two new cytotoxic furoquinoline alkaloids isolated from Aegle marmelos (Linn.) Correa. Nat Prod Res 2016. January 4:1–8. doi: 10.1080/14786419.2015.1126262. [DOI] [PubMed] [Google Scholar]
- Moult PJ, Sherlock S. Halothane-related hepatitis. A clinical study of twenty-six cases. Q J Med. 1975. January;44(173):99–114. [PubMed] [Google Scholar]
- Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 2016. March;7(2):27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narender T, Shweta S, Tiwari P, Papi Reddy K, Khaliq T, Prathipati P, Puri A, Srivastava AK, Chander R, Agarwal SC, Raj K. Antihyperglycemic and antidyslipidemic agent from Aegle marmelos. Bioorg Med Chem Lett. 2007. March 15;17(6):1808–11. [DOI] [PubMed] [Google Scholar]
- Navarro VJ, Barnhart H, Bonkovsky HL, Davern T, Fontana RJ, Grant L, Reddy KR, Seeff LB, Serrano J, Sherker AH, Stolz A, Talwalkar J, Vega M, Vuppalanchi R. Liver injury from herbals and dietary supplements in the U.S. Drug-Induced Liver Injury Network. Hepatology. 2014. October;60(4):1399–408. doi: 10.1002/hep.27317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro VJ, Khan I, Björnsson E, Seeff LB, Serrano J, Hoofnagle JH. Liver injury from herbal and dietary supplements. Hepatology. 2017. January;65(1):363–373. doi: 10.1002/hep.28813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehlig A, Daval JL, Debry G. Caffeine and the central nervous system: mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res Brain Res Rev. 1992. May-Aug;17(2):139–70. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States. NCHS Data Brief No. 82, January 2012. [PubMed]
- Pan HJ, Reifsnyder P, Vance DE, Xiao Q, Leiter EH. Pharmacogenetic analysis of rosiglitazone-induced hepatosteatosis in new mouse models of type 2 diabetes. Diabetes. 2005. June;54(6):1854–62. [DOI] [PubMed] [Google Scholar]
- Powell DR, Gay JP, Smith M, Wilganowski N, Harris A, Holland A, Reyes M, Kirkham L, Kirkpatrick LL, Zambrowicz B, Hansen G, Platt KA, van Sligtenhorst I, Ding ZM, Desai U. Fatty acid desaturase 1 knockout mice are lean with improved glycemic control and decreased development of atheromatous plaque. Diabetes Metab Syndr Obes. 2016. June 22;9:185–99. doi: 10.2147/DMSO.S106653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randesi M, Levran O, Correa da Rosa J, Hankins J, Rule J, Kreek MJ, Lee WM; Acute Liver Failure Study Group. Association of Variants of Arginine Vasopressin and Arginine Vasopressin Receptor 1A With Severe Acetaminophen Liver Injury. Cell Mol Gastroenterol Hepatol 2017. January 24;3(3):500–505. doi: 10.1016/j.jcmgh.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratziu V, Charlotte F, Bernhardt C, Giral P, Halbron M, Lenaour G, Hartmann-Heurtier A, Bruckert E, Poynard T; LIDO Study Group. Long-term efficacy of rosiglitazone in nonalcoholic steatohepatitis: results of the fatty liver improvement by rosiglitazone therapy (FLIRT 2) extension trial. Hepatology. 2010. February;51(2):445–53. doi: 10.1002/hep.23270. [DOI] [PubMed] [Google Scholar]
- Rigter H, Crabbe JC. Vasopressin and ethanol preference. I. Effects of vasopressin and the fragment DGAVP on altered ethanol preference in Brattleboro diabetes insipidus rats. Peptides. 1985. Jul-Aug;6(4):669–76. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- Ronis MJJ, Pedersen KB, Watt J. Adverse Effects of Nutraceuticals and Dietary Supplements. Annu Rev Pharmacol Toxicol. 2018. January 6;58:583–601. doi: 10.1146/annurev-pharmtox-010617-052844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roytman MM, Pörzgen P, Lee CL, Huddleston L, Kuo TT, Bryant-Greenwood P, Wong LL, Tsai N. Outbreak of severe hepatitis linked to weight-loss supplement OxyELITE Pro. Am J Gastroenterol. 2014. August;109(8):1296–8. doi: 10.1038/ajg.2014.159. [DOI] [PubMed] [Google Scholar]
- Rull A, Geeraert B, Aragonès G, Beltrán-Debón R, Rodríguez-Gallego E, García-Heredia A, Pedro-Botet J, Joven J, Holvoet P, Camps J. Rosiglitazone and fenofibrate exacerbate liver steatosis in a mouse model of obesity and hyperlipidemia. A transcriptomic and metabolomic study. J Proteome Res. 2014. March 7;13(3):1731–43. doi: 10.1021/pr401230s. [DOI] [PubMed] [Google Scholar]
- Sanbe A, Takagi N, Fujiwara Y, Yamauchi J, Endo T, Mizutani R, Takeo S, Tsujimoto G, Tanoue A. Alcohol preference in mice lacking the Avpr1a vasopressin receptor. Am J Physiol Regul Integr Comp Physiol. 2008. May;294(5):R1482–90. doi: 10.1152/ajpregu.00708.2007. [DOI] [PubMed] [Google Scholar]
- Saphner T, Triest-Robertson S, Li H, Holzman P. The association of nonalcoholic steatohepatitis and tamoxifen in patients with breast cancer. Cancer. 2009. July 15;115(14):3189–95. doi: 10.1002/cncr.24374. [DOI] [PubMed] [Google Scholar]
- Schilter B, Andersson C, Anton R, Constable A, Kleiner J, O’Brien J, Renwick AG, Korver O, Smit F, Walker R; Natural Toxin Task Force of the European Branch of the International Life Sciences Institute. Guidance for the safety assessment of botanicals and botanical preparations for use in food and food supplements. Food Chem Toxicol. 2003. December;41(12):1625–49. [DOI] [PubMed] [Google Scholar]
- Shetty A, Cho W, Alazawi W, Syn WK. Methotrexate Hepatotoxicity and the Impact of Nonalcoholic Fatty Liver Disease. Am J Med Sci. 2017. August;354(2):172–181. doi: 10.1016/j.amjms.2017.03.014. [DOI] [PubMed] [Google Scholar]
- Somanathan R, Aguilar HR, Ventura GR, Smith KM. Syntheses of natural hydroxyamides using trimethylsilyl cyanide. Synth Commun. 1983, 13 (4), 273–80. [Google Scholar]
- Sun P, Zhu JJ, Wang T, Huang Q, Zhou YR, Yu BW, Jiang HL, Wang HY. Benzbromarone aggravates hepatic steatosis in obese individuals. Biochim Biophys Acta. 2018. June;1864(6 Pt A):2067–2077. doi: 10.1016/j.bbadis.2018.03.009. [DOI] [PubMed] [Google Scholar]
- Temple JL, Bernard C, Lipshultz SE, Czachor JD, Westphal JA, Mestre MA. The Safety of Ingested Caffeine: A Comprehensive Review. Front Psychiatry 2017. May 26;8:80. doi: 10.3389/fpsyt.2017.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Jackson Laboratory, Mouse strain datasheet – 002105. https://www.jax.org/strain/002105 (accessed 18 September 2018).
- US Food and Drug Administration, Final rule declaring dietary supplements containing ephedrine alkaloids adulterated because they present an unreasonable risk. Docket number: 1995N-0304. 2004 [PubMed]
- Venhuis B, Keizers P, van Riel A, de Kaste D. A cocktail of synthetic stimulants found in a dietary supplement associated with serious adverse events. Drug Test Anal 2014. June;6(6):578–81. doi: 10.1002/dta.1664. [DOI] [PubMed] [Google Scholar]
- Waluga M, Janusz M, Karpel E, Hartleb M, Nowak A. Cardiovascular effects of ephedrine, caffeine and yohimbine measured by thoracic electrical bioimpedance in obese women. Clin Physiol 1998. January;18(1):69–76. [DOI] [PubMed] [Google Scholar]
- Wang Y, Yu X, Wang F, Wang Y, Wang Y, Li H, Lv X, Lu D, Wang H. Yohimbine promotes cardiac NE release and prevents LPS-induced cardiac dysfunction via blockade of presynaptic α2A-adrenergic receptor. PLoS One. 2013. May14;8(5):e63622. doi: 10.1371/journal.pone.0063622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins SM, Reifsnyder PR, Pan HJ, German JB, Leiter EH. Lipid metabolome-wide effects of the PPARgamma agonist rosiglitazone. J Lipid Res. 2002. November;43(11):1809–17. [DOI] [PubMed] [Google Scholar]
- Wojcikowski K, Gobe G. Animal studies on medicinal herbs: predictability, dose conversion and potential value. Phytother Res 2014. January;28(1):22–7. doi: 10.1002/ptr.4966. [DOI] [PubMed] [Google Scholar]
- Wong KK, Lo CF, Chen CM. Endothelium-dependent higenamine-induced aortic relaxation in isolated rat aorta. Planta Med. 1997. April;63(2):130–2. [DOI] [PubMed] [Google Scholar]
- Wu MP, Zhang YS, Zhou QM, Xiong J, Dong YR, Yan C. Higenamine protects ischemia/reperfusion induced cardiac injury and myocyte apoptosis through activation of β2-AR/PI3K/AKT signaling pathway. Pharmacol Res. 2016. February;104:115–23. doi: 10.1016/j.phrs.2015.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu GR, Wang BY, Zheng DS, Huang MX, Gu BK, Chen YL, Shang M, Jin ZJ, Cheng TO. Effect of higenamine on action potential of ventricular myocardial cells. J Electrocardiol. 1985. Jan;18(1):97–103. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- Zaretsky DV, Zaretskaia MV, DiMicco JA, Rusyniak DE. Yohimbine is a 5-HT1A agonist in rats in doses exceeding 1 mg/kg. Neurosci Lett. 2015. October 8;606:215–9. doi: 10.1016/j.neulet.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Tong X, VanDommelen K, Gupta N, Stamper K, Brady GF, Meng Z, Lin J, Rui L, Omary MB, Yin L. Lipogenic transcription factor ChREBP mediates fructose-induced metabolic adaptations to prevent hepatotoxicity. J Clin Invest. 2017. June 30;127(7):2855–2867. doi: 10.1172/JCI89934. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.