Abstract
Purpose
To study deep gray matter susceptibility in multiple sclerosis (MS) by using quantitative susceptibility mapping (QSM) and to assess the relationship between susceptibility and clinical disability.
Materials and Methods
For this prospective study between March 2009 and November 2013, 600 participants with MS (452 with relapsing-remitting MS and 148 with secondary progressive MS) and 250 age- and sex-matched healthy control participants were imaged with 3.0-T MRI to measure magnetic susceptibility. Deep gray matter susceptibility (in parts per billion) was analyzed by using region of interest and voxelwise methods. QSM and MRI volumetric differences between study groups and associations with clinical outcomes were assessed. Analysis of covariance, multivariable linear regression, and voxelwise analyses, controlling for age and sex, were used to compare study groups and to explore associations between MRI and clinical outcomes.
Results
Compared with control participants, participants with MS presented with lower thalamic susceptibility (−7.5 ppb vs −1.1 ppb; P < .001) and higher susceptibility of basal ganglia (62 ppb vs 54.8 ppb; P < .001). Lower thalamic susceptibility was associated with longer disease duration (β = −0.42; P = .002), higher degree of disability (β = −0.64; P = .03), and secondary-progressive course (β = −4.3; P = .009). Higher susceptibility of the globus pallidus was associated with higher disability (β = 2; P = .03). After correcting for each individual structural volume in voxelwise analysis, lower thalamic susceptibility and higher susceptibility of the globus pallidus remained associated with clinical disability (P < .05).
Conclusion
Quantitative susceptibility mapping (QSM) suggests that altered deep gray matter iron is associated with the evolution of multiple sclerosis (MS) and on disability accrual, independent of tissue atrophy.
© RSNA, 2018
See also the editorial by Barkhof and Thomas.
Introduction
The pathogenesis of multiple sclerosis (MS) is multifactorial and involves both genetic and environmental factors, many of which are still a matter of ongoing research. A growing body of literature has investigated the role of iron and accumulation of white matter and gray matter damage in MS (1–3). It has yet to be elucidated, however, whether local changes in brain tissue iron concentrations are a causal factor in neurodegeneration or an epiphenomenon of cell death (4). Histopathologic and MRI data have consistently shown profound changes in iron concentration in all the central nervous system compartments, with reduced iron content in newly forming white matter lesions, cortical lesions, thalamus and normal-appearing white matter (2,4) and with higher iron content in the rim of chronic active lesions (5) and in some structures of the basal ganglia (6–8).
The recent availability of quantitative MRI techniques, such as quantitative susceptibility mapping (QSM), has facilitated iron quantification in vivo (9–11) globally as well as in specific regions of interest, such as the basal ganglia and the thalamus (4,6,7,12,13). QSM has been shown to be exquisitely sensitive to paramagnetic and diamagnetic tissue components and can depict specific subcortical gray matter structures with an excellent level of anatomic detail. Although literature data consistently show higher iron content in other deep gray matter structures (6,12–14), results are more contradictory with respect to the thalamus. Some studies show higher iron content (6,13), whereas others report lower iron load (4,7,8,15,16). However, comparisons between different studies are challenging because of the use of different MRI acquisition and postprocessing techniques, as well as the strong association between aging and brain iron levels (4,17).
Against this background, we aimed to better characterize the susceptibility of the thalamus and of other deep gray matter structures (caudate, putamen, globus pallidus [GP]), applying both regions of interest–based analysis and voxelwise analysis of QSM data to a large cohort of participants with relapsing-remitting MS and secondary progressive MS, and to explore the relationship between susceptibility and clinical disability. We hypothesized we would find higher magnetic susceptibility in the deep gray matter structures because MS leads to demyelination and atrophy. Furthermore, because thalamic atrophy occurs early in the course of MS, we expected to find higher magnetic susceptibility because of higher iron content in the thalamic tissue.
Materials and Methods
Participants
This prospective study enrolled participants between March 2009 and November 2013. The study was approved by our institutional review board; written informed consent and Health Insurance Portability and Accountability Act approval were obtained from all participants.
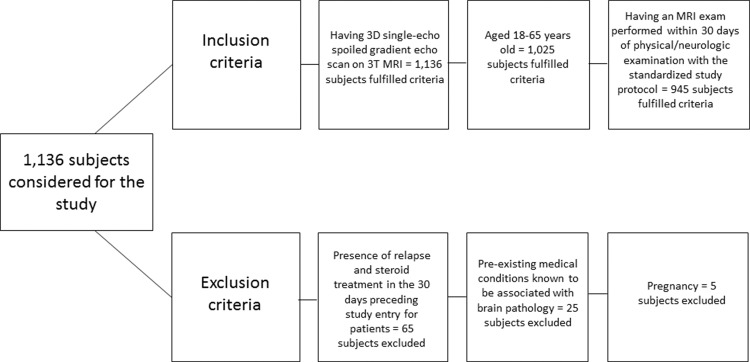
The inclusion criteria for this study were as follows: age between 18 and 65 years, undergoing three-dimensional single-echo spoiled gradient-echo sequence imaging on the same 3.0-T imager with standardized study protocol, and undergoing an MRI examination within 30 days of physical and/or neurologic examination. Exclusion criteria were as follows: presence of relapse and steroid treatment in the 30 days preceding study entry for participants with clinically isolated syndrome and MS, preexisting medical conditions known to be associated with brain pathologic processes (cerebrovascular disease, positive history of alcohol abuse), and pregnancy. In total, 600 participants with MS (452 with relapsing-remitting MS and 148 with secondary progressive MS) and 250 age- and sex-matched healthy control participants fulfilled inclusion and exclusion criteria (Fig 1). The mean age for participants with MS was 44.9 years (age range, 18–65 years) and for healthy control participants was 45 years (age range, 18–65 years). No significant age differences were found between men and women in the examined study groups.
Figure 1:
Flowchart shows inclusion and exclusion criteria. 3D = three-dimensional.
Clinical disability in participants was quantified by using the Expanded Disability Status Scale and disease course of MS was classified according to the revised McDonald criteria (18).
MR Image Acquisition
All images were acquired on the same 3.0-T Signa Excite HD 12.0 unit (General Electric, Milwaukee, Wis) with an eight-channel head and neck coil. The imaging system did not undergo any major hardware or software upgrades during the study. Data for QSM were acquired by using an unaccelerated three-dimensional single-echo spoiled gradient-recalled-echo sequence with first-order flow compensation in read and section directions, matrix of 512 × 192 × 64, nominal resolution of 0.5 × 1 × 2 mm3, field of view of 256 × 192 × 128 mm3, flip angle of 12°, and repetition time msec/echo time msec of 40/22.
A T2-weighted fast fluid-attenuated inversion-recovery sequence was acquired: repetition time msec/echo time msec/inversion time msec, 8500/120/2100; flip angle of 90°; echo train length of 24; matrix of 256 × 192 (frequency × phase); field of view of 256 × 192 mm2; and 48 sections of 3-mm thickness without gap for a nominal resolution of 1 × 1 × 3 mm3. A magnetization-prepared three-dimensional T1-weighted fast spoiled three-dimensional single-echo spoiled gradient-echo sequence was also acquired with 5.9/2.8/900, flip angle of 10°, matrix of 256 × 256 × 180, and isotropic resolution of 1 mm.
QSM
QSM images were reconstructed (F.S., with 13 years of experience) as previously described (1,4,7). In brief, we reconstructed susceptibility maps from raw k-space data through scalar phase matching of multichannel phase images (19), best-path phase unwrapping (20), sophisticated harmonic artifact reduction for phase data (SHARP) background field correction (20) with variable-radius SHARP (V-SHARP) (21), and field-to-source inversion through homogeneity-enabled incremental dipole inversion (HEIDI) (22). Magnetic susceptibility was referenced (0 ppb) to the average susceptibility of the brain, with the assumption that a larger reference region would reduce additional interparticipant variability compared with a smaller reference region. In-house developed algorithms for QSM processing were written in Matlab (version 2013b; MathWorks, Natick, Mass). To confirm that the chosen reference region did not have an impact on our findings, magnetic susceptibility was also referenced (0 ppb) to the lateral ventricles.
Lesion and Tissue Volumetry
T2 lesion volume was obtained (D.P., with 15 years of experience) by using a semiautomated reliable edge detection contouring-thresholding technique with Jim software (version 6.0; Xinapse Systems, Northants, United Kingdom; http://www.xinapse.com). Three-dimensional T1-weighted images were lesion filled prior to tissue volumetry analysis by using the “lesion_filling” tool from FSL (FMRIB Software Library; https://www.fmrib.ox.ac.uk/fsl), which replaces lesional voxels with values drawn from the normal-appearing white matter (N.B., with 15 years of experience). Normalized whole brain, total gray matter volume, and total white matter volume were calculated by using SIENAX (part of FSL) (23), whereas basal ganglia structures (defined as the caudate, putamen, and pallidus) and the thalamus were segmented by using FIRST (part of FSL) (24). The resulting masks were used to measure magnetic susceptibility within the deep gray matter structures. The resulting values were expressed in parts per billion.
QSM Template Construction and Registration
Sixty QSM images (demographically matched between participants with MS and healthy control participants and not part of our study population) were randomly selected to construct a study-specific QSM template based on the diffeomorphic Greedy-SyN transformation model in Advanced Normalization Tools (version 2.1; https://stnava.github.io/ANTs) (25) (F.L., with 16 years of experience). The coregistrations were driven by cross-correlation minimizations and performed after intensity rescaling of the susceptibility maps. After template construction, the original QSM data for each participant was registered to the QSM template by using the diffeomorphic Greedy-SyN transformation model in Advanced Normalization Tools. QSM data were used for driving the registration because of better deep gray matter tissue contrast, and thus more accurate spatial alignment compared with three-dimensional T1-weighted images.
Whole-Brain Voxel-based QSM Analyses
Spatially registered QSM data were analyzed by using a nonparametric permutation-based analysis, as implemented in the Randomize program (26), with 5000 permutations per analysis and age and sex included as covariates (F.L.). Additional analyses were performed including whole brain volume, T2 lesion volume, or individual structural volumes as additional covariates. For each deep gray matter region, we performed a separate analysis including the corresponding structural volume. Finally, a single model including all of the aforementioned covariates was analyzed. Threshold-free cluster enhancement (27) was used to obtain the differences between groups at P < .05, controlling for familywise error rate. Analysis was restricted to the thalamus, caudate, putamen, and GP by using a standard-space mask derived from the Harvard-Oxford Subcortical Structural Atlas. Group comparisons between healthy control participants and participants with MS were also repeated after referencing magnetic susceptibility to the lateral ventricles. For this analysis, we included age, sex, and individual structural volumes as covariates.
Statistical Analyses
Statistical analyses were performed by using SPSS (version 24; IBM, Armonk, NY) (J.H., with 12 years of experience). Differences in demographic characteristics between the groups were assessed by using the Student t test and χ2, as appropriate. Comparisons between imaging measures were made by using analysis of covariance models, correcting for age and sex, as well as disease duration when comparing participants with relapsing-remitting MS and secondary progressive MS. Multivariable linear regression models, controlling for age and sex, were then used to evaluate associations of global and regional susceptibility with disease duration, clinical disability, and disease course. Coefficients are reported as unstandardized β, representing the degree of change in magnetic susceptibility (in parts per billion) with a one-unit change of the independent measure (change in MS course, a year of disease duration, an Expanded Disability Status Scale point). Outcome P values of regions of interest–based analyses were adjusted by using false discovery rate, after which an adjusted P value of < .05 was considered to indicate statistical significance (28).
Results
Demographic, clinical, and conventional MRI features of participants with MS (relapsing-remitting MS and secondary progressive MS) versus healthy control participants are reported in Table 1. The mean disease duration for the whole cohort of patients was 13.3 years (11.3 years for relapsing-remitting MS, 19.5 years for secondary progressive MS). The median Expanded Disability Status Scale score was 2.5 (interquartile range, 1.5–5.0) for participants with MS, 2.0 (interquartile range, 1.5–3.5) for those with relapsing-remitting MS, and 6.0 (interquartile range, 5.0–6.5) for those secondary progressive MS. Four hundred ninety-three (82.5%) participants with MS (373 [82.5%] with relapsing-remitting MS and 122 [82.4%] with secondary progressive MS) were undergoing disease-modifying treatment. Participants with MS showed a higher T2 lesion volume (P < .001), as well as lower volumes of white matter (P < .001), gray matter (P < .001), and deep gray matter (P < .001) structures with respect to healthy control participants. The same results were found when participants with secondary progressive MS were compared with those with relapsing-remitting MS.
Table 1:
Demographic, Clinical, and MRI Characteristics in Participants with MS and Healthy Control Participants

Note.—Unless otherwise indicated, data are means ± standard deviations. All MRI volume measures are normalized. Differences between MRI measures are adjusted for age, sex, and disease duration when comparing participants with relapse-remitting multiple sclerosis (MS) and those with secondary progressive MS. P values comparing MRI measures were corrected for false discovery rate.
*Indicates significant P values.
†Data are the number of patients, with percentages in parentheses.
‡Data are medians, with interquartile ranges in parentheses.
§Basal ganglia was defined as caudate, putamen, and globus pallidus.
Region of Interest–based QSM Analysis
Results pertaining to the region of interest–based QSM analysis are shown in Table 2. The cohort of participants with MS showed lower thalamic susceptibility (−7.5 ppb vs −1.1 ppb; P < .002) and higher susceptibility in the basal ganglia (62 ppb vs 54.8 ppb; P < .001), as well as separately within the caudate (44.5 ppb vs 37.2 ppb; P < .001), putamen (51.9 ppb vs 47.9 ppb; P < .001), and GP (124.3 ppb vs 107.3 ppb; P < .001) when compared with healthy control participants. The comparison between both relapsing-remitting MS and secondary progressive MS separately versus healthy control participants are shown in Table E1 (online).
Table 2:
Relative QSM Values in Participants with MS and Healthy Control Participants

Note.—Data are means ± standard deviations. Differences between relative quantitative susceptibility mapping (QSM) measures are adjusted for age, sex, and disease duration by using analysis of covariance models. P values were corrected for false discovery rate. MS = multiple sclerosis.
*Indicates significant P values.
†Basal ganglia was defined as caudate, putamen, and globus pallidus.
Finally, compared with participants with relapsing-remitting MS, those with secondary progressive MS showed lower thalamic susceptibility (−10.9 ppb vs −6.4 ppb; P = .011) and higher in the basal ganglia as a whole (66.7 ppb vs 60.5 ppb; P = .041), but not in the individual components.
Results from the regression analyses are presented in Table 3. Lower thalamic susceptibility was associated with a longer disease duration (β = −0.42; P = −.002), a higher degree of disability (β = −0.64; P = .03,) and secondary-progressive MS course (β = −4.25; P = .009). Higher susceptibility of the GP was associated with higher disability (β = 2; P = .03). No associations were found between any of the analyzed clinical variables and the susceptibility of the basal ganglia as a whole, or when considering caudate or putamen separately. Moreover, the interaction effect between disease course and thalamic susceptibility showed that the latter is lower in participants with relapsing-remitting MS while tending to be more stable in participants with secondary progressive MS (β = 0.21; P = .021).
Table 3:
Regression Analyses Showing Independent Associations between QSM of Different Deep Gray Matter Structures and Clinical Variables in Participants with MS
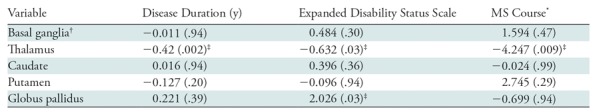
Note.—Data are β coefficients, with P values in parentheses. P values were corrected for false discovery rate. MS = multiple sclerosis, QSM = quantitative susceptibility mapping.
*Coded with relapsing-remitting MS as 1 and secondary progressive MS as 2, such that a negative β coefficient indicates lower susceptibility in participants with secondary progressive MS.
†Basal ganglia was defined as caudate, putamen, and globus pallidus.
‡Indicates significant P values.
Correlation coefficients between QSM and normalized volumes in participants with MS and healthy control participants are shown in Table E2 (online). In both participants with MS and healthy control participants, we found negative correlations in all explored regions (P < .01) except the thalamus, where positive correlations were detected (P < .001). No correlation was found in the GP for participants with secondary progressive MS (P = .93). Correlation coefficients between clinical outcomes in MS, and participants with relapsing-remitting MS and secondary progressive MS are shown in Table E3 (online).
Voxelwise QSM Analysis
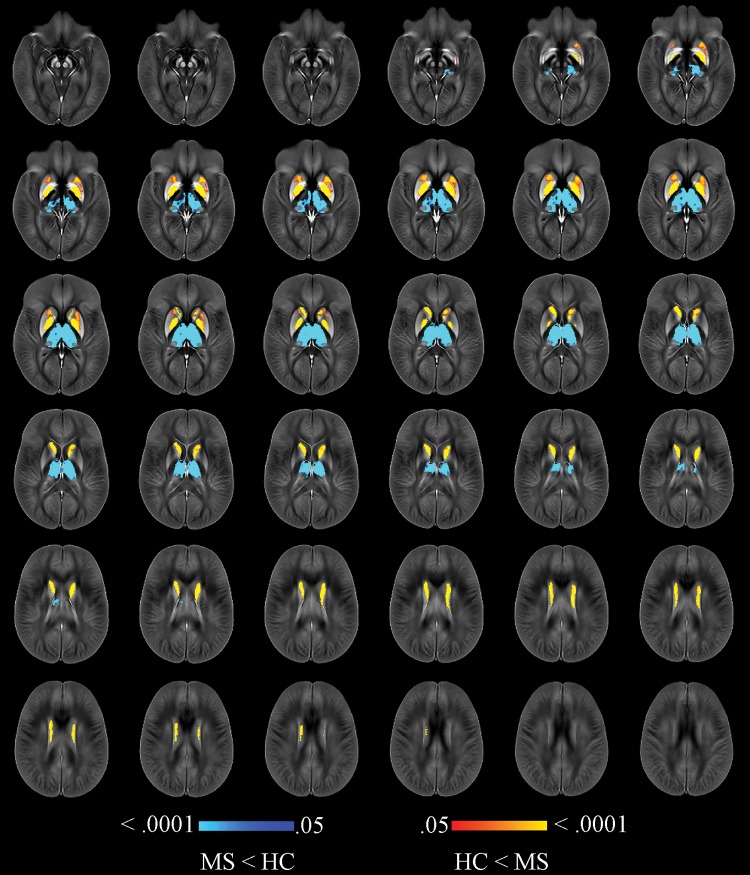
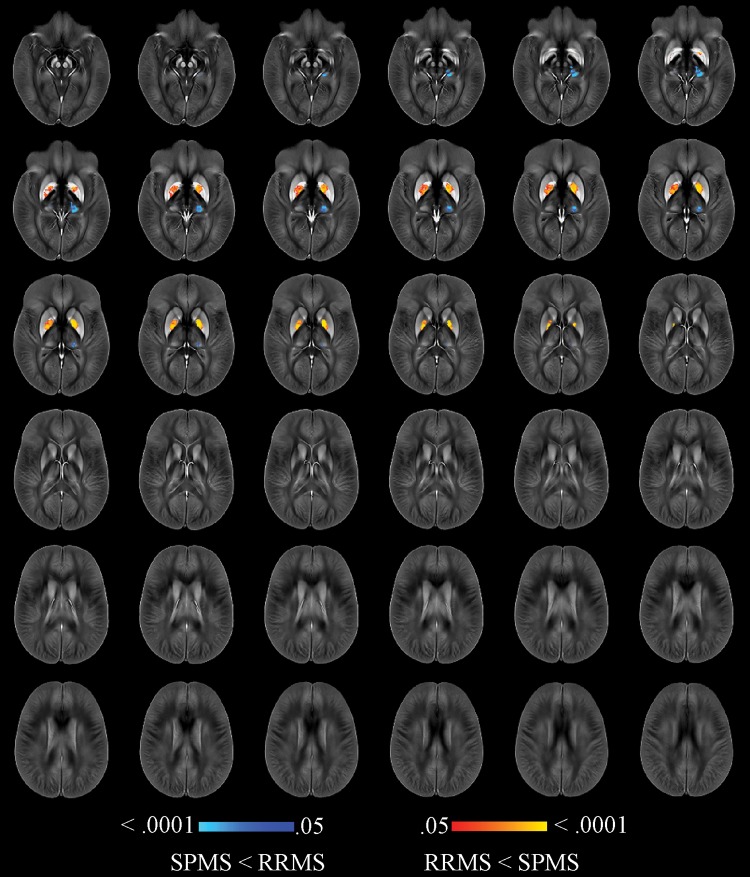
Participants with MS showed higher susceptibility in the body of the caudate, rostral putamen, and GP, along with lower susceptibility in the whole thalamus when compared with healthy control participants (Fig E1 [online]), even after correcting for each individual structural volume (Fig 2). Compared with the relapsing-remitting group, participants with secondary progressive MS had lower thalamic susceptibility as well as higher susceptibility in the caudate, putamen, and GP. After correcting for individual structural volumes, reduced thalamic susceptibility (left pulvinar) together with higher susceptibility in the dorsal GP was found in the group with secondary progressive MS (Fig 3).
Figure 2:
Image shows voxelwise analysis of quantitative susceptibility maps within thalamus, caudate, globus pallidus, and putamen comparing all participants with multiple sclerosis (MS) to healthy control (HC) participants. Results were corrected for age, sex, and individual structural volumes. They are overlaid on study-specific susceptibility template and are shown at P < .05, corrected for familywise error rate. Areas of higher susceptibility in participants with MS compared with HC participants are shown in red-yellow. Areas of lower susceptibility in participants with MS compared with HC participants are shown in blue-light blue. Warmer colors are indicative of smaller P values.
Figure 3:
Image shows voxelwise analysis of quantitative susceptibility maps within thalamus, caudate, globus pallidus, and putamen comparing participants with secondary progressive (SP) multiple sclerosis (MS) with participants with relapsing-remitting (RR) MS. Results were corrected for age, sex, and individual structural volumes. They are overlaid on study-specific susceptibility template and are shown at P < .05, corrected for familywise error rate. Areas of higher susceptibility in participants with SPMS compared with participants with RRMS are shown in red-yellow. Areas of lower susceptibility in participants with SPMS compared with participants with RRMS are shown in blue-light blue. Warmer colors are indicative of smaller P values.
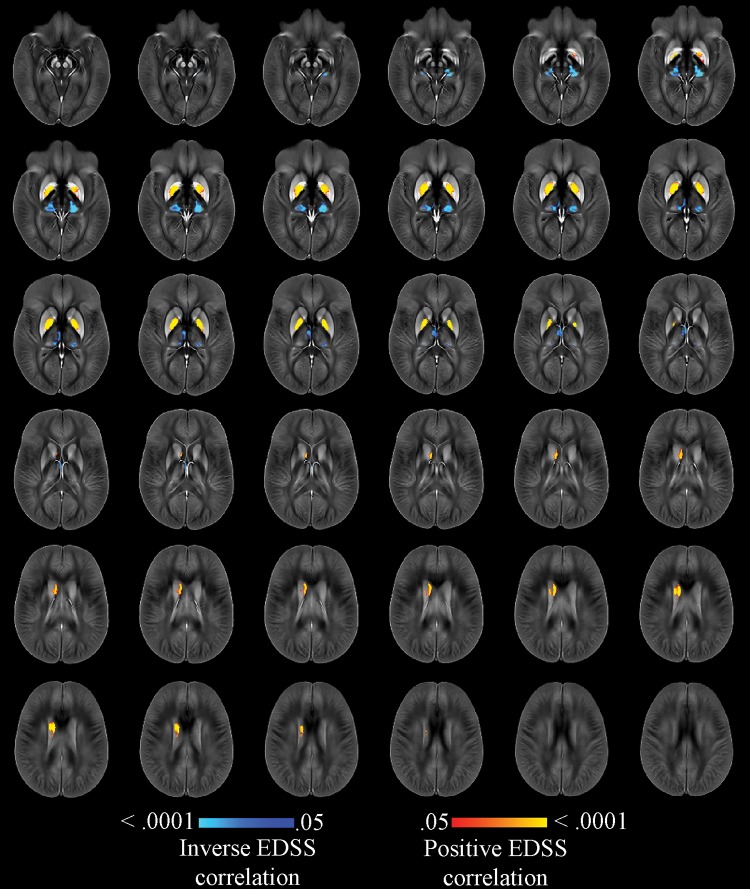
Furthermore, lower thalamic susceptibility in the bilateral pulvinar and the medial nuclear regions, as well as higher susceptibility in the GP interna (but not externa) and the body of the caudate, showed an association with a higher degree of disability in the whole cohort of participants with MS. The same results were obtained after correcting for whole brain volume (Fig E2 [online]) or T2 lesion volume (except left caudate) (Fig E3 [online]). When correcting for individual structural volumes, we observed an association of clinical disability with lower susceptibility in pulvinar and medial nuclear regions and with higher susceptibility in the whole GP and in the body of the right caudate (Fig 4). Finally, after correcting for T2 lesion volume, whole brain volume, and individual structural volumes together, we found an association between Expanded Disability Status Scale score and lower susceptibility in the left pulvinar, as well as higher susceptibility in small clusters of the dorsal GP bilaterally and of the right body of the caudate (Fig 5).
Figure 4:
Image shows voxelwise analysis of quantitative susceptibility maps within thalamus, caudate, globus pallidus, and putamen showing association with Expanded Disability Status Scale (EDSS), correcting for age, sex, and individual structural volumes in whole population with multiple sclerosis. Results are overlaid on study-specific susceptibility template and are shown at P < .05, corrected for the familywise error rate. Areas of higher susceptibility associated with higher EDSS are shown in red-yellow. Areas of lower susceptibility associated with higher EDSS are shown in blue-light blue. Warmer colors are indicative of smaller P values.
Figure 5:
Image shows voxelwise analysis of quantitative susceptibility maps within thalamus, caudate, globus pallidus, and putamen showing association with Expanded Disability Status Scale (EDSS), correcting for age, sex, individual structural volume, whole brain volume, and T2 lesion volume in whole population with multiple sclerosis. Results are overlaid on study-specific susceptibility template and are shown at P < .05, corrected for familywise error rate. Areas of higher susceptibility associated with higher EDSS are shown in red-yellow. Areas of lower susceptibility associated with higher EDSS are shown in blue-light blue. Warmer colors are indicative of smaller P values.
When referencing magnetic susceptibility to the lateral ventricles, higher susceptibility was still observed in the caudate, putamen, and GP, along with lower susceptibility of the thalamus in participants with MS compared with healthy control participants (Fig E4 [online]).
Discussion
In this study, reduced thalamic susceptibility was found both in the MS group considered as a whole and also in participants with relapsing-remitting MS and secondary progressive MS. The biologic explanation of a reduced susceptibility can reside both in lower paramagnetic iron components and in higher diamagnetic components, such as myelin and calcium. However, because evidence of altered iron levels in all the brain compartments in MS comes from both histopathologic data (3) and different iron-sensitive MRI techniques (4,6,7,29), we can assume that the reduction of susceptibility observed in the thalamus is mainly related to a lower iron content, despite the lack of histopathologic studies on this topic. The results obtained with the regions of interest–based QSM analysis were confirmed when using the voxelwise statistics. Our study sheds further light on the dynamics of iron within deep gray matter, demonstrating lower thalamic susceptibility and confirming previous findings of higher iron content in other deep gray matter structures.
One of the most critical issues to understand is whether the reduction of thalamic iron is the indirect consequence of tissue loss (30) or because of an active process of iron removal or depletion. Our study yields insight in this regard; we showed that even after correcting for structural tissue volume in the voxelwise analysis, susceptibility was still lower in the MS cohort as a whole and in participants with secondary progressive MS compared with those with relapsing-remitting MS. These findings seem to suggest that although thalamic atrophy is known to occur since the early stages of disease (30), and as such may partially contribute to iron changes, some other mechanisms are involved. In fact, iron depletion might derive from a reduced iron content of damaged oligodendrocytes that would release iron under stress conditions, subsequently scavenged by activated microglia (31). This mechanism might contribute to perpetuating a state of chronic inflammation, with further oligodendrocytic damage and iron depletion, finally leading to a definite loss of iron-containing cells (such as oligodendrocytes and neurons), as demonstrated by the presence of intrathalamic demyelinated lesions showing axonal transaction and neuronal degeneration (32). Although these interpretations are certainly speculative at this time, further preclinical and clinical studies should explore them in more detail.
Iron loss might also derive from pathologic changes of other central nervous system compartments, considering that the thalamus is a major relay center of the central nervous system and highly connected to both cortical and subcortical areas. For example, a recent study (33) investigated cerebral blood flow in nonatrophic gray matter areas and found hypoperfusion only in the thalamus. Normal-appearing white matter and thalamus both show reduced iron content (2), and white matter impairment has also been implicated in the pathogenesis of thalamic damage (34). Another recent study (16) described a gradient of reduced susceptibility within the thalamus, with lower values nearby white matter in relapsing-remitting MS, as opposed to secondary progressive MS that showed homogenously reduced iron content within the whole thalamus.
By comparing relapsing-remitting MS with secondary progressive MS, we also showed that thalamic susceptibility was lower in the latter, as well as associated with a longer disease duration. All these data, taken together, point toward the notion that iron-related thalamic damage starts earlier in the disease course, in line with previous findings (8), and as such is more likely associated with the inflammatory environment typical of the earlier phases of the disease. Indeed, a recent study (15) comparing primary progressive MS, which is characterized by a low degree of inflammation, with age-matched relapsing-remitting MS showed that lower thalamic susceptibility in the latter was the only MR metric able to differentiate the two MS phenotypes.
Reduced iron content within the thalamus was also associated with higher clinical disability, as quantified by Expanded Disability Status Scale, even after correcting for other MRI parameters in the voxelwise analysis, such as thalamic volume, whole brain volume, and T2 lesion volume. This suggests a direct contribution of altered iron homeostasis in the process of disability accumulation in MS.
Contrarily to the thalamus, GP, caudate and putamen showed higher susceptibility in participants with MS. Demyelination likely contributes in this regard, as evidenced by histopathologic findings (31,32) and MRI data (12,14). Although, considering the relatively low myelin content within the deep gray matter (except for the GP), it seems reasonable to hypothesize that the main cause of higher susceptibility is iron accumulation. A higher susceptibility was still significant after correcting for individual structure volumes. An active process of iron deposition might be a causal factor in the basal ganglia, which are known to have the highest iron content in the brain (17). The mechanisms involved in this process are currently unknown, but taking into consideration the relatively high perfusion of deep gray matter and the recent findings of a positive correlation between iron levels in the deep gray matter and serum (35), a blood-brain iron translocation within the basal ganglia might be speculated.
Furthermore, susceptibility in deep gray matter structures other than the thalamus was not different between relapsing-remitting MS and secondary progressive MS. This finding, together with results from other studies showing higher susceptibility in patients with clinically isolated syndrome (8,36) and progressive iron accumulation over time in MS (14), suggests that altered iron homeostasis occurs early in the disease, as indicated also by the aforementioned rapid lowering of thalamic iron content in relapsing-remitting MS with respect to secondary progressive MS. Khalil et al (8) showed also that whereas susceptibility changes within the basal ganglia seem to be more pronounced in patients with clinically isolated syndrome and slowed down in those with relapsing-remitting MS, atrophy follows an opposite evolution. All of these findings support the association between iron and neurodegeneration but a causal role remains speculative, although consistent with similar findings in other neurodegenerative diseases (1).
Higher susceptibility of the basal ganglia was also associated with disability even after correcting for whole brain volume and T2 lesion volume. Previous studies (29) have shown a stronger association between higher deep gray matter iron content and higher disability in MS than conventional MRI measures, underlining the clinical relevance of iron accumulation in gray matter structures highly involved in the maintenance of a properly functioning sensorimotor system. When correcting for structural volumes, however, only the findings within the GP survived. This structure naturally has one of the highest iron concentrations within the deep gray matter (17,37,38) and as such, further pathologic iron deposition might make it more susceptible to damage and impact on its functionality than are other deep gray matter regions, mainly related to the motor system. It should be noted, however, that the relatively high myelin content with respect to other deep gray matter regions renders interpretation somewhat more complex because demyelination also leads to higher susceptibility.
Our voxelwise analysis revealed an involvement of specific subregions of the major deep gray matter nuclei. The body of the caudate, the pulvinar, and the medial nuclear regions of the thalamus are the primarily associative territories of the caudate and thalamus, respectively, which connect to cortical regions. The GP interna and externa are part of the direct and indirect pathways of the basal ganglia circuitry, respectively, and the GP interna maintains a feedback loop providing direct input to the thalamus. In particular, pulvinar, the body of the caudate, and GP interna are part of the corticostriatal visual loop, in line with previous speculations that susceptibility changes in the thalamus reflect remote damage of the visual system (4). The ventrorostral part of the putamen, which showed higher iron concentration when comparing participants with MS and control participants but was not associated with disability, is a partly associative territory integrated in the cortico-striato-thalamo-cortical circuit that targets medial, orbital and prefrontal cortices.
The volume loss of deep gray matter structures and of thalamic subnuclei with age in participants with MS and control participants is consistent with previous findings (38). The lower magnetic susceptibility with age in most regions is likely a reflection of the known decrease in thalamic iron concentration after the characteristic peak during the fourth decade of life (17,37–41). With a mean age exceeding the midforties, the relapsing-remitting and secondary-progressive MS groups in the current study (and the corresponding healthy control groups were above this particular age.
Our study had some limitations. First, the cross-sectional design did not allow any conclusion on the temporal evolution of iron changes in the basal ganglia and thalamus and on the possible causal relationship between volume changes and altered iron homeostasis. In addition, we did not include any participants with clinically isolated syndrome in our study and our relapsing-remitting MS cohort had a mean disease duration of 11.3 years, limiting our capability to draw firm conclusions on iron alterations in the very early phases of the disease. Finally, regional gray matter volumes were calculated by using FIRST, which does not incorporate partial volume correction into the resulting structural volumes. As such, higher partial volume averaging with progressive atrophy may have had some impact on our results.
In conclusion, our study contributes to the understanding of the dynamics of iron within subcortical gray matter by using sophisticated techniques such as QSM and voxelwise analysis in a large sample of participants with relapsing-remitting MS and secondary progressive MS. Moreover, our results shed further light on the potential mechanisms underlying thalamic iron reduction as well as iron accumulation within basal ganglia, suggesting that these processes are not merely indirect consequences of structural atrophy.
Summary
Reduced thalamic susceptibility and higher iron content in other deep gray matter structures are present in multiple sclerosis and are associated with clinical disability.
Implications for Patient Care
■ Deep gray matter susceptibility in multiple sclerosis is a putative imaging marker of disease severity in multiple sclerosis.
■ Monitoring deep gray matter susceptibility may be relevant for identifying patients with multiple sclerosis at higher risk for developing physical disability.
SUPPLEMENTAL TABLES
SUPPLEMENTAL FIGURES
Supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) (UL1TR001412). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Supported by internal resources of the Buffalo Neuroimaging Analysis Center and the Jacquemin Family Foundation.
Author contributions: Guarantors of integrity of entire study, R.Z., F.L., C.K., D.R., J.D.; study concepts/study design or data acquisition or data analysis/interpretation, all authors; manuscript drafting or manuscript revision for important intellectual content, all authors; approval of final version of submitted manuscript, all authors; agrees to ensure any questions related to the work are appropriately resolved, all authors; literature research, R.Z., E.T., N.B., J.H., F.L., D.H., J.D., B.W.G., F.S.; clinical studies, R.Z., N.B., F.L., E.C., C.K., D.H., B.W.G., F.S.; statistical analysis, R.Z., N.B., J.H., F.L., D.R., J.D.; and manuscript editing, all authors.
Disclosures of Conflicts of Interest: R.Z. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a consultant for Claret Medical, Celgene, Genzyme-Sanofi, and Novartis; has grants/grants pending with Claret Medical, Celgene, Genzyme-Sanofi, IMS Health, Novartis, and Protembis; received payment for lectures including service on speakers bureaus from Celgene, Genzyme-Sanofi, and Novartis; received payment for development of educational presentations from Celgene. Other relationships: disclosed no relevant relationships. E.T. disclosed no relevant relationships. N.B. disclosed no relevant relationships. J.H. disclosed no relevant relationships. F.L. disclosed no relevant relationships. M.G.D. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a consultant for Claret Medical and EMD Serono; has grants/grants pending with Novartis. Other relationships: disclosed no relevant relationships. E.C. disclosed no relevant relationships. C.K. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a member of advisory board for Biogen, EMD Serono, and TEVA; received payment for lectures including service on speakers bureaus from Biogen, EMD Serono, Mallinckrodt, Novartis, and TEVA. Other relationships: disclosed no relevant relationships. D.H. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a consultant for and received payment for lectures including service on speakers bureaus from Biogen, EMD Serono, Genentech, and TEVA. Other relationships: disclosed no relevant relationships. D.R. disclosed no relevant relationships. J.D. disclosed no relevant relationships. B.W.G. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a consultant for Biogen, Celgene, EMD Serono, Genentech, Novartis, and TEVA; has grants/grants pending with Biogen, EMD Serono, and TEVA; received payment for lectures including service on speakers bureaus from Biogen, Genentech, and TEVA. Other relationships: disclosed no relevant relationships. F.S. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: received payment for expert testimony from Goodwin Procter; has grants/grants pending with SynchroPET; received payment for lectures including service on speakers bureaus from Toshiba Canada Medical Systems; received payment for travel/accommodations/meeting expenses unrelated to activities listed from GE Healthcare and SynchroPET. Other relationships: disclosed no relevant relationships.
Abbreviations:
- GP
- globus pallidus
- MS
- multiple sclerosis
- QSM
- quantitative susceptibility mapping
References
- 1.Hagemeier J, Geurts JJ, Zivadinov R. Brain iron accumulation in aging and neurodegenerative disorders. Expert Rev Neurother 2012;12(12):1467–1480. [DOI] [PubMed] [Google Scholar]
- 2.Hametner S, Wimmer I, Haider L, Pfeifenbring S, Brück W, Lassmann H. Iron and neurodegeneration in the multiple sclerosis brain. Ann Neurol 2013;74(6):848–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stüber C, Pitt D, Wang Y. Iron in multiple sclerosis and its noninvasive imaging with quantitative susceptibility mapping. Int J Mol Sci 2016;17(1):E100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schweser F, Raffaini Duarte Martins AL, Hagemeier J, et al. Mapping of thalamic magnetic susceptibility in multiple sclerosis indicates decreasing iron with disease duration: a proposed mechanistic relationship between inflammation and oligodendrocyte vitality. Neuroimage 2018;167:438–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehta V, Pei W, Yang G, et al. Iron is a sensitive biomarker for inflammation in multiple sclerosis lesions. PLoS One 2013;8(3):e57573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rudko DA, Solovey I, Gati JS, Kremenchutzky M, Menon RS. Multiple sclerosis: improved identification of disease-relevant changes in gray and white matter by using susceptibility-based MR imaging. Radiology 2014;272(3):851–864. [DOI] [PubMed] [Google Scholar]
- 7.Hagemeier J, Zivadinov R, Dwyer MG, et al. Changes of deep grey matter magnetic susceptibility over 2 years in multiple sclerosis and healthy control brain. Neuroimage Clin 2017 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khalil M, Langkammer C, Pichler A, et al. Dynamics of brain iron levels in multiple sclerosis: a longitudinal 3T MRI study. Neurology 2015;84(24):2396–2402. [DOI] [PubMed] [Google Scholar]
- 9.Haacke EM, Liu S, Buch S, Zheng W, Wu D, Ye Y. Quantitative susceptibility mapping: current status and future directions. Magn Reson Imaging 2015;33(1):1–25. [DOI] [PubMed] [Google Scholar]
- 10.Schweser F, Deistung A, Lehr BW, Reichenbach JR. Quantitative imaging of intrinsic magnetic tissue properties using MRI signal phase: an approach to in vivo brain iron metabolism? Neuroimage 2011;54(4):2789–2807. [DOI] [PubMed] [Google Scholar]
- 11.Schweser F, Deistung A, Reichenbach JR. Foundations of MRI phase imaging and processing for quantitative susceptibility mapping (QSM). Z Med Phys 2016;26(1):6–34. [DOI] [PubMed] [Google Scholar]
- 12.Langkammer C, Liu T, Khalil M, et al. Quantitative susceptibility mapping in multiple sclerosis. Radiology 2013;267(2):551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cobzas D, Sun H, Walsh AJ, Lebel RM, Blevins G, Wilman AH. Subcortical gray matter segmentation and voxel-based analysis using transverse relaxation and quantitative susceptibility mapping with application to multiple sclerosis. J Magn Reson Imaging 2015;42(6):1601–1610. [DOI] [PubMed] [Google Scholar]
- 14.Elkady AM, Cobzas D, Sun H, Blevins G, Wilman AH. Progressive iron accumulation across multiple sclerosis phenotypes revealed by sparse classification of deep gray matter. J Magn Reson Imaging 2017;46(5):1464–1473. [DOI] [PubMed] [Google Scholar]
- 15.Burgetova A, Dusek P, Vaneckova M, et al. Thalamic iron differentiates primary-progressive and relapsing-remitting multiple sclerosis. AJNR Am J Neuroradiol 2017;38(6):1079–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Louapre C, Govindarajan ST, Giannì C, et al. Heterogeneous pathological processes account for thalamic degeneration in multiple sclerosis: insights from 7 T imaging. Mult Scler 2017 Aug 1 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 17.Hallgren B, Sourander P. The effect of age on the non-haemin iron in the human brain. J Neurochem 1958;3(1):41–51. [DOI] [PubMed] [Google Scholar]
- 18.Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammond KE, Lupo JM, Xu D, et al. Development of a robust method for generating 7.0 T multichannel phase images of the brain with application to normal volunteers and patients with neurological diseases. Neuroimage 2008;39(4):1682–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdul-Rahman HS, Gdeisat MA, Burton DR, Lalor MJ, Lilley F, Moore CJ. Fast and robust three-dimensional best path phase unwrapping algorithm. Appl Opt 2007;46(26):6623–6635. [DOI] [PubMed] [Google Scholar]
- 21.Li W, Wu B, Liu C. Quantitative susceptibility mapping of human brain reflects spatial variation in tissue composition. Neuroimage 2011;55(4):1645–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schweser F, Sommer K, Deistung A, Reichenbach JR. Quantitative susceptibility mapping for investigating subtle susceptibility variations in the human brain. Neuroimage 2012;62(3):2083–2100. [DOI] [PubMed] [Google Scholar]
- 23.Smith SM, Zhang Y, Jenkinson M, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage 2002;17(1):479–489. [DOI] [PubMed] [Google Scholar]
- 24.Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A. Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage 2011;56(3):907–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 2011;54(3):2033–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage 2014;92:381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 2009;44(1):83–98. [DOI] [PubMed] [Google Scholar]
- 28.Benjamani Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser A Stat Soc 1995;57(1):289–300. [Google Scholar]
- 29.Hagemeier J, Weinstock-Guttman B, Heininen-Brown M, et al. Gray matter SWI-filtered phase and atrophy are linked to disability in MS. Front Biosci (Elite Ed) 2013;5(2):525–532. [DOI] [PubMed] [Google Scholar]
- 30.Azevedo CJ, Cen SY, Khadka S, et al. Thalamic atrophy in multiple sclerosis: a magnetic resonance imaging marker of neurodegeneration throughout disease. Ann Neurol 2018;83(2):223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haider L, Simeonidou C, Steinberger G, et al. Multiple sclerosis deep grey matter: the relation between demyelination, neurodegeneration, inflammation and iron. J Neurol Neurosurg Psychiatry 2014;85(12):1386–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vercellino M, Masera S, Lorenzatti M, et al. Demyelination, inflammation, and neurodegeneration in multiple sclerosis deep gray matter. J Neuropathol Exp Neurol 2009;68(5):489–502. [DOI] [PubMed] [Google Scholar]
- 33.Doche E, Lecocq A, Maarouf A, et al. Hypoperfusion of the thalamus is associated with disability in relapsing remitting multiple sclerosis. J Neuroradiol 2017;44(2):158–164. [DOI] [PubMed] [Google Scholar]
- 34.Kipp M, Wagenknecht N, Beyer C, Samer S, Wuerfel J, Nikoubashman O. Thalamus pathology in multiple sclerosis: from biology to clinical application. Cell Mol Life Sci 2015;72(6):1127–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bergsland N, Agostini S, Laganà MM, et al. Serum iron concentration is associated with subcortical deep gray matter iron levels in multiple sclerosis patients. Neuroreport 2017;28(11):645–648. [DOI] [PubMed] [Google Scholar]
- 36.Al-Radaideh AM, Wharton SJ, Lim SY, et al. Increased iron accumulation occurs in the earliest stages of demyelinating disease: an ultra-high field susceptibility mapping study in clinically isolated syndrome. Mult Scler 2013;19(7):896–903. [DOI] [PubMed] [Google Scholar]
- 37.Pfefferbaum A, Adalsteinsson E, Rohlfing T, Sullivan EV. MRI estimates of brain iron concentration in normal aging: comparison of field-dependent (FDRI) and phase (SWI) methods. Neuroimage 2009;47(2):493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hagemeier J, Dwyer MG, Bergsland N, et al. Effect of age on MRI phase behavior in the subcortical deep gray matter of healthy individuals. AJNR Am J Neuroradiol 2013;34(11):2144–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keuken MC, Bazin PL, Backhouse K, et al. Effects of aging on T1, T2*, and QSM MRI values in the subcortex. Brain Struct Funct 2017;222(6):2487–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu M, Liu S, Ghassaban K, et al. Assessing global and regional iron content in deep gray matter as a function of age using susceptibility mapping. J Magn Reson Imaging 2016;44(1):59–71. [DOI] [PubMed] [Google Scholar]
- 41.Habib CA, Liu M, Bawany N, et al. Assessing abnormal iron content in the deep gray matter of patients with multiple sclerosis versus healthy controls. AJNR Am J Neuroradiol 2012;33(2):252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.