Abstract
Malaria is caused by Plasmodium parasites that proliferate in the bloodstream. During each replication cycle some parasites differentiate into gametocytes, the only forms able to infect the mosquito vector and transmit malaria. Sexual commitment is triggered by activation of AP2-G, the master transcriptional regulator of gametocytogenesis. Heterochromatin protein 1 (HP1)-dependent silencing of ap2-g prevents sexual conversion in proliferating parasites. Here, we identified Plasmodium falciparum gametocyte development 1 (GDV1) as an upstream activator of sexual commitment. We found that GDV1 targeted heterochromatin and triggered HP1 eviction thus de-repressing ap2-g. Expression of GDV1 was responsive to environmental triggers of sexual conversion and controlled via a gdv1 antisense RNA. Hence, GDV1 appears to act as an effector protein that induces sexual differentiation by antagonizing HP1-dependent gene silencing.
Heterochromatin protein 1 (HP1) is a conserved regulator of heterochromatin formation, heritable gene silencing and variegated gene expression (1). In Plasmodium falciparum, HP1-dependent clonally variant expression allows parasites to adapt rapidly to environmental challenges encountered during infection (2–4). For example, immune evasion via antigenic variation of var/PfEMP1 is the hallmark of Plasmodium survival. Other processes, such as expression of red blood cell (RBC) invasion ligands or nutrient transporters, are similarly regulated in this parasite (4). Most clonally variant genes cluster in subtelomeric domains but some also occur in chromosome-internal heterochromatic regions. In addition, HP1 forms microdomains at some euchromatic genes (2). One of these encodes the transcription factor AP2-G that is required for sexual conversion and differentiation (2, 5–7). HP1-dependent regulation of ap2-g controls the rate at which parasites commit to sexual differentiation (7).
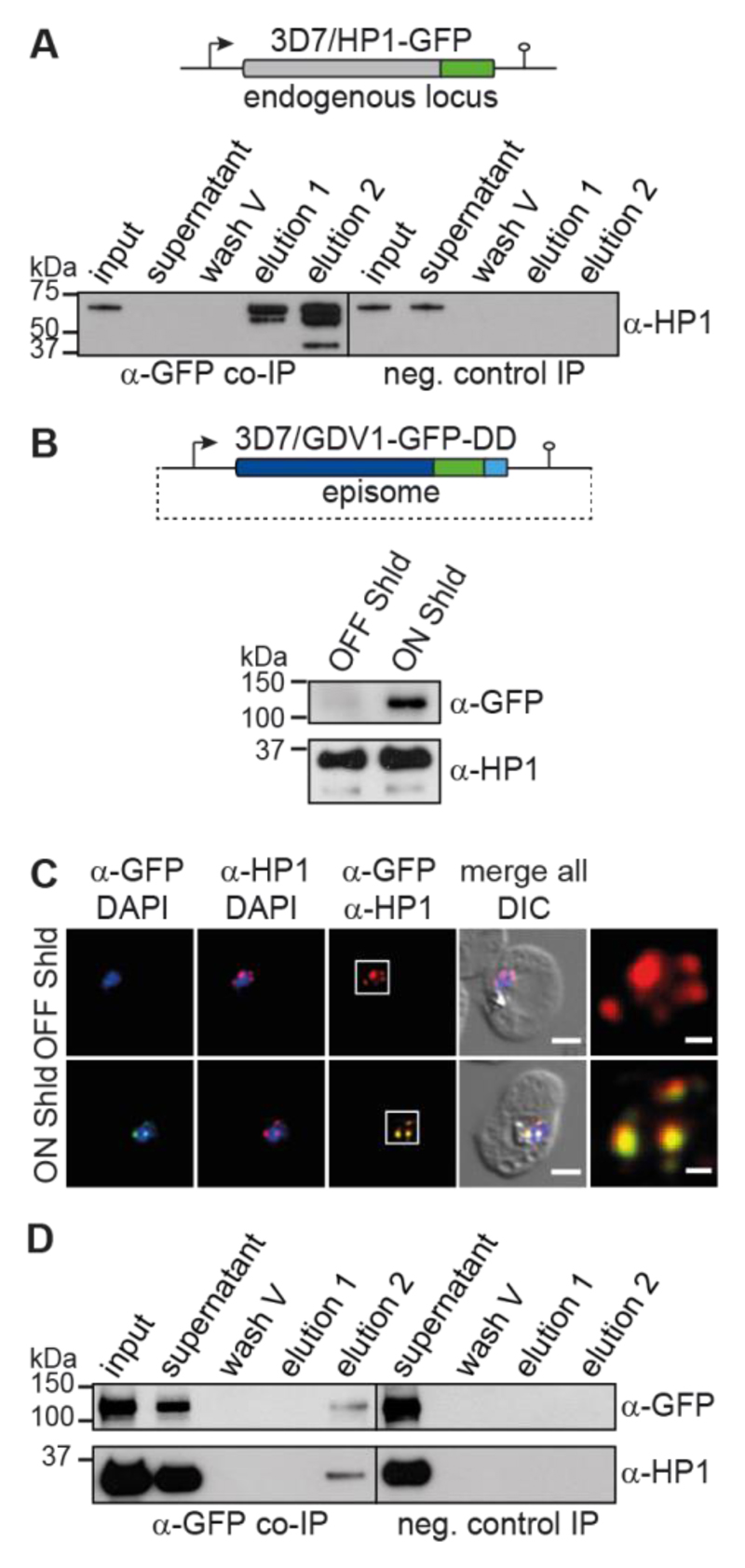
To explore the mechanisms regulating HP1 occupancy in P. falciparum we identified HP1-interacting proteins by liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis of native HP1 complexes that were purified by co-immunoprecipitation (co-IP) from parasites expressing GFP-tagged HP1 (7) (Fig. 1A and Table S3). Interestingly, we consistently observed GDV1 among the potential HP1 interaction partners (Table S1). GDV1 is a nuclear protein implicated in sexual commitment and early gametocytogenesis but its exact function remains unknown (8). We therefore created a parasite line for the conditional expression of fluorescently labelled ectopic GDV1 (GDV1-GFP-DD) (Fig. 1B). Proteins tagged with the immunophilin protein-folding chaperone FKBP destabilisation domain (DD) are proteolytically degraded unless cells are cultured in presence of Shield-1, a small molecule ligand stabiliser (9, 10). Thus, GDV1-GFP-DD is barely detectable in parasites cultured in absence of Shield-1 (3D7/GDV1-GFP-DDOFF), but its expression is markedly induced in parasites grown in presence of Shield-1 (3D7/GDV1-GFP-DDON) (Fig. 1, B and C). In agreement with the co-IP results GDV1-GFP-DD co-localizes with HP1 at the nuclear periphery (Fig. 1C and Fig. S1). Furthermore, we found that recombinant HP1 and GDV1 formed a complex (Fig. S1) and that HP1 co-purified with GDV1-GFP-DD in reverse co-IPs (Fig. 1D, Tables S2 and S4). The chromodomain-helicase-DNA-binding protein 1 (CHD1) and a protein of unknown function (PF3D7_1451200) also consistently co-purified with both HP1 and GDV1-GFP-DD (Tables S1 and S2). Given that CHD1 plays important roles in cell fate decision and heterochromatin remodelling in other organisms (11, 12) and that GDV1 is implicated in gametocytogenesis (8) it appears that this putative regulatory complex may function in activating sexual commitment.
Fig. 1. GDV1 interacts with HP1.
(A) Endogenous hp1 locus in 3D7/HP1-GFP parasites and α-HP1 Western blots of the α-HP1-GFP co-IP and negative control samples. Results are representative of three biological replicates. (B) gdv1-gfp-dd expression plasmid and α-GFP Western blots of 3D7/GDV1-GFP-DDOFF and 3D7/GDV1-GFP-DDON parasites. α-HP1 antibodies served as loading control. (C) GDV1-GFP-DD/HP1 co-localisation IFAs in 3D7/GDV1-GFP-DDOFF and 3D7/GDV1-GFP-DDON trophozoites (24-32 hpi). DIC, differential interference contrast. Scale bar, 2.5 μm (0.5 μm for the magnified views in the rightmost images). Results are representative of three biological replicates. (D) α-GFP and α-HP1 Western blots of the α-GDV1-GFP-DD co-IP and negative control samples. Results are representative of three biological replicates.
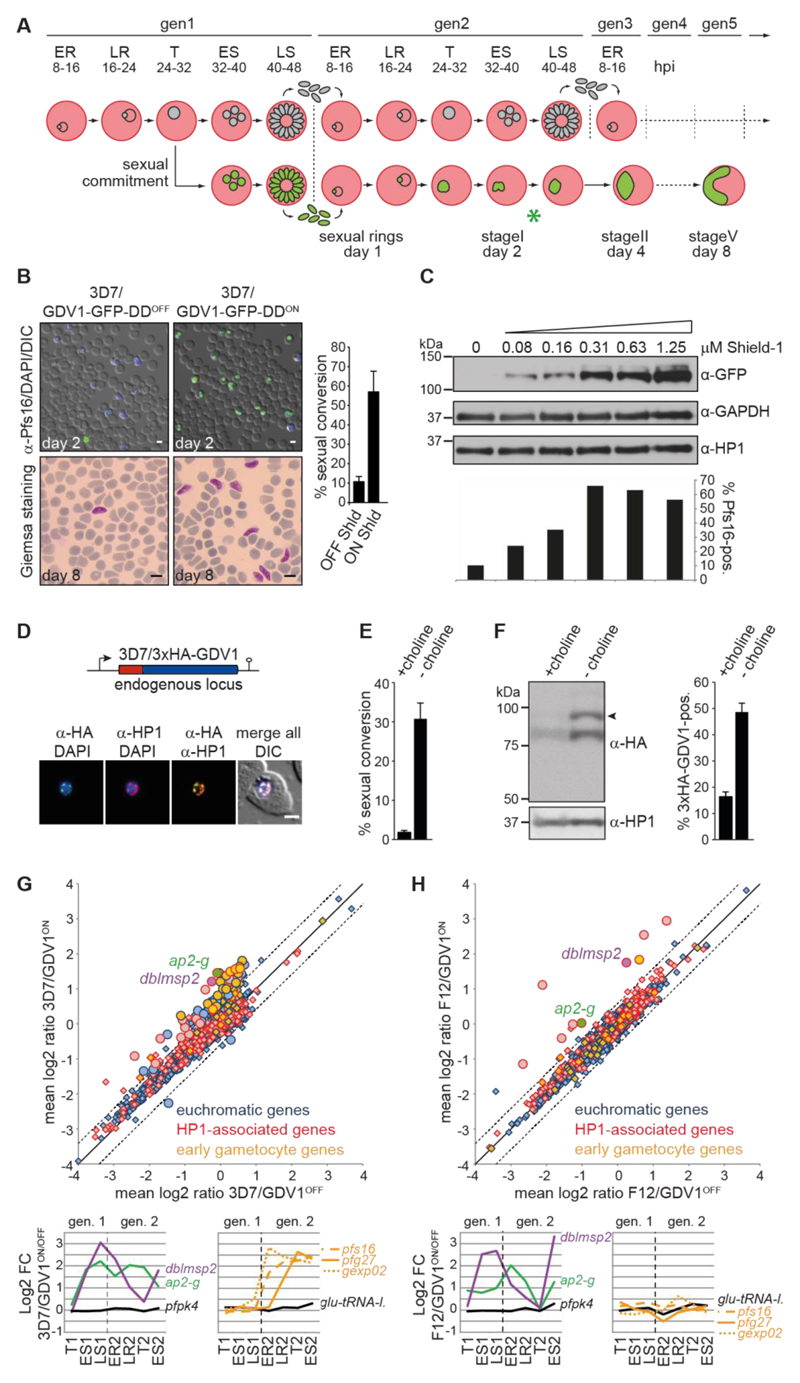
Malaria parasites proliferate by iterative rounds of intra-erythrocytic replication through schizogony, merozoite release and RBC re-invasion. The decision to enter gametocytogenesis is made in the cell cycle prior to sexual differentiation; sexually committed schizonts release merozoites that invade RBCs and differentiate all into either female or male gametocytes (13, 14) (Fig. 2A). To test if GDV1 triggers sexual commitment, 3D7/GDV1-GFP-DDOFF parasites were split and cultured in the absence or presence of Shield-1. After re-invasion, stage I gametocytes were quantified by immuno-fluorescence assays (IFA) using antibodies against the gametocyte marker Pfs16 (15). Strikingly, the 3D7/GDV1-GFP-DDON population displayed a sexual conversion rate of 57.2% (+/- 10.0 SD) compared to 11.0% (+/- 2.4 SD) in 3D7/GDV1-GFP-DDOFF parasites, and these gametocytes differentiated normally into both male and female gametocytes and showed a typical female-biased sex ratio (Fig. 2B and Fig. S2). Moreover, Shield-1 titration revealed a positive correlation between ectopic GDV1-GFP-DD expression levels and sexual conversion rates (Fig. 2C). To test if endogenous GDV1 levels similarly correlate with gametocyte conversion we used CRISPR/Cas9-based gene editing to append a triple hemagglutinin (HA) tag to the N-terminus of GDV1 (3D7/3xHA-GDV1) (Fig. S3). Endogenous 3xHA-GDV1 co-localised with HP1 as expected (Fig. 2D and Fig. S3) but was only expressed in some parasites. We next quantified 3xHA-GDV1 expression under conditions that either suppress or favour sexual conversion. To this end, we made use of the recent discovery of choline as an inhibitor of sexual commitment (16). 3D7/3xHA-GDV1 parasites cultured in the presence or absence of 2 mM choline displayed sexual commitment rates of 1.8% (+/- 0.3 SD) or 30.9% (+/- 3.8 SD), respectively (Fig. 2E). Strikingly, parasites cultured in the absence of choline showed markedly increased 3xHA-GDV1 expression levels (Fig. 2F). This was accounted for by a higher proportion of 3xHA-GDV1-positive cells (48.6% (+/- 3.4 SD) in absence compared to 16.4% (+/- 1.8 SD) in presence of choline) (Fig. 2F) and comparatively higher 3xHA-GDV1 expression levels in individual 3xHA-GDV1-positive parasites (Fig. S3). Together, these results show that GDV1 activates sexual conversion in a dose-dependent manner and that endogenous GDV1 expression can be induced by environmental signals triggering sexual commitment.
Fig. 2. GDV1 induces sexual commitment and differentiation.
(A) Schematic illustrating the iterative cycles of schizogony and RBC re-invasion (top) or sexual commitment, RBC re-invasion and gametocyte differentiation (bottom). ER/LR, early/late ring stages; T, trophozoites; ES/LS, early/late schizonts; gen, generation; hpi, hours post-invasion; asterisk, time point of α-Pfs16 IFAs. (B) Top panel: α-Pfs16 IFAs identifying stage I gametocytes. Quantification of Pfs16-positive parasites is shown at the right (results are the mean of three biological replicates (200 infected RBCs counted per sample); error bars indicate SD). Bottom panel: Giemsa-stained blood smears showing stage V gametocytes. Scale bars, 5 μm. (C) Western blot showing GDV1-GFP-DD expression in presence of increasing Shield-1 concentrations. α-GAPDH and α-HP1 antibodies served as loading controls. Percentages of Pfs16-positive parasites are shown at the bottom (400 infected RBCs counted per sample). (D) Endogenous gdv1 locus in 3D7/3xHA-GDV1 parasites and 3xHA-GDV1/HP1 co-localisation IFAs in trophozoites (24-32 hpi). Scale bar, 2.5 μm. (E) Sexual conversion rates in 3D7/3xHA-GDV1 parasites cultured in presence or absence of choline (results are the mean of three biological replicates (>190 infected RBCs counted per sample); error bars indicate SD). (F) Left panel: Western blot showing 3xHA-GDV1 expression levels in 3D7/3xHA-GDV1 parasites cultured in presence or absence of choline. α-HP1 antibodies served as loading control. Right panel: Percentages of 3xHA-GDV1-positive parasites in presence or absence of choline (results are the mean of three biological replicates (>100 infected RBCs counted per sample); error bars indicate SD). (G,H) Comparison of mean expression levels of all genes in 3D7/GDV1-GFP-DDON versus 3D7/GDV1-GFP-DDOFF (G) and F12/GDV1-GFP-DDON versus F12/GDV1-GFP-DDOFF parasites (H). Significantly de-regulated genes are indicated by circles (mean fold change cut-off >1.5; q-value (fdr) cut-off <0.15). Known early gametocyte markers (7, 8, 17) are labelled orange. Line graphs show fold changes in expression across seven consecutive TPs. pfs16/pfg27/gexp02, early gametocyte markers (15, 22, 32); pk4 (PF3D7_0628200)/glu-tRNA-l. (PF3D7_1331700), control genes (7).
We next performed comparative transcriptome analyses using two-colour microarrays. 3D7/GDV1-GFP-DDOFF ring stage parasites were split, cultured separately in absence or presence of Shield-1 and total RNA was harvested at seven paired time points spanning the remaining 24 hours of generation 1 (24-32 hours post-invasion (hpi); 32-40 hpi, 40-48 hpi) and the first 40 hours after re-invasion in generation 2 (8-16 hpi, 16-24 hpi, 24-32 hpi, 32-40 hpi) (Fig. 2A). As expected, GDV1-GFP-DD expression triggered a transcriptional response characteristic of sexual commitment and early differentiation. This was evident from the induction of ap2-g in generation 1, followed by activation of early gametocyte markers (5, 7, 8, 17) after re-invasion (Fig. 2G, Fig. S4 and Table S5). In F12 parasites, a 3D7-derived gametocyte-deficient clone carrying a loss-of-function mutation in ap2-g (5, 18), GDV1-GFP-DD expression still activated ap2-g but failed to launch a sexual differentiation response (Fig. 2H and Table S6). Next to ap2-g only eight other genes were significantly induced in F12/GDV1-GFP-DDON parasites, all of which are marked by HP1. This set included dblmsp2, which was also induced in 3D7/GDV1-GFP-DDON parasites (Fig. 2, G and H). Given that DBLMSP2 is a merozoite surface antigen expressed only in a small subpopulation of schizonts (19, 20) the GDV1-dependent activation of the dblmsp2 locus suggests it may be expressed specifically in sexually committed schizonts. In summary, these findings show that GDV1 is an upstream activator of sexual commitment and likely triggers this process by antagonising HP1-dependent silencing of ap2-g.
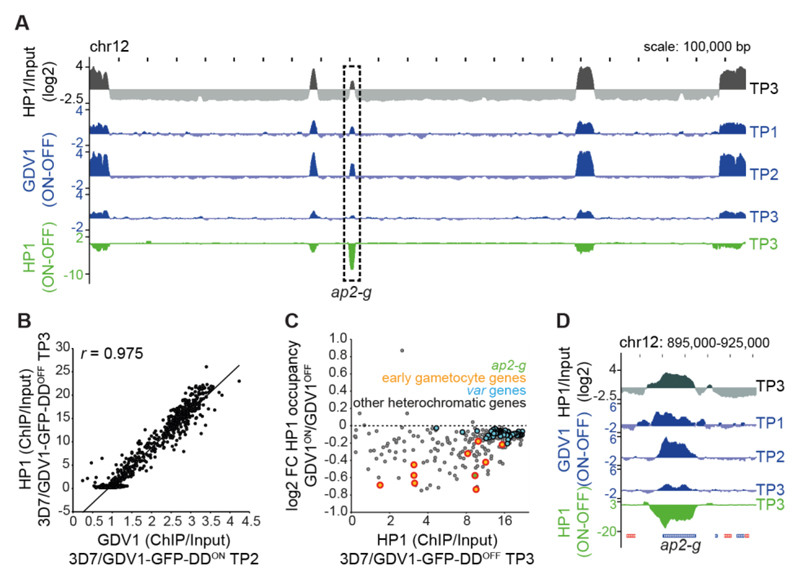
To test if GDV1 associates with heterochromatin in vivo we conducted comparative ChIP-seq experiments. 3D7/GDV1-GFP-DDOFF parasites were split at 28-34 hpi, cultured in parallel in the absence or presence of Shield-1 and paired chromatin samples were harvested two (30-36 hpi), six (34-40 hpi) and ten (38-44 hpi) hours after Shield-1 addition. We found that (1) GDV1-GFP-DD associates specifically with heterochromatin throughout the genome (Fig. 3A, Fig. S5, Table S7); (2) GDV1-GFP-DD occupancy was markedly higher in 3D7/GDV1-GFP-DDON compared to 3D7/GDV1-GFP-DDOFF parasites (Fig. S5, Table S7); and (3) GDV1-GFP-DD occupancy is highly correlated with that of HP1 (Fig. 3B). Moreover, GDV1-GFP-DD occupancy peaked six hours post-induction and decreased substantially thereafter (Fig. 3A, Fig. S5, Table S7). This drop in GDV1-GFP-DD signal coincided with a reduced HP1 occupancy over heterochromatic genes in 3D7/GDV1-GFP-DDON compared to 3D7/GDV1-GFP-DDOFF parasites (Fig. 3, A and C, Table S7). While the vast majority of heterochromatic loci, in particular those displaying high HP1 occupancy such as var genes, displayed only slightly decreased HP1 levels, some genes exhibited as much as 40% reduced HP1 occupancy (Fig. 3C and Table S7). This group of genes includes ap2-g and most known HP1-associated early gametocyte markers including geco (21), pfgexp17 (22) and pfg14_748 (8, 17) (Fig. 3, C and D, Table S7). These data are consistent with the microarray results, where GDV1-GFP-DD expression activated ap2-g and early gametocyte genes but had no effect on the expression of the bulk of heterochromatic loci including var genes (Fig. 2, G and H). Of note, given the 50-60% sexual conversion rate observed for 3D7/GDV1-GFP-DDON parasites (see above), a 30-40% reduction in HP1 occupancy indicates that HP1 may be depleted at these loci specifically in sexually committed parasites but single cell approaches are required to confirm this hypothesis. Overall, we suggest that GDV1 destabilises heterochromatin and thus allows specific transcription factors to activate expression of ap2-g and other gametocyte-specific heterochromatic genes, and this may play an important role in the positive auto-regulatory feedback loop proposed to reinforce AP2-G expression in committed parasites (5, 6, 23). How GDV1 achieves specificity in unlocking specific HP1-associated genes despite binding heterochromatin genome-wide is a challenging question to be addressed in the future.
Fig. 3. GDV1 associates with heterochromatin throughout the genome and triggers HP1 removal at ap2-g.
(A) HP1 over input ratio track from 3D7/GDV1-GFP-DDOFF schizonts (38-44 hpi, TP3) (grey). ChIP-seq subtraction tracks display relative enrichment of GDV1-GFP-DD in 3D7/GDV1-GFP-DDON schizonts two (30-36 hpi, TP1), six (34-40 hpi, TP2) and ten (38-44 hpi, TP3) hours after Shield-1 addition (blue), and relative depletion of HP1 in 3D7/GDV1-GFP-DDON parasites at TP3 (green). (B) Correlation between GDV1-DD-GFP enrichment in 3D7/GDV1-GFP-DDON (34-40 hpi, TP2) and HP1 enrichment in 3D7/GDV1-GFP-DDOFF schizonts at each coding region. r, Pearson correlation coefficient. (C) Fold change in HP1 enrichment upon GDV1-GFP-DD overexpression in relation to HP1 enrichment in 3D7/GDV1-GFP-DDOFF schizonts for each heterochromatic gene. (D) Zoom-in view of the enrichment/subtraction tracks at the ap2-g locus.
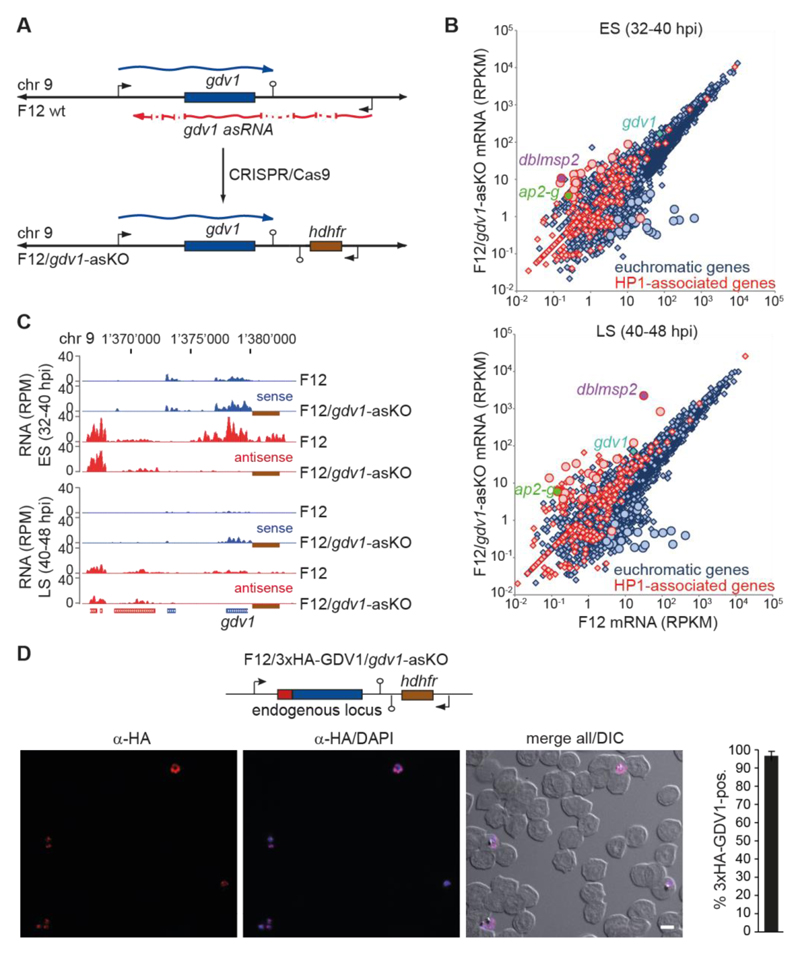
Since GDV1 activates sexual commitment, the question arises of how parasites limit GDV1 expression to prevent sexual conversion in asexual schizonts. A recent study identified a multi-exon long non-coding gdv1 antisense RNA (asRNA) that initiates downstream of the gdv1 locus and overlaps with the ATG start codon of gdv1 (24), which is a hallmark feature of regulatory asRNAs (25). To investigate if the gdv1 asRNA participates in regulating sexual commitment we created a gdv1 asRNA loss-of-function mutant in F12 parasites (F12/gdv1-asKO) (Fig. 4A and Fig. S6). Strand-specific RNA-seq analysis identified a small set of genes that were consistently differentially expressed between F12/gdv1-asKO and F12 wild-type parasites (17 up- and 23 down-regulated genes) (Fig. 4B, Table S8). Strikingly, and similar to F12 parasites expressing ectopic GDV1-GFP-DD (Fig. 2H), ap2-g, dblmsp2 and two early gametocyte genes (pfg14_748, PF3D7_1477400) (8, 17) were markedly induced in F12/gdv1-asKO parasites, and all except one up-regulated gene are HP1-associated genes (Fig. 4B, Fig. S6, Table S8). gdv1 sense transcripts were slightly increased in the F12/gdv1-asKO population, while gdv1 antisense transcripts were undetectable as expected (Fig. 4, B and C, Fig. S6, Table S8). These results indicated that the gdv1 asRNA acts as a negative regulator of GDV1 expression. To confirm this hypothesis, we tagged endogenous GDV1 in these parasites (F12/3xHA-GDV1/gdv1-asKO) and observed that indeed almost all parasites expressed 3xHA-GDV1 (96.7% +/- 2.5 SD) (Fig. S7). Lastly, we show that deletion of the gdv1 asRNA locus in a conditional AP2-G mutant resulted in a markedly increased production of gametocytes (Fig. S8 and Supplementary text). Together, these findings demonstrate a central role for the gdv1-asRNA in regulating GDV1-dependent activation of sexual commitment. We anticipate this mechanism likely involves inhibiting GDV1 expression by interference with gdv1 mRNA transcription, stability or translation, similar to asRNA-mediated gene regulation in other organisms (26).
Fig. 4. A gdv1 antisense RNA antagonises GDV1-dependent sexual commitment.
(A) gdv1 locus in F12 wild-type and F12/gdv1-asKO parasites. The gdv1 sense transcript (blue), five-exon gdv1-asRNA (24) (red) and hdhfr resistance marker (brown) are highlighted. (B) Comparison of gene expression levels in F12 wild-type and F12/gdv1-asKO early (ES) and late (LS) schizonts. Genes de-regulated > 5-fold in both TPs are indicated by circles. (C) UCSC genome browser screenshots of RNA-seq coverage plots over the gdv1 locus in F12 wild-type and F12/gdv1-asKO early (ES) and late (LS) schizonts. The hdhfr resistance cassette downstream of the gdv1 locus in F12/gdv1-asKO parasites and absent in the 3D7 reference genome is indicated by a brown box. (D) Endogenous gdv1 locus in F12/3xHA-GDV1/gdv1-asKO parasites and α-HA overview IFA in early schizonts (ES, 32-40 hpi). DIC, differential interference contrast. Scale bar, 5 μm. Percentage of 3xHA-GDV1-positive parasites is shown at the right (results are the mean of three biological replicates (100 infected RBCs counted per sample); error bars indicate SD).
We identified GDV1-mediated heterochromatin destabilisation as an epigenetic control strategy regulating sexual cell fate decision in P. falciparum. Our discovery of the gdv1-asRNA as a negative regulator of sexual commitment is reminiscent of lncRNA-mediated control of gametogenesis in yeasts (27, 28). In S. cerevisiae, nutritional stress triggers gametogenesis by activating the transcriptional regulator Inducer of Meiosis 1 (IME1) (28). A lncRNA in the ime1 promoter and antisense transcription of ime4 are key factors in preventing IME1 expression under non-inducing conditions (29, 30). These parallels raise the exciting possibility that evolutionary divergent unicellular eukaryotes may employ a conceptually similar regulatory logic to control entry into the sexual phases of their life cycles. Interestingly, all Plasmodium species infecting humans possess a GDV1 ortholog suggesting the GDV1-based regulation of sexual commitment is conserved in all human-infective malaria parasites. In conclusion, our study contributes to understanding the molecular pathway underlying the formation of malaria transmission stages and provides opportunities for the development of intervention strategies targeting transmission of human malaria.
Supplementary Material
One sentence summary.
The nuclear factor GDV1 induces gametocyte differentiation by activating expression of the master transcription factor AP2-G
Acknowledgements
We are grateful to M. van de Vegte-Bolmer and R. Sauerwein for determining gametocyte sex ratios and providing α-Pfs16 antibodies, to D. Richard for providing the pL6-3HA_glmS-246 plasmid and to T. Haefliger for technical assistance. This work was supported by the Swiss National Science Foundation (grant numbers 31003A_143916, 31003A_163258, BSCGI0_157729), the Foundation Pasteur Suisse and the Netherlands Organization for Scientific Research (NWO-Vidi 864.11.007). All data and code to understand and assess the conclusions of this research are available in the main text, supplementary materials and via the following repository: Gene Expression Omnibus (www.ncbi.nlm.nih.gov/geo) (31) accession GSE95549 (microarray data) and GSE94901 (ChIP-seq and RNA-seq data). M.F. designed and performed experiments, analysed data, prepared illustrations and wrote the paper. S.A.F. performed and analysed ChIP-Seq and RNA-seq experiments. I.N. designed and cloned CRISPR/Cas9 mother plasmids and performed experiments involving recombinant proteins. N.M.B.B. performed experiments related to the 3D7/3xHA-GDV1 and F12/3xHA-GDV1/gdv1-asKO parasites. E. Carrington performed and analysed RT-qPCR experiments. E. Carrió performed experiments involving 3D7/AP2-G-GFP-DDglmS parasites. S.M. performed LC-MS/MS experiments. P.J. provided conceptual advice. P.J., R.B and T.S.V. provided resources. R.B. designed, supervised and analysed experiments. T.S.V. conceived of the study and designed, supervised, and analysed experiments and wrote the paper. All authors contributed to editing of the manuscript.
References and Notes
- 1.Kwon SH, Workman JL. The heterochromatin protein 1 (HP1) family: put away a bias toward HP1. Mol Cells. 2008;26:217–227. [PubMed] [Google Scholar]
- 2.Flueck C, et al. Plasmodium falciparum heterochromatin protein 1 marks genomic loci linked to phenotypic variation of exported virulence factors. PLoS Pathog. 2009;5:e1000569. doi: 10.1371/journal.ppat.1000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rovira-Graells N, et al. Transcriptional variation in the malaria parasite Plasmodium falciparum. Genome Res. 2012;22:925–938. doi: 10.1101/gr.129692.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voss TS, Bozdech Z, Bartfai R. Epigenetic memory takes center stage in the survival strategy of malaria parasites. Curr Opin Microbiol. 2014;20:88–95. doi: 10.1016/j.mib.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Kafsack BF, et al. A transcriptional switch underlies commitment to sexual development in malaria parasites. Nature. 2014;507:248–252. doi: 10.1038/nature12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sinha A, et al. A cascade of DNA-binding proteins for sexual commitment and development in Plasmodium. Nature. 2014;507:253–257. doi: 10.1038/nature12970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brancucci NM, et al. Heterochromatin protein 1 secures survival and transmission of malaria parasites. Cell Host Microbe. 2014;16:165–176. doi: 10.1016/j.chom.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Eksi S, et al. Plasmodium falciparum gametocyte development 1 (Pfgdv1) and gametocytogenesis early gene identification and commitment to sexual development. PLoS Pathog. 2012;8:e1002964. doi: 10.1371/journal.ppat.1002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banaszynski LA, et al. A rapid, reversible, and tunable method to regulate protein function in living cells using synthetic small molecules. Cell. 2006;126:995–1004. doi: 10.1016/j.cell.2006.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armstrong CM, Goldberg DE. An FKBP destabilization domain modulates protein levels in Plasmodium falciparum. Nat Methods. 2007;4:1007–1009. doi: 10.1038/nmeth1132. [DOI] [PubMed] [Google Scholar]
- 11.Gaspar-Maia A, et al. Chd1 regulates open chromatin and pluripotency of embryonic stem cells. Nature. 2009;460:863–868. doi: 10.1038/nature08212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bugga L, McDaniel IE, Engie L, Armstrong JA. The Drosophila melanogaster CHD1 chromatin remodeling factor modulates global chromosome structure and counteracts HP1a and H3K9me2. PLoS ONE. 2013;8:e59496. doi: 10.1371/journal.pone.0059496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruce MC, Alano P, Duthie S, Carter R. Commitment of the malaria parasite Plasmodium falciparum to sexual and asexual development. Parasitology. 1990;100(Pt 2):191–200. doi: 10.1017/s0031182000061199. [DOI] [PubMed] [Google Scholar]
- 14.Silvestrini F, Alano P, Williams JL. Commitment to the production of male and female gametocytes in the human malaria parasite Plasmodium falciparum. Parasitology. 2000;121(Pt 5):465–471. doi: 10.1017/s0031182099006691. [DOI] [PubMed] [Google Scholar]
- 15.Bruce MC, et al. Cellular location and temporal expression of the Plasmodium falciparum sexual stage antigen Pfs16. Mol Biochem Parasitol. 1994;65:11–22. doi: 10.1016/0166-6851(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 16.Brancucci NMB, et al. Lysophosphatidylcholine Regulates Sexual Stage Differentiation in the Human Malaria Parasite Plasmodium falciparum. Cell. 2017;171 doi: 10.1016/j.cell.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eksi S, et al. Identification of a subtelomeric gene family expressed during the asexual-sexual stage transition in Plasmodium falciparum. Mol Biochem Parasitol. 2005;143:90–99. doi: 10.1016/j.molbiopara.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 18.Alano P, et al. Plasmodium falciparum: parasites defective in early stages of gametocytogenesis. Exp Parasitol. 1995;81:227–235. doi: 10.1006/expr.1995.1112. [DOI] [PubMed] [Google Scholar]
- 19.Pearce JA, et al. Characterisation of two novel proteins from the asexual stage of Plasmodium falciparum, H101 and H103. Mol Biochem Parasitol. 2005;139:141–151. doi: 10.1016/j.molbiopara.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 20.Amambua-Ngwa A, et al. Population genomic scan for candidate signatures of balancing selection to guide antigen characterization in malaria parasites. PLoS Genet. 2012;8:e1002992. doi: 10.1371/journal.pgen.1002992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morahan BJ, et al. Functional analysis of the exported type IV HSP40 protein PfGECO in Plasmodium falciparum gametocytes. Eukaryot Cell. 2011;10:1492–1503. doi: 10.1128/EC.05155-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silvestrini F, et al. Protein export marks the early phase of gametocytogenesis of the human malaria parasite Plasmodium falciparum. Mol Cell Proteomics. 2010;9:1437–1448. doi: 10.1074/mcp.M900479-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poran A, et al. Single-cell RNA sequencing reveals a signature of sexual commitment in malaria parasites. Nature. 2017;551:95–99. doi: 10.1038/nature24280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Broadbent KM, et al. Strand-specific RNA sequencing in Plasmodium falciparum malaria identifies developmentally regulated long non-coding RNA and circular RNA. BMC Genomics. 2015;16:454. doi: 10.1186/s12864-015-1603-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huber F, et al. Protein Abundance Control by Non-coding Antisense Transcription. Cell Rep. 2016;15:2625–2636. doi: 10.1016/j.celrep.2016.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pelechano V, Steinmetz LM. Gene regulation by antisense transcription. Nat Rev Genet. 2013;14:880–893. doi: 10.1038/nrg3594. [DOI] [PubMed] [Google Scholar]
- 27.Hiriart E, Verdel A. Long noncoding RNA-based chromatin control of germ cell differentiation: a yeast perspective. Chromosome Res. 2013;21:653–663. doi: 10.1007/s10577-013-9393-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Werven FJ, Amon A. Regulation of entry into gametogenesis. Philos Trans R Soc Lond B Biol Sci. 2011;366:3521–3531. doi: 10.1098/rstb.2011.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gelfand B, et al. Regulated antisense transcription controls expression of cell-type-specific genes in yeast. Mol Cell Biol. 2011;31:1701–1709. doi: 10.1128/MCB.01071-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Werven FJ, et al. Transcription of two long noncoding RNAs mediates mating-type control of gametogenesis in budding yeast. Cell. 2012;150:1170–1181. doi: 10.1016/j.cell.2012.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alano P, Premawansa S, Bruce MC, Carter R. A stage specific gene expressed at the onset of gametocytogenesis in Plasmodium falciparum. Mol Biochem Parasitol. 1991;46:81–88. doi: 10.1016/0166-6851(91)90201-g. [DOI] [PubMed] [Google Scholar]
- 33.Trager W, Jensen JB. Cultivation of malarial parasites. Nature. 1978;273:621–622. doi: 10.1038/273621a0. [DOI] [PubMed] [Google Scholar]
- 34.Lambros C, Vanderberg JP. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979;65:418–420. [PubMed] [Google Scholar]
- 35.Witmer K, et al. Analysis of subtelomeric virulence gene families in Plasmodium falciparum by comparative transcriptional profiling. Mol Microbiol. 2012;84:243–259. doi: 10.1111/j.1365-2958.2012.08019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voss TS, et al. A var gene promoter controls allelic exclusion of virulence genes in Plasmodium falciparum malaria. Nature. 2006;439:1004–1008. doi: 10.1038/nature04407. [DOI] [PubMed] [Google Scholar]
- 37.Ghorbal M, et al. Genome editing in the human malaria parasite Plasmodium falciparum using the CRISPR-Cas9 system. Nat Biotechnol. 2014;32:819–821. doi: 10.1038/nbt.2925. [DOI] [PubMed] [Google Scholar]
- 38.Montague TG, et al. CHOPCHOP: a CRISPR/Cas9 and TALEN web tool for genome editing. Nucleic Acids Res. 2014;42:W401–W407. doi: 10.1093/nar/gku410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Labun K, et al. CHOPCHOP v2: a web tool for the next generation of CRISPR genome engineering. Nucleic Acids Res. 2016;44:W272–W276. doi: 10.1093/nar/gkw398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas JA, et al. Development and Application of a Simple Plaque Assay for the Human Malaria Parasite Plasmodium falciparum. PLoS ONE. 2016;11:e0157873. doi: 10.1371/journal.pone.0157873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Voss TS, et al. Identification of nuclear proteins that interact differentially with Plasmodium falciparum var gene promoters. Mol Microbiol. 2003;48:1593–1607. doi: 10.1046/j.1365-2958.2003.03528.x. [DOI] [PubMed] [Google Scholar]
- 42.Schindelin J, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Daubenberger CA, et al. The N'-terminal domain of glyceraldehyde-3-phosphate dehydrogenase of the apicomplexan Plasmodium falciparum mediates GTPase Rab2-dependent recruitment to membranes. Biol Chem. 2003;384:1227–1237. doi: 10.1515/BC.2003.135. [DOI] [PubMed] [Google Scholar]
- 44.Malakhov MP, et al. SUMO fusions and SUMO-specific protease for efficient expression and purification of proteins. J Struct Funct Genomics. 2004;5:75–86. doi: 10.1023/B:JSFG.0000029237.70316.52. [DOI] [PubMed] [Google Scholar]
- 45.Flueck C, et al. A major role for the Plasmodium falciparum ApiAP2 protein PfSIP2 in chromosome end biology. PLoS Pathog. 2010;6:e1000784. doi: 10.1371/journal.ppat.1000784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Studier FW. Protein production by auto-induction in high density shaking cultures. Protein Expr Purif. 2005;41:207–234. doi: 10.1016/j.pep.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 47.Ponnudurai T, Lensen AH, Meis JF, Meuwissen JH. Synchronization of Plasmodium falciparum gametocytes using an automated suspension culture system. Parasitology. 1986;93(Pt 2):263–274. doi: 10.1017/s003118200005143x. [DOI] [PubMed] [Google Scholar]
- 48.Carter R, Graves PM. Gametocytes. In: Wernsdorfer WH, McGregor I, editors. Malaria: Principles and Practice of Malariology. Vol. 1. Churchill Livingstone; Edinburgh: 1988. pp. 253–305. [Google Scholar]
- 49.Hoeijmakers WA, Bartfai R, Francoijs KJ, Stunnenberg HG. Linear amplification for deep sequencing. Nat Protoc. 2011;6:1026–1036. doi: 10.1038/nprot.2011.345. [DOI] [PubMed] [Google Scholar]
- 50.Kensche PR, et al. The nucleosome landscape of Plasmodium falciparum reveals chromatin architecture and dynamics of regulatory sequences. Nucleic Acids Res. 2016;44:2110–2124. doi: 10.1093/nar/gkv1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bozdech Z, et al. The transcriptome of the intraerythrocytic developmental cycle of Plasmodium falciparum. PLoS Biol. 2003;1:E5. doi: 10.1371/journal.pbio.0000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Painter HJ, Altenhofen LM, Kafsack BF, Llinas M. Whole-genome analysis of Plasmodium spp. Utilizing a new agilent technologies DNA microarray platform. Methods Mol Biol. 2013;923:213–219. doi: 10.1007/978-1-62703-026-7_14. [DOI] [PubMed] [Google Scholar]
- 53.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saldanha AJ. Java Treeview--extensible visualization of microarray data. Bioinformatics. 2004;20:3246–3248. doi: 10.1093/bioinformatics/bth349. [DOI] [PubMed] [Google Scholar]
- 55.Hoeijmakers WA, Bartfai R, Stunnenberg HG. Transcriptome analysis using RNA-Seq. Methods Mol Biol. 2013;923:221–239. doi: 10.1007/978-1-62703-026-7_15. [DOI] [PubMed] [Google Scholar]
- 56.Prommana P, et al. Inducible knockdown of Plasmodium gene expression using the glmS ribozyme. PLoS ONE. 2013;8:e73783. doi: 10.1371/journal.pone.0073783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Watson PY, Fedor MJ. The glmS riboswitch integrates signals from activating and inhibitory metabolites in vivo. Nat Struct Mol Biol. 2011;18:359–363. doi: 10.1038/nsmb.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Winkler WC, et al. Control of gene expression by a natural metabolite-responsive ribozyme. Nature. 2004;428:281–286. doi: 10.1038/nature02362. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.