Abstract
In the transition from B cells to antibody secreting cells (ASCs) many genes are induced, like ELL2, Irf4, Prdm1, Xbp1, while other mRNAs do not change in abundance. Nonetheless using splicing array technology and mouse splenic B cells +/− LPS we found that induced and “un-induced” genes can show large differences in splicing patterns between the cell stages which could influence ASC development. We found that about 55% of these splicing changes depend on ELL2, a transcription elongation factor that influences expression levels and splicing patterns of ASC signature genes, genes in the cell-cycle and N-glycan biosynthesis and processing pathways, and the secretory vs membrane forms of the Ig heavy chain mRNA. Some of these changes occur when ELL2 binds directly to the genes encoding those mRNAs while some of the changes are indirect. To attempt to account for the changes that occur in RNA splicing before or without ELL2 induction, we examined the amount of the snRNA molecules (small nuclear RNA) and found that they were significantly decreased within 18 hours of LPS stimulation and stayed low until 72 hours. Correlating with this, at 18 hours after LPS, endo-reticular (ER) stress and Ire1 phosphorylation are induced. Inhibiting the regulated Ire1 dependent mRNA decay pathway (RIDD) with 4u8C correlates with the reduction in snRNA and changes in the normal splicing patterns at 18 hours. Thus we conclude that the RNA splicing patterns in ASCs are shaped early by ER stress and Ire1 phosphorylation and later by ELL2 induction.
Introduction
The majority of multi-exon containing mammalian genes are alternatively spliced, thereby producing on average 4–5 differently spliced products (1) with a large variation in proteins expressed (2). Alternative mRNA isoforms play important roles in normal development and physiology. Yet little is known beyond the Igh gene itself and the U1A small nuclear RNA (snRNA) protein expression about the overall landscape of alternative splicing and its effect on the path from B cells to antibody secreting cells. Upon stimulation by antigen, cytokines, or lipopolysaccharide, naïve B cells drastically alter gene expression in order to become antibody secreting cells (ASCs) (3). Thus far RNA processing reactions have been implicated only in a small number of the changes seen in the differentiation to antibody secretion but are expected to play significant roles in others.
Extensive work from our lab (4) and that of others (5, 6) has shown that alternative 5’ donor and 3’ acceptor splice sites in the Igh mu gene are used in B cells while in ASCs the weak 5’ splice site embedded in Igh mu CH4 is ignored to make secreted Igh mu mRNA and protein. Concomitantly there is a 10–100 fold increase in abundance of that mRNA over B cells because the RNA polymerase II (RNAPII) more efficiently transits the Igh mu gene, allows better recognition of the secretory-specific poly(A) site, while engaging the highly induced transcription elongation factor ELL2 (eleven-nineteen lysine-rich leukemia gene), an important part of the super elongation complex (7–11). This led us to ask if ELL2 could influence the splicing of genes other than Igh in the B cell to ASC transition.
Meanwhile we had also found a significant decrease in the amount of snRNP-associated U1A following stimulation to produce ASCs (12). This observation led us to ask if there were changes in the small nuclear RNAs that might also be involved in altering splicing when B cells differentiate into ASCs. Previous studies showing that snRNAs could change splicing patterns include during Drosophila development (13), in Alzheimer’s disease where the changed levels of U1 snRNA lead to altered RNA processing of several mRNAs (14), and human diseases like hemato-lymphoid neoplasia, retinitis pigmentosa, and microcephalic osteodysplastic primoerdial dwarfism type 1 (MOPD1) associated with a loss of U4atac snRNA (15).
When B cells are stimulated to secrete antibody, the primary pathway for endoplasmic reticulum (ER) remodeling (aka the unfolded protein response, or UPR) appears to uniquely include only the phosphorylation of Ire1 (inositol-requiring enzyme 1 alpha) (16, 17) not the Perk or Atf6 pathways seen in other cells. We had previously shown that mouse splenic B cells deficient in ELL2 are unable to secrete Ig after LPS stimulation but still maintain Ire1 phosphorylation, an ER stress sensor (9). Interestingly the phosphorylation of Ire1 occurs even when Igh mu secretion is rendered moot by mutations in the Igh mu gene itself and in the activation-induced cytidine deaminase AID/Aicda gene to prevent subclass-switching (18). Therefore, much of the ER stress that occurs in B cell activation precedes Ig secretion. The phosphorylation of Ire1 results in acquisition of a regulated IRE dependent mRNA decay (19, 20) activity (RIDD). We wondered if this pathway could play a role in the stark transcriptional divide between B cells and antibody secreting cells and influence splicing patterns in some way.
Here we analyzed the changes in splicing between resting primary splenic mouse B cells and their resultant LPS stimulated ASCs. We sought to understand the forces which could alter the splicing patterns. We compared splicing patterns in B cell conditional knockouts of ELL2 with ELL2 positive litter mates and found that some but not all of the changes in alternative splicing were altered by eliminating that induced elongation factor. We explored what else might be responsible for the altered splicing. We found that the levels of all the classical and non-canonical (AT/AC) RNAPII transcribed snRNAs were reduced following LPS stimulation. Eliminating the RIDD activity of Ire1 changed snRNA levels and changed splicing patterns. Overall then, ER stress and ELL2 induction contribute to the changes seen in RNA splicing in the development of antibody secreting cells.
Methods and Materials
Affymetrix Clariom D RNA microarray and analysis
RNA samples from at least three independent biological samples were obtained as described below and sent to the University of Pittsburgh Genomics Research Core where the Affymetrix array was performed. Analysis of the data was performed using Transcriptome Analysis Console (TAC) software from Affymetrix. Samples were analyzed using default parameters. An alternative splicing event was defined as a differentially expressed exon with at least a 2-fold change in expression, at a p value of p=0.05 or better. The TAC software was set to identify the change in exon expression as one of the 5 classifications for alternative splicing based on the expression patterns of the surrounding exons. For gene enrichment analysis, gene lists were submitted to the ConsensusPathwayDB website developed by the Max Planck Institute for Molecular Genetics. Over-representation analysis returned pathway-based set results. The most enriched pathways were grouped and tabulated.
Mice
The derivation and characterization of the ELL2 loxp/loxp exon3 mice crossed with the C57BL/6 CD19cre mice (Jackson Labs #006785) to inhibit Ig secretion was described previously (9). Naïve splenic B cells were extracted from those mice that had been crossed with either CD19+/+ (designated wild type littermates) or CD19+/cre (exhibiting a conditional knock out of exon 3 in the ELL2 gene). Deletion of the exon 3 of ELL2 in cre+ mice in LPS stimulated B cells and reduction in Igh secretory mRNA were verified by a real time PCR assay with the DNA and mRNA respectively. All mice were maintained at the University of Pittsburgh animal facilities, and experiments were undertaken and conducted in accordance with institutional policies as per Animal Welfare Assurance number A3187–01. The ELL2 exon 3 loxp/loxp mice have been deposited at Jackson Labs as stock #029528 and are available for unrestricted use.
B cell isolation and stimulation
Naïve splenic B cells were extracted from 6 to 20 week old ELL2 loxp/loxp exon 3 mice, with either CD19+/+ or CD19+/cre, and isolated using the autoMACS system (#130–090-862; Miltenyi Biotec) as previously described (9). The cells were cultured in medium on day zero at 5 × 106 cells/ml. The cells were counted via hemocytometer, split, and cultured at 1×106 cells/ml for up to 72 hours with LPS at 20μg/ml in RPMI 1640 medium containing 50 μM 2-ME, 2 mM glutamine, 10% FBS, 1 mM sodium pyruvate, nonessential amino acids, 1X Pen/Strep, and 1mM HEPES buffer. CD138+ cells were isolated using CD138 MACS MicroBeads from MiltenyiBiotec (Cat# 130–098-257) as per the manufacturer’s instructions.
ChIP seq and ChIP Quantitative PCR
ChIP seq arrays were performed by Active Motif (Carlsbad, CA). DNA was prepared for amplification by converting overhangs into phosphorylated blunt ends and adding an adenine to the 3’-ends. Illumina genomic adapters were ligated and the sample was size-fractionated (200–300 bp) on an agarose gel. After a final PCR amplification step (18 cycles), the resulting DNA libraries were quantified and sequenced on HiSeq 2000. Sequences (50 nt reads, single end) were aligned to the mouse genome (mm10) using the BWA algorithm. Alignments were extended in silico at their 3’-ends to a length of 150 bp. Active Motif used their proprietary software to analyze the data. More than 17 million reads were obtained and peaks were called using the MACS (v.1.4.2) algorithm and a cutoff p-value of p=0.00005. Reads were aligned to the mouse genome (mm10) and visualized in the Integrated Genome Browser (UCSC). The anti-ELL2 mouse polyclonal anti-peptide antibody 4502 (10) was used in triplicate for the positive signal and compared with a control non-specific rabbit antibody with the AxJ mouse plasma cell line (IgG2a, k, lambda) we have studied extensively(7). The AxJ cells were verified by sequencing the heavy chain V-region. Confirmation of a portion of the ChIP seq data was performed with conventional chromatin immunoprecipitations as previously described (9) using the Q-PCR probes in the list of primers in Supplemental Table1.
IRE1 inhibitor treatments
Primary splenic B cells were treated with Ire1 ribo nuclease inhibitor 4¼8c (Sigma) at a concentration of 5 microM (21) in DMSO in the presence of LPS as described above. Samples were taken at 0, 6, 18 and 24 hrs. The 18 hour samples were used for the array and western data. Control cells were treated with equivalent amount of DMSO and taken at the same time points.
RNA isolation and quantitative RT-QPCR
RNA was isolated from cells using the RNeasy Mini Kit (Qiagen #74106) or Macherey-Nagel nucleospin RNA/ protein kit (Cat# 740933). RNA was copied using Superscript First Strand Synthesis for RT-QPCR following the random oligonucleotide procedure (Invitrogen #11904–018). Quantification by QPCR was performed using Sybr® Green PCR Master Mix (Applied Biosystems #4909155) along with the identified primers (Supplemental Table 1). The instrument used was a StepOnePlus Real-time QPCR System from Applied Biosystems. The Ct values were normalized as log fold change relative Hprt as a constant, and GraphPad Prism 7 software was used for graphing and statistical analyses. B cell and Go values were normalized to 100.
Statistics
Data are shown with S.E.M. error bars and significance calculated using GraphPad Prism 7 software with a 2 way ANOVA such that *= P<0.05; ** =P<0.01, and ***=P<0.001. Experiments in Figures 1B, 2A, 2C, 4B, all of 5 and 6 were performed at least three times with at least three independent biological samples. We used Hprt, Gapdh and Actb mRNAs as internal controls; the level of Hprt mRNA was previously shown to remain constant through LPS stimulation of B cells (22, 23).
Western blotting
Protein samples were obtained from whole cell extracts that were sonicated briefly to reduce viscosity. Western protocol from the Bio-Rad General Protocol (Bulletin 6376 Rev A) was followed. Molecular weight markers were the Precision Protein Kaleidoscope markers from Bio-Rad (#161–0375). Bio-Rad precast Mini-PROTEAN gels were used. Antibodies used include: anti-Ire1 Abcam #37073; anti-Ire1ser724P from Thermo Fisher PA1–16927 (Invitrogen); anti-SnapC/43 kDa (k-16; sc20241) goat polyclonal from Santa Cruz; other primary and secondary antibodies as previously described (9).
Availability of the data
The ChIP-seq and Affymetrix splicing array data have been deposited at the NCBI GEO (Gene Expression Omnibus GSE 113317, GSE113475 and GSE114435) publicly available web sites. Affymetrix splicing array data accession # GSE113317 on 4/18/18 for +/− LPS 72 hrs and +/− ELL2 is viewable at: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE113317
Microarray data # GSE 113475 for the 0, 18 hr LPS +/− 4u8C deposited on 4/21/18 is viewable at: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE113475
Raw data for ChIP-seq data (GSE114435) is deposited and viewable at: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE114435 the data for ChIP seq is associated with Figure 4 and Supplemental Table 2. Affymetrix splicing data are associated with Figures 1, 2, 3, 7 and Supplemental Table 2. There are no restrictions on the availability of the datasets.
Figure 4.
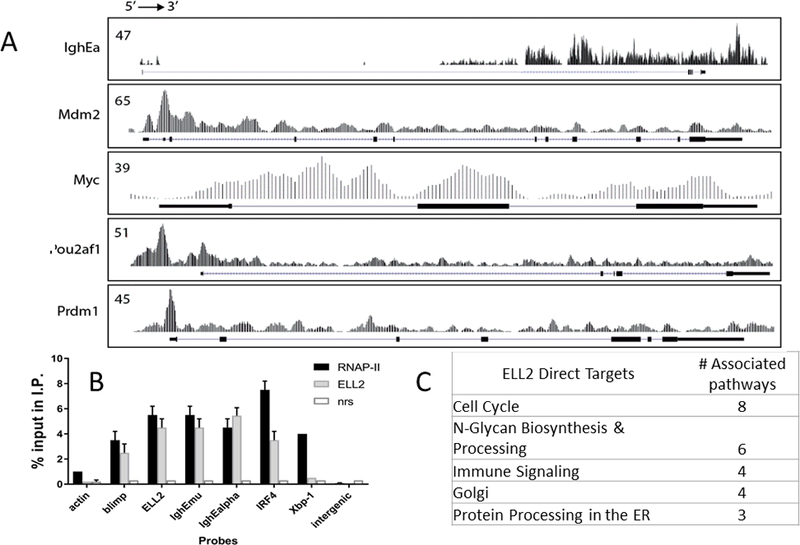
ELL2 binds to many genes (a) ChIPseq tracks of ELL2 on the Igh enhancer alpha region, along with other genes of interest. Track numbers on left indicate the highest number of tags per peak. mRNA coding region of each gene is shown below the tracks, exons in dark boxes. Direct targets of ELL2 with an influence on mRNA abundance and or splicing are enumerated in Supplemental Table 2. (b) Chromatin immunoprecipitation analysis of LPS stimulated antigen secreting cell chromatin from ELL2+/+ immunoprecipitated with antibody to RNA polymerase II (RNAP-II) or ELL2. Normal rabbit anti-sera (nrs) is a control. Immunoprecipitated (I.P.) DNA was analyzed by real time PCR relative to the input sample taken before the ChIP steps. At least three independent biological replicates were used for ChIP and the QPCRs were performed in triplicate.(c) Tally of pathway analyses of 343 alternatively spliced ELL2 direct targets by ChIP that are also alternatively spliced between the ELL2 +/+ and ELL2 cKO +LPS, with a significance p-value cutoff at p=0.001.
Figure 1.

Major splicing changes occur when B cells become antibody secreting cells(a)Transcriptome Analysis Console (TAC) software analysis of alternative splicing tracks of B cell example genes before and after LPS. The overall abundance of these sample mRNAs in this panel did not change. B cell (-LPS) splicing enrichment is indicated by orange, +LPS enrichment is represented by blue, with the intensity of the color corresponding to fold change in expression of that region of mRNA, depicted by the scale at the bottom. The indicated P7275/6 forward primer is in the standard exon, while the reverse is in the retained intron. The J5320 forward primer spans the junction between section 3b and 3d, while the reverse sits downstream in an exon. (b) Myd88 expression levels with validation primers in panel a, relative to Hprt, RT-QPCR are shown. The expression ratios (B: CD138+) of validation primers between the unstimulated B cells and stimulated CD138+ cells. Three independent biological samples were used with triplicate determinations in the QPCRs.(c) TAC software classifiers for differentially spliced events, broken down by number of genes and number of exons in each class. Comparison in ELL2+/+ cells in B cells vs LPS stimulated antibody secreting cells. Results determined averaging five independent biological replicates for the array data, see methods.
Figure 2.
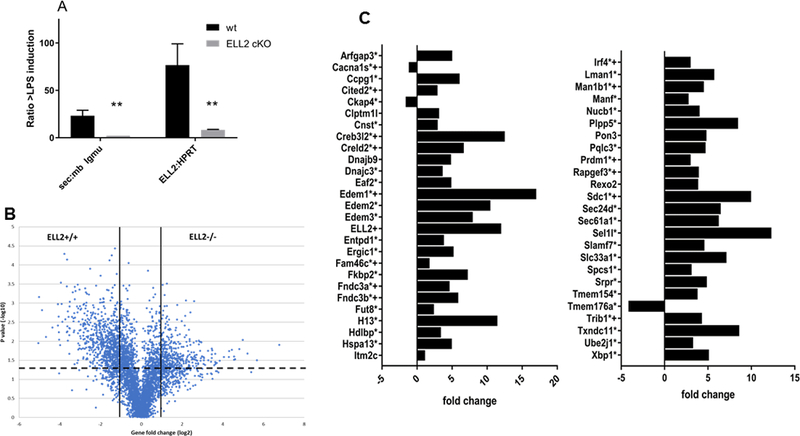
Loss of ELL2 changes gene expression levels in antibody secreting cells (a) mRNA levels in splenic B cells plus and minus ex vivo LPS for 72 hr. The ELL2 conditional knockout (ELL2 cKO) mice are ELL2 loxp/loxp CD19cre/+ and the wild type (wt) are their ELL2+/+ litter mates. RNA was converted to cDNA and real time PCR conducted using the secretory specific or membrane specific probes for Igmu heavy chain or a probe for exon 3 of ELL2 normalized to Hprt. Error bars indicate SEM and ** p=0.01 from three independent biological replicates and triplicate QPCRs.(b) mRNA levels in LPS stimulated ELL2+/+ and ELL2 −/− (conditional knockout) cells 72 hrs after LPS addition. Dashed line indicates p-value = 0.05 (ANOVA), solid lines show linear fold change of +/− 2. Genes are named in Supplemental Table 2.(c)Expression profile changes in 48 signature antibody secreting cell genes, plus Prdm1 and Irf4. Bars represent the linear fold change in expression of each gene, with positive fold-change values indicating higher levels in the ELL2+/+ than the ELL2 cKO cells. Error bars are omitted for clarity. The “+” notation indicates that the gene binds ELL2 in ChIP, * indicates genes that mRNAs are alternatively spliced after LPS stimulation in ELL2+/+ cells. Three independent biological samples were used for each type of mouse and QPCRs performed in triplicate.
Figure 3.
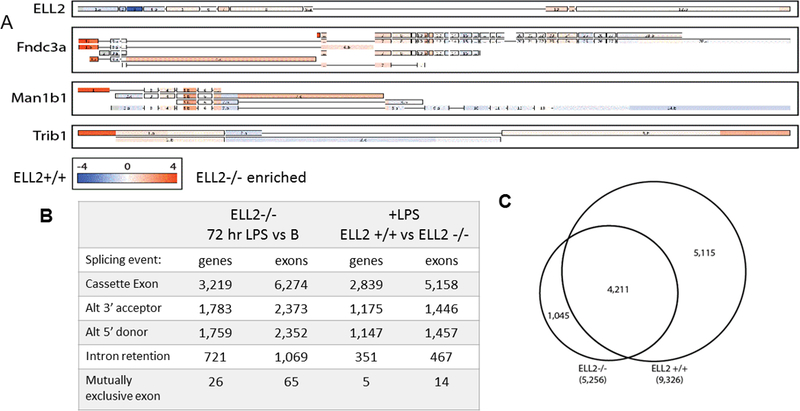
ELL2 impacts alternative splicing (a) TAC software generated splicing tracks of selected antibody secreting cell signature genes comparing stimulated ELL2 sufficient and deficient cells. ELL2+/+ enrichment is indicated by blue, ELL2 cKO enrichment is represented by orange, with the intensity of the color corresponding to fold change in expression, depicted by the scale at the bottom.(b) TAC software classifiers for differentially spliced events, broken down by number of genes and number of exons. Comparison of ELL2−/− cells plus LPS for 72hr versus B cells and 72 hr LPS stimulated cells comparing ELL2+/+ versus ELL2 cKO. At least three independent biological samples were used for each genotype of mouse for the array data.(c)Venn diagram depicting the amount of overlap between alternatively spliced genes (B cells vs 72hr plus LPS) in the ELL2 +/+ and ELL2 cKO data sets.
Results
Major splicing changes occur when B cells become antibody secreting cells
While it is well known there are changes in the genes expressed between B cells and antibody secreting cells (ASCs), we wanted to determine if overall splicing changes were also occurring. We used splicing arrays and the Transcriptome Analysis Console (TAC) software from Affymetrix™ to answer this question. These arrays enable one to assess both the differences in overall expression levels as well as alternative splicing of individual mRNAs. First we validated the differentiation of our samples from B cells into ASCs by 72 hrs of LPS stimulation by observing their increased Ig heavy chain secretory mRNA, ELL2 mRNA production and CD138 surface protein acquisition, by Q-PCR and flow cytometry; these RNA changes in expression were also seen in the array data. In addition, in the data we obtained and deposited at the NCBI GEO site we noted that many key genes are induced to higher expression levels in the transition after 72 hrs of LPS treatment to the ASCs, like Irf4, blimp1 (Prdm1) and the ASC “signature genes” previously described (3). Meanwhile, many other genes do not change mRNA abundance, nonetheless we noted that they change their splicing patterns. We show the splicing tracks for three such genes in which mRNA abundance does not change, yet splicing does (Figure 1A); orange means that an exon is enriched in B cells while blue means it is enriched in ASCs. The more intense the color the larger the enrichment. These genes have been reported to have mRNA isoforms that can encode different proteins based on the splicing alterations. For example Hdac9, histone deacetylase 9, that is missing exon 7 is reported to be retained in the cytoplasm while proteins encoded by mRNA with exon 7 (dark blue), enriched here in ASCs, are reported to enter the nucleus to acetylate histones to activate gene transcription (24). Ptbp3, polypyrimidine track binding protein 3, is an RNA binding protein that mediates pre-mRNA alternative splicing. Exon 2 inclusion (seen here in orange), previously shown to be enriched in B cells, is known to result in greater nuclear localization and an extra RNA binding domain (25). As shown here, forms without exon 2 are enriched in ASCs. Myd88, myeloid differentiation primary response gene 88, a universal adapter protein used by almost all TLRs (except TLR 3) to transmit signals and activate the transcription factor NF-κB, has been shown to exist in a long form (with the orange retained intron) that activates immunity while the short form, without the intron, inhibits TLR signals and usually arises after LPS treatment (26, 27). We see these short forms after 72 hrs of LPS stimulation. Panel 1B validates the splicing array differences by QPCR for myD88. The array results for several other genes were also validated by QPCR (data not shown). Thus changing splicing patterns can influence mRNA function and in the examples illustrated the changed splicing patterns were shown to generate alternative forms of the protein. We saw changes in the splicing patterns when comparing B cells to ASCs (plus LPS for 72 hrs) in other genes that have previously been reported to be capable of producing different isoforms of mRNA and protein. Among those genes where the splicing patterns are changed at least 3-fold after 72 hrs of LPS in our microarray studies (see GEO data) are: Traf7, Zbtb20, DNAjb6 (hsp40), IL-2 receptor gamma chain, Ppp2r5c, Srsf1, Ikzf1, Cmip (c-maf), Elmo, Ugp2, Picalm, Tmem209, Hspa4 (hsp70), Mapk1, Xiap, Eifak3, Cstf3, and Srpk2. We have not pursued potential changes in protein expression by mass spectroscopy analyses for these, but the finding that these genes could be encoding alternative protein forms provides insight into the potential importance of altered RNA splicing during the B cell to ASC transition.
In the totality of mRNA coding genes that were expressed in both B and ASCs at 72 hrs, many have different splicing patterns that have not yet been shown to result in different protein isoforms. There are 9,326 genes that show RNA splicing differences in the two conditions as summarized in Figure 1C; note, some genes may have several types of splicing alterations so there are many more changes on summing the events than the total number of genes involved. The majority of genes with altered splicing show changes in a “Cassette Exon” event (6,533) which denotes the inclusion or skipping of a single exon in an mRNA isoform. Since there are 16,411 exons included or skipped in 6,533 genes where this happens, there are roughly 2.5 of these changes per gene. This is a remarkable number of changes. Use of alternative 5’ donor and 3’ splice acceptor sites are the next most common alternative splicing events. Intron retention can occur as well, with mutually exclusive exon usage occurring, but less frequently than the rest (0.7%). The enormity of the changes we see in B versus ASCs at 72 hrs after LPS indicates that the splicing landscape is significantly different between the two.
Loss of ELL2 changes gene expression levels in antibody secreting cells
While numerous splicing changes that were heretofore unappreciated could significantly impact the gene expression profile of antibody secreting cells, we focused not on the consequence of all those changes but rather on how these changes in the splicing milieu of the cells are brought about. Since transcription elongation rate and alternative splicing had been previously linked (28), we first hypothesized that based on its role as a transcription elongation factor, ELL2 might have a significant role in changing the landscape of alternative splicing in ASCs. We examined global changes in mRNA levels that might accompany loss of ELL2. As shown in Figure 2A when the ELL2 sufficient splenic B cells were stimulated with LPS for 72 hrs they produce 25-fold more of the secreted form of Ig mu heavy chain versus the membrane-specific form. Meanwhile, ELL2 deficient cells (cKO Figure 2A) were impaired in the production of the secretory specific form as we had previously shown (9, 29). ELL2 induction is also impaired in the conditional knockout cells. These were the samples used for our subsequent analyses. In data not shown we found that ELL2 is not significantly induced until 24 to 36 hrs post LPS treatment of B cells.
We assessed global expression after 72 hrs of LPS stimulation of splenic B cells and as shown in Figure 2B, upper left and right quadrants, in plus vs minus ELL2, a large number of genes (>1,000) are altered in their abundance. The majority show decreased levels in the absence of ELL2. Of these, 192 were V regions genes for heavy or light Ig chains. A complete list of the 851 non-V regions induced by ELL2 is shown in Table 2 in supplemental data while the complete data set is deposited at the NCBI GEO web site. ELL2 induction therefore has a broad effect on mRNA abundance in ASCs at 72 hrs post LPS.
Among the genes whose mRNA abundances are increased when ELL2 is present, we concentrated first on 48 “signature ASC genes” described as expressed at higher levels after all modes of stimulation to produce ASCs by Shi et al (3); Irf4 and Prdm1 (blimp1) are included for completion. As shown in Figure 2C a positive fold-change value is shown for the majority of the signature genes indicating higher levels of expression in the ELL2+/+ than the ELL2 cKO cells. There are just three exceptions. These data show that ELL2 has a major impact on the expression of the signature ASC genes, as well as Irf4 and Prdm1.
ELL2 impacts alternative splicing
We then asked how ELL2 would change the landscape of alternative splicing. The “ASC signature genes” marked with an asterisk (*) in Figure 2C show alternative splicing with ELL2 in the microarray studies (45/52) and are listed as such in Table 2 in supplemental material with the other ELL2 induced genes. A few examples of alternative splicing TAC (transcriptome analysis console) patterns of non-signature genes, also listed in Table 2, are shown in Figure 3A to indicate the specific ELL2 dependent changes in splicing patterns. These three have mRNAs isoforms with more abundant, extended 5’ UTRs when ELL2 is knocked out (orange). Longer 5’ UTRs have been shown to lead to mRNAs bearing them to be less effectively translated (30). Thus when ELL2 is absent the 5’ end of the mRNA is retained more often. The splicing pattern of ELL2 mRNA itself is shown in Figure 3A; when comparing the wild type (blue) to the knockout cells, exon 3 is much more abundant in the wild type since this is the exon flanked by loxP sites in the conditional knockout.
Extending beyond the 851 ELL2 induced genes we next turned to the global splicing profile in cells 72hrs post LPS when ELL2 is missing, summarized in Figure 3B. We see that the number of genes affected by differential splicing events in cells stimulated by LPS for 72 hrs in ELL2 deficient cells is significantly reduced in all categories relative to when ELL2 is present (Figure 3B); there are only 5,256 genes affected and there are fewer multiple splicing events per gene. Loss of ELL2 results in many changes in all the splicing types (cassette, 3’ acceptor, 5’ donor, intron retention, mutually exclusive exons), compare Figure 3B versus the ELL2 sufficient cells Figure 1C. Proportionally, the mutually exclusive exon category is most impacted by the loss of ELL2 (down to 0.2%). In order to understand how much of the alternatively splicing in ELL2 sufficient cells at 72 hrs requires the action of ELL2, we created the Venn diagram (Figure 3C). It shows that mRNA from 9,326 genes are alternatively spliced in cells 72hrs after LPS addition when ELL2 is present but among these only 4,211 are alternatively spliced when ELL2 is absent. Thus we conclude that significantly more than half of the 9,326 genes (that is the remaining 5,115) require ELL2 to be present to have the proper alternative splicing occur.
ELL2 binds to many genes for elongation
Next, we asked which mRNAs have genes that are direct targets of ELL2. In order to address this we performed ChIP-seq experiments using antibody to ELL2. We previously showed that ELL2 is found on chromatin with RNAPII through its association with the super elongation complex (31) (10). Chromatin immunoprecipitation was performed on a mouse plasma cell line and the products subjected to deep sequencing and analysis with the help of Active Motif and their ChIp-ITR software. There are ~2,000 candidates with values >10 tags per peak. Genes that bind ELL2 and that also show increased abundance in the presence of ELL2 are shown in Table 2 in supplementary data. The complete data set of 2,000 ELL2 ChIP targets is deposited at the NCBI GEO web site. ELL2 protein binds to a number of highly expressed genes including Atf6, Pou2af1 (aka OcaB, Bob1, Obf1), several heat shock genes, Ig heavy chain enhancers, both near the mu C region (Igh Emu) and downstream of the Igh alpha genes (Igh Ealpha), Igh constant regions, Ig heavy & light chain genes, Prdm1 (blimp1), Irf4, several ASC signature genes and Mdm2. The tracks of the ELL2 protein on several of the genes are shown in Figure 4A. In the tracks, ELL2 is often associated strongly with the region near the promoter where RNAPII is found in abundance on an active gene. The results from the ChIP-seq were confirmed using standard ChIP QPCR on several of the genes as shown in Figure 4B using LPS stimulated primary splenic B cells that were harvested 72 hrs after LPS stimulation and selected as CD138+ (i.e. ASCs). Therefore the changes in splicing expression of some genes we see are a direct result of ELL2 association after induction; meanwhile some changes after ELL2 induction are indirect. Some ASC signature genes shown in Figure 2C are direct targets of ELL2 (indicated by a + sign in that figure) along with more of the upregulated RNAs shown in the ELL2+/+ area in Figure 2B, see supplemental Table 2.
A pathway analysis (Figure 4C) of 343 of the ELL2 direct targets (from ChIP seq) that are also induced in expression and most highly alternatively spliced as shown here indicates that the genes are in eight cell cycle pathways, six N-glycan biosynthesis and processing pathways, four immune signaling pathways, four in Golgi pathways and five in protein processing in the ER. ELL2 binds to genes in these pathways, increases the abundance of their mRNAs, and changes their splicing patterns.
snRNA expression decreases after LPS stimulation
Having previously seen changes in U1A expression after stimulation of B cells to ASC (12) we wondered if the small RNAs themselves which directly participate in splicing are influencing and perhaps influenced by the differentiation of B cells to ASCs. Changing the level of the snRNAs might contribute to the additional splicing changes seen here as it has in the examples cited in the introduction. Splicing arrays and several other mRNA deep sequencing techniques under-represent the snRNAs, so we used RT-QPCR to investigate their amounts.
We examined the levels of the snRNAs before and after 72 hrs of LPS treatment of mouse splenic B cells by making cDNA using random primers and then RT-QPCR with U snRNA gene specific primers. As shown in Figure 5A, the levels of most of the small RNAs falls off in cells treated + LPS for 72 hrs (ASCs) relative to B cells (BC), set as 100% to normalize for the different basal levels of expression, with the levels of most of the Uatac snRNAs (U11, U4atac, U6atac) falling to less than 10% of that seen in B cells. The U6 snRNA gene is transcribed by RNAPIII in contrast to the others (32).
Figure 5.

snRNAs decrease after LPS stimulation(a) Quantitative PCR was performed with snRNA primers on random-primed cDNA from either ELL2+/+ B cells (BC) or from ELL2+/+ cells stimulated with LPS for 72 hours (+LPS). The amount of PCR product was normalized relative to the determination of Hprt mRNA in the same sample on the same PCR plate. The amount of product in the B cell (BC) sample was set to 100% and the amount of product +LPS was calculated as a % so that the data could be presented succinctly on one graph. A minimum of three independent biological replicates were used and QPCRs were performed at least three times at each time point for each snRNA.(b) Primers for the indicated mRNAs were used to determine the amount of each mRNA in BC and +LPS samples. Results are plotted as % time zero and performed multiple times as described in(c)Primers for the SnapC subunits were used in RT-QPCR and plotted and performed multiple times as described in (d)Western blot of protein samples from B cells or cells treated for 24 or 72 hours with LPS. SnapC1 mRNA encodes a 43 kDa protein. Xbp1 long and short forms are indicated. Actin was used as a loading control. This represents one sample of the representative data from among three independent biological replicates.
Next, we investigated whether the low levels of the Uatac snRNAs in particular might correlate with the expression of some genes known to contain the AT/AC style introns like U1A, HnrnpLL and Ints10. As shown in Figure 5B, the level of these mRNAs was significantly reduced after 72 hours of LPS. Other genes with AT/AC introns like Fyn were previously shown to decrease in ASCs (33, 34). When U4atac is mutated in humans, a condition called MOPD1 results and AT/AC introns are not properly spliced (35). This suggests that the drop we see in U snRNAs between splenic B cells and ASCs could influence the splicing of some AT/AC intron containing genes and perhaps other genes as well, either directly or indirectly.
We examined the levels of the mRNA encoding some of the snRNA transcription factors. The transcription of snRNAs requires cooperative binding of five SnapC subunit proteins with Oct1 (and or Oct2) to the octamer sequence for each of the snRNA enhancers (36, 37). A little elongation complex including Ice1 and Zc3h8 with ELL1 forms and travels with RNAPII to synthesize the snRNAs (38). The Integrator complex of at least five proteins is necessary for snRNA transcription termination. We showed that the mRNA for the Ints10 gene is decreased in Figure 5B and in Figure 5C we show that the level of mRNA for SnapC1 and SnapC2 are also decreased. This is also true for the SnapC1 43 kDa subunit protein as shown in Figure 5D.
Loss of snRNAs occurs early with ER stress
The data indicate a marked drop off of snRNAs between B cells and cells treated with LPS for 72 hrs. We did a time course of expression of the U RNAs and saw that the drop off begins within 18 hrs of LPS treatment (Figure 6A) before ELL2 is induced which occurs around 24–36 hrs. We also examined the relative loss of the small RNAs in blimp1 (Prdm1) knockout mice and saw a reduction similar to that seen in blimp1+ mice (data not shown). Thus the changes start early and seem to be Prdm1/ blimp1 independent.
Figure 6.

Loss of snRNA occurs early with ER stress (a) Quantitative PCR was performed on RNA isolated from ELL2+/+ B cells (0 hour), cells treated for 18 hour +LPS with or without 4u8C, an inhibitor of Ire1 phosphorylation and the RIDD activity. Non-canonical Xbp1 splicing to the short form (Xbp1-S) as indicated. Three independent biological replicates were assay in triplicate.(b) Western blot of protein levels from B cells or cells treated for 18 hours plus LPS with or without 4u8C. SnapC1 mRNA encodes a 43 kDa protein. Xbp1 long and short forms are indicated. Actin was used as a loading control. Representative of three independent biological replicates.
It seemed possible that the decrease in snRNA levels could be attributed to decreases in the SnapC mRNAs that we saw, the ER stress induced by LPS treatment, and/ or the Ire1 linked RIDD pathway (20). The phosphorylation of Ire1 is apparent by 18 and 24 hours post LPS treatment and also at 72 hours, see Figures 5D and6B, and as expected, Xbp1 expression and the short form of Xbp1 increases with time after LPS. Meanwhile SnapC1 (Snap43), protein is decreased by 18 hours like its mRNA.
To determine if the decrease in SnapC1 & 2 might be correlated with the RIDD activity of Ire1, we cultured primary splenic B cells stimulated for 18hrs with LPS plus and minus the RIDD inhibitor, 4μ8c (21). With 18hrs of LPS the splicing of Xbp1 to create the short form of the protein is increased, indicating that RIDD is active; but in LPS plus the inhibitor 4u8C there is little to no Xbp1 splicing (Figure 6). The analysis of the Ire1 protein showed that its auto phosphorylation was occurring without 4u8C but not in its presence Figure 6B. Meanwhile at 18 hours, with LPS and no inhibitor, the SnapC1 mRNA is decreased while in the presence of the drug there is a significant increase in its level. Likewise in LPS and no 4u8c the U1 and U11 snRNA levels are slightly decreased at 18 hrs while the addition of the RIDD inhibitor dramatically increases their amount (Figure 6A). Altering the snRNA content in the cell could cause changes in expression of other regulators like U1A, HnrnpLL and Ints10 and could thus contribute to the altered splicing patterns in ASCs.
ER stress influences splicing patterns
Next we sought to determine if the ER stress response was accompanied by alterations in the B cell splicing patterns at 18 hours post LPS. The Affymetrix splicing arrays were used to analyze splenic B cell samples at time zero and 18 hours after LPS stimulation, plus and minus 4u8C, the drug shown above to influence the RIDD activity of Ire1-P (21). We compiled the differential splicing events and report them in Figure 7A and on the NCBI GEO web site. Blocking the RIDD function of Ire1-P by 4u8C was correlated with significantly reduced overall number of splicing events in mRNA in all categories. Mutually exclusive exon splicing, in particular, was reduced by 3-fold. The number of genes that were alternatively spliced was reduced from 11,144 without inhibitor to 7,248 with the 4u8C inhibitor.
Figure 7.

ER stress influences splicing patterns (a) TAC software generated classifiers for differentially spliced events, broken down by number of genes and number of exons. Two-way comparison of each of the three 4u8c conditions (0hr, 18hr+4u8c, and 18hr-4u8c). Results determined with three independent biological replicates for the array data, see methods.(b) Overlap between alternatively spliced genes in the ELL2 independent genes (genes differentially spliced in LPS stimulated ELL2−/− versus ELL2+/+) and + and – 4u8c at 18 hours after stimulation.(c) Tally of pathway analyses of alternatively spliced genes mined from the 18hr + LPS-4u8c dataset (significance p-value cutoff at p=1E-9) and the 18hr+LPS+4u8c dataset (significance p-value cutoff at p=1E-5).
When we cross referenced the 4,211 genes that are alternatively spliced at 72 hrs of LPS regardless of ELL2, the “ELL2 independent genes”, with the 0–18 hour family of alternatively spliced genes, we see that many of the ELL2 independent genes (~90%, 3783/4211) are included in the 0–18 hour group (Figure 7B). Thus they change early and before ELL2 is induced as we predicted. We conclude that many splicing changes occur before ELL2 is induced, but while RIDD is activated and U snRNA levels are falling; some of these alternative splicing events persist until the 72hr plus LPS stage, others do not.
As shown in the pathway analyses (Figure 7C), RNAs in 15 cell cycle pathways are altered in 18 hours after LPS with immune signaling and metabolism represented by 9 and 6 pathways respectively. There are several pathways in the alternative splicing and mRNA processing pathways induced early after LPS. Addition of 4u8C and LPS modifies not only the number of genes alternatively spliced but also the pathways in which those genes map. Thus we conclude that the Ire1-P pathway and RIDD have profound effects on the differentiation of the B cell into an ASC.
Discussion
We have shown that large changes in mRNA splicing occur in the B to ASC transition. In Figure 8, we summarized the many factors that may contribute to the large number of changes in splicing seen including ELL2 up-regulation and ER stress. While genes that get induced to higher levels in one stage or another have been the focus of many studies, it is important to realize that some genes have mRNAs that do not increase in abundance and yet they can show alternative splicing. Many of these changes may be missed in flow cytometry and standard western blotting using antibodies that recognize only one exon. The genome project found that most multi-exon genes can produce 4 to 5 different splicing products. Not all of these changes result in different proteins, yet many do. In our array studies we have seen examples of such genes that have been documented in the literature to result in proteins with different amino or carboxyl termini, altered cellular location, or altered translation based on inclusion of alternative exons. We see splicing changes in exons where the differences are 3-fold or more in Hadc9, Ptbp3, Myd88, and not illustrated: Traf7, Zbtb20, DNAjb6 (hsp40), IL-2 receptor gamma chain, Ppp2r5c, Srsf1, Ikzf1, Cmip (c-maf), Elmo, Ugp2, Picalm, Tmem209, Hspa4 (hsp70), Mapk1, Xiap, Eifak3, Cstf3, and Srpk2 listed in order of descending splicing indexes. These genes fall in many pathways and it will be important for future research to determine the relevance of these changes in gene expression to the development of antibody secreting cells.
Figure 8.

Summary.Data presented in this work are summarized in a time line depicting alternative splicing at 18 and 72 hrs post LPS treatment and the potential influences on it. The exon splicing patterns are figuratively meant to show increasing complexity with time. We hypothesize that SnapC and snRNA decreases may be linked to the RIDD activity induced by ER stress although this is speculative at this point.
We show here that ELL2 has a widespread influence on gene expression beyond the Igh gene. We found changes in the splicing of many genes both between B cells and ASCs in ELL2 sufficient mice and between cells that are ELL2 sufficient versus deficient especially pronounced in the mutually exclusive exon category. ELL2 induction causes increased expression of most of the ASC signature genes, Irf4, Prdm1, Pou2af2, and Xbp1 mRNAs, all previously shown to influence gene expression to ASCs many of which bind ELL2 in our ChIP-seq studies. But many more genes in toto (5,115) undergo alternative splicing without changes in abundance when ELL2 is induced and the number of splicing changes per gene are more numerous with ELL2 present. When we performed an overlap analysis as shown in the Venn diagram (Figure 3 C) and see that in about 55% of the genes, splicing events are dependent on the presence of ELL2.
Of the 1,039 genes with increased mRNA levels after the transition from B cells to ASCs at 72hrs after LPS in ELL2 sufficient cells, 188 were V regions genes. Out of the remaining 851 non-V region genes, 635 showed changes in splicing both between B cells and ASC in ELL2+/+ mice and between ELL2+/+ and ELL2 cKO mice (Supplemental Table 2).Only some of those were on the list of 2,000 strong ChIP targets for ELL2 [see NCBI GEO list](35).Therefore, we conclude that some mRNAs are influenced straight-forwardly by ELL2 binding to their gene. Some are induced in the presence of ELL2 either indirectly or fall below the strict cut off of 10 tags per gene that we imposed.
We did a pathway analysis on the 343 genes that are induced by 72 hrs of LPS treatment when ELL2 is present and that are also differentially spliced between B cells and the ASCs. These genes are primarily associated with the cell cycle, N-glycan synthesis and processing, immune signaling, Golgi associated, and ER protein processing genes. A number of the genes in which ELL2 deficiency influences splicing show extended 5’ untranslated regions. This phenomena of extended 5’UTRs was observed in meiotic differentiation in budding yeast where these RNAs were poorly translated (30). This is yet another way in which mRNA sequence could influence the resulting protein products aside from overt changes in coding regions by alternative exon use.
Genome-wide association studies of altered IgG N-glycosylation phenotypes uncovered ELL2 as a significant gene for IgG glycosylation in humans (39) consistent with the results we found here. ELL2 is lacking in certain kinds of non-secreting multiple myelomas (40) and an analysis of 505 patients with an ELL2 deficiency revealed decreased expression of mRNAs for Bip, Atf6 in the unfolded protein response pathway, decreased Pou2af1 and ELL1 (41), all of which we see here in the ELL2 altered pathways, either directly or indirectly. Pou2af1 is especially interesting since the gene is found associated with ELL2 by ChIP seq and it encodes a protein that binds to Oct1 and Oct2 to stimulate transcription of genes that have the octamer sequence associated (42).
At 18 hrs after LPS stimulation many genes are being alternatively spliced relative to the B cells at zero hours, more than the number seen at 72 hours when the ASCs are focused on the task of Ig production and export. Many of these 18 hour genes are involved in cell division and immune signaling pathways. Ninety percent of the genes that are alternatively spliced independently of ELL2 in the 72 hr plus LPS samples overlap with 0–18 hour alternatively spliced genes. Since ELL2 is not significantly upregulated until more than 24 hours post LPS this is understandable.
The number of genes scored as alternatively spliced 0 hour versus 18 hours after LPS represents a combination of newly synthesized mRNAs made after LPS, loss of some 0 hour RNAs by the RIDD process, and persistence of the previously made, un-degraded, B cell mRNAs. When the ER phosphorylation of Ire1 is blocked by 4u8C, the number of genes displaying alternative splicing events in all categories relative to B cells is decreased. The RNAs in 0 versus 18 hour + 4u8C show fewer cell cycle genes, but an increase in immune system signaling and intracellular signaling pathways. Non-canonical splicing of Xbp1 to the short form is inhibited in 4u8C. Since it is the short form of Xbp1 that encodes a transcription factor causing induction of the unfolded protein response genes, these genes would not be turned on in the presence of 4u8C and thus they would be under-represented. The levels of the snRNAs rise in 4u8C which could be expected to perturb splicing patterns. Comparing genes that are expressed both plus and minus 4u8C, >4,990 cassette exon splicing changes and changes in all the other categories occur. Ire1 phosphorylation appears to have a large effect on alternative splicing patterns, some of it perhaps through changes in the levels of the RNAs in the snRNA pathway.
RIDD appears to require both Ire1-P activity and ER stress (43). Under severe stress, Ire1 mediated RNA decay can even promote apoptosis (44). Through RIDD, selective mRNA decay is able to alleviate some of the stress on the ER and presumably make space for the massive upregulation of UPR proteins and their mRNAs (43); this may then contribute to the diversity of the splicing pattern of the RNAs that survive at 18 hours since RIDD inhibition reduces diversity of alternatively spliced products.
We have shown that changing the splicing pattern of RNAs in the B to ASC transition is a complex process that depends on induction of ELL2 and may require Ire1-phosphorylation and RIDD. ELL2 may act with either direct binding to genes or indirect effects on their expression. Clearly more work needs to be done to understand the interplay of all of these factors and the effect of the changing levels of the snRNAs which may contribute.
Supplementary Material
Acknowledgments
Grant support:
This work was supported by AI124241 from the National Institutes of Health NIAID to C.M. and a shared resources grant P30CA047904 to the University of Pittsburgh Cancer Institute.
Abbreviations used in this article:
- ASC
antibody secreting cell
- BC
B cell
- cKO
conditional knockout
- ELL2
eleven nineteen lysine-rich leukemia gene
- ER
endoplasmic reticulum
- LPS
lipopolysaccharide
- QPCR
quantitative PCR
- RIDD
regulated Ire1 dependent mRNA decay pathway
- RNAPII
RNA polymerase II
- snRNA
small nuclear RNAs involved in splicing
- UPR
unfolded protein response
- wt
wild type.
References:
- 1.Johnson JM, Castle J, Garrett-Engele P, Kan Z, Loerch PM, Armour CD, Santos R, Schadt EE, Stoughton R, and Shoemaker DD. 2003. Genome-wide survey of human alternative pre-mRNA splicing with exon junction microarrays. Science 302: 2141–2144. [DOI] [PubMed] [Google Scholar]
- 2.Rozen S, Tieri A, Ridner G, Stark A-K, Schmaler T, Ben-Nissan G, Dubiel W, and Sharon M. 2013. Exposing the subunit diversity within protein complexes: A mass spectrometry approach. Methods 59: 270–277. [DOI] [PubMed] [Google Scholar]
- 3.Shi W, Liao Y, Willis SN, Taubenheim N, Inouye M, Tarlinton DM, Smyth GK, Hodgkin PD, Nutt SL, and Corcoran LM. 2015. Transcriptional profiling of mouse B cell terminal differentiation defines a signature for antibody-secreting plasma cells. Nat Immunol 16: 663–673. [DOI] [PubMed] [Google Scholar]
- 4.Bayles I, and Milcarek C. 2014. Plasma cell formation, secretion, and persistence: the short and the long of it. Critical reviews in immunology 34: 481–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peterson ML 2007. Mechanisms controlling production of membrane and secreted immunoglobulin during B cell development. Immunol Res 37: 33–48. [DOI] [PubMed] [Google Scholar]
- 6.Ma J, Gunderson SI, and Phillips C. 2006. Non-snRNP U1A levels decrease during mammalian B-cell differentiation and release the IgM secretory poly(A) site from repression. RNA 12: 122–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shell SA, Martincic K, Tran J, and Milcarek C. 2007. Increased phosphorylation of the carboxyl-terminal domain of RNA polymerase II and loading of polyadenylation and cotranscriptional factors contribute to regulation of the Ig heavy chain mRNA in plasma cells. J Immunol 179: 7663–7673. [DOI] [PubMed] [Google Scholar]
- 8.Milcarek C, Albring M, Langer C, and Park KS. 2011. The Eleven-Nineteen Lysine-rich Leukemia gene (ELL2) influences the histone H3 modifications accompanying the shift to secretory Immunoglobulin heavy chain mRNA production. J. Biol. Chem. 286: 33795–33803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park KS, Bayles I, Szlachta-McGinn A, Paul J, Boiko J, Santos P, Liu J, Wang Z, Borghesi L, and Milcarek C. 2014. Transcription elongation factor ELL2 drives immunoglobulin secretory specific mRNA production and the unfolded protein response. J. Immunol. 193: 4663–4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martincic K, Alkan SA, Cheatle A, Borghesi L, and Milcarek C. 2009. Transcription elongation factor ELL2 directs immunoglobulin secretion in plasma cells by stimulating altered RNA processing. Nat Immunol 10: 1102–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson AM, and Milcarek C. 2017. Transcription elongation factor ELL2 in antibody secreting cells, myeloma, and HIV infection: a full measure of activity. Current Trends in Immunology 18: 1–11. [Google Scholar]
- 12.Milcarek C, Martincic K, Chung-Ganster L-H, and Lutz CS. 2003. The snRNP-associated U1A levels change following IL-6 stimulation of human B-cells. Mol Immunol 39: 809–814. [DOI] [PubMed] [Google Scholar]
- 13.Ray R, Ray K, and Panda CK. 1997. Differential alterations in metabolic pattern of the six major U snRNAs during development. Mol. Cell Biochem 177: 79–88. [DOI] [PubMed] [Google Scholar]
- 14.Cheng Z, Du Z, Shang Y, Zhang Y, and Zhang T. 2017. A preliminary study: PS1 increases U1 snRNA expression associated with AD. J. Mol. Neuroscience: 269–275. [DOI] [PubMed] [Google Scholar]
- 15.Singh RK, and Cooper TA. 2012. Pre-mRNA splicing in disease and therapeutics. Trends in Molecular Medicine 18: 472–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aragon IV, Barrington RA, Jackowski S, Mori K, and Brewer JW. 2012. The specialized unfolded protein response of B lymphocytes: ATF6α-independent development of antibody-secreting B cells. Mol. Immunol. 51: 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aronov M, and Tirosh B. 2016. Metabolic control of plasma cell differentiation- what we know and what we don’t know. J. Clinical Immunol. 36: 12–17. [DOI] [PubMed] [Google Scholar]
- 18.Kumazaki K, Tirosh B, Maehr R, Boes M, Honjo T, and Ploegh HL. 2007. AID−/−μs−/− mice are agammaglobulinemic and fail to maintain B220−CD138+ plasma cells. J. Immunol. 178: 2192–2203. [DOI] [PubMed] [Google Scholar]
- 19.Tirasophon W, Lee K, Callaghan B, Welihinda A, and Kaufman RJ. 2000. The endoribonuclease activity of mammalian IRE1 autoregulates its mRNA and is required for the unfolded protein response. Genes & development 14: 2725–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.So J-S, Hur KY, Tarrio M, Ruda V, Frank-Kamenetsky M, Fitzgerald K, Koteliansky V, Lichtman AH, Iwawaki T, Glimcher LH, and Lee A-H. 2012. Silencing of lipid metabolism genes through IRE1α-mediated mRNA decay lowers plasma lipids in mice. Cell Metabolism 16: 487–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cross BCS, Bond PJ, Sadowski PG, Jha BK, Zak J, Goodman JM, Silverman RH, Neubert TA, Baxendale IR, Ron D, and Harding HP. 2012. The molecular basis for selective inhibition of unconventional mRNA splicing by an IRE1-binding small molecule. Proc. Nat. Acad. Sci. USA 109: E869–E878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Genovese C, and Milcarek C. 1990. Increased half-life of mu Ig mRNA during mouse B-cell development increases abundancy. Mol Immunol 17: 69–81. [DOI] [PubMed] [Google Scholar]
- 23.Genovese C, Harrold S, and Milcarek C. 1991. Differential mRNA stabilities affect mRNA levels in mutant mouse myeloma cells. Som Cell & Mol Genet 17: 69–81. [DOI] [PubMed] [Google Scholar]
- 24.Petrie K, Guidez F, Howell L, Healy L, Waxman S, Greaves M, and Zelent A. 2003. The Histone Deacetylase 9 Gene Encodes Multiple Protein Isoforms. J. Biol. Chem. 278: 16059–16072. [DOI] [PubMed] [Google Scholar]
- 25.Tan L-Y, Whitfield P, Llorian M, Monzon-Casanova E, Diaz-Munoz MD, Turner M, and Smith CWJ. 2015. Generation of functionally distinct isoforms of PTBP3 by alternative splicing and translation initiation. Nucleic Acids Research 43: 5586–5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Arras L, and Alper S. 2013. Limiting of the innate immune response by SF3A-dependent control of MyD88 alternative mRNA splicing. PLoS Genetics 9: e1003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janssens S, Burns K, Tschopp J, and Beyaert R. 2002. Regulation of Interleukin-1- and Lipopolysaccharide-Induced NF-κB Activation by Alternative Splicing of MyD88. Current Biology 12: 467–471. [DOI] [PubMed] [Google Scholar]
- 28.de la Mata M, Alonso CR, Kadener S, Fededa JP, Blaustein M, Pelisch JP, Cramer P, Bentley D, and Kornblihtt AR. 2003. A slow RNA polymerase II affects alternative splicing in vivo. Mol. Cell 12: 525–532. [DOI] [PubMed] [Google Scholar]
- 29.Martincic K, Campbell R, Edwalds-Gilbert G, Souan L, Lotze M, and Milcarek C. 1998. Increase in the 64-kDa subunit of the polyadenylation/cleavage stimulatory factor during the Go to S phase transition. Proc Natl Acad Sci USA 95: 11095–11100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng Z, Otto GM, Powers EN, Keskin A, Mertins P, Carr SA, Jovanovic M, and Brar GA. 2018. Pervasive, coordinated protein-level changes driven by transcript isoform switching during meiosis. Cell 172: 910–923.e916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith E, Lin C, and Shilatifard A. 2011. The super elongation complex (SEC) and MLL in development and disease. Genes Dev 25: 661–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Listerman I, Bledau AS, G. I., and Neugebauer KM. 2007. Extragenic accumulation of RNA Polymerase II enhances transcription by RNA Polymerase III. PLoS Genet 3: e212 doi:210.1371/journal.pgen.0030212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Underhill GH, George D, Bremer EG, and Kansas GS. 2003. Gene expression profiling reveals a highly specialized genetic program of plasma cells. Blood 101: 4013–4021. [DOI] [PubMed] [Google Scholar]
- 34.Underhill GH, Kolli KP, and Kansas GS. 2003. Complexity within the plasma cell compartment of mice deficient in both E- and P-selectin: implications for plasma cell differentiation. Blood 102: 3867–3873. [DOI] [PubMed] [Google Scholar]
- 35.Baumgartner M, Lemoine C, Al Seesi S, Karunakaran DKP, Sturrock N, Banday AR, Kilcollins AM, Mandoiu I, and Kanadia RN. 2014. Minor splicing snRNAs are enriched in the developing mouse CNS and are crucial for survival of differentiating retinal neurons. Dev Neurobiology 75: 895–907. [DOI] [PubMed] [Google Scholar]
- 36.Murphy S, Yoon JB, Gerster T, and Roeder RG. 1992. Oct-1 and Oct-2 potentiate functional interactions of a transcription factor with the proximal sequence element of small nuclear RNA genes. Molecular and Cellular Biology 12: 3247–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henry RW, Mittal V, Ma B, Kobayashi R, and Hernandez N. 1998. SNAP19 mediates the assembly of a functional core promoter complex (SNAP(c)) shared by RNA polymerases II and III. Genes Dev 12: 2664–2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith Edwin R., Lin C, Garrett Alexander S., Thornton J, Mohaghegh N, Hu D, Jackson J, Saraf A, Swanson Selene K., Seidel C, Florens L, Washburn Michael P., Eissenberg Joel C., and Shilatifard A. 2011. The little elongation complex regulates small nuclear RNA transcription. Molecular Cell 44: 954–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen X, Klarić L, Sharapov S, Mangino M, Ning Z, Wu D, Trbojević-Akmačić I, Pučić-Baković M, Rudan I, Polašek O, Hayward C, Spector TD, Wilson JF, Lauc G, and Aulchenko YS. 2017. Multivariate discovery and replication of five novel loci associated with Immunoglobulin G N-glycosylation. Nat. Commun. 8: 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swaminathan B, Thorleifsson G, Jöud M, Ali M, Johnsson E, Ajore R, Sulem P, Halvarsson B-M, Eyjolfsson G, Haraldsdottir V, Hultman C, Ingelsson E, Kristinsson SY, Kähler AK, Lenhoff S, Masson G, Mellqvist U-H, Månsson R, Nelander S, Olafsson I, Sigurðardottir O, Steingrimsdóttir H, Vangsted A, Vogel U, Waage A, Nahi H, Gudbjartsson DF, Rafnar T, Turesson I, Gullberg U, Stefánsson K, Hansson M, Thorsteinsdóttir U, and Nilsson B. 2015. Variants in ELL2 influencing immunoglobulin levels associate with multiple myeloma. Nat. Commun. 6: 7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li N, Johnson DC, Weinhold N, Kimber S, Dobbins SE, Mitchell JS, Kinnersley B, Sud A, Law PJ, Orlando G, Scales M, Wardell CP, Försti A, Hoang PH, Went M, Holroyd A, Hariri F, Pastinen T, Meissner T, Goldschmidt H, Hemminki K, Morgan GJ, Kaiser M, and Houlston RS. 2017. Genetic predisposition to multiple myeloma at 5q15 is mediated by an ELL2 enhancer polymorphism. Cell Reports 20: 2556–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greiner A, Müller KB, Hess J, Pfeffer K, Müller-Hermelink HK, and Wirth T. 2000. Up-regulation of BOB.1/OBF.1 expression in normal germinal center B cells and germinal center-derived lymphomas. American J. Pathology 156: 501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hollien J, Lin JH, Li H, Stevens N, Walter P, and Weissman JS. 2009. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. The Journal of Cell Biology 186: 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han D, Lerner AG, Vande Walle L, Upton JP, Xu W, Hagen A, Backes BJ, Oakes SA, and Papa FR. 2009. IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell 138: 562–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


